Introduction
One of the major issues in the field of oncology is
tumor heterogeneity. Tumor heterogeneity is described as
differences between tumors of the same type in different patients
(inter-tumor heterogeneity), as well as between cancer cells in a
single tumor of one patient [intra-tumor heterogeneity (ITH)].
These differences may be morphological, physiological and/or
genetic and may result in differences in progression, metastasis
and response to treatment (1,2).
In sporadic colorectal cancers (CRC), inter-tumor
heterogeneity is widely present, resulting in cases without any
identified well-known or with numerous different genetic
aberrations, suggesting that there are still undiscovered
mechanisms (3,4). One of the challenging fields in CRC
progression is ITH, which is frequently a source of variability in
tumors. ITH may be characterized by temporal (variations within a
given tumor over time) and spatial (variations in distinctive
regions of a tumor) differences in genetic mutations, epigenetic
regulation and expression of coding and non-coding genes, which may
be specific to each individual patient. ITH may be clonal and
related to different types of genomic instability that may also
explain certain differences between the primary tumor and its
metastasis. ITH is also closely related to cancer progression,
aggressiveness, therapy resistance and recurrences (4).
Tumor progression is thought to rely on a minority
of cells in a given tumor recognized as cancer stem cell (CSC)-like
cells, which are capable of self-renewal and differentiation. It is
thought that CSCs represent the basis for tumor growth and
metastatic spread (3), since they
have a higher propensity towards invasion, suggesting that they are
enriched in all stages of metastasis. CSCs may be found as a
sub-population on the invasive tumor front as well as based on
genes related to CSCs, supporting this hypothesis (5–7).
Certain studies reported the potential involvement of CSCs in the
progression of CRC (8,9). Genes associated with CSC features may
be promising prognostic and therapeutic markers. It has been
previously indicated that CSC-associated molecular profiles may
predict tumour regeneration and disease relapse after conventional
therapy in patients with CRC (9–14).
Several potential anti-CSC targeted drugs have emerged in previous
studies, with some of them making their way to the clinic (15). Furthermore, studying microRNAS
(miRNAs) regulating selected genes is a promising therapeutic
approach (16), with miRNAs having
anti- or pro-metastatic effects, while they may also be considered
as potential biomarkers for metastasis (17).
Clonal selection of CSCs is considered the main
mechanism underlying differences between primary tumors and
metastasis, suggesting that ITH is present in metastatic spread.
Polyclonal seeding of CSCs and inter-metastatic exchange of cancer
clones are also possible and may contribute to ITH (4). In addition, in most CRC cases, the
sub-clonal origin of the local lymph node metastases is thought to
be different from that of distant metastases (18), where liver metastases is most
commonly due to anatomical factors related to the portal
circulation (19), resulting in a
complex picture of potential expression patterns for CSC-related
genes and regulatory miRNAs.
Sampling from the border of the tumor, including the
surrounding stroma and the sub-border in comparison to the central
part, may provide distinctive information, since different areas of
the same tumor may have different patterns of gene expression.
Differences in expression patterns between the central part of
primary CRC and its invasive tumor front, as well as between
primary tumor and lymph node and liver metastasis, have been
reported (4). A total of four
CSC-related genes LINE1 type transposase domain containing 1
(L1TD1), SLIT and NTRK like family member 6 (SLITRK6), ST6
N-acetylgalactosaminide alpha-2,6-sialyltransferase 1 (ST6GALNAC1)
and transcription elongation factor A3 (TCEA3) (20–27)
and their potential regulatory miRNAs (miR-199a-3p, miR-425-5p,
miR-1225-3p, miR-1233-3p and miR-1303) that were indicated to be
involved in the cancerogenesis of CRC according to a previous study
by our group (28) were now
analyzed for spatial and temporal expression changes to investigate
their involvement in the development of metastatic CRC.
Materials and methods
Tissue samples
All tissue samples were fixed for 24 h in 10%
buffered formalin prior to paraffin embedding. After this step,
tissues were cut into 3–4 µm slices and stained with haematoxylin
and eosin for routine histopathological examination. CRC specimens
were histopathologically examined and classified according to the
pathologic Tumor-Nodes-Metastasis system (29). For the purposes of the present
study, representative paraffin blocks from the years 2006 and
2015–2019 were collected retrospectively from the archives of the
Institute of Pathology, Faculty of Medicine, University of
Ljubljana (Ljubljana, Slovenia).
In all cases, three representative tissue cores were
punched using a 0.6-mm inner diameter needle. Tissue cores were
taken from the central part of the tumor, invasive tumor front and
from lymph node metastases, liver metastases or both. Sporadic CRC
cases with corresponding lymph node/liver metastases were included
in the study. Only samples that passed the subsequent RNA quality
assessment were included in the study.
RNA isolation from formalin-fixed
paraffin-embedded (FFPE) tissue cores
Isolation of total RNA from three tissue cores per
region was performed using a MagMax FFPE DNA/RNA Ultra kit (Applied
Biosystems; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol with a modification. Protease digestion was
performed overnight at 56°C with shaking for 15 sec at 300 rpm
every 4 min on an Eppendorf ThermoMixer®C (Eppendorf
SE). A NanoDrop®−1000 Spectrophotometer (Thermo Fisher
Scientific, Inc.) was used to determine the concentration and
assess the quality of the isolates at the wavelengths of 260, 280
and 230 nm.
RNA quality assessment
Reverse transcription (RT) of RNU6B, a housekeeping
small nuclear RNA gene, was used as the quality control, followed
by amplification using quantitative real-time PCR (qPCR) and TaqMan
methodology (Thermo Fisher Scientific, Inc.). All of the samples
included in the study had passed this quality control step.
Positive and negative amplification of RNU6B was in positive
correlation with positive and negative amplification of GAPDH (100
bp) initially used as quality control in previous studies (data not
shown) (30,31). In addition, TaqMan primers and
probes that amplify and detect PCR products <100 bp in length
were chosen, as indicated in Table
I.
 | Table I.Probes used for miRNA and mRNA
quantification using reverse-transcription quantitative PCR. |
Table I.
Probes used for miRNA and mRNA
quantification using reverse-transcription quantitative PCR.
| Gene/miRNA
name | Probe ID/miRNA
ID | Sequence (probe
sequence or mature miRNA sequence in 5′-3′ direction) |
|---|
| B2M | Hs99999907_m1 |
GTTAAGTGGGATCGAGACATGTAAG |
| IPO8 | Hs00183533_m1 |
GGGGAATTGATCAGTGCATTCCACT |
| L1TD1 | Hs00219459_m1 |
TTTTTCGCCAGGCACCAAGGCACAG |
| SLITRK6 | Hs00536106_s1 |
TTTCCATGGACTGGAAAACCTGGAA |
|
ST6GALNAC1 | Hs01027885_m1 |
AGGAGGCCTTCAGACGACTTGCCCT |
| TCEA3 | Hs00957468_m1 |
GAAATCGAAGATCATATCTACCAAG |
|
hsa-mir-199a-3p | 002304 |
ACAGUAGUCUGCACAUUGGUUA |
| hsa-mir-425-5p | 001516 |
AAUGACACGAUCACUCCCGUUGA |
|
hsa-miR-1225-3p | 002766 |
UGAGCCCCUGUGCCGCCCCCAG |
|
hsa-mir-1233-3p | 002768 |
UGAGCCCUGUCCUCCCGCAG |
| hsa-mir-1274b | 002884 |
UCCCUGUUCGGGCGCCA |
| hsa-mir-1303 | 002792 |
UUUAGAGACGGGGUCUUGCUCU |
| RNU6B | 001093 |
CGCAAGGATGACACGCAAATTCGTGAAGCGTTCCATATTTTT |
Efficiency testing
A pre-designed mixture of probes and primers
specific for miRNAs or target genes (mRNAs) was used. Three pools
of RNA samples were created, obtained from primary colorectal
tumors, lymph node metastases and liver metastases prior to qPCR.
The obtained cDNA of miRNAs and pre-amplified cDNA of mRNAs was
diluted in four steps, ranging from 5-point dilution to 625-point
dilution, and the probes were tested for qPCR efficiency. The qPCR
efficiency reactions were performed on a RotorGene Q (Qiagen GmbH)
in triplicate. The efficiency was calculated as follows:
E=10(−1/slope)-1 (32).
RT of miRNAs
Looped primers for specific RT of miRNAs and a
MicroRNA TaqMan RT kit (Applied Biosystems; Thermo Fisher
Scientific, Inc.) were used as per the manufacturer's protocol for
RT. RNU6B and miR-1247b were used as reference genes (RGs). miRNAs
were tested relative to the geometric mean of the expression of
RNU6B and miR-1247b (Table I). The
RT reaction mix (10 µl) was prepared with 10 ng of total RNA
sample, 1.0 µl of MultiScribe Reverse Transcriptase (50 U/µl), 1.0
µl of RT Buffer (10X), 0.1 µl of dNTP (100 mM), 0.19 µl RNAase
inhibitor (20 U/µl) and 2.0 µl of RT primer (5X). The reaction
conditions were: 16°C for 30 min, 42°C for 30 min and 85°C for 5
min.
qPCR of miRNAs
qPCR for miRNAs was performed in a PCR mixture (10
µl) containing 5.0 µl TaqMan 2X FastStart Essential DNA Probe
Master (Roche Diagnostics), 0.5 µl TaqMan assay 20X and 4.5 µl RT
products diluted 100-fold. qPCR was performed on a RotorGene Q
(Qiagen GmbH) in duplicate, as follows: Initial denaturation at
95°C for 10 min and 40 cycles of 15 sec at 95°C (denaturation) and
60 sec at 60°C (primer annealing and elongation). The signal was
collected at the endpoint of every cycle.
RT of mRNAs
The mRNAs summarized in Table I were analyzed relative to the
geometric mean of the RGs IPO8 and B2M. They were reverse
transcribed using a OneTaq RT-PCR Kit (New England BioLabs, Inc.)
using random primers according to the manufacturer's instructions.
RT reactions were performed with 3.0 µl (60 ng) of total RNA and
1.0 µl of Random Primer Mix incubated at 70°C for 5 min. The 10 µl
RT mixture included 5.0 µl of M-MuLV Reaction Mix, 1.0 µl of M-MuLV
reverse transcriptase and 4.0 µl of reaction mix after random
priming. The reaction conditions were as follows: 25°C for 5 min,
42°C for 60 min and 80°C for 4 min.
Pre-amplification and qPCR of
mRNAs
Following RT, pre-amplification was performed using
a TaqMan PreAmp Master Mix (Applied Biosystems; Thermo Fisher
Scientific, Inc.) in a 10-µl reaction according to the
manufacturer's protocol. The obtained PreAmp reaction was diluted
5-fold in all cases, except when investigating lymph-node
metastases, where it was diluted 25-fold. For the qPCR, 4.5 µl of
the diluted sample was used in a 10-µl reaction volume with 5.0 µl
of 2X FastStart Essential DNA Probe Master Mix (Roche Diagnostics)
and 0.5 µl of TaqMan 20X probe. The thermal cycling conditions were
as follows: 50°C for 2 min, initial denaturation at 95°C for 10 min
and 40 cycles of denaturation at 95°C for 15 sec and annealing at
60°C for 1 min. All qPCR analyses were performed on a Rotor Gene Q
(Qiagen GmbH) in duplicate. The signal was collected at the
endpoint of each cycle.
Statistical analysis
The results were presented as relative gene
expression. All quantification cycle values (Cqs) were corrected
for PCR efficiencies and the expression of the gene of interest was
calculated relative to a geometric mean of RGs, named ΔCq using the
∆∆Cq method (32). In CRC samples,
expression differences in mRNAs and miRNAs were compared between
the central part and invasive front, lymph node metastases or liver
metastases, using ΔCq and the Wilcoxon Signed-Rank test. For all of
the investigated correlations/associations, Spearman rank-order
correlation was used. An additional Bonferroni correction was
performed for the investigated comparisons after the Wilcoxon
Signed Rank test and comparisons that failed to pass the adjusted
α-value were mentioned accordingly. Statistical analysis of data
was performed using SPSS version 24 (IBM Corporation). Differences
were considered significant at P≤0.05.
Results
Patients and tissue samples
A total of 19 patients with CRC were included in the
study, namely seven patients with lymph node but not liver
metastasis (N+ M0 group; mean age, 76.0±13.5 years; age range,
54–91 years; males/females, 6:1), three patients without lymph node
but with liver metastases (N0 M+ group; mean age, 72.0±6.1 years;
age range, 68–79 years; males/females, 2:1) and nine patients with
both lymph node and liver metastases (N+ M+ group; mean age,
69.3±16.5 years; age range, 31–88 years; male/female, 5:4).
Demographic data and clinicopathological features for each patient
are also available in previously published work (33). In total, 63 tissue samples from 19
patients with CRC, with lymph node metastases and/or liver
metastases, were analyzed. The invasive tumor front, lymph node and
liver metastases were compared to the corresponding central part of
the primary tumor.
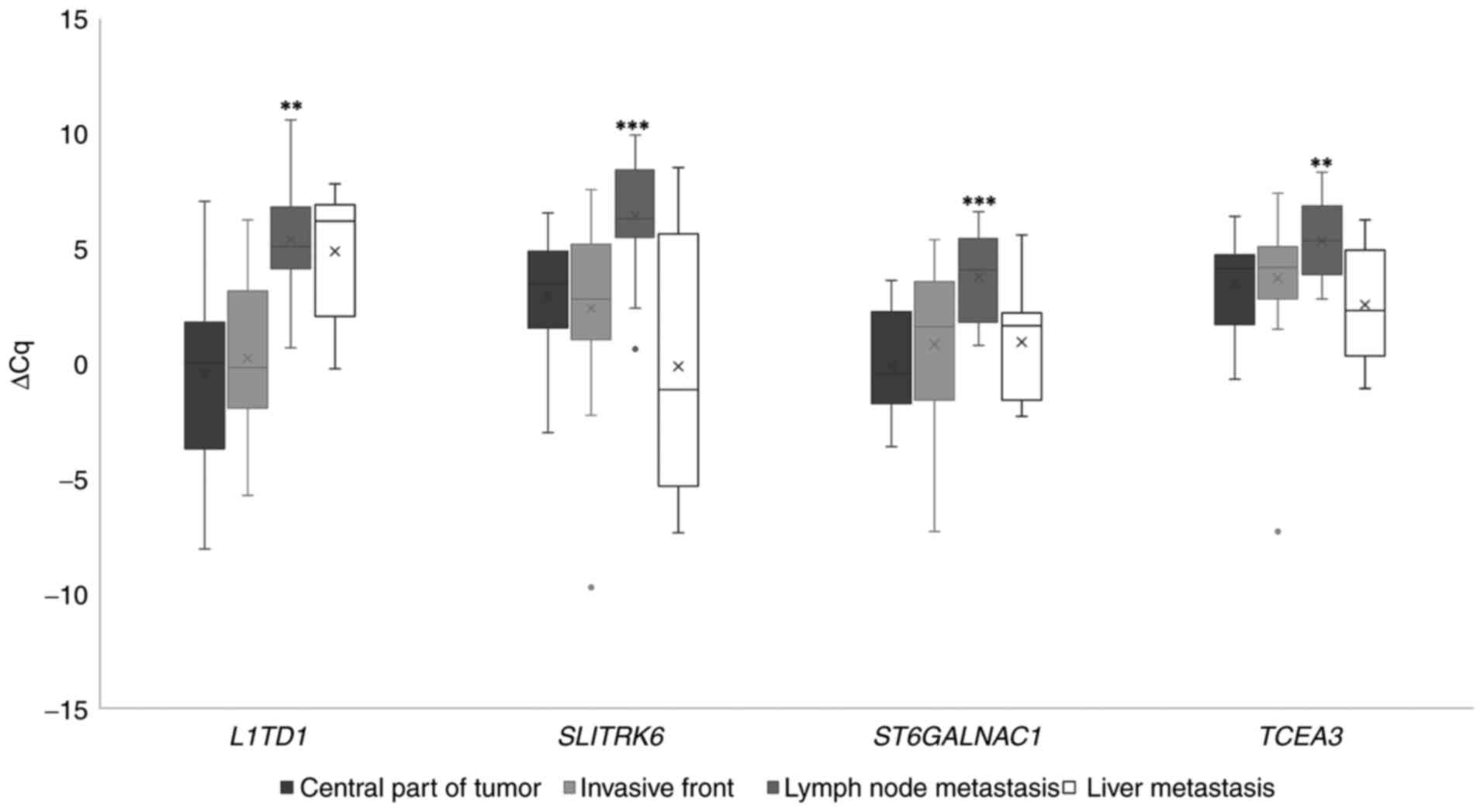
Expression of mRNAs
When comparing the invasive tumor front to the
central part of the primary tumor, ST6GALNAC1 and TCEA3 exhibited a
statistically significant difference in expression prior to
Bonferroni correction; downregulation in this case. Regarding
comparisons between the central part of the primary tumor and lymph
node metastases, all genes investigated were downregulated with
statistical significance. No statistically significant differences
were observed when comparing liver metastases to the central part
of the primary tumor for the investigated genes (Fig. 1).
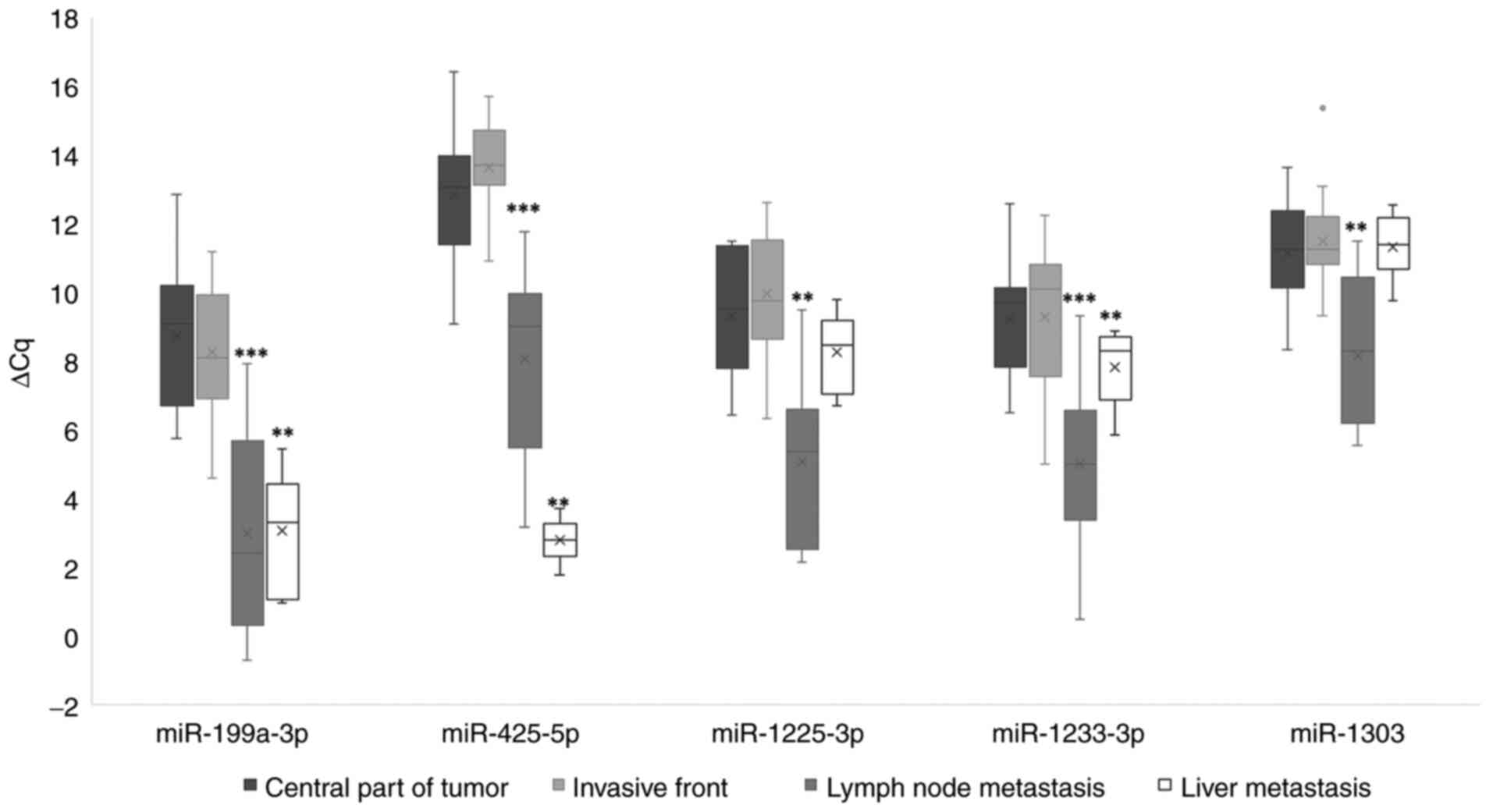
Expression of miRNAs
When comparing the invasive tumor front to the
central part of the primary tumor, miR-199a-3p exhibited a
statistically significant upregulation only prior to Bonferroni
correction. Regarding comparisons between the central part of the
primary tumor and lymph node metastases, all miRNAs investigated
were upregulated, with statistical significance. In liver
metastases, significantly upregulated expression was observed when
comparing to the central part of the primary tumor for miR-199a-3p,
miR-425-5p and miR-1233-3p, as indicated in Fig. 2.
A heatmap of the expression changes of all
investigated genes and miRNAs in each patient is additionally
available in Table SI. Fold
changes and P-values for all investigated comparisons between
groups on gene/miRNA expression are available in Table SII for gene expression and
Table SIII for miRNA
expression.
Correlations between the investigated
mRNAs and miRNAs
Spearman coefficients of the correlation revealed
negative correlations of a weak or moderate nature for all
significant correlations between the investigated genes and miRNAs.
miR-1303 did not correlate significantly with any of the
investigated genes. TCEA3 did not correlate significantly with any
of the investigated miRNAs. L1TD1 exhibited a significant, negative
moderate correlation with miR-199a-3p, miR-425-5p, miR-1225-3p and
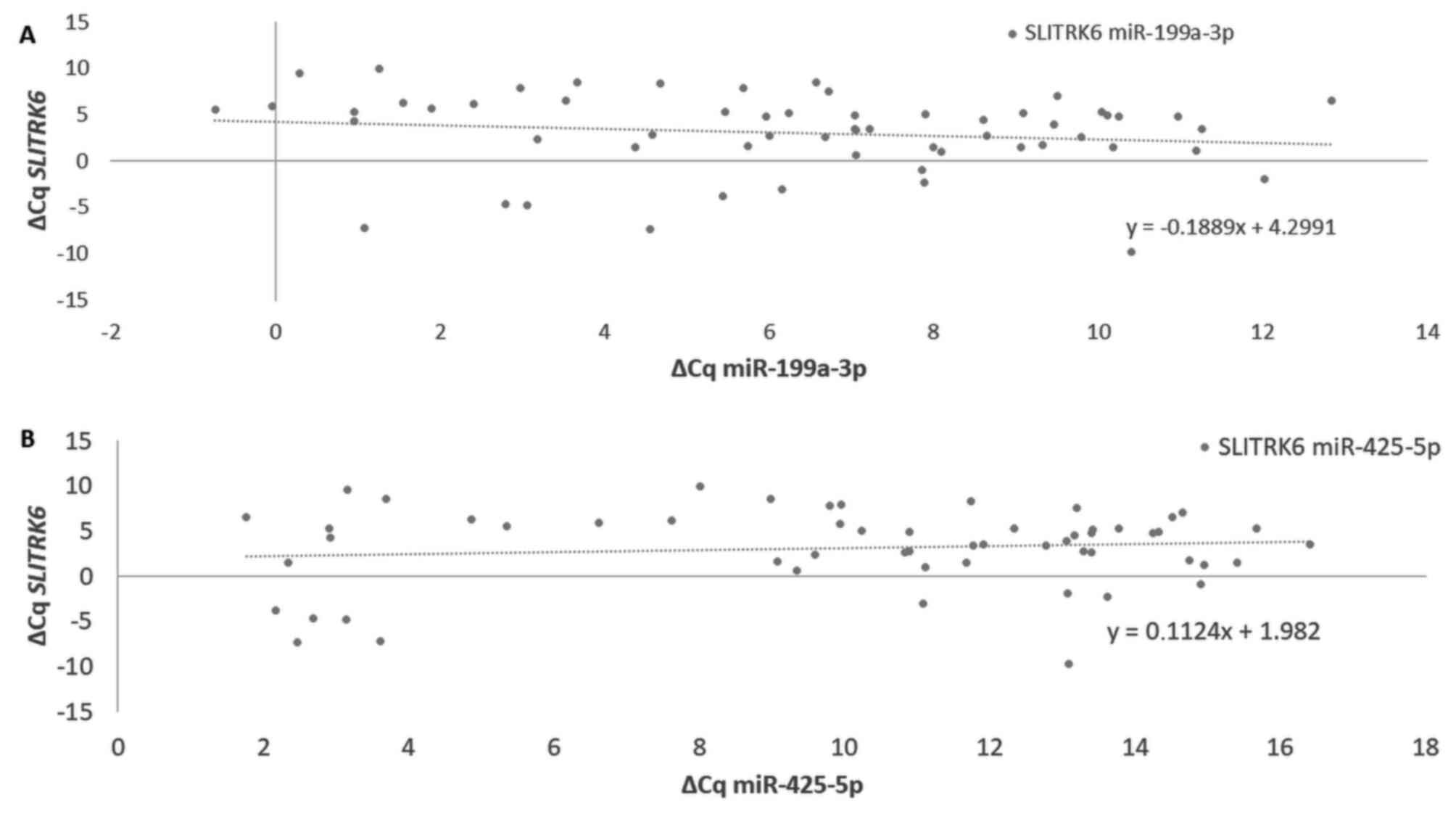
miR-1233-3p. SLITRK6 had a weak negative significant correlation
with its proposed regulatory miRNA miR-199a-3p. In addition,
ST6GALNAC1 had weak negative significant correlations with
miR-199a-3p, miR-425-5p, miR-1225-3p and miR-1233-3p. ∆Cq
comparisons between the potential regulatory miRNAs miR-199a-3p and
miR-425-5p and target gene SLITRK6 are presented in Fig. 3. Additional expression comparisons
for L1TD1, TCEA3 and their potential regulatory miRNAs are
available in Fig. S1. Results
including P-values are summarized in Table II.
 | Table II.Significant Spearman correlation
coefficients and corresponding P-values for comparisons between the
investigated genes and miRNAs. |
Table II.
Significant Spearman correlation
coefficients and corresponding P-values for comparisons between the
investigated genes and miRNAs.
| Gene | miR-199a-3p | miR-425-5p | miR-1225-3p | miR-1233-3p | miR-1303 |
|---|
| L1TD1 | −0.474
(P=0.001) | −0.538
(P<0.001) | −0.443
(P=0.004) | −0.496
(P<0.001) | / |
| SLITRK6 | −0.259
(P=0.048) | / | / | / | / |
|
ST6GALNAC1 | −0.358
(P=0.006) | −0.266
(P=0.050) | −0.385
(P=0.006) | −0.397
(P=0.002) | / |
| TCEA3 | / | / | / | / | / |
As presented in Table
III, significant positive Spearman correlation coefficients
were obtained between all of the investigated miRNAs. The
correlations were either weak (e.g. miR-425-5p and miR-1303,
rs=0.2-0.39), moderate (e.g. miR-199a-3p and miR-1303,
rs=0.40-0.59), strong (e.g. miR-199a-3p and miR-1225-3p,
rs=0.6-0.79) or very strong (e.g. miR-199a-3p and miR-425-5p,
rs>0.8).
 | Table III.Significant Spearman correlation
coefficients and corresponding P-values for investigated
comparisons between the miRNAs. |
Table III.
Significant Spearman correlation
coefficients and corresponding P-values for investigated
comparisons between the miRNAs.
| miRNA | miR-199a-3p | miR-425-5p | miR-1225-3p | miR-1233-3p | miR-1303 |
|---|
| miR-199a-3p | 1 | 0.823
(P<0.001) | 0.653
(P<0.001) | 0.633
(P<0.001) | 0.460
(P<0.001) |
| miR-425-5p |
| 1 | 0.587
(P<0.001) | 0.592
(P<0.001) | 0.314
(P=0.025) |
| miR-1225-3p |
|
| 1 | 0.886
(P<0.001) | 0.699
(P<0.001) |
| miR-1233-3p |
|
|
| 1 | 0.714
(P<0.001) |
| miR-1303 |
|
|
|
| 1 |
Discussion
In the present study, the expression of four genes
related to CSC and CSC-like properties were validated in CRC tissue
samples, obtained from the central part of primary tumors, invasive
tumor front, lymph node and liver metastases. The investigated
genes (L1TD1, SLITRK6, ST6GALNAC1, TCEA3) were previously
identified as differentially expressed between normal mucosa,
adenomas and CRC using bioinformatics analysis of publicly
available microarray data (34),
and validated to be involved in CRC carcinogenesis together with
their potential regulatory miRNAs (28).
Regarding the expression of the investigated mRNAs,
two different patterns were observed. The first pattern was
observed in lymph node metastases compared to the central part of
the primary tumor, where all investigated genes were downregulated.
The second one was observed in liver metastases, where none of the
investigated genes was differentially expressed when compared to
the central part of the primary tumor. By contrast, their
regulatory miRNAs exhibited variable expression, except in the case
of lymph node metastases when compared to the central part of the
tumor, where all of the investigated miRNAs exhibited the opposite
expression trend to their target mRNAs.
In lymph node metastases, when compared to the
central part of the primary tumor, all investigated genes were
downregulated. Bioinformatics analysis indicated that a higher
expression of L1TD1 in CRC was associated with longer disease-free
survival (35), confirming the
negative trend of expression of L1TD1 in relation to invasiveness
observed in the present study. Small inhibitory RNA-mediated
silencing of ST6GALNAC1 in gastric cancer cells was previously
reported to lead to reduced growth, migration and invasion of
cancer cells (36), whereas its
overexpression enhanced their metastatic ability (37). High levels of ST6GALNAC1 were
observed in ovarian CSCs and silencing of ST6GALNAC1 was indicated
to reduce cell proliferation, migration, invasion, self-renewal
ability and tumorigenicity (38).
For TCEA3, a previous study by our group reported significantly
different expression between CRC without and with lymph node
metastases, which suggested a role in lymph node metastasis
development (28). TCEA3 has also
been associated with gastric cancer, in which high expression has
been associated with better prognosis, lower proliferation of
carcinoma cells and induction of apoptosis (39). The results thus suggest that higher
expression of these genes is necessary for cancer progression to
lymph nodes. To the best of our knowledge, the present study was
the first to report that the SLITRK6 gene was downregulated in
lymph node metastases of CRC, indicating its possible role in
metastasizing CRC, which may be further investigated in future
studies.
In liver metastases, none of the investigated genes
exhibited differential expression when compared to the central
part. This observation is not surprising, since in a previous
study, gene expression profiling using microarrays clearly
distinguished normal colon mucosa and normal liver from primary CRC
and liver metastases, respectively; the authors observed moderate
variations in expression of most differentially expressed genes, or
their dysregulation limited to one individual (40). The observation, that primary CRC
and liver metastases have a similar expression pattern was further
supported by high-throughput transcriptome sequencing (41). This finding suggests that
expression changes consistently occur during CRC development, but
only a small number of them may be associated with metastatic
progression to the liver (40). In
liver metastases, only two different subpopulations of CSCs were
identified based on the expression of OCT4, one in the peritumoral
stroma and the other in tumor nests (42). The same group reported that the
only two populations of CSCs in primary tumor may also be
stratified by OCT4 expression (43). The present expression analysis
supports this observation of a small number of CSC subclones in
primary tumor and liver metastases.
Based on 213 archival biopsy samples investigating
genetic changes between primary tumor, lymph node and distant
metastases from 17 patients, it has been recently indicated that in
65% of cases of lymph node and liver metastases, they arise from
independent sub-clones, whereas in 35% of cases, they share a
common origin (18). The same
group further confirmed that lymph node and distant metastases
develop through different evolutionary mechanisms, with a higher
inter-lesion heterogeneity of lymph node metastases (44). Furthermore, the KRAS mutation
status in CRC confirmed a much lower level of concordance when
comparing primary tumor and matched lymph node metastases; however,
a high concordance rate was observed between primary and matched
distant (e.g., liver) metastases (3). The observations of the present study
further support the hypothesis that not only on the chromosomal,
genetic and epigenetic levels, but also on the expression level,
there is a higher ITH between primary tumor and lymph node
metastases than between primary tumor and liver metastases, further
confirming different developmental pathways of lymph node and liver
metastases.
In lymph node as well as in liver metastases, all
investigated miRNAs were up-regulated when compared to the center
of the primary tumor, except miR-1303, which was downregulated in
liver metastases. miR-199a-3p was indicated to target stemness and
mitogenic-related pathways to suppress the expansion and
tumorigenic capabilities of prostate CSCs in vitro (45). Its expression was observed to
significantly differ between metastatic and low metastatic groups
of patients with uveal melanoma (46). It was not able to promote tumor
cell proliferation in melanoma; however, it may regulate metastatic
invasion of melanoma, angiogenesis and endothelial cell recruitment
(47). An analysis of clinical and
pathologic data revealed that a higher miR-199a-3p expression
contributed to more advanced lymphatic invasion and lymph node
metastases, as well as liver metastases, in CRC (48). miR-425-5p facilitates
epithelial-mesenchymal transition and extracellular matrix
degradation and promotes hepatocellular carcinoma cell metastasis
(49). Of note, it has been
indicated that miR-1225-5p suppresses gastric cancer invasion and
metastases (50) and that it
inhibits apoptosis of pancreatic cancer cells (51). Furthermore, overexpression of
miR-1233-3p promoted the migration and invasion of human breast
cancer cells (52). Downregulation
of miR-1303 inhibited the proliferation, migration and invasion of
gastric cancer cells (53) and it
also suppressed the proliferation, migration and invasion of
prostate cancer cells (54). In
addition, miR-1303 was one of the five miRNAs, where the expression
signature was an independent predictor of poor metastasis-free
survival of patients with breast cancer (55). It was also upregulated in a
bioinformatics analysis of microarray data between primary CRC
tumors and liver metastases (56).
In non-small cell lung cancer, high expression of miR-1303 was
associated with TNM stage, lymph node metastasis and shorter
survival time, and its overexpression in H1299 and A549 cells
promoted cell proliferation, migration and invasion (57). These data suggest that all five
genes and all investigated miRNAs are involved in the formation of
CRC metastases in lymph nodes, but only three out of five miRNAs
are involved in the formation of liver metastases of CRC.
An advantage of the present study is the use of the
punching technique, which enabled us to obtain tissue from the
locations of interest, determined by microscopic analysis by a
pathologist. A limitation of the present study is the relatively
small sample size, as cases that fit the inclusion criteria are not
very common. Another limitation is that only samples that
successfully passed the initial quality control and samples with
stable expression of the RGs were selected for further analysis,
thus limiting the number of included samples. As another
limitation, patients were sub-grouped as patients with only lymph
node metastases (N+ M0), patients with only liver metastases (N0
M+) and patients with both lymph node and liver metastases (N+ M+),
resulting in a small number of patients with only liver metastases.
However, such cases are rare and it is difficult to collect
sufficient sample numbers; therefore, these results should be
interpreted with caution. Furthermore, there was an unequal
male/female ratio; however, there are no public data available that
investigated genes and miRNAs are differentially regulated in
different genders in CRC. Another limitation of the present study
is that the results were not validated further through a functional
study using a CRC cell line and experimental animals. Finally, the
study lacks direct validation of a regulatory role of the
investigated miRNAs on the analyzed genes; however, inverse
co-expression analysis may provide indirect support for the
predicted regulation.
In conclusion, the present study analyzed the
expression patterns of the L1TD1, SLITRK6, ST6GALNAC1 and TCEA3
genes and their potential regulatory miRNAs in tissues obtained
using a punching technique to enable the validation of ITH of these
genes in CRC. The central part of the primary tumor, the invasive
tumor front, as well as lymph node and liver metastases were
compared. Of note, all genes and all miRNAs were differentially
expressed in lymph node metastases. However, none of the
investigated genes were differentially expressed in liver
metastases, whereas the majority of miRNAs were. The present
results thus indicate a role of all of the investigated genes in
the development of lymph node metastases, but for none of them in
the development of liver metastases. As CSCs are involved in
treatment resistance and disease recurrence, analysis of CSC
markers has prognostic and therapeutic potential (9–14).
Their potential regulatory miRNAs are easily delivered in vivo due
to their small size. Synthetic miRNAs may therefore be administered
systematically and may thus serve as therapeutic agents in the
future (16,17). Future perspectives include
validation of obtained results through a functional study both
in vivo and in vitro.
Supplementary Material
Supporting Data
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
This research was funded by the Slovenian Research Agency
(research core funding grant no. P3-0054; project funding grant no.
J3-1754; PhD research funding for KU).
Availability of data and materials
The ∆∆Cq data that support the findings of the
present study are available in Table
SI. The full ∆Cq data are available from the corresponding
author upon reasonable request. Demographic data and
clinicopathological features for each patient are available upon
reasonable request from the authors or in previously published work
by our group (33).
Authors' contributions
Conceptualization of article: KU, EB and NZ.
Methodology: KU and EB. Data acquisition: KU and AT. KU and EB
checked and approved the authenticity of the raw data. Formal
analysis: KU. Original draft preparation, KU and EB. Review and
editing: EB, NZ and AT. Visualization: KU. Supervision: EB, AT and
NZ. All authors have read and agreed to the published version of
the manuscript.
Ethics approval and consent to
participate
The present study was performed according to the
tenets of the Declaration of Helsinki. The study is retrospective,
observational, performed on tissue samples that were obtained
during routine diagnostic/therapeutic procedures, consisting of
either excision or resection. Sufficient tissue was available for
routine analysis and research; furthermore, tissue is still
available for any additional routine analysis in the future. Prior
to excision, or resection, informed consent was obtained for the
routine surgical procedure. The National Medical Ethics Committee
(Ljubljana, Slovenia) approved the study and any further
requirement for informed consent for participation in scientific
studies was waived (approval no. 0120-54/2020/7).
Patient consent for publication
Further need for consent for participation in
scientific studies was waived by the National Medical Ethics
Committee (approval number 0120-54/2020/7) due to the nature of the
study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Marusyk A and Polyak K: Tumor
heterogeneity: Causes and consequences. Biochim Biophys Acta.
1805:105–117. 2010.
|
|
2
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar
|
|
3
|
Blank A, Roberts DE II, Dawson H, Zlobec I
and Lugli A: Tumor heterogeneity in primary colorectal cancer and
corresponding metastases. Does the apple fall far from the tree?
Front Med (Lausanne). 5:2342018.
|
|
4
|
Stanta G and Bonin S: Overview on clinical
relevance of intra-tumor heterogeneity. Front Med (Lausanne).
5:852018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Prager BC, Xie Q, Bao S and Rich JN:
Cancer stem cells: The architects of the tumor ecosystem. Cell Stem
Cell. 24:41–53. 2019. View Article : Google Scholar
|
|
6
|
Grillet F, Bayet E, Villeronce O, Zappia
L, Lagerqvist EL, Lunke S, Charafe-Jauffret E, Pham K, Molck C,
Rolland N, et al: Circulating tumour cells from patients with
colorectal cancer have cancer stem cell hallmarks in ex vivo
culture. Gut. 66:1802–1810. 2017. View Article : Google Scholar
|
|
7
|
Toloudi M, Apostolou P, Chatziioannou M
and Papasotiriou I: Correlation between cancer stem cells and
circulating tumor cells and their value. Case Rep Oncol. 4:44–54.
2011. View Article : Google Scholar
|
|
8
|
Saiki Y, Ishimaru S, Mimori K, Takatsuno
Y, Nagahara M, Ishii H, Yamada K and Mori M: Comprehensive analysis
of the clinical significance of inducing pluripotent
stemness-related gene expression in colorectal cancer cells. Ann
Surg Oncol. 16:2638–2644. 2009. View Article : Google Scholar
|
|
9
|
Dylla SJ, Beviglia L, Park IK, Chartier C,
Raval J, Ngan L, Pickell K, Aguilar J, Lazetic S, Smith-Berdan S,
et al: Colorectal cancer stem cells are enriched in xenogeneic
tumors following chemotherapy. PLoS One. 3:e24282008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Giampieri R, Scartozzi M, Loretelli C,
Piva F, Mandolesi A, Lezoche G, Del Prete M, Bittoni A, Faloppi L,
Bianconi M, et al: Cancer stem cell gene profile as predictor of
relapse in high risk stage II and stage III, radically resected
colon cancer patients. PLoS One. 8:e728432013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Merlos-Suárez A, Barriga FM, Jung P,
Iglesias M, Céspedes MV, Rossell D, Sevillano M, Hernando-Momblona
X, da Silva-Diz V, Muñoz P, et al: The intestinal stem cell
signature identifies colorectal cancer stem cells and predicts
disease relapse. Cell Stem Cell. 8:511–524. 2011. View Article : Google Scholar
|
|
12
|
Colak S, Zimberlin CD, Fessler E, Hogdal
L, Prasetyanti PR, Grandela CM, Letai A and Medema JP: Decreased
mitochondrial priming determines chemoresistance of colon cancer
stem cells. Cell Death Differ. 21:1170–1177. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lombardo Y, Scopelliti A, Cammareri P,
Todaro M, Iovino F, Ricci-Vitiani L, Gulotta G, Dieli F, de Maria R
and Stassi G: Bone morphogenetic protein 4 induces differentiation
of colorectal cancer stem cells and increases their response to
chemotherapy in mice. Gastroenterology. 140:297–309. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lotti F, Jarrar AM, Pai RK, Hitomi M,
Lathia J, Mace A, Gantt GA Jr, Sukhdeo K, DeVecchio J, Vasanji A,
et al: Chemotherapy activates cancer-associated fibroblasts to
maintain colorectal cancer-initiating cells by IL-17A. J Exp Med.
210:2851–2872. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zeuner A, Todaro M, Stassi G and De Maria
R: Colorectal cancer stem cells: From the crypt to the clinic. Cell
Stem Cell. 15:692–705. 2014. View Article : Google Scholar
|
|
16
|
Wang V and Wu W: MicroRNA-based
therapeutics for cancer. Biodrugs. 23:15–23. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang D, Liu J, Huo T, Tian Y and Zhao L:
The role of microRNAs in colorectal liver metastasis: Important
participants and potential clinical significances. Tumour Biol.
39:10104283177096402017. View Article : Google Scholar
|
|
18
|
Naxerova K, Reiter JG, Brachtel E, Lennerz
JK, van de Wetering M, Rowan A, Cai T, Clevers H, Swanton C, Nowak
MA, et al: Origins of lymphatic and distant metastases in human
colorectal cancer. Science. 357:55–60. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gonzalez-Villarreal CA, Quiroz-Reyes AG,
Islas JF and Garza-Treviño EN: Colorectal cancer stem cells in the
progression to liver metastasis. Front Oncol. 10:15112020.
View Article : Google Scholar
|
|
20
|
Wong RC, Ibrahim A, Fong H, Thompson N,
Lock LF and Donovan PJ: L1TD1 is a marker for undifferentiated
human embryonic stem cells. PLoS One. 6:e193552011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Närvä E, Rahkonen N, Emani MR, Lund R,
Pursiheimo JP, Nästi J, Autio R, Rasool O, Denessiouk K, Lähdesmäki
H, et al: RNA-binding protein L1TD1 interacts with LIN28 via RNA
and is required for human embryonic stem cell self-renewal and
cancer cell proliferation. Stem Cells. 30:452–460. 2012. View Article : Google Scholar
|
|
22
|
Emani MR, Närvä E, Stubb A, Chakroborty D,
Viitala M, Rokka A, Rahkonen N, Moulder R, Denessiouk K, Trokovic
R, et al: The L1TD1 protein interactome reveals the importance of
post-transcriptional regulation in human pluripotency. Stem Cell
Reports. 4:519–528. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sandberg CJ, Vik-Mo EO, Behnan J, Helseth
E and Langmoen IA: Transcriptional profiling of adult neural
stem-like cells from the human brain. PLoS One. 9:e1147392014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Farahani E, Patra HK, Jangamreddy JR,
Rashedi I, Kawalec M, Rao Pariti RK, Batakis P and Wiechec E: Cell
adhesion molecules and their relation to (cancer) cell stemness.
Carcinogenesis. 35:747–759. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ambriz X, de Lanerolle P and Ambrosio JR:
The mechanobiology of the actin cytoskeleton in stem cells during
differentiation and interaction with biomaterials. Stem Cells Int.
2018:28919572018. View Article : Google Scholar
|
|
26
|
Ogawa T, Hirohashi Y, Murai A, Nishidate
T, Okita K, Wang L, Ikehara Y, Satoyoshi T, Usui A, Kubo T, et al:
ST6GALNAC1 plays important roles in enhancing cancer stem
phenotypes of colorectal cancer via the Akt pathway. Oncotarget.
8:112550–112564. 2017. View Article : Google Scholar
|
|
27
|
Park KS, Cha Y, Kim CH, Ahn HJ, Kim D, Ko
S, Kim KH, Chang MY, Ko JH, Noh YS, et al: Transcription elongation
factor Tcea3 regulates the pluripotent differentiation potential of
mouse embryonic stem cells via the lefty1-nodal-smad2 pathway. Stem
Cells. 31:282–292. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Urh K, Žlajpah M, Zidar N and Boštjančič
E: Identification and validation of new cancer stem cell-related
genes and their regulatory microRNAs in colorectal cancerogenesis.
Biomedicines. 9:1792021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Brierley JD, Gospodarowicz MK and
Wittekind C: TNM Classification of Malignant Tumours. 8th edition.
Wiley-Blackwell; Oxford, UK: 2017
|
|
30
|
Ranković B, Zidar N, Žlajpah M and
Boštjančič E: Epithelial-mesenchymal transition-related microRNAs
and their target genes in colorectal cancerogenesis. J Clin Med.
8:16032019. View Article : Google Scholar
|
|
31
|
Žlajpah M, Hauptman N, Boštjančič E and
Zidar N: Differential expression of extracellular matrix-related
genes DCN, EPHA4, FN1, SPARC, SPON2 and SPP1 in colorectal
carcinogenesis. Oncol Rep. 42:1539–1548. 2019.
|
|
32
|
Latham GJ: Normalization of microRNA
quantitative RT-PCR data in reduced scale experimental designs.
Methods Mol Biol. 667:19–31. 2010. View Article : Google Scholar
|
|
33
|
Pavlič A, Urh K, Štajer K, Boštjančič E
and Zidar N: Epithelial-mesenchymal transition in colorectal
carcinoma: Comparison between primary tumor, lymph node and liver
metastases. Front Oncol. 11:6628062021. View Article : Google Scholar
|
|
34
|
Hauptman N, Boštjančič E, Žlajpah M,
Ranković B and Zidar N: Bioinformatics analysis reveals most
prominent gene candidates to distinguish colorectal adenoma from
adenocarcinoma. Biomed Res Int. 2018:94165152018. View Article : Google Scholar
|
|
35
|
Chakroborty D, Emani MR, Klén R, Böckelman
C, Hagström J, Haglund C, Ristimäki A, Lahesmaa R and Elo LL:
L1TD1-a prognostic marker for colon cancer. BMC Cancer. 19:7272019.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tamura F, Sato Y, Hirakawa M, Yoshida M,
Ono M, Osuga T, Okagawa Y, Uemura N, Arihara Y, Murase K, et al:
RNAi-mediated gene silencing of ST6GalNAc I suppresses the
metastatic potential in gastric cancer cells. Gastric Cancer.
19:85–97. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ozaki H, Matsuzaki H, Ando H, Kaji H,
Nakanishi H, Ikehara Y and Narimatsu H: Enhancement of metastatic
ability by ectopic expression of ST6GalNAcI on a gastric cancer
cell line in a mouse model. Clin Exp Metastasis. 29:229–238. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang WY, Cao YX, Zhou X, Wei B, Zhan L and
Sun SY: Stimulative role of ST6GALNAC1 in proliferation, migration
and invasion of ovarian cancer stem cells via the Akt signaling
pathway. Cancer Cell Int. 19:862019. View Article : Google Scholar
|
|
39
|
Li J, Jin Y, Pan S, Chen Y, Wang K, Lin C,
Jin S and Wu J: TCEA3 attenuates gastric cancer growth by apoptosis
induction. Med Sci Monit. 21:3241–3246. 2015. View Article : Google Scholar
|
|
40
|
Koehler A, Bataille F, Schmid C, Ruemmele
P, Waldeck A, Blaszyk H, Hartmann A, Hofstaedter F and Dietmaier W:
Gene expression profiling of colorectal cancer and metastases
divides tumours according to their clinicopathological stage. J
Pathol. 204:65–74. 2004. View Article : Google Scholar
|
|
41
|
Lee JR, Kwon CH, Choi Y, Park HJ, Kim HS,
Jo HJ, Oh N and Park do Y: Transcriptome analysis of paired primary
colorectal carcinoma and liver metastases reveals fusion
transcripts and similar gene expression profiles in primary
carcinoma and liver metastases. BMC Cancer. 16:5392016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Humphries HN, Wickremesekera SK, Marsh RW,
Brasch HD, Mehrotra S, Tan ST and Itinteang T: Characterization of
cancer stem cells in colon adenocarcinoma metastasis to the liver.
Front Surg. 4:762018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Munro MJ, Wickremesekera SK, Peng L, Marsh
RW, Itinteang T and Tan ST: Cancer stem cell subpopulations in
primary colon adenocarcinoma. PLoS One. 14:e02219632019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Reiter JG, Hung WT, Lee IH, Nagpal S,
Giunta P, Degner S, Liu G, Wassenaar ECE, Jeck WR, Taylor MS, et
al: Lymph node metastases develop through a wider evolutionary
bottleneck than distant metastases. Nat Genet. 52:692–700. 2020.
View Article : Google Scholar
|
|
45
|
Liu R, Liu C, Zhang D, Liu B, Chen X,
Rycaj K, Jeter C, Calhoun-Davis T, Li Y, Yang T, et al: miR-199a-3p
targets stemness-related and mitogenic signaling pathways to
suppress the expansion and tumorigenic capabilities of prostate
cancer stem cells. Oncotarget. 7:56628–56642. 2016. View Article : Google Scholar
|
|
46
|
Worley LA, Long MD, Onken MD and Harbour
JW: Micro-RNAs associated with metastasis in uveal melanoma
identified by multiplexed microarray profiling. Melanoma Res.
18:184–190. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Pencheva N, Tran H, Buss C, Huh D,
Drobnjak M, Busam K and Tavazoie SF: Convergent multi-miRNA
targeting of ApoE drives LRP1/LRP8-dependent melanoma metastasis
and angiogenesis. Cell. 151:1068–1082. 2012. View Article : Google Scholar
|
|
48
|
Wan D, He S, Xie B, Xu G, Gu W, Shen C, Hu
Y, Wang X, Zhi Q and Wang L: Aberrant expression of miR-199a-3p and
its clinical significance in colorectal cancers. Med Oncol.
30:3782013. View Article : Google Scholar
|
|
49
|
Fang F, Song T, Zhang T, Cui Y, Zhang G
and Xiong Q: MiR-425-5p promotes invasion and metastasis of
hepatocellular carcinoma cells through SCAI-mediated dysregulation
of multiple signaling pathways. Oncotarget. 8:31745–31757. 2017.
View Article : Google Scholar
|
|
50
|
Zheng H, Zhang F and Lin X, Huang C, Zhang
Y, Li Y, Lin J, Chen W and Lin X: MicroRNA-1225-5p inhibits
proliferation and metastasis of gastric carcinoma through
repressing insulin receptor substrate-1 and activation of β-catenin
signaling. Oncotarget. 7:4647–4663. 2016. View Article : Google Scholar
|
|
51
|
Zhong R, Li S, Fang K, Yang L and Wang L:
microRNA-1225 inhibit apoptosis of pancreatic cancer cells via
targeting JAK1. Cell Cycle. 18:990–1000. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lu M, Wu Y, Zeng B, Sun J, Li Y, Luo J,
Wang L, Yi Z, Li H and Ren G: CircEHMT1 inhibits metastatic
potential of breast cancer cells by modulating
miR-1233-3p/KLF4/MMP2 axis. Biochem Biophys Res Commun.
526:306–313. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhang SJ, Feng JF, Wang L, Guo W, Du YW,
Ming L and Zhao GQ: miR-1303 targets claudin-18 gene to modulate
proliferation and invasion of gastric cancer cells. Dig Dis Sci.
59:1754–1763. 2014. View Article : Google Scholar
|
|
54
|
Liu B, Zhou W, Jiang H, Xiang Z and Wang
L: miR-1303 promotes the proliferation, migration and invasion of
prostate cancer cells through regulating the Wnt/β-catenin pathway
by targeting DKK3. Exp Ther Med. 18:4747–4757. 2019.PubMed/NCBI
|
|
55
|
Lerebours F, Cizeron-Clairac G, Susini A,
Vacher S, Mouret-Fourme E, Belichard C, Brain E, Alberini JL,
Spyratos F, Lidereau R and Bieche I: miRNA expression profiling of
inflammatory breast cancer identifies a 5-miRNA signature
predictive of breast tumor aggressiveness. Int J Cancer.
133:1614–1623. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Torres S, Garcia-Palmero I, Bartolomé RA,
Fernandez-Aceñero MJ, Molina E, Calviño E, Segura MF and Casal JI:
Combined miRNA profiling and proteomics demonstrates that different
miRNAs target a common set of proteins to promote colorectal cancer
metastasis. J Pathol. 242:39–51. 2017. View Article : Google Scholar
|
|
57
|
Chen J, Jiang T, Yu B, Li T, Zhao P, Yuan
L and Qi J: Upregulation of microRNA-1303 is a potential prognostic
marker of non-small cell lung cancer. Cancer Biomark. 28:439–446.
2020. View Article : Google Scholar : PubMed/NCBI
|