Introduction
Over the past several decades, colorectal cancer
(CRC) has become one of the most common solid tumour types, and is
predicted to become more prevalent in the future, with a projected
global incidence of 2,500,000 in 2035 (1,2).
Furthermore, CRC has become increasingly common among individuals
<50 years of age, which is partially due to unhealthy dietary
habits, a sedentary lifestyle and obesity (3). Therefore, many patients are in the
advanced stages of disease before receiving treatment. In a
clinical setting, the management of patients with CRC primarily
includes extensive surgery, ablation, radiotherapy, chemotherapy,
targeted therapy and immunotherapy (4,5).
However, despite the development of novel drugs and precision
medicine, the overall patient prognosis has not improved
considerably, as patient survival is largely dependent on the
disease stage at first diagnosis. Additionally, though rectal
bleeding is a common clinical feature, the majority of CRC cases
are asymptomatic, which further complicates early detection.
Moreover, cancer stem cells (CSCs) also play a critical role in
promoting CRC progression. CSCs are a class of highly
undifferentiated cancer cells that share features with normal stem
cells, are pluripotent and have the ability to self-renew (6). To date, CSCs have been reported to be
involved in multiple pathogenic processes in CRC, and the most
predominant findings include the correlation of CSCs with tumour
growth, metastasis and chemoresistance (7–10).
Therefore, the discovery of novel and efficient therapies is
critical to enhancing or facilitating the treatment response, and
ultimately prolonging the survival of patients with CRC.
The Hedgehog (Hh) signalling pathway is responsible
for promoting the progression of various malignancies, such as lung
cancer and clear renal cell carcinoma (11–13).
Notably, the Hh pathway is also critically involved in the
regulation of CRC pathogenesis, via, for example, regulating drug
resistance to fluorouracil and irinotecan, cancer cell metastasis
and stemness. Studies have also illustrated that this pathway is a
potential biomarker for the management of patients with CRC
(14–16). GLI family zinc finger 1 (Gli1; also
known as glioma-associated oncogene-1) is an important
transcription factor in Hh signalling whose levels represent Hh
signalling activity (17,18). As a selective inhibitor of Gli1,
GANT61 inhibits the transactivation modulated by Gli1, and
experiments have identified GANT61-D as its bioactive form
(19,20). GANT61 has increased in popularity as
a potential targeting agent for the treatment of carcinomas. For
instance, it has been revealed that GANT61 promotes the sensitivity
of prostate cancer cells to ionizing radiation in vitro and
in vivo (21). In addition,
GANT61 also exerts anticancer and anti-CSC effects in
triple-negative breast cancer (22).
Hence, based on the fact that GANT61 is a promising potential
therapeutic target for carcinoma, and the selective inhibitor of
Gli (a transcription factor in the Hh signalling pathway that is
closely involved in CRC pathology), we hypothesised that GANT61 may
effectively diminish both CRC cells and CRC-CSCs.
Therefore, the aim of the present study was to
elucidate the cytotoxic effects of GANT61 on cancer cells and CSCs,
and its regulatory role on the Wnt/β-catenin and Notch signalling
pathways in CRC.
Materials and methods
Cell culture
The HT-29 and HCT-116 human CRC cell lines (both
ATCC) were cultured in McCoy's 5A medium supplemented with 10%
foetal bovine serum (both Gibco; Thermo Fisher Scientific, Inc.),
and maintained at 37°C in a humidified incubator containing 95% air
and 5% CO2. Both cell lines were authenticated by STR
profiling.
Treatment of CRC cells with
GANT61
GANT61 was dissolved in dimethyl sulfoxide (DMSO)
(both MilliporeSigma) to prepare stock solutions of 2.5, 5, 10, 20
and 40 mM. HT-29 and HCT-116 cells were cultured with different
concentrations of GANT61 for 24, 48 and 72 h, and cell viability
was evaluated by Cell Counting Kit-8 (CCK-8) assay (Dojindo
Molecular Technologies, Inc.). At 48 h, the expression levels of
Gli1, β-catenin and Notch pathway proteins were evaluated by
western blotting, and the percentage of CD133+ cells was
determined by flow cytometry.
Co-treatment of CRC cells with GANT61,
HLY78 and JAG1
The Wnt/β-catenin pathway agonist HLY78
(MilliporeSigma) was dissolved in DMSO to produce a 10-mM solution.
The Notch pathway agonist JAG1 peptide (23–25)
(amino acid sequence, CDDYYYGFGCNKFCRPR; Sino Biological, Inc.) was
dissolved in phosphate-buffered saline (PBS) to a concentration of
30 mM. The cells were treated with HLY78 and JAG1 at concentrations
of 10 (26,27) and 30 µM (25,28,29),
respectively. For co-treatment, 20 µM GANT61 was also added. Then,
48 h after incubation, cell viability was determined by CCK-8
assay, and the apoptotic rate was assessed by annexin V/propidium
iodide (AV/PI) staining. Finally, the expression levels of
β-catenin, transcription factor 7 (TCF7), Notch1 and Hes1 were
detected by western blotting at 48 h.
CSC culture
HT-29 and HCT-116 cells were cultured in DMEM/F12
medium supplemented with 2% B27 and 20 ng/ml bFGF (all Gibco;
Thermo Fisher Scientific, Inc.), as well as 20 ng/ml EGF and 4
ug/ml heparin (MilliporeSigma), for 10 days. The spheres were then
harvested, trypsinized and cultured as CSCs (30,31).
Next, the expression of CD133 was assessed by immunofluorescence
(IF), while the expression levels of β-catenin, TCF7, Notch1 and
Hes1 were detected by western blotting.
Co-treatment of CRC-CSCs with GANT61,
HLY78 and JAG1
After isolation, the CSCs were treated with GANT61,
HLY78 and JAG1, and subsequent detection was performed in the same
manner as that described in the section on ‘co-treatment of CRC
cells with GANT61, HLY78 and JAG1’.
CCK-8 assay
In brief, cells were incubated in 100 µl DMEM mixed
with 10 µl CCK-8 reagent for 2 h at 37°C. The optical density value
was recorded using a microplate reader (BioTek Instruments,
Inc.).
Flow cytometry
Cells were harvested and washed with PBS, prior to
incubation with a rabbit CD133 monoclonal antibody (1:50 dilution)
(1:50; cat. no. ab216323; Abcam) at room temperature for 30 min.
After further washing with PBS, the cells were incubated with Alexa
Fluor® 488-conjugated anti-rabbit IgG (H+L) (1:500; cat.
no. #4412; Cell Signaling Technology, Inc.,) at room temperature
for 30 min in the dark. Finally, a FACSCalibur 2 flow cytometer (BD
Biosciences) and FlowJo 7.6 software (FlowJo LLC) were used to
assess the percentage of CD133+ cells.
Western blotting
After harvesting, total protein was extracted from
the cells using RIPA Lysis and Extraction Buffer (Thermo Fisher
Scientific, Inc.), after which the protein was quantified using a
BCA Protein Assay Kit (Pierce; Thermo Fisher Scientific, Inc.).
Then, gel electrophoresis was performed using 4–12% NuPAGE Bis-Tris
Gels (20 µg total protein/lane) and the proteins were transferred
to nitrocellulose membranes. The membranes were blocked with 5% BSA
(MilliporeSigma) at 37°C for 1.5 h), and then incubated with
primary antibodies (4°C, overnight) followed by secondary
antibodies (37°C, 1.5 h); the bands were detected using enhanced
chemiluminescence (ECL Plus western blotting Substrate, Pierce;
Thermo Fisher Scientific, Inc.) in the dark. The antibodies used
for the western blot analysis are listed in Table I.
 | Table I.Antibodies. |
Table I.
Antibodies.
| Antibody | Cat. no. | Dilution |
|---|
| Primary |
|
|
| Gli
rabbit mAb | 3538 | 1:1,000 |
|
β-catenin rabbit mAb | 9582 | 1:1,000 |
| TCF7
rabbit mAb | 2206 | 1:1,000 |
| Notch1
rabbit mAb | 3608 | 1:1,000 |
| Hes1
rabbit mAb | 11988 | 1:1,000 |
| GAPDH
rabbit mAb | 5174 | 1:1,000 |
| Secondary |
|
|
| Goat
Anti-Rabbit IgG-HRP | 14708 | 1:3,000 |
Apoptosis analysis
An Annexin V Apoptosis Detection Kit (Thermo Fisher
Scientific, Inc.) was used for co-annexin V/propidium iodide
(AV/PI) detection, per the manufacturer's instructions. Briefly,
the cells were collected and incubated with AV and PI for 15 min at
room temperature. Next, the apoptotic rate was evaluated by flow
cytometry and analysed with FlowJo software, as aforementioned.
IF
Briefly, the cells were fixed in 4% paraformaldehyde
(at room temperature for 10 min) and permeabilized with 0.5% Triton
X-100 (both MilliporeSigma) (room temperature for 3 min). After
blocking nonspecific protein binding with 5% BSA (MilliporeSigma;
room temperature for 30 min), the cells were incubated with a
rabbit mAb against CD133 (1:200 dilution) for 2 h at room
temperature, followed by incubation with Alexa Fluor®
488-conjugated anti-rabbit IgG (H+L) (1:500 dilution) at room
temperature for 1.5 h in the dark. Images were then obtained using
an inverted microscope (Olympus, Japan).
Statistical analysis
All data are expressed as the mean ± standard
deviation. GraphPad Prism Software version 7.0 (GraphPad Software
Inc.,) was used for data analysis and graph plotting. Comparisons
between the control and other treatment groups were determined by
one-way ANOVA followed by Dunnett's multiple comparisons test.
Multiple comparisons among groups were determined by one-way ANOVA
followed by Tukey's multiple comparisons test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Impact of GANT61 on viability, Gli1,
β-catenin, Notch1 and CD133+ proportion in CRC
cells
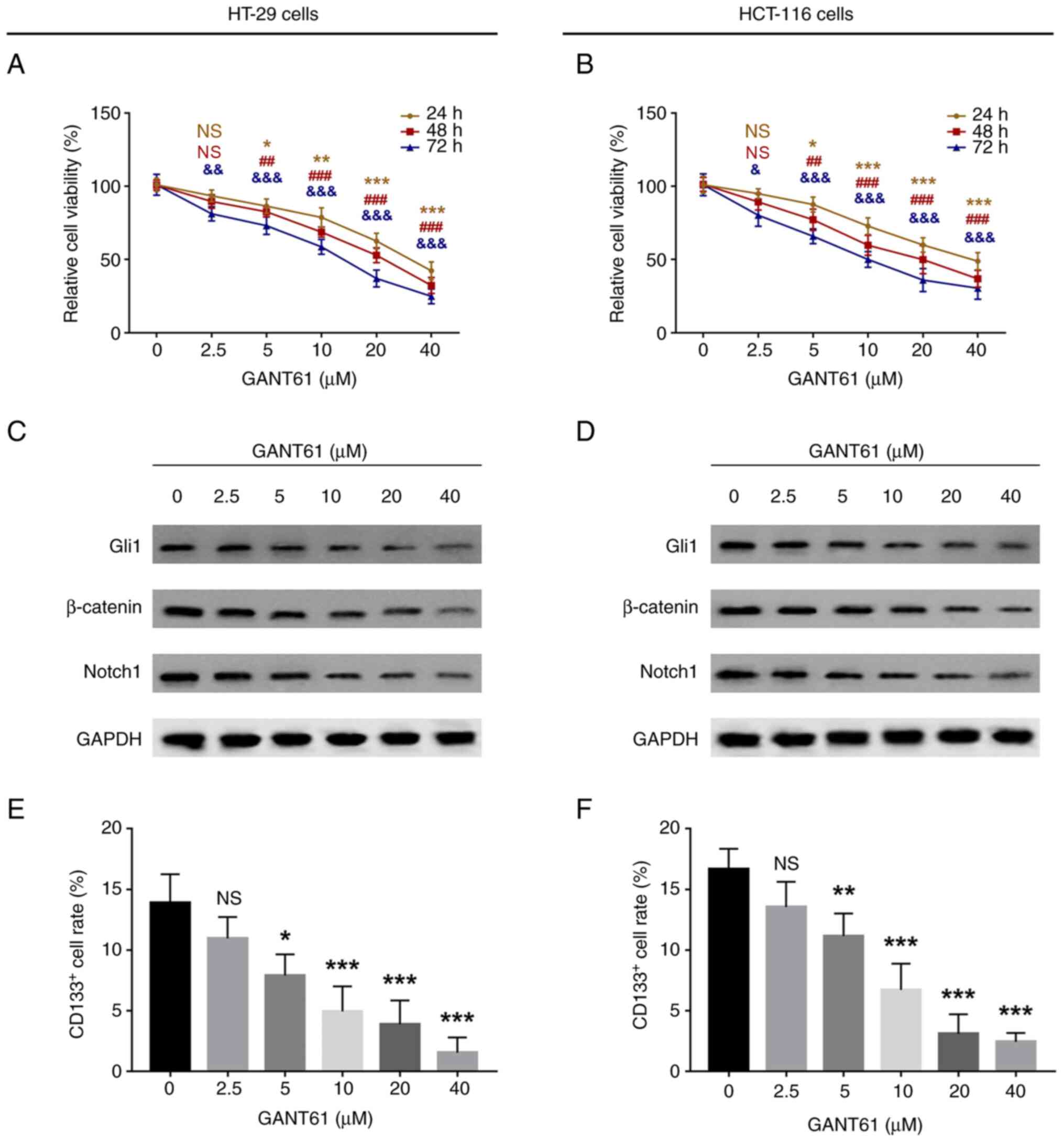
GANT61 decreased the relative viability of both
HT-29 and HCT-116 cells in a time- and dose-dependent manner
(P<0.05; Fig. 1A and B).
Furthermore, GANT61 decreased the protein expression levels of
Gli1, β-catenin and Notch1 in a dose-dependent manner in HT-29
(Fig. 1C) and HCT-116 (Fig. 1D) cells. In addition, in HT-29 cells,
GANT61 reduced the proportion of CD133+ cells in a
dose-dependent manner (P<0.05; Figs.
1E and S1A). Similarly, in
HCT-116 cells, GANT61 also decreased the percentage of
CD133+ cells in a dose-dependent manner (P<0.05;
Figs. 1F and S1B).
GANT61 exhibits killing effects on CRC
cells by regulating the Wnt/β-catenin pathway
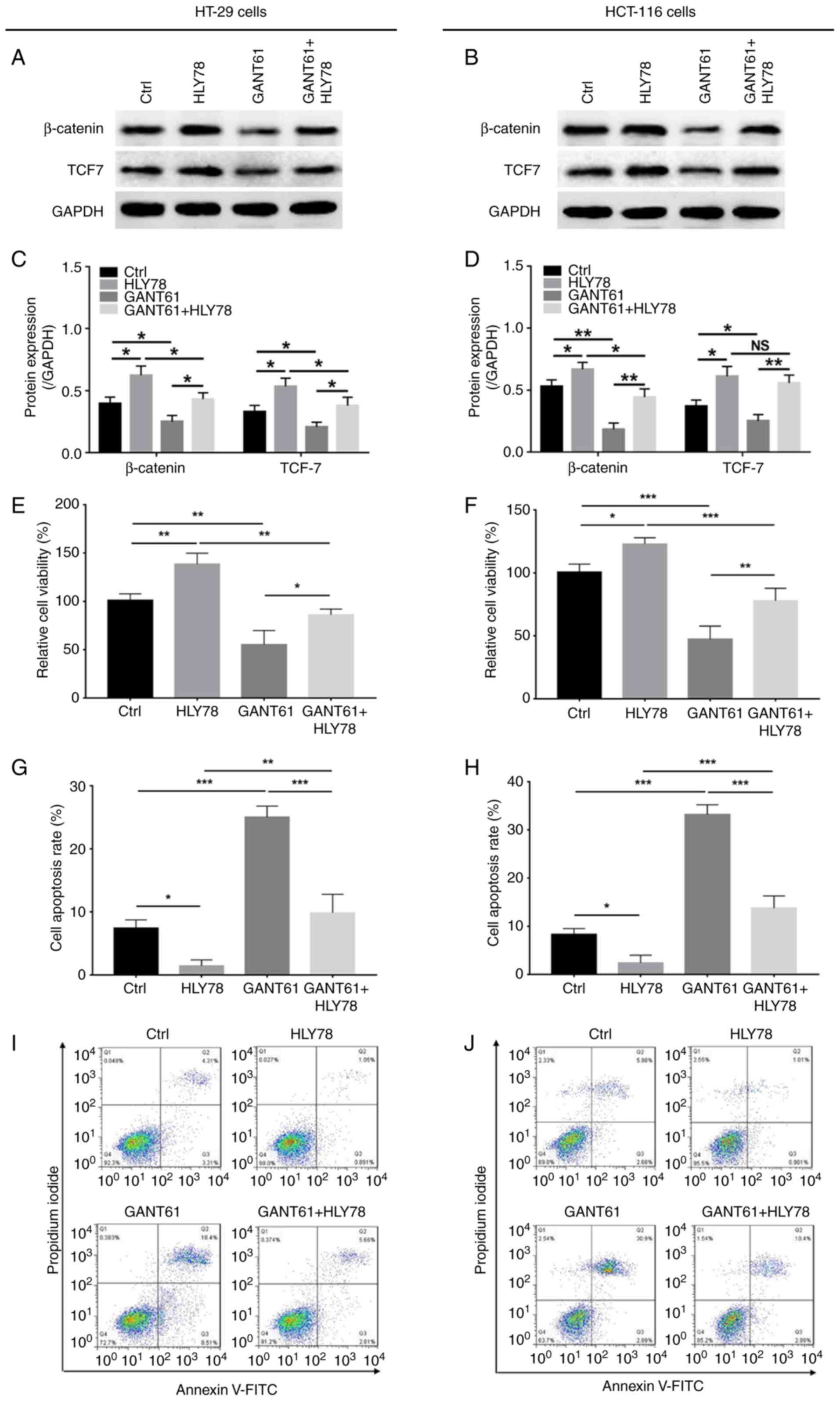
Furthermore, GANT61 decreased, while HLY78
increased, the protein expression levels of β-catenin and TCF7 in
HT-29 and HCT-116 cells; HLY78 also reversed the decrease in
β-catenin and TCF7 protein expression in GANT61-treated HT-29 and
HCT-116 cells (P<0.05) (Fig.
2A-D). Also, GANT61 decreased (P<0.01), while HLY78
increased (P<0.01), the relative viability of HT-29 cells, and
HLY78 also retained the viability of GANT61-treated HT-29 cells
(P<0.05) (Fig. 2E). In addition,
GANT61 promoted (P<0.001), but HLY78 inhibited (P<0.05) the
apoptosis of HT-29 cells, which was prevented by HLY78 in
GANT61-treated HT-29 cells (P<0.001) (Fig. 2G and I). Furthermore, GANT61
downregulated (P<0.001), while HLY78 upregulated (P<0.05) the
relative cell viability of HCT-116 cells, and HLY78 also retained
the viability GANT61-treated HCT-116 cells (P<0.01) (Fig. 2F). Furthermore, GANT61 (P<0.001)
increased HCT-116 cell apoptosis in, which was decreased by HLY78
(P<0.05); subsequently, HLY78 also reduced apoptosis in
GANT61-treated HCT-116 cells (P<0.001) (Fig. 2H and J). These data suggested that
HLY78 curtailed the effects of GANT61 on CRC cell viability and
apoptosis.
GANT61 exhibits killing effects on CRC
cells by regulating the Notch pathway
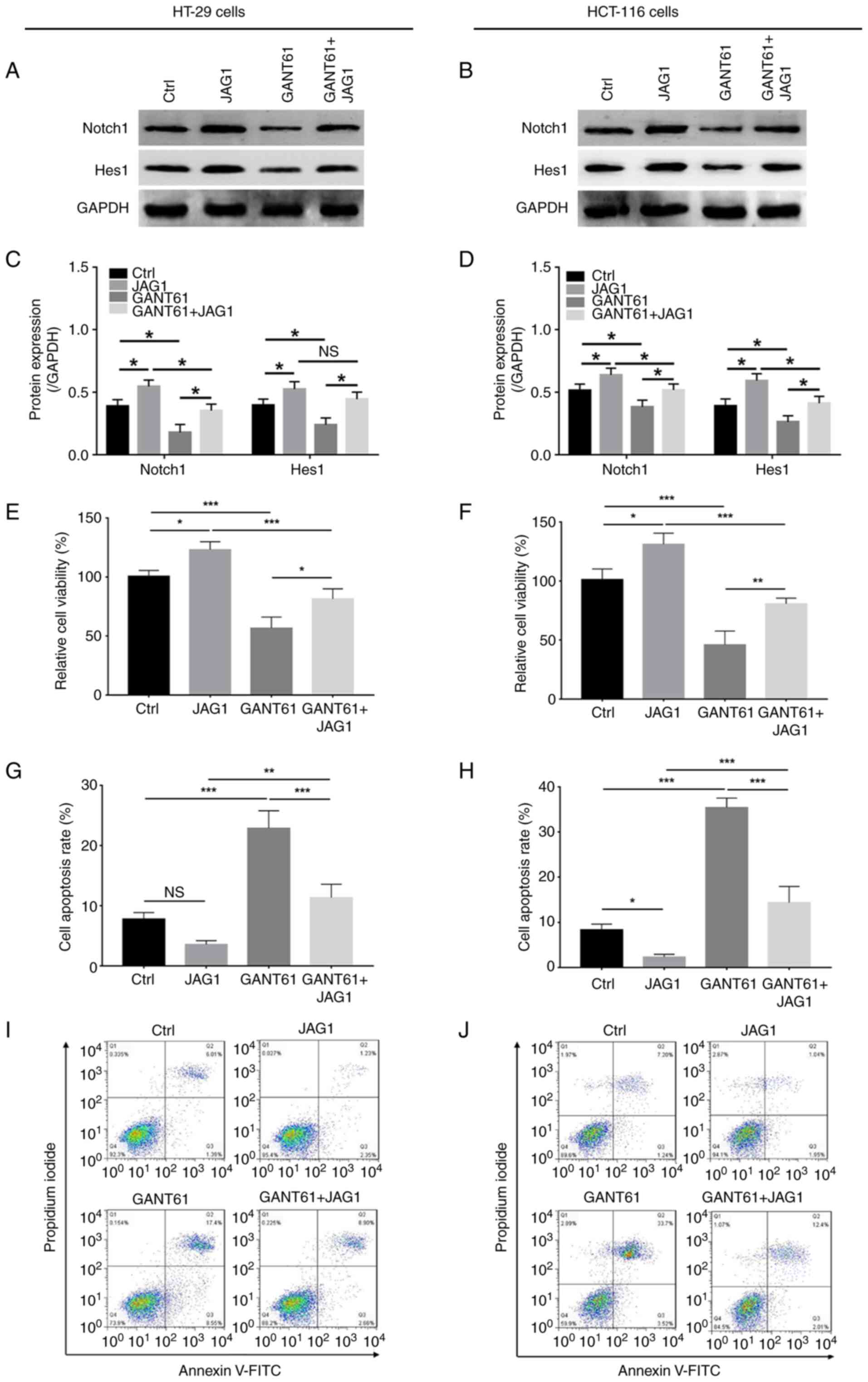
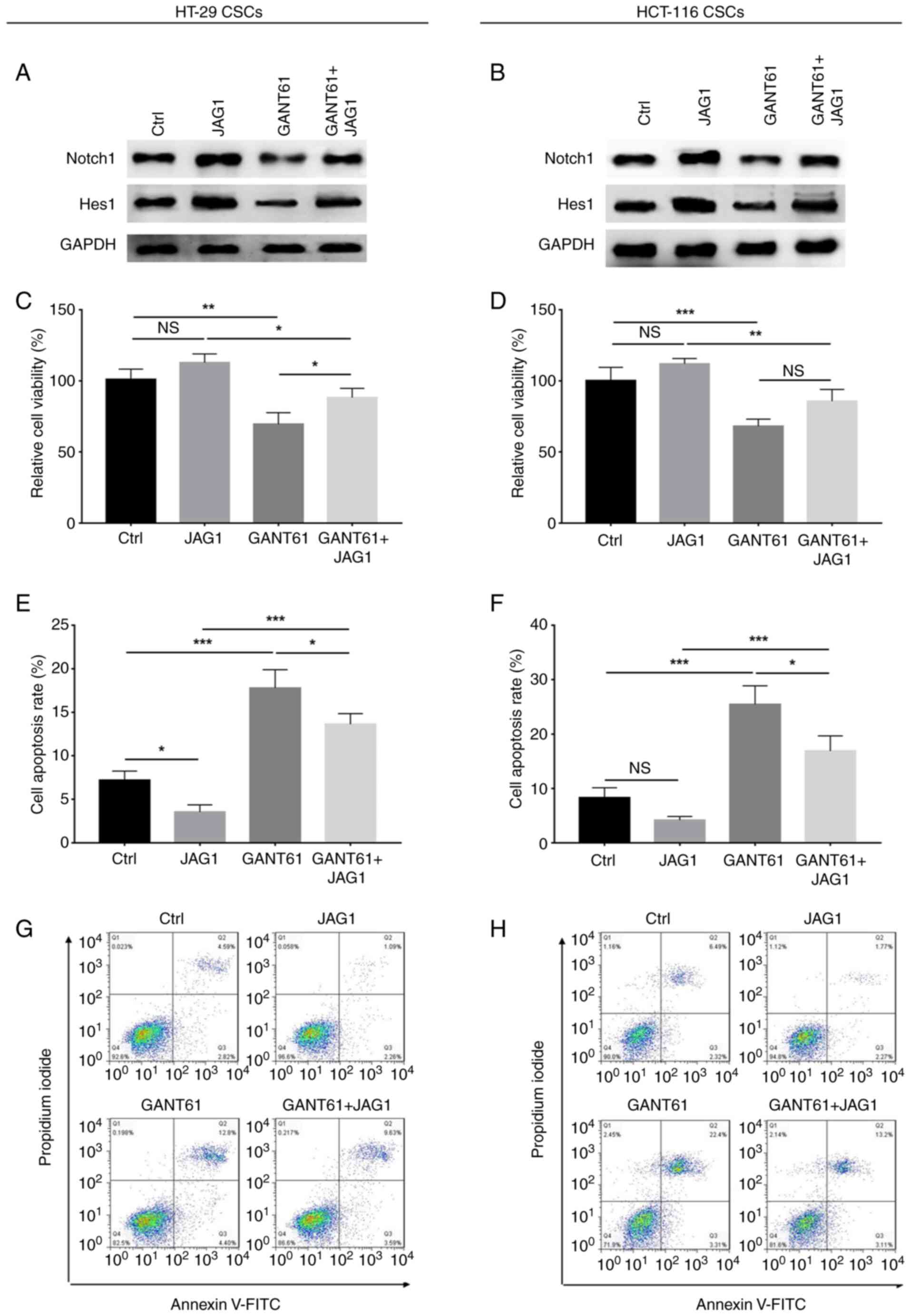
GANT61 decreased, while JAG1 increased Notch1 and
Hes1 protein expression in HT-29 and HCT-116 cells, and JAG1 also
retained the expression of these proteins in GANT61-treated HT-29
and HCT-116 cells (Fig. 3A-D). In
regard to relative cell viability and apoptosis, GANT61 decreased
(P<0.001), while JAG1 increased (P<0.05) the relative
viability of HT-29 cells; in addition, JAG1 retarded the reduction
in viability of GANT61-treated HT-29 cells (P<0.05) (Fig. 3E). Furthermore GANT61 increased
(P<0.001), while JAG1 had no significant impact on apoptosis in
HT-29 cells. However, JAG1 did reduce apoptosis in GANT61-treated
HT-29 cells (P<0.001) (Fig. 3G and
I). In addition, GANT61 downregulated (P<0.001), but JAG1
upregulated (P<0.05) the relative viability of HCT-116 cells,
and JAG1 revived the viability of GANT61-treated HCT-116 cells
(P<0.01) (Fig. 3F); additionally,
GANT61 increased apoptosis in HCT-116 cells (P<0.001), while
JAG1 decreased apoptotic rate (P<0.05). As with HT-29 cells,
JAG1 also reduced apoptosis in HCT-116 cells following treatment
with GANT61 (P<0.001) (Fig. 3H and
J). These data implied that JAG1 reduced the effects of GANT61
on CRC cell viability and apoptosis.
β-catenin, TCF7, Notch1 and Hes1
expression in CRC-CSCs
Considering that GANT61 was found to regulate
stemness markers, as well as the Wnt/β-catenin and Notch1
signalling pathways, activation of the Wnt/β-catenin and Notch
signalling pathways was evaluated in CRC-CSCs. The results showed
that CD133 was abundantly expressed in CSCs derived from both HT-29
and HCT-116 cells, compared with the control cells (Fig. 4A and C), suggesting that CSC
generation was successful. Moreover, the protein expression levels
of β-catenin, TCF7, Notch1 and Hes1 were upregulated in CRC-CSCs
compared with the control cells (Fig.
4B, D, E and F), suggesting that the Wnt/β-catenin and Notch1
signalling pathways were activated in CRC-CSCs.
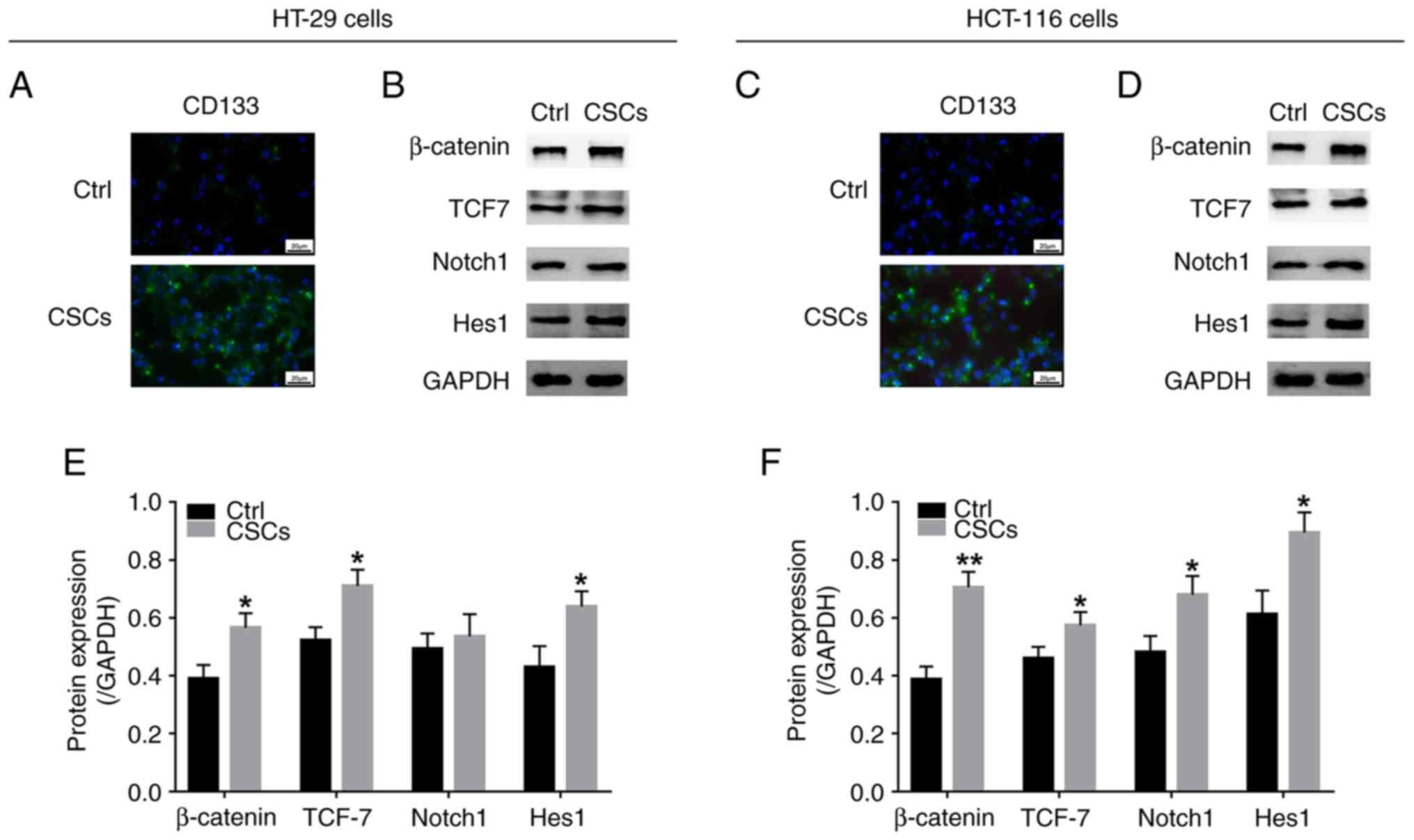 | Figure 4.Wnt/β-catenin and Notch signalling
pathways in colorectal cancer CSCs. (A) CD133+ cells in
Ctrl cells and HT-29 cell-derived CSCs and (B) western blot to
assess the protein expression levels of β-catenin, TCF7, Notch1 and
Hes1. (C) CD133+ cells and (D) protein expression levels
of β-catenin, TCF7, Notch1 and Hes1 in Ctrl cells and HCT-116
cell-derived CSCs of the line. Quantified expression levels of
β-catenin, TCF7, Notch1 and Hes1 in in Ctrl cells and derived CSCs
of (E) HT-29 and (F) HCT-116 cells. *P<0.05, **P<0.01. CSC,
cancer stem cell; TCF7, transcription factor 7; Ctrl, control. |
GANT61 exhibits killing effects on
CRC-CSCs by modulating the Wnt/β-catenin pathway
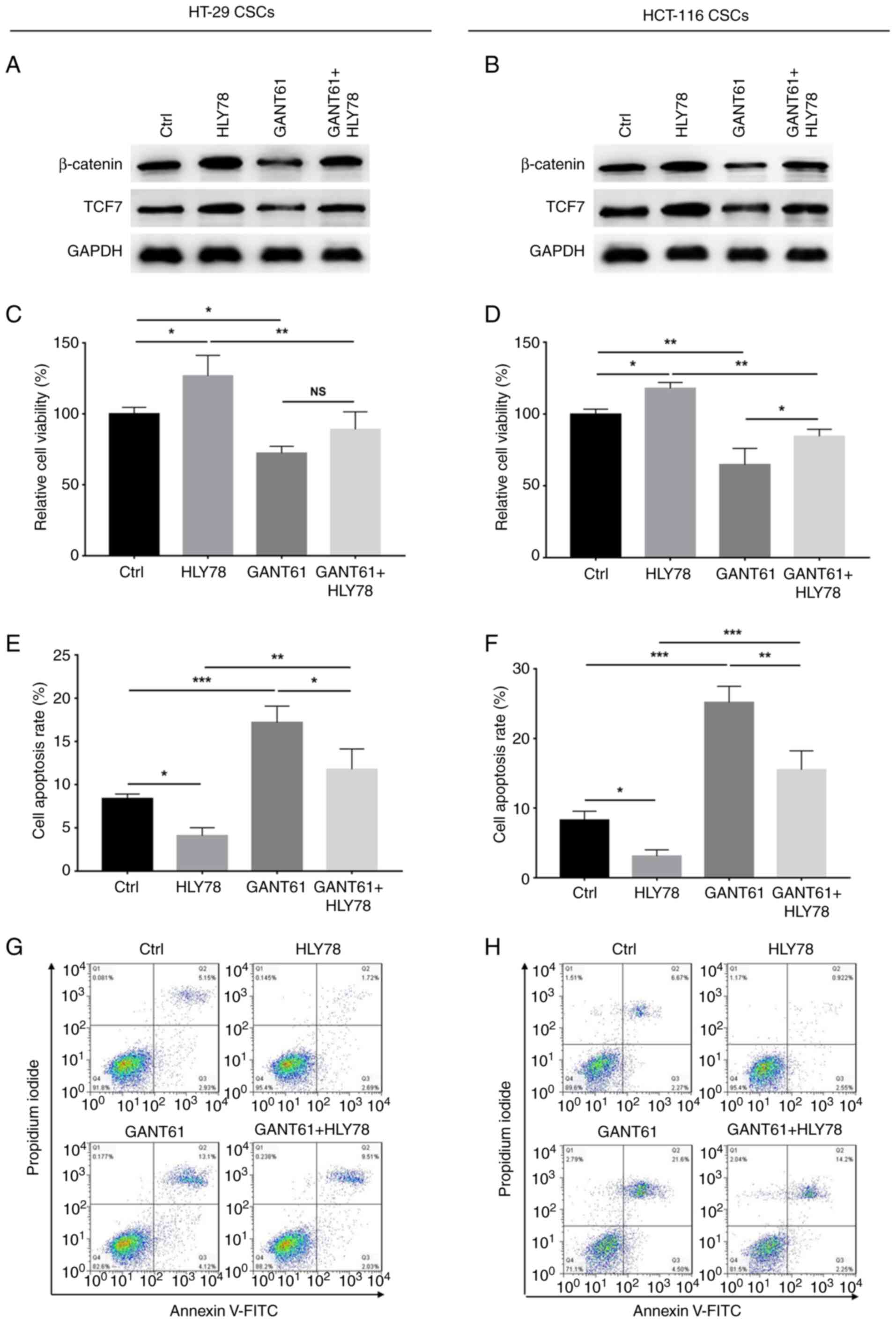
GANT61 was found to decrease the protein expression
levels of β-catenin and TCF7 in HT-29 and HCT-116 CSCs, which were
increased by HLY78; HLY78 also increased the expression of
β-catenin and TCF7 in HT-29 and HCT-116 CSCs following GANT61
treatment (Fig. 5A and B).
Furthermore, the relative viability of HT-29 CSCs was decreased by
GANT61, but increased by HLY78 (both P<0.05), and HLY78 had no
significant impact on the viability of GANT61-treated HT-29 CSCs
(Fig. 5C). In addition, GANT61
elevated (P<0.001), while HLY78 decreased (P<0.05) the number
of apoptotic HT-29 CSCs; HLY78 also decreased apoptosis in
GANT61-treated HT-29 CSCs (P<0.05) (Fig. 5E and G). In HCT-116 CSCs, GANT61
decreased (P<0.01) while HLY78 increased (P<0.05) relatively
viability; HLY78 also increased the viability of GANT61-treated
HCT-116 CSCs (P<0.05) (Fig. 5D).
Additionally, GANT61 upregulated (P<0.001), while HLY78
downregulated (P<0.05) apoptosis in HCT-116 CSCs, and HLY78 also
downregulated apoptosis in GANT61-treated HCT-116 CSCs (P<0.01)
(Fig. 5F and H). These data
suggested that the cytotoxic effects of GANT61 in CRC-CSCs were
exerted via the regulation of the Wnt/β-catenin pathway.
Killing effects of GANT61 on CRC-CSCs
via modulation of the Notch pathway
GANT61 reduced, while JAG1 elevated Notch1 and Hes1
protein expression levels in HT-29 and HCT-116 CSCs, and JAG1 also
elevated the levels of these proteins in GANT61-treated HT-29 and
HCT-116 CSCs (Fig. 6A and B).
Furthermore, GANT61 decreased relative cell viability (P<0.01),
while JAG1 had no significant effect on HT-29 CSCs; however, JAG1
did elevate the relative viability of GANT61-treated HT-29 CSCs
(P<0.05) (Fig. 6C). Moreover,
GANT61 elevated apoptosis in HT-29 CSCs (P<0.001), while JAG1
decreased this (P<0.05), as well as reducing apoptosis in
GANT61-treated HT-29 CSCs (P<0.05) (Fig. 6E and G). In addition, GANT61
downregulated (P<0.001), while JAG1 did not significantly impact
relative HCT-116 CSC or GANT61-treated HCT-116 CSC viability
(Fig. 6D). Moreover, GANT61
upregulated the levels of apoptosis (P<0.001), while JAG1 did
not significantly affect the apoptotic rate of HCT-116 CSCs.
However, JAG1 did reduce apoptosis in GANT61-treated HCT-116 CSCs
(P<0.05) (Fig. 6F and H). The
results indicated that JAG1 eliminated the effects of GANT61 on CSC
viability and apoptosis.
Discussion
Although CRC patient prognosis has improved, the
poor survival rate of patients with metastatic disease necessitates
the discovery of novel drugs and additional therapies, among which
biological (such as anti-VEGF agents) and targeted therapies are
the most promising. In a clinical setting, several novel drugs,
such as anti-VEGF agents and MEK inhibitors, have demonstrated
favourable therapeutic effects; however, these are not sufficient
to fulfil the need for more efficacious therapies (32,33).
Previous studies have clarified that the Hh signalling pathway
promotes progression and mediates chemoresistance in CRC (14,34,35).
However, a limited number of studies have been published on the
role of the Hh pathway and its inhibitor GANT61 in CRC. Therefore,
the present study was performed to evaluate the effects of GANT61
on CRC cells and CRC-CSCs, which revealed the following: i) GANT61
treatment decreased CRC cell viability and the expression of Gli1,
β-catenin and Notch1, as well as the proportion of
CD133+ CRC cells, in a dose-dependent manner; and (ii)
GANT61 exhibited good cytotoxic activity against CRC cells and
CRC-CSCs by regulating the Wnt/β-catenin and Notch signalling
pathways.
Studies performed on multiple carcinomas have
suggested GANT61 as a potentially promising antitumour drug, since
the increasingly important role of the Hh signalling pathway has
been established in cancer. For instance, a study performed in nude
mice involving the implantation of HeLa cells revealed that GANT61
represses the growth and apoptosis of allograft tumours, and that
this compound is also tolerated in mice, as shown by white blood
cell count, haemoglobin level, platelet count, and the levels of
alanine aminotransferase, aspartate aminotransferase and creatine
(36). Moreover, in a Ewing's
sarcoma family-derived tumour cell line (SK-N-LO), GANT61 treatment
induced cellular and morphological changes (such as cell shrinkage,
chromatin condensation and nuclear fragmentation) in a
dose-dependent manner; GANT61 also increased the percentage of
apoptotic cells (37). Another study
illustrated that GANT61 decreased cell viability, but enhanced
apoptosis in three T-cell lymphoma cell lines (Jurkat, Karpass299
and Myla3676 cells) by reducing the levels of STAT3 phosphorylation
and suppressor of cytokine signalling 3 (SOCS3) (38). These studies indicate the cytotoxic
effect of GANT61 in multiple cancers. As for its role in enhancing
drug sensitivity, it has been reported that GANT61 increases the
response of prostate cancer cells to ionizing radiation both in
vitro and in vivo (21).
Another study revealed that the combination of phospholipase
Cε-knockdown and GANT61 administration in castration-resistant
prostate cancer cells enhanced their sensitivity to enzalutamide
via inhibition of the androgen receptor pathway (39). Collectively, these findings indicate
that in combination with other antitumour drugs, GANT61 can
sensitize carcinomas to various treatment types.
Compared with the present study, these previous
studies were primarily conducted in cancers other than CRC; the
present study revealed that GANT61 reduced CRC cell viability,
β-catenin and Notch1 protein expression levels, and the percentage
of CD133+ CRC cells, in a dose-dependent manner. Some
possible explanations for these results are as follows: i) GANT61
potentially inhibited the malignant behaviour of CRC cells,
including decreasing viability and stemness, and enhancing
apoptosis by targeting Gli1, which subsequently reduced the Hh
pathway activity involved in the promotion of CRC pathology
(14,34,35); ii)
GANT61 may also have regulated the functions and stemness of CRC
cells by mediating p-STAT3, SOCS3 or other related factors, as
reported in previous studies (21,22,37–42); and
iii) in later experiments, it was shown that GANT61 decreased the
relative viability of CRC cells, while enhancing apoptosis by
regulating the Wnt/β-catenin and Notch signalling pathways, which
could provide another explanation of the current results.
Several pathways have been implicated in the
regulation of CRC pathogenesis, including those assessed in the
current study, the Wnt/β-catenin and Notch signalling pathways.
With regards to Wnt/β-catenin, a study reported that
Fusobacterium nucleatum promotes the progression of CRC by
enhancing annexin A1, which is a Wnt/β-catenin modulator (43). It has also been reported that MEK
inhibitors, including those of MEK1 and 2, activate Wnt signalling
and increase the plasticity of CSCs (44). Furthermore, the epithelial Notch
signalling pathway was found to be associated with a poor-prognosis
subtype and metastasis in CRC, due to its ability to rewire the
tumour microenvironment in vitro (including regulating
multiple processes, such as neutrophil recruitment) (45). Given the aforementioned findings, and
those of the present study suggesting that GANT61 regulates
Wnt/β-catenin and Notch signalling pathways in CRC cells, rescue
experiments when then performed, which indicated that GANT61
induced cytotoxicity in CRC cells by downregulating the
Wnt/β-catenin and Notch signalling pathways. These results suggest
a potential therapeutic mechanism of GANT61 in the treatment of
CRC.
As for the modulatory role of GANT61 in CSCs, a
study revealed that GANT61 and GDC-0449 (a SMO receptor inhibitor
of the Hh pathway) elevate apoptosis in prostate CSCs by repressing
the GLI family of transcription factors in a direct or indirect
manner; moreover, GANT61 induced apoptosis in prostate CSCs more
effectively than GCD-0449 (42).
Additionally, in CRC organoid culture, GANT61 treatment suppressed
the expression of stem cell markers, including c-Myc, CD44 and
Nanog, possibly by inhibiting the expression of its transcription
factor Gli1 (14). Another study
revealed that combining GANT61 administration with mTOR suppression
markedly reduces the proportion of CSCs among pancreatic cancer
cells (46). Collectively, these
findings indicate that GANT61 inhibits stemness and diminishes CSC
numbers in multiple carcinomas.
Studies have also revealed that the Notch pathways
are involved in the regulation of stemness in CRC and CRC-CSCs. For
instance, β-catenin interacts with Tribbles homolog 3 and TCF4 to
increase CSC-related gene expression in intestinal cells (47). In addition, another study reported
that inhibition of the Notch signalling pathway with a γ-secretase
inhibitor N-[N-(3,5-difluorophenacetyl)-l-alanyl]-S-phenylglycine
t-butyl ester (DAPT) resulted in a killing effect on CRC-CSCs
(48). In consideration of the
cytotoxic effect of GANT61 on CSCs found in other cancers, and the
impact of Wnt/β-catenin and Notch signalling pathways on CRC-CSCs
discovered in other studies and in the present study (where GANT61
presented with anticancer ability by blocking Wnt/β-catenin and
Notch signalling pathways in CRC), we hypothesized that GANT61 may
also effectively destroy CRC-CSCs via the Wnt/β-catenin and Notch
signalling pathways. Therefore, further rescue experiments were
performed, and the results showed that GANT61 demonstrated good
cytotoxicity in CRC-CSCs by blocking the Wnt/β-catenin and Notch
signalling pathways. Possible explanations for these findings
include the following: GANT61 might inhibit its target Gli1 (as
reported in studies conducted in other cancers), which subsequently
blocks the Wnt/β-catenin and Notch signalling pathways, and then
represses stemness in CRC cells, reduces viability, but also
enhances apoptosis of CRC-CSCs (21,22,37–42). To
further clarify these conclusions, in vivo experiments may
be required to validate the present study findings; however, due to
insufficient financial support and limited lab conditions, these
in vivo experiments were not performed.
In conclusion, the Hh signalling pathway/Gli1
inhibitor, GANT61, effectively eliminates cancer cells and CSCs by
blocking the Wnt/β-catenin and Notch signalling pathways in CRC.
These findings suggest that GANT61 may serve as a potential
treatment option for patients with CRC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HT conceived and designed the experiments, analysed
the data and revised the manuscript. YS and LL collected and
analysed the data. WZ and XL performed data analysis and provided
interpretation. QL provided technical support and analysed and
interpreted the results. BL critically revised the article and
interpreted the data. YS and HT confirm the authenticity of all the
raw data. All authors approved the final version of the
article.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CRC
|
colorectal cancer
|
|
CSCs
|
cancer stem cells
|
|
Hh
|
Hedgehog
|
|
Gli1
|
GLI family zinc finger 1
|
|
CD133+
|
CD133 positive
|
|
VEGF
|
vascular endothelial growth factor
|
|
MEK
|
methyl ethyl ketone
|
|
SOCS3
|
suppressor of cytokine signalling
3
|
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Arnold M, Sierra MS, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global patterns and trends in
colorectal cancer incidence and mortality. Gut. 66:683–691. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
World Cancer Research Fund
International/American Institute for Cancer Research, . Continuous
update project report: Diet, nutrition, physical activity, and
colorectal cancer. American Institute for Cancer Research.
https://www.aicr.org/wp-content/uploads/2020/01/colorectal-cancer-2017-report.pdf
|
|
4
|
Marmol I, Sanchez-de-Diego C, Pradilla
Dieste A, Cerrada E and Rodriguez Yoldi MJ: Colorectal Carcinoma: A
general overview and future perspectives in colorectal cancer. Int
J Mol Sci. 18:1972017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brody H: Colorectal cancer. Nature.
521:S12015. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nassar D and Blanpain C: Cancer stem
cells: Basic concepts and therapeutic implications. Annu Rev
Pathol. 11:47–76. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Quarni W, Dutta R, Green R, Katiri S,
Patel B, Mohapatra SS and Mohapatra S: Mithramycin a inhibits
colorectal cancer growth by targeting cancer stem cells. Sci Rep.
9:152022019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Park SY, Lee CJ, Choi JH, Kim JH, Kim JW,
Kim JY and Nam JS: The JAK2/STAT3/CCND2 Axis promotes colorectal
Cancer stem cell persistence and radioresistance. J Exp Clin Cancer
Res. 38:3992019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhou Y, Xia L, Wang H, Oyang L, Su M, Liu
Q, Lin J, Tan S, Tian Y, Liao Q and Cao D: Cancer stem cells in
progression of colorectal cancer. Oncotarget. 9:33403–33415. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Elbadawy M, Usui T, Yamawaki H and Sasaki
K: Emerging Roles of C-Myc in cancer stem cell-related signaling
and resistance to cancer chemotherapy: A potential therapeutic
target against colorectal cancer. Int J Mol Sci. 20:23402019.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dimou A, Bamias A, Gogas H and Syrigos K:
Inhibition of the Hedgehog pathway in lung cancer. Lung Cancer.
133:56–61. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kotulak-Chrzaszcz A, Klacz J, Matuszewski
M, Kmiec Z and Wierzbicki PM: Expression of the Sonic Hedgehog
pathway components in clear cell renal cell carcinoma. Oncol Lett.
18:5801–5810. 2019.PubMed/NCBI
|
|
13
|
Carpenter RL and Ray H: Safety and
tolerability of sonic hedgehog pathway inhibitors in cancer. Drug
Saf. 42:263–279. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Usui T, Sakurai M, Umata K, Elbadawy M,
Ohama T, Yamawaki H, Hazama S, Takenouchi H, Nakajima M, Tsunedomi
R, et al: Hedgehog signals mediate anti-cancer drug resistance in
three-dimensional primary colorectal cancer organoid culture. Int J
Mol Sci. 19:10982018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim BR, Na YJ, Kim JL, Jeong YA, Park SH,
Jo MJ, Jeong S, Kang S, Oh SC and Lee DH: RUNX3 suppresses
metastasis and stemness by inhibiting Hedgehog signaling in
colorectal cancer. Cell Death Differ. 27:676–694. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Papadopoulos V, Tsapakidis K, Riobo Del
Galdo NA, Papandreou CN, Del Galdo F, Anthoney A, Sakellaridis N,
Dimas K and Kamposioras K: The prognostic significance of the
Hedgehog signaling pathway in colorectal cancer. Clin Colorectal
Cancer. 15:116–127. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lu J, Liu L, Zheng M, Li X, Wu A, Wu Q,
Liao C, Zou J and Song H: MEKK2 and MEKK3 suppress Hedgehog
pathway-dependent medulloblastoma by inhibiting GLI1 function.
Oncogene. 37:3864–3878. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lu L, Wu M, Zhao F, Fu W, Li W, Li X and
Liu T: Prognostic and clinicopathological value of Gli-1 expression
in gastric cancer: A meta-analysis. Oncotarget. 7:69087–69096.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lauth M, Bergstrom A, Shimokawa T and
Toftgard R: Inhibition of GLI-mediated transcription and tumor cell
growth by small-molecule antagonists. Proc Natl Acad Sci USA.
104:8455–8460. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Calcaterra A, Iovine V, Botta B, Quaglio
D, D'Acquarica I, Ciogli A, Iazzetti A, Alfonsi R, Lospinoso
Severini L, Infante P, et al: Chemical, computational and
functional insights into the chemical stability of the Hedgehog
pathway inhibitor GANT61. J Enzyme Inhib Med Chem. 33:349–358.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gonnissen A, Isebaert S, McKee CM, Dok R,
Haustermans K and Muschel RJ: The hedgehog inhibitor GANT61
sensitizes prostate cancer cells to ionizing radiation both in
vitro and in vivo. Oncotarget. 7:84286–84298. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Koike Y, Ohta Y, Saitoh W, Yamashita T,
Kanomata N, Moriya T and Kurebayashi J: Anti-cell growth and
anti-cancer stem cell activities of the non-canonical hedgehog
inhibitor GANT61 in triple-negative breast cancer cells. Breast
Cancer. 24:683–693. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zlobin A, Jang M and Miele L: Toward the
rational design of cell fate modifiers: Notch signaling as a target
for novel biopharmaceuticals. Curr Pharm Biotechnol. 1:83–106.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nickoloff BJ, Qin JZ, Chaturvedi V,
Denning MF, Bonish B and Miele L: Jagged-1 mediated activation of
notch signaling induces complete maturation of human keratinocytes
through NF-kappaB and PPARgamma. Cell Death Differ. 9:842–855.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wen F, Wong HK, Tay CY, Yu H, Li H, Yu T,
Tijore A, Boey FY, Venkatraman SS and Tan LP: Induction of myogenic
differentiation of human mesenchymal stem cells cultured on Notch
agonist (Jagged-1) modified biodegradable scaffold surface. ACS
Appl Mater Interfaces. 6:1652–1661. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yakisich JS, Azad N, Kaushik V, O'Doherty
GA and Iyer AK: Nigericin decreases the viability of
multidrug-resistant cancer cells and lung tumorspheres and
potentiates the effects of cardiac glycosides. Tumour Biol.
39:10104283176943102017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Guo X, Zhang L, Fan Y, Zhang D, Qin L,
Dong S and Li G: Oxysterol-binding protein-related protein 8
inhibits gastric cancer growth through induction of ER stress,
inhibition of wnt signaling, and activation of apoptosis. Oncol
Res. 25:799–808. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhou Y, An Q, Guo RX, Qiao YH, Li LX,
Zhang XY and Zhao XL: MiR424-5p functions as an anti-oncogene in
cervical cancer cell growth by targeting KDM5B via the Notch
signaling pathway. Life Sci. 171:9–15. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Karimzadeh F, Modarres Mousavi SM, Alipour
F, Hosseini Ravandi H, Kovac S and Gorji A: Developmental changes
in Notch1 and NLE1 expression in a genetic model of absence
epilepsy. Brain Struct Funct. 222:2773–2785. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hemmati HD, Nakano I, Lazareff JA,
Masterman-Smith M, Geschwind DH, Bronner-Fraser M and Kornblum HI:
Cancerous stem cells can arise from pediatric brain tumors. Proc
Natl Acad Sci USA. 100:15178–15183. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang L, Hirohashi Y, Ogawa T, Shen M,
Takeda R, Murai A, Yamamoto E, Kubo T and Nakatsugawa M: LY6/PLAUR
domain containing 3 has a role in the maintenance of colorectal
cancer stem-like cells. Biochem Biophys Res Commun. 486:232–238.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Roth AD, Tejpar S, Delorenzi M, Yan P,
Fiocca R, Klingbiel D, Dietrich D, Biesmans B, Bodoky G, Barone C,
et al: Prognostic role of KRAS and BRAF in stage II and III
resected colon cancer: Results of the translational study on the
PETACC-3, EORTC 40993, SAKK 60–00 trial. J Clin Oncol. 28:466–474.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rahbari NN, Kedrin D, Incio J, Liu H, Ho
WW, Nia HT, Edrich CM, Jung K, Daubriac J, Chen I, et al: Anti-VEGF
therapy induces ECM remodeling and mechanical barriers to therapy
in colorectal cancer liver metastases. Sci Transl Med.
8:360ra1352016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tiwari A, Saraf S, Verma A, Panda PK and
Jain SK: Novel targeting approaches and signaling pathways of
colorectal cancer: An insight. World J Gastroenterol. 24:4428–4435.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Park SH, Jo MJ, Kim BR, Jeong YA, Na YJ,
Kim JL, Jeong S, Yun HK, Kim DY, Kim BG, et al: Sonic hedgehog
pathway activation is associated with cetuximab resistance and
EPHB3 receptor induction in colorectal cancer. Theranostics.
9:2235–2251. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chang Y, Chen H, Duan J, Wu W, Le F and
Mou F: The inhibitory effect and safety of GANT61 on HeLa cells in
nude mice. Exp Mol Pathol. 113:1043522020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Matsumoto T, Tabata K and Suzuki T: The
GANT61, a GLI inhibitor, induces caspase-independent apoptosis of
SK-N-LO cells. Biol Pharm Bull. 37:633–641. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Geng L, Lu K, Li P, Li X, Zhou X, Li Y and
Wang X: GLI1 inhibitor GANT61 exhibits antitumor efficacy in T-cell
lymphoma cells through down-regulation of p-STAT3 and SOCS3.
Oncotarget. 8:48701–48710. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sun W, Li L, Du Z, Quan Z, Yuan M, Cheng
H, Gao Y, Luo C and Wu X: Combination of phospholipase Cε knockdown
with GANT61 sensitizes castrationresistant prostate cancer cells to
enzalutamide by suppressing the androgen receptor signaling
pathway. Oncol Rep. 41:2689–2702. 2019.PubMed/NCBI
|
|
40
|
Kurebayashi J, Koike Y, Ohta Y, Saitoh W,
Yamashita T, Kanomata N and Moriya T: Anti-cancer stem cell
activity of a hedgehog inhibitor GANT61 in estrogen
receptor-positive breast cancer cells. Cancer Sci. 108:918–930.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Vlckova K, Reda J, Ondrusova L, Krayem M,
Ghanem G and Vachtenheim J: GLI inhibitor GANT61 kills melanoma
cells and acts in synergy with obatoclax. Int J Oncol. 49:953–960.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tong W, Qiu L, Qi M, Liu J, Hu K, Lin W,
Huang Y and Fu J: GANT-61 and GDC-0449 induce apoptosis of prostate
cancer stem cells through a GLI-dependent mechanism. J Cell
Biochem. 119:3641–3652. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Rubinstein MR, Baik JE, Lagana SM, Han RP,
Raab WJ, Sahoo D, Dalerba P, Wang TC and Han YW: Fusobacterium
nucleatum promotes colorectal cancer by inducing Wnt/β-catenin
modulator Annexin A1. EMBO Rep. 20:e476382019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhan T, Ambrosi G, Wandmacher AM, Rauscher
B, Betge J, Rindtorff N, Häussler RS, Hinsenkamp I, Bamberg L,
Hessling B, et al: MEK inhibitors activate Wnt signalling and
induce stem cell plasticity in colorectal cancer. Nat Commun.
10:21972019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Jackstadt R, van Hooff SR, Leach JD,
Cortes-Lavaud X, Lohuis JO, Ridgway RA, Wouters VM, Roper J,
Kendall TJ, Roxburgh CS, et al: Epithelial NOTCH signaling rewires
the tumor microenvironment of colorectal cancer to drive
poor-prognosis subtypes and metastasis. Cancer Cell. 36:319–336.e7.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Miyazaki Y, Matsubara S, Ding Q, Tsukasa
K, Yoshimitsu M, Kosai K and Takao S: Efficient elimination of
pancreatic cancer stem cells by hedgehog/GLI inhibitor GANT61 in
combination with mTOR inhibition. Mol Cancer. 15:492016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hua F, Shang S, Yang YW, Zhang HZ, Xu TL,
Yu JJ, Zhou DD, Cui B, Li K, Lv XX, et al: TRIB3 interacts With
β-Catenin and TCF4 to increase stem cell features of colorectal
cancer stem cells and tumorigenesis. Gastroenterology.
156:708–721.e15. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Huang R, Wang G, Song Y, Tang Q, You Q,
Liu Z, Chen Y, Zhang Q, Li J, Muhammand S and Wang X: Colorectal
cancer stem cell and chemoresistant colorectal cancer cell
phenotypes and increased sensitivity to Notch pathway inhibitor.
Mol Med Rep. 12:2417–2424. 2015. View Article : Google Scholar : PubMed/NCBI
|