Introduction
Liver cancer is one of the most common forms of
carcinomas diagnosed worldwide. In recent years, the morbidity and
mortality of liver cancer have steadily increased (1). In the early stages of liver cancer,
patients are treated using either surgical resection of the
carcinoma or liver transplantation. However, the early phase of
liver cancer is often asymptomatic, which means that diagnosis
mostly occurs at the later stages of the disease (2). For most liver cancer patients,
chemotherapy remains the primary therapeutic strategy, of which
sorafenib is the current first-line therapy. However, sorafenib
exhibits limited efficacy, and long-term treatment with the drug
results in little benefit to the patient's long-term survival. The
latter of which is due to the development of drug resistance
through multiple mechanisms (3,4). Thus,
new therapeutic methods that can effectively treat refractory liver
cancer are urgently needed.
Hedgehog (Hh) signaling is a pathway that directs
the development of embryonic cells in animals, from invertebrates
to vertebrates. Hh induces the differentiation of endodermal
progenitors into hepatocytes (5).
The pathway is also reported to play a substantial role in the
carcinogenesis and progression of liver cancer. Aberrant activation
of Hh occurs in chronic liver damage and across various stages of
liver cancer development (6). The
central role of Hh in the development of liver cancer has resulted
in a search for potential therapeutic targets to mitigate this
pathway. Smoothened (SMO) and the glioma-associated oncogene
homolog (Gli) family of zinc-finger transcription factors are both
downstream effectors of Hh signaling. They are both regarded as
important targets for cancer therapeutics. Gli activators bind to
the GACCACCCA motif to regulate transcription of GLI1, PTCH1,
PTCH2, HHIP1, MYCN, CCND1, CCND2, BCL2, CFLAR, FOXF1, FOXL1, PRDM1
(BLIMP1), JAG2, GREM1, and Follistatin (7). Recently, we identified Rho guanine
nucleotide exchange factor 16 (ARHGEF16) as a new downstream target
of Gli2 (8). In recent years, a
large number of small-molecule inhibitors targeting the Hh
signaling pathway have been developed. Of these, vismodegib
(GDC-0449) and sonidegib (LDE225) received FDA approval for the
treatment of basal cell carcinoma, while glasdegib (PF-04449913)
was approved for treating acute myeloid leukemia in combination
therapy. However, acquired resistance to vismodegib was observed in
clinical trials of the compound. At present, preclinical studies
and clinical trials continue to evaluate the efficacy of Hh
inhibitors across multiple types of cancer.
Over the past decade, several naturally occurring
compounds capable of inhibiting the aberrant activation of Hh
signaling have also been investigated for their preventative and
therapeutic potential (9,10). For example, berberine, cyclopamine,
and vitamin D3 target SMO to directly inhibit Hh signaling, while
glabrescione B acts via Gli1 (11–14).
Curcumin, genistein and resveratrol also hold potential as Hh
inhibitors and are currently under investigation (15). Natural compounds often target
multiple signaling pathways, rather than acting as specific or
direct modulators of individual signaling pathways. Therefore, the
inhibitors of Hh signaling may function in a cell type-dependent
manner. The mechanisms of these Hh inhibitors in certain cancers
remain problematic, and additional natural compounds capable of
inhibiting Hh signaling remain undiscovered.
Cepharanthine, a biscoclaurine alkaloid isolated
from the roots of Stephania cephalantha Hayata, has been
used clinically to treat radiation-induced leukopenia, alopecia,
and snakebites for decades. It is not associated with any severe
side effects (16). Cepharanthine
has also been reported to possess antitumor activity in multiple
types of cancer, including liver cancer (17–22).
Cepharanthine acts to inhibit cellular proliferation and promote
apoptosis. The mechanisms by which it does this are complex, and
likely cell type-dependent. Mechanistic studies of cepharanthine
suggest that the compound induces: the suppression of NF-κB
activity (23), chromatin
condensation, nuclear fragmentation, JNK1/2 activation (24), upregulation of p21Waf1/Cip1,
downregulation of cyclin A and Bcl-2, induction of reactive oxygen
species (ROS) (25), and
mitochondrial dysfunctions (26).
However, more potential antitumor mechanisms of cepharanthine are
still under investigation.
In the present study, cepharanthine hydrochloride
(CH), a semi-synthetic derivative of cepharanthine, was utilized to
investigate the association of CH and the Wnt/Hh/Gli1 signaling
pathways for the treatment of liver cancer. This study may provide
insight into the clinical potential of CH as an Hh signaling
inhibitor for the treatment of liver cancer or other Hh-driven
cancers.
Materials and methods
Cell culture
Hepatocellular carcinoma cell lines Huh7 and HepG2
were purchased from the Cell Bank of the Shanghai Biological
Institute (Shanghai, China). Cells were cultured in DMEM (41965120,
Gibco) with 10% fetal bovine serum (FBS; 1027-106, Gibco; Thermo
Fisher Scientific, Inc.) and 100 U/ml penicillin/streptomycin
(15140163, Invitrogen; Thermo Fisher Scientific, Inc.). Both cell
lines were incubated in a humidified incubator at 37°C with 5%
CO2. All cell lines were authenticated using STR method
by a third-party testing organization.
Chemicals and antibodies
CH, a semi-synthetic derivative of cepharanthine was
developed by Guangzhou Jinan Biomedicine Research and Development
Center Co. Ltd. Wnt signaling inhibitor IWR-1 (S7086) and Gli1
antagonist GANT61 (S8075) were both purchased from Selleck
Chemicals.
Primary antibodies for Gli1 (#2643), PARP (#9532),
GAPDH (#2118), Snail (#3879), c-myc (#5605) and cyclin D1 (#2978)
were all purchased from Cell Signaling Technology (CST). Antibodies
for Gli1 (ab92611), caspase 3 (NB100-56708), SMO (20787-1-AP) and
β-catenin (sc-7963) were purchased from Abcam, NOVUS Biologicals,
Proteintech and Santa Cruz Biotechnology, Inc., respectively.
Cell viability
Cells (20,000) were seeded into 96-well plates and
allowed to attach overnight. The following morning, the supernatant
was discarded and replaced with the complete medium containing 0,
5, 10, 20, 40 and 60 µM of CH. Cells were cultured at 37°C and 5%
CO2 for either 24 or 48 h. Following this, 10 µl of MTT
(M2128, Sigma-Aldrich; Merck KGaA) at a final concentration of 5
mg/ml was added and incubated for 4 h at 37°C. The supernatant
fraction was carefully discarded and 100 µl DMSO was added to
dissolve the water-insoluble MTT formazan. The optical density (OD)
was subsequently measured using a wavelength of 570 nm, and a
microplate reader (Epoch, Bio-tek). The OD570 of the control cells
was used to represent 100% viability.
Cell invasion assay
The invasive ability of the cells was evaluated
using 6.5-mm Transwell chambers (8-µm pore size, Corning Inc.).
These chambers were pre-coated with 1 mg/ml Matrigel®
Basement Membrane Matrix (Corning Inc.) according to the
manufacturer's protocol. The cells (4,000) were suspended in 200 µl
serum-free medium and seeded in the upper chambers. The wells under
the chambers were filled with 600 µl medium containing 10% FBS with
or without CH. After 48 h, the residual cells in the top surface of
the chambers were removed. The invading cells on the bottom were
fixed with 4% paraformaldehyde (P1110, Solarbio) and stained using
1% crystal violet. The cells were then photographed using the Leica
DMi1 inverted microscope with a total magnification of ×100.
Colony formation assay
Colony formation assays were undertaken by seeding
Huh7 or HepG2 cells (4,000 cells/well) into 6-well plates. The
cells were allowed to form colonies for 7–10 days before different
concentrations of CH were added and incubated with the cells for an
additional 72 h. The medium was renewed every three days. Following
this, the cells were washed with PBS, and fixed in 4%
paraformaldehyde for 15 min. Fixed cells were then stained with 1%
crystal violet for 15 min and then rinsed with distilled water. The
colonies formed were photographed using the Leica DMi1 inverted
microscope with a total magnification of ×40.
Western blot analysis
The cells were lysed in RIPA buffer containing 50 mM
Tris (pH 7.4), 150 mM NaCl, 1% NP40, 0.5% sodium deoxycholate and
0.1% SDS (P0013C, Beyotime Institute of Biotechnology). Total
protein concentration was quantified using the BCA Protein Assay
Kit (P0011, Beyotime Institute of Biotechnology). The lysates of
equal protein concentrations (total 30 µg protein) were separated
using 8–12% sodium dodecyl sulfate-polyacrylamide gels and then
transferred to PVDF membranes (ISEQ00010, Millipore). Next, 5% skim
milk was used to block the membranes before they were probed with
the primary antibodies (1:1,000) and incubated at 4°C overnight on
a shaker. The species-specific HRP-conjugated secondary antibodies
(1:4,000) were incubated with the membranes for 1 h at room
temperature. The immunoreactive bands were detected using an
enhanced chemiluminescence kit (34580, Thermo Fisher Scientific,
Inc.). GAPDH was used as a loading control.
Flow cytometry
CH-induced apoptosis was assessed by the detection
of cells stained with Annexin V Alexa Fluor488/propidium iodide
(PI) reagent (FXP022-050, 4A Biotech Co.) using a FACSCalibur flow
cytometer (BD Biosciences). Flow cytometry was conducted in
accordance with the manufacturer's protocol. In short, cells were
cultured with various concentrations of CH for 24 h before being
digested in EDTA-free trypsin, and collected by centrifugation at
800 × g for 5 min. The cells were then washed with cold PBS before
being resuspended by the mixture of binding buffer and
Annexin-V-FITC. This mixture was then incubated for 5 min at room
temperature and away from light. The mixture of binding buffer and
PI was then added for an additional 15 min. Apoptotic cells were
subsequently detected using flow cytometry, and the data were
analyzed using FlowJo v10 software (FlowJo LLC).
RNA extraction and real-time
quantitative (q)PCR
Cells were treated with various concentrations of CH
for 24 h. Total RNA was extracted using Trizol reagent (15596-026,
Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. Briefly, the cells were harvested and
soaked in Trizol at room temperature for 10 min. The RNA was then
precipitated using isopropanol before being rinsed with 75% ethanol
and dissolved in RNAase-free water. The purity and concentration of
the RNA sample were then assessed using an UV spectrophotometer
(NanoPhotometer® P330, IMPLEN). Next, 1 µg of RNA was
used to reversely transcribe into cDNA with a PrimeScript RT
reagent kit (RR047A, TaKaRa). The assay was performed in a Bio-Rad
CFX96 RT-PCR systems (Bio-Rad Laboratories) using an SYBR Premix
DimerEraser (RR091A, TaKaRa). The reaction conditions were as
follows: pre-denaturation at 95°C for 30 sec; 40 cycles of
denaturation at 95°C for 5 sec; annealing and extension at 55°C for
30 sec. Levels of mRNA were normalized to the housekeeping gene,
GAPDH, using the comparative Cq method. The sequences of the
primers used are listed in Table
I.
 | Table I.Primer sequences for qPCR. |
Table I.
Primer sequences for qPCR.
| Gene name |
| Primer sequence
(5′-3′) |
|---|
| GLI1 | Forward |
CTACATCAACTCCGGCCAAT |
|
| Reverse |
CGGCTGACAGTATAGGCAGA |
| SMO | Forward |
GGGAGGCTACTTCCTCATCC |
|
| Reverse |
GGCAGCTGAAGGTAATGAGC |
| PTCH1 | Forward |
CTCTGGAGCAGATTTCCAAGG |
|
| Reverse |
TGCCGCAGTTCTTTTGAATG |
| GAPDH | Forward |
GAGTCAACGGATTTGGTCGT |
|
| Reverse |
GACAAGCTTCCCGTTCTCAG |
Dual-luciferase reporter assay
HepG2 cells were seeded in a 24-well culture plate.
Plasmids of pGL3 basic or pGL3 basic-ARHGEF16 (0.75 µg) and
pUB6/V5-HisB-Gli1 (0.25 µg), mixed with 0.025 µg pRL-TK-luc, were
co-transfected into the cells using Lipofectamine™ 3000
transfection reagent (L3000015, Thermo Fisher Scientific, Inc.).
The ARHGEF16 gene is a downstream target of Gli2 (8). This was conducted in triplicate,
according to the manufacturer's instructions. After a 48-h
transfection, the cells were treated with different concentrations
of CH for 6 h. Cell lysates were extracted, and luciferase activity
was measured using the Luciferase Report Assay System (E1910,
Promega, Corp.).
Xenograft tumor assay
All animal experiments were performed in accordance
with national ethical guidelines and following the approval
(approval no. IACUC-20180904-05) from the Institutional Animal Care
and Use Committee of Jinan University (Guangzhou, Guangdong,
China). Briefly, 10 female BALB/c-nu mice (5-weeks-old, BW 17.3±0.6
g, Institute of Laboratory Animal Sciences, Beijing, China) were
housed in a specific pathogen-free environment with a 12 h light/12
h dark cycle and constant temperature of 23°C. All the mice were
given a standard chow diet and sterilized water ad libitum.
Huh7 cells (5105) resuspended in 200 µl Matrigel/PBS (6
mg/ml) were injected subcutaneously into the flank of the mice.
Tumors were allowed to grow until reaching approximately 100
mm3, before the mice were randomly assigned to either
the control or the treatment group (n=5 mice per group). Mice
received daily peritoneal injections of either CH (20 mg/ml)
dissolved in saline (purchased from the First Affiliated Hospital
of Jinan University) or saline only for 12 days. Tumor sizes (long
tumor diameter, L; short tumor diameter, S) and body weights were
measured on alternate days. The tumor volume (V) was calculated
using the formula V=1/2×LxW2. On day 13, the mice were
euthanized through cervical dislocation performing by experienced
experimenters. We verified death by signs of no breathing and no
corneal reflex. Their xenograft tumors and livers were collected
and fixed in 4% paraformaldehyde for further analysis.
Immunohistochemistry assay
Fixed xenograft tumors and livers were embedded into
a paraffin block and sliced into 3-µm-thick sections. Paraffin
sections were then de-waxed, rehydrated and incubated with 0.3%
H2O2 to block endogenous peroxidase activity.
Following this, the sections were autoclaved in 10 mM sodium
citrate buffer (pH 6.0) for 15 min, and then incubated with 10%
goat serum for 30 min. Tissue sections were then exposed to the
primary Gli1 antibody (1:200) and incubated overnight. The next
day, sections were rinsed with PBS and the slides were immersed in
the HRP-conjugated secondary antibodies before
3,3′-diaminobenzidine (DAB) was added. The staining of the slides
was then visualized and photographed using Nikon Ti-E microscopy
(Tokyo, Japan). The German semiquantitative scoring method was
adopted to score the expression level of Gli1 (27). Each specimen was assigned a score
according to both the intensity of the staining (0=no staining, not
detected; 1=weak staining, light yellow; 2=moderate staining,
yellowish-brown; 3=strong staining, brown) and the extent of cells
stained (0=no staining; 1=1-24% stained; 2=25-49% stained; 3=50-74%
stained; 4=75-100% stained). The immunoreactive score of the
stained cells was then calculated using the following equation:
Total score=intensity score × extent score.
Staining scores were conducted based on observations
by at least two independent investigators blinded to the treatment
conditions.
Statistical analysis
All data consist of the results of at least three
independent experiments. Statistical significance was determined by
the Mann-Whitney U test for nonparametric analysis of the scoring
of Gli1 expression in xenograft tissue. Two-tailed Student's
t-tests and one-way ANOVA were used to compare the results of other
assays. For the luciferase assay, Scheffe's post hoc test was used
to compare the differences between every 2 groups. For comparison
among three groups, LSD's post hoc test was used. All data are
presented as mean ± SD. The statistical differences are shown as
*P<0.05; **P<0.01; ***P<0.001, as indicated in the figures
and legends.
Results
CH inhibits cellular function and
induces the apoptosis of liver cancer cells
Cepharanthine hydrochloride (CH) is a semi-synthetic
derivative of cepharanthine, and its chemical structure is shown as
Fig. 1A. Cell viability assays were
conducted using Huh7 and HepG2 cells to assess the effect of CH on
liver cancer. As shown in Fig. 1B,
CH inhibited the growth of Huh7 and HepG2 in a dose-dependent
manner. The half maximal inhibitory concentration (IC50)
values indicated that the inhibitory effect of CH was more evident
in Huh7 cells than in HepG2 cells. However, no significant
differences were observed between 24 and 48 h in these cells. To
further investigate the effects of CH on cellular function of these
liver cancer cells, Transwell and colony formation assays were
performed. As shown in Fig. 1C and
D, CH markedly inhibited the invasive and proliferative
activities of both cell lines. Although the concentrations of CH
used in the cell viability and colony formation assays were the
same, the treatment durations in the two assays are different. The
cells were treated with CH for 24 or 48 h in the viability assay,
but 72 h in the colony formation assay. In addition, the different
confluence of the cells before drug treatment was also another
influencing factor. Note that the cleaved forms of
apoptosis-related proteins poly(ADP-ribose) polymerase (PARP) and
caspase 3 were increased in a dose-dependent manner in both Huh7
and HepG2 cells (Fig. 1E). It was
difficult to obtain a clear band of cleaved caspase 3 in the Huh7
cells. We suppose that caspase 3 is not a sensitive marker of
CH-induced apoptosis in Huh7 cells. Flow cytometry was then used to
assess apoptosis. As shown in Fig.
1F, significantly more apoptotic cells were observed in the
CH-treated groups, in particulary the groups treated with high
concentrations of CH (10 µM for Huh7 cells and 20 µM for HepG2
cells). These results revealed that, consistent with previous
reports of cepharanthine in liver cancer (24), CH functioned to suppress liver cancer
in vitro.
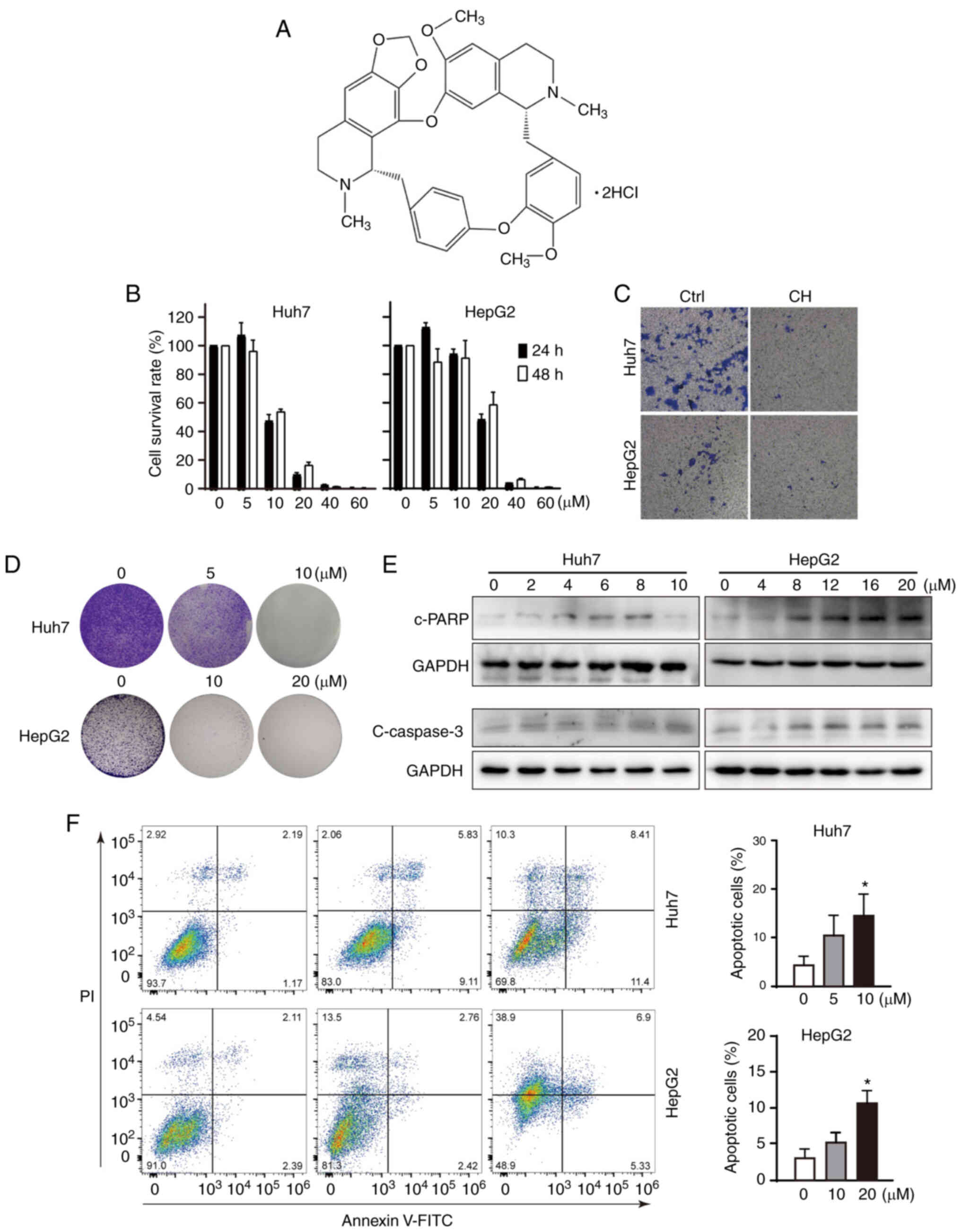 | Figure 1.Cepharanthine hydrochloride (CH)
inhibits the cell functions of liver cancer cells. (A) Chemical
structure of CH. (B) Cell viability assays of CH-treated liver
cancer cells. Huh7 and HepG2 cells were exposed to different
concentrations (0–60 µM) of CH for 24 and 48 h. Cell viability was
detected using the MTT method. (C) Transwell assay. Transwell
chambers were precoated with 1 mg/ml Matrigel, and 4,000 cells were
suspended in 200 µl DMEM with no FBS and seeded in the upper
chamber. The lower well was filled with DMEM containing 10% FBS in
the presence or absence of CH (Huh7: 10 µM; HepG2: 20 µM) for 48 h.
The cells that invaded the outer side of the chamber bottom were
fixed and stained with crystal violet, and then photographed
(×100). (D) Colony formation assay. Cells (4,000 cells per well)
were seeded into a 6-well plate. The cells were allowed to form
colonies for 7–10 days before different concentrations of CH were
added and incubated with the cells for an additional 72 h.
Following fixation with 4% paraformaldehyde, the cells were stained
with 1% crystal violet and photographed (×40). (E) Detection of
apoptotic proteins using western blot analysis. Cells were treated
with increasing concentrations of CH. The lysates were subjected to
western blotting and probed with primary and corresponding
species-specific secondary antibodies, as indicated. (F) Flow
cytometry assay. Cells were treated with different concentrations
of CH for 24 h, and then stained using Annexin V and PI reagents
for flow cytometric analysis. Data are shown as mean ± SD of three
independent experiments. *P<0.05, compared to the untreated
group. c-, cleaved; PARP, poly(ADP-ribose) polymerase. |
CH regulates the Hh signaling pathway
in liver cancer cells
The Hh pathway is involved in the development of
liver cancer, and its downregulation has been shown to suppress the
growth of various types of cancer (28). In the present study, it was
investigated whether the effects of CH on liver cancer cells were
due to the inhibition of the Hh pathway. First, the qPCR assay
demonstrated that CH treatment downregulated the transcription of
both GLI1 and SMO, two downstream effectors of Hh
signaling (Fig. 2A). Moreover,
PTCH1, the negative regulator of Hh signaling, was found to
be slightly increased in the HepG2 cells. Protein levels of Gli1,
SMO and Snail, a rapidly induced protein of Gli1, were reduced in
both cell types in a concentration-dependent and time-dependent
manner (Fig. 2B and C). To further
verify the effect of CH on Hh signaling activation, a
dual-luciferase reporter assay was used to detect the
transcriptional regulatory activity of Gli1 in CH-treated cells.
HepG2 cells were used to perform this experiment given their
transfection efficiency and capacity to cope with the burden of
multiple plasmids. The use of lower CH concentrations avoided
further cytotoxicity to the cells. As shown in Fig. 2D, CH reduced the transcription
activity of Gli1 in HepG2 cells. These results suggested that CH
was functioning as an inhibitor of Hh by suppressing the
transcription and transcriptional activity of Gli1 in liver cancer
cells.
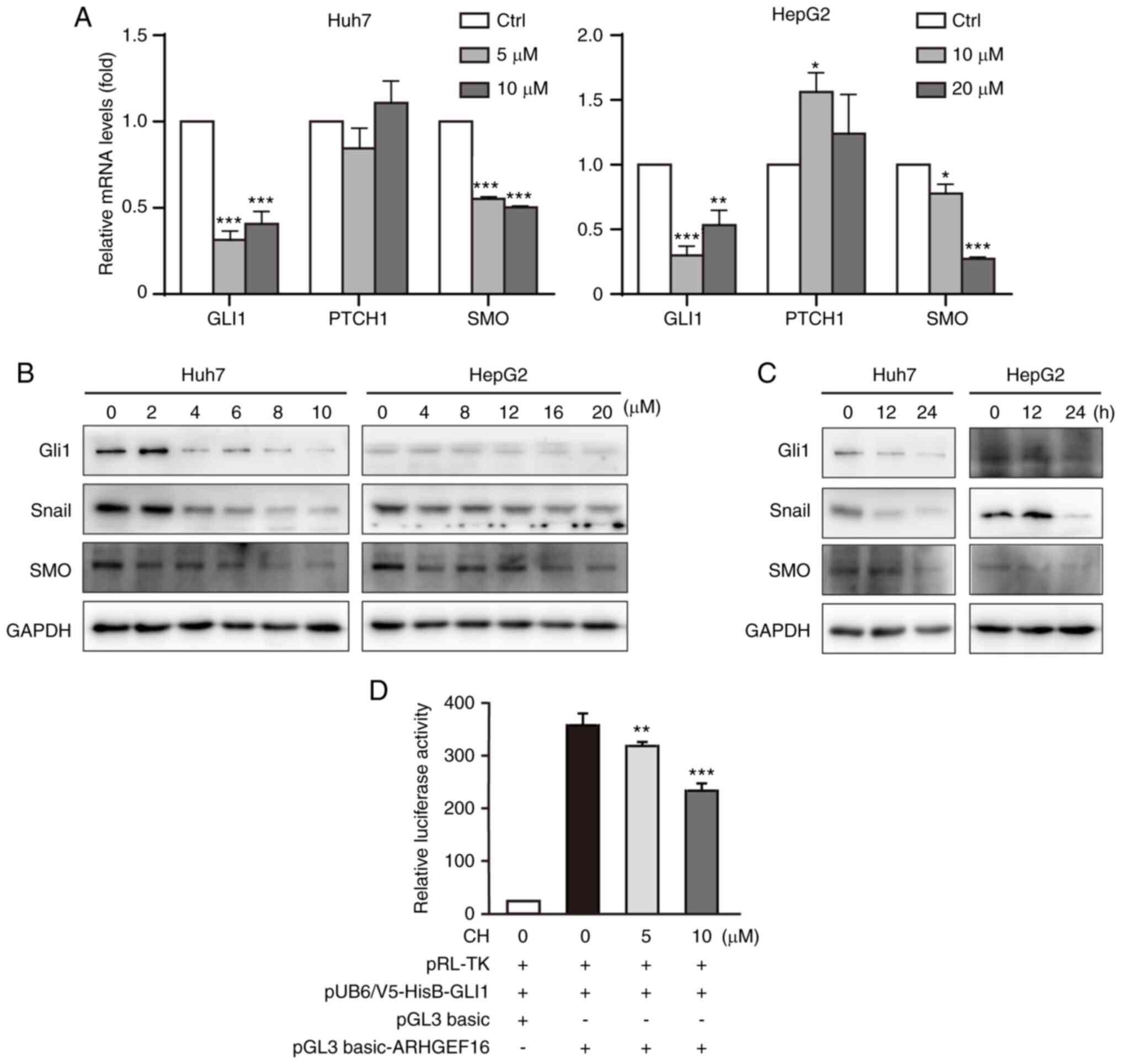 | Figure 2.Cepharanthine hydrochloride (CH)
inhibits Hedgehog (Hh) signaling. (A) Real-time quantitative PCR
assay. Cells were treated with different concentrations of CH for
24 h. Total RNA was extracted by Trizol before undergoing reverse
transcription into cDNA. The mRNA levels were normalized to levels
of the housekeeping gene GAPDH. (B) Proteins of Hh signaling
respond to different concentrations of CH. Cells were treated with
increasing concentrations of CH for 24 h. The lysates were
subjected to western blotting and probed with primary and
corresponding species-specific secondary antibodies, as indicated.
*P<0.05, **P<0.01, ***P<0.001, significance reported
relative to the control (Ctrl). (C) Proteins of Hh signaling across
the time course of CH treatment. Cells were treated with CH (10 µM
for Huh7 cells; 20 µM for HepG2 cells) for 12 or 24 h. The lysates
were subjected to western blotting and probed with primary and
corresponding species-specific second antibodies, as indicated. (D)
Dual-luciferase assays. HepG2 cells were co-transfected with
pRL-TK-luc, pUB6/V5-HisB-Gli1, pGL3 basic/basic-ARHGEF16 plasmids
for 48 h. Following this, cells were treated with CH (5 or 10 µM)
for an additional 6 h. Luciferase activity was measured in the cell
lysates using the Luciferase Report Assay System. **P<0.01,
***P<0.001, significance reported relative to the control
(Ctrl). GLI1, glioma-associated oncogene homolog 1; PTCH1, patched
1; SMO, smoothened. |
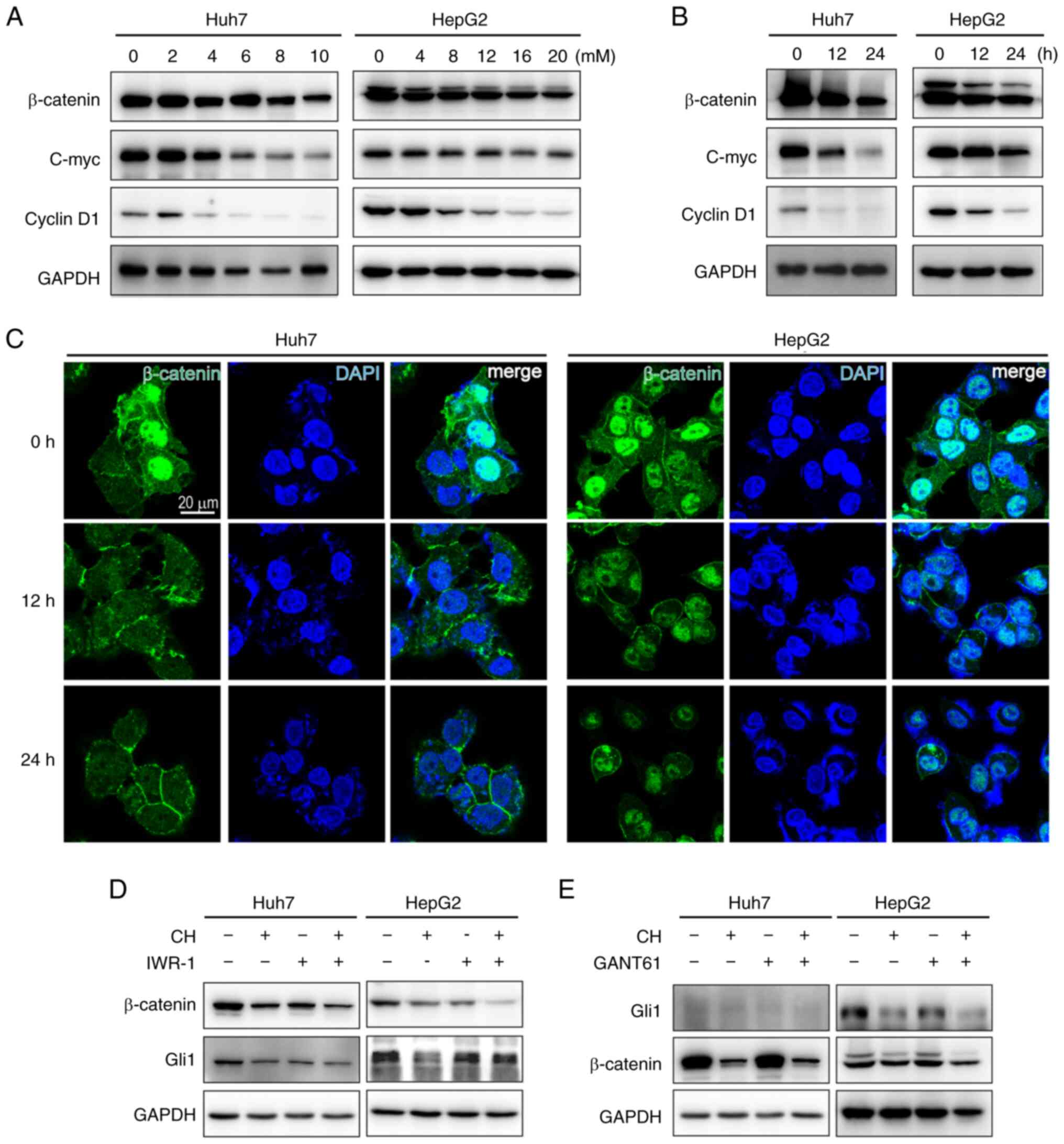
CH inhibits upstream Wnt/β-catenin
signaling
To determine the mechanism by which CH regulates Hh
signaling, another essential regulator of embryonic development and
tissue homeostasis, Wnt signaling was assessed. Crosstalk between
Wnt and Hh signaling has been reported in multiple types of cancer.
Here, it was demonstrated that CH inhibited the Wnt co-activator,
β-catenin, in addition to two of its targets, proto-oncogene c-myc
and cyclin D1 in a concentration-dependent and time-dependent
manner (Fig. 3A and B). Images of
the immunofluorescence in liver cancer cells showed that β-catenin
was mainly located in the nucleus and was diminished under CH
treatment (Fig. 3C). The regulatory
relationships of these signaling pathways were then assessed using
the Wnt inhibitor, IWR-1, and the Gli1/2 antagonist, GANT61. When
Wnt signaling was inhibited, as indicated by the diminished level
of β-catenin proteins, Gli1 levels in the IWR-1-treated cells were
also decreased (Fig. 3D). On the
other hand, GANT61 did not affect the level of β-catenin proteins
(Fig. 3E). This indicated that Wnt
signaling was occurring upstream of Hh signaling, and CH was acting
to inhibit the Wnt/β-catenin/Hh signaling cascade in liver cancer
cells.
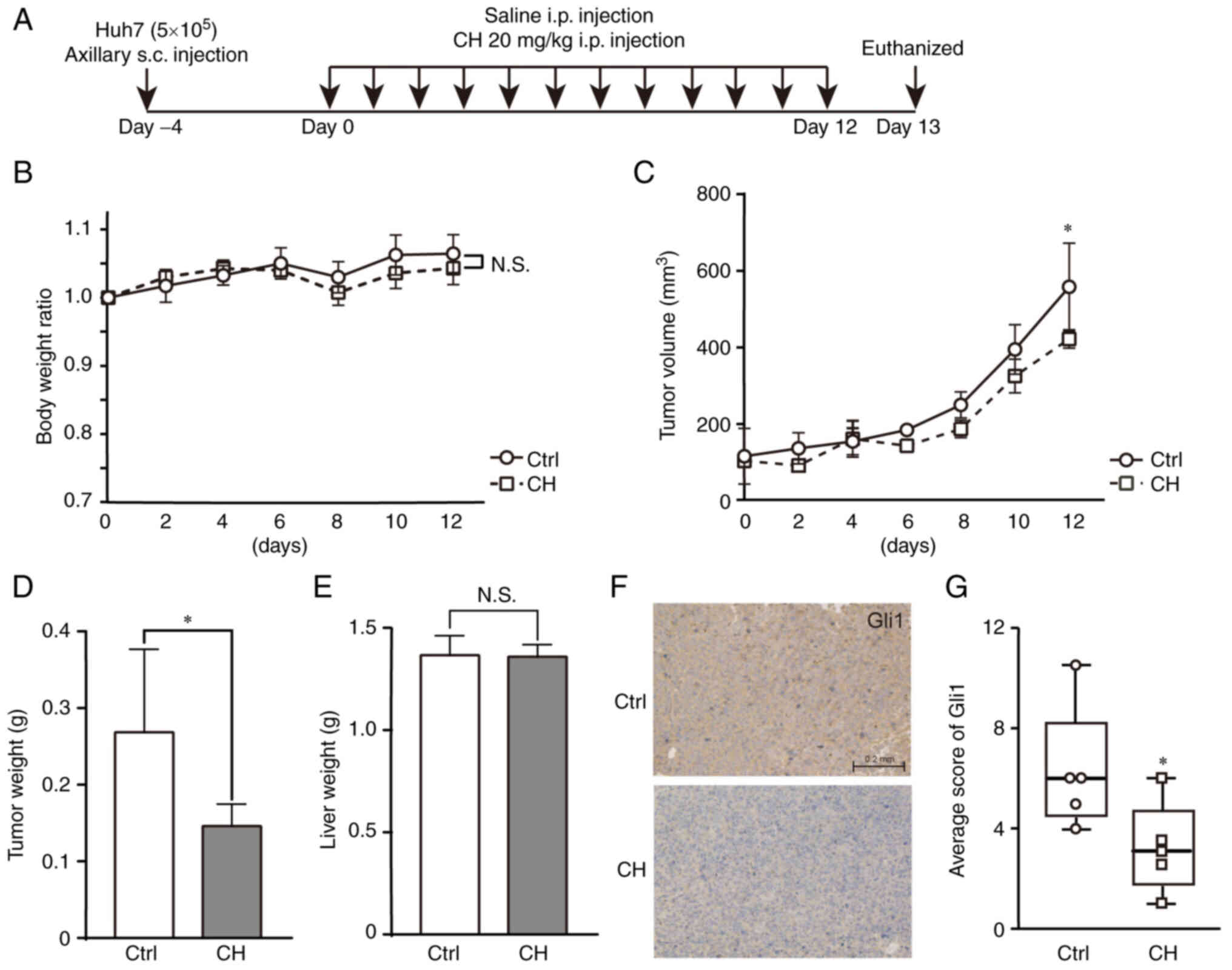
CH inhibits tumorigenesis through Hh
signaling in vivo
We next examined the antitumor activity of CH in
vivo. Xenograft mouse models were established by injecting Huh7
cells subcutaneously (s.c.) into the flank of nude mice. Xenografts
were allowed to grow to ~100 mm3 before animals were
treated with daily intraperitoneal (i.p) injections of CH or saline
for 12 days (Fig. 4A). No
significant difference in body weight was observed between the two
groups, indicating that CH was well-tolerated by these mice
(Fig. 4B). In the control mice,
xenograft growth increased dramatically from the 6th day after
saline administration. Xenograft growth rates in the CH-treated
group were slower than those observed in the control group. This
difference became significant at the end of the experiment
(Fig. 4C). The maximum long (L) and
short (S) tumor diameters are shown in Table II. Following the sacrifice of these
animals at the end of the study, the weights of the xenografts in
the CH-treated animals were found to be decreased compared with
those in the control group (Fig.
4D). Many chemicals are known to induce hepatotoxicity and
subsequently increase the weight of the liver; as such, liver
weights were also compared across treatment groups. No significant
differences in liver weights were observed, suggesting that the
administration of CH did not induce hepatotoxicity during the
12-day treatment in these nude mice (Fig. 4E). Hematoxylin and eosin staining of
the livers confirmed the safety of CH (data not shown). Xenograft
levels of Gli1 were assessed using immunohistochemistry assay. This
analysis demonstrated that Gli1 protein expression was suppressed
in the CH-treated xenografts compared with the saline-treated
xenografts (Fig. 4F and G). The
median score of the control group was 6 [interquartile range; IQR
(3.75-8.25)], while the median score of the CH-treated group was 3
[interquartile range; IQR (1.75-4.75)]. Taken together, these
results revealed that Hh signaling may be an essential mechanism by
which CH exerts its antitumor activity in liver cancer cells.
 | Table II.The maximum long (L) and short (S)
tumor diameters (in mm). |
Table II.
The maximum long (L) and short (S)
tumor diameters (in mm).
|
|
Day |
|---|
|
|
|
|---|
|
| 0 | 2 | 4 | 6 | 8 | 10 | 12 |
|---|
|
|
|
|
|
|
|
|
|
|---|
|
| L | S | L | S | L | S | L | S | L | S | L | S | L | S |
|---|
| Ctrl group | 8.9 | 7.3 | 8.2 | 6.3 | 9.5 | 5.3 | 9.1 | 6.1 | 9.7 | 6.4 | 11.1 | 7.5 | 13.2 | 8.7 |
|
| 8.9 | 4.2 | 8.1 | 3.9 | 8.5 | 4.9 | 10.4 | 6.1 | 10.9 | 7.2 | 13.3 | 8.3 | 12.7 | 8.4 |
|
| 4.8 | 4.3 | 7.7 | 6.1 | 7.8 | 6.4 | 7.8 | 6.8 | 9.8 | 7.3 | 10.8 | 8.9 | 11.7 | 9.9 |
|
| 7.0 | 6.0 | 7.1 | 6.8 | 8.0 | 6.7 | 8.0 | 6.5 | 8.1 | 7.4 | 10.8 | 8.9 | 13.4 | 10.5 |
|
| 7.4 | 4.8 | 7.4 | 6.1 | 7.6 | 7.1 | 7.4 | 7.3 | 9.2 | 7.8 | 9.6 | 8.4 | 11.4 | 9.5 |
| CH group | 6.0 | 6.0 | 6.4 | 5.7 | 10.7 | 6.4 | 8.2 | 5.9 | 10.3 | 6.3 | 9.8 | 8.0 | 13.8 | 7.5 |
|
| 6.0 | 5.0 | 6.6 | 5.1 | 7.0 | 6.3 | 7.0 | 6.4 | 7.2 | 6.5 | 10.7 | 7.9 | 9.4 | 9.3 |
|
| 6.1 | 5.6 | 5.6 | 5.4 | 6.5 | 5.4 | 6.5 | 6.4 | 7.8 | 6.7 | 9.4 | 7.5 | 11.5 | 8.8 |
|
| 7.7 | 5.7 | 7.0 | 5.5 | 9.8 | 5.7 | 9.2 | 5.5 | 7.8 | 6.9 | 10.5 | 7.8 | 9.9 | 9.5 |
|
| 6.7 | 5.6 | 5.8 | 4.8 | 7.6 | 6.9 | 7.5 | 6.3 | 7.8 | 7.3 | 10.1 | 8.8 | 12.0 | 8.3 |
Discussion
In the present study, we revealed a novel antitumor
mechanism of cepharanthine hydrochloride (CH) in cell and animal
models of liver cancer. This was achieved by suppressing the
Hedgehog (Hh) signaling pathway and its upstream signaling via
Wnt/β-catenin. In vitro and in vivo experiments
verified each other. The results propose the application of CH in
Wnt/Hedgehog-driven tumors, including medulloblastoma, basal cell
carcinoma, glioblastoma, leukemia, lymphoma, and esophageal, lung,
gastric, pancreatic, colorectal, prostate, ovarian, and liver
cancer (29). Therefore, the present
study findings may contribute to the drug development of
cepharanthine for tumor therapy.
Dozens of compounds capable of inhibiting Hh
signaling have been identified. These molecules target several key
steps in the Hh activation process. Most Hh pathway antagonists
target smoothened (SMO); however, SMO is susceptible to mutation
and is a frequent contributor to the development of
chemoresistance. Therefore, compounds that target the cascade
downstream of SMO may represent a more promising therapeutic
strategy. As with SMO, multiple compounds have been identified as
either direct or indirect inhibitors of glioma-associated oncogene
homolog (Gli) transcription. One such compound is GANT61. GANT61 is
a highly efficient antagonist that acts by binding to the zinc
finger regions of Gli1 and Gli2. Several preclinical studies have
demonstrated that GANT61 has antitumor activity in a number of
cancer types, including acute myeloid leukemia, rhabdomyosarcoma,
neuroblastoma, melanoma, and cancers of the lung, breast, colon,
prostate, and pancreas (30).
Despite its broad spectrum of antitumor activity, the instability
of GANT61 at a physiological pH results in the compound hydrolyzing
into an inactive benzaldehyde species (31). Arsenic trioxide (ATO) is another Gli
inhibitor and is known for its use in acute promyelocytic leukemia
(32). In recent preclinical studies
and clinical trials, ATO has shown promising antitumor effects in
several solid tumors, such as breast, lung, glioma, pancreas, and
liver cancer (30,33,34). ATO
is recommended for clinical use in situations where conventional
chemotherapies cannot be used. Although Gli is a promising
antitumor target, synthetic compounds targeting Gli have been
reported to result in frequent adverse events and show low
bioavailability in human patients. A number of existing, naturally
derived compounds have shown promise as Gli inhibitors. These
clinically approved therapies have better bioavailability, fewer
toxic side effects, and an existing clinical trial dataset. They
also have the ability to inhibit the Hh pathway via multiple
targets. Metformin is a good example of such a compound, and has
been shown to inhibit Hh signaling by suppressing sonic hedgehog,
SMO, Patched, and Gli1 expression in various cancer cells (35–37).
Cepharanthine, a biscoclaurine alkaloid isolated from the roots of
Stephania cephalantha Hayata, is another example. This
compound has been used in Japan since the 1950s to treat multiple
acute and chronic diseases. Cepharanthine has also recently been
reported to possess antitumor activity via a number of mechanisms;
these oncogenic pathways or signaling nodes include NF-κB,
multidrug resistance protein 1 (MRP1), PI3K/Akt, AMPK, JNK1/2, and
DNA damage repair (22). Here, the
present study revealed for the first time that cepharanthine, and
its semi-synthetic derivative CH, are also able to suppress
oncogenesis by inhibiting the Hh signaling pathway.
The Wnt/β-catenin pathway and the Hh signaling
pathway are both correlated with the presence and progression of
drug resistance in liver cancer, and crosstalk between these
pathways has been frequently reported (38). More specially, the Hh transcription
factor Gli1 is reported to induce secreted frizzled-related protein
1 (sFRP-1), which is a negative regulator of Wnt signaling. Thus,
sFRP-1 inhibits the transduction of Wnt signaling by interacting
selectively and non-covalently with both the Wnt protein and the
Frizzled receptors (39,40). Therefore, the inhibition of Hh
signaling using a Gli antagonist may act to activate Wnt signaling
via the upregulation of sFRP-1 in some cancers. This indicates that
strategies targeting Hh signaling only, may not achieve the desired
therapeutic effect. Co-activation of both Hh and Wnt pathways is
associated with earlier recurrence and shorter overall survival
times in patients with triple-negative breast cancer in a cohort
study (41). Therefore, the findings
that CH inhibits Wnt/β-catenin signaling and Hh signaling
simultaneously highlights its therapeutic potential not only in
liver cancer, but also in other Wnt/β-catenin and Hh associated
cancers.
According to the literature, the regulatory
relationship of Hh and Wnt signaling pathways remains
controversial. In the present study, it was found that
Wnt/β-catenin functions upstream of Hh signaling in the two liver
cancer cell lines Huh7 and HepG2. The antagonist of Wnt (IWR-1)
reduced Gli1 proteins; however, the Hh inhibitor GANT61 was not
found to affect the levels of the β-catenin protein. Therefore, the
suppression of Hh signaling may be the major downstream effect of
the CH-mediated inactivation of Wnt/β-catenin signaling and the
diminished transcription of Gli1. It should also be noted that
combined treatment with CH and IWR-1 exhibited synergistic effects
on the suppression of β-catenin protein levels. As such, CH may
have potential as an adjuvant drug for Wnt inhibitors in cases
where the inhibition of Wnt signaling may be beneficial. Unlike Hh
signaling, elevated Wnt signaling does not correlate with reduced
patient survival in some types of cancer (42). However, Wnt signaling cannot be
targeted using a single universal strategy, but rather it remains
reliant on personalized clinical decision-making.
Wnt signaling is read out through total β-catenin
levels, which are highly cell-type dependent. The effect of CH on
cancers mediated by Wnt signaling (nuclear β-catenin levels,
endogenous gene expression) should thus be assessed on a
cancer-by-cancer basis. The β-catenin protein binds to a broad
spectrum of transcription factors allowing it to modulate multiple
downstream biological processes. With the exception of the nuclear
messenger for Wnt signaling, β-catenin acts as the core link
between cadherins and the cytoskeleton. Therefore, the effect of CH
on tumor cell migration and invasion may rely on a diminished
β-catenin response.
The antitumor effect of cepharanthine was
demonstrated in the case of a patient suffering from multiple
myeloma and showing no respond to preceding chemotherapy, who
coincidently received therapy with cepharanthine due to
thrombocytopenia. The case showed a marked reduction of tumor load.
This is the only reported clinical case, to the best of our
knowledge, that has demonstrated antitumor effect of cepharanthine
(43). Since then, several in
vivo studies were performed using xenograft animal models to
research the pharmaceutical effect of cepharanthine on cancers,
including lung cancer, breast cancer, and oral squamous cell
carcinoma (18,44,45). In
these studies, the doses of cepharanthine varied from 20 to 50
mg/kg, and were administered over experiments lasting between 21
and 46 days. Significantly, in these studies, cepharanthine alone
did not demonstrate any marked antitumor effects. However, when it
was used as an adjuvant, cepharanthine enhanced the antitumor
activity of both chemotherapy and radiation therapy. In our liver
cancer mouse model, CH monotherapy of 20 mg/kg/day showed
significance when calculating tumor sizes on the 12th day. However,
the capacity of CH to inhibit tumors seemed to be limited via an
unknown mechanism. The underlying mechanisms inhibiting the
antitumor effects of CH thus require additional research. These
investigations would also help to identify which precise
combination of therapeutic strategies incorporating CH would
provide the greatest benefit as an antitumor therapeutic.
In conclusion, the present study identified that the
semi-synthetic cepharanthine derivative CH inhibits Hh signaling in
liver cancer cells. The newly described antitumor
mechanism-of-action of CH provides further support for its
application in cancer therapy.
Acknowledgements
Plasmids for the dual-luciferase assay were kind
gifts from Professor Shi-Wen Luo of Nanchang University.
Funding
This work was supported by the Natural Science Foundation of
Guangdong Province (grant no. 2018030310401) and the China
Postdoctoral Science Foundation (grant no. 2017M622922).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding authors on reasonable
request.
Authors' contributions
GFS carried out the experiments, visualization, and
preparation for the experiments. ZXH carried out the experiments
and preparation of the experiments. DLH designed the study
methodology and carried out the experiments. PXC conducted the
preparation and designed the methodology used in the experiments.
YW was responsible for the conceptualization of the research
design, methodology, data curation, formal analysis, funding
acquisition, experiments, visualization, and writing of the
original draft. YFW was the project administration in charge of the
resource acquisition, validation of the data and supervision. All
authors read and approved the manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
All animal experiments were performed in accordance
with national ethical guidelines and with approval (approval no.
IACUC-20180904-05) from the Institutional Animal Care and Use
Committee of Jinan University (Guangzhou, Guangdong, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CH
|
cepharanthine hydrochloride
|
|
Hh
|
Hedgehog
|
|
SMO
|
Smoothened
|
|
Gli
|
glioma-associated oncogene homolog
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Daher S, Massarwa M, Benson AA and Khoury
T: Current and future treatment of hepatocellular carcinoma: An
updated comprehensive review. J Clin Transl Hepatol. 6:69–78. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nishida N, Kitano M, Sakurai T and Kudo M:
Molecular mechanism and prediction of sorafenib chemoresistance in
human hepatocellular carcinoma. Dig Dis. 33:771–779. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Niu L, Liu L, Yang S, Ren J, Lai PBS and
Chen GG: New insights into sorafenib resistance in hepatocellular
carcinoma: Responsible mechanisms and promising strategies. Biochim
Biophys Acta Rev Cancer. 1868:564–570. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Della Corte CM, Viscardi G, Papaccio F,
Esposito G, Martini G, Ciardiello D, Martinelli E, Ciardiello F and
Morgillo F: Implication of the Hedgehog pathway in hepatocellular
carcinoma. World J Gastroenterol. 23:4330–4340. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Che L, Yuan YH, Jia J and Ren J:
Activation of sonic hedgehog signaling pathway is an independent
potential prognosis predictor in human hepatocellular carcinoma
patients. Chin J Cancer Res. 24:323–331. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Katoh Y and Katoh M: Hedgehog target
genes: Mechanisms of carcinogenesis induced by aberrant hedgehog
signaling activation. Curr Mol Med. 9:873–886. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang D, Wang Y, Xu L, Chen L, Cheng M,
Shi W, Xiong H, Zalli D and Luo S: GLI2 promotes cell proliferation
and migration through transcriptional activation of ARHGEF16 in
human glioma cells. J Exp Clin Cancer Res. 37:2472018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mayank .Jaitak V: Molecular docking study
of natural alkaloids as multi-targeted hedgehog pathway inhibitors
in cancer stem cell therapy. Comput Biol Chem. 62:145–154. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Drenkhahn SK, Jackson GA, Slusarz A,
Starkey NJ and Lubahn DB: Inhibition of hedgehog/Gli signaling by
botanicals: A review of compounds with potential hedgehog pathway
inhibitory activities. Curr Cancer Drug Targets. 13:580–595. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang J, Peng Y, Liu Y, Yang J, Ding N and
Tan W: Berberine, a natural compound, suppresses Hedgehog signaling
pathway activity and cancer growth. BMC Cancer. 15:5952015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen JK, Taipale J, Cooper MK and Beachy
PA: Inhibition of Hedgehog signaling by direct binding of
cyclopamine to Smoothened. Genes Dev. 16:2743–2748. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bijlsma MF, Peppelenbosch MP and Spek CA:
(Pro-)vitamin D as treatment option for hedgehog-related
malignancies. Med Hypotheses. 70:202–203. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Infante P, Mori M, Alfonsi R, Ghirga F,
Aiello F, Toscano S, Ingallina C, Siler M, Cucchi D, Po A, et al:
Gli1/DNA interaction is a druggable target for Hedgehog-dependent
tumors. EMBO J. 34:200–217. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bao C, Kramata P, Lee HJ and Suh N:
Regulation of hedgehog signaling in cancer by natural and dietary
compounds. Mol Nutr Food Res. 62:2018.doi: 10.1002/mnfr.201700621.
View Article : Google Scholar
|
|
16
|
Rogosnitzky M and Danks R: Therapeutic
potential of the biscoclaurine alkaloid, cepharanthine, for a range
of clinical conditions. Pharmacol Rep. 63:337–347. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhou P, Zhang R, Wang Y, Xu D, Zhang L,
Qin J, Su G, Feng Y, Chen H, You S, et al: Cepharanthine
hydrochloride reverses the mdr1 (P-glycoprotein)-mediated
esophageal squamous cell carcinoma cell cisplatin resistance
through JNK and p53 signals. Oncotarget. 8:111144–111160. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tang ZH, Cao WX, Guo X, Dai XY, Lu JH,
Chen X, Zhu H and Lu JJ: Identification of a novel autophagic
inhibitor cepharanthine to enhance the anti-cancer property of
dacomitinib in non-small cell lung cancer. Cancer Lett. 412:1–9.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gao S, Li X, Ding X, Qi W and Yang Q:
Cepharanthine induces autophagy, apoptosis and cell cycle arrest in
breast cancer cells. Cell Physiol Biochem. 41:1633–1648. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Harada K, Ferdous T, Itashiki Y, Takii M,
Mano T, Mori Y and Ueyama Y: Cepharanthine inhibits angiogenesis
and tumorigenicity of human oral squamous cell carcinoma cells by
suppressing expression of vascular endothelial growth factor and
interleukin-8. Int J Oncol. 35:1025–1035. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Seubwai W, Vaeteewoottacharn K, Hiyoshi M,
Suzu S, Puapairoj A, Wongkham C, Okada S and Wongkham S:
Cepharanthine exerts antitumor activity on cholangiocarcinoma by
inhibiting NF-kappaB. Cancer Sci. 101:1590–1595. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bailly C: Cepharanthine: An update of its
mode of action, pharmacological properties and medical
applications. Phytomedicine. 62:1529562019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tamatani T, Azuma M, Motegi K, Takamaru N,
Kawashima Y and Bando T: Cepharanthin-enhanced radiosensitivity
through the inhibition of radiation-induced nuclear factor-kappa B
activity in human oral squamous cell carcinoma cells. Int J Oncol.
31:761–768. 2007.PubMed/NCBI
|
|
24
|
Biswas KK, Tancharoen S, Sarker KP,
Kawahara K, Hashiguchi T and Maruyama I: Cepharanthine triggers
apoptosis in a human hepatocellular carcinoma cell line (HuH-7)
through the activation of JNK1/2 and the downregulation of Akt.
FEBS Lett. 580:703–710. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rattanawong A, Payon V, Limpanasittikul W,
Boonkrai C, Mutirangura A and Wonganan P: Cepharanthine exhibits a
potent anticancer activity in p53-mutated colorectal cancer cells
through upregulation of p21Waf1/Cip1. Oncol Rep. 39:227–238.
2018.PubMed/NCBI
|
|
26
|
Hua P, Sun M, Zhang G, Zhang Y, Tian X, Li
X, Cui R and Zhang X: Cepharanthine induces apoptosis through
reactive oxygen species and mitochondrial dysfunction in human
non-small-cell lung cancer cells. Biochem Biophys Res Commun.
460:136–142. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shi C, Huang D, Lu N, Chen D, Zhang M, Yan
Y, Deng L, Lu Q, Lu H and Luo S: Aberrantly activated Gli2-KIF20A
axis is crucial for growth of hepatocellular carcinoma and predicts
poor prognosis. Oncotarget. 7:26206–26219. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xin M, Ji X, De La Cruz LK, Thareja S and
Wang B: Strategies to target the Hedgehog signaling pathway for
cancer therapy. Med Res Rev. 38:870–913. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Onishi H and Katano M: Hedgehog signaling
pathway as a therapeutic target in various types of cancer. Cancer
Sci. 102:1756–1760. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Girardi D, Barrichello A, Fernandes G and
Pereira A: Targeting the hedgehog pathway in cancer: Current
evidence and future perspectives. Cells. 8:1532019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Infante P, Alfonsi R, Botta B, Mori M and
Di Marcotullio L: Targeting GLI factors to inhibit the Hedgehog
pathway. Trends Pharmacol Sci. 36:547–558. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang ZY and Chen Z: Differentiation and
apoptosis induction therapy in acute promyelocytic leukaemia.
Lancet Oncol. 1:101–106. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lv XH, Wang CH and Xie Y: Arsenic trioxide
combined with transarterial chemoembolization for primary liver
cancer: A meta-analysis. J Gastroenterol Hepatol. 32:1540–1547.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Owonikoko TK, Zhang G, Kim HS, Stinson RM,
Bechara R, Zhang C, Chen Z, Saba NF, Pakkala S, Pillai R, et al:
Patient-derived xenografts faithfully replicated clinical outcome
in a phase II co-clinical trial of arsenic trioxide in relapsed
small cell lung cancer. J Transl Med. 14:1112016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Song Z, Wei B, Lu C, Huang X, Li P and
Chen L: Metformin suppresses the expression of Sonic hedgehog in
gastric cancer cells. Mol Med Rep. 15:1909–1915. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fan C, Wang Y, Liu Z, Sun Y, Wang X, Wei G
and Wei J: Metformin exerts anticancer effects through the
inhibition of the Sonic hedgehog signaling pathway in breast
cancer. Int J Mol Med. 36:204–214. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nakamura M, Ogo A, Yamura M, Yamaguchi Y
and Nakashima H: Metformin suppresses sonic hedgehog expression in
pancreatic cancer cells. Anticancer Res. 34:1765–1769.
2014.PubMed/NCBI
|
|
38
|
Chatterjee S and Sil PC: Targeting the
crosstalks of Wnt pathway with Hedgehog and Notch for cancer
therapy. Pharmacol Res. 142:251–261. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ding M and Wang X: Antagonism between
Hedgehog and Wnt signaling pathways regulates tumorigenicity. Oncol
Lett. 14:6327–6333. 2017.PubMed/NCBI
|
|
40
|
Katoh Y and Katoh M: WNT antagonist,
SFRP1, is Hedgehog signaling target. Int J Mol Med. 17:171–175.
2006.PubMed/NCBI
|
|
41
|
Arnold KM, Pohlig RT and Sims-Mourtada J:
Co-activation of Hedgehog and Wnt signaling pathways is associated
with poor outcomes in triple negative breast cancer. Oncol Lett.
14:5285–5292. 2017.PubMed/NCBI
|
|
42
|
Anastas JN and Moon RT: WNT signalling
pathways as therapeutic targets in cancer. Nat Rev Cancer.
13:11–26. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kikukawa Y, Okuno Y, Tatetsu H, Nakamura
M, Harada N, Ueno S, Kamizaki Y, Mitsuya H and Hata H: Induction of
cell cycle arrest and apoptosis in myeloma cells by cepharanthine,
a biscoclaurine alkaloid. Int J Oncol. 33:807–814. 2008.PubMed/NCBI
|
|
44
|
Lyu J, Yang EJ, Head SA, Ai N, Zhang B, Wu
C, Li RJ, Liu Y, Yang C, Dang Y, et al: Pharmacological blockade of
cholesterol trafficking by cepharanthine in endothelial cells
suppresses angiogenesis and tumor growth. Cancer Lett. 409:91–103.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Harada T, Harada K and Ueyama Y: The
enhancement of tumor radioresponse by combined treatment with
cepharanthine is accompanied by the inhibition of DNA damage repair
and the induction of apoptosis in oral squamous cell carcinoma. Int
J Oncol. 41:565–572. 2012. View Article : Google Scholar : PubMed/NCBI
|