Introduction
Pancreatic cancer is a highly metastatic cancer with
a poor prognosis, causing >300,000 deaths annually (1). Currently, gemcitabine and
5-fluorouracil are the standard chemotherapy regimens for
pancreatic cancer, and combinations such as gemcitabine plus
nanoparticle albumin-bound paclitaxel and FOLFIRINOX (5-FU,
leucovorin, irinotecan, oxaliplatin) therapy are also used.
However, the response rate to chemotherapy is very low, with a
5-year survival rate of <10% (2,3).
This is due to the low drug transferability into the tumor, because
the blood flow in the pancreas and its tumor are very low and, the
formation of stroma around the tumor forms a barrier (4,5).
Nitric oxide (NO) is an important biosignaling
molecule that regulates various physiological and pathological
responses, and is involved in maintaining blood pressure (6), balancing thrombus and thrombolytic
homeostasis (7) and suppressing
inflammatory responses (8). On the
other hand, high concentrations of NO act in an inhibitory manner
against the growth of cancer cells. In the past two decades,
various NO donor drugs have been synthesized and have attracted
attention for their anti-malignant tumor effects. NO-donating
nonsteroidal anti-inflammatory drugs (NO-NSAIDs),
(Z)-1-[N-(2-aminoethyl)-N-(2-ammonioethyl) amino]
diazen-1-ium-1,2-diolate (DETA/NONOate), and sodium nitroprusside
(SNP) induce apoptotic cell death (9–11),
and S-nitroso-N-acetyl-DL-penicillamine (SNAP) induces apoptotic
and necrotic cell growth (12). In
addition, O2−3-aminopropyl diazeniumdiolate has
been reported to inhibit tumor invasion and metastasis (13). S-nitrosylated human serum albumin
has been reported to shrink peritumoral stroma (14). Thus, although various efficacies of
NO donor drugs have been reported to date, none have reached the
stage of clinical use. One of the reasons for this is that the
half-life of NO or NO donor drugs is very short, and its effects
are transient.
Phenylbutyrate (PB) is an orphan drug used for the
treatment of urea cycle disorders. Previously, it was determined
that PB binds to human serum albumin (HSA) with a high affinity
(15,16). By binding drugs and endogenous
substances such as fatty acids, HSA is able to maintain their blood
retention and control tissue distribution. Recently, a drug
delivery system utilizing this property was developed. Detemir and
degludec are insulin analogues and liraglutide is a glucagon-like
peptide 1, which are acylated with fatty acids. Binding of the
fatty acid moieties of these to HSA improves their kinetic
properties without affecting the affinity for their receptors
(17–19). In fact, they have been reported to
exhibit a significant sustainable pharmacodynamic effect due to
protraction of the half-life in blood in clinical use. Moreover, in
tumor tissue, vascular permeability is significantly higher than in
normal tissue. In addition, because the lymphatic system is not
well developed, substances that reach the tumor tissue accumulate
(20). This characteristic is
called the enhanced permeation and retention (EPR) effect and is an
important factor for passive targeting of cancer cells. Therefore,
macromolecules such as HSA are more likely to flow out from tumor
blood vessels. Kinoshita et al reported that S-nitrosylated
HSA tends to accumulate in tumor tissue (14). These findings suggest that the
nitrated form of phenylbutyrate (NPB) and HSA complex selectively
migrates to and accumulates in tumors.
In the present study, to develop a sustained NO
donor drug with an antitumor effect for treatment of pancreatic
cancer, NPB based on chlorambucil, a PB analogue, was synthesized
and the effects in vitro and in vivo were
investigated.
Materials and methods
Reagents and antibodies
PB was purchased from TCI (Shanghai) Development
Co., Ltd. Chlorambucil was obtained from Tokyo Chemical Industry
(TCI) Co., Ltd. Dihydroxy chlorambucil was purchased from SynZeal
Research Pvt Ltd. Z-VAD-FMK was obtained from Promega Corporation.
Diaminofluorescein-FM diacetate (DAF-FM DA) was purchased from
Goryo Chemical, Inc. Necrostatin and N-acetyl-L-cysteine (NAC) were
procured from FUJIFILM Wako Pure Chemical Corporation. GSK872 was
obtained from Abcam. Necrosulfonamide was purchased from Funakoshi
Co., Ltd. Antibodies against caspase-3 (product no. 9662S),
caspase-7 (product no. 9492S), poly (ADP-ribose) polymerase
(PARP)-1 (product no. 9542S), CHOP (product no. 2895S), β-actin
(cat. no. 3700S), and HRP-conjugated anti-rabbit (product no.
7074P2) were purchased from Cell Signaling Technology, Inc.
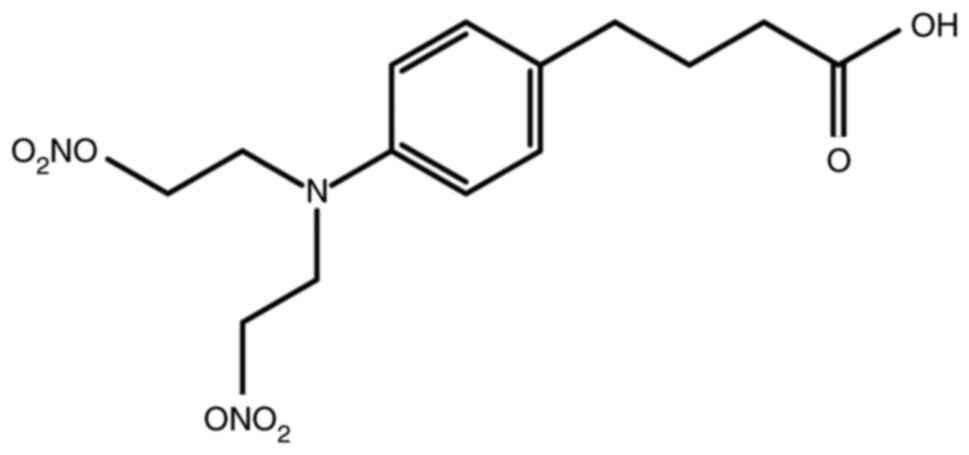
Synthesis of NPB
(4-(4-(bis(2-(nitrooxy)ethyl)amino)phenyl)butanoic acid)
A mixture of chlorambucil (250 mg, 0.822 mmol) and
AgNO3 (558 mg, 3.29 mmol) in CH3CN (16 ml)
was stirred at 70°C overnight. After being cooled to room
temperature, the suspension was filtered and the solvent was
evaporated. The residue was purified by flash column chromatography
on silica gel (DCM/MeOH, 99:1 to 92:8, v/v) to yield NPB (Fig. 1) (254 mg, 0.711 mmol, 86% yield) as
a pale yellow oil. 1H-NMR (500 MHz, CDCl3):
δ=7.10 (d, J=8.6 Hz, 2H), 6.66 (d, J=8.6 Hz, 2H), 4.60 (t, J=6.0
Hz, 4H), 3.71 (t, J=5.7 Hz, 4H), 2.59 (t, J=7.4 Hz, 2H), 2.37 (t,
J=7.4 Hz, 2H), 1.90-1.96 (m, 2H). 13C-NMR (126 MHz,
CDCl3): δ=179.8, 144.1, 131.2, 129.8, 112.9, 69.9, 48.9,
33.8, 33.2, 26.3. MS (ESI): m/z calculated for
C14H20N3O8
[M+H]+ 358.1250, found 358.1241.
Cell culture
The human pancreatic cancer cell lines, AsPC1
(CRL-1682) and BxPC3 (CRL-1687), were obtained from the American
Type Culture Collection. The cells were cultured in the recommended
medium, consisting of RPMI-1640 (FUJIFILM Wako Pure Chemical
Corporation), supplemented with 10% heat-inactivated fetal calf
serum (Capricorn Scientific GmbH), penicillin (100 U/ml), and
streptomycin (100 µg/ml) (FUJIFILM Wako Pure Chemical Corporation),
and grown at 37°C in 95% humidified air with 5% carbon dioxide.
Cell death
Live and apoptotic cell numbers were determined
using the MUSE Annexin V and Dead Cell kit (Luminex Corporation)
according to the manufacturer's instructions. Briefly, AsPC1 and
BxPC3 cells were seeded in a 6-well plate with 1×105
cells per well, and then, incubated at 37°C overnight. Following
incubation, the cells were exposed with various concentrations of
NPB (100, 300, and 500 µM) for 48 h or with 500 µM for 24, 48 and
72 h. Necrostatin, GSK872, Necrosulfonamide and NAC were used at
concentrations of 20, 1 and 1 µM and 1 mM respectively. After
treatment, the cells were washed twice with phosphate-buffered
saline (PBS), trypsinized, and mixed well with the Muse Annexin V
and Dead Cell Assay kit reagents. Reactions, which were conducted
in triplicate, and analyzed using a MUSE Cell Analyzer (Luminex
Corporation).
Effect of NPB on growth of
spheroids
AsPC1 and BxPC3 were seeded in round bottom 96-well
plates with 5×105 cells per well. After confirming the
formation of spheroids, 500 M of NPB was added to the each well,
and then incubated for 1 week at 37°C. Spheroid structure and the
spheroid area were analyzed by fluorescence microscopy.
NO release from NPB
The nitrite and nitrate (NOx) levels were quantified
by the Griess method [NO2/NO3 Assay kit-C II
(Colorimetric); Dojindo Laboratories, Inc.]. The NOx levels were
assessed at 0, 3, 24, 48, 72 and 96 h after 100 µM of NPB was
dissolved in PBS containing 10% MeOH. Samples were read at 540 nm
in a 96-well plate using a Spectra Microplate Auto reader (Bio-Rad
Model 680; Bio-Rad Laboratories, Inc.). For microscopic
observation, AsPC1 and BxPC3 cells were seeded in a 6-well plate
with 5×105 cells per well. Following overnight
incubation, the culture medium was replaced with 10 µM of DAF-FM
DA, and then cells were incubated at 37°C for 1 h. After
incubation, cells were washed with PBS three times. Following
washing, the cells were treated with 500 µM NPB for 5 min and
observed using a fluorescence microscope. For assessment of
fluorescence intensity of DAF-FM DA, cells were seeded in a black
96-well plate with 5×104 cells per well. Following
overnight incubation at 37°C, the culture medium was replaced with
10 µM DAF-FM DA, followed by incubation at 37°C for 1 h. Following
incubation, the cells were washed with PBS three times. After
washing, the cells were treated with 500 µM of NPB with PBS for 5
min and measured by using fluorescence plate reader (ex. 495 nm and
em. 515 nm).
Caspase-3/7 activity
BxPC3 cells were seeded in a black 96-well plate
with 1×104 cells per well. Following overnight
incubation at 37°C, the cells were treated with 500 µM of NPB for 6
and 24 h. Subsequently, 100 µl of Caspase-Glo 3/7 Reagent (Promega
Corporation) was added to each well. After mixing gently, the cells
were incubated at room temperature for 1 h. Finally, luminescence
of each sample was measured by a multifunctional microplate reader
(Infinite 200 Pro; Tecan Group, Ltd.).
Western blotting
BxPC3 cells were seeded in a 6-well plate with
5×105 cells per well. After overnight incubation at
37°C, the cells were treated 500 µM of NPB for 6 and 24 h.
Following treatment, the cells were lysed with RIPA buffer (Thermo
Fisher Scientific, Inc.), including a Protease/Phosphatase
Inhibitor Cocktail (Thermo Fisher Scientific, Inc.). Protein
concentration of each lysate was determined by BCA method. Aliquots
of protein (30–40 µg) were subjected to SDS-PAGE (12% of acrylamide
for PARP and 10% for caspase-3/7 and β-actin), transferred to a
polyvinylidene difluoride, (PVDF) membrane. After blocking with 5%
skim milk in PBS including 0.1% Tween-20 for 3 h at 37°C, the
membrane was processed for incubation with caspase-3, caspase-7,
PARP, CHOP or β-actin antibody (all 1:1,000) for 12 h at 4°C,
followed by anti-rabbit IgG antibodies for 1 h at 4°C. Membranes
were reacted with a chemiluminescence reagent (GE Healthcare UK
Ltd.; Cytiva). Band density values were normalized to β-actin.
Assessment of the intracellular
adenosine 5′-triphosphate (ATP) levels
BxPC3 cells were seeded in a white 96-well plate
with 1×104 cells per well. Following overnight
incubation at 37°C, cells were treated with 500 µM of NPB for 48 h,
and then 100 µl of intracellular ATP Reagent (TOYO-B Net Co., Ltd.)
was added to each well. After being gently mixed, luminescence of
each sample was measured using a plate-reading luminometer
(Infinite 200 Pro; Tecan Group, Ltd.). To correct for variations in
cell number, the protein content of each sample was measured using
the BCA protein assay kit (Thermo Fisher Scientific, Inc.) and the
ATP content was normalized to the protein content.
Cell cycle
For the cell cycle analysis, propidium iodide-based
nuclear staining was carried out using Muse Cell Cycle Kit (Luminex
Corporation) according to the manufacturer's protocol. Briefly,
AxPC1 and BxPC3 cells were cultured in a 6-well plate for 24 h, and
then treated with 500 µM of NPB for 24 h. After the treatment,
cells were fixed in 70% ethanol and stored at −20°C for at least 3
h. Fixed cells were washed with cold PBS and centrifuged at 300 × g
for 5 min and stained using a Muse™ Cell Cycle Kit for 30 min at
room temperature in dark conditions. After the staining, analysis
was performed by Muse Cell Analyzer (Luminex Corporation).
Animal studies
A total of 10 male, six-week-old BALB/c nude mice
(20–25 g) were obtained from Japan SLC, Inc., and raised in a
laminar mouse house, with 50±5% humidity, 25°C and a 12-h
light/dark cycle. The mice were fed standard rodent food and
mineral water. BxPC3 cells (5×106 cells/mouse) were s.c.
injected into the right flank. When tumor volumes reached 50
mm3, NPB (10 mg/kg) or saline were administered via the
tail vein once. Following treatment, tumor formation was monitored
by measuring the width and length of the mass, and the tumor volume
(TV) was calculated as follows: TV (mm3)=(L ×
W2)/2, with L as the longest and W as the shortest
radius of the tumor. Animals were euthanized by cervical
dislocation after seven weeks from the administration of NPB. The
death of the animals was confirmed by checking for cardiac arrest,
decreased body temperature, and no movement. All experiments were
approved by the Animal Ethics Committee of Sojo University
(Kumamoto, Japan) and carried out according to the Laboratory
Protocol for Animal Handling of Sojo University.
Statistical analyses
For continuous variables, unpaired Student's t-test
or one-way analysis of variance (one-way ANOVA) was performed. The
pairwise t-test with Holm's adjustment for post hoc test was
employed after one-way ANOVA. In addition, a linear mixed model was
used to analyze longitudinal data such as tumor volume and body
weight. The mean ± standard deviation was used to present
statistical outcome.
These analyses were performed using R version 4.0.3
(The R Foundation for Statistical Computing; http://www.r-project.org/foundation/). P<0.05 was
considered to indicate a statistically significant difference.
Results
Cell death-inducing effect of NPB
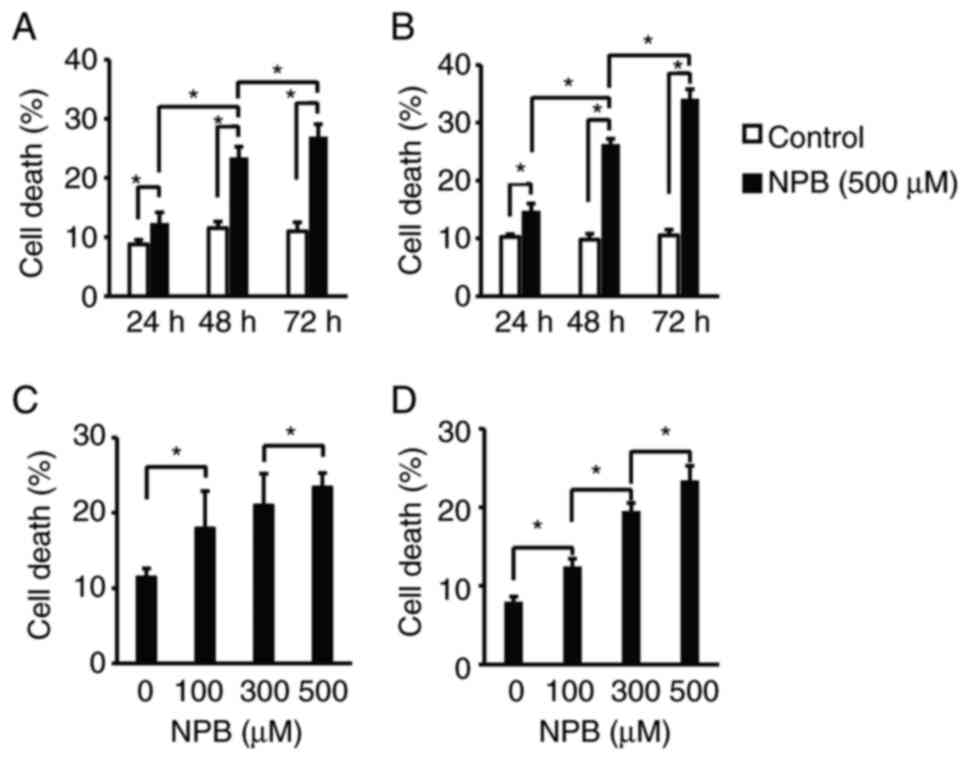
First, the effects of NPB on the cell death of human
pancreatic cancer cells were examined. Human pancreatic cancer cell
lines, AsPC1 and BxPC3, were exposed to 500 µM NPB for 24-72 h
(Fig. 2A and B) or 100-500 µM for
48 h (Fig. 2C and D) and the
number of Annexin-positive cells was determined. In both cell
lines, NPB significantly induced cell death in a time- and
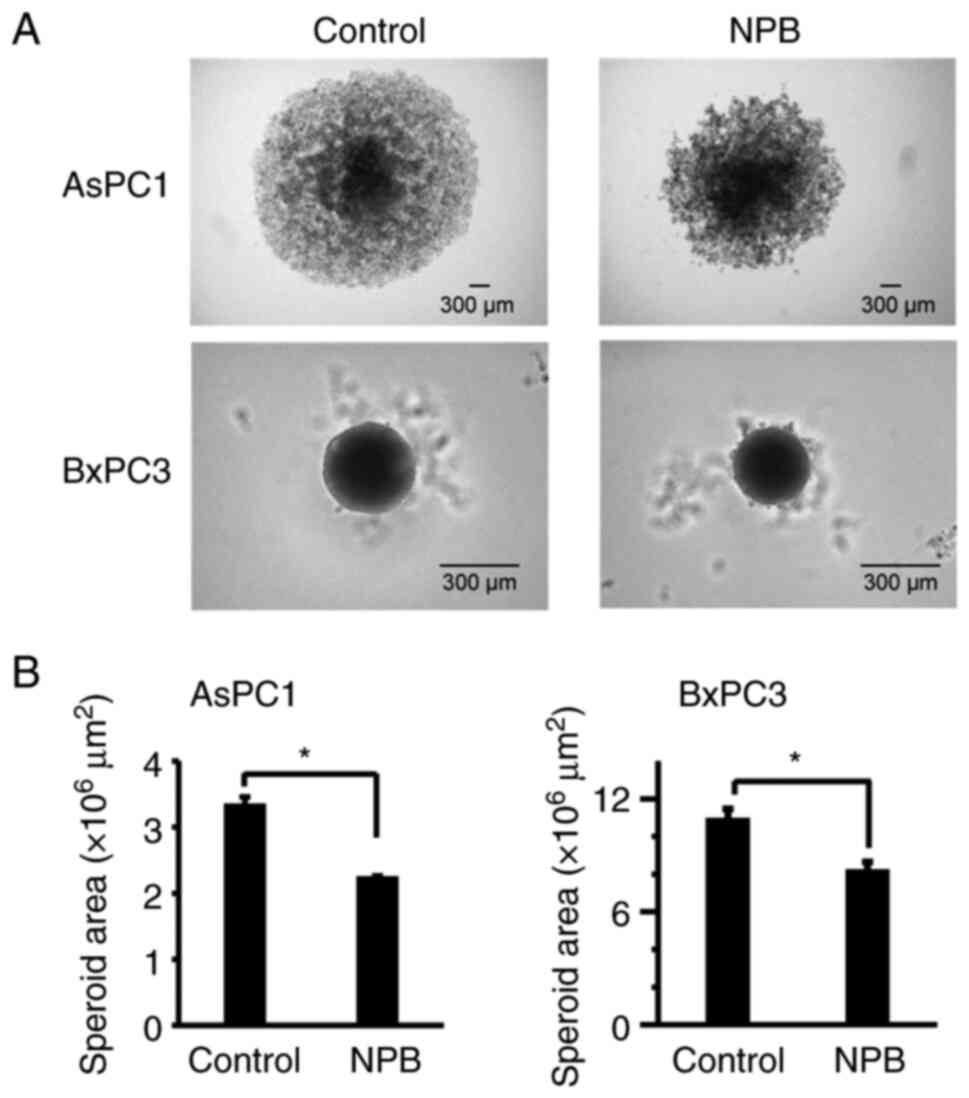
concentration-dependent manner. Next, the effect of NPB on the
growth inhibition of AsPC1 and BxPC3 spheroids was examined. Even 7
days after the addition of 500 µM of NPB, spheroid growth was
significantly inhibited in both cell lines compared to their
controls. In addition, disruption of spheroid surface structure was
observed in AsPC1 cells (Fig.
3).
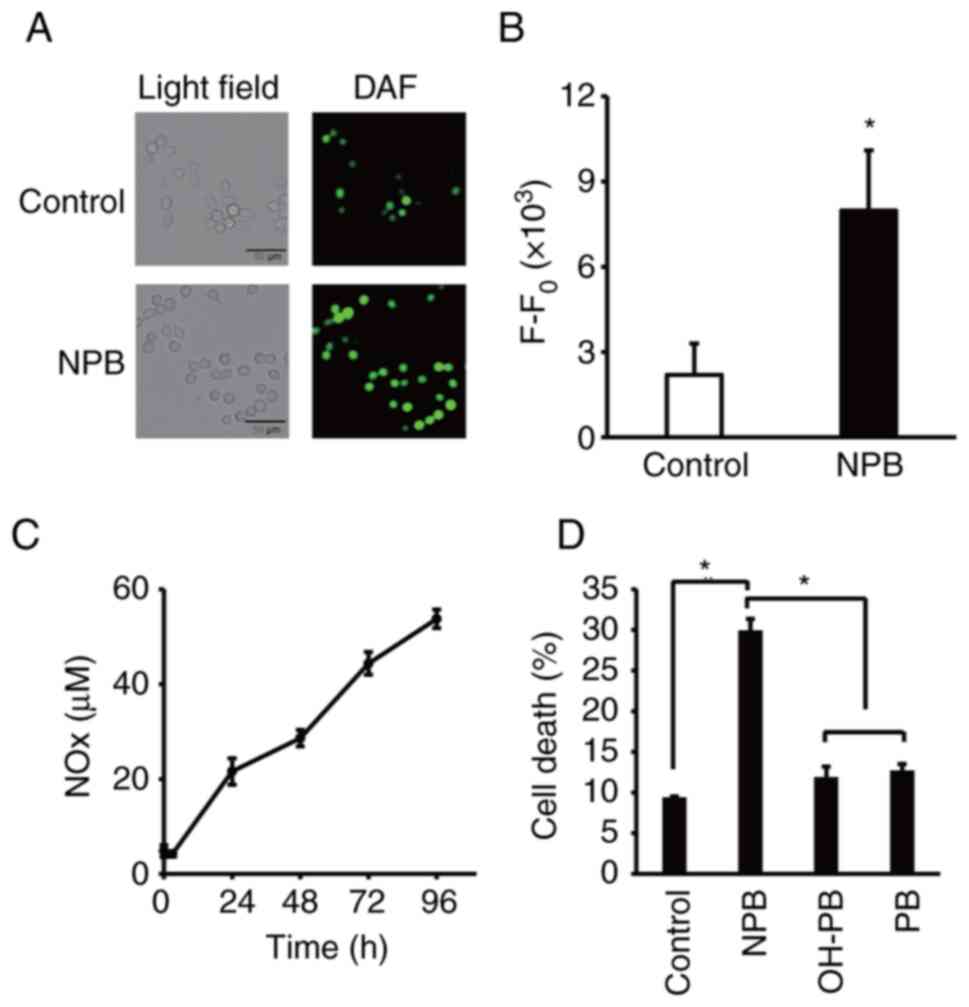
NO release from NPB
NO radical detecting agent, DAF-FM DA, was used to
examine intracellular NO release from NPB. As revealed, more cells
in the NPB-exposed cells had DAF-FM DA-derived fluorescence
compared with the control, indicating that NO radical is generated
within NPB-exposed cells (Fig. 4A and
B). It is known that a part of nitrite ions released from the
NO-donor compound are reduced to NO under anaerobic conditions in
tumors but are oxidized to nitrate ions under aerobic conditions.
Quantitative assessment of NOx released from NPB was performed
(Fig. 4C). Notably, increased NOx
was observed at least up to 96 h after dissolving NPB in PBS.
Moreover, no cell death-inducing effect was observed for OH-PB, in
which the NO2 moiety of NPB was replaced by an OH group,
and PB (Fig. 4D), suggesting that
NO released from NPB was mainly involved in the cell death-inducing
effect of NPB.
Cell death mechanism of NPB
Cell death is induced by various pathways, including
apoptosis and necrosis. To date, NO donors have been reported to
induce apoptosis (9–11,23–25).
To investigate the involvement of caspase, which plays a central
role in apoptosis, the cell death effects of NPB in the presence of
a caspase inhibitor, Z-VAD-FMK (Fig.
5A) were examined. The results revealed no significant effect
of Z-VAD-FMK on cell death induction by NPB. Assessment of
caspase-3/7 activity after NPB exposure exhibited no activation at
6 h or 24 h after the addition of NPB (Fig. 5B). Results of western blotting also
showed no degradation of PARP or caspase-3/7 (Fig. 5C). These results indicated that
apoptosis was not involved in the induction of cell death by NPB.
Necrosis is characterized by cell swelling and a decrease and
depletion of intracellular ATP (26). Thus, the amount of ATP after the
addition of NPB was determined. A total of 48 h after addition of
NPB, the amount of ATP was significantly decreased compared with
the control (Fig. 5D), suggesting
cell death was due to necrosis. Necrostatin, GSK872 and
Necrosulfonamide, which are necroptosis inhibitors, did not
suppress the cell death effect by NPB (Fig. S1).
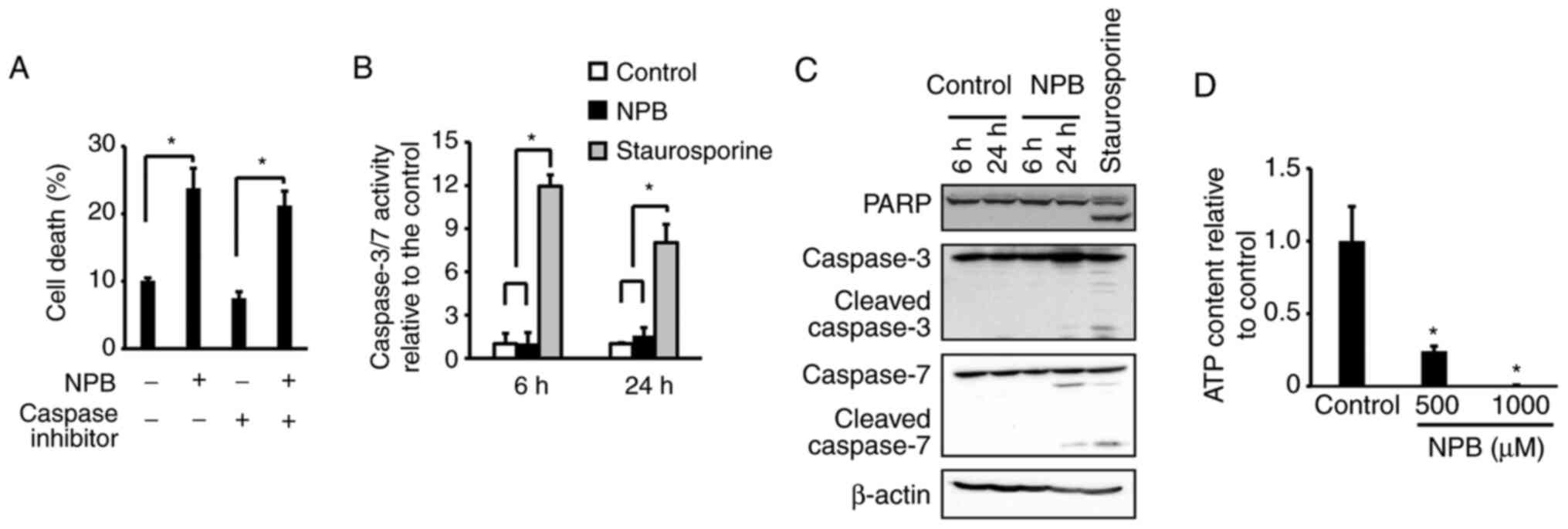 | Figure 5.Mechanism of NPB-induced cell death.
(A) Effect of Z-VAD-FMK, a pan-caspase inhibitor, on death of
NPB-treated BxPC3 cells. The cells were treated with 10 µM
Z-VAD-FMK and 500 µM NPB for 48 h. (B) Activation of caspase-3/7 in
BxPC3 cells after treatment with 500 µM NPB for 6 and 24 h. (C)
Western blot analysis of PARP, caspase-3, and caspase-7 in BxPC3
cells after treatment with 500 µM NPB for 6 and 24 h. (D) Content
of intracellular ATP in BxPC3 cells after treatment with 500 and
1,000 µM NPB for 48 h. Averages and SD of three separate
experiments are shown, *P<0.05). In A, B and D, data were
analyzed using one-way ANOVA and the pairwise t-test with Holm's
adjustment. NPB, nitrated form of phenylbutyrate; PARP, poly
(ADP-ribose) polymerase; ATP, adenosine 5′-triphosphate. |
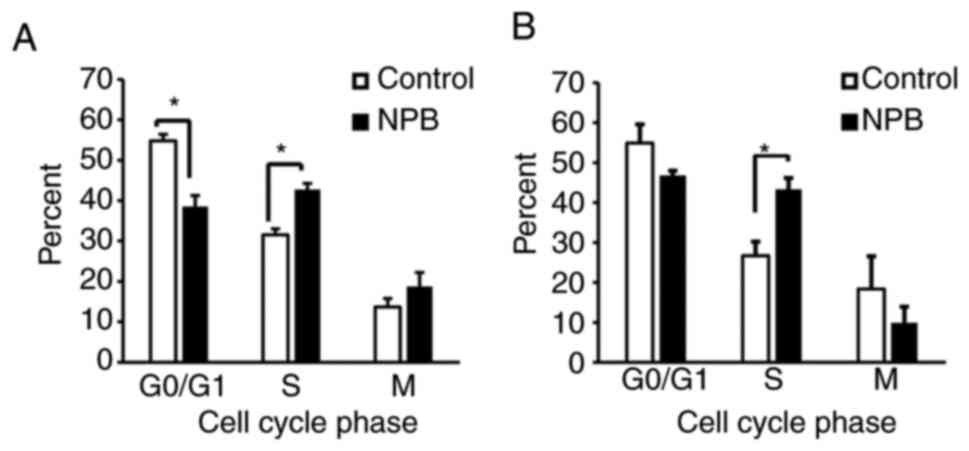
Effects of NPB on the cell cycle
Since NPB was observed to inhibit cell proliferation
(Fig. S2), the effect of NPB on
the cell cycle was examined and it was determined that cells
exposed to NPB for 24 h exhibited a significant accumulation of
S-phase cells compared with the control (Fig. 6). This indicated that NPB also
induced cell cycle arrest.
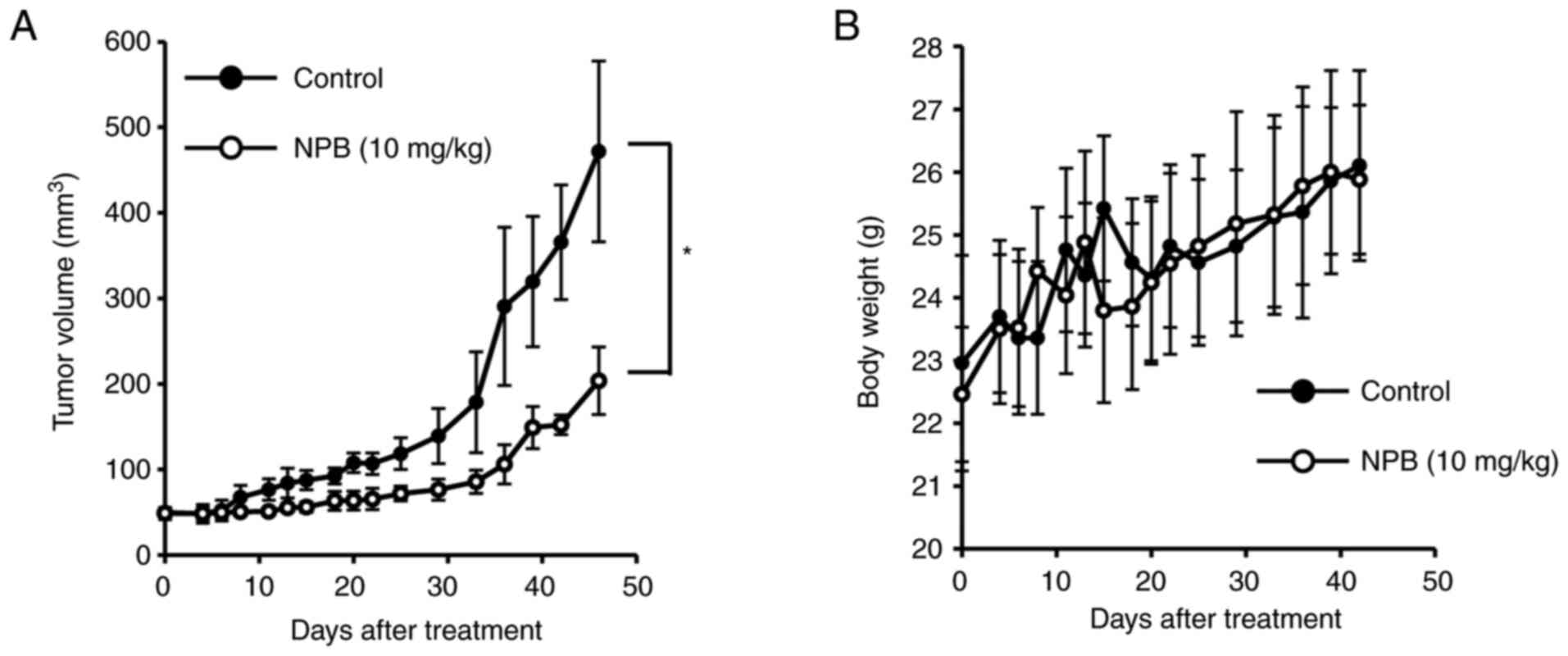
Antitumor effect of NPB in vivo
The antitumor effect of NPB was investigated in
vivo (Fig. 7). Tumor-bearing
mice with BxPC3 tumors (50 mm3) implanted subcutaneously
were prepared, and after a single dose of NPB (10 mg/kg) by
intravenous tail injection, the tumor volumes and body weights were
evaluated. There were no significant differences in body weight and
no mice succumbed during the observation period. Notably,
significant tumor suppression was observed even up to 7 weeks after
NPB administration compared with the control group.
Discussion
Pancreatic cancer is known to have a poor response
rate to chemotherapy due to low drug distribution caused by low
blood flow and abundant stroma around the tumor. In addition to the
vasodilation effect of NO, it has also been revealed to induce
cancer cell death, suppress stromal tissue fibrosis, and shrink
stromal cells themselves (21–25).
Therefore, NO donors are anticipated as new anticancer drugs for
the treatment of pancreatic cancer.
In vitro, NPB caused cell death in a time-
and concentration-dependent manner without activation of
caspase-3/7, degradation of PARP, indicating that apoptosis is not
the major pathway of cell death by NPB. On the other hand, NPB
caused a marked decrease in intracellular ATP, suggesting that
necrosis is mainly involved in cell death by NPB. In fact, in the
flow cytometric assay with Annexin, NPB increased the population of
late apoptosis, which is characteristic of necrotic cells (Fig. S3). Necrosis is recognized as
unregulated cell death, but recently, necroptosis, a form of
necrosis regulated by receptor-interacting protein kinase (26), has emerged as another mechanism of
cell death. However, significant suppressive effects of necroptosis
inhibitors (Necrostatin, GSK872 and Necrosulfonamide) were not
observed on the cell death effects by NPB (Fig. S1). Moreover, the factors that
caused the induction of cancer cell death by NPB were investigated.
Reactive oxygen oxide species (ROS) become more reactive nitrogen
species (RNS), by reacting with NO. RNS oxidizes and nitrates
biomolecules (27) such as nucleic
acids, proteins and lipids, causing various intracellular events
such as endoplasmic reticulum stress (28) and autophagy, followed by cell
death. No effect was identified using ROS scavenger, NAC, on the
effect of NPB, and the endoplasmic reticulum stress marker, CHOP,
was not observed in BxPC3 cells exposed to NPB (Fig. S4). NPB not only causes cell death
but also inhibits cell proliferation. In fact, it has been reported
that nitrated aspirin causes accumulation of S-phase cells
(29), and a similar tendency was
also observed in NPB. The fact that NPB exerts a cancer cell death
effect in a cell cycle-dependent manner suggests that the effect of
NPB is marked on cancer cells which are under active cell
proliferation. In addition, in the present study, toxicity to
non-tumor cells was not evaluated, but the cell cycle results
suggest that NPB has a selective effect on cancer cells with a fast
cell cycle. In an experiment using sodium nitrite, which has low
cell membrane permeability, sodium nitrite did not exhibit
significant toxicity to pancreatic cancer cells. This result
suggests that NO is released from NPB after NPB is uptaken into the
cell membrane (data not shown).
To determine a preclinical antitumor effect, the
effect of NPB in vivo was evaluated using a BxPC3 ×enograft
model. Single-dose administration of NPB significantly inhibited
tumor growth up to 7 weeks without no significant change in body
weight. In general, the retention of small molecule compounds in
blood is low. In fact, numerous chemotherapeutic drugs have caused
various side effects due to drug delivery to non-targeted tissues.
Therefore, focusing on the high affinity of PB to HSA (15), an NO donor compound, NPB, was newly
designed to bind to HSA. Notably, it was determined that NPB has an
equivalent HSA binding property as PB (Table SI). The binding of NPB to HSA is
also considered to be effective in selectively transporting drugs
to tumors using the EPR effect. Although the binding of NPB to
mouse albumin has not been confirmed, it is considered that NPB
also has high binding to mouse albumin because the homology of
mouse albumin with HSA is extremely high. Moreover, there is
another issue in NO donor compounds, which is that the half-life of
NO itself is also very short. Interestingly, NPB released NOx very
gradually even up to 96 h after dissolution, whereas numerous NO
compounds release most of their NOx immediately after dissolution
in aqueous solution (30–34). Combined with the prolonged
elimination half-life of NPB and the EPR effect by binding to HSA,
gradual NOx release, and the long-term growth inhibitory effect
observed in spheroid experiments, these findings demonstrated the
preclinical antitumor effect as observed in vivo.
In the present study, a novel nitric compound, NPB,
was successfully synthesized and it was revealed that NPB is a new
type of chemotherapeutic agent, unlike conventional nitro
compounds. The cell death-inducing effect of NPB on pancreatic
cancer cells is comparable or milder than that of other nitro
compounds. However, NPB is characterized by its affinity for human
serum albumin and the release of NOx is extremely slow. Therefore,
these are considered to be a great advantage of NPB in terms of
blood retention and tumor accumulation. Indeed, it was observed
that the antitumor effect of NPB on the xenograft lasted much
longer despite a single dose. Further detailed antitumor mechanisms
in vitro and in vivo are required for clinical
application.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by JSPS KAKENHI (grant no.
20K07193) and in part by Nagai Memorial Research Scholarship from
the Pharmaceutical Society of Japan.
Availability of data and materials
The data that support the findings of the present
study are available from the corresponding author, KY, upon
reasonable request.
Authors' contributions
TB contributed to the experiments, the design of
this study, data collection, interpretation, and wrote the initial
draft of the manuscript. KN contributed to the design of this
study, and data collection and interpretation, and wrote the
initial draft of the manuscript. SI contributed to the synthesis
and the structural validation of NPB. WA, IS, AU, NS and YI
contributed to data collection. TI contributed to the statistical
analysis. MO and KY contributed to the design of this study,
interpretation, and critically reviewed the manuscript. KN and KY
confirm the authenticity of all the raw data. All authors approved
the final version of the manuscript and agree to be accountable for
all aspects of the work in ensuring that questions related to the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Animal Care and Use Committee of Sojo University (Kumamoto, Japan)
and was carried out according to the Laboratory Protocol for Animal
Handling of Sojo University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
NO
|
nitric oxide
|
|
NPB
|
nitrated form of phenylbutyrate
|
|
NOx
|
nitrite and nitrate
|
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Le Large TY, Bijlsma MF, Kazemier G, van
Laarhoven HW, Giovannetti E and Jimenez CR: Key biological
processes driving metastatic spread of pancreatic cancer as
identified by multi-omics studies. Semin Cancer Biol. 44:153–169.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ercan G, Karlitepe A and Ozpolat B:
Pancreatic cancer stem cells and therapeutic approaches. Anticancer
Res. 37:2761–2775. 2017.PubMed/NCBI
|
|
5
|
Hwang RF, Moore T, Arumugam T,
Ramachandran V, Amos KD, Rivera A, Ji B, Evans DB and Logsdon CD:
Cancer-associated stromal fibroblasts promote pancreatic tumor
progression. Cancer Res. 68:918–926. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rees DD, Palmer RM and Moncada S: Role of
endothelium-derived nitric oxide in the regulation of blood
pressure. Proc Natl Acad Sci USA. 86:3375–3378. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Loscalzo J: Nitric oxide insufficiency,
platelet activation, and arterial thrombosis. Circ Res. 88:756–762.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Korhonen R, Lahti A, Kankaanranta H and
Moilanen E: Nitric oxide production and signaling in inflammation.
Curr Drug Targets Inflamm Allergy. 4:471–479. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Williams JL, Borgo S, Hasan I, Castillo E,
Traganos F and Rigas B: Nitric oxide-releasing nonsteroidal
anti-inflammatory drugs (NSAIDs) alter the kinetics of human colon
cancer cell lines more effectively than traditional NSAIDs:
Implications for colon cancer chemoprevention. Cancer Res.
61:3285–3289. 2001.PubMed/NCBI
|
|
10
|
Pervin S, Singh R, Gau CL, Edamatsu H and
Tamanoi F: Potentiation of nitric oxide-induced apoptosis of
MDA-MB-468 cells by farnesyltransferase inhibitor: Implications in
breast cancer. Cancer Res. 61:4701–4706. 2001.PubMed/NCBI
|
|
11
|
Yang L, Lan C, Fang Y, Zhang Y, Wang J,
Guo J, Wan S, Yang S, Wang R and Fang D: Sodium nitroprusside (SNP)
sensitizes human gastric cancer cells to TRAIL-induced apoptosis.
Int Immunopharmacol. 17:383–389. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mitrovic B, Ignarro LJ, Vinters HV, Akers
MA, Schmid I, Uittenbogaart C and Merrill JE: Nitric oxide induces
necrotic but not apoptotic cell death in oligodendrocytes.
Neuroscience. 65:531–539. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kang F, Zhu J, Wu J, Lv T, Xiang H, Tian
J, Zhang Y and Huang Z: O 2-3-Aminopropyl diazeniumdiolates
suppress the progression of highly metastatic triple-negative
breast cancer by inhibition of microvesicle formation via nitric
oxide-based epigenetic regulation. Chem Sci. 9:6893–6898. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kinoshita R, Ishima Y, Ikeda M,
Kragh-Hansen U, Fang J, Nakamura H, Chuang VT, Tanaka R, Maeda H,
Kodama A, et al: S-Nitrosated human serum albumin dimer as novel
nano-EPR enhancer applied to macromolecular anti-tumor drugs such
as micelles and liposomes. J Control Release. 217:1–9. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Enokida T, Yamasaki K, Okamoto Y, Taguchi
K, Ishiguro T, Maruyama T, Seo H and Otagiri M: Tyrosine411 and
Arginine410 of human serum albumin play an important role in the
binding of sodium 4-phenylbutyrate to site II. J Pharm Sci.
105:1987–1994. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Krach-Hansen U, Chuang VT and Otagiri M:
Practical aspects of the ligand-binding and enzymatic properties of
human serum albumin. Biol Pharm Bull. 25:695–704. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Heise T and Mathieu C: Impact of the mode
of protraction of basal insulin therapies on their pharmacokinetic
and pharmacodynamic properties and resulting clinical outcomes.
Diabetes Obes Metab. 19:3–12. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Heinemann L, Sinha K, Weyer C, Loftager M,
Hirschberger S and Heise T: Time-action profile of the soluble,
fatty acid acylated, long-acting insulin analogue NN304. Diabet
Med. 16:332–338. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Knudsen LB, Nielsen PF, Huusfeldt PO,
Johansen NL, Madsen K, Pedersen FZ, Thøgersen H, Wilken M and
Agersø H: Potent derivatives of glucagon-like peptide-1 with
pharmacokinetic properties suitable for once daily administration.
J Med Chem. 43:1664–1669. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Matsumura Y and Maeda H: A new concept for
macromolecular therapeutics in cancer chemotherapy: Mechanism of
tumoritropic accumulation of proteins and the antitumor agent
smancs. Cancer Res. 46:6387–6392. 1986.PubMed/NCBI
|
|
21
|
Durante M, Frosini M, Fusi F, Neri A,
Sticozzi C and Saponara S: In vitro vascular toxicity assessment of
NitDOX, a novel NO-releasing doxorubicin. Eur J Pharmacol.
880:1731642020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Maeda H, Akaike T, Yoshida M and Suga M:
Multiple functions of nitric oxide in pathophysiology and
microbiology: Analysis by a new nitric oxide scavenger. J Leukoc
Biol. 56:588–592. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang Z, Liu L, Chen J, Cao M and Wang J:
JS-K as a nitric oxide donor induces apoptosis via the
ROS/Ca2+/caspase-mediated mitochondrial pathway in HepG2 cells.
Biomed Pharmacother. 107:1385–1392. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Millet A, Bettaieb A, Renaud F, Prevotat
L, Hammann A, Solary E, Mignotte B and Jeannin JF: Influence of the
nitric oxide donor glyceryl trinitrate on apoptotic pathways in
human colon cancer cells. Gastroenterology. 123:235–246. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu L, Li T, Tan J, Fu J, Guo Q, Ji H and
Zhang Y: NG as a novel nitric oxide donor induces apoptosis by
increasing reactive oxygen species and inhibiting mitochondrial
function in MGC803 cells. Int Immunopharmacol. 23:27–36. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kroemer G, Galluzzi L, Vandenabeele P,
Abrams J, Alnemri ES, Baehrecke EH, Blagosklonny MV, El-Deiry WS,
Golstein P, Green DR, et al: Classification of cell death:
Recommendations of the nomenclature committee on cell death 2009.
Cell Death Differ. 16:3–11. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Eiserich JP, Patel RP and O'Donnell VB:
Pathophysiology of nitric oxide and related species: Free radical
reactions and modification of biomolecules. Mol Aspects Med.
19:221–357. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gotoh T and Mori M: Nitric oxide and
endoplasmic reticulum stress. Arterioscler Thromb Vasc Biol.
26:1439–1446. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kashfi K, Rayyan Y, Qiao LL, Williams JL,
Chen J, Del Soldato P, Traganos F, Rigas B and Ryann Y: Nitric
oxide-donating nonsteroidal anti-inflammatory drugs inhibit the
growth of various cultured human cancer cells: Evidence of a tissue
type-independent effect. J Pharmacol Exp Ther. 303:1273–1282. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dunlap T, Abdul-Hay SO, Chandrasena RE,
Hagos GK, Sinha V, Wang Z, Wang H and Thatcher GR: Nitrates and
NO-NSAIDs in cancer chemoprevention and therapy: In vitro evidence
querying the NO donor functionality. Nitric Oxide. 19:115–124.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wu W, Gaucher C, Fries I, Hu XM, Maincent
P and Sapin-Minet A: Polymer nanocomposite particles of
S-nitrosoglutathione: A suitable formulation for protection and
sustained oral delivery. Int J Pharm. 495:354–361. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang C, Hwang HH, Jeong S, Seo D, Jeong Y,
Lee DY and Lee K: Inducing angiogenesis with the controlled release
of nitric oxide from biodegradable and biocompatible copolymeric
nanoparticles. Int J Nanomedicine. 13:6517–6530. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Laschak M, Spindler KD, Schrader AJ,
Hessenauer A, Streicher W, Schrader M and Cronauer MV: JS-K, a
glutathione/glutathione S-transferase-activated nitric oxide
releasing prodrug inhibits androgen receptor and WNT-signaling in
prostate cancer cells. BMC Cancer. 12:1302012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nishi K, Imoto S, Beppu T, Uchibori S,
Yano A, Ishima YU, Ikeda T, Tsukigawa K, Otagiri M and Yamasaki K:
The nitrated form of nateglinide induces apoptosis in human
pancreatic cancer cells through a caspase-dependent mechanism.
Anticancer Res. 42:1333–1338. 2022. View Article : Google Scholar : PubMed/NCBI
|