Introduction
Cervical cancer is a global malignancy burden among
females; it ranks 4th in both incidence and mortality worldwide
based on GLOBOCAN 2020 (1). It is
estimated that lower-to-middle income countries account for ~84–90%
of the global cervical cancer cases (2). Particularly in Indonesia, cervical
cancer is the second most common and the second deadliest
malignancy reported (1). It was
also noted that the incidence rate of cervical cancer in Indonesia
rose by ~17% between 1990 to 2017; however, the death rate remains
relatively similar (3). Increased
efforts are required to improve the treatment of cervical
cancer.
Radiotherapy has an essential role in the treatment
of cervical cancer. Concurrent chemoradiotherapy may yield a 5-year
overall survival rate of almost 70% for locally advanced cervical
cancer (4). In comparison with
radiotherapy alone, chemoradiotherapy also leads to a significant
6% improvement in 5-year-survival (5). Adjuvant radiotherapy may decrease
disease recurrence with a relative risk of 0.53 compared to no
treatment. Although chemoradiation is the mainstay treatment for
locally advanced cervical cancer, the results of this treatment
modality remain unsatisfactory.
Based on radiobiology, the effect of radiation
increases if the cell is in the radiosensitive phase when exposure
is given. Radiotherapy failure occurs when the proportion of
radioresistant cells is more significant, so that tumour
proliferation cannot be prevented.
It is critical to remember that cancer cells are
most sensitive to radiation during the G2/M phase of the cell
cycle. However, clinical identification and assessment of cell
kinetics to obtain the timing of G2/M phases are difficult and
impractical (6). A previous study
by our group examined the DNA content using flow cytometry to
assess the proportion of G2/M and S phases and to analyse whether
the proportion of sensitive phases had a circadian pattern
(7). However, the study was not
able to provide objective results due to the limited number of
samples.
It is worthwhile to explore factors affecting
radiotherapy and cellular kinetics, which may potentially increase
radiosensitivity in cervical cancer. The circadian rhythm and the
melatonin concentration are two such factors, which happen to be
interrelated. The circadian cycle is a biorhythmic daily period in
various body systems, featuring a specific, intricate, harmonious
pattern. The circadian and cell cycles are two critical systems
initially considered separate, but several studies have proven a
close relationship between them (8–14).
Furthermore, melatonin protects cells against the
side effects of radiation because it is a scavenger of
OH-containing molecules (15).
However, it has remained elusive whether the circadian rhythm and
the level of melatonin may clinically affect the radiation
response.
The current standard conventional radiation therapy
guideline does not specify the timing for radiation treatment
(morning, afternoon or evening) and there is no adjustment or
preference for the timing of radiation among individual patients.
Differences in response to radiation are expected in patients with
cervical cancer between the morning and afternoon radiation groups
due to the circadian rhythm and melatonin levels.
In the present study, it was hypothesised that the
circadian cycle influences tumour radiosensitivity, including that
of cervical cancer. A previous study by our group indicated that
the melatonin concentration in patients with cervical cancer was
significantly different when measured in the morning and in the
afternoon (7). Based on these
initial findings, a further study was performed and patients with
cervical cancer were allocated into two groups: Patients who were
irradiated in the morning and those irradiated in the afternoon.
The pre-treatment melatonin concentration in the blood was measured
exactly prior to irradiation to closely represent the melatonin
concentration in the two groups.
The present study tested the hypothesis that a
difference in radiation sensitivity of cervical cancer is present,
depending on the time of day within 24 h. Based on previous
findings, a study was designed to examine the effect of radiation
administration at two different times in the circadian pattern on
tumour response. The melatonin levels in each subject in the two
groups were checked immediately prior to radiation to determine the
melatonin levels corresponding to the time of radiation
administration. Melatonin levels were examined three times in the
irradiation period from the beginning of external radiation until
the end of brachytherapy. It included the time-points prior to the
initial irradiation, in the middle of the radiation period (at the
15–20th fraction) and after brachytherapy (7).
A case-control study was herein performed following
on from the previous research, aiming to enhance the significance
of the results and evaluate the 5-year survival rate. The present
study aimed to identify prognostic factors, including the circadian
cycle and melatonin levels, which may affect the response to
radiation in patients with cervical cancer.
Patients and methods
Study population
The present study was conducted at the Radiotherapy
Department at Cipto Mangunkusumo Hospital (Jakarta, Indonesia) in
cooperation with the Department of Obstetrics and Gynaecology of
the Faculty of Medicine, Universitas Indonesia-Cipto Mangunkusumo
Hospital (Jakarta, Indonesia). The subjects were initially enrolled
in this study between March 2012 and August 2014. The target
population included patients with International Federation of
Gynaecology and Obstetrics (FIGO) stage IIB-IIIB cervical cancer
who received no previous treatment and had histopathologically
confirmed squamous cell carcinoma. Patients with recurrent cancer,
HIV-positive status, and chronic diseases, including diabetes
mellitus and hypertension, were excluded. Subjects were patients
who completed regular standard radiotherapy treatment either in the
morning (06.00-10.00 am) or the afternoon (04.00-06.00 pm).
According to World Health Organization tumour response criteria
(16), the subjects were
classified based on post-radiotherapy tumour response into subjects
with a good response (complete and near-complete response/<1 cm)
and poor response (partial response, progressive disease or stable
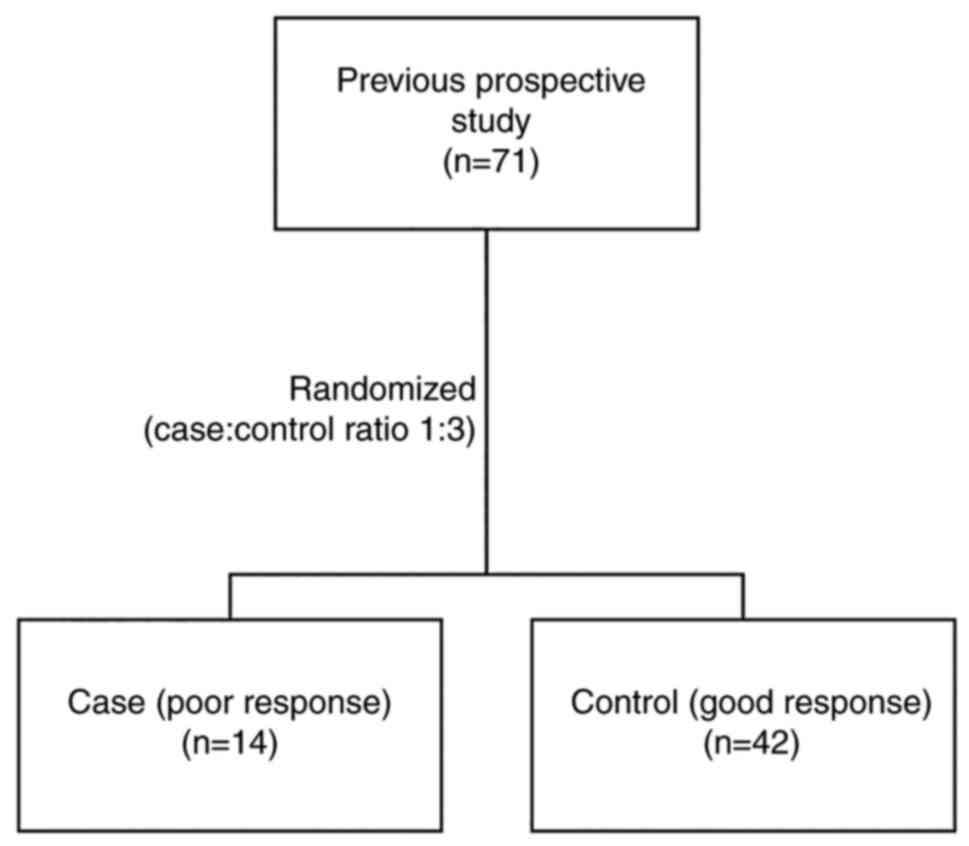
disease). Poor response cases were assigned to the case group. The
remaining patients with complete melatonin data were randomized
into the control group. To ensure good statistical power in the
study, a ratio of 1:3 between the case and control groups was used
(Fig. 1) (17,18).
All other possible confounding variables were denoted and included
in the analysis.
The patients included were aged 25–70 years with a
Karnofsky Performance Status >70, haemoglobin (Hb) levels >10
g/dl and had provided written informed consent to participate in
the study. The standard treatment in this study was radiation alone
or combined with chemotherapy. Radiotherapy was administered based
on the protocol adopted by our department, which was five times a
week (25×2 Gy) followed by intracavitary brachytherapy (3×7 Gy)
once a week for three weeks. The decision to add chemotherapy or
not was at the clinician's discretion. Patients were excluded if
they did not complete at least a regular irradiation schedule
comprising 20 sessions of external beam radiation therapy and two
sessions of brachytherapy or if the patients received <25
fractions of the radiotherapy regimen. The response to radiation in
the present study was assessed four weeks after the completion of
brachytherapy. Blood samples for melatonin workup were taken in the
morning or afternoon based on irradiation time. Melatonin was
measured by ELISA using a melatonin kit (cat. no. IBL-RE54021; IBL
International GmbH) and peripheral blood specimens were obtained
before the start of the first session of radiotherapy (19).
Other variables investigated that may contribute to
treatment response included age, time of radiotherapy, overall
treatment time, Hb level, pathological findings, and initial
clinical tumour size. Time of radiation was defined as when the
external irradiation or brachytherapy was performed; each patient
had been randomly assigned for irradiation treatment at 6–10 am for
the morning group or at 4–6 pm for the afternoon group. The
treatment was stationary and treatment time was according to the
hospital's schedule. The time windows for the morning and afternoon
groups were set according to the common melatonin level curve in
the body. The overall treatment time was defined as the days
between the first irradiation and the completion of brachytherapy.
The Hb level was measured immediately before the initial
irradiation treatment by using the standard Hb-cyanide
spectrophotometric method at the Department of Clinical Pathology,
Dr Cipto Mangunkusumo Hospital (Jakarta, Indonesia) and patients
were divided into normal Hb (>10 g/dl) and low Hb (≤10 g/dl)
groups. The pathologists performed a histopathological examination
based on cell differentiation and keratinisation. The initial
clinical tumour size was recorded at a gynaecological examination
of the local tumour measuring the clinical tumour volume in the
anteroposterior, latero-lateral and craniocaudal aspects in
centimetres at the initial visit. Other variables, including age,
the combination of chemotherapy, body weight, blood transfusion,
erythrocyte sedimentation rate and intravenous pyelography results,
were collected from medical records.
Statistical analysis
The statistical analysis was performed using SPSS
version 20.0 software (IBM Corporation). The relationship between
potential prognostic factors and tumour response after irradiation
was investigated by performing a univariate analysis using the χ2
test or Fisher's exact test. P<0.05 was considered to indicate
statistical significance. In the univariate analysis, variables
with P<0.25 were deemed suitable and included for multivariate
analysis using binary logistic regression and Nagelkerke's R2 to
identify the prognostic factors for radiation responses in patients
with cervical cancer. Multicollinearity was assessed by correlation
matrix. Kaplan-Meier curves were drawn, and log-rank tests were
used to determine the 5-year survival rate.
Results
Characteristics of subjects in the two
groups
The complete medical records of 71 patients with
cervical cancer were collected between March 2012 and August 2014
in Dr Cipto Mangunkusumo Hospital (Jakarta, Indonesia). Most
patients were <50 years old; 57.1% had poor response and 64.3%
with good response. From the 71 patients, the proportion of
subjects in each group was adjusted using a 1:3 ratio of
cases/controls. A total of 14 patients with poor response were
included in the case group, while the control group consisted of 42
of 57 patients with good response who were randomly selected from
the control group. The clinical and laboratory data of the
patients, including age, initial weight and the presence of pain,
are presented in Table I. The
tumour size was significantly related to the tumour response after
irradiation (P=0.002).
 | Table I.Subject characteristics (percentage
based on column). |
Table I.
Subject characteristics (percentage
based on column).
|
| Radiation
response |
|
|---|
|
|
|
|
|---|
| Variable | Poor (n=14) | Good (n=42) | P-value |
|---|
| Age, years |
|
| 0.633a |
|
≤50 | 8 (57.1) | 27 (64.3) |
|
|
>50 | 6 (42.9) | 15 (35.7) |
|
| Initial body
weight, kg |
|
| 0.106b |
|
≤50 | 2 (14.3) | 17 (40.5) |
|
|
>50 | 12 (85.7) | 25 (59.5) |
|
| Pain |
|
| 0.518b |
| No | 8 (57.1) | 29 (69.0) |
|
|
Yes | 6 (42.9) | 13 (31.0) |
|
| Clinical tumour
size, cm3 |
|
| 0.002a |
| ≤40
(small) | 2 (14.3) | 26 (61.9) |
|
| >40
(large) | 12 (85.7) | 16 (38.1) |
|
| Initial Hb level
(g/dl) |
|
| 0.058b |
|
>10 | 10 (71.4) | 39 (92.9) |
|
|
≤10 | 4 (28.6) | 3 (7.1) |
|
| ESR |
|
| 0.097b |
|
≤40 | 10 (71.4) | 38 (90.5) |
|
|
>40 | 4 (28.6) | 4 (9.5) |
|
| Pathological
differentiation |
|
| 0.119b |
|
Moderate/well | 9 (64.3) | 36 (85.7) |
|
|
Poor/moderate | 5 (35.7) | 6 (14.3) |
|
| Pathological
keratinisation |
|
| 1.000b |
| No | 13 (92.9) | 39 (92.9) |
|
|
Yes | 1 (7.1) | 3 (7.1) |
|
A comparison between clinical tumour size and stage
is included in Table II. Most
patients were categorized into the stage III group, as they came
for treatment after their symptoms developed into a more advanced
stage. The patients with stage II and stage III were equally
distributed based on clinical tumour sizes, as cervical cancer
staging is determined by tumour size and parametrium invasion;
thus, tumour size alone is insufficient to determine stages.
 | Table II.Association between tumor size and
stage. |
Table II.
Association between tumor size and
stage.
| Stage | Small tumor, ≤40
cm3 (n=28) | Large tumor, >40
cm3 (n=28) |
|---|
| Undefined | 2 (7.1) | 0 (0) |
| II | 6 (21.4) | 5 (17.9) |
| III | 20 (71.4) | 23 (82.1) |
In addition, the comparison of tumour responses
according to melatonin levels between the subjects irradiated in
the morning and the afternoon was presented in Table III. The results suggested that
the influence of melatonin levels on the tumour response in both
groups was insignificant.
 | Table III.Relationship between melatonin levels
and tumour response. |
Table III.
Relationship between melatonin levels
and tumour response.
| Group/melatonin
levels, pg/ml | Good response | Poor response | P-value |
|---|
| Morning |
|
| 0.298 |
| ≤13
(low) | 10 (66.7) | 5 (33.3) |
|
| >13
(high) | 10 (83.3) | 2 (16.7) |
|
| Afternoon |
|
| 0.223 |
| ≤13
(low) | 7 (63.6) | 4 (36.4) |
|
| >13
(high) | 15 (83.3) | 3 (4.3) |
|
Potential factors affecting the
radiation response
Univariate analysis was performed to identify
variables significantly predictive of the response to radiation. As
indicated in Table IV, tumour
size (P=0.002) and transfusion during radiation (P=0.004) were
significantly associated with the response to radiation. Other
predictive variables significantly related to tumour response were
the time of radiation (P=0.045) and post-treatment body weight
(P=0.027).
 | Table IV.Prognostic factors affecting the
radiation response of patients (n=56) four weeks after
brachytherapy. |
Table IV.
Prognostic factors affecting the
radiation response of patients (n=56) four weeks after
brachytherapy.
|
| Clinical
response |
|
|---|
|
|
|
|
|---|
| Variable | Good (n=42) | Poor (n=14) | P-value |
|---|
| Time of
radiation |
|
| 0.045a |
|
Morning | 25 (59.5) | 4 (28.6) |
|
|
Afternoon | 17 (40.5) | 10 (71.4) |
|
| Chemotherapy |
|
| 0.356b |
|
Yes | 23 (54.8) | 11 (78.6) |
|
| No | 19 (45.2) | 3 (21.4) |
|
| Pathological
keratinisation |
|
| 1.000b |
|
Yes | 3 (7.1) | 1 (7.1) |
|
| No | 39 (92.9) | 13 (92.9) |
|
| Differentiation
status |
|
| 0.119b |
|
Moderate/well | 36 (85.7) | 9 (64.3) |
|
|
Poor/moderate | 6 (14.3) | 5 (35.7) |
|
| Body weight after
treatment, kg |
|
| 0.027a |
|
>50 | 22 (52.4) | 12 (85.7) |
|
|
≤50 | 20 (47.6) | 2 (14.3) |
|
| Reduction of body
weight >5 kg |
|
| 0.089a |
| No | 23 (54.8) | 4 (28.6) |
|
|
Yes | 19 (45.2) | 10 (71.4) |
|
| IVP |
|
| 0.070a |
|
Normal | 26 (89.7) | 19 (70.4) |
|
|
Abnormal | 3 (10.3) | 8 (29.6) |
|
| Reduction of
Hb |
|
| 0.310a |
| No | 10 (34.5) | 6 (22.2) |
|
|
Yes | 19 (65.5) | 21 (77.8) |
|
| Pre-radiation blood
transfusion |
|
| 0.080a |
| No | 29 (69.0) | 6 (42.9) |
|
|
Yes | 13 (31.0) | 8 (57.1) |
|
| Blood transfusion
during radiation |
|
| 0.004a |
| No | 30 (71.4) | 4 (28.6) |
|
|
Yes | 12 (28.6) | 10 (71.4) |
|
| Alignment with
radiation time |
|
| 1.000b |
|
Yes | 36 (85.7) | 12 (85.7) |
|
| No | 6 (14.3) | 2 (14.3) |
|
| OTT |
|
| 1.751b |
|
On-time | 29 (69.0) | 9 (64.3) |
|
| Not on
time | 13 (31) | 5 (35.7) |
|
Time of radiation affects the
radiation response
According to the multivariate analysis, radiation
time in the morning [adjusted odds ratio (OR)=8.70, 95%
CI=1.25-60.73, P=0.023], normal initial Hb level (OR=13.53, 95%
CI=1.38-132.25, P=0.017) and small clinical tumour size (OR=8.85,
95% CI=1.45-54.16, P=0.039) were associated with a significantly
better tumour response to treatment at 4 weeks after brachytherapy.
(Table V). The results of the
multivariate analysis were based on this model with the Hosmer
Lemeshow test (P=0.803), and Nagelkerke's R2 value was 0.441. It
was revealed that the model was a good fit with 44.1% variability
observed in the target variable. There were no multicollinearity
assumptions among the independent variables based on the
correlation matrix.
 | Table V.Factors affecting the tumour response
after irradiation (n=56). |
Table V.
Factors affecting the tumour response
after irradiation (n=56).
|
| Clinical
response |
|
|
|---|
|
|
|
|
|
|---|
| Variable | Good (n=42) | Poor (n=14) | Adj. OR | 95% CI |
|---|
| Radiation time,
morning vs. afternoon | 25 (59.5) | 4 (28.6) | 8.70 | (1.25-60.73) |
| Initial clinical
tumour size, <40 cm3 (small) | 26 (61.9) | 2 (14.3) | 8.85 | (1.45-54.16) |
| Initial Hb level,
>10 g/dl (normal) | 39 (92.9) | 10 (71.4) | 13.52 | (1.38-132.25) |
Relationship between time of radiation
and 5-year overall survival
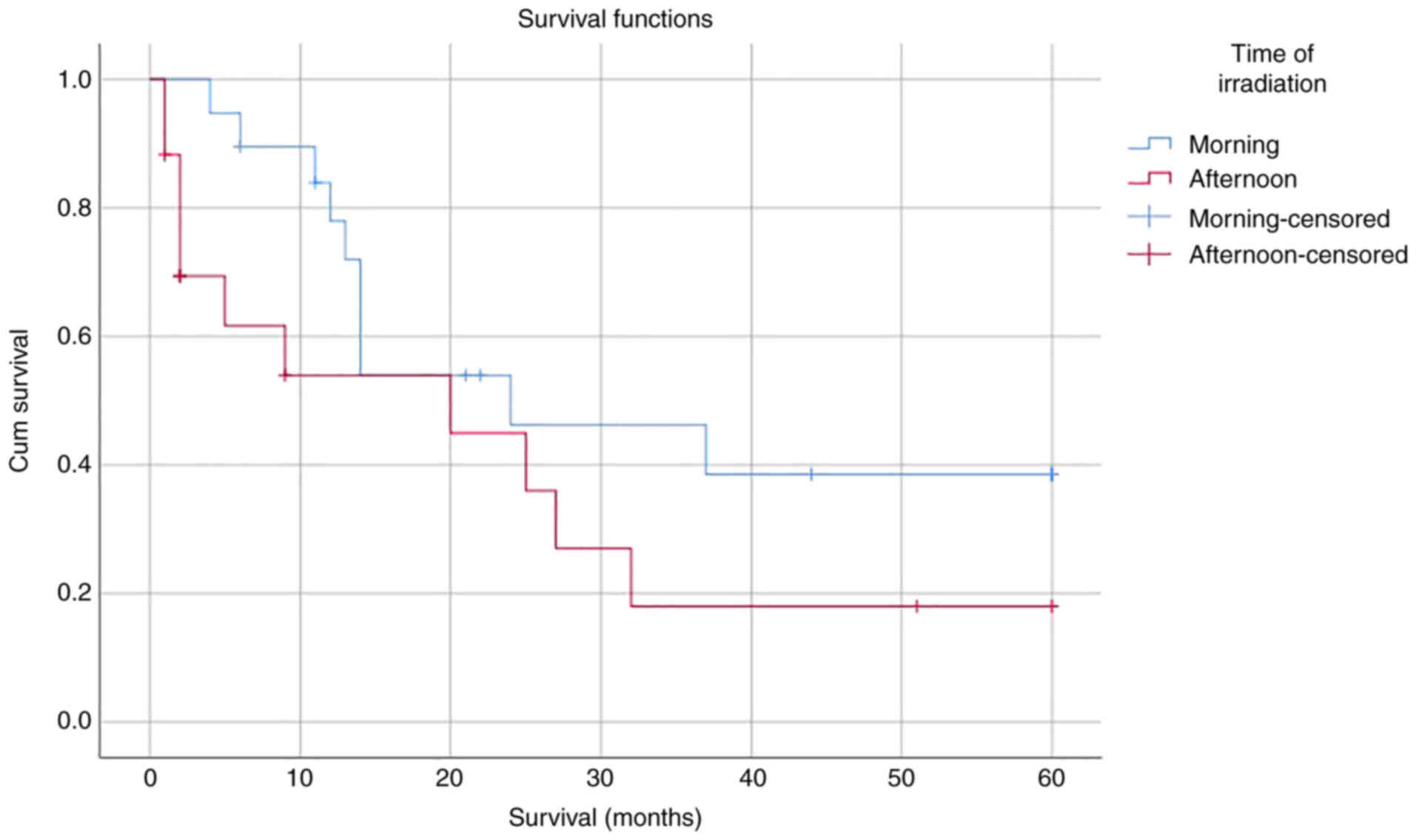
A total of 56 patients were included in the survival
analysis. Among the subjects, three patients were lost to
follow-up. The median follow-up duration was 11 months
(interquartile range, 2.0-24.0 months). During the 5-year follow-up
period, 25 subjects died. Based on the Kaplan-Meier curves
(Fig. 2), the median survival time
of the subjects irradiated in the morning was 24 months (95% CI:
6.30-41.70); meanwhile, the median survival time of the subjects
irradiated in the afternoon was 20 months (95% CI: 0.00-44.06;
P=0.121). In the Kaplan-Meier curves, there was a trend of
irradiation in the morning being associated with increased
survival, but overall, the difference was not significant.
Discussion
The present study aimed to prove the role of the
circadian cycle in the treatment of cervical cancer by examining
the effects of different timing of administering radiation therapy.
Radiotherapy administered in the morning achieved a better tumour
size reduction than radiotherapy administered in the afternoon.
However, there was no significant difference in survival results
between morning and afternoon radiation. This may be worth further
investigating. The initial response in the cohort of the present
study is certainly more representative of factors directly related
to radiosensitivity, whereas survival may be influenced by numerous
confounding factors, including disease severity, metastatic
process, immune response, and side effects of treatment.
It has been proven that the difference in expression
of genes between morning and afternoon is regulated by several
CLOCK genes that work in accordance with circadian rhythms
(20). The concept of
circadian-related radiotherapy aims to deliver radiation with
maximum synergy with the radiosensitive atmosphere provided by the
time system from the ‘internal’ body clock and the world clock. In
a study using zebrafish, Peyric et al (21) demonstrated cell cycle regulation by
the circadian clock. The M phase of the cell cycle occurs
rhythmically and under circadian control (21). This may explain the better
radiation response in the morning, as the probability of cancer
cell death is higher when cells are in the G2M phase. Bjarnason
et al (8) reported that
mucous cells and human skin cells mainly divide in the evening
between 6:00 pm to 12:00 am. Furthermore, three different studies
by Klevec et al (22),
Lakatua et al (23) and
Smaaland et al (24)
indicated that tumour-cell division occurs at an opposite time to
that of healthy cell division. Based on these results, it may be
assumed that cancer cells are more likely to be in the
radiosensitive G2/M phase between 6:00 pm and 12:00 am, whereas
normal cell proliferation occurs in the afternoon. This circadian
pattern is evident in the melatonin hormone levels (22–24).
The levels of melatonin produced by the pineal gland
depend on the circadian patterns: Gradually increasing from ~08.00
pm, reaching a peak at 03.00 am, gradually decreasing until 11.00
am, and reaching their lowest levels between 11.00 am and 08.00 pm
(25–29). Certain studies revealed the
different roles, functions and potential activities of melatonin,
including its utility as a circadian biomarker, function in cancer
development associated with circadian disruption (30,31),
antioxidant activity and inhibition of cancer growth (15,32,33).
Prior studies reported the role and function of
melatonin in cancer in the absence of radiation; to the best of our
knowledge, no study has examined the effects of melatonin levels
and the timing of radiotherapy (morning vs. afternoon) on the
radiation response. Vijayalaxmi et al (33) suspected a role of pineal gland
products in cancer development, particularly melatonin, which
inhibited carcinogenesis in an in vitro study using MCF-7
breast cancer cells. This hormone was specifically demonstrated to
increase the number of apoptotic cells and inhibit metastasis
(34). The cancer-inhibiting
effects of melatonin are influenced by various factors, including
the melatonin concentration in culture media, the pattern of
melatonin administration, the oestrogen receptor status (35,36),
growth hormone levels in culture media and the rate of cell
proliferation (35). Melatonin
inhibits tumour transduction signals and the metabolic activity of
cancer cells through MT1 receptor activity. Even though melatonin
levels were noted to be higher in the morning compared to the
afternoon, the present study does not sufficiently prove the effect
of melatonin on the treatment response. This may be due to the
difference in the concentration of melatonin among individuals, and
thus, cut-off values for high or low concentrations of melatonin
should be individualised. Such a study design may be able to negate
factors with a low influence. The present study has not been able
to provide sufficiently objective results due to the limited number
of samples.
The present study illustrated that Hb levels affect
the radiation response, in line with prior findings that anaemia
and decreased Hb levels are prognostic indicators (7). Decreased Hb levels result in hypoxia,
making cancer cells resistant to radiation. Oxygen increases
radiosensitivity through direct and indirect effects; it is
generally known that oxygenation increases the sensitivity of cells
to radiation (6).
The tumour volume is an essential factor influencing
the success of cervical cancer treatment. Lee et al
(37) assessed the outcomes of 75
patients with stage IIB cervical cancer treated with
chemoradiotherapy using MRI and overall survival was strongly
related to the tumour volume. Specifically, the 5-year overall
survival of patients with tumour volumes of 2.5-10, 10–50 and
>50 ml were 75, 70 and 48%, respectively (38). This is consistent with the results
of the present study that a smaller tumour size increases the
success of therapy. However, the present study analysed the tumour
size only, which was insufficient to determine the stage of
cervical cancer.
In the present study, the tumour response was
measured after 20–25 fractions of radiation, immediately after
radiation and 2–4 weeks after radiation. This is in line with the
time-points selected by Mayr et al (39), who performed MRI in 68 patients
with advanced-stage IB2-IVB cervical cancer prior to radiation,
after 10–12 fractions of radiation, after 20–25 fractions of
radiation and 1–2 months after the completion of radiation.
According to their research, the best time to perform MRI in the
context of outcomes, namely the tumour regression rate, was after
25 fractions of radiation. Their study determined that this
measurement most accurately predicted local control (84 vs. 22%,
P<0.0001) and disease-free survival (63 vs. 20%, P=0.0005).
Based on preliminary research on patients irradiated
in the morning (7), it was
indicated that the melatonin concentration 2 h before radiation was
high, even though its levels were already sharply declining. This
phenomenon does not apply to patients irradiated in the afternoon.
Although multivariate analysis did not indicate that melatonin
levels affected clinical responses, it is possible that melatonin
indirectly contributes to good responses, as the hormone influences
variables that meaningfully predict response. It is also possible
that the combination of radiation in the morning and melatonin
levels influence the response to radiation.
One of the factors that may have induced bias in the
present study was that during Hb level measurement, the patient's
clinical status and blood transfusion status were not considered.
The application of the study results may be generalised to patients
with cervical cancer with FIGO stage IIB-IIIB who are indicated to
receive radiotherapy.
The present study also did not implement the
administration of radiotherapy during dawn (02.00-06.00 am), when
the level of melatonin is theoretically the highest, as it is
impractical in a clinical setting. It was observed that the
circadian cycle has an essential role in radiosensitivity; however,
the present study did not find any significant difference in
melatonin levels between groups. Other factors related to the
circadian cycle should be investigated that may support the
increased radiosensitivity in the morning (06.00-10.00 am).
It was not the primary goal of the present study to
find the highest levels of melatonin in an individual and then
provide radiation at that time because, clinically, this would be
difficult to apply. The present study simply aimed to prove the
existence of a difference in radiosensitivity within a reasonable
time in the daily clinical practice of radiation so that the
results of the present study may be applied.
There are several limitations to the present study.
The initial response may reflect a more specific intrinsic
radiosensitivity that is unaffected by the stage and severity of
the disease. Furthermore, this study did not assess numerous other
factors that may influence the course of the disease, such as
nutritional conditions, vitamin intake, immunity, and
comorbidities, which may be associated with overall survival. The
patients with comorbidities were excluded, so that it was not
possible to further analyse this. Furthermore, the differences in
adrenocorticotropic hormone levels between groups, which has an
essential role in the circadian rhythm and may influence the tumour
response, were not investigated (40). Those limitations should be
considered in a future study.
In conclusion, the present case-control study is a
further step to analyse the strength of the significance of the
results of the study, following up with a 5-year survival analysis.
While the study had a relatively small sample size, it pointed out
that the circadian cycle may affect radiation treatment in cervical
cancer. The circadian cycle, large tumour size and Hb levels
affected the response of cervical cancer to radiation. Small tumour
size, normal initial Hb level, and irradiation in the morning were
associated with better tumour response after radiotherapy. However,
the 5-year survival analysis indicated no significant difference
between irradiation in the morning and afternoon, which was
probably due to the small sample size and certain confounding
factors related to overall survival that were not possible to
control in this study.
Further research with a larger sample size is
required to identify the optimal treatment for patients with
radioresistant features. More sophisticated radiotherapy techniques
such as Intensity-Modulated Radiation Therapy, hyper-fractionation
and radiotherapy combined with chemosensitizers, and other methods
should be applied to achieve a better treatment response. More
accurate evaluations of the initial Hb level and tumour volume will
be beneficial for designing treatment strategies and determining
prognosis.
Acknowledgements
The authors convey their appreciation to Dr Handoko;
Dr Vito Filbert Jayalie; and Dr Yoga Dwi Oktavianda from the
Department of Radiation Oncology, Dr Cipto Mangunkusumo Hospital
(Jakarta, Indonesia) for their assistance during manuscript writing
and statistical analysis.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
IR: Conceptualization, study design, data
collection, data analysis, drafting and finalisation of the
manuscript. SS, LN, ARH and SIW: Conceptualization, study design,
supervision, finalisation of the manuscript. MM: Conceptualization,
study design, data analysis, drafting and finalisation of the
manuscript. SS and NCS: Conceptualization, finalisation of the
manuscript. IR and SS checked and approved the authenticity of the
raw data. All authors read and approved the final version before
manuscript submission.
Ethics approval and consent to
participate
This research was part of a previous prospective
study approved by the Ethics Committee of the Faculty of Medicine
Universitas Indonesia (Jakarta, Indonesia; no.
27/PT02.FK/ETIK/2010). All subjects provided written informed
consent for participating in this study. The protocol was
registered in the National Clinical Trial (NCT) registry (no.
NCT05511740; registration date, 08/20/2022).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hull R, Mbele M, Makhafola T, Hicks C,
Wang SM, Reis RM, Mehrotra R, Mkhize-Kwitshana Z, Kibiki G, Bates
DO and Dlamini Z: Cervical cancer in low and middle-income
countries. Oncol Lett. 20:2058–2074. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wahidin M, Febrianti R and Susanty F:
Burden of cervical cancer in Indonesia: Findings from the global
burden of disease study 1990–2017. Proceedings of the 4th
International Symposium on Health Research. Atlantis Press; Bali:
2020
|
|
4
|
Pearcey R, Brundage M, Drouin P, Jeffrey
J, Johnston D, Lukka H, MacLean G, Souhami L, Stuart G and Tu D:
Phase III trial comparing radical radiotherapy with and without
cisplatin chemotherapy in patients with advanced squamous cell
cancer of the cervix. J Clin Oncol. 20:966–972. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cohen PA, Jhingran A, Oaknin A and Denny
L: Cervical cancer. Lancet. 393:169–182. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Joiner M and van der Kogel A: Basic
Clinical Radiobiology. 4th edition. Hodder Arnold; London: pp.
p3752009
|
|
7
|
Ramli I: Influence of radiation patterns
following circadian rhythm upon response of radiotherapy of uterine
cervix cancer: Melatonin and cycle cell phase as biological marker
and radiosensitivity (unpublished dissertation). Universitas
Indonesia. (Jakarta). 2019.
|
|
8
|
Bjarnason GA, Jordan RC, Wood PA, Li Q,
Lincoln DW, Sothern RB, Hrushesky WJ and Ben-David Y: Circadian
expression of clock genes in human oral mucosa and skin:
Association with specific cell-cycle phases. Am J Pathol.
158:1793–1801. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wood PA, Du-Quiton J, You S and Hrushesky
WJ: Circadian clock coordinates cancer cell cycle progression,
thymidylate synthase, and 5-fluorouracil therapeutic index. Mol
Cancer Ther. 5:2023–2033. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nagoshi E, Saini C, Bauer C, Laroche T,
Naef F and Schibler U: Circadian gene expression in individual
fibroblasts. Cell. 119:693–705. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Warman GR, Tripp HM, Warman VL and Arendt
J: Circadian neuroendocrine physiology and electromagnetic field
studies: Precautions and complexities. Radiat Prot Dosimetry.
106:369–373. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Arjona A and Sarkar DK: Circadian
oscillations of clock genes, cytolytic factors, and cytokines in
rat NK cells. J Immunol. 174:7618–7624. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Granda TG, Liu XH, Smaaland R, Cermakian
N, Filipski E, Sassone-Corsi P and Lévi F: Circadian regulation of
cell cycle and apoptosis proteins in mouse bone marrow and tumor.
FASEB J. 19:304–306. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lis CG, Grutsch JF, Wood P, You M, Rich I
and Hrushesky WJ: Circadian timing in cancer treatment: The
biological foundation for an integrative approach. Integr Cancer
Ther. 19:1–22. 2003.
|
|
15
|
Karbownik M and Reiter RJ: Melatonin
protects against oxidative stress caused by delta-aminolevulinic
acid: Implications for cancer reduction. Cancer Invest. 20:276–286.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Therasse P, Arbuck SG, Eisenhauer EA,
Wanders J, Kaplan RS, Rubinstein L, Verweij J, Glabbeke MV, van
Oosterom AT, Christian MC, et al: New guidelines to evaluate the
response to treatment in solid tumors. J Natl Cancer Inst.
92:205–216. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Biesheuvel CJ, Vergouwe Y, Oudega R, Hoes
AW, Grobbee DE and Moons KG: Advantages of the nested case-control
design in diagnostic research. BMC Med Res Methodol. 8:482008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Langholz B and Clayton D: Sampling
strategies in nested case-control studies. Environ Health Perspect.
102 (Suppl 8):47–51. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kennaway DJ: Measuring melatonin by
immunoassay. J Pineal Res. 69:e126572020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hunt T and Sassone-Corsi P: Riding tandem:
Circadian clocks and the cell cycle. Cell. 129:461–464. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Peyric E, Moore HA and Whitmore D:
Circadian clock regulation of the cell cycle in the zebrafish
intestine. PLoS One. 8:e732092013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Klevecz RR, Shymko RM, Blumenfeld D and
Braly PS: Circadian gating of S phase in human ovarian cancer.
Cancer Res. 47:6267–6271. 1987.PubMed/NCBI
|
|
23
|
Lakatua DJ, White M, Sackett-Lundeen LL
and Haus E: Change in phase relations of circadian rhythms in cell
proliferation induced by time-limited feeding in BALB/c X DBA/2F1
mice bearing a transplantable Harding-Passey tumor. Cancer Res.
43:4068–4072. 1983.PubMed/NCBI
|
|
24
|
Smaaland R, Lote K, Sothern RB and Laerum
OD: DNA synthesis and ploidy in non-Hodgkin's lymphomas demonstrate
intrapatient variation depending on circadian stage of cell
sampling. Cancer Res. 53:3129–3138. 1993.PubMed/NCBI
|
|
25
|
Florez JC and Takahashi JS: Biological
rhythm and the pineal gland. Greger R and Windhorst U:
Comprehensive human physiology: From cellular mechanisms to
integration Berlin, Heidelberg: Springer; 1996, pp. 1199–1214.
View Article : Google Scholar
|
|
26
|
Arendt J: Melatonin and the pineal gland:
Influence on mammalian seasonal and circadian physiology. Rev
Reprod. 3:13–22. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jung B and Ahmad N: Melatonin in cancer
management: Progress and promise. Cancer Res. 66:9789–9793. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Srinivasan V, Spence DW, Pandi-Perumal SR,
Trakht I and Cardinali DP: Therapeutic actions of melatonin in
cancer: Possible mechanisms. Integr Cancer Ther. 7:189–203. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Aulinas A: Physiology of the pineal gland
and melatonin. Feingold KR, Anawalt B, Boyce A, Chrousos G, de
Herder WW, Dhatariya K, et al: Endotext (Internet) South Dartmouth
(MA): MDText.com, Inc.; 2000, (cited 2022 Jul 26). Available from:.
http://www.ncbi.nlm.nih.gov/books/NBK550972/
|
|
30
|
Brzezinski A: Melatonin in humans. N Engl
J Med. 336:186–195. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Karasek M and Winczyk K: Melatonin in
humans. J Physiol Pharmacol. 57 (Suppl 5):19–39. 2006.PubMed/NCBI
|
|
32
|
Martín V, Herrera F, Carrera-Gonzalez P,
García-Santos G, Antolín I, Rodriguez-Blanco J and Rodriguez C:
Intracellular signaling pathways involved in the cell growth
inhibition of glioma cells by melatonin. Cancer Res. 66:1081–1088.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Vijayalaxmi, Thomas CR Jr, Reiter RJ and
Herman TS: Melatonin: From basic research to cancer treatment
clinics. J Clin Oncol. 20:2575–2601. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Blask DE and Hill SM: Effects of melatonin
on cancer: Studies on MCF-7 human breast cancer cells in culture. J
Neural Transm Suppl. 21:433–449. 1986.PubMed/NCBI
|
|
35
|
Hill SM and Blask DE: Effects of the
pineal hormone melatonin on the proliferation and morphological
characteristics of human breast cancer cells (MCF-7) in culture.
Cancer Res. 48:6121–6126. 1988.PubMed/NCBI
|
|
36
|
Sánchez-Hidalgo M, Guerrero JM, Villegas
I, Packham G and de la Lastra CA: Melatonin, a natural programmed
cell death inducer in cancer. Curr Med Chem. 19:3805–3821. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lee DW, Kim YT, Kim JH, Kim S, Kim SW, Nam
EJ and Kim JW: Clinical significance of tumor volume and lymph node
involvement assessed by MRI in stage IIB cervical cancer patients
treated with concurrent chemoradiation therapy. J Gynecol Oncol.
21:18–23. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Feldman J, Goldwasser R, Mark S and
Schwartz J: A mathematical model for tumor volume evaluation using
two dimensions. J Appl Quant Methods. 4:455–462. 2008.
|
|
39
|
Mayr NA, Taoka T, Yuh WT, Denning LM, Zhen
WK, Paulino AC, Gaston RC, Sorosky JI, Meeks SL, Walker JL, et al:
Method and timing of tumor volume measurement for outcome
prediction in cervical cancer using magnetic resonance imaging. Int
J Radiat. 52:14–22. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nicolaides NC, Charmandari E, Chrousos GP
and Kino T: Circadian endocrine rhythms: The
hypothalamic-pituitary-adrenal axis and its actions. Ann NY Acad
Sci. 1318:71–80. 2014. View Article : Google Scholar : PubMed/NCBI
|