Introduction
Lung cancer is the leading cause of cancer-related
mortality worldwide, characterized by drug resistance and poor
prognosis (1). Of all lung cancer
cases, lung adenocarcinoma (LUAD) accounts for >50% (2). Studies have made significant progress
in LUAD targeted therapy, such as the various tyrosine kinase
inhibitors used to effectively prolong the survival of patients
with advanced LUAD (3). Therefore,
it is of great significance to explore the intrinsic molecular
mechanism of lung cancer progression.
Chinese medicine herb (CMH) is one of the
alternative options for treating lung cancer. Among them, icariin
(ICA) has been found to have lung-protective effects. However, the
molecular mechanism of action remains to be elucidated (4,5). In
addition, the dysregulation of micro (mi)RNAs expression levels is
closely associated with the progression of lung cancer and some
CMHs can exert antitumor effects by targeting aberrant miRNAs. For
example, Panax notoginseng saponins significantly decreased the
level of miR-222 regulated by Met and exhibited a therapeutic
effect on lung cancer (6). It is
also found that cinnamaldehyde can downregulate miR-1252 and
increase the apoptosis of non-small cell lung cancer (NSCLC) cells
(7). Additionally, a total of 162
miRNAs displayed abnormal expression in Radix tetrastigma
hemsleyani flavonoids-treated lung cancer cells (8). The above findings indicate that
exploring aberrant miRNAs expression levels might be of clinical
value for lung cancer treatment. Therefore, it is promising to
explore the regulatory relationship between CMHs, such as ICA and
abnormally-expressed miRNAs in lung cancer.
Phosphatase and tensin homolog deleted on chromosome
ten (PTEN) is known to exhibit antitumor effects in various
cancers. For instance, PTEN is abnormally downregulated in ovarian
cancer and its overexpression can inhibit the malignant progression
of ovarian cancer (9). In bladder
cancer, PTEN is significantly downregulated and is regulated by
circular (circ) SLC8A1 to inhibit the malignant behavior of cancer
cells (10). PTEN is been shown to
suppress NSCLC and may be used as a prognostic marker for those
patients (11–13). Based on the above studies, it was
hypothesized that it was important to decipher the level and
regulation mechanism of PTEN in tumors, especially in lung cancer.
However, most current studies focus on the regulatory relationship
between PTEN and other genes and studies on the mechanism of PTEN
in lung cancer based on CMHs are severely lacking.
In the present study, ICA was been found to
downregulate the level of miR-205-5p and inhibit lung cancer cells
proliferation. The downstream molecular mechanism of miR-205-5p was
further explored in combination with bioinformatics prediction
results. Taken together, the present study provided new insights
into the use of ICA to target miR-205-5p as a potential therapeutic
strategy for lung cancer.
Materials and methods
Cell culture
The human lung cancer cell line A549, NCI-H1975 and
human bronchial epithelial cell line BEAS-2B were purchased from
China General Microbiological Culture Collection Center. All cells
were cultured in RPMI-1640 medium (Gibco; Thermo Fisher Scientific,
Inc.) supplemented with 10% FBS (Gibco; Thermo Fisher Scientific,
Inc.) at 37°C with 5% CO2. 293T cells (American Type
Culture Collection) were grown in Dulbecco's Modified Eagle Medium
with 1% L-glutamine, 1% penicillin-streptomycin (Beyotime Institute
of Biotechnology) and 10% FBS at 37°C with 5% CO2. The
cells used in the subsequent experiments were all passaged 3
times.
Cell transfection and drug
treatment
miR-205-5p mimic (50 nM,
5′-UCCUUCAUUCCACCGGAGUCUG-3′), miRNA mimic negative control (mimic
NC; 50 nM, 5′-UUCUCCGAACGUGUCACGUTT-3′), miR-205-5p inhibitor (50
nM, 5′-CAGACUCCGGUGGAAUGAAGGA-3′), miRNA inhibitor NC (inhibitor
NC) (50 nM, 5′-CAGUACUUUUGUGUAGUACAA-3′), small interfering
(si)-PTEN (50 nM, 5′-GACGGGAAGACAAGUUCAUTT-3′) and siRNA NC (si-NC;
50 nM, 5′-GCACAGTTAACCGCATAAA-3′) were all purchased from Guangzhou
RiboBio Co., Ltd. Cell transfections were performed using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.), 0.5×106 cells (NCI-H1975 or A549)
were inoculated into 6-well plates and incubated at room
temperature for 5 min. Following transfection, the cells were used
for subsequent experiments 24 h later.
Lung cancer cells (NCI-H1975 or A549) in the
logarithmic growth phase were treated with different concentrations
of ICA (The concentration range was 5–40 µM and the concentrations
of the four treatment groups were 5, 10, 20 and 40 µM,
respectively) or control for 24 h at 37°C (14). ICA was obtained from Shanghai Tauto
Biotech Co., Ltd.
Reverse transcription-quantitative PCR
(RT-qPCR)
The experimental steps were carried out according to
the manufacturers' instructions. Total RNA in cells (NCI-H1975 or
A549) at 80% confluence was extracted using TRIzol®
(Thermo Fisher Scientific, Inc.). PrimeScript RT kit (Takara
Biotechnology Co., Ltd.) and SYBR Select Master Mix (Invitrogen;
Thermo Fisher Scientific, Inc.) were used for reverse transcription
and qPCR. The PCR conditions were as follows: Initial denaturation
at 95°C for 2 min; followed by 40 cycles of denaturation at 95°C
for 15 sec, annealing at 60°C for 60 sec and elongation at 72°C for
30 sec and final extension at 72°C for 5 min. U6 and GAPDH were
used for the standardization of miR-205-5p and PTEN. The sequences
of primers were: miR-205-5p F, 5′-CTTGTCCTTCATTCCACCGGA-3′ and R,
5′-TGCCGCCTGAACTTCACTCC-3′; PTEN F, 5′-TGTGGGTGTGGTTGGAAGTC-3′ and
R, 5′-CCTCAAGGTCAGACCCTTCC-3′; GAPDH F, 5′-GGAGCGAGATCCCTCCAAAAT-3′
and R, 5′-GGCTGTTGTCATACTTCTCATGG-3′; and U6 F,
5′-CTCGCTTCGGCAGCACA-3′ and R, 5′-AACGCTTCACGAATTTGCGT-3′ The
quantitative analysis was performed using the 2−ΔΔCq
method (15). All experiments were
repeated 3 times.
Western blotting
The cells (NCI-H1975 or A549 at 80% confluence in
6-well plates) were lysed with RIPA buffer (Thermo Fisher
Scientific, Inc.) and the protein concentration was determined
using the bicinchoninic acid kit (Beyotime Institute of
Biotechnology). Proteins (20 µg) were separated by 10% sodium
dodecyl sulfate polyacrylamide gel electrophoresis and transferred
to polyvinylidene fluoride membranes (MilliporeSigma). Following
blocking (with 5% nonfat milk in TBST at room temperature for 1 h),
the membrane was incubated overnight at 4°C with the primary
antibodies: PTEN (1:1,000; cat. no. ab267787), p-Akt (1:500; cat.
no. ab131443), Akt (1:10,000; cat. no. ab179463), p-PI3K (1:500;
cat. no. ab182651), PI3K (1:1,000; cat. no. ab191606) and GAPDH
(1:1,000; cat. no. ab9485; all Abcam). Subsequently, PBST was used
to rinse the membranes three times. Secondary antibody goat
anti-rabbit IgG H&L (HRP; cat. no. ab6721; Abcam) was used to
incubate the membranes at room temperature for 1 h. All protein
bands were visualized using ECL chemiluminescence (Thermo Fisher
Scientific, Inc.). Protein bands were visualized in a gel imaging
system (MG8600, Bio-Rad). Images were using Image Pro Plus software
(Version 7.0, Media Cybernetics).
Dual-luciferase assay
A luciferase vector pSicheck (Sangon Biotech Co.,
Ltd.) for wild-type (WT) and mutated (MUT) PTEN 3′UTR was
constructed. The binding sites between PTEN and miR-155-5p were
predicted using miRbase (https://mirbase.org). miR-205-5p mimic/mimic NC and
PTEN-psicheck WT/MUT were co-transfected into 293T cells using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). After 48 h transfection, luciferase activity was
measured using the dual-luciferase reporter gene assay kit
(Shanghai Qcbio Science & Technologies Co., Ltd.). Luciferase
activity was normalized to Renilla activity.
Colony formation assay
Cells (1×103/well) were inoculated on
Petri dishes and cultured until colonies were visible. Colonies
were fixed with 4% formaldehyde (Thermo Fisher Scientific, Inc.)
for 15 min at room temperature, stained with 0.1% crystal violet
for 15 min (Thermo Fisher Scientific, Inc.) at room temperature and
quantified under a light microscope (magnification, ×40, three
randomly selected fields). A colony was defined as an agglomeration
of >50 cells.
Cell Counting Kit-8 (CCK-8) assay
After inoculating for 0, 24, 48, 72 and 96 h, CCK8
solution (10 µl/well) (Dojindo Molecular Technologies, Inc.) was
added to the cells. After 4 h, the absorbance of cells at 450 nm
was measured using a microplate reader (BioTek Instruments,
Inc.).
Transwell assay
Cell suspension (200 µl) was added to the chamber.
For induction, 5% FBS was added to the lower chamber. After 24 h,
the upper cells were gently scraped off and the migratory cells
were stained with 0.1% crystal violet for 30 min at room
temperature (Thermo Fisher Scientific, Inc.), observed and counted.
The upper chamber was pre-coated with Matrigel (BD Biosciences) at
37°C for 30 min to detect the invasiveness of cells and the other
operations were basically the same as the migration assay.
Flow cytometry (FCM)
FCM was used to detect apoptosis, as previously
described by Xu et al (16). In short, in the presence of 50
ug/ml RNase A (MilliporeSigma), cells were stained with Annexin
V/FITC and propidium (PI; Thermo Fisher Scientific, Inc.) and
analyzed by flow cytometer (CytoFLEX; Beckman Coulter). The data
were analyzed using FlowJo software (version 10.0.2, FlowJo LLC).
Apoptosis rate=percentage of early + late apoptotic cells. For cell
cycle detection, cells were treated with trypsin, fixed for 2 h at
4°C with 70% ethanol which was precooled in a 4°C refrigerator,
then stained with PI for 15 min at 4°C. Cell cycle was analyzed by
FCM.
Nude mouse transplantation tumor
experiment
BALB/C nude mice (n=15, female, aged 6 weeks,
weighed 16–18 g, purchased from Laboratory Animal Resources,
Chinese Academy of Sciences) were raised in a 12-h light/dark cycle
at a temperature of 25°C±1°C and relative humidity of 50±10%. All
the mice were given standard diet and sterile water ad
libitum. Mice were randomly divided into A, B and C groups with
5 mice in each group. Mice in group A were inoculated with A549
cells transfected with NC Agomir (Guangzhou RiboBio Co., Ltd.) and
mice in groups B and C were inoculated with A549 cells transfected
with miR-205-5p Agomir (Guangzhou RiboBio Co., Ltd.). Mice in group
C were additionally injected with ICA (10 mg/kg) every 3 days
(4).
Tumor diameter was measured every 7 days. After the
experiment, the mice were sacrificed by inhalation of
CO2 (50% of the chamber volume/min) and the tumors were
resected and weighed. The flow of CO2 was maintained for
at least 2 min after respiratory arrest. All animal experiments
were approved by the Institutional Animal Care and Use Committee of
the First Affiliated Hospital of Zhejiang Chinese Medical
University (approval No. 2021026) in accordance with the National
Research Council Guide for Care and Use of Laboratory Animals.
Immunohistochemistry (IHC)
IHC was performed using the principle of antigen and
antibody-specific binding. The tumor tissue was fixed with 4%
paraformaldehyde at room temperature for 24 h, embedded with
paraffin and sectioned. The paraffin sections were dewaxed with
xylene, washed with 100% ethanol twice, 95% ethanol twice and
dH2O twice. The sections were then boiled in 10 mM
sodium citrate buffer for 10 min. After 3 washes with
dH2O, the slices were incubated with 3%
H2O2 for 10 min and washed for twice. The
sections were blocked in 5% goat serum (Beijing Solarbio Science
& Technology Co., Ltd.) for 1 h at room temperature. Primary
antibody Ki-67 (cat. no. ab15580; Rabbit; Abcam) was added and
incubated overnight in a 4°C refrigerator, followed by secondary
antibody IgG (cat. no. ab6721; Goat Anti-Rabbit; Abcam) for half an
hour at room temperature. Specific immunostaining was performed
with a 3,3′-diaminobenzidine substrate (MilliporeSigma) at room
temperature and monitored under a light microscope, until the
target tissue appeared brownish yellow when staining was stopped
and counterstained with hematoxylin (~1–2 min) at room temperature.
Finally, five fields were randomly selected using a light
microscope (Olympus Corporation; magnification, ×200).
Statistical analysis
At least three biological and technical duplications
were independently performed. All data were expressed as Mean ±
Standard deviation (SD) using GraphPad Prism 6 software (La Jolla,
USA). Differences between two or multiple groups were conducted
using the Student t-test or one-way ANOVA followed by a post hoc
Tukey's test. P<0.05 was considered to indicate a statistically
significant difference.
Results
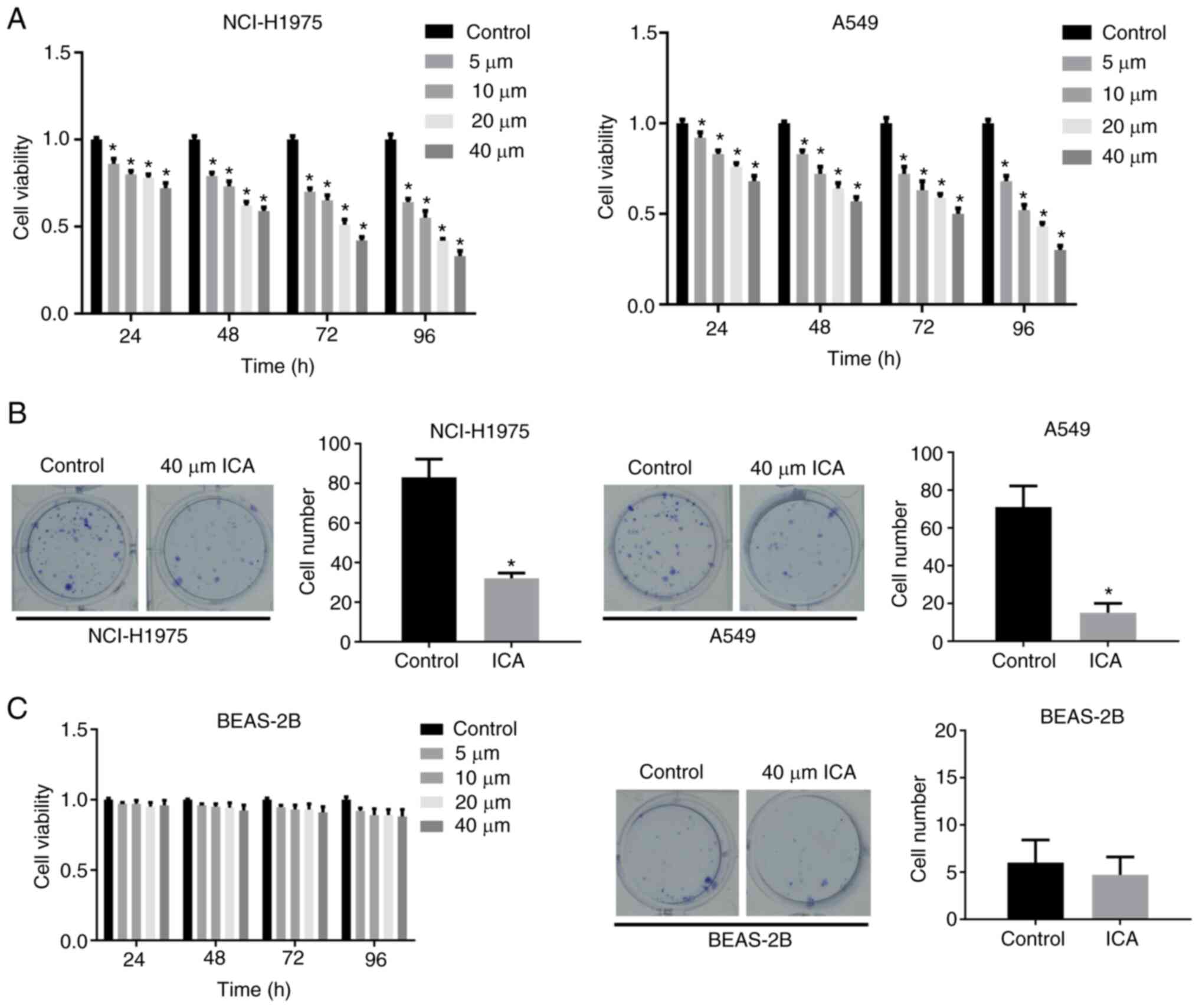
Icariin suppresses the viability and
colony formation ability of lung cancer cells but had little effect
on lung epithelial cells
Lung cancer cell lines, A549 and H1975, were exposed
to different ICA concentrations. The results of the CCK-8 assay
revealed that ICA could markedly reduce cell viability in a time
and dose-dependent manner compared with the control group (Fig. 1A). In addition, ICA could
significantly reduce the cell colony-forming ability of cancer cell
lines compared with the control group (Fig. 1B). At the same time, the above
experiments were also performed with BEAS-2B cells and the results
showed that the viability and proliferation ability of BEAS-2B were
almost unaffected by the increase in ICA treatment time and
concentration (Fig. 1C). These
results suggest that ICA inhibited the growth of lung cancer cells
but had almost no toxic effects on normal cells.
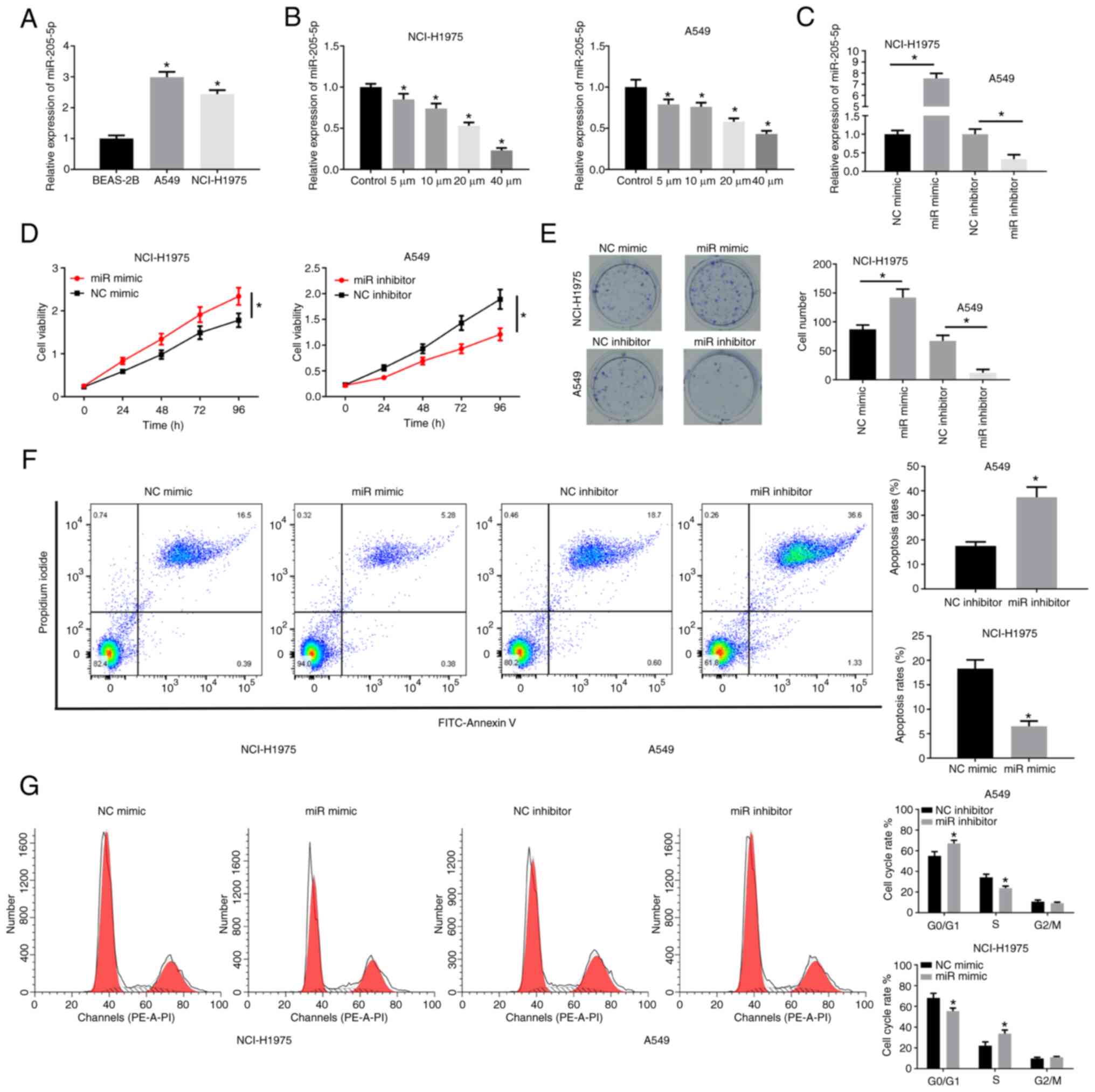
miR-205-5p is highly expressed in lung
cancer and promotes cell proliferation
miR-205-5p has been shown to be upregulated in
various cancers (17,18). In the present study, miR-205-5p was
highly expressed in NCI-H1975 and A549 cell lines (Fig. 2A). Subsequently, the cancer cells
were treated with different concentrations of ICA and it was found
that miR-205-5p decreased as the dose increased (Fig. 2B). NCI-H1975 and A549 were selected
for follow-up research. miR-205-5p mimic (Fig. 2C) could markedly increase the
viability and colony formation of lung cancer cells, while
miR-205-5p inhibitor treatment had the opposite effect (Fig. 2D and E). FCM results revealed that
miR-205-5p inhibited apoptosis but increased cell cycle progression
(Fig. 2F and G). Together, these
results further suggested the tumor-promoting effect of miR-205-5p
in lung cancer cells.
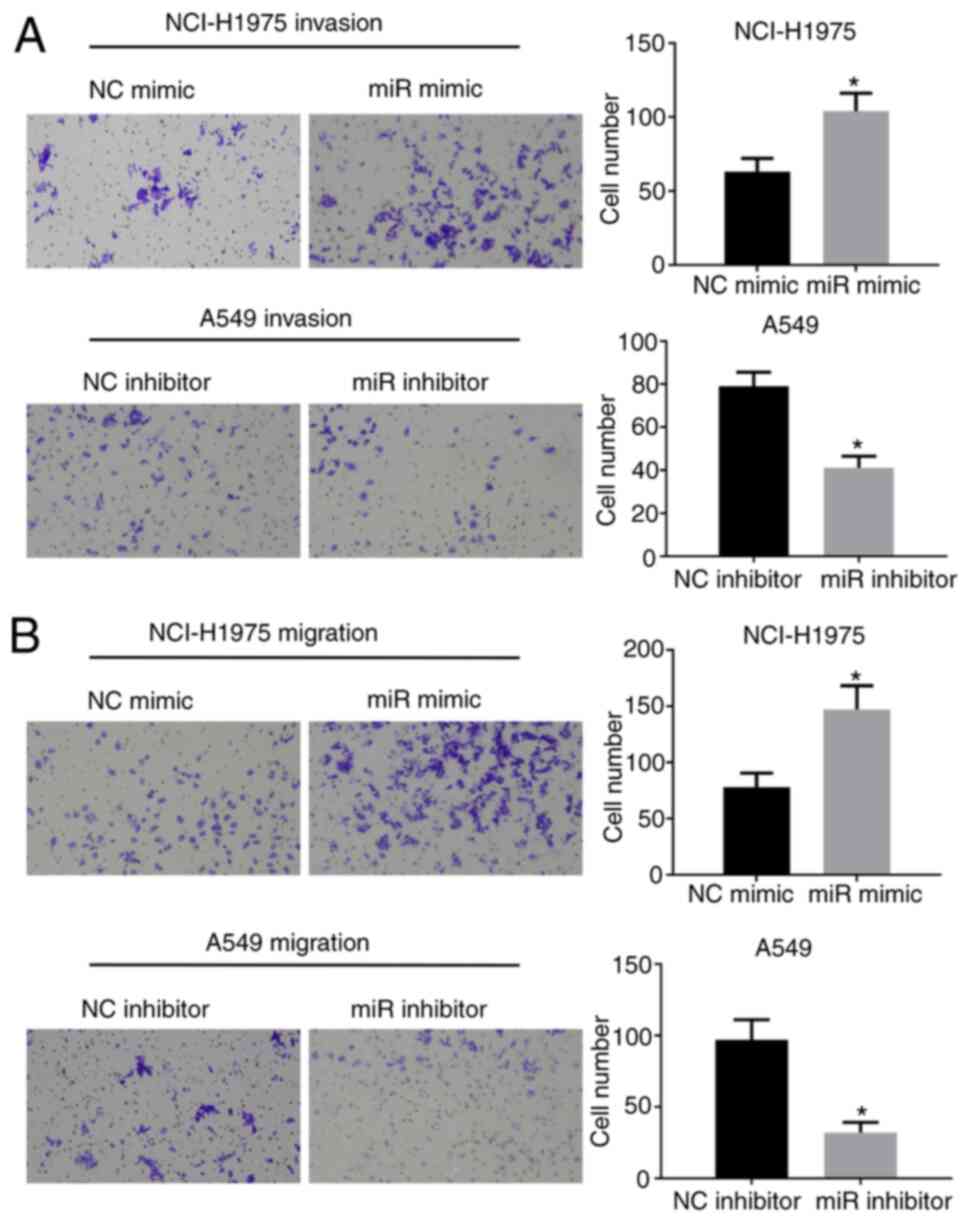
miR-205-5p can promote the invasion
and migration of lung cancer cells
Next, a Transwell migration and invasion assay was
performed to determine if miR-205-5p could promote the migration
and invasiveness of lung cancer cells. It was found that miR-205-5p
overexpression could facilitate the invasion and migratory
abilities of lung cancer cells (Fig.
3A and B), while inhibition of miR-205-5p could suppress these
effects.
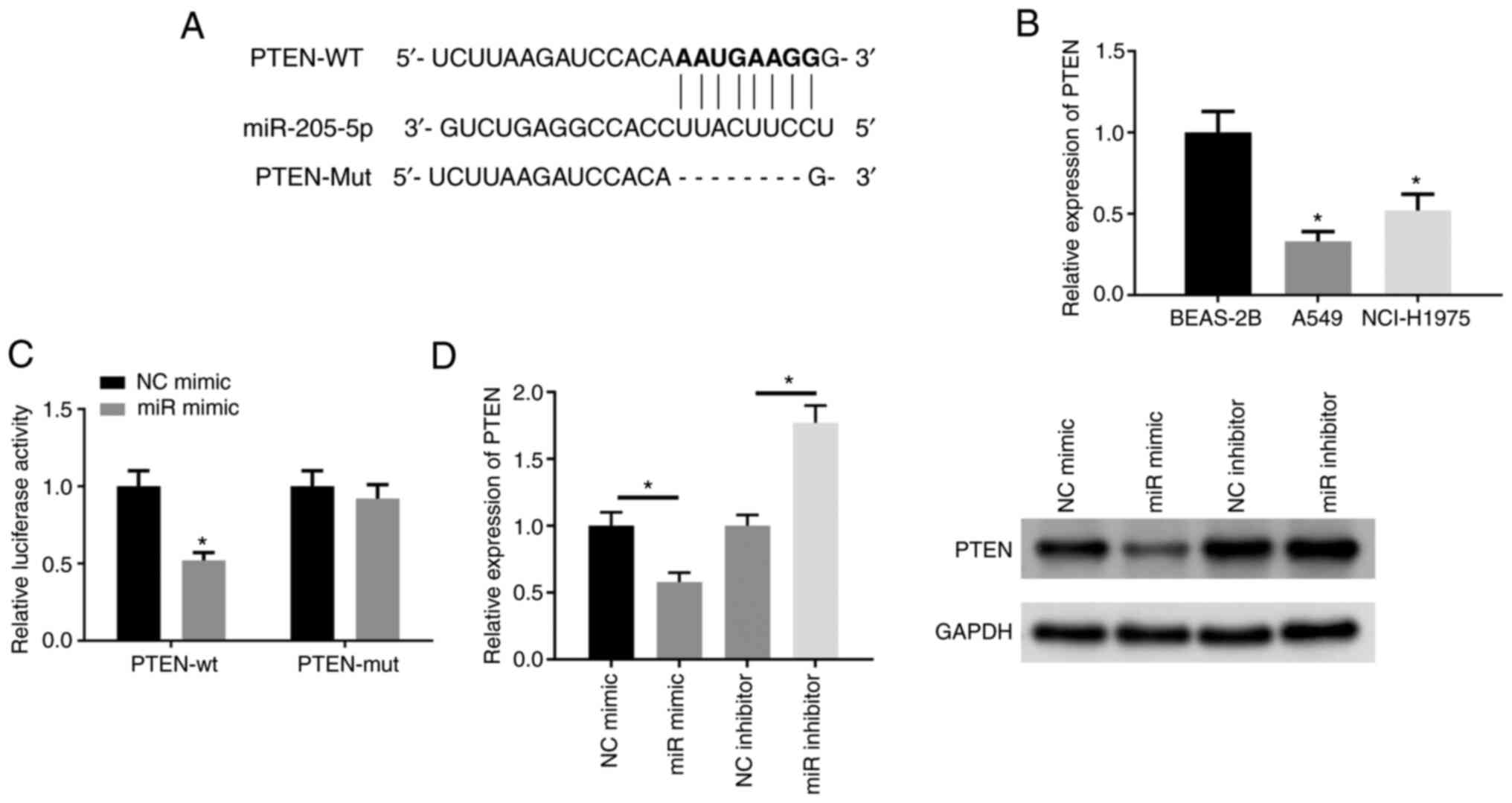
miR-205-5p translationally represses
PTEN by targeting specific sequences in the 3′UTR of PTEN
To further explore the mechanism of action of
miR-205-5p in lung cancer, the targeted binding sites of miR-205-5p
and PTEN were predicted (Fig. 4A).
PTEN mRNA expression was found to be significantly downregulated in
lung cancer cell lines (Fig. 4B).
Dual-luciferase reporter gene assay was applied to explore the
binding relationship between miR-205-5p and PTEN (Fig. 4C). The expression of PTEN in
different treatment groups was determined. PTEN was notably
downregulated in cells overexpressing miR-205-5p, while cells
depleted of miR-205-5p showed the opposite trend (Fig. 4D). The above results thus indicated
that miR-205-5p could suppress PTEN in lung cancer.
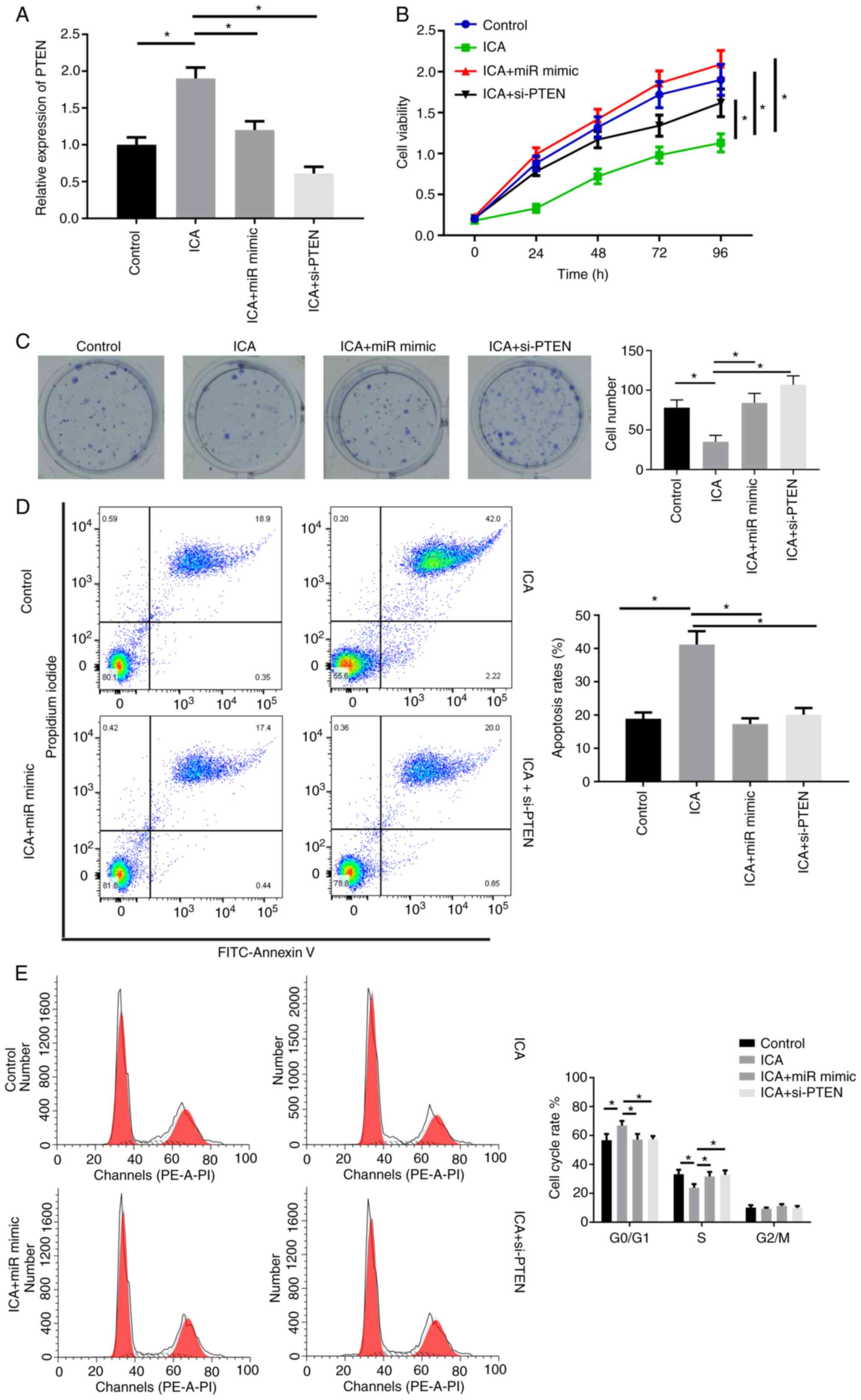
ICA inhibits the proliferation of lung
cancer cells through the miR-205-5p/PTEN axis
Having demonstrated that PTEN is a target gene of
miR-205-5p, the present study next investigated whether ICA could
regulate the expression of PTEN by inhibiting miR-205-5p. In A549
cells, ICA treatment could upregulate the level of PTEN mRNA, while
overexpression of miR-205-5p or downregulation of PTEN reversed
that effect (Fig. 5A). In A549
cells, ICA treatment could suppress the cell vitality and colony
formation effects of miR-mimic or si-PTEN transfection (Fig. 5B and C). Moreover, FCM results
showed that ICA could promote cell apoptosis and induce cell cycle
arrest, while miR-205-5p or inhibition of PTEN attenuated this
effect (Fig. 5D and E).
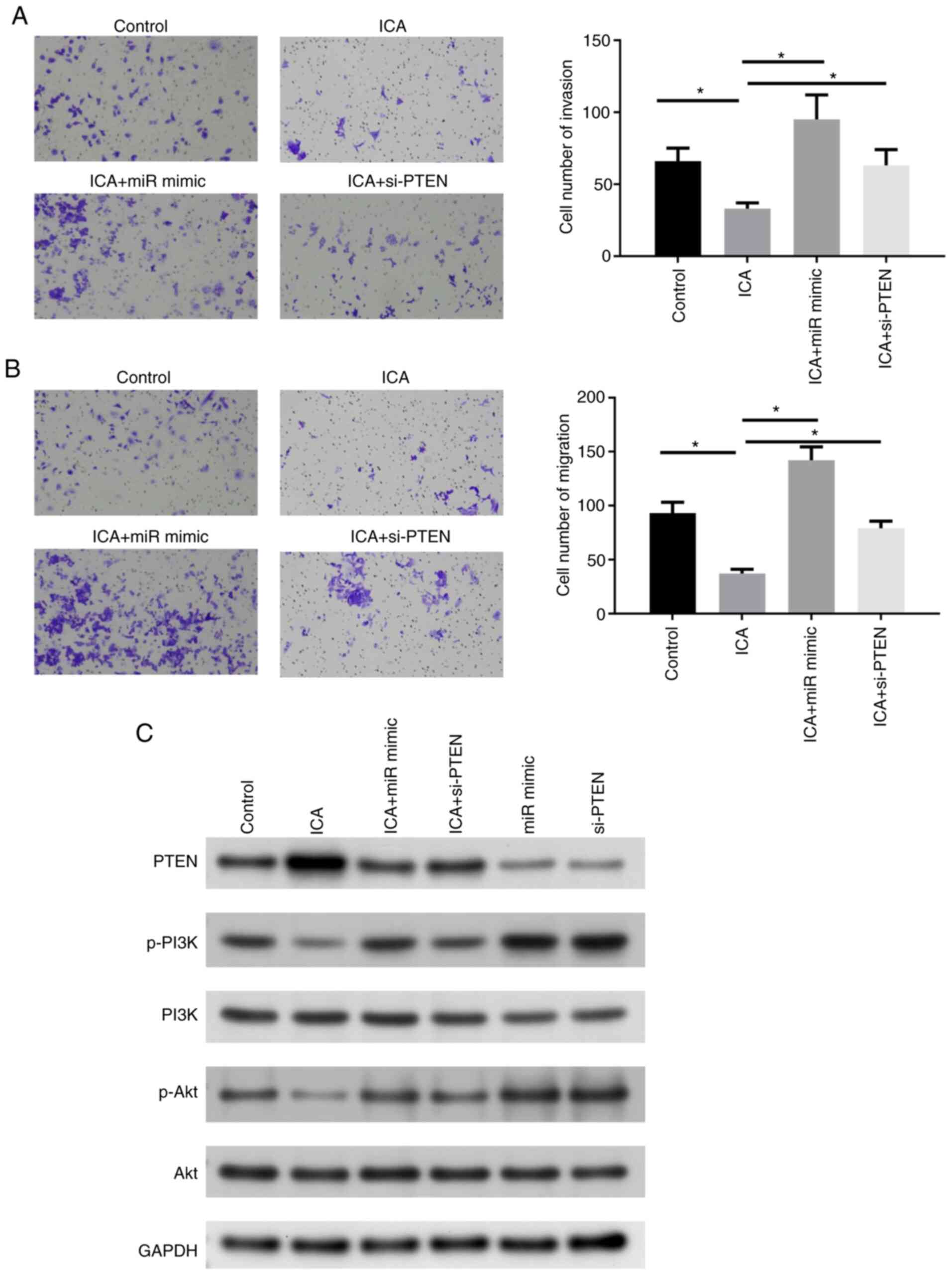
ICA can affect the migration and
invasion ability of cancer cells and regulate the PI3K/Akt
signaling pathway through the miR-205-5p/PTEN axis
Next, the migratory and invasive abilities of A549
cells were detected. Transwell assay revealed that ICA could
significantly decrease the migratory ability and invasiveness of
cells, but following transfection with miR mimic or si-PTEN, the
above trends were restored (Fig. 6A
and B). To explore the regulatory effect of the axis identified
in this study on the PI3K/Akt signaling pathway, the
pathway-related proteins (Akt, PI3K and its corresponding
phosphorylation level) were measured. It was found that PTEN
protein expression was decreased and the PI3K/Akt pathway was
activated when cells were transfected with miR mimic and si-PTEN.
However, phosphorylation of Akt and PI3K decreased following ICA
treatment, while it was markedly increased in the ICA + miR mimic
or the ICA + si-PTEN group compared with the ICA treatment group
(Fig. 6C). In short, ICA could
inhibit the malignant phenotype of lung cancer cells through the
miR-205-5p/PTEN axis.
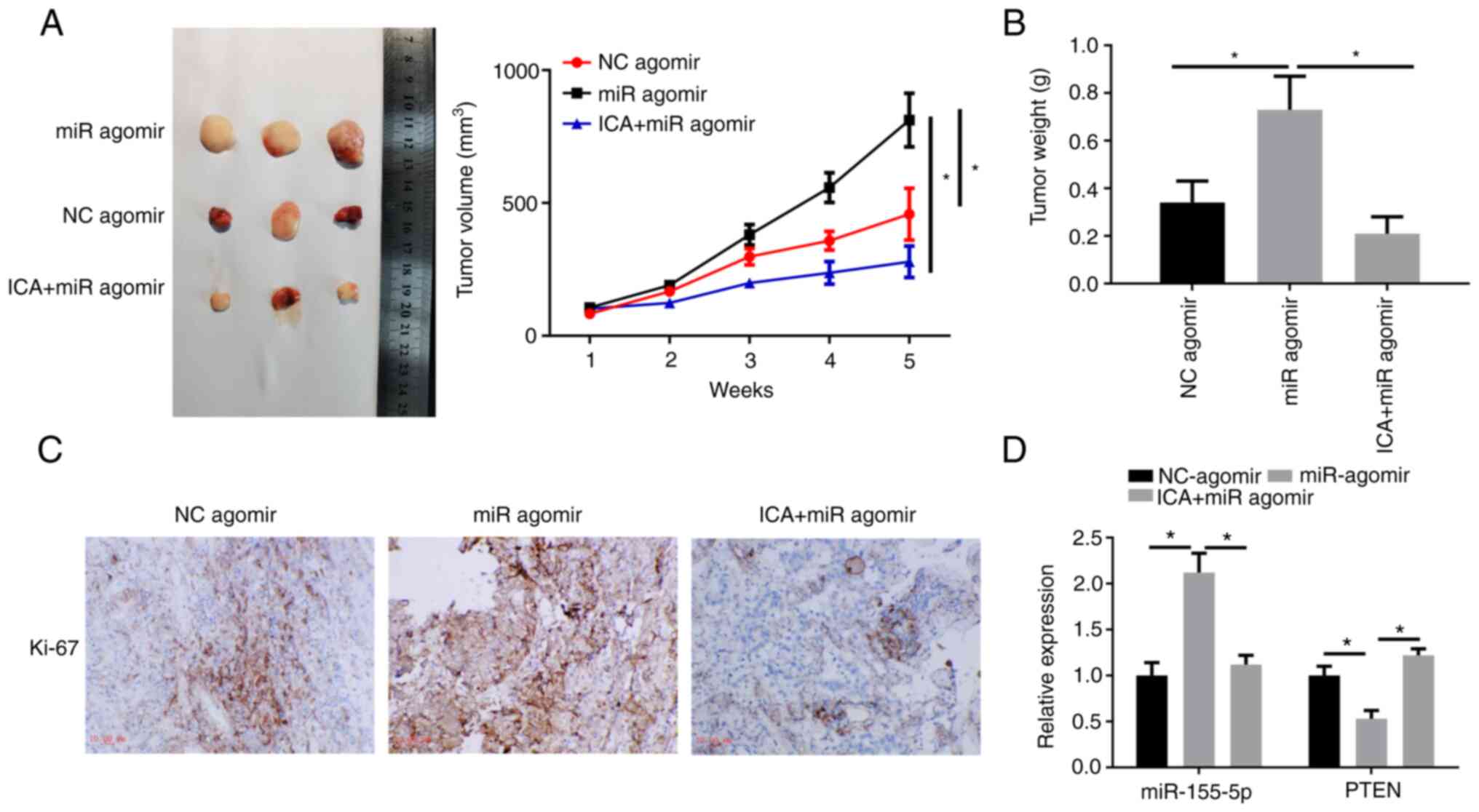
ICA impairs the promoting effect of
exogenous miR-205-5p on tumor growth
In addition to in vitro studies, a xenograft
model was also employed to explore the effect of ICA on miR-205-5p
mediated cancer promotion. The tumor growth curve showed that tumor
growth was faster in the miR agomir group compared with the NC
group, with ICA administration significantly suppressing the tumor
growth rate (Fig. 7A). Tumor
weight was significantly increased in mice treated with miR agomir
when compared with control mice. Following ICA administration,
tumor weight was significantly reduced compared with the miR agomir
group (Fig. 7B). IHC staining
revealed that Ki67 was highly expressed in miR agomir xenograft
tumor, while Ki67 was downregulated following ICA administration
(Fig. 7C). Moreover, miR-205-5p
was upregulated and PTEN mRNA was downregulated in the miR agomir
group, while the above-mentioned phenomena were inhibited in the
ICA + miR agomir group (Fig. 7D).
The above findings revealed that miR-205-5p could promote lung
cancer progression, while this promoting effect was reversed when
ICA was added concurrently.
Discussion
At present, a number of CMHs have shown good
efficacy in lung cancer treatment. For example, the xihuang pill
can inhibit lung cancer by regulating the amino acid metabolism
pathway in the Lewis lung cancer animal model (19). In addition, Rg3 is shown to
regulate DNA damage in NSCLC cells, thereby inhibiting the
development of cancer cells (20).
Salidroside can also suppress the malignant phenotype of human lung
cancer cells (21). However, the
clinical application of CMHs has been greatly limited because of
their elusive mechanisms of action to prevent tumor
progression.
As a flavonoid glycoside from genus Epimedium
plants, ICA has been shown to promote the blood flow of
cardiovascular and cerebrovascular vessels, hematopoiesis, immune
function, anticancer effects (22,23).
The present study found that ICA could suppress the viability and
colony formation ability of lung cancer cells, which is similar to
the results of a previous study (4). However, to further strengthen the
theoretical basis of ICA to facilitate future clinical application,
the present study also used BEAS-2B cells and found that ICA had
almost no effect on the growth of BEAS-2B cells, signifying that
ICA has little to no toxicity effect on normal cells. Several
studies have previously shown that ICA can exert an important role
via miRNAs or mRNAs in multiple diseases. For instance, in liver
fibrosis, ICA can regulate miR-875-5p to attenuate the
epithelial-mesenchymal transition process by targeting the hedgehog
signaling pathway (24). ICA can
also regulate the biological function of ovarian cancer cells by
regulating miR-21 (25). In
addition, a study found that ICA could target miR-625-3p and
inhibit thyroid cancer cell growth (26). The results of previous studies
suggest that exploring the mechanism of the anticancer activity of
ICA might help to expand its clinical application. The present
study revealed for the first time, to the best of the authors'
knowledge, that miR-205-5p was upregulated in lung cancer cells and
that ICA could inhibit its level, miR-205 is located at the 1q32.2
locus of the human genome and plays an important role in multiple
diseases. miR-205-5p is reported to inhibit apoptosis and promote
invasion of A549 cells (27).
Furthermore, miR-205-5p can also inhibit LRP1 and regulate NSCLC
proliferation (28). In
vitro functional studies in the present study showed that the
overexpression of miR-205-5p could significantly enhance the
progression of lung cancer cells, in line with previous
reports.
The present study also predicted that miR-205-5p and
PTEN had binding sites and a double luciferase assay further
verified the relationship between the two. PTEN is considered a
common tumor suppressor and expression loss often leads to abnormal
activation of the PI3K signaling pathway (29). In bladder cancer, long non-coding
RNA LINC00641 is shown to target miR-197-3p/KLF10/PTEN/PI3K/Akt and
inhibit bladder cancer (30). In
NSCLC, the PTEN/PI3K/Akt signaling affects a variety of cellular
functions (11). Aberrant
activation of the PI3K/Akt axis is often one of the inducing
factors for the development of a number of tumors. Therefore,
targeting the PI3K/Akt signaling pathway can also be considered a
potential strategy for treating tumors (31). The present study found that ICA
could inhibit the progression of lung cancer cells through the
PI3K/Akt pathway in a miR-205-5p/PTEN axis-dependent manner.
Further corroboration was obtained through in vivo
experiments. Moreover, it was also learned that there are a number
of other targets (including SMAD4) downstream of miR-205-5p and a
number of other miRNAs (including miR-382-5p) upstream of PTEN
(32–34). Whether these pathways are the
molecular mechanisms through which ICA regulates lung cancer
progression warrants further exploration.
In conclusion, the present study confirmed that ICA
could significantly slow lung cancer progression to a certain
extent. Notably, it revealed that ICA could suppress lung cancer
progression via the miR-205-5p/PTEN and PI3K/Akt pathways in
vivo and in vitro. Together, the findings provided novel
molecular targets and the foundation for the clinical treatment of
lung cancer. Further research into other possible molecular
mechanisms of ICA affecting the progression of lung cancer will
further improve the assessment of the therapeutic potential of ICA
for lung cancer.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
The two authors designed the experiments, wrote the
manuscript and acquired and analyzed the data. The two authors
approved the manuscript and are responsible for confirming the
authenticity of the raw data. The two authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The experiments were approved by the Experimental
Animal Ethics Committee of the First Affiliated Hospital of
Zhejiang Chinese Medical University (Approval No. 2021026).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jordan EJ, Kim HR, Arcila ME, Barron D,
Chakravarty D, Gao J, Chang MT, Ni A, Kundra R, Jonsson P, et al:
Prospective comprehensive molecular characterization of lung
adenocarcinomas for efficient patient matching to approved and
emerging therapies. Cancer Discov. 7:596–609. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pakkala S and Ramalingam SS: Personalized
therapy for lung cancer: Striking a moving target. JCI Insight.
3:e1208582018. View Article : Google Scholar
|
|
4
|
Wu X, Kong W, Qi X, Wang S, Chen Y, Zhao
Z, Wang W, Lin X, Lai J, Yu Z and Lai G: Icariin induces apoptosis
of human lung adenocarcinoma cells by activating the mitochondrial
apoptotic pathway. Life Sci. 239:1168792019. View Article : Google Scholar
|
|
5
|
Handoussa H, AbdAllah W and AbdelMohsen M:
UPLC-ESI-PDA-Msn Profiling Of phenolics involved in
biological activities of the medicinal plant halocnemum
strobilaceum (Pall.). Iran J Pharm Res. 18:422–429. 2019.PubMed/NCBI
|
|
6
|
Yang Q, Wang P, Cui J, Wang W, Chen Y and
Zhang T: Panax notoginseng saponins attenuate lung cancer growth in
part through modulating the level of Met/miR-222 axis. J
Ethnopharmacol. 193:255–265. 2016. View Article : Google Scholar
|
|
7
|
Tian F, Yu CT, Ye WD and Wang Q:
Cinnamaldehyde induces cell apoptosis mediated by a novel circular
RNA hsa_circ_0043256 in non-small cell lung cancer. Biochem Biophys
Res Commun. 493:1260–1266. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu P, Yang X, Zhang H, Pu J and Wei K:
Analysis of change in microRNA expression profiles of lung cancer
A549 cells treated with Radix tetrastigma hemsleyani flavonoids.
Onco Targets Ther. 11:4283–4300. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhao W, Han T, Li B, Ma Q, Yang P and Li
H: MiR-552 promotes ovarian cancer progression by regulating PTEN
pathway. J Ovarian Res. 12:1212019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lu Q, Liu T, Feng H, Yang R, Zhao X, Chen
W, Jiang B, Qin H, Guo X, Liu M, et al: Circular RNA circSLC8A1
acts as a sponge of miR-130b/miR-494 in suppressing bladder cancer
progression via regulating PTEN. Mol Cancer. 18:1112019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Perez-Ramirez C, Canadas-Garre M, Molina
MA, Faus-Dader MJ and Calleja-Hernandez MA: PTEN and PI3K/AKT in
non-small-cell lung cancer. Pharmacogenomics. 16:1843–1862. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zang X, Gu J, Zhang J, Shi H, Hou S, Xu X,
Chen Y, Zhang Y, Mao F, Qian H, et al: Exosome-transmitted lncRNA
UFC1 promotes non-small-cell lung cancer progression by
EZH2-mediated epigenetic silencing of PTEN expression. Cell Death
Dis. 11:2152020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang H, Ma Z, Liu X, Zhang C, Hu Y, Ding
L, Qi P, Wang J, Lu S and Li Y: MiR-183-5p is required for
non-small cell lung cancer progression by repressing PTEN. Biomed
Pharmacother. 111:1103–1111. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao J, Ohba S, Shinkai M, Chung UI and
Nagamune T: Icariin induces osteogenic differentiation in vitro in
a BMP- and Runx2-dependent manner. Biochem Biophys Res Commun.
369:444–448. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu F, Li Q, Wang Z and Cao X: Sinomenine
inhibits proliferation, migration, invasion and promotes apoptosis
of prostate cancer cells by regulation of miR-23a. Biomed
Pharmacother. 112:1085922019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu M, Duan Q, Liu X, Zhang P, Fu Y, Zhang
Z, Liu L, Cheng J and Jiang H: MiR-155-5p promotes oral cancer
progression by targeting chromatin remodeling gene ARID2. Biomed
Pharmacother. 122:1096962020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li N, Cui T, Guo W, Wang D and Mao L:
MiR-155-5p accelerates the metastasis of cervical cancer cell via
targeting TP53INP1. Onco Targets Ther. 12:3181–3196. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu N, Ma M, Qu N, Wang R, Chen H, Hu F,
Gao S and Shan F: Low-dose naltrexone inhibits the
epithelial-mesenchymal transition of cervical cancer cells in vitro
and effects indirectly on tumor-associated macrophages in vivo. Int
Immunopharmacol. 86:1067182020. View Article : Google Scholar
|
|
20
|
Liu T, Zuo L, Guo D, Chai X, Xu J, Cui Z,
Wang Z and Hou C: Ginsenoside Rg3 regulates DNA damage in non-small
cell lung cancer cells by activating VRK1/P53BP1 pathway. Biomed
Pharmacother. 120:1094832019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ren M, Xu W and Xu T: Salidroside
represses proliferation, migration and invasion of human lung
cancer cells through AKT and MEK/ERK signal pathway. Artif Cells
Nanomed Biotechnol. 47:1014–1021. 2019. View Article : Google Scholar
|
|
22
|
Shen R and Wang JH: The effect of icariin
on immunity and its potential application. Am J Clin Exp Immunol.
7:50–56. 2018.
|
|
23
|
Fang J and Zhang Y: Icariin, an
Anti-atherosclerotic Drug from Chinese Medicinal Herb Horny Goat
Weed. Front Pharmacol. 8:7342017. View Article : Google Scholar
|
|
24
|
Ye L, Yu Y and Zhao Y: Icariin-induced
miR-875-5p attenuates epithelial-mesenchymal transition by
targeting hedgehog signaling in liver fibrosis. J Gastroenterol
Hepatol. 35:482–491. 2020. View Article : Google Scholar
|
|
25
|
Li J, Jiang K and Zhao F: Icariin
regulates the proliferation and apoptosis of human ovarian cancer
cells through microRNA-21 by targeting PTEN, RECK and Bcl-2. Oncol
Rep. 33:2829–2836. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fang L, Xu W and Kong D: Icariin inhibits
cell proliferation, migration and invasion by down-regulation of
microRNA-625-3p in thyroid cancer cells. Biomed Pharmacother.
109:2456–2463. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jiang M, Zhong T, Zhang W, Xiao Z, Hu G,
Zhou H and Kuang H: Reduced expression of miR2055p promotes
apoptosis and inhibits proliferation and invasion in lung cancer
A549 cells by upregulation of ZEB2 and downregulation of erbB3. Mol
Med Rep. 15:3231–3238. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang P, Chen D, Ma H and Li Y: Long
non-coding RNA MEG3 regulates proliferation and apoptosis in
non-small cell lung cancer via the miR-205-5p/LRP1 pathway. RSC
Adv. 7:49710–49719. 2017. View Article : Google Scholar
|
|
29
|
Wise HM, Hermida MA and Leslie NR:
Prostate cancer, PI3K, PTEN and prognosis. Clin Sci (Lond).
131:197–210. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li Z, Hong S and Liu Z: LncRNA LINC00641
predicts prognosis and inhibits bladder cancer progression through
miR-197-3p/KLF10/PTEN/PI3K/AKT cascade. Biochem Biophys Res Commun.
503:1825–1829. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ebrahimi S, Hosseini M, Shahidsales S,
Maftouh M, Ferns GA, Ghayour-Mobarhan M, Hassanian SM and Avan A:
Targeting the Akt/PI3K signaling pathway as a potential therapeutic
strategy for the treatment of pancreatic cancer. Curr Med Chem.
24:1321–1331. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chu P, Liang A, Jiang A and Zong L:
MiR-205 regulates the proliferation and invasion of ovarian cancer
cells via suppressing PTEN/SMAD4 expression. Oncol Lett.
15:7571–7578. 2018.
|
|
33
|
Liu D, Zhong L, Yuan Z, Yao J, Zhong P,
Liu J, Yao S, Zhao Y, Liu L, Chen M, et al: MiR-382-5p modulates
the ATRA-induced differentiation of acute promyelocytic leukemia by
targeting tumor suppressor PTEN. Cell Signal. 54:1–9. 2019.
View Article : Google Scholar
|
|
34
|
Zhao YS, Yang WC, Xin HW, Han JX and Ma
SG: MiR-182-5p knockdown targeting PTEN inhibits cell proliferation
and invasion of breast cancer cells. Yonsei Med J. 60:148–157.
2019. View Article : Google Scholar : PubMed/NCBI
|