Introduction
Gastric cancer is the fifth most frequently
diagnosed cancer and the third leading cause of cancer-related
death (1). Various biomarkers
targeting gastric cancer cells have been studied; however, there
are no standardized and established biomarkers, particularly for
scirrhous gastric cancer (2–4). A
large amount of stroma containing fibroblasts is present around
scattered gastric cancer cells; this feature is remarkable in
scirrhous gastric cancer, which, among the subtypes of gastric
cancer, has a poor prognosis (5,6).
Thus, an analysis that focuses solely on the expression in cancer
cells themselves may not be sufficient to identify biomarkers that
reflect the condition of patients with scirrhous gastric
cancer.
The stromal cells around a cancer lesion are called
cancer-associated fibroblasts (CAFs) and it has been reported that
CAFs are involved in cancer progression and the metastatic
potential of gastric cancer (7,8).
There are several CAF markers in gastric cancer, including α-smooth
muscle actin (α-SMA), fibroblast activation protein (FAP) and
podoplanin (PDPN) (9,10). Previous research by our group
focused on PDPN, which is expressed in lymphatic endothelium, as a
CAF marker in gastric cancer and the results indicated that high
PDPN expression in CAFs is a poor prognostic factor in patients
with gastric cancer (11).
Furthermore, it was observed that PDPN is expressed in CAFs but
rarely in gastric cancer cells (11). In the present study, it was
hypothesized that it may be useful to focus on molecules present in
gastric cancer stromal tissue rather than gastric cancer cells as
biomarkers to indicate the pathology of scirrhous gastric
cancer.
Using liquid biopsy instead of tissue biopsy may
enable a comprehensive evaluation of patient pathology. The present
study aimed to use liquid biopsy to search for biomarkers targeting
CAFs. In addition, the clinical significance of soluble PDPN was
investigated in patients with gastric cancer by assessing the
relationship between plasma PDPN levels and patients'
clinicopathological factors and prognosis. Furthermore, the
biological significance of soluble PDPN was examined by assessing
the association between tissue and plasma PDPN expression and by
evaluating the function of soluble PDPN in vitro.
Materials and methods
Patients and samples
The present study was performed as a joint research
project of the Faculty of Medicine, University of Yamanashi
(Yamanashi, Japan) and the Division of Digestive Surgery,
Department of Surgery, Kyoto Prefectural University of Medicine
(Kyoto, Japan). A total of 46 and 84 patients with gastric cancer
were respectively included in a development cohort (Table I) and an independent validation
cohort (Table SI) to assess the
clinicopathological characteristics of plasma PDPN levels.
Furthermore, the analysis was performed using the data of all 130
patients from both cohorts (Table
II). The development cohort included patients who underwent
radical resection at Yamanashi University Hospital (Yamanashi,
Japan) between December 2017 and May 2020. The independent
validation cohort included patients with stage I–III gastric cancer
who underwent radical resection at Kyoto Prefectural University of
Medicine Hospital (Kyoto, Japan) between June 2010 and December
2015. The prognostic impact of plasma PDPN expression was also
investigated by determining the 5-year overall survival (OS) rate
and 5-year the recurrence-free survival (RFS) rate in the
validation cohort with stage I–III gastric cancer. Patient
demographic data and details of tumor recurrence and subsequent
management were recorded. The pathological classification of tumors
was determined according to the Union for International Cancer
Control classification (12).
 | Table I.Clinicopathological features and their
relationship with plasma PDPN levels in patients with gastric
cancer from the development cohort (n=46). |
Table I.
Clinicopathological features and their
relationship with plasma PDPN levels in patients with gastric
cancer from the development cohort (n=46).
|
| PDPN levels in
plasma |
|---|
|
|
|
|---|
| Variable | Low | High | P-value |
|---|
| Sex |
|
| 0.434 |
| Male | 10 (50.0) | 16 (61.5) |
|
|
Female | 10 (50.0) | 10 (38.5) |
|
| Age, years | 74.6±8.3 | 70.6±12.3 | 0.225 |
| Tumor size, mm | 62.4±39.7 | 65.7±49.2 | 0.805 |
| Depth of tumor |
|
| 0.212 |
| T2-3 | 16 (80.0) | 16 (61.5) |
|
| T4 | 4
(20.0) | 10 (38.5) |
|
| Lymph node
metastasis |
|
| 0.350 |
|
Negative | 5
(25.0) | 11 (42.3) |
|
|
Positive | 15 (75.0) | 15 (57.7) |
|
| Lymphatic
invasion |
|
| 0.883 |
|
Negative | 5
(25.0) | 7
(26.9) |
|
|
Positive | 15 (75.0) | 19 (73.1) |
|
| Venous
invasion |
|
| 0.802 |
|
Negative | 4
(20.0) | 6
(23.1) |
|
|
Positive | 16 (80.0) | 20 (76.9) |
|
| pStage |
|
| 0.208 |
| I | 1 (5.0) | 4
(15.4) |
|
| II | 11 (55.0) | 17 (65.4) |
|
|
III | 8
(40.0) | 5 (19.2) |
|
| Lauren
classification |
|
| 0.079 |
|
Intestinal type | 14 (70.0) | 11 (42.3) |
|
| Diffuse
type | 6
(30.0) | 15 (57.7) |
|
 | Table II.Clinicopathological features and
their relationship with plasma PDPN expression in patients with
gastric cancer (n=130). |
Table II.
Clinicopathological features and
their relationship with plasma PDPN expression in patients with
gastric cancer (n=130).
|
| PDPN expression in
plasma |
| Multivariate |
|---|
|
|
|
|
|
|---|
| Variable | Low | High | P-value
Univariate | Odds ratio | 95% CI | P-value |
|---|
| Sex |
|
| 0.031 | 1.80 | 0.852-3.788 | 0.124 |
|
Male | 48 (68.6) | 30 (50.0) |
|
|
|
|
|
Female | 22 (31.4) | 30 (50.0) |
|
|
|
|
| Age, years | 71.8±8.1 | 68.0±12.5 | 0.085 |
|
|
|
| Tumor size, mm | 58.9±32.3 | 70.5±43.1 | 0.186 |
|
|
|
| Depth of tumor |
|
| 0.976 |
|
|
|
|
T2-3 | 50 (71.4) | 43 (71.7) |
|
|
|
|
| T4 | 20 (28.6) | 17 (28.3) |
|
|
|
|
| Nodal status |
|
| 0.846 |
|
|
|
|
Negative | 28 (40.0) | 23 (38.3) |
|
|
|
|
|
Positive | 42 (60.0) | 37 (61.7) |
|
|
|
|
| Lymphatic
invasion |
|
| 0.926 |
|
|
|
|
Negative | 18 (25.7) | 15 (25.0) |
|
|
|
|
|
Positive | 52 (74.3) | 45 (75.0) |
|
|
|
|
| Venous
invasion |
|
| 0.185 |
|
|
|
|
Negative | 25 (35.7) | 15 (25.0) |
|
|
|
|
|
Positive | 45 (64.3) | 45 (75.0) |
|
|
|
|
| Lauren
classification |
|
| 0.002 | 2.73 | 1.308-5.703 | 0.008 |
|
Intestinal | 48 (68.6) | 25 (41.7) |
|
|
|
|
|
Diffuse | 22 (31.4) | 35 (58.3) |
|
|
|
|
Preparation of plasma samples
From each patient, a 5-ml blood sample was collected
in an EDTA tube prior to surgery. Plasma was immediately separated
from the cellular fraction by centrifugation as described elsewhere
(13) and then stored at −80°C for
further processing.
Plasma PDPN analysis using ELISA
Quantification of PDPN expression in the plasma was
performed using the Human Podoplanin ELISA kit (cat. no.
ELH-PDPN-1; RayBiotech, Inc.) according to the manufacturer's
protocol. In brief, 100 µl of plasma from each patient was pipetted
into wells coated with an antibody specific for human PDPN. The
plate was incubated for 2.5 h at room temperature to allow binding
of the immobilized antibody to PDPN in the plasma. After washing
the wells, biotinylated anti-human PDPN antibody was added,
followed by incubation for 1 h at room temperature. After washing,
bound PDPN was incubated with horseradish peroxidase
(HRP)-conjugated streptavidin for 45 min at room temperature; the
binding of HRP-conjugated streptavidin was detected with
3,3′,5,5′-tetramethylbenzidine substrate solution. The intensity of
the color was measured at a wavelength of 450 nm.
Immunohistochemical procedures and
evaluation
Immunohistochemistry was performed using 32 tissue
samples of stages II and III from the Yamanashi University cohort.
Formalin-fixed, paraffin-embedded tissue was cut into 4-µm slices
that were placed on glass slides. Slides were deparaffinized using
xylene and rehydrated using a graded series of ethanol solutions.
Antigen retrieval was performed by heating the samples in Dako
Target Retrieval Solution (Agilent Technologies, Inc.) for 20 min
at 120°C. Endogenous peroxidases were quenched using peroxidase
blocking reagent (Dako; Agilent Technologies, Inc.). Sections were
incubated overnight at 4°C with D2-40 monoclonal antibody (cat. no.
413451; not diluted; Nichirei Biosciences, Inc.). After washing,
immunoperoxidase staining was performed using a Vectastain ABC
elite kit (Vector Laboratories) and 3,3′-diaminobenzidine tablet
(Fujifilm) according to the manufacturers' instructions, followed
by counterstaining with hematoxylin. Lymphatic epithelium stained
positive for PDPN was used as a positive control for each slide.
The investigators were blinded to the clinicopathological data of
the patients. PDPN expression was evaluated using high-power
microscopy (magnification, ×100) in five different fields. PDPN
expression in the stroma surrounding the cancer cells was
evaluated. Samples with the presence of immunoreactivity in >10%
of stromal cells were considered positive. The CAFs at the
peritumoral and tumor invasion fronts were assessed.
Cell culture
The human gastric cancer cell lines NUGC-3 and MKN74
were used in the present study. Cell lines were obtained from the
Japanese Collection of Research Bioresources Cell Bank and were
cultured in RPMI 1640 medium (Thermo Fisher Scientific, Inc.)
supplemented with 100 U/ml penicillin (Sigma-Aldrich; Merck KGaA),
100 µg/ml streptomycin (Sigma-Aldrich; Merck KGaA) and 10% fetal
bovine serum (FBS; Thermo Fisher Scientific, Inc.). Cells were
incubated in a 5% carbon dioxide atmosphere at 37°C.
Biological functional analysis of
soluble PDPN in gastric cancer cells
To assess whether soluble PDPN is able to alter the
phenotype of gastric cancer cells, cells were treated with PDPN
protein and then subjected to the assays specified below. As the
PDPN protein, the Podoplanin/Fc Chimera, Human, Recombinant,
carrier-free (R&D Systems, Inc.) was used.
Migration and invasion assays
A migration assay was performed using Falcon cell
culture inserts with 8-µm pore membranes for use with 12-well
plates (Corning, Inc.). Furthermore, an invasion assay was
performed using Falcon cell culture inserts with 8-µm pore
membranes for use in 24-well plates (Corning, Inc.), for which the
insert was coated with Biocoat Matrigel® (BD
Biosciences) prior to use.
In the migration and invasion assays, gastric cancer
cells (2×105 cells/ml) were seeded in the upper chambers
in FBS-free medium with or without PDPN. The medium volume in the
migration assay was 1 ml and the medium volume in the invasion
assay was 500 µl. PDPN protein was used at a concentration of 2
µg/ml. Medium containing 10% FBS was added to the lower
chambers.
After incubation for 24 h, cells that had not
migrated or invaded through the pores were removed using cotton
swabs. The migrated or invaded cells were fixed and stained with
Diff-Quick staining reagent. Cells were counted in four independent
fields at a magnification of ×100 using a BZ-X9000 All-in-One
fluorescence microscope and BZ-X Analyzer Software Hybrid cell
count (Keyence Corporation).
Proliferation assay
Gastric cancer cells were seeded into 12-well plates
at a concentration of 2.0×105/ml and then treated with
PDPN at 2 µg/ml. Cells were then incubated for 48 h and
subsequently peeled off and counted by the Automated cell counter
Countess (Invitrogen; Thermo Fisher Scientific, Inc.).
Statistical analysis
Continuous variables in each group were compared
using the Wilcoxon signed-rank test. The χ2 test and
Fisher's test were used to compare the categories of each group.
The Cox proportional hazards model was used to investigate the
clinicopathological factors that were significantly associated with
plasma PDPN. Survival curves were constructed using the
Kaplan-Meier method and compared using the log-rank test.
Differences were assessed using a two-sided test P<0.05 was
considered to indicate a statistically significant difference.
Results
PDPN detection in plasma
The range of the plasma PDPN concentration was
0-678.6 ng/ml. In the present study, the cut-off value of the
plasma-PDPN concentration was set to the median plasma-PDPN
concentration in the development cohort that was then divided into
the high-PDPN and low-PDPN groups. The cut-off value was 0.6
ng/ml.
The associations between the clinicopathological
characteristics of patients with gastric cancer and the results of
the plasma PDPN status determined with PDPN ELISAs are provided in
Tables I and II. In the development cohort, there were
no significant differences in clinicopathological parameters
between the two groups; however, the high-PDPN group tended to have
a higher proportion of diffuse-type gastric cancer according to
their Lauren classification (14)
(P=0.079; Table I). Multivariate
logistic regression analysis of all 130 patients in both cohorts
revealed that a high-PDPN status was significantly associated with
diffuse-type gastric cancer (P=0.008; Table II).
The prognostic impact of PDPN in the plasma was then
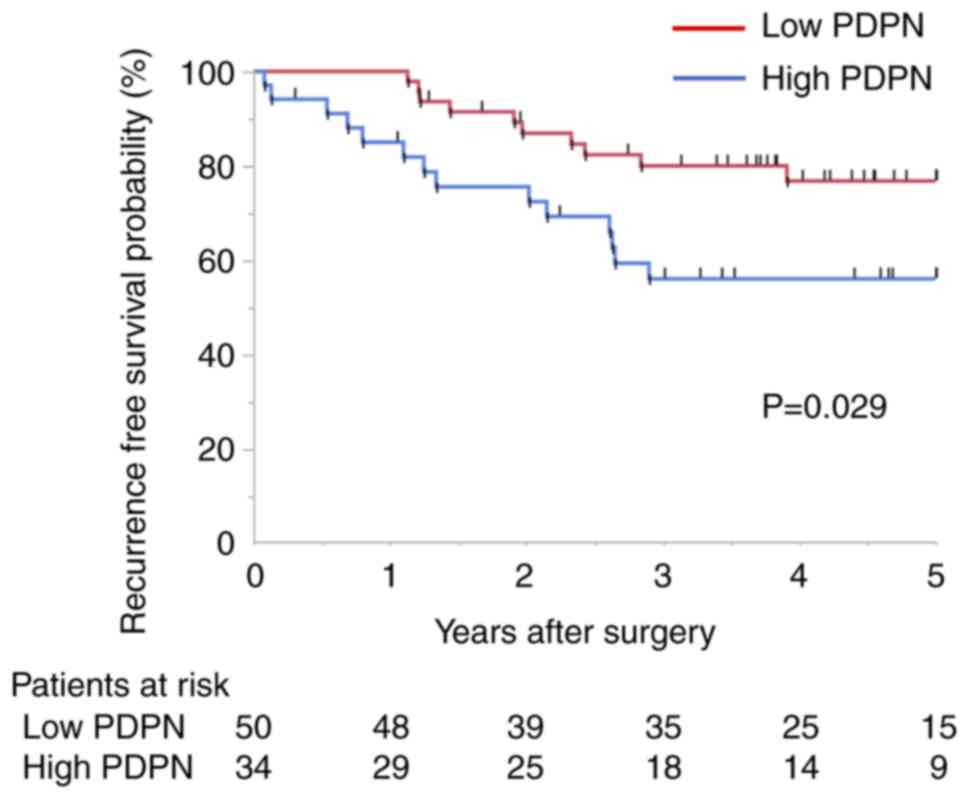
investigated in the validation cohort (Fig. 1). Kaplan-Meier survival estimates
indicated that the recurrence-free survival (RFS) rate was
significantly lower (P=0.029) and the overall survival rate tended
to be lower (P=0.126) in the high-PDPN group (Fig. S1). The 5-year RFS rate in the
PDPN-positive group was 56.1% and that in the negative group was
76.8%. There was no significant difference in recurrence patterns
between the high- and low-PDPN groups. A subgroup analysis of 36
patients with diffuse-type gastric cancer according to the levels
of plasma PDPN expression revealed that the RFS rate tended to be
lower in the high-PDPN group than in the low-PDPN group, but it was
not significant (P=0.166; Fig.
S1).
Association between tissue and plasma
PDPN expression
To investigate the origin of soluble plasma PDPN,
the association between plasma PDPN expression and tissue PDPN
expression was examined through immunohistochemical staining.
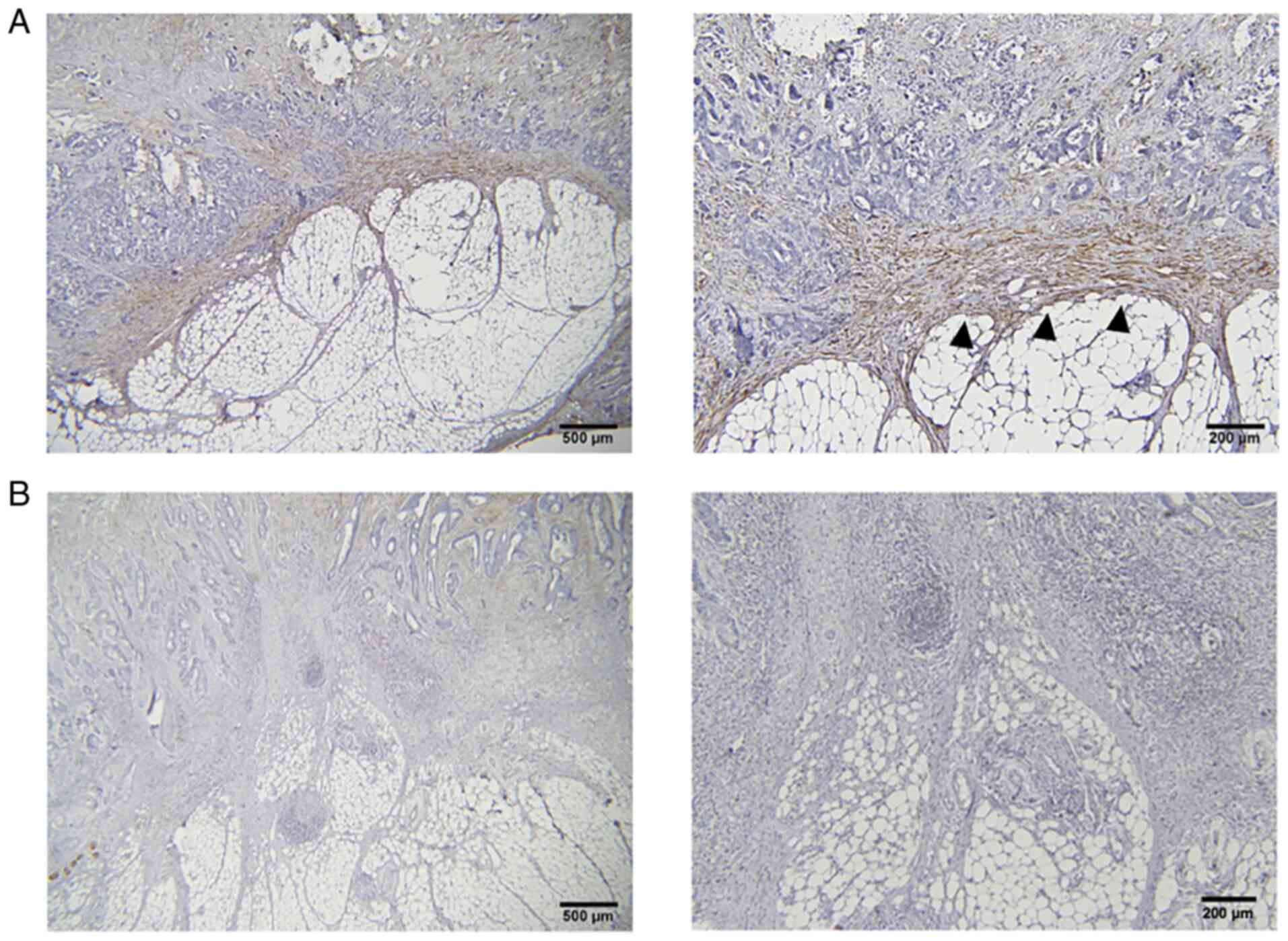
Representative results of the immunohistochemical detection of the
PDPN protein are provided in Fig.
2. Of the 46 patients in the development cohort, the data of 32
patients, for whom both tissue and plasma samples were obtained,
were examined. Among these, 15 patients had high plasma PDPN
expression. PDPN expression on the tumor invasion front was
observed in 12 patients, of whom 9 (75%) had high plasma PDPN
expression. By contrast, 14 (70%) of the 20 patients who did not
exhibit PDPN expression on the tumor invasion front had low plasma
PDPN expression (P=0.027; Table
III). These results indicated that plasma PDPN may reflect PDPN
expression in CAFs at the tumor invasion front.
 | Table III.Clinicopathological characteristics
of patients with Stage II/III gastric cancer with both tissue and
plasma samples from the development cohort (n=32). |
Table III.
Clinicopathological characteristics
of patients with Stage II/III gastric cancer with both tissue and
plasma samples from the development cohort (n=32).
|
| Podoplanin
expression in plasma |
|
|---|
|
|
|
|
|---|
| Variable | Low | High | P-value |
|---|
| Sex |
|
| 0.760 |
|
Male | 8 (47.1) | 8 (53.3) |
|
|
Female | 9 (52.9) | 7 (46.7) |
|
| Age, years | 75.1±8.2 | 69.7±12.8 | 0.162 |
| Tumor size, mm | 68.1±41.6 | 69.7±53.4 | 0.925 |
| Depth of tumor |
|
| 0.062 |
|
T2,3 | 14 (82.4) | 7 (46.7) |
|
| T4 | 3 (17.7) | 8 (53.3) |
|
| Lymph node
metastasis |
|
| 0.243 |
|
Negative | 3
(17.7) | 6 (40.0) |
|
|
Positive | 14 (82.4) | 9 (60.0) |
|
| Lymphatic
invasion |
|
| 0.736 |
|
Negative | 3
(17.7) | 2
(13.3) |
|
|
Positive | 14 (82.4) | 13 (86.7) |
|
| Venous
invasion |
|
| 0.499 |
|
Negative | 4
(23.5) | 2
(13.3) |
|
|
Positive | 13 (76.5) | 13 (86.7) |
|
| Lauren
classification |
|
| 0.280 |
|
Intestinal type | 12 (70.6) | 7 (46.7) |
|
| Diffuse
type | 5
(29.4) | 8 (53.3) |
|
| IHC of tumor
invasion front |
|
| 0.027 |
|
Negative | 14 (82.4) | 6 (40.0) |
|
|
Positive | 3
(17.6) | 9 (60.0) |
|
Biological analysis of soluble
PDPN
To investigate whether soluble PDPN itself is able
to affect phenotypic changes in gastric cancer cells, NUGC-3 and
MKN74 cells were treated with soluble PDPN.
In the migration and invasion assays, the addition
of PDPN did not enhance the migratory ability compared with the
control. In addition, there was no significant difference in
proliferative ability between the two groups (Figs. S2 and S3).
Discussion
Diffuse-type gastric cancer, also known as scirrhous
gastric cancer, has high invasion and metastatic potential, causes
peritoneal metastasis at an early stage and is considered to have a
poor prognosis despite various treatment strategies (15). Diffuse-type gastric cancer is
characterized by a larger number of stromal cells, called CAFs,
than the number of cancer cells in the tissue (16). The present study focused on CAFs in
gastric cancer and examined their potential as a blood biomarker.
Identification of PDPN, one of the representative CAF markers in
gastric cancer, in the plasma was significantly correlated with
diffuse-type gastric cancer and patients with gastric cancer with
high plasma PDPN expression had poor prognosis.
Various molecules, such as α-SMA, FAP and PDPN, have
been reported as CAF markers for gastric cancer (9,10). A
previous study by our group reported that PDPN is a CAF marker for
gastric cancer and demonstrated the prognostic impact of PDPN
expression in patients with gastric cancer (11). Liu et al (17) also demonstrated that high PDPN
expression in tissues reduces the sensitivity to adjuvant
chemotherapy and correlates with poor prognosis of patients with
gastric cancer. PDPN is a mucin-like transmembrane glycoprotein
that is widely used as a marker for the lymphatic endothelium
(18). PDPN expression in cancer
cells has been reported in various tumor types, including brain
tumors, esophageal squamous cell carcinoma, angiosarcoma,
mesothelioma, squamous cell carcinoma of the lung and cervical
carcinoma (19,20). By contrast, in gastric and
colorectal cancers, PDPN expression is low in cancer cells but high
in CAFs (11,21).
In the present study, the association between plasma
PDPN expression and tissue PDPN expression was examined to
investigate the origin of soluble plasma PDPN. Plasma PDPN
expression was associated with PDPN expression at the tumor
invasion front, suggesting that plasma PDPN may reflect the number
of CAFs at the tumor invasion front of the tissue and may be a
surrogate marker for CAFs in patients with gastric cancer. Neri
et al (22) reported that
PDPN-positive CAFs at the tumor invasion front lead and enhance the
local invasion of cancer cells in vitro. Thus, plasma PDPN
may be an indication of CAF activity, which promotes tumor
progression.
Zhao et al (23) demonstrated that the plasma levels
of soluble PDPN reflect tumor dynamics prior to and after treatment
in patients with gastric cancer. In the present study, it was also
demonstrated that soluble PDPN not only reflects the amount of
stroma in patients with gastric cancer but also has potential as a
prognostic biomarker. Kemi et al (24) reported that the amount of stroma in
gastric cancer tissues correlates with poor prognosis in patients
with gastric cancer. Furthermore, the present results not only
demonstrated that plasma PDPN expression may help determine the
prognosis of patients with diffuse-type gastric cancer but
indicated that patients with intestinal-type gastric cancer with
high plasma PDPN expression may also tend to have unfavorable
prognosis, although there was no significant influence (data not
shown). These results suggest the possibility that PDPN-based
liquid biopsy has the potential to detect hidden diffuse-type
gastric cancers. The plasma-based PDPN liquid biopsy performed in
the present study may not only reflect the amount of stroma in
patients with gastric cancer but may also enable comprehensive
assessment of a patient's status without being affected by
intra-tissue heterogeneity.
It remains elusive whether the soluble PDPN examined
in the present study is a functional secretory protein or
CAF-derived membrane debris. In the molecular biological analysis,
no phenotypic changes were observed in cancer cells due to the
direct administration of soluble PDPN. However, the mechanisms
underlying platelet-mediated cancer progression may also be
considered. Suzuki-Inoue et al (25) reported that PDPN is an in
vivo ligand of the platelet activation receptor CLEC-2.
Platelets have a major role in cancer progression by adhering to
cancer cells and supporting metastasis (26). Soluble PDPN may indirectly affect
cancer progression via platelet activation. In addition, cell-cell
communication through microvesicles may be considered.
Carrasco-Ramírez et al (27) reported that PDPN protein was
transmitted between cells as a component of extracellular vesicles.
It may be necessary to examine the functional changes of gastric
cancer cells by cell-cell communication of PDPN using extracellular
vesicles instead of direct administration of PDPN protein.
The present study has certain limitations. The study
had a small sample size and a retrospective design; therefore, bias
was likely to be present and influence the clinicopathological
findings. Furthermore, since the observation period in the
development cohort was short and it was not possible to analyze the
survival data, the survival outcome was analyzed in a small sample
using the validation cohort; thus, it was not possible to draw any
concrete conclusions regarding the prognostic impact of a
plasma-based PDPN liquid biopsy.
In conclusion, the present study focused on PDPN, a
representative marker of CAFs in gastric cancer, and investigated
its potential as a blood biomarker. Plasma soluble PDPN was
indicated to be a surrogate marker for diffuse-type gastric cancer
and may reflect the prognosis of gastric cancer.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This work was partially supported by the JSPS KAKENHI (grant no.
21K16442).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
Conceptualization: KS and DI; Methodology: KoT, KS,
TN, KaT, RS, AY, SF; Formal analysis and investigation: KoT, KS,
HidenoA, NH, YK, HidetA, HiromK, SI, HirosK, HirotK and EO; Writing
- original draft preparation: KoT; Writing - review and editing:
KS, DI and EO. KS and KoT checked and approved the authenticity of
the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the ethics
committee of the University of Yamanashi Hospital (Yamanashi,
Japan; approval no. 2301) and Kyoto Prefectural University of
Medicine (Kyoto, Japan) and was performed in accordance with the
tenets of the Declaration of Helsinki. Written informed consent was
obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Izumi D, Zhu Z, Chen Y, Toden S, Huo X,
Kanda M, Ishimoto T, Gu D, Tan M, Kodera Y, et al: Assessment of
the diagnostic efficiency of a liquid biopsy assay for early
detection of gastric cancer. JAMA Netw Open. 4:e21211292021.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shoda K, Ichikawa D, Fujita Y, Masuda K,
Hiramoto H, Hamada J, Arita T, Konishi H, Kosuga T, Komatsu S, et
al: Clinical utility of circulating cell-free Epstein-Barr virus
DNA in patients with gastric cancer. Oncotarget. 8:28796–28804.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shoda K, Ichikawa D, Fujita Y, Masuda K,
Hiramoto H, Hamada J, Arita T, Konishi H, Komatsu S, Shiozaki A, et
al: Monitoring the HER2 copy number status in circulating tumor DNA
by droplet digital PCR in patients with gastric cancer. Gastric
Cancer. 20:126–135. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Miki Y, Yashiro M, Moyano-Galceran L,
Sugimoto A, Ohira M and Lehti K: Crosstalk between cancer
associated fibroblasts and cancer cells in scirrhous type gastric
cancer. Front Oncol. 10:5685572020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huang KH, Chen MH, Fang WL, Lin CH, Chao
Y, Lo SS, Li AF, Wu CW and Shyr YM: The clinicopathological
characteristics and genetic alterations of signet-ring cell
carcinoma in gastric cancer. Cancers (Basel). 12:23182020.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shi H, Qi C, Meng L, Yao H, Jiang C, Fan
M, Zhang Q, Hou X and Lin R: Bone marrow-derived mesenchymal stem
cells promote Helicobacter pylori-associated gastric cancer
progression by secreting thrombospondin-2. Cell Prolif.
54:e131142021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jeong HY, Ham IH, Lee SH, Ryu D, Son SY,
Han SU, Kim TM and Hur H: Spatially distinct reprogramming of the
tumor microenvironment based on tumor invasion in diffuse-type
gastric cancers. Clin Cancer Res. 27:6529–6542. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Venning FA, Zornhagen KW, Wullkopf L,
Sjölund J, Rodriguez-Cupello C, Kjellman P, Morsing M, Hajkarim MC,
Won KJ, Erler JT and Madsen CD: Deciphering the temporal
heterogeneity of cancer-associated fibroblast subpopulations in
breast cancer. J Exp Clin Cancer. 40:1752021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nurmik M, Ullmann P, Rodriguez F, Haan S
and Letellier E: In search of definitions: Cancer-associated
fibroblasts and their markers. Int J Cancer. 146:895–905. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Maruyama S, Furuya S, Shiraishi K, Shimizu
H, Akaike H, Hosomura N, Kawaguchi Y, Amemiya H, Kawaida H, Sudo M,
et al: Podoplanin expression as a prognostic factor in gastric
cancer. Anticancer Res. 38:2717–2722. 2018.PubMed/NCBI
|
|
12
|
Brierley JD, Gospodarowicz MK and
Wittekind C: TNM classification of malignant tumours. 8th ed. John
Wiley & Sons, Ltd.; 2017
|
|
13
|
Tomita H, Ichikawa D, Ikoma D, Sai S, Tani
N, Ikoma H, Fujiwara H, Kikuchi S, Okamoto K, Ochiai T and Otsuji
E: Quantification of circulating plasma DNA fragments as tumor
markers in patients with esophageal cancer. Anticancer Res.
27:2737–2741. 2007.PubMed/NCBI
|
|
14
|
Lauren P: The two histological main types
of gastric carcinoma: Diffuse and so-called intestinal-type
carcinoma. An attempt at a histo-clinical classification. Acta
Pathol Microbiol Scand. 64:31–49. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen Y, Zhou Q, Wang H, Zhuo W, Ding Y, Lu
J, Wu G, Xu N and Teng L: Predicting peritoneal dissemination of
gastric cancer in the Era of precision medicine: Molecular
characterization and biomarkers. Cancers (Basel). 12:22362020.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kasashima H, Yashiro M, Nakamae H,
Kitayama K, Masuda G, Kinoshita H, Fukuoka T, Hasegawa T, Nakane T,
Hino M, et al: CXCL1-Chemokine (C-X-C Motif) receptor 2 signaling
stimulates the recruitment of bone marrow-derived mesenchymal cells
into diffuse-type gastric cancer stroma. Am J Pathol.
186:3028–3039. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu X, Cao Y, Lv K, Gu Y, Jin K, He X,
Fang H, Fei Y, Shi M, Lin C, et al: Tumor-infiltrating
podoplanin+ cells in gastric cancer: Clinical outcomes
and association with immune contexture. Oncoimmunology.
9:18450382020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cimini M and Kishore R: Role of
podoplanin-positive cells in cardiac fibrosis and angiogenesis
after ischemia. Front Physiol. 12:6672782021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Suzuki-Inoue K: Platelets and
cancer-associated thrombosis: Focusing on the platelet activation
receptor CLEC-2 and podoplanin. Blood. 134:1912–1918. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Quintanilla M, Montero-Montero L, Renart J
and Martín-Villar E: Podoplanin in Inflammation and Cancer. Int J
Mol Sci. 20:7072019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sugai T, Uesugi N, Kitada Y, Yamada N,
Osakabe M, Eizuka M, Sugimoto R, Fujita Y, Kawasaki K, Yamamoto E,
et al: Analysis of the expression of cancer-associated fibroblast-
and EMT-related proteins in submucosal invasive colorectal cancer.
J Cancer. 9:2702–2712. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Neri S, Ishii G, Hashimoto H, Kuwata T,
Nagai K, Date H and Ochiai A: Podoplanin-expressing
cancer-associated fibroblasts lead and enhance the local invasion
of cancer cells in lung adenocarcinoma. Int J Cancer. 137:784–796.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao X, Pan Y, Ren W, Shen F, Xu M, Yu M,
Fu J, Xia L, Ruan C and Zhao Y: Plasma soluble podoplanin is a
novel marker for the diagnosis of tumor occurrence and metastasis.
Cancer Sci. 109:403–411. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kemi N, Eskuri M, Herva A, Leppänen J,
Huhta H, Helminen O, Saarnio J, Karttunen TJ and Kauppila JH:
Tumour-stroma ratio and prognosis in gastric adenocarcinoma. Br J
Cancer. 119:435–439. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Suzuki-Inoue K, Inoue O, Ding G, Nishimura
S, Hokamura K, Eto K, Kashiwagi H, Tomiyama Y, Yatomi Y, Umemura K,
et al: Essential in vivo roles of the C-type lectin receptor
CLEC-2: Embryonic/neonatal lethality of CLEC-2-deficient mice by
blood/lymphatic misconnections and impaired thrombus formation of
CLEC-2-deficient platelets. J Biol Chem. 285:24494–24507. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Suzuki-Inoue K: Roles of the
CLEC-2-podoplanin interaction in tumor progression. Platelets. 1–7.
2018.(Epub ahead of print). PubMed/NCBI
|
|
27
|
Carrasco-Ramírez P, Greening DW, Andrés G,
Gopal SK, Martín-Villar E, Renart J, Simpson RJ and Quintanilla M:
Podoplanin is a component of extracellular vesicles that reprograms
cell-derived exosomal proteins and modulates lymphatic vessel
formation. Oncotarget. 7:16070–16089. 2016. View Article : Google Scholar : PubMed/NCBI
|