Introduction
Ovarian cancer is the seventh most common cancer in
women, and its incidence is the highest among all types of cancer
in women worldwide. Ovarian cancer is the most lethal
gynaecological cancer, with a 5-year survival rate of only 50%
(1). Until recently, there has
been slow progress in the development of treatment strategies for
this deadly disease. A combination of surgery and chemotherapy is
used as a standard therapy for patients with ovarian cancer.
Paclitaxel, a widely used first-line chemotherapeutic agent against
ovarian cancer, causes apoptosis of tumour cells by arresting the
G2/M phase in the cell cycle, which is important for cell division
(2). However, several clinical
studies have reported the occurrence of resistance to and toxicity
of paclitaxel, with limited results in the treatment of ovarian
cancer (3,4). Therefore, there is a need to develop
low-toxicity formulations with distinct molecular targets to
improve ovarian cancer treatment outcomes.
Inflammatory cytokines have been shown to play
several roles in cancerous tumour growth in the ovaries and in the
production of pre-inflammatory cytokines. Interleukin-6 (IL-6) is a
major immune-regulatory cytokine that has been implicated in
ovarian cancer growth (5,6). Glycoprotein 130 (GP130) is a
co-receptor subunit of the IL-6 cytokine family that contains IL-6,
IL-11, IL-27, oncostatin M (OSM), leukaemia inhibitory factor
(LIF), ciliary neurotrophic factor (CNTF), cardiotrophin 1 (CT-1),
and cardiotrophin-like cytokine factor (CLCF1). GP130 mediates a
wide variety of biological processes and plays an important role in
resistance to apoptosis, and promotion of angiogenesis,
proliferation, metastasis, and tumourigenesis (7–11).
Thus, inhibition of GP130 is a promising strategy for cancer
treatment.
The IL-6/GP130/signal transducer and activator of
transcription 3 (STAT3) pathway is involved in a variety of
oncogenic processes (12–15). In addition, constitutive activation
of STAT3 is maintained in ovarian cancer and plays an important
role in cancer cell growth, cell cycle progression, and invasion
(16,17). GP130 acts as a signal transducer
for this signalling axis, which is closely related to cancer
progression. However, the use of direct GP130 antagonists is
extremely limited. A recent study found that bazedoxifene, an
FDA-approved drug, targets the GP130 D1 domain and interferes with
the IL-6/GP130 interaction. Thus, inactivating the dimerisation of
the IL-6/IL-6R/GP130 heterotrimer can inhibit downstream STAT3
phosphorylation (18). Recently,
it has been reported that bazedoxifene exhibits anticancer activity
by inhibiting STAT3 signalling in cervical cancer, breast cancer,
pancreatic cancer, and colon cancer (19–23).
Research on bazedoxifene is expected to accelerate as well as
clinical treatment with bazedoxifene on a variety of cancers
dependent on IL-6/GP130/STAT3. However, the potential therapeutic
effects in regards to ovarian cancer have not yet been
investigated.
In the present study, combination therapy with
bazedoxifene and paclitaxel was found to inhibit the IL-6-mediated
GP130/STAT3 signalling pathway, induced apoptosis in ovarian cancer
cells, inhibited epithelial-mesenchymal transition (EMT), and
inhibited tumour growth in human ovarian cancer xenografts.
Targeting the GP130/STAT3 signalling pathway could act as a new
therapeutic strategy for ovarian cancer.
Materials and methods
Surface plasmon resonance (SPR)
analysis
SPR analysis was performed using the BIAcore T200
model (GE Healthcare) at room temperature with PBSTT buffer (1X
PBS, 0.05% Triton X-100, 0.05% Tween-20) containing 5% dimethyl
sulfoxide (DMSO; Sigma-Aldrich; Merck KGaA). The pH scouting for
GP130 (Sino Biological, China, or ANRT, Korea) and IL-6Rα
(PeproTech) immobilisation was performed in 10 mM acetate buffer at
pH 4.0, 4.5, 5.0, and 5.5. GP130 and IL-6Rα were immobilised on a
CM5 chip to 2,000 and 1,000 response units (RU) with standard amine
coupling at pH 4.5. Bazedoxifene was injected into the GP130 and
IL-6Rα-immobilised flow cell at concentrations of 50, 100, 125,
150, 175, and 200 µM with a flow rate of 20 µl/min for 250 sec and
allowed to dissociate for 600 sec. The T-200 BIAevaluation software
v3.1 (GE Healthcare) was used to subtract the references and
determine steady-state KD. Between the sample series, a
solvent correction cycle was run to adjust for referencing errors
caused by refractive index mismatches between the running buffer
and samples (24).
Bioassay with IL-6-dependent DS-1
cells
The DS-1 cell line was obtained from the American
Type Culture Collection (ATCC). DS-1 cells were cultured in
RPMI-1640 medium (Welgene) containing 10% FBS (HyClone; Thermo
Fisher Scientific, Inc.), 1% penicillin-streptomycin (Corning,
Inc.), 1 ng/ml recombinant human IL-6 (BioLegend). After starvation
of the DS-1 cells without IL-6 for 24 h, various concentrations of
IL-6 (0, 0.01, 0.05, 0.1, 0.5, 1, 5, and 10 ng/ml) were added to
5×04 cells/well and incubated for 72 h. The D-Plus™ Cell
Viability Assay Kit (DonginBiotech) was used to assess the cell
proliferation. Next, 10 µl of the kit reagent was added to each
well and incubated for 4 h in a CO2 incubator.
After incubation, the optical density (OD) was measured at 450
nm.
IL-6 inhibitory bioassay
After starvation of DS-1 cells without IL-6 for 24
h, various concentrations of bazedoxifene (1, 2.5, 5, 7.5, 10,
12.5, and 15 µM) (Sigma-Aldrich; Sigma-Aldrich; Merck KGaA) in the
presence of IL-6 (10 ng/ml) were added to 5×104
cells/well and incubated for 72 h. The D-Plus™ Cell Viability Assay
Kit (DonginBiotech) was used to measure cell proliferation. Next,
10 µl of the kit reagent was added to each well and incubated for 4
h in a CO2 incubator. After incubation, the OD was
measured at 450 nm wavelenth.
Transfection of small interfering RNA
(siRNA)
siRNAs against negative control siRNA (siNC) and
STAT3 (siSTAT3) were purchased from Genolution (Genolution
Pharmaceutical Inc.). Cells were seeded in 96-well plates at a
density of 1×104 cells/well. Cells were transfected with
30 nM siRNA in phosphate-buffered saline (1X PBS); a G-Fectin Kit
(Genolution Pharmaceutical Inc.) was used according to the
manufacturer's instructions. siRNA-transfected cells were used in
the in vitro assays 48 h after transfection. The target
sequences of siNC and siSTAT3 are listed in Table SI. The efficiency of siRNA-based
STAT3 knockdown transfection was assessed by western blotting and
then the cells were used for subsequent experiments.
MTT cell viability assay in ovarian
cancer cell lines
The A2780 cell line was purchased from the European
Collection of Cell Cultures (ECACC, London, UK). The OVCA433 cell
line was provided by the Korea Gynecologic Cancer Bank through the
Bio and Medical Technology Development Program of the Ministry of
Science, Information and Communication Technology, and Future
Planning (MSIP). SKOV3 and TOV112D cell lines were purchased from
the Korean Cell Line Bank (KCLB) and American Type Culture
Collection (ATCC). The subtypes of the ovarian cancer cell lines
were as follows: A2780, not specified; OVCA433, serous; SKOV3,
serous; and TOV112D, endometrioid (25). Ovarian cancer cell lines were
seeded in 96-well plates at a density of 5,000 cells/well. The next
day, bazedoxifene (1, 2, 4, 6, 8, and 10 µM), paclitaxel (0.005,
0.01, and 0.05 µg/ml) (Bristol-Myers Squibb), and a combination of
bazedoxifene and paclitaxel was added in triplicate to the plates
for 48 h. The cells were incubated at 37°C. Then, 100 µl of
N,N-dimethylformamide (Sigma-Aldrich; Merck KGaA) solubilisation
solution was added to each well and incubated for 2 h. After 2 h,
the solution was removed by suction, and the cells stained with
DMSO were lysed. The OD was measured at 450 nm wavelength. The
half-maximal inhibitory concentration (IC50) was
analysed using the GraphPad Prism software (version 7.0; GraphPad
Software, Inc.). The combination index (CI) was calculated using
CompuSyn software ver 1.0 (ComboSyn, Inc.). The CI values indicate
an antagonistic effect when >1, an additive effect when equal to
1, and a synergistic effect when <1 (23).
Clonogenic formation assay
Each ovarian cancer cell line was seeded at
approximately 50,000 cells in a 60-mm dish. After 6 h, bazedoxifene
and paclitaxel, and the combination of bazedoxifene and paclitaxel
were added to the cell medium at each concentration (B: 10 µM, P:
0.05 µg/ml, OVCA433; B: 6 µM, P: 0.005 µg/ml, SKOV3; B: 6 µM, P:
0.01 µg/ml, A2780; B: 6 µM, P: 0.01 µg/ml, TOV112D). Cells that
formed colonies within 1 to 3 weeks were fixed with glutaraldehyde
(6.0% v/v), stained with crystal violet (0.5% w/v), and
photographed using a ChemiDoc imaging system (Bio-Rad
Laboratories). The intensities of the images were quantified using
ImageJ software 1.5 a (National Institutes of Health).
Wound-healing assay and Matrigel
invasion assay
Ovarian cancer cell lines were grown to
approximately 90% in each 6-well plate. After starvation in
serum-free medium for 24 h, a wound-healing assay was performed in
the same medium. A single layer of cells was scraped to form an
artificially homogeneous wound. Afterwards, each concentration of
the drug (B: 10 µM, P: 0.05 µg/ml, OVCA433; B: 6 µM, P: 0.005
µg/ml, SKOV3; B: 6 µM, P: 0.01 µg/ml, A2780; B: 6 µM, P: 0.01
µg/ml, TOV112D) was administered, and images were captured under a
microscope [Primo Vert (Carl Zeiss Inc.; ×100 magnification] at 0,
24, and 48 h, and the degree of cell migration was measured using
ImageJ software. All experiments were conducted independently in
triplicates (19).
Matrigel invasion assays were performed using a BD
BioCoat Matrigel Invasion Chamber (BD Bioscience). Ovarian cancer
cell lines (5×104 cells) were seeded inside the chamber
in serum-free medium containing 300 µl of drugs. Five hundred
microlitres of the medium containing serum was placed outside the
chamber. After 48 h of incubation, the cells that infiltrated the
chamber were stained using the Differential Quik Stain Kit (Diff
Quik, Sysmex). Images were captured with a microscope [Primo Vert
(Carl Zeiss Inc.; ×100 magnification], and the number of cells
invading the chamber was measured using ImageJ software. All
experiments were independently conducted in triplicate.
Western blotting
Cells were lysed in 100 µl of
radioimmunoprecipitation assay (RIPA) buffer (Thermo Fisher
Scientific Inc.) and centrifuged at 13,000 × g for 15 min at 4°C.
Protein concentration was measured using the Pierce BCA Protein
Assay Kit (Thermo Fisher Scientific, Inc.). Proteins were
quantified to 30 ng and boiled in 5X sample buffer, separated on a
10% SDS-polyacrylamide gel, and transferred to polyvinylidene
difluoride (PVDF) membranes (Millipore) by electrophoresis. The
membranes were blocked for 1 h at room temperature with 3% BSA in
1X Tris-buffered saline containing 0.1% Tween 20 (Sigma-Aldrich;
Merck KGaA) and anti-β-actin (cat. no. 8457S; dilution 1:1,000),
anti-estrogen receptor α (ERα) (cat. no. 13258S; 1:500), anti-Mcl-1
(cat. no. 5354S; 1:1,000), anti-Bcl-xl (cat. no. 2764S; 1:500),
anti-Bcl-2 (cat. no. 3498S; 1:500), anti-Bax (cat. no. 2772S;
1:500), anti-E-cadherin (cat. no. 3195S; 1:500), anti-N-cadherin
(cat. no. 4061S; 1:500), anti-β-catenin (cat.no. 9562S; 1:1,000),
anti-vimentin (cat. no. 5741S; 1:1,000), anti-Snail (cat. no.
3879S; 1:500), anti-Twist (cat. no. 69366S; 1:500), anti-Claudin-1
(cat. no. 4933S; 1:500), anti-phospho-STAT3 (Tyr705) (cat. no.
9131S; 1:500), anti-STAT3 (cat. no. 12640S; 1:500),
anti-phospho-ERK 1/2 (cat. no. 4695S; 1:1,000), anti-ERK 1/2 (cat.
no. 9101S; 1:1,000), anti-phospho-p38 (cat. no. 4511S; 1:1,000),
anti-p38 (cat. no. 3690S; 1:1,000), anti-phospho-Akt (cat. no.
4060L; 1:1,000), anti-Akt (cat. no. 2920S; 1:1,000), anti-cyclin D1
(cat. no. 2978T; 1:1,000), anti-CDK4 (cat. no. 12790T; 1:1,000),
anti-CDK6 (cat. no. 3136T; 1:1,000), anti-p21waf1 (cat.
no. 2947T; 1:1,000), anti-p27kipl (cat. no. 3686T;
1:1,000), anti-PARP (cat. no. 9542S; 1:1,000), and anti-cleaved
caspase-3 (cat. no. 9661S; 1:1,000) (all obtained from Cell
Signalling Technologies, Inc.), anti-estrogen receptor β (ERβ)
(cat. no. ab3576; 1:1,000) (Abcam, Inc,), and anti-phospho-GP130
(cat. no. SC-377572; 1:500), and anti-GP130 (cat. no. SC-376280;
1:500) (Santa Cruz Biotechnology, Inc.) antibody was stored at 4°C
overnight. An anti-β-actin antibody was used as an internal
control. Membranes were washed with 1X Tris-buffered saline
containing 0.1% Tween-20 (TBST) and incubated with AffiniPure goat
anti-rabbit IgG secondary antibody (Jackson Immunoresearch) and
goat anti-mouse IgG secondary antibody (Bethyl Laboratories) for 2
h at room temperature. After washing again with TBST, an enhanced
chemiluminescence (ECL) kit (Thermo Fisher Scientific, Inc.) was
used to detect the signal, and the intensity was quantified using
ImageJ software. Data were obtained from at least three biological
replicates (23).
Flow cytometric analysis of
apoptosis
For cell death analysis, after treatment with
bazedoxifene, paclitaxel, or their combination for 48 h in ovarian
cancer cell lines, the level of apoptosis was quantified by flow
cytometry. Experiments were conducted using the Annexin V-FITC
Apoptosis Detection Kit (BD Pharmingen), and apoptosis was analysed
by flow cytometry. Cells were sorted using a FACSCanto II (BD
Biosciences), and apoptotic cells were analysed using BD FACS Diva
software version 6.2 (BD Biosciences). The analysis was performed
in triplicate (20).
Tumour xenografts in mice
A total of 24 female BALB/c nude mice (5 weeks of
age, body weight 18–20 g, Orient Bio) were maintained under aseptic
conditions with constant at 25°C temperature and 50% humidity with
a 12/12-h light/dark cycle. (Catholic University protocol). Water
and sterile food were provided daily at the Catholic University.
The animals had access to water and food ad libitum. Animal
experiments were approved by the Institutional Animal Care and Use
Committee (IACUC) of the Catholic University of Korea (approval no.
CUK-IACUC-2019-026-01). All experimental work complied with the
legal obligations and federal guidelines for the care and
maintenance of laboratory animals.
Each mouse received a subcutaneous injection of
1×107 OVCA433 cells in 100 µl PBS into the dorsal
scapula area, and the tumour volumes continued to be monitored. The
tumour size was measured every 3 days by using calipers, while the
bodyweight of the mice was measured. The tumours developed 10 days
after implantation, and the tumour size reached approximately 200
mm3. A total of 24 mice were randomly assigned into four
groups (n=6 per group). Next, the mice were randomly divided into
vehicle control, bazedoxifene, paclitaxel, and combination groups.
Bazedoxifene (4 mg/kg) was administered by gavage five times a
week, and paclitaxel (10 mg/kg) was administered by intraperitoneal
injection twice weekly (19,26).
Calipers were used to measure the tumour size once every 2 days.
Tumour volume was calculated using a simplified equation to
estimate the rotational ellipsoid (length × width2 ×
0.5). At the end of the experiment, the mice were then sacrificed
by CO2 inhalation (50% of the chamber volume/min).
Whenever the gradual displacement method is used, the
CO2 flow must be maintained for at least 1 min after
respiratory arrest. Each tumour was harvested 16 days
post-treatment and measured, and the maximum tumor diameter did not
exceed 2 cm.
Hematoxylin and eosin (H&E)
staining
The mice were sacrificed 16 days after the drug
administration. Tumour tissues were collected, fixed in 4%
paraformaldehyde for 24 h, washed in 1X PBS, and embedded in
paraffin. Sections (2-µm) were stained with H&E following
standard procedures. After staining, dehydration was performed.
After fixing with mineral oil, cover slipping was performed.
Data analysis
Results are presented as mean ± standard deviation
(SD). Data were assessed using Excel and GraphPad Prism software
(version 7.0; GraphPad Software, Inc.). Two-way ANOVA followed by
Bonferroni's post hoc test (multiple comparisons) were performed
with GraphPad Prism 7.0. P<0.05 was considered to indicate a
statistically significant difference.
Results
Characterisation of bazedoxifene and
anti-IL-6 activity in vitro
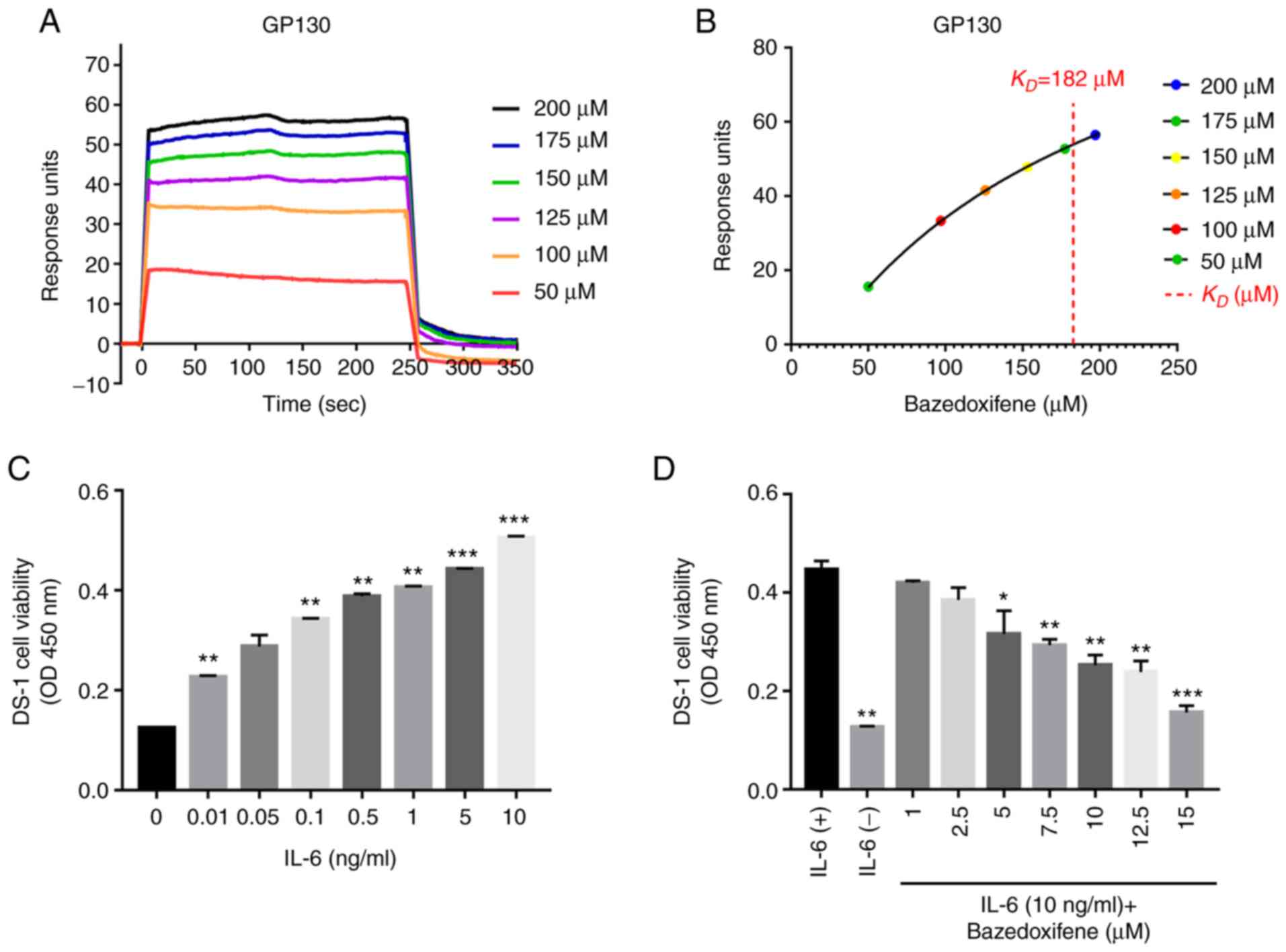
Bazedoxifene targets GP130, and in the present
study, surface plasmon resonance (SPR) analysis was performed to
monitor its binding affinity to GP130. Recombinant GP130 and
rIL-6Rα were covalently cross-linked to the dextran matrix of the
CM5 chip, and various concentrations of bazedoxifene were passed
through the surface and the reference surface. To investigate the
association between GP130 binding affinity and anticancer activity,
response units (RU) for each concentration of bazedoxifene were
detected by SPR analysis (Fig.
1A). An established SPR-based biosensor assay was used to
investigate the ability of bazedoxifene to bind GP130. A single
binding site model was fitted to the data to calculate the
KD value of bazedoxifene at 182.7 µM (Fig. 1B). Solvent-corrected binding curve
fit was analysed using the Biacore evaluation software. The IL-6Rα
data are shown in Fig. S1.
Interestingly, bazedoxifene has a higher binding affinity for
GP130. The IL-6-dependent DS-1 cell line was confirmed to be
dependent on IL-6, and the optimal IL-6 concentration for
inhibition analysis was determined to be 10 ng/ml (Fig. 1C). To confirm whether bazedoxifene
inhibited cell proliferation by blocking the binding of IL-6/GP130,
different concentrations of bazedoxifene were added to DS-1 cells
treated with IL-6 (10 ng/ml). The proliferation of cells was
inhibited by bazedoxifene in a concentration-dependent manner
(Fig. 1D). The results showed that
bazedoxifene inhibited cell proliferation by inhibiting the binding
of IL-6 to GP130.
Bazedoxifene, paclitaxel and their
combination inhibit cell viability and proliferation of ovarian
cancer cells
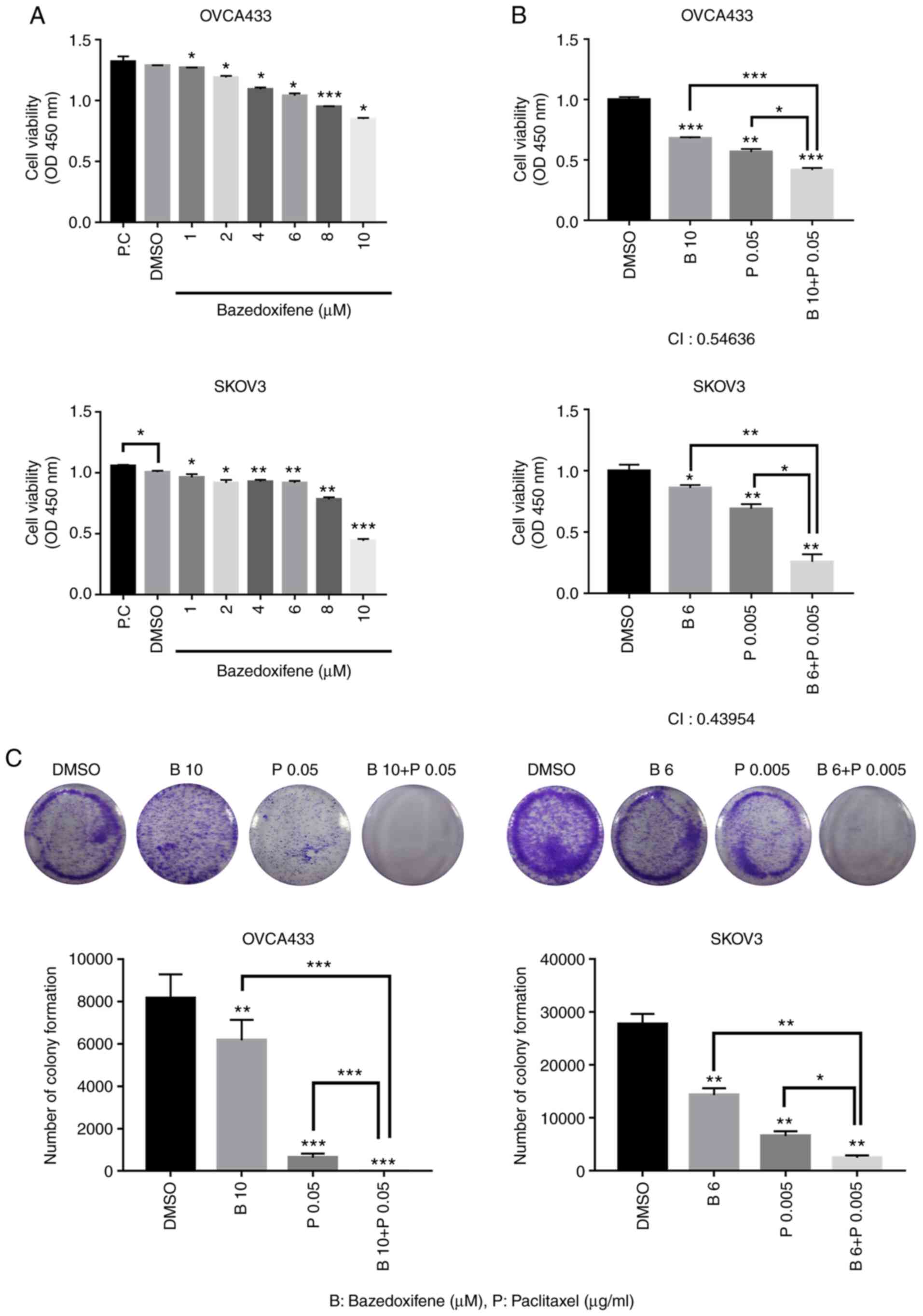
It was next investigated whether bazedoxifene
inhibits the growth of human ovarian cancer cell lines. OVCA433,
SKOV3, A2780, and TOV112D cells were treated with bazedoxifene at
different concentrations in triplicate for 48 h and processed for
MTT assay to analyse cell viability (Figs. 2A and S2A). Cell viability of OVCA433, SKOV3,
A2780, and TOV112D cells were decreased by bazedoxifene in a
concentration-dependent manner. To explore the synergistic
inhibitory effects of bazedoxifene and paclitaxel on ovarian cancer
cells, cells were treated with bazedoxifene, paclitaxel, or their
combination in triplicate for 48 h. Cell viability was further
inhibited in the combined group compared to the monotherapy group,
with respective IC50 values of 17.2, 8.99, 14.7 and 4.56
µM (Figs. 2B and S2B). The combination index (CI) values
of all combination treatments were <1, suggesting that combined
treatment with bazedoxifene and paclitaxel and
bazedoxifene-sensitized ovarian cancer cells were synergistic in
combination treatment with paclitaxel. To confirm the anticancer
ability of the drug in ovarian cancer cell lines, a clonogenic
formation assay was performed (Figs.
2C and S2C). The clonogenic
ability was significantly reduced in the group treated with
bazedoxifene and paclitaxel alone, compared to the group treated
with DMSO. In the group treated with bazedoxifene and paclitaxel,
colony formation was significantly suppressed compared to that in
the group treated with either drug alone. This suggests that the
combination treatment of bazedoxifene and paclitaxel exerts a
synergistic effect on ovarian cancer cells.
Bazedoxifene and paclitaxel or their
combination inhibits migration and invasion in ovarian cancer
cells
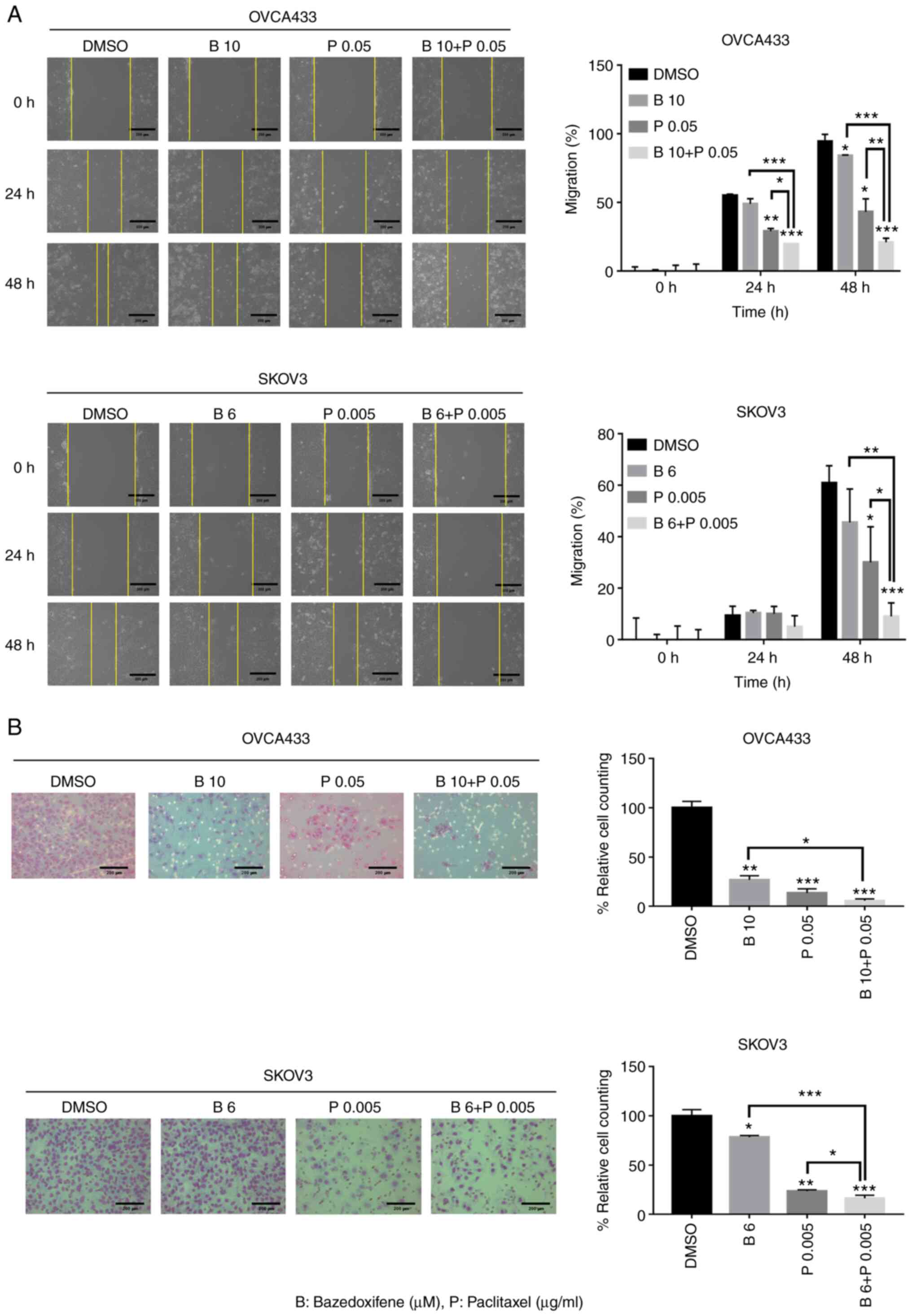
OVCA433 and SKOV3 cells were treated with
bazedoxifene, paclitaxel, or their combination and allowed to
migrate to the scratched area for 0, 24, and 48 h. The yellow line
indicates the width of the wound. The percentage of the migrating
area in the wound-healing assay was quantified in OVCA433 and SKOV3
cells. Cell migration was significantly inhibited at 48 h in both
bazedoxifene and paclitaxel, and bazedoxifene and paclitaxel
combination treatment groups compared with the DMSO-treated control
group. Among them, the group treated with bazedoxifene and
paclitaxel showed more significant inhibition of cell migration
(Fig. 3A). The Matrigel invasion
assay was used to determine the invasion after 48 h in OVCA433 and
SKOV3 cells treated with the combination of drugs. Compared with
the control group treated with DMSO, cell invasion was
significantly suppressed in both bazedoxifene and paclitaxel alone
and bazedoxifene and paclitaxel combination treatment groups. In
the group treated with bazedoxifene and paclitaxel, cell invasion
was significantly suppressed compared to that in groups treated
with bazedoxifene and paclitaxel alone (Fig. 3B). A2780 and TOV112D cells showed
significant results (Fig. S3),
suggesting that bazedoxifene and paclitaxel inhibited migration and
invasion, respectively. However, their combination exerted more
effective inhibitory effects on migration and invasion.
Bazedoxifene suppresses the expression
of p-STAT3 and estrogen receptors in ovarian cancer cells
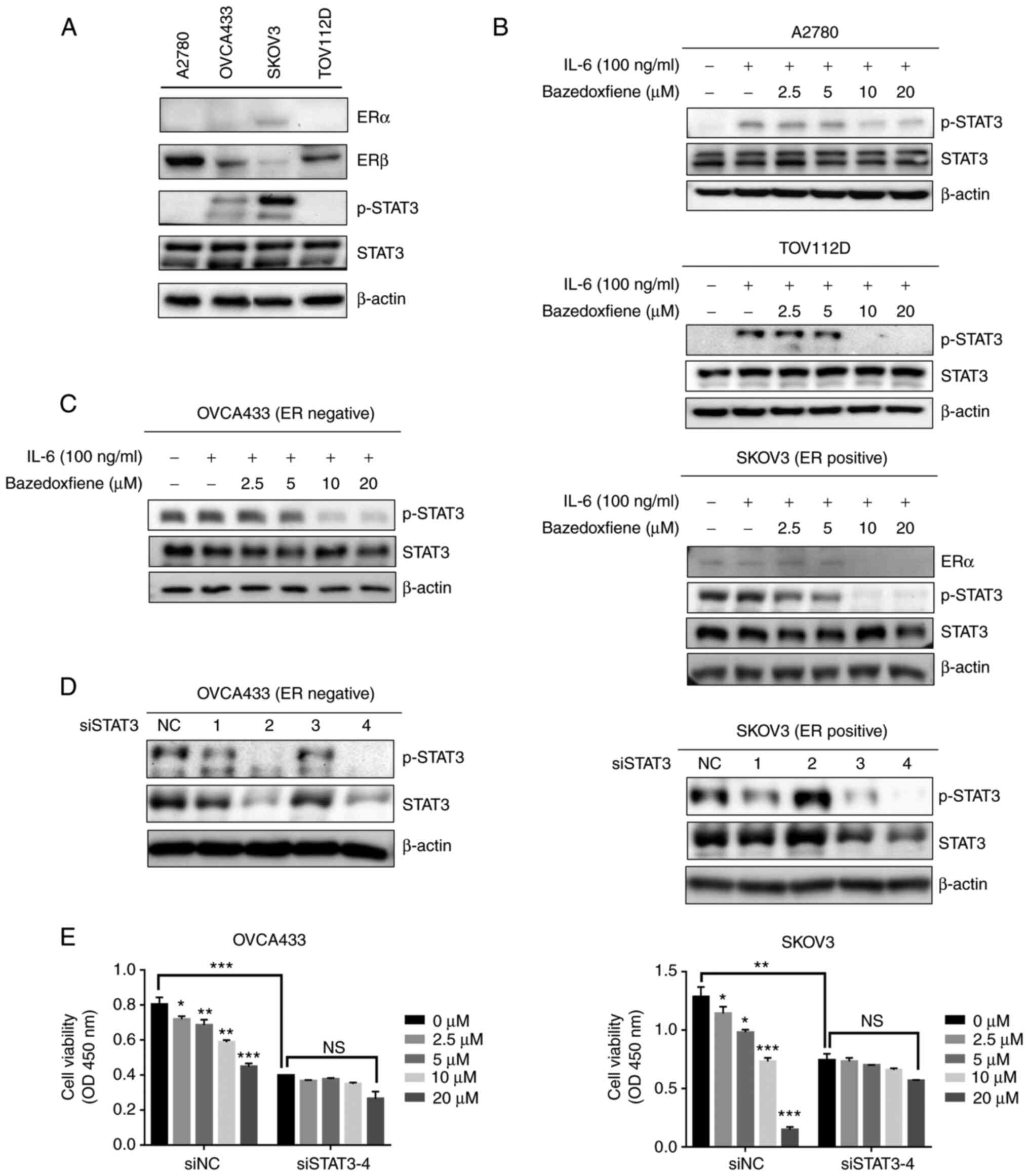
Bazedoxifene is a selective estrogen receptor (ER)
modulator, and research on potential novel GP130 inhibitors is
currently underway. We aimed to elucidate the mechanism by which
bazedoxifene inhibits STAT3 signalling by targeting GP130 in
ovarian cancer. As shown in Fig.
4A, SKOV3 cells were found to express ERα, while the other cell
lines tested exhibited no ERα expression. In addition, STAT3
phosphorylation (p-STAT3) was not observed in the A2780 and TOV112D
cell lines. Accordingly, it is thought that there is a difference
in the degree of drug response depending on the presence or absence
of ERs. IL-6 is known to induce STAT3 phosphorylation, and we
attempted to determine whether bazedoxifene can inhibit
IL-6-induced STAT3 phosphorylation. A2780 and TOV112D cells that
did not express phosphorylated STAT3 in serum-free medium for 24 h
were used. In this study. It was found that IL-6 stimulated the
phosphorylation of STAT3 and that bazedoxifene decreased
phosphorylation in a dose-dependent manner (Fig. 4B). The mechanism by which
bazedoxifene inhibits the phosphorylation of STAT3 in the
ER-positive ovarian cancer cell line SKOV3 was subsequently
investigated. Bazedoxifene downregulated the expression of ERα and
p-STAT3 induced by IL-6 in SKOV3 cells. It was also confirmed that
phosphorylation of STAT3 induced by IL-6 was inhibited as the
concentration of bazedoxifene increased in OVCA433 cells, which are
ER-negative cells (Fig. 4C). Thus,
we found that bazedoxifene inhibited STAT3 phosphorylation with and
without ERs.
OVCA433 and SKOV3 cells were transfected with four
different sequences of siSTAT3 (si1, si2, si3, and si4), and
siSTAT3-4 showed the maximum inhibitory activity against STAT3 in
the OVCA433 and SKOV3 cell lines (Fig.
4D). To confirm the ability of bazedoxifene to inhibit cell
proliferation and migration and to test whether this effect is
dependent on STAT3, the inhibitory effect of bazedoxifene on the
proliferation and migration of OVCA433 and SKOV3 cells transfected
with siSTAT3 was investigated (Figs.
4E and S4). We found that
bazedoxifene had no significant effect on siSTAT3 cells. These
results indicate that bazedoxifene is an inhibitory target of the
STAT3 signalling pathway in ER-positive and -negative ovarian
cancer cells. The whole western membrane and expression levels are
shown in Fig. S5.
Combined bazedoxifene and paclitaxel
inhibits IL-6-induced phosphorylation of GP130, STAT3, ERK1/2 and
EMT in ovarian cancer cells
The effect of bazedoxifene binding to GP130 on STAT3
signalling, a sub-signaling pathway of GP130, was investigated.
Ovarian cancer cell lines were starved for 24 h and treated with
bazedoxifene, paclitaxel, and a combination of bazedoxifene and
paclitaxel for 6 h. Next, to evaluate the effect on sub-signaling,
IL-6 (100 ng/ml) was added for 15 min, and p-GP130, p-STAT3, p-Akt,
p-p38 MAPK, p-ERK 1/2, and EMT-related antibodies were detected by
western blotting (Fig. 5).
Compared with the control group treated with only IL-6, the
phosphorylation of GP130 (p-GP130), STAT3 (p-STAT3), ERK1/2
(p-ERK1/2), Akt (p-Akt), and p38 MAPK (p-p38-MAPK) was decreased in
the bazedoxifene and paclitaxel alone group, and further decreased
in the combined treatment group (Fig.
5A). The whole western membrane and expression levels are shown
in Figs. S6 and S7. A2780 and TOV112D cells also showed
significant results (Fig. S8A).
The whole western membrane and expression levels are shown in
Figs. S9 and S10. To confirm whether STAT3 signalling
by drugs affected EMT, the expression of EMT markers N-cadherin,
E-cadherin, β-catenin, vimentin, Snail, Twist, and Claudin-1 was
assessed by western blotting. The combination treatment of
bazedoxifene and paclitaxel inhibited EMT-related protein
expression, with significant differences in the expression of
vimentin, Snail, Twist, and Claudin-1 in OVCA433 and SKOV3 cells
(Fig. 5B). The whole western
membrane and expression levels are shown in Figs. S11 and S12. A2780 and TOV112D cells showed
significant results (Fig. S8B).
The whole western membrane and expression levels are shown in
Figs. S13 and S14. These results showed that
bazedoxifene and paclitaxel inhibited the signalling of GP130/STAT3
and EMT, and the combination treatment significantly inhibited this
effect.
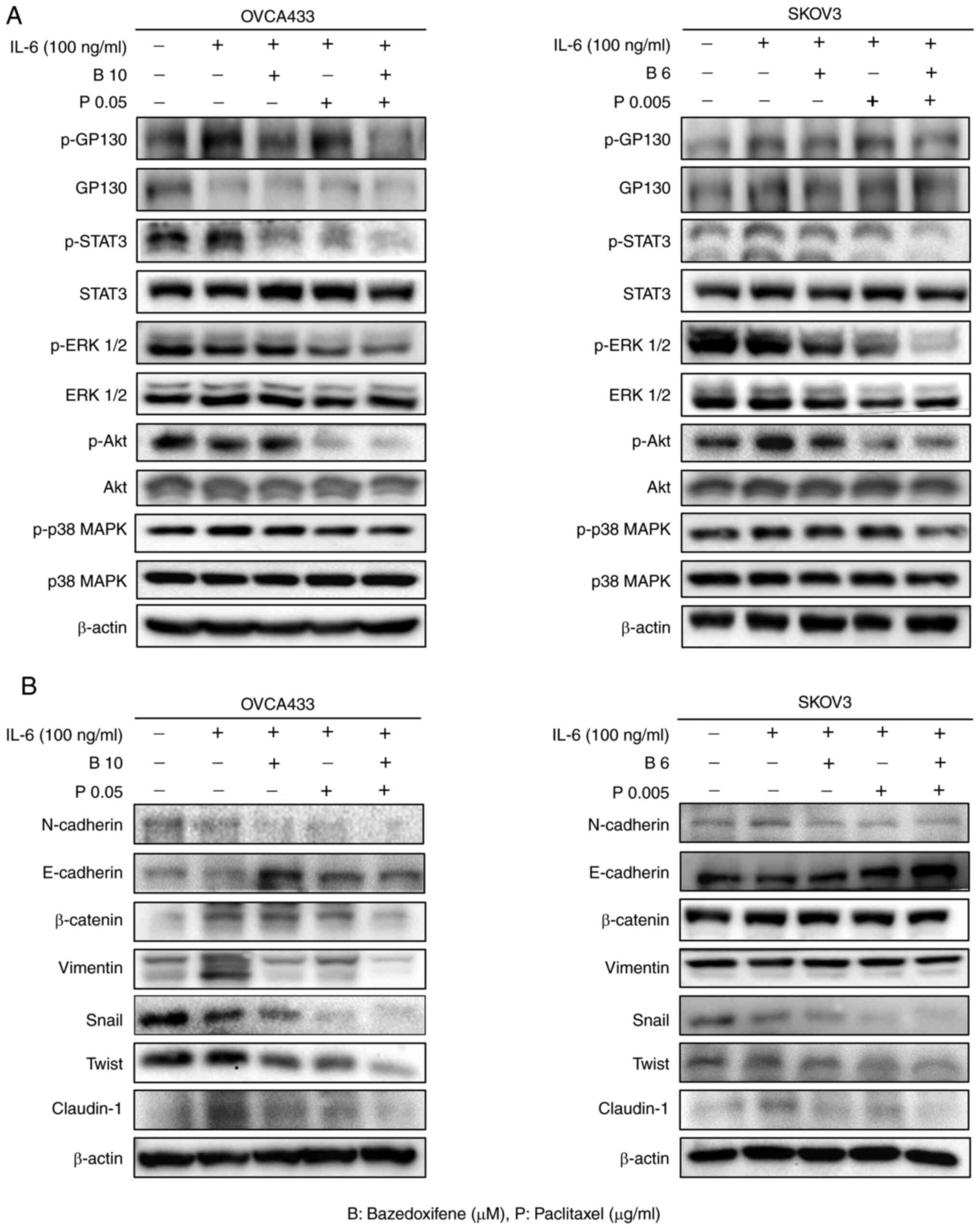 | Figure 5.The combination of bazedoxifene and
paclitaxel inhibits the expression of phosphorylated (p)-GP130,
p-STAT3, p-ERK1/2 and EMT-related proteins in ovarian cancer cells.
(A) Combined bazedoxifene and paclitaxel decreased the expression
of p-GP130, p-STAT3 and p-ERK 1/2 in ovarian cancer OVCA433 and
SKOV3 cells. (B) Effect of combined treatment with bazedoxifene and
paclitaxel on N-cadherin, E-cadherin, β-catenin, vimentin, Snail,
Twist, and Claudin-1 on EMT marker in OVCA433 and SKOV3 cells. EMT,
epithelial-mesenchymal transition |
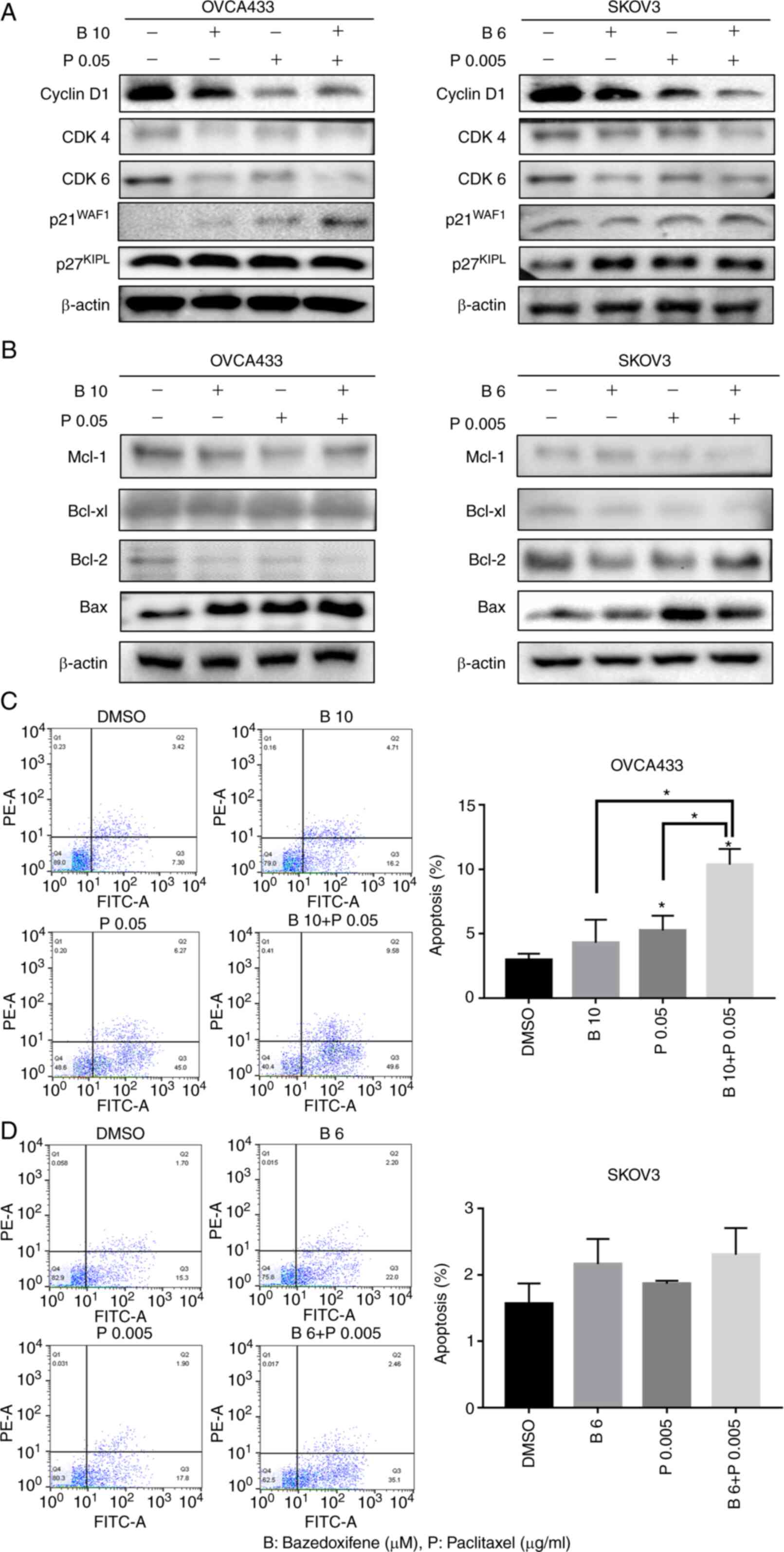
Bazedoxifene combined with paclitaxel
induces growth arrest and apoptosis
It was demonstrated that bazedoxifene inhibits the
survival, migration, and metastasis of cancer cells by inhibiting
IL-6/GP130/STAT3 signalling. Furthermore, whether bazedoxifene
affects the cell cycle and apoptosis in ovarian cancer cells was
assessed. As observed through western blot analysis, cyclin D1,
CDK4, and CDK6 were significantly decreased, and p21 and p27 were
slightly increased in OVCA433 and SKOV3 cells treated with
bazedoxifene and paclitaxel alone and the combination treatment.
These results showed that treatment with bazedoxifene alone or in
combination with paclitaxel induced cell cycle arrest (Fig. 6A). In addition, we demonstrated an
apoptotic effect in ovarian cancer cells treated with bazedoxifene
and paclitaxel alone or in combination. Paclitaxel, an anticancer
drug, is an anthracycline drug that suppresses cancer by inducing
apoptosis. In the above experiments, bazedoxifene significantly
inhibited the viability of ovarian cancer cell lines. The
combination of bazedoxifene and paclitaxel reduced the levels of
Mcl-1, Bcl-xl, and Bcl-2, and significantly increased the levels of
Bax in the ovarian cancer OVCA433 and SKOV3 cells (Fig. 6B). The whole western membrane and
expression levels are shown in Figs.
S15 and S16. Moreover, flow
cytometry analysis using Annexin V-fluorescein isothiocyanate
(FITC)/propidium iodide (PI) double staining revealed that the
combined treatment of bazedoxifene and paclitaxel significantly
induced the apoptosis of OVCA433 and SKOV3 cells (Fig. 6C and D). A2780 and TOV112D cells
also showed significantly increased apoptosis following treatment
with the combination of paclitaxel and bazedofixene (Fig. S17). These results confirmed that
both bazedoxifene and paclitaxel induced apoptosis, and apoptosis
increased significantly when both drugs were used together.
Combined bazedoxifene and paclitaxel
inhibits OVCA433 tumour growth in vivo
OVCA433 cells (1×107) were injected
subcutaneously into BALB/c nude mice with an equal volume of
phosphate-buffered saline (PBS). When tumours reached a volume of
200 mm3, bazedoxifene (4 mg/kg) was administered with
0.05% CMC and 5% DMSO as a vehicle by gavage five times a week, and
paclitaxel (10 mg/kg) was administered by intraperitoneal injection
twice weekly. Tumour volumes were calculated using caliper
measurements (n=6 mice per treatment group) (Fig. 7A). After 16 days of treatment, all
mice were sacrificed, and the total mass of each tumour was
determined at autopsy. The tumour mass was excised for comparison
between groups (Fig. 7B and C).
There was no change in the body weight of the mice during the drug
treatment period (Fig. 7D). To
assess IL-6 production per serum in mouse blood, the total amount
of IL-6 was normalised to that of the vehicle (Fig. 7E). Histological examination
revealed fewer cells with large nucleoli and irregular nuclear
membranes in the drug-treated group than in the vehicle group,
especially in the drug combination group (Fig. 7F). The phosphorylation levels of
GP130, STAT3, and ERK were determined by western blotting of the
harvested tumour tissue. β-actin served as a loading control. The
protein levels of N-cadherin, E-cadherin, β-catenin, vimentin,
Snail, Slug and Claudin-1 were determined by western blotting of
the harvested tumour tissue. β-actin served as a loading control
(Fig. 7G and H). The whole western
membrane and expression levels are shown in Figs. S18 and S19. The results of experiments using a
xenograft mouse model showed that the combination treatment of
bazedoxifene and paclitaxel significantly reduced the tumour volume
and weight. In addition, GP130/STAT3 signalling and EMT signalling
were inhibited by the combination treatment in mouse tumour
tissues. This supports the results of the in vitro
experiments.
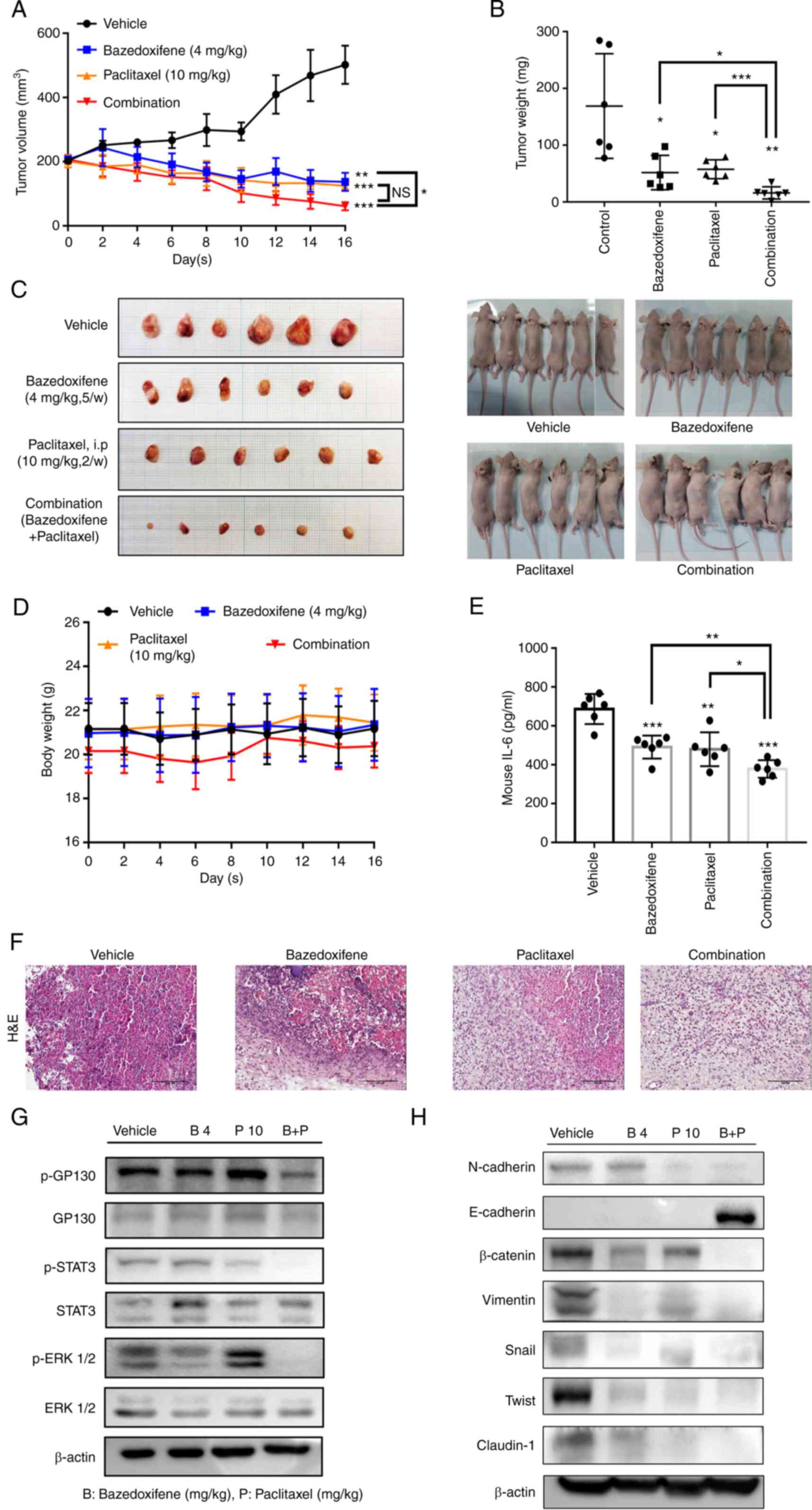 | Figure 7.Combined bazedoxifene and paclitaxel
inhibits ovarian cancer OVCA433 tumour growth in vivo.
OVCA433 cells (1×107) were injected subcutaneously into
Balb/c-nude mice with an equal volume of PBS. When tumours reached
a volume of 200 mm3, bazedoxifene (4 mg/kg) with a
vehicle of 0.05% CMC and 5% DMSO was administered by gavage five
time a week, and paclitaxel (10 mg/kg) was administered by
intraperitoneal (i.p.) injection twice weekly. (A) Tumour volumes
were calculated from caliper measurements. (B) After 16 days of
treatment, all mice were sacrificed, and the total mass (in mg) of
each tumour was determined at autopsy (n=6 mice per treatment
group). (C) The tumour masses were excised for comparison among the
groups. (D) The mouse body weights were assessed on the days
indicated. (E) To assess IL-6 production per serum in mouse blood,
the total amount of IL-6 was normalised by the vehicle in the
different treatment groups (*P<0.05, **P<0.01,
***P<0.001). (F) Hematoxylin and eosin (H&E) staining
results show the anticancer effect of combined bazedoxifene and
paclitaxel on ovarian cancer. (G) The phosphorylation levels (p) of
GP130, STAT3 and ERK1/2 were determined using western blotting of
the harvested tumour tissue. β-actin served as the loading control.
(H) The protein levels of N-cadherin, E-cadherin, β-catenin,
Vimentin, Snail, Twist and Claudin-1 were determined using western
blotting of the harvested tumour tissue. β-actin served as the
loading control. STAT3, signal transducer and activator of
transcription 3; GP130, glycoprotein 130. |
Discussion
Interleukin-6 (IL-6) plays several roles in
haematopoiesis and regeneration. In addition, its function plays a
central role in cancer progression and development. The expression
of IL-6 is upregulated in several malignancies, and enhanced IL-6
signalling increases IL-6 expression by inducing sustained
activation of the transcription factor signal transducer and
activator of transcription 3 (STAT3). Therefore, it provides a
tumour microenvironment that promotes tumour growth (27). IL-6 is known to be involved in
cancer progression and resistance in a variety of cancers and is
involved in sub-STAT3 signalling (28,29).
IL-6 signalling is induced by the binding of IL-6 to IL-6R. IL-6,
which is complexed with IL-6R, binds to glycoprotein 130 (GP130),
known as IL-6R, with high affinity and activates the homodimer
(30). Because GP130 is located in
the middle of this oncogenic signalling network, blocking GP130 can
be an important treatment method for suppressing carcinogenesis and
tolerance. However, several drugs that inhibit GP130 have not been
developed, and studies on cancer have not been conducted.
Currently, SC144 is an inhibitor of GP130 in ovarian cancer, and
studies have been conducted on cancer inhibition and resistance
(31).
Bazedoxifene, a GP130 inhibitor, has been used as a
selective estrogen modulator (SERM) drug to prevent and treat
osteoporosis in women after menopause. Recently, it was discovered
that it selectively inhibits IL-6-induced STAT3 phosphorylation in
the GP130/JAK/STAT3 signalling pathway in cancer (18). Using surface plasmon resonance
(SPR), we confirmed that bazedoxifene binds to GP130 through
intermolecular interactions. To confirm that binding to GP130
inhibited the binding of IL-6, we examined whether IL-6 was
inhibited by the IL-6-dependent cell line DS-1. The results showed
that IL-6 inhibition increased with increasing concentrations of
bazedoxifene.
We aimed to determine whether bazedoxifene
selectively inhibits IL-6-induced STAT3 phosphorylation through the
GP130/JAK/STAT3 signalling pathway in ovarian cancer. IL-6-mediated
STAT3 phosphorylation in A2780 and SKOV3 cells, where STAT3
phosphorylation is absent in ovarian cancer, was inhibited by
bazedoxifene. In addition, it was confirmed that STAT3
phosphorylation was inhibited through inhibition of the STAT3
signalling pathway even in the absence of estrogen receptors (ERs),
which are the targets of bazedoxifene. This demonstrates that
bazedoxifene inhibits the IL-6/GP130/STAT3 signalling pathway with
and without ERs in ovarian cancer.
To confirm the effect of bazedoxifene on ovarian
cancer cell lines, the cells were treated with bazedoxifene at each
concentration. The survival of ovarian cancer cells was inhibited
by increasing the concentration of bazedoxifene. Thus, bazedoxifene
could be used to inhibit the growth of ovarian cancer cells, and
the combined effect of bazedoxifene and paclitaxel could be used as
an anticancer agent. Subsequently, we investigated the binding
effect of bazedoxifene and paclitaxel on the migration and invasion
of cancer cells. Both migration and invasion showed decreased
results compared with the control group, even with single
treatment, and decreased further with the combination treatment.
The results showed that bazedoxifene suppressed survival,
migration, and invasion by a combination treatment with
paclitaxel.
Survival, migration, and invasion were inhibited by
bazedoxifene-mediated inhibition of GP130 through the STAT3
signalling system. The results demonstrated that the
phosphorylation of GP130, STAT3, and ERK1/2 was inhibited in
combination with bazedoxifene, paclitaxel, and bazedoxifene and
paclitaxel. STAT3 is continuously activated in several types of
cancers, and is associated with the incidence and progression of
cancer, as well as the prognosis of cancer patients (28,32).
Constitutive STAT3 activation not only has intrinsic consequences
for tumour cells but also affects the extracellular matrix (ECM) of
the tumour microenvironment and stromal cells, thereby increasing
the survival, proliferation, migration, and invasiveness of tumour
cells and their tumor-promoting activity (33). Thus, bazedoxifene inhibited
IL-6-induced phosphorylation of STAT3 and inhibited the expression
of genes downstream of IL-6. Because it could affect the tumour
microenvironment, the effect on EMT in the tumour microenvironment
was confirmed. In the EMT signalling pathway, bazedoxifene and
paclitaxel inhibited EMT, and the combination treatment showed
greater inhibition.
We demonstrated that bazedoxifene inhibits the
survival, migration, and metastasis of cancer cells by inhibiting
IL-6/GP130/STAT3 signalling. STAT3 plays a central role in
Gp130-mediated cell growth, survival, and differentiation. STAT3 is
activated by various oncogenes and is involved in cell growth and
differentiation through G1 to S phase cell cycle regulation
(34). Apoptosis is an essential
sign of cell death and is associated with the development of
several tumours (35). Paclitaxel,
an anticancer drug, induces apoptosis by stabilising microtubule
dynamics (36–38). In addition, constitutive activation
of STAT3 induces resistance to apoptosis, presumably by
upregulating the expression of survivin, Bcl-xl, and Bcl-2
(39). We investigated whether
bazedoxifene affected cell growth and differentiation through
GP130/STAT3 inhibition. Thus, it was confirmed that the drug alone
or combination treatment inhibited the growth and differentiation
of cells. We found that cyclin D1, CDK4, and CDK6 decreased
significantly, and p21 and p27 increased slightly in the cell
treated with bazedoxifene and paclitaxel alone and in the
combination group. We also investigated the apoptotic effects of
bazedoxifene and paclitaxel. In the above experiment, bazedoxifene
significantly inhibited the viability of ovarian cancer cell lines.
These results confirmed that both bazedoxifene and paclitaxel
induced apoptosis, and apoptosis significantly increased when both
drugs were used together.
In addition, we found that bazedoxifene inhibited
the growth of transplanted tumours in immunosuppressed mice,
improved the sensitivity of traditional anticancer drugs, and
exerted synergistic effects. The expression of STAT3 signalling and
EMT was more inhibited in the group treated with the combination of
bazedoxifene and paclitaxel as compared to the single treatment
group.
In this study, we investigated the effect of
bazedoxifene on cancer cell growth and metastasis by inhibiting
IL-6/GP130/STAT3 signalling in ovarian cancer cells. Furthermore,
we investigated the effect of bazedoxifene alone or in combination
with paclitaxel on the growth and metastasis inhibition of ovarian
cancer. Bazedoxifene increased the sensitivity of ovarian cancer
cells to paclitaxel. The antitumour effect of the combination of
bazedoxifene and paclitaxel significantly improved the therapeutic
efficacy of ovarian cancer. This suggests its potential as an
adjuvant for anticancer drugs.
In conclusion, in this study, bazedoxifene inhibited
IL-6 by binding to GP130. Bazedoxifene inhibited cell survival in
ovarian cancer cells, and combination therapy with bazedoxifene and
paclitaxel further inhibited cell survival, migration, and invasion
compared to treatment with either drug alone. In addition,
combination treatment with bazedoxifene and paclitaxel inhibited
the IL-6-mediated GP130/STAT3 signalling pathway, induced apoptosis
in ovarian cancer cells, and inhibited EMT. Tumour growth was
suppressed in human ovarian cancer xenografts. These results
suggest that bazedoxifene can be a novel therapeutic agent for
ovarian cancer treatment, and can be used as an adjunct to the
existing anticancer drug, paclitaxel.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This work was supported by the Basic Science Research Program
through the National Research Foundation of Korea (NRF), funded by
the Ministry of Education, Science, and Technology (grant nos.
NRF-2018R1D1A1B07049780, 2018R1A6A1A03025108, 2021R1A2C2009782 and
2021R1A6A3A1303840811).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
SAP was responsible for the data curation, writing
of the original draft, visualization, investigation, software,
validation of the data, writing of the review, and editing. LKK was
responsible for the data curation, methodology, and validation of
the data and results. HMP was responsible for the research
methodology. HJK was responsible for the data curation,
conceptualization, methodology, software, supervision, project
administration, writing of the review, and editing. THH was
responsible for the conceptualization, data curation and
validation, visualization, supervision, project administration, and
funding acquisition. All authors have read and agreed to the
published version of the manuscript.
Ethics approval and consent to
participate
Animal experiments were approved by the
Institutional Animal Care and Use Committee (IACUC) of the Catholic
University of Korea (approval no. CUK-IACUC-2019-026-01). All
experimental work complied with the legal obligations and federal
guidelines for the care and maintenance of laboratory animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that there are no competing
interests for any of the authors.
Glossary
Abbreviations
Abbreviations:
|
IL-6
|
interleukin-6
|
|
STAT3
|
signal transducer and activator of
transcription 3
|
|
GP130
|
glycoprotein 130
|
|
EMT
|
epithelial-mesenchymal transition
|
|
SPR
|
surface plasmon resonance
|
|
ECACC
|
European Collection of Cell
Culture
|
|
KCLB
|
Korean Cell Line Bank
|
|
ATCC
|
American Type Culture Collection
|
|
CCK-8
|
Cell Counting Kit-8
|
|
RIPA
|
radioimmunoprecipitation assay
|
|
NRF
|
National Research Foundation of
Korea
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Markman M and Mekhail TM: Paclitaxel in
cancer therapy. Expert Opin Pharmacother. 3:755–766. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Richardson DL, Sill MW, Coleman RL, Sood
AK, Pearl ML, Kehoe SM, Carney ME, Hanjani P, Van Le L, Zhou XC, et
al: Paclitaxel with and without pazopanib for persistent or
recurrent ovarian cancer: A randomized clinical trial. JAMA Oncol.
4:196–202. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pignata S, Lorusso D, Scambia G, Sambataro
D, Tamberi S, Cinieri S, Mosconi AM, Orditura M, Brandes AA,
Arcangeli V, et al: Pazopanib plus weekly paclitaxel versus weekly
paclitaxel alone for platinum-resistant or platinum-refractory
advanced ovarian cancer (MITO 11): A randomised, open-label, phase
2 trial. Lancet Oncol. 16:561–568. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Browning L, Patel M, Bring Horvath E,
Tawara K and Jorcyk C: IL-6 and ovarian cancer: Inflammatory
cytokines in promotion of metastasis. Cancer Manag Res.
10:6685–6693. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Heo TH, Wahler J and Suh N: Potential
therapeutic implications of IL-6/IL-6R/gp130-targeting agents in
breast cancer. Oncotarget. 7:154602016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ernst M and Jenkins BJ: Acquiring
signalling specificity from the cytokine receptor gp130. Trends
Genet. 20:23–32. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rebouissou S, Amessou M, Couchy G, Poussin
K, Imbeaud S, Pilati C, Izard T, Balabaud C, Bioulac-Sage P and
Zucman-Rossi J: Frequent in-frame somatic deletions activate gp130
in inflammatory hepatocellular tumours. Nature. 457:200–204. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Murakami M, Kamimura D and Hirano T:
Pleiotropy and specificity: Insights from the interleukin 6 family
of cytokines. Immunity. 50:812–831. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ernst M and Putoczki TL: Molecular
pathways: IL11 as a tumor-promoting cytokine-translational
implications for cancers. Clin Cancer Res. 20:5579–5588. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rose-John S: Interleukin-6 family
cytokines. Cold Spring Harb Perspect Biol. 10:a0284152018.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bromberg J and Wang TC: Inflammation and
cancer: IL-6 and STAT3 complete the link. Cancer Cell. 15:79–80.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Haan C, Heinrich PC and Behrmann I:
Structural requirements of the interleukin-6 signal transducer
gp130 for its interaction with Janus kinase 1: The receptor is
crucial for kinase activation. Biochem J. 361:105–111. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Taher MY, Davies DM and Maher J: The role
of the interleukin (IL)-6/IL-6 receptor axis in cancer. Biochem Soc
Trans. 46:1449–1462. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Skiniotis G, Boulanger MJ, Garcia KC and
Walz T: Signaling conformations of the tall cytokine receptor gp130
when in complex with IL-6 and IL-6 receptor. Nat Struct Mol Biol.
12:545–551. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dijkgraaf EM, Welters MJ, Nortier JW, van
der Burg SH and Kroep JR: Interleukin-6/interleukin-6 receptor
pathway as a new therapy target in epithelial ovarian cancer. Curr
Pharm Des. 18:3816–3827. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Levy DE and Darnell JE Jr: Stats:
Transcriptional control and biological impact. Nat Rev Mol Cell
Biol. 3:651–662. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li H, Xiao H, Lin L, Jou D, Kumari V, Lin
J and Li C: Drug design targeting protein-protein interactions
(PPIs) using multiple ligand simultaneous docking (MLSD) and drug
repositioning: Discovery of raloxifene and bazedoxifene as novel
inhibitors of IL-6/GP130 interface. J Med Chem. 57:632–641. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fu S, Chen X, Lo HW and Lin J: Combined
bazedoxifene and paclitaxel treatments inhibit cell viability, cell
migration, colony formation, and tumor growth and induce apoptosis
in breast cancer. Cancer Lett. 448:11–19. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim L, Park S, Park H, Kim H and Heo TH:
Bazedoxifene, a GP130 inhibitor, modulates EMT signaling and
exhibits antitumor effects in HPV-positive cervical cancer. Int J
Mol Sci. 22:86932021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu X, Cao Y, Xiao H, Li C and Lin J:
Bazedoxifene as a novel GP130 inhibitor for pancreatic cancer
therapy. Mol Cancer Ther. 15:2609–2619. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tian J, Chen X, Fu S, Zhang R, Pan L, Cao
Y, Wu X, Xiao H, Lin HJ, Lo HW, et al: Bazedoxifene is a novel
IL-6/GP130 inhibitor for treating triple-negative breast cancer.
Breast Cancer Res Treat. 175:553–566. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wei J, Ma L, Lai YH, Zhang R, Li H, Li C
and Lin J: Correction to: Bazedoxifene as a novel GP130 inhibitor
for colon cancer therapy. J Exp Clin Cancer Res. 38:3742019.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hong SS, Choi JH, Lee SY, Park YH, Park
KY, Lee JY, Kim J, Gajulapati V, Goo JI, Singh S, et al: A novel
small-molecule inhibitor targeting the IL-6 receptor β subunit,
glycoprotein 130. J Immunol. 195:237–245. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Beaufort CM, Helmijr JC, Piskorz AM,
Hoogstraat M, Ruigrok-Ritstier K, Besselink N, Murtaza M, van IJcken
WF, Heine AA, Smid M, et al: Ovarian cancer cell line panel (OCCP):
Clinical importance of in vitro morphological subtypes. PLoS One.
9:e1039882014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fu W, Zhao P, Li H, Fu H, Liu X, Liu Y, Wu
J and Fu W: Bazedoxifene enhances paclitaxel efficacy to suppress
glioblastoma via altering Hippo/YAP pathway. J Cancer. 11:657–667.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chang Q, Daly L and Bromberg J: The IL-6
feed-forward loop: A driver of tumorigenesis. Semin Immunol.
26:48–53. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huang M, Page C, Reynolds RK and Lin J:
Constitutive activation of stat 3 oncogene product in human ovarian
carcinoma cells. Gynecol Oncol. 79:67–73. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Duan Z, Ames RY, Ryan M, Hornicek FJ,
Mankin H and Seiden MV: CDDO-Me, a synthetic triterpenoid, inhibits
expression of IL-6 and Stat3 phosphorylation in multi-drug
resistant ovarian cancer cells. Cancer Chemother Pharmacol.
63:681–689. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wolf J, Rose-John S and Garbers C:
Interleukin-6 and its receptors: A highly regulated and dynamic
system. Cytokine. 70:11–20. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xu S, Grande F, Garofalo A and Neamati N:
Discovery of a novel orally active small-molecule gp130 inhibitor
for the treatment of ovarian cancer. Mol Cancer Ther. 12:937–949.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Huang F, Tong X, Fu L and Zhang R:
Knockdown of STAT3 by shRNA inhibits the growth of CAOV3 ovarian
cancer cell line in vitro and in vivo. Acta Biochim Biophys Sin
(Shanghai). 40:519–525. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yu H, Kortylewski M and Pardoll D:
Crosstalk between cancer and immune cells: Role of STAT3 in the
tumour microenvironment. Nat Rev Immunol. 7:41–51. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hirano T, Ishihara K and Hibi M: Roles of
STAT3 in mediating the cell growth, differentiation and survival
signals relayed through the IL-6 family of cytokine receptors.
Oncogene. 19:2548–2556. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Evan GI and Vousden KH: Proliferation,
cell cycle and apoptosis in cancer. Nature. 411:342–348. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ling YH, Yang Y, Tornos C, Singh B and
Perez-Soler R: Paclitaxel-induced apoptosis is associated with
expression and activation of c-Mos gene product in human ovarian
carcinoma SKOV3 cells. Cancer Res. 58:3633–3640. 1998.PubMed/NCBI
|
|
37
|
Orr GA, Verdier-Pinard P, McDaid H and
Horwitz SB: Mechanisms of taxol resistance related to microtubules.
Oncogene. 22:7280–7295. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jones NA, Turner J, McIlwrath AJ, Brown R
and Dive C: Cisplatin-and paclitaxel-induced apoptosis of ovarian
carcinoma cells and the relationship between bax and bak
up-regulation and the functional status of p53. Mol Pharmacol.
53:819–826. 1998.PubMed/NCBI
|
|
39
|
Nielsen M, Kaestel CG, Eriksen K, Woetmann
A, Stokkedal T, Kaltoft K, Geisler C, Röpke C and Odum N:
Inhibition of constitutively activated Stat3 correlates with
altered Bcl-2/Bax expression and induction of apoptosis in mycosis
fungoides tumor cells. Leukemia. 13:735–738. 1999. View Article : Google Scholar : PubMed/NCBIPubMed/NCBIPubMed/NCBIPubMed/NCBIPubMed/NCBIPubMed/NCBIPubMed/NCBIPubMed/NCBIPubMed/NCBIPubMed/NCBIPubMed/NCBIPubMed/NCBIPubMed/NCBIPubMed/NCBIPubMed/NCBI
|