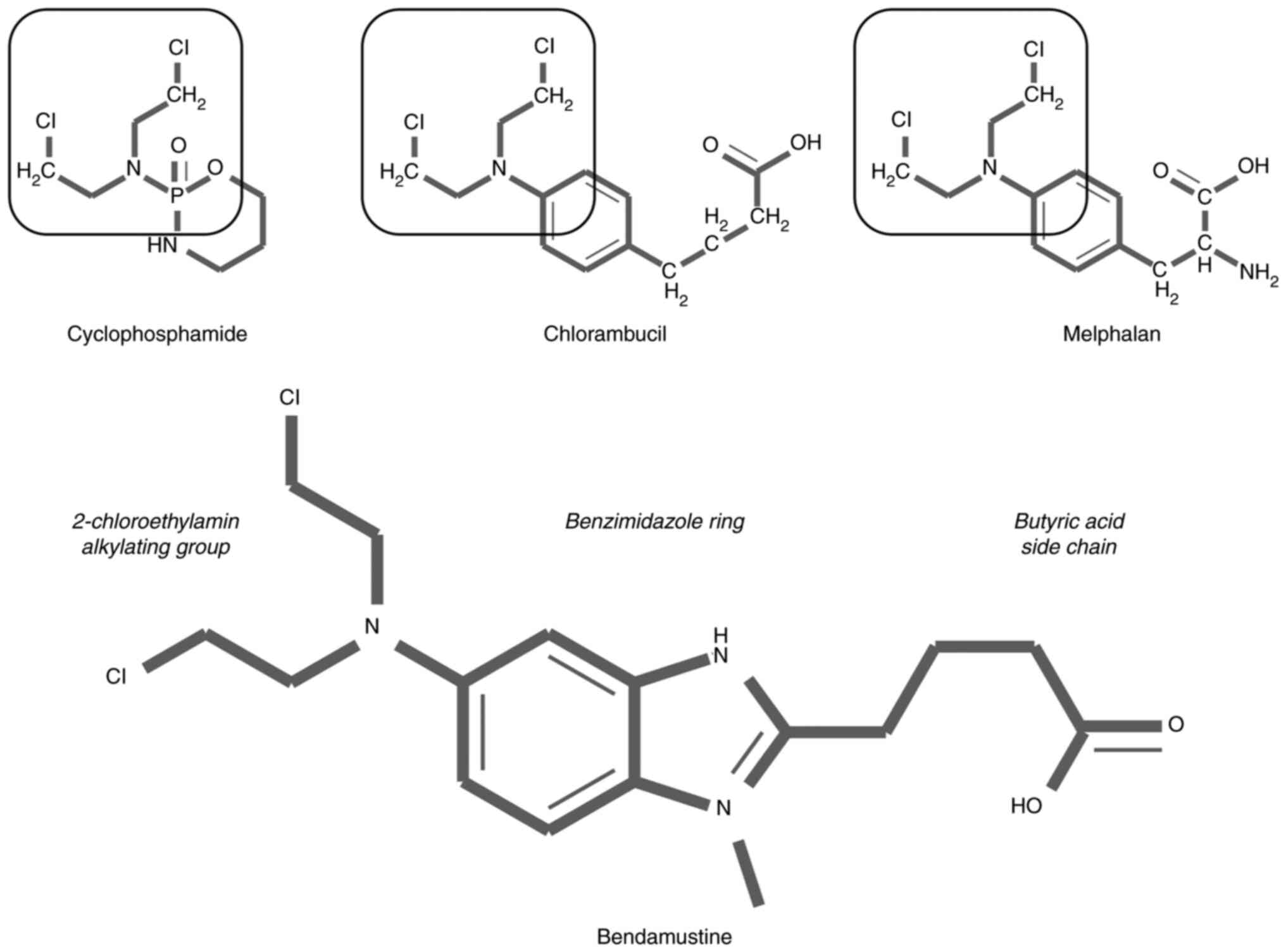
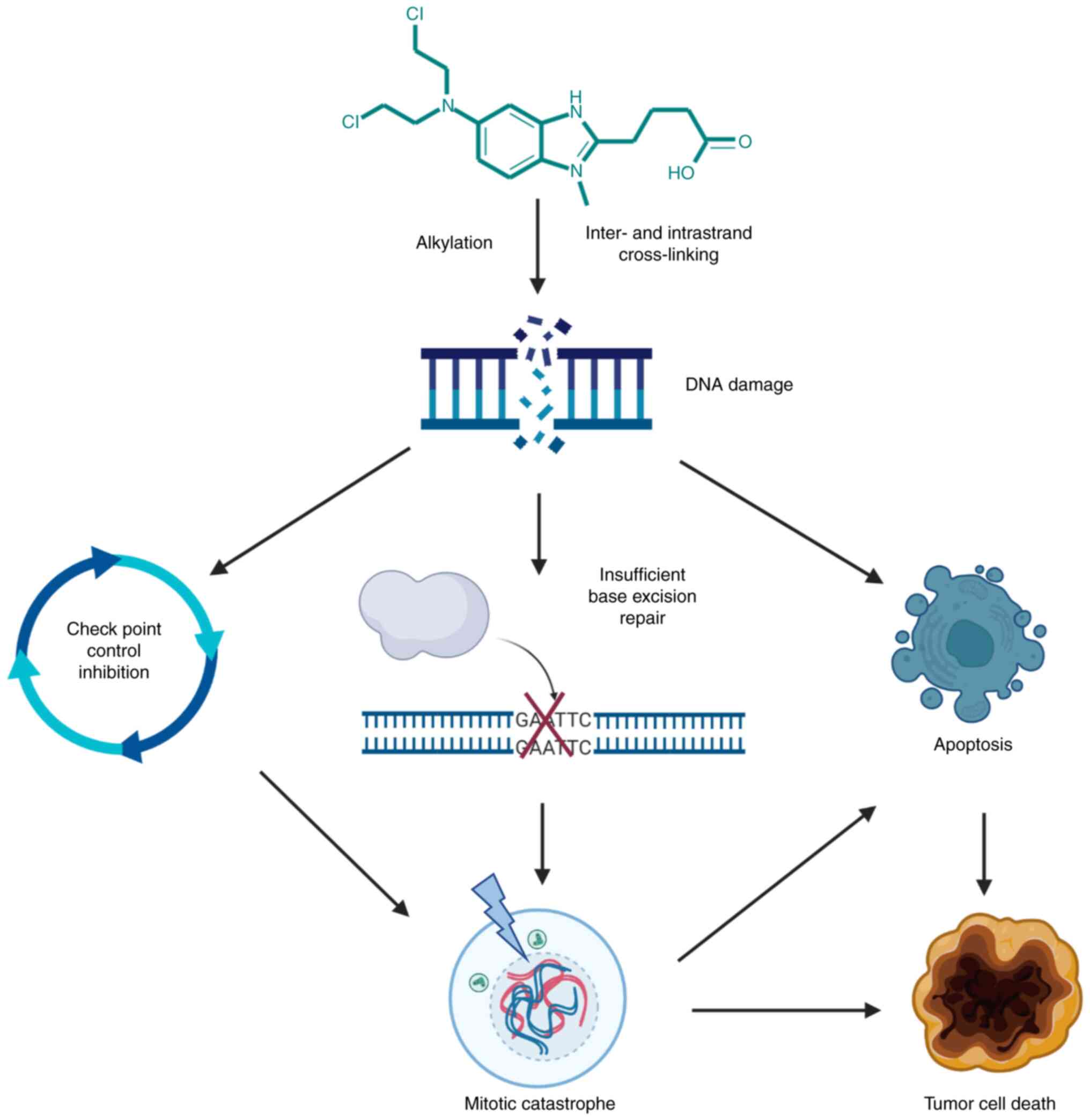
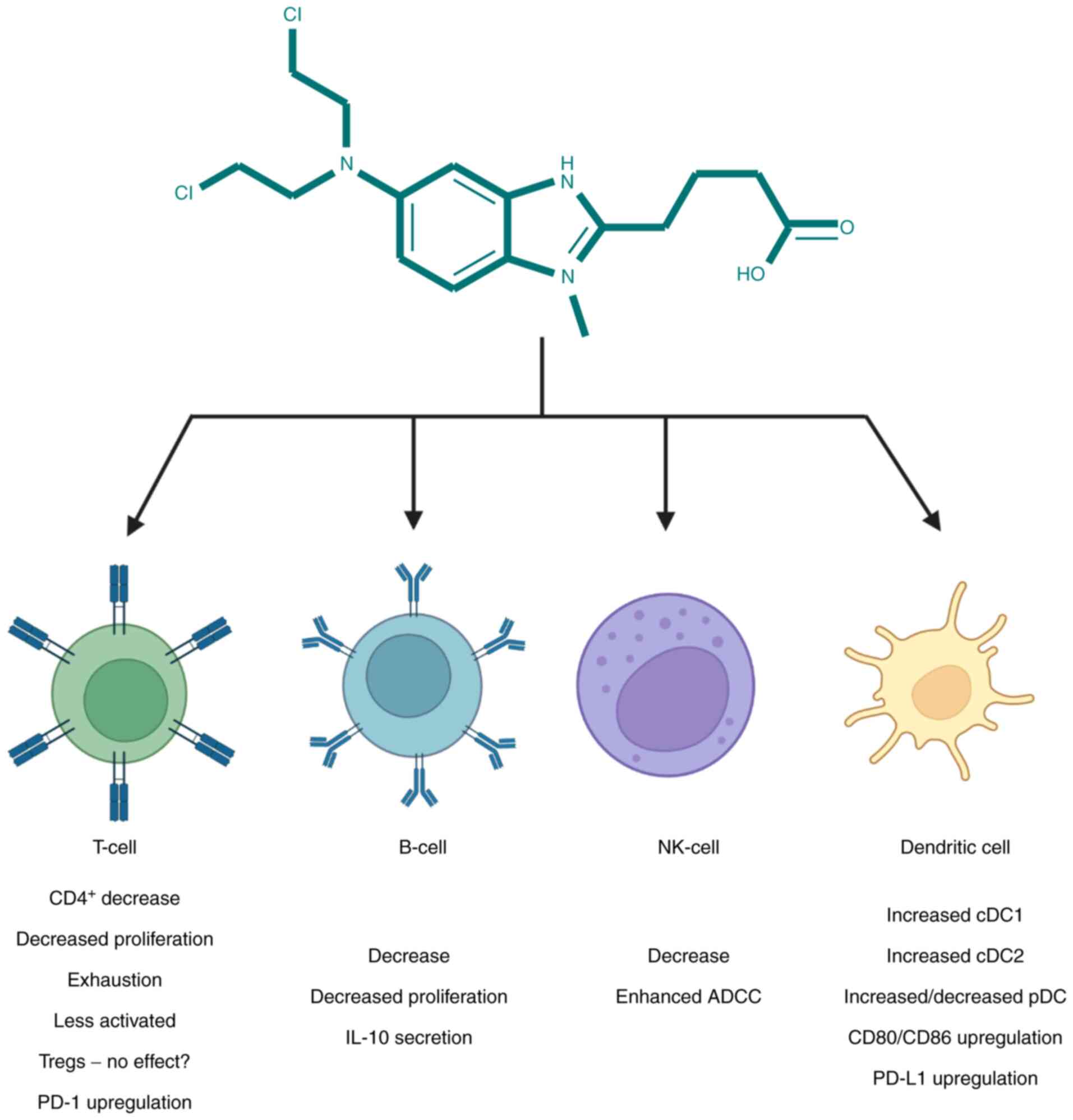
|
1
|
WHO collaborating centre for drug
statistics methodology, . ATC classification index with DDDs. Oslo,
Norway: 2021
|
|
2
|
Cheson BD and Rummel MJ: Bendamustine:
Rebirth of an Old Drug. J Clin Oncol. 27:1492–1501. 2009.
View Article : Google Scholar
|
|
3
|
Cheson BD and Leoni L: Bendamustine:
Mechanism of action and clinical data. Clin Adv Hematol Oncol. 9 (8
Suppl 19):S1–S11. 2011.
|
|
4
|
World Health Organization model list of
essential medicines, 21st list 2019, . Geneva: World Health
Organization; 2019
|
|
5
|
Gandhi V and Burger JA: Bendamustine in
B-cell malignancies: The new 46-year-old kid on the block. Clin
Cancer Res. 15:7456–7461. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Garnock-Jones KP: Bendamustine: A review
of its use in the management of indolent non-Hodgkin's lymphoma and
mantle cell lymphoma. Drugs. 70:1703–1718. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tageja N: Bendamustine: Safety and
efficacy in the management of indolent non-hodgkins lymphoma. Clin
Med Insights Oncol. 5:145–156. 2011. View Article : Google Scholar
|
|
8
|
Hoy SM: Bendamustine: A review of its use
in the management of chronic lymphocytic leukaemia,
rituximab-refractory indolent non-Hodgkin's lymphoma and multiple
myeloma. Drugs. 72:1929–1950. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fu D, Calvo JA and Samson LD: Balancing
repair and tolerance of DNA damage caused by alkylating agents. Nat
Rev Cancer. 12:104–120. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Leoni LM, Bailey B, Reifert J, Bendall HH,
Zeller RW, Corbeil J, Elliott G and Niemeyer CC: Bendamustine
(Treanda) displays a distinct pattern of cytotoxicity and unique
mechanistic features compared with other alkylating agents. Clin
Cancer Res. 14:309–317. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Leoni LM and Hartley JA: Mechanism of
action: The unique pattern of bendamustine-induced cytotoxicity.
Semin Hematol. 48 (Suppl 1):S12–S23. 2011. View Article : Google Scholar
|
|
12
|
Leoni LM: Bendamustine: Rescue of an
effective antineoplastic agent from the mid-twentieth century.
Semin Hematol. 48 (Suppl 1):S4–S11. 2011. View Article : Google Scholar
|
|
13
|
Hiraoka N, Kikuchi J, Yamauchi T, Koyama
D, Wada T, Uesawa M, Akutsu M, Mori S, Nakamura Y, Ueda T, et al:
Purine analog-like properties of bendamustine underlie rapid
activation of DNA damage response and synergistic effects with
pyrimidine analogues in lymphoid malignancies. PLoS One.
9:e906752014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Arimany-Nardi C, Montraveta A, Lee-Vergés
E, Puente XS, Koepsell H, Campo E, Colomer D and Pastor-Anglada M:
Human organic cation transporter 1 (hOCT1) as a mediator of
bendamustine uptake and cytotoxicity in chronic lymphocytic
leukemia (CLL) cells. Pharmacogenomics J. 15:363–371. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hagos Y, Hundertmark P, Shnitsar V, Marada
VV, Wulf G and Burckhardt G: Renal human organic anion transporter
3 increases the susceptibility of lymphoma cells to bendamustine
uptake. Am J Physiol Physiol. 308:F330–F338. 2015. View Article : Google Scholar
|
|
16
|
Schwänen C, Hecker T, Hübinger G, Wölfle
M, Rittgen W, Bergmann L and Karakas T: In vitro evaluation of
bendamustine induced apoptosis in B-chronic lymphocytic leukemia.
Leukemia. 16:2096–2105. 2002. View Article : Google Scholar
|
|
17
|
Matthews HK, Bertoli C and de Bruin RAM:
Cell cycle control in cancer. Nat Rev Mol Cell Biol. 23:74–88.
2022. View Article : Google Scholar
|
|
18
|
Vitale I, Galluzzi L, Castedo M and
Kroemer G: Mitotic catastrophe: A mechanism for avoiding genomic
instability. Nat Rev Mol Cell Biol. 12:385–392. 2011. View Article : Google Scholar
|
|
19
|
Cai B, Lyu H, Huang J, Wang S, Lee CK, Gao
C and Liu B: Combination of bendamustine and entinostat
synergistically inhibits proliferation of multiple myeloma cells
via induction of apoptosis and DNA damage response. Cancer Lett.
335:343–350. 2013. View Article : Google Scholar
|
|
20
|
Cai B, Wang S, Huang J, Lee CK, Gao C and
Liu B: Cladribine and bendamustine exhibit inhibitory activity in
dexamethasone-sensitive and -resistant multiple myeloma cells. Am J
Transl Res. 5:36–46. 2013.PubMed/NCBI
|
|
21
|
Visco C, Castegnaro S, Chieregato K,
Bernardi M, Albiero E, Zanon C, Madeo D and Rodeghiero F: The
cytotoxic effects of bendamustine in combination with cytarabine in
mantle cell lymphoma cell lines. Blood Cells Mol Dis. 48:68–75.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gaul L, Mandl-Weber S, Baumann P, Emmerich
B and Schmidmaier R: Bendamustine induces G2 cell cycle arrest and
apoptosis in myeloma cells: The role of ATM-Chk2-Cdc25A and
ATM-p53-p21-pathways. J Cancer Res Clin Oncol. 134:245–253. 2008.
View Article : Google Scholar
|
|
23
|
Kaneko N, Mitsuoka K, Amino N, Yamanaka K,
Kita A, Mori M, Miyoshi S and Kuromitsu S: Combination of YM155, a
survivin suppressant, with bendamustine and rituximab: A new
combination therapy to treat relapsed/refractory diffuse large
B-cell lymphoma. Clin Cancer Res. 20:1814–1822. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Beeharry N, Rattner JB, Bellacosa A, Smith
MR and Yen TJ: Dose dependent effects on cell cycle checkpoints and
DNA repair by bendamustine. PLoS One. 7:e403422012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Darwish M, Bond M, Hellriegel E, Robertson
P Jr and Chovan JP: Pharmacokinetic and pharmacodynamic profile of
bendamustine and its metabolites. Cancer Chemother Pharmacol.
75:1143–1154. 2015. View Article : Google Scholar
|
|
26
|
Dubbelman AC, Rosing H, Darwish M,
D'Andrea D, Bond M, Hellriegel E, Robertson P Jr, Beijnen JH and
Schellens JH: Pharmacokinetics and excretion of 14C-bendamustine in
patients with relapsed or refractory malignancy. Drugs R D.
13:17–28. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim T, Choi HY, Lee HS, Jung SH, Ahn JS,
Kim HJ, Lee JJ, Yoo HD and Yang DH: Clinical response and
pharmacokinetics of bendamustine as a component of salvage R-B(O)AD
therapy for the treatment of primary central nervous system
lymphoma (PCNSL). BMC Cancer. 18:7292018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cephalon Inc., . Treanda (bendamustine
hydrochloride for injection) for intravenous infusion. US
prescribing information. 2021.
|
|
29
|
Knauf WU, Lissitchkov T, Aldaoud A,
Liberati AM, Loscertales J, Herbrecht R, Juliusson G, Postner G,
Gercheva L, Goranov S, et al: Bendamustine compared with
chlorambucil in previously untreated patients with chronic
lymphocytic leukaemia: Updated results of a randomized phase III
trial. Br J Haematol. 159:67–77. 2012. View Article : Google Scholar
|
|
30
|
Eichhorst B, Fink AM, Bahlo J, Busch R,
Kovacs G, Maurer C, Lange E, Köppler H, Kiehl M, Sökler M, et al:
First-line chemoimmunotherapy with bendamustine and rituximab
versus fludarabine, cyclophosphamide, and rituximab in patients
with advanced chronic lymphocytic leukaemia (CLL10): An
international, open-label, randomised, phase 3, non-inferiority
trial. Lancet Oncol. 17:928–942. 2016. View Article : Google Scholar
|
|
31
|
Woyach JA, Ruppert AS, Heerema NA, Zhao W,
Booth AM, Ding W, Bartlett NL, Brander DM, Barr PM, Rogers KA, et
al: Ibrutinib regimens versus chemoimmunotherapy in older patients
with untreated CLL. N Engl J Med. 379:2517–2528. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ghia P, Pluta A, Wach M, Lysak D, Kozak T,
Simkovic M, Kaplan P, Kraychok I, Illes A, de la Serna J, et al:
ASCEND: Phase III, randomized trial of acalabrutinib versus
idelalisib plus rituximab or bendamustine plus rituximab in
relapsed or refractory chronic lymphocytic leukemia. J Clin Oncol.
38:2849–2861. 2020. View Article : Google Scholar
|
|
33
|
Fraser GAM, Chanan-Khan A, Demirkan F,
Santucci Silva R, Grosicki S, Janssens A, Mayer J, Bartlett NL,
Dilhuydy MS, Loscertales J, et al: Final 5-year findings from the
phase 3 HELIOS study of ibrutinib plus bendamustine and rituximab
in patients with relapsed/refractory chronic lymphocytic
leukemia/small lymphocytic lymphoma. Leuk Lymphoma. 61:3188–3197.
2020. View Article : Google Scholar
|
|
34
|
Seymour JF, Kipps TJ, Eichhorst B, Hillmen
P, D'Rozario J, Assouline S, Owen C, Gerecitano J, Robak T, De la
Serna J, et al: Venetoclax-rituximab in relapsed or refractory
chronic lymphocytic leukemia. N Engl J Med. 378:1107–1120. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Marcus R, Davies A, Ando K, Klapper W,
Opat S, Owen C, Phillips E, Sangha R, Schlag R, Seymour JF, et al:
Obinutuzumab for the first-line treatment of follicular lymphoma. N
Engl J Med. 377:1331–1344. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hiddemann W, Barbui AM, Canales MA,
Cannell PK, Collins GP, Dürig J, Forstpointner R, Herold M,
Hertzberg M, Klanova M, et al: Immunochemotherapy with obinutuzumab
or rituximab for previously untreated follicular lymphoma in the
GALLIUM study: Influence of chemotherapy on efficacy and safety. J
Clin Oncol. 36:2395–2404. 2018. View Article : Google Scholar
|
|
37
|
Rummel MJ, Niederle N, Maschmeyer G, Banat
GA, von Grünhagen U, Losem C, Kofahl-Krause D, Heil G, Welslau M,
Balser C, et al: Bendamustine plus rituximab versus CHOP plus
rituximab as first-line treatment for patients with indolent and
mantle-cell lymphomas: An open-label, multicentre, randomised,
phase 3 non-inferiority trial. Lancet. 381:1203–1210. 2013.
View Article : Google Scholar
|
|
38
|
Flinn IW, van der Jagt R, Kahl B, Wood P,
Hawkins T, MacDonald D, Simpson D, Kolibaba K, Issa S, Chang J, et
al: First-line treatment of patients with indolent non-Hodgkin
lymphoma or mantle-cell lymphoma with bendamustine plus rituximab
versus R-CHOP or R-CVP: Results of the BRIGHT 5-year follow-up
study. J Clin Oncol. 37:984–991. 2019. View Article : Google Scholar
|
|
39
|
Sehn LH, Chua N, Mayer J, Dueck G, Trněný
M, Bouabdallah K, Fowler N, Delwail V, Press O, Salles G, et al:
Obinutuzumab plus bendamustine versus bendamustine monotherapy in
patients with rituximab-refractory indolent non-Hodgkin lymphoma
(GADOLIN): A randomised, controlled, open-label, multicentre, phase
3 trial. Lancet Oncol. 17:1081–1093. 2016. View Article : Google Scholar
|
|
40
|
Cheson BD, Chua N, Mayer J, Dueck G,
Trněný M, Bouabdallah K, Fowler N, Delwail V, Press O, Salles G, et
al: Overall survival benefit in patients with rituximab-refractory
indolent non-Hodgkin lymphoma who received obinutuzumab plus
bendamustine induction and obinutuzumab maintenance in the GADOLIN
study. J Clin Oncol. 36:2259–2266. 2018. View Article : Google Scholar
|
|
41
|
Rummel M, Kaiser U, Balser C, Stauch M,
Brugger W, Welslau M, Niederle N, Losem C, Boeck HP, Weidmann E, et
al: Bendamustine plus rituximab versus fludarabine plus rituximab
for patients with relapsed indolent and mantle-cell lymphomas: A
multicentre, randomised, open-label, non-inferiority phase 3 trial.
Lancet Oncol. 17:57–66. 2016. View Article : Google Scholar
|
|
42
|
Visco C, Chiappella A, Nassi L, Patti C,
Ferrero S, Barbero D, Evangelista A, Spina M, Molinari A, Rigacci
L, et al: Rituximab, bendamustine, and low-dose cytarabine as
induction therapy in elderly patients with mantle cell lymphoma: A
multicentre, phase 2 trial from Fondazione Italiana Linfomi. Lancet
Haematol. 4:e15–e23. 2017. View Article : Google Scholar
|
|
43
|
McCulloch R, Visco C, Eyre TA, Frewin R,
Phillips N, Tucker DL, Quaglia FM, McMillan A, Lambert J, Crosbie N
and Rule S: Efficacy of R-BAC in relapsed, refractory mantle cell
lymphoma post BTK inhibitor therapy. Br J Haematol. 189:684–688.
2020. View Article : Google Scholar
|
|
44
|
Kamdar M, Li H, Chen RW, Rimsza LM,
Leblanc ML, Fenske TS, Shea TC, Barr PM, Phillips TJ, Leonard JP,
et al: Five-year outcomes of the S1106 study of R-hyper-CVAD vs
R-bendamustine in transplant-eligible patients with mantle cell
lymphoma. Blood Adv. 3:3132–3135. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Park SI, Grover NS, Olajide O, Asch AS,
Wall JG, Richards KL, Sobol AL, Deal AM, Ivanova A, Foster MC, et
al: A phase II trial of bendamustine in combination with rituximab
in older patients with previously untreated diffuse large B-cell
lymphoma. Br J Haematol. 175:281–289. 2016. View Article : Google Scholar
|
|
46
|
Flinn IW, Erter J, Daniel DB, Mace JR and
Berdeja JG: Phase II study of bendamustine and ofatumumab in
elderly patients with newly diagnosed diffuse large B-cell lymphoma
who are poor candidates for R-CHOP chemotherapy. Oncologist.
24:1035–e623. 2019. View Article : Google Scholar
|
|
47
|
Sehn LH, Hertzberg M, Opat S, Herrera AF,
Assouline S, Flowers CR, Kim TM, McMillan A, Ozcan M, Safar V, et
al: Polatuzumab vedotin plus bendamustine and rituximab in
relapsed/refractory DLBCL: Survival update and new extension cohort
data. Blood Adv. 6:533–543. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
O'Connor OA, Lue JK, Sawas A, Amengual JE,
Deng C, Kalac M, Falchi L, Marchi E, Turenne I, Lichtenstein R, et
al: Brentuximab vedotin plus bendamustine in relapsed or refractory
Hodgkin's lymphoma: An international, multicentre, single-arm,
phase 1–2 trial. Lancet Oncol. 19:257–266. 2018. View Article : Google Scholar
|
|
49
|
Broccoli A, Argnani L, Botto B, Corradini
P, Pinto A, Re A, Vitolo U, Fanti S, Stefoni V and Zinzani PL;
Fondazione Italiana Linfomi ONLUS, : First salvage treatment with
bendamustine and brentuximab vedotin in Hodgkin lymphoma: A phase 2
study of the Fondazione Italiana Linfomi. Blood Cancer J.
9:1002019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
LaCasce AS, Bociek RG, Sawas A, Caimi P,
Agura E, Matous J, Ansell SM, Crosswell HE, Islas-Ohlmayer M,
Behler C, et al: Three-year outcomes with brentuximab vedotin plus
bendamustine as first salvage therapy in relapsed or refractory
Hodgkin lymphoma. Br J Haematol. 189:e86–e90. 2020. View Article : Google Scholar
|
|
51
|
Hueso T, Gastinne T, Garciaz S, Tchernonog
E, Delette C, Casasnovas RO, Durot E, Houot R, Tessoulin B,
Tournilhac O, et al: Bendamustine-EAM versus BEAM regimen in
patients with mantle cell lymphoma undergoing autologous stem cell
transplantation in the frontline setting: A multicenter
retrospective study from lymphoma study association (LYSA) centers.
Bone Marrow Transplant. 55:1076–1084. 2020. View Article : Google Scholar
|
|
52
|
Gomez-Arteaga A, Mark TM, Guarneri D,
Christos PJ, Gergis U, Greenberg JD, Hsu J, Mayer SA, Niesvizky R,
Pearse RN, et al: High-dose bendamustine and melphalan conditioning
for autologous stem cell transplantation for patients with multiple
myeloma. Bone Marrow Transplant. 54:2027–2038. 2019. View Article : Google Scholar
|
|
53
|
Lee HC, Feng L, Oriabure O, Graham V, Chen
W, Badillo M, Lu R, Lee HJ, Jain P, Manasanch EE, et al: A phase
one trial of carfilzomib, bendamustine, and dexamethasone in
relapsed and/or refractory multiple myeloma. Am J Hematol.
96:E243–E246. 2021. View Article : Google Scholar
|
|
54
|
Chanan-Khan A, Cramer P, Demirkan F,
Fraser G, Silva RS, Grosicki S, Pristupa A, Janssens A, Mayer J,
Bartlett NL, et al: Ibrutinib combined with bendamustine and
rituximab compared with placebo, bendamustine, and rituximab for
previously treated chronic lymphocytic leukaemia or small
lymphocytic lymphoma (HELIOS): A randomised, double-blind, phase 3
study. Lancet Oncol. 17:200–211. 2016. View Article : Google Scholar
|
|
55
|
Fraser G, Cramer P, Demirkan F, Silva RS,
Grosicki S, Pristupa A, Janssens A, Mayer J, Bartlett NL, Dilhuydy
MS, et al: Updated results from the phase 3 HELIOS study of
ibrutinib, bendamustine, and rituximab in relapsed chronic
lymphocytic leukemia/small lymphocytic lymphoma. Leukemia.
33:969–980. 2019. View Article : Google Scholar
|
|
56
|
Cheson BD, Brugger W, Damaj G, Dreyling M,
Kahl B, Kimby E, Ogura M, Weidmann E, Wendtner CM and Zinzani PL:
Optimal use of bendamustine in hematologic disorders: Treatment
recommendations from an international consensus panel-an update.
Leuk Lymphoma. 57:766–782. 2016. View Article : Google Scholar
|
|
57
|
Flinn IW, van der Jagt R, Kahl BS, Wood P,
Hawkins TE, Macdonald D, Hertzberg M, Kwan YL, Simpson D, Craig M,
et al: Randomized trial of bendamustine-rituximab or R-CHOP/R-CVP
in first-line treatment of indolent NHL or MCL: The BRIGHT study.
Blood. 123:2944–2952. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Martin P, Chen Z, Cheson BD, Robinson KS,
Williams M, Rajguru SA, Friedberg JW, van der Jagt RH, LaCasce AS,
Joyce R, et al: Long-term outcomes, secondary malignancies and stem
cell collection following bendamustine in patients with previously
treated non-Hodgkin lymphoma. Br J Haematol. 178:250–256. 2017.
View Article : Google Scholar
|
|
59
|
Fung M, Jacobsen E, Freedman A, Prestes D,
Farmakiotis D, Gu X, Nguyen PL and Koo S: Increased risk of
infectious complications in older patients with indolent
non-Hodgkin lymphoma exposed to bendamustine. Clin Infect Dis.
68:247–255. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Pezzullo L, Giudice V, Serio B, Fontana R,
Guariglia R, Martorelli MC, Ferrara I, Mettivier L, Bruno A, Bianco
R, et al: Real-world evidence of cytomegalovirus reactivation in
non-Hodgkin lymphomas treated with bendamustine-containing
regimens. Open Med (Wars). 16:672–682. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Chen RW, Li H, Bernstein SH, Kahwash S,
Rimsza LM, Forman SJ, Constine L, Shea TC, Cashen AF, Blum KA, et
al: RB but not R-HCVAD is a feasible induction regimen prior to
auto-HCT in frontline MCL: Results of SWOG study S1106. Br J
Haematol. 176:759–769. 2017. View Article : Google Scholar
|
|
62
|
Smith A, Roman E, Appleton S, Howell D,
Johnson R, Burton C and Patmore R: Impact of novel therapies for
mantle cell lymphoma in the real world setting: A report from the
UK's haematological malignancy research network (HMRN). Br J
Haematol. 181:215–228. 2018. View Article : Google Scholar
|
|
63
|
Bašić-Kinda S, Mišura Jakobac K,
Sinčić-Petričević J, Deak D, Vodanović M, Jakić-Bubalo M, Mitrović
Z, Grubešić A, Dreta B, Županić Krmek D, et al: Improvement in the
outcomes of mantle cell lymphoma in the last decade: A real-life
non interventional study of the croatian cooperative group for
hematologic diseases. Croat Med J. 62:455–463. 2021. View Article : Google Scholar
|
|
64
|
Roué G, López-Guerra M, Milpied P,
Pérez-Galán P, Villamor N, Montserrat E, Campo E and Colomer D:
Bendamustine is effective in p53-deficient B-cell neoplasms and
requires oxidative stress and caspase-independent signaling. Clin
Cancer Res. 14:6907–6915. 2008. View Article : Google Scholar
|
|
65
|
Aurer I: Mantle cell lymphoma in patients
not eligible for autologous stem cell transplantation. Curr Opin
Oncol. 31:374–379. 2019. View Article : Google Scholar
|
|
66
|
Aukema SM, Hoster E, Rosenwald A, Canoni
D, Delfau-Larue MH, Rymkiewicz G, Thorns C, Hartmann S,
Kluin-Nelemans H, Hermine O, et al: Expression of TP53 is
associated with the outcome of MCL independent of MIPI and Ki-67 in
trials of the European MCL network. Blood. 131:417–420. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
FDA approves polatuzumab vedotin-piiq for
diffuse large B-cell lymphoma. 2019.
|
|
68
|
Nikitorowicz-Buniak J: CHMP recommends EMA
approval of polatuzumab vedotin for the treatment of adult patients
with R/R DLBCL. 2019.
|
|
69
|
Moskowitz AJ, Hamlin PA Jr, Perales MA,
Gerecitano J, Horwitz SM, Matasar MJ, Noy A, Palomba ML, Portlock
CS, Straus DJ, et al: Phase II study of bendamustine in relapsed
and refractory Hodgkin lymphoma. J Clin Oncol. 31:456–460. 2013.
View Article : Google Scholar
|
|
70
|
Rinnerthaler G, Gampenrieder SP, Petzer A,
Hubalek M, Petru E, Sandholzer M, Andel J, Balic M, Melchardt T,
Hauser-Kronberger C, et al: Capecitabine in combination with
bendamustine in pretreated women with HER2-negative metastatic
breast cancer: Results of a phase II trial (AGMT MBC-6). Ther Adv
Med Oncol. 13:175883592110423012021. View Article : Google Scholar
|
|
71
|
Lammers PE, Shyr Y, Li CI, Hutchison AS,
Sandler A, Carbone DP, Johnson DH, Keedy VL and Horn L: Phase II
study of bendamustine in relapsed chemotherapy sensitive or
resistant small-cell lung cancer. J Thorac Oncol. 9:559–562. 2014.
View Article : Google Scholar
|
|
72
|
Hartmann JT, Mayer F, Schleicher J, Horger
M, Huober J, Meisinger I, Pintoffl J, Käfer G, Kanz L and Grünwald
V; German sarcoma group, : Bendamustine hydrochloride in patients
with refractory soft tissue sarcoma: A noncomparative multicenter
phase 2 study of the German sarcoma group (AIO-001). Cancer.
110:861–866. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Chambers SK: Open trial of bendamustine
hydrochloride in women with advanced ovarian cancer. NCT00867503.
2012.
|
|
74
|
Friedberg JW, Cohen P, Chen L, Robinson
KS, Forero-Torres A, La Casce AS, Fayad LE, Bessudo A, Camacho ES,
Williams ME, et al: Bendamustine in patients with
rituximab-refractory indolent and transformed non-Hodgkin's
lymphoma: Results from a phase II multicenter, single-agent study.
J Clin Oncol. 26:204–210. 2008. View Article : Google Scholar
|
|
75
|
Knauf WU, Lissichkov T, Aldaoud A,
Liberati A, Loscertales J, Herbrecht R, Juliusson G, Postner G,
Gercheva L, Goranov S, et al: Phase III randomized study of
bendamustine compared with chlorambucil in previously untreated
patients with chronic lymphocytic leukemia. J Clin Oncol.
27:4378–4384. 2009. View Article : Google Scholar
|
|
76
|
Cheson BD, Friedberg JW, Kahl BS, Van der
Jagt RH and Tremmel L: Bendamustine produces durable responses with
an acceptable safety profile in patients with rituximab-refractory
indolent non-Hodgkin lymphoma. Clin Lymphoma Myeloma Leuk.
10:452–457. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Stokes J, Molina MS, Hoffman EA, Simpson
RJ and Katsanis E: Immunomodulatory effects of bendamustine in
hematopoietic cell transplantation. Cancers (Basel). 13:17022021.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Allegra A, Tonacci A, Musolino C, Pioggia
G and Gangemi S: Secondary immunodeficiency in hematological
malignancies: Focus on multiple myeloma and chronic lymphocytic
leukemia. Front Immunol. 12:7389152021. View Article : Google Scholar
|
|
79
|
Gafter-Gvili A, Gurion R, Raanani P,
Shpilberg O and Vidal L: Bendamustine-associated
infections-systematic review and meta-analysis of randomized
controlled trials. Hematol Oncol. 35:424–431. 2017. View Article : Google Scholar
|
|
80
|
Lamure S, Duléry R, Di Blasi R, Chauchet
A, Laureana C, Deau-Fischer B, Drenou B, Soussain C, Rossi C, Noël
N, et al: Determinants of outcome in Covid-19 hospitalized patients
with lymphoma: A retrospective multicentric cohort study.
EClinicalMedicine. 27:1005492020. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Fraser C, Brown P, Megason G, Ahn HS, Cho
B, Kirov I, Frankel L, Aplenc R, Bensen-Kennedy D, Munteanu M, et
al: Open-label bendamustine monotherapy for pediatric patients with
relapsed or refractory acute leukemia: Efficacy and tolerability. J
Pediatr Hematol Oncol. 36:e212–e218. 2014. View Article : Google Scholar
|
|
82
|
García Muñoz R, Izquierdo-Gil A, Muñoz A,
Roldan-Galiacho V, Rabasa P and Panizo C: Lymphocyte recovery is
impaired in patients with chronic lymphocytic leukemia and indolent
non-Hodgkin lymphomas treated with bendamustine plus rituximab. Ann
Hematol. 93:1879–1887. 2014. View Article : Google Scholar
|
|
83
|
Saito H, Maruyama D, Maeshima AM, Makita
S, Kitahara H, Miyamoto K, Fukuhara S, Munakata W, Suzuki T,
Kobayashi Y, et al: Prolonged lymphocytopenia after bendamustine
therapy in patients with relapsed or refractory indolent B-cell and
mantle cell lymphoma. Blood Cancer J. 5:e3622015. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Coutre SE, Flinn IW, de Vos S, Barrientos
JC, Schreeder MT, Wagner-Johnson ND, Sharman JP, Boyd TE, Fowler N,
Dreiling L, et al: Idelalisib in combination with rituximab or
bendamustine or both in patients with relapsed/refractory chronic
lymphocytic leukemia. Hemasphere. 2:e392018. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Rummel MJ, Janssens A, MacDonald D,
Keating MM, Zaucha JM, Davis J, Lasher J, Babanrao Pisal C,
Izquierdo M and Friedberg JW: A phase 3, randomized study of
ofatumumab combined with bendamustine in rituximab-refractory iNHL
(COMPLEMENT A + B study). Br J Haematol. 193:1123–1133. 2021.
View Article : Google Scholar
|
|
86
|
Schöffski P, Seeland G, Engel H, Grünwald
V, Paul H, Merkle K, Kowalski R and Ganser A: Weekly administration
of bendamustine: A phase I study in patients with advanced
progressive solid tumours. Ann Oncol. 11:729–734. 2000. View Article : Google Scholar
|
|
87
|
Bremer K: High rates of long-lasting
remissions after 5-day bendamustine chemotherapy cycles in
pre-treated low-grade non-Hodgkin's-lymphomas. J Cancer Res Clin
Oncol. 128:603–609. 2002. View Article : Google Scholar
|
|
88
|
Klippstein A, Schneider CP, Sayer HG and
Höffken K: Pneumocystis carinii pneumonia as a complication of
bendamustine monotherapy in a patient with advanced progressive
breast cancer. J Cancer Res Clin Oncol. 129:316–319. 2003.
View Article : Google Scholar
|
|
89
|
Layman RM, Ruppert AS, Lynn M, Mrozek E,
Ramaswamy B, Lustberg MB, Wesolowski R, Ottman S, Carothers S,
Bingman A, et al: Severe and prolonged lymphopenia observed in
patients treated with bendamustine and erlotinib for metastatic
triple negative breast cancer. Cancer Chemother Pharmacol.
71:1183–1190. 2013. View Article : Google Scholar
|
|
90
|
Burotto M, Stetler-Stevenson M, Arons E,
Zhou H, Wilson W and Kreitman RJ: Bendamustine and rituximab in
relapsed and refractory hairy cell leukemia. Clin Cancer Res.
19:6313–6321. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Martínez-Calle N, Hartley S, Ahearne M,
Kasenda B, Beech A, Knight H, Balotis C, Kennedy B, Wagner S, Dyer
MJS, et al: Kinetics of T-cell subset reconstitution following
treatment with bendamustine and rituximab for low-grade
lymphoproliferative disease: A population-based analysis. Br J
Haematol. 184:957–968. 2019.
|
|
92
|
Stokes J, Hoffman EA, Zeng Y, Larmonier N
and Katsanis E: Post-transplant bendamustine reduces GvHD while
preserving GvL in experimental haploidentical bone marrow
transplantation. Br J Haematol. 174:102–116. 2016. View Article : Google Scholar
|
|
93
|
Cona A, Tesoro D, Chiamenti M, Merlini E,
Ferrari D, Marti A, Codecà C, Ancona G, Tincati C, d'Arminio
Monforte A and Marchetti G: Disseminated cytomegalovirus disease
after bendamustine: A case report and analysis of circulating B-
and T-cell subsets. BMC Infect Dis. 19:8812019. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Stokes J, Hoffman EA, Molina MS, Kummet N,
Simpson RJ, Zeng Y and Katsanis E: Bendamustine with total body
irradiation conditioning yields tolerant T-cells while preserving
T-cell-dependent graft-versus-leukemia. Oncoimmunology.
9:17580112020. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
García M, Bellosillo B, Sánchez-González
B, García-Payarols F, Seoane A, Ferrer AM, Gimeno E, Barranco LE,
Torner A, Solé F, et al: Study of regulatory T-cells in patients
with gastric malt lymphoma: Influence on treatment response and
outcome. PLoS One. 7:e516812012. View Article : Google Scholar
|
|
96
|
Molina MS, Hoffman EA, Stokes J, Kummet N,
Smith KA, Baker F, Zúñiga TM, Simpson RJ and Katsanis E: Regulatory
dendritic cells induced by bendamustine are associated with
enhanced Flt3 expression and alloreactive T-cell death. Front
Immunol. 12:6991282021. View Article : Google Scholar
|
|
97
|
Lepik KV, Mikhailova NB, Kondakova EV,
Zalyalov YR, Fedorova LV, Tsvetkova LA, Kotselyabina PV, Borzenkova
ES, Babenko EV, Popova MO, et al: A study of safety and efficacy of
nivolumab and bendamustine (NB) in patients with
relapsed/refractory Hodgkin lymphoma after nivolumab monotherapy
failure. Hemasphere. 4:e4012020. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Montraveta A, Lee-Vergés E, Roldán J,
Jiménez L, Cabezas S, Clot G, Pinyol M, Xargay-Torrent S, Rosich L,
Arimany-Nardí C, et al: CD69 expression potentially predicts
response to bendamustine and its modulation by ibrutinib or
idelalisib enhances cytotoxic effect in chronic lymphocytic
leukemia. Oncotarget. 7:5507–5520. 2016. View Article : Google Scholar
|
|
99
|
Gafter-Gvili A and Polliack A:
Bendamustine associated immune suppression and infections during
therapy of hematological malignancies. Leuk Lymphoma. 57:512–519.
2016. View Article : Google Scholar
|
|
100
|
Yamasaki S, Matsushima T, Minami M,
Kadowaki M, Takase K and Iwasaki H: Clinical impact of bendamustine
exposure on lymphopenia risk after bendamustine and rituximab
combination therapy for follicular lymphoma: A single-institute
retrospective study. Ann Hematol. 101:209–211. 2022. View Article : Google Scholar
|
|
101
|
Lu L, Yoshimoto K, Morita A, Kameda H and
Takeuchi T: Bendamustine increases interleukin-10 secretion from B
cells via p38 MAP kinase activation. Int Immunopharmacol.
39:273–279. 2016. View Article : Google Scholar
|
|
102
|
Stenger EO, Turnquist HR, Mapara MY and
Thomson AW: Dendritic cells and regulation of graft-versus-host
disease and graft-versus-leukemia activity. Blood. 119:5088–5103.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Barbarroja-Escudero J, Sanchez-Gonzalez
MJ, Antolin-Amerigo D, Rodriguez-Rodriguez M and Alvarez-Mon M:
Hypersensitivity reactions and drug fever by bendamustine: A case
report of three patients. Allergol Int. 64:109–111. 2015.
View Article : Google Scholar
|
|
104
|
Chan M, Silverstein WK, Nikonova A,
Pavenski K and Hicks LK: Bendamustine-induced immune hemolytic
anemia: A case report and systematic review of the literature.
Blood Adv. 4:1756–1759. 2020. View Article : Google Scholar : PubMed/NCBI
|