Introduction
Lung cancer is one of the most common malignant
tumors and ~25% of all cancer-related deaths are related to lung
cancer (1). Lung cancer can be
divided into small cell lung cancer and non-small cell lung cancer
(NSCLC) according to its histopathological features (2). The proportion of NSCLC cases is
80–85% of all the lung cancer cases (3). In the past decades, several effective
novel strategies have been emerged in NSCLC treatment research,
such as immunotherapy (4).
Moreover, anti-angiogenic agents have been also discovered to
improve the outcomes of patients with NSCLC (5). Previously, drug-loaded nanoparticle
treatment and nanotechnology drug delivery strategies in the lungs
have been reported to be effective in the treatment of lung cancer
(6–8). However, the 5-year survival rate is
only ~20% despite improved treatment methods (2). exploration and elucidation of its
molecular mechanisms is key to improving the prognosis.
MicroRNAs (miRNAs or miRs) are a class of non-coding
small RNAs (9). They play a
crucial role in regulating biological processes, such as apoptosis,
invasion, autophagy and proliferation (10,11).
miR-491-3p plays an indispensable role in regulating development in
certain human tumors. For instance, invasion, migration, and
proliferation were weakened and apoptosis was exacerbated in
retinoblastoma cells after miR-491-3p was upregulated (12). miR-491-3p acts as a tumor
suppressor in osteosarcoma by inhibiting the growth and invasion of
the osteosarcoma cells (13). Zhao
et al (14) showed that
miR-491-3p attenuated the multidrug resistance of hepatocellular
carcinoma. Simultaneously, low miR-491-3p expression was associated
with poor outcomes in patients with tongue cancer. The inhibition
of miR-491-3p expression enhanced the chemotherapy resistance of
the tongue cancer cells (15).
However, the role of miR-491-3p in NSCLC remains unclear. In our
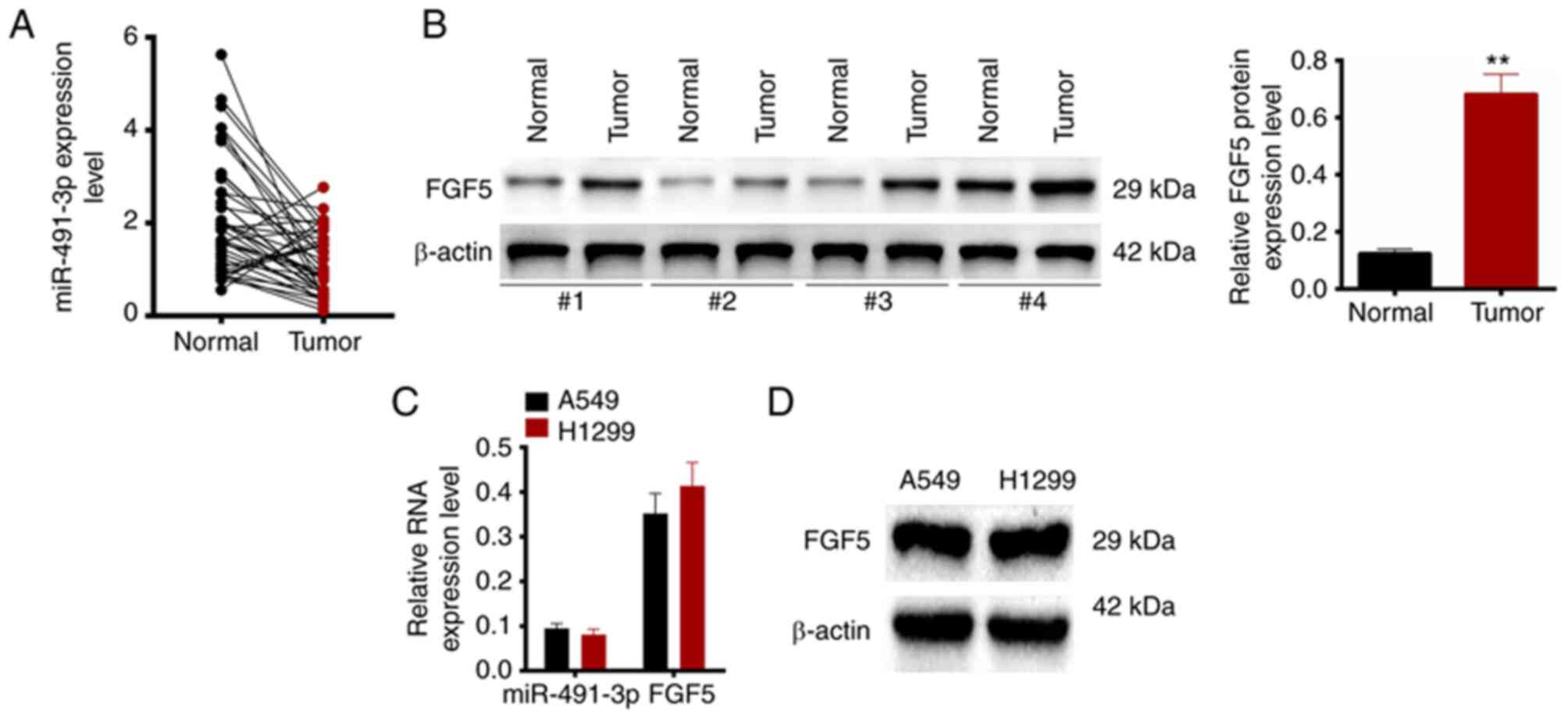
preliminary research (Fig. 1A), it
was found that miR-491-3p expression was abnormally low expressed
in patients with NSCLC. Therefore, it was hypothesized that
miR-491-3p may be involved in regulating the progression of NSCLC.
The present study was then executed to identify the function of
miR-491-3p in NSCLC progression.
It has been revealed that one of the main ways of
miRNAs to regulate diseases development is to regulate coding genes
expression via binding to specific mRNA targets (16). through TargetScan version 7.1
(http://www.targetscan.org/vert_71/)
prediction, it was observed that fibroblast growth factor 5 (FGF5)
had the binding site for miR-491-3p in the 3′-untranslated (UTR)
region. Notably, our preliminary research (Fig. 1B) indicated that FGF5 was
aberrantly up-regulated in patients with NSCLC. FGF5 has been
reported to be overexpressed in NSCLC. The silencing of FGF5
reduced proliferation, migration and invasion and enhanced
apoptosis in NSCLC cells (17).
Moreover, high FGF5 expression was reported to be associated with
poor overall survival and relapse-free survival patients with in
lung adenocarcinoma (18). Taking
together, it was hypothesized that miR-491-3p may regulate NSCLC
progression by targeting FGF5. In the present study, a series of
experiments were performed to identify this hypothesis. The present
findings may provide a novel molecular target for NSCLC
treatment.
Materials and methods
Patients and tissues
Patients with NSCLC (n=43) from Yijishan Hospital
(Wuhu, China) participated in the present study. Patients with
previous history of cancer-related treatments were not included.
Patients with other severe organic diseases were excluded. All
patients with NSCLC were treated with surgical resection from April
2018 to November 2019 at the First Affiliated Hospital of Wannan
Medical College Yijishan Hospital (Wuhu, China). tumor tissues and
adjacent normal tissues were collected and stored in liquid
nitrogen. Clinicopathological characteristics of all the patients
were recorded. The research protocol was reviewed and approved
(approval no. TCH036) by the Ethics Committee of the First
Affiliated Hospital of Wannan Medical College Yijishan Hospital
(Wuhu, China) and was in line with the Declaration of Helsinki.
Written informed consent was obtained from all patients. Details of
all were de-identified patients. The document of the Ethics
Committee was related to the human studies of the present
study.
Cell culture and transfection
A549 (CCL-185) and H1299 cells (CRL-5803) were
commercially obtained from Shanghai Institute of Cell Biology
(Shanghai, China). Cells were maintained in Dulbecco's Modified
Eagle's medium (DMEM) with 10% fetal bovine serum (FBS; both from
Beijing Solarbio Science & Technology Co., Ltd.), 100 U/ml
penicillin and 100 µg/ml streptomycin at 37°C with 5%
CO2. The medium was changed every three days.
At ~80% confluence, cells were harvested and a cell
suspension with serum-free DMEM was prepared. The concentration of
the cell suspension was 1×106 cells/ml. Then, 1 ml of
the cell suspension was added to each well of 6-well plates.
miR-491-3p mimic (5′-CUUAUGCAAGAUUCCCUUCUAC-3′), mimic negative
control (NC, 5′-GUAAUGCUAGAUUCGGUACUUG-3′), miR-491-3p inhibitor
(5′-AGTAGAAGGGAATCTTGCATAAG-3′), and inhibitor NC
(5′-ACAGAGCTATAGATGTAAGTGAG-3′) (Shanghai GenePharma Co., Ltd.)
were transfected into A549 and H1299 cells according to the
manufacturer's protocol using Lipofectamine® 2000
(Thermo Fisher Scientific, Inc.). pcDNA3.1-FGF5 plasmid and control
vector (Shanghai Zeye Biotechnology, Co., Ltd.) were separately
transfected into A549 cells similarly. Moreover, A549 cells were
cotransfected with mimic NC and pcDNA3.1-FGF5 plasmid, or mimic NC
and control vector, or miR-491-3p mimic and pcDNA3.1-FGF5 plasmid,
or miR-491-3p mimic and control vector. The transfection was
performed according to the manufacturer's protocol of Lipofectamine
2000. Cells were transfected for 8 h at 37°C with 5%
CO2. Then, DMEM with 10% FBS was used to treat the cells
for 48 h at 37°C with 5% CO2.
Cell counting kit-8 (CCK-8) assay
Cell viability was evaluated using a CCK-8 assay.
A549 and H1299 cells were prepared into a cell suspension
(1×105 cells/ml) with DMEM containing 10% FBS. The cell
suspension (100 µl) was added to a 96-well plate for culturing at
37°C with 5% CO2. Cell viability was observed every 24
h. CCK-8 solution (10 µl) was added into each well and incubated
for 2 h at 37°C. The optical density (OD) was measured at 450 nm
using a microplate reader (BioTek Instruments, Inc.).
Ethynyldeoxyuridine (EdU)
experiment
cell proliferation was observed using the Edu
experiment. The suspension (1×104 cells/ml, 1 ml) of
A549 and H1299 cells was seeded in a 6-well plate and cultured at
37°C with 5% CO2 for 48 h. Subsequently, cell
proliferation was observed using the EdU detection kit (Guangzhou
Ribobio Co., Ltd.) according to the manufacturer instructions.
Cells were stained using an anti-EdU working solution (100 µl, for
2 h at 37°C) and 4′6-diamidino-2-phenylindole (DAPI, 1 mg/ml) was
used to stain the nucleus (15 min at room temperature). Cells were
observed and images were captured under a confocal laser scanning
microscope (Olympus Corporation). The Edu staining positive cells
(red fluorescent) and DAPI staining positive cells (blue
fluorescence) were calculated using ImageJ software (Version 1.45s;
National Institutes of Health). Five randomly-selected fields were
observed in each group.
Matrigel experiment
The upper chamber (8-µm pore size) was precoated
with Matrigel (30 min at 37°C), followed by seeding with
2×104 cells (dispersed in 200 µl serum-free DMEM). DMEM
containing 10% FBS (600 µl) was added into the lower chamber. Cells
were cultured for 24 h at 37°C with 5% CO2. Cells on the
upper surface were gently scraped off using a cotton swab.
Paraformaldehyde (4%) was used to fix (15 min at room temperature)
the cells on the lower surface. Then, the invasive cells were
stained with 0.1% crystal violet for 20 min at room temperature.
The invasive cells in five randomly-selected fields were counted
under a confocal microscope (Olympus Corporation).
Wound healing assay
A549 and H1299 cells were prepared into cell
suspension with DMEM without FBS. The suspensions (1×106
cells/ml, 1 ml) were seeded onto a 6-well plate and incubated at
37°C with 5% CO2. When the cells attached to the bottom
of the wells, they were scratched using a 100-µl sterile pipette
tip. The initial wound width was measured and recorded. the
residual liquid was replaced with fresh DMEM (without FBS). Cells
were cultured at 37°C with 5% CO2. After 24 h, the final
wound width was measured. The relative wound width was calculated
(final wound width/the initial wound width).
Flow cytometric analysis
A549 and H1299 cells (1×106 cells/ml)
were added into flow tubes. The cells were washed in pre-cooled
phosphate buffer solution (PBS) three times. Then, they were washed
in 1X binding buffer once. apoptosis (early + late) was detected
using the Annexin V- fluorescein isothiocyanate apoptosis detection
kit (Biovision, Inc.) according to the manufacturer's protocol. The
percentage of apoptosis in cells was evaluated using flow cytometry
(FACScan; BD Biosciences) and analyzed by the Diva software
(version 8.0, Becton, Dickinson and Company).
Dual-luciferase reporter gene
assay
Using TargetScan version 7.1. (http://www.targetscan.org/vert_71/), it was
observed that FGF5 and miR-491-3p had common binding site in the
3′-UTR region. Based on this, dual-luciferase reporter gene assay
was performed with A549 and H1299 cells to establish a relationship
between miR-491-3p and FGF5. The cells (1×105 cells/ml)
were seeded in a 6-well plate with serum-free DMEM. They were
transfected with miR-491-3p mimic, mimic NC, miR-491-3p inhibitor,
and inhibitor NC. Using Lipofectamine 2000, pmirGLO-FGF5-wild-type
(WT) and pmirGLO-FGF5-mutant type (MUT) luciferase reporter vectors
(Shanghai GenePharma Co., Ltd.) were individually transfected into
A549 and H1299 cells. After incubating for 48 h at 37°C, the
luciferase activity was measured using the dual-luciferase reporter
assay system (Promega Corporation). Renilla luciferase
activity was used as control.
In vivo study
Animal experiments were approved (approval no.
AE041A) by the Animal Ethics Committee of First Affiliated Hospital
of Wannan Medical College Yijishan Hospital (Wuhu, China). The
document of the Animal Ethics Committee was related to the animal
experiments of the present study. A total of 12 BALB/c nude mice
(male, 4 weeks old, weight 220–240 g, obtained from Vital River
Laboratory Animal Technology; Beijing, China) were randomly divided
into two groups: NC group (n=6) and miR-491-3p group (n=6).
Lentiviruses (hU6-MCS-CMV-Puromycin) were purchased from Shanghai
GeneChem Co., Ltd. The 293T cells were seeded at a density of
6×106 cells/ml in a 15-cm culture dish, cultured at 37°C
with 5% CO2 to 70–80% confluence, and the lentivirus
plasmids were co-transfected into 293T cells using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.), at 37°C for 6 h. The supernatant of 293T cells
transfected for 72 h was collected, centrifuged at 4,000 × g for 10
min at 4°C to remove cell debris, filtered, centrifuged at 7,000 ×
g for 5 min at 4°C, resuspended in ice-cold PBS to detect the
titer, and stored at- 80°C. Based on the transfection, the cells
were divided into miR-491-3p and NC groups. The A549 cells at a
density of 6×105 cells/well in a six-well culture plate
were infected with the miR-491-3p with a multiplicity of infection
of 20, and the miR-491-3p gene expression sequence carried by the
lentivirus was integrated into the cell to obtain stable
overexpression. A549 cells were transfected with lentivirus/medium
at a ratio of 1:50. Stable cell lines were selected by puromycin
(Sigma-Aldrich; Merck KGaA) at 5 µg/ml for 2 weeks. The lentiviral
vectors contained enhanced green fluorescent protein (eGFP).
Lentiviral-transfected A549 cells were harvested and dispersed into
PBS to prepare cell suspension. The density of each cell suspension
samples was 1×107 cells/ml. Nude mice of NC group and
miR-491-3p group were injected subcutaneously with 100 µl of the
corresponding cell suspension samples. The injection site was on
the back. After injection, nude mice were kept at 22±2°C for 28
days in a 12 h day/night cycle room with free access to food and
water. The tumor volume was measured at 7-day intervals by (length
× width2)/2. Nude mice were then euthanized by rapid
cervical dislocation after deep anesthesia with 5% isoflurane. The
xenograft tumor tissues were collected, weighted and stored in a
refrigerator at −80°C. During the 28-day, the humane endpoint of
mice was defined as the following symptoms: hunched posture, pale
extremities, inactivity and dyspnea; and the tumor size exceeded 20
mm in one dimension. Mice with these aforementioned symptoms were
then immediately euthanized. The regularly tumor progression was
checked every day by using vernier caliper in order to ensure the
tumor size was within the allowable range of humane endpoints.
Immunohistochemistry (IHC)
The protein expression of FGF5 and Ki67 in the
xenograft tumor tissues was detected by performing IHC. Briefly,
the xenograft tumor tissues were embedded into paraffin before
being cut into 4-µm sections. After dewaxing in xylene and
rehydration in descending ethanol series, the sections were treated
with 3% H2O2 for 10 min at room temperature
and then subjected to antigen retrieval in boiled citrate buffer.
The blockage of the sections was implemented by 5% normal goat
serum. Thereafter, the sections were incubated overnight with
rabbit anti-FGF5 primary antibody (1:100; cat. no. ab88118) and
rabbit anti-Ki67 primary antibody (1:100; cat. no. ab15580; both
from Abcam) at 4°C. PBS was utilized to wash the sections twice.
The sections were then treated for 30 min with horseradish
peroxidase-conjugated goat anti-rabbit secondary antibody (1:200;
cat. no. ab6721; Abcam) at 37°C. Post twice washing with PBS, the
sections were stained with 3,3′-diaminobenzidine (for 5 min at room
temperature) and hematoxylin (for 30 sec at room temperature).
After dehydration, the sections were sealed in neutral resin and
observed under a light microscope (Olympus Corporation;
magnification, ×200; scale bar: 100 µm).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
TRIzol® reagent (Wuhan Boster Biological
Technology, Ltd.) was added into tissues and cells to extract total
RNA. The total RNA sample was reverse transcribed to obtain the
cDNA template. It was reverse transcribed using the PrimeScript RT
Master Mix (Takara Bio, Inc.) according to the manufacturer's
protocol. RT-qPCR was performed using the SYBR Premix Ex Taq
(Takara Bio, Inc.) in an ABI 7500 Real-Time PCR system (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The thermocycling
conditions were as follows: 40 cycles at 95°C for 5 min, 95°C for
30 sec, 60°C for 45 sec, and 72°C for 30 min. The primers were
designed by Shanghai GenePharma Co., Ltd. and the sequences were as
follows: miR-491-3p forward, 5′-AGTGGGGAACCCTTCC-3′ and reverse,
5′-GAACATGTCTGCG-TATCTC-3′; U6 forward, 5′-AAAGCAAATCATCGGACGACC-3′
and reverse, 5′-GTACAACACATTGTTTCCTCGGA-3′; FGF5 forward,
5′-TTCTCTTTCACAGCACCAAA-3′ and reverse, 5′-CTCCTTGCTTCTAACCCATC-3′;
and β-actin forward, 5′-AGCGAGCATCCCCCAAAGTT-3′ and reverse,
5′-GGGCACGAAGGCTCATCATT-3′. U6 was used as control for miR-491-3p
relative expression using the 2−ΔΔCq method (19).
Western blot analysis
Total proteins were extracted from tissues and cells
using RIPA lysis buffer (Beyotime Institute of Biotechnology)
containing protease inhibitor. The concentration of total proteins
was determined using a BCA kit (Beyotime Institute of
Biotechnology). A 10 µg protein sample was mixed with 1X loading
buffer and separated using 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis. the proteins were
transferred onto a polyvinylidene fluoride (PVDF) membrane and
blocked with 5% skimmed milk for 1 h at room temperature. Rabbit
anti-FGF5 primary antibody (1:1,000) and rabbit anti-β-actin
primary antibody (1:1,000; cat. no. ab8227; Abcam) were used to
probe the PVDF membrane for 12 h at 4°C. horseradish
peroxidase-conjugated goat anti-rabbit secondary antibody (1:2,000)
was used to treat the PVDF membrane for 1 h at room temperature.
The protein blots were visualized using enhanced chemiluminescence
detection reagent (Beyotime Institute of Biotechnology). The
quantitative analysis of proteins was performed using Quantity one
software (version 4.6.3; Bio-Rad Laboratories, Inc.). β-actin was
used as the control for the relative expression of FGF5
protein.
Statistical analysis
All experiments were performed in triplicate. SPSS
19.0 (IBM Corp.) and GraphPad Prism 5.0 software (GraphPad
Software, Inc.) were used to analyze the data (mean ± standard
deviation). The two-tailed paired Student's t-test was used for the
comparison between two groups. more than two groups were compared
using One-way ANOVA followed by Tukey's post hoc test. Pearson's
correlation coefficient test was employed to identify the
correlation between miR-491-3p and FGF5 mRNA in tumor tissues of
patients with NSCLC. P<0.05 was considered to indicate a
statistically significant difference.
Results
miR-491-3p is downregulated and FGF5
is upregulated in patients with NSCLC
In the present study, the expression of miR-491-3p
and FGF5 protein was evaluated in 43 patients with NSCLC.
miR-491-3p expression was distinctly low in tumor tissues compared
with the adjacent normal tissues (P<0.01) (Fig. 1A). By contrast, FGF5 protein
expression was higher in tumor tissues of patients with NSCLC
compared with normal adjacent tissues (P<0.01) (Fig. 1B). The relationship between
miR-491-3p expression and clinicopathological characteristics of
patients was demonstrated in Table
I. NSCLC patients with low miR-491-3p expression exhibited
advanced tumor stages and lymphatic metastasis (P<0.05).
Additionally, the relationship between FGF5 mRNA expression and
clinicopathological characteristics of NSCLC patients was listed in
Table II. High FGF5 mRNA
expression was associated with advanced TNM stage and lymphatic
metastasis of NSCLC patients (P<0.05). Therefore, miR-491-3p was
downregulated and FGF5 was upregulated in tumor tissues of NSCLC
patients. Low miR-491-3p expression and high FGF5 mRNA expression
was associated with poor outcomes in NSCLC patients, including
advanced TNM stage and lymph node metastasis.
 | Table I.The relationship between miR-491-3p
expression and clinicopathological characteristics of patients with
non-small cell lung cancer. |
Table I.
The relationship between miR-491-3p
expression and clinicopathological characteristics of patients with
non-small cell lung cancer.
|
|
| Expression level of
miR-491-3p |
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
characteristics | Number of
patients | Low
(<median) | High (≥
median) | P-value |
|---|
| Number | 43 | 21 | 22 |
|
| Age, years |
|
|
| 0.625 |
|
<60 | 18 | 8 | 10 |
|
|
≥60 | 25 | 13 | 12 |
|
| Sex |
|
|
| 0.658 |
|
Female | 19 | 10 | 9 |
|
|
Male | 24 | 11 | 13 |
|
| TNM stage |
|
|
| 0.004 |
|
I/II | 20 | 5 | 15 |
|
|
III/IV | 23 | 16 | 7 |
|
| Pathological
type |
|
|
| 0.745 |
|
Squamous | 22 | 12 | 10 |
|
|
Adenocarcinoma | 14 | 6 | 8 |
|
| Large
cell lung cancer | 7 | 3 | 4 |
|
| Smoking status |
|
|
| 0.850 |
|
Non-smoker | 17 | 8 | 9 |
|
|
Smoker | 26 | 13 | 13 |
|
| Lymphatic
Metastasis |
|
|
| 0.002 |
| No | 16 | 3 | 13 |
|
|
Yes | 27 | 18 | 9 |
|
 | Table II.The relationship between FGF5
expression and clinicopathological characteristics of patients with
non-small cell lung cancer. |
Table II.
The relationship between FGF5
expression and clinicopathological characteristics of patients with
non-small cell lung cancer.
|
|
| Expression level of
FGF5 |
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
characteristics | Number of
patients | Low
(<median) | High (≥
median) | P-value |
|---|
| Number | 43 | 21 | 22 |
|
| Age, years |
|
|
| 0.429 |
|
<60 | 18 | 8 | 10 |
|
|
≥60 | 25 | 13 | 12 |
|
| Sex |
|
|
| 0.227 |
|
Female | 19 | 11 | 8 |
|
|
Male | 24 | 10 | 14 |
|
| TNM stage |
|
|
| 0.04 |
|
I/II | 20 | 14 | 6 |
|
|
III/IV | 23 | 7 | 16 |
|
| Pathological
type |
|
|
| 0.745 |
|
Squamous | 22 | 10 | 12 |
|
|
Adenocarcinoma | 14 | 8 | 6 |
|
| Large
cell lung cancer | 7 | 3 | 4 |
|
| Smoking status |
|
|
| 0.760 |
|
Non-smoker | 17 | 10 | 7 |
|
|
Smoker | 26 | 11 | 15 |
|
| Lymphatic
Metastasis |
|
|
| 0.012 |
| No | 16 | 12 | 4 |
|
|
Yes | 27 | 9 | 16 |
|
Additionally, the expression of miR-491-3p, FGF5
mRNA and protein expression in A549 and H1299 cells were monitored
by RT-qPCR and western blotting (Fig.
1C and D). An opposite trend was found between miR-491-3p and
FGF5 expression levels in A549 and H1299 cells.
miR-491-3p overexpression inhibits
viability, proliferation, migration and invasion, and promotes
apoptosis in NSCLC cells
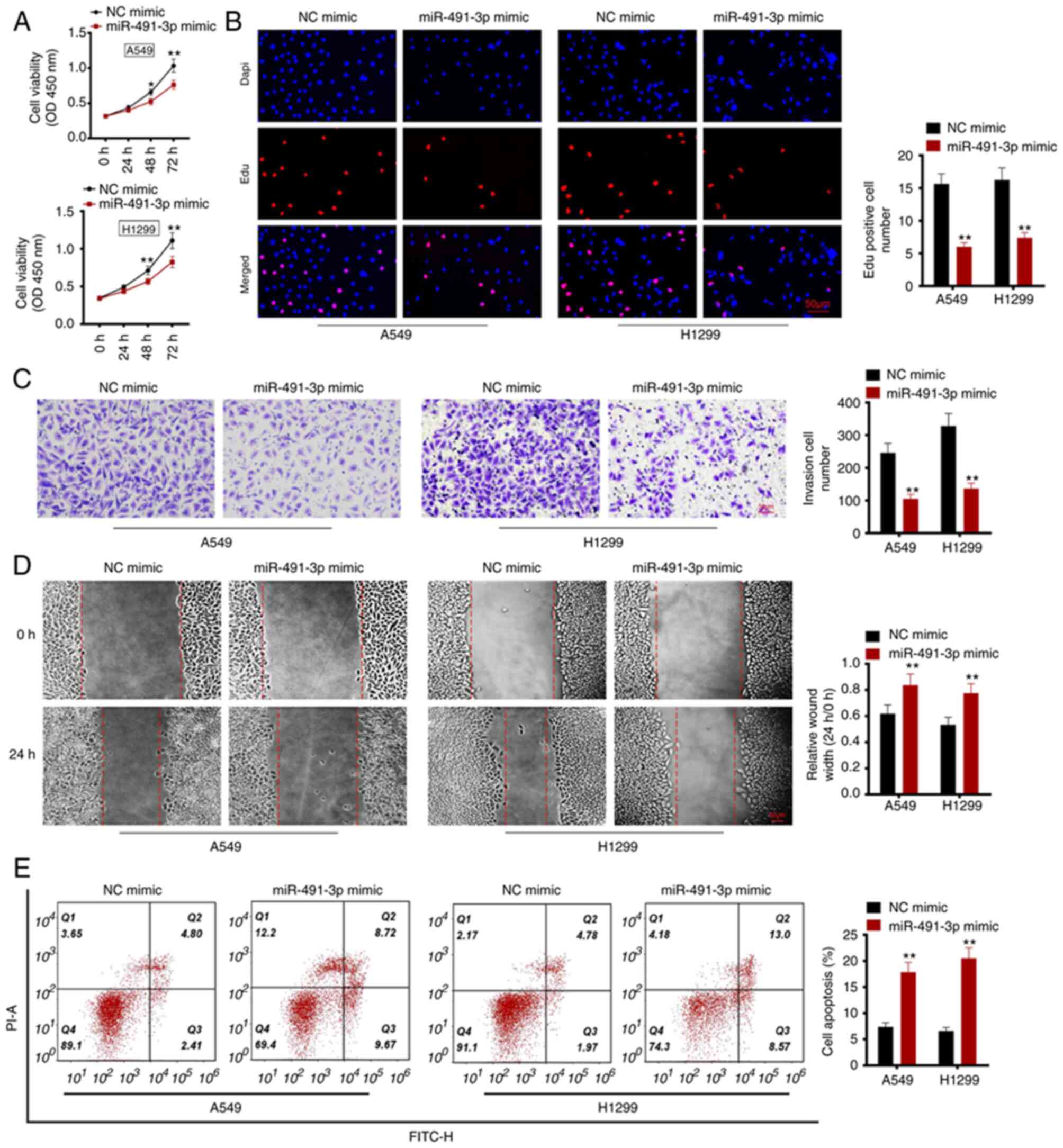
A549 and H1299 cells were transfected with
miR-491-3p mimic and corresponding NC. After transfection, cell
viability and proliferation were investigated using CCK-8 assay and
Edu experiment, respectively. After 48 h, the OD (at 450 nm) values
for A549 and H1299 cells in the miR-491-3p mimic group were
significantly lower compared with the NC mimic group (P<0.05 or
P<0.01; Fig. 2A). in the
miR-491-3p mimic group, the number of Edu positive cells was
decreased compared with the NC mimic group (P<0.01; Fig. 2B). Therefore, miR-491-3p
overexpression inhibited viability and proliferation of NSCLC
cells.
The Matrigel experiment was used to study the
invasion ability of the NSCLC cells. As revealed in Fig. 2C, compared with the NC mimic group,
A549 and H1299 cells of the miR-491-3p mimic group exhibited a
significantly lower invasive cell number (P<0.01). Wound healing
assay demonstrated a significantly higher relative wound width in
A549 and H1299 cells of the miR-491-3p mimic group compared with
the NC mimic group (P<0.01; Fig.
2D). These data suggested that miR-491-3p overexpression
inhibits invasion and migration of NSCLC cells.
Flow cytometric analysis was utilized for observing
cell apoptosis. The percentage of apoptosis was subsequently
investigated. A549 and H1299 cells in the miR-491-3p mimic group
showed a higher cell apoptotic percentage, compared with the NC
mimic group (P<0.01; Fig. 2E).
Therefore, miR-491-3p overexpression facilitated apoptosis in NSCLC
cells.
miR-491-3p directly inhibits the
expression of FGF5
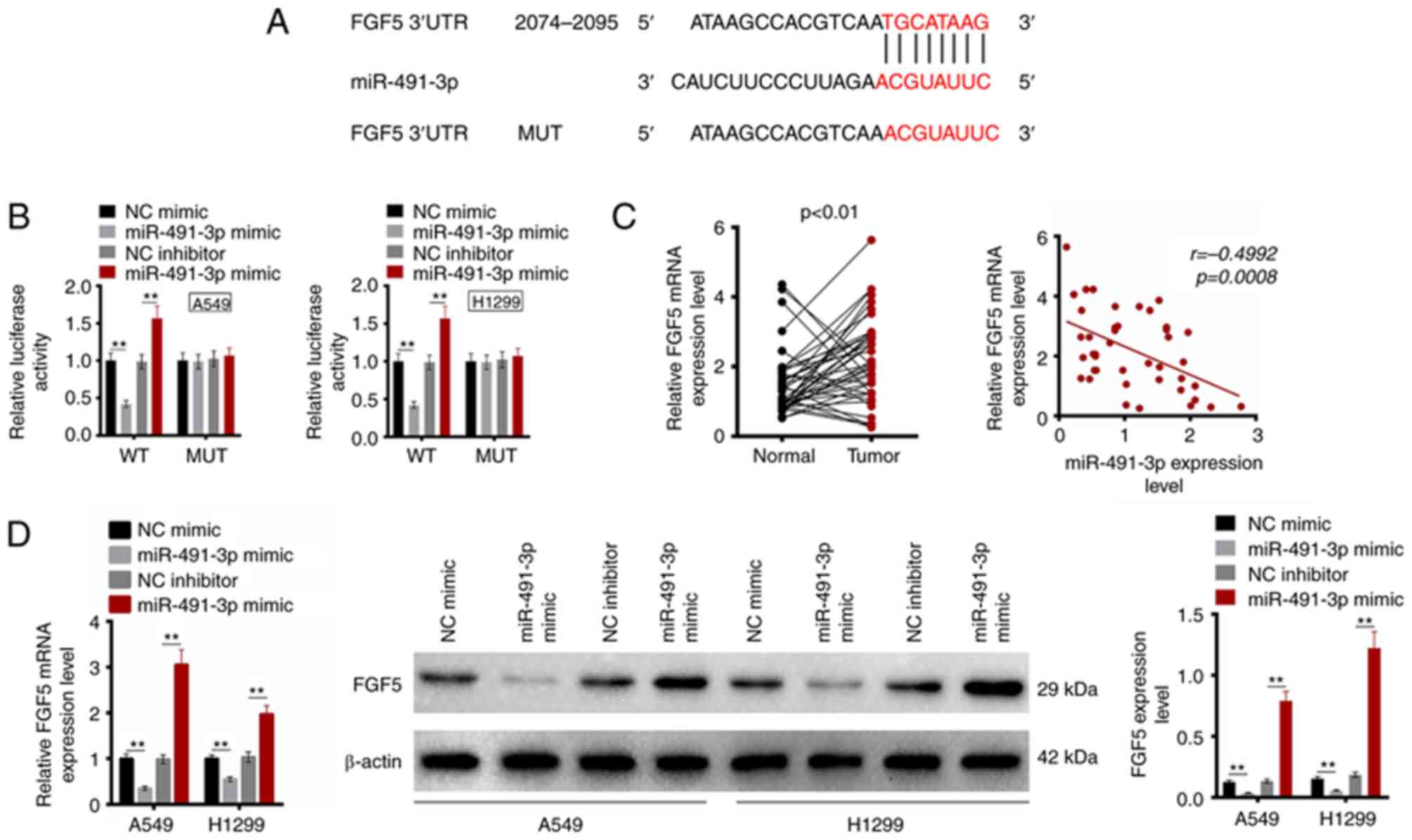
Using TargetScan 7.1. online prediction software, it
was identified that FGF5 possessed a binding site for miR-491-3p in
the 3′-UTR region (Fig. 3A).
Subsequently, the dual-luciferase reporter gene assay was used to
detect the relationship between miR-491-3p and FGF5. As revealed in
Fig. 3B, the relative luciferase
activity of WT-FGF5 reporter in A549 and H1299 cells was lower in
the miR-491-3p mimic group compared with the NC mimic group
(P<0.01). By contrast, the miR-491-3p inhibitor group revealed
higher relative luciferase activity of WT-FGF5 reporter in A549 and
H1299 cells compared with the NC inhibitor (P<0.01). However, no
significant changes in relative luciferase activity of MUT-FGF5
reporter were observed among the four groups. The FGF5 mRNA
expression in clinical samples of patients with NSCLC was monitored
using RT-qPCR. Significantly upregulated FGF5 mRNA was detected in
tumor tissues compared with that in adjacent normal tissues
(P<0.01). The miR-491-3p and FGF5 mRNA level in tumor tissues of
patients with NSCLC exhibited a negative correlation (P=0.0008;
Fig. 3C). Additionally, FGF5 mRNA
and protein expression was detected in A549 and H1299 cells of the
four groups. As a result, the FGF5 mRNA and protein expression was
decreased in A549 and H1299 cells of the miR-491-3p mimic group in
comparison with the NC mimic group (P<0.01). Compared with the
NC inhibitor group, FGF5 mRNA and protein expression significantly
increased in A549 and H1299 cells of the miR-491-3p inhibitor group
(P<0.01; Fig. 3D). These
results indicated that FGF5 expression is directly inhibited by
miR-491-3p.
FGF5 upregulation reverses the
inhibitory effects of miR-491-3p on NSCLC cell malignant
phenotype
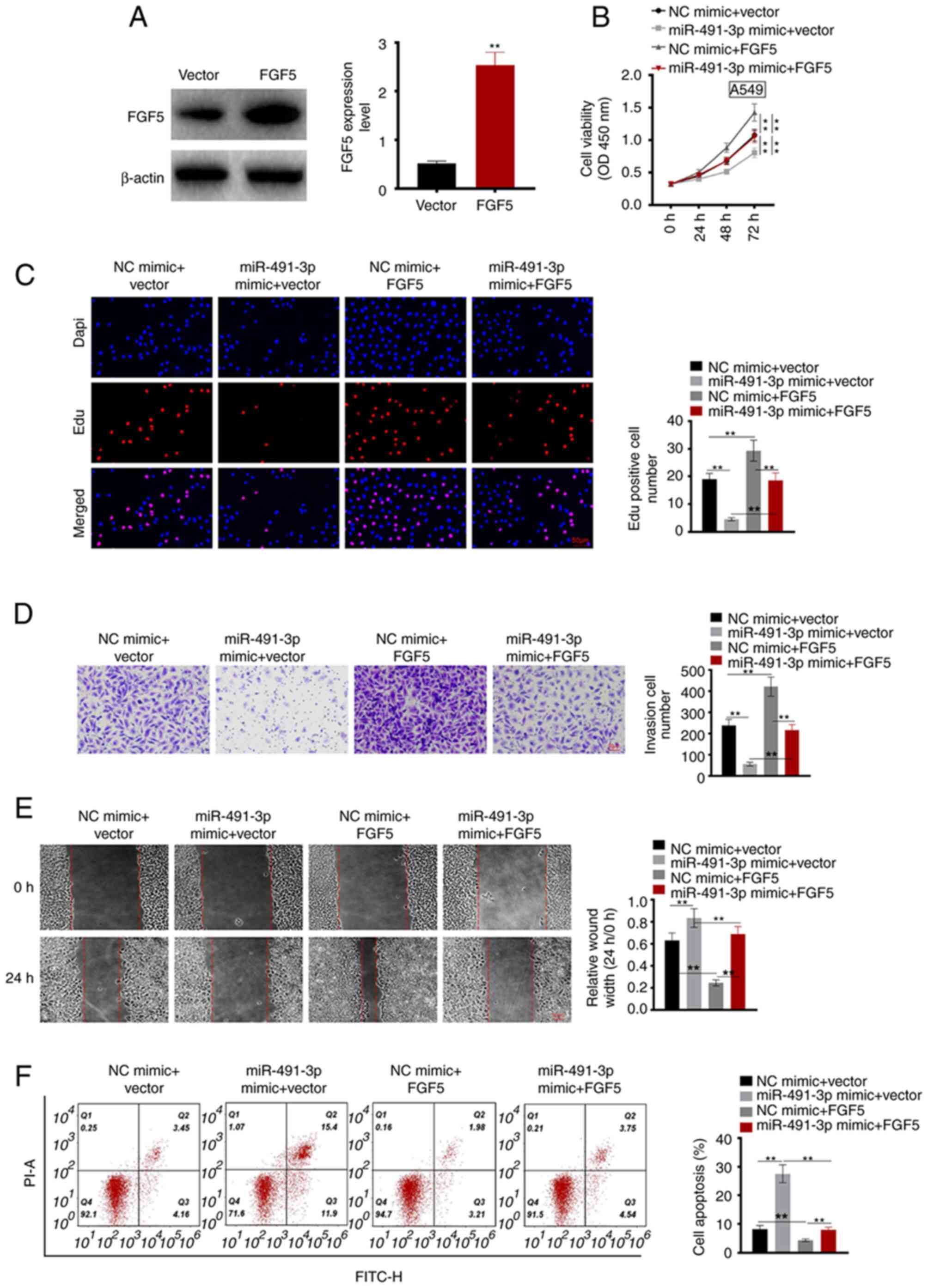
pcDNA3.1-FGF5 plasmid and control vectors were
transfected into A549 cells. The transfection efficiency was
analyzed using RT-qPCR. A549 cells of the FGF5 group exhibited
significantly higher FGF5 protein expression compared with the
vector group (P<0.01; Fig.
4A).
After 72 h, compared with the NC mimic + vector
group, the OD value of A549 cells decreased in the miR-491-3p mimic
+ vector and increased in NC mimic + FGF5 groups, respectively
(P<0.01). Meanwhile, compared with the miR-491-3p mimic + FGF5
group, the OD value of A549 cells in the miR-491-3p mimic + vector
group decreased, and in the NC mimic + FGF5 group increased
(P<0.01; Fig. 4B).
Compared with the NC mimic + vector group, the Edu
positive cell number decreased in the miR-491-3p mimic + vector and
increased in the NC mimic + FGF5 groups, respectively (P<0.01).
Compared with the miR-491-3p mimic + FGF5 group, the Edu positive
cell number decreased in the miR-491-3p mimic + vector group and
increased in the NC mimic + FGF5 group, respectively (P<0.01;
Fig. 4C).
In the Matrigel experiment, compared with the NC
mimic + vector group the invasive cell numbers were lower in the
miR-491-3p mimic + vector and higher in the NC mimic + FGF5 groups,
respectively (P<0.01). Similarly, compared with the miR-491-3p
mimic + FGF5 group, the invasive cell numbers decreased in the
miR-491-3p mimic + vector and increased in the NC mimic + FGF5
groups, respectively (P<0.01; Fig.
4D).
Wound healing assay was performed to evaluate the
cell migration ability. The results showed that compared with the
NC mimic + vector group, the relative wound width was larger in the
miR-491-3p mimic + vector and smaller in the NC mimic + FGF5
groups, respectively (P<0.01). Similarly, compared with the
miR-491-3p mimic + FGF5 group, the relative wound width was larger
in the miR-491-3p mimic + vector and smaller in the NC mimic + FGF5
groups, respectively (P<0.01; Fig.
4E).
Flow cytometric analysis was used to detect
apoptosis. Compared with the NC mimic + vector group, the cell
apoptosis percentage was higher in the miR-491-3p mimic + vector
and lower in the NC mimic + FGF5 groups, respectively (P<0.01).
Moreover, compared with the miR-491-3p mimic + FGF5 group, the cell
apoptosis percentage of A549 cells was higher in the miR-491-3p
mimic + vector and lower in the NC mimic + FGF5 groups,
respectively (P<0.01; Fig. 4F).
These results suggested that FGF5 upregulation reversed the
inhibitory effect of miR-491-3p on the NSCLC cell malignant
phenotype.
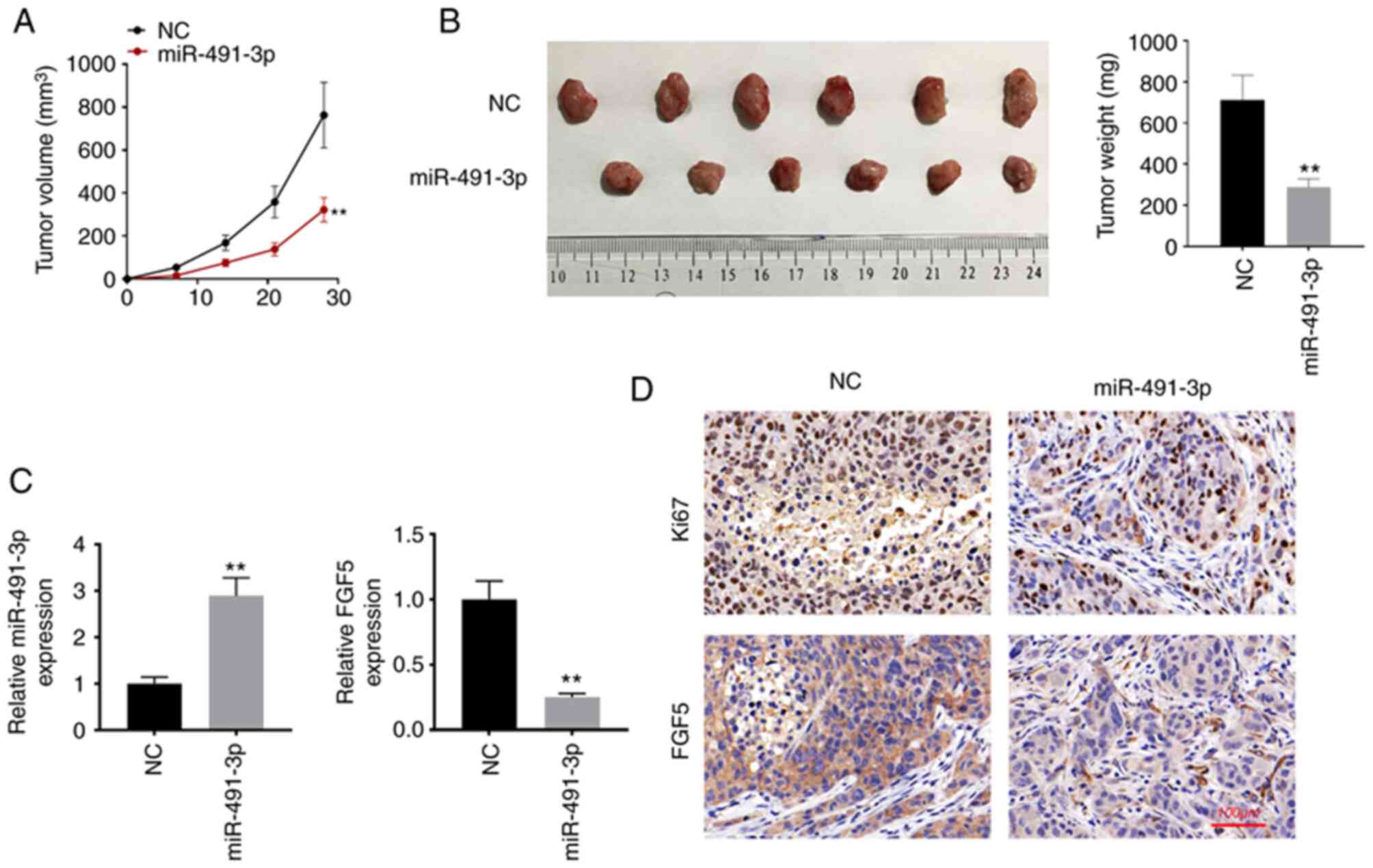
miR-491-3p overexpression suppresses
the in vivo growth of NSCLC
In vivo study was performed to monitor the
effect of miR-491-3p on the in vivo development of NSCLC. As
revealed in Fig. 5A and B,
miR-491-3p overexpression suppressed the in vivo growth of
NSCLC cells, as proved by the lower tumor volume and weight in
miR-491-3p group compared with the NC group (P<0.01). Xenograft
tumor tissues were subjected to RT-qPCR to detect the expression of
miR-491-3p and FGF5 mRNA. As a result, higher miR-491-3p expression
as well as lower FGF5 mRNA expression was revealed in the xenograft
tumor tissues of miR-491-3p group when compared with NC group
(P<0.01; Fig. 5C).
Simultaneously, less Ki67 and FGF5 protein expression was
identified in the xenograft tumor tissues of miR-491-3p group
compared with the NC group (Fig.
5D). Thus, miR-491-3p suppressed the in vivo growth of
NSCLC.
Discussion
The expression of miRNA is tissue-specific and is
usually dysregulated in multiple human types of cancer (20). In the present study, the
tumor-suppressive role of miR-491-3p was demonstrated in NSCLC. Low
miR-491-3p expression was associated with poor outcomes in patients
with NSCLC, including advanced TNM stage as well as lymph node
metastasis. The overexpression of miR-491-3p promoted apoptosis and
inhibited viability, proliferation, migration and invasion of the
NSCLC cells. Additionally, miR-491-3p overexpression suppressed the
in vivo growth of NSCLC. This is the first time, to the best
of our knowledge, that miR-491-3p is identified as a tumor
suppressor in NSCLC.
miRNAs are important regulators of tumorigenesis.
They can promote degradation in mRNA or suppress translation of
proteins by interacting with the 3′-UTR region of the mRNA of
target genes (21). In recent
years, the role of miRNAs in the progression of NSCLC has attracted
extensive attention. For instance, miR-605-5p was revealed to be
overexpressed in NSCLC. The malignant phenotype of NSCLC cells,
such as migration and invasion, were intensified after the
overexpression of miR-605-5p (22). miR-512-5p played a
tumor-suppressive effect on NSCLC. The upregulation of miR-512-5p
enhanced the apoptosis and suppressed the invasion of NSCLC cells
(23). By contrast, miR-148b was
identified to be poorly expressed in NSCLC. NSCLC patients with low
miR-148b expression showed shorter overall survival. The elevated
miR-148b expression suppressed NSCLC cell growth, migration and
invasion (24). The decreased
miR-654-3p expression was associated with node metastasis in NSCLC.
NSCLC cells with overexpressed miR-654-3p possessed the attenuated
proliferation ability and the enhanced apoptotic capacity (25). Heretofore, the function of
miR-491-3p in NSCLC has never been explored. Previous studies have
identified that miR-491-3p exerted the tumor suppressor role in
human malignant tumors, such as hepatocellular carcinoma and
osteosarcoma (13,14). Similarly, the tumor-suppressing
function of miR-491-3p in NSCLC was confirmed in the present study.
the present study was the first to identify the tumor suppressor
function of miR-491-3p in NSCLC by directly targeting and
inhibiting FGF5. In clinical practice, the effective delivery of
miRNAs to the tumor sites remains an important challenge in the
transition of miRNAs therapy. Notably, recently, it has been
demonstrated that nano-formulations of miRNAs not only reduce
systemic and cytotoxicity, but also elevate the bioavailability of
miRNAs and the accumulation of miRNAs at the tumor site (26). Therefore, in the future, the
development of nano-formulations of miR-491-3p for NSCLC target
therapy will be a promising direction to realize the clinical use
of miR-491-3p.
FGF5 is involved in multiple biological processes,
such as tissue growth and repair (27). A recent study has proved that FGF5
is a cancer promoting factor. FGF5 upregulation in breast cancer
was associated with poor prognosis (28). Han et al (29) reported that FGF5 was required for
the proliferation of osteosarcoma cells. The addition of exogenous
FGF5 enhanced proliferation and suppressed apoptosis in
osteosarcoma cells by activating the mitogen-activated protein
kinase (MAPK) signaling pathway. the upregulation of FGF5 partially
reverses the suppression of miR-567 in osteosarcoma cell migration
and invasion (30). Moreover, FGF5
was verified to be a target of miR-188-5p. restoring FGF5
expression reversed the inhibition effect of miR-188-5p on the
metastasis of hepatocellular carcinoma (31). FGF5 expression is aberrantly
elevated in pancreatic cancer. The growth of pancreatic cancer
cells is significantly exacerbated after treatment with exogenous
FGF5. However, the addition of exogenous FGF5 enhanced the activity
of the MAPK signaling pathway (32). Similarly, it was demonstrated that
FGF5 was highly expressed in patients with NSCLC. miR-491-3p
directly restrained the expression of FGF5. Moreover, restoring the
FGF5 expression abrogated the inhibition of miR-491-3p on the
malignant phenotype of NSCLC cells. Thus, miR-491-3p could suppress
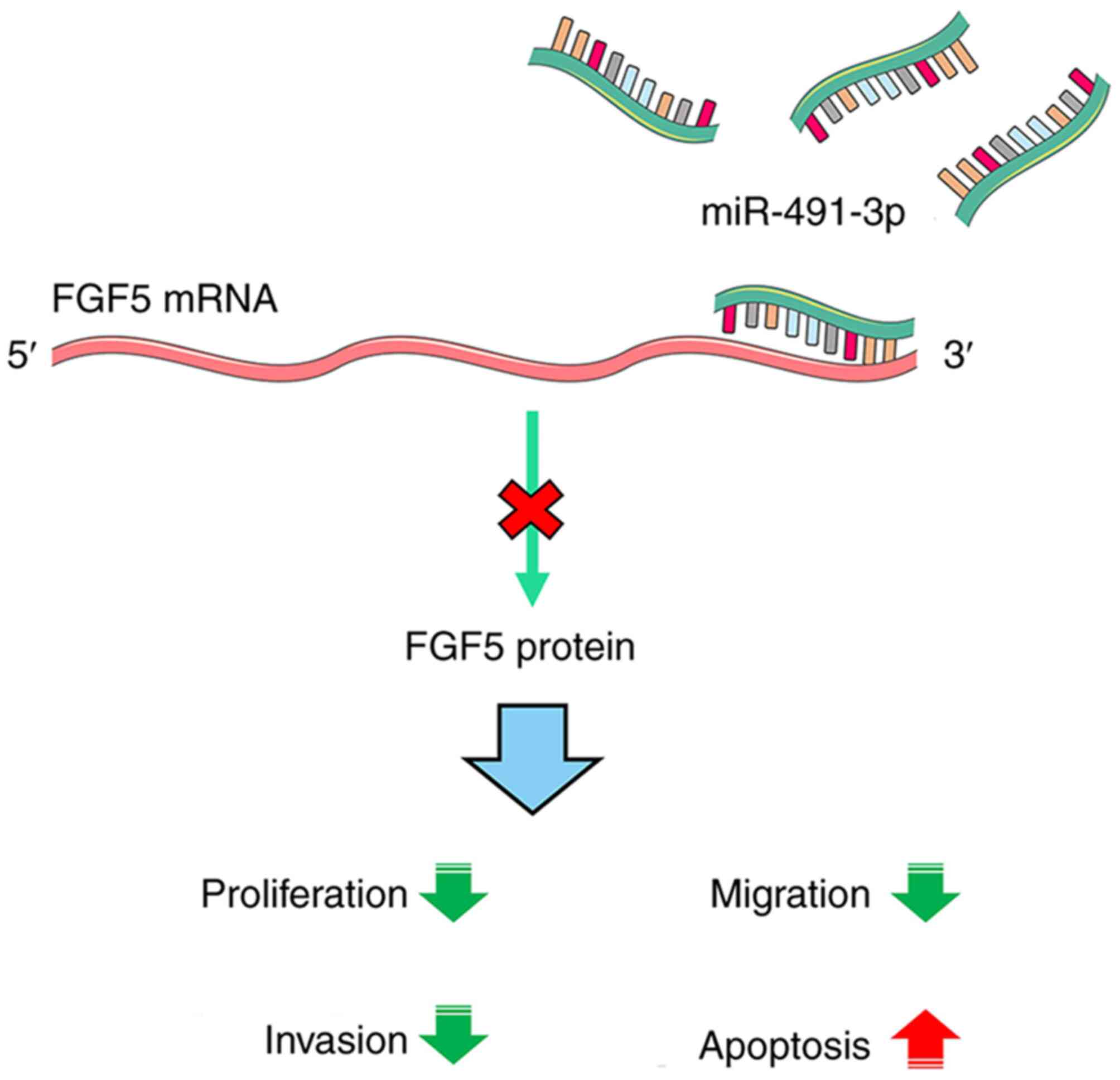
the progression of NSCLC by targeting FGF5. The mechanism diagram
is revealed in Fig. 6.
Nevertheless, there are certain limitations to the
present study. First, the sample size/power analysis was not
performed. Moreover, a previous study has reported that FGF5 could
promote the activity of the MAPK signaling pathway (29). However, due to laboratory
limitations, it could not be confirmed whether FGF5 promoted NSCLC
progression via regulating the MAPK signaling pathway activity.
Furthermore, the present study focused on miR-491-3p and its
downstream gene in NSCLC. It should be better to explore whether
miR-491-3p expression correlates with driver gene status.
Meanwhile, the concomitant mutations or co-mutations should also be
investigated. These aforementioned issues will be addressed in
future studies.
These findings indicated that miR-491-3p acts as a
tumor suppressor in NSCLC. It weakens the proliferation, migration,
invasion, and enhances the apoptosis of NSCLC cells by targeting
FGF5. restoring FGF5 expression reverses the suppression role of
miR-491-3p on the NSCLC cell malignant phenotype. More importantly,
miR-491-3p overexpression suppresses the in vivo growth of
NSCLC. Therefore, miR-491-3p may be a potential target for the
treatment of NSCLC.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
GZ was responsible for study design and data
collection. LW contributed to data analysis. GZ and HZ were in
charge of clinical data recording and data analysis. GZ, HZ and LW
performed the experiments. GZ, HZ and LW wrote the manuscript. All
authors read and approved the final manuscript. GZ and LW confirm
the authenticity of all the raw data.
Ethics approval and consent to
participate
The research protocol was reviewed and approved
(approval no. TCH036) by the Ethics Committee of the First
Affiliated Hospital of Wannan Medical College Yijishan Hospital
(Wuhu, China) and was in line with the Declaration of Helsinki.
Animal experiments were approved (approval no. AE041A) by the
Animal Ethics Committee of First Affiliated Hospital of Wannan
Medical College Yijishan Hospital (Wuhu, China). Written informed
consent was obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Khanmohammadi A, Aghaie A, Vahedi E,
Qazvini A, Ghanei M, Afkhami A, Hajian A and Bagheri H:
Electrochemical biosensors for the detection of lung cancer
biomarkers: A review. Talanta. 206:1202512020. View Article : Google Scholar
|
|
2
|
Yu Q, Xu L, Chen L, Sun B and Yang Z, Lu K
and Yang Z: Vinculin expression in non-small cell lung cancer. J
Int Med Res. 48:3000605198395232020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gelatti AC, Drilon A and Santini FC:
Optimizing the sequencing of tyrosine kinase inhibitors (TKIs) in
epidermal growth factor receptor (EGFR) mutation-positive non-small
cell lung cancer (NSCLC). Lung Cancer. 137:113–122. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brozos-Vázquez EM, Díaz-Peña R,
García-González J, León-Mateos L, Mondelo-Macía P, Peña-Chilet M
and López-López R: Immunotherapy in nonsmall-cell lung cancer:
Current status and future prospects for liquid biopsy. Cancer
Immunol Immunother. 70:1177–1188. 2021. View Article : Google Scholar
|
|
5
|
Alexander M, Kim SY and Cheng H: Update
2020: Management of non-small cell lung cancer. Lung. 198:897–907.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen X, Zhao L, Kang Y, He Z, Xiong F,
Ling X and Wu J: Significant suppression of non-small-cell lung
cancer by hydrophobic poly(ester amide) nanoparticles with high
docetaxel loading. Front Pharmacol. 9:1182018. View Article : Google Scholar
|
|
7
|
Zhong W, Zhang X, Zeng Y, Lin D and Wu J:
Recent applications and strategies in nanotechnology for lung
diseases. Nano Res. 14:2067–2089. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xiong F, Ling X, Chen X, Chen J, Tan J,
Cao W, Ge L, Ma M and Wu J: Pursuing specific chemotherapy of
orthotopic breast cancer with lung metastasis from docking
nanoparticles driven by bioinspired exosomes. Nano Lett.
19:3256–3266. 2019. View Article : Google Scholar
|
|
9
|
Naeli P, Yousefi F, Ghasemi Y,
Savardashtaki A and Mirzaei H: The role of MicroRNAs in lung
cancer: Implications for diagnosis and therapy. Curr Mol Med.
20:90–101. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Grossi I, Salvi A, Abeni E, Marchina E and
De Petro G: Biological function of microRNA193a-3p in health and
disease. Int J Genomics. 2017:59131952017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shan C, Chen X, Cai H, Hao X, Li J, Zhang
Y, Gao J, Zhou Z, Li X, Liu C, et al: The emerging roles of
autophagy-related MicroRNAs in cancer. Int J Biol Sci. 17:134–150.
2021. View Article : Google Scholar
|
|
12
|
Hu Y, Zhao M, Li L, Ding J, Gui YM and Wei
TW: miR-491-3p is downregulated in retinoblastoma and inhibit tumor
cells growth and metastasis by targeting SNN. Biochem Genet.
59:453–474. 2021. View Article : Google Scholar
|
|
13
|
Duan J, Liu J, Liu Y, Huang B and Rao L:
miR-491-3p suppresses the growth and invasion of osteosarcoma cells
by targeting TSPAN1. Mol Med Rep. 16:5568–5574. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao Y, Qi X, Chen J, Wei W, Yu C, Yan H,
Pu M, Li Y, Miao L, Li C and Ren J: The miR-491-3p/Sp3/ABCB1 axis
attenuates multidrug resistance of hepatocellular carcinoma. Cancer
Lett. 408:102–111. 2017. View Article : Google Scholar
|
|
15
|
Zheng G, Jia X, Peng C, Deng Y, Yin J,
Zhang Z, Li N, Deng M, Liu X, Liu H, et al: The
miR-491-3p/mTORC2/FOXO1 regulatory loop modulates chemo-sensitivity
in human tongue cancer. Oncotarget. 6:6931–6943. 2015. View Article : Google Scholar
|
|
16
|
Wang X, He Y, Mackowiak B and Gao B:
MicroRNAs as regulators, biomarkers and therapeutic targets in
liver diseases. Gut. 70:784–795. 2021. View Article : Google Scholar
|
|
17
|
Zhou Y, Yu Q, Chu Y, Zhu X, Deng J, Liu Q
and Wang Q: Downregulation of fibroblast growth factor 5 inhibits
cell growth and invasion of human nonsmall-cell lung cancer cells.
J Cell Biochem. Dec 5–2018.(Epub ahead of print). doi:
10.1002/jcb.28107.
|
|
18
|
Zhao T, Qian K and Zhang Y: High
expression of FGF5 is an independent prognostic factor for poor
overall survival and relapse-free survival in lung adenocarcinoma.
J Comput Biol. 27:948–957. 2020. View Article : Google Scholar
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liang G, Meng W, Huang X, Zhu W, Yin C,
Wang C, Fassan M, Yu Y, Kudo M, Xiao S, et al: miR-196b-5p-mediated
downregulation of TSPAN12 and GATA6 promotes tumor progression in
non-small cell lung cancer. Proc Natl Acad Sci USA. 117:4347–4357.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Correia de Sousa M, Gjorgjieva M, Dolicka
D, Sobolewski C and Foti M: Deciphering miRNAs' action through
miRNA editing. Int J Mol Sci. 20:62492019. View Article : Google Scholar
|
|
22
|
Liao Y, Cao L, Wang F and Pang R:
miR-605-5p promotes invasion and proliferation by targeting TNFAIP3
in non-small-cell lung cancer. J Cell Biochem. 121:779–787. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang Z, Zhu X, Zhang T and Yao F:
miR-512-5p suppresses the progression of non-small cell lung cancer
by targeting β-catenin. Oncol Lett. 19:415–423. 2020.
|
|
24
|
Jiang Z, Zhang J, Chen F and Sun Y:
miR-148b suppressed non-small cell lung cancer progression via
inhibiting ALCAM through the NF-κB signaling pathway. Thorac
Cancer. 11:415–425. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pu JT, Hu Z, Zhang DG, Zhang T, He KM and
Dai TY: miR-654-3p suppresses non-small cell lung cancer
tumourigenesis by inhibiting PLK4. Onco Targets Ther. 13:7997–8008.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ganju A, Khan S, Hafeez BB, Behrman SW,
Yallapu MM, Chauhan SC and Jaggi M: miRNA nanotherapeutics for
cancer. Drug Discov Today. 22:424–432. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kehler JS, David VA, Schäffer AA, Bajema
K, Eizirik E, Ryugo DK, Hannah SS, O'Brien SJ and Menotti-Raymond
M: Four independent mutations in the feline fibroblast growth
factor 5 gene determine the long-haired phenotype in domestic cats.
J Hered. 98:555–566. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huang Y, Wang Y and Yang Y: Expression of
fibroblast growth factor 5 (FGF5) and its influence on survival of
breast cancer patients. Med Sci Monit. 24:3524–3530. 2018.
View Article : Google Scholar
|
|
29
|
Han D, Wang M, Yu Z, Yin L, Liu C, Wang J,
Liu Y, Jiang S, Ren Z and Yin J: FGF5 promotes osteosarcoma cells
proliferation via activating MAPK signaling pathway. Cancer Manag
Res. 11:6457–6466. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu D, Zhang C, Li X, Zhang H, Pang Q and
Wan A: MicroRNA-567 inhibits cell proliferation, migration and
invasion by targeting FGF5 in osteosarcoma. EXCLI J. 17:102–112.
2018.PubMed/NCBI
|
|
31
|
Fang F, Chang RM, Yu L, Lei X, Xiao S,
Yang H and Yang LY: MicroRNA-188-5p suppresses tumor cell
proliferation and metastasis by directly targeting FGF5 in
hepatocellular carcinoma. J Hepatol. 63:874–885. 2015. View Article : Google Scholar
|
|
32
|
Kornmann M, Ishiwata T, Beger HG and Korc
M: Fibroblast growth factor-5 stimulates mitogenic signaling and is
overexpressed in human pancreatic cancer: Evidence for autocrine
and paracrine actions. Oncogene. 15:1417–1424. 1997. View Article : Google Scholar : PubMed/NCBI
|