Introduction
Lung cancer is a common malignant tumor type with a
high morbidity and mortality in the world, particularly in males
(1). The onset of lung cancer is
usually due to the interaction of multiple factors, including
genetic and environmental factors such as cigarette smoking and air
pollution in particular. Cigarette smoking and air pollution could
induce the inhalation of multiple carcinogens, particularly
benzo[a]pyrene, which could lead to DNA damage and further tumor
onset. Therefore, a lower DNA repair capacity (DRC) may be
associated with lung cancer susceptibility (2). A significant difference in individual
DRC has been observed, and it has been suggested to be associated
with genetic variations in the human genome (3). To disclose the potential genetic
contribution to DRC, genome-wide association studies (GWAS) have
been performed, and one single nucleotide polymorphism (SNP) in
chromosome 6q24.2, rs9390123, was identified to be associated with
DRC in Caucasians and thus has been suggested to contribute to lung
cancer (4). Since this SNP is
located on the intron of phosphatase and actin regulator 2
(PHACTR2), it was proposed that the association was through
the function of this gene (4).
However, to the best of our knowledge, this mechanism has not been
investigated to date. Due to the limited capacity of microarrays,
only ~317K SNPs were selected as tags to represent the human genome
in a previous study (4).
Consequently, the causal SNP(s) for DRC and lung cancer may be not
only the genetic marker rs9390123, but also the one(s) that were
not present in the microarray but exhibited linkage disequilibrium
(LD) with rs9390123, despite the fact that there were no other SNPs
in LD with rs9390123 according to the International HapMap Project
(5). However, to the best of our
knowledge, the LD pattern in this locus has not been investigated
thus far.
In the present study, the genotype data for the
rs9390123 surrounding region were downloaded from the 1000 Genomes
Project (6), and the LD pattern
was analyzed. The function of these SNPs and the mechanism were
evaluated by functional genomics assay. Through chromosome
conformation capture (3C), the regulatory target genes were
identified, one of which is PHACTR2-antisense RNA 1
(AS1), a long non-coding RNA (lncRNA). The function of
PHACTR2-AS1 in lung cancer was further evaluated in the
present study. The current findings identified a novel oncogene for
lung cancer and illustrated the potential genetic mechanism for
DRC.
Materials and methods
Genotype data download and
analysis
The genotype of rs9390123 surrounding 1-Mb segment
(chr6: 143443314-144443314 relative to human genome build 37) was
downloaded from the 1000 Genomes Project for three representative
populations in the world, namely CEU (Utah Residents with European
Ancestry), CHB (Han Chinese in Beijing) and YRI (Yoruba in Ibadan),
by using an online program, VCF (Variant Call Format) to PED
(pedigree) converter (http://grch37.ensembl.org/Homo_sapiens/Tools/VcftoPed).
The obtained genotype data file (PED format) and locus information
file (INFO format) were loaded into Haploview 4.2 (Broad Institute)
(7) and r2 was
calculated with default parameter. When r2≥0.8,
the SNPs were supposed to be in strong LD.
Tissue culture
The human lung epithelial cell line Beas-2B (cat.
no. KCB200922YJ), lung cancer cell line A549 (cat# KCB200434YJ) and
293TN cells (cat. no. KCB2013068YJ) were maintained in high-glucose
DMEM (HyClone; Cytiva) supplemented with 10% fetal bovine serum
(FBS, Biological Industries; Sartorius AG) in 5% CO2 at
37°C. All cell lines were purchased from Kunming Cell Bank of Type
Culture Collection of Chinese Academy of Sciences (http://www.kmcellbank.com/). The cell bank performed
short tandem repeat genotyping, karyotype and isoenzyme analyses to
verify the cell identity in order to avoid potential
contamination.
Luciferase plasmid construction and
transfection
To include all the potential cis-regulatory elements
and amplify the segment efficiently, rs9390123 and its nearby
region (~1.5 kb; chr6: 143942517-143944088) were amplified via PCR
with the forward primer 5′-GGCAAATCCTTCCCATAGTTCC-3′ and the
reverse one 5′-TAGGGCCAAATTTCACAGGTCTTA −3′ containing MluI
and BglII restriction sites, respectively. The DNA isolated
from Beas-2B cells was utilized as a template. The thermocycling
conditions are as follows: 98°C for 30 sec; 35 cycles of 98°C for
10 sec, 65°C for 30 sec, 72°C for 30 sec; 72°C for 2 min. To avoid
potential PCR errors, amplification was performed by utilizing Q5
High-Fidelity DNA Polymerase (New England BioLabs, Inc.). Upon
digestion with the aforementioned restriction enzymes (New England
BioLabs, Inc.), the PCR product was inserted into the cloning site
of the pGL3-promoter plasmid (Promega Corp.). The plasmid
containing another allele was produced for each SNP by using a Q5
Site-Directed Mutagenesis kit (New England BioLabs, Inc.) and the
primers listed in Table SI.
After seeding (~105 cells) into a 24-well
plate and culture for 24 h as described above, 475 ng plasmid with
the aforementioned inserted segment was transfected into the
Beas-2B cells by using Lipofectamine® 2000 (Thermo
Fisher Scientific, Inc.). After additional 36 h of culture and cell
harvest, the luciferase expression was determined by using a Dual
Luciferase Reporter Assay System (Promega Corp.). A total of 25 ng
pRL-TK plasmid (Promega Corp.) was transfected simultaneously as an
internal control to normalize the transfection efficiency, and the
luciferase ratio between firefly and Renilla was utilized to
represent the activity of the enhancer containing rs9390123 and
rs9399451. In total, six replicates were performed for each plasmid
transfection.
3C
The spatial conformation of the enhancer and the
related gene promoter was examined by using 3C as previously
described (8). Briefly, after
subjecting Beas-2B cells (~108) to crosslink by using
formaldehyde and subsequently lysing the cells, the chromatin was
directly digested with BsrGI (New England BioLabs, Inc.) and
further ligated with T4 DNA ligase (New England BioLabs, Inc.). The
ligation product was purified by using the standard
phenol-chloroform method (9).
Simultaneously, a BAC (bacterial artificial chromosome)
RP11-1012I24 (BACPAC Genomics; http://bacpacresources.org/) harboring the 6q24.2
segment was grown in LB (Luria-Bertani) medium and purified by
Large-Construct Kit (Qiagen Corp.) according to the manufacturer's
protocol. After digestion, ligation and purification as
aforementioned, the product was utilized as a control to normalize
the primer efficiency. The relative enrichment of the 3C product
was assessed by quantitative PCR (qPCR) with the unidirectional
primers shown in Table SII and
iTaq Universal SYBR Green Supermix (Biorad Corp.). The
thermocycling conditions are as follows: 95°C for 10 min; 40 cycles
of 95°C for 5 sec, 60°C for 30 sec. The experiment was repeated in
triplicate. All 3C PCR products were verified using sequencing with
constant primer (Table SII) and
BigDye® Terminator v3.1 (Thermo Fisher Scientific, Inc.)
according to the manufacturer's recommendation.
Chromatin immunoprecipitation
(ChIP)
Potential transcription factors (TFs) were predicted
by an online program Match (http://www.gene-regulation.com/cgi-bin/pub/programs/match/bin/match.cgi).
ChIP was performed with EZ-ChIP kit (MilliporeSigma). In brief,
cells (~107) were crosslinked by formaldehyde, lysed by
lysis buffer (MilliporeSigma) and fragmented into segments of
200–800 bp by using sonication. After diluting by dilution buffer
and preclearing by Protein A beads (MilliporeSigma), the
chromatin/protein complex was captured overnight at 4°C by addition
of 2 µg anti-mouse POU class 2 homeobox 1 (POU2F1) antibody (Santa
Cruz Biotechnology, Inc.; cat. no. sc-53830) or the same amount of
normal mouse IgG (Santa Cruz Biotechnology, Inc.; cat. no. sc-2025)
and further immunoprecipitated by using Protein A Beads
(MilliporeSigma). After washing, resuspending, crosslink reversing
and protein digestion with related buffer (MilliporeSigma), the DNA
was recovered by using a column (MilliporeSigma), and quantified
via qPCR as described above with the primers shown in Table SIII. The experiment was repeated
in triplicate, and the PCR product was sequenced for validation by
using forward PCR primer in Table
SIII and BigDye® Terminator v3.1 as described
above.
TF overexpression and gene expression
analysis
The POU2F1 coding region was obtained by using
nested PCR with Q5 High-Fidelity DNA Polymerase. The PCR primer
sequences, annealing temperature and target region are displayed in
Table SIV. After dual enzymatic
cleavage with KpnI and HindIII (New England BioLabs,
Inc.), the POU2F1 coding region was inserted into the pEGFP-N1
overexpression vector (Clontech Laboratories; Takara Bio USA,
Inc.). A total of 500 ng POU2F1 overexpression plasmid was
transfected into Beas-2B cells with Lipofectamine® 2000
as aforementioned. pEGFP-N1 was utilized as a negative control.
After 48 h of culture, the relative mRNA levels of POU2F1
and target genes were evaluated by qPCR with primers in Table SV. In total, three independent
repeats were carried out.
Electrophoretic mobility shift assay
(EMSA)
The probes for both alleles of rs9390123 and
rs9399451 (Table SVI) were
labeled with biotin. Nuclear extracts were isolated from Beas-2B
cells by using a Nuclear and Cytoplasmic Protein Extraction kit
(Beyotime Institute of Biotechnology) and incubated with
biotin-labeled probes (Sangon Biotech; 10 fmol). The probe-protein
complexes were separated via electrophoresis in 4.9% non-denatured
polyacrylamide gel and transferred to nylon membranes with positive
charge (Beyotime Institute of Biotechnology). For each SNP allele,
electrophoresis of only biotin-labeled probes and probe-protein
complexes incubated with non-labeled probes (competitor
oligonucleotides) was performed as a control. After incubation with
7.5 µg streptavidin-HRP (horseradish peroxidase) conjugate
(Beyotime Institute of Biotechnology; cat. no. A0303) at room
temperature for 15 min with 150 rpm rotation, the membrane was
visualized with enhanced chemiluminescence.
Nuclear and cytoplasmic RNA
isolation
RNA in the nucleus and cytoplasm of Beas-2B cells
was separated and isolated by using an RNA subcellular isolation
kit (Active Motif, Inc.). Reverse transcription (RT) was performed
with SuperScript III First-Strand Synthesis System (Thermo Fisher
Scientific, Inc.). PHACTR2-AS1 expression was determined by
using qPCR with primer pairs from the literature (10–12)
(Table SV). U6 and
GAPDH (glyceraldehyde-3-phosphate dehydrogenase) are known
to be expressed mainly in the nucleus (13) and cytoplasm (14), respectively. Therefore, their
expression was also determined by qPCR with primer pairs shown in
Table SV, and it was used as
positive controls to verify the separation of nucleus and
cytoplasm.
Short hairpin RNA (shRNA) design,
plasmid construction, lentivirus packaging and transfection
shRNA (5′-GCCCTGCATACTGTGGATTCA −3′) for
PHACTR2-AS1 was designed with the online software BLOCK-iT
RNAi Designer (https://rnaidesigner.thermofisher.com/rnaiexpress/;
Thermo Fisher Scientific, Inc.). The restrictive sites for
EcoRI and BamHI were added into shRNA sequence and
both forward and reverse strands were synthesized (Sangon Biotech).
After annealing, the two strands formed double-strand DNA with
sticky end. After EcoRI and BamHI (New England
BioLabs, Inc.) digestion, the pGreenPuro vector (System
Biosciences, LCC) was ligated with the DNA segment containing shRNA
by T4 DNA ligase. The lentiviral pGreenPuro vector (3 µg) was
transfected into 293TN cells by Lipofectamine® 2000
along with packaging (psPAX2; addgene# 12260; 2.5 µg) and envelope
(pMD2.G; addgene# 12259; 2.5 µg) plasmids to produce viruses. 293TN
is a genetically modified cell line to produce high titer virus.
After 48 h of culture, the viruses were collected via
centrifugation. The empty pGreenPuro vector (3 µg) was also
transfected into 293TN cells along with packaging and envelope
plasmids to produce viruses, which were further used as a negative
control (sh-NC). Beas-2B and A549 cells were transfected with the
aforementioned lentivirus and screened with puromycin (2 µg/ml
final concentration) for 2 weeks in order to obtain a stable cell
line.
Total RNA was purified from Beas-2B and A549 cells
using TRIzol® (Thermo Fisher Scientific, Inc.) according
to the manufacturer's instructions, quantified by using Nanodrop
2000 (Thermo Fisher Scientific, Inc.) and RT was carried out as
aforementioned. PHACTR2-AS1 expression after knockdown was
evaluated via PCR or qPCR with the primer pair listed in Table SV. GAPDH was also amplified
as a positive control by using the primer pair listed in Table SV.
Cell proliferation assay
Beas-2B and A549 cells with lentiviral transfection
were cultured for 4 days in 96-well plates (6,000 cells per well).
Every 24 h, 20 µl MTT (5 mg/ml; Beijing Solarbio Science &
Technology Co., Ltd.) was added to the cells and incubated for 4 h
at 37°C. Four time points were included in this assay. After
removing the supernatant and adding 150 µl DMSO to each well, the
plate was covered with foil and placed on an orbital shaker for 15
min at room temperature. Next, the optical density (OD) value was
examined at a wavelength of 490 nm. A total of three independent
repeats were carried out for each time point.
Colony formation assay
Beas-2B and A549 cells were adjusted to a density
500 cells per well and cultured for 14 days in a 6-well plate.
After washing with PBS, the cells were fixed with paraformaldehyde
and stained with 1% crystal violet (Beijing Solarbio Science &
Technology Co., Ltd.) for 20 min at room temperature. The colony
number was then determined visually using a DMi8 Automated Inverted
Phase Contrast Fluorescence Microscope (Leica Microsystems). Three
independent repeats were performed.
Cell migration assay
Beas-2B and A549 cells (104) were
resuspended into DMEM without FBS and placed in the upper chamber
of a Transwell cell culture plate (MilliporeSigma), while DMEM with
10% FBS was placed in the lower chamber. After 2 days of culture at
37°C, paraformaldehyde fixing and crystal violet staining of
Beas-2B and A549 cells were performed as aforementioned, and the
number of cells was examined using a DMi8 Automated Inverted Phase
Contrast Fluorescence Microscope as described above. Three
independent repeats were carried out.
Wound healing assay
Beas-2B and A549 cells were adjusted to a density of
7×105 cells per well and maintained to near full (95%)
confluence in DMEM with 10% FBS. After scratching the cell
monolayer with a pipette tip, the cells were washed with PBS for 2
min at room temperature (twice), and fresh DMEM without FBS was
added. The wound areas were imaged at 0 and 48 h using an inverted
microscope, and were analyzed using ImageJ software v1.51
(https://imagej.net/). Three independent repeats
were performed.
Statistical analysis
The luciferase activity for each plasmid, ChIP
enrichment, gene expression after POU2F1 overexpression, and cell
proliferation, colony formation, cell migration and wound healing
abilities were presented as mean ± standard deviation (SD).
Independent (unpaired) Student's t-test was utilized to evaluate
difference in the above data between two groups. All statistical
analyses were performed with SPSS 20.0 (IBM Corp.). P<0.05 was
considered to indicate a statistically significant difference.
Results
SNPs and LD pattern in the rs9390123
surrounding region
To identify the potential SNP(s) associated with
DRC, the genotype data surrounding rs9390123 in its nearby region
were obtained from the 1000 Genomes Project for three
representative populations in the world. For the 1M segment
surrounding rs9390123, 3,927, 4,285 and 6,800 SNPs were found to
exist in the CEU, CHB and YRI populations, respectively. However,
only one SNP, rs9399451, presented high LD with rs9390123 in CEU
(r2=0.979) and YRI (r2=0.851;
Fig. S1), thus suggesting that
this SNP may also be associated with DRC in these two populations.
In the CHB population, the LD between these two SNPs was found to
be moderate (r2=0.645; Fig. S1). Except rs9399451, all other
SNPs within this region showed relatively low LD with rs9390123
(all r2≤0.668, 0.633 and 0.675 in CEU, CHB and
YRI, respectively; Fig. S1).
There was a distance of only 560 bp between these two SNPs,
suggesting that they both could form part of the same functional
element. For the entire human population, T is the minor allele for
both rs9390123 and rs9399451, and their frequencies were found to
be ~38% and 30% for CEU and YRI populations, respectively. In
contrast, T frequencies in CHB were ~58 and ~68% for rs9390123 and
rs9399451, respectively.
Function of rs9390123 and
rs9399451
Since neither rs9390123 nor rs9399451 are within
protein-coding regions, it was hypothesized that these two SNPs
could influence target gene expression. To evaluate the function of
rs9390123 and rs9399451 on gene expression, the segment surrounding
rs9390123 and rs9399451 was inserted into the cloning site of the
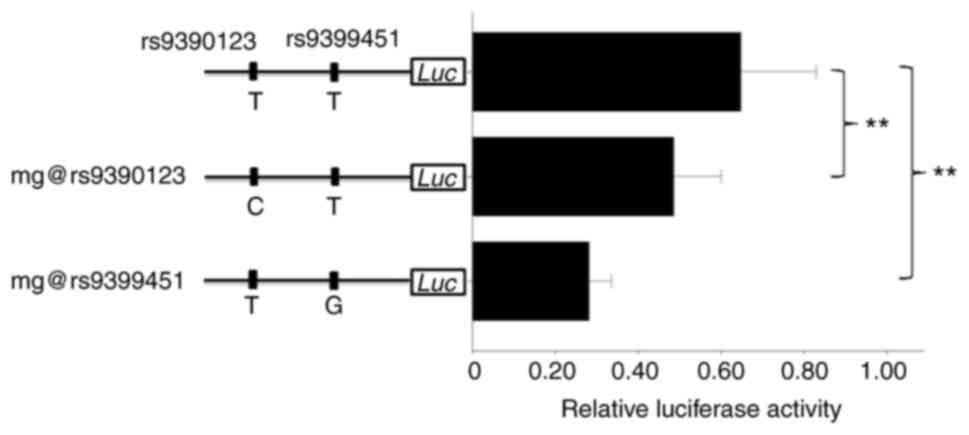
pGL3-promoter vector. The sequencing indicated that the nucleotide
is T for both rs9390123 and rs9399451 in the plasmid construct
(Fig. 1). The plasmids with
corresponding allele, i.e., C at rs9390123 and G at rs9399451, were
generated via mutagenesis, and subsequently all plasmids were
transfected. Since most lung cancer events derive from normal
epithelial cells, the lung epithelial cell line Beas-2B was used in
following functional genomics work.
As shown in Fig. 1,
the C allele of rs9390123 showed ~24.8% lower relative luciferase
activity than the T allele (P=0.0041), while the G allele of
rs9399451 presented ~56.7% lower luciferase activity than the T one
(P<10−5; Fig. 1),
which indicated that both SNPs are functional in lung cells, and
rs9399451 may have a more important effect than rs9390123 in
regulating target gene expression.
Interaction between the enhancer
containing rs9390123 and rs9399451, and the PEX3 and PHACTR2-AS1
promoter
Since rs9390123 and rs9399451 are not located at the
promoter of any known genes, it was proposed that these two SNPs
may be located within an enhancer region and could regulate the
expression of target genes. This suggestion could be supported by
the histone modification signal. Searching the data from the
Encyclopedia of DNA Elements project (15) in the UCSC genome browser
(http://genome.ucsc.edu) revealed that there are
clear H3K27Ac (acetylation at the 27th lysine residue of the
histone H3 protein) and H3K4me1 (mono-methylation at the 4th lysine
residue of the histone H3 protein) peaks, which are two common
histone modifications around an active enhancer (16), near this region in the human lung
cancer cell line A549 (Fig. S2).
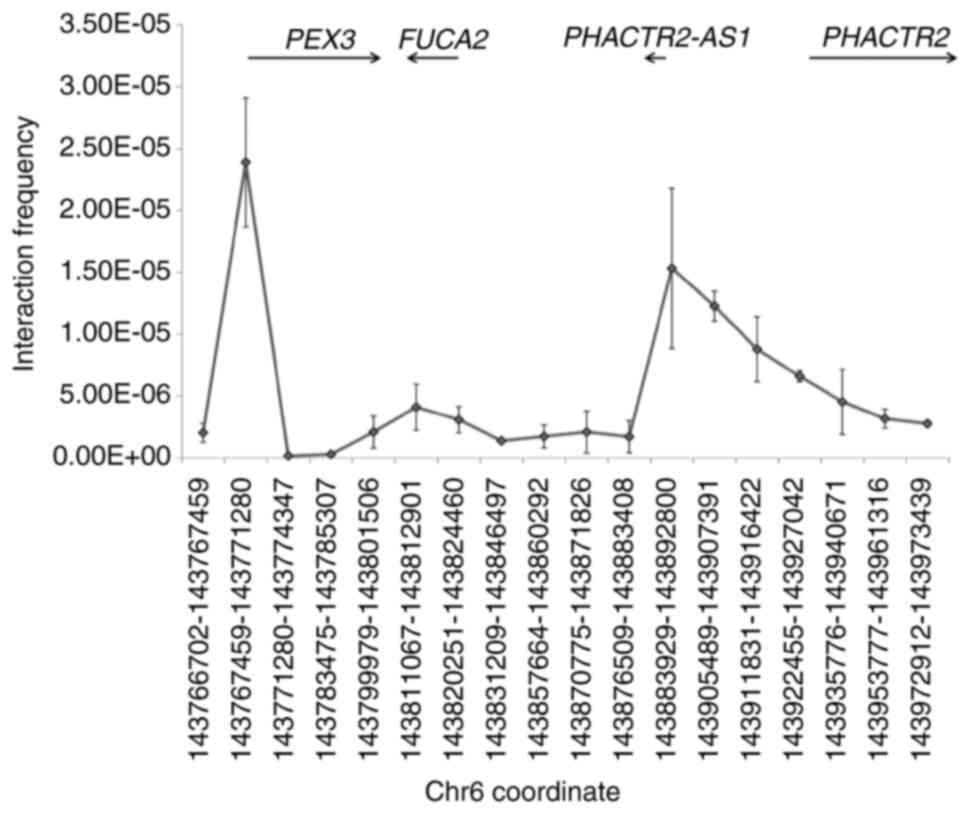
However, the target gene remained unclear. To identify the
potential target gene for this enhancer, 3C was used. The rationale
of 3C is that the enhancer is far away from its target gene(s) in
sequence but close in space. Thus, after 3C library construction,
the target gene promoter can be ligated in higher efficiency with
enhancer than other random genome regions, which can be disclosed
by qPCR. To normalize the primer efficiency, the BAC RP11-1012I24
including nearby region was used as a control. There are only three
protein-coding genes [PEX3, FUCA2 (α-L-fucosidase 2) and
PHACTR2] and one lncRNA PHACTR2-AS1 in this BAC.
Therefore, in our 3C assay, unidirectional primers were designed to
anchor the promoter of these four genes, the aforementioned
enhancer and several random regions. As shown in Fig. 2, no increase was observed in
ligation frequency at the PHACTR2 promoter (corresponding to
the 15th point in the x-axis, ~17.9 kb away from the enhancer),
suggesting that the regulatory target of this enhancer is not
PHACTR2. The FUCA2 promoter region (7th point in the
x-axis) exhibited a similar result. By contrast, a higher ligation
efficiency was obtained in the PEX3 promoter region (2nd
point in the x-axis, ~173.6 kb away from the enhancer). In
addition, the promoter of the lncRNA PHACTR2-AS1 (12th point
in the x-axis, ~50.5 kb away from the enhancer), also presented a
high ligation efficiency, indicating that PEX3 and
PHACTR2-AS1 are the regulatory targets of this enhancer.
TF binding to rs9390123 and
rs9399451
Since rs9390123 and rs9399451 are located within a
non-coding region of the genome, it could be hypothesized that
these two SNPs were located at a TF binding region, and could
influence the interaction between TF and DNA. Since TFs usually
bind to DNA through recognizing specific motif, the potential TF
can be predicted by bioinformatics approach. If one TF can interact
with specific DNA segment, the TF antibody can immunoprecipitate
more DNA-TF complex than IgG, since IgG presents random interaction
with TF. The surrounding region containing T of rs9390123 and
rs9399451 are matching with the motif of POU2F1 (see https://jaspar.genereg.net/ for detail). In contrast,
both C of rs9390123 and G of rs9399451 can disrupt the recognizing
site for POU2F1, thus resulting much lower scores for POU2F1
interaction in prediction (data not shown). Therefore, it was
hypothesized that rs9390123 and rs9399451 effect through
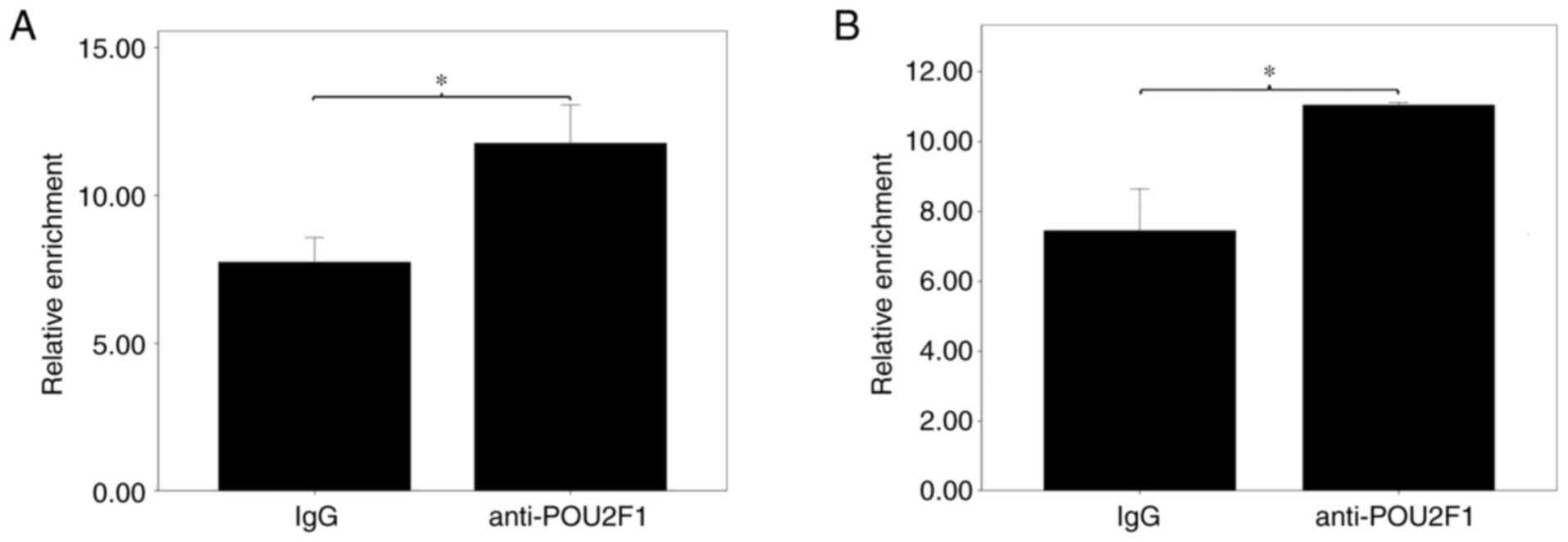
influencing POU2F1 binding. To confirm this suggestion, ChIP with
POU2F1 antibodies was utilized to identify the surrounding region
of the potential binding TF. As shown in Fig. 3, the anti-POU2F1 antibody
significantly pulled down more chromatin for the region surrounding
rs9390123 and rs9399451 compared with that pulled down by IgG
(P=0.001 and 0.004, respectively), thus supporting that POU2F1 has
the ability to bind these two regions in lung cells.
Effect of POU2F1 on gene
expression
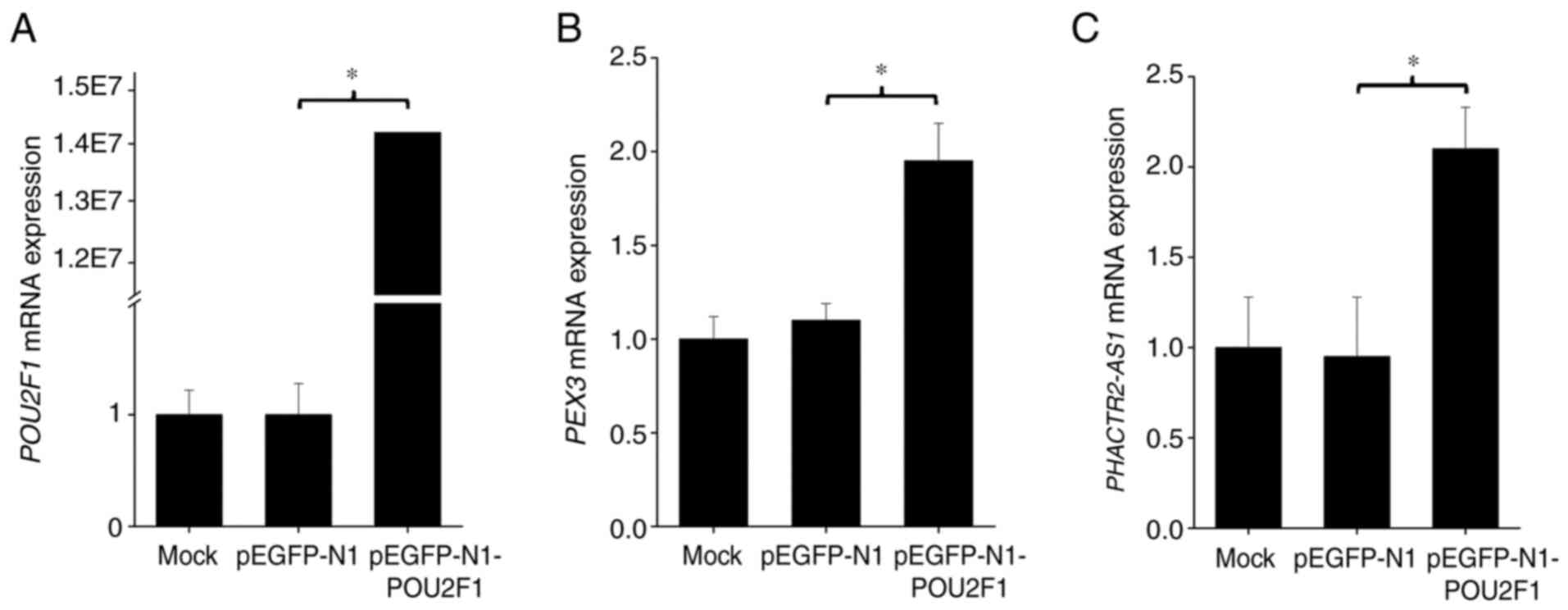
To further examine the function of POU2F1 on gene
transcription, a POU2F1 overexpression vector was constructed and
transfected into Beas-2B cells and mRNA levels were evaluated by
RT-qPCR. After transfection of the overexpression plasmid, POU2F1
expression was increased ~1.4×107 fold
(P<10−7; Fig. 4A),
which verified that the transfection efficiency was very high. This
could further induce ~95.0 and ~110% increase in mRNA expression of
PEX3 (P=0.0002; Fig. 4B)
and PHACTR2-AS1 (P=0.012; Fig.
4C) in lung cells, respectively, suggesting that POU2F1 can
influence the expression of target genes.
Difference in TF binding affinity
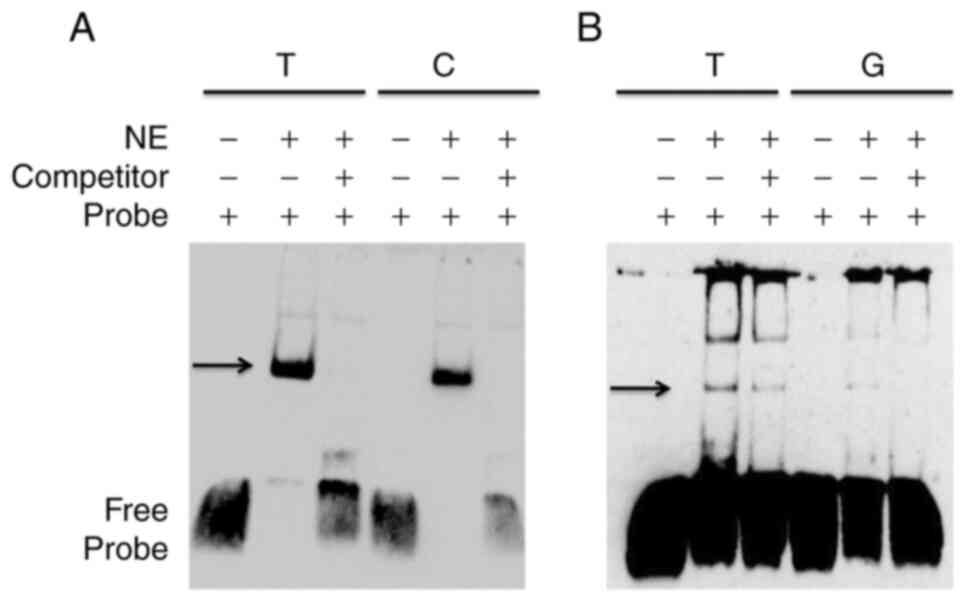
between alleles at the rs9390123 and rs9399451 SNPs
Our prediction, ChIP and overexpression assay verify
the effect of POU2F1 in the expression of these two genes. To
further investigate the binding efficiency between two alleles at
these two SNPs, EMSA was performed based on biotin-labeled probes.
As shown in Fig. 5, the two
alleles for rs9390123 and rs9399451 showed an apparent different
affinity to nuclear proteins from Beas-2B cells. Furthermore, these
patterns could be abolished by adding competitor oligonucleotides
(Fig. 5), which verified the
hypothesis that these two SNPs could alter TF binding affinity.
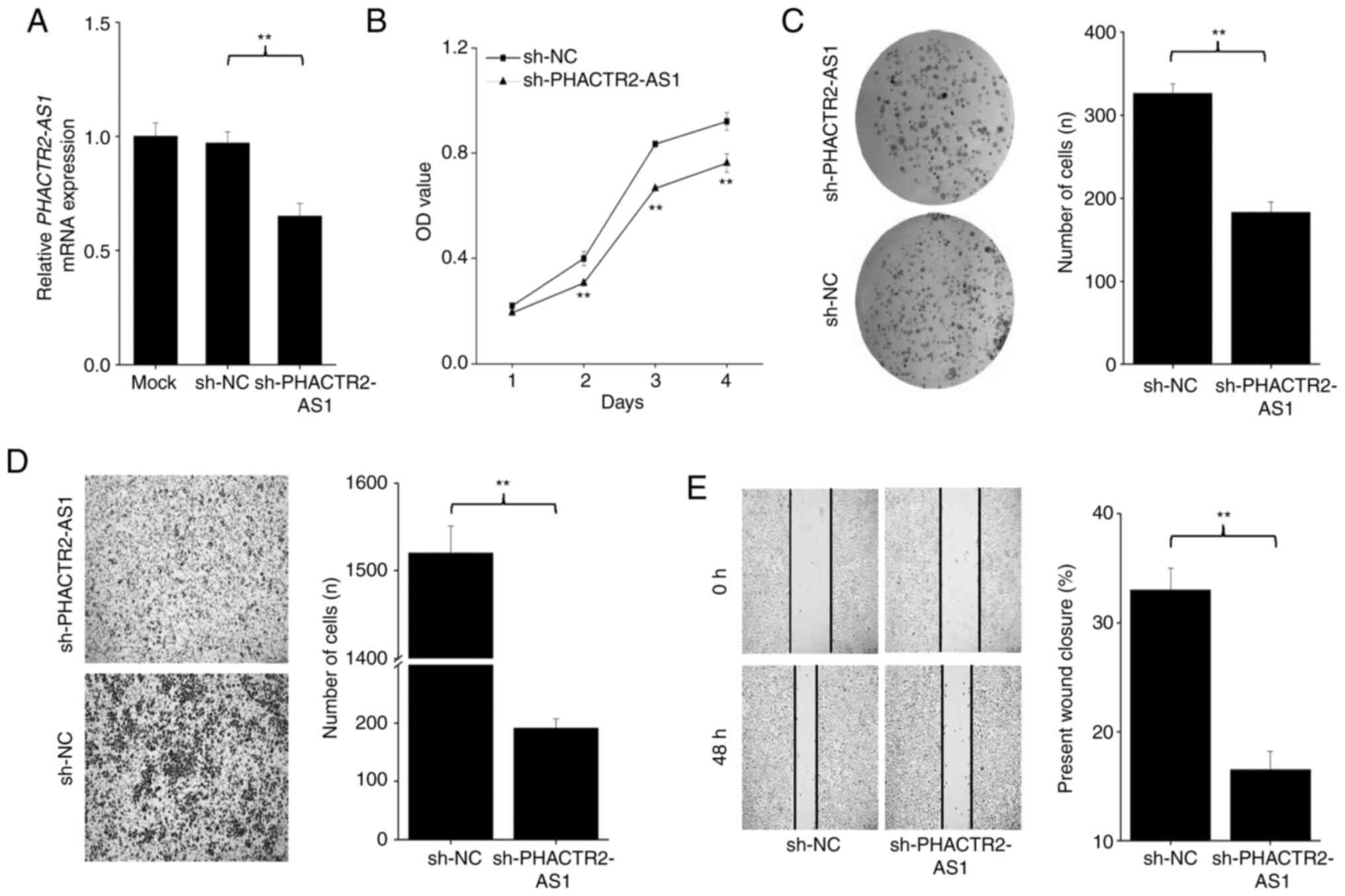
Transcript location and effect of
PHACTR2-AS1 on cell proliferation, colony formation, migration and
wound healing
Since PHACTR2-AS1 is the regulatory target of
the enhancer containing rs9390123 and rs9399451, the function and
transcript location of this gene in lung cells were investigated.
To determine the location of the lncRNA PHACTR2-AS1, nuclear
and cytoplasmic RNA was isolated and quantified separately. As
shown in Fig. S3, this lncRNA is
predominantly expressed in the nucleus. To investigate the function
of PHACTR2-AS1 in lung cells, a stable knockdown cell line
was constructed using Beas-2B cells by transfecting a lentivirus
containing shRNA, and the knockdown efficiency is displayed in
Fig. 6A. In Fig. 6A, the left panel is the PCR result
for GAPDH and the right panel is for PHACTR2-AS1. For
each part, the four lanes, from left to right, are ladder (L), Mock
(MC), negative control (NC) and knockdown (KD) cells, respectively.
The arrow in Fig. 6A points out
the position of the PHACTR2-AS1 PCR product. As shown in
Fig. 6A, PHACTR2-AS1
expression is persistent in the NC but decreased to undetectable
amount in the KD cells, which indicates that the transfection
efficiency was high and the knockdown was successful. Cell
proliferation was evaluated with an MTT assay. As shown in Fig. 6B, cell proliferation was
significantly inhibited in the KD group compared with that of the
control group (P=0.0017 and <10−5 for days 3 and 4,
respectively). The number of formed clones was also significantly
decreased after PHACTR2-AS1 knockdown (P=0.00085; Fig. 6C). Migration and wound healing
abilities were also significantly attenuated after knockdown
(P=0.00048 and 0.00024, respectively; Fig. 6D and E).
 | Figure 6.Results of (A) amplification and its
effect on the (B) cell proliferation, (C) colony formation, (D)
cell migration and (E) wound healing of PHACTR2-AS1 stable
knockdown Beas-2B cells (sh-PHACTR2-AS1). In panel A, the
left side corresponds to GAPDH, while the right side
corresponds to PHACTR2-AS1. For each side, the first lane is
the DNA ladder, while the second and third lanes contain the cDNA
from sh-NC and sh-PHACTR2-AS1, respectively. The arrow
indicates the position of the PHACTR2-AS1 PCR product, which
was undetectable after knockdown. In panel B, the x-axis designates
culture time, while the y-axis represents relative cell number. In
panel D, the background for sh-PHACTR2-AS1 is relatively
lighter, which may induce an overlook of some cells in vision.
Three independent repeats are performed in panel B-E. Data are
shown as the mean ± SD. **P<0.01. sh, small hairpin;
PHACTR2, phosphatase and actin regulator 2; AS1, antisense
RNA 1; MC, Mock; L, ladder; NC, Negative control; KD,
knockdown. |
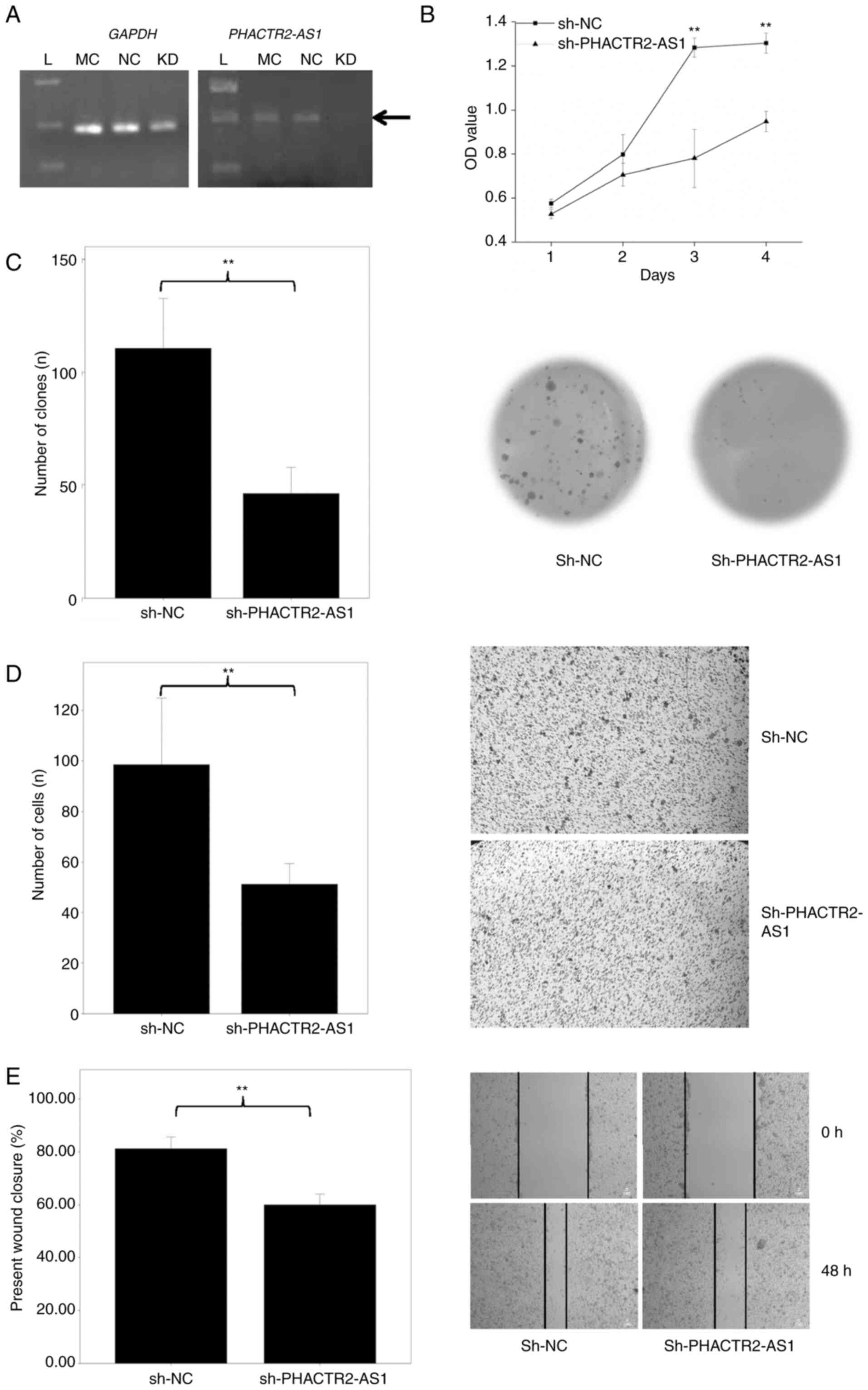
To substantiate the function of PHACTR2-AS1
in tumorigenic lung cells, we further transfected lung cancer cell
line A549 with the lentivirus. As shown in Fig. 7A, PH2ACTR2-AS1 expression
was similar in the mock and negative control (NC) (P>0.05).
After RNA interference, PH2ACTR2-AS1 expression was
decreased ~40% (Fig. 7A) compared
with the mock and NC, which indicates that the transfection
efficiency was high. Consequently, the cell count was reduced
significantly at day 2, 3 and 4 (all P=0.0012, <10−5
and 0.0011, respectively; Fig.
7B). Meanwhile, the clone formation (P=0.00012; Fig. 7C), migration (P<10−6;
Fig. 7D) and wound healing
(P=0.00062; Fig. 7E) abilities
were also significantly inhibited. Overall, these results indicated
that PHACTR2-AS1 can promote lung cell proliferation and
migration in vitro, and suggested that it could be a novel
oncogene for lung cancer.
Discussion
The present study aimed to clarify the underlying
mechanism between the genetic marker rs9390123 and DNA repair
capacity (DRC). Data from the 1000 Genomes Project were utilized to
analyze the linkage disequilibrium (LD) spectrum, and another
single nucleotide polymorphism (SNP), rs9399451, was identified to
be in strong LD with rs9390123, particularly in Caucasians. Dual
luciferase assay results suggested that both SNPs could alter
enhancer activity. Chromosome conformation capture (3C) revealed
that the regulatory target genes may be PEX3 and lncRNA
PHACTR2-AS1 instead of PHACTR2. By using ChIP and
EMSA, the associated transcription factors (TFs) and molecular
mechanism were identified. The function of PHACTR2-AS1 in
lung cancer was further evaluated. The present findings suggest an
association between the genetic marker rs9390123 and DRC.
Phosphatase and actin regulator 2 (PHACTR2)
was reported to be mainly expressed in the nervous system (17). Since rs9390123 is located within
the intron of PHACTR2, this gene was proposed to be the
regulatory target of this enhancer (4). However, the present 3C results
rejected a potential spatial interaction between the enhancer
containing rs9390123 and the PHACTR2 promoter, which
indicated that PHACTR2 is not the regulatory target of this
enhancer.
Instead, the results of 3C suggested a clear spatial
contact between this enhancer and the promoter of PEX3 and
PHACTR2-AS1. The peroxisome is an organelle containing
numerous enzymes (18), which
plays a central role in the metabolism of multiple substrates,
particularly reactive oxygen species (ROS) (19). Active ROS in cells can induce DNA
damage and further cancer onset (20). Therefore, an association between
the peroxisome and cancer has been proposed (21). Peroxisomal biogenesis factor 3
(PEX3) is a membrane import receptor that plays an important
role in peroxisome biogenesis (22). Therefore, high PEX3
expression could benefit DRC and further reduce the possibility of
lung cancer onset. A search for PEX3 expression in The
Cancer Genome Atlas database by online software TANRIC (23) indicated that the expression of this
gene is significantly suppressed in lung adenocarcinoma tissues
compared with that in normal tissues (P=0.0059; Fig. S4). However, in lung squamous cell
carcinoma, no significant difference was observed (P=0.17; data not
shown), which may be due to the relatively low sample size of the
normal group (n=17). Considering the function of PEX3, this
gene should have a tumor-suppressor effect through a long-term
pattern, and knockdown of this gene would not directly influence
the cell cycle, particularly in culture condition.
PHACTR2-AS1 (previously known as lncIHS or
NR027113) (10–12), another regulatory target of this
enhancer containing rs9390123 and rs9399451 besides PEX3, is
a novel lncRNA with various cellular locations and functions. In
breast tissue, PHACTR2-AS1 is suggested to be a
tumor-suppressor gene for breast cancer, and injecting this lncRNA
fragment into mice could inhibit tumor growth and metastasis
(24). By contrast, this lncRNA
was suggested to be an oncogene for hepatocellular, gastric and
tongue squamous cell carcinoma (10–12,25).
In tongue squamous carcinoma cells, PHACTR2-AS1 is mainly
expressed in the cytoplasm and functions by interacting with
microRNA-137 (25). In
hepatocellular and gastric carcinoma cells, this lncRNA was
proposed to be present in the nucleus and is able to regulate the
activity of the ERK and AKT signaling pathways (10–12).
Considering the same location and function, and the wide
distribution of members of the ERK and AKT signaling pathways in
human tissues, it could be hypothesized that PHACTR2-AS1
could also activate the ERK and AKT signaling pathways in lung
tissues. Furthermore, it has been proposed that the ERK and AKT
signaling pathways could activate DNA repair (26–31),
and inhibition of either pathway could impair DRC in several cancer
types (32–35). Therefore, the association between
the genetic marker rs9390123 and DRC in lung tissue (4) may also be interpreted by the
capability of regulating PHACTR2-AS1 expression.
Due to the important role of DRC in the onset of
lung cancer, it could be concluded that rs9390123 may be associated
with lung cancer by regulating PEX3 and PHACTR2-AS1
expression and further DRC. However, the association between
rs9390123 and lung cancer risk failed to reach the genome-wide
significance threshold (4). This
inconsistency may be due to the function of the PHACTR2-AS1
transcript. Indeed, higher PHACTR2-AS1 expression would not
only benefit DRC but also promote cell proliferation, as shown in
the present knockdown results, which may attenuate the association
between rs9390123 and lung cancer risk.
The present work has some limitations in the lack of
evidence for an association between rs9399451 or the two target
genes and DRC. Since measuring DRC is beyond our ability, we cannot
provide direct evidence for this issue. However, it is notable that
the r2 between rs9399451 and rs9390123 was as
high as 0.979 in the CEU population, which is near a complete LD
and suggests the same result of these two SNPs in association
study. Moreover, there has been no evidence for the involvement of
PEX3 and PHACTR2-AS1 in DRC. However, considering the
function of the peroxisome (18)
and the role of ERK and AKT pathway in DNA repair activation
(26–31), a lower expression of these two
genes induced by genetic factors will definitely decrease
individual DRC, which deserves further investigation.
Considering the function of rs9390123 and rs9399451
and the present 3C results, it could be proposed that these two
SNPs may be an expression quantitative trait locus (eQTL) for
PEX3 and PHACTR2-AS1. To verify this hypothesis, a
search was performed in GTEx (https://gtexportal.org/), a database including data on
the regulation of gene expression in multiple tissues (36). However, no association was observed
between this locus and the expression of the PEX3 and
PHACTR2-AS1 genes. This discrepancy may be interpreted by
the fact that the sensitivity and power of eQTL analysis could be
influenced by environmental and physiological effects (37). This effect is more serious for
genes involved in the cell cycle and response to exogenous
treatment (38).
In conclusion, our research effort indicates that
rs9390123 and rs9399451 influence DRC of lung cancer through
regulating PEX3 and PHACTR2-AS1 expression, which
illuminates the mechanism for the association in GWAS and
guarantees the usage of the expression levels of these two genes to
measure individual DRC.
Supplementary Material
Supporting Data
Acknowledgements
We thank Professor Huanjie Shao (Shaanxi Normal
University) for technical advice.
Funding
This research was supported by the Fundamental Research Funds
for the Central Universities (nos. 2018CBLY005 and GK202001004),
National Natural Science Foundation of China (no. 31370129) and
Qinzhu Grant (no. KY2019YB017).
Availability of data and materials
The datasets used and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
QS and CS designed the project and applied for the
funding. QS, QNS, HYW, YJL, XXZ, JWX, YHF, RHT, RJ and CCL
performed the experiments. QS analyzed the data. CS composed the
manuscript. All authors read and approved the manuscript and ensure
the integrity of the collected data and agree to be accountable for
all aspects of the research in ensuring that the accuracy or
integrity of any part of the work are appropriately investigated
and resolved.
Ethics approval and consent to
participate
Ethics approval was not applicable to the current
study as no human subjects were involved. The data in Fig. S4 are from a public database.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
Dr Chang Sun: ORCID: 0000-0002-2425-9485.
Glossary
Abbreviations
Abbreviations:
|
FUCA2
|
alpha-L-fucosidase 2
|
|
BAC
|
bacterial artificial chromosome
|
|
CDS
|
coding DNA sequence
|
|
ChIP
|
chromatin immunoprecipitation
|
|
3C
|
chromosome conformation capture
|
|
CHB
|
Han Chinese in Beijing
|
|
DRC
|
DNA repair capacity
|
|
EMSA
|
electrophoretic mobility shift
assay
|
|
EXO1
|
exonuclease 1
|
|
eQTL
|
expression quantitative trait
locus
|
|
FBS
|
fetal bovine serum
|
|
FPKM
|
fragments per kilobase of transcripts
per million fragments mapped
|
|
GWAS
|
genome-wide association studies
|
|
GAPDH
|
glyceraldehyde-3-phosphate
dehydrogenase
|
|
LD
|
linkage disequilibrium
|
|
LUAD
|
lung adenocarcinoma
|
|
LUSC
|
lung squamous cell carcinoma
|
|
PED
|
pedigree
|
|
PEX3
|
peroxisomal biogenesis factor 3
|
|
PHACTR2-AS1
|
PHACTR2 antisense RNA 1
|
|
PHACTR2
|
phosphatase and actin regulator 2
|
|
POU2F1
|
POU class 2 homeobox 1
|
|
ROS
|
reactive oxygen species
|
|
SNP
|
single nucleotide polymorphism
|
|
TCGA
|
The Cancer Genome Atlas
|
|
TF
|
transcription factor
|
|
CEU
|
Utah Residents with Northern and
Western European Ancestry
|
|
VCF
|
Variant Call Format
|
|
YRI
|
Yoruba in Ibadan, Nigeria
|
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wei Q, Cheng L, Amos CI, Wang LE, Guo Z,
Hong WK and Spitz MR: Repair of tobacco carcinogen-induced DNA
adducts and lung cancer risk: A molecular epidemiologic study. J
Natl Cancer Inst. 92:1764–1772. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wei Q and Spitz MR: The role of DNA repair
capacity in susceptibility to lung cancer: A review. Cancer
Metastasis Rev. 16:295–307. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang LE, Gorlova OY, Ying J, Qiao Y, Weng
SF, Lee AT, Gregersen PK, Spitz MR, Amos CI and Wei Q: Genome-wide
association study reveals novel genetic determinants of DNA repair
capacity in lung cancer. Cancer Res. 73:256–264. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
International HapMap Consortium, . The
International HapMap Project. Nature. 426:789–796. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
1000 Genomes Project Consortium, . Auton
A, Brooks LD, Durbin RM, Garrison EP, Kang HM, Korbel JO, Marchini
JL, McCarthy S, McVean GA and Abecasis GR: A global reference for
human genetic variation. Nature. 526:68–74. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Barrett JC, Fry B, Maller J and Daly MJ:
Haploview: Analysis and visualization of LD and haplotype maps.
Bioinformatics. 21:263–265. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang YC, Fu WP, Zhang J, Zhong L, Cai SX
and Sun C: rs401681 and rs402710 confer lung cancer susceptibility
by regulating TERT expression instead of CLPTM1L in east Asian
populations. Carcinogenesis. 39:1216–1221. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kochl S, Niederstatter H and Parson W: DNA
extraction and quantitation of forensic samples using the
phenol-chloroform method and real-time PCR. Methods Mol Biol.
297:13–30. 2005.PubMed/NCBI
|
|
10
|
Chen Z, Zhou ZY, He CC, Zhang JL, Wang J
and Xiao ZY: Down-regulation of LncRNA NR027113 inhibits cell
proliferation and metastasis via PTEN/PI3K/AKT signaling pathway in
hepatocellular carcinoma. Eur Rev Med Pharmacol Sci. 22:7222–7232.
2018.PubMed/NCBI
|
|
11
|
Chen QF, Hu CF, Sun K and Lang YP: LncRNA
NR027113 promotes malignant progression of gastric carcinoma via
EMT signaling pathway. Eur Rev Med Pharmacol Sci. 23:4746–4755.
2019.PubMed/NCBI
|
|
12
|
Chen Z, Yu W, Zhou Q, Zhang J, Jiang H,
Hao D, Wang J, Zhou Z, He C and Xiao Z: A novel lncRNA IHS promotes
tumor proliferation and metastasis in HCC by regulating the ERK-
and AKT/GSK-3β-signaling pathways. Mol Ther Nucleic Acids.
16:707–720. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pessa HK, Will CL, Meng X, Schneider C,
Watkins NJ, Perälä N, Nymark M, Turunen JJ, Lührmann R and
Frilander MJ: Minor spliceosome components are predominantly
localized in the nucleus. Proc Natl Acad Sci USA. 105:8655–8660.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tristan C, Shahani N, Sedlak TW and Sawa
A: The diverse functions of GAPDH: Views from different subcellular
compartments. Cell Signal. 23:317–323. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
ENCODE Project Consortium: An integrated
encyclopedia of DNA elements in the human genome. Nature.
489:57–74. 2012. View Article : Google Scholar
|
|
16
|
Calo E and Wysocka J: Modification of
enhancer chromatin: What, how, and why? Mol Cell. 49:825–837. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Allen PB, Greenfield AT, Svenningsson P,
Haspeslagh DC and Greengard P: Phactrs 1-4: A family of protein
phosphatase 1 and actin regulatory proteins. Proc Natl Acad Sci
USA. 101:7187–7192. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
de Duve C: The peroxisome: A new
cytoplasmic organelle. Proc R Soc Lond B Biol Sci. 173:71–83. 1969.
View Article : Google Scholar
|
|
19
|
Fransen M, Lismont C and Walton P: The
peroxisome-mitochondria connection: How and why? Int J Mol Sci.
18:11262017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Prasad S, Gupta SC and Tyagi AK: Reactive
oxygen species (ROS) and cancer: Role of antioxidative
nutraceuticals. Cancer Lett. 387:95–105. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dahabieh MS, Di Pietro E, Jangal M,
Goncalves C, Witcher M, Braverman NE and Del Rincón SV: Peroxisomes
and cancer: The role of a metabolic specialist in a disease of
aberrant metabolism. Biochim Biophys Acta Rev Cancer. 1870:103–121.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sugiura A, Mattie S, Prudent J and McBride
HM: Newly born peroxisomes are a hybrid of mitochondrial and
ER-derived pre-peroxisomes. Nature. 542:251–254. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li J, Han L, Roebuck P, Diao L, Liu L,
Yuan Y, Weinstein JN and Liang H: TANRIC: An interactive open
platform to explore the function of lncRNAs in cancer. Cancer Res.
75:3728–3737. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chu W, Zhang X, Qi L, Fu Y, Wang P, Zhao
W, Du J, Zhang J, Zhan J, Wang Y, et al: The
EZH2-PHACTR2-AS1-Ribosome axis induces genomic instability and
promotes growth and metastasis in breast cancer. Cancer Res.
80:2737–2750. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yuan F, Miao Z, Chen W, Wu F, Wei C, Yong
J and Xiao C: Long non-coding RNA PHACTR2-AS1 promotes tongue
squamous cell carcinoma metastasis by regulating Snail. J Biochem.
168:651–657. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Golding SE, Morgan RN, Adams BR, Hawkins
AJ, Povirk LF and Valerie K: Pro-survival AKT and ERK signaling
from EGFR and mutant EGFRvIII enhances DNA double-strand break
repair in human glioma cells. Cancer Biol Ther. 8:730–738. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sato A, Sunayama J, Matsuda K, Seino S,
Suzuki K, Watanabe E, Tachibana K, Tomiyama A, Kayama T and
Kitanaka C: MEK-ERK signaling dictates DNA-repair gene MGMT
expression and temozolomide resistance of stem-like glioblastoma
cells via the MDM2-p53 axis. Stem Cells. 29:1942–1951. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
de Laval B, Pawlikowska P, Barbieri D,
Besnard-Guerin C, Cico A, Kumar R, Gaudry M, Baud V and Porteu F:
Thrombopoietin promotes NHEJ DNA repair in hematopoietic stem cells
through specific activation of Erk and NF-κB pathways and their
target, IEX-1. Blood. 123:509–519. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu Q, Turner KM, Alfred Yung WK, Chen K
and Zhang W: Role of AKT signaling in DNA repair and clinical
response to cancer therapy. Neuro Oncol. 16:1313–1323. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sun Y, Zhai L, Ma S, Zhang C, Zhao L, Li
N, Xu Y, Zhang T, Guo Z, Zhang H, et al: Down-regulation of RIP3
potentiates cisplatin chemoresistance by triggering HSP90-ERK
pathway mediated DNA repair in esophageal squamous cell carcinoma.
Cancer Lett. 418:97–108. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rezatabar S, Karimian A, Rameshknia V,
Parsian H, Majidinia M, Kopi TA, Bishayee A, Sadeghinia A, Yousefi
M, Monirialamdari M and Yousefi B: RAS/MAPK signaling functions in
oxidative stress, DNA damage response and cancer progression. J
Cell Physiol. 2019.(Online Ahead of Print). View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Toulany M, Kasten-Pisula U, Brammer I,
Wang S, Chen J, Dittmann K, Baumann M, Dikomey E and Rodemann HP:
Blockage of epidermal growth factor receptor-phosphatidylinositol
3-kinase-AKT signaling increases radiosensitivity of K-RAS mutated
human tumor cells in vitro by affecting DNA repair. Clin Cancer
Res. 12:4119–4126. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kao GD, Jiang Z, Fernandes AM, Gupta AK
and Maity A: Inhibition of phosphatidylinositol-3-OH kinase/Akt
signaling impairs DNA repair in glioblastoma cells following
ionizing radiation. J Biol Chem. 282:21206–21212. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen YR, Liu MT, Chang YT, Wu CC, Hu CY
and Chen JY: Epstein-Barr virus latent membrane protein 1 represses
DNA repair through the PI3K/Akt/FOXO3a pathway in human epithelial
cells. J Virol. 82:8124–8137. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Marampon F, Gravina GL, Di Rocco A,
Bonfili P, Di Staso M, Fardella C, Polidoro L, Ciccarelli C,
Festuccia C, Popov VM, et al: MEK/ERK inhibitor U0126 increases the
radiosensitivity of rhabdomyosarcoma cells in vitro and in vivo by
downregulating growth and DNA repair signals. Mol Cancer Ther.
10:159–168. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Battle A, Brown CD, Engelhardt BE and
Montgomery SB; GTEx Consortium; Laboratory, Data Analysis&
Coordinating Center (LDACC)-Analysis Working Group; Statistical
Methods groups-Analysis Working Group; Enhancing GTEx (eGTEx)
groups; NIH Common Fund; NIH/NCI; NIH/NHGRI; NIH/NIMH; NIH/NIDA
Biospecimen Collection Source Site-NDRI; Biospecimen Collection
Source Site-RPCI, : Genetic effects on gene expression across human
tissues. Nature. 550:204–213. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gagneur J, Stegle O, Zhu C, Jakob P,
Tekkedil MM, Aiyar RS, Schuon AK, Pe'er D and Steinmetz LM:
Genotype-environment interactions reveal causal pathways that
mediate genetic effects on phenotype. PLoS Genet. 9:e10038032013.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Umans BD, Battle A and Gilad Y: Where are
the disease-associated eQTLs? Trends Genet. 37:109–124. 2021.
View Article : Google Scholar : PubMed/NCBI
|