Introduction
Osteosarcoma (OS), the primary malignant bone tumor,
is found in children, adolescents and young adults (1). OS exhibits high destructive and
metastatic potential in patients (2,3). A
total of ~15–20% of OS patients have clinically detectable
metastases, more than 85% of the metastatic site occurs in the lung
(4), and the other is the distant
bone (5). Therefore, OS metastasis
is an obstacle to disease treatment. After clinical treatment, ~80%
of OS patients with metastatic disease have suffered relapse
(6). The current treatment for OS
patients, including radiotherapy, surgery, chemotherapy,
bisphosphonates, calcitonin and analgesics (7,8), is
accompanyed with adverse side effects (9,10).
Thus, it has been with the addition of adjuvant chemotherapy after
surgery. Although both surgical techniques with adjuvant
chemotherapy have been improved for patient survival, OS remains a
primary cause of mortality for patients (11). Therefore, new drugs from natural
products are attractive for patients with OS.
Tumor metastasis, the primary problem for tumor
therapy (5), is a complicated,
multi-step process and accounts for the vast majority of
cancer-related deaths (12).
Metastasis is a series of sequential and interrelated multi-step
processes where tumor cells disseminate from the primary tumor to
colonize distant organs (13).
These steps include cancer cells must detach and move from the
primary tumor and survive. Then these cells intravasate into the
circulatory and lymphatic systems and evade immune attacks at
distant capillary beds. Subsequently, cancer cells exit the
bloodstream to colonize a distant target organ and finally
proliferate and grow at different organ sites resulting in
malignant secondary tumors (14–19).
Urokinase and matrix metalloproteinases (MMPs) that degrade the
extracellular matrix and basement membrane are involved in
metastasis (20) for cell movement
(18) and metastasize (21). The epithelial-mesenchymal
transition (EMT), which involves cell polarity and cell-cell
junction, plays a vital role in the process of metastasis (22). Both MMP-2 and MMP-9 are
overexpressed and associated with enhanced metastatic ability in
human OS cell lines (23).
Inhibiting MMPs and their related pathways may be the potential
strategies for inhibiting OS metastasis.
Numerous pharmaceutical drugs are obtained from
natural products, and numerous studies have focused on screening
phytochemicals for treating human diseases. Curcuminoids, yellow,
lipid-soluble polyphenols, are extracted from the rhizome of
turmeric (Curcuma longa). Three major components of
curcuminoids are curcumin (Cur), demethoxycurcumin and
bisdemethoxycurcumin (BDMC) (24).
Curcuminoids exist in various biological activities, including
anti-oxidant, anti-inflammatory and cytotoxic in numerous human
cancer cell types (25). The lack
of methoxyl groups at the ortho position on the BDMC aromatic ring
renders it more stable in physiological media than Cur (26). BDMC presents anticancer effects on
human breast cancer MCF-7 cells (27) and gastric adenocarcinoma cells
(28). In addition, BDMC prevents
kidney fibrosis by activating fibroblast apoptosis (29). Previously, BDMC was revealed to
suppress migration and invasion in human cervical cancer HeLa cells
(30) and highly metastatic lung
cancer 95D cells (31).
BDMC has been revealed to inhibit the proliferation
and increase the apoptotic rate of cancer cells. U-2 OS cells with
functional p53 and pRb genes result in the lowest
level of chromosomal numerical variations compared with other OS
(32). In addition, abundant
osteoid production and infiltrating immune cells were detected in
U-2 OS-derived tumors. Therefore, the U-2 OS cells are widely used
for studying the cancer treatment, bone formation arthritis, and
the interaction between immune system and tumor (33,34).
However, there is no available information to show the effect and
molecular mechanism of BDMC on cell migration and invasion in human
OS cells. Therefore, the present study investigated the possible
effects and molecular mechanisms of BDMC on cell migration and
invasion of U-2 OS cells in vitro. The results indicated
that BDMC inhibited cell migration and invasion by suppressing
MMP-2 and MMP-9 signaling pathways in U-2 OS cells. This
information may provide the clinical trial treatment of human OS,
which is similar to U-2 OS cell line, metastasis in the future.
Materials and methods
Test chemicals, reagents, antibodies
and culture medium
BDMC, dimethyl sulfoxide (DMSO), EDTA, gelatin,
Tris-HCl, trypan blue, trypsin, propidium iodide (PI) and Coomassie
blue R-250 with purity higher than 98% were purchased from
Sigma-Aldrich; Merck KGaA. All chemicals were used as received
without any further purification. McCoy's 5A medium,
penicillin-streptomycin and fetal bovine serum (FBS) were obtained
from Gibco; Thermo Fisher Scientific, Inc. The antibodies against
Akt (1:1,000; cat. no. 4691), E-cadherin (1:1,000; cat. no. 14472),
EGFR (1:1,000; cat. no. 4267), ERK1/2 (1:1,000; cat. no. 4695), JNK
(1:1,000; cat. no. 9252), MMP-2 (1:1,000; cat. no. 87809),
N-cadherin (1:1,000; cat. no. 14215), NF-κB (1:1,000; cat. no.
8242), P38 (1:1,000; cat. no. 8690), phosphorylated
(p)-AktThr308 (1:1,000; cat. no. 4056),
p-EGFRTyr1068 (1:1,000; cat. no. 2234), vimentin
(1:1,000; cat. no. 3932), goat anti-rabbit IgG, horseradish
peroxidase (HRP)-linked antibody (1:5,000; cat. no. 7074) and horse
anti-mouse IgG, HRP-linked antibody (1:5,000; cat. no. 7076) were
purchased from Cell Signaling Technology, Inc. The antibodies
against c-Jun (1:4,000; cat. no. 558036), GRB2 (1:5,000; cat. no.
610112), PI3K (1:2,500; cat. no. 610046), protein kinase C (PKC;
1:250; cat. no. 554207), Ras (1:500; cat. no. 610002) and SOS1
(1:250; cat. no. 610095) were obtained from BD Pharmingen; BD
Biosciences. The antibodies against p-ERK1/2 (1:1,000; cat. no.
sc-16982-R), p-JNK (1:1,000; cat. no. sc-6254), p-P38 (1:1,000;
cat. no. sc-17852-R), Rho A (1:1,000; cat. no. sc-418) and uPA
(1:1,000; cat. no. sc-14019) were purchased from Santa-Cruz
Biotechnology, Inc. The antibodies against GSK3β (1:500; cat. no.
05-412) and p-AktSer473 (1:500; cat. no. 05-669), were
obtained from Millipore. The antibodies against β-catenin (1:2,000;
cat. no. GTX101435), MMP-9 (1:1,000; cat. no. GTX62122) and MMP-13
(1:500; cat. no. GTX55707) were purchased from GeneTex, Inc. The
antibodies against β-actin (1:5,000; cat. no. A5316), p-c-Jun
(1:1,000; cat. no. J2253) and VE-cadherin (1:500; cat. no. V1514)
were obtained from Sigma-Aldrich; Merck KGaA. BDMC was dissolved in
DMSO (carrier solvent), and 1% DMSO was used in control groups (as
0 concentration).
Cell culture
The human OS cell line (U-2 OS) was purchased from
the Food Industry Research and Development Institute (Hsinchu,
Taiwan, R.O.C.). U-2 OS cells were cultured in McCoy's 5A medium
containing 10% FBS, 2 mM L-glutamine, 100 µg/ml streptomycin and
100 units/ml penicillin at 37°C with 5% CO2 in a
humidified atmosphere as previously described (35).
Cell viability assays
U-2 OS cells (8×104 cells/well) were
placed in each well of 12-well plates with McCoy's 5A medium for 24
h. Cells were treated with 0, 2.5, 5, 7.5, 10, 15, 20 and 40 µM
BDMC in triplicate for 24 and 48 h. Cells were harvested, washed,
and stained with PI (5 µg/ml) on the ice and then immediately to
determine cell viability by using flow cytometry (Becton Dickinson
and Company) as previously described (35).
Cell proliferation assays
U-2 OS cells (5×103 cells/well) were
placed in each well of 96-well plates with McCoy's 5A medium for 24
h. Cells were treated with 0, 2.5, 5, 7.5, 10, 15, 20 and 40 µM
BDMC in triplicate for 24 and 48 h. After treatment, 10 µl MTT
reagent (5 mg/ml) was added for another 4 h, and then 10% SDS
solution (in 0.4 N HCl) was used to dissolve the formazan crystals
overnight. The absorbance was measured at OD595 nm for
analyzing cell proliferation by Bio-Rad Model 680 microplate reader
as previously described (36).
Wound healing motility assay
U-2 OS cells were placed in a 12-well plate at
8×104 cells/well and cultured in McCoy's 5A medium
containing 10% FBS to almost 100% confluence of the cell monolayer.
After 12 h of starvation (McCoy's 5A medium containing 2% FBS), the
cell monolayers were carefully wounded using a 200-µl pipette tip,
and cell debris was removed and then treated with 0, 5 and 10 µM of
BDMC in serum-free medium for 12 and 24 h. Cell healing images were
captured under a phase-contrast microscope in the denuded zone at
different periods (0, 12 and 24 h) as previously described
(35).
Gelatin zymography assay
U-2 OS cells (8×104 cells/well) were
plated in 12-well plates in McCoy's 5A culture medium overnight and
replaced with serum-free medium containing BDMC (0, 5 and 10 µM)
for 48 h. The conditioned medium from each treatment was harvested
for gelatinase activity assay on 10% SDS-polyacrylamide gel
electrophoresis (SDS-PAGE) gel containing 0.2% gelatin and run in
the SDS running buffer. At the end of the process, all gels were
washed twice with the renaturing solution containing 2.5% Triton
X-100 for 45 min and then incubation with zymogen developing buffer
[550 mM Tris (pH 7.5), 200 mM NaCl, 5 mM CaCl2, 1 µM
ZnCl2, and 0.02% Brij-35] followed for 24 h at 37°C. The
bands corresponding to MMP-2 and MMP-9 activities in gels were
stained using 0.2% Coomassie Brilliant Blue. Then the gels were
destained using 50% methanol and 10% acetic acid and images were
captured. All bands (gelatinolytic activity) were measured using
NIH ImageJ software (version 1.47; National Institutes of Health)
(35,37).
Transwell assay
A commercial Transwell chamber insert (8-µm pore
size; Millipore) was used to measure cell migration and invasion
ability. For measuring cell migration and invasion ability,
chambers were precoated with collagens (Sigma-Aldrich; Merck KGaA)
and with Matrigel, reapectively, then put it in the incubator at
37°C overnight. U-2 OS cells (2×104 cells/well) were
suspended in serum-free McCoy's 5A medium containing 0, 5 and 10 µM
of BDMC and seeded in the upper chamber. The lower chambers were
filled with 800 µl of McCoy's 5A medium supplemented with 10% FBS
for 24 h. After treatment, cells adhere to the upper surface of the
cham swab. Cells on the underside of the membrane (migratory cells)
were fixed with 100% methanol at room temperature for 10 min,
stained with 0.1% crystal violet solution at room temperature for
10 min, examined and images were captured under a light microscope.
In the cell invasion experiment, all subsequent steps were
performed as the cell migration assay, except for the fact that
chamber membranes were coated with Matrigel as previously described
(35,37).
Western blot analysis
The U-2 OS cells (1×106 cells/dish) were
placed in 10-cm culture dishes for 24 h and incubated with 0, 5 and
10 µM of BDMC for 24 and 48 h. Cells were collected, washed with
cold PBS and lysed with lysis buffer (50 mM Tris-HCl pH 7.5, 400 mM
NaCl, 2 mM EGTA, 1 mM EDTA, 1 mM DTT) containing protease inhibitor
cocktail (Roche Diagnostics) for 30 min on ice. Then total protein
concentration was quantitated following the guideline of the
protein assay kit (cat. no. 5000006; Bio-Rad Laboratories, Inc.). A
defined amount (40 µg) of proteins were electrophoresed on 8–12%
SDS-PAGE gels and transferred to PVDF membranes. After blocked in
blocking buffer (PBS with 2% FBS and 0.1% Tween 20) at room
temperature for 1 h, the membranes were probed with specific
primary antibodies against Akt, β-actin, β-catenin, c-Jun,
E-cadherin, EGRF, ERK1/2, GRB2, GSK3β, JNK, MMP-2, MMP-9, MMP-13,
N-cadherin, NF-κB, P38, p-AktThr308,
p-AktSer473, p-c-Jun, p-EGFRTyr1068, p-c-Jun,
p-ERK1/2, PI3K, PKC, p-JNK, p-P38, Ras, Rho A, SOS1, uPA,
VE-cadherin and vimentin at 4°C overnight. After washing, the
membranes were incubated with horseradish peroxidase-conjugated
secondary antibodies at room temperature for 1 h and visualized by
the ECL detection system (cat. no. WBKLS0500; MilliporeSigma).
Finally, the ImageJ software was used for densitometry of the
respective protein band intensity in all blots (35,37).
Statistical analysis
Data are presented as the mean ± SD from three
independent experiments. The statistical analysis was performed by
one-way ANOVA analysis of variance, and then the Dunnett's post-hoc
test was used to compare all groups against control, or the Tukey's
post hoc test was used for multiple group comparisons (SigmaPlot
for Windows version 12.0; Systat Software, Inc.). P<0.05 was
considered to indicate a statistically significant difference.
Results
BDMC decreases the total cell
viability and cell proliferation of U-2 OS cells
After U-2 OS cells were treated with BDMC (0, 2.5,
5, 7.5, 10, 15, 20, and 40 µM) for 24 and 48 h, the total viable
cell number was counted (Figs.
S1, 1A and B). Tretament with
20 and 40 µM BDMC at 24 and 48 h significantly decreased the
percentage of cell viability. However, 2.5–15 µM treatment of BDMC
did not exhibit a significant decrease in total cell viability of
U-2 OS cells.
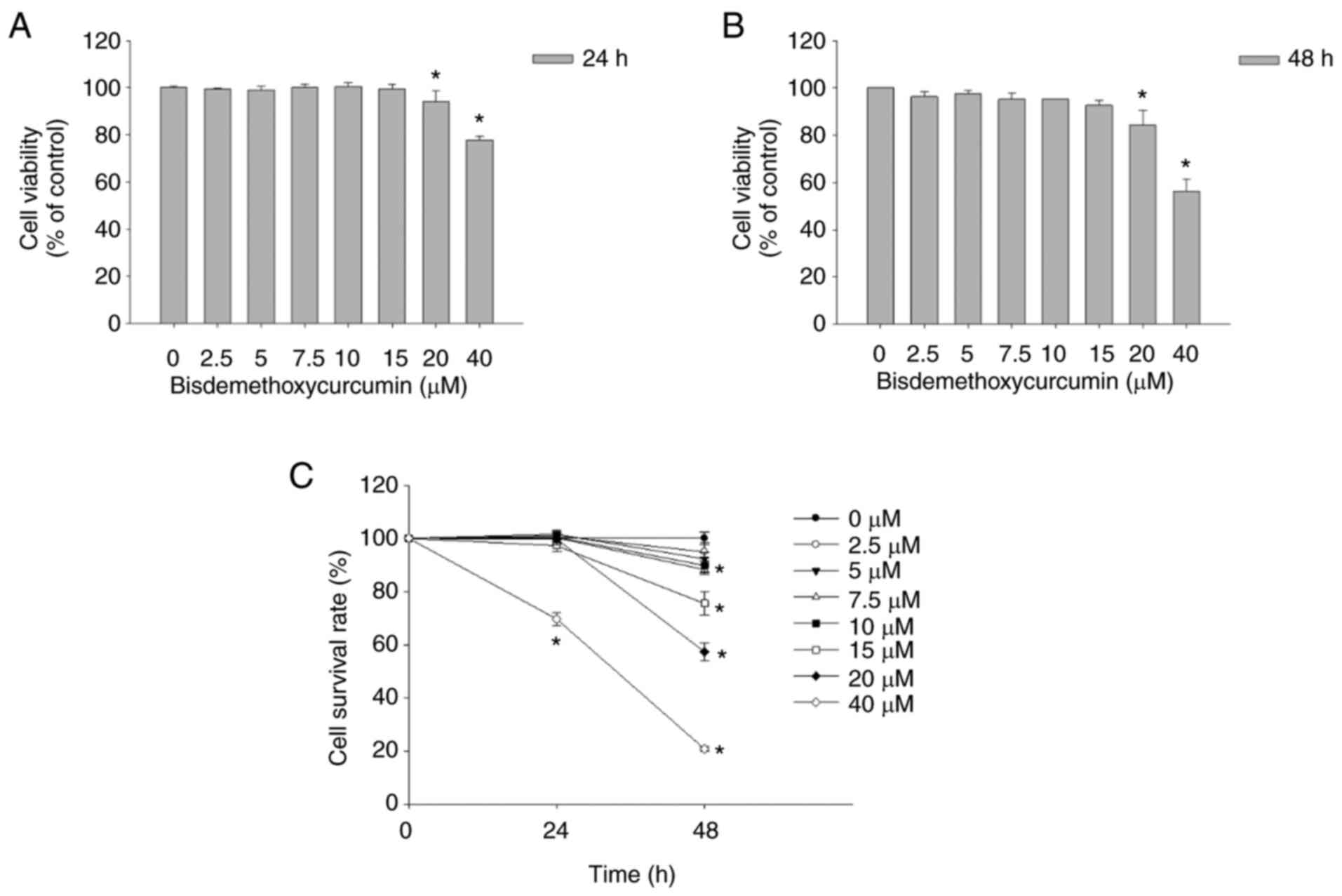 | Figure 1.BDMC decreases cell viability and
cell proliferation of U-2 OS cells. (A and B) Cells (8×104
cells/well) were incubated with BDMC (0, 2.5, 5, 7.5, 10, 15, 20
and 40 µM) for (A) 24 and (B) 48 h and harvested to measure the
total cell viability. (C) Cells (5×103 cells/well) were incubated
with BDMC (0, 2.5, 5, 7.5, 10, 15, 20 and 40 µM) for 48 h. After
treatment, cell solutions were added MTT reagent and the cell
proliferation was determined. *P<0.05 (one-way ANOVA
followed by Dunnett's post hoc test). BDMC,
bisdemethoxycurcumin. |
For the cell proliferation assay, U-2 OS cells were
treated with BDMC (0, 2.5, 5, 7.5, 10, 15, 20 and 40 µM) for 24 and
48 h and subsequently the cell proliferation was determined. As
revealed in Fig. 1C, BDMC at 10–40
µM significantly reduced the proliferation (16–84%, respectively).
According to the results of cell viability and cell proliferation
assays, 5 and 10 µM of BDMC were selected for further experiments,
as these concentrations did not influence the cell viability but
slightly inhibited the cell proliferation.
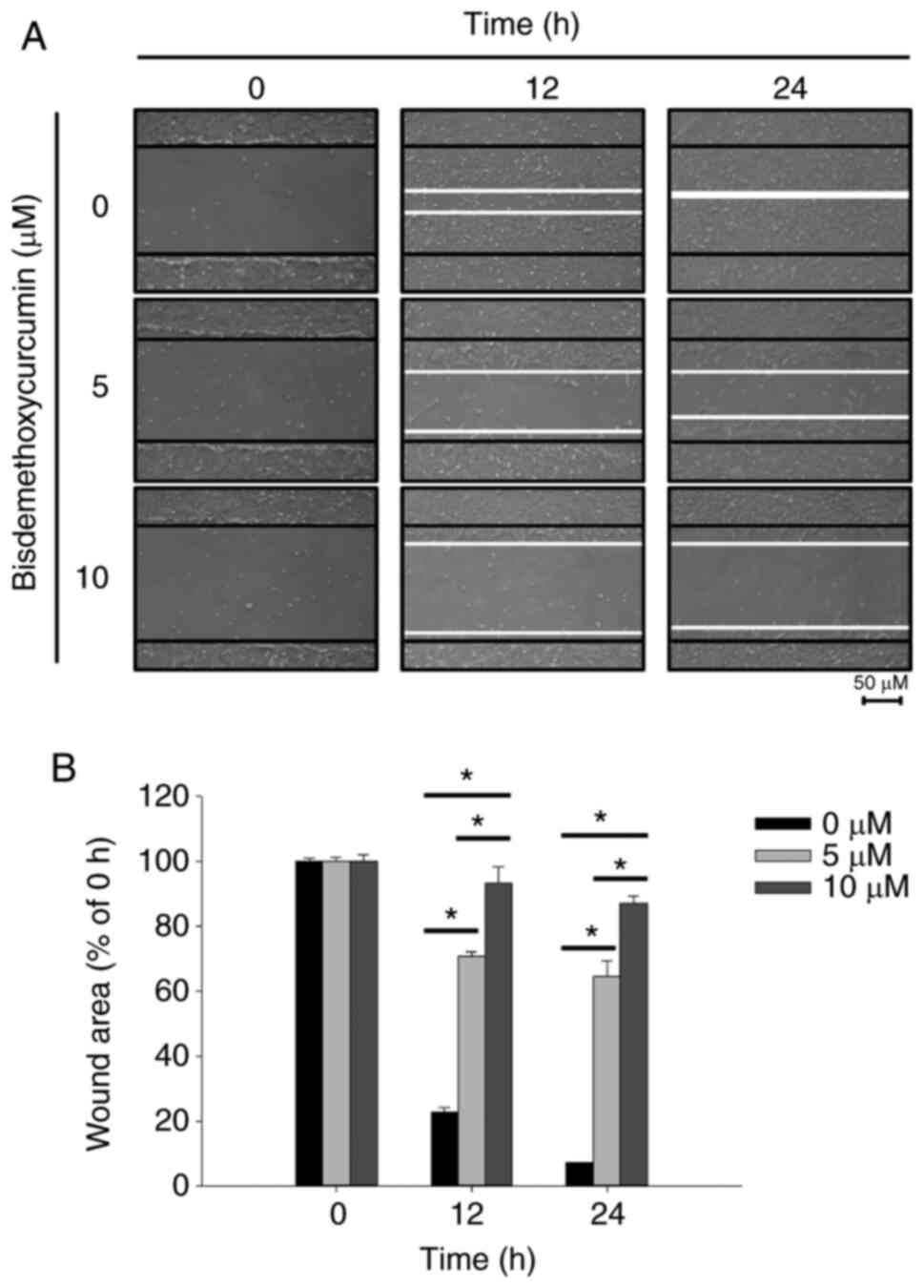
BDMC inhibits cell motility in U-2 OS
cells
After U-2 OS cells were treated with BDMC (0, 5 and
10 µM) for 12 and 24 h, cell motility was observed and images were
captured (Fig. 2A). The percentage
of inhibition of cell motility was calculated (Fig. 2B). After 12 and 24 h of treatment,
both cell migration movement and the scratch in the control group
were basically covered; however, the scratch areas of the higher
dose (10 µM) of BDMC treatment were more evident than that of lower
dose (5 µM). Moreover, the wound areas of the BDMC-treated groups
were higher than that of the control group (Fig. 2A and B). The results revealed that
BDMC significantly inhibited the motility of U-2 OS cells.
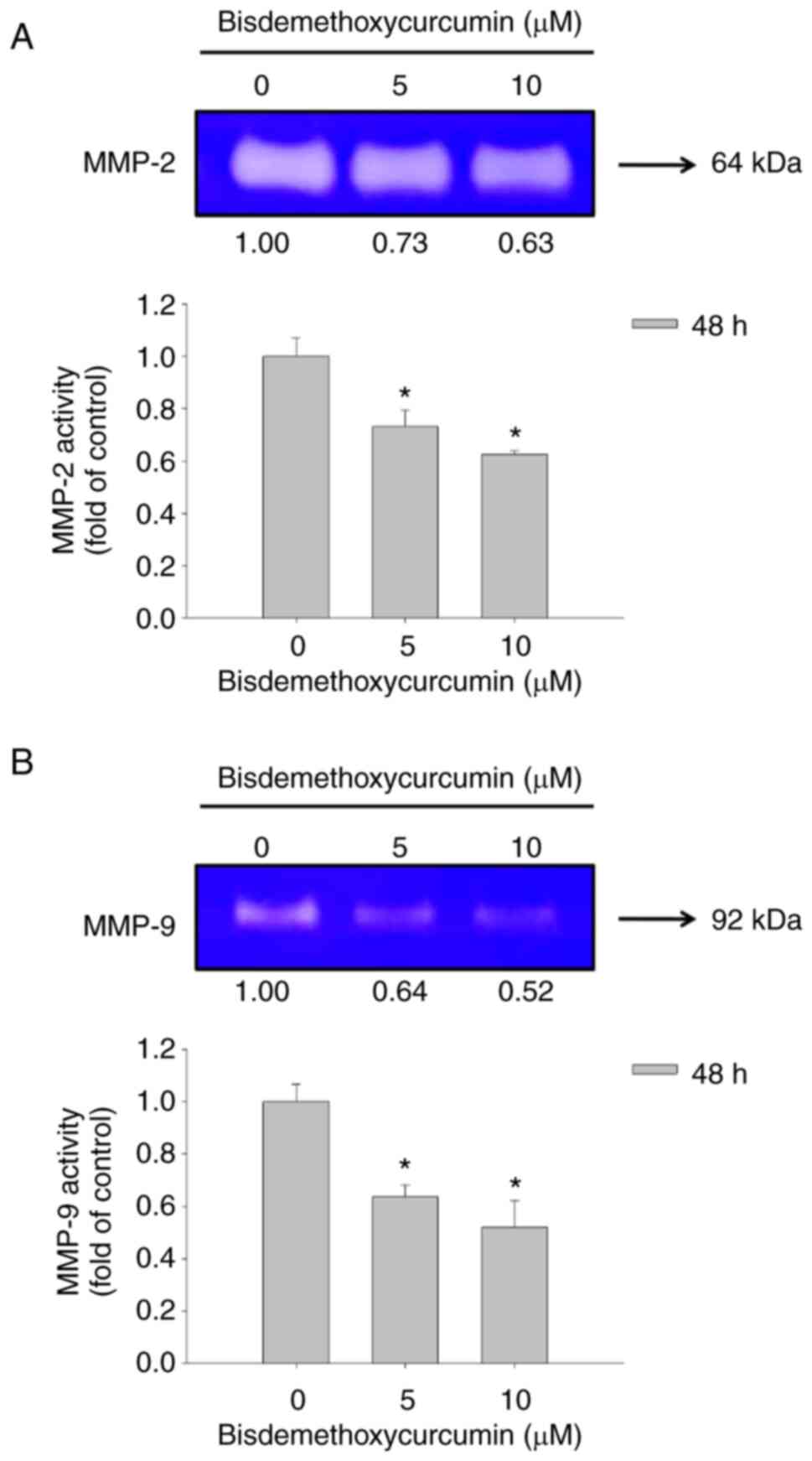
BDMC affects matrix metalloproteinase
activity in U-2 OS cells
After U-2 OS cells were treated with 0, 5 and 10 µM
of BDMC for 48 h, conditioned medium in the well was harvested for
examining the gelatinase activities of MMP-2 and MMP-9. Both
activities were measured using gelatin zymography assay (Fig. 3). BDMC at 5 and 10 µM significantly
inhibited MMP-2 (active form; 64 kDa) and MMP-9 (pro-form; 92 kDa)
activity at 48 h of treatment (Fig. 3A
and B). Moreover, the higher dose (10 µM) of BDMC demonstrated
a higher inhibition of MMP-2 (active form) and MMP-9 (pro-form)
activities than the lower dose (5 µM) of BDMC at 48 h of treatment
in U-2 OS cells.
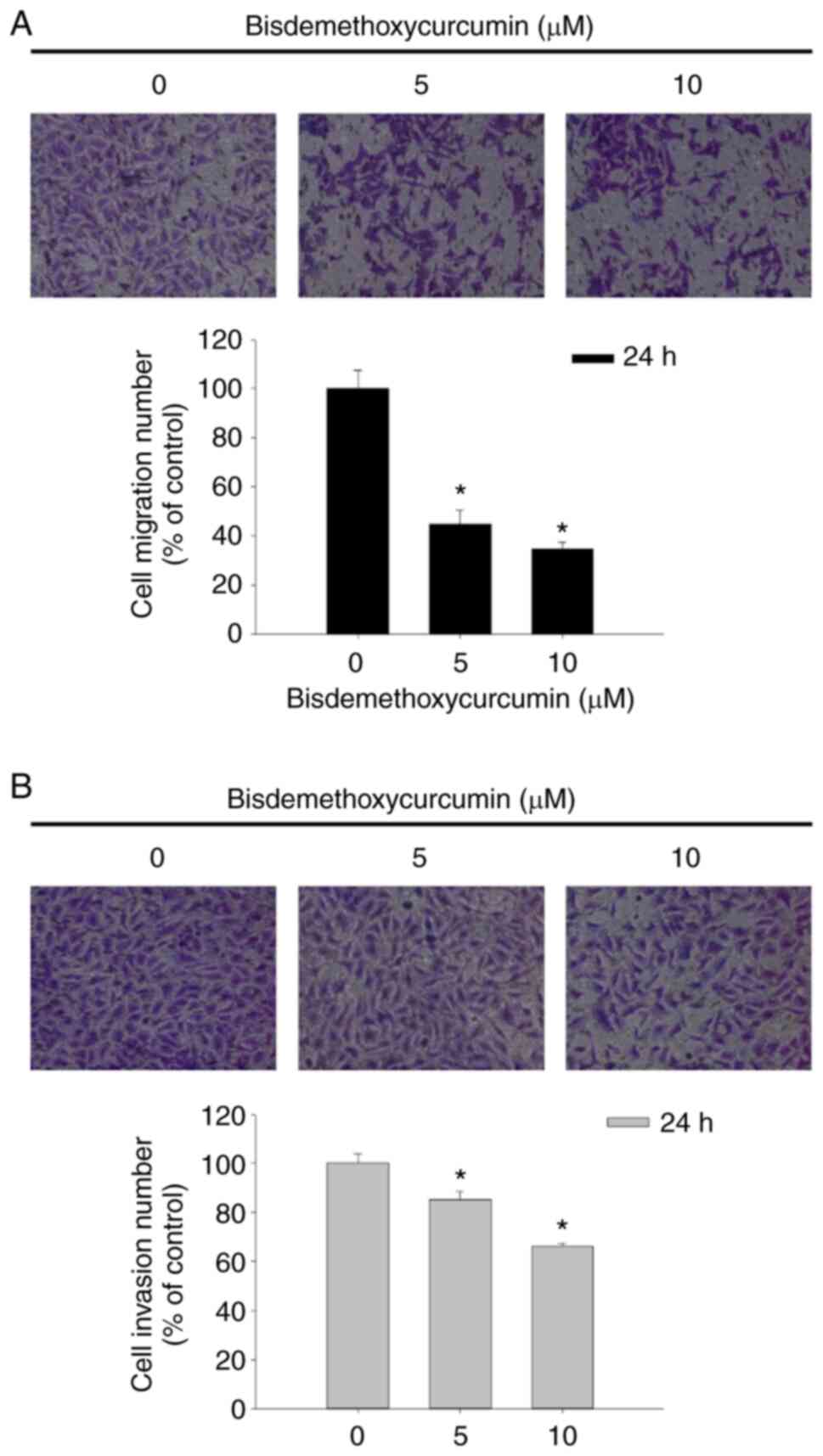
BDMC affects cell migration and
invasion in U-2 OS cells
After being exposed to BDMC at the final
concentrations of 0, 5 and 10 µM for 24 h, cells were assayed for
cell migration and invasion by using the Transwell chambers. As
revealed in Fig. 4A, BDMC at 5 and
10 µM significantly inhibited cell migration of U-2 OS cells
~56–66% compared with untreated cells. The results indicated that
BDMC at 5 and 10 µM significantly inhibited cell invasion of U-2 OS
cells ~16–34% compared with untreated groups (Fig. 4B). Both results indicated that BDMC
reduced cell migration and invasion in a dose-dependent manner.
BDMC affects key metastasis-related
proteins in U-2 OS cells
In order to understand the effects and mechanism of
BDMC on inhibiting cell migration and invasion of U-2 OS cells,
cells were treated with 0, 5 and 10 µM of BDMC for 24 and 48 h and
harvested for western blotting. The results indicated that BDMC (5
and 10 µM) at 24 h of treatment increased p-EGFRTyr1068,
but 48 h treatment led to a decrease in the expression of
p-EGFRTyr1068 (Fig.
5A). The ratio of p-EGFRTyr1068/EGFR only decreased
at 24 h. BDMC at 5 and 10 µM decreased the expression of SOS1,
GRB2, Ras, PKC, Rho A and uPA at 24 and 48 h treatment. BDMC (5 and
10 µM) inhibited the expression of p-ERK1/2, p-JNK, p-P38, p-c-Jun
and c-Jun (Fig. 5B). These results
indicated that BDMC affected the protein expression levels of the
MAPK signaling pathway. In addition, BDMC (5 and 10 µM) reduced the
expression levels of PI3K, p-AktThr308,
p-AktSer473, NF-κB, GSK3β and β-catenin at both periods,
indicating the effects of BDMC on the PI3K/Akt/NF-κB and
PI3K/Akt/GSK3β signaling pathways in U-2 OS cells (Fig. 5C). BDMC (5 and 10 µM) significantly
decreased the expression levels of MMP-2, MMP-9 and MMP-13 at both
periods (Fig. 5D). Furthermore,
BDMC (5 and 10 µM) increased E-cadherin and decreased N-cadherin,
VE-cadherin and vimentin at both periods in U-2 OS cells (Fig. 5E).
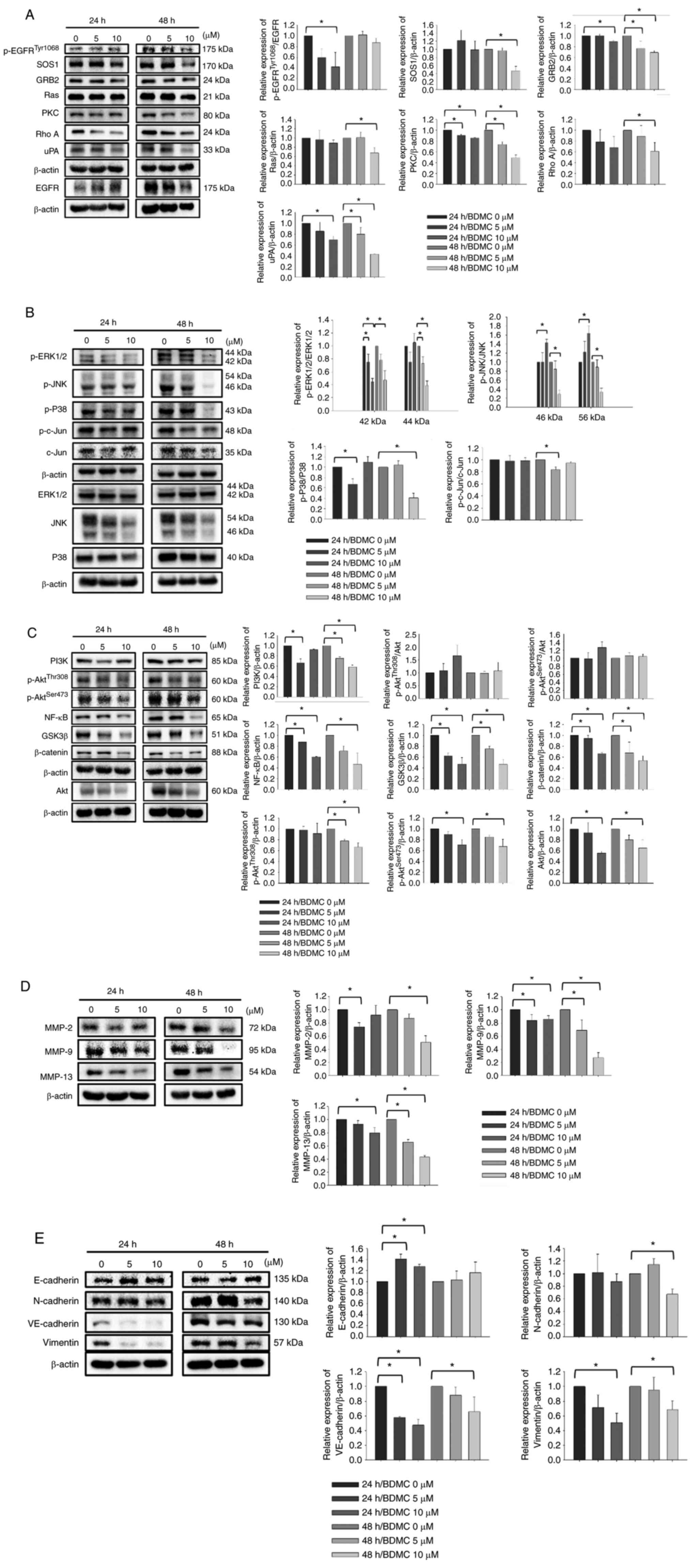 | Figure 5.BDMC affects the levels of
metastasis-associated proteins in U-2 OS cells. (A-E) Cells (1×106
cells/dish) were treated with BDMC (0, 5 and 10 µM) for 24 and 48
h. Cells were harvested for total protein evaluation using western
blotting and the band intensity was quantitated by ImageJ software.
The protein levels of (A) p-EGFRTyr1068, SOS1, GRB2, Ras, PKC, Rho
A, uPA and EGFR; (B) p-ERK1/2, p-JNK, p-p38, p-c-Jun, c-Jun,
ERK1/2, JNK and p38; (C) PI3K, p-AktThr308, p-AktSer473, NF-κB,
GSK3β, β-catenin and Akt; (D) MMP-2, MMP-9 and MMP-13; and (E)
E-cadherin, N-cadherin, VE-cadherin and Vimentin were analyzed.
*P<0.05 (one-way ANOVA followed by Dunnett's post hoc
test). BDMC, bisdemethoxycurcumin; p-, phosphorylated; PKC, protein
kinase C. |
Discussion
Cell metastasis involves multi-step processes in
which tumor cells disseminate from the primary tumor and colonize
distant organs (13). Cancer cell
metastasis has been recognized to account for more than 90% of all
cancer-related deaths (38,39).
Thus, investigating the mechanisms driving cancer cell motility and
invasion is crucial to understanding metastasis and inhibiting
cancer cell growth in other organs. To prevent bone metastasis, the
molecular pathways involved in bone metastasis need to be
comprehended (40,41). Thus, agents that block cancer cell
migration and invasion or inhibit metastasis-associated molecular
pathways may be potential strategies to inhibit cancer metastasis.
BDMC, one of the natural plants, has been identified to induce
cancer cell apoptosis and inhibit cell migration and invasion in
numerous human cancer cell lines. At present, there are no studies
revealing that BDMC suppresses cell migration and invasion in human
OS cells. Herein, the present studies were focused on BDMC and
whether or not it could inhibit U-2 OS cell migration and invasion
in vitro.
The U-2 OS cells were treated with various
concentrations of BDMC for 24 and 48 h and the results indicated
that BDMC significantly decreased total viable cell number (cell
viability) and the cell proliferation of U-2 OS cells. Therefore,
for further experiments, the concentrations of 5 and 10 µM of BDMC
were selected, which did not influence cell survival and slightly
inhibited cell proliferation. Cancer cell motility is involved in
tumor cell metastasis and wound healing cell motility assay is used
to measure cell motility (35,42).
The results indicated that BDMC inhibited cell motility of U-2 OS
cells in a dose-dependent manner. The present study, to the best of
our knowledge, is the first to identify that BDMC suppresses the
cell motility of U-2 OS cells in vitro.
MMPs play a critical cascade in cancer cell
migration and invasion. BDMC shows excellent effects on
degradation-associated proteins in several cells, including uPA,
MMP-2, MMP-9, membrane Type 1 MMP (MT1-MMP) and tissue inhibitors
of MMPs (TIMP-2) (30,43,44).
Whether or not reduced motility regarding the MMP-2 and MMP-9
activities was affected by BDMC in U-2 OS cells, the gelatin
zymography method was assayed in U-2 OS cells after exposure to
BDMC. Gelatin zymography detects proteolytic enzymes, including
MMP-2 (gelatinase A) and MMP-9 (gelatinase B), based on both
enzymes having potent gelatin-degrading activity (45). Both MMP-2 and MMP-9 were found to
be overexpressed in OS cells (35). Moreover, the increased expression
of MMP-2 in the tumor tissue has been shown to involve clinical
stages, including cancer cell metastases, recurrence and survival
(46). The present results
indicated that BDMC significantly reduced MMP-2 (active form) and
MMP-9 (pro-form) activities. Furthermore, the Transwell system for
examining cell migration and invasion across endothelial monolayer
in vitro was used to evaluate cancer cell metastasis ability
(46–48). BDMC significantly inhibited cell
migration and invasion in U-2 OS cells in vitro. These
effects are in a dose-dependent manner.
For further investigating the protein expression
levels regarding BDMC suppressing cell migration and invasion of
U-2 OS cells in vitro, western blot analysis was used.
Previous studies reported that numerous signaling pathways,
including PI3K/Akt/mTOR, ERK/MAPK and Slit-Robo pathways, were
involved in tumor metastasis (49,50).
The results indicated that BDMC decreased p-EGFRTyr1068,
SOS1, GRB2, Ras, PKC and Rho A after 48 h treatment. The Rho
GTPases and downstream effector proteins have been shown to mediate
tumor cell migration, invasion and metastasis via the cytoskeleton
(51). Moreover, BDMC
significantly inhibited the expression levels of p-ERK1/2, p-JNK,
p-p38, p-c-Jun and c-Jun in U-2 OS cells. These were consistent
with previous studies that indicated that the MAPK pathway
(including ERK, Jun and p38) (52)
and the Ras/Raf/MEK/ERK pathways are associated with OS-lung
metastasis (53). The present
results may suggest that these pathways were associated with the
U-2 OS cell migration and invasion. In addition, the p38/MAPK
signaling pathways are involved in cell metastasis (54). Therefore, a feasible and promising
approach for OS treatment is to block the Ras/MAPK kinase cascade
(55).
BDMC reduced the expression of PI3K,
p-AktSer473, NF-κB, GSK3β and β-catenin in U-2 OS cells.
In cancer cells, overexpressed PI3K/Akt/GSK3β signaling pathways
will promote cancer cell invasion and metastasis (56,57).
Furthermore, NF-κB induces the expression of diverse target genes
to stimulate cancer cell invasion and metastasis (58). Notably, Aurora-B has also been
revealed to activate the PTK2/PI3K/Akt/NF-κB pathway to promote the
malignant phenotype of OS cells (59). Thus, if agents could inhibit the
PI3K/Akt/GSK3β and PI3K/Akt/NF-κB signaling pathways, that may
benefit treating cancer patients with advanced metastasis. BDMC
inhibited the expression of β-catenin in U-2 OS cells. The
activation of Wnt/β-catenin has been revealed to induce actin to
alter the cytoskeleton to acquire a migratory phenotype (60). Thus, BDMC inhibiting U-2 OS cell
migration and invasion may also mediate the inhibition of
β-catenin.
BDMC significantly inhibited MMP-2, MMP-9 and MMP-13
in U-2 OS cells. It was also confirmed that MMP-2 and MMP-9
activities were suppressed by BDMC, and MMP-2 and MMP-9 were
involved in cancer invasion and metastasis (61,62).
The regulation of EMT, a decrease or the loss of E-cadherin
expression, or the induction of N-cadherin or vimentin in cancer
cells associated with cell migration and invasion is an evaluated
strategy for agents to affect cancer cell metastasis. In the
present study, E-cadherin, N-cadherin and VE-cadherin were analyzed
in U-2 OS cells after exposure to BDMC. The results indicated that
BDMC increased E-cadherin but decreased N-cadherin, VE-cadherin and
vimentin in U-2 OS cells. In prostate cancer cells, cells migrated
to bone metastases often switched the cadherin type from E-cadherin
to Cad11 by EMT (63). The EMT
process was associated with decreased E-cadherin (cell adhesion
molecule) and increased vimentin and N-cadherin expression
(64,65). Furthermore, inhibition of EMT
activation, including the downregulation of N-cadherin, vimentin,
MMP-2 and MMP-9, or the upregulation of E-cadherin and tissue
inhibitor of MMPs (TIMP-2), could lead to suppressing cancer cell
migration and invasion in cervical cancer (66).
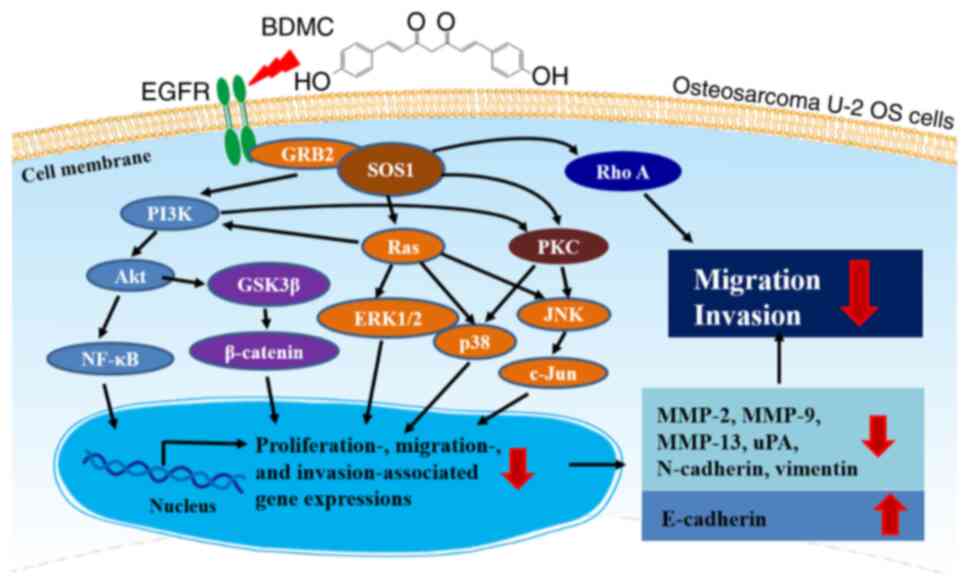
BDMC affects the PI3K/Akt/GSK3β, PI3K/Akt/NF-κB and
Ras/MAPK signaling pathways in U-2 OS cells and these pathways are
cross-talked in the present study. PI3K is one of the main Ras
effectors and regulates important cellular functions, including
cell viability or angiogenesis upon oncogenic Ras activation
(67). PKC is a family of
serine/threonine kinases and stimulates survival- or proliferation-
or metastasis-associated signaling pathways, including the
Ras/Raf/MEK/ERK or PI3K/Akt/mTOR pathways (68). Therefore, PI3K, Ras and PKC play a
cross-talked role in connecting each other (Fig. 6). BDMC, targeting PI3K, Ras and
PKC, may indicate potential therapies in the metastasis inhibition
of OS U-2 OS cells in the future.
In the present study, BDMC inhibited the migration
and invasion of U-2 OS cells by affecting the PI3K/Akt/NF-κB,
PI3K/Akt/GSK3β and MAPK signaling pathways in vitro.
However, there are certain limitations to the present study. The
associated signaling pathway of BDMC on U-2 OS cells was not
confirmed by related inhibitors. In addition, further research will
be needed to investigate the effects of cell migration and invasion
of BDMC in other OS with different genetic backgrounds.
In conclusion, in the present study, BDMC
significantly inhibited cell motility, migration and invasion of
U-2 OS cells in vitro involved in the inhibitions of the
PI3K/Akt/GSK3β, PI3K/Akt/NF-κB and Ras/MAPK signaling pathways.
Furthermore, it also reduced the levels of MMP-2, MMP-9, MMP-13,
N-cadherin, vimentin and uPA but increased E-cadherin. Therefore,
BDMC or BDMC nanocarrier which improve its water solubility may
become a potential drug or adjuvant for treating other OS with
wild-type p53 and pRb genes in the future.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported (grant no. EDAHP-108005) by the
Research Fund of E-Da Hospital (Kaohsiung, Taiwan). Experiments and
data analysis were performed in part through the use of the Medical
Research Core Facilities Center, Office of Research &
Development at China Medical University, Taichung, Taiwan.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
YSM, CLL, and TCH conceived and designed the study.
SFP, RSCW, FSC and WWH acquired the data. PYC, CLK and ACH analyzed
and interpretated the data. CLL and TCH wrote the draft of the
manuscript. CLL and TCH critically revised the manuscript. CLL and
TCH confirm the authenticity of all the raw data. All Authors
discussed the results and commented on the article. All authors
read and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Taran SJ, Taran R and Malipatil NB:
Pediatric osteosarcoma: An updated review. Indian J Med Paediatr
Oncol. 38:33–43. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Deng Z, Liu X, Jin J, Xu H, Gao Q, Wang Y
and Zhao J: Histone deacetylase inhibitor trichostatin a promotes
the apoptosis of osteosarcoma cells through p53 signaling pathway
activation. Int J Biol Sci. 12:1298–1308. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Moirangthem A, Bondhopadhyay B, Mukherjee
M, Bandyopadhyay A, Mukherjee N, Konar K, Bhattacharya S and Basu
A: Simultaneous knockdown of uPA and MMP9 can reduce breast cancer
progression by increasing cell-cell adhesion and modulating EMT
genes. Sci Rep. 6:219032016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lu J, Song G, Tang Q, Zou C, Han F, Zhao
Z, Yong B, Yin J, Xu H, Xie X, et al: IRX1 hypomethylation promotes
osteosarcoma metastasis via induction of CXCL14/NF-κB signaling. J
Clin Invest. 125:1839–1856. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bielack SS, Kempf-Bielack B, Delling G,
Exner GU, Flege S, Helmke K, Kotz R, Salzer-Kuntschik M, Werner M,
Winkelmann W, et al: Prognostic factors in high-grade osteosarcoma
of the extremities or trunk: An analysis of 1,702 patients treated
on neoadjuvant cooperative osteosarcoma study group protocols. J
Clin Oncol. 20:776–790. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang JY, Wu PK, Chen PC, Yen CC, Hung GY,
Chen CF, Hung SC, Tsai SF, Liu CL, Chen TH and Chen WM:
Manipulation therapy prior to diagnosis induced primary
osteosarcoma metastasis-from clinical to basic research. PLoS One.
9:e965712014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mercadante S: Malignant bone pain:
Pathophysiology and treatment. Pain. 69:1–18. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mercadante S and Fulfaro F: Management of
painful bone metastases. Curr Opin Oncol. 19:308–314. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Harris JD: Management of expected and
unexpected opioid-related side effects. Clin J Pain. 24 (Suppl
10):S8–S13. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Weber M and Huber C: Documentation of
severe pain, opioid doses, and opioid-related side effects in
outpatients with cancer: A retrospective study. J Pain Symptom
Manage. 17:49–54. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yi XJ, Zhao YH, Qiao LX, Jin CL, Tian J
and Li QS: Aberrant Wnt/β-catenin signaling and elevated expression
of stem cell proteins are associated with osteosarcoma side
population cells of high tumorigenicity. Mol Med Rep. 12:5042–5048.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gupta GP and Massagué J: Cancer
metastasis: Building a framework. Cell. 127:679–695. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Talmadge JE and Fidler IJ: AACR centennial
series: The biology of cancer metastasis: Historical perspective.
Cancer Res. 70:5649–5669. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bacac M and Stamenkovic I: Metastatic
cancer cell. Annu Rev Pathol. 3:221–247. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chambers AF, Groom AC and MacDonald IC:
Dissemination and growth of cancer cells in metastatic sites. Nat
Rev Cancer. 2:563–572. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Duffy MJ, McGowan PM and Gallagher WM:
Cancer invasion and metastasis: Changing views. J Pathol.
214:283–293. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fidler IJ: The pathogenesis of cancer
metastasis: The ‘seed and soil’ hypothesis revisited. Nat Rev
Cancer. 3:453–458. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Steeg PS: Tumor metastasis: Mechanistic
insights and clinical challenges. Nat Med. 12:895–904. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tarin D: Cell and tissue interactions in
carcinogenesis and metastasis and their clinical significance.
Semin Cancer Biol. 21:72–82. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yadav L, Puri N, Rastogi V, Satpute P,
Ahmad R and Kaur G: Matrix metalloproteinases and cancer-roles in
threat and therapy. Asian Pac J Cancer Prev. 15:1085–1091. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yilmaz M, Christofori G and Lehembre F:
Distinct mechanisms of tumor invasion and metastasis. Trends Mol
Med. 13:535–541. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nieto MA, Huang RY, Jackson RA and Thiery
JP: EMT: 2016. Cell. 166:21–45. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kansara M and Thomas DM: Molecular
pathogenesis of osteosarcoma. DNA Cell Biol. 26:1–18. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jayaprakasha GK, Jaganmohan Rao L and
Sakariah KK: Antioxidant activities of curcumin, demethoxycurcumin
and bisdemethoxycurcumin. Food Chem. 98:720–724. 2006. View Article : Google Scholar
|
|
25
|
Shishodia S: Molecular mechanisms of
curcumin action: Gene expression. Biofactors. 39:37–55. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Masuda T, Hidaka K, Shinohara A, Maekawa
T, Takeda Y and Yamaguchi H: Chemical studies on antioxidant
mechanism of curcuminoid: Analysis of radical reaction products
from curcumin. J Agric Food Chem. 47:71–77. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li YB, Gao JL, Zhong ZF, Hoi PM, Lee SM
and Wang YT: Bisdemethoxycurcumin suppresses MCF-7 cells
proliferation by inducing ROS accumulation and modulating
senescence-related pathways. Pharmacol Rep. 65:700–709. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Luo C, Du Z, Wei X, Chen G and Fu Z:
Bisdemethoxycurcumin attenuates gastric adenocarcinoma growth by
inducing mitochondrial dysfunction. Oncol Lett. 9:270–274. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hongtao C, Youling F, Fang H, Huihua P,
Jiying Z and Jun Z: Curcumin alleviates ischemia
reperfusion-induced late kidney fibrosis through the APPL1/Akt
signaling pathway. J Cell Physiol. 233:8588–8596. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liao CL, Chu YL, Lin HY, Chen CY, Hsu MJ,
Liu KC, Lai KC, Huang AC and Chung JG: Bisdemethoxycurcumin
suppresses migration and invasion of human cervical cancer HeLa
cells via inhibition of NF-ĸB, MMP-2 and −9 pathways. Anticancer
Res. 38:3989–3997. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xu J, Yang H, Zhou X, Wang H, Gong L and
Tang C: Bisdemethoxycurcumin suppresses migration and invasion of
highly metastatic 95D lung cancer cells by regulating E-cadherin
and vimentin expression, and inducing autophagy. Mol Med Rep.
12:7603–7608. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Isfort RJ, Cody DB, Lovell G and Doersen
CJ: Analysis of oncogenes, tumor suppressor genes, autocrine
growth-factor production, and differentiation state of human
osteosarcoma cell lines. Mol Carcinog. 14:170–178. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gorgoulis VG, Vassiliou LV, Karakaidos P,
Zacharatos P, Kotsinas A, Liloglou T, Venere M, Ditullio RA Jr,
Kastrinakis NG, Levy B, et al: Activation of the DNA damage
checkpoint and genomic instability in human precancerous lesions.
Nature. 434:907–913. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mancini L, Paul-Clark MJ, Rosignoli G,
Hannon R, Martin JE, Macintyre I and Perretti M: Calcitonin and
prednisolone display antagonistic actions on bone and have
synergistic effects in experimental arthritis. Am J Pathol.
170:1018–1027. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shih YL, Au MK, Liu KL, Yeh MY, Lee CH,
Lee MH, Lu HF, Yang JL, Wu RS and Chung JG: Ouabain impairs cell
migration, and invasion and alters gene expression of human
osteosarcoma U-2 OS cells. Environ Toxicol. 32:2400–2413. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen HY, Yang MD, Chou YC, Ma YS, Peng SF,
Liao CL, Chen PY, Hsia TC, Lien JC and Chen CH: Ouabain suppresses
cell migration and invasion in human gastric cancer AGS cells
through the inhibition of MMP signaling pathways. Anticancer Res.
41:4365–4375. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ma YS, Hsiao YT, Lin JJ, Liao CL, Lin CC
and Chung JG: Phenethyl isothiocyanate (PEITC) and benzyl
isothiocyanate (BITC) inhibit human melanoma A375.S2 cell migration
and invasion by affecting MAPK signaling pathway in vitro.
Anticancer Res. 37:6223–6234. 2017.PubMed/NCBI
|
|
38
|
Siegel R, Ward E, Brawley O and Jemal A:
Cancer statistics, 2011: The impact of eliminating socioeconomic
and racial disparities on premature cancer deaths. CA Cancer J
Clin. 61:212–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sporn MB: The war on cancer. Lancet.
347:1377–1381. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Roato I, Caldo D, Godio L, D'Amico L,
Giannoni P, Morello E, Quarto R, Molfetta L, Buracco P, Mussa A and
Ferracini R: Bone invading NSCLC cells produce IL-7: Mice model and
human histologic data. BMC Cancer. 10:122010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Santini D, Galluzzo S, Zoccoli A, Pantano
F, Fratto ME, Vincenzi B, Lombardi L, Gucciardino C, Silvestris N,
Riva E, et al: New molecular targets in bone metastases. Cancer
Treat Rev. 36 (Suppl 3):S6–S10. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu Y, Bi T, Shen G, Li Z, Wu G, Wang Z,
Qian L and Gao Q: Lupeol induces apoptosis and inhibits invasion in
gallbladder carcinoma GBC-SD cells by suppression of EGFR/MMP-9
signaling pathway. Cytotechnology. 68:123–133. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Pei H, Yang Y, Cui L, Yang J, Li X, Yang Y
and Duan H: Bisdemethoxycurcumin inhibits ovarian cancer via
reducing oxidative stress mediated MMPs expressions. Sci Rep.
6:287732016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yodkeeree S, Chaiwangyen W, Garbisa S and
Limtrakul P: Curcumin, demethoxycurcumin and bisdemethoxycurcumin
differentially inhibit cancer cell invasion through the
down-regulation of MMPs and uPA. J Nutr Biochem. 20:87–95. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Toth M, Sohail A and Fridman R: Assessment
of gelatinases (MMP-2 and MMP-9) by gelatin zymography. Methods Mol
Biol. 878:121–135. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Grzelczyk WL, Szemraj J and
Józefowicz-Korczyńska M: The matrix metalloproteinase in larynx
cancer. Postepy Hig Med Dosw (Online). 70:1190–1197.
2016.PubMed/NCBI
|
|
47
|
Hendrix MJ, Seftor EA, Seftor RE and
Fidler IJ: A simple quantitative assay for studying the invasive
potential of high and low human metastatic variants. Cancer Lett.
38:137–147. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Li YH and Zhu C: A modified Boyden chamber
assay for tumor cell transendothelial migration in vitro. Clin Exp
Metastasis. 17:423–429. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Huang T, Kang W, Cheng AS, Yu J and To KF:
The emerging role of Slit-Robo pathway in gastric and other gastro
intestinal cancers. BMC Cancer. 15:9502015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yang M and Huang CZ: Mitogen-activated
protein kinase signaling pathway and invasion and metastasis of
gastric cancer. World J Gastroenterol. 21:11673–11679. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Fife CM, McCarroll JA and Kavallaris M:
Movers and shakers: Cell cytoskeleton in cancer metastasis. Br J
Pharmacol. 171:5507–5523. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Koul HK, Pal M and Koul S: Role of p38 MAP
kinase signal transduction in solid tumors. Genes Cancer.
4:342–359. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Yu Y, Luk F, Yang JL and Walsh WR:
Ras/Raf/MEK/ERK pathway is associated with lung metastasis of
osteosarcoma in an orthotopic mouse model. Anticancer Res.
31:1147–1152. 2011.PubMed/NCBI
|
|
54
|
Wada T and Penninger JM: Mitogen-activated
protein kinases in apoptosis regulation. Oncogene. 23:2838–2849.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wang C, Zhou X, Li W, Li M, Tu T, Ba X, Wu
Y, Huang Z, Fan G, Zhou G, et al: Macrophage migration inhibitory
factor promotes osteosarcoma growth and lung metastasis through
activating the RAS/MAPK pathway. Cancer Lett. 403:271–279. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Liu Y, Yuan X, Li W, Cao Q and Shu Y:
Aspirin-triggered resolvin D1 inhibits TGF-β1-induced EMT through
the inhibition of the mTOR pathway by reducing the expression of
PKM2 and is closely linked to oxidative stress. Int J Mol Med.
38:1235–1242. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Wang H, Zhang C, Xu L, Zang K, Ning Z,
Jiang F, Chi H, Zhu X and Meng Z: Bufalin suppresses hepatocellular
carcinoma invasion and metastasis by targeting HIF-1α via the
PI3K/AKT/mTOR pathway. Oncotarget. 7:20193–20208. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Mirzaei S, Saghari S, Bassiri F, Raesi R,
Zarrabi A, Hushmandi K, Sethi G and Tergaonkar V: NF-κB as a
regulator of cancer metastasis and therapy response: A focus on
epithelial-mesenchymal transition. J Cell Physiol. 237:2770–2795.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Pi WS, Cao ZY, Liu JM, Peng AF, Chen WZ,
Chen JW, Huang SH and Liu ZL: Potential molecular mechanisms of
AURKB in the oncogenesis and progression of osteosarcoma cells: A
label-free quantitative proteomics analysis. Technol Cancer Res
Treat. 18:15330338198532622018.PubMed/NCBI
|
|
60
|
Akiyama T and Kawasaki Y: Wnt signalling
and the actin cytoskeleton. Oncogene. 25:7538–7544. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Birkedal-Hansen H, Moore WG, Bodden MK,
Windsor LJ, Birkedal-Hansen B, DeCarlo A and Engler JA: Matrix
metalloproteinases: A review. Crit Rev Oral Biol Med. 4:197–250.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Dutta A, Li J, Lu H, Akech J, Pratap J,
Wang T, Zerlanko BJ, FitzGerald TJ, Jiang Z, Birbe R, et al:
Integrin αvβ6 promotes an osteolytic program in cancer cells by
upregulating MMP2. Cancer Res. 74:1598–1608. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Chu K, Cheng CJ, Ye X, Lee YC, Zurita AJ,
Chen DT, Yu-Lee LY, Zhang S, Yeh ET, Hu MC, et al: Cadherin-11
promotes the metastasis of prostate cancer cells to bone. Mol
Cancer Res. 6:1259–1267. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Chang JW, Kang SU, Shin YS, Seo SJ, Kim
YS, Yang SS, Lee JS, Moon E, Lee K and Kim CH: Combination of NTP
with cetuximab inhibited invasion/migration of cetuximab-resistant
OSCC cells: Involvement of NF-κB signaling. Sci Rep. 5:182082015.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Guarino M, Rubino B and Ballabio G: The
role of epithelial-mesenchymal transition in cancer pathology.
Pathology. 39:305–318. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Yuan Y, Ye HQ and Ren QC: Upregulation of
the BDNF/TrKB pathway promotes epithelial-mesenchymal transition,
as well as the migration and invasion of cervical cancer. Int J
Oncol. 52:461–472. 2018.PubMed/NCBI
|
|
67
|
Cuesta C, Arévalo-Alameda C and Castellano
E: The importance of being PI3K in the RAS signaling network. Genes
(Basel). 12:10942021. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Matsuoka H, Tsubaki M, Yamazoe Y, Ogaki M,
Satou T, Itoh T, Kusunoki T and Nishida S: Tamoxifen inhibits tumor
cell invasion and metastasis in mouse melanoma through suppression
of PKC/MEK/ERK and PKC/PI3K/Akt pathways. Exp Cell Res.
315:2022–2032. 2009. View Article : Google Scholar : PubMed/NCBI
|