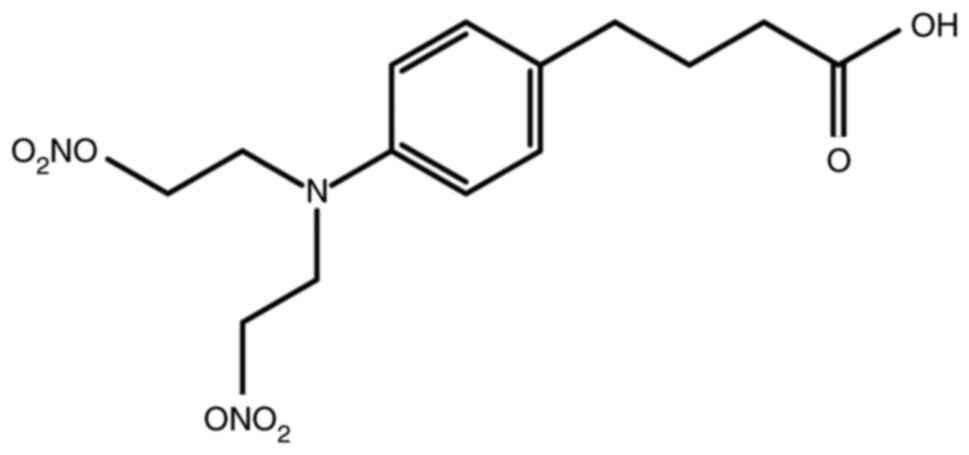
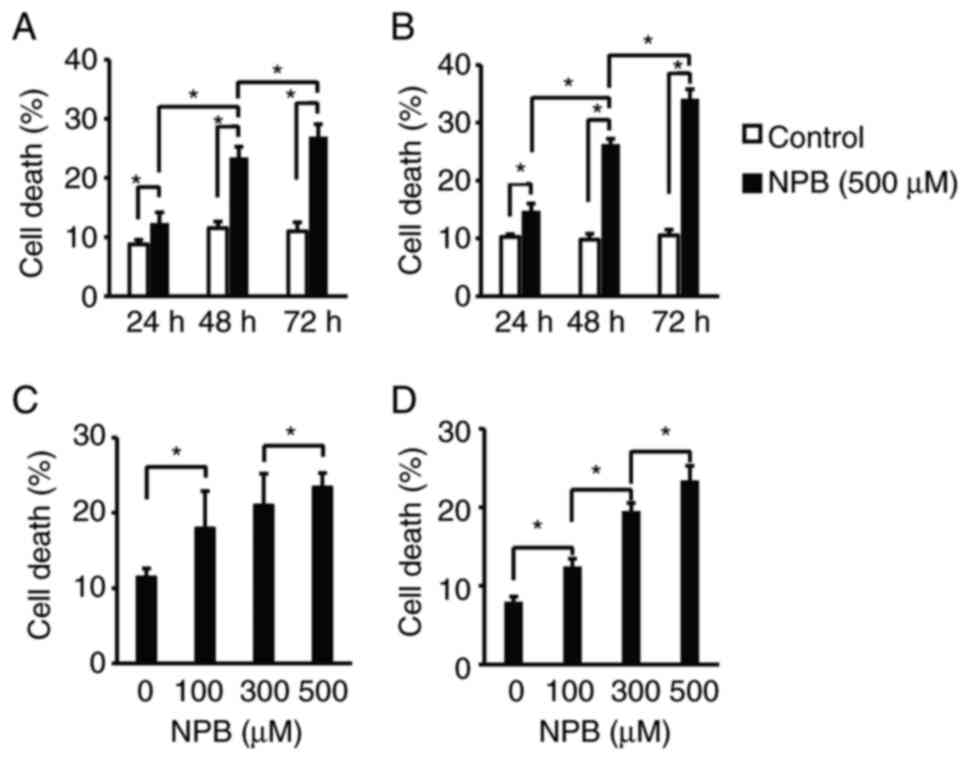
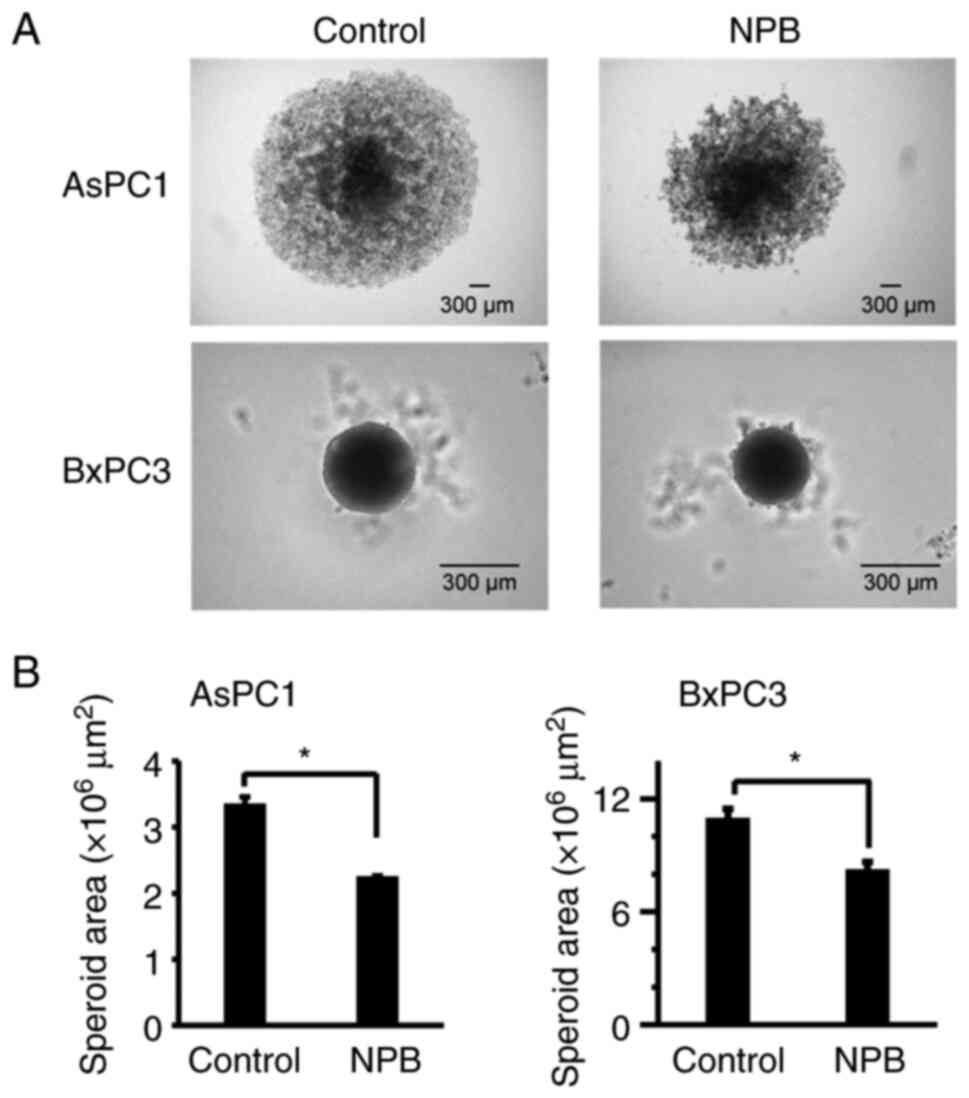
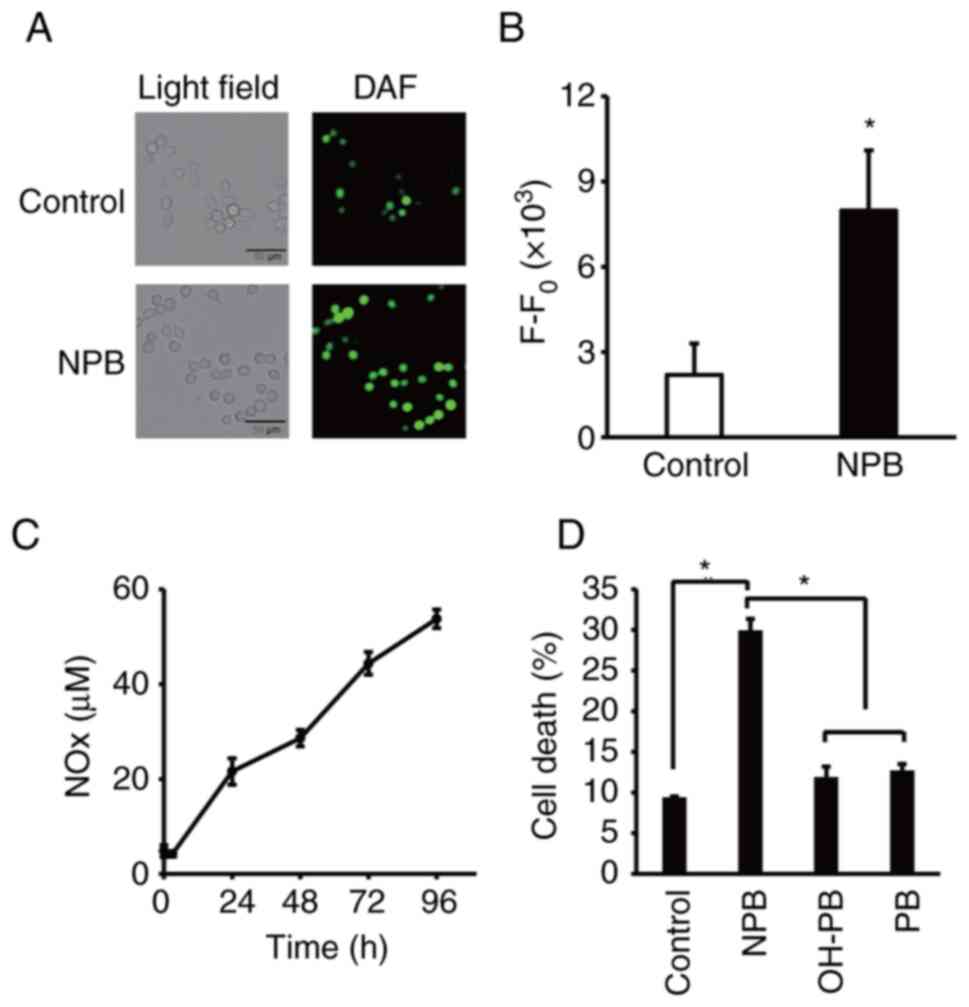
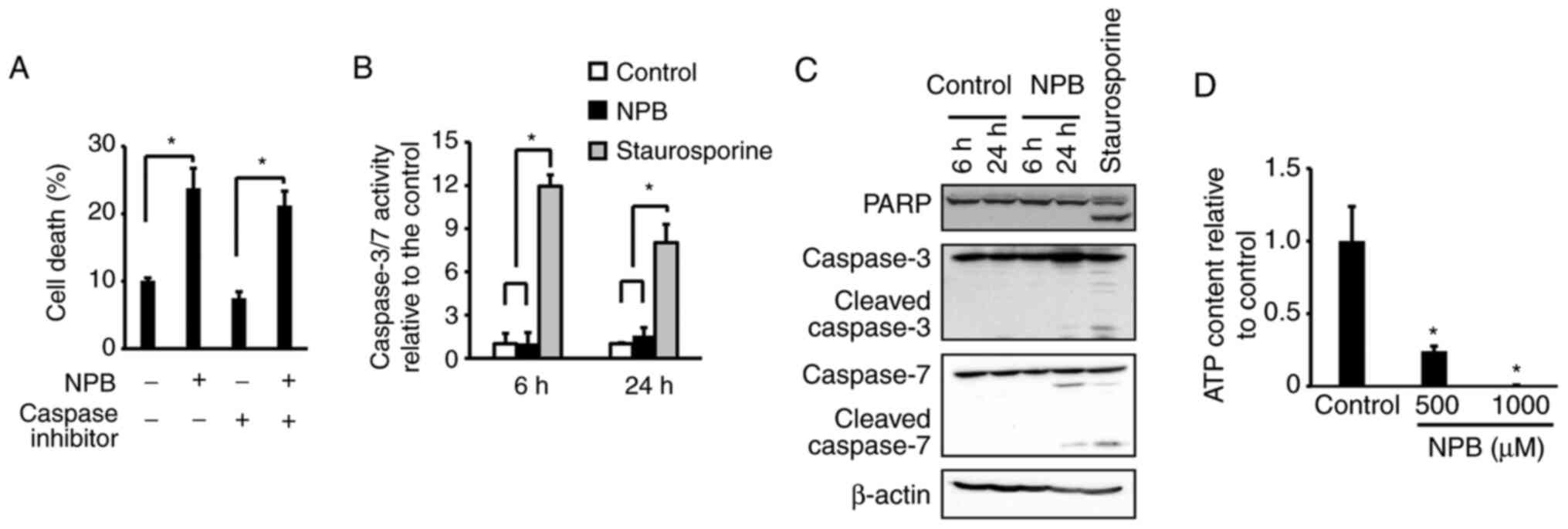
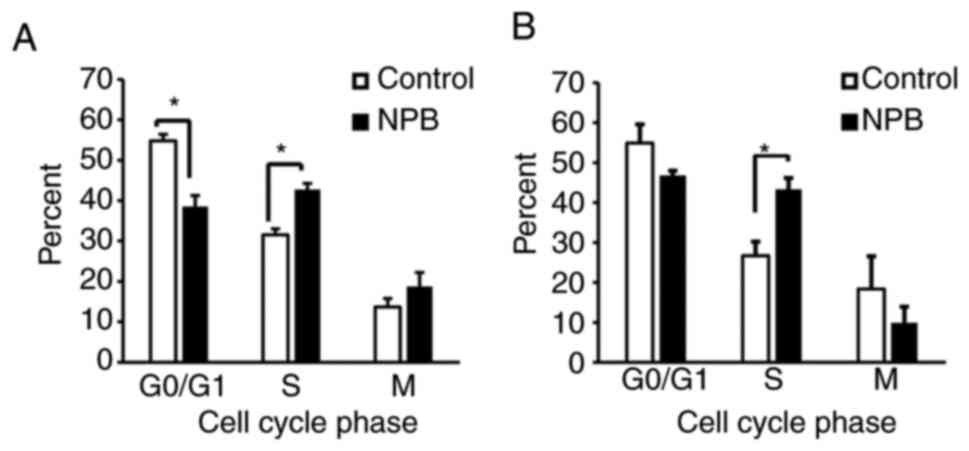
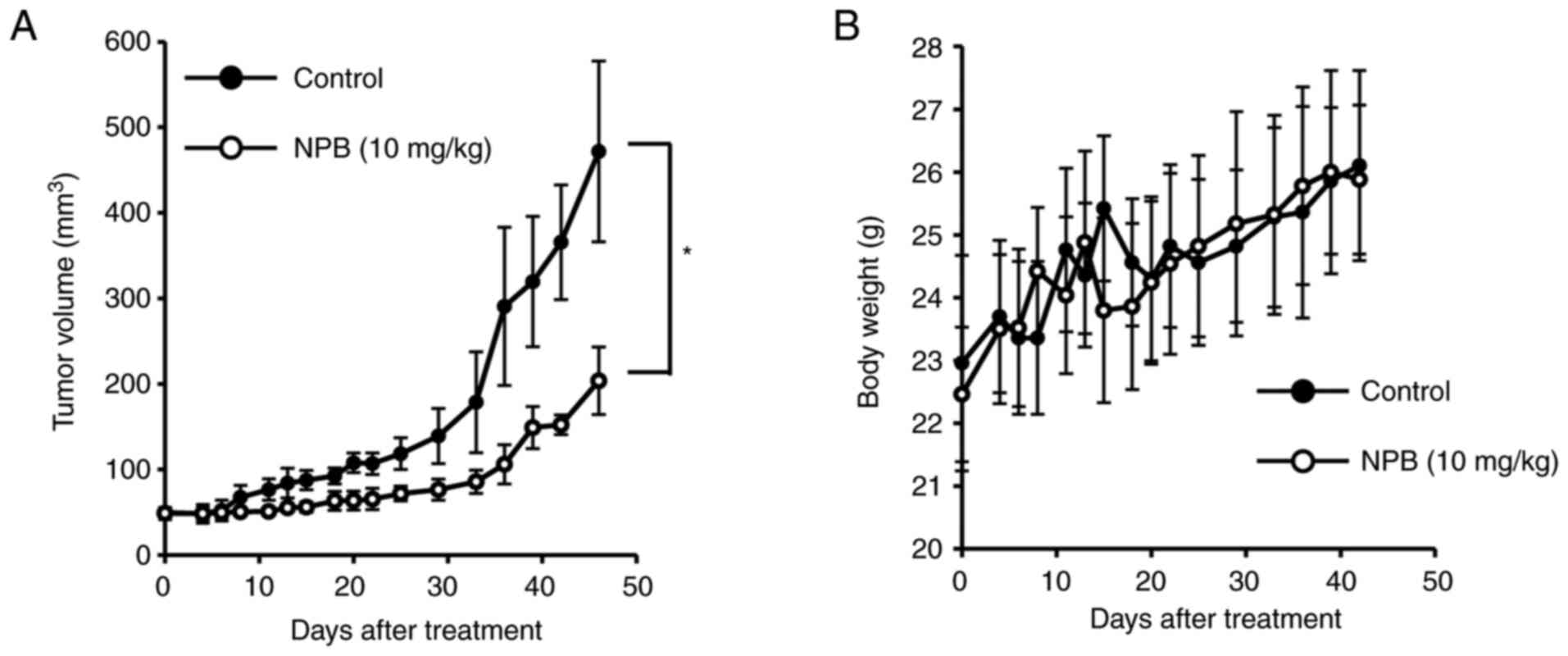
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Le Large TY, Bijlsma MF, Kazemier G, van
Laarhoven HW, Giovannetti E and Jimenez CR: Key biological
processes driving metastatic spread of pancreatic cancer as
identified by multi-omics studies. Semin Cancer Biol. 44:153–169.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ercan G, Karlitepe A and Ozpolat B:
Pancreatic cancer stem cells and therapeutic approaches. Anticancer
Res. 37:2761–2775. 2017.PubMed/NCBI
|
|
5
|
Hwang RF, Moore T, Arumugam T,
Ramachandran V, Amos KD, Rivera A, Ji B, Evans DB and Logsdon CD:
Cancer-associated stromal fibroblasts promote pancreatic tumor
progression. Cancer Res. 68:918–926. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rees DD, Palmer RM and Moncada S: Role of
endothelium-derived nitric oxide in the regulation of blood
pressure. Proc Natl Acad Sci USA. 86:3375–3378. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Loscalzo J: Nitric oxide insufficiency,
platelet activation, and arterial thrombosis. Circ Res. 88:756–762.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Korhonen R, Lahti A, Kankaanranta H and
Moilanen E: Nitric oxide production and signaling in inflammation.
Curr Drug Targets Inflamm Allergy. 4:471–479. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Williams JL, Borgo S, Hasan I, Castillo E,
Traganos F and Rigas B: Nitric oxide-releasing nonsteroidal
anti-inflammatory drugs (NSAIDs) alter the kinetics of human colon
cancer cell lines more effectively than traditional NSAIDs:
Implications for colon cancer chemoprevention. Cancer Res.
61:3285–3289. 2001.PubMed/NCBI
|
|
10
|
Pervin S, Singh R, Gau CL, Edamatsu H and
Tamanoi F: Potentiation of nitric oxide-induced apoptosis of
MDA-MB-468 cells by farnesyltransferase inhibitor: Implications in
breast cancer. Cancer Res. 61:4701–4706. 2001.PubMed/NCBI
|
|
11
|
Yang L, Lan C, Fang Y, Zhang Y, Wang J,
Guo J, Wan S, Yang S, Wang R and Fang D: Sodium nitroprusside (SNP)
sensitizes human gastric cancer cells to TRAIL-induced apoptosis.
Int Immunopharmacol. 17:383–389. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mitrovic B, Ignarro LJ, Vinters HV, Akers
MA, Schmid I, Uittenbogaart C and Merrill JE: Nitric oxide induces
necrotic but not apoptotic cell death in oligodendrocytes.
Neuroscience. 65:531–539. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kang F, Zhu J, Wu J, Lv T, Xiang H, Tian
J, Zhang Y and Huang Z: O 2-3-Aminopropyl diazeniumdiolates
suppress the progression of highly metastatic triple-negative
breast cancer by inhibition of microvesicle formation via nitric
oxide-based epigenetic regulation. Chem Sci. 9:6893–6898. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kinoshita R, Ishima Y, Ikeda M,
Kragh-Hansen U, Fang J, Nakamura H, Chuang VT, Tanaka R, Maeda H,
Kodama A, et al: S-Nitrosated human serum albumin dimer as novel
nano-EPR enhancer applied to macromolecular anti-tumor drugs such
as micelles and liposomes. J Control Release. 217:1–9. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Enokida T, Yamasaki K, Okamoto Y, Taguchi
K, Ishiguro T, Maruyama T, Seo H and Otagiri M: Tyrosine411 and
Arginine410 of human serum albumin play an important role in the
binding of sodium 4-phenylbutyrate to site II. J Pharm Sci.
105:1987–1994. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Krach-Hansen U, Chuang VT and Otagiri M:
Practical aspects of the ligand-binding and enzymatic properties of
human serum albumin. Biol Pharm Bull. 25:695–704. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Heise T and Mathieu C: Impact of the mode
of protraction of basal insulin therapies on their pharmacokinetic
and pharmacodynamic properties and resulting clinical outcomes.
Diabetes Obes Metab. 19:3–12. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Heinemann L, Sinha K, Weyer C, Loftager M,
Hirschberger S and Heise T: Time-action profile of the soluble,
fatty acid acylated, long-acting insulin analogue NN304. Diabet
Med. 16:332–338. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Knudsen LB, Nielsen PF, Huusfeldt PO,
Johansen NL, Madsen K, Pedersen FZ, Thøgersen H, Wilken M and
Agersø H: Potent derivatives of glucagon-like peptide-1 with
pharmacokinetic properties suitable for once daily administration.
J Med Chem. 43:1664–1669. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Matsumura Y and Maeda H: A new concept for
macromolecular therapeutics in cancer chemotherapy: Mechanism of
tumoritropic accumulation of proteins and the antitumor agent
smancs. Cancer Res. 46:6387–6392. 1986.PubMed/NCBI
|
|
21
|
Durante M, Frosini M, Fusi F, Neri A,
Sticozzi C and Saponara S: In vitro vascular toxicity assessment of
NitDOX, a novel NO-releasing doxorubicin. Eur J Pharmacol.
880:1731642020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Maeda H, Akaike T, Yoshida M and Suga M:
Multiple functions of nitric oxide in pathophysiology and
microbiology: Analysis by a new nitric oxide scavenger. J Leukoc
Biol. 56:588–592. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang Z, Liu L, Chen J, Cao M and Wang J:
JS-K as a nitric oxide donor induces apoptosis via the
ROS/Ca2+/caspase-mediated mitochondrial pathway in HepG2 cells.
Biomed Pharmacother. 107:1385–1392. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Millet A, Bettaieb A, Renaud F, Prevotat
L, Hammann A, Solary E, Mignotte B and Jeannin JF: Influence of the
nitric oxide donor glyceryl trinitrate on apoptotic pathways in
human colon cancer cells. Gastroenterology. 123:235–246. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu L, Li T, Tan J, Fu J, Guo Q, Ji H and
Zhang Y: NG as a novel nitric oxide donor induces apoptosis by
increasing reactive oxygen species and inhibiting mitochondrial
function in MGC803 cells. Int Immunopharmacol. 23:27–36. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kroemer G, Galluzzi L, Vandenabeele P,
Abrams J, Alnemri ES, Baehrecke EH, Blagosklonny MV, El-Deiry WS,
Golstein P, Green DR, et al: Classification of cell death:
Recommendations of the nomenclature committee on cell death 2009.
Cell Death Differ. 16:3–11. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Eiserich JP, Patel RP and O'Donnell VB:
Pathophysiology of nitric oxide and related species: Free radical
reactions and modification of biomolecules. Mol Aspects Med.
19:221–357. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gotoh T and Mori M: Nitric oxide and
endoplasmic reticulum stress. Arterioscler Thromb Vasc Biol.
26:1439–1446. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kashfi K, Rayyan Y, Qiao LL, Williams JL,
Chen J, Del Soldato P, Traganos F, Rigas B and Ryann Y: Nitric
oxide-donating nonsteroidal anti-inflammatory drugs inhibit the
growth of various cultured human cancer cells: Evidence of a tissue
type-independent effect. J Pharmacol Exp Ther. 303:1273–1282. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dunlap T, Abdul-Hay SO, Chandrasena RE,
Hagos GK, Sinha V, Wang Z, Wang H and Thatcher GR: Nitrates and
NO-NSAIDs in cancer chemoprevention and therapy: In vitro evidence
querying the NO donor functionality. Nitric Oxide. 19:115–124.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wu W, Gaucher C, Fries I, Hu XM, Maincent
P and Sapin-Minet A: Polymer nanocomposite particles of
S-nitrosoglutathione: A suitable formulation for protection and
sustained oral delivery. Int J Pharm. 495:354–361. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang C, Hwang HH, Jeong S, Seo D, Jeong Y,
Lee DY and Lee K: Inducing angiogenesis with the controlled release
of nitric oxide from biodegradable and biocompatible copolymeric
nanoparticles. Int J Nanomedicine. 13:6517–6530. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Laschak M, Spindler KD, Schrader AJ,
Hessenauer A, Streicher W, Schrader M and Cronauer MV: JS-K, a
glutathione/glutathione S-transferase-activated nitric oxide
releasing prodrug inhibits androgen receptor and WNT-signaling in
prostate cancer cells. BMC Cancer. 12:1302012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nishi K, Imoto S, Beppu T, Uchibori S,
Yano A, Ishima YU, Ikeda T, Tsukigawa K, Otagiri M and Yamasaki K:
The nitrated form of nateglinide induces apoptosis in human
pancreatic cancer cells through a caspase-dependent mechanism.
Anticancer Res. 42:1333–1338. 2022. View Article : Google Scholar : PubMed/NCBI
|