Introduction
Colorectal cancer (CRC) is currently the third most
common cause of cancer-related mortality in the economically
developed world and is on track to increase in ranking in the
coming decades (1). Surgical
resection in combination with systemic chemotherapy offers the only
hope of cure or long-term survival for patients with CRC. However,
the disease recurs in ~30% of patients and better treatment options
are required to improve prognosis (2). Only a small number of specific
diagnostic or therapeutic tools are currently available, to a large
part due to the currently limited understanding of the molecular
pathogenesis of the disease. Although certain molecular targeted
therapies have proven efficacious in CRC (3–7),
there is an urgent requirement to identify novel therapeutic
targets. Furthermore, as CRC has a high relapse rate even early
after radical resection, there is a requirement to identify
additional biomarkers that may complement those currently available
to predict early postoperative recurrence and poor prognosis for
patients with CRC (3,5,6,8).
Previous studies by our group reported that
sarcopenia is an independent unfavorable prognostic factor after
curative resection in patients with CRC (9,10).
Sarcopenia, defined as a decrease in muscle mass associated with
aging and disease, is common in cancer patients (11), and other studies also indicated
that it is a poor prognostic factor for various types of cancer
(10,12–14).
Sarcopenia is associated with decreased survival in patients with
CRC undergoing curative resection (9), suggesting that understanding the
molecular events underlying skeletal muscle degradation may
identify potential novel therapeutic targets for these
patients.
One molecule associated with the regulation of
skeletal muscle mass is activin A, a member of the transforming
growth factor-β (TGF-β) family of proteins. In addition to
promoting skeletal muscle degradation and atrophy, activin A
displays an array of biological activities (15). Under physiological conditions,
activin A exerts its effects on cells by binding to type II
receptors, which induces its dimerization with type I receptors.
Once engaged, the activated type I receptor complex, which has
serine/threonine kinase activity, phosphorylates SMAD2/3 and
recruits SMAD4, the main signal transducers of the TGF-β family
receptors. The SMAD complex then translocates to the nucleus where
it promotes transcription of a panel of genes involved in the
regulation of cell development and proliferation, including muscle
catabolism (16).
Activin A is expressed and secreted by a number of
human cancer cell lines (17) and
its overexpression has been associated with poor prognosis in
various malignant tumor types, including esophageal adenocarcinoma,
lung cancer and gastric cancer (18–22).
In the present study, activin A expression was determined in CRC
tissues and its association with skeletal muscle mass and its
prognostic significance were examined. In addition, the mechanisms
underlying the involvement of activin A in CRC were explored in
vitro.
Materials and methods
Patients and tissue samples
The study population consisted of 157 patients with
CRC who underwent surgical resection at the Department of
Gastroenterological Surgery, Kumamoto University Hospital
(Kumamoto, Japan) between January 2008 and December 2012. The mean
observation period for the cohort was 57 months (range, 1–91
months). The clinical characteristics of the 157 patients are
summarized in Table I. This study
included 83 males and 74 females ranging in age from 34 to 86
years. The patients underwent imaging examination, such as
colonoscopy and enhanced computed tomography, for CRC diagnosis and
staging prior to surgery. The diagnosis was pathologically
confirmed using biopsy specimens. Patients who had received
preoperative chemotherapy or emergency surgery were excluded. CRC
tissue or paired normal epithelial tissue was obtained at the time
of surgical resection, snap-frozen and stored at −80°C until use.
The present retrospective, non-interventional, observational study
was approved by the institutional ethics committee of Kumamoto
University Hospital (14 June 2019/approval no. 1047) and performed
in accordance with the Declaration of Helsinki from 1975.
 | Table I.Characteristics of the patients
(n=157). |
Table I.
Characteristics of the patients
(n=157).
| Factor | Value |
|---|
| Age, years | 64 (34–86) |
| Sex |
|
|
Male | 83 |
|
Female | 74 |
| Body mass index,
kg/m2 | 22.6
(15.7-34.2) |
| SMI,
cm2/m2 | 47.0
(28.4-85.0) |
| Location |
|
|
Colon | 113 |
|
Rectum | 44 |
| Tumor stage |
|
| T1 | 24 |
| T2 | 28 |
| T3 | 73 |
| T4 | 32 |
| Lymph node
metastasis |
|
|
Present | 59 |
|
Absent | 98 |
| Lymphatic
invasion |
|
|
Present | 51 |
|
Absent | 106 |
| Venous
invasion |
|
|
Present | 67 |
|
Absent | 90 |
| pStage |
|
| I | 33 |
| II | 49 |
|
III | 39 |
| IV | 36 |
| CEA, ng/ml | 24.7
(0.6-18021) |
| CA19-9, U/ml | 39.7
(0.6-34708) |
Validation analysis in The Cancer
Genome Atlas (TCGA) database
To validate the association between activin A
expression and prognosis for patients with CRC, the activin
expression data and related clinical information of patients with
CRC were obtained from the TCGA database (http://www.cbioportal.org). The patients with CRC in
the TCGA dataset were divided into two groups according to the
median activin A expression. The cumulative overall survival (OS)
rate of the patients was determined using Kaplan-Meier survival
analysis with a log-rank test.
Cell lines and cell culture
The human CRC cell lines LoVo and SW480 were
purchased from RIKEN Bioresource Center Cell Bank and the Japanese
Collection of Research Bioresource Cell Bank, respectively. LoVo
and SW480 cells were cultured in Ham's and RPMI media (both from
Wako Pure Chemical Industries, Ltd.), respectively, supplemented
with 10% fetal bovine serum (Mediatech, Inc.). Cells were cultured
at 37°C in a humidified atmosphere with 5% CO2 and were
confirmed to be negative for mycoplasma infection prior to use.
Measurement of skeletal muscle
area
The skeletal muscle area was retrospectively
measured on preoperative computed tomography scans at the third
lumbar vertebra (L3) level in the inferior direction with the
patient in the supine position. In brief, a three-dimensional image
analysis system (Volume Analyzer SYNAPSE VINCENT; Fujifilm Medical)
was used to measure pixels using a window width of −30 to 150 HU to
delineate the muscle compartments and compute the cross-sectional
area of each in centimeters squared (cm2). The
cross-sectional area of the muscle (cm2) at the L3 level
computed from each image was normalized by the square of the height
(m2) to obtain the skeletal muscle index (SMI) expressed
in cm2/m2 (9,10).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from frozen tissue samples
or CRC cell lines using TRIzol (Invitrogen; Thermo Fisher
Scientific, Inc.) and the concentration of purified RNA was
measured by comparing absorbance at 260 nm (A260) and A280 using a
Nanodrop® 2000 spectrophotometer (Thermo Fisher
Scientific, Inc.). cDNA was generated from total RNA using a
ReverTra Ace qPCR RT kit (Toyobo Life Science) according to the
manufacturer's protocol and was subsequently used as a PCR
template. Real-time q-PCR was performed as described previously
(21). mRNA levels were measured
in technical duplicates and the relative level of activin A mRNA
was calculated as the fold change relative to β-actin (ACTB) mRNA.
The samples were quantified using the 2−ΔΔCq method
(23). The primers used were as
follows: Activin A (INHBA) forward, 5′-CCTCGGAGATCATCACGTTT-3′ and
reverse, 5′-CCCTTTAAGCCCACTTCCTC-3′; and ACTB forward,
5′-ATTGGCAATGAGCGGTTC-3′ and reverse, 5′-CGTGGATGCCACAGGACT-3′.
Immunohistochemical (IHC)
staining
CRC and normal epithelial samples were
formalin-fixed and paraffin-embedded. Blocks were cut into 3-µm
sections, which were deparaffinized and rehydrated. Activin A
antigen was retrieved by autoclaving in a pH 9 buffer solution for
15 min. Subsequently, the sections were incubated overnight at 4°C
with goat anti-activin A antibody (1:100 dilution; cat. no. A1594;
MilliporeSigma). The sections were washed in PBS and incubated with
horseradish peroxidase-conjugated mouse anti-goat secondary
antibody (1:50 dilution; cat. no. K8000; EnVision goat; Dako;
Agilent Technologies, Inc.) at room temperature for 30 min. Color
development was achieved by the addition of 3,3′-diaminobenzidine
[Wako Tablet; cat. no. 040-27001 (5 mg); Dako; Agilent
Technologies, Inc.] followed by counterstaining with
hematoxylin.
Cell transfection
A total of two activin A-specific small interfering
RNAs (siRNAs; siActivin A #1 and #2; Silencer Select s7434 and
s7436; Thermo Fisher Scientific, Inc.) and a negative control siRNA
(siCtrl, Stealth RNAi; Invitrogen; Thermo Fisher Scientific, Inc.)
were employed. Pilot experiments were performed to determine the
optimal siRNA concentration (10 µM) for inhibition of activin A
expression to <30% of the levels in siCtrl-transfected cells.
Cells were seeded in 6-well plates at a density of 105
cells/well in 2.5 ml medium and incubated for 24 h. The cells were
then transfected with 10 µM siActivin A or siCtrl using
Lipofectamine® RNAiMAX Transfection Reagent (Thermo
Fisher Scientific, Inc.) in accordance with the manufacturer's
protocol. After 48 h of transfection, the supernatant was removed,
the cells were washed with PBS and the experiments were
performed.
Cell proliferation assay
Cell proliferation was measured using a Cell
Counting Kit-8 (CCK-8; Dojindo Molecular Technologies, Inc.)
according to the manufacturer's protocol. LoVo and SW480 cells were
seeded in 96-well plates at a density of 3.0×103
cells/well in 100 µl medium and incubated overnight at 37°C.
Aliquots of 10 µl/well CCK-8 solution were then added to the cells
after 0, 24, 48, 72 or 96 h of incubation and the plates were
incubated for an additional 90 min. The absorbance at 450 nm was
then read using a microplate reader (SPECTRAmax PLUS 384 microplate
spectrophotometer; Scientific Equipment Source). Each experiment
was performed in triplicate.
Invasion assay
Cell invasion was measured assay using BioCoat
Matrigel invasion chambers (24-well plates, 8-µm pore size; BD
Biosciences) according to the manufacturer's protocol. In brief,
LoVo and SW480 cells were resuspended in medium at a concentration
of 105 cells/ml and 500 µl of the cell suspension was
placed in the upper chambers. The same medium supplemented with 10%
fetal bovine serum (FBS; Mediatech) was placed in the lower chamber
and the plates were incubated for 22 h. The cells on the upper
surface of the membrane were then removed using a cotton swab and
the cells on the lower surface were fixed with 100% ice-cold
methanol for 2 min, followed by staining with toluidine blue for 2
min at room temperature. The membrane was rinsed with water and
examined using a microscope (MRP-3001; R&D Systems, Inc.). The
number of invaded cells was counted in five microscopic fields per
membrane (magnification, ×40).
Migration assay
Migration was measured using 6-well plates coated
with 200 µl/well Matrigel® (BD Biosciences). LoVo and
SW480 cells were resuspended at a concentration of
4.0×104 cells/ml in each medium, plated at 200 µl/well
and allowed to adhere for 12 h. The plates were then imaged with a
KEYENCE BZ-X700 all-in-one fluorescence microscope equipped with a
CO2 and temperature-controlled chamber and time-lapse
tracking system (Keyence Corporation). Phase contrast images were
acquired every 10 min for 24 h and converted to video files using a
BZ-X Analyzer (Keyence Corporation). Cell migration was analyzed
using video editing analysis software VW-H2MA (Keyence Corporation)
and the tracking data were processed using Excel 2010 (Microsoft
Corporation) to generate xy coordinate plots and allow measurement
of the distance moved. Migration distance was calculated by
randomly selecting three cells in each well, tracking their
movement for 15 sec and plotting the average value (n=3) of the
distances moved on a graph.
Statistical analysis
Continuous variables are expressed as the median and
range. Continuous and categorical variables were compared using the
Mann-Whitney U-test and χ2 test, respectively. Survival
analyses were performed using the Kaplan-Meier method with the
log-rank test. The correlation between activin A mRNA levels and
SMI was assessed by calculating Spearman's rank correlation
coefficient ρ. OS was calculated as the duration from the date of
surgery until death or the last follow-up. Cancer-specific survival
(CSS) was calculated from the time of diagnosis to the time of
death from any cancer or last follow-up. Variables with
significance at P<0.05 in the univariate analysis were included
in multivariate analysis using stepwise backward elimination
procedures. The Cox proportional hazards model for multivariate
analysis was used. All statistical analyses were performed using
JMP version 13.1 (SAS Institute, Inc.). All P-values were two-sided
and P<0.05 was considered to indicate statistical significance.
The term ‘prognostic marker’ is used according to the REMARK
guidelines (24).
Results
Associations between activin A
expression in CRC tissues and clinicopathological
characteristics
To determine whether the expression level of activin
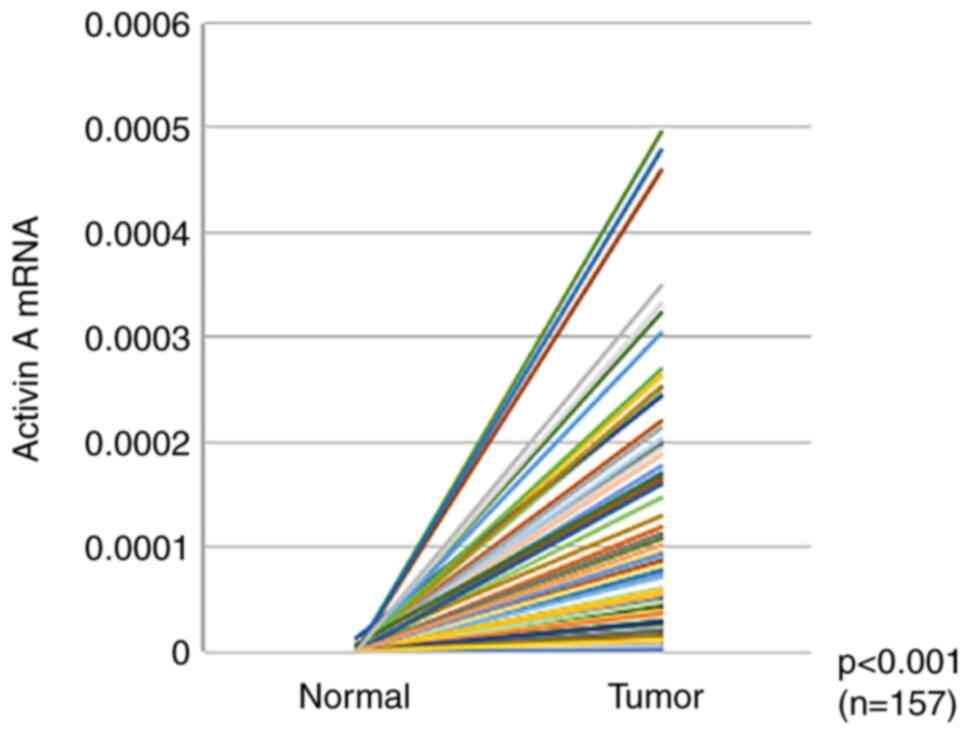
A is elevated in CRC, RT-qPCR analysis of 157 matched pairs of CRC
and normal epithelial tissue samples was performed. Activin A mRNA
expression was significantly higher in CRC tissues than in normal
epithelia (P<0.001; Fig. 1). To
assess the associations between activin A mRNA levels and
clinicopathological factors, patients were assigned to high (n=78)
and low (n=79) activin A expression groups using the median value
as the cut-off. However, none of the clinicopathological factors
examined, including tumor location and metastasis/invasion status,
was significantly associated with activin A mRNA levels in tumor
tissues (Table II).
 | Table II.Patients' characteristics and
clinicopathological factors in patients with colorectal cancer
according to activin A expression. |
Table II.
Patients' characteristics and
clinicopathological factors in patients with colorectal cancer
according to activin A expression.
|
| Activin A mRNA
expression |
|
|---|
|
|
|
|
|---|
| Factor | Low (n=78) | High (n=79) | P-value |
|---|
| Age ≥75 years | 25 (32) | 30 (38) | 0.585 |
| Female sex | 39 (50) | 35 (44) | 0.162 |
| Body mass index
≥25.0 kg/m2 | 19 (24) | 19 (24) | 0.933 |
| Location in
rectum | 13 (17) | 29 (37) | 0.055 |
| Tumor stage
T3-T4 | 55 (71) | 60 (76) | 0.221 |
| Lymph node
metastasis | 29 (37) | 30 (38) | 0.778 |
| Lymphatic
invasion | 26 (33) | 25 (32) | 0.502 |
| Venous
invasion | 30 (38) | 37 (47) | 0.425 |
| CEA >3.4
ng/ml | 24 (31) | 28 (35) | 0.440 |
| CA19-9 >37.0
U/ml | 12 (15) | 15 (19) | 0.590 |
Correlation between tumor expression
of activin A and SMI
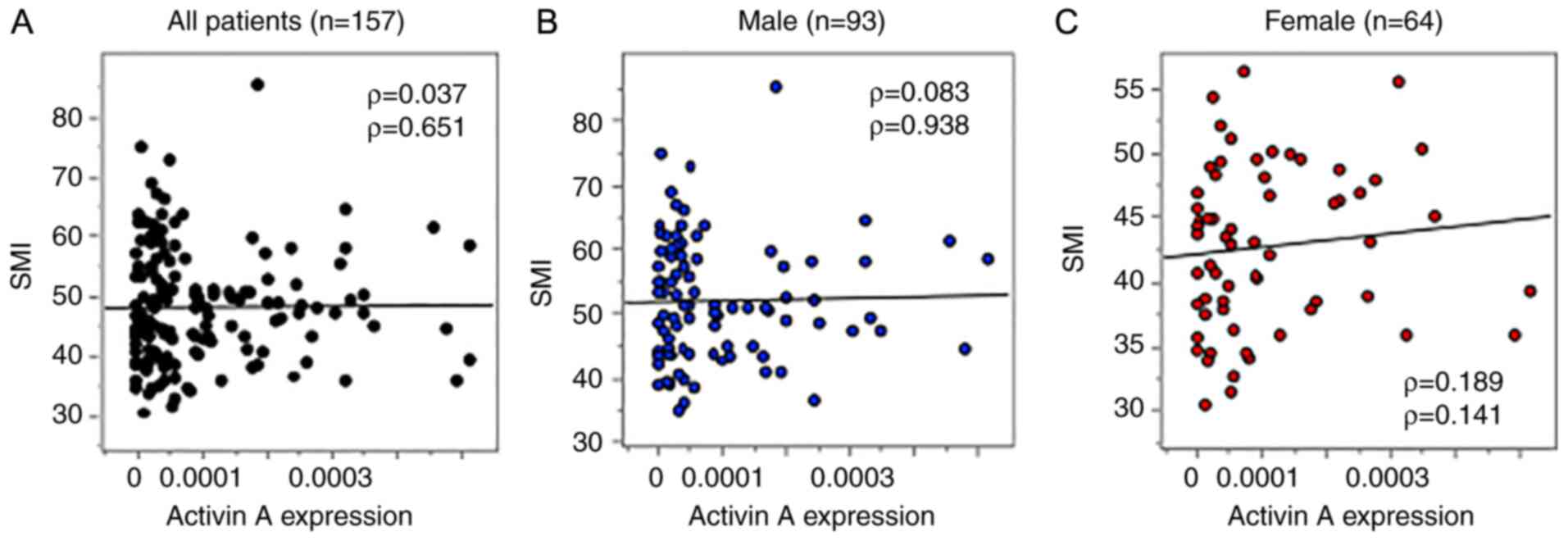
Next, the association between activin A mRNA
expression and the SMI was assessed using Spearman's rank
correlation analysis. As presented in Fig. 2, there were no significant
correlations between activin A mRNA expression in CRC tissues and
the SMI for the full patient cohort (n=157, ρ=0.037, P=0.651),
males (n=93, ρ=0.083, P=0.938) or females (n=64, ρ=0.189, P=0.141).
Thus, the elevated expression of activin A in CRC tumors appeared
to be unrelated to the SMI.
Association between activin A
expression and patient survival
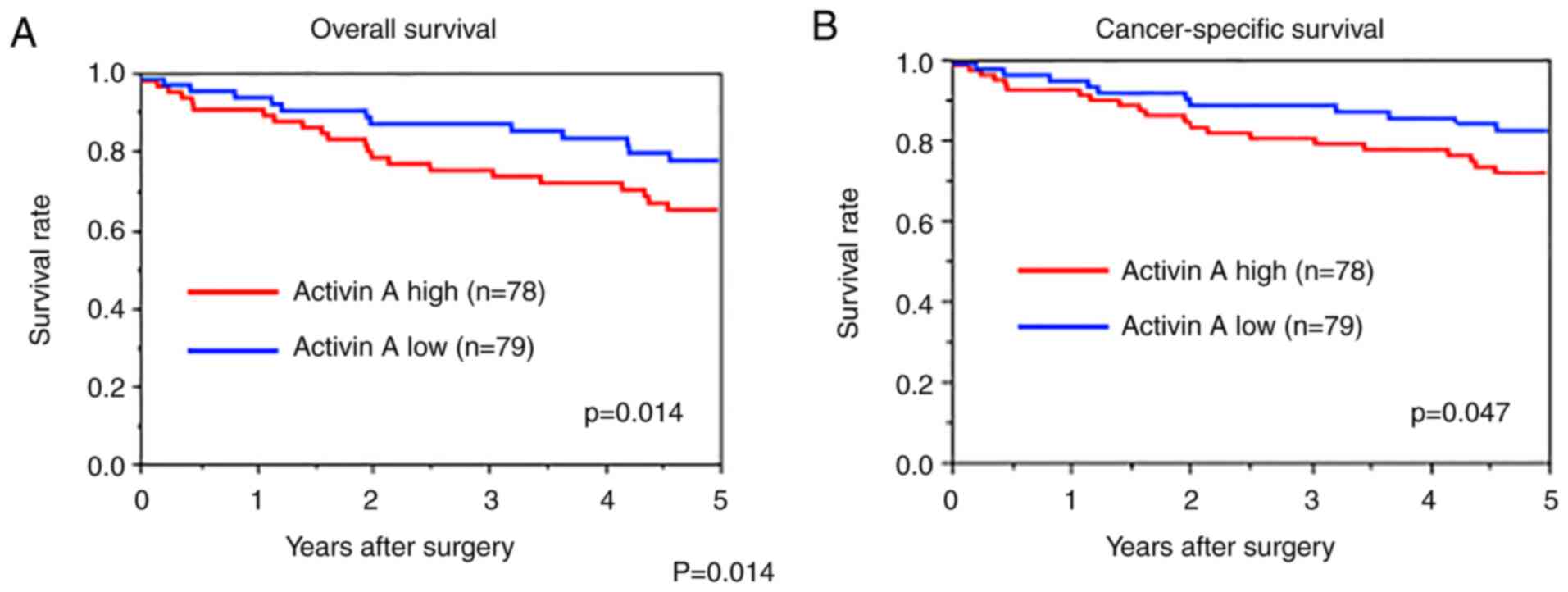
Kaplan-Meier curves were generated to assess the OS
and CSS of patients with CRC according to activin A tumor
expression levels (Fig. 3). It was
indicated that patients with high tumor expression of activin A had
significantly poorer OS (P=0.014) and CSS (P=0.047, log-rank test)
than patients with low tumor expression. Next, factors associated
with poor OS were evaluated by univariate and multivariate Cox
regression analyses. Univariate analysis revealed that an age of
≥75 years, tumor stage, lymph node metastasis, CA19-9 level >37
U/l and high activin A expression were significantly associated
with poor OS (Table III) and
multivariate analysis demonstrated that an age of ≥75 years [hazard
ratio (HR)=4.678, P=0.009], lymph node metastasis (HR=3.372,
P=0.009), CA19-9 level >37 (HR=3.591, P=0.015) and high activin
A expression (HR=4.287, P=0.001) were independent risk factors for
poor OS (Table III). To validate
the present results, the association of activin A mRNA levels with
survival of 443 patients with CRC was determined using a dataset
from the TCGA database. Kaplan-Meier analysis confirmed that high
activin A expression (n=333) was significantly associated with poor
OS (P=0.039; Fig. S1).
 | Table III.Univariate and multivariate analyses
of factors influencing overall survival in colorectal cancer. |
Table III.
Univariate and multivariate analyses
of factors influencing overall survival in colorectal cancer.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Factor | HR | 95%CI | P-value | HR | 95%CI | P-value |
|---|
| Age ≥75 years | 1.801 | 1.108-2.898 | 0.018 | 4.678 | 2.018-11.15 | 0.009 |
| Female sex | 1.185 | 0.733-1.902 | 0.485 |
|
|
|
| Body mass index
≥25.0 kg/m2 | 0.564 | 0.271-1.052 | 0.073 |
|
|
|
| Location in
rectum | 0.933 | 0.552-1.529 | 0.788 |
|
|
|
| Tumor stage
T3-T4 | 2.949 | 1.218-9.704 | 0.014 | 2.711 | 0.839-12.12 | 0.100 |
| Lymph node
metastasis | 2.835 | 1.754-4.673 | <0.001 | 3.372 | 1.344-9.028 | 0.009 |
| CEA >3.4
ng/ml | 1.005 | 0.624-1.614 | 0.982 |
|
|
|
| CA19-9 >37.0
U/ml | 2.034 | 1.166-3.408 | 0.014 | 3.591 | 1.313-9.048 | 0.015 |
| Activin A high | 2.543 | 1.157-5.982 | 0.020 | 4.287 | 1.776-11.14 | 0.001 |
Proliferation, invasion and migration
of human CRC cell lines exposed to activin A in vitro
Next, in vitro experiments were performed to
clarify the biological activities of activin A in CRC cells. The
subcellular pattern of activin A expression in CRC cells was
determined by IHC staining of sections of resected specimens.
Activin A expression was not present in normal epithelium but in
certain CRC tissues. Activin A staining was observed throughout the
cytoplasm of CRC cells (Fig. S2).
The present results thus indicated that high activin A expression
is associated with unfavorable patient outcomes, but not with
sarcopenia, as indicated by the SMI analysis. Therefore, it was
next queried whether exposure to activin A directly affects the
malignant behaviors of CRC cells in vitro. First, RT-qPCR
analysis of a panel of human CRC cell lines was performed, which
indicated that LoVo and SW480 cell lines expressed significantly
higher levels of endogenous activin A mRNA than the other cell
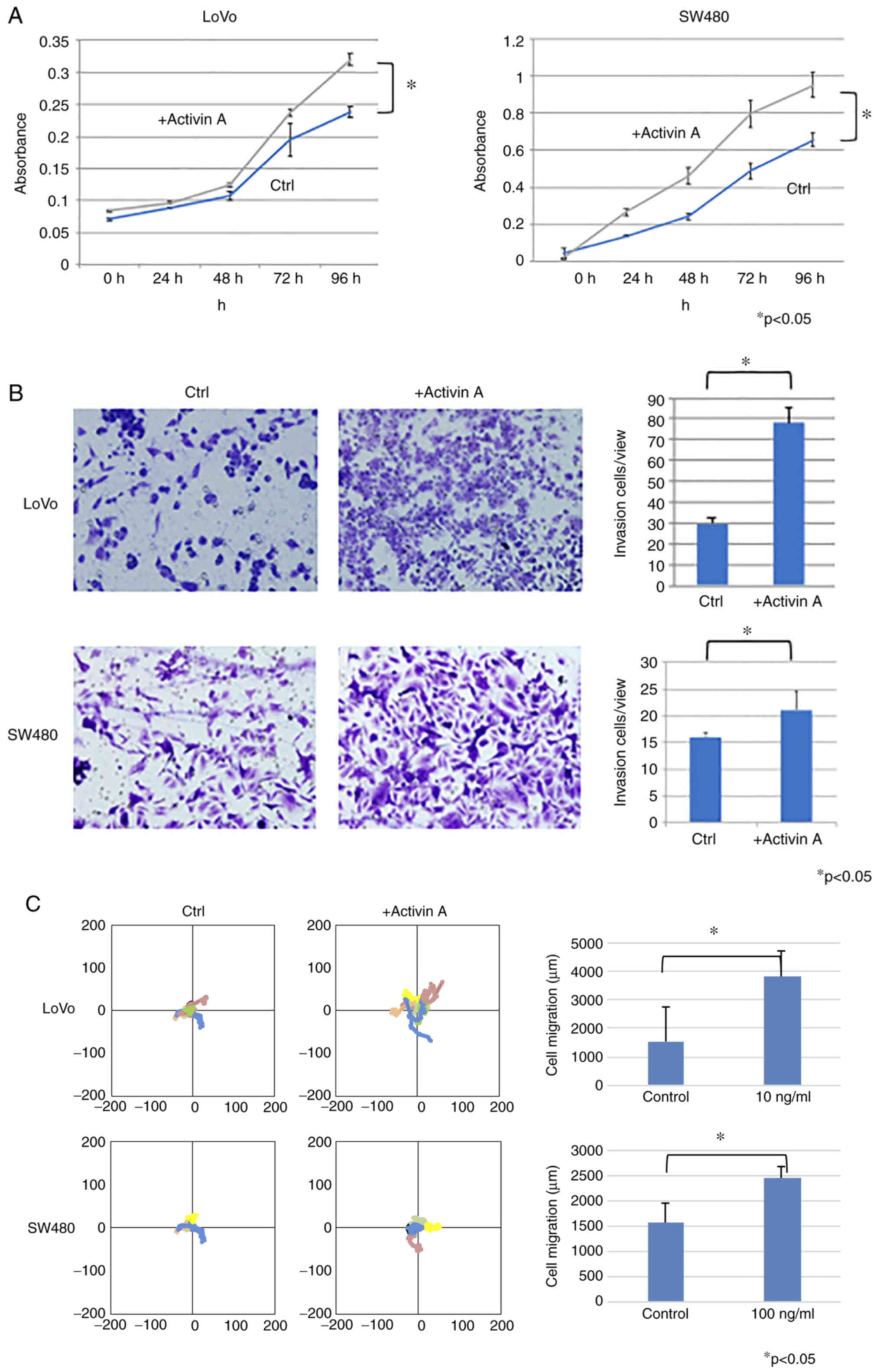
lines tested (Fig. S3). LoVo and
SW480 cells were exposed to exogenous activin A at 10 ng/ml for up
to 96 h and the effects on cell proliferation, migration and
invasion were analyzed. Cells exposed to activin A displayed
significantly increased proliferation (P<0.05; Fig. 4A), invasion (P<0.05; Fig. 4B) and migration (P<0.0; Fig. 4C) compared with untreated control
cells. Similar results were obtained in SW620 cells, which had
lower activin A expression than LoVo cells (Fig. S4). Thus, activin A may act on CRC
cells to promote behaviors associated with malignancy.
Proliferation, invasion and migration
of human CRC cell lines subjected to activin A knockdown
As LoVo and SW480 cells express high endogenous
levels of activin A (Fig. S3), it
was next investigated whether siRNA-mediated knockdown of activin A
affected malignant cell phenotypes in vitro. Two independent
activin A-targeting siRNAs (siActivin A #1 and #2) were evaluated
and confirmed by RT-qPCR analysis to effectively suppress activin A
mRNA expression to levels <30% of those in LoVo or SW480 cells
transfected with an siCtrl sequence (Fig. S5).
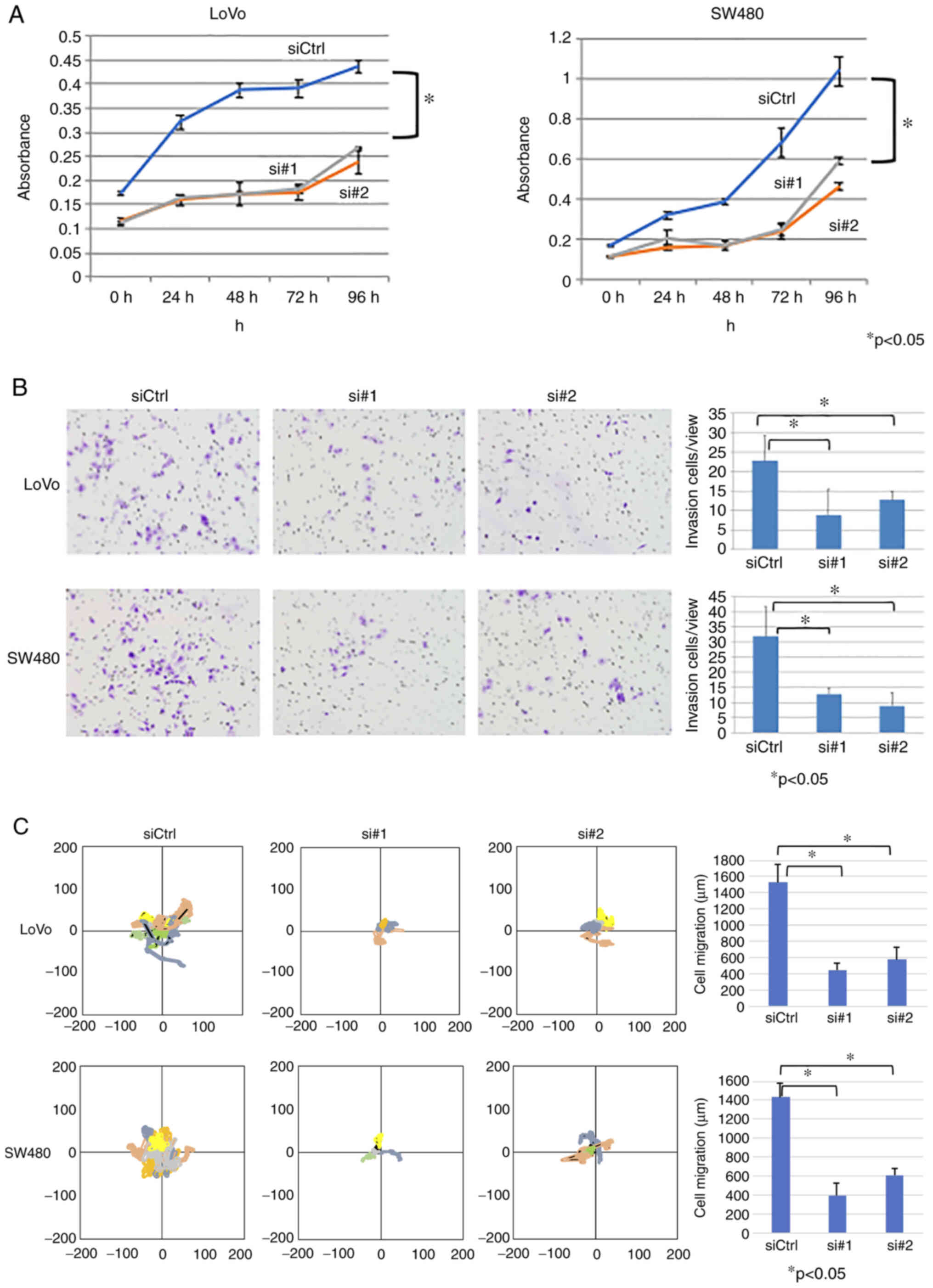
Compared with siCtrl-transfected cells, siActivin A
#1- or #2-transfected LoVo and SW480 cells exhibited significantly
decreased proliferation (P<0.05; Fig. 5A), invasion (P<0.05; Fig. 5B) and migration (P<0.05;
Fig. 5C). These results suggested
that endogenous activin A expression contributed to the malignant
behavior of CRC cells.
Discussion
Activin A has a number of important physiological
roles, including induction of differentiation during vertebrate
embryogenesis, neuronal differentiation and skeletal muscle cell
degradation. Activin A circulates in the blood and is secreted by
the gonads, pituitary gland and placenta. Previous studies reported
that high tumor expression of activin A is associated with poor
prognosis in patients with certain types of gastrointestinal cancer
(18–21). Consistent with this, the present
study demonstrated that activin A was highly expressed in CRC
tissues compared with matched normal intestinal epithelium and that
high expression was an independent predictor of poor prognosis for
patients with CRC.
In the early stages of epithelial cancers, TGF-β
family cytokines function as tumor suppressors and inhibit cell
proliferation. However, as the cancer progresses, TGF-β cytokines
become tumor promoters and induce cancer metastasis by enhancing
cell migration and invasion. Numerous studies have examined the
involvement of activin A in cancer malignancy (22,25,26).
For instance, Hoda et al (25) reported that activin A is involved
in the malignant transformation of pleural mesothelioma through
regulation of cyclin D. The results of the present study provide
support for these earlier findings.
Antagonism of Activin A receptor type IIB, the main
cell surface receptor for activin A, has been reported to suppress
cachexia and prolong survival in a mouse model (26). This is consistent with the role of
activin A in regulating skeletal muscle degradation, suggesting
that activin A released from cancer tissues may be associated with
the sarcopenia observed in numerous patients with CRC. However, in
the present study, no significant correlation between tumor activin
A expression levels and SMI was obtained. One possible explanation
is that SMI is strongly affected by age and sex, with higher SMIs
observed in males and young adults compared with those observed in
females and older individuals, respectively (27). In addition, Loumaye et al
(28) reported that the blood
level of activin A was associated with skeletal muscle density in
patients with CRC or lung cancer and high blood levels were
associated with poor prognosis. Zhong et al (29) reported that serum activin A
secreted by pancreatic adenocarcinoma cells was associated with
cachexia and poor prognosis. Thus, activin A secreted by tumor
cells and other tissues may be involved in the regulation of
skeletal muscle mass.
The present study suggested that high expression of
activin A was associated with poor prognosis but not with SMI,
which suggested that any contribution of activin A to CRC would
occur via alternative mechanisms. Indeed, exposure of two CRC cell
lines to exogenous activin A directly enhanced the proliferation,
invasion and migration of the cells, while knockdown of endogenous
activin A had the opposite effects. Furthermore, IHC staining
suggested that activin A protein was detected predominantly in the
cytoplasm, suggesting that it may be secreted by the CRC cells.
These data indicated that CRC cells may both secrete activin A and
respond to extracellular activin A, and it may be speculated that
activin A therefore functions as both an autocrine and paracrine
modulator of CRC malignancy.
Matsuzaki (30)
reported that activin A is involved in the metastasis and invasion
of cancer cells by promoting c-Myc transcription factor activity
and matrix metalloproteinase-9 production via its effects on SMAD
signaling. The mechanism by which activin A exposure and knockdown
affects the behavior of the CRC cells assessed in the present study
remains to be elucidated; however, it is reasonable to assume that
it may have effects on SMAD pathway activity. The results of the
present study support those of previous reports indicating that
activin A contributes to the malignant behaviors of CRC cell lines,
including their proliferation, migration and invasion.
The current study has certain limitations, including
its retrospective design and the fact that it was performed at a
single institution. However, the association between activin A
expression in CRC tumors and OS was validated using a dataset from
the TCGA. Furthermore, only SMI was used as an indicator of
sarcopenia, although there are other indicators of sarcopenia.
However, the method using abdominal CT scan was considered to be
the best means of evaluating sarcopenia in patients with
gastrointestinal cancer, as CT has the great advantage of being
routinely performed for staging and follow-up of cancer.
Furthermore, Albano et al (31) reported that CT is probably the
easiest and most promising modality for the evaluation of
sarcopenia. In addition, there are certain reports on the prognosis
of CRC (9,10). Furthermore, the results of the
in vitro experiments were also confirmed with clinical
samples. The effect of activin A should be evaluated in a
dose-dependent manner. In additional invasion and migration assays,
treatment of LoVo and SW620 cells with 10 ng/ml activin A
significantly increased their invasive ability compared with
treatment with 100 ng/ml activin A; activin A did not increase
invasion or migration in a dose-dependent manner (data not shown).
These findings may be associated with activin A receptors and their
nuclear translocation. Another limitation is that activin A
secreted by the CRC cell lines into the culture medium was not
removed prior to the experiments, which may have affected the
results. However, given that exogenous activin A was added at a
high concentration and that the opposite effects on cell phenotypes
were observed upon activin A knockdown, it may be assumed that
activin A secreted by the tumor cells would have only had a minor
effect on the results.
In conclusion, the present study demonstrated that
activin A promotes the proliferation, invasion and migration of CRC
cell lines and that high expression of activin A in tumor tissues
correlates with poor prognosis in patients with CRC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding authors on reasonable
request.
Authors' contributions
YM and HB designed and directed the study. ND
performed the laboratory experiments and collected all the
clinicopathological data. YH, RT and YS assisted with the
collection of clinicopathological data. HS, TI, YB, NY supported
the experiments. ND and YM were responsible for the statistical
analysis and wrote the manuscript. TI, YB, NY and HB supervised the
study. ND, YM and HB confirmed the authenticity of all the raw
data. All authors have read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
This retrospective and observational study was
approved by the institutional ethics committee of Kumamoto
University Hospital (14 June 2019/approval no. 1047) and performed
in accordance with the Declaration of Helsinki from 1975. Informed
consent for sample use was obtained from the patients and families
according to institutional review board protocols.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
van der Stok EP, Spaander MCW, Grunhagen
DJ, Verhoef C and Kuipers EJ: Surveillance after curative treatment
for colorectal cancer. Nat Rev Clin Oncol. 14:297–315. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tokunaga R, Imamura Y, Nakamura K,
Uchihara T, Ishimoto T, Nakagawa S, Iwatsuki M, Baba Y, Sakamoto Y,
Miyamoto Y, et al: Carbohydrate antigen 19-9 is a useful prognostic
marker in esophagogastric junction adenocarcinoma. Cancer Med.
4:1659–1666. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Daitoku N, Miyamoto Y, Tokunaga R,
Sakamoto Y, Hiyoshi Y, Iwatsuki M, Baba Y, Iwagami S, Yoshida N and
Baba H: Controlling nutritional status (CONUT) score is a
prognostic marker in metastatic colorectal cancer patients
receiving first-line chemotherapy. Anticancer Res. 38:4883–4888.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Daitoku N, Miyamoto Y, Sakamoto Y,
Tokunaga R, Hiyoshi Y, Nagai Y, Iwatsuki M, Iwagami S, Yoshida N
and Baba H: Prognostic significance of serum p53 antibody according
to KRAS status in metastatic colorectal cancer patients. Int J Clin
Oncol. 25:651–659. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Taniguchi H, Yamazaki K, Yoshino T, Muro
K, Yatabe Y, Watanabe T, Ebi H, Ochiai A, Baba E and Tsuchihara K;
Japanese Society of Medical Oncology, : Japanese society of medical
oncology clinical guidelines: RAS (KRAS/NRAS) mutation testing in
colorectal cancer patients. Cancer Sci. 106:324–327. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yoshino T, Arnold D, Taniguchi H,
Pentheroudakis G, Yamazaki K, Xu RH, Kim TW, Ismail F, Tan IB, Yeh
KH, et al: Pan-Asian adapted ESMO consensus guidelines for the
management of patients with metastatic colorectal cancer: A
JSMO-ESMO initiative endorsed by CSCO, KACO, MOS, SSO and TOS. Ann
Oncol. 29:44–70. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Forones NM and Tanaka M: CEA and CA 19-9
as prognostic indexes in colorectal cancer. Hepatogastroenterology.
46:905–908. 1999.PubMed/NCBI
|
|
9
|
Miyamoto Y, Baba Y, Sakamoto Y, Ohuchi M,
Tokunaga R, Kurashige J, Hiyoshi Y, Iwagami S, Yoshida N, Yoshida
M, et al: Sarcopenia is a negative prognostic factor after curative
resection of colorectal cancer. Ann Surg Oncol. 22:2663–2668. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Miyamoto Y, Baba Y, Sakamoto Y, Ohuchi M,
Tokunaga R, Kurashige J, Hiyoshi Y, Iwagami S, Yoshida N, Watanabe
M and Baba H: Negative impact of skeletal muscle loss after
systemic chemotherapy in patients with unresectable colorectal
cancer. PLoS One. 10:e01297422015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Evans WJ: Skeletal muscle loss: Cachexia,
sarcopenia, and inactivity. Am J Clin Nutr. 91:1123S–1127S. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kitano Y, Yamashita YI, Saito Y, Nakagawa
S, Okabe H, Imai K, Komohara Y, Miyamoto Y, Chikamoto A, Ishiko T
and Baba H: Sarcopenia affects systemic and local immune system and
impacts postoperative outcome in patients with extrahepatic
cholangiocarcinoma. World J Surg. 43:2271–2280. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Harada K, Ida S, Baba Y, Ishimoto T,
Kosumi K, Tokunaga R, Izumi D, Ohuchi M, Nakamura K, Kiyozumi Y, et
al: Prognostic and clinical impact of sarcopenia in esophageal
squamous cell carcinoma. Dis Esophagus. 29:627–633. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Levolger S, van Vugt JL, de Bruin RW and
JN IJ: Systematic review of sarcopenia in patients operated on for
gastrointestinal and hepatopancreatobiliary malignancies. Br J
Surg. 102:1448–1458. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gaddykurten D, Tsuchida K and Vale W:
Activins and the receptor serine kinase superfamily. Recent Prog
Horm Res. 50:109–129. 1995.PubMed/NCBI
|
|
16
|
Miyamoto Y, Hanna DL, Zhang W, Baba H and
Lenz HJ: Molecular pathways: Cachexia signaling-a targeted approach
to cancer treatment. Clin Cancer Res. 22:3999–4004. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lokireddy S, Wijesoma IW, Bonala S, Wei M,
Sze SK, McFarlane C, Kambadur R and Sharma M: Myostatin is a novel
tumoral factor that induces cancer cachexia. Biochem J. 446:23–36.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lyu S, Jiang C, Xu R, Huang Y and Yan S:
INHBA upregulation correlates with poorer prognosis in patients
with esophageal squamous cell carcinoma. Cancer Manag Res.
10:1585–1596. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Oshima T, Yoshihara K, Aoyama T, Hasegawa
S, Sato T, Yamamoto N, Akito N, Shiozawa M, Yoshikawa T, Numata K,
et al: Relation of INHBA gene expression to outcomes in gastric
cancer after curative surgery. Anticancer Res. 34:2303–2309.
2014.PubMed/NCBI
|
|
20
|
Katayama Y, Oshima T, Sakamaki K, Aoyama
T, Sato T, Masudo K, Shiozawa M, Yoshikawa T, Rino Y, Imada T and
Masuda M: Clinical significance of INHBA gene expression in
patients with gastric cancer who receive curative resection
followed by adjuvant S-1 chemotherapy. In Vivo. 31:565–571. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Okano M, Yamamoto H, Ohkuma H, Kano Y, Kim
H, Nishikawa S, Konno M, Kawamoto K, Haraguchi N, Takemasa I, et
al: Significance of INHBA expression in human colorectal cancer.
Oncol Rep. 30:2903–2908. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hoda MA, Rozsas A, Lang E, Klikovits T,
Lohinai Z, Torok S, Berta J, Bendek M, Berger W, Hegedus B, et al:
High circulating activin a level is associated with tumor
progression and predicts poor prognosis in lung adenocarcinoma.
Oncotarget. 7:13388–13399. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sauerbrei W, Taube SE, McShane LM,
Cavenagh MM and Altman DG: Reporting recommendations for tumor
marker prognostic studies (REMARK): An abridged explanation and
elaboration. J Natl Cancer Inst. 110:803–811. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hoda MA, Munzker J, Ghanim B, Schelch K,
Klikovits T, Laszlo V, Sahin E, Bedeir A, Lackner A, Dome B, et al:
Suppression of activin a signals inhibits growth of malignant
pleural mesothelioma cells. Br J Cancer. 107:1978–1986. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou X, Wang JL, Lu J, Song Y, Kwak KS,
Jiao Q, Rosenfeld R, Chen Q, Boone T, Simonet WS, et al: Reversal
of cancer cachexia and muscle wasting by ActRIIB antagonism leads
to prolonged survival. Cell. 142:531–543. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Janssen I, Heymsfield SB and Ross R: Low
relative skeletal muscle mass (sarcopenia) in older persons is
associated with functional impairment and physical disability. J Am
Geriatr Soc. 50:889–896. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Loumaye A, de Barsy M, Nachit M, Lause P,
van Maanen A, Trefois P, Gruson D and Thissen JP: Circulating
activin A predicts survival in cancer patients. J Cachexia
Sarcopenia Muscle. 8:768–777. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhong XL, Pons M, Poirier C, Jiang Y, Liu
J, Sandusky GE, Shahda S, Nakeeb A, Schmidt CM, House MG, et al:
The systemic activin response to pancreatic cancer: Implications
for effective cancer cachexia therapy. J Cachexia Sarcopenia
Muscle. 10:1083–1101. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Matsuzaki K: Smad phosphoisoform signaling
specificity: The right place at the right time. Carcinogenesis.
32:1578–1588. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Albano D, Messina C, Vitale J and
Sconfienza LM: Imaging of sarcopenia: Old evidence and new
insights. Eur Radiol. 30:2199–2208. 2020. View Article : Google Scholar : PubMed/NCBI
|