Introduction
An important initial process involved in the
multistep mechanism of cancer metastasis is the migration of cancer
cells. During the process of migration, the cancer cells detach
from the primary tumor and enter the lymphatic and blood vessels,
which subsequently can result in the formation of tumors at the
secondary sites (1,2). Additionally, the stromal extracellular
matrix (ECM) undergoes remodeling during cancer cell migration. The
cancer cells interact with the ECM and promote ECM remodeling. This
interaction affects tissue stiffness and migration of the cancer
cells. The matrix metalloproteinases (MMPs), which are released by
cancer and stromal cells, play a key role in remodeling the ECM
(2–6). The proteolytic activity of MMPs is
regulated by the tissue inhibitors of metalloproteinases (TIMPs).
The TIMPs are reported to exhibit anticancer activity as they are
natural endogenous inhibitors of MMPs. Recently, the function of
TIMP-1, which is one of the four identified members of the TIMP
family (TIMP-1, −2, −3, and −4), in cancer progression has been
gaining attention. Interestingly, recent studies have demonstrated
that TIMP-1 may exhibit cancer-promoting effects, such as
regulation of cell proliferation, induction of anti-apoptotic
signaling, and promotion of angiogenesis, which are independent of
MMPs (7–11). Several studies have reported that
TIMP-1 promotes cancer cell migration and metastasis (12,13).
However, the role of TIMP-1 in cancer cell migration has not been
fully elucidated.
An increasing number of studies have focused on the
interaction between cancer cells and stromal cancer-associated
fibroblasts (CAFs), which is called cancer-stromal interaction
(CSI), during the cancer progression (14–17).
CAFs maintain an optimal tumor microenvironment for the cancer
cells by secreting cytokines and tumor growth and angiogenic
factors (6,14,18–26).
Recent studies have suggested that CAFs also play an important role
in cancer metastasis through ECM remodeling (6,17,27).
Furthermore, several studies suggest that TIMP-1 is derived from
the stromal CAFs and that TIMP-1 may regulate the CAF activity
during cancer progression (25,28,29).
However, the synergistic function of TIMP-1 and CAFs, which is
induced through CSI, in cancer cell migration and metastasis is not
completely understood.
Hence, in the present study, we focused on the
potential role of TIMP-1 in mediating the interaction between
cancer cells and CAFs. The aim of the present study was to evaluate
the role of CAFs in colon cancer cell migration through CSI via
TIMP-1.
Materials and methods
Cell lines
In the present study, we used the human colon cancer
cell lines (LoVo, HT29, and HCT116) and CAF cells derived from
patients with cancer. All cancer cell lines were purchased from the
American Type Culture Collection (ATCC). The cancer cell lines were
cultured in DMEM (Merck) supplemented with 10% FBS (Thermo Fisher
Scientific, Inc.) and 1% antibiotic-antimycotic solution (Merck).
The CAFs were cultured in DMEM supplemented with 5% FBS and 1%
antibiotic-antimycotic solution. All the cells were cultured at
37°C in a humidified 5% CO2 atmosphere.
Isolation and culture of human colon
fibroblasts
The human colon fibroblast cell lines were
established using the resected tumor specimens obtained from
patients with colorectal cancer who underwent surgery at the Nagoya
City University Hospital (Nagoya, Japan). The study protocol was
approved by the Institutional Review Board of Nagoya City
University Hospital (Institutional code, 70-00-0071). The technical
procedure for establishing the fibroblast cell lines was similar to
that described in previous studies (14,22,24).
Briefly, the colorectal cancer tissues and nonmalignant tissues
were collected from the patients after obtaining written informed
consent. The tissues were cut into 2–3 mm3 cubes using a
scalpel. The tissues were cultured in DMEM supplemented with 1,000
U/ml dispase (Godo Shusei) for 2 h. Next, the tissues were cultured
in DMEM supplemented with 5% FBS and 1% antibiotic-antimycotic
solution at 37°C in a humidified 5% CO2 atmosphere. The
fibroblasts that were isolated from the cancer tissues were defined
as CAFs and those isolated from the nonmalignant tissues were
defined as normal fibroblasts (NFs) as described previously
(14,22,24). The
CAFs and NFs were used for analysis between passages 3 and 6.
Antibodies
The primary mouse anti-vimentin (M0725) and mouse
anti-cytokeratin (M3515) antibodies, and the anti-mouse
HRP-conjugated secondary antibody (Dako Envision+System, K4001)
were purchased from Dako for immunohistochemical staining. The
primary mouse anti-α-smooth muscle actin (α-SMA) (ab7817; Abcam)
and rabbit anti-fibroblast activation protein α (FAP) (ab28244;
Abcam), and the secondary Alexa flour 488-conjugated anti-mouse
(ab150113; Abcam) and cyanine (Cy) 3-conjugated anti-rabbit
(ab6939; Abcam) antibodies were used for immunofluorescence
staining. Primary mouse anti-CD63 (ab59479; Abcam) and rabbit
anti-GAPDH (#2118; Cell Signaling, Inc.) antibodies and secondary
anti-mouse HRP-conjugated antibody (P0447; Dako) and anti-rabbit
HRP-conjugated antibody (P0448; Dako) were purchased for western
blotting analysis. The human recombinant TIMP-1 (410–01) was
purchased from PeproTech and reconstituted in PBS containing 0.1%
BSA to a concentration of 0.2 mg/ml. The human TIMP-1 neutralizing
antibody (AF970) was purchased from R&D Systems Inc. and
reconstituted in PBS to a concentration of 0.2 mg/ml.
Immunohistochemical and
immunofluorescence staining
The isolated fibroblasts were confirmed as CAFs
through immunohistochemical and immunofluorescence staining as
described previously (14). For
immunohistochemical staining, the isolated fibroblasts were fixed
with 10% formalin for 10 min. The fixed fibroblasts were blocked
with 3% BSA prepared in PBS. Next, the fibroblasts were probed with
the primary mouse anti-vimentin (1:80) and mouse anti-cytokeratin
(1:80) antibodies for 60 min at 37°C. The fibroblasts were then
probed with the HRP-conjugated secondary antibody for 60 min at
37°C. The cells were stained with a DAB substrate (Dako) and
counterstained with hematoxylin.
For immunofluorescence staining, the fibroblasts
were fixed with 10% formalin for 10 min and treated with 0.2%
Triton X-100 (MP Biomedicals) for 10 min. The fibroblasts were
blocked with 1% BSA prepared in PBS. The fibroblasts were probed
with the primary mouse anti-α-SMA (1:200) and rabbit anti-FAP
(1:100) antibodies for 60 min at room temperature. Next, the
fibroblasts were probed with the Alexa flour 488-conjugated
anti-mouse (1:200) and cyanine (Cy) 3-conjugated anti-rabbit
(1:1,000) secondary antibodies for 30 min. The fibroblasts were
washed with PBS and treated with the ProLong™ Gold
Antifade Mountant containing DAPI (P36941; Thermo Fisher
Scientific, Inc.) for 10 min at room temperature. The images were
captured and analyzed using the KEYENCE BZ-X700 Fluorescence
Microscope and BZ-X700 Analyzer (Keyence).
Co-culturing CAFs with the colon
cancer cell lines
The CAFs were seeded in 6-well plates at a cell
density of 1.0×105 cells/well. The LoVo, HT29, and
HCT116 cells were seeded in the Transwell inserts (Falcon Permeable
Support for 6-well plate with 0.4 µm Transparent PET Membrane,
353090, Corning Incorporated) at a cell density of
1.0×105 cells/insert. The cells were incubated in DMEM
supplemented with 2% FBS for 24 h. Next, each insert was placed in
the 6-well plates and co-cultured in DMEM supplemented with 2% FBS.
After 48 h co-culture, the conditioned medium was collected. The
cancer cell lines and CAFs were monocultured in DMEM supplemented
with 2% FBS in the 6-well plates and the respective conditioned
medium was collected.
Cytokine antibody array
The secretion of various cytokines by the CAFs was
screened through human cytokine antibody array using a commercially
available array system for the human MMPs and TIMPs (ab134004;
Abcam). The secretion of cytokines in the collected monoculture
conditioned medium by the HT29 and CAF cells and the co-culture
conditioned medium was analyzed, following the manufacturer's
instructions.
Cell survival assay
The effect of TIMP-1 and human TIMP-1 neutralizing
antibody on the viability and proliferation of cancer cell lines
was evaluated using the Premix WST-1 Cell Proliferation Assay
System (Takara Bio), following the manufacturer's instruction. The
LoVo, HT29, and HCT116 cells (3.0×104 cells/well) were
placed in 96-well plates and allowed to attach overnight at 37°C.
The growth medium was replaced with a medium containing human
recombinant TIMP-1 or human TIMP-1 neutralizing antibody. The
number of viable cells was examined at 0, 24, 48 and 72 h by
measuring the absorbance at 450 nm with the reference wavelength at
650 nm using a microplate reader (Molecular Devices).
ELISA
The secretion levels of TIMP-1 in the monoculture
conditioned medium of cancer cells, CAFs, and the respective
co-culture conditioned medium were evaluated by ELISA. The levels
of TIMP-1 in the collected conditioned medium were measured using
the Human TIMP-1 ELISA Kit (DTM100; R&D Systems Inc.),
following the manufacturer's instructions.
Wound-healing assay for colon cancer
cell lines in the presence or absence of TIMP-1 and TIMP-1
antibodies
To evaluate cell migration ability, several
approaches, such as Transwell assays and wound healing assays, can
be used. In this study, we used wound healing assays, which have
been used in many other studies, to evaluate the effects of TIMP-1
on the colon cancer cell migration (30–32). The
LoVo, HT29, and HCT116 cells were cultured in DMEM supplemented
with 2% FBS in 24-well plates until confluency. The wounds were
carefully generated by scratching the confluent cells with the
200-µl pipette tips. The cells were washed with PBS. Next, the
cells were cultured in DMEM containing 2% FBS and human recombinant
TIMP-1 or the control DMEM containing equivalent amount of BSA
prepared in PBS. The images from the wound-healing assay were
captured and analyzed using the BZ-X700 and BZ-X700 Analyzer
(Keyence) at 0, 24, 48, and 72 h.
The effect of TIMP-1 neutralization on cell
migration was evaluated by wound-healing assay. The assay was
performed in the presence of human recombinant TIMP-1 and in the
presence or absence of human TIMP-1 neutralizing antibody or in
control DMEM/PBS.
Wound-healing assay for cancer cells
co-cultured with CAF
The effect of co-culturing CAFs with cancer cells on
promoting cancer cell migration was evaluated by wound-healing
assay. The LoVo monoculture was used as the control in the
wound-healing assay. The wound-healing assay was performed for the
cancers cells co-cultured with CAFs, which were seeded and
incubated in the Transwell inserts (Corning Inc.) at the cell
density of 1.0×105 cells/well. Furthermore, the role of
TIMP-1 secreted by the CAFs in promoting the migration of LoVo
cancer cells co-cultured with CAFs was evaluated using the TIMP-1
neutralizing antibody.
Western blot analysis
Western blot analysis was performed as described
previously (14). Protein samples
were prepared in RIPA lysis and extraction buffer with Protease
Inhibitor Single-Use Cocktail and Phosphatase Inhibitor Cocktail
(all from Thermo Fisher Scientific, Inc.). The concentration of
each protein was measured using a BCA protein assay kit (Thermo
Fisher Scientific, Inc.). Equal amounts of protein were denatured
by boiling at 90°C for 5 min. Proteins (30 µg) were fractionated on
10% Mini-PROTEAN TGX gels and transferred to nitrocellulose
membranes (Bio-Rad Laboratories, Inc.). Primary and secondary
antibody reactions were performed using an iBind Flex Western
System (Thermo Fisher Scientific, Inc.). Membranes were incubated
with iBind Flex Solution (iBind Flex Buffer, iBind Flex Additive,
and distilled water) for 10 min at room temperature to block
nonspecific binding. Primary (CD63 and GAPDH) and secondary
(polyclonal goat anti-mouse IgGs conjugated to HRP and polyclonal
goat anti-rabbit IgGs conjugated to HRP) antibody reactions were
performed at room temperature for 2.5 h, following the
manufacturer's protocol. Protein-antibody complexes were visualized
using SuperSignal West Pico Chemiluminescent Substrate (Thermo
Fisher Scientific, Inc.). The immunoreactive protein band was
detected, and band density was quantified via densitometry using an
Amersham Imager 600 (GE Healthcare Life Sciences).
Statistical analysis
Each experiment was repeated at least three times.
The data are presented as the mean ± SEM. All statistical analyses
were performed using the JMP software version 14 (SAS Institute).
The differences between the groups were compared using the
Student's t-test or ANOVA followed by post hoc test with Dunnett's
test and Tukey's test. The difference was considered statistically
significant when the P-value was <0.05.
Results
Isolation and characterization of
primary CAFs and NFs
The spindle-shaped cells with large cytoplasm were
established from the fresh colorectal cancer tissues and the
nonmalignant tissues. The cells were subjected to immunostaining to
detect the markers of fibroblasts and CAFs as described previously
(14,22). The immunohistochemical staining
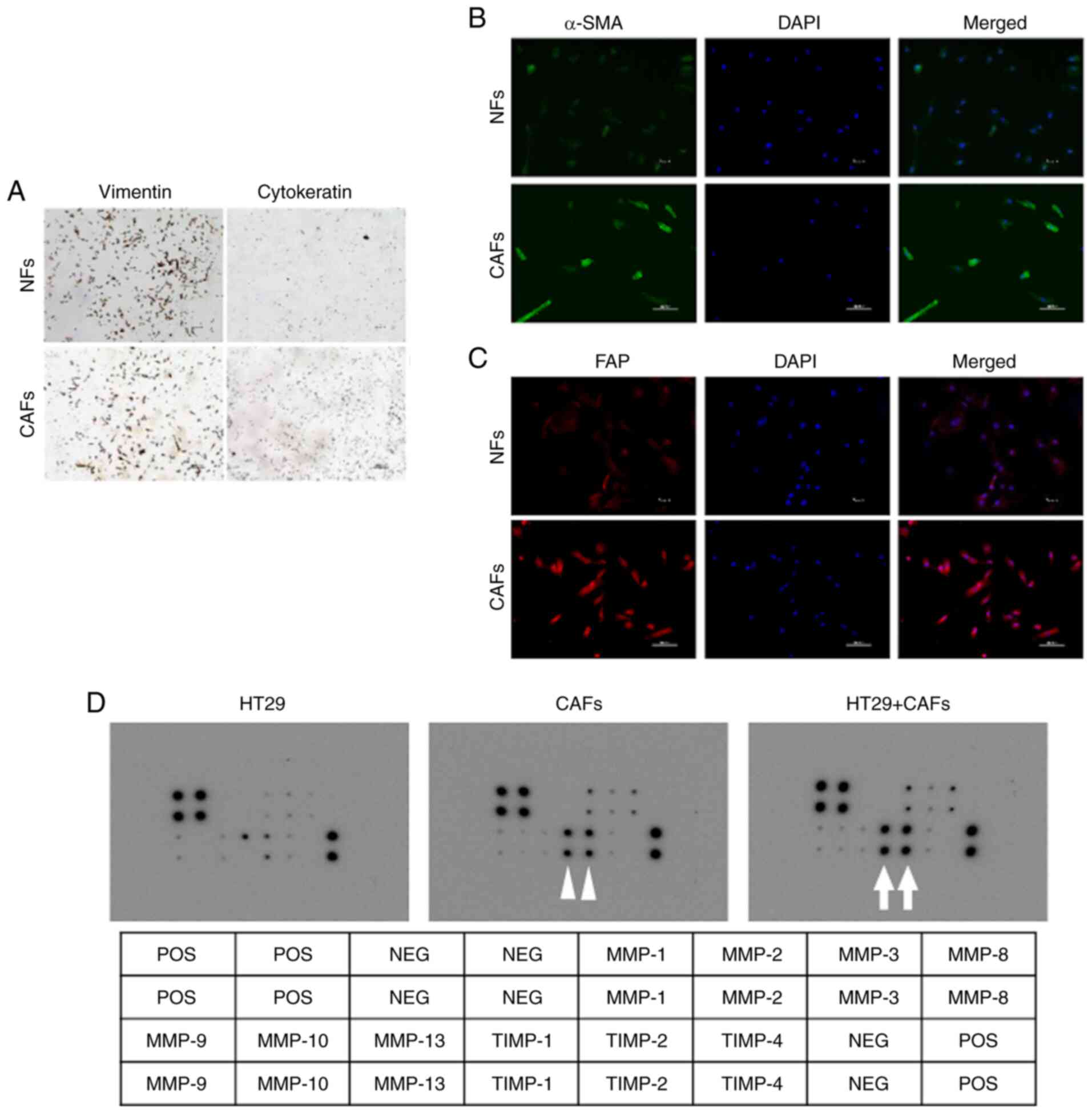
analysis confirmed that the established cells tested positive for
vimentin and tested negative for cytokeratin expression (Fig. 1A). The immunofluorescence staining
demonstrated that the cells derived from the cancerous lesions
exhibited enhanced expression of α-SMA and FAP, whereas those
derived from nonmalignant tissues exhibited weak expression
(Fig. 1B and C). These results
confirmed that the established cells were fibroblasts.
Additionally, the cells derived from cancerous lesions were
confirmed as CAFs, while those derived from nonmalignant tissues
were confirmed as NFs. The characteristics of patients from whom
cancerous lesions and nonmalignant tissues were obtained to isolate
the CAFs and NFs are shown in Table
I.
 | Table I.Characteristics of the patients whose
tissues were used to isolate the CAFs and NFs and the figure
numbers in which the patient samples were used for experiments. |
Table I.
Characteristics of the patients whose
tissues were used to isolate the CAFs and NFs and the figure
numbers in which the patient samples were used for experiments.
| Patient no. | Age (years) | Sex | Histological
type | pTNMa | Successfully
established fibroblasts | Figure numbers in
which the patient samples were used for experiments |
|---|
| #1 | 68 | M | Well-differentiated
adenocarcinoma | T3N0M0 | CAF#1, NF#1 | 1B, 1C, 1D, 5A,
5B |
| #2 | 61 | M | Moderately
differentiated adenocarcinoma | T4bN0M0 | CAF#2 | 1A, 5A, 5B |
| #3 | 71 | F | Moderately
differentiated adenocarcinoma | T4bN0M0 | CAF#3, NF#3 | 5A, 5B |
| #4 | 50 | F | Moderately
differentiated adenocarcinoma | T3N1bM0 | CAF#4 | 6B |
| #5 | 65 | M | Moderately
differentiated adenocarcinoma | T4aN0M0 | CAF#5, NF#5 | 5A, 5B, 6A, 6B |
Cytokine secretion by the CAFs
The cytokine antibody array was used to analyze the
MMPs and TIMPs in the monoculture conditioned medium of HT29 cells
and CAFs and the co-culture conditioned medium. The analysis
revealed that the levels of TIMP-1 and TIMP-2 in the monoculture
conditioned medium of CAFs were higher than those in the
monoculture conditioned medium of HT29 cells. Additionally, the
co-culture of HT29 cells and CAFs enhanced the secretion of TIMP-1
and TIMP-2 by the CAFs (Fig. 1D).
Among these two TIMPs, we focused on TIMP-1 in this study.
TIMP-1 enhances the migration of LoVo
cells
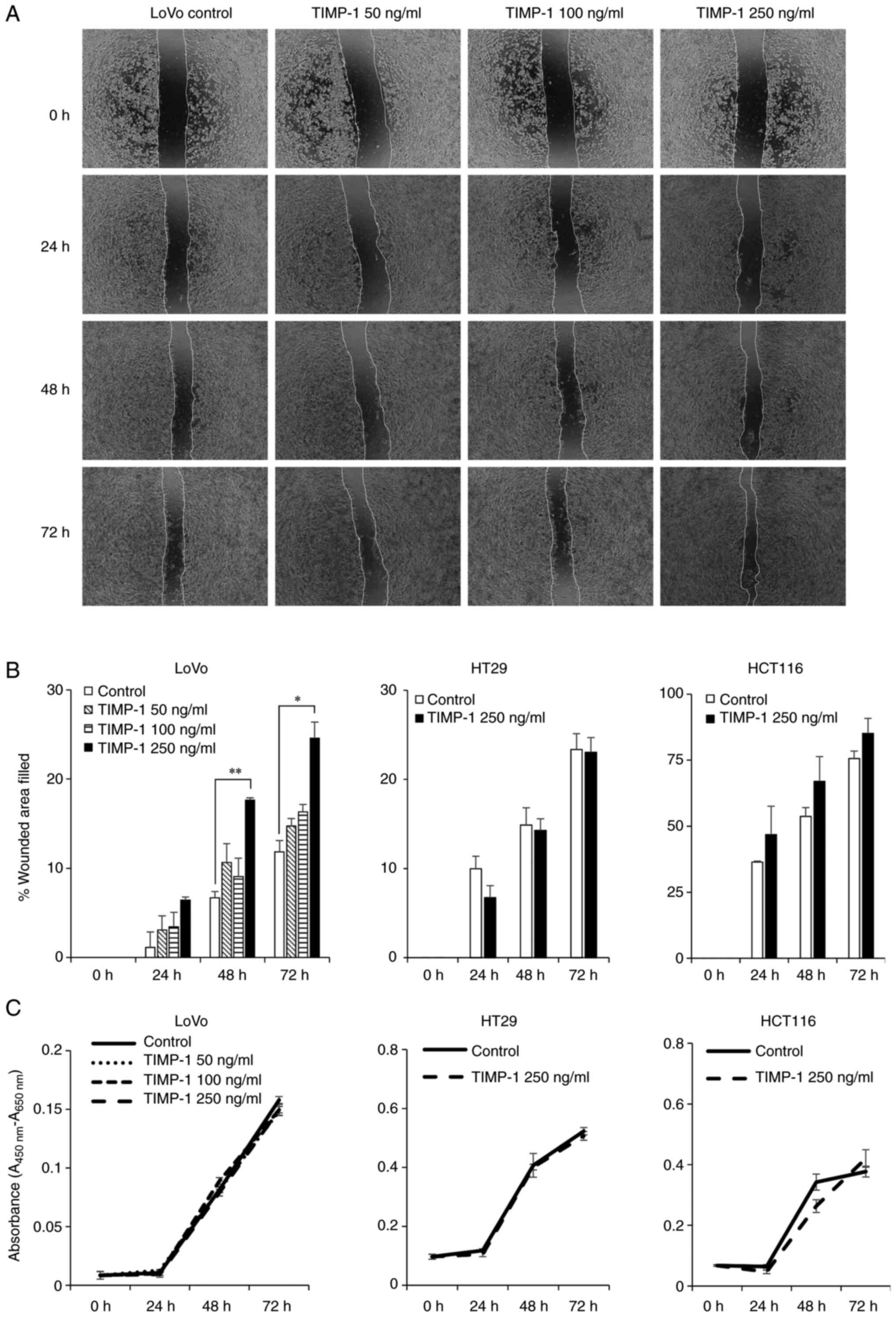
The effect of TIMP-1 on colon cancer cell migration
was investigated using wound-healing assay, which is an in
vitro assay for cell migration. We investigated the effect of
increasing concentrations of human recombinant TIMP-1 (50, 100, and
250 ng/ml) on the migration of LoVo colon cancer cells. As shown in
Fig. 2A and B, TIMP-1 at a
concentration of 250 ng/ml significantly enhanced the migration of
LoVo cells at 48 h (P<0.01) and 72 h (P<0.05). The effects of
human recombinant TIMP-1 on the survival or proliferation of LoVo
cells were evaluated at different concentrations of TIMP-1 using
WST-1 assays at 48 and 72 h. The tested concentrations of TIMP-1
did not affect the survival of LoVo cells (Fig. 2C). Next, we assessed the effects of
250 ng/ml TIMP-1 on the migration of HT29 and HCT116 colon cancer
cells. The migration of HT29 and HCT116 colon cancer cells was not
significantly affected after treatment with 250 ng/ml TIMP-1
(Fig. 2B). As observed in LoVo
cells, TIMP-1 did not affect the survival of HT29 and HCT116 cells
(Fig. 2C).
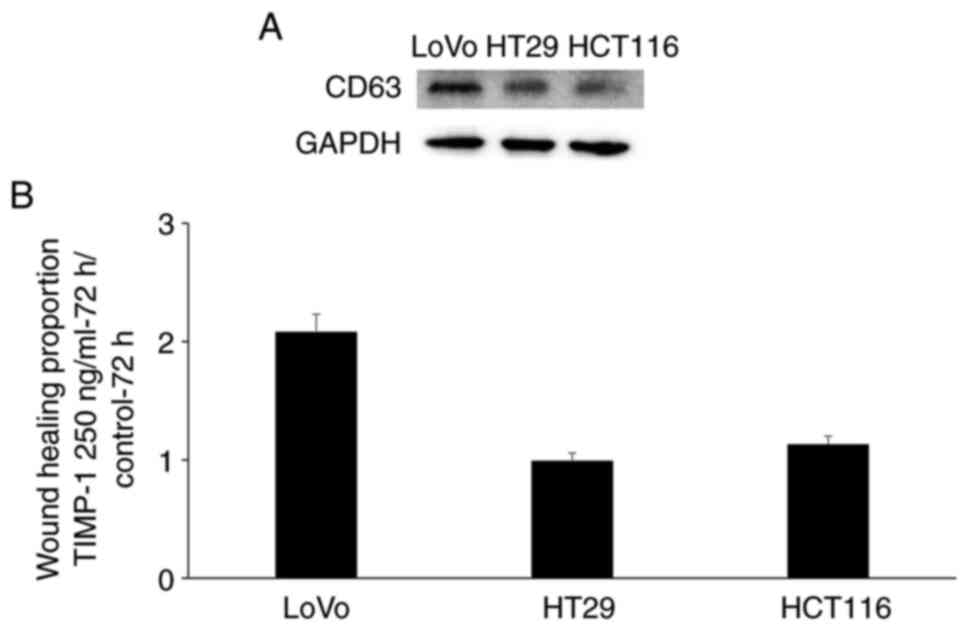
To determine whether the differential effect of
TIMP-1 on cell migration was due to the differential expression of
CD63, the expression of CD63 in the cancer cell lines was evaluated
via western blotting analysis. The LoVo cancer cells exhibited
higher CD63 expression than that found in the HT29 and HCT116 cells
(Fig. 3A). The enhanced
wound-healing rate between the LoVo cells treated with 250 ng/ml
TIMP-1 and untreated LoVo cells at 72 h was approximately twice as
that between the 250 ng/ml TIMP-1-treated and untreated HT29 and
HCT116 cells (Fig. 3B). This
differential effect of TIMP-1 on cell migration may be correlated
with the differential CD63 expression in these cancer cell
lines.
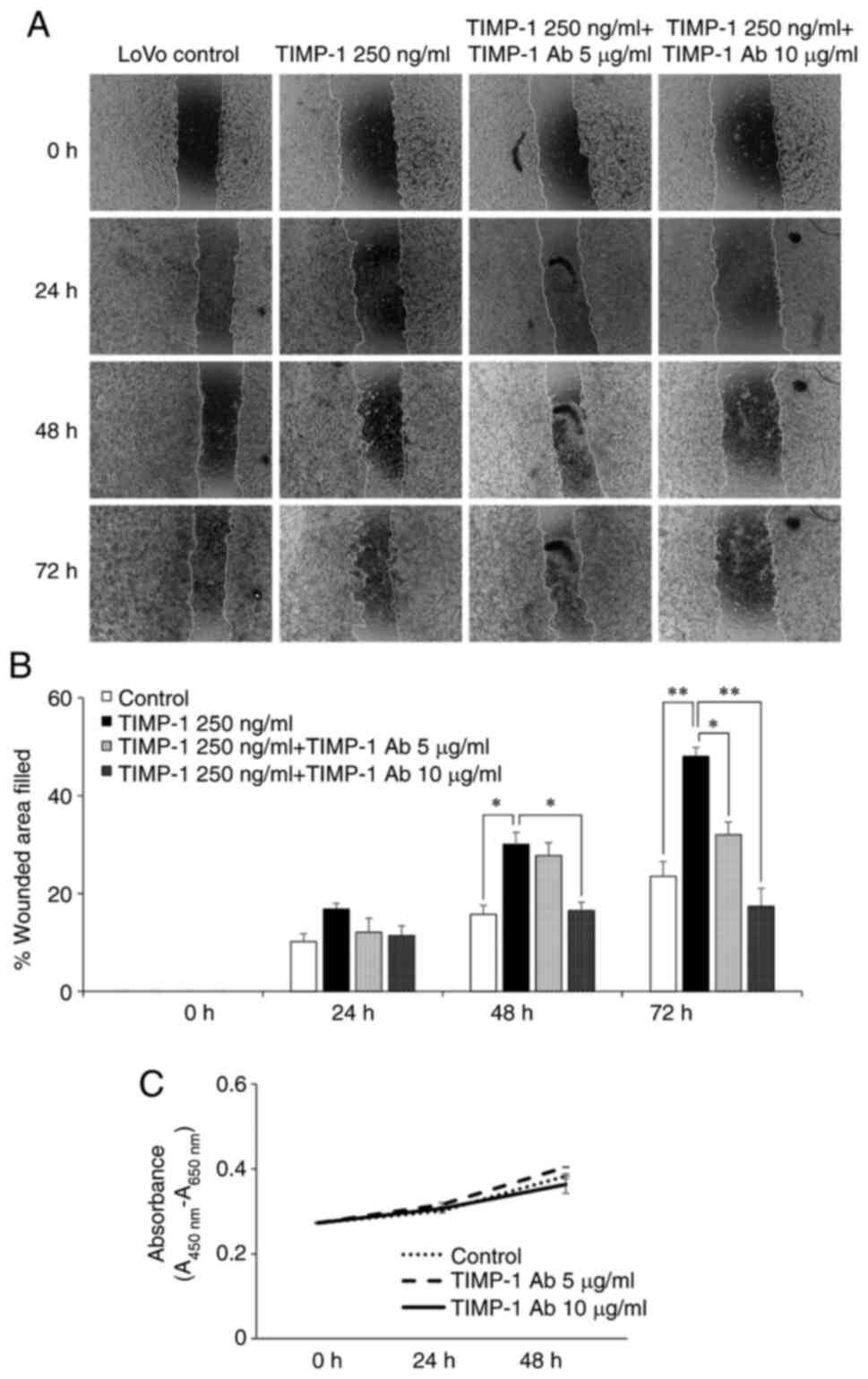
TIMP-1 neutralization inhibits the
TIMP-1-mediated enhanced migration of LoVo cells
The inhibitory effect of TIMP-1 neutralization on
TIMP-1-mediated LoVo cell migration was evaluated by wound-healing
assay. The migration of LoVo cells treated with 250 ng/ml TIMP-1
was evaluated in the presence or absence of human TIMP-1
neutralizing antibody. TIMP-1-mediated migration of LoVo cells was
inhibited by 10 µg/ml of human TIMP-1 neutralizing antibody at 48 h
(P<0.05), and by 5 µg/ml (P<0.05) and 10 µg/ml (P<0.01) of
TIMP-1 antibody at 72 h (Fig. 4A and
B). Next, WST-1 assay was performed to confirm that the
inhibition of LoVo migration by different concentrations of TIMP-1
antibody was not due to its inhibitory effect on the LoVo cell
survival. The results of WST-1 assay revealed that none of the
concentrations of TIMP-1 antibody affected the LoVo cell survival
(Fig. 4C).
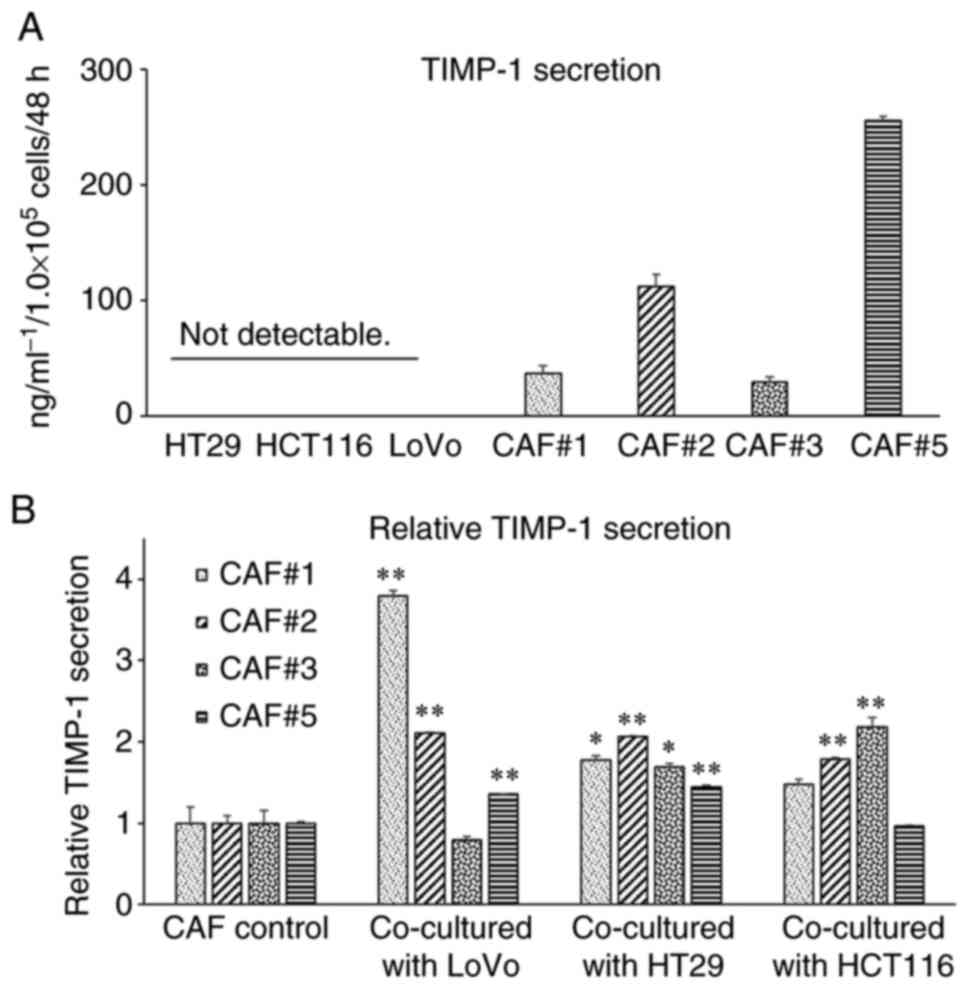
TIMP-1 secretion levels from cancer
cells and CAFs
Previous studies have demonstrated that elevated
plasma TIMP-1 levels are associated with adverse long-term outcomes
in patients with colorectal cancer (33–35) and
that the protein expression of TIMP-1 is upregulated in the stroma
of several types of cancer (25,28,36,37).
However, the potential source of TIMP-1 in colorectal cancer tissue
is poorly elucidated. Hence, we examined whether the source of
TIMP-1 secretion is the cancer cells or CAFs in vitro. The
secreted levels of TIMP-1 in the monoculture conditioned medium of
colon cancer cell lines and established CAFs were evaluated using
ELISA. As shown in Fig. 5A, the
secreted levels of TIMP-1 in the monoculture conditioned medium of
the cancer cell lines were too low to measure. However, the
secreted levels of TIMP-1 in the monoculture conditioned medium of
CAFs were higher than those in the monoculture conditioned medium
of cancer cell lines. The secreted levels of TIMP-1 varied
depending on the established cell lines. These results suggest that
CAFs are the main source of TIMP-1 secretion in the colon cancer
tissues.
Co-culturing CAFs with the colon
cancer cell lines enhances TIMP-1 secretion
The effect of interaction between cancer cells and
CAFs on TIMP-1 secretion was evaluated by measuring the secreted
levels of TIMP-1 in the monoculture medium of CAFs and those in the
co-culture conditioned medium of CAFs and cancer cell lines. As
shown in Fig. 5B, the secretion of
TIMP-1 by the CAFs increased when they were co-cultured with the
LoVo, HT29, and HCT116 cells. These results indicated that TIMP-1
secretion by the CAFs was enhanced through the interaction between
cancer cells and CAFs in the colon cancer tissues.
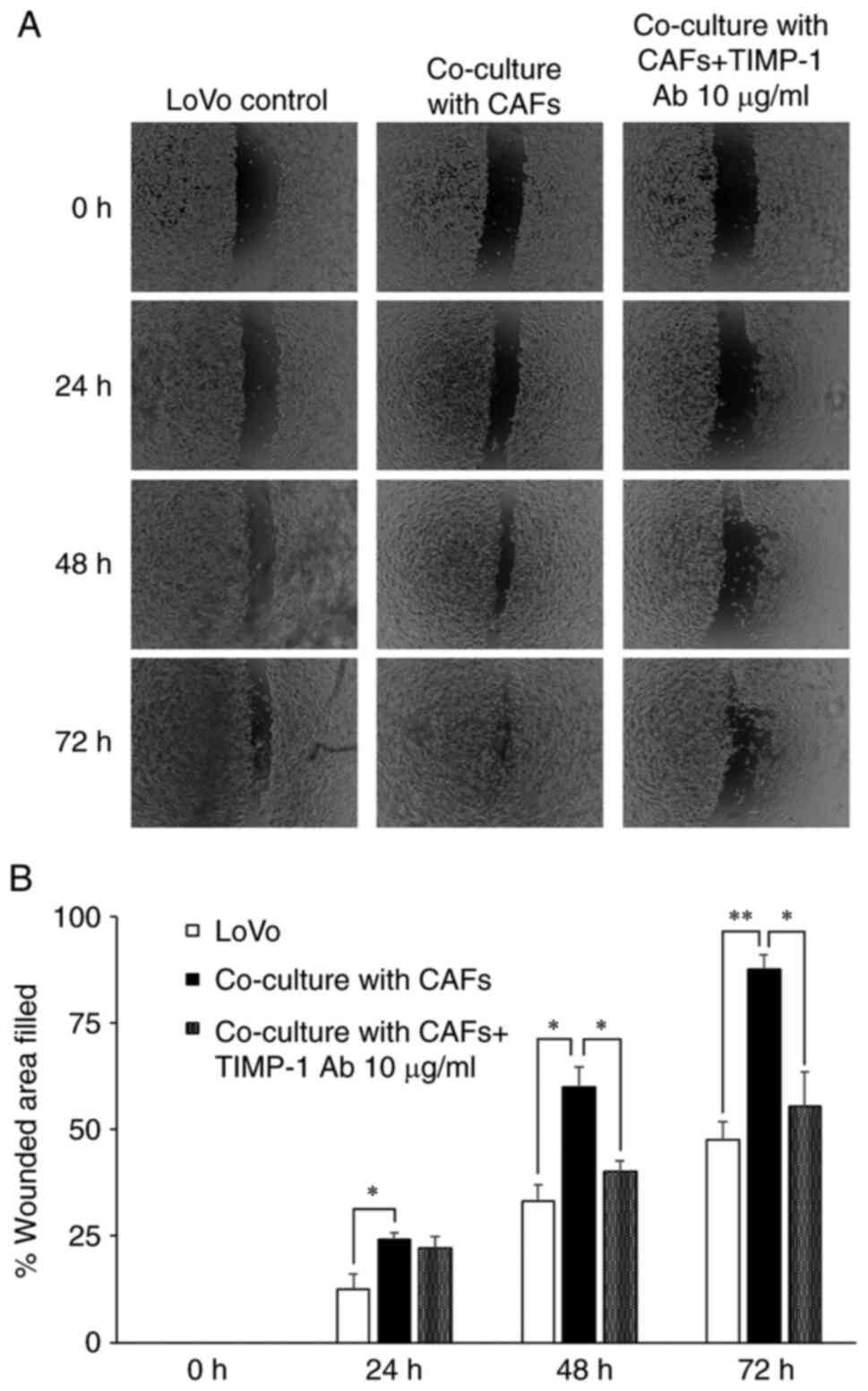
Co-culturing CAFs with the cancer
cells enhances LoVo cell migration and TIMP-1 neutralization
inhibits the enhanced cell migration
Although previous studies have demonstrated that the
CAFs enhance cancer cell migration in in vitro co-culture
models in several cancer types (27,38,39), the
effects of CAF co-culture and the role of TIMP-1 in colon cancer
cell migration have not been examined. Hence, we assessed the
effects of co-culturing CAFs with cancer cells and the role of
TIMP-1 on LoVo cell migration by neutralizing the TIMP-1 in the
co-culture model. As shown in Fig.
6, we observed that co-culturing enhanced LoVo cell migration
at all tested time points (24, 48, and 72 h). Furthermore,
treatment with 10 µg/ml TIMP-1 antibody inhibited the enhanced LoVo
cell migration at 48 h (P<0.05), and partially inhibited the
enhanced migration at 72 h (P<0.05, while P<0.01 in CAF
co-culture vs. control). These observations indicate the secretion
of TIMP-1 by the CAFs is enhanced through the interaction with
cancer cells, which is one of the important mechanisms underlying
colon cancer cell migration.
Discussion
In the last decade, increasing number of studies
have demonstrated that the cancer stromal cells promote cancer
progression even though they are not malignant cells. Various
processes, such as migration, invasion, adhesion, and angiogenesis
are necessary for cancer metastasis. The interaction between the
cancer cells and stromal cells is reported to be important for
progression, angiogenesis, and chemoresistance. This interaction
promotes tumor progression more than the cancer cells alone. Among
the various stromal cells, cancer-associated fibroblasts (CAFs) are
reported to play a potentially important role in tumor metastasis
(6,17,36,40).
Chemokines and cytokines secreted from the CAFs provide the
microenvironment suitable for cancer cell survival, proliferation,
invasion, and migration (6,15,16,20,21,23,24,27).
However, the role of stromal cells in cancer cell migration and
invasion has been poorly studied.
In the present study, we demonstrated that in
addition to promoting angiogenesis and cancer progression, CAFs
also promoted cancer cell migration. As shown in the present study,
co-culturing CAFs with the cancer cells markedly promoted the
migration of cancer cells when compared to the monocultured cancer
cells. These results concurred with those of a previous study
(27). The cell migration rate of
cancer cells into the wounded area when co-cultured with CAFs was
almost twice as that of the monocultured cancer cells (mean values
in 72 h, monocultured cancer cells: 47.6% and cancer cells
co-cultured with CAFs: 87.6%).
The migration properties of cancer cells enable the
metastasis of cancer cells to other organs. Several studies have
demonstrated that cancer cell migration involves multistep
processes, such as protrusion of the leading edge of the cell,
focal contact formation through cell-matrix interaction, focalized
proteolysis through the recruitment of surface proteases to
extracellular matrix (ECM) contacts, cell contraction by
actomyosin, the cell trailing edge detachment, and the proteolytic
remodeling of the ECM (2–4). Matrix metalloproteinases (MMPs) play a
key role in remodeling the ECM. The proteolytic activity of MMPs is
regulated by the tissue inhibitors of metalloproteinase (TIMPs).
TIMP-1, a glycoprotein that is detected in various body fluids and
the extracellular compartment in various tissues, is a human
natural endogenous inhibitor of MMPs. The TIMP-1 forms a
noncovalent 1:1 stoichiometric complexes with the MMPs (4). However, increasing evidence indicates
that TIMP-1 also exhibits tumor-promoting effects, such as
regulation of cell proliferation, anti-apoptotic function, cell
migration, angiogenesis, and chemoresistance (7,8,12,28,36). The
correlation between high plasma TIMP-1 levels and poor prognosis is
reported clinically in various malignant neoplasms (8,29,33–35,37).
In the present study, we investigated whether TIMP-1
enhanced colon cancer cell migration. The concentration of TIMP-1
in the conditioned medium of CAF monoculture or CAFs co-cultured
with cancer cells at a cell density of 1.0×105 cells/48
h was 200 to 300 ng/ml at most. Additionally, previous studies have
reported that the plasma TIMP-1 concentration in patients with
several malignancies ranged from approximately 100 to 300 ng/ml
(8,12,28,33–35).
Therefore, we investigated the effect of TIMP-1 on cancer cell
migration with a maximum concentration of 250 ng/ml, a value that
was also used in a study by Gong et al (28). In contrast to the results with LoVo,
the same concentration of TIMP-1 did not enhance the migration of
HT29 and HCT116 cells. Considering a previous study in which
enhanced migration of another colon cancer cell line, DLD-1, was
induced by as much as 5 µg/ml of TIMP-1, the reason that TIMP-1 did
not enhance HT29 and HCT116 migration may be the use of low
concentrations of TIMP-1; however, TIMP-1 concentration above 500
ng/ml appeared to be too high as a component of the tumor
microenvironment, considering our previous results and previous
studies.
Furthermore, we demonstrated that co-culturing
cancer cells with CAFs promoted the migration of cancer cells. The
migration of co-cultured cancer cells was similar to that of the
cancer cells treated with TIMP-1. Furthermore, treatment with
TIMP-1 neutralizing antibody decreased the migration of cancer
cells to almost the same level as cancer cells (mean value with
antibody: 55.4% and cancer cells alone: 47.6%). These results
indicated that TIMP-1 secreted from the CAFs promoted cancer cell
migration.
The mechanism underlying the cancer-promoting effect
of TIMP-1 involves the conformational activation of integrin β1 and
activation of mitogen-activated protein kinase signaling induced by
the interaction between TIMP-1 and the TIMP-1 interacting cell
surface protein CD63 (11,41). CD63 is a member of the tetraspanins,
which are a superfamily of cell surface-associated membrane
proteins involved in cell activation, adhesion, differentiation,
migration, and invasion. CD63 is detected in the late endosomes,
lysosomes, secretory vesicles, and plasma membrane (10). Western blot analysis revealed that
the LoVo cells exhibited enhanced expression of CD63, while the
HT29 and HCT116 cells exhibited weak CD63 expression; these
observations concurred with the results of an earlier study
(41). Hence, TIMP-1 may enhance the
migration of only LoVo cells. Currently, there are limited data on
the expression of CD63 and the effect of TIMP-1 on the migration of
various colon cancer cell lines. The results of the present study
suggest that the differential effect of TIMP-1 on cell migration
can be attributed to differential CD63 expression.
The main source of TIMP-1 secretion in the
colorectal cancer tissue has not been well studied. Our study
revealed that TIMP-1 was mainly secreted from the CAFs and that the
TIMP-1 secretion from the cancer cells was low. Alpizar-Alpizar
et al and Gong et al demonstrated enhanced TIMP-1
expression in the cancer stroma through immunohistochemical
staining, which is consistent with our results (25,28).
However, Niewiarowska et al reported enhanced TIMP-1
expression in cancer cells (29).
Moreover, simultaneous TIMP-1 expression in both cancer cells and
stromal fibroblasts was also demonstrated by Kahlert et al
(36). These discordant results may
be due to the methodological differences. However, both cancer
cells and CAFs may be the potential source of TIMP-1 (25). Although TIMP-1 secretion levels
varied between the established CAFs, the correlation between TIMP-1
secretion levels and patient clinicopathological characteristics is
unclear. The CAFs are generally a heterogeneous population in each
individual. Hence, the secretion of TIMP-1 from the CAFs derived
from different individuals may vary (15,16).
Interestingly, the levels of TIMP-1 secreted from
the CAFs in the present study were shown to be significantly higher
than those secreted from the CAFs co-cultured with the cancer
cells. The cytokine antibody array for MMPs and TIMPs revealed that
the CAFs secreted various cytokines and that their secretion was
influenced by the presence of cancer cells. Enhanced secretion of
CAF-derived tumor-promoting factors, such as chemokines,
interleukins, growth factors, and transcription factors, is
mediated by the interaction between CAFs and cancer cells (14–17,24,27). Our
previous studies also demonstrated a similar phenomenon in
angiogenesis, where the secretion of VEGFA by the CAFs co-cultured
with the cancer cells was significantly higher than that by the
monocultured CAFs (14). The results
of this study also demonstrated that co-culturing cancer cells
potentiates the ability of CAFs to promote cancer cell progression.
Additionally, cancer-stromal interaction (CSI) was observed not
only during angiogenesis and proliferation but also during cancer
cell migration. Consistent with our results, several previous
immunohistochemical studies demonstrated the enhanced TIMP-1
secretion from CAFs in the tumor tissues (25,28,36).
However, the role of cancer cells in promoting TIMP-1 secretion by
the CAFs in vitro has never been reported. Our results
demonstrated that cancer cells promote enhanced TIMP-1 secretion by
the CAFs in vitro using simple co-culture models. Further
studies are needed to determine the underlying factors and their
role in enhancing the TIMP-1 production from CAFs.
In conclusion, this study demonstrated that CAFs
exhibit cancer-promoting activity in CD63-positive colon cancer
through the secretion of TIMP-1, which can potentially promote
colon cancer cell migration. The secretion of TIMP-1 by the CAFs
was further enhanced through the interaction with the colon cancer
cells. Thus, CAFs and TIMP-1 could be potential novel therapeutic
targets for the clinical treatment of patients with colon
cancer.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
NN, AM, TS, KT and NA performed most of the
experiments. NN, TY, YMae, TH and KS collected the cultured
fibroblasts. NN, MH and HT designed the study. NN, MH, RO, YMat and
HT analyzed the obtained data. ST conducted the entire study. All
authors read and approved the manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
The study protocol was approved by Nagoya City
University Graduate School of Medical Sciences and Nagoya City
University Hospital Institutional Review Board (Institutional code,
70-00-0071). Written informed consent was obtained from the
patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ECM
|
extracellular matrix
|
|
MMP
|
matrix metalloproteinase
|
|
TIMP
|
tissue inhibitors of
metalloproteinase
|
|
CAFs
|
cancer-associated fibroblasts
|
|
CSI
|
cancer-stromal interaction
|
References
|
1
|
Valastyan S and Weinberg RA: Tumor
metastasis: Molecular insights and evolving paradigms. Cell.
147:275–292. 2011. View Article : Google Scholar
|
|
2
|
Friedl P and Wolf K: Tumour-cell invasion
and migration: Diversity and escape mechanisms. Nat Rev Cancer.
3:362–374. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bourboulia D and Stetler-Stevenson WG:
Matrix metalloproteinases (MMPs) and tissue inhibitors of
metalloproteinases (TIMPs): Positive and negative regulators in
tumor cell adhesion. Semin Cancer Biol. 20:161–168. 2010.
View Article : Google Scholar
|
|
4
|
Chirco R, Liu XW, Jung KK and Kim HR:
Novel functions of TIMPs in cell signaling. Cancer Metastasis Rev.
25:99–113. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Egeblad M and Werb Z: New functions for
the matrix metalloproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Koliaraki V, Pallangyo CK, Greten FR and
Kollias G: Mesenchymal cells in colon cancer. Gastroenterology.
152:964–979. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Park SA, Kim MJ, Park SY, Kim JS, Lim W,
Nam JS and Yhong Sheen Y: TIMP-1 mediates TGF-β-dependent crosstalk
between hepatic stellate and cancer cells via FAK signaling. Sci
Rep. 5:164922015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tarpgaard LS, Ørum-Madsen MS, Christensen
IJ, Nordgaard C, Noer J, Guren TK, Glimelius B, Sorbye H, Ikdahl T,
Kure EH, et al: TIMP-1 is under regulation of the EGF signaling
axis and promotes an aggressive phenotype in KRAS-mutated
colorectal cancer cells: A potential novel approach to the
treatment of metastatic colorectal cancer. Oncotarget.
7:59441–59457. 2016. View Article : Google Scholar
|
|
9
|
Luparello C, Avanzato G, Carella C and
Pucci-Minafra I: Tissue inhibitor of metalloprotease (TIMP)-1 and
proliferative behaviour of clonal breast cancer cells. Breast
Cancer Res Treat. 54:235–244. 1999. View Article : Google Scholar
|
|
10
|
Jung KK, Liu XW, Chirco R, Fridman R and
Kim HR: Identification of CD63 as a tissue inhibitor of
metalloproteinase-1 interacting cell surface protein. EMBO J.
25:3934–3942. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Grunwald B, Schoeps B and Krüger A:
Recognizing the molecular multifunctionality and interactome of
TIMP-1. Trends Cell Biol. 29:6–19. 2019. View Article : Google Scholar
|
|
12
|
Forte D, Salvestrini V, Corradi G, Rossi
L, Catani L, Lemoli RM, Cavo M and Curti A: The tissue inhibitor of
metalloproteinases-1 (TIMP-1) promotes survival and migration of
acute myeloid leukemia cells through CD63/PI3K/Akt/p21 signaling.
Oncotarget. 8:2261–2274. 2017. View Article : Google Scholar
|
|
13
|
Song G, Xu S, Zhang H, Wang Y, Xiao C,
Jiang T, Wu L, Zhang T, Sun X, Zhong L, et al: TIMP1 is a
prognostic marker for the progression and metastasis of colon
cancer through FAK-PI3K/AKT and MAPK pathway. J Exp Clin Cancer
Res. 35:1482016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nagasaki T, Hara M, Nakanishi H, Takahashi
H, Sato M and Takeyama H: Interleukin-6 released by colon
cancer-associated fibroblasts is critical for tumour angiogenesis:
Anti-interleukin-6 receptor antibody suppressed angiogenesis and
inhibited tumour-stroma interaction. Br J Cancer. 110:469–478.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shiga K, Hara M, Nagasaki T, Sato T,
Takahashi H and Takeyama H: Cancer-associated fibroblasts: Their
characteristics and their roles in tumor growth. Cancers (Basel).
7:2443–2458. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tao L, Huang G, Song H, Chen Y and Chen L:
Cancer associated fibroblasts: An essential role in the tumor
microenvironment. Oncol Lett. 14:2611–2620. 2017. View Article : Google Scholar
|
|
17
|
Erdogan B and Webb DJ: Cancer-associated
fibroblasts modulate growth factor signaling and extracellular
matrix remodeling to regulate tumor metastasis. Biochem Soc Trans.
45:229–236. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cirri P and Chiarugi P:
Cancer-associated-fibroblasts and tumour cells: A diabolic liaison
driving cancer progression. Cancer Metastasis Rev. 31:195–208.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Santi A, Kugeratski FG and Zanivan S:
Cancer associated fibroblasts: The architects of stroma remodeling.
Proteomics. 18:e17001672018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ostman A and Augsten M: Cancer-associated
fibroblasts and tumor growth-bystanders turning into key players.
Curr Opin Genet Dev. 19:67–73. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shimoda M, Mellody KT and Orimo A:
Carcinoma-associated fibroblasts are a rate-limiting determinant
for tumour progression. Semin Cell Dev Biol. 21:19–25. 2010.
View Article : Google Scholar
|
|
22
|
Wei L, Ye H, Li G, Lu Y, Zhou Q, Zheng S,
Lin Q, Liu Y, Li Z and Chen R: Cancer-associated fibroblasts
promote progression and gemcitabine resistance via the SDF-1/SATB-1
pathway in pancreatic cancer. Cell Death Dis. 9:10652018.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gaggioli C, Hooper S, Hidalgo-Carcedo C,
Grosse R, Marshall JF, Harrington K and Sahai E: Fibroblast-led
collective invasion of carcinoma cells with differing roles for
RhoGTPases in leading and following cells. Nat Cell Biol.
9:1392–1400. 2007. View Article : Google Scholar
|
|
24
|
Tyan SW, Kuo WH, Huang CK, Pan CC, Shew
JY, Chang KJ, Lee EY and Lee WH: Breast cancer cells induce
cancer-associated fibroblasts to secrete hepatocyte growth factor
to enhance breast tumorigenesis. PLoS One. 6:e153132011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Alpizar-Alpizar W, Laerum OD, Christensen
IJ, Ovrebo K, Skarstein A, Høyer-Hansen G, Ploug M and Illemann M:
Tissue inhibitor of metalloproteinase-1 is confined to
tumor-associated myofibroblasts and is increased with progression
in gastric adenocarcinoma. J Histochem Cytochem. 64:483–494. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zheng X, Xu M, Yao B, Wang C, Jia Y and
Liu Q: IL-6/STAT3 axis initiated CAFs via up-regulating TIMP-1
which was attenuated by acetylation of STAT3 induced by PCAF in HCC
microenvironment. Cell Signal. 28:1314–1324. 2016. View Article : Google Scholar
|
|
27
|
Salvatore V, Teti G, Focaroli S, Mazzotti
MC, Mazzotti A and Falconi M: The tumor microenvironment promotes
cancer progression and cell migration. Oncotarget. 8:9608–9616.
2017. View Article : Google Scholar
|
|
28
|
Gong Y, Scott E, Lu R, Xu Y, Oh WK and Yu
Q: TIMP-1 promotes accumulation of cancer associated fibroblasts
and cancer progression. PLoS One. 8:e773662013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Niewiarowska K, Pryczynicz A,
Dymicka-Piekarska V, Gryko M, Cepowicz D, Famulski W, Kemona A and
Guzińska-Ustymowicz K: Diagnostic significance of TIMP-1 level in
serum and its immunohistochemical expression in colorectal cancer
patients. Pol J Pathol. 65:296–304. 2014. View Article : Google Scholar
|
|
30
|
Valster A, Tran NL, Nakada M, Berens ME,
Chan AY and Symons M: Cell migration and invasion assays. Methods.
37:208–215. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Guy JB, Espenel S, Vallard A,
Battiston-Montagne P, Wozny AS, Ardail D, Alphonse G, Rancoule C,
Rodriguez-Lafrasse C and Magne N: Evaluation of the cell invasion
and migration process: A comparison of the video microscope-based
scratch wound assay and the boyden chamber assay. J Vis Exp.
129:563372017.
|
|
32
|
Grada A, Otero-Vinas M, Prieto-Castrillo
F, Obagi Z and Falanga V: Research techniques made simple: Analysis
of collective cell migration using the wound healing assay. J
Invest Dermatol. 137:e11–e16. 2017. View Article : Google Scholar
|
|
33
|
Yukawa N, Yoshikawa T, Akaike M, Sugimasa
Y, Rino Y, Masuda M and Imada T: Impact of plasma tissue inhibitor
of matrix metalloproteinase-1 on long-term survival in patients
with colorectal cancer. Oncology. 72:205–208. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Böckelman C, Beilmann-Lehtonen I, Kaprio
T, Koskensalo S, Tervahartiala T, Mustonen H, Stenman UH, Sorsa T
and Haglund C: Serum MMP-8 and TIMP-1 predict prognosis in
colorectal cancer. BMC Cancer. 18:6792018. View Article : Google Scholar
|
|
35
|
Birgisson H, Nielsen HJ, Christensen IJ,
Glimelius B and Brünner N: Preoperative plasma TIMP-1 is an
independent prognostic indicator in patients with primary
colorectal cancer: A prospective validation study. Eur J Cancer.
46:3323–3331. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kahlert C, Bandapalli OR, Schirmacher P,
Weitz J and Brand K: Invasion front-specific overexpression of
tissue inhibitor of metalloproteinase-1 in liver metastases from
colorectal cancer. Anticancer Res. 28:1459–1465. 2008.PubMed/NCBI
|
|
37
|
Lee SY, Park SY, Kim SH and Choi EC:
Expression of matrix metalloproteinases and their inhibitors in
squamous cell carcinoma of the tonsil and their clinical
significance. Clin Exp Otorhinolaryngol. 4:88–94. 2011. View Article : Google Scholar
|
|
38
|
Medeiros M, Ribeiro AO, Lupi LA, Romualdo
GR, Pinhal D, Chuffa LGA and Delella FK: Mimicking the tumor
microenvironment: Fibroblasts reduce miR-29b expression and
increase the motility of ovarian cancer cells in a co-culture
model. Biochem Biophys Res Commun. 516:96–101. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen Z, Yan X, Li K, Ling Y and Kang H:
Stromal fibroblast-derived MFAP5 promotes the invasion and
migration of breast cancer cells via Notch1/slug signaling. Clin
Transl Oncol. 22:522–531. 2020. View Article : Google Scholar
|
|
40
|
Nakagawa H, Liyanarachchi S, Davuluri RV,
Auer H, Martin EW Jr, de la Chapelle A and Frankel WL: Role of
cancer-associated stromal fibroblasts in metastatic colon cancer to
the liver and their expression profiles. Oncogene. 23:7366–7377.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sordat I, Decraene C, Silvestre T,
Petermann O, Auffray C, Piétu G and Sordat B: Complementary DNA
arrays identify CD63 tetraspanin and alpha3 integrin chain as
differentially expressed in low and high metastatic human Colon
Carcinoma Cells. Lab. Invest. 82:1715–1724. 2002.
|