Introduction
Cervical cancer is the global leading cause of
morbidity and mortality in women and has been listed as one of the
most critical issues affecting women's health (1,2).
Over the past few decades, the advancements made in screening
programs, vaccinations to avoid human papillomavirus (HPV)
infection, targeted therapies and immunotherapies for cervical
cancer have markedly reduced the disease burden (3–6).
However, it should be noted that the current overall prognosis of
patients with cervical cancer remains far from satisfactory
(1,3). Cisplatin (DDP) is widely used in the
treatment of cervical cancer; it is not only used as a
chemotherapeutic agent for patients with advanced cervical cancer,
but is also applied for neoadjuvant/adjuvant treatment (7,8).
However, DDP monotherapy has been indicated as insufficient for the
treatment of cervical cancer (8).
Thalidomide (THD), an immunomodulatory and
anti-angiogenic agent, also potentially induces the apoptosis of
cancer cells and has been applied for the treatment of several
types of cancer over the past few decades (9–12).
Notably, the combination of THD with other chemotherapeutic
reagents (including DDP) has been shown to exert a synergistic
anticancer effect. For instance, a previous study demonstrated that
THD plus DDP exerted a synergistic inhibitory effect on tumor
growth and angiogenesis in head and neck squamous cell carcinoma
model mice (13). Furthermore,
another study found that THD plus DDP exerted a more prominent
suppressive effect on tumor volume than DDP monotherapy in glioma
model rats (14). Similar results
were also found in breast tumor model mice and colorectal tumor
model mice (15). More
importantly, a previous randomized controlled trial revealed that
THD plus DDP improved the 3-year overall survival and
progression-free survival rate of patients with advanced esophageal
cancer (16). Based on these
findings, it was hypothesized that THD plus DDP may also exert a
synergistic effect on cervical cancer. However, relative
information is lacking.
The phosphoinositide 3-kinase (PI3K)/protein kinase
B (AKT) and Janus kinase 1 (JAK1)/signal transducer and activator
of transcription 3 (STAT3) pathways regulate various biological
processes, including cell survival, metabolism and protein
synthesis (17,18). In cervical cancer, it has been
suggested that the PI3K/AKT and JAK1/STAT3 pathways critically
participate in cancer pathogenesis and progression (19,20).
In addition, previous studies have indicated that both THD and DDP
exert antitumor effects by modulating the PI3K/AKT and JAK1/STAT3
pathways (21–24). Therefore, the present study aimed
to evaluate the effects of THD plus DDP on cell viability,
apoptosis, as well as the activation of the PI3K/AKT and JAK1/STAT3
pathways in HeLa and SiHa cervical cancer cell lines.
Materials and methods
Cells and cell culture
The human cervical carcinoma cell lines, HeLa
(TCHu187) and SiHa (SCSP-5058), were purchased from The Cell Bank
of Type Culture Collection of the Chinese Academy of Sciences. Both
HeLa and SiHa cells were cultured in 90% Eagle's minimum essential
medium (Nissui Pharmaceutical Co., Ltd.) and 10% fetal bovine serum
(FBS) (Gibco; Thermo Fisher Scientific, Inc.). Normal human
cervical epithelial (HCerEpiC) (product no. FC-0080) cells were
purchased from Lifeline Cell Technology, LLC. HCerEpiC cells were
cultured with 90% cervical epithelial medium (Lifeline Cell
Technology, LLC) and 10% FBS. The culture was conducted in a cell
incubator with a humidified atmosphere at 37°C and 5%
CO2. Cells in the exponential growth phase were selected
for use in the following experiments.
Treatments and detections
DDP and THD were purchased from MedChemExpress and
were prepared into gradient solutions with dimethyl sulfoxide
(MedChemExpress) for use in the following experiments. After the
preparation of DDP and THD, the following treatments were carried
out:
i) Single-drug treatment: Both HeLa and SiHa cells
were respectively treated with a single-drug solution at various
concentrations in medium containing 10% FBS for 24 h, and the
concentration gradient was set as follows: DDP: 0, 2, 4, 8, 16, 32
and 64 µM; and THD: 0, 5, 10, 20, 40, 80 and 160 µM. Following 24 h
of treatment with the single-drug solution, cell viability was
analyzed using a Cell Counting Kit-8 (CCK-8) (Beyotime Institute of
Biotechnology).
ii) Two-drug treatment: Both HeLa and SiHa cells
were respectively treated with the two drugs in 12 combinations at
various concentrations in medium containing 10% FBS for 24 h, and
the concentration gradient was set as follows: DDP: 0, 4, 16 and 64
µM; THD: 0, 20 and 160 µM. Following 24 h of treatment with the
two-drug solution, cell viability was analyzed using CCK-8
(Beyotime Institute of Biotechnology). Furthermore, the combination
index (CI) was estimated to determine the optimal combination
concentration, which was calculated as follows: The relative cell
viability of combination treatment divided by the product of the
relative cell viability of two single-drug treatments.
iii) Synergistic treatment: Both HeLa and SiHa cells
were respectively categorized into four groups: Group A, cells were
treated with 0 µM DDP and 0 µM THD dissolved in medium containing
10% FBS for 24 h; group B, cells were treated with 16 µM DDP and 0
µM THD dissolved in medium containing 10% FBS for 24 h; group C,
cells were treated with 0 µM DDP and 160 µM THD dissolved in medium
containing 10% FBS for 24 h; group D, cells were treated with 16 µM
DDP and 160 µM THD dissolved in medium containing 10% FBS for 24 h.
Following 24 h of treatment, in each cell group, cell viability was
analyzed using CCK-8 (Beyotime Institute of Biotechnology); cell
apoptosis was assessed using the Annexin V-fluorescein
Isothiocyanate (FITC) Apoptosis Detection kit (Beyotime Institute
of Biotechnology); the expression levels of the PI3K/AKT pathway
and the JAK/STAT pathway in each group were determined using
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR) and western blot analysis.
iv) PI3K and JAK activation: The HeLa and SiHa cells
were divided into three groups as follows: Group A, cells were
treated 16 µM DDP and 160 µM THD for 24 h; group B, cells were
treated with 16 µM DDP, 160 µM THD and 20 µM 740Y-P
(MedChemExpress) for 24 h; group C, cells were treated with 16 µM
DDP, 160 µM THD and 20 µM RO8191 (MedChemExpress) for 24 h. Cell
viability and apoptosis were measured using CCK-8 assay and the
Annexin V-FITC Apoptosis Detection kit, respectively.
Cell viability determination
The cells were seeded at 1×104 per well
in a 96-well plate. Following treatment, the old culture solution
in the experimental wells was discarded and 90 µl medium and 10 µl
CCK-8 solution were added to each well of the 96-well plate,
followed by incubation for 2 h at 37°C. Finally, a microplate
reader (BioTek Instruments, Inc.) was applied to measure the
absorbance of each experimental well at 450 nm and the relative
cell viability was calculated based on the optical density
value.
Cell apoptosis determination
The cells were seeded at 4×105 per well
in a 6-well plate. Following the treatment, the cells were digested
by trypsin at 37°C and the supernatant was removed by
centrifugation (1,500 × g for 3 min at room temperature). The cells
were stained with trypan blue solution (Beyotime Institute of
Biotechnology) at room temperature for 2 min, followed by cell
counting under an inverted microscope (Motic China Group Co.,
Ltd.). Following the adjustment of the cell density, 5 µl Annexin V
and 5 µl propidium iodide were added to a 100-µl cell suspension,
which was then maintained at room temperature for 15 min in the
dark. After the cells were passed through 400-mesh sieves, a
FACSCalibur flow cytometer (BD Biosciences) was applied to analyze
cell apoptosis. The data were analyzed using Flowjo 7.6 (BD
Biosciences).
RT-qPCR
The expression levels of JAK1, STAT3 and PI3K in
each group were assayed using RT-qPCR. The cells were seeded at
4×105 per well in a six-well plate. Following treatment,
the isolation of total RNA was completed using TRIzol reagent
(Beyotime Institute of Biotechnology). qPCR was performed on an
AFD9600 real-time fluorescence quantitative PCR instrument (AGS)
using ReverTra Ace® qPCR RT kit (Toyobo Co., Ltd.) (at
37°C for 15 min, followed by 98°C for 5 min) and
THUNDERBIRD® SYBR® qPCR Mix (Toyobo Co.,
Ltd.) (95°C for 1 min, 1 cycle; followed by 95°C for 15 sec, 61°C
for 30 sec, 40 cycles) as per the manufacturer's protocol. The
internal reference was glyceraldehyde 3-phosphate dehydrogenase
(GAPDH). The 2−ΔΔCq method was used to calculate the
relative expression of each gene (25). The primer sequences used for
RT-qPCR are presented in Table
I.
 | Table I.Primers sequences used for
RT-qPCR. |
Table I.
Primers sequences used for
RT-qPCR.
| Gene | Forward
(5′->3′) | Reverse
(5′->3′) |
|---|
| JAK1 |
TGGATTACAAGGATGACGAAGGAA |
CGGACACAGACGCCATAGAG |
| STAT3 |
GAGAAGGACATCAGCGGTAAGAC |
GGATAGAGATAGACCAGTGGAGACA |
| PI3K |
TTCTCAACTGCCAATGGACTGT |
AGCACGAGGAAGATCAGGAATG |
| GAPDH |
GAGTCCACTGGCGTCTTCAC |
ATCTTGAGGCTGTTGTCATACTTCT |
Western blot analysis
The procedures of western blot analysis were
conducted according to those described in a previous study with
certain modifications (26).
Briefly, the cells were seeded at 4×105 per well in a
six-well plate. Following treatment, the cells were collected and
protein was extracted using RIPA Lysis Buffer (Beyotime Institute
of Biotechnology) on ice for 30 min and quantified using a Pierce™
BCA Protein Assay kit (Thermo Fisher Scientific, Inc.).
Subsequently, 20 µg protein was separated by 4–20% sodium dodecyl
sulfate polyacrylamide gel electrophoresis, transferred to a
polyvinylidene fluoride membrane and incubated with 5% non-fat milk
powder at room temperature for 1 h. Subsequently, the membrane was
incubated with primary antibodies at 4°C overnight, followed by
incubation with HRP-conjugated goat anti-rabbit secondary
antibodies (1:20,000; product code ab6721; Abcam) at room
temperature for 1 h and visualization using Pierce™ ECL Plus
Western Blotting Substrate (Thermo Fisher Scientific, Inc.). The
primary and secondary antibodies for JAK1 (monoclonal antibody;
1:5,000; product code ab133666), phosphorylated (p)-JAK1
(monoclonal antibody; 1:5,000; product code ab138005), STAT3
(monoclonal antibody; 1:2,000; product code ab68153), p-STAT3
(monoclonal antibody; 1:1,000; product code ab267373), PI3K
(monoclonal antibody; 1:1,500; product code ab40755), AKT
(polyclonal antibody; 1:500; product code ab8805), p-AKT
(polyclonal antibody; 1:1,000; product code ab38449) and GAPDH
(polyclonal antibody; 1:2,500; product code ab9485) were all
purchased from Abcam. The quantification of the western blots was
carried out with ImageJ 1.8 (National Institutes of Health).
Statistical analysis
All data processing and analysis were completed
using GraphPad Prism 7.02 (GraphPad Software Inc.) and are
presented in bar plots, representing the mean value and standard
deviation (error bar). Multiple comparisons were performed using
one-way analysis of variance (ANOVA) followed by Tukey's or
Dunnett's test. A P-value <0.05 was considered to indicate a
statistically significant difference. The Shapiro-Wilk normality
test was used for normality test of data. The data were parametric
distribution.
Results
Sensitivity of cervical cancer cell
lines to DDP monotherapy and THD monotherapy
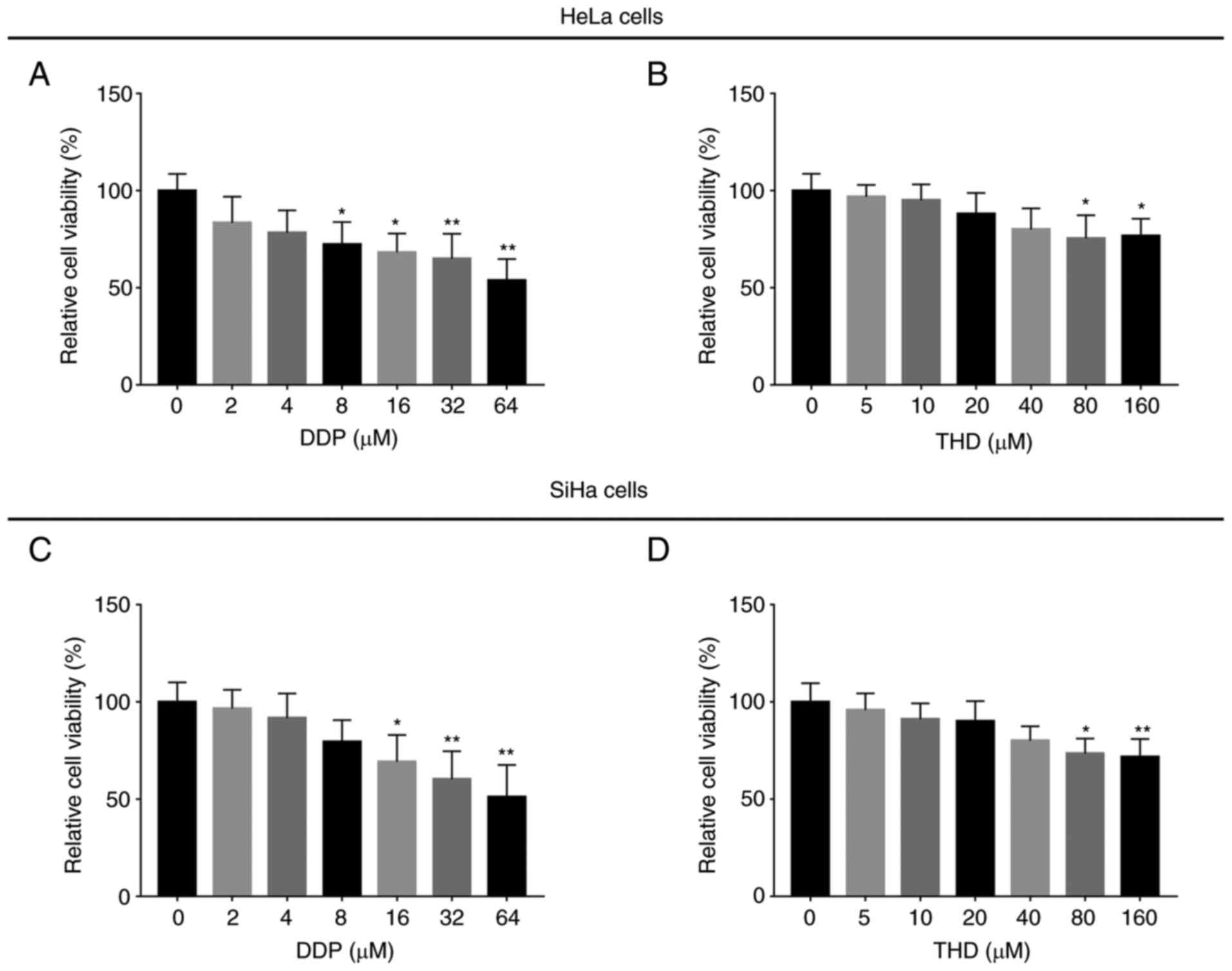
DDP treatment suppressed the relative viability of
HeLa cells (Fig. 1A) (P<0.05
and P<0.01 when DDP ≥8 µM) and SiHa cells (Fig. 1C) (P<0.05 and P<0.01 when DDP
≥16 µM) in a concentration-dependent manner. Moreover, THD
treatment suppressed the relative viability of HeLa cells (Fig. 1B) (both P<0.05 when THD ≥80 µM)
and SiHa cells (Fig. 1D)
(P<0.05 and P<0.01 when THD ≥80 µM) in a
concentration-dependent manner.
Synergized effect of THD and DDP on
cervical cancer cell lines
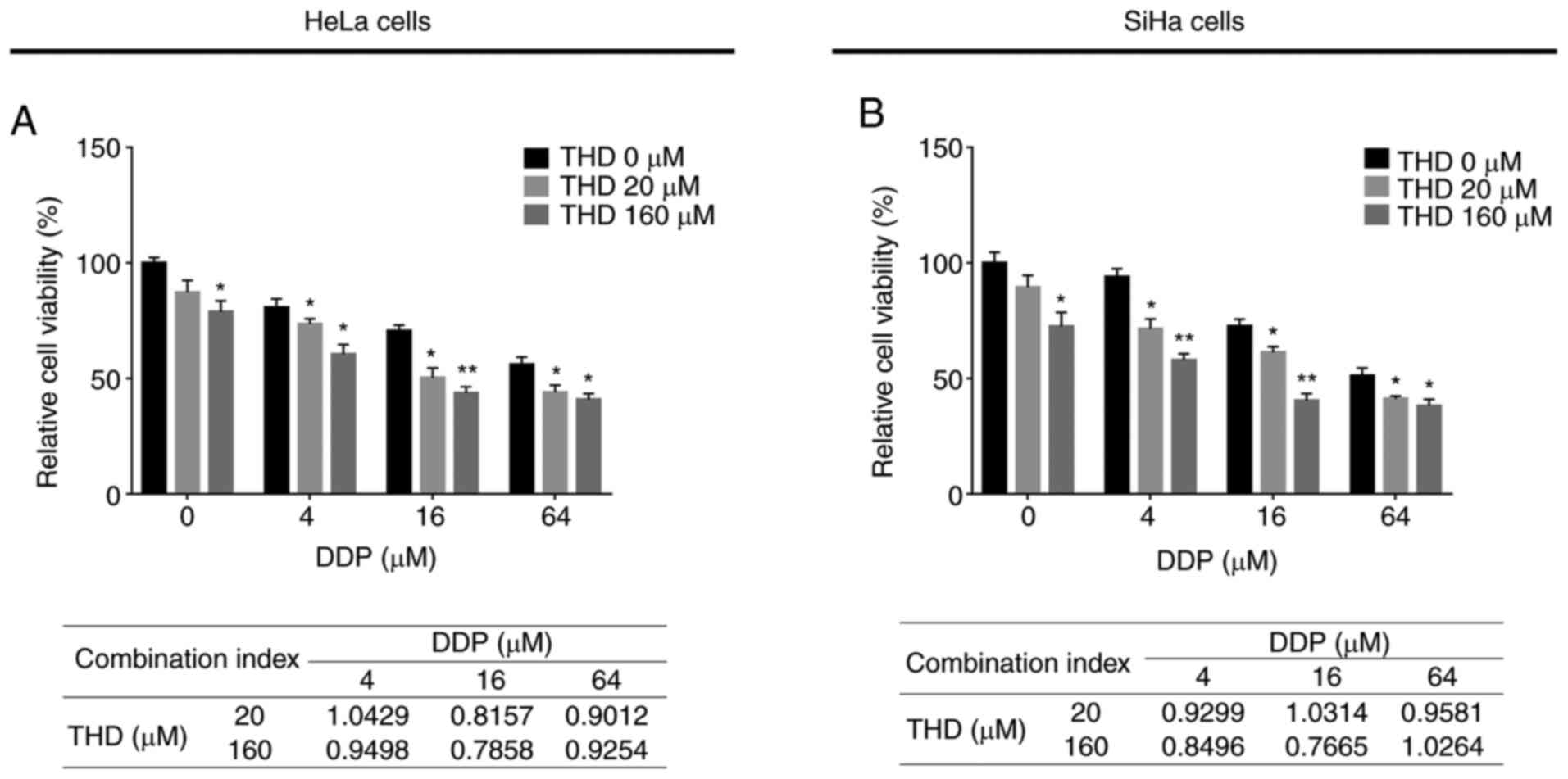
THD plus DDP treatment exerted a more prominent
suppressive effect on the relative viability of HeLa cells
(Fig. 2A) and SiHa cells (Fig. 2B) compared to DDP monotherapy at
several concentration settings (P<0.05 and P<0.01).
Furthermore, after calculating the CI (the lower value, the more
prominent the synergistic effect), it was determined that the
optimal combination concentration was 16 µM for DDP and 160 µM for
THD (synergistic treatment) in both cell lines (Fig. 2A and B); thus, these settings were
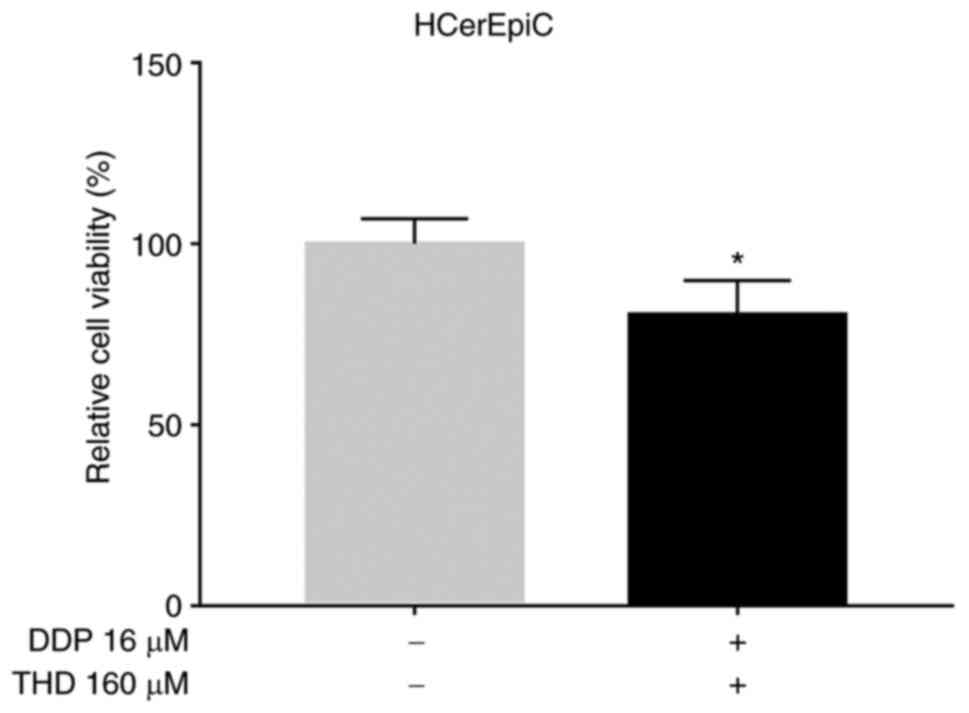
applied in the following experiments. In addition, THD plus DDP
suppressed the relative viability of HCerEpiC cells, although the
inhibitory effect was weaker than that observed in the cervical
cancer cells (P<0.05; Fig.
3).
Synergistic effects of THD and DDP on
the relative viability and apoptosis of cervical cancer cell
lines
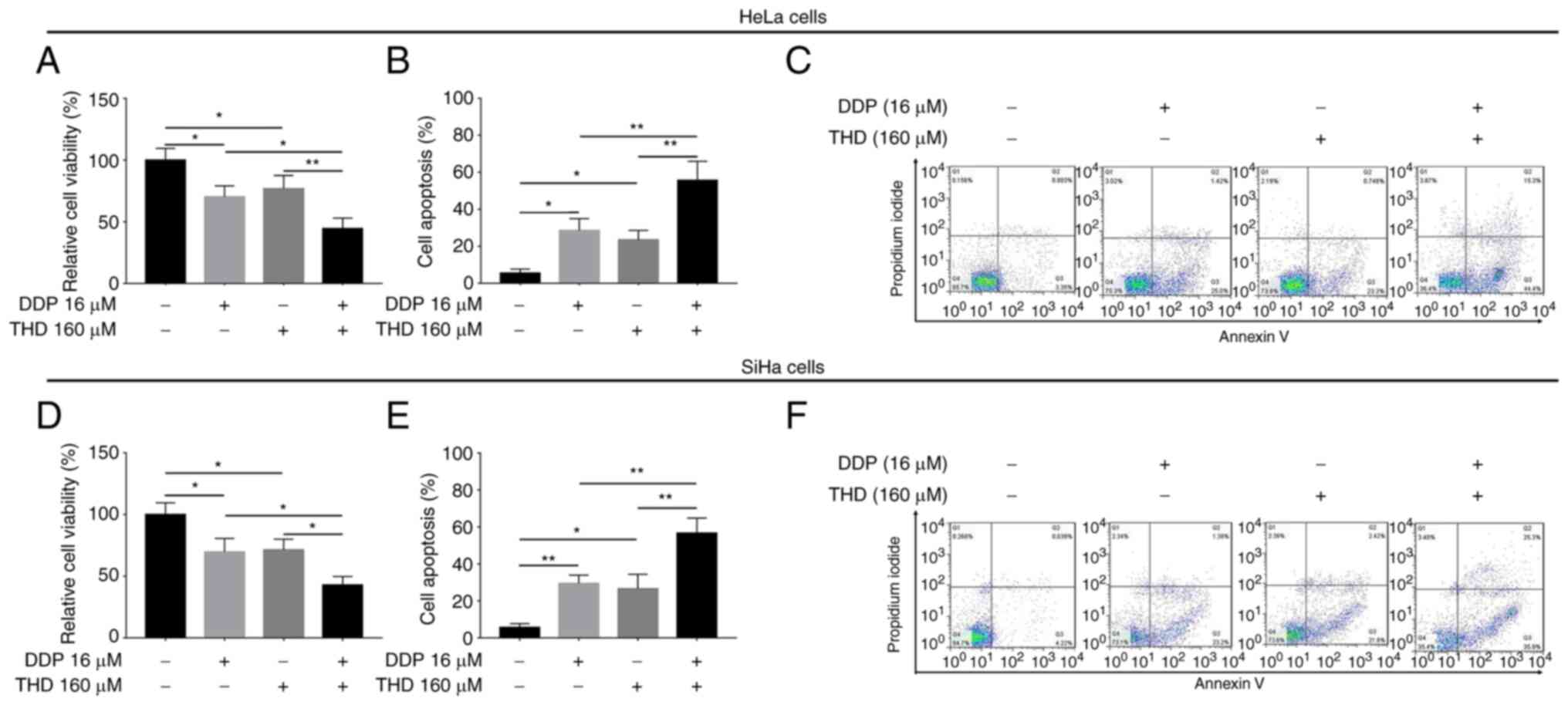
In HeLa cells, treatment with both THD and DDP
suppressed the relative cell viability (P<0.05 and P<0.01;
Fig. 4A), while it promoted cell
apoptosis (both P<0.01) (Fig. 4B
and C) compared with DPP or THD monotherapy. In addition, THD
and DDP exerted a similar synergistic effect on the relative
viability (both P<0.05; Fig.
4D) and apoptosis (both P<0.01; Fig. 4E and F) of SiHa cells.
Synergistic effects of THD and DDP on
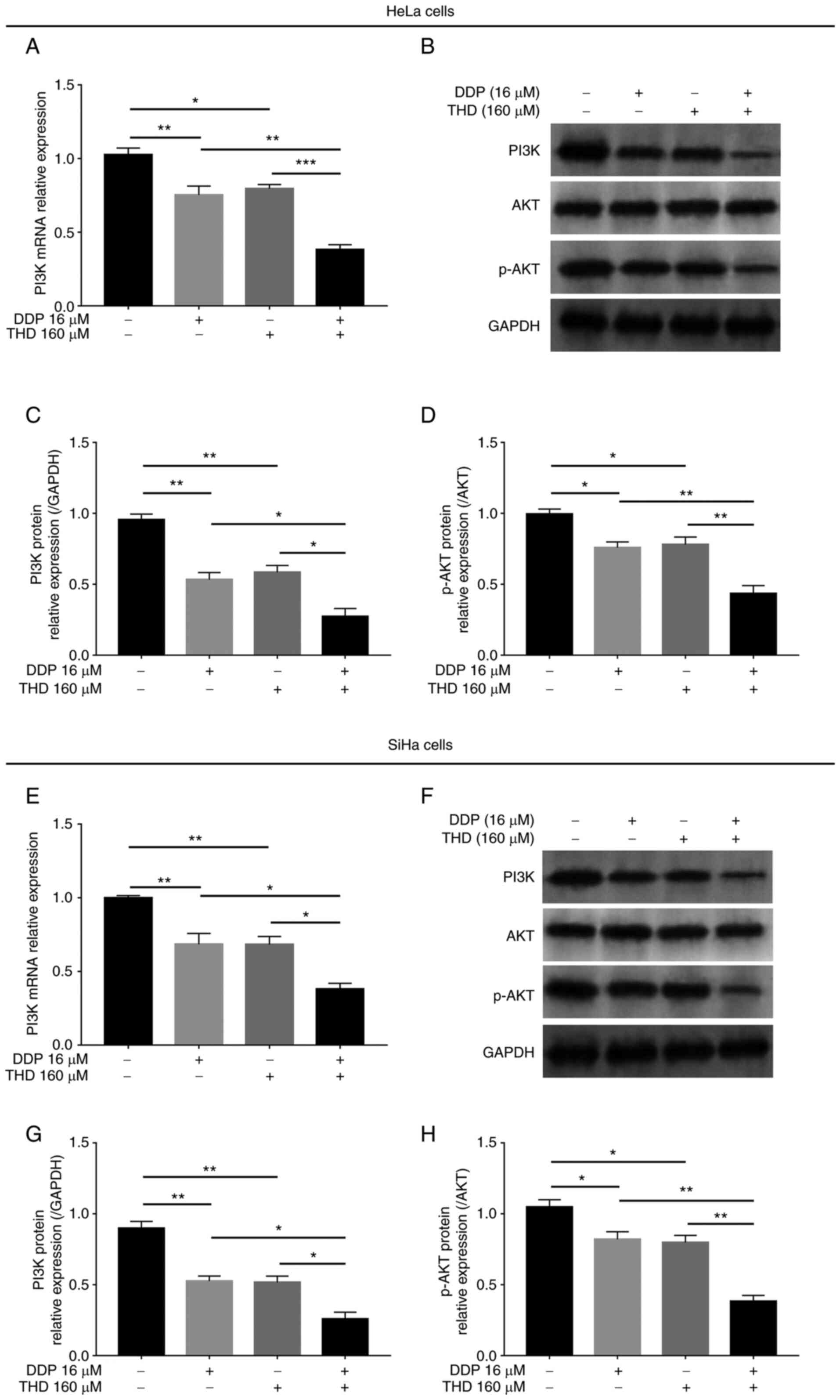
the PI3K/AKT pathway in cervical cancer cell lines
In both HeLa cells and SiHa cells, THD or DDP
monotherapy reduced the mRNA and protein levels of PI3K, as well as
the phosphorylation levels of AKT (P<0.05 and P<0.01;
Fig. 5A-H). Moreover, combined
treatment with THD and DDP exerted a more prominent suppressive
effect on the mRNA and protein levels of PI3K, as well as the on
the phosphorylation levels of AKT, compared with DDP or THD
monotherapy (P<0.05, P<0.01 and P<0.001; Fig. 5A-H).
Synergistic effects of THD and DDP on
the JAK1/STAT3 pathway in cervical cancer cell lines
In HeLa cells, the mRNA (P<0.05, P<0.01 and
P<0.001; Fig. 6A and B) and
protein levels (P<0.05 and P<0.01; Fig. 6C-E) of JAK1 and STAT3 were
decreased by THD or DDP monotherapy. Furthermore, the mRNA and
protein levels of JAK1 and STAT3 were further reduced by THD and
DDP combined treatment compared with DDP or THD monotherapy
(P<0.05, P<0.01 and P<0.001; Fig. 6A-E). Moreover, the levels of
phosphorylated JAK1 and STAT3 were also decreased by THD and DDP
combined treatment (P<0.05 and P<0.01; Fig. 6F and G). In SiHa cells, mRNA
(P<0.05 and P<0.01; Fig. 6H and
I) and protein levels (all P<0.05; Fig. 6J-L) of JAK1 and STAT3 were
decreased by THD or DDP monotherapy and further reduced by THD and
DDP combined treatment (P<0.05 and P<0.01; Fig. 6H-L). In addition, the levels of
phosphorylated JAK1 and STAT3 were also decreased by THD and DDP
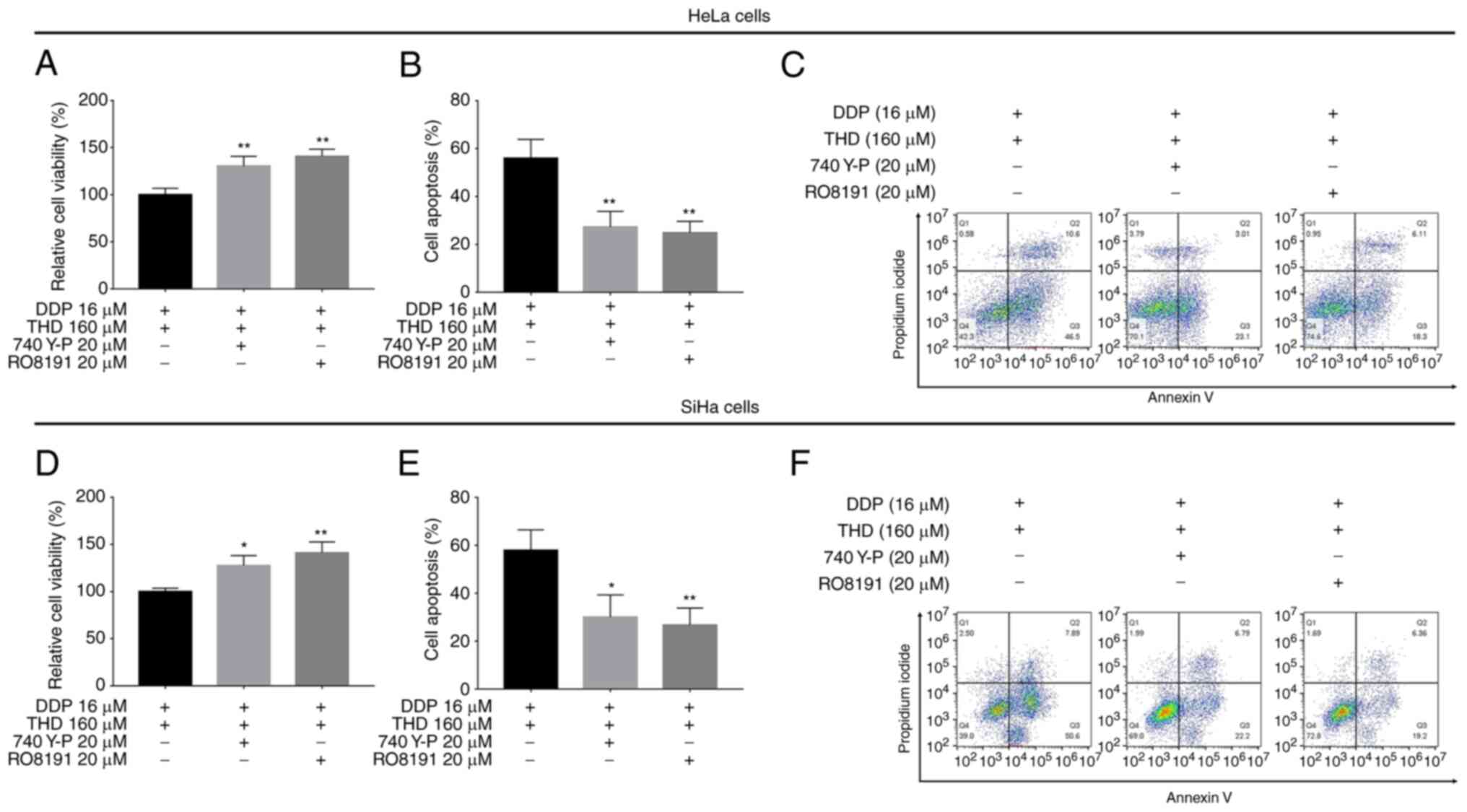
combined treatment (P<0.05 and P<0.01; Fig. 6M and N). Furthermore, when the
PI3K/AKT or JAK1/STAT3 pathway was activated, the effects of THD
plus DDP on reducing cell viability and increasing apoptosis were
attenuated (P<0.05 and P<0.01; Fig. 7A-F), indicating that the PI3K/AKT
and JAK1/STAT3 pathways are required for the killing effects of THD
plus DDP on cervical cancer cells.
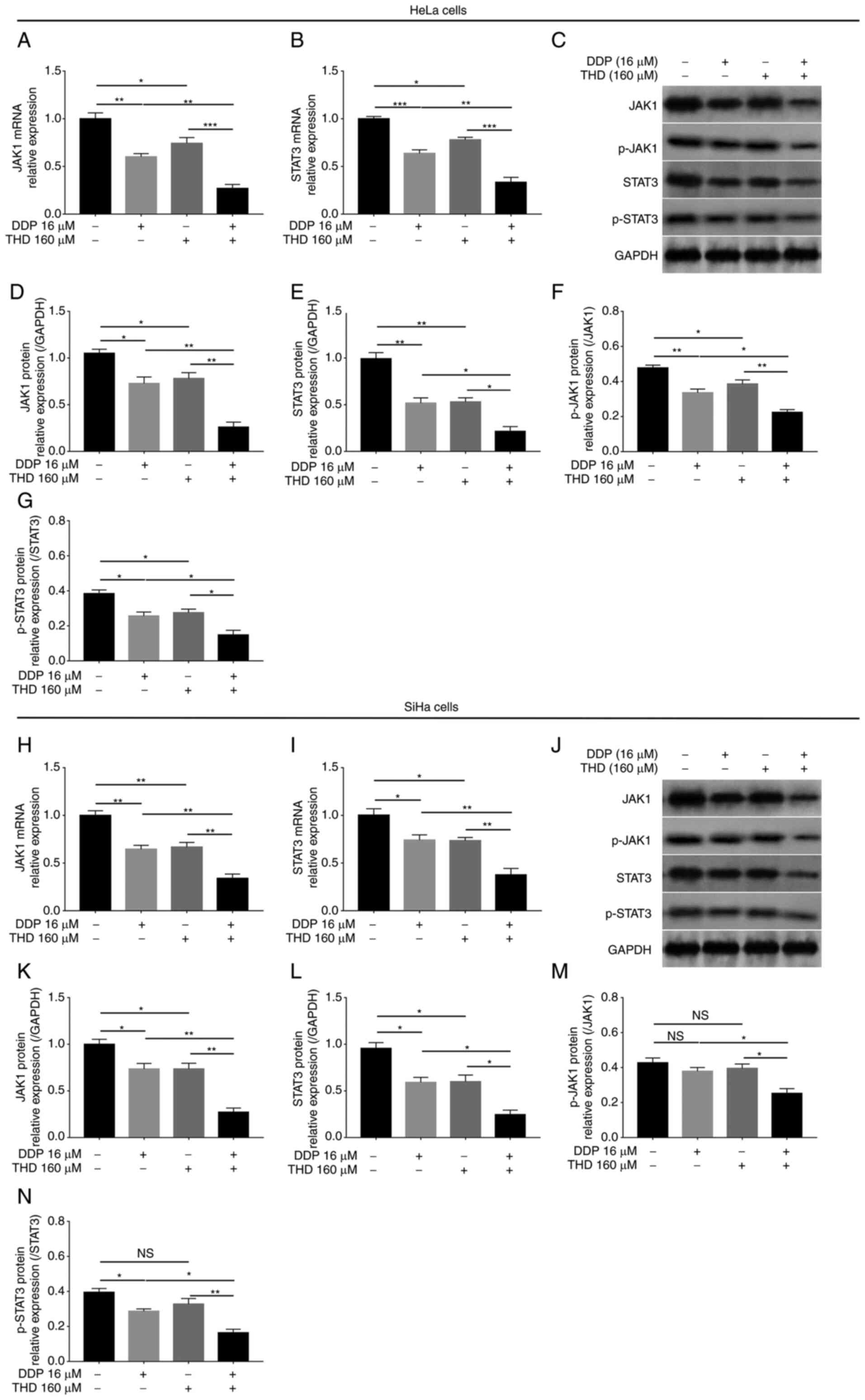 | Figure 6.Comparison of JAK1 and STAT3
expression levels. Comparison of (A) JAK1 and (B) STAT3 mRNA
expression levels by THD/DDP monotherapy and their synergistic
treatment in HeLa cells. (C) Representative images of JAK1 and
STAT3 protein expression detection by western blotting in HeLa
cells. Comparison of (D) JAK1, (E) STAT3, (F) p-JAK1 and (G)
p-STAT3 protein expression levels by THD/DDP monotherapy and their
synergistic treatment in HeLa cells. Comparison of (H) JAK1 and (I)
STAT3 mRNA expression levels by THD/DDP monotherapy and their
synergistic treatment in SiHa cells. (J) Representative images of
JAK1 and STAT3 protein expression detection by western blotting in
SiHa cells. Comparison of (K) JAK1, (L) STAT3, (M) p-JAK1 and (N)
p-STAT3 protein expression levels by THD/DDP monotherapy and their
synergistic treatment in SiHa cells. *P<0.05, **P<0.01 and
***P<0.001. JAK1, Janus kinase 1; STAT3, signal transducer and
activator of transcription 3; THD, thalidomide; DDP, cisplatin; p-,
phosphorylated; NS, not significant. |
Discussion
DDP has long been included in the treatment regimen
of cervical cancer; however, its use as a monotherapy has not
proven to be effective (5,7). Previous studies have demonstrated
that the combination of cisplatin with other therapeutic agents
exhibits an adequate treatment efficacy in several types of cancer,
including non-small cell lung, bladder and cervical cancer
(27–29). On the other hand, THD exerts a
synergistic effect when combined with other therapeutic agents in
the treatment of cancer patients. For instance, a previous study
demonstrated that THD plus chemo-radiotherapy improved the 3-year
overall survival rate, progression-free survival rate and median
progression-free survival time compared with chemo-radiotherapy
alone in patients with esophageal cancer (16). Moreover, another systematic review
indicated that THD plus docetaxel improved patient prognosis and
exerted a more prominent suppressive effect on prostate-specific
antigen levels than docetaxel in patients with androgen-independent
prostate cancer (30). In
addition, it has been reported that THD plus chemotherapy exerts an
improved treatment effect compared with chemotherapy alone in
patients with advanced non-small cell lung or small cell lung
cancer (31).
Although the aforementioned studies have indicated
that the combination of THD or DDP with other treatment strategies
enhances their effects on several types of cancer, including
cervical cancer (13,27,29),
it remains unclear whether THD plus DDP can exert a synergistic
therapeutic effect on cervical cancer. Therefore, the present study
found that DDP or THD monotherapy both suppressed the relative
viability of cervical cancer cell lines in a
concentration-dependent manner. These data were partially in line
with those of previous studies (32,33).
In addition, the present study demonstrated that DDP plus THD
exerted a more prominent suppressive effect on the relative cell
viability of cervical cancer cell lines than DDP or THD
monotherapy, and the optimal combination concentrations were 16 µM
for DDP and 160 µM for THD. It was hypothesized that THD may
regulate several pathways, such as the PI3K/AKT and JAK1/STAT3
pathways (as demonstrated using western blot analysis in the
present study) (34) to enhance
the suppressive effects of cisplatin on the viability of cervical
cancer cell lines. Moreover, it was found that combined treatment
with THD and DDP enhanced apoptosis compared with DDP or THD
monotherapy in cervical cancer cell lines. These data also suggest
that THD may regulate several pathways to enhance the cytotoxic
effects of DDP (as aforementioned).
The PI3K/AKT and JAK1/STAT3 pathways are two classic
pathways that regulate cell survival in cervical cancer. Previous
studies have indicated that activating the PI3K/AKT or JAK1/STAT3
pathways promotes the proliferation, whereas it suppresses the
apoptosis of cervical cancer cell lines (35,36).
In addition, the PI3K/AKT and JAK1/STAT3 pathways are associated
with HPV infection, which is a critical risk factor of cervical
cancer (37–39). In the present study, it was found
that combined treatment suppressed PI3K, AKT, JAK1 and STAT3 gene
expression. Concurrently, combined treatment also inhibited PI3K,
p-AKT, JAK1 and STAT3 protein expression, suggesting that it
suppressed the PI3K/AKT and JAK1/STAT3 pathways in cervical cancer
cell lines. However, these assays did not include the human normal
cervical epithelial cell line, which was a limitation of the
present study.
Previous studies have demonstrated that the
combination of THD and DDP exerts a suppressive synergistic effect
on tumor progression in breast tumor model mice, colorectal tumor
model mice and head and neck squamous cell carcinoma model mice
(13,15). However, to the best of our
knowledge, only one in vitro study found that the
combination between THD and DDP exerted a synergistic suppressive
effect on the proliferation of head and neck squamous cell
carcinoma cells (13). Currently,
studies investigating the treatment efficacy of THD plus DDP in
patients with cancer are relatively limited; to the best of our
knowledge, to date, only one randomized controlled trial revealed
that DDP plus THD improved the prognosis of patients with advanced
esophageal cancer (16).
Furthermore, the use of THD plus DDP in patients with cancer also
lacks pre-clinical research and theoretical support. The findings
of the present study potentially contribute to this issue. However,
further pilot clinical trials are required to explore the efficacy
of THD plus DDP in cervical cancer patients.
In conclusion, the present study demonstrated that
THD synergized with DDP in killing cervical cancer cell lines,
which also inactivated the PI3K/AKT and JAK1/STAT3 pathways; this
suggests their potential for use in cervical cancer treatment.
However, further studies are warranted to determine whether THD and
DDP also exert synergistic suppressive effects on tumor progression
in cervical cancer model mice.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Hebei Provincial Health
Committee: Effects of thalidomide or cisplatin on proliferation
inhibition of human cervical cancer HeLa cells and its mechanism
(grant no. 20181674).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
CL and LY contributed to the conception of the
study. HF, LS, SL and YW contributed to data acquisition and data
analysis. CL, HF, LS and YW drafted the manuscript. SL and LY
revised the manuscript. CL and LY confirm the authenticity of all
the raw data. All authors have approved the final version to be
published and agree to be accountable for all aspects of the work
in ensuring that questions related to the accuracy or integrity of
any part of the work are appropriately investigated and
resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
THD
|
thalidomide
|
|
CI
|
combination index
|
|
DDP
|
cisplatin
|
References
|
1
|
Vu M, Yu J, Awolude OA and Chuang L:
Cervical cancer worldwide. Curr Probl Cancer. 42:457–465. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cohen PA, Jhingran A, Oaknin A and Denny
L: Cervical cancer. Lancet. 393:169–182. 2019. View Article : Google Scholar
|
|
3
|
Lei J, Ploner A, Elfstrom KM, Wang J, Roth
A, Fang F, Sundström K, Dillner J and Sparén P: HPV vaccination and
the risk of invasive cervical cancer. N Engl J Med. 383:1340–1348.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sawaya GF, Smith-McCune K and Kuppermann
M: Cervical cancer screening: More choices in 2019. JAMA.
321:2018–2019. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Johnson CA, James D, Marzan A and Armaos
M: Cervical cancer: An overview of pathophysiology and management.
Semin Oncol Nurs. 35:166–174. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Scarth JA, Patterson MR, Morgan EL and
Macdonald A: The human papillomavirus oncoproteins: A review of the
host pathways targeted on the road to transformation. J Gen Virol.
102:0015402021. View Article : Google Scholar
|
|
7
|
Koh WJ, Abu-Rustum NR, Bean S, Bradley K,
Campos SM, Cho KR, Chon HS, Chu C, Clark R, Cohn D, et al: Cervical
cancer, version 3.2019, NCCN clinical practice guidelines in
oncology. J Natl Compr Canc Netw. 17:64–84. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Marth C, Landoni F, Mahner S, McCormack M,
Gonzalez-Martin A and Colombo N; ESMO Guidelines Committee, :
Cervical cancer: ESMO clinical practice guidelines for diagnosis,
treatment and follow-up. Ann Oncol. 28:iv72–iv83. 2017. View Article : Google Scholar
|
|
9
|
Eleutherakis-Papaiakovou V, Bamias A and
Dimopoulos MA: Thalidomide in cancer medicine. Ann Oncol.
15:1151–1160. 2004. View Article : Google Scholar
|
|
10
|
Tamalunas A, Sauckel C, Ciotkowska A, Rutz
B, Wang R, Huang R, Li B, Stief CG, Gratzke C, Hennenberg M, et al:
Inhibition of human prostate stromal cell growth and smooth muscle
contraction by thalidomide: A novel remedy in LUTS? Prostate.
81:377–389. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhu J, Yang Y, Liu S, Xu H, Wu Y, Zhang G,
Wang Y, Wang Y, Liu Y and Guo Q: Anticancer effect of thalidomide
in vitro on human osteosarcoma cells. Oncol Rep. 36:3545–3551.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang Y, Zhu YQ, Jiang L, Li LF and Ge JP:
Thalidomide induces apoptosis in human oral squamous cell carcinoma
cell line with altered expression of tumor necrosis factor-related
apoptosis-inducing ligand (TRAIL). Oral Oncol. 47:927–928. 2011.
View Article : Google Scholar
|
|
13
|
Vasvari GP, Dyckhoff G, Kashfi F, Lemke B,
Lohr J, Helmke BH, Schirrmacher V, Plinkert PK, Beckhove P,
Herold-Mende CC, et al: Combination of thalidomide and cisplatin in
an head and neck squamous cell carcinomas model results in an
enhanced antiangiogenic activity in vitro and in vivo. Int J
Cancer. 121:1697–1704. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Murphy S, Davey RA, Gu XQ, Haywood MC,
McCann LA, Mather LE and Boyle FM: Enhancement of cisplatin
efficacy by thalidomide in a 9L rat gliosarcoma model. J
Neurooncol. 85:181–189. 2007. View Article : Google Scholar
|
|
15
|
Shen Y, Li S, Wang X, Wang M, Tian Q and
Yang J, Wang J, Wang B, Liu P and Yang J: Tumor vasculature
remolding by thalidomide increases delivery and efficacy of
cisplatin. J Exp Clin Cancer Res. 38:4272019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang J, Yu J, Wang J, Ni X, Sun Z, Sun W,
Sun S and Lu Y: Thalidomide combined with chemo-radiotherapy for
treating esophageal cancer: A randomized controlled study. Oncol
Lett. 18:804–813. 2019.
|
|
17
|
Khezri MR, Jafari R, Yousefi K and
Zolbanin NM: The PI3K/AKT signaling pathway in cancer: Molecular
mechanisms and possible therapeutic interventions. Exp Mol Pathol.
127:1047872022. View Article : Google Scholar
|
|
18
|
Jin W: Role of JAK/STAT3 signaling in the
regulation of metastasis, the transition of cancer stem cells, and
chemoresistance of cancer by epithelial-mesenchymal transition.
Cells. 9:2172020. View Article : Google Scholar
|
|
19
|
Zheng X, Zhu Y, Wang X, Hou Y and Fang Y:
Silencing of ITGB6 inhibits the progression of cervical carcinoma
via regulating JAK/STAT3 signaling pathway. Ann Transl Med.
9:8032021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang L, Wu J, Ling MT, Zhao L and Zhao
KN: The role of the PI3K/Akt/mTOR signalling pathway in human
cancers induced by infection with human papillomaviruses. Mol
Cancer. 14:872015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kian MM, Salemi M, Bahadoran M, Haghi A,
Dashti N, Mohammadi S, Rostami S, Chahardouli B, Babakhani D and
Nikbakht M: Curcumin combined with thalidomide reduces expression
of STAT3 and Bcl-xL, leading to apoptosis in acute myeloid leukemia
cell lines. Drug Des Devel Ther. 14:185–194. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sun X, Xu Y, Wang Y, Chen Q, Liu L and Bao
Y: Synergistic inhibition of thalidomide and icotinib on human
non-small cell lung carcinomas through ERK and AKT signaling. Med
Sci Monit. 24:3193–3203. 2018. View Article : Google Scholar
|
|
23
|
Lee JH, Chung KS, Lee HH, Ko D, Kang M,
Yoo H, Ahn J, Lee JY and Lee KT: Improved tumor-suppressive effect
of OZ-001 combined with cisplatin mediated by mTOR/p70S6K and STAT3
inactivation in A549 human lung cancer cells. Biomed Pharmacother.
142:1119612021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang Y, Yang Z, Zhang R, Jia C, Mao R,
Mahati S, Zhang Y, Wu G, Sun YN, Jia XY, et al: MiR-27a-3p enhances
the cisplatin sensitivity in hepatocellular carcinoma cells through
inhibiting PI3K/Akt pathway. Biosci Rep. 41:BSR201920072021.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xu F, Li Q, Wang Z and Cao X: Sinomenine
inhibits proliferation, migration, invasion and promotes apoptosis
of prostate cancer cells by regulation of miR-23a. Biomed
Pharmacother. 112:1085922019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhong WZ, Wang Q, Mao WM, Xu ST, Wu L,
Shen Y, Liu YY, Chen C, Cheng Y, Xu L, et al: Gefitinib versus
vinorelbine plus cisplatin as adjuvant treatment for stage II–IIIA
(N1-N2) EGFR-mutant NSCLC (ADJUVANT/CTONG1104): A randomised,
open-label, phase 3 study. Lancet Oncol. 19:139–148. 2018.
View Article : Google Scholar
|
|
28
|
Coen JJ, Zhang P, Saylor PJ, Lee CT, Wu
CL, Parker W, Lautenschlaeger T, Zietman AL, Efstathiou JA, Jani
AB, et al: Bladder preservation with twice-a-day radiation plus
fluorouracil/cisplatin or once daily radiation plus gemcitabine for
muscle-invasive bladder cancer: NRG/RTOG 0712-a randomized phase II
trial. J Clin Oncol. 37:44–51. 2019. View Article : Google Scholar
|
|
29
|
Kitagawa R, Katsumata N, Shibata T, Kamura
T, Kasamatsu T, Nakanishi T, Nishimura S, Ushijima K, Takano M,
Satoh T and Yoshikawa H: Paclitaxel plus carboplatin versus
paclitaxel plus cisplatin in metastatic or recurrent cervical
cancer: The open-label randomized phase III trial JCOG0505. J Clin
Oncol. 33:2129–2135. 2015. View Article : Google Scholar
|
|
30
|
Chen L, Qiu X, Wang R and Xie X: The
efficacy and safety of docetaxel plus thalidomide vs. docetaxel
alone in patients with androgen-independent prostate cancer: A
systematic review. Sci Rep. 4:48182014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li L and Huang XE: Thalidomide combined
with chemotherapy in treating patients with advanced lung cancer.
Asian Pac J Cancer Prev. 17:2583–2585. 2016.PubMed/NCBI
|
|
32
|
Downs LS Jr, Rogers LM, Yokoyama Y and
Ramakrishnan S: Thalidomide and angiostatin inhibit tumor growth in
a murine xenograft model of human cervical cancer. Gynecol Oncol.
98:203–210. 2005. View Article : Google Scholar
|
|
33
|
Mohanty S, Huang J and Basu A: Enhancement
of cisplatin sensitivity of cisplatin-resistant human cervical
carcinoma cells by bryostatin 1. Clin Cancer Res. 11:6730–6737.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hernandez MO, Fulco TO, Pinheiro RO,
Pereira RM, Redner P, Sarno EN, Lopes UG and Sampaio EP:
Thalidomide modulates Mycobacterium leprae-induced NF-κB pathway
and lower cytokine response. Eur J Pharmacol. 670:272–279. 2011.
View Article : Google Scholar
|
|
35
|
Che Y, Li Y, Zheng F, Zou K, Li Z, Chen M,
Hu S, Tian C, Yu W, Guo W, et al: TRIP4 promotes tumor growth and
metastasis and regulates radiosensitivity of cervical cancer by
activating MAPK, PI3K/AKT, and hTERT signaling. Cancer Lett.
452:1–13. 2019. View Article : Google Scholar
|
|
36
|
Yan CM, Zhao YL, Cai HY, Miao GY and Ma W:
Blockage of PTPRJ promotes cell growth and resistance to 5-FU
through activation of JAK1/STAT3 in the cervical carcinoma cell
line C33A. Oncol Rep. 33:1737–1744. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Morgan EL, Chen Z and Waes CV: Regulation
of NFκB signalling by ubiquitination: A potential therapeutic
target in head and neck squamous cell carcinoma? Cancers (Basel).
12:28772020. View Article : Google Scholar
|
|
38
|
Morgan EL and Macdonald A: Autocrine STAT3
activation in HPV positive cervical cancer through a virus-driven
Rac1-NFκB-IL-6 signalling axis. PLoS Pathog. 15:e10078352019.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cochicho D, Esteves S, Rito M, Silva F,
Martins L, Montalvão P, Cunha M, Magalhães M, da Costa RMG and
Felix A: PIK3CA gene mutations in HNSCC: Systematic review and
correlations with HPV status and patient survival. Cancers (Basel).
14:12862022. View Article : Google Scholar : PubMed/NCBI
|