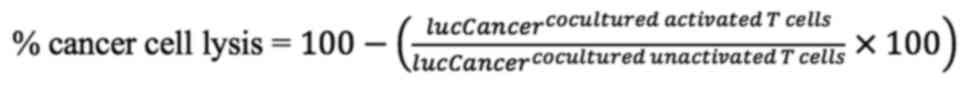Introduction
Triple negative breast cancer (TNBC) is a subtype of
breast cancer accounting for 15–20% of all breast cancer and
characterized by the absence of estrogen receptor (ER),
progesterone receptor (PR) and human epidermal growth factor
receptor 2 (HER2) (1). TNBC is
predominant in younger patients and demonstrates aggressive
clinical features with poor prognosis (1). Due to lack of hormonal receptors and
HER2 expression, TNBC is less sensitive to hormonal- and
HER2-targeted treatment (1). The
current treatment standard for TNBC is surgical resection and
chemotherapy (1). Although,
several studies have shown that TNBC is more sensitive to
chemotherapy than other subtypes, a portion of patients experience
recurrence at the visceral organs within five years after treatment
(1,2). A number of biomarkers are identified
as the theranostic biomarkers for breast cancer, such as microRNAs,
checkpoint molecules, epigenetic profiles and aberrant signaling
molecules, though the effective utilization of these markers in the
management of patients with TNBC is limited (3–5).
Therefore, developing a novel treatment approach to improve the
treatment outcome for patients with TNBC is required.
Dendritic cells (DCs)-based immunotherapy aims to
induce an antigen-specific immune response through DCs presenting
antigens to stimulate cancer-specific T cells returned to the
patients to eliminate cancer cells (6,7). Our
group has previously demonstrated the ability of monocyte-derived
DCs generated by lentiviral transduction of granulocyte-macrophage
colony stimulating factor (GM-CSF), interleukin-4 (IL-4) and
protein kinase cAMP-dependent type I-alpha regulatory subunit
(PRKAR1A) to efficiently induce PRKAR1A-specific T cells against
cholangiocarcinoma cells (8).
These DCs are called self-differentiated myeloid derived
antigen-presenting cells reactive against tumor (SmartDCs) and can
produce the cytokines required for DCs differentiation in an
autocrine manner and the tumor-associated antigen processing for
cytotoxic T cells activation (8).
The SmartDC platform has been demonstrated an efficient
antigen-specific T cells in leukemia, melanoma and
cholangiocarcinoma (8–12).
Mesothelin (MSLN) is a
glycophosphatidylinositol-linked glycoprotein limitedly expressed
in mesothelial cells but reported to be aberrantly expressed in
various types of cancer, such as ovarian cancer, pancreatic cancer,
mesothelioma and TNBC (13–16).
Notably, it has been recognized as a potential target for cancer
immunotherapy (15–17). In breast cancer, several studies
have reported the overexpression of MSLN specifically in TNBC and
its high expression is associated with poor prognosis (14,18–22).
The overexpression of MSLN in TNBC but limited expression in normal
tissue highlight the potential of MSLN as a target for T cell
therapy.
The efficacy of T cells activated by DCs is greatly
affected by the maturation status of DCs including the human
leukocyte antigens (HLAs), co-stimulatory molecules and cytokines
expressions (6). In a steady
state, DCs express these molecules limitedly but on encountering
factors derived from pathogen or cancer cells, DCs can be activated
via the pattern recognition receptors signaling pathway such as
toll-like receptors (TLRs) (23).
TLR ligands are used as adjuvant to induce immune response in
several cancers (23–25). A TLR4 ligand called 40s ribosomal
protein subunit 3 (RPS3) can induce DCs maturation (26). Taken together, the present study
aimed to investigate the expression of MSLN in breast cancer
tissues and the potential of genetically manipulated MSLN-SmartDC
to induce MSLN-specific T cells responding against TNBC cells.
Moreover, the effect of RPS3 treatment on MSLN-SmartDC
immunophenotype and T cells activity to enhance cytolytic activity
of T cells were reported. These findings suggested the effect of
MSLN as a potential TNBC antigen and MSLN-specific T cells produced
from RPS3-MSLN-SmartDC presented effective capability to destroy
MSLN-positive TNBC cells.
Materials and methods
Immunohistochemistry
A total of 351 cases of paraffin-embedded breast
cancer tissues and clinicopathological data (Table I) were collected under Siriraj
Institutional Review Board ethical approval (COA no. Si 580/2018).
Following antigen retrieval in pH 6 citrate buffer, the samples
were stained with anti-human MSLN antibody (cat. no. sc-271540;
Santa Cruz Biotechnology, Inc.) at 4°C overnight. Then incubated
with goat anti-mouse Envision (cat. no. K4001; Dako; Agilent
Technologies, Inc.) for 30 min and HRP activity was detected by
Dako-HRP detection kit (Dako; Agilent Technologies, Inc.) before
counterstained with Mayer's hematoxylin. The samples were scanned
at 400× by Scanscope slide scanner (Aperio Technologies, Inc.).
MSLN expression was scored by multiplying the intensity [I; graded:
0 (negative), 1 (weak), 2 (moderate) and 3 (strong)], with the
percentage of MSLN positive cells [P; graded: as 0 (no positive
cells), 1 (1–25%), 2 (26–50%), 3 (51–75%) and 4 (76–100%)]. The
MSLN expression scores calculated by multiplying I and P resulting
in score between 0 to 12. The samples were classified into low if
the score is less than median (which was 0) and rated as high if
the score was equal or more than median.
 | Table I.Relationship between MSLN expression
and clinicopathological factors analyzed by Fisher's exact test.
(Some clinical data were not available for some patient
samples). |
Table I.
Relationship between MSLN expression
and clinicopathological factors analyzed by Fisher's exact test.
(Some clinical data were not available for some patient
samples).
|
|
| Full cohort
(n=351) | HER2 cohort
(n=134) | TNBC cohort
(n=165) |
|---|
|
|
|
|
|
|
|---|
| Characteristic |
| Low | High | P-value | Low | High | P-value | Low | High | P-value |
|---|
| Age (years)
(n=351) | ≤50 | 83 | 46 | 0.410 | 28 | 5 | 1.000 | 24 | 41 | 0.260 |
|
| >50 | 153 | 69 |
| 86 | 15 |
| 47 | 53 |
|
| pT (n=351) | 1-2 | 210 | 107 | 0.255 | 103 | 19 | 0.693 | 63 | 87 | 0.424 |
|
| 3-4 | 26 | 8 |
| 11 | 1 |
| 8 | 7 |
|
| pN (n=350) | 0 | 121 | 71 | 0.086 | 56 | 9 | 0.810 | 41 | 62 | 0.331 |
|
| 1-3 | 114 | 44 |
| 57 | 11 |
| 30 | 32 |
|
| pM (n=348) | 0 | 226 | 114 | 0.175 | 112 | 20 | 1.000 | 71 | 94 | NA |
|
| 1 | 9 | 1 |
| 1 | 0 |
| 0 | 0 |
|
| Clinical staging
(n=348) | 1-2 | 168 | 92 | 0.087 | 80 | 14 | 1.000 | 53 | 78 | 0.244 |
|
| 3-4 | 66 | 22 |
| 32 | 5 |
| 18 | 16 |
|
| Subtype
(n=351) | TNBC | 71 | 94 | <0.001 | - | - | NA | - | - | NA |
|
| HER2 | 114 | 20 |
| - | - |
| - | - |
|
|
| Luminal | 51 | 1 |
| - | - |
| - | - |
|
| ER (n=351) | Neg | 185 | 114 | <0.001 | - | - | NA | - | - | NA |
|
| Pos | 51 | 1 |
| - | - |
| - | - |
|
| PR (n=351) | Neg | 190 | 114 | <0.001 | - | - | NA | - | - | NA |
|
| Pos | 46 | 1 |
| - | - |
| - | - |
|
| HER2 (n=342) | Neg | 69 | 63 | <0.001 | - | - | NA | - | - | NA |
|
| Pos | 158 | 52 |
| - | - |
| - | - |
|
| LN metastasis
(n=350) | Neg | 129 | 73 | 0.136 | 55 | 10 | 1.000 | 44 | 63 | 0.515 |
|
| Pos | 106 | 42 |
| 58 | 10 |
| 27 | 31 |
|
| Perineural
metastasis (n=351) | Neg | 185 | 99 | 0.111 | 83 | 15 | 1.000 | 59 | 83 | 0.370 |
|
| Pos | 51 | 16 |
| 31 | 5 |
| 12 | 11 |
|
Cell culture
MDA-MB-231, HCC70 and T2 cell lines were from
American Type Culture Collection. Lenti-X™ 293T cells were from
Takara Bio (Takara Bio, San Jose, CA). The Lenti-X™ 293T cells,
MDA-MB-231 and MSLN-MDA-MB-231, produced by lentiviral
transduction, were maintained in DMEM with 10% fetal bovine serum
(FBS; Invitrogen; Thermo Fisher Scientific, Inc.). HCC70 cells were
cultured in RPMI-1640 with 10% FBS (Invitrogen; Thermo Fisher
Scientific, Inc.). The T2 cell line was cultured in RPMI-1640 with
10% FBS and 2 mM L-glutamine (Invitrogen; Thermo Fisher Scientific,
Inc.). All cell lines were cultured at 37°C with 5%
CO2.
MSLN-SmartDC lentiviral vector
construction and production
The construction of lentiviral vector was performed
as previously described (8).
Briefly, the full-length MSLN cDNA with restriction cut
sites was amplified from HCC70 cells and cloned into
pCDH1/GM-CSF/IL-4 plasmid. The sequence integrity of
MSLN-SmartDC plasmid was confirmed by Sanger's sequencing. A mock
control pCDH1/GM-CSF/IL-4 containing an irrelevant
red fluorescence protein (IRFP), IRFP-SmartDC, was constructed
(8). MSLN-SmartDC lentiviral
particle was produced by transfecting the 30 µg of MSLN-SmartDC
plasmid along with 21 µg of lentiviral envelop plasmid, 6 µg of
pMD2.G and lentiviral packaging plasmid, psPAX2 into
8×106 Lenti-X™ 293T using calcium phosphate method at
room temperature (RT). The lentiviral particle in the culture
medium was collected 3 days after transfection and concentrated by
20,000 × g ultracentrifugation at 4°C before measured for viral
titer using the qualitative (q)PCR Lentivirus Titration kit
(Applied Biological Materials, Inc.).
Generation of MSLN-SmartDC and
rhRPS3-treated MSLN-SmartDC
The peripheral blood mononuclear cells (PBMCs) were
isolated from 30 ml of HLA-A2+ healthy donor blood with
written consent under Siriraj Institutional Review Board ethical
approval (COA no. Si 580/2018) by density centrifugation at 800 × g
for 20 min at RT in lymphocyte separating medium (Corning Life
Sciences). The monocytes were isolated and incubated for 1 h at
37°C. The non-adherence cells were collected and cryopreserved in
human AB serum (MilliporeSigma) containing 10% dimethyl sulfoxide
until use. The monocytes were transduced with IRFP-SmartDC or
MSLN-SmartDC at 75 multiplicity of infection (MOI) together with 10
µg/ml protamine sulfate in AIM-V medium (Invitrogen; Thermo Fisher
Scientific, Inc.). On day 5 post-transduction, 1 µg/ml of
recombinant human RPS3 (cat. no. NBP2-90977; Novus Biologicals,
LLC) was added. Monocytes were cultured in 100 ng/ml of rhGM-CSF
(cat. no. 11343125; ImmunoTools GmbH) and 50 ng/ml of rhIL-4 (cat.
no. 11340045; ImmunoTools GmbH) for five days and treated with 100
ng/ml of rhIFN-γ (cat. no. 11343536; ImmunoTools GmbH) and rhTNF-α
(cat. no. 11343015; ImmunoTools GmbH) for additional 48 h served as
positive control or conventional DC (conv. DC). All DCs were
harvested on day 7 to check the activated characters.
Activation and expansion of T cells by
MSLN-SmartDC
The cryopreserved T cells were thawed and
co-cultured with DCs for 48 h at a 10:1 ratio to support T cell
activation and proliferation (27)
in AIM-V medium at 37°C with 5% CO2. T cells were
expanded in AIM-V with 5% human AB serum (MilliporeSigma), 20 ng/ml
of rhIL-2 (cat. no. 11340025, ImmunoTools GmbH), 10 ng/ml of rhIL-7
(cat. no. 11340075, ImmunoTools GmbH) and 20 ng/ml of rhIL-15 (cat.
no. 11340155, ImmunoTools GmbH) for seven days at 37°C with 5%
CO2.
Flow cytometry analysis
The immunophenotype of monocytes and DCs were
assessed by anti-CD14 (cat. no. 21620143, ImmunoTools GmbH),
anti-CD40 (cat. no. 21270403, ImmunoTools GmbH) and anti-human
leukocyte antigen (HLA)-DR (cat. no. 21278993, ImmunoTools GmbH),
anti-CD80 (cat. no. 12-0809-42, Thermo Fisher Scientific, Rockford,
IL), anti-CD83 (cat. no. 12-0839-42, Thermo Fisher Scientific) and
anti-CD86 antibodies (cat. no. 12-0869-42, Thermo Fisher
Scientific). All antibodies were used at 1:50 dilution for 30 min
at 4°C. Isotype antibodies were used as negative controls.
The memory T cell subsets were stained by anti-CD3
(cat. no. 21620033, ImmunoTools GmbH), anti-CD45RA (cat. no.
21819456, ImmunoTools GmbH) and anti-CD62L (cat. no. 21819624,
ImmunoTools GmbH), anti-CD4 (cat. no. 56-0049-42, Thermo Fisher
Scientific) and anti-CD8 (cat. no. A15448, Thermo Fisher
Scientific). For intracellular cytokines, the activated T cells
were re-stimulated with 10 µg of MSLN antigenic peptides (SLLFLLFSL
and VLPLTVAEV) (GenScript, Jiangsu, China) (13,28)
for 6 h in the presence of BD GolgiPlug (BD Biosciences, San Jose,
CA) at 37°C with 5% CO2. The cells were stained with
anti-CD3, anti-CD4, anti-CD8 and anti-CD69 (cat. no. MA1-10277,
Thermo Fisher Scientific). Cells were fixed in BD Cytofix/Cytoperm
(BD Biosciences) for 20 min at 4°C, stained with anti-IFN-γ
antibody (cat. no. 17-7319-82, Thermo Fisher Scientific). All
antibodies were stained at 1:100 dilution for 30 min at 4°C.
The flow cytometry data of DCs and T cell
immunophenotypes were acquired by CytoFLEX (Beckman Coulter, Inc.);
the intracellular cytokine staining was acquired by BD LSRFortessa
(BD Biosciences). The data were analyzed by FlowJo version 10.7
(FlowJo LLC) and shown as geometric mean fluorescence intensity
(MFI) of each marker normalized by isotype control.
Enzyme-linked immunosorbent assay
(ELISA)
GM-CSF, IL-4 and IL-12p70 were measured using Human
GM-CSF (cat. no. DGM00), IL-4 (cat. no. D4050) and IL-12p70 (cat.
no. D1200) Quantikine ELISA kits (R&D systems, Inc.). IFN-γ was
measured in medium collected from activated T cells co-cultured
with target cancer cells using Human IFN-γ Quantikine ELISA kit
(cat. no. DIF50, R&D systems, Inc.).
Enzyme-linked immunosorbent spot assay
(ELISpot)
The IFN-γ ELISpot assay was performed using a Human
IFN-γ ELISpotBASIC kit (Mabtech AB). Briefly, 15 µg/ml
of IFN-γ capture antibody was coated for overnight at 4°C. The
2×105 activated T cells were then re-stimulated with
1×104 MSLN peptides-pulsed HLA-A2+ T2 cells.
T cells treated with 20 ng/ml of phorbol 12-myristate 13-acetate
and 1 µg/ml of ionomycin (MilliporeSigma) served as positive
controls. After removing these T cells, 1 µg/ml of
biotinylated-IFN-γ antibody was incubated for 2 h and
ALP-conjugated streptavidin for 1 h at RT. The IFN-γ spots were
evaluated by 5-bromo-4-chloro-3-indolyl phosphate/nitro blue
tetrazolium plus substrate (Mabtech AB) and captured by ELISpot
plate reader (BIOREADER® 5000 Fγ, BIOSyS). The numbers
of spots were counted by CellCounter software (https://github.com/nghiaho12/CellCounter) version
0.2.1.
Western blot analysis
Lenti-X™ 293T and cancer cell lysates were prepared
in RIPA Lysis Buffer System (Santa Cruz Biotechnology). The protein
concentration was determined by Bradford assay and 30 µg of protein
lysates were separated in 12% SDS-PAGE before transferred to PVDF
membrane (GE Healthcare, Buckinghamshire, UK). The membrane was
blocked in 5% skimmed milk (MilliporeSigma) for 30 min at RT. The
membrane was incubated with 1:500 anti-MSLN and 1:5,000
anti-β-actin antibodies (cat. no. sc-47778; Santa Cruz
Biotechnology, Inc.) at 4°C overnight. The 1:1,000 HRP-conjugated
goat anti-mouse antibody (cat. no. 7076; Cell Signaling Technology,
Inc.) was added and incubated for 1 h at RT. The signal was
detected by Clarity™ western ECL substrate (Bio-Rad Laboratories,
Inc.) using a Gel Doc instrument (G:Box Chemi XR5; Syngene Europe).
The band intensity was analyzed by ImageJ software version 1.53
(NIH).
2D cancer killing by luciferase
assay
T cells were co-cultured with 5,000 cells of
luciferase expressing HCC70, MDA-MB-231 and MSLN-MDA-MB-231 cells
at Effector: Target ratio of 1:1, 5:1 and 10:1 for 24 h at 37°C
with 5% CO2. The luciferase activity was determined
using Pierce Firefly Luciferase Glow Assay kit (Pierce; Thermo
Fisher Scientific, Inc.) and Lumat LB 9507 Ultra-sensitive
Luminometer (Berthold Technologies GmbH & Co. KG). After
normalizing the luciferase activity of every condition with target
cell alone condition, the percentage of cancer cell lysis was
calculated. The luciferase activity of target cell alone was served
as an internal control.
3D-spheroid cancer killing assay
The 1×103 target mWasabi-transduced
cancer cells were formed into spheroid in 96-well ultra-low
attachment plates (Corning Life Sciences) in 200 µl culture medium
containing 2.5% cold Matrigel (BD Biosciences) by centrifugation at
300 × g for 3 min at 4°C. The spheroid was incubated for 4 days at
37°C with 5% CO2 with CellTracker™ Orange CMRA Dye
(Invitrogen; Thermo Fisher Scientific, Inc.)-labelled activated T
cells at Effector: Target ratios of 1:1, 5:1, 10:1 and 20:1 for 48
h at 37°C with 5% CO2. The mWasabi and CMRA fluorescence
signals were detected by inverted fluorescence microscope and
cellSens standard program version 1.15 (Olympus Corporation).
Statistical analysis
The association between MSLN score and
clinicopathological data were accessed by Fisher's exact test.
One-way ANOVA and Tukey's post-hoc test was used for all
experiments except in the intracellular cytokine staining for
comparison of control- and peptide-challenged T cells from the same
condition in which Student's t-test was used. The Fisher's exact
test was performed in SPSS 17.0 (SPSS, Inc.), whereas GraphPad
prism V (GraphPad Software, Inc.) was used for one-way ANOVA and
Student's t-test. All results were shown as mean ± standard
deviation from at least three independent experiments. P<0.05
was considered to indicate a statistically significant
difference.
Results
MSLN expression in breast cancer
tissues
All patient cases were female with mean age of
54±11.5 years diagnosed as luminal, HER2-positive and TNBC subtypes
for 52 (15%), 154 (38%) and 165 (47%) cases, respectively (Table I). The cytoplasmic and membranous
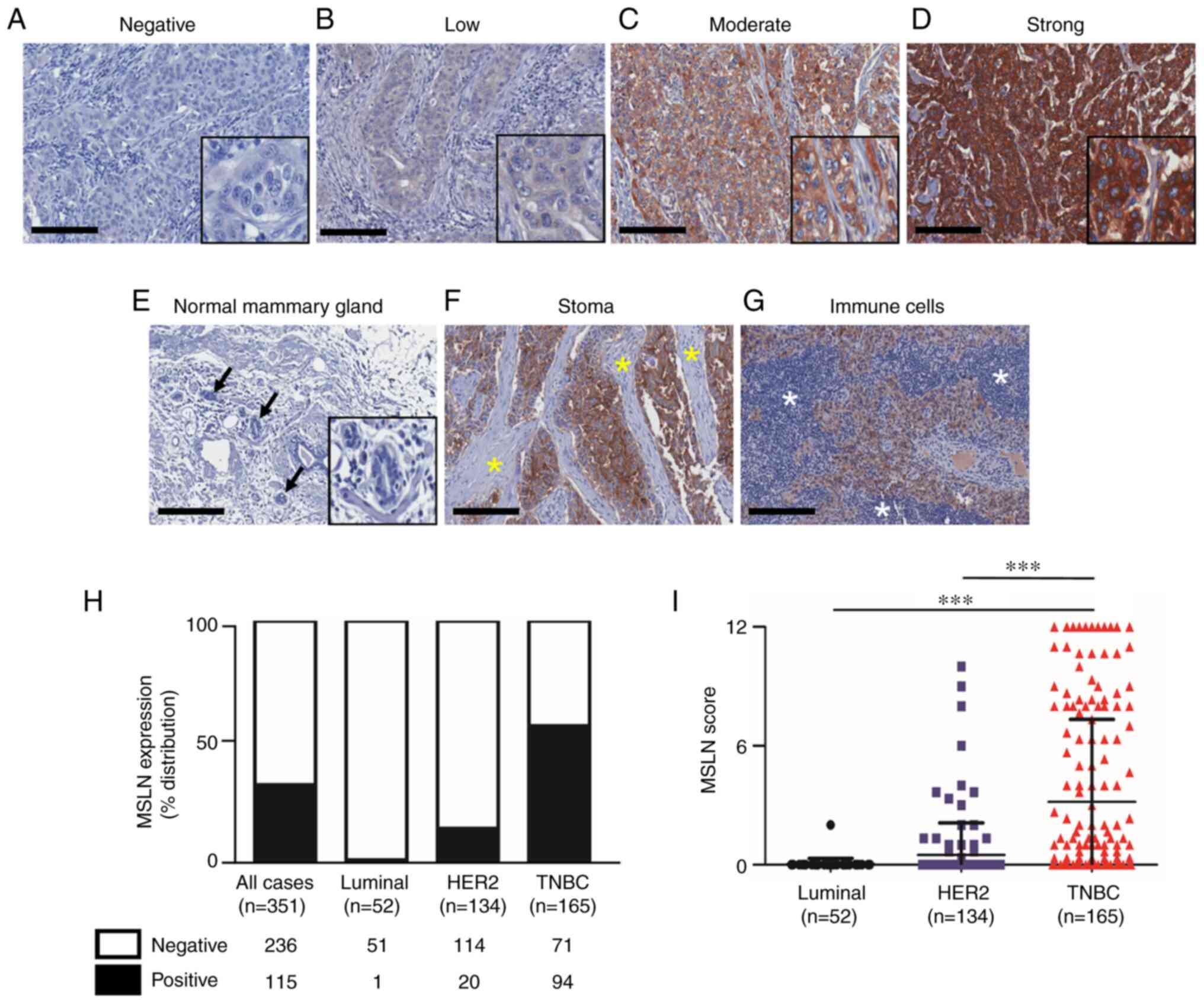
patterns of MSLN positive were detected in cancer cells with
various levels (Fig. 1A-D). No
MSLN expression was observed in the adjacent normal mammary cells
(Fig. 1E), stromal cells (Fig. 1F) and immune cells (Fig. 1G). Among 351 total cases, 115 cases
(32.8%) were positive for MSLN. When stratifying the patients
according to the subtypes, MSLN expression was found in 94 of 165
cases (57%) for TNBC subtype, 20 of 154 cases (14.9%) in
HER2-positive and 1 of 52 cases (1.9%) in luminal subtypes
(Fig. 1H). The mean MSLN
expression score was significantly higher in TNBC subtype compared
with that in HER2-positive and luminal subtype (Fig. 1I). MSLN was significantly
correlated with the absence of ER, PR and HER2 and TNBC subtype
(Table 1). There was no
association between MSLN and overall or disease-free survival in
all samples, HER2-positive and TNBC subtypes (data not shown).
Generation and immunophenotyping of
MSLN-SmartDC and RPS3-MSLN-SmartDC
MSLN-SmartDC was generated by transducing the
lentiviral vector containing GM-CSF, IL-4 and MSLN
(Fig. 2A) into PBMCs-derived
monocytes from four HLA-A2 positive healthy donors. The expression
of MSLN protein from MSLN-SmartDC lentiviral vector was confirmed
in Lenti-X™ 293T (Fig. 2B). All
DCs demonstrated dendritic-like morphology (Fig. 2C). The GM-CSF was significantly
detected higher levels in both MSLN-SmartDC and RPS3-MSLN-SmartDC
compared with monocytes (Fig. 2D).
MSLN-SmartDC and RPS3-MSLN-SmartDC also secreted IL-4 significantly
higher than the monocytes but significantly lower compared with
IRFP-SmartDC (Fig. 2E). Production
of IL-12p70 was detected in significantly higher levels in
RPS3-MSLN-SmartDC compared with other conditions (Fig. 2F). The significant reduction of
CD14 monocyte marker in all DCs conditions (Fig. 2G) and mature DC markers including
CD40, HLA-DR, CD80, CD83 and CD86 (Fig. 2H-L) were significantly increased
compared with monocytes. The addition of RPS3 to MSLN-SmartDC could
significantly increase CD40, CD80 and CD83 (Fig. 2H and J-K) compared with those of
MSLN-SmartDC.
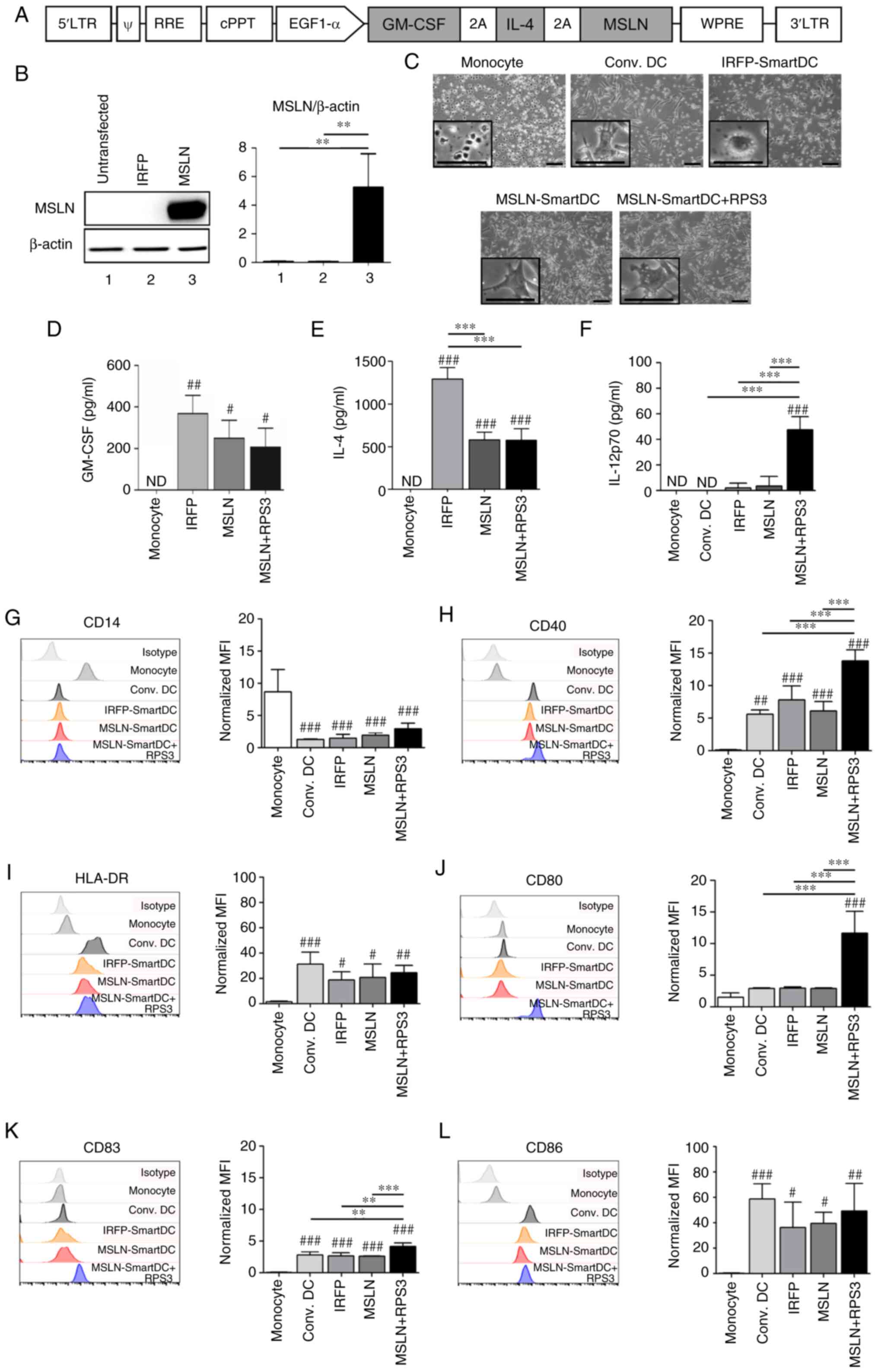 | Figure 2.The lentiviral vector schematic maps
and immunophenotype of MSLN-SmartDC and RPS3-MSLN-SmartDC. (A)
Construction of MSLN-SmartDC lentiviral vector. (B) Expression of
MSLN protein in Lenti-X™ 293T transfected with IRFP-SmartDC and
MSLN-SmartDC lentiviral vectors. (C) Representative images of
monocytes at day 0 and DCs at day 7 of the experiment. Production
of (D) GM-CSF, (E) IL-4 and (F) IL-12p70 by monocytes and different
DCs. The geometric mean fluorescence intensity of surface markers
of DCs in different treatment conditions including (G) CD14, (H)
CD40, (I) HLA-DR, (J) CD80, (K) CD83 and (L) CD86 in monocytes and
DCs. Original magnification, ×100 and scale bar=50 µm. The results
were collected from four independent experiments. ND, not detected.
#P<0.05, ##P<0.01 and
###P<0.001 vs. monocytes. **P<0.01 and
***P<0.001. MSLN, mesothelin; MSLN-SmartDC, MSLN dendritic
cells; RPS3, ribosomal protein subunit 3; GM-CSF,
granulocyte-macrophage colony stimulating factor; DCs, dendritic
cells; HLA, human leukocyte antigen. |
Immunophenotype of T cells activated
by MSLN-SmartDC and RPS3-MSLN-SmartDC
The results showed slight changes in CD4+
and CD8+ T cells frequencies and memory T cells subsets
compared with those in PBMCs at day 0 (Fig. S1A and B). Re-stimulation with MSLN
antigenic peptides in T cells activated by MSLN-SmartDC and
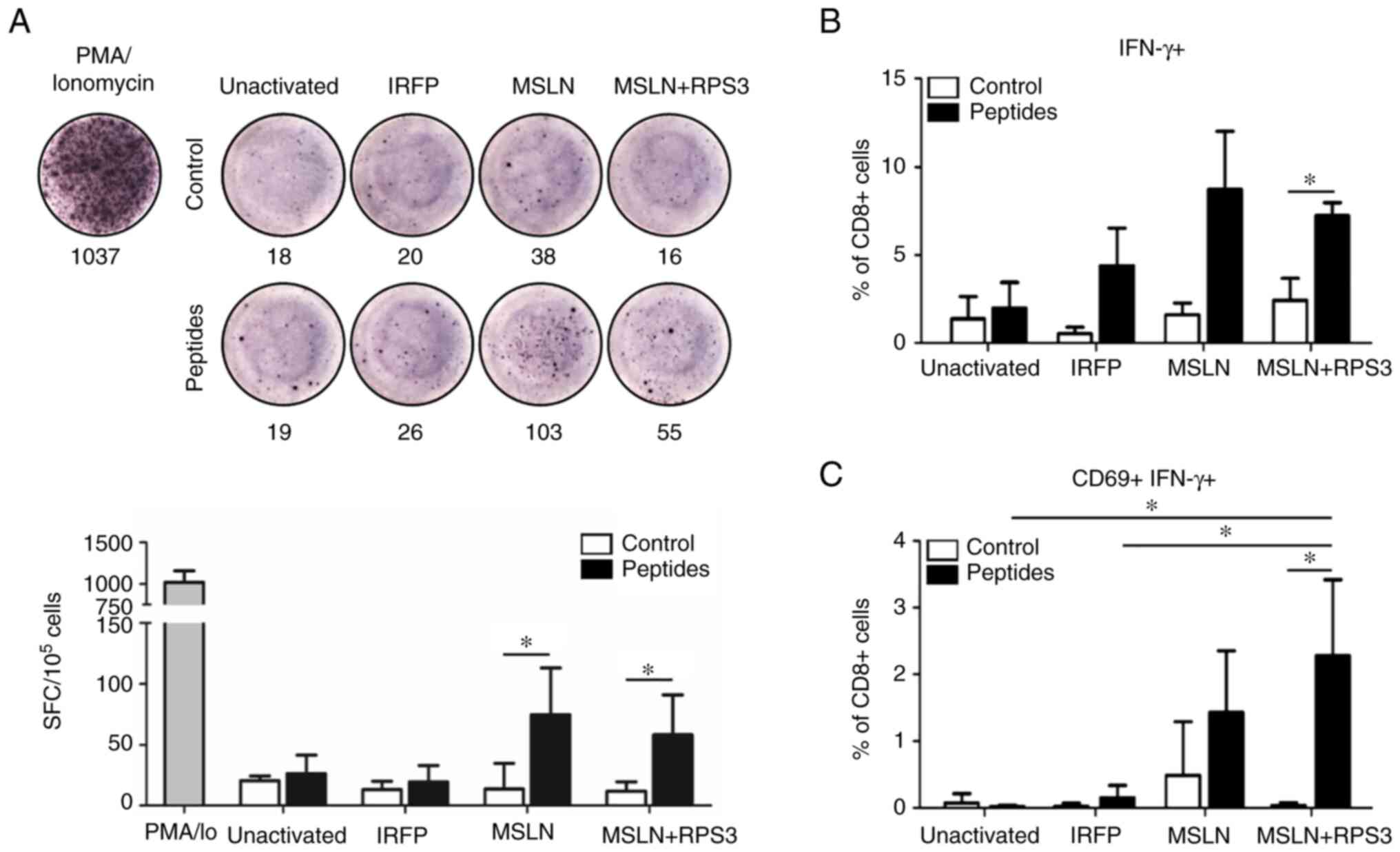
RPS3-MSLN-SmartDC showed significantly higher number of IFN-γ than
those without peptide treatment (Fig.
3A). The frequency of IFN-γ+ CD8+ T cells
after MSLN peptides challenging were found at higher levels in
MSLN-SmartDC- and RPS3-MSLN-activated T cells than unactivated and
IRFP-activated T cells (Fig. 3B).
However, only the RPS3-MSLN-activated T cells revealed significant
increased IFN-γ+ CD8+ T cells (Fig. 3B). The MSLN-SmartDC-activated T
cells result did not achieved a statistically significant level.
The dual expressions of CD69 and IFN-γ were observed in MSLN- and
RPS3-MSLN-activated T cells, but very low level in T cells treated
with unactivated- and IRFP-activated T cells (Fig. 3C). The frequency of MSLN-specific
CD69+ IFN-γ+ CD8+ T cells
activated by RPS3-MSLN-SmartDC was clearly higher than unactivated
and IRFP-activated T cells. Moreover, CD69+
IFN-γ+ CD8+ T cells in RPS3-MSLN-activated T
cells was significantly higher compared with those without peptides
re-stimulation.
2D cytolytic activity of MSLN-SmartDC-
and RPS3-MSLN-SmartDC-activated T cells
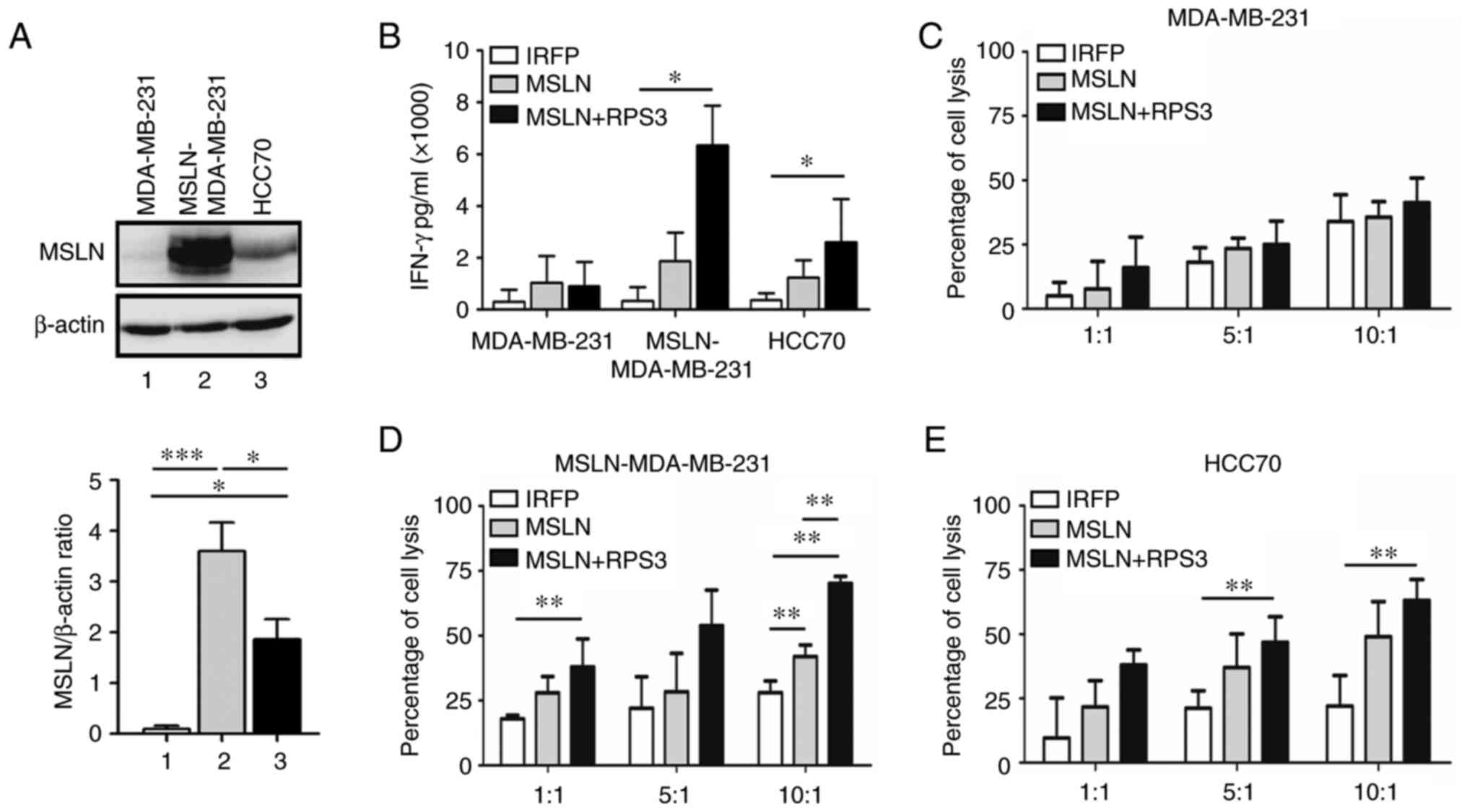
The MSLN showed the highest level in
MSLN-MDA-MB-231, followed by HCC70, whereas MDA-MB-231 had no MSLN
(Fig. 4A). The IFN-γ releasing
from RPS3-MSLN-activated T cells after co-culturing with
MSLN-MDA-MB-231 and HCC70 cells was higher than MSLN-activated T
cells and significantly higher than those of IRFP-activated T cells
(Fig. 4B). No changes of IFN-γ
secreted from T cells cocultured with MDA-MB-231 were observed.
The results exhibited no difference in MDA-MB-231
cell lysis co-cultured with both MSLN-activated T cells and
RPS3-MSLN-activated T cells at Effector: Target ratios of 1:1, 5:1
and 10:1 (Fig. 4C). T cells
activated by MSLN-SmartDC demonstrated significantly higher
MSLN-MDA-MB-231 cell cytotoxicity compared with IRFP-activated T
cells at 10:1 ratio (Fig. 4D). At
10:1, T cells activated by RPS3-MSLN-SmartDC could significantly
induce MSLN-MDA-MB-231 cell lysis more than MSLN-activated T cells
(Fig. 4D). In HCC70, T cells
activated by MSLN-SmartDC and RPS3-MSLN-SmartDC demonstrated higher
killing activity than IRFP-activated T cells (Fig. 4E). However, only
RPS3-MSLN-activated T cells achieved statistical significance of
cancer cell killing compared with that of IRFP-activated T cells at
ratios of 5:1 and 10:1 (Fig.
4E).
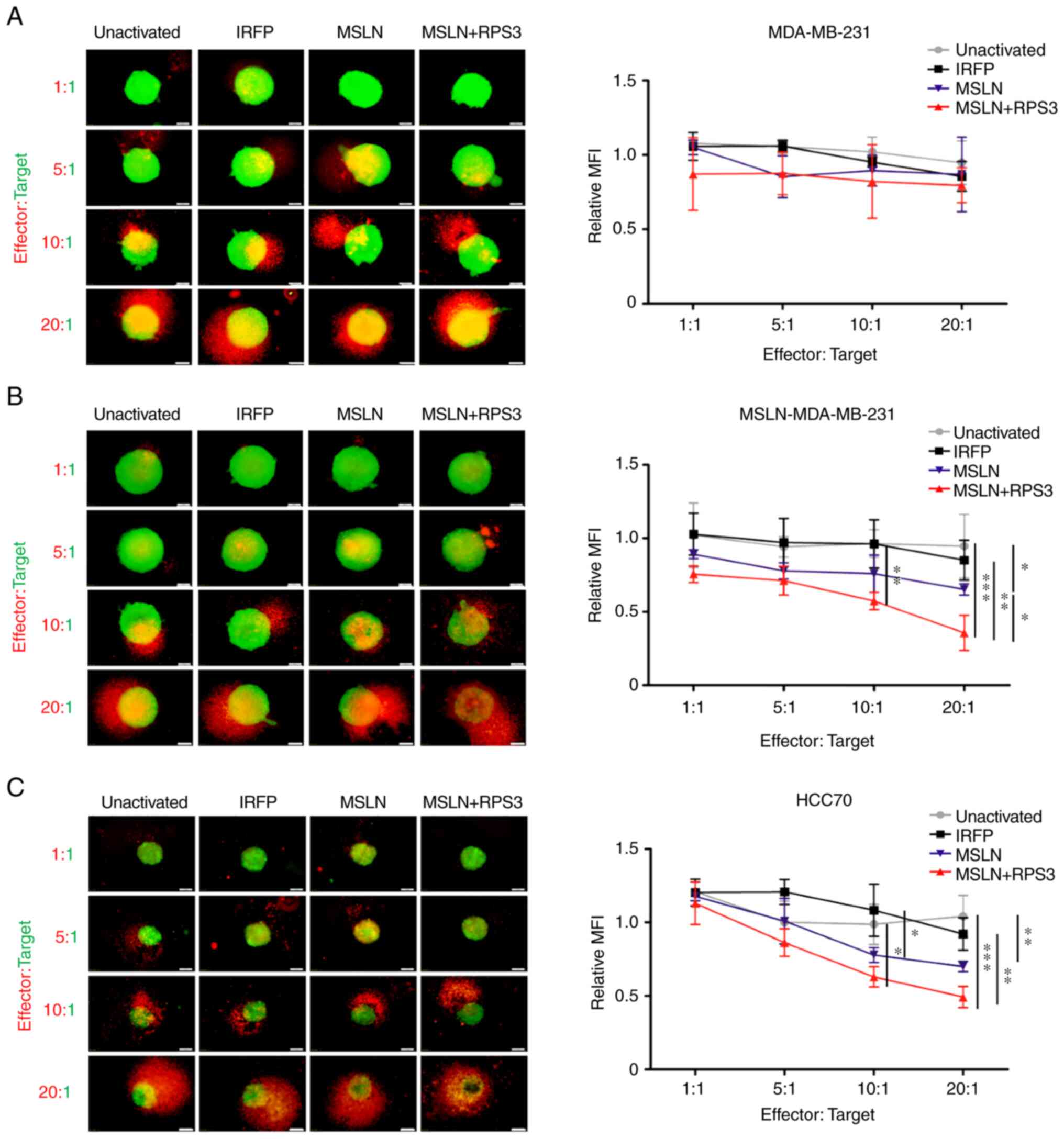
3D cytolytic activity of T cells
activated by MSLN-SmartDC and RPS3-MSLN-SmartDC
MDA-MB-231 spheroids co-cultured with activated T
cells or unactivated T cells for 48 h revealed no differences in
mWasabi green fluorescence signals representing viable cancer cells
(Fig. 5A). MSLN-MDA-MB-231
spheroid co-cultured with T cells activated by MSLN-SmartDC
significantly exhibited lower viable cells compared with those of
unactivated T cells at 20:1 (Fig.
5B). T cells activated by RPS3-MSLN-SmartDC had significant
decrease viable cells compared with unactivated T cells
(P<0.001), IRFP-activated and MSLN-activated T cells at 20:1
(Fig. 5B). RPS3 could enhance DCs
activation that subsequently increase T cells capability to
recognize MSLN-MDA-MB-231 cells. For HCC70 spheroid, the
significant decreased fluorescence signal was observed in
MSLN-SmartDC-activated T cells at ratio 20:1 compared with
unactivated T cells. Moreover, T cells activated by
RPS3-MSLN-SmartDC demonstrated significant reduction of mWasabi
fluorescence signal compared with both unactivated and
IRFP-activated T cells at 10:1 and 20:1 (Fig. 5C).
Discussion
The lack of ER/PR and HER2 in TNBC limits the
available treatment to systemic chemotherapy and surgical resection
(1). Targeting the protein
overexpressed in TNBC such as MSLN is currently an active area of
immunotherapy (14,29). The high expression of MSLN in TNBC
reported in the present study was consistent with previous studies,
with the range of MSLN ~37–67% (14,18–22).
The DCs-based immunotherapy is another potential approach for the
TNBC treatment that could promote the antigen-specific immune
response which leading to clinical response as observed in various
types of cancer including melanoma, leukemia, cholangiocarcinoma
and pancreatic cancer (6,8–10,13,24).
In the present study, self-differentiated DCs presenting MSLN
antigen, termed MSLN-SmartDC, was developed and shown to promote
the MSLN-specific immune response against TNBC. The effect of RPS3,
a TLR4 ligand, on MSLN-SmartDC immunophenotype and T cells
activation capability was significantly enhanced. As high MSLN
expression was confirmed in most TNBC cases, the MSLN-specific T
cell production by MSLN-SmartDC platform is suggested as an
alternative T cell treatment in patients with TNBC.
Several studies have reported the utility of DCs
generated by lentiviral transduction of cytokine genes for DCs
differentiation and by tumor-associated antigen gene to induce
antigen-specific immune response leading to the tumor growth
inhibition (8–12). The MSLN-SmartDC of the present
study demonstrated consistent immunophenotypes with DCs generated
by recombinant GM-CSF and IL-4 observed by the significant
downregulation of CD14 monocyte marker, while the markers of DCs
such as CD40, CD83, CD86 and HLA-DR were significantly upregulated.
Moreover, the increased MSLN-SmartDC maturation profile by
upregulation of the costimulatory molecules; CD40, CD80, CD83 and
IL-12p70 production were observed. Although the secretion of GM-CSF
and IL-4 were found to be higher in IRFP-SmartDC compared with
MSLN-SmartDC which may be explained by the smaller tri-cistronic
mRNA size produced by IRFP-SmartDC (30). Nevertheless, these higher GM-CSF
and IL-4 levels secreted by IRFP-SmartDC did not confer the
immunophenotype differences as seen by a comparable mature DCs
markers expression compared with MSLN-SmartDC. CD40, CD80 and CD83
increment in RPS3-MSLN-SmartDC compared with MSLN-SmartDC supported
previous findings that RPS3 could activate DC maturation (26). These characteristics suggested the
potential of higher T cell activation of RPS3-MSLN-SmartDC compared
with MSLN-SmartDC. The upregulation of CD40 in matured DCs is
required for the DCs licensing by CD40L-expressing T cells which
further augment the costimulatory molecules and cytokine production
initiated by RPS3 treatment (31–33).
This is the limitation of the present study, but it may be
explained that RPS3 induces MSLN-SmartDC maturation and the
upregulation of CD40 can further augment the maturation initiated
by TLR4 signaling pathway (33).
The T cells characteristics following co-culturing
with MSLN-SmartDC with or without RPS3 showed slightly changed in
frequencies of CD4+, CD8+ and the memory T
cells subsets. This may be due to the effect of cytokines used
during T cells expansion process which can non-specifically promote
T cell proliferation (34,35). However, the presence of
MSLN-specific CD8+ T cells recognizes HLA-A2 restricted
MSLN antigenic peptides (13,28)
in cells activated by MSLN-SmartDC and RPS3-MSLN-SmartDC compared
with the negative control conditions. Although there was a trend
toward increased MSLN-specific T cells in RPS3-MSLN-SmartDC
compared with other conditions, it did not achieve the
statistically significant levels. The addition of RPS3 to
MSLN-SmartDC could not affect the frequency of MSLN-specific T
cells. It is possible that the upregulated factors found in
RPS3-MSLN-SmartDC, including CD40, CD80 and IL-12p70, exert their
effect on the quality of antigen-specific T cells; in particular
the cytotoxicity function rather than the quantity or the frequency
of T cells (31,36,37).
This may be supported by the significant increase of
IFN-γ+ and CD69+ IFN-γ+ T cells
driven by RPS3-MSLN-SmartDC. T cells activated by MSLN-SmartDC or
RPS3-MSLN-SmartDC promoted TNBC cell killing in effector cells and
antigen-dependent manners. These findings are in agreement with
previous studies in SmartDC system in different antigens and cancer
models (8–11). Moreover, T cells activated by
RPS3-MSLN-SmartDC demonstrated enhanced cytolytic activity against
MSLN expressing cancer cells. This was associated with the
increased IFN-γ production and may explain effective target cells
lysis by RPS3-MSLN-activated T cells.
The cytolytic activity of T cells activated by
MSLN-SmartDC in 3D-cancer spheroid was consistent with that
observed in 2D culture system. To minimize the effect of
non-specific T cells killing mediated by HLA-mismatched between the
target cells and T cells, healthy donors with HLA-A2 partially
matched with MDA-MB-231, but not HCC70 (HLA-A3), were selected.
Using MSLN-MDA-MB-231 in comparison with parental MDA-MB-231 could
eliminate the intrinsic factors of target cells that may interfere
with the T cells cytolytic activity, except the presence of MSLN.
Future study using the ex vivo generated SmartDC and T cells
to kill patient-derived TNBC cells can eliminate this limitation.
The addition of RPS3 in MSLN-SmartDC trend toward increased cancer
cell cytotoxicity in HCC70. Collectively, the obtained findings of
the present study highlighted the efficacy of T cells activated by
MSLN-SmartDC to eliminate MSLN-expressing TNBC cells and the
addition of RPS3 to MSLN-SmartDC prior co-cultured with T cells
enhances T cells cytolytic activity against the TNBC cells.
The MSLN-specific T cells in cancer patients have
been reported (28,38,39).
Different approaches of MSLN targeted treatment demonstrate safety
and efficacy in several types of cancer (16,29,40).
Targeting MSLN in TNBC using T cells activated by MSLN-SmartDC and
RPS3-MSLN-SmartDC may provide a potential safe and effective
treatment for patients with TNBC. It is known that the immune
response against the cancer cells gradually declines with age
(41,42). The use of RPS3-MSLN-SmartDC
treatment which utilizes patient's immune cells in younger patients
might be both compatible with more aggressive nature of TNBC and
also the more competent immune response in young patients with
TNBC. The response of MSLN-SmartDC or MSLN-specific T cell
treatment in luminal and HER2+ patients should be the same as
patients with TNBC, if they expressed high level of MSLN in the
cancer cells. The current trend in breast cancer treatment approach
has now shifted from the monotherapy to the combinational treatment
involving several interventions such as surgery, radiation,
chemotherapy and immunotherapy (1). Combination of treatments targeting
the bulk tumor mass or the stromal cells and reducing the
immunosuppressive signal in tumor microenvironment via surgery,
chemotherapy and checkpoint inhibitor together with the DCs-based
immunotherapy that promote the antigen-specific T cells could
result in significant improved clinical outcome (43–46).
Therefore, further investigation of MSLN-SmartDC and
RPS3-MSLN-SmartDC in combination with other treatment approaches is
a great promise for the novel treatment modality in patients with
TNBC.
In conclusion, the efficacy of MSLN-SmartDC
promoting MSLN-specific immune response killing MSLN-expressing
TNBC cells was successfully developed. The MSLN-SmartDC maturation
enhancement by RPS3 treatment can improve the cytolytic activity of
T cells against high MSLN TNBC cells. Though the clinical use of
MSLN-SmartDC and RPS3-MSLN-SmartDC needs more convincing data in
the in vivo where the effect of tumor microenvironment on
this treatment could be assessed and in the ex vivo system
where the fully-matched HLAs type could be done, the present
findings demonstrated the potential of these DCs to activate
MSLN-specific T cells as an alternative treatment for patients with
TNBC.
Supplementary Material
Supporting Data
Acknowledgements
The authors gratefully thank Miss Surat Phumphuang
(Department of Immunology, Faculty of Medicine Siriraj Hospital,
Mahidol University, Thailand) for her effort in clinicopathological
data collection. The authors would also like to thank Emeritus
Professor James A. Will (senior editor for the Faculty of Medicine,
Khon Kaen University) for the English version of the present
manuscript.
Funding
This work has been funded by Midcareer Research Grant (grant no.
RSA6280091), National Research Council of Thailand to CT; Graduate
Grant, National Research Council of Thailand (grant no. N41D640036)
and Siriraj Graduate Scholarship to NJ and TRF-IRN Scholarship
(Scholarship number IRN5801PHDW05) to WC.
Availability of data and materials
The datasets used and/or analyzed during the
current study are available from the corresponding author upon
reasonable request.
Authors' contributions
CT and PY conceived the study. NJ, TC, PT, PY and
CT designed the experiments. NJ, ST and WC performed the
experiments. DS and MW performed data resource and analysis. NJ, ST
and CT analyzed and interpreted the data. CT supervised the overall
research. NJ and CT confirm the authenticity of all raw data. NJ,
ST and CT wrote the manuscript. CT reviewed and/or edited the
manuscript. All authors reviewed and approved the final
manuscript.
Ethics approval and consent to
participate
All experiments in this study were approved by
Siriraj Institutional Review Board (COA no. Si580/2018) and with
the 1964 Helsinki declaration and its later amendments or
comparable ethical standards. All blood donors in this study
provided written informed consent for the use of blood samples for
research.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Waks AG and Winer EP: Breast cancer
treatment: A review. JAMA. 321:288–300. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Reddy SM, Barcenas CH, Sinha AK, Hsu L,
Moulder SL, Tripathy D, Hortobagyi GN and Valero V: Long-term
survival outcomes of triple-receptor negative breast cancer
survivors who are disease free at 5 years and relationship with low
hormone receptor positivity. Br J Cancer. 118:17–23. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cheung KL: Treatment strategies and
survival outcomes in breast cancer. Cancers (Basel). 12:7352020.
View Article : Google Scholar
|
|
4
|
Arnedos M, Roulleaux Dugage M,
Perez-Garcia J and Cortes J: Window of opportunity trials for
biomarker discovery in breast cancer. Curr Opin Oncol. 31:486–492.
2019. View Article : Google Scholar
|
|
5
|
Falzone L, Grimaldi M, Celentano E,
Augustin LSA and Libra M: Identification of modulated micrornas
associated with breast cancer, diet, and physical activity. Cancers
(Basel). 12:25552020. View Article : Google Scholar
|
|
6
|
Saxena M and Bhardwaj N: Re-emergence of
dendritic cell vaccines for cancer treatment. Trends Cancer.
4:119–137. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bernhard H, Neudorfer J, Gebhard K, Conrad
H, Hermann C, Nährig J, Fend F, Weber W, Busch DH and Peschel C:
Adoptive transfer of autologous, HER2-specific, cytotoxic T
lymphocytes for the treatment of HER2-overexpressing breast cancer.
Cancer Immunol Immunother. 57:271–280. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Panya A, Thepmalee C, Sawasdee N,
Sujjitjoon J, Phanthaphol N, Junking M, Wongkham S and
Yenchitsomanus PT: Cytotoxic activity of effector T cells against
cholangiocarcinoma is enhanced by self-differentiated
monocyte-derived dendritic cells. Cancer Immunol Immunother.
67:1579–1588. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sundarasetty BS, Chan L, Darling D, Giunti
G, Farzaneh F, Schenck F, Naundorf S, Kuehlcke K, Ruggiero E,
Schmidt M, et al: Lentivirus-induced ‘smart’ dendritic cells:
Pharmacodynamics and GMP-compliant production for immunotherapy
against TRP2-positive melanoma. Gene Ther. 22:707–720. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sundarasetty BS, Singh VK, Salguero G,
Geffers R, Rickmann M, Macke L, Borchers S, Figueiredo C, Schambach
A, Gullberg U, et al: Lentivirus-induced dendritic cells for
immunization against high-risk WT1(+) acute myeloid leukemia. Hum
Gene Ther. 24:220–237. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bialek-Waldmann JK, Domning S, Esser R,
Glienke W, Mertens M, Aleksandrova K, Arseniev L, Kumar S,
Schneider A, Koenig J, et al: Induced dendritic cells co-expressing
GM-CSF/IFN-α/tWT1 priming T and B cells and automated manufacturing
to boost GvL. Mol Ther Methods Clin Dev. 21:621–641. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pincha M, Sundarasetty BS, Salguero G,
Gutzmer R, Garritsen H, Macke L, Schneider A, Lenz D, Figueiredo C,
Blasczyk R, et al: Identity, potency, in vivo viability, and
scaling up production of lentiviral vector-induced dendritic cells
for melanoma immunotherapy. Hum Gene Ther Methods. 23:38–55. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
de Goeje PL, Klaver Y, Kaijen-Lambers MEH,
Langerak AW, Vroman H, Kunert A, Lamers CHJ, Aerts JGJV, Debets R
and Hendriks RW: Autologous dendritic cell therapy in mesothelioma
patients enhances frequencies of peripheral CD4 T cells expressing
HLA-DR, PD-1, or ICOS. Front Immunol. 9:20342018. View Article : Google Scholar
|
|
14
|
Tchou J, Wang LC, Selven B, Zhang H,
Conejo-Garcia J, Borghaei H, Kalos M, Vondeheide RH, Albelda SM,
June CH and Zhang PJ: Mesothelin, a novel immunotherapy target for
triple negative breast cancer. Breast Cancer Res Treat.
133:799–804. 2012. View Article : Google Scholar
|
|
15
|
Le K, Wang J, Zhang T, Guo Y, Chang H,
Wang S and Zhu B: Overexpression of mesothelin in pancreatic ductal
adenocarcinoma (PDAC). Int J Med Sci. 17:422–427. 2020. View Article : Google Scholar
|
|
16
|
Haas AR, Tanyi JL, O'Hara MH, Gladney WL,
Lacey SF, Torigian DA, Soulen MC, Tian L, McGarvey M, Nelson AM, et
al: Phase I study of lentiviral-transduced chimeric antigen
receptor-modified T cells recognizing mesothelin in advanced solid
cancers. Mol Ther. 27:1919–1929. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hassan R and Ho M: Mesothelin targeted
cancer immunotherapy. Eur J Cancer. 44:46–53. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Parinyanitikul N, Blumenschein GR, Wu Y,
Lei X, Chavez-Macgregor M, Smart M and Gonzalez-Angulo AM:
Mesothelin expression and survival outcomes in triple receptor
negative breast cancer. Clin Breast Cancer. 13:378–384. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tozbikian G, Brogi E, Kadota K, Catalano
J, Akram M, Patil S, Ho AY, Reis-Filho JS, Weigelt B, Norton L, et
al: Mesothelin expression in triple negative breast carcinomas
correlates significantly with basal-like phenotype, distant
metastases and decreased survival. PLoS One. 9:e1149002014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bayoglu IV, Kucukzeybek BB, Kucukzeybek Y,
Varol U, Yildiz I, Alacacioglu A, Akyol M, Demir L, Dirican A,
Yildiz Y, et al: Prognostic value of mesothelin expression in
patients with triple negative and HER2-positive breast cancers.
Biomed Pharmacother. 70:190–195. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li YR, Xian RR, Ziober A, Conejo-Garcia J,
Perales-Puchalt A, June CH, Zhang PJ and Tchou J: Mesothelin
expression is associated with poor outcomes in breast cancer.
Breast Cancer Res Treat. 147:675–684. 2014. View Article : Google Scholar
|
|
22
|
Suzuki T, Yamagishi Y, Einama T, Koiwai T,
Yamasaki T, Fukumura-Koga M, Ishibashi Y, Takihata Y, Shiraishi T,
Miyata Y, et al: Membrane mesothelin expression positivity is
associated with poor clinical outcome of luminal-type breast
cancer. Oncol Lett. 20:1932020. View Article : Google Scholar
|
|
23
|
Vermaelen K: Vaccine strategies to improve
anti-cancer cellular immune responses. Front Immunol. 10:82019.
View Article : Google Scholar
|
|
24
|
Mehrotra S, Britten CD, Chin S,
Garrett-Mayer E, Cloud CA, Li M, Scurti G, Salem ML, Nelson MH,
Thomas MB, et al: Vaccination with poly(IC:LC) and peptide-pulsed
autologous dendritic cells in patients with pancreatic cancer. J
Hematol Oncol. 10:822017. View Article : Google Scholar
|
|
25
|
Chow LQM, Morishima C, Eaton KD, Baik CS,
Goulart BH, Anderson LN, Manjarrez KL, Dietsch GN, Bryan JK,
Hershberg RM, et al: Phase Ib trial of the Toll-like receptor 8
agonist, motolimod (VTX-2337), combined with cetuximab in patients
with recurrent or metastatic SCCHN. Clin Cancer Res. 23:2442–2450.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Park HJ, Jang GY, Kim YS, Park JH, Lee SE,
Vo MC, Lee JJ, Han HD, Jung ID, Kang TH and Park YM: A novel TLR4
binding protein, 40S ribosomal protein S3, has potential utility as
an adjuvant in a dendritic cell-based vaccine. J Immunother Cancer.
7:602019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Höpken UE, Lehmann I, Droese J, Lipp M,
Schüler T and Rehm A: The ratio between dendritic cells and T cells
determines the outcome of their encounter: Proliferation versus
deletion. Eur J Immunol. 35:2851–2863. 2005. View Article : Google Scholar
|
|
28
|
Thomas AM, Santarsiero LM, Lutz ER,
Armstrong TD, Chen YC, Huang LQ, Laheru DA, Goggins M, Hruban RH
and Jaffee EM: Mesothelin-specific CD8(+) T cell responses provide
evidence of in vivo cross-priming by antigen-presenting cells in
vaccinated pancreatic cancer patients. J Exp Med. 200:297–306.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Del Bano J, Florès-Florès R, Josselin E,
Goubard A, Ganier L, Castellano R, Chames P, Baty D and Kerfelec B:
A bispecific antibody-based approach for targeting mesothelin in
triple negative breast cancer. Front Immunol. 10:15932019.
View Article : Google Scholar
|
|
30
|
Fernandes LD, Moura APS and Ciandrini L:
Gene length as a regulator for ribosome recruitment and protein
synthesis: Theoretical insights. Sci Rep. 7:174092017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ara A, Ahmed KA and Xiang J: Multiple
effects of CD40-CD40L axis in immunity against infection and
cancer. Immunotargets Ther. 7:55–61. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tay NQ, Lee DCP, Chua YL, Prabhu N,
Gascoigne NRJ and Kemeny DM: CD40L expression allows
CD8+ T cells to promote their own expansion and
differentiation through dendritic cells. Front Immunol. 8:14842017.
View Article : Google Scholar
|
|
33
|
Michael Dohnal A, Luger R, Paul P, Fuchs D
and Felzmann T: CD40 ligation restores type 1 polarizing capacity
in TLR4-activated dendritic cells that have ceased interleukin-12
expression. J Cell Mol Med. 13((8B)): 1741–1750. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ross SH and Cantrell DA: Signaling and
function of interleukin-2 in T lymphocytes. Annu Rev Immunol.
36:411–433. 2018. View Article : Google Scholar
|
|
35
|
Drake A, Kaur M, Iliopoulou BP, Phennicie
R, Hanson A and Chen J: Interleukins 7 and 15 maintain human T cell
proliferative capacity through STAT5 signaling. PLoS One.
11:e01662802016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Agarwal P, Raghavan A, Nandiwada SL,
Curtsinger JM, Bohjanen PR, Mueller DL and Mescher MF: Gene
regulation and chromatin remodeling by IL-12 and type I IFN in
programming for CD8 T cell effector function and memory. J Immunol.
183:1695–1704. 2009. View Article : Google Scholar
|
|
37
|
Chen L and Flies DB: Molecular mechanisms
of T cell co-stimulation and co-inhibition. Nat Rev Immunol.
13:227–242. 2013. View Article : Google Scholar
|
|
38
|
Chen Y, Ayaru L, Mathew S, Morris E,
Pereira SP and Behboudi S: Expansion of anti-mesothelin specific
CD4+ and CD8+ T cell responses in patients with pancreatic
carcinoma. PLoS One. 9:e881332014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhenjiang L, Rao M, Luo X, Sandberg E,
Bartek J Jr, Schoutrop E, von Landenberg A, Meng Q, Valentini D,
Poiret T, et al: Mesothelin-specific immune responses predict
survival of patients with brain metastasis. EBioMedicine. 23:20–24.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang J, Khanna S, Jiang Q, Alewine C,
Miettinen M, Pastan I and Hassan R: Efficacy of anti-mesothelin
immunotoxin RG7787 plus nab-paclitaxel against mesothelioma
patient-derived xenografts and mesothelin as a biomarker of tumor
response. Clin Cancer Res. 23:1564–1574. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zirbes A, Joseph J, Lopez JC, Sayaman RW,
Basam M, Seewaldt VL and LaBarge MA: Changes in immune cell types
with age in breast are consistent with a decline in immune
surveillance and increased immunosuppression. J Mammary Gland Biol
Neoplasia. 26:247–261. 2021. View Article : Google Scholar
|
|
42
|
Hamilton JAG and Henry CJ: Aging and
immunotherapies: New horizons for the golden ages. Aging Cancer.
1:30–44. 2020. View Article : Google Scholar
|
|
43
|
Waldman AD, Fritz JM and Lenardo MJ: A
guide to cancer immunotherapy: From T cell basic science to
clinical practice. Nat Rev Immunol. 20:651–668. 2020. View Article : Google Scholar
|
|
44
|
Bulgarelli J, Tazzari M, Granato AM,
Ridolfi L, Maiocchi S, de Rosa F, Petrini M, Pancisi E, Gentili G,
Vergani B, et al: Dendritic cell vaccination in metastatic melanoma
turns ‘non-T cell inflamed’ into ‘T-cell inflamed’ tumors. Front
Immunol. 10:23532019. View Article : Google Scholar
|
|
45
|
Sawasdee N, Thepmalee C, Sujjitjoon J,
Yongpitakwattana P, Junking M, Poungvarin N, Yenchitsomanus PT and
Panya A: Gemcitabine enhances cytotoxic activity of effector
T-lymphocytes against chemo-resistant cholangiocarcinoma cells. Int
Immunopharmacol. 78:1060062020. View Article : Google Scholar
|
|
46
|
Kodumudi KN, Ramamoorthi G, Snyder C, Basu
A, Jia Y, Awshah S, Beyer AP, Wiener D, Lam L, Zhang H, et al:
Sequential anti-PD1 therapy following dendritic cell vaccination
improves survival in a HER2 mammary carcinoma model and identifies
a critical role for CD4 T cells in mediating the response. Front
Immunol. 10:19392019. View Article : Google Scholar
|