Introduction
Renal cell carcinoma (RCC), the most common
malignancy found in adult kidneys, is the tenth leading cause of
cancer-related mortality in Western countries, and is known to be
resistant to chemotherapy and radiation therapy (1,2).
Although agents used for molecular-targeted therapy, including
sunitinib, axitinib, temsirolimus, and pazopanib, have
significantly prolonged the survival of patients with advanced RCC,
responses induced by these drugs are transient (3–5).
Additionally, the recent advent of immunotherapy with the use of an
immune checkpoint inhibitor (ICI), such as nivolumab,
pembrolizumab, ipilimumab, and avelumab, has resulted in the
possibility of obtaining significant antitumor activity with
prolonged and durable responses in metastatic RCC patients,
although reported complete response rates induced by these drugs
range only from 4 to 10% (6–8).
Furthermore, a related concern is the occurrence of immune-related
adverse events (irAEs), which can have effects on nearly all organs
with varying frequency and severity, such as hypophysitis,
thyroiditis, adrenalitis, hepatitis, interstitial pneumonitis,
colitis, and interstitial nephritis (9). Therefore, development of novel and
effective therapeutic strategies for metastatic RCC is an urgent
need.
Apigenin, a natural flavonoid, is widely distributed
in a variety of fruits and vegetables, and this particular natural
compound has been shown to have a low level of toxicity as well as
potential antioxidant, anti-inflammatory, and anticancer properties
(10–14). It has also been reported that
apigenin inhibited tumor proliferation in vitro and in
vivo in examinations of several different types of human cancer
cell lines, including those associated with lung (15), prostate (16,17),
leukemia (18), breast (19), pancreatic (20), and oral cancer (21). This flavonoid is considered to be a
potentially effective anticancer agent because it exhibits
selective induction of cell cycle arrest and apoptosis in tumor
cells without affecting normal cells (22,23).
The present study was conducted to investigate the
anticancer activity of apigenin toward RCC cells in experiments
conducted with three human RCC cell lines as well as primary RCC
cells obtained from five patients. In addition, molecular
mechanisms possibly involved in the anticancer activity of apigenin
toward RCC cells were explored.
Materials and methods
Reagents
Apigenin was purchased from Sigma-Aldrich/Merck KGaA
and a Cell Counting Kit-8 (CCK-8) was obtained from Dojindo
Laboratories. Apigenin was dissolved in dimethylsulfoxide (DMSO)
and subsequently diluted in culture medium. The DMSO concentration
did not exceed 0.1% during treatment.
RCC cell lines and primary RCC
cells
The human RCC cell lines ACHN, Caki-1, and NC65
(ATCC) were used. Primary RCC cells were separated from surgical
specimens obtained from five patients with untreated RCC, as
previously described (24).
Pathologic stage and grade were consistent with the 2004 WHO
criteria (https://www.patologi.com/WHO%20kidney%20testis.pdf),
as follows: T2N0M0 grade 1 in patient 1 (70 years, male); T3bN0M0
grade 2 in patient 2 (68 years, female); T2N0M0 grade 1 in patient
3 (70 years, male); T3bN0M1b grade 2 in patient 4 (76 years,
female); and T2N0M0 grade 2 in patient 5 (63 years, male) at Hyogo
College of Medicine Hospital between March 1997 and February 1998.
All cells were cultured in RPMI-1640 medium supplemented with 10%
FBS and 1% penicillin and streptomycin, and then maintained at 37°C
in 5% CO2.
Ethical approval for the use of human tissue was
granted by the Hyogo College of Medicine (Hyogo, Japan). All
patients provided individual written informed consent for the use
of their sampled tissues.
Cytotoxicity assays
Cytotoxicity was determined based on colorimetric
assay findings of cell viability obtained with a CCK-8 Kit (Dojindo
Laboratories). Briefly, a 100-µl suspension of 0.5×104
cells was seeded into a 96-well flat bottom microtiter plate. After
incubation for 24 h, a drug solution or medium alone (control) was
added to the plates in triplicate, and then each plate was
incubated for an additional 24 h. Subsequently, 10 µl of CCK-8
solution was added for 3 h. Absorbance (A) in each well was
measured using a SPECTRAmax PLUS384 (Molecular Devices, LLC) at 450
nm as the reference, and cell viability was determined based on the
percentage of control cells using the following formula: [Percent
cell viability = (A of treated wells/A of control wells) ×100]
(25).
Cell viability and morphologic changes were
evaluated using trypan blue dye exclusion test and phase-contrast
microscopy findings, respectively. Cells were seeded into a 6-well
plate at 1.5×105 cells per well and cultured for 24 h,
and then treated in duplicate with apigenin for 24 h. After
treatment, the cells were harvested and viability was assessed
after 0.5% trypan blue dye (Sigma-Aldrich/Merck KGaA) staining for
1 min at room temperature, and then counted using a hemocytometer
under a phase contrast microscope as previously reported (26). Cell death was observed and
photographs of adherent cells were obtained using phase-contrast
microscopy after removal of medium containing floating cells.
Cell cycle analysis
Cells were treated with apigenin for 24 h, then
harvested, washed twice with cold assay buffer, and processed for
cell cycle analysis. Briefly, the cells were fixed in cell cycle
phase determination fixative at room temperature and stored at
−20°C overnight for later analysis according to the protocol of the
manufacturer (cell cycle phase determination kit, no. 10009349,
Cayman Chemical). Fixed cells were centrifuged at 1,500 rpm and
washed with cold PBS twice. Next, RNase A (20 µg/ml final
concentration) and propidium iodide (PI) staining solution (20
µg/ml final concentration) were added, and the cells were incubated
for 30 min at room temperature in the dark. Analysis was performed
using a LSRFortessa™ X-20 instrument (BD Biosciences)
equipped with BD FACSDiva software. Furthermore, FlowJo v10.7.1
(FlowJo LLC) trial cell cycle analysis software was used to
determine the percentage of cells in each of the different cell
cycle phases.
Western blot analysis
Cells were plated in 10-cm plates for 24 h and then
treated with apigenin for 6–24 h in a cell culture incubator at
37°C. Following the indicated treatment, cells were lysed for 30
min on ice in lysis buffer with a protease inhibitor cocktail, and
then protein concentrations were determined using a Bradford Assay
Kit (Bio-Rad Laboratories). Next, 20 µg of protein was separated
using 10% SDS-PAGE and transferred to a PVDF membrane. After
blocking nonspecific binding sites for 2 h at room temperature with
5% skim milk in TBS with 0.1% Tween-20, the membranes were
incubated overnight at 4°C with the following primary antibodies:
cyclin-dependent kinase 1 (CDK1; bs-0542R, Bioss Inc.), cyclin A
(sc-271682), cyclin B1 (sc-245), cyclin D1 (sc-6281), cyclin D3
(sc-6283), and cyclin E (sc-247) from Santa Cruz Biotechnology,
Inc. at 1:200 dilution, and β-actin mouse polyclonal [E4D9Z, Cell
Signaling Technology, Inc. (CST)] at 1:2,000 dilution. The
membranes were washed three times by TBST buffer for 30 min at room
temperature and then incubated with horseradish peroxidase
(HRP)-conjugated anti-mouse IgG secondary antibody (code. 330, MBL)
at 1:2,000 dilution and HRP-conjugated goat anti-rabbit IgG
secondary antibody (sc-2004, Santa Cruz Biotechnology, Inc.) at
1:1,000 dilution for 1 h at room temperature. Finally, the
membranes were washed three times with TBST buffer for 30 min at
room temperature and signals were detected using chemiluminescence
ECL kit (GE Healthcare) with an ImageQuant LAS 4010 system (GE
Healthcare).
Statistical analysis
All determinations were conducted at least three
times, and the results are expressed as the mean ± SD of three
experiments. All analyses were performed using Graphpad Prism V8
for Mac (GraphPad Software, Inc.). A two-tailed value of P<0.05
was considered to indicate statistical significance. Differences
between treatment groups and controls were analyzed by one-way
ANOVA analysis of variance, followed by Dunnett's multiple
comparison test.
Results
Apigenin inhibits cell
proliferation
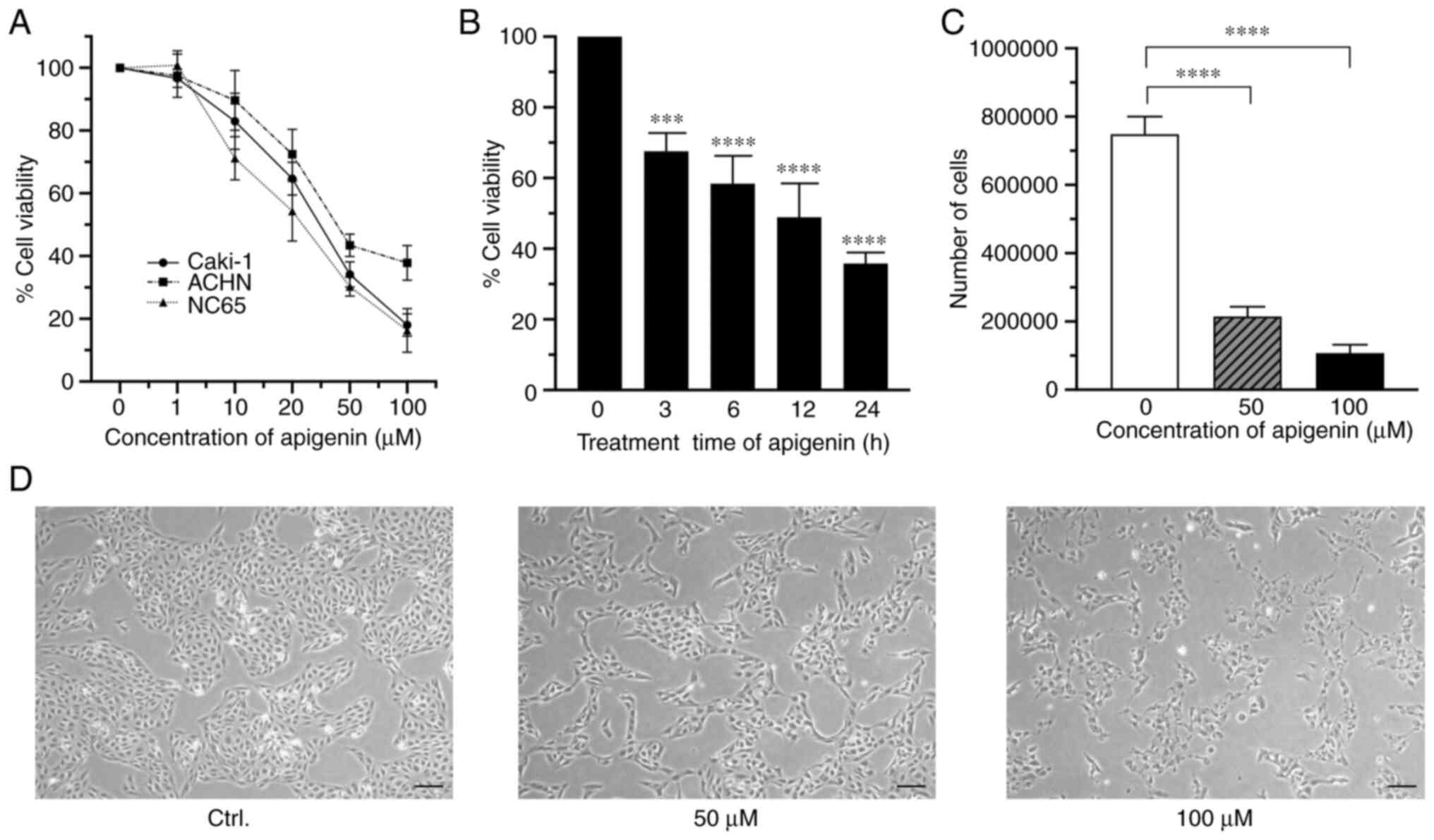
First, the effects of different concentrations of
apigenin on cell viability were examined using Caki-1, a human RCC
cell line. For cells treated for 24 h, apigenin inhibited
proliferation in a dose-dependent manner with a 50% inhibition
concentration (IC50) value of 27.02 µM. Similar
antiproliferative effects were also noted with the RCC cell lines
ACHN and NC65 (Fig. 1A), which
showed IC50 values of 50.40 and 23.34 µM, respectively.
Furthermore, apigenin inhibited proliferation of Caki-1 cells in a
time-dependent manner (Fig. 1B).
This antiproliferative activity of apigenin was further confirmed
by findings obtained with a trypan blue dye-exclusion test
(Fig. 1C). Furthermore, a marked
decrease in cell numbers, cell swelling, and destruction of cells
were also observed using phase-contrast microscopy when the cells
were treated with apigenin (Fig.
1D).
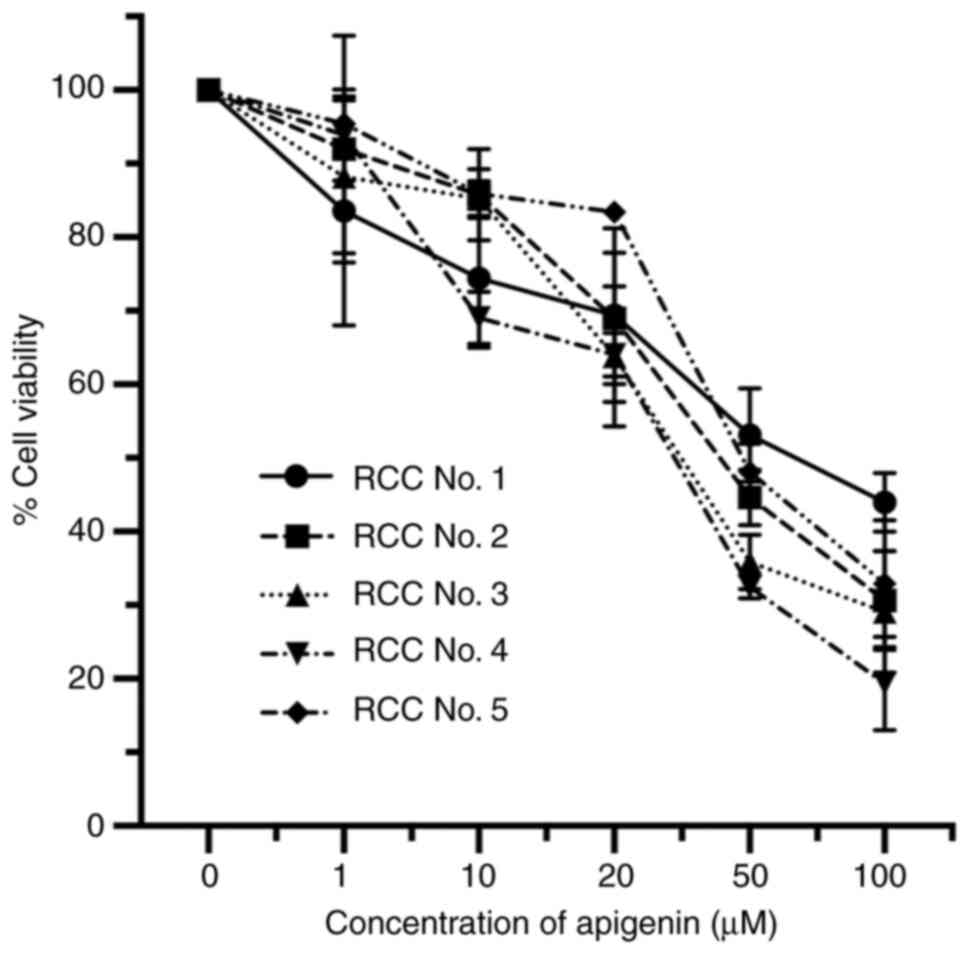
To further examine the antiproliferative effect of
apigenin on RCC cells, we examined primary RCC cells obtained from
five patients. In all patient samples, a marked dose-dependent
antiproliferative effect was achieved, regardless of the varying
sensitivity of the RCC cells (Fig.
2). The IC50 values for apigenin with cells from
those five cases (patient 1–5) were 73.02, 43.74, 35.63, 26.80, and
53.51 µM, respectively.
Taken together, these findings clearly demonstrated
that treatment of human RCC lines as well as primary RCC cells with
apigenin provides an antiproliferative effect.
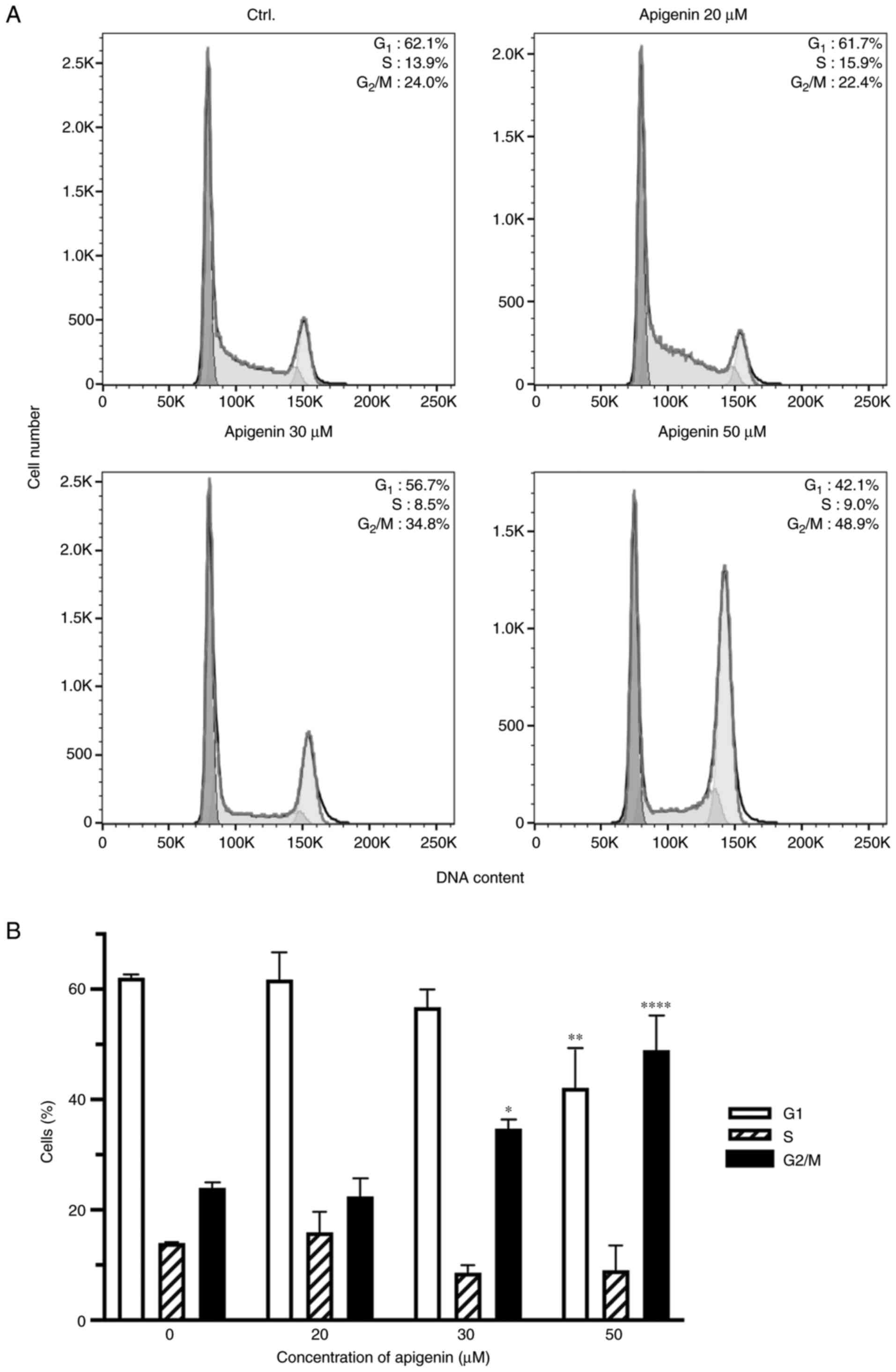
Apigenin induces G2/M phase
cell cycle arrest
In order to better understand the mechanism of cell
proliferation inhibition, cell cycle distribution in different
phases of the cell cycle was analyzed following apigenin treatment.
There were marked changes in the cell cycle shown by Caki-1 cells
treated with apigenin including an increase in percentage of cells
in the G2/M phase, along with a concomitant decrease in
those in the G0/G1 and S phases as compared
with untreated cells (Fig. 3A).
Following treatment with apigenin at 20, 30, and 50 µM, the
percentage of Caki-1 cells in the G2/M phase was 22.4,
34.8, and 48.9%, respectively, while only 24% of the control cells
were found to be in the G2/M phase (Fig. 3B).
Apigenin modulates expression of
cyclin A, B1, D3, and E
To further assess the molecular mechanisms related
to inhibition of cell proliferation, the effects of apigenin on
expression levels of cyclin A, B1, D1, D3, and E, as well as CDK1
in RCC cells were evaluated. Apigenin significantly reduced cyclin
A, B1, D3 and E expression levels in Caki-1 cells (Fig. 4A-D), whereas there was no effect on
cyclin D1 or CDK1 expression noted. Additionally, downregulation of
cyclin A, B1, D3, and E expression was also observed in the primary
RCC cells (Fig. 4E and F).
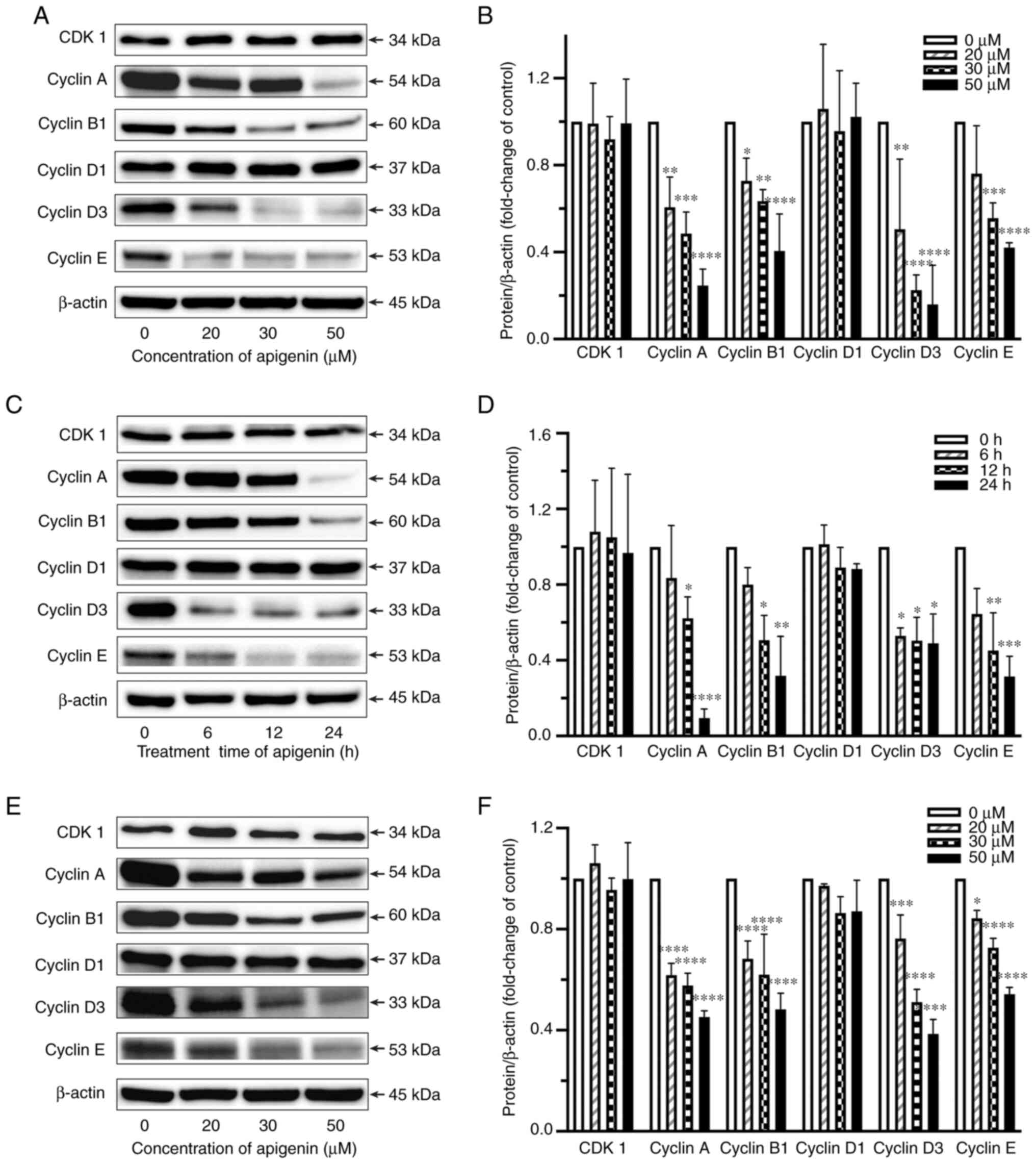 | Figure 4.Effects of apigenin on expression
levels of CDK1 and cyclin A, B1, D1, D3, and E in RCC cells. Caki-1
cells were treated with apigenin at 20–50 µM for 24 h (A and B) or
at 30 µM for 6–24 h. (C and D) Primary RCC cells (RCC No. 1) were
treated with apigenin at 20–50 µM for 24 h. (E and F) Expression
levels of CDK1 and cyclin A1, B1, D1, D3, and E were assessed by
western blotting. β-actin was used as the loading control.
Representative findings from one of three individual experiments
are shown. Densitometric analysis of blots of protein were
normalized to the corresponding β-actin levels. Values are shown as
the mean±SD of three individual experiments. *P<0.05,
**P<0.01, ***P<0.001, ****P<0.0001 vs. untreated
control. |
Discussion
Renal cell carcinoma (RCC) malignancy is highly
resistant to conventional chemotherapy and radiation therapy.
Durable responses to targeted agents are rare, and most patients
with metastatic RCC eventually progress and die from the disease,
even though several molecular-targeted drugs administered to slow
RCC growth are currently being used, with some instances of success
reported (27,28). Recently, immune checkpoint
inhibitors (ICIs) have been shown to have significant antitumor
activity, with prolonged and durable responses noted in metastatic
RCC cases, although the complete response rate induced by these
drugs is less than 10% (6,7). Furthermore, a matter of concern is
the wide range of immune-related adverse events (irAEs) that can
affect nearly all organs with varying frequency and severity in
patients receiving ICIs (9). Thus,
development of novel and effective therapeutic strategies for
metastatic RCC is an urgent need.
The present results demonstrated that apigenin, a
flavonoid widely present in fruits and vegetables, has
antiproliferative effects toward RCC cells, which were observed in
experiments with not only established human RCC cell lines but also
human primary RCC cells. The antiproliferative activity of apigenin
was demonstrated by induction of G2/M phase cell cycle arrest. From
a clinical perspective, our findings suggest that apigenin is a
promising agent.
The anticancer action of apigenin is dependent on
various mechanisms that can vary according to cell type and involve
apoptosis, modulation of the cell cycle, and alteration of kinase
pathways (14). In the present
study, analysis of cell cycle distribution revealed a marked
increase in the percentage of RCC cells in the G2/M phase following
apigenin treatment. These results support those of previous studies
showing that apigenin inhibits the cell cycle of various types of
human cancer cells in the G2/M phase (20,29–32).
Cell cycle checkpoints at G2/M as well as G1/S are
critical for maintaining DNA integrity, and also regulating the
passage of cells through the cell cycle. It is well known that loss
of these checkpoints is involved in the process of transformation
into cancer cells and disease progression. A protein kinase complex
consisting of CDK1, a catalytic subunit, and cyclin B has a central
rate-limiting function in the transition from G2 to M phase
(33,34). This CDK1/cyclin B complex responds
to DNA damage and causes a delay in cell cycle progression, which
allows for DNA repair before the cells enter mitosis. Several
investigators have also shown that the combination of CDK1 with
cyclin A and B is critical for G2/M phase transition (35,36).
On the other hand, it has been reported that keratinocyte cells do
not show modulation of the CDK1 level after apigenin treatment
(21), thus indicating that
apigenin induces G2/M arrest through a variety of mechanisms in
different cells, and that cell growth deregulation in cancer cells
may be dependent on the downregulation of cyclin E expression
(21,37). In the present experiments, apigenin
remarkably reduced the expression of cyclin A, B1, D3 and E in RCC
cells, whereas it had no effect on expression of CDK1 or cyclin D1.
Thus, downregulation of cyclin B1, as well as cyclin A, D3 and E by
apigenin may have been the main cause of G2/M phase arrest observed
in the RCC cells. This decrease in quantity of cyclins observed as
a result of apigenin treatment is consistent with arrest during the
G2/M phase, because these proteins are not expressed in resting
cells (38).
Based on findings demonstrating a relatively
selective growth inhibitory effect toward cancer cells as compared
with normal cells, apigenin is considered to be an attractive
candidate for cancer treatment (22). Shukla et al reported
significantly delayed development of prostate cancer in mice
following apigenin administration as well as delayed occurrence of
death from prostate cancer (17).
It was also demonstrated that apigenin inhibits melanoma lung
metastasis by impairing the interaction of tumor cells with the
endothelium (39). Recently, Meng
et al reported reduced tumor growth and volume in
vivo in an ACHN cell xenograft mouse model administered
apigenin treatment (33). In
addition, no severe side effects of apigenin administration have
been observed in studies of mice that used therapeutic doses
(17,33,38,40).
The present results demonstrated that apigenin has
antiproliferative effects, not only in human RCC cell lines but
also human primary RCC cells. Notably, even when the treatment time
was shortened from 24 to 3 h, the same cytostatic effect was shown
in the RCC cells. These results suggest that apigenin may be useful
for development as an effective therapeutic agent for advanced RCC.
Additionally, the present study also observed that the
IC50 values for apigenin in primary RCC cells from those
five cases (patient 1–5) were the strong different, thus may
indicate different sensitivities of apigenin in different patients.
Furthermore, the present findings showing a cytostatic effect on
RCC cells treated with apigenin for a short period of time may
provide a foundation for optimizing administration in clinical
applications. Further study will be needed to confirm its antitumor
activity using primary RCC cell xenograft mouse models, as the
limitations of the current study include lack of an in vivo
mouse model.
In conclusion, apigenin was shown to have an
antiproliferative effect and induce G2/M phase arrest in RCC cells,
possibly through direct downregulation of cyclin A, B1, D3 and E.
Together, the present findings suggest treatment of RCC with
apigenin as a promising potential clinical application.
Acknowledgements
The authors wish to thank the members of the
Joint-Use Research facilities, Hyogo College of Medicine (Japan),
for providing technical assistance.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in the current published article.
Authors' contributions
YB performed the cell proliferation and western blot
assays, and statistical analysis of the data obtained in all of the
experiments. XJ and AK performed the trypan blue staining and cell
cycle analysis. MN and YK analyzed and interpreted the cell
proliferation assay data. SY and XW planned, analyzed, and
interpreted all of the experiments and validity of the data, and
drafted the manuscript. All authors read and approved the
manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work and data are appropriately investigated and resolved.
Ethics approval and consent to
participate
Ethical approval for the use of human tissue was
granted by the Hyogo College of Medicine (approval no. 202104-07,
Hyogo, Japan). All patients provided individual written informed
consent for the use of their sampled tissues.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2020. CA Cancer J Clin. 70:7–30. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hudes G, Carducci M, Tomczak P, Dutcher J,
Figlin R, Kapoor A, Staroslawska E, Sosman J, McDermott D, Bodrogi
I, et al: Temsirolimus, interferon alfa, or both for advanced
renal-cell carcinoma. N Engl J Med. 356:2271–2281. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Albiges L, Oudard S, Negrier S, Caty A,
Gravis G, Joly F, Duclos B, Geoffrois L, Rolland F, Guillot A, et
al: Complete remission with tyrosine kinase inhibitors in renal
cell carcinoma. J Clin Oncol. 30:482–487. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mitchell AP, Hirsch BR, Harrison MR,
Abernethy AP and George DJ: Deferred systemic therapy in patients
with metastatic renal cell carcinoma. Clin Genitourin Cancer.
13:e159–166. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Motzer RJ, Rini BI, McDermott DF, Arén
Frontera O, Hammers HJ, Carducci MA, Salman P, Escudier B,
Beuselinck B, Amin A, et al: Nivolumab plus ipilimumab versus
sunitinib in first-line treatment for advanced renal cell
carcinoma: Extended follow-up of efficacy and safety results from a
randomised, controlled, phase 3 trial. Lancet Oncol. 20:1370–1385.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Powles T, Plimack ER, Soulières D, Waddell
T, Stus V, Gafanov R, Nosov D, Pouliot F, Melichar B, Vynnychenko
I, et al: Pembrolizumab plus axitinib versus sunitinib monotherapy
as first-line treatment of advanced renal cell carcinoma
(KEYNOTE-426): Extended follow-up from a randomised, open-label,
phase 3 trial. Lancet Oncol. 21:1563–1573. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Uemura M, Tomita Y, Miyake H, Hatakeyama
S, Kanayama HO, Numakura K, Takagi T, Kato T, Eto M, Obara W, et
al: Avelumab plus axitinib vs sunitinib for advanced renal cell
carcinoma: Japanese subgroup analysis from JAVELIN Renal 101.
Cancer Sci. 111:907–923. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Martins F, Sofiya L, Sykiotis GP, Lamine
F, Maillard M, Fraga M, Shabafrouz K, Ribi C, Cairoli A,
Guex-Crosier Y, et al: Adverse effects of immune-checkpoint
inhibitors: Epidemiology, management and surveillance. Nat Rev Clin
Oncol. 16:563–580. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang M, Firrman J, Liu L and Yam K: A
review on flavonoid apigenin: Dietary intake, ADME, antimicrobial
effects, and interactions with human gut microbiota. Biomed Res
Int. 2019:70104672019.PubMed/NCBI
|
|
11
|
Salehi B, Venditti A, Sharifi-Rad M,
Kręgiel D, Sharifi-Rad J, Durazzo A, Lucarini M, Santini A, Souto
EB, Novellino E, et al: The therapeutic potential of apigenin. Int
J Mol Sci. 20:13052019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shukla S and Gupta S: Apigenin: A
promising molecule for cancer prevention. Pharm Res. 27:962–978.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou Y, Zheng J, Li Y, Xu DP, Li S, Chen
YM and Li HB: Natural polyphenols for prevention and treatment of
cancer. Nutrients. 8:5152016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Patel D, Shukla S and Gupta S: Apigenin
and cancer chemoprevention: Progress, potential and promise
(review). Int J Oncol. 30:233–245. 2007.PubMed/NCBI
|
|
15
|
Lu HF, Chie YJ, Yang MS, Lee CS, Fu JJ,
Yang JS, Tan TW, Wu SH, Ma YS, Ip SW, et al: Apigenin induces
caspase-dependent apoptosis in human lung cancer A549 cells through
Bax- and Bcl-2-triggered mitochondrial pathway. Int J Oncol.
36:1477–1484. 2010.PubMed/NCBI
|
|
16
|
Ganai SA: Plant-derived flavone Apigenin:
The small-molecule with promising activity against therapeutically
resistant prostate cancer. Biomed Pharmacother. 85:47–56. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shukla S, MacLennan GT, Flask CA, Fu P,
Mishra A, Resnick MI and Gupta S: Blockade of beta-catenin
signaling by plant flavonoid apigenin suppresses prostate
carcinogenesis in TRAMP mice. Cancer Res. 67:6925–6935. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ruela-de-Sousa RR, Fuhler GM, Blom N,
Ferreira CV, Aoyama H and Peppelenbosch MP: Cytotoxicity of
apigenin on leukemia cell lines: Implications for prevention and
therapy. Cell Death Dis. 1:e192010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee HH, Jung J, Moon A, Kang H and Cho H:
Antitumor and anti-invasive effect of apigenin on human breast
carcinoma through suppression of IL-6 expression. Int J Mol Sci.
20:31432019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ujiki MB, Ding XZ, Salabat MR, Bentrem DJ,
Golkar L, Milam B, Talamonti MS, Bell RH Jr, Iwamura T and Adrian
TE: Apigenin inhibits pancreatic cancer cell proliferation through
G2/M cell cycle arrest. Mol Cancer. 5:762006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Maggioni D, Garavello W, Rigolio R,
Pignataro L, Gaini R and Nicolini G: Apigenin impairs oral squamous
cell carcinoma growth in vitro inducing cell cycle arrest and
apoptosis. Int J Oncol. 43:1675–1682. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gupta S, Afaq F and Mukhtar H: Selective
growth-inhibitory, cell-cycle deregulatory and apoptotic response
of apigenin in normal versus human prostate carcinoma cells.
Biochem Biophys Res Commun. 287:914–920. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chiang LC, Ng LT, Lin IC, Kuo PL and Lin
CC: Anti-proliferative effect of apigenin and its apoptotic
induction in human Hep G2 cells. Cancer Lett. 237:207–214. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu XX, Mizutani Y, Kakehi Y, Yoshida O and
Ogawa O: Enhancement of Fas-mediated apoptosis in renal cell
carcinoma cells by adriamycin. Cancer Res. 60:2912–2918.
2000.PubMed/NCBI
|
|
25
|
Jin X, Wu XX, Abdel-Muneem Nouh MA and
Kakehi Y: Enhancement of death receptor 4 mediated apoptosis and
cytotoxicity in renal cell carcinoma cells by subtoxic
concentrations of doxorubicin. J Urol. 177:1894–1899. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu XX, Jin XH, Zeng Y, El Hamed AM and
Kakehi Y: Low concentrations of doxorubicin sensitizes human solid
cancer cells to tumor necrosis factor-related apoptosis-inducing
ligand (TRAIL)-receptor (R) 2-mediated apoptosis by inducing
TRAIL-R2 expression. Cancer Sci. 98:1969–1976. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Coppin C, Le L, Porzsolt F and Wilt T:
Targeted therapy for advanced renal cell carcinoma. Cochrane
Database Syst Rev. Cd0060172008.PubMed/NCBI
|
|
28
|
Zhou L, Liu XD, Sun M, Zhang X, German P,
Bai S, Ding Z, Tannir N, Wood CG, Matin SF, et al: Targeting MET
and AXL overcomes resistance to sunitinib therapy in renal cell
carcinoma. Oncogene. 35:2687–2697. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang W, Heideman L, Chung CS, Pelling JC,
Koehler KJ and Birt DF: Cell-cycle arrest at G2/M and growth
inhibition by apigenin in human colon carcinoma cell lines. Mol
Carcinog. 28:102–110. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim SM, Vetrivel P, Ha SE, Kim HH, Kim JA
and Kim GS: Apigetrin induces extrinsic apoptosis, autophagy and
G2/M phase cell cycle arrest through PI3K/AKT/mTOR pathway in AGS
human gastric cancer cell. J Nutr Biochem. 83:1084272020.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shendge AK, Chaudhuri D and Mandal N: The
natural flavones, acacetin and apigenin, induce Cdk-Cyclin mediated
G2/M phase arrest and trigger ROS-mediated apoptosis in
glioblastoma cells. Mol Biol Rep. 48:539–549. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shendge AK, Chaudhuri D, Basu T and Mandal
N: A natural flavonoid, apigenin isolated from Clerodendrum
viscosum leaves, induces G2/M phase cell cycle arrest and apoptosis
in MCF-7 cells through the regulation of p53 and caspase-cascade
pathway. Clin Transl Oncol. 23:718–730. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Meng S, Zhu Y, Li JF, Wang X, Liang Z, Li
SQ, Xu X, Chen H, Liu B, Zheng XY, et al: Apigenin inhibits renal
cell carcinoma cell proliferation. Oncotarget. 8:19834–19842. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Norbury C and Nurse P: Animal cell cycles
and their control. Annu Rev Biochem. 61:441–470. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Morla AO, Draetta G, Beach D and Wang JY:
Reversible tyrosine phosphorylation of cdc2: dephosphorylation
accompanies activation during entry into mitosis. Cell. 58:193–203.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jessus C and Beach D: Oscillation of MPF
is accompanied by periodic association between cdc25 and
cdc2-cyclin B. Cell. 68:323–332. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yamada S, Sumrejkanchanakij P, Amagasa T
and Ikeda MA: Loss of cyclin E requirement in cell growth of an
oral squamous cell carcinoma cell line implies deregulation of its
downstream pathway. Int J Cancer. 111:17–22. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Choi EJ and Kim GH: Apigenin causes G(2)/M
arrest associated with the modulation of p21(Cip1) and Cdc2 and
activates p53-dependent apoptosis pathway in human breast cancer
SK-BR-3 cells. J Nutr Biochem. 20:285–290. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Piantelli M, Rossi C, Iezzi M, La Sorda R,
Iacobelli S, Alberti S and Natali PG: Flavonoids inhibit melanoma
lung metastasis by impairing tumor cells endothelium interactions.
J Cell Physiol. 207:23–29. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li J, Tan G, Cai Y, Liu R, Xiong X, Gu B,
He W, Liu B, Ren Q, Wu J, et al: A novel Apigenin derivative
suppresses renal cell carcinoma via directly inhibiting wild-type
and mutant MET. Biochem Pharmacol. 190:1146202021. View Article : Google Scholar : PubMed/NCBIPubMed/NCBIPubMed/NCBIPubMed/NCBIPubMed/NCBIPubMed/NCBIPubMed/NCBIPubMed/NCBIPubMed/NCBIPubMed/NCBIPubMed/NCBIPubMed/NCBIPubMed/NCBIPubMed/NCBIPubMed/NCBIPubMed/NCBIPubMed/NCBIPubMed/NCBIPubMed/NCBIPubMed/NCBIPubMed/NCBI
|