Introduction
Tumor angiogenesis is a well-known mechanism by
which blood is supplied to promote tumor progression through oxygen
and nutrient intake (1,2). Several angiogenesis inhibitors have
been developed, but some have been unable to overcome drug
resistance and provide sufficient benefit for cancer patients
(3). In certain cases, tumors can
grow even when angiogenesis is inhibited, suggesting the existence
of distinct blood supply pathways.
Recent studies have indicated that vasculogenic
mimicry (VM), the formation of blood vessel-like structures only by
cancer cells, constitutes an alternative blood supply system in
malignant tumors (4,5). VM has been reported in a wide variety
of malignancies, including melanoma, breast cancer, and lung
cancer, and this phenomenon is considered to contribute to tumor
growth and metastasis (4,6–8).
Although some reports indicate that VM correlates with reduced
overall survival in cancer patients (9,10),
the underlying mechanisms of VM have not been detailed
completely-an urgent area of study as a potential therapeutic
target in cancer.
An association between angiogenesis and VM has been
implicated in the past 2 decades. For example, the transcription
factor hypoxia-inducible factor (HIF)-1α is activated under
hypoxia. The increase in HIF-1α activity promotes tumor-associated
angiogenesis in solid tumors by stimulating the transcription of
angiogenesis-related genes (11);
furthermore, HIF-1α was found to mediate epithelial-to-mesenchymal
transition (EMT) and VM by upregulating LOXL2 in hepatocellular
carcinoma (12). Matrix
metalloproteinase (MMP)-9 facilitates angiogenesis during
carcinogenesis (13), and many
studies have reported that high expression of MMP-9 correlates
positively with VM in many types of cancer cells (14,15).
These studies suggest that the mechanisms of VM resemble in part
those of angiogenesis and are regulated by certain angiogenic
factors.
N-terminal methionine processing of nascent proteins
is a critical event in cells for maintaining the proper growth of
organisms, and methionine aminopeptidases (MetAPs) are central in
the removal of N-terminal methionine (16,17).
MetAPs consist of 2 members: MetAP1 and MetAP2. In contrast to
MetAP1, MetAP2 is associated with other molecules, in addition to
having aminopeptidase activity, modulating protein synthesis by
regulating the phosphorylation of eukaryotic initiation factor-2α
(eIF2α) (18). These studies
indicate that MetAP2 has several functions and is crucial for cell
growth.
Fumagillin (FUM) was first isolated from
Aspergillus sp. as an antibiotic (19), and FUM and its derivative, TNP-470
(TNP), have been used as potent angiogenesis inhibitors (20,21).
Because these inhibitors commonly target MetAP2 (22,23),
angiogenesis has been considered to be regulated by MetAP2. In
point of fact, a disruption in MetAP2 suppresses the proliferation
of endothelial cells and effects an abnormal vasculature in embryos
(24), suggesting that MetAP2
promotes tumor growth by mediating angiogenesis. Moreover, tumor
cells such as mesothelioma and colorectal, breast, and lung
adenocarcinoma, highly express MetAP2, and ectopic expression of
MetAP2 stimulates fibrosarcoma cell proliferation (25–27).
Although the functions of MetAP2 in endothelial
cells are well known, that of MetAP2 in tumor cells has not been
determined. A MetAP2 inhibitor was found to suppress proliferation
in a subset of tumor cells and in endothelial cells (28), suggesting that MetAP2 regulates
cell growth in cancers. However, van der Schaft et al
reported that TNP does not prevent VM in melanoma cells, despite it
inhibiting endothelial tube formation at the same concentration
(29). Collectively, MetAP2
inhibitors suppress the growth and tube formation of endothelial
cells, but their inhibitory effects on VM in tumor cells has not
been examined.
In the present study, we used human fibrosarcoma
HT1080 cells, which have high potential for VM, to determine the
relationship between MetAP2 and VM. We found that MetAP2 inhibitors
significantly suppressed VM in the HT1080 cells. Using the
CRISPR/Cas9 system, we established MetAP2-knockout (KO) HT1080
cells and observed that deletion of MetAP2 inhibited VM.
Re-expression of wild-type (wt) MetAP2 recovered VM, but an
inactive mutant form of MetAP2 failed to effect VM in MetAP2-KO
cells. Moreover, TNP inhibited VM in several tumor cell lines.
These results indicate that the angiogenic factor MetAP2 is pivotal
in VM and suggest that treatment with a MetAP2 inhibitor is an
effective strategy against VM-positive tumors.
Materials and methods
Cell culture
Human fibrosarcoma HT1080 (Japanese Collection of
Research Bioresources Cell Bank, Osaka, Japan) and human breast
cancer MDA-MB-231 cells (gifted by Professor Masakazu Toi, Kyoto
University Graduate School of Medicine, Kyoto, Japan) were cultured
in Dulbecco's modified Eagle's medium (DMEM; Nissui Pharmaceutical
Co., Ltd.) that was supplemented with 5% (v/v) fetal bovine serum
(FBS), 100 U/ml penicillin G, 100 mg/l kanamycin, 600 mg/l
L-glutamine, and 2.25 g/l NaHCO3 at 37°C in a humidified
incubator with 5% CO2. Human melanoma SK-MEL-28 and
human breast cancer T47D cells were obtained from RIKEN BioResource
Center and cultured in DMEM that was supplemented with 8% (v/v)
FBS, 100 U/ml penicillin G, 100 mg/l kanamycin, 600 mg/l
L-glutamine, and 2.25 g/l NaHCO3 at 37°C in a humidified
incubator with 5% CO2.
Reagents
TNP-470 (Cayman Chemical) and fumagillin (FUJIFILM
Wako Pure Chemical Corp.) were dissolved in dimethyl sulfoxide
(DMSO). The concentrations of the stock solution (TNP and FUM) were
10 mg/ml. In all experiments, the level of DMSO was 0.1% in the
vehicle control.
In vitro VM assay
HT1080, SK-MEL-28, T47D, and MDA-MB-231 cells,
suspended in culture medium, were seeded at 1.6×104
cells/well in a 96-well plate that was precoated with 40 µl/well
Matrigel (Corning Inc.). The seeded cells were cultured at 37°C and
photographed under a phase-contrast microscope (Leica DMi1; Leica
Microsystems GmbH) 3 h later.
MTT assay
MTT assay was performed to measure cell
proliferation rates with thiazolyl blue tetrazolium bromide (Merck
KGaA). Cells were seeded at 2.0×103 cells/well in a
96-well plate and cultured for 24 h. Then, thiazolyl blue
tetrazolium bromide was added to the cells and incubated for 4 h at
37°C. After incubation, the medium was removed, and the MTT
formazan product was dissolved with 100 µl DMSO. The levels of
these products were measured by absorbance at 570 nm.
WST assay
WST assay was performed to measure living cell
numbers with the Cell Counting Kit-8 (FUJIFILM Wako Pure Chemical
Corp.). Cells were seeded at 1.6×104 cells/well in a
96-well plate that was precoated with 40 µl/well Matrigel and
cultured for 1 h at 37°C. Then, the Cell Counting Kit-8 was added
to the cells. After incubation for 2 h at 37°C, absorbance at 450
nm was measured.
Generation of MetAP2-KO cell lines by
CRISPR/Cas9
MetAP2-KO HT1080 cells were established using the
CRISPR/Cas9 system as previously described (30). To avoid off-target effects, we used
the pSpCas9n(BB)-2A-Puro (PX462) V2.0 plasmid (Addgene), a gift
from Feng Zhang, to coexpress Cas9n [D10A nickase mutant (31)]. Target sequences were designed
within exon 1 of human MetAP2, with the following
oligonucleotides: forward 1, 5′-CACCGGGAAGAAGGAGCTGCCTCTA-3′ and
reverse 1, 5′-AAACTAGAGGCAGCTCCTTCTTCCC-3′; forward 2,
5′-CACCGCTGGATCCAGGTCGCCATTC and reverse 2,
5′-AAACGAATGGCGACCTGGATCCAGC-3′.
Each pair of oligonucleotides was annealed and then
inserted into the BbsI restriction site of the Cas9n
expression vector. HT1080 cells were cotransfected with these
plasmids and selected with 2 µg/ml puromycin dihydrochloride (Merck
KGaA) for 2 weeks. After selection, clonal cell lines were isolated
by limiting dilution method, and KO of MetAP2 was confirmed by
western blot analysis.
Western blot analysis
We performed western blot analysis as previously
described (32–35). Cells were cultured in a 60-mm dish
and lysed in lysis buffer [50 mM Tris-HCl, pH 7.5, 150 mM NaCl,
0.1% (w/v) sodium dodecyl sulfate, 1% (v/v) Triton X-100, 1% (w/v)
sodium deoxycholate, and 1 mM phenylmethylsulfonyl fluoride] at 4°C
with sonication. The cell lysates were centrifuged at 15,000 × g
for 10 min to eliminate debris. Protein concentrations were
measured by Coomassie Brilliant Blue G-250 staining (Bio-Rad
Laboratories), and loading buffer [350 mM Tris-HCl, pH 6.8, 30%
(w/v) glycerol, 0.012% (w/v) bromophenol blue, 6% (w/v) SDS and 30%
(v/v) 2-mercaptoethanol] was added to each lysate. After
electrophoresis, proteins (15 µg/lane) were transferred to
polyvinylidene fluoride membranes and immunoblotted with
anti-MetAP2 (#12547S; Cell Signaling Technology, Inc.) or
anti-α-tubulin (#T5168; Merck KGaA). Signals were detected by ECL
using Western Lightning Plus-ECL (PerkinElmer, Inc.) or Immobilon
Western Chemiluminescent HRP substrate (Merck KGaA).
MetAP2-rescued cell lines
Human MetAP2 cDNA was amplified from an
HT1080 cell cDNA library using the following primers: forward,
5′-TTTTCTCGAGATGGCGGGTGTGGAGGAGGTAGC-3′ and reverse,
5′-TTTTACGCGTTTAATAGTCATCTCCTCTGCTG-3′.
To prevent recognition and cleavage by Cas9n, we
designed Cas9n-resistant MetAP2 cDNA by codon optimization
with no amino acid substitutions, using the following primers:
forward,
5′-ATAGAGAGGAGGGGGCCGCTTCCACAGCTGAGGAAGCAGCCAAGAAAAAAAGAC-3′ and
reverse, 5′-CATCGGGGTCAAGATCTCCGTTTAAGTGGCTCCCGGAGGCCGCTACCTC-3′.
The resulting DNA was subcloned into the XhoI/MluI
sites of pCI-neo (Promega Corp.).
A point mutation (D251A) was introduced into the
MetAP2 cDNA by site-directed mutagenesis using the following
primers: forward, 5′-ATCTGTAAAATAGCCTTTGGAACACATATAAGTGGTAGG-3′ and
reverse, 5′-GTGTTCCAAAGGCTATTTTACAGATGTCATCATACTGTAATACTG-3′. The
resulting DNA was subcloned into the XhoI/MluI sites
of pCI-neo.
MetAP2-KO HT1080 cells were transfected with these
plasmids and selected with 500 µg/ml G418 (FUJIFILM Wako Pure
Chemical Corp.) for 2 weeks. After selection, the clonal cell lines
were isolated by limiting dilution method, and re-expression of
MetAP2 was confirmed by western blot analysis.
Wound-healing assay
Cells were seeded in 12-well plates at
1.5×105 cells/well and cultured for 24 h. One hundred
percent of the cells were wounded using a 200-µl pipette tip
(Watson). After being washed twice with PBS to remove floating
cells, the cells were cultured in serum-free DMEM as described
previously (36). Photographs of 4
independent areas were taken, and the migrated areas were
quantified using ImageJ 1.52q (National Institutes of Health)
(37).
Trypan blue dye exclusion assay
Suspended cells were mixed with trypan blue solution
(Merck KGaA), and unstained (alive) and blue (dead) cells were
counted on a hemocytometer (ERMA) as previously described (38).
Statistical analysis
Statistical analysis was performed using SPSS
Statistics 27.0 [International Business Machines (IBM) Corp.].
Unpaired one-way ANOVA with Dunnett's multiple comparisons test was
performed to confirm statistical significance, and the results are
expressed as means ± standard deviation (SD). P-values <0.05
were considered to be statistically significant.
Results
Fumagillin and TNP-470 suppress VM in
HT1080 cells
Based on its function as a methionine
aminopeptidase, we continuously treated tumor cell lines with
inhibitors to evaluate the effects of MetAP2 on VM. As shown in
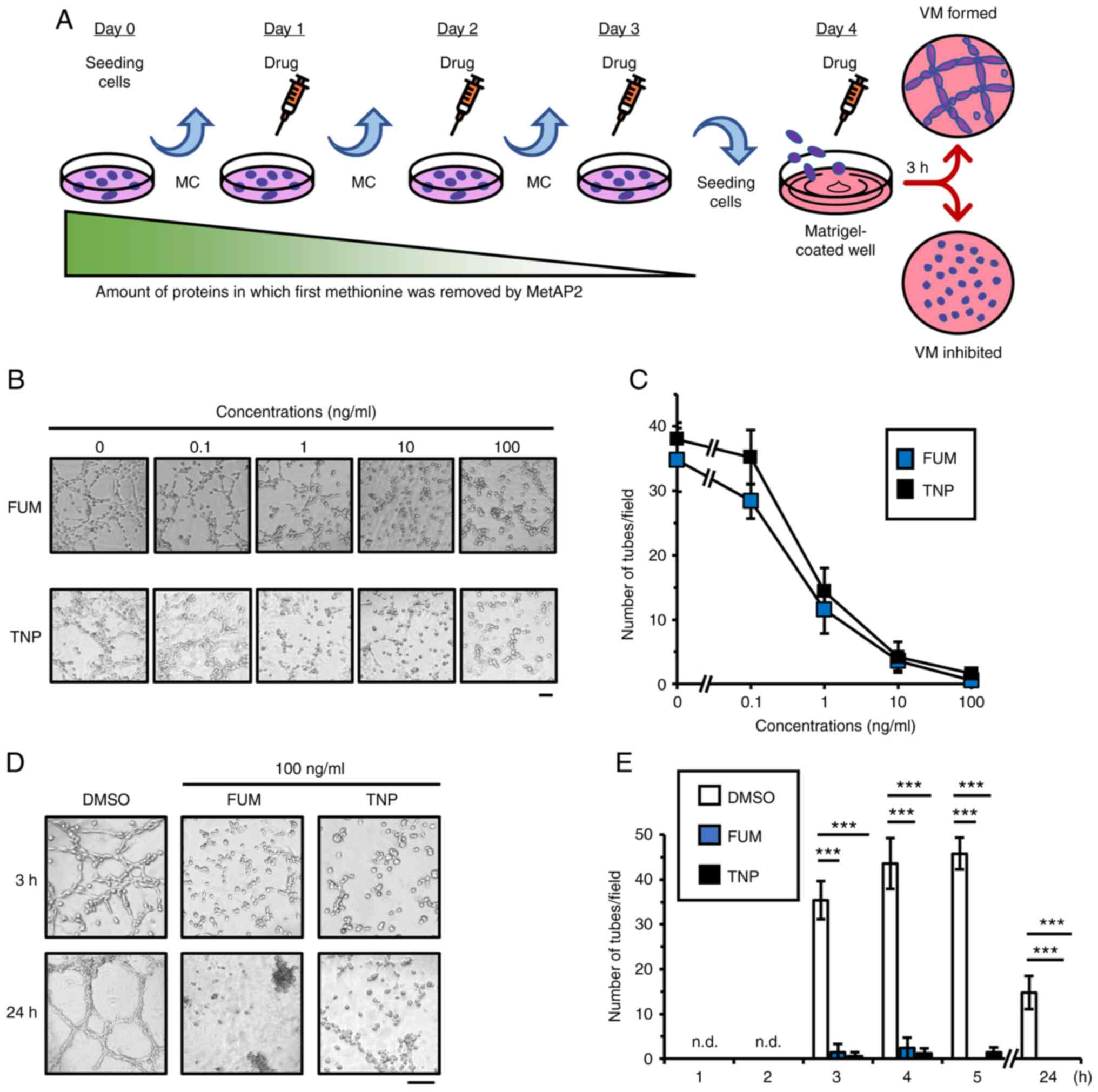
Fig. 1A, on Day 0, tumor cells
were seeded in plates and cultured for 24 h. On Days 1, 2, and 3,
the culture media was changed, and cells were treated with vehicle
control (DMSO) or drugs (FUM or TNP). On Day 4, we suspended the
cells and seeded them on Matrigel-coated well plates in the
presence of drugs, thus inhibiting MetAP2 activities.
To determine the inhibitory effects of FUM and TNP
on VM, we used HT1080 cells in which VM was previously found to
occur in a short-time culture (30). We administered these inhibitors to
HT1080 cells in which VM and performed in vitro VM assay, as
described above (Fig. 1A). FUM and
TNP suppressed VM in HT1080 cells dose-dependently (IC50
of FUM: 0.80 ng/ml, IC50 of TNP: 0.69 ng/ml; Fig. 1B and C). Because these inhibitors
can be cytotoxic, we confirmed living cell numbers on Matrigel
matrices. By WST assay, the number of living cells among the
control and inhibitor-treated cells did not change significantly
(Fig. S1A and B), indicating that
the inhibition of VM by FUM and TNP was not due to their
cytotoxicity. We further analyzed their effects by monitoring
short-term and long-term VM. Whereas control cells formed tube
structures from 3 h that were maintained until 24 h after seeding,
FUM and TNP significantly disrupted VM at all periods (Fig. 1D and E). These results suggest that
inhibition of MetAP2 causes a significant decrease in VM in HT1080
cells.
MetAP2 is pivotal for VM in HT1080
cells
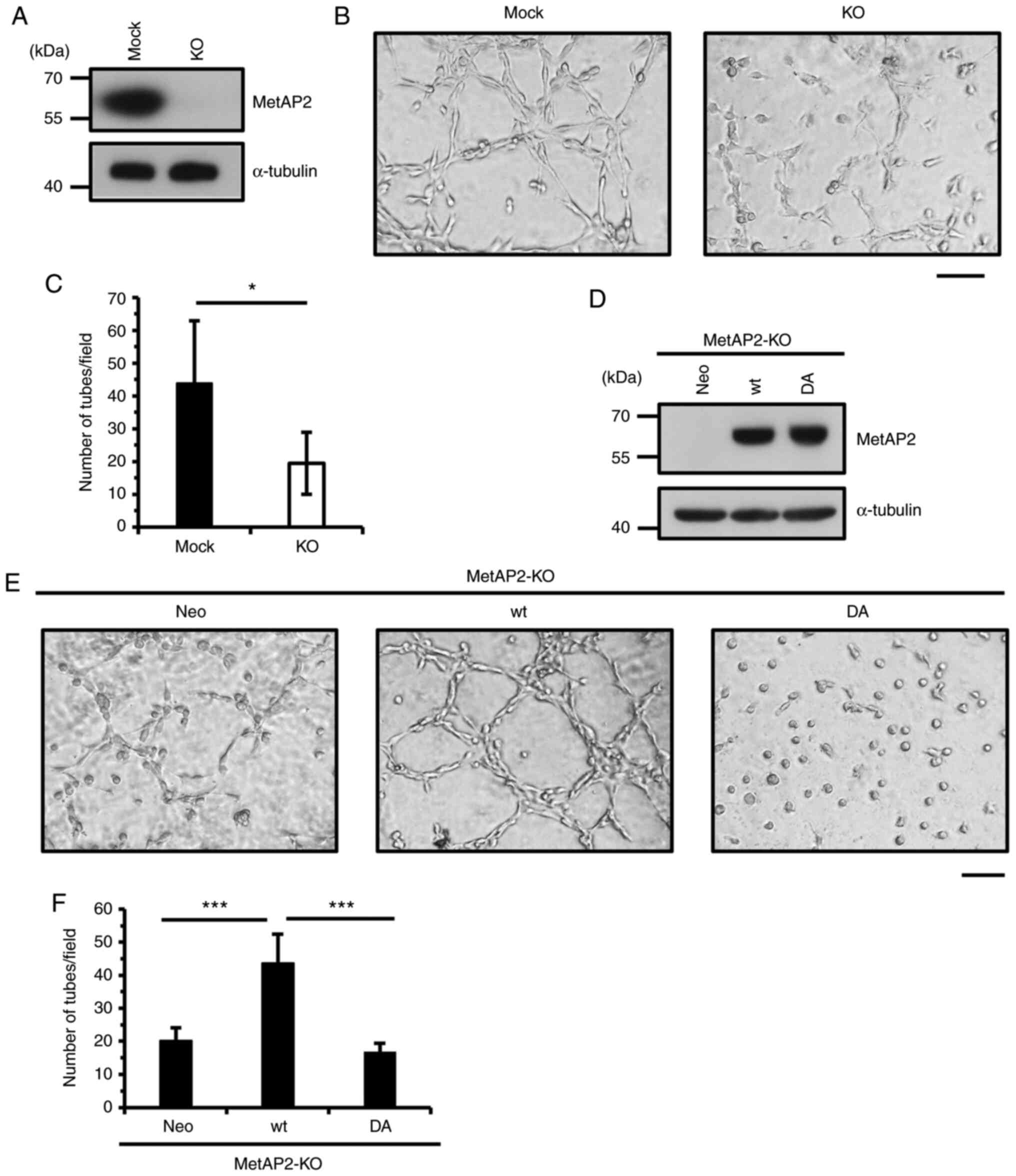
Because MetAP2 inhibitors impaired VM in HT1080
cells, we verified that MetAP2 regulates VM using a genetic
approach, generating MetAP2-KO HT1080 cells by CRISPR/Cas9
(Fig. 2A). As expected, network
formation was significantly inhibited upon KO of MetAP2 (Fig. 2B and C). To confirm this effect, we
performed rescue experiment in HT1080 cells by using wild-type and
enzymatically-inactive MetAP2. A previous study indicated that FUM
covalently targets H231 residue of MetAP2 and inactivates enzymatic
activity of MetAP2 (39). H231N
mutation lacks catalytic activity of MetAP2 (39), however overexpression of this
mutant causes serious cell growth inhibition in HT1080 cells
(27), therefore, we replaced
other crucial residues of MetAP2. D251 residue locates conserved
metal-binding site of MetAP2 and that replacement of this aspartic
acid is considered to affect its catalytic activity. Thus, we
re-expressed the D251A mutant form (DA) of MetAP2 and wild-type
(wt) MetAP2 in MetAP2-KO HT1080 cells (Fig. 2D). As a result, whereas wild-type
MetAP2 recovered VM, D251A MetAP2 failed to enhance network
formation (Fig. 2E and F),
indicating that MetAP2 regulates VM. Although the activity of
MetAP2 is crucial for VM, the expression of wild-type or D251A
MetAP2 did not significantly affect the proliferation of HT1080
cells (Fig. S2). These results
suggest that MetAP2 promotes VM without altering cell
proliferation.
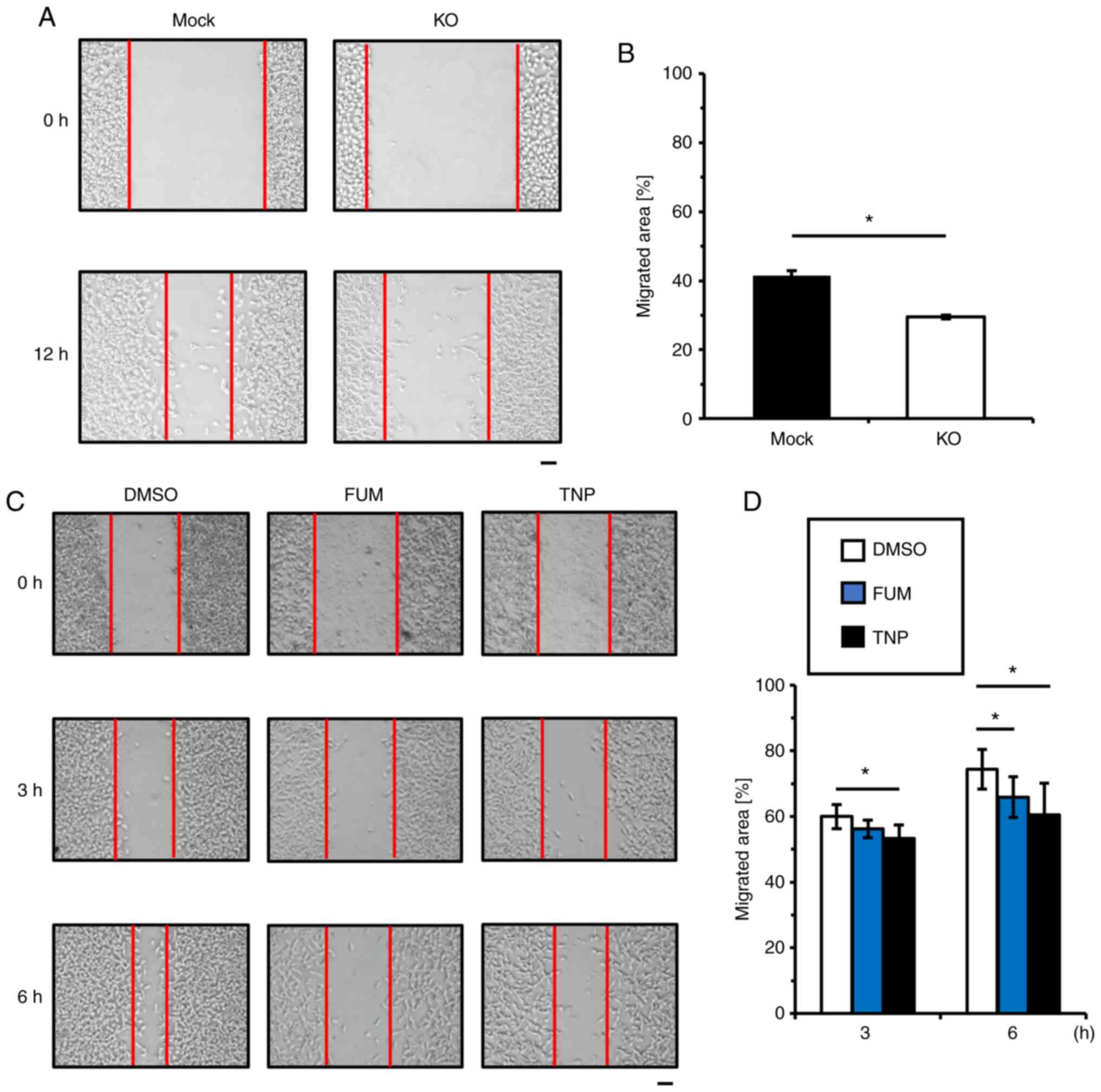
Because VM is associated with the high metastatic
phenotype of tumor cells, we assessed cell migration in MetAP2-KO
HT1080 cells. By wound-healing assay, KO of MetAP2 significantly
reduced cell migration compared with the mock cells (Fig. 3A and B). Consistent with this
result, FUM and TNP decreased HT1080 cell migration (Fig. 3C and D). These results indicate
that MetAP2 is important for cell migration and that
MetAP2-mediated VM is upregulated through the promotion of cell
motility.
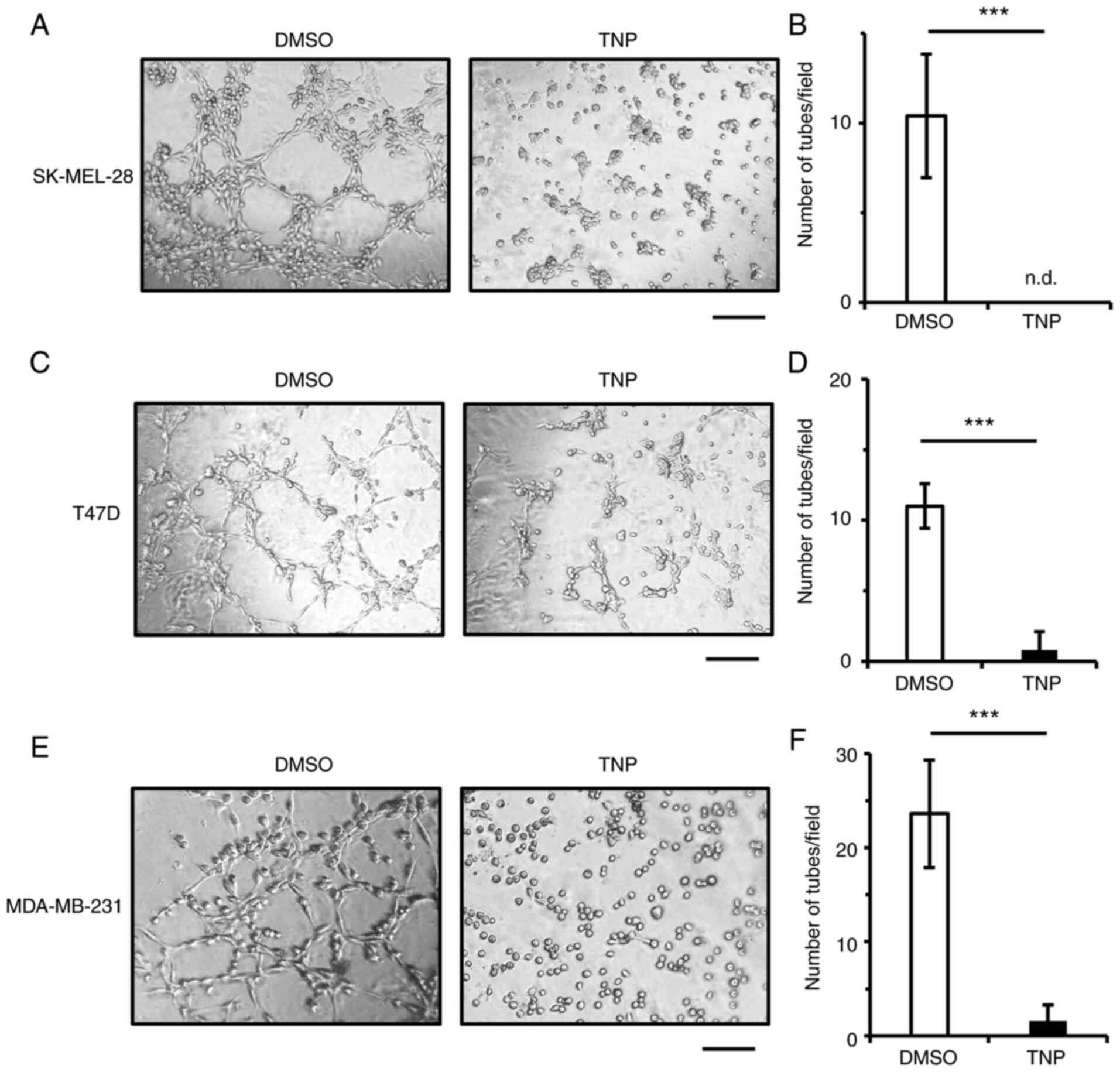
TNP suppresses VM in several cancer
cell lines
MetAP2 is central to VM in HT1080 cells. Thus, to
determine whether MetAP2 is involved in VM in other types of cancer
cells, we treated several types of cancer cells with TNP. As shown
in Fig. 1A, upon treatment of
human melanoma SK-MEL-28 and human breast cancer T47D and
MDA-MB-231 cells with TNP, VM was significantly inhibited (Fig. 4). Viable cell numbers in
TNP-treated cells on Matrigel were unchanged compared with the
control cells (Fig. S3),
confirming that the inhibition of VM was not caused by cell
cytotoxicity. These results demonstrate that MetAP2 is critical for
VM in human cancer cells and implicate MetAP2 inhibitors as
promising therapeutics for cancer patients.
Discussion
Tumor cells acquire aggressiveness and invasiveness
through various processes, such as angiogenesis and
epithelial-to-mesenchymal transition (EMT), constituting targets in
the development of drugs for cancer treatment (40,41).
Recently, vasculogenic mimicry (VM) has been indicated as a new
malignant phenotype that supports tumor development (4,10).
In VM-positive tumors, cells take in nutrients and oxygen through
their blood vessel-like tubes, rendering VM a potential novel
target for antitumor therapy (42). However, the mechanisms of VM have
not been detailed extensively. In the present study, we examined
methionine aminopeptidase-2 (MetAP2), an important
angiogenesis-related protein, and its regulation of VM in human
cancer cells.
Van der Schaft et al administered the MetAP2
inhibitor TNP to several endothelial and melanoma cell lines,
reporting that TNP inhibited tube formation by endothelial cells
but that VM of melanoma cells was not impaired (29). In their study, TNP (100 ng/ml) was
given only once; thus, we hypothesized that MetAP2 activity was not
completely inhibited. To test this hypothesis, we treated human
fibrosarcoma HT1080 cells with FUM and TNP for 3 consecutive days
to fully suppress the effects of MetAP2 (Fig. 1A).
As a result, complete inhibition of MetAP2 disrupted
VM in several cancer cell lines (Figs.
1 and 4). MetAP2 displays two
major functions: removing the N-terminal methionine of nascent
proteins and promoting eukaryotic initiation factor 2α
(eIF2α)-mediated protein synthesis (16–18).
Given that tube formation by endothelial cells was inhibited by a
single TNP treatment (29), the
translation of critical factors of angiogenesis might be regulated
by MetAP2. Conversely, VM by cancer cells was inhibited by
continuous treatment with FUM or TNP in our study. Under these
conditions, the N-terminal methionine of the substrates of MetAP2
is not cleaved, suggesting that processing by MetAP2 is pivotal for
VM.
Although MetAP2 has 4 conserved amino acid residues,
D251, D262, E364 and E459, D251 has been reported to influence its
activity (43). The D251A MetAP2
mutant loses its autoproteolytic activity and increases the
phosphorylation of eIF2α (44,45);
thus, protein synthesis is partially inhibited in D251A
MetAP2-expressing cells. In HT1080 cells, the D251A mutation
impaired the ability of MetAP2 to promote VM (Fig. 2E and F). This result indicates that
eIF2α regulates the translation of VM-related genes and that the
inhibition of translation by mutations in MetAP2 induces a defect
in the VM machinery.
In the present study, we demonstrated that MetAP2
inhibitors significantly suppressed VM in human fibrosarcoma,
melanoma, and breast cancer cell lines. Using genetic methods, it
was confirmed that the activity of MetAP2 was pivotal for VM. Thus,
targeting MetAP2 might be an ideal strategy for the treatment of
VM-positive cancers.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by JSPS KAKENHI (grant no.
JP20J11197).
Availability of data and materials
The datasets of this study are available from the
corresponding author on reasonable request.
Authors' contributions
SShi, RK and SSim designed the study. SSim performed
all of the experiments. SShi, RK and SSim wrote the original draft.
All authors confirmed the accuracy of the data, read and approved
the manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
EMT
|
epithelial-to-mesenchymal
transition
|
|
eIF2α
|
eukaryotic translation initiation
factor-2α
|
|
FUM
|
fumagillin
|
|
HIF
|
hypoxia-inducible factor
|
|
MetAP2
|
methionine aminopeptidase-2
|
|
MMP
|
matrix metalloproteinase
|
|
TNP
|
TNP-470
|
|
VM
|
vasculogenic mimicry
|
References
|
1
|
Folkman J: Role of angiogenesis in tumor
growth and metastasis. Semin Oncol. 29 (6 Suppl 16):S15–S18. 2002.
View Article : Google Scholar
|
|
2
|
Folkman J: Angiogenesis: An organizing
principle for drug discovery? Nat Rev Drug Discov. 6:273–286. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Aalders KC, Tryfonidis K, Senkus E and
Cardoso F: Anti-angiogenic treatment in breast cancer: Facts,
successes, failures and future perspectives. Cancer Treat Rev.
53:98–110. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Maniotis AJ, Folberg R, Hess A, Seftor EA,
Gardner LM, Pe'er J, Trent JM, Meltzer PS and Hendrix MJ: Vascular
channel formation by human melanoma cells in vivo and in vitro:
Vasculogenic mimicry. Am J Pathol. 155:739–752. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wei X, Chen Y, Jiang X, Peng M, Liu Y, Mo
Y, Ren D, Hua Y, Yu B, Zhou Y, et al: Mechanisms of vasculogenic
mimicry in hypoxic tumor microenvironments. Mol Cancer. 20:72021.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Williamson SC, Metcalf RL, Trapani F,
Mohan S, Antonello J, Abbott B, Leong HS, Chester CP, Simms N,
Polanski R, et al: Vasculogenic mimicry in small cell lung cancer.
Nat Commun. 7:133222016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shirakawa K, Tsuda H, Heike Y, Kato K,
Asada R, Inomata M, Sasaki H, Kasumi F, Yoshimoto M, Iwanaga T, et
al: Absence of endothelial cells, central necrosis, and fibrosis
are associated with aggressive inflammatory breast cancer. Cancer
Res. 61:445–451. 2001.PubMed/NCBI
|
|
8
|
Wagenblast E, Soto M, Gutiérrez-Ángel S,
Hartl CA, Gable AL, Maceli AR, Erard N, Williams AM, Kim SY,
Dickopf S, et al: A model of breast cancer heterogeneity reveals
vascular mimicry as a driver of metastasis. Nature. 520:358–362.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cao Z, Bao M, Miele L, Sarkar FH, Wang Z
and Zhou Q: Tumour vasculogenic mimicry is associated with poor
prognosis of human cancer patients: A systemic review and
meta-analysis. Eur J Cancer. 49:3914–3923. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang JP, Liao YD, Mai DM, Xie P, Qiang YY,
Zheng LS, Wang MY, Mei Y, Meng DF, Xu L, et al: Tumor vasculogenic
mimicry predicts poor prognosis in cancer patients: A
meta-analysis. Angiogenesis. 19:191–200. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li W, Zong S, Shi Q, Li H, Xu J and Hou F:
Hypoxia-induced vasculogenic mimicry formation in human colorectal
cancer cells: Involvement of HIF-1a, Claudin-4, and E-cadherin and
Vimentin. Sci Rep. 6:375342016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang M, Zhao X, Zhu D, Liu T, Liang X, Liu
F, Zhang Y, Dong X and Sun B: HIF-1α promoted vasculogenic mimicry
formation in hepatocellular carcinoma through LOXL2 up-regulation
in hypoxic tumor microenvironment. J Exp Clin Cancer Res.
36:602017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bergers G, Brekken R, McMahon G, Vu TH,
Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z and
Hanahan D: Matrix metalloproteinase-9 triggers the angiogenic
switch during carcinogenesis. Nat Cell Biol. 2:737–744. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Meng J, Chen S, Lei YY, Han JX, Zhong WL,
Wang XR, Liu YR, Gao WF, Zhang Q, Tan Q, et al: Hsp90β promotes
aggressive vasculogenic mimicry via epithelial-mesenchymal
transition in hepatocellular carcinoma. Oncogene. 38:228–243. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cai HP, Wang J, Xi SY, Ni XR, Chen YS, Yu
YJ, Cen ZW, Yu ZH, Chen FR, Guo CC, et al: Tenascin-c mediated
vasculogenic mimicry formation via regulation of MMP2/MMP9 in
glioma. Cell Death Dis. 10:8792019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chang YH, Teichert U and Smith JA:
Molecular cloning, sequencing, deletion, and overexpression of a
methionine aminopeptidase gene from Saccharomyces cerevisiae. J
Biol Chem. 267:8007–8011. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li X and Chang YH: Amino-terminal protein
processing in Saccharomyces cerevisiae is an essential function
that requires two distinct methionine aminopeptidases. Proc Natl
Acad Sci USA. 92:12357–12361. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Datta B, Chakrabarti D, Roy AL and Gupta
NK: Roles of a 67-kDa polypeptide in reversal of protein synthesis
inhibition in heme-deficient reticulocyte lysate. Proc Natl Acad
Sci USA. 85:3324–3328. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
McCowen MC, Callender ME and Lawlis JF Jr:
Fumagillin (H-3), a new antibiotic with amebicidal properties.
Science. 113:202–203. 1951. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ingber D, Fujita T, Kishimoto S, Sudo K,
Kanamaru T, Brem H and Folkman J: Synthetic analogues of fumagillin
that inhibit angiogenesis and suppress tumour growth. Nature.
348:555–557. 1990. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kusaka M, Sudo K, Matsutani E, Kozai Y,
Marui S, Fujita T, Ingber D and Folkman J: Cytostatic inhibition of
endothelial cell growth by the angiogenesis inhibitor TNP-470
(AGM-1470). Br J Cancer. 69:212–216. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sin N, Meng L, Wang MQ, Wen JJ, Bornmann
WG and Crews CM: The anti-angiogenic agent fumagillin covalently
binds and inhibits the methionine aminopeptidase, MetAP-2. Proc
Natl Acad Sci USA. 94:6099–6103. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Griffith EC, Su Z, Turk BE, Chen S, Chang
YH, Wu Z, Biemann K and Liu JO: Methionine aminopeptidase (type 2)
is the common target for angiogenesis inhibitors AGM-1470 and
ovalicin. Chem Biol. 4:461–471. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yeh JJ, Ju R, Brdlik CM, Zhang W, Zhang Y,
Matyskiela ME, Shotwell JD and Crews CM: Targeted gene disruption
of methionine aminopeptidase 2 results in an embryonic gastrulation
defect and endothelial cell growth arrest. Proc Natl Acad Sci USA.
103:10379–10384. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Catalano A, Romano M, Robuffo I, Strizzi L
and Procopio A: Methionine aminopeptidase-2 regulates human
mesothelioma cell survival: Role of Bcl-2 expression and telomerase
activity. Am J Pathol. 159:721–731. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Selvakumar P, Lakshmikuttyamma A, Kanthan
R, Kanthan SC, Dimmock JR and Sharma RK: High expression of
methionine aminopeptidase 2 in human colorectal adenocarcinomas.
Clin Cancer Res. 10:2771–2775. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tucker LA, Zhang Q, Sheppard GS, Lou P,
Jiang F, McKeegan E, Lesniewski R, Davidsen SK, Bell RL and Wang J:
Ectopic expression of methionine aminopeptidase-2 causes cell
transformation and stimulates proliferation. Oncogene.
27:3967–3976. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang J, Sheppard GS, Lou P, Kawai M,
BaMaung N, Erickson SA, Tucker-Garcia L, Park C, Bouska J, Wang YC,
et al: Tumor suppression by a rationally designed reversible
inhibitor of methionine aminopeptidase-2. Cancer Res. 63:7861–7869.
2003.PubMed/NCBI
|
|
29
|
Van der Schaft DW, Seftor RE, Seftor EA,
Hess AR, Gruman LM, Kirschmann DA, Yokoyama Y, Griffioen AW and
Hendrix MJ: Effects of angiogenesis inhibitors on vascular network
formation by human endothelial and melanoma cells. J Natl Cancer
Inst. 96:1473–1477. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kawahara R, Niwa Y and Simizu S: Integrin
β1 is an essential factor in vasculogenic mimicry of human cancer
cells. Cancer Sci. 109:2490–2496. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ran FA, Hsu PD, Wright J, Agarwala V,
Scott DA and Zhang F: Genome engineering using the CRISPR-Cas9
system. Nat Protoc. 8:2281–2308. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Simizu S, Umezawa K, Takada M, Arber N and
Imoto M: Induction of hydrogen peroxide production and Bax
expression by caspase-3(−like) proteases in tyrosine kinase
inhibitor-induced apoptosis in human small cell lung carcinoma
cells. Exp Cell Res. 238:197–203. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yasukagawa T, Niwa Y, Simizu S and Umezawa
K: Suppression of cellular invasion by glybenclamide through
inhibited secretion of platelet-derived growth factor in ovarian
clear cell carcinoma ES-2 cells. FEBS Lett. 586:1504–1509. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Komai K, Niwa Y, Sasazawa Y and Simizu S:
Pirin regulates epithelial to mesenchymal transition independently
of Bcl3-Slug signaling. FEBS Lett. 589:738–743. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Katsuyama S, Sugino K, Sasazawa Y, Nakano
Y, Aono H, Morishita K, Kawatani M, Umezawa K, Osada H and Simizu
S: Identification of a novel compound that inhibits
osteoclastogenesis by suppressing nucleoside transporters. FEBS
Lett. 590:1152–1162. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ishida K, Wierzba MK, Teruya T, Simizu S
and Osada H: Novel heparan sulfate mimetic compounds as antitumor
agents. Chem Biol. 11:367–377. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Schneider CA, Rasband WS and Eliceiri KW:
NIH Image to ImageJ: 25 years of image analysis. Nat Methods.
9:671–675. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Simizu S, Imoto M and Umezawa K: Induction
of apoptosis by erbstatin in mouse leukemia L1210 cells. Biosci
Biotechnol Biochem. 58:1549–1552. 1994. View Article : Google Scholar
|
|
39
|
Griffith EC, Su Z, Niwayama S, Ramsay CA,
Chang YH and Liu JO: Molecular recognition of angiogenesis
inhibitors fumagillin and ovalicin by methionine aminopeptidase 2.
Proc Natl Acad Sci USA. 95:15183–15188. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kerbel RS: Tumor angiogenesis. N Engl J
Med. 358:2039–2049. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Davis FM, Stewart TA, Thompson EW and
Monteith GR: Targeting EMT in cancer: Opportunities for
pharmacological intervention. Trends Pharmacol Sci. 35:479–488.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Delgado-Bellido D, Serrano-Saenz S,
Fernández-Cortés M and Oliver FJ: Vasculogenic mimicry signaling
revisited: Focus on non-vascular VE-cadherin. Mol Cancer.
16:652017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Datta B, Majumdar A, Datta R and Balusu R:
Treatment of cells with the angiogenic inhibitor fumagillin results
in increased stability of eukaryotic initiation factor 2-associated
glycoprotein, p67, and reduces phosphorylation of extracellular
signal-regulated kinases. Biochemistry. 43:14821–14831. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Datta B and Datta R: Mutation at the
acidic residue-rich domain of eukaryotic initiation factor 2
(eIF2alpha)-associated glycoprotein p67 increases the protection of
eIF2alpha phosphorylation during heat shock. Arch Biochem Biophys.
413:116–122. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Datta B, Ghosh A, Majumdar A and Datta R:
Autoproteolysis of rat p67 generates several peptide fragments: The
N-terminal fragment, p26, is required for the protection of
eIF2alpha from phosphorylation. Biochemistry. 46:3465–3475. 2007.
View Article : Google Scholar : PubMed/NCBI
|