Introduction
Cancer has become a global public concern and
according to data available in CA: A Cancer Journal for
Clinicians, the number of new cancer cases around the world for
the year 2020 reached 19.3 million while the number of deaths
related to this condition was 10 million (1). It is a well-established fact that the
human genome possesses oncogenes that promote tumor formation as
well as tumor-suppressor genes that, instead, inhibit tumor
development. However, in addition to these, the potential role of
non-coding RNAs (ncRNAs) in tumor occurrence and development has
also been reported. ncRNAs represent one type of RNAs that do not
code for proteins; and although they makes up 90% of all RNAs in
the human body, their role has only been appreciated during the
past decade (2).
MicroRNAs (miRNAs/miRs) are a class of ncRNAs,
encoded by endogenous genes, that typically consist of 18–24
nucleotides. The process of miRNA synthesis starts when the
microprocessor complex, composed of Drosha and DGCR8, first
recognize and splice the miRNA's primary transcripts (pri-MiRNAs)
to produce precursor miRNAs (pre-miRNAs) which are basically
hairpin-like RNAs of about 70 bp (3). Exportin 5 then transports these
pre-miRNAs into the cytoplasm where they undergo cleavage by Dicer
ribonuclease to produce mature miRNAs (3), with the latter subsequently binding
to Argonaute (Ago) proteins to yield the RNA-induced silencing
complex (RISC). Eventually, on binding to the miRNA response
elements (MREs), this RISC complex silences target mRNAs. miRNAs
influence various biological processes of cells and hence, they
influence a number of cancer-related processes such as tumor
growth, cell death, metastasis, stemness, angiogenesis and
chemotherapy resistance.
One type of microRNA is the miR-200b family which
includes miR-200a, miR-200b, miR-200c, miR-429, and miR-141. Given
that this family affects many malignant phenotypes of tumors, this
review provides a summary of the functions of miR-200b-3p in
different types of tumors, including its regulatory mechanism.
In pubMed, Web of Science and other relative
databases, we searched the literature studies of 10 years
(2012–2022) with ‘miR-200b-3p’ as the key word. We selected the
articles that were related to tumors and identified the finding
concerning the mechanism of miR-200b-3p and tumor development and
the tumor microenvironment.
Mechanism of action of miR-200b-3p in
cancer
miR-200b-3p, along with other members of the miR-200
family, are generally considered to be part of the tumor-suppressor
gene group due to their suppressive effects on most tumors,
especially in inhibiting their metastasis. Although metastatic
inhibition can take place through several mechanisms, miR-200b-3p
is best known to exert this effect by inhibiting
epithelial-mesenchymal transition (EMT) through zinc finger
E-box-binding homeobox 1/2 (ZEB1/2). In addition, further studies
have suggested that this miRNA can also act on other genes such as
microfibril-associated glycoprotein 2 (MAGP2), mothers
against decapentaplegic homolog 2 (SMAD2) and high mobility
group box 3 (HMGB3) to silence their mRNAs, thereby
inhibiting tumor proliferation, apoptosis, invasion as well as
migration.
However, even though members of the miR-200b family
are known as a group of tumor-suppressor genes, some researchers
have pointed out that miR-200b-3p may even have oncogenic
functions. Indeed, miR-200b-3p can actually promote tumor
progression by downregulating a number of tumor suppressor genes,
including ATP binding cassette subfamily A member 1 (ABAC1),
large tumor suppressor kinase 2 (LATS2) and transcriptional
intermediary factor 1 γ (TIF1γ).
In addition, miR-200b-3p can also improve cancer
treatment by influencing tumor resistance to chemotherapy. Unlike
the case for tumor progression, most reports suggest that
miR-200b-3p can reduce tumor resistance to chemotherapy by
inhibiting the expression of downstream targets such as tubulin β 3
class III (TUBB3), AKT serine/threonine kinase 2
(AKT2), and fibronectin-1 (FN1).
miR-200b-3p function is dependent on cancer
type
miR-200b-3p can exert different functions depending
on the type of tumor. For instance, in most cases, it exhibits
tumor-suppressive effects and is therefore downregulated, with
examples of such tumors being colorectal cancer (CRC) (4–7),
hepatocellular carcinoma (HCC) (8–12),
pancreatic cancer (13,14), gastrointestinal stromal tumors
(15), breast cancer (16–18),
melanoma (19), glioblastoma
(20), glioma (21), thyroid carcinoma (22), cemento-ossifying fibroma (23), esophageal cancer (24,25),
oral squamous cell carcinoma (26), prostate cancer (27,28),
renal cell carcinoma (29),
bladder cancer (30), and
malignant mesothelioma (31).
However, as already noted, this microRNA can also be oncogenic in
various types of cancers, with examples of such tumors being
prostate cancer (32,33), lung cancer (34–38),
oral squamous cell carcinoma (39), esophageal squamous cell carcinoma
(40), breast cancer (41), colorectal cancer (42,43),
neck cancer (44),
cholangiocarcinoma (45), and
hepatocellular carcinoma (46).
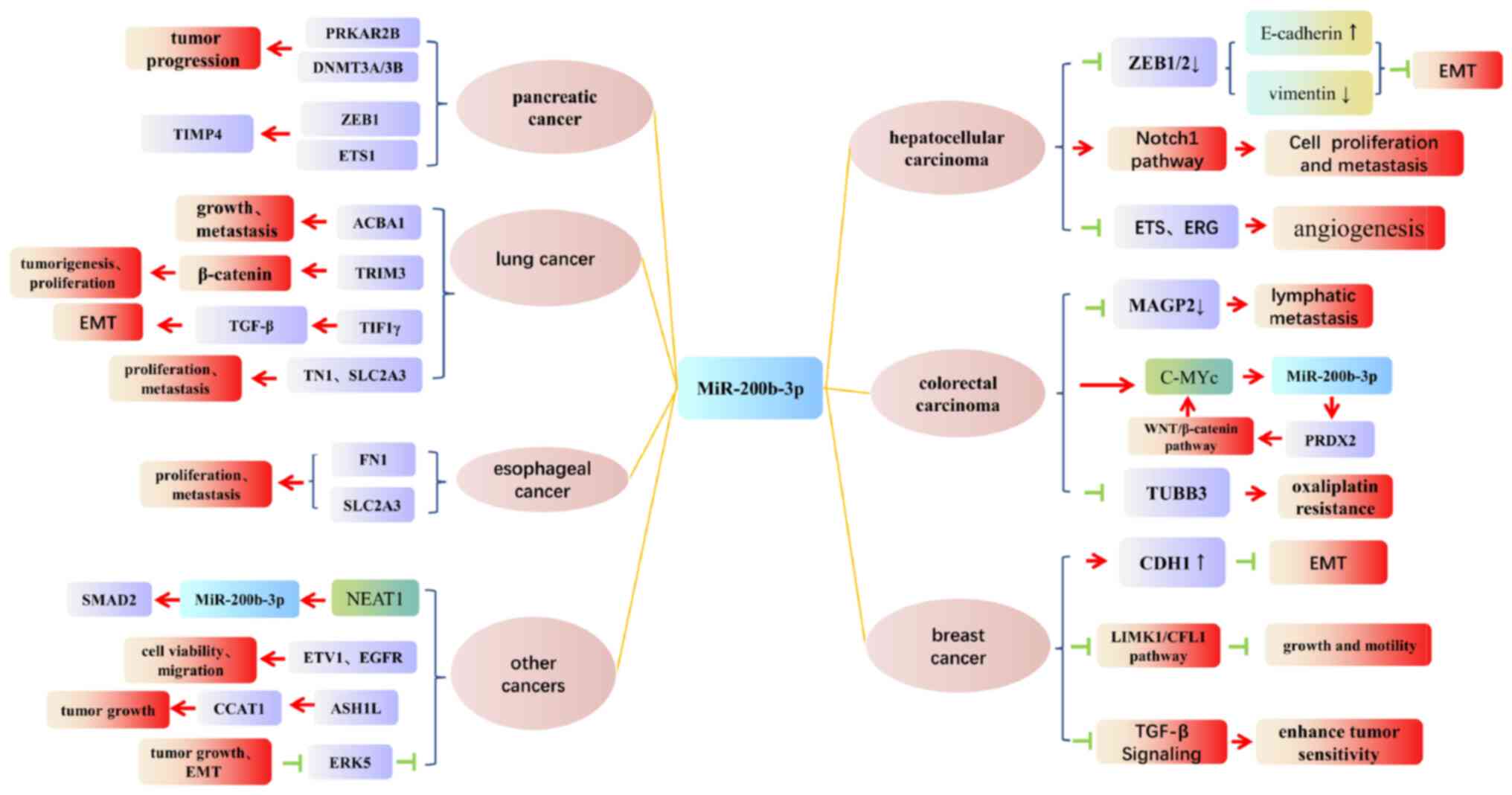
Table I and Fig. 1 summarize the effects of
miR-200b-3p dysregulation.
 | Table I.Functions and mechanism of
miR-200b-3p in various types of cancer. |
Table I.
Functions and mechanism of
miR-200b-3p in various types of cancer.
| Types of
cancer | Expression | Target gene | Pathway | Activity | (Refs.) |
|---|
| Anaplastic thyroid
carcinoma | Down | ASH1L | / | Inhibition of
proliferation | (22) |
| Bladder cancer | Down | ZEB1 | miR-200/ZEB
axis | Inhibition of
invasion and migration | (30) |
| Breast cancer | Down | TGF-β2 and
ZEB1 | TGF-β2/ZEB1
signaling axis | Inhibition of
invasion and migration; enhancement of chemotherapy resistance | (17) |
|
|
| LIMK1 | LIMK1/CFL1 | Inhibition of
proliferation, invasion and migration | (16) |
|
|
| RHOA and PRKCA | RHOGDI signaling
pathway | Inhibition of
invasion and migration | (18) |
| Colorectal
cancer | Down | TUBB3 | / | Enhancement of
chemotherapy resistance | (51) |
|
|
| Wnt1 | Wnt signaling
pathway | Inhibition of
proliferation | (4) |
|
|
| MAGP2 | / | Inhibition of
proliferation, invasion and migration | (5) |
|
|
| PRDX2 |
c-Myc/miR-200b-3p/PRDX2 pathway;
WNT/β-catenin pathway | Inhibition of
invasion and migration; enhancement of chemotherapy resistance | (6) |
|
|
| ZEB1 | miR-200/ZEB
axis | Inhibition of
invasion and migration | (7) |
| Esophageal
cancer | Down | SLC2A3 | / | Inhibition of
proliferation, invasion and migration | (25) |
|
|
| Fibronectin-1 | mTOR signaling
pathway | Inhibition of
proliferation, invasion and migration | (24) |
| Gastric
adenocarcinoma | Down | ETV1 and EGFR | / | Inhibition of
invasion and migration | (30) |
| Glioma | Down | HMGB3 | MAPK signaling
pathway | Inhibition of
proliferation, invasion and migration | (20) |
|
|
| / |
HIF1-α/VEGF/MMP9 | Inhibition of
proliferation, invasion and migration | (55) |
|
|
| ERK5 | / | Inhibition of
proliferation, invasion and migration | (21) |
| Hepatocellular
carcinoma | Down | ERG | / | Inhibition of
angiogenesis | (8) |
|
|
| Notch1 | / | Inhibition of
proliferation, invasion and migration | (9) |
|
|
| ZEB1/2 | miR-200/ZEB
axis | Inhibition of
invasion and migration | (10) |
|
|
| ZEB1 | miR-200/ZEB
axis | Inhibition of
invasion and migration | (12) |
|
|
| ZEB1 | miR-200/ZEB
axis | Inhibition of
invasion and migration | (50) |
| Lung cancer | Up | ABCA1 | / | Promote
proliferation, invasion and migration | (34) |
|
|
| LATS2 and
SOCS6 | / | Promote
proliferation, invasion and migration | (36) |
|
|
| TIF1γ | Wnt pathway | Promote invasion
and migration | (35) |
| Melanoma | Down | SMAD2 | TGF-β signaling
pathway | Inhibition of
invasion and migration | (19) |
| Osteosarcoma | Down | AKT2 | PI3K/Akt
pathway | Enhancement of
chemotherapy resistance | (56) |
|
|
| Fibronectin-1 | / | Inhibition of
proliferation; enhancement of chemotherapy resistance | (57) |
| Pancreatic
cancer | Down | Notch | Notch
signaling | Inhibition of
proliferation | (13) |
|
|
| ZEB1 | miR-200/ZEB
axis | Inhibition of
invasion and migration | (14) |
| Prostate
cancer | Down | PRKAR2B | / | Inhibition of
proliferation | (53) |
|
|
| DNMT3A/3B | PPARG2/AKAP12
axis | Inhibition of
proliferation | (27) |
|
| Up | TIMP4 | / | Promote
proliferation, invasion and migration | (33) |
miR-200b-3p and its functions in
hepatocellular carcinoma
For HCC, microRNAs can display tumor-suppressive
effects, with its most important mechanism of action being the
inhibition of epithelial-mesenchymal transition (EMT) by acting on
ZEB1/2. Indeed, ZEB1, as an important regulator in
tumors (47), promotes EMT by
downregulating or upregulating E-cadherin (48) and vimentin (49) respectively and it has been shown
that, by acting on both ZEB1 and ZEB2, miR-200b-3p
can silence their mRNAs to downregulate their expression, thus
suppressing EMT as well as reducing metastasis in HCC (10,12,50).
Interestingly, similar mechanisms have also been reported for CRC
(7), pancreatic cancer (14), breast carcinoma (17), and bladder cancer (30). In addition, Notch1, an
oncogene which regulates biological processes in most cancers, is
also targeted by miR-200b-3p (9).
In this case, the Notch signaling pathway can be activated when
miR-200b-3p is expressed at low levels and this can lead to both
tumor growth and metastasis in HCC. In fact, since miR-200b-3p can
also inhibit the expression of the endothelial erythroblast
transformation-specific (ETS)-related gene (ERG), its
downregulation and subsequent low expression can further promote
tumor progression by inducing angiogenesis in HCC (8).
miR-200b-3p and its effects in colorectal
carcinoma
Regarding CRC, in addition to ZEB1/2, the
c-Myc/miR-200b-3p/peroxiredoxin-2 (PRDX2) regulatory pathway
is also targeted by miR-200b-3p to inhibit cancer progression, with
this regulatory mechanism controlling the activation of c-Myc
through the WNT/β-catenin pathway (6). However, in CRC, reduced expression of
miR-200b-3p can disrupt this regulatory loop, resulting in the
activation of c-Myc and subsequent tumor metastasis (6), with miR-200b-3p even able to
influence the expression of Wnt1 within this pathway
(4). Low expression levels of the
miRNA can, in fact, further lead to the overexpression of
microfibril-associated glycoprotein 2 (MAGP2) which forms part of
the extracellular matrix and this process can be associated with
local lymphatic metastasis in CRC.
Finally, miR-200b-3p can cause CRC to become more
resistant to chemotherapeutic agents. In this context, Lv et
al suggested that disruptions to the abovementioned
c-Myc/miR-200b-3p/PRDX2 regulatory pathway due to reduced
miR-200b-3p expression can cause colorectal cancer to develop
greater resistance to oxaliplatin (6). Moreover, as an additional target of
miR-200b-3p, tubulin β 3 class III (TUBB3) can also be
involved in oxaliplatin resistance (51) and in this case, the downregulation
of miR-200b-3p can ultimately result in enhanced oxaliplatin
resistance by reducing the presence of TUBB3 in CRC
(51).
miR-200b-3p and its involvement in breast
cancer
For this type of cancer, in addition to
ZEB1/2, miR-200b-3p can also influence the expression of the
LIM domain kinase 1 (LIMK1) gene. More specifically, low
levels of the miRNA tend to reduce inhibition of the
LIMK1/phosphorylation of cofilin 1 (CFL1) pathway, thereby
promoting the proliferation and metastasis of breast cancer
(16). Furthermore, in
triple-negative breast cancer, miR-200b-3p can target the Rho
GDP-dissociation inhibitor (RHOGDI) signaling pathway to being
about increased CDH1 expression as well as EMT suppression
(18). In terms of treatment,
miR-200b-3p can also be helpful for inhibiting the transforming
growth factor (TGF)-β signaling pathway which lead to enhanced
tumor sensitivity to tamoxifen (17), and in this case, research has shown
that upregulation of miR-200b-3p can lead to improved pathologic
responses to preoperative chemotherapy which, in turn, is more
beneficial for implementing surgery in triple-negative breast
cancer (52).
Role of miR-200b-3p in prostate
cancer
For prostate cancer, the functions of miR-200b-3p
are less understood due to contrasting effects. Indeed, in some
instances, it has been shown to regulate tumor progression by
targeting the regulatory subunit RIIβ (PRKAR2B) and DNA
methyltransferase 3A/3B (DNMT3A/3B). The former, as an
oncogene (53), is not only
involved in adipogenesis but is also significantly upregulated in
metastatic lesions compared with primary tumors in prostate cancer
(53). In contrast, the protein
products of DNMT3A/3B are both enzymes that act on DNA
sequences to catalyze the methylation of CpG and by targeting
DNMT3A/3B, miR-200b-3p facilitates interactions between
peroxisome proliferator-activated receptor γ 2 (PPARG2) and
demethylated A-kinase anchoring protein 12 (AKAP12) gene promoter
to suppress the growth of prostate cancer (27).
However, Janiak et al found that, in prostate
cancer, miR-200b-3p could even regulate the level of tissue
inhibitor of metalloproteinase 4 (TIMP4) expression
(33), with this regulation being
mediated by ZEB1 and ETS proto-oncogene 1 (ETS1)
which represent two targets of miR-200b-3p (33). In addition, Samli et al
reported that, compared with parental cells, prostate cancer cells
that were resistant to paclitaxel displayed a significantly higher
expression of miR-200b-3p. Yet, even though the results of that
study indicated that miR-200b-3p could be involved in the
paclitaxel resistance of prostate cancer (54), the specific mechanism behind this
process remains unknown (54).
miR-200b-3p and its functions in lung
cancer
In the case of lung cancer, miR-200b-3p mainly acts
as an oncogene by regulating the expression of the ATP-binding
cassette transporter A member 1 (ABCA1) (34), the transcriptional intermediary
factor 1 γ (TIF1γ) (35),
the large tumor suppressor kinase 2 (LATS2) as well as the
suppressor of cytokine signaling 6 (SOCS6) (36), all of which are involved in tumor
progression. Among these, the tumor-suppressor gene ABCA1
encodes a transmembrane protein that transports cholesterol to the
outside of the cell and a study by Liu et al demonstrated
that, by acting on this gene, miR-200b-3p could encourage
non-small-cell lung cancer to grow and metastasize (34). Similarly, TIF1γ, also known
as tripartite motif-containing 33 (TRIM33), inhibits tumorigenesis
and proliferation by degrading β-catenin, a key component of the
Wnt pathway but when miR-200b-3p targets this gene, TGF-β-induced
EMT is promoted along with tumor invasion (35). Finally, even though both
LATS2 and SOCS6 act as tumor suppressors in various
tumors, the upstream miR-200b-3p can regulate their expression to
promote the proliferation of lung cancer as well as metastasis
(36).
Even though miR-200b-3p is known for its
tumor-suppressive effects in most studies, its ability to act as an
oncogene in lung cancer could actually be attributed to differences
in the molecular backgrounds of different tumors. For example,
according to The Cancer Genome Atlas (TCGA) database, TIF1γ
expression levels in lung cancer are higher than those in HCC or
CRC, and therefore, its inhibition under the influence of
miR-200b-3p may result in greater effects.
miR-200b-3p and its functions in
esophageal cancer
Regarding esophageal cancer, tumor progression is
regulated when miR-200b-3p act on both fibronectin-1 (FN1)
(24) as well as the solute
carrier family 2 member 3 (SLC2A3) (25). FN1 has been shown to enhance
tumor growth by activating the mTOR signaling pathway while
metastasis can be induced through the overexpression of genes such
as MMP2 that encode matrix metalloproteinases. Similarly,
SLC2A3, also referred to as glucose transporter 3
(GLUT3), is not only associated with proliferation and EMT
but it can even act as immune signatures in various cancer. In
esophageal cancer, the downregulation of miR-200b-3p causes both
FN1 and SLC2A3 to be overexpressed, thereby promoting
tumor proliferation and metastasis (24,25).
Role of miR-200b-3p in glioma
The hypoxic environment present in tumors causes a
key transcription factor known as the hypoxia-inducible factor-1
(HIF-1) to be produced. This allows interactions between
HIF1-α, vascular endothelial growth factor (VEGF) and
matrix metallopeptidase 9 (MMP9) which largely contribute to
tumor growth and metastasis (55);
but by inhibiting such interactions, miR-200b-3p suppresses both
tumorigenesis and invasion in glioblastoma multiforme (55). Moreover, miR-200b-3p also targets
the high mobility group box 3 (HMGB3) which regulates the
MAPK signaling pathway as well as the extracellular-regulated
protein kinase 5 (ERK5) which forms part of the mitogen
activated protein kinase (MAPK) family. For the first gene,
miR-200b-3p acts by regulating glioblastoma multiforme progression
(20) while in the latter case,
both the growth of tumors and the EMT process is inhibited when
miR-200b-3p suppresses ERK5 expression (21).
miR-200b-3p and its functions in other
cancers
The signal transductor SMAD family member 2 (SMAD2)
mediates a number of signaling pathways (19) and in melanoma, it is involved in
the nuclear enriched abundant transcript 1
(NEAT1)/miR-200b-3p/SMAD2 axis that promotes the progression of
tumors through EMT activation (19). On the other hand, in the case of
gastrointestinal stromal tumors (GISTs), Gyvyte et al
(15) pointed out that cell
viability and migration was reduced when miR-200b-3p downregulated
the expression of ETV1 and EGFR. Finally, in cancers
such as anaplastic thyroid carcinoma (ATC), miR-200b-3p can inhibit
the growth of tumors by silencing the ASH1L/CCAT1
axis (22) while for osteosarcoma,
the downregulation of miR-200b-3p mediates doxorubicin resistance
through its effects on AKT2, a member of the AKT family, as
well as on FN1 (56,57).
In summary, although miR-200b-3p acts as a tumor
suppressors in most cancers, it may also act as an oncogene in some
of them, depending on the tumor microenvironment. However, by
understanding its role and mode of action in different tumors,
miR-200b-3p can prove to be a useful biomarker for diagnostic
purposes or even for applications in targeted therapy.
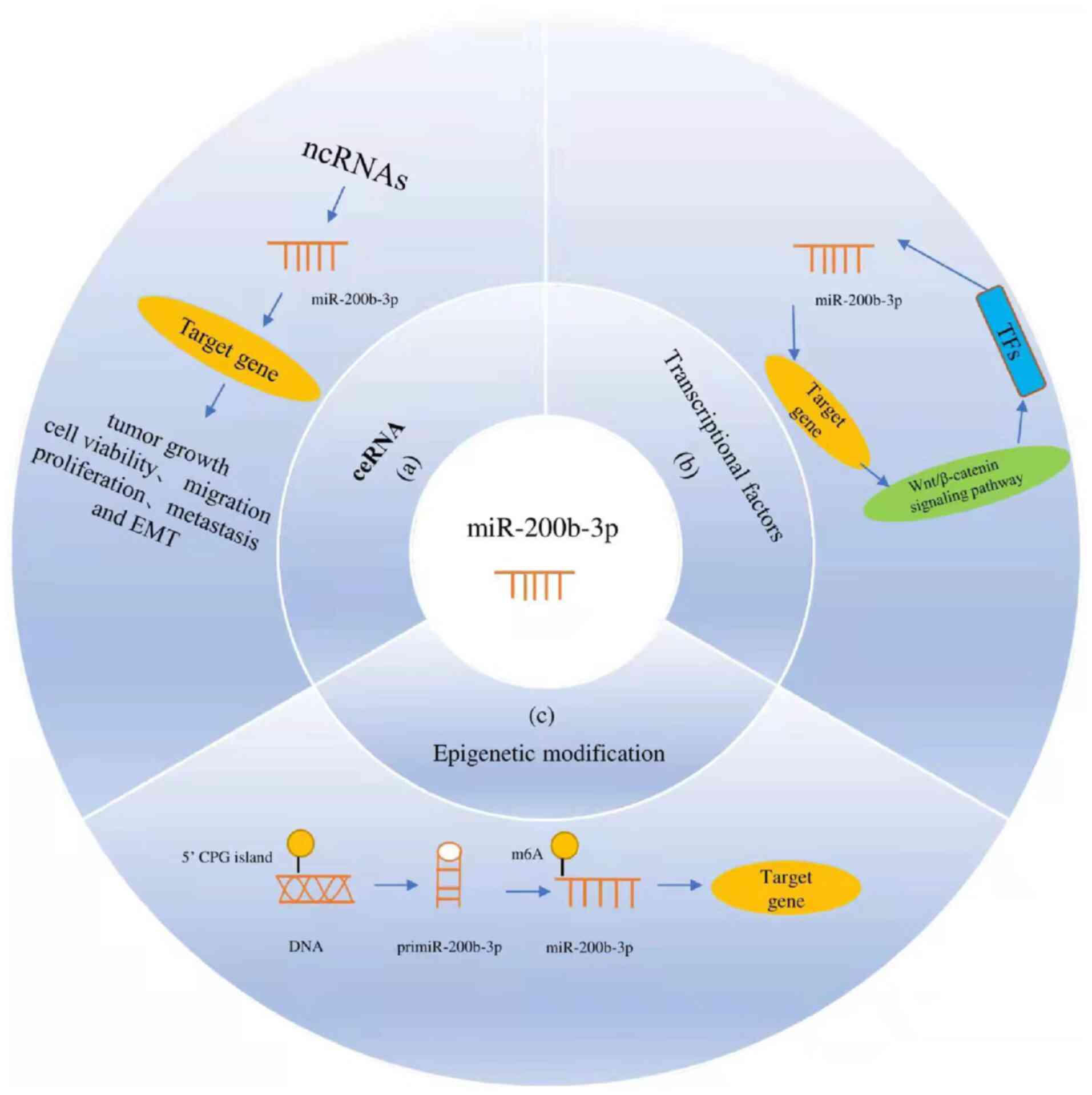
Mechanism of miR-200b-3p regulation
Being an miRNA, regulation of miR-200b-3p is
achieved by various mechanisms, of which the most important include
the competing endogenous RNA (ceRNA) mechanism, regulation at
transcription factor levels as well as epigenetic modifications
(Fig. 2).
Mechanism of ceRNA regulation
In our summary, lncRNA XIST is the most mentioned
ceRNA-based regulation of miR-200b-3p, and it can induce HCC as
well as CRC progression by inhibiting miR-200b-3p (7,10,11).
ZEB1-AS1 can also act as a ceRNA to inhibit miR-200b-3p as
reported by Liu et al (50), with similar inhibitory effects on
the miRNA observed in other cancers. For instance, H19 participates
in the metastasis of HCC by reducing miR-200b-3p levels (12). Similarly, in osteosarcoma, both
lncRNA MEG3 (56) and OIP5-AS1
(57) act upstream of miR-200b-3p
to promote tumor development, while in the case of esophageal
cancer, tumor progression occurs as LINC00667 (25) and LNC-ABCA12-3 (24) act on the miRNA. Finally, for
melanoma as well as breast cancer, miR-200b-3p is respectively
targeted by NEAT1 and LINC00894-002 that inhibit its functions,
thereby promoting tumor progression (17,19).
In addition to the above lncRNA, circRNAs can also
be an important part of the ceRNA-based mechanisms, with studies
showing that circPTK2 (35),
circ_103820 (36) as well as
circKDM4C (30) can exert
inhibitory effects on miR-200b-3p, resulting in the development of
breast and bladder cancer.
Transcription factor-based
regulation
The ZEB/miR-200 feedback loop is the most common
transcription factor that regulates miR-200b-3p, and this mutual
inhibitory relationship can enhance EMT progression in various
cancers (48). P53, being a
well-known tumor-suppressor gene, can also upregulate all genes
from the microRNA-200 family (58)
while, c-Myc, as part of the c-Myc/miR-200b-3p/PRDX2 regulatory
loop as mentioned earlier, can reduce miR-200b-3p levels (6). Finally, for androgen-independent
prostate cancer (AIPC), it has been reported that p73 is positively
correlated with miR-200b-3p (28).
Epigenetic modification
Methylation is the most common epigenetic
modification regulating miR-200b-3p, and it has been reported that
the methylation of its adenosine at the N6-position can interfere
with its inhibitory effects on downstream targets by preventing the
miRNA/3UTRmRNA duplex from being generated (58,59).
In addition, studies have shown that high methylation of CpG
islands in DNA can promote EMT by silencing genes belonging to the
miR-200 family (60).
Conclusion
In summary, miR-200b-3p is an miRNA closely related
to human tumors. By inhibiting the expression of downstream mRNAs,
miR-200b-3p participates in regulating various tumor processes.
Among them, the most noteworthy mechanism is that miR-200b-3p
inhibits the tumor EMT process through ZEB1/2 interaction. ZEB1/2
is an important factor in the EMT process, and it can affect the
tumor EMT process by influencing downstream molecules such as
E-cadherin and vimentin. miR-200b-3p and ZEB1/2 can inhibit the
expression of each other, respectively. The disruption of this
delicate balance may play a key role in the EMT process, which is
helpful to our understanding of the mechanism of EMT. In addition,
miR-200b-3p can also regulate tumor proliferation, apoptosis,
invasion, migration and chemotherapy resistance through the
WNT/β-catenin pathway, MAPK signaling pathway, PI3K/Akt pathway and
others.
In most cancers, miR-200b-3p always shows tumor
inhibition, but due to the difference in the tumor
microenvironment, miR-200b-3p shows differential functions in
different tumors. Especially in lung cancer, in the statistics of
this review, several reports have pointed out that miR-200b-3p can
promote the occurrence and development of lung cancer. However, we
summarized some studies and found that even in the same tumor,
miR-200b-3p may show different dysregulations and functions. This
may be because they belong to different tumor stages or subtypes,
and therefore have different microenvironments and molecular
backgrounds.
In addition to the function of miR-200b-3p in
tumors, we also summarized the regulatory mechanism of miR-200b-3p
expression. miR-200b-3p is mainly regulated by the ceRNA mechanism,
transcription factor-involved regulation and epigenetic
modification, while the most important one is the ceRNA
mechanism.
However, the specific mechanism by which miR-200b-3p
plays a role in tumor, as well as its own regulatory mechanism, is
not well understood. Many questions remain to be answered before it
can be applied clinically. For example, we need to use different
analytic strategies for different tumors because of the different
regulatory patterns of miR-200b-3p in various tumors.
By further studying its mechanism, miR-200b-3p may
play a valuable role in the diagnosis and treatment of tumors. The
expression of miR-200b-3p can help us determine the type and
malignancy of the tumor. In the future, with the development of
targeted therapy, miR-200b-3p can also be used as a potential
therapeutic target to provide effective treatment of tumors.
Acknowledgements
Not applicable.
Funding
This research was supported by the Natural Science Foundation of
Zhejiang Province (China) (LY18H160041).
Availability of data and materials
All information provided in this review is
documented by relevant references.
Authors' contributions
SC and YT were the guarantors and designed the
study. HY and ZS participated in the acquisition, analysis, and
interpretation of the data. YG and WG drafted the initial
manuscript after interpretation of the literature data. ST revised
the article critically for important intellectual content in light
of the literature findings. This manuscript is a review of the
literature and thus no novel data were collected.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kozomara A, Birgaoanu M and
Griffiths-Jones S: miRBase: From microRNA sequences to function.
Nucleic Acids Res. 47(D1): D155–D162. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ha M and Kim VN: Regulation of microRNA
biogenesis. Nat Rev Mol Cell Biol. 15:509–524. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen L, Wang X, Zhu Y, Zhu J and Lai Q:
miR-200b-3p inhibits proliferation and induces apoptosis in
colorectal cancer by targeting Wnt1. Mol Med Rep. 18:2571–2580.
2018.PubMed/NCBI
|
|
5
|
Feifei W, Hui G, Ruiqiang Z, Qunxiang J
and Yu'an X: MAGP2, a component of extracellular matrix, is
upregulated in colorectal cancer and negatively modulated by
miR-200b-3p. Technol Cancer Res Treat. 18:15330338198707772019.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lv Z, Wei J, You W, Wang R, Shang J, Xiong
Y, Yang H, Yang X and Fu Z: Disruption of the
c-Myc/miR-200b-3p/PRDX2 regulatory loop enhances tumor metastasis
and chemotherapeutic resistance in colorectal cancer. J Transl Med.
15:2572017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen DL, Chen LZ, Lu YX, Zhang DS, Zeng
ZL, Pan ZZ, Huang P, Wang FH, Li YH, Ju HQ and Xu RH: Long
noncoding RNA XIST expedites metastasis and modulates
epithelial-mesenchymal transition in colorectal cancer. Cell Death
Dis. 8:e30112017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Moh-Moh-Aung A, Fujisawa M, Ito S,
Katayama H, Ohara T, Ota Y, Yoshimura T and Matsukawa A: Decreased
miR-200b-3p in cancer cells leads to angiogenesis in HCC by
enhancing endothelial ERG expression. Sci Rep. 10:104182020.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Qiu W, Wang Z, Chen R, Shi H, Ma Y and
Zhou H, Li M, Li W, Chen H and Zhou H: Xiaoai jiedu recipe
suppresses hepatocellular carcinogenesis through the
miR-200b-3p/Notch1 axis. Cancer Manag Res. 12:11121–11131. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu L, Jiang H, Pan H and Zhu X: LncRNA
XIST promotes liver cancer progression by acting as a molecular
sponge of miR-200b-3p to regulate ZEB1/2 expression. J Int Med Res.
49:30006052110162112021.PubMed/NCBI
|
|
11
|
Liu WG and Xu Q: Long non-coding RNA XIST
promotes hepatocellular carcinoma progression by sponging
miR-200b-3p. Eur Rev Med Pharmacol Sci. 23:9857–9862.
2019.PubMed/NCBI
|
|
12
|
Huang Z, Chu L, Liang J, Tan X, Wang Y,
Wen J, Chen J, Wu Y, Liu S, Liao J, et al: H19 promotes HCC bone
metastasis through reducing osteoprotegerin expression in a protein
phosphatase 1 catalytic subunit alpha/p38 mitogen-activated protein
kinase-dependent manner and sponging microRNA 200b-3p. Hepatology.
74:214–232. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nwaeburu CC, Abukiwan A, Zhao Z and Herr
I: Quercetin-induced miR-200b-3p regulates the mode of
self-renewing divisions in pancreatic cancer. Mol Cancer.
16:232017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gui Z, Luo F, Yang Y, Shen C, Li S and Xu
J: Oridonin inhibition and miR-200b-3p/ZEB1 axis in human
pancreatic cancer. Int J Oncol. 50:111–120. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gyvyte U, Lukosevicius R, Inciuraite R,
Streleckiene G, Gudoityte G, Bekampyte J, Valentini S, Salteniene
V, Ruzgys P, Satkauskas S, et al: The role of miR-375-3p and
miR-200b-3p in gastrointestinal stromal tumors. Int J Mol Sci.
21:51512020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li D, Wang H, Song H, Xu H, Zhao B, Wu C,
Hu J, Wu T, Xie D, Zhao J, et al: The microRNAs miR-200b-3p and
miR-429-5p target the LIMK1/CFL1 pathway to inhibit growth and
motility of breast cancer cells. Oncotarget. 8:85276–85289. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang X, Wang M, Sun H, Zhu T and Wang X:
Downregulation of LINC00894-002 contributes to tamoxifen resistance
by enhancing the TGF-β signaling pathway. Biochemistry (Mosc).
83:603–611. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rhodes LV, Martin EC, Segar HC, Miller DF,
Buechlein A, Rusch DB, Nephew KP, Burow ME and Collins-Burow BM:
Dual regulation by microRNA-200b-3p and microRNA-200b-5p in the
inhibition of epithelial-to-mesenchymal transition in
triple-negative breast cancer. Oncotarget. 6:16638–16652. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou WJ, Wang HY, Zhang J, Dai HY, Yao ZX,
Zheng Z, Meng-Yan S and Wu K: NEAT1/miR-200b-3p/SMAD2 axis promotes
progression of melanoma. Aging (Albany NY). 12:22759–22775.
2020.PubMed/NCBI
|
|
20
|
Liu J, Wang L and Li X: HMGB3 promotes the
proliferation and metastasis of glioblastoma and is negatively
regulated by miR-200b-3p and miR-200c-3p. Cell Biochem Funct.
36:357–365. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu J, Cui H, Zhu Z and Wang L:
MicroRNA-200b-3p suppresses epithelial-mesenchymal transition and
inhibits tumor growth of glioma through down-regulation of ERK5.
Biochem Biophys Res Commun. 478:1158–1164. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xu B, Qin T, Yu J, Giordano TJ, Sartor MA
and Koenig RJ: Novel role of ASH1L histone methyltransferase in
anaplastic thyroid carcinoma. J Biol Chem. 295:8834–8845. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pereira TDSF, Brito JAR, Guimarães ALS,
Gomes CC, de Lacerda JCT, de Castro WH, Coimbra RS, Diniz MG and
Gomez RS: MicroRNA profiling reveals dysregulated microRNAs and
their target gene regulatory networks in cemento-ossifying fibroma.
J Oral Pathol Med. 47:78–85. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ma J, Xiao Y, Tian B, Chen S, Zhang B, Wu
J, Wu Z, Li X, Tang J, Yang D, et al: Long noncoding RNA
lnc-ABCA12-3 promotes cell migration, invasion, and proliferation
by regulating fibronectin 1 in esophageal squamous cell carcinoma.
J Cell Biochem. 121:1374–1387. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pan J and Zang Y: LINC00667 promotes
progression of esophageal cancer cells by regulating
miR-200b-3p/SLC2A3 axis. Dig Dis Sci. Jul 27–2021.(Epub ahead of
print). doi: 10.1007/s10620-021-07145-5. View Article : Google Scholar
|
|
26
|
Masaoka T, Shinozuka K, Ohara K, Tsuda H,
Imai K and Tonogi M: Bioinformatics analysis of dysregulated
exosomal microRNAs derived from oral squamous cell carcinoma cells.
J Oral Sci. 63:174–178. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li F, Lu T, Liu D, Zhang C, Zhang Y and
Dong F: Upregulated PPARG2 facilitates interaction with
demethylated AKAP12 gene promoter and suppresses proliferation in
prostate cancer. Cell Death Dis. 12:5282021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
He M, Liu Y, Deng X, Qi S, Sun X, Liu G,
Liu Y, Liu Y and Zhao M: Down-regulation of miR-200b-3p by low p73
contributes to the androgen-independence of prostate cancer cells.
Prostate. 73:1048–1056. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dong Y, Zhai W and Xu Y: Bioinformatic
gene analysis for potential biomarkers and therapeutic targets of
diabetic nephropathy associated renal cell carcinoma. Transl Androl
Urol. 9:2555–2571. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ma X, Ying Y, Sun J, Xie H, Li J, He L,
Wang W, Chen S, Shen H, Yi J, et al: circKDM4C enhances bladder
cancer invasion and metastasis through miR-200bc-3p/ZEB1 axis. Cell
Death Discov. 7:3652021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Micolucci L, Akhtar MM, Olivieri F, Rippo
MR and Procopio AD: Diagnostic value of microRNAs in asbestos
exposure and malignant mesothelioma: Systematic review and
qualitative meta-analysis. Oncotarget. 7:58606–58637. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pełka K, Klicka K, Grzywa TM, Gondek A,
Marczewska JM, Garbicz F, Szczepaniak K, Paskal W and Włodarski PK:
miR-96-5p, miR-134-5p, miR-181b-5p and miR-200b-3p heterogenous
expression in sites of prostate cancer versus benign prostate
hyperplasia-archival samples study. Histochem Cell Biol.
155:423–433. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Janiak M, Paskal W, Rak B, Garbicz F,
Jarema R, Sikora K and Włodarski P: TIMP4 expression is regulated
by miR-200b-3p in prostate cancer cells. APMIS. 125:101–105. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu K, Zhang W, Tan J, Ma J and Zhao J:
MiR-200b-3p functions as an oncogene by targeting ABCA1 in lung
adenocarcinoma. Technol Cancer Res Treat. 18:15330338198925902019.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang L, Tong X, Zhou Z, Wang S, Lei Z,
Zhang T, Liu Z, Zeng Y, Li C, Zhao J, et al: Circular RNA
hsa_circ_0008305 (circPTK2) inhibits TGF-β-induced
epithelial-mesenchymal transition and metastasis by controlling
TIF1γ in non-small cell lung cancer. Mol Cancer. 17:1402018.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chi Y, Zheng W, Bao G, Wu L, He X, Gan R,
Shen Y, Yin X and Jin M: Circular RNA circ_103820 suppresses lung
cancer tumorigenesis by sponging miR-200b-3p to release LATS2 and
SOCS6. Cell Death Dis. 12:1852021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang Y, Xu YM, Zou YQ, Lin J, Huang B, Liu
J, Li J, Zhang J, Yang WM, Min QH, et al: Identification of
differential expressed PE exosomal miRNA in lung adenocarcinoma,
tuberculosis, and other benign lesions. Medicine (Baltimore).
96:e83612017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xie K, Wang C, Qin N, Yang J, Zhu M, Dai
J, Jin G, Shen H, Ma H and Hu Z: Genetic variants in regulatory
regions of microRNAs are associated with lung cancer risk.
Oncotarget. 7:47966–47974. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sun G, Cao Y, Wang P, Song H, Bie T, Li M
and Huai D: miR-200b-3p in plasma is a potential diagnostic
biomarker in oral squamous cell carcinoma. Biomarkers. 23:137–141.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ishibashi O, Akagi I, Ogawa Y and Inui T:
MiR-141-3p is upregulated in esophageal squamous cell carcinoma and
targets pleckstrin homology domain leucine-rich repeat protein
phosphatase-2, a negative regulator of the PI3K/AKT pathway.
Biochem Biophys Res Commun. 501:507–513. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Amorim M, Lobo J, Fontes-Sousa M,
Estevão-Pereira H, Salta S, Lopes P, Coimbra N, Antunes L, Palma de
Sousa S, Henrique R and Jerónimo C: Predictive and prognostic value
of selected MicroRNAs in luminal breast cancer. Front Genet.
10:8152019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Guo L, Yang G, Kang Y, Li S, Duan R, Shen
L, Jiang W, Qian B, Yin Z and Liang T: Construction and analysis of
a ceRNA network reveals potential prognostic markers in colorectal
cancer. Front Genet. 11:4182020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Della Vittoria Scarpati G, Calura E, Di
Marino M, Romualdi C, Beltrame L, Malapelle U, Troncone G, De
Stefano A, Pepe S, De Placido S, et al: Analysis of differential
miRNA expression in primary tumor and stroma of colorectal cancer
patients. Biomed Res Int. 2014:8409212014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Patil S and Warnakulasuriya S: Blood-based
circulating microRNAs as potential biomarkers for predicting the
prognosis of head and neck cancer-a systematic review. Clin Oral
Investig. 24:3833–3841. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Shen L, Chen G, Xia Q, Shao S and Fang H:
Exosomal miR-200 family as serum biomarkers for early detection and
prognostic prediction of cholangiocarcinoma. Int J Clin Exp Pathol.
12:3870–3876. 2019.PubMed/NCBI
|
|
46
|
Livingstone MC, Johnson NM, Roebuck BD,
Kensler TW and Groopman JD: Profound changes in miRNA expression
during cancer initiation by aflatoxin B1 and their
abrogation by the chemopreventive triterpenoid CDDO-Im. Mol
Carcinog. 56:2382–2390. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Usman S, Waseem NH, Nguyen TKN, Mohsin S,
Jamal A, Teh MT and Waseem A: Vimentin is at the heart of
epithelial mesenchymal transition (EMT) mediated metastasis.
Cancers (Basel). 13:49852021. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhang P, Sun Y and Ma L: ZEB1: At the
crossroads of epithelial-mesenchymal transition, metastasis and
therapy resistance. Cell Cycle. 14:481–487. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Qin Y, Yu J, Zhang M, Qin F and Lan X:
ZEB1 promotes tumorigenesis and metastasis in hepatocellular
carcinoma by regulating the expression of vimentin. Mol Med Rep.
19:2297–2306. 2019.PubMed/NCBI
|
|
50
|
Liu J, Cao L, Meng J, Li Y, Deng P, Pan P,
Hu C and Yang H: The fibrotic microenvironment promotes the
metastatic seeding of tumor cells into the lungs via mediating the
ZEB1-AS1/miR-200b-3p/ ZEB1 signaling. Cell Cycle. 19:2701–2719.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wu YZ, Lin HY, Zhang Y and Chen WF:
miR-200b-3p mitigates oxaliplatin resistance via targeting TUBB3 in
colorectal cancer. J Gene Med. 22:e31782020. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kolacinska A, Morawiec J, Fendler W,
Malachowska B, Morawiec Z, Szemraj J, Pawlowska Z, Chowdhury D,
Choi YE, Kubiak R, et al: Association of microRNAs and pathologic
response to preoperative chemotherapy in triple negative breast
cancer: Preliminary report. Mol Biol Rep. 41:2851–2857. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Xia L, Han Q, Chi C, Zhu Y, Pan J, Dong B,
Huang Y, Xia W, Xue W and Sha J: Transcriptional regulation of
PRKAR2B by miR-200b-3p/200c-3p and XBP1 in human prostate cancer.
Biomed Pharmacother. 124:1098632020. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Samli H, Samli M, Vatansever B, Ardicli S,
Aztopal N, Dincel D, Sahin A and Balci F: Paclitaxel resistance and
the role of miRNAs in prostate cancer cell lines. World J Urol.
37:1117–1126. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Doğanlar O, Doğanlar ZB, Delen E and Doğan
A: The role of melatonin in angio-miR-associated inhibition of
tumorigenesis and invasion in human glioblastoma tumour spheroids.
Tissue Cell. 73:1016172021. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhu KP, Zhang CL, Ma XL, Hu JP, Cai T and
Zhang L: Analyzing the interactions of mRNAs and ncRNAs to predict
competing endogenous RNA networks in osteosarcoma chemo-resistance.
Mol Ther. 27:518–530. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Kun-Peng Z, Chun-Lin Z, Xiao-Long M and
Lei Z: Fibronectin-1 modulated by the long noncoding RNA
OIP5-AS1/miR-200b-3p axis contributes to doxorubicin resistance of
osteosarcoma cells. J Cell Physiol. 234:6927–6939. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Jones M and Lal A: MicroRNAs, wild-type
and mutant p53: More questions than answers. RNA Biol. 9:781–791.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Briand J, Sérandour AA, Nadaradjane A,
Bougras-Cartron G, Heymann D, Ory B, Vallette FM and Cartron PF:
N6-adenosine methylation of miRNA-200b-3p influences its
functionality and is a theranostic tool. Mol Ther Nucleic Acids.
22:72–83. 2020.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Davalos V, Moutinho C, Villanueva A, Boque
R, Silva P, Carneiro F and Esteller M: Dynamic epigenetic
regulation of the microRNA-200 family mediates epithelial and
mesenchymal transitions in human tumorigenesis. Oncogene.
31:2062–2074. 2012. View Article : Google Scholar : PubMed/NCBI
|