Introduction
The tumor microenvironment (TME) plays a crucial
role in cancer occurrence and progression. The TME consists of
endothelial cells, fibroblasts, vascular components, macrophages,
immune cells and secreted cytokines (1,2).
Targeting the TME represents a potential approach for the treatment
of cancer at the tumor and cellular level simultaneously.
Tumor-associated macrophages (TAMs) are one of the
most common populations of tumor-infiltrating immune cells in the
TME (3). Macrophages can be
classified into two subtypes, the M1 phenotype (classically
activated) and M2 phenotype (alternatively activated). TAMs are
usually polarized toward the tumor-promoting M2 phenotype rather
than the tumoricidal M1 phenotype (4). Several studies have demonstrated that
high numbers of TAMs in the TME are associated with a poor
prognosis in various types of cancer, including ovarian, pancreatic
and lung cancer (5–8). Through the secretion of various
cytokines, TAMs can promote tumor growth, angiogenesis and
epithelial-mesenchymal transition (EMT) and reduce immune cell
antitumor activity, leading to the suppression of antitumor immune
responses in the TME (9–11).
Vascular endothelial growth factor (VEGF) is an
important cytokine in the TME, and cancer cells and TAMs are the
major sources of VEGF (12,13).
During tumor progression, VEGF secreted by cancer cells binds to
the VEGF receptor (VEGFR) of endothelial cells, thereby enhancing
endothelial cell proliferation and migration, and promoting
angiogenesis near the tumor site (14). Furthermore, it has been reported
that VEGF secreted by cancer cells functions in an autocrine and
paracrine manner, and enhances tumor growth via the activation of
the VEGF/VEGFR signaling pathway in cancer cells (15). Cancer cells have been also reported
to produce cytokines and chemokines, such as interleukin (IL)-4,
IL-10, and C-C motif chemokine ligand 2 (CCL2) to induce TAM
infiltration. CCL2 is involved in the recruitment of monocytes,
IL-4 and IL-10, polarizing monocytes toward the M2 phenotype
(termed TAMs) (16). TAMs that are
recruited and activated by cancer cells have been reported to
secrete VEGF and promote tumor progression (17). It has been previously reported by
the authors that VEGF secreted by TAMs may promote the EMT of
cancer cells via transcription factor nuclear factor
(erythroid-derived 2)-like 2 (Nrf2) activation in cancer cells
(18). However, the findings of
the effects of VEGF on macrophages have thus far been controversial
(19,20). Wheeler et al (19) reported that VEGF treatment
significantly enhanced the upreglation of M2 markers of
macrophages, while Linde et al (20) reported that VEGF was not involved
in the M2 polarization of macrophages in vitro. Thus, the
effects of VEGF secreted by cancer cells on TAM polarization remain
unclear.
Programmed death-ligand 1 (PD-L1) plays a crucial
role in the TME by suppressing the anti-tumor T cell-mediated
immune response. Novel therapies targeting the PD-L1/programmed
cell death protein 1 axis have been developed for cancer treatment.
In the treatment of liver cancer, bevacizumab (a VEGF-A inhibitor)
and atezolizumab (a PD-L1 inhibitor) have already been clinically
applied as cancer treatments (21,22).
Previous studies have suggested that the tumor stroma, including
TAMs, is a regulator of PD-L1 expression in cancer cells (1–3).
However, whether VEGF secreted by TAMs is involved in PD-L1
regulation in the TME remains unclear.
In the present study, the role of VEGF in the TME of
liver cancer was investigated with particular focus on the effects
of VEGF inhibition on TAM function. It was observed that a
VEGF-depleted environment attenuated the tumor-promoting function
of TAMs and the ability of TAMs to enhance PD-L1 expression in
cancer cells through the decreased cytokine secretion of TAMs, and
via the inactivation of the VEGFR2/Akt/mTOR pathway.
Materials and methods
Cells and cell culture
Huh-7 (RCB1366) and HepG2 (RCB1648) cells (human
liver cancer cell lines) were obtained from the RIKEN BioResource
Center Cell Bank. THP-1 cells (a human monocyte cell line) were
obtained from the Culture Collections of Public Health England
(https://www.phe-culturecollections.org.uk/). The Huh-7
and HepG2 cells were maintained in DMEM (Thermo Fisher Scientific,
Inc.), and the THP-1 cells were maintained in RPMI-1640 (Wako Pure
Chemical Industries, Ltd.). Media were supplemented with 10% FBS
(Thermo Fisher Scientific, Inc.) and 1% penicillin/streptomycin
(Gibco; Thermo Fisher Scientific, Inc.). To induce cell
differentiation into M0 macrophages, the THP-1 cells were exposed
to 150 nmol/l phorbol 12-myristate 13-acetate (PMA; MilliporeSigma)
for 48 h. All cells were maintained under a 5% CO2
atmosphere at 37°C.
Preparation of conditioned medium (CM)
and TAMs
The Huh-7 and HepG2 cells were cultured to 80%
confluency in 100-mm culture dishes, in order to obtain CM. All
cells were washed with pre-warmed PBS twice and then incubated with
fresh medium without FBS. Following 48 h of incubation at 37°C, the
supernatant was collected, centrifuged at 800 × g for 5 min at
22°C, and filtered through a 0.2-µm sterile filter. The cancer
cell-derived CM (Huh-7-CM and HepG2-CM) was used without additional
FBS.
To obtain TAMs, M0 macrophages were treated with
cancer cell-derived CM (Huh-7-CM and HepG2-CM). CM was added to the
culture medium at a ratio of 1:1, and the cells were stimulated for
48 h at 37°C. TAMs induced from Huh-7-CM were defined as TAM(Huh7),
and TAMs induced from HepG2-CM were defined as TAM(HepG2). To
obtain modified TAMs, M0 macrophages were treated with cancer
cell-derived CM containing VEGF antibody. VEGF antibody (cat. no.
MAB293; R&D Systems, Inc.) was added to the medium at the
concentration of 60 ng/ml.
The M0-CM, TAM-CM and modified TAM-CM were collected
from the M0 macrophage culture, TAM culture and modified TAM
culture, respectively, in the same manner as described above. In
the proliferation and migration assays, the CMs were added to the
culture medium at a ratio of 1:1. The same concentration of human
IgG (cat. no. 1-001-A; R&D Systems, Inc.) was used as a
control.
Cell proliferation assay
Cell Counting Kit-8 (CCK-8) assay (Dojindo Molecular
Technologies, Inc.) was performed in accordance with the
manufacturer's protocol. Briefly, 10% CCK-8 reagent was added to
each well. The plates were incubated at 37°C for 2 h, and the
absorbance was analyzed at 450 nm using a microplate reader
(SpectraMax i3; Molecular Devices, LLC).
Migration assay
Transwell migration assays were performed using
24-well plates with 8-µm pore membrane inserts (Corning, Inc.) in
accordance with the manufacturer's protocol. Huh-7 and HepG2 cells
were seeded in the upper chamber at a concentration of 20,000 cells
in 100 µl of medium containing 1% FBS. In the lower chamber,
corresponding CM was added to the 10% FBS DMEM culture medium at a
ratio of 1:1; the final FBS concentration was 5%. Following 24 h of
incubation at 37°C, cells that had migrated to the bottom of the
Transwell membrane were fixed with 4% paraformaldehyde for 15 min.
The membrane was stained using 0.2% crystal violet solution (Wako
Pure Chemical Industries, Ltd.) for 20 min at room temperature, and
stained cells were counted using a phase-contrast microscope (BX43;
Olympus Corporation) in three random fields per membrane (×200
magnification).
Wound healing assay
Huh-7 and HepG2 cells were seeded in six-well plates
and grown to 90% confluency. The cell monolayer was scratched using
a plastic pipette tip across the well to create a 1-mm-wide gap.
The detached cells were removed by washing with PBS twice. The well
was then replenished with fresh DMEM medium containing 1% FBS
followed by the addition of corresponding CM at a ratio of 1:1, the
final FBS concentration was 0.5%, the cancer cells were cultured
for a further 24 h at 37°C. Images of the wound areas were captured
using a phase-contrast microscope (magnification, ×40; DP22-CU;
Olympus Corporation) at 0 and 24 h after scratching. The wound
healing rates were calculated using ImageJ v1.46r software
(National Institutes of Health) and using the following equation:
Wound healing rate (%)=[area (0 h)-area (24 h)]/area (0 h)
×100.
Cytokine array
Cytokines in M0-CM and TAM-CM were detected using a
Proteome Profiler Human Cytokine Array kit (cat. no. ARY005B;
R&D Systems, Inc.) following the manufacturer's protocol. The
chemiluminescence signal intensities on the membranes were detected
using a Lumino Image Analyzer (Amersham Imager; Cytiva).
ELISA
The concentrations of VEGF and MMP-9 in the CM were
detected using a VEGF ELISA kit (cat. no. DVE00; R&D Systems,
Inc.) and an MMP-9 ELISA kit (cat. no. DMP900; R&D Systems,
Inc.), respectively, following the manufacturer's protocol. The
absorbance was measured at 450 nm using a microplate reader
(SpectraMax i3; Molecular Devices, LLC).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The RNeasy Mini Kit (Qiagen GmbH) was used to
extract total RNA from the cells following the manufacturer's
instructions. The total RNA concentration was determined using a
spectrophotometer (NanoDrop 2000; Thermo Fisher Scientific, Inc.)
according to manufacturer's instructions. cDNA was synthesized from
2.5 µg total RNA using a High-Capacity cDNA Reverse Transcription
kit (4368813, Applied Biosystems; Thermo Fisher Scientific Inc.) in
a final volume of 50 µl. The cycling conditions for the reverse
transcription were as follows: Incubation at 25°C for 10 min, 37°C
for 120 min, 85°C for 5 min. All samples were kept at −20°C until
ready for use. The StepOnePlus Real-Time PCR System (Applied
Biosystems; Thermo Fisher Scientific Inc.) was used to conduct
qPCR. The qPCR conditions consisted of an Initial denaturation for
3 min at 95°C, followed by 40 cycles of 30 sec denaturation at
95°C, annealing for 30 sec at 58°C and extension at 72°C for 45
sec. The final extension was carried out at 72°C for 10 min. The
primers from TaqMan assays (assay identification number) used in
the present study are as follows: CD163 (Hs00174705_m1), CD206
(Hs00267207_m1), VEGFR2 (Hs00911700_m1) and PD-L1 (Hs00204257_m1).
GAPDH (4326317E) was used as an internal control. All primers were
purchased from Thermo Fisher Scientific, Inc. The data were
analyzed using the 2−ΔΔCq method (23). The results are presented as the
fold changes of the relative mRNA expression for each experimental
group compared with that in the control group.
Western blot analysis
Cell lysates were obtained, and western blot
analysis was performed as previously described (18,24).
Briefly, total proteins were extracted by lysing the cells using
RIPA lysis buffer (Thermo Fisher Scientific, Inc.), containing a
protease inhibitor cocktail (Sigma-Aldrich; Merck KGaA) and a
PhosSTOP phosphatase inhibitor cocktail (Roche Diagnostics). The
protein concentration was measured using a BCA kit (Thermo Fisher
Scientific Inc.). Equal amounts (20 µg) of extracted proteins were
separated on 10% SDS-PAGE gels and transferred onto PVDF membranes
(Bio-Rad Laboratories, Inc.). The membranes were incubated with the
indicated primary antibody overnight at 4°C. The membranes were
then incubated with appropriate HRP-conjugated secondary antibody
for 1 h at room temperature. The proteins were detected using ECL
reagents (Cytiva). Western blot densitometry band quantification
was performed using ImageJ v1.46r software (National Institutes of
Health). The primary antibodies used in the present study, along
with the corresponding dilutions, are listed in Table I. The p-Akt antibody used in the
present study detected endogenous Akt phosphorylated at Ser473.
 | Table I.Details of antibody sources and
concentrations used for western blot analysis. |
Table I.
Details of antibody sources and
concentrations used for western blot analysis.
| Antibody | Company | Cat. no. | Dilution |
|---|
| Akt (total-Akt)
mAb | Cell Signaling
Technology, Inc. | 4691S | 1:1,000 |
| Phospho-Akt (p-Akt)
mAb | Cell Signaling
Technology, Inc. | 4060S | 1:1,000 |
| mTOR (total-mTOR)
mAb | Abcam | ab32028 | 1:1,000 |
| Phospho-mTOR
(p-mTOR) mAb | Abcam | ab109268 | 1:1,000 |
| β-actin mAb | Cell Signaling
Technology, Inc. | 4970S | 1:1,000 |
| HRP-linked
anti-rabbit antibody | Cell Signaling
Technology, Inc. | 7074S | 1:3,000 |
Statistical analysis
All statistical analyses were performed using JMP
software (version 13; SAS Campus Drive). The Student's t-test was
used for statistical comparisons between two groups, and one-way
ANOVA with the Tukey-Kramer test was used for statistical
comparisons among three or more groups. All experiments were
repeated more than three times. Data are expressed as the mean ±
SD. A P-value <0.05 was considered to indicate a statistically
significant difference.
Results
Polarization of M0 macrophages toward
the M2 phenotype (termed TAMs) by cancer cell-CM
To generate TAMs, THP-1 cells were first stimulated
with PMA for 48 h to induce their differentiation into M0
macrophages. The M0 macrophages were then cultured with Huh-7-CM or
HepG2-CM for a further 48 h (Fig.
S1A). The two generated TAM lines, TAM(Huh-7) and TAM(HepG2),
expressed increased mRNA levels of the M2 macrophage markers, CD163
and CD206, as compared with the expression levels in M0 macrophages
(P<0.05, Fig. S1B).
TAMs enhance liver cancer cell line
proliferation and migration
Subsequently, the effects of TAMs on the malignant
potential of Huh-7 and HepG2 cells were investigated. TAMs were
established using CM from Huh-7 and HepG2 cells; CM from the two
sets of TAMs was then collected, and the Huh-7 and HepG2 cells were
cultured with the TAM-CM (Fig.
S2A). TAM-CM from both cell lines significantly increased the
proliferation and migration of the Huh-7 and HepG2 cells compared
with the effects of M0-CM (P<0.05, Fig. S2B-D). These results indicated that
secreted factors from TAMs may play a crucial role in the effects
of TAMs on the proliferation and migration of cancer cells.
Inhibition of VEGF secretion by TAMs
attenuates liver cancer cell proliferation and migration
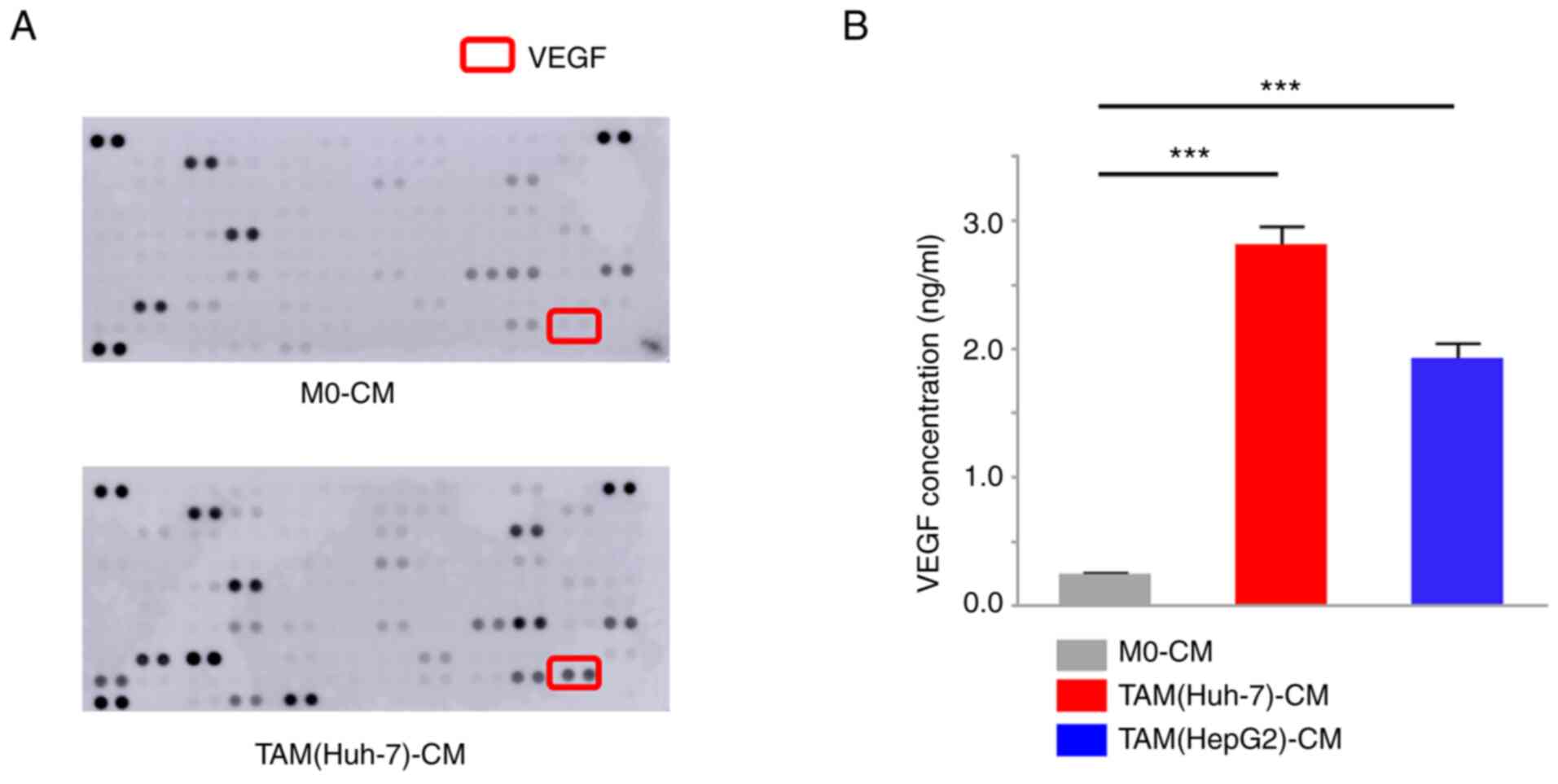
A cytokine array using TAM(Huh-7)-CM was used to
compare the differences in cytokine secretion between TAMs and M0
macrophages. The results illustrated that TAMs secreted increased
levels of VEGF in comparison with the M0 macrophages (Fig. 1A). The VEGF concentrations in the
TAM(Huh-7)-CM and TAM(HepG2)-CM were then examined using ELISA. The
results revealed that both TAM cell lines secreted significantly
higher VEGF levels than the M0 macrophages (P<0.001, Fig. 1B). To investigate the effects of
VEGF secretion by TAMs on cancer cells, the Huh-7 and HepG2 cells
were cultured with TAM-CM or M0-CM, with the addition of VEGF
antibody (Fig. S3A). The effects
of TAM-CM on the Huh-7 and HepG2 cell proliferation and migration
activities were attenuated at almost the same levels with M0-CM
upon VEGF inhibition (P<0.05, Fig.
S3B-D). These results indicated that VEGF secreted by TAMs may
promote cancer cell malignancy, further supporting its critical
role in the TME.
VEGF inhibition induces the functional
attenuation of TAMs without affecting M2 polarization
To clarify the critical role of VEGF in the
interaction of TAMs and cancer cells, the effects of VEGF secreted
by cancer cells on TAM polarization and function were investigated.
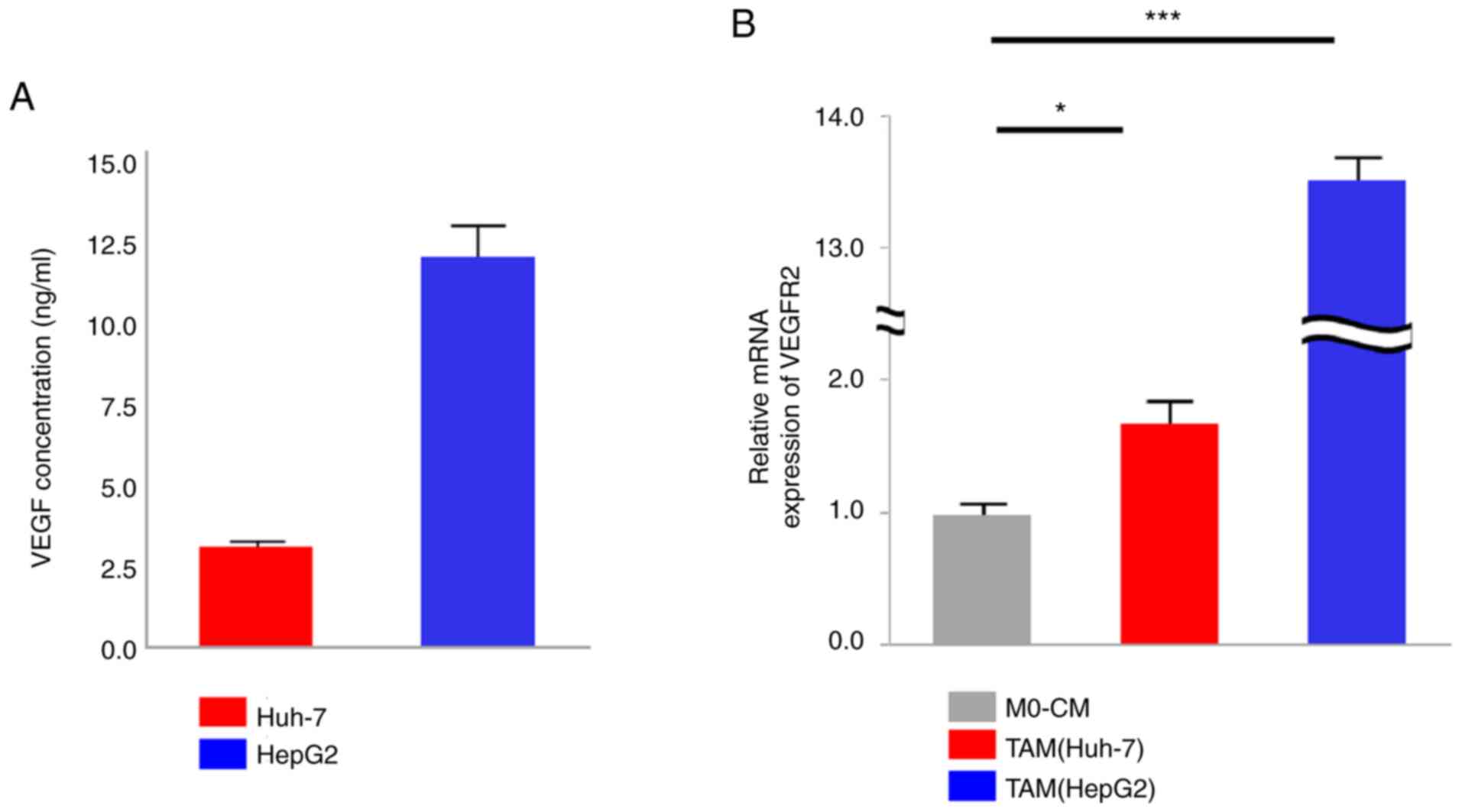
VEGF secretion from cancer cells was confirmed (Fig. 2A), as well as VEGFR2 expression
upregulation in TAMs from the Huh-7 and HepG2 cells, as compared
its expression in M0 macrophages (P=0.04 and P<0.001,
respectively, Fig. 2B). In order
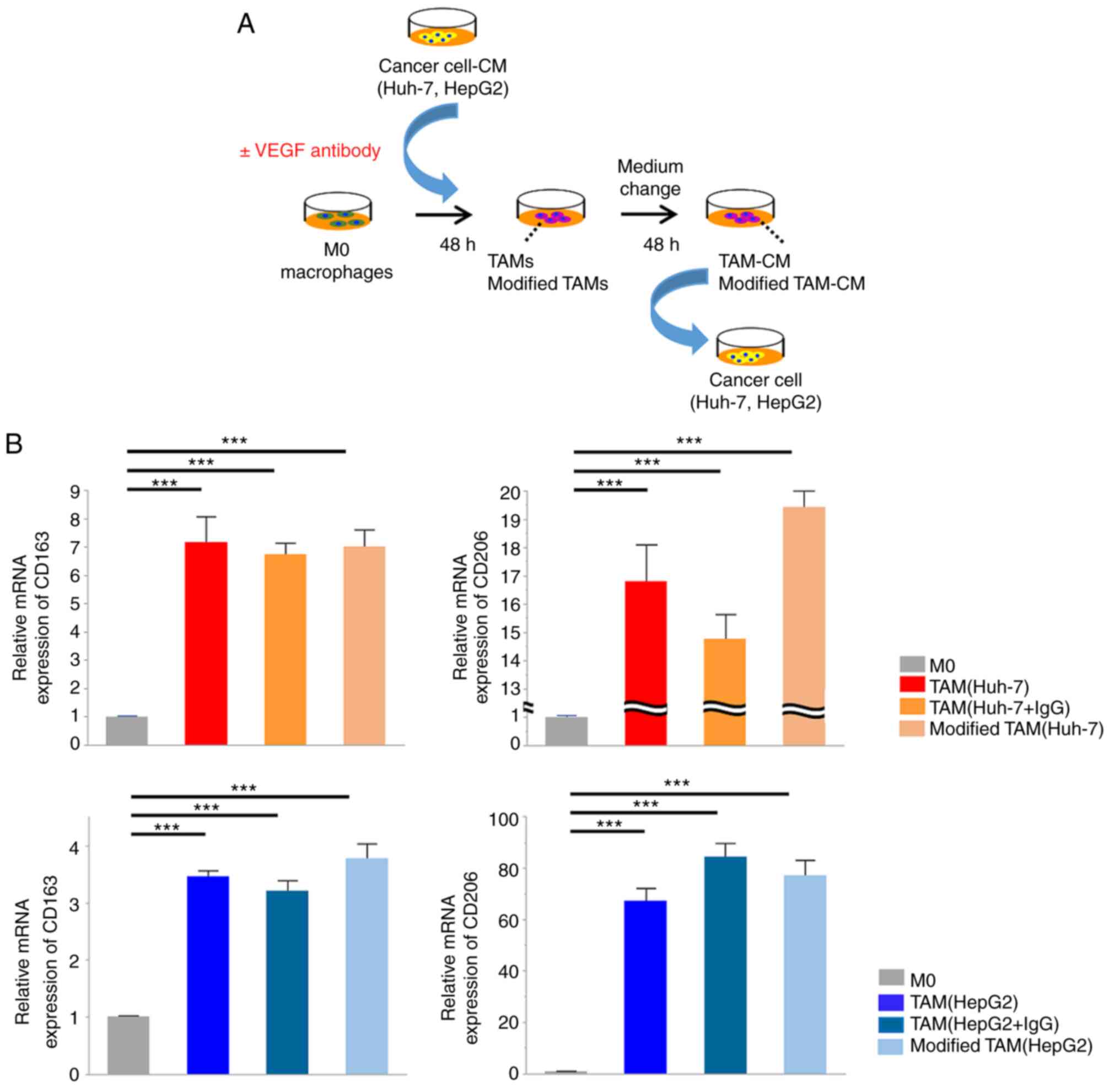
to investigate whether VEGF is involved in the establishment of TAM
function during M2 polarization, M0 macrophages were cultured in
cancer cell-CM in the presence or absence of VEGF antibody for 48 h
(Fig. 3A). TAMs induced in the
presence of the VEGF antibody (modified TAMs) displayed M2-like
spindle-shaped morphological changes (data not shown), indicating
that the inhibition of VEGF during M2 polarization did not affect
TAM morphology. Likewise, modified TAMs exhibited similar mRNA
expression levels of CD163 and CD206 as TAMs stimulated by cancer
cell-CM without anti-VEGF antibody. These findings revealed that
the inhibition of VEGF secreted by cancer cells did not affect the
upregulation of the expression of CD163 and CD206, which are M2
macrophage markers (Fig. 3B).
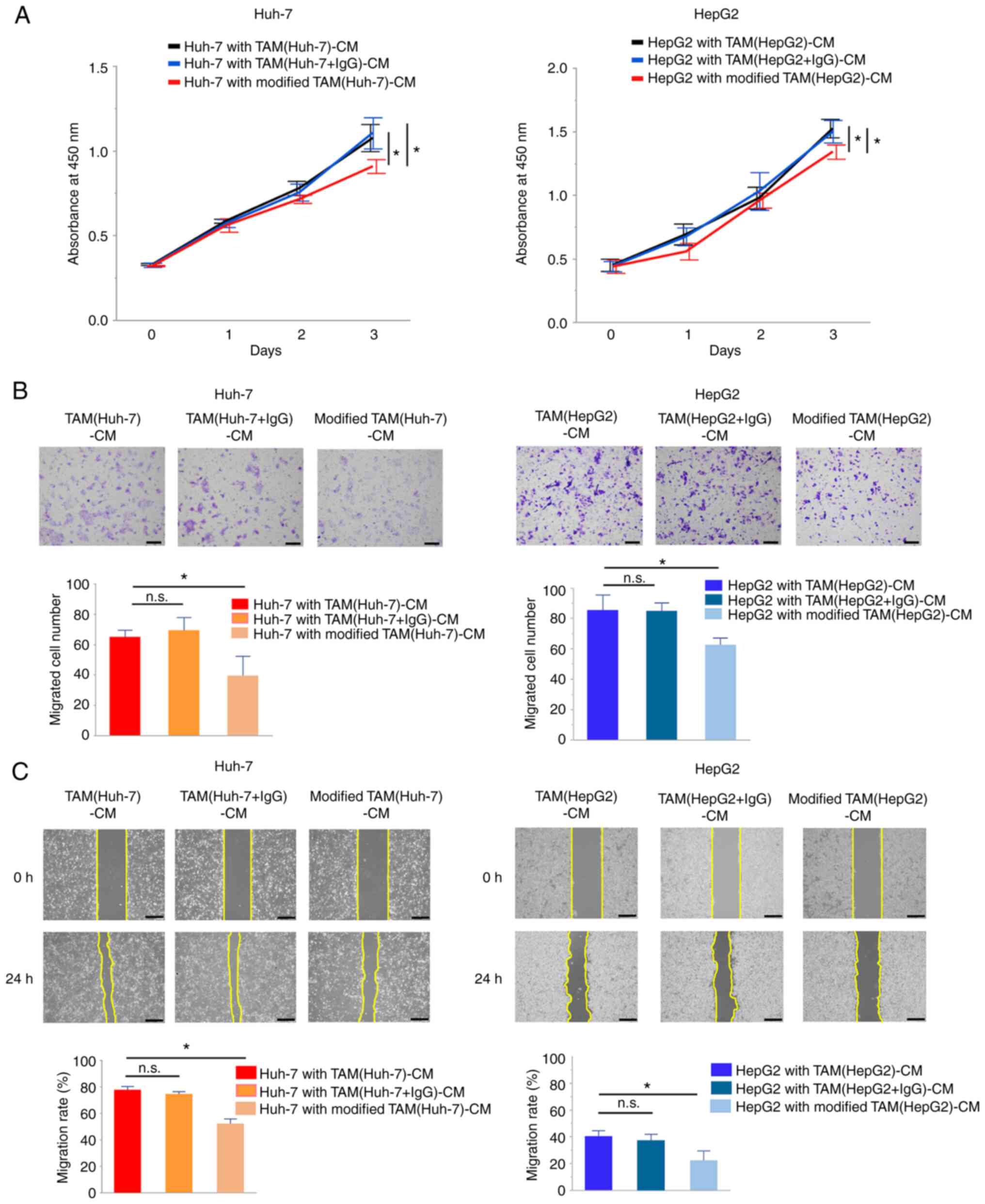
However, modified TAM-CM exerted weaker effects on cancer cell
proliferation and migration compared with TAM-CM (P<0.05,
Fig. 4A-C). VEGF secretion by
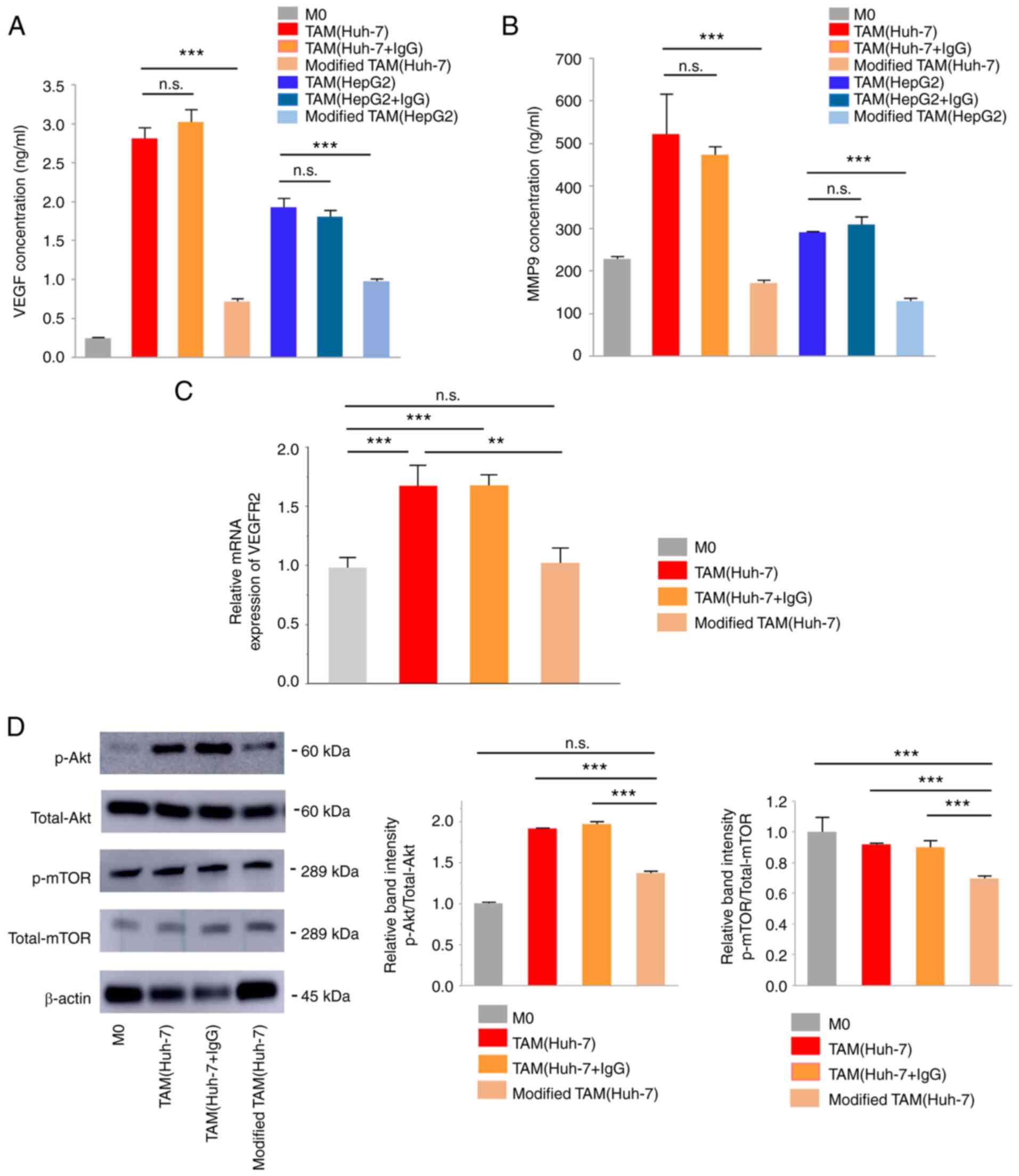
modified TAMs was then investigated and it was revealed that
modified TAMs displayed a significantly reduced VEGF secretion
compared with TAMs (P<0.001, Fig.
5A). Additionally, the secretion of MMP-9, which has been
reported as one of the major factors secreted by TAMs to be
positively correlated with VEGF secretion (25), was increased in TAMs and decreased
in modified TAMs (P<0.05, Fig.
5B).
Subsequently, the signaling pathways associated with
VEGF secretion were examined in TAMs and modified TAMs. VEGFR2 mRNA
expression was significantly downregulated in modified TAM(Huh-7)
compared with the levels in TAM(Huh-7) (P=0.001, Fig. 5C). The p-Akt and p-mTOR levels were
also decreased in the modified TAM(Huh-7) compared with levels in
TAM(Huh-7), as revealed using western blot analysis (P<0.001,
Fig. 5D). These results suggested
that VEGF inhibition during M2 polarization resulted in a decreased
VEGF secretion in modified TAMs via the inactivation of the
VEGFR2/Akt/mTOR pathway.
Modified TAMs have a reduced ability
to upregulate PD-L1 expression
Several studies have previously reported that M2
macrophages may enhance PD-L1 expression in lung cancer, pancreatic
cancer, and liver cancer (26–28).
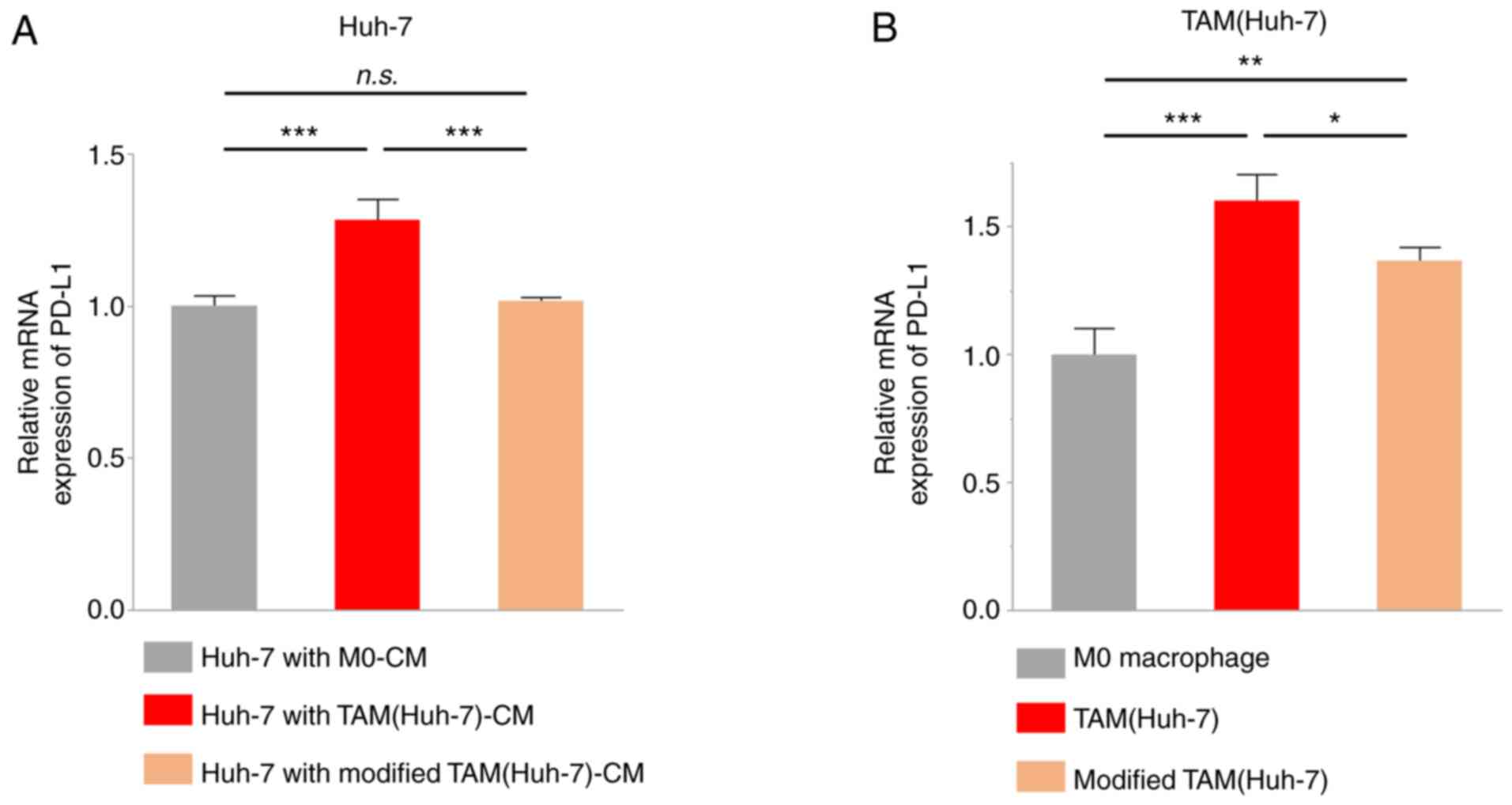
Thus, in the present study, Huh-7 cells were cultured with
TAM(Huh-7)-CM or modified TAM(Huh-7)-CM for 48 h, and PD-L1 mRNA
expression in cancer cells was examined using RT-qPCR. The results
demonstrated that TAM(Huh-7)-CM increased the mRNA expression of
PD-L1 in the Huh-7 cells; however, modified TAM(Huh-7)-CM had no
effect compared with M0-CM (Fig.
6A). Another study previously reported that TAMs not only
promoted PD-L1 expression in cancer cells, but also expressed PD-L1
(29). Therefore, in the present
study, PD-L1 mRNA expression was examined and its upregulation in
TAMs was detected, while a weaker upregulation was detected in
modified TAM(Huh-7) compared with TAM(Huh-7) (Fig. 6B). These findings illustrated that
VEGF inhibition attenuated the expression of PD-L1 and the tumor
immunosuppression function in TAMs.
Discussion
In the present study, it was demonstrated that the
inhibition of VEGF secreted by cancer cells did not alter the M2
polarization of macrophages in the TME; however, the
tumor-promoting characteristics of TAMs were attenuated though the
suppression of VEGF secretion in TAMs. TAMs induced in a
VEGF-depleted environment exhibited reduced activation of the
Akt/mTOR signaling pathway and decreased secretion of humoral
factors, including VEGF and MMP-9. Furthermore, the increased PD-L1
expression in cancer cells was not achieved in the presence of TAMs
induced in a VEGF-depleted environment, with the PD-L1 expression
of these modified TAMs also being suppressed.
Furthermore, the effect of VEGF inhibition on the M2
polarization of macrophages in the TME was investigated. TAMs are
mainly M2 macrophages that are activated by tumor-derived IL-4,
IL-13, IL-10, macrophage colony-stimulating factor and lactic acid
in the TME. M2 macrophages produce anti-inflammatory cytokines,
such as IL-10, IL-13 and transforming growth factor-β to promote
tumor development and growth (30–32).
It has been previously reported by the authors that TAMs and cancer
cells may interact via the Nrf2 pathway, VEGF secretion by TAMs may
enhance EMT of cancer cells and that lactic acid secreted by cancer
cells may polarize macrophages toward the M2 phenotype (18). However, contrary to the current
expectations, the present study demonstrated that VEGF inhibition
by VEGF antibody in the cancer cell-CM did not contribute to M2
macrophage polarization inhibition.
The function of TAMs stimulated by cancer cell-CM in
a VEGF-depleted environment was further investigated using VEGF
antibody. Previous studies have demonstrated that TAMs may
contribute to tumor neovascularization through the upregulation of
VEGF secretion, and the MMP-induced degradation of extracellular
matrix surrounding cancer cells, resulting in the release of
heparin-bound growth factors, including VEGF, to further support
angiogenesis and tumor progression (33,34).
The results of the present study indicated that modified TAMs,
which were induced in a VEGF-depleted environment, exhibited a
weaker ability to promote the proliferation and migration of cancer
cells due to a reduction in the VEGF secretion level. VEGFR2 is
expressed on macrophages, and its expression is upregulated on M2
macrophages (35). VEGF secreted
by TAMs has been reported to function simultaneously in a paracrine
and autocrine manner through the VEGFR2 signaling pathway in TAMs,
as also observed in cancer cells. Recently, several studies have
reported that VEGFR2 expression on M2 TAMs plays a crucial role in
tumor immune tolerance within the TME (19,35).
Other studies have previously suggested that humoral factor
secretion by M2 macrophages may be regulated via the VEGF/VEGFR2
signaling pathway (36–38). The present study confirmed that
VEGF depletion suppressed Akt/mTOR pathway activation, which is a
main downstream pathway of VEGF/VEGFR2 signaling, in modified TAMs.
The inactivation of the Akt/mTOR pathway has been previously
reported to reduce VEGFR2 expression in glioma cells (39), and the inhibition of the mTOR
pathway has been reported to downregulate the production of VEGF
and MMP-9 in macrophages and cancer cells (40,41).
These reports are in support of the present results, concerning the
modified TAMs demonstrating attenuated cytokine secretion via
inactivation of VEGFR2/Akt/mTOR pathway. This finding suggests that
the inhibition of autocrine and paracrine VEGF secretion by TAMs
and cancer cells may represent a treatment strategy for inhibiting
the TME, thereby suppressing tumor progression.
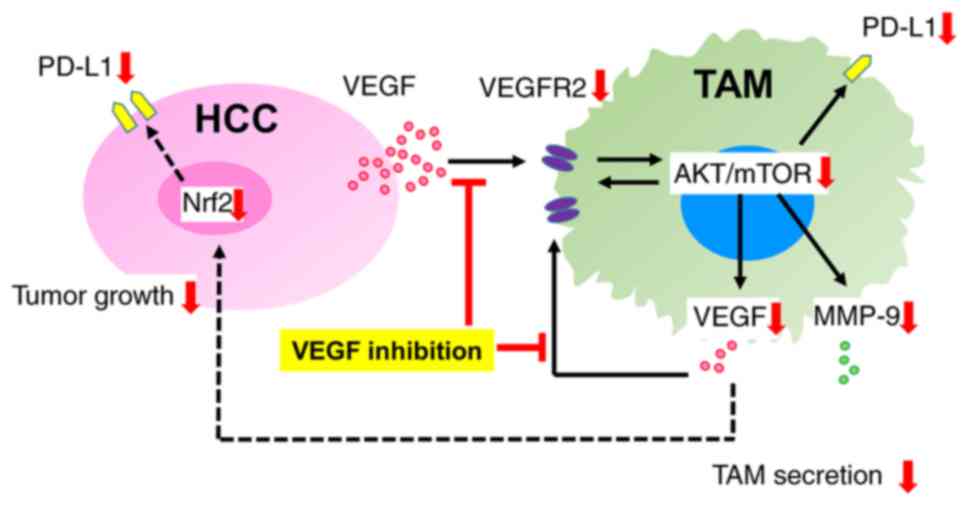
Finally, the present study investigated whether the
VEGF-depleted environment was associated with tumor immune
tolerance via PD-L1 expression in cancer cells and TAMs. Shima
et al (28) reported that
macrophages significantly expressed PD-L1 during TAM-like M2
differentiation, and TGF-β produced by TAMs induced PD-L1
expression in lung cancer cells. Yao et al (42) reported that PD-L1 expression by
macrophages was positively regulated by the PI3K/Akt signaling
pathway, in support of the current results. Another study
previously reported that PD-L1 expression by liver cancer cells was
regulated by the Nrf2 pathway (43), and a previous report by the authors
previously revealed that VEGF secreted by TAMs may activate the
Nrf2 pathway in liver cancer cells (18). Therefore, VEGF inhibition can
suppress the VEGF autocrine loop in macrophages and reduce PD-L1
expression in cancer cells through the inactivation of the AKT/mTOR
pathway in TAMs, possibly due to the suppression of the Nrf2
pathway in cancer cells.
Recent large-scale randomized clinical trials have
demonstrated that the concomitant use of anti-VEGF treatment
enhances the efficacy of anti-PD-L1 therapy in advanced liver
cancer, and this strategy has already been clinically introduced
globally. The results of clinical trials have demonstrated that
treatment with the combination of anti-PD-L1 and anti-VEGF therapy
is effective due to their synergistic effects on tumor growth, as
well as due to their ability to reprogram the immunosuppressive
environment to enhance anti-tumor immune responses (21,22).
Although the effects of combination therapy have been clinically
confirmed, only a limited number of studies support the
co-association between VEGF and PD-L1. Schmidinger (44) reported that PD-L1 expression, as
detected by immunohistochemistry, was associated with VEGF
expression, which reflected poor pathological features in patients
with clear cell renal cell carcinoma. The findings of the present
study suggested that the TAMs induced in a VEGF-depleted
environment had a weaker ability to promote tumor progression and
that VEGF inhibition in the TME regulates PD-L1 expression in TAMs
and cancer cells (Fig. 7). This
concept could help clarify the mechanisms of the effects of the
combined anti-PD-L1 and anti-VEGF therapy.
The present study has several limitations. Firstly,
the results were based on in vitro experiments using VEGF
antibody. To further investigate the effects of the inhibition of
the VEGF signal pathway in TAMs, genetic modification technology
targeting VEGF, including shRNA are required, as well as
experiments using a liver cancer animal model.
In conclusion, the importance of VEGF signaling for
the malignant potential of TAMs in the TME of liver cancer was
demonstrated in the present study. VEGF inhibitors may not affect
M2 polarization; however, VEGF inhibition impedes tumor growth and
attenuates TAM function. These effects may suppress tumor
progression and reduce tumor immune escape through the inactivation
of the VEGFR2/AKT/mTOR pathway. Therefore, the present study
revealed that VEGF inhibition can lead to the functional deficiency
of TAMs, in support of the potential efficacy of the combined
anti-VEGF and anti-PD-L1 treatment.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported in part by the Research Program
on Hepatitis from the Japanese Foundation for Multidisciplinary
Treatment of Cancer, the Japan Agency for Medical Research and
Development (grant nos. JP19fk0210048 and JP20fk0210048), and
Grants-in-Aid for Scientific Research (grant nos. 20K08957 and
18K02871). This study was also funded by Taiho Pharmaceutical Co.,
Ltd. (Tokyo, Japan).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SO, KT, YM and MS designed the experiments. SO,
SYamas, KM and HT performed the experiments and collected the data.
YS, SYamad, TI and SI analyzed and interpreted the data. SO drafted
the manuscript. YM and MS revised the paper critically for
important intellectual content. SO and YM confirmed the
authenticity of all the raw data. SO and YM agree to be accountable
for all aspects of the work in ensuring that questions related to
the accuracy or integrity of any part of the work are appropriately
investigated and resolved. All the authors have read and approved
the final version of the manuscript for publication.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
All the authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CCL2
|
C-C motif chemokine ligand 2
|
|
CM
|
conditioned medium
|
|
EMT
|
epithelial-mesenchymal transition
|
|
IL
|
interleukin
|
|
Nrf2
|
nuclear factor (erythroid-derived
2)-like 2
|
|
PMA
|
phorbol 12-myristate 13-acetate
|
|
PD-L1
|
programmed death-ligand 1
|
|
TAM
|
tumor-associated macrophage
|
|
TME
|
tumor microenvironment
|
|
VEGF
|
vascular endothelial growth factor
|
|
VEGFR
|
vascular endothelial growth factor
receptor
|
References
|
1
|
Casey SC, Li Y, Fan AC and Felsher DW:
Oncogene withdrawal engages the immune system to induce sustained
cancer regression. J Immunother Cancer. 2:242014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kenny PA, Lee GY and Bissell MJ: Targeting
the tumor microenvironment. Front Biosci. 12:3468–3474. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhou K, Cheng T, Zhan J, Peng X, Zhang Y,
Wen J, Chen X and Ying M: Targeting tumor-associated macrophages in
the tumor microenvironment. Oncol Lett. 20:2342020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mantovani A, Sozzani S, Locati M, Allavena
P and Sica A: Macrophage polarization: Tumor-associated macrophages
as a paradigm for polarized M2 mononuclear phagocytes. Trends
Immunol. 23:549–555. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lan C, Huang X, Lin S, Huang H, Cai Q, Wan
T, Lu J and Liu J: Expression of M2-polarized macrophages is
associated with poor prognosis for advanced epithelial ovarian
cancer. Technol Cancer Res Treat. 12:259–267. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kurahara H, Shinchi H, Mataki Y, Maemura
K, Noma H, Kubo F, Sakoda M, Ueno S, Natsugoe S and Takao S:
Significance of M2-polarized tumor-associated macrophage in
pancreatic cancer. J Surg Res. 167:e211–e219. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang B, Yao G, Zhang Y and Gao J, Yang B,
Rao Z and Gao J: M2-polarized tumor-associated macrophages are
associated with poor prognoses resulting from accelerated
lymphangiogenesis in lung adenocarcinoma. Clinics (Sao Paulo).
66:1879–1886. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chanmee T, Ontong P, Konno K and Itano N:
Tumor-associated macrophages as major players in the tumor
microenvironment. Cancers (Basel). 6:1670–1690. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Qian BZ and Pollard JW: Macrophage
diversity enhances tumor progression and metastasis. Cell.
141:39–51. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Condeelis J and Pollard JW: Macrophages:
Obligate partners for tumor cell migration, invasion, and
metastasis. Cell. 124:263–266. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pollard JW: Tumour-educated macrophages
promote tumour progression and metastasis. Nat Rev Cancer. 4:71–78.
2004. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Valković T, Dobrila F, Melato M, Sasso F,
Rizzardi C and Jonjić N: Correlation between vascular endothelial
growth factor, angiogenesis, and tumor-associated macrophages in
invasive ductal breast carcinoma. Virchows Arch. 440:583–588. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shieh YS, Hung YJ, Hsieh CB, Chen JS, Chou
KC and Liu SY: Tumor-associated macrophage correlated with
angiogenesis and progression of mucoepidermoid carcinoma of
salivary glands. Ann Surg Oncol. 16:751–760. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Deryugina EI and Quigley JP: Tumor
angiogenesis: MMP-mediated induction of intravasation- and
metastasis-sustaining neovasculature. Matrix Biol. 44–46. 94–112.
2015.PubMed/NCBI
|
|
15
|
Lin Y, Zhai E, Liao B, Xu L, Zhang X, Peng
S, He Y, Cai S, Zeng Z and Chen M: Autocrine VEGF signaling
promotes cell proliferation through a PLC-dependent pathway and
modulates Apatinib treatment efficacy in gastric cancer.
Oncotarget. 8:11990–12002. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Capece D, Fischietti M, Verzella D,
Gaggiano A, Cicciarelli G, Tessitore A, Zazzeroni F and Alesse E:
The inflammatory microenvironment in hepatocellular carcinoma: A
pivotal role for tumor-associated macrophages. Biomed Res Int.
2013:1872042013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu K, Lin K, Li X, Yuan X, Xu P, Ni P and
Xu D: Redefining tumor-associated macrophage subpopulations and
functions in the tumor microenvironment. Front Immunol.
11:17312020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Feng R, Morine Y, Ikemoto T, Imura S,
Iwahashi S, Saito Y and Shimada M: Nrf2 activation drive
macrophages polarization and cancer cell epithelial-mesenchymal
transition during interaction. Cell Commun Signal. 16:542018.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wheeler KC, Jena MK, Pradhan BS, Nayak N,
Das S, Hsu CD, Wheeler DS, Chen K and Nayak NR: VEGF may contribute
to macrophage recruitment and M2 polarization in the decidua. PLoS
One. 13:e01910402018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Linde N, Lederle W, Depner S, van Rooijen
N, Gutschalk CM and Mueller MM: Vascular endothelial growth
factor-induced skin carcinogenesis depends on recruitment and
alternative activation of macrophages. J Pathol. 227:17–28. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Finn RS, Qin S, Ikeda M, Galle PR, Ducreux
M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, et al: Atezolizumab
plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J
Med. 382:1894–1905. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cheng AL, Qin S, Ikeda M, Galle P, Ducreux
M, Zhu A, Kim TY, Kudo M, Breder V, Merle P, et al: IMbrave150:
Efficacy and safety results from a ph III study evaluating
atezolizumab (atezo) + bevacizumab (bev) vs sorafenib (Sor) as
first treatment (tx) for patients (pts) with unresectable
hepatocellular carcinoma (HCC). Ann Oncol. 30:ix186–ix187. 2019.
View Article : Google Scholar
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yoshimoto T, Morine Y, Takasu C, Feng R,
Ikemoto T, Yoshikawa K, Iwahashi S, Saito Y, Kashihara H, Akutagawa
M, et al: Blue light-emitting diodes induce autophagy in colon
cancer cells by Opsin 3. Ann Gastroenterol Surg. 2:154–161. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Riabov V, Gudima A, Wang N, Mickley A,
Orekhov A and Kzhyshkowska J: Role of tumor associated macrophages
in tumor angiogenesis and lymphangiogenesis. Front Physiol.
5:752014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tsukamoto M, Imai K, Ishimoto T, Komohara
Y, Yamashita YI, Nakagawa S, Umezaki N, Yamao T, Kitano Y, Miyata
T, et al: PD-L1 expression enhancement by infiltrating
macrophage-derived tumor necrosis factor-α leads to poor pancreatic
cancer prognosis. Cancer Sci. 110:310–320. 2019.PubMed/NCBI
|
|
27
|
Wei Y, Zhao Q, Gao Z, Lao XM, Lin WM, Chen
DP, Mu M, Huang CX, Liu ZY, Li B, et al: The local immune landscape
determines tumor PD-L1 heterogeneity and sensitivity to therapy. J
Clin Invest. 129:3347–3360. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shima T, Shimoda M, Shigenobu T, Ohtsuka
T, Nishimura T, Emoto K, Hayashi Y, Iwasaki T, Abe T, Asamura H and
Kanai Y: Infiltration of tumor-associated macrophages is involved
in tumor programmed death-ligand 1 expression in early lung
adenocarcinoma. Cancer Sci. 111:727–738. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lai YS, Wahyuningtyas R, Aui SP and Chang
KT: Autocrine VEGF signalling on M2 macrophages regulates PD-L1
expression for immunomodulation of T cells. J Cell Mol Med.
23:1257–1267. 2019.PubMed/NCBI
|
|
30
|
Sica A and Mantovani A: Macrophage
plasticity and polarization: In vivo veritas. J Clin Invest.
122:787–795. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mantovani A, Biswas SK, Galdiero MR, Sica
A and Locati M: Macrophage plasticity and polarization in tissue
repair and remodelling. J Pathol. 229:176–185. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Petty AJ and Yang Y: Tumor-associated
macrophages: Implications in cancer immunotherapy. Immunotherapy.
9:289–302. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hughes R, Qian BZ, Rowan C, Muthana M,
Keklikoglou I, Olson OC, Tazzyman S, Danson S, Addison C, Clemons
M, et al: Perivascular M2 macrophages stimulate tumor relapse after
chemotherapy. Cancer Res. 75:3479–3491. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Osterberg N, Ferrara N, Vacher J, Gaedicke
S, Niedermann G, Weyerbrock A, Doostkam S, Schaefer HE, Plate KH
and Machein MR: Decrease of VEGF-A in myeloid cells attenuates
glioma progression and prolongs survival in an experimental glioma
model. Neuro Oncol. 18:939–949. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Min AKT, Mimura K, Nakajima S, Okayama H,
Saito K, Sakamoto W, Fujita S, Endo H, Saito M, Saze Z, et al:
Therapeutic potential of anti-VEGF receptor 2 therapy targeting for
M2-tumor-associated macrophages in colorectal cancer. Cancer
Immunol Immunother. 70:289–298. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chu M, Xu L, Zhang MB, Chu ZY and Wang YD:
Role of baicalin in anti-influenza virus A as a potent inducer of
IFN-gamma. Biomed Res Int. 2015:2636302015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yu FY, Huang SG, Zhang HY, Chi HG, Zou Y,
Lu RX and Zheng XB: Effect of baicalin on signal transduction and
activating transcription factor expression in ulcerative colitis
patients. Zhongguo Zhong Xi Yi Jie He Za Zhi. 35:419–424. 2015.(In
Chinese). PubMed/NCBI
|
|
38
|
Zhang CL, Zhang S, He WX, Lu JL, Xu YJ,
Yang JY and Liu D: Baicalin may alleviate inflammatory infiltration
in dextran sodium sulfate-induced chronic ulcerative colitis via
inhibiting IL-33 expression. Life Sci. 186:125–132. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kessler T, Sahm F, Blaes J, Osswald M,
Rübmann P, Milford D, Urban S, Jestaedt L, Heiland S, Bendszus M,
et al: Glioma cell VEGFR-2 confers resistance to chemotherapeutic
and antiangiogenic treatments in PTEN-deficient glioblastoma.
Oncotarget. 6:31050–31068. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Faes S, Santoro T, Demartines N and
Dormond O: Evolving significance and future relevance of
anti-angiogenic activity of mTOR inhibitors in cancer therapy.
Cancers (Basel). 9:1522017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mercurio A, Lipscomb E and Bachelder R:
Non-angiogenic functions of VEGF in breast cancer. J Mammary Gland
Biol Neoplasia. 10:283–290. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yao X, Tu Y, Xu Y, Guo Y, Yao F and Zhang
X: Endoplasmic reticulum stress-induced exosomal miR-27a-3p
promotes immune escape in breast cancer via regulating PD-L1
expression in macrophages. J Cell Mol Med. 24:9560–9573. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li D, Sun FF, Wang D, Wang T, Peng JJ,
Feng JQ, Li H, Wang C, Zhou DJ, Luo H, et al: Programmed death
ligand-1 (PD-L1) regulated by NRF-2/MicroRNA-1 regulatory axis
enhances drug resistance and promotes tumorigenic properties in
sorafenib-resistant hepatoma cells. Oncol Res. 28:467–481. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Schmidinger M: Clinical decision-making
for immunotherapy in metastatic renal cell carcinoma. Curr Opin
Urol. 28:29–34. 2018. View Article : Google Scholar : PubMed/NCBI
|