Introduction
Malignant mesothelioma is a highly aggressive tumor
with poor prognosis. It arises from mesothelial cells lining the
serous cavities (pleura, pericardium, peritoneum and tunica
vaginalis). The incidence of mesothelioma is increasing worldwide
due to previous occupational and/or environmental exposure to
asbestos (1,2). The incidence of malignant
mesothelioma in Japan is predicted to reach a peak between 2030 and
2034. In developing countries, the incidence of this disease is
predicted to increase due to the continued use of asbestos
(3,4). Currently available treatments have a
limited effect on malignant mesothelioma management (5). Therefore, there is a need to identify
feasible and effective therapeutic targets.
Non-coding RNAs are RNA molecules that are
transcribed from the genome but do not encode proteins. They have
been revealed to play structural and functional roles within the
cell (6–10). They are primarily grouped into two
classes based on transcript size: Small non-coding RNAs and long
non-coding RNAs (lncRNAs) (11).
Small non-coding RNAs include microRNAs (miRNAs) that function as
major regulators of gene expression and complex components of
cellular gene expression networks. In contrast to miRNAs, lncRNAs
are a class of RNA transcripts that are over 200 nucleotides in
length (12). lncRNAs have been
associated with various biological processes, including
epigenetics, alternative splicing, and nuclear import;
additionally, they function as precursors of small non-coding RNAs,
and regulators of mRNA decay (13–15).
Dysregulated lncRNA expression has been reported in numerous
cancers, suggesting that lncRNAs are a newly emerging class of
oncogenic and tumor-suppressor genes (16).
Plasmacytoma variant translocation 1 (PVT1)
is an oncogenic lncRNA located at chromosomal region 8q24 (17). The carcinogenicity of PVT1
has been identified in various human cancers, including non-small
cell lung (18), leukemia
(19), hepatocellular (20), colon (21), breast (22), and ovarian cancer (23). Non-coding RNA expression data from
Human Transcriptome 2.0 GeneChip Array analysis performed in our
previous study revealed increased PVT1 expression in
epithelioid mesothelioma and lung adenocarcinoma (24). In the present study, the biological
function of PVT1 in mesothelioma was elucidated.
Materials and methods
PVT1 expression database
Affymetrix mRNA expression subset data were obtained
from the Cancer Cell Line Encyclopedia (CCLE) website (data created
from https://www.broadinstitute.org/ccle/ on December 7,
2019). The CCLE project dataset is a compilation of gene expression
data from human cancer cell lines (25).
Mesothelioma cell lines
ACC-MESO-1 (Expasy ID: CVCL_5113) and ACC-MESO-4
(Expasy ID: CVCL_5114) mesothelioma cell lines were purchased from
RIKEN BioResource Research Center (Tsukuba, Japan), and NCI-H2052
(CRL-5915) and NCI-H2452 (CRL-5946) mesothelioma cell lines were
purchased from the American Type Culture Collection (ATCC). In
addition, two lung adenocarcinoma cell lines, A549 and PC9,
purchased from the European Collection of Authenticated Cell
Cultures, were also used to confirm PVT1 expression in lung
adenocarcinoma. Cells were cultured in Roswell Park Memorial
Institute-1640 (RPMI-1640) medium supplemented with 1% kanamycin,
1% amphotericin B, and 10% fetal bovine serum (FBS; all from Thermo
Fisher Scientific, Inc.). Cells were maintained in culture dishes
at 37°C in a humidified incubator supplied with 5%
CO2.
Transfection of mesothelioma
cells
PVT1 small interfering (si)RNA (Lincode Human
PVT1 siRNA - SMARTpool; cat. no. R-029357-00-0005) and its
negative control (NC) siRNA (Lincode Non-targeting Pool; cat. no.
D-001320-10-05) were purchased from GE Healthcare Dharmacon, Inc.
Forkhead box M1 (FOXM1) siRNA (FOXM1 Silencer Select
Pre-designed siRNA; cat. no. 4427037 ID# s5248) and its NC siRNA
(Silencer Select Negative Control No. siRNA; cat. no. 4390843) were
purchased from Thermo Fisher Scientific, Inc. Cells cultured until
attaining 70–80% confluency, were transfected with 50 nM of
PVT1/NC siRNA, 25 nM of FOXM1/NC siRNA, or both,
using Lipofectamine RNAiMAX (Thermo Fisher Scientific, Inc.) in
Opti-Mem Reduced Serum Medium (Thermo Fisher Scientific, Inc.) at
37°C in a humidified incubator supplied with 5% CO2
according to the manufacturer's recommended protocols. The images
of morphological change of the transfected mesothelioma cells were
captured at 0 and 72 h using a CKX53 inverted light microscope with
a DP21 digital camera (Olympus Corporation).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Mesothelioma cell lines (3×105 cells)
were transfected with 15 pmol of PVT1/NC siRNA or 7.5 pmol
of FOXM1/NC siRNA in 6-well plates at 37°C in a humidified
incubator supplied with 5% CO2 for 72 h. RNA was
extracted from the cells using Maxwell® RSC simplyRNA
Cells Kit and Maxwell® RSC Instrument (both from Promega
Corporation) according to the manufacturer's protocols. The
extracted RNA was reverse-transcribed with SuperScript IV VILO
Master Mix (Thermo Fisher Scientific, Inc.) and amplified using
Power Up SYBR Green Master Mix (Thermo Fisher Scientific, Inc.) on
an AriaMx Real-Time PCR System (Agilent Technologies, Inc.)
according to the manufacturer's recommended protocols. In brief,
qPCR was performed with initial denaturation at 95°C for 10 min
followed by 40 cycles of denaturation at 95°C for 15 sec and
annealing and elongation at 60°C for 1 min, and a dissociation
curve condition from 95°C to 60°C. Relative expression levels were
calculated using the comparative 2-ΔΔCq method (26). Expression levels were normalized
against those of glyceraldehyde 3-phosphate dehydrogenase (GAPDH).
The primer sequences used for RT-qPCR were as follows: PVT1
forward, 5′-TGAGAACTGTCCTTACGTGACC-3′ and reverse,
5′-AGAGCACCAAGACTGGCTCT-3′; FOXM1 forward,
5′-GGAGCAGCGACAGGTTAAGG-3′ and reverse,
5′-GTTGATGGCGAATTGTATCATGG-3′; and GAPDH forward,
5′-ACAACTTTGGTATCGTGGAAGG-3′ and reverse,
5′-GCCATCACGCCACAGTTTC-3′.
Cell proliferation assay
Mesothelioma cell lines (3×103 cells)
were incubated with 1 pmol PVT1/NC siRNA or 0.5 pmol
FOXM1/NC siRNA in 96-well plates at 37°C in a humidified
incubator supplied with 5% CO2 for 3 days. The
proliferation rate was determined at 24, 48 and 72 h using 100 µl
of 2X Cell Titer-Glo 2.0 reagent (Promega Corporation), which
assesses the number of viable cells relative to the ATP level, with
a GloMax Explorer microplate reader (Promega Corporation) according
to the manufacturer's protocols.
Cell cycle assay
Mesothelioma cell lines (1×105 cells)
were transfected with 5 pmol PVT1/NC siRNA in 24-well plates
for 3 days, and subsequently the cells were collected after
trypsinization and fixed in 70% ethanol in 15-ml centrifuge tubes
at room temperature for ~3 h. After ethanol removal, the cells were
stained with 200 ml of Guava Cell Cycle Reagent (Luminex
Corporation) at room temperature shielding away from the light for
30 min. The reagent containing propidium iodide discriminates the
cells at different stages of the cell cycle by labeling cellular
DNA. The labeling signal intensity was evaluated using a Guava
EasyCyte Mini flow cytometer (Guava Technologies) according to the
manufacturer's protocols. Analysis of raw data was performed with
FCS express 5.0 (De Novo Software).
Wound healing assay
The migration ability of all four mesothelioma cells
was analyzed using a wound scratch assay. Serum starved
mesothelioma cell lines grown to 80% confluence were incubated
overnight with 5 pmol PVT1/NC siRNA in collagen-coated
24-well plates at 37°C in a humidified incubator supplied with 5%
CO2. Wounds were created by scratching the cells with
1-ml micropipette tips. The wells were washed twice to remove
floating cells. Images of the gap area (wound) were captured every
24 h (for ACC-MESO-1 every 12 h) using a CKX53 inverted light
microscope equipped with a DP21 digital camera (Olympus
Corporation), and the gap area was further analyzed using T Scratch
software version 1.0 downloaded from https://github.com/cselab/TScratch (27).
Cell invasion assay
BD FluroBlok culture inserts containing 8-μm pores
(BD Biosciences) were coated with 100 µl of 10X diluted Geltrex
Matrigel (Thermo Fisher Scientific, Inc.) at 37°C in a humidified
incubator supplied with 5% CO2 for 3 h. Mesothelioma
cell lines (3×104 ACC-MESO-1 cells, and 5×104
ACC-MESO-4, CRL-5915, CRL-5946 cells) were incubated with 3 pmol
siRNA in 500 µl RPMI-1640 medium (without FBS) in the upper chamber
of culture inserts and 750 µl RPMI-1640 medium containing 5% FBS in
the lower chamber of culture inserts according to the
manufacturer's protocols. Cells were incubated at 37°C in a
humidified incubator supplied with 5% CO2 for 72 h (48 h
for ACC-MESO-1 cells), and invading cells were stained with
addition of 50 µl of 1 µg/ml solution of Hoechst 33324 (Thermo
Fisher Scientific, Inc.) at room temperature for 10 min, and
subsequently the imaged area of the insert membrane was visualized
using a fluorescence microscope. The total number of invading cells
was analyzed using the CellProfiler cell imaging software version
2.1.0 downloaded from https://cellprofiler.org (28).
Western blot analysis
Mesothelioma cell lines (3×105 cells)
were transfected with 15 pmol PVT1/NC siRNA in 6-well plates
for 72 h. Cell lysates were obtained from the cells using RIPA
Lysis Buffer System (Santa Cruz Biotechnology, Inc.), and total
protein was determined with Qubit™ Protein Assay Kit
using a Qubit Fluorometer (Thermo Fisher Scientific, Inc.). Total
proteins (20 µg) were electrophoresed on a 10% sodium dodecyl
sulfate-polyacrylamide gel (SureCast Acrylamide Gel; Thermo Fisher
Scientific, Inc.) at 200 V for 40 min and transferred onto
polyvinylidene difluoride (PVDF) membranes using a Mini Blot Module
(Thermo Fisher Scientific, Inc.) at 20 V for 60 min. Following
blocking with 2% bovine serum albumin (Sigma Aldrich; Merck KGaA)
in 1X TBS with 0.05% Tween-20 at room temperature for 1 h, the
membranes were incubated overnight at 4°C with primary antibodies
[anti-FOXM1 rabbit monoclonal antibody (1:4,000; product no.
20459S) and an anti-GAPDH rabbit monoclonal antibody (1:4,000;
product no. 2118S; both from Cell Signaling Technology, Inc.)]. The
membranes were then incubated with the anti-rabbit IgG, HRP-linked
secondary antibody (1:4,000; cat. no. 7074P2; Cell Signaling
Technology, Inc.) at room temperature for 40 min. The membranes
were stained with ImmunoStar LD (Wako Pure Chemical Industries) at
room temperature for 1 min and images were captured using a c-Digit
Blot Scanner (LI-COR). Scanned images were analyzed by Image Studio
Digits software version 5.2 (LI-COR Biosciences).
Statistical analysis
The experiments were performed at least three times
in triplicate. Experimental data are expressed as the mean ±
standard deviation. The statistical significance of the difference
between two groups was analyzed using unpaired Student's t-test
with the default function of Microsoft Excel version 16.53.
P<0.05 was considered to indicate a statistically significant
difference.
Results
PVT1 expression in mesothelioma and
lung adenocarcinoma cell lines
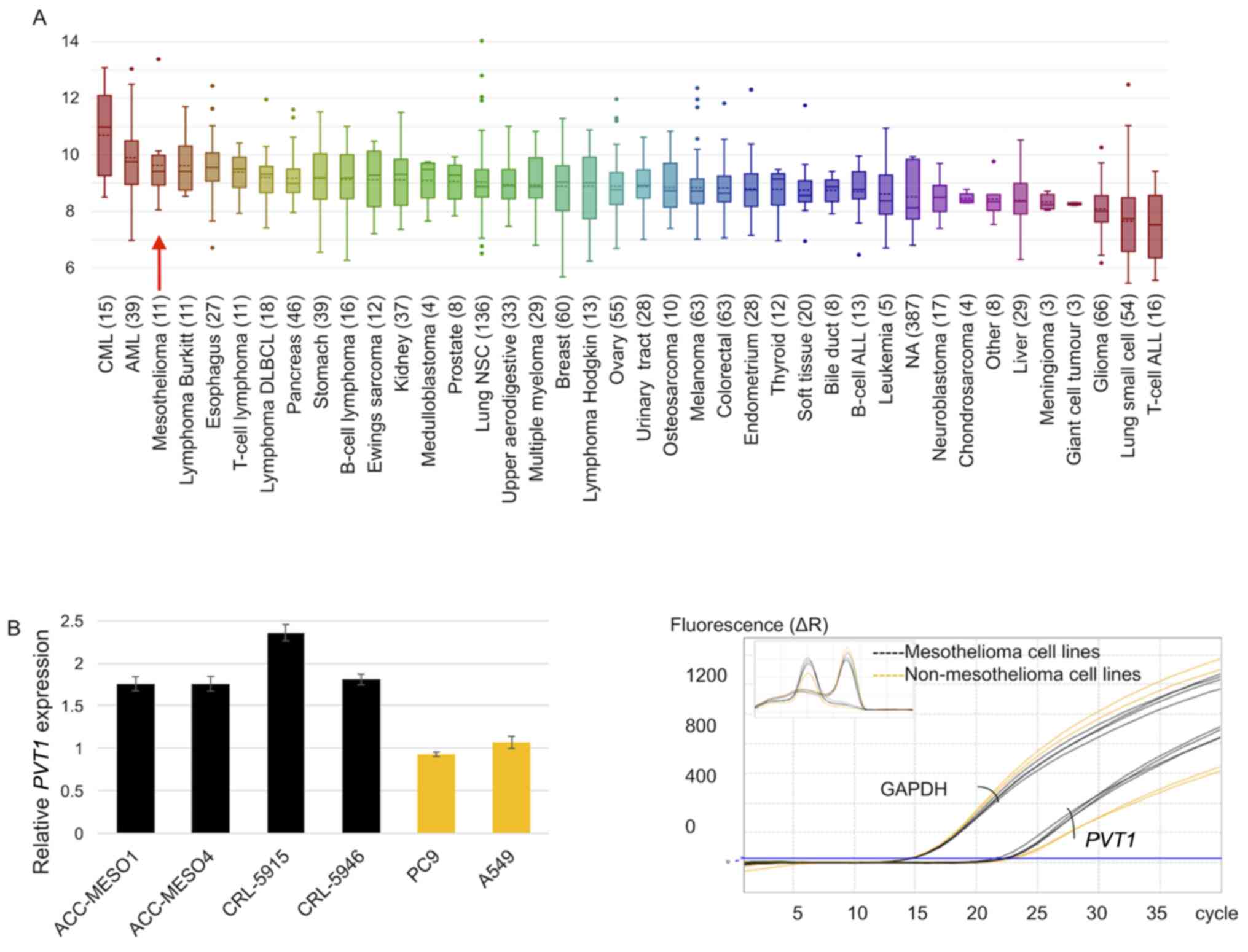
PVT1 expression was high in mesothelioma and
non-small cell cancers, in addition to different human cancers
(Fig. 1A). RT-qPCR analysis
results revealed that PVT1 was expressed in all four
mesothelioma cell lines and two lung adenocarcinoma cell lines.
Compared with the average PVT1 expression in the two lung
adenocarcinoma cell lines, PVT1 expression was increased by
1.8-, 1.8-, 2.4-, and 1.8-fold in ACC-MESO-1, ACC-MESO-4, CRL-5915
and CRL-5946 cell lines, respectively (Fig. 1B).
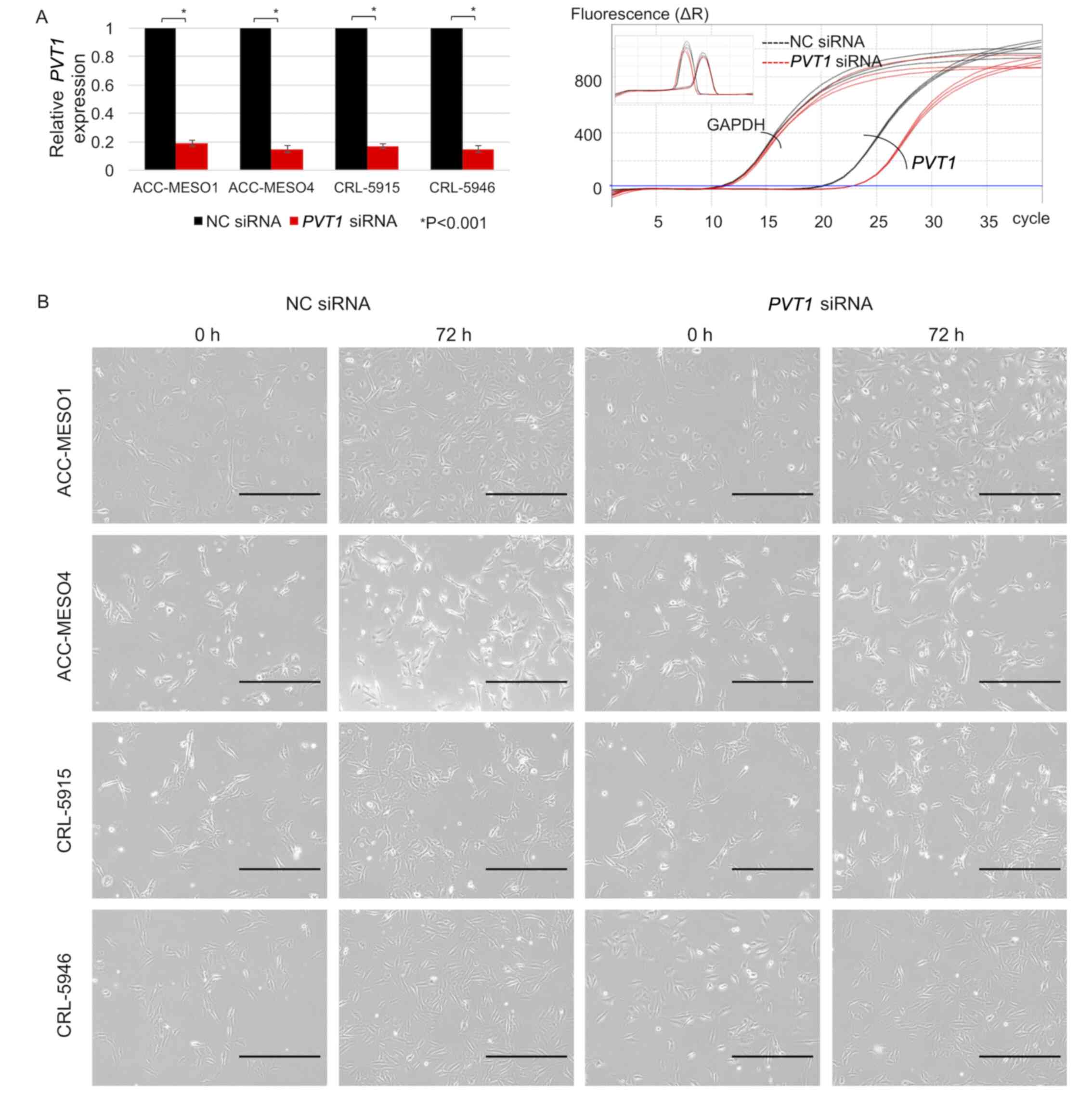
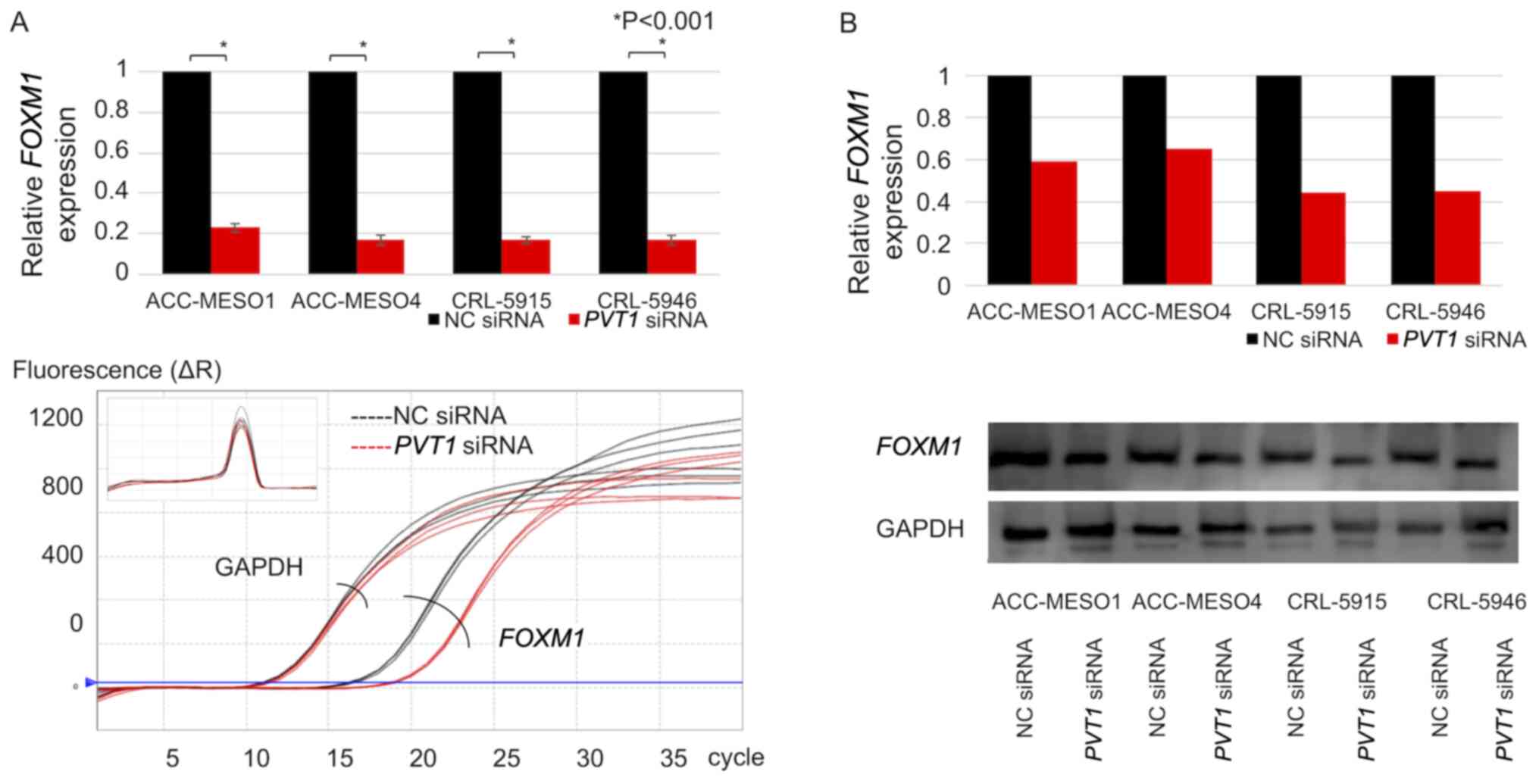
PVT1 expression is reduced by siRNA
transfection
PVT1 expression was downregulated by >80%
following PVT1 siRNA transfection in all mesothelioma cell
lines compared with that in cells transfected with NC siRNA
(Fig. 2A). Morphological changes
were not observed in PVT1 siRNA-transfected mesothelioma
cell lines compared with NC siRNA-transfected mesothelioma cell
lines (Fig. 2B).
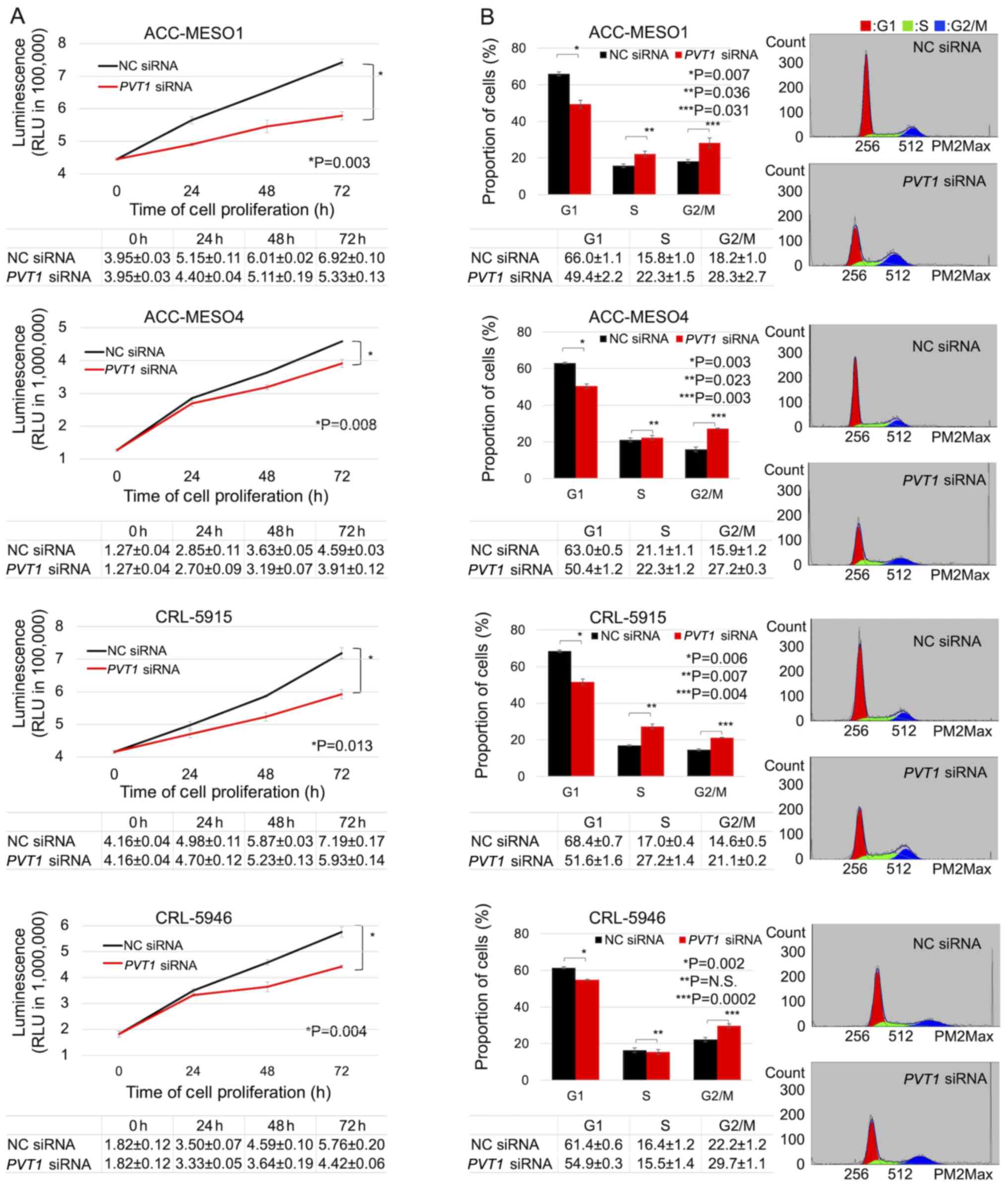
PVT1 knockdown reduces mesothelioma
cell proliferation and increases the G2/M phase of the cell
cycle
Knockdown of PVT1 significantly reduced the
proliferation of all mesothelioma cells compared with NC
siRNA-transfected cells. Following 3 days of treatment, the
inhibition of PVT1 expression significantly reduced the
viability of ACC-MESO-1 cells by 22.9%, ACC-MESO-4 cells by 14.8%,
CRL-5915 cells by 17.6%, and CRL-5946 cells by 23.3% (Fig. 3A). The proportion of cells in the
G2/M phase in the PVT1 siRNA-transfected mesothelioma cell
lines (28.7, 27.4, 21.0 and 30.3% in ACC-MESO-1, ACC-MESO-4,
CRL-5915, and CRL-5946 cell lines, respectively) was significantly
higher than with the NC siRNA-transfected mesothelioma cell lines
(17.5, 15.6, 14.4, and 22.8%). The proportion of cells in the G1
phase in the PVT1 siRNA-transfected mesothelioma cell lines
(49.6, 50.2, 52.5 and 55.0%, in ACC-MESO-1, ACC-MESO-4, CRL-5915,
and CRL-5946 cell lines, respectively) was significantly lower than
with the NC siRNA-transfected mesothelioma cell lines (66.3, 63.2,
68.8 and 61.1%) (Fig. 3B).
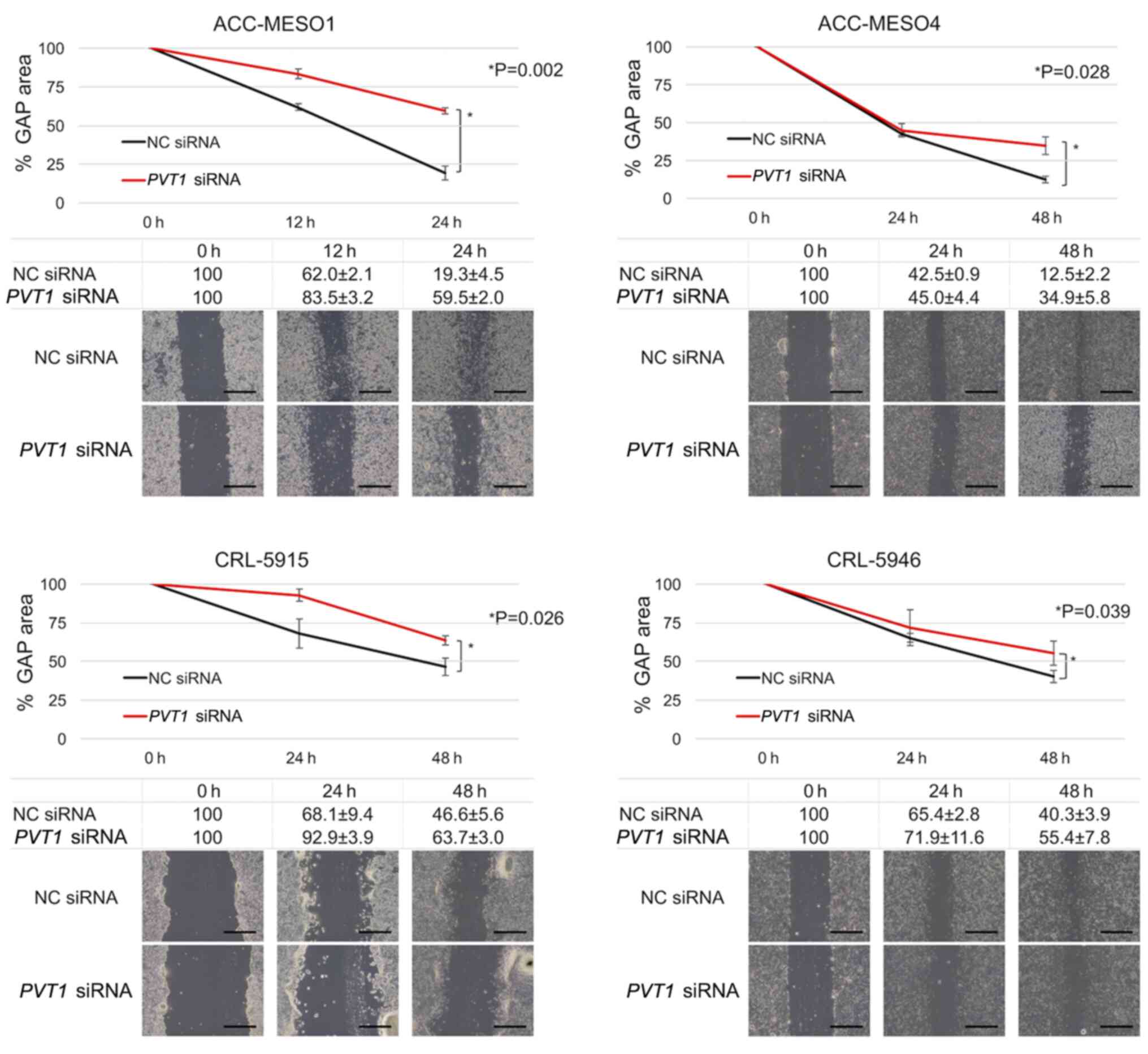
PVT1 knockdown reduces mesothelioma
cell migration but not invasion
In the PVT1 siRNA-transfected cells, the gap
area decreased more slowly than in the NC siRNA-transfected cell
lines in all four cell lines. The migration of ACC-MESO-1 cells
after 24 h of PVT1 knockdown was reduced by 67.5% and that
of ACC-MESO-4, CRL5915, and CRL5946 cell lines after 48 h of
PVT1 knockdown was reduced by 64.2, 26.8 and 27.3%,
respectively (Fig. 4). PVT1
was inhibited by siRNA, but it was not significantly associated
with the invasion of all four mesothelioma cell lines (data not
shown).
PVT1 knockdown downregulates FOXM1
expression
All four mesothelioma cell lines exhibited
FOXM1 expression. FOXM1 mRNA expression in cells
transfected with PVT1 siRNA compared with cells transfected
with NC siRNA was downregulated by 77, 83, 84 and 82% in ACC-MESO1,
ACC-MESO4, CRL-5915, and CRL-5946 cell lines, respectively
(Fig. 5A). Similarly, FOXM1
protein was downregulated by 41% in ACC-MESO-1 cells, 35% in
ACC-MESO-4 cells, 56% in CRL-5915 cells, and 55% in CRL-5946 cells
(Fig. 5B).
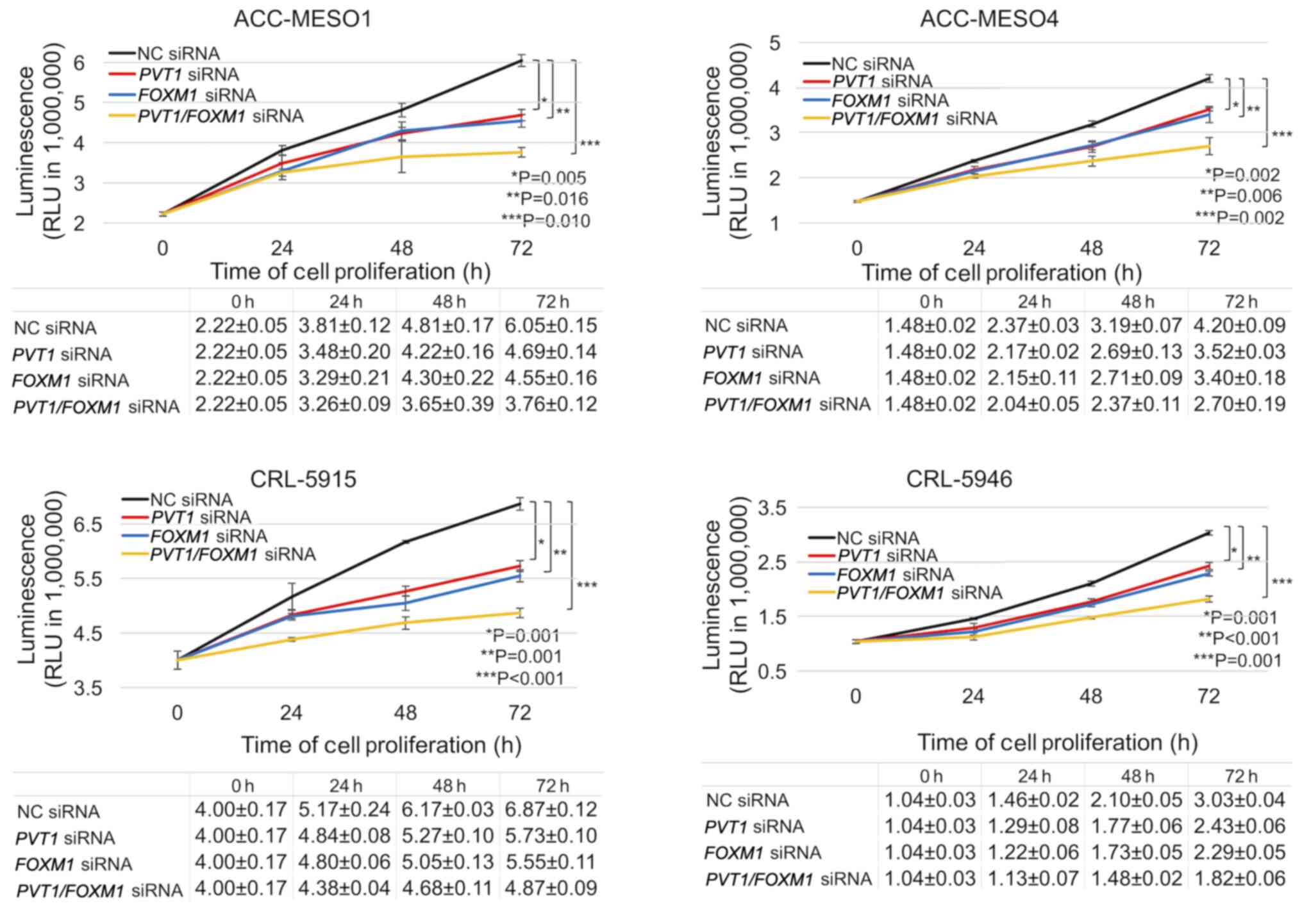
FOXM1 and PVT1 knockdown reduces
mesothelioma cell proliferation
Transfection with either FOXM1 or PVT1
siRNA revealed a similar decrease in the proliferation of
mesothelioma cells. However, combined FOXM1 and PVT1
siRNA transfection further decreased the proliferation of
mesothelioma cells. Following 3 days of treatment, FOXM1
knockdown significantly reduced the viability of ACC-MESO-1,
ACC-MESO-4, CRL-5915, and CRL-5946 cells by 24.8, 19.0, 19.2 and
24.4%, respectively. Furthermore, inhibition of both PVT1
and FOXM1 expression significantly reduced the viability of
ACC-MESO-1, ACC-MESO-4, CRL-5915, and CRL-5946 cells by 37.9, 35.7,
29.1 and 39.9%, respectively (Fig.
6).
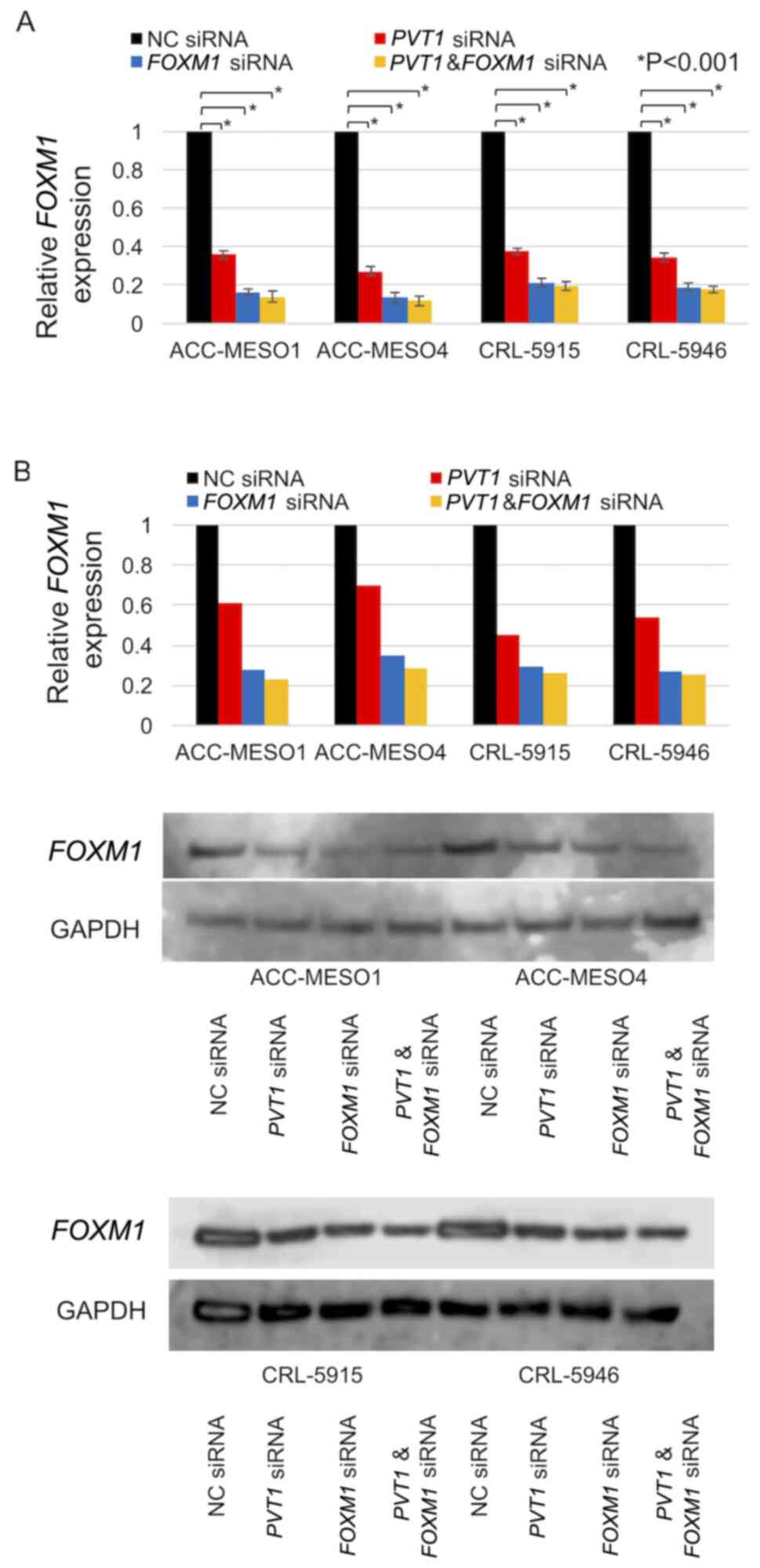
PVT1 and FOXM1 knockdown downregulates
FOXM1 expression
Downregulation of FOXM1 mRNA expression in
cells transfected with combined PVT1 and FOXM1 siRNA
compared with cells transfected with NC siRNA (86, 88, 80 and 82%
in ACC-MESO-1, ACC-MESO-4, CRL-5915, and CRL-5946 cell lines,
respectively) was markedly lower than that in cells transfected
with PVT1 siRNA alone (64, 73, 62 and 65%) but similar to
that in cells transfected with FOXM1 siRNA alone (84, 86, 79
and 81%) (Fig. 7A). Downregulation
of FOXM1 protein expression in cells transfected with
combined PVT1 and FOXM1 siRNA compared with cells
transfected with NC siRNA (77, 72, 74 and 75% in ACC-MESO-1,
ACC-MESO-4, CRL-5915, and CRL-5946 cell lines, respectively) was
lower than that in cells transfected with PVT1 siRNA alone
(39, 30, 54 and 46%) but similar to that in cells transfected with
FOXM1 siRNA alone (72, 65, 70 and 73%) (Fig. 7B).
Discussion
Malignant pleural mesothelioma (MPM) is an
aggressive form of cancer. Patients with malignant mesothelioma are
treated with surgery, radiotherapy, chemotherapy, and targeted drug
therapy. However, the survival rates of MPM patients remain
extremely low, with survival ranging from 5 to 13.2 months
(29).
In a previous study, the median survival period did
not improve beyond 13–29 months with extended
pleurectomy/decortication and 12–22 months with extrapleural
pneumonectomy (30). Therefore,
feasible and effective therapeutic targets need to be identified.
In the present study, the biological function of
PVT1-FOXM1 was investigated as a possible novel
target in malignant mesothelioma.
As they regulate gene expression and function at the
transcriptional, translational, and post-translational levels,
lncRNAs are important in tumor growth and metastasis (31,32).
Wright et al have previously revealed various dysregulated
lncRNAs involved in the pathogenesis of malignant mesothelioma
using NCode long noncoding microarrays and their potential to serve
as biomarkers in MPM (33).
However, the mechanisms of these lncRNAs have not yet been
described in detail. Non-coding transcripts from our previous gene
expression microarray analysis of malignant mesothelioma and lung
adenocarcinoma were extracted and analyzed and numerous upregulated
lncRNAs were identified, including PVT1, MEG3, and H19
(24). Riquelme et al
previously suggested that c-Myc and PVT1 copy number gain
may promote a malignant phenotype of mesothelioma with PVT1,
demonstrating a tendency to upregulate proliferation and inhibit
apoptosis (34). The biological
functions of PVT1 in malignant mesothelioma have not been
fully established; however, previous studies have revealed that
PVT1 knockdown inhibits cell proliferation and induces
apoptosis through suppression of c-Myc in leukemia (19) and breast cancer (22). PVT1 binds competitively with
microRNA-424, which has been reported to increase radiosensitivity
by regulating CARM1 in non-small cell lung cancer (18). PVT1 led to increased
proliferation and invasion of glioma (35) and hepatocellular carcinoma
(20) by targeting EZH2. In the
present study, increased expression of PVT1 in mesothelioma
and lung adenocarcinoma cell lines was revealed by RT-qPCR, and PVT
expression was revealed to be ~2 times higher in mesothelioma than
in lung adenocarcinoma cell lines. PVT1 knockdown of
mesothelioma cell lines revealed reduced cell proliferation with
G2/M arrest and migration.
FOXM1, a member of the FOX transcription
factor family 1, is associated to cell viability and is considered
a key gene in the carcinogenic pathway. Previous studies have
indicated that FOXM1 participates in drug resistance,
cancer, and metastasis of cancers (36–38).
Several previous studies have demonstrated that FOXM1 is
overexpressed in multiple cancers, such as ovarian (39), colon (40), gastrointestinal (41), and non-small cell lung cancer
(42). Increased FOXM1
expression was also observed in mesothelioma cell lines, and
knockdown of mesothelioma cell lines decreased their
proliferation.
PVT1 was revealed to promote tumor
progression by interacting with FOXM1 in ovarian and gastric
cancer (43,44). In the present study, it was also
revealed that PVT1 knockdown in mesothelioma cell lines
downregulated FOXM1 expression.
Our study also revealed that PVT1 knockdown
reduced FOXM1 expression. Furthermore, knockdown of both
FOXM1 and PVT1 in mesothelioma cell lines
demonstrated more reduced proliferation of mesothelioma cell lines
compared with knockdown of PVT1 or FOXM1 alone.
FOXM1 expression in mesothelioma cell lines with combined
PVT1 and FOXM1 knockdown was lower than that with
PVT1 knockdown alone. Further studies such as spheroid
formations and in-vivo experiments which are limited
in this study are necessary to clarify the function of
PVT1-FOXM1 in mesothelioma cell lines.
In conclusion, it was revealed in the present study
that lncRNA PVT1 was upregulated in mesothelioma cell lines,
and knockdown of PVT1 decreased the proliferation and
migration of mesothelioma cells and downregulated FOXM1
expression. Furthermore, concurrent knockdown of FOXM1 and
PVT1 in mesothelioma cell lines demonstrated more reduced
proliferation compared with knockdown of PVT1 or
FOXM1 alone. PVT1 and FOXM1 may be considered
as candidate targets for the therapy of malignant mesothelioma.
Acknowledgements
The authors would like to thank Ms Yukari Go and Mr
Tatsuya Nakagawa of the Technical Center, Hiroshima University for
their excellent technical assistance and Ms Naomi Fukuhara
(Department of Pathology, Hiroshima University) for administrative
support.
Funding
Funding: This research received no specific grant from any
funding agency in the public, commercial, or not-for-profit
sectors.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YF, VJA and YT designed the study. VJA and YT
supervised and facilitated the study. YF, RS, KK, YK and TK
performed the experiments. YF and VJA confirm the authenticity of
all the raw data. YF analyzed the data. YF and VJA interpreted the
results, and YF wrote the manuscript. All authors read and approved
the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Frost G: The latency period of
mesothelioma among a cohort of British asbestos workers
(1978–2005). Br J Cancer. 109:1965–1973. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Delgermaa V, Takahashi K, Park EK, Le GV,
Hara T and Sorahan T: Global mesothelioma deaths reported to the
World Health Organization between 1994 and 2008. Bull World Health
Organ. 89:716–724. 724A–724C. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Murayama T, Takahashi K, Natori Y and
Kurumatani N: Estimation of future mortality from pleural malignant
mesothelioma in Japan based on an age-cohort model. Am J Ind Med.
49:1–7. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Joshi TK and Gupta RK: Asbestos in
developing countries: Magnitude of risk and its practical
implications. Int J Occup Med Environ Health. 17:179–185.
2004.PubMed/NCBI
|
|
5
|
Yap TA, Aerts JG, Popat S and Fennell DA:
Novel insights into mesothelioma biology and implications for
therapy. Nat Rev Cancer. 17:475–488. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ponting CP, Oliver PL and Reik W:
Evolution and functions of long noncoding RNAs. Cell. 136:629–641.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mattick JS: The genetic signatures of
noncoding RNAs. PLoS Genet. 5:e10004592009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kapranov P, St Laurent G, Raz T, Ozsolak
F, Reynolds CP, Sorensen PH, Reaman G, Milos P, Arceci RJ, Thompson
JF, et al: The majority of total nuclear-encoded non-ribosomal RNA
in a human cell is ‘dark matter’ un-annotated RNA. BMC Biol.
8:1492010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gibb EA, Brown CJ and Lam WL: The
functional role of long non-coding RNA in human carcinomas. Mol
Cancer. 10:382011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gibb EA, Vucic EA, Enfield KS, Stewart GL,
Lonergan KM, Kennett JY, Becker-Santos DD, MacAulay CE, Lam S,
Brown CJ, et al: Human cancer long non-coding RNA transcriptomes.
PLoS One. 6:e259152011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brosnan CA and Voinnet O: The long and the
short of noncoding RNAs. Curr Opin Cell Biol. 21:416–425. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mercer TR, Dinger ME and Mattick JS: Long
non-coding RNAs: Insights into functions. Nat Rev Genet.
10:155–159. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Statello L, Guo CJ, Chen LL and Huarte M:
Gene regulation by long non-coding RNAs and its biological
functions. Nat Rev Mol Cell Biol. 22:96–118. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guttman M, Amit I, Garber M, French C, Lin
MF, Feldser D, Huarte M, Zuk O, Carey BW, Cassady JP, et al:
Chromatin signature reveals over a thousand highly conserved large
non-coding RNAs in mammals. Nature. 458:223–227. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Costa FF: Non-coding RNAs: Meet thy
masters. BioEssays. 32:599–608. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huarte M and Rinn JL: Large non-coding
RNAs: Missing links in cancer? Hum Mol Genet. 19:R152–R161. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cui M, You L, Ren X, Zhao W, Liao Q and
Zhao Y: Long non-coding RNA PVT1 and cancer. Biochem Biophys
Res Commun. 471:10–14. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang D and Hu Y: Long Non-coding RNA
PVT1 Competitively Binds MicroRNA-424-5p to Regulate CARM1
in Radiosensitivity of Non-Small-Cell Lung Cancer. Mol Ther Nucleic
Acids. 16:130–140. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hu J, Han Q, Gu Y, Ma J, McGrath M, Qiao
F, Chen B, Song C and Ge Z: Circular RNA PVT1 expression and
its roles in acute lymphoblastic leukemia. Epigenomics. 10:723–732.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guo J, Hao C, Wang C and Li L: Long
noncoding RNA PVT1 modulates hepatocellular carcinoma cell
proliferation and apoptosis by recruiting EZH2. Cancer Cell Int.
18:982018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang R, Li J, Yan X, Jin K, Li W, Liu X,
Zhao J, Shang W and Liu Y: Long Noncoding RNA Plasmacytoma Variant
Translocation 1 (PVT1) Promotes Colon Cancer Progression via
Endogenous Sponging miR-26b. Med Sci Monit. 24:8685–8692. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Guan Y, Kuo WL, Stilwell JL, Takano H,
Lapuk AV, Fridlyand J, Mao JH, Yu M, Miller MA, Santos JL, et al:
Amplification of PVT1 contributes to the pathophysiology of
ovarian and breast cancer. Clin Cancer Res. 13:5745–5755. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang Q, Yu Y, Sun Z and Pan Y: Long
non-coding RNA PVT1 promotes cell proliferation and invasion
through regulating miR-133a in ovarian cancer. Biomed Pharmacother.
106:61–67. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kuraoka M, Amatya VJ, Kushitani K, Mawas
AS, Miyata Y, Okada M, Kishimoto T, Inai K, Nishisaka T, Sueda T,
et al: Identification of DAB2 and Intelectin-1 as Novel Positive
Immunohistochemical Markers of Epithelioid Mesothelioma by
Transcriptome Microarray Analysis for Its Differentiation From
Pulmonary Adenocarcinoma. Am J Surg Pathol. 41:1045–1052. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Barretina J, Caponigro G, Stransky N,
Venkatesan K, Margolin AA, Kim S, Wilson CJ, Lehár J, Kryukov GV,
Sonkin D, et al: The Cancer Cell Line Encyclopedia enables
predictive modelling of anticancer drug sensitivity. Erratum.
Nature. 492:2902012. View Article : Google Scholar
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Δ Δ C(T)) Method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gebäck T, Schulz MM, Koumoutsakos P and
Detmar M: TScratch: A novel and simple software tool for automated
analysis of monolayer wound healing assays. Biotechniques.
46:265–274. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lamprecht MR, Sabatini DM and Carpenter
AE: CellProfiler: Free, versatile software for automated biological
image analysis. Biotechniques. 42:71–75. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Montanaro F, Rosato R, Gangemi M, Roberti
S, Ricceri F, Merler E, Gennaro V, Romanelli A, Chellini E,
Pascucci C, et al: Survival of pleural malignant mesothelioma in
Italy: A population-based study. Int J Cancer. 124:201–207. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cao C, Tian D, Park J, Allan J, Pataky KA
and Yan TD: A systematic review and meta-analysis of surgical
treatments for malignant pleural mesothelioma. Lung Cancer.
83:240–245. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bunch H: Gene regulation of mammalian long
non-coding RNA. Mol Genet Genomics. 293:1–15. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chi Y, Wang D, Wang J, Yu W and Yang J:
Long Non-Coding RNA in the Pathogenesis of Cancers. Cells.
8:10152019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wright CM, Kirschner MB, Cheng YY, O'Byrne
KJ, Gray SG, Schelch K, Hoda MA, Klebe S, McCaughan B, van Zandwijk
N, et al: Long non coding RNAs (lncRNAs) are dysregulated in
Malignant Pleural Mesothelioma (MPM). PLoS One. 8:e709402013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Riquelme E, Suraokar MB, Rodriguez J, Mino
B, Lin HY, Rice DC, Tsao A and Wistuba II: Frequent coamplification
and cooperation between C-MYC and PVT1 oncogenes promote
malignant pleural mesothelioma. J Thorac Oncol. 9:998–1007. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang A, Wang H and Yang X: Long non-coding
RNA PVT1 indicates a poor prognosis of glioma and promotes
cell proliferation and invasion via target EZH2. Biosci Rep.
37:BSR201708712017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fei BY, He X, Ma J, Zhang M and Chai R:
FOXM1 is associated with metastasis in colorectal cancer
through induction of the epithelial-mesenchymal transition. Oncol
Lett. 14:6553–6561. 2017.PubMed/NCBI
|
|
37
|
Shi C and Zhang Z: MicroRNA-320 suppresses
cervical cancer cell viability, migration and invasion via directly
targeting FOXM1. Oncol Lett. 14:3809–3816. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang J, Chen XY, Huang KJ, Wu WD, Jiang
T, Cao J, Zhou LS, Qiu ZJ and Huang C: Expression of FOXM1
and the EMT-associated protein E-cadherin in gastric cancer and its
clinical significance. Oncol Lett. 12:2445–2450. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tassi RA, Todeschini P, Siegel ER, Calza
S, Cappella P, Ardighieri L, Cadei M, Bugatti M, Romani C, Bandiera
E, et al: FOXM1 expression is significantly associated with
chemotherapy resistance and adverse prognosis in non-serous
epithelial ovarian cancer patients. J Exp Clin Cancer Res.
36:632017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang H, Zhong H, Li L, Ji W and Zhang X:
Overexpressed transcription factor FOXM1 contributes to the
progression of colorectal cancer. Mol Med Rep. 13:2696–2700. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang J, Niu Y and Huang C: Role of
FOXM1 in the Progression and Epithelial to Mesenchymal
Transition of Gastrointestinal Cancer. Recent Patents Anticancer
Drug Discov. 12:247–259. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kong FF, Qu ZQ, Yuan HH, Wang JY, Zhao M,
Guo YH, Shi J, Gong XD, Zhu YL, Liu F, et al: Overexpression of
FOXM1 is associated with EMT and is a predictor of poor
prognosis in non-small cell lung cancer. Oncol Rep. 31:2660–2668.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li M, Chi C, Zhou L, Chen Y and Tang X:
Circular PVT1 regulates cell proliferation and invasion via
miR-149-5p/FOXM1 axis in ovarian cancer. J Cancer.
12:611–621. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Xu MD, Wang Y, Weng W, Wei P, Qi P, Zhang
Q, Tan C, Ni SJ, Dong L, Yang Y, et al: A Positive Feedback Loop of
lncRNA-PVT1 and FOXM1 Facilitates Gastric Cancer
Growth and Invasion. Clin Cancer Res. 23:2071–2080. 2017.
View Article : Google Scholar : PubMed/NCBI
|