Introduction
Epileptic seizures are the first symptom in 14% of
patients with glioblastoma (1) and
40–60% of patients eventually suffer epileptic seizures even if
none were observed at the time of diagnosis (2). Prevention of epileptic seizures is
almost as important as the treatment of the glioblastoma and
numerous patients receive antiepileptic therapy in actual clinical
practice. Consequently, an agent that is able to prevent
symptomatic epilepsy and exert antitumor effects may be beneficial
to patients with glioblastoma.
Antiepileptic drugs reported to have antitumor
effects include sodium valproate (VPA), carbamazepine (CBZ),
levetiracetam (LEV), talampanel and perampanel, which achieve
anticonvulsant effects through various mechanisms of action. In
particular, VPA and LEV were indicated to improve overall survival
in patients with glioblastoma (3–5), but
no survival benefit was subsequently reported (6). Talampanel and perampanel are
selective non-competitive antagonists of the
α-amino−3-hydroxyl-5-methyl-4-isoxazole-propionate
(AMPA)-type glutamate receptor and inhibit the influx of cations
(Na+, K+) to suppress nerve excitement and
exert antiepileptic activity (7).
Talampanel administration increased the overall
survival time to 20.3 months compared to 14.6 months in the control
group in a phase II clinical trial of primary glioblastoma
(8). However, talampanel
administered as a single agent achieved no significant prolongation
of progression-free survival in a phase II study of recurrent
glioblastoma (9).
Investigation of the antitumor effects of
perampanel, VPA, LEV and CBZ on glioblastoma cells indicated that
only perampanel suppressed cell proliferation (10). The mechanism of action of
perampanel depends on the reduction of cell metabolism caused by
decreased glucose uptake (10).
Perampanel exhibited a synergistic antitumor effect with
temozolomide (TMZ), a standard chemotherapeutic agent for malignant
glioma, on malignant glioma cell lines (11). Furthermore, perampanel suppressed
cell proliferation by induction of apoptosis, but the synergistic
effect with TMZ was not observed in all cell lines (11). However, the perampanel
concentration used in those studies was 100 and 250 µM,
respectively (10,11), which was much higher than the blood
concentration of 1.48 µM achieved by administration of the
perampanel maintenance dose of 8 mg (12).
A recent study by our group indicated that
perampanel has a dose-dependent antitumor effect on malignant
glioma cell lines, and induces apoptosis and inhibits cell
proliferation in combination with TMZ at clinical blood
concentrations as an antiepileptic drug (13). Clearly, the antitumor effect of
perampanel on malignant glioma apparently depends on inhibition of
cell proliferation, whereas any effects on cell migration or
invasion have remained undetermined. Cell migration is the process
of movement of cells from one location to another. Cell invasion
implies that the cells denature the extracellular matrix (ECM) and
settle in another location, which requires the secretion of
proteolytic enzymes such as matrix metalloproteinases (MMPs) that
denature the adjacent ECM (14).
Various signaling pathways are involved in cell
migration and invasion of glioblastoma. Ca2+-permeable
AMPA receptors expressed in glioblastoma cells increase
intracellular Ca2+ levels, allowing both tumor cell
proliferation and migration through cascades such as
phosphatidylinositol 3-kinase (PI3K)/Akt (15,16).
In addition, activation of the AMPA receptors increases the
expression of integrins and transmembrane receptors and enhances
the focal adhesion kinase/steroid receptor coactivator (FAK/Src)
pathway (17). These signals
enhance the activity of molecules of the Rho family small
GTP-binding protein, rac family small GTPase 1 (Rac1), cell
division cycle 42 (Cdc42) and ras homolog family member A (RhoA),
which are involved in the transformation of the cytoskeleton
(18). On the other hand, the
epithelial-mesenchymal transition (EMT) process is also important
for the migration and invasion of glioblastoma cells. EMT-related
molecules include E-cadherin and N-cadherin (19,20).
E-cadherin is an epithelial marker and increases in its expression
result in enhanced cell-cell adhesion and reduced cell motility. By
contrast, N-cadherin is a mesenchymal marker and increased
expression weakens cell-cell binding, facilitating cell separation
and enhancing motility. EMT is induced by signaling pathways such
as FAK/Src and PI3K/Akt. Glioblastoma cells, which exhibit
mesenchymal morphology and migrate, secrete MMPs, destroy ECM and
create pathways for migration (18,21).
However, the mechanisms of cell migration and cell invasion remain
to be fully elucidated due to the complex involvement of numerous
factors in intracellular signaling pathways.
The present study compared the antitumor effects of
perampanel, CBZ, VPA and LEV, which are commonly used antiepileptic
drugs with different mechanisms of action, at therapeutic blood
levels on the proliferation, migration and infiltration of
malignant glioma cell lines. Changes in the expression of genes
that affect cell migration and infiltration were evaluated using
reverse transcription-quantitative (RT-q)PCR. Furthermore, the
combination of these antiepileptic drugs with TMZ was also
investigated to examine whether they exhibit any synergistic
antitumor effect.
Materials and methods
Cell lines, culture conditions and
materials
The human malignant glioma cell lines A-172 (cat.
no. JCRB0228; lot no. 021999), AM-38 (cat. no. IFO50492; lot no.
12082003), T98G (cat. no. IFO50303; lot no. 1007), U-251MG (cat.
no. IFO50288; lot no. 12132002) and YH-13 (cat. no. IFO50493; lot
no. 1164) were purchased from the Health Science Research Resources
Bank of Japan. U-138MG (cat. no. HTB-16; lot no. 1104428) was
purchased from the American Type Culture Collection. A previous
study by our group confirmed that O6-methylguanine-DNA
methyltransferase (MGMT), a key factor of alkylating agents, was
expressed in the T98G, U-138MG and YH-13 cell lines via RT-qPCR and
western blot analyses (22).
Consistent with an earlier study (23), it was also confirmed that the T98G
(237 Met→Ile) and U-251MG (273 Arg→His) cell lines have a point
mutation in the TP53 gene (data not shown).
Cells were cultured in Dulbecco's modified Eagle's
medium (Nissui Pharmaceutical) supplemented with 10% fetal calf
serum (Thermo Fisher Scientific, Inc.) using plastic culture flasks
(Corning, Inc.) in a 37°C incubator in a humidified (>95%)
atmosphere containing 5% CO2.
The antiepileptic drugs perampanel (kindly gifted by
Eisai Co., Ltd.), CBZ (Tokyo Chemical Industry), VPA (Tokyo
Chemical Industry) and LEV (Tokyo Chemical Industry) and the
anti-cancer agent TMZ (Tokyo Chemical Industry) were employed for
this study.
Cell culture growth studies
The growth inhibitory effects of the four
antiepileptic drugs (perampanel, CBZ, VPA and LEV) on malignant
glioma cells were evaluated by quantifying the numbers of cells. In
brief, cells were seeded in 24-well, flat-bottomed plates (Iwaki)
at 1×104 cells/well and incubated with medium for 24 h.
The cells were subsequently washed twice with medium and further
incubated with fresh medium (control) or medium containing
perampanel (0.01, 0.1, 1.0 and 10 µM), CBZ (0.5, 5.0, 50 and 500
µM), VPA (1.0, 10, 100 and 1,000 µM) and LEV (1.0, 10, 100 and
1,000 µM). After exposure to the various concentrations of
antiepileptic drugs for 72 h, the cells were detached by
trypsinization and counted using a Coulter Counter Z1 (Beckman
Coulter, Inc.). The experiments were repeated at least 12 times at
each concentration. For certain experiments regarding the effect of
perampanel on cell growth, certain experimental repeats included
the research results of another study of our group by Tatsuoka
et al (13).
The molecular weights of perampanel, CBZ, VPA and
LEV are 349.4, 236.3, 166.2 and 170.2 g/mol; the maintenance doses
are 8–12, 400–1,200, 400–1,200 and 1,000–3,000 mg; and the
therapeutic ranges of blood concentrations are 0.14–1.14, 17–50,
300–600 and 70–270 µM, respectively (24). The treatment dose of each
anticonvulsant was determined using this information.
Since T98G and U-251MG cells display a marked
antitumor response after being treated with a small amount of
perampanel (13) and are widely
used in brain tumor experiments, these cells were employed in the
subsequent experiments to investigate the effects of perampanel
combined with TMZ on cell migration and invasion. The 50%
inhibitory concentration (IC50) for perampanel on
U-251MG cells was close to the blood concentration used for
antiepileptic therapy (see Results section). In addition, as
mentioned above, T98G cells expressed MGMT, but U-251MG cells did
not.
Enhanced effects of TMZ and
antiepileptic drugs
The additive antitumor effect of the combination of
TMZ and antiepileptic drugs compared to TMZ alone in malignant
glioma cells was assessed. T98G and U-251MG cells were plated at
1×104 cells/well in 24-well, flat-bottom plates and
incubated with medium for 24 h. Subsequently, they were incubated
with medium containing various concentrations of perampanel (0–10
µM), CBZ (0–500 µM), VPA (0–1,000 µM), and LEV (0–1,000 µM) with or
without TMZ (10 µM). The TMZ concentration (10 µM) was chosen as a
representative of a clinically relevant concentration (25). After exposure to the various
concentrations of antiepileptic drugs with/without TMZ for 72 h,
cells were detached using 0.25% Trypsin-EDTA solution (Invitrogen;
Thermo Fisher Scientific, Inc.) and counted. The experiments were
repeated at each concentration in at least four independent systems
and cell numbers were quantified at least four times in total.
Certain experiments regarding the effect of a combination
perampanel with TMZ on cell growth were included; those experiments
were reported in part (but not in detail) by co-author Tatsuoka
et al (13).
Inhibitory effect of antiepileptic
drugs on cell migration and cell infiltration
Cell migration was evaluated in the T98G and U-251MG
cell lines using the CytoSelect 24-well Cell Migration Assay (Cell
Biolabs, Inc.). Among the cells seeded in the upper chamber filled
with serum-free medium, only migrating cells pass through the
membrane with pores and move to the lower chamber side filled with
serum-containing medium. Cell migration may be evaluated by
quantifying the migrated cells. The cell migration study was
performed using the following method: T98G or U-251MG cells,
adjusted to 1×105 cells/300 µl/well in serum-free
medium, were seeded in the upper chamber equipped with the
polycarbonate membrane with 8-µm pores. The antiepileptic drug
concentration was set to the blood concentration level within the
maximum therapeutic range of 1.0 µM for perampanel, 50 µM for CBZ,
600 µM for VPA and 270 µM for LEV. Medium containing 10% calf serum
was placed in the lower chamber. The following was performed
according to the manufacturer's instructions for the Cell Migration
Assay (Cell Biolabs, Inc.). After culturing for 24 h, the
non-migrating cells remaining in the upper chamber were wiped off
with a cotton swab and the migrating cells that had passed through
the pores of the upper chamber and moved to the bottom surface of
the filter were treated with a cell stain solution. After drying,
the stain was extracted with extraction solution and measured for
absorbance at 560 nm using a microplate reader (Model 680; Bio-Rad
Laboratories, Inc.). The experiments were repeated for each
antiepileptic drug in at least five independent systems and
quantified at least five times in total.
Cell invasion was evaluated using the CytoSelect
24-well Cell Invasion Assay (Cell Biolabs, Inc.). Unlike that in
the Transwell assay used in the cell migration experiment, the
polycarbonate membrane was coated with an extracellular matrix
layer. This allows the cell invasion ability to be measured if
extracellular matrix degradation occurs. Apart from this, the same
procedure as that for the cell migration experiment was used.
mRNA expression analysis of genes
involved in cell migration and infiltration using RT-qPCR
Perampanel was observed to cause significant
suppression of cell migration and tended to suppress cell invasion.
Therefore, the changes in mRNA expression levels after perampanel
treatment were evaluated using RT-qPCR to identify factors related
to the induction of EMT, which is an important process for cell
migration and infiltration. Cells were cultured in 75
cm2 flasks, treated with 1.0 µM of perampanel for 4 h,
and total RNA was extracted from 1×106 cells by
employing the RNeasy Mini kit (Qiagen, Inc.). After determination
of the RNA contents with a NanoDrop (Thermo Fisher Scientific,
Inc.), mRNA expression levels were analyzed using the Step-One
Real-time PCR system (Applied Biosystems; Thermo Fisher Scientific,
Inc.) with RNA-direct SYBR Green Realtime PCR Master Mix (Toyobo
Life Science) according to the manufacturer's instructions. The
primer sequences for each gene are presented in Table I. In the Step-One Real time PCR
system, real-time PCR assay, RT and PCR amplification were
performed in the same reaction tube. The total reaction volume of
20 µl containing 0.8 µg of RNA, 2.0 µl each of forward and reverse
primers (10 pmol), 1.0 µl of 50 mM Mn(OAc)2, 10 µl of
RNA-direct SYBR Green Realtime PCR Master Mix (Toyobo Life Science)
and RNase-free water. The thermocycling conditions were as follows:
1st stage, 95°C for 30 sec, 61°C for 20 min, and 95°C for 1 min;
2nd stage, 45 cycles at 95°C for 15 sec, 55°C for 15 sec, and 74°C
for 45 sec; and 3rd stage, 95°C for 15 sec, 60°C for 1 min and 95°C
for 15 sec. GAPDH mRNA expression levels were employed as the
quantitative internal control. The expression levels were
calculated employing the following equations by comparing the
threshold cycle (Cq): ∆Cq=Cq of β1 integrin, FAK, Src, PI3K, Akt,
Rac1, RhoA, Cdc42, MMP-2, E-cadherin or N-cadherin, -Cq of GAPDH,
∆∆Cq (target cell line)-∆Cq (reference cell line), and
ratio=2−∆∆Cq (26). The
experiments were repeated three times for each condition.
 | Table I.Primer sets. |
Table I.
Primer sets.
| Gene/direction | Sequence |
|---|
| GAPDH |
|
|
Forward |
5′-CAGAACATCATCCCTGCCTCT-3′ |
|
Reverse |
5′-GCTTGACAAAGTGGTCGTTGAG-3′ |
| β1
integrin |
|
|
Forward |
5′-AATGGGAACAACGAGGTCATGGTT-3′ |
|
Reverse |
5′-TTGTGGGATTTGCACGGGCAGTAC-3′ |
| FAK |
|
|
Forward |
5′-GGTGCAATGGAGCGAGTATT-3′ |
|
Reverse |
5′-GCCAGTGAACCTCCTCTGA-3′ |
| Src |
|
|
Forward |
5′-GGGTAGCAACAAGAGCAA-3′ |
|
Reverse |
5′-GAGTTGAAGCCTCCGAACAG-3′ |
| PI3K |
|
|
Forward |
5′-CCCTGCTCATCAACTAGGAAACC-3′ |
|
Reverse |
5′-TTGCCGTAAATCATCCCCATT-3′ |
| Akt |
|
|
Forward |
5′-TGCCCTTCTACAACCAGGAC-3′ |
|
Reverse |
5′-ACACGATACCGGCAAAGAAG-3′ |
| Rac1 |
|
|
Forward |
5′-CTGCCAATGATATGGTAGATG-3′ |
|
Reverse |
5′-CCGCACCTCAGGATACCA-3′ |
| RhoA |
|
|
Forward |
5′-TCAAGCCGGAGGTCAACAAC-3′ |
|
Reverse |
5′-ACGAGCTGCCCATAGCAGAA-3′ |
| Cdc42 |
|
|
Forward |
5′-GAAGGCTGTCAAGTATGTGG-3′ |
|
Reverse |
5′-CTCTTCTTCGGTTCTGGAGG-3′ |
| MMP-2 |
|
|
Forward |
5′-CCGTCGCCCATCATCAAGTTC-3′ |
|
Reverse |
5′-GCAGCCATAGAAGGTGTTCAGG-3′ |
|
E-cadherin |
|
|
Forward |
5′-ATTGCTCACATTTCCCAACTCC-3′ |
|
Reverse |
5′-CTCTGTCACCTTCAGCCATCCT-3′ |
|
N-cadherin |
|
|
Forward |
5′-TTTGATGGAGGTCTCCTAACACC-3′ |
|
Reverse |
5′-ACGTTTAACACGTTGGAAATGTG-3′ |
Statistical analysis
Values are expressed as the mean ± standard error of
the mean. Student's t-test (unpaired) was used to compare the data
between two groups and one-way analysis of variance followed by the
Tukey-Kramer method was used to compare three or more groups using
the software SPSS (v.21.0; IBM Corporation). P<0.05 was
considered to indicate a statistically significant difference.
Results
Antitumor effect of antiepileptic
drugs in human malignant glioma cells
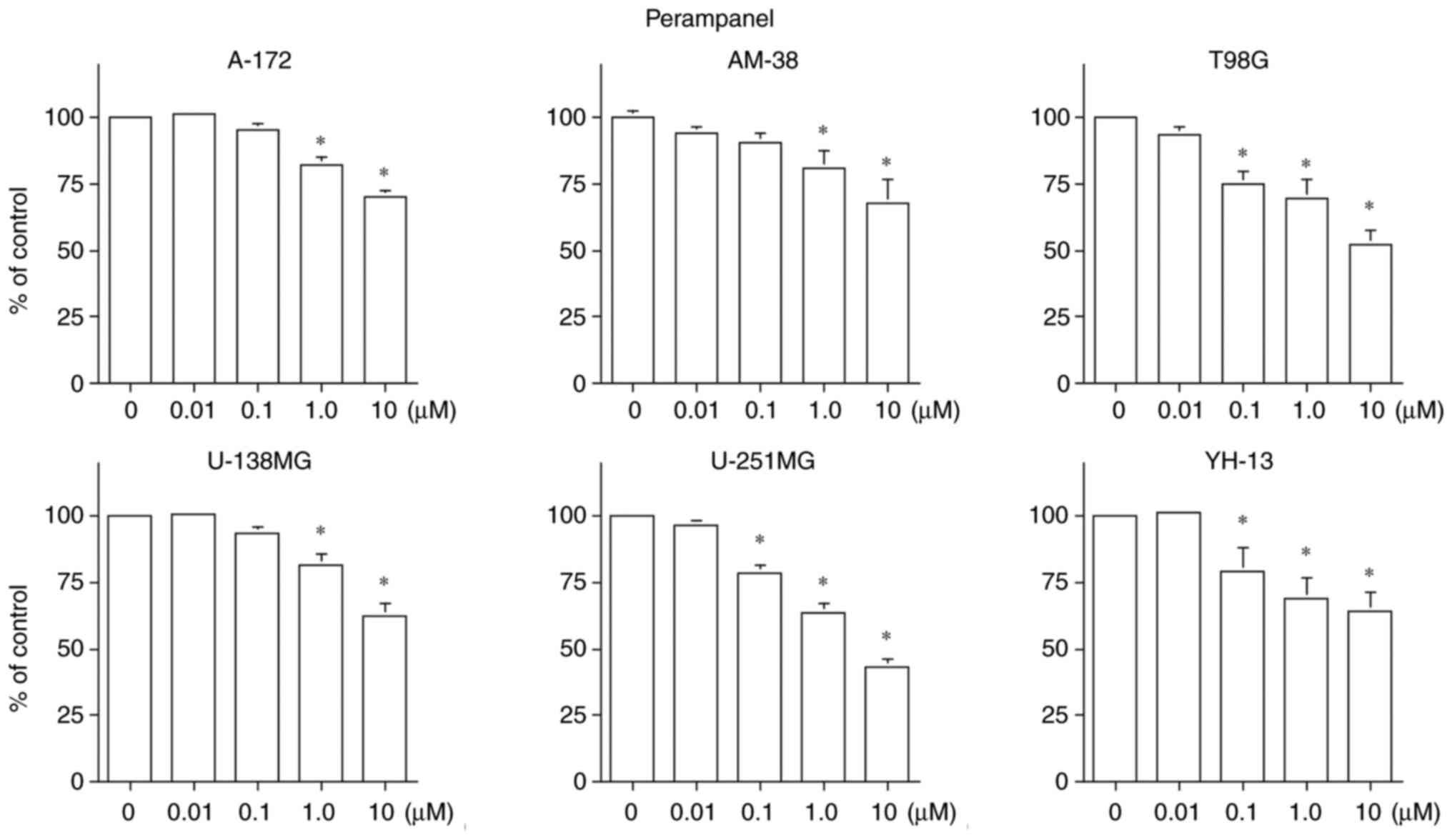
Fig. 1 indicates
that the cell growth inhibitory effects of perampanel were
dose-dependent in all tumor cell lines. However, the sensitivity of
the cell lines varied, as also suggested by the previous study
(13). The therapeutic blood
concentration range of perampanel as an antiepileptic drug is 8–12
µM (24). Only U-251MG cells had
an IC50 of <10 µM for perampanel.
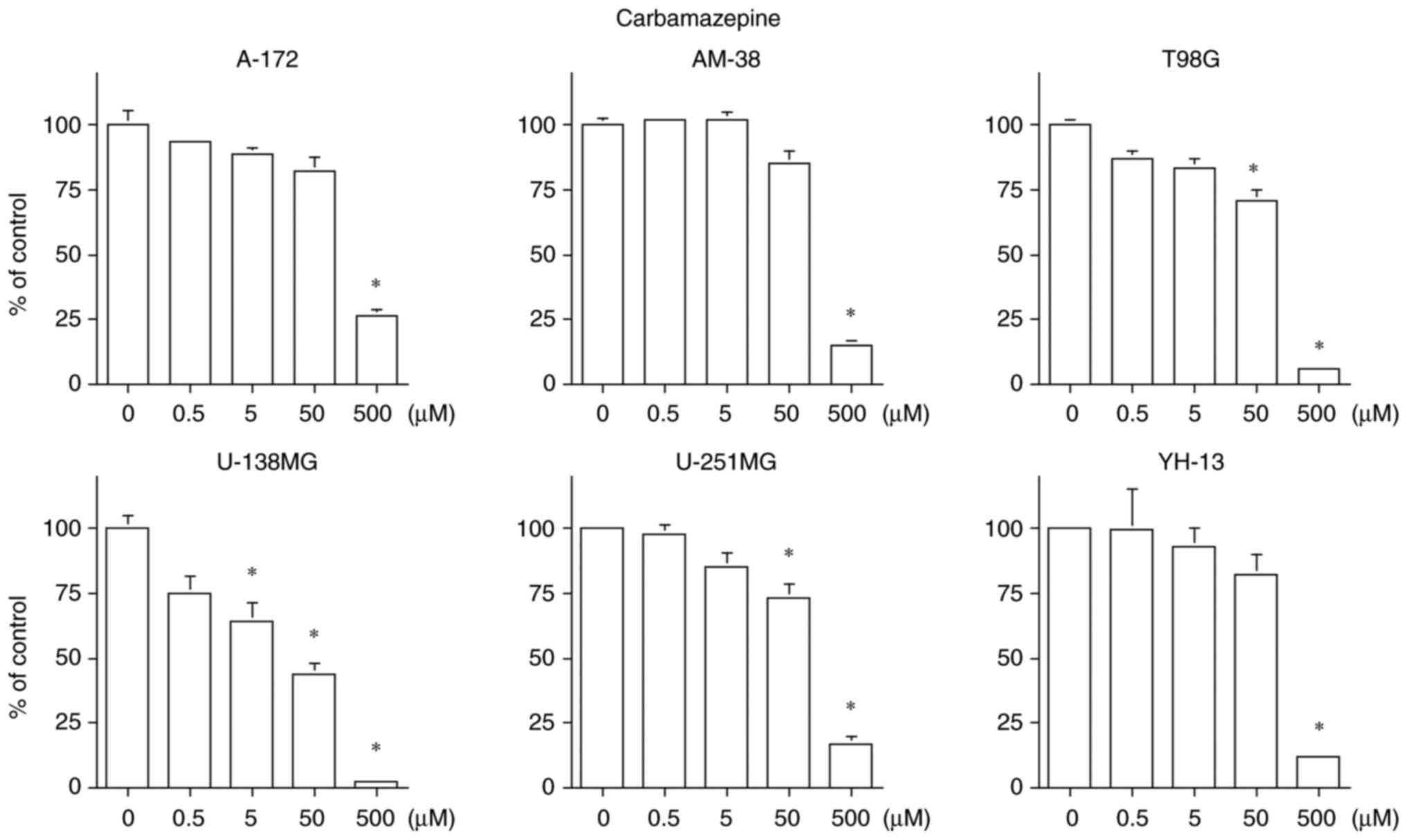
CBZ inhibited the cell proliferation in all six cell
lines, but suppressed the cell proliferation only at a
concentration of 500 µM in the A-172, AM-38 and YH-13 cell lines.
CBZ exhibited a concentration-dependent cell proliferation
inhibition in the T98G, U-138MG and U-251MG cell lines. The U-138MG
cell line was particularly sensitive, with an IC50 of 25
µM. The therapeutic blood concentration range of CBZ as an
antiepileptic drug is 17 to 50 µM (24). Only the U-138MG cell line had
IC50 of 50 µM or less for CBZ (Fig. 2).
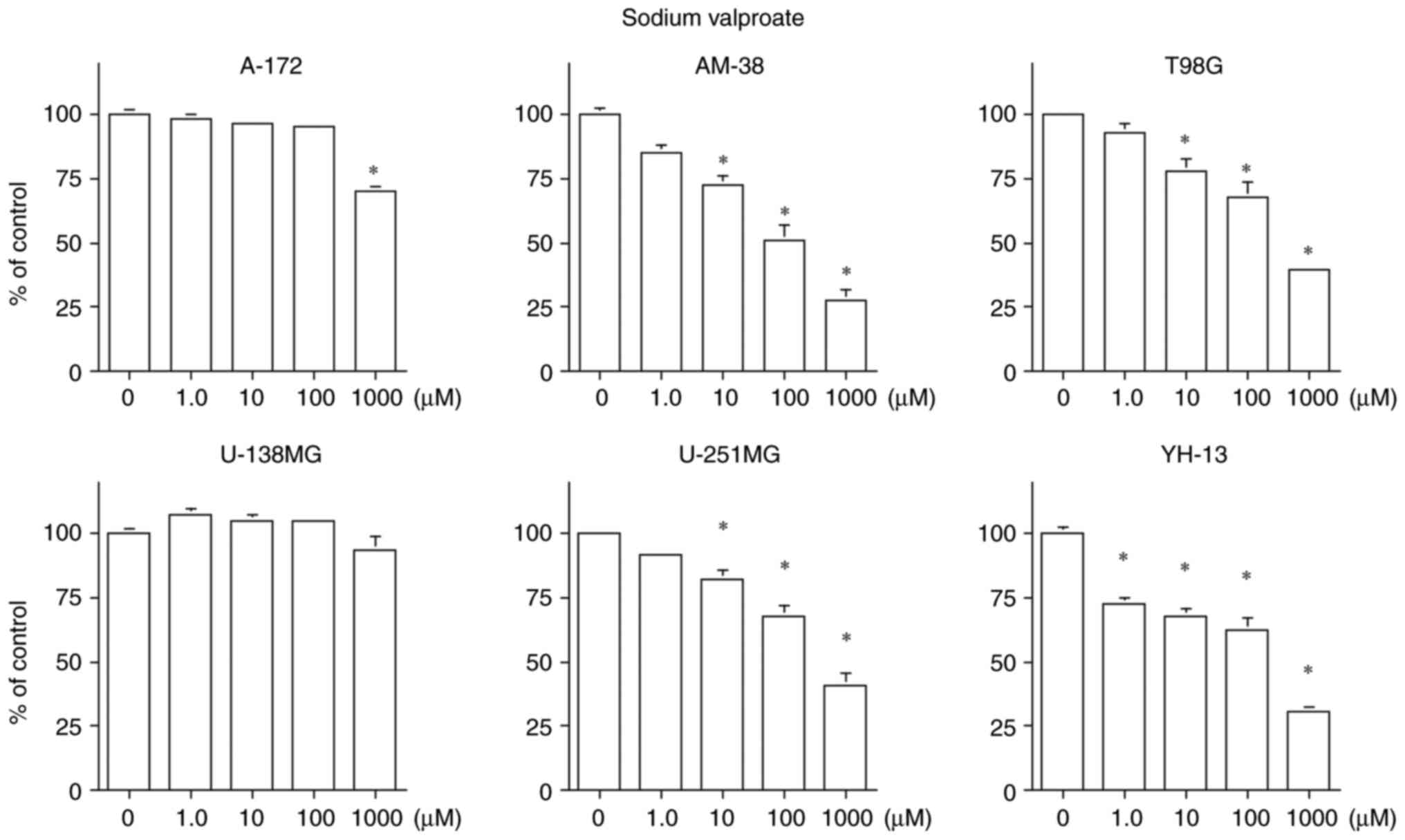
VPA inhibited cell proliferation in all cell lines
except U-138MG. Growth inhibition was observed only at a
concentration of 1,000 µM in the A-172 cell line, which is higher
than the maximum therapeutic range of the blood concentration of
600 µM (24). The cell
proliferation inhibition was concentration-dependent in the other
four cell lines, with IC50 values below the therapeutic
blood level range of 300–600 µM for VPA, including 91 µM in AM-38,
463 µM in T98G, 546 µM in U-251MG and 139 µM in YH-13 cells
(Fig. 3).
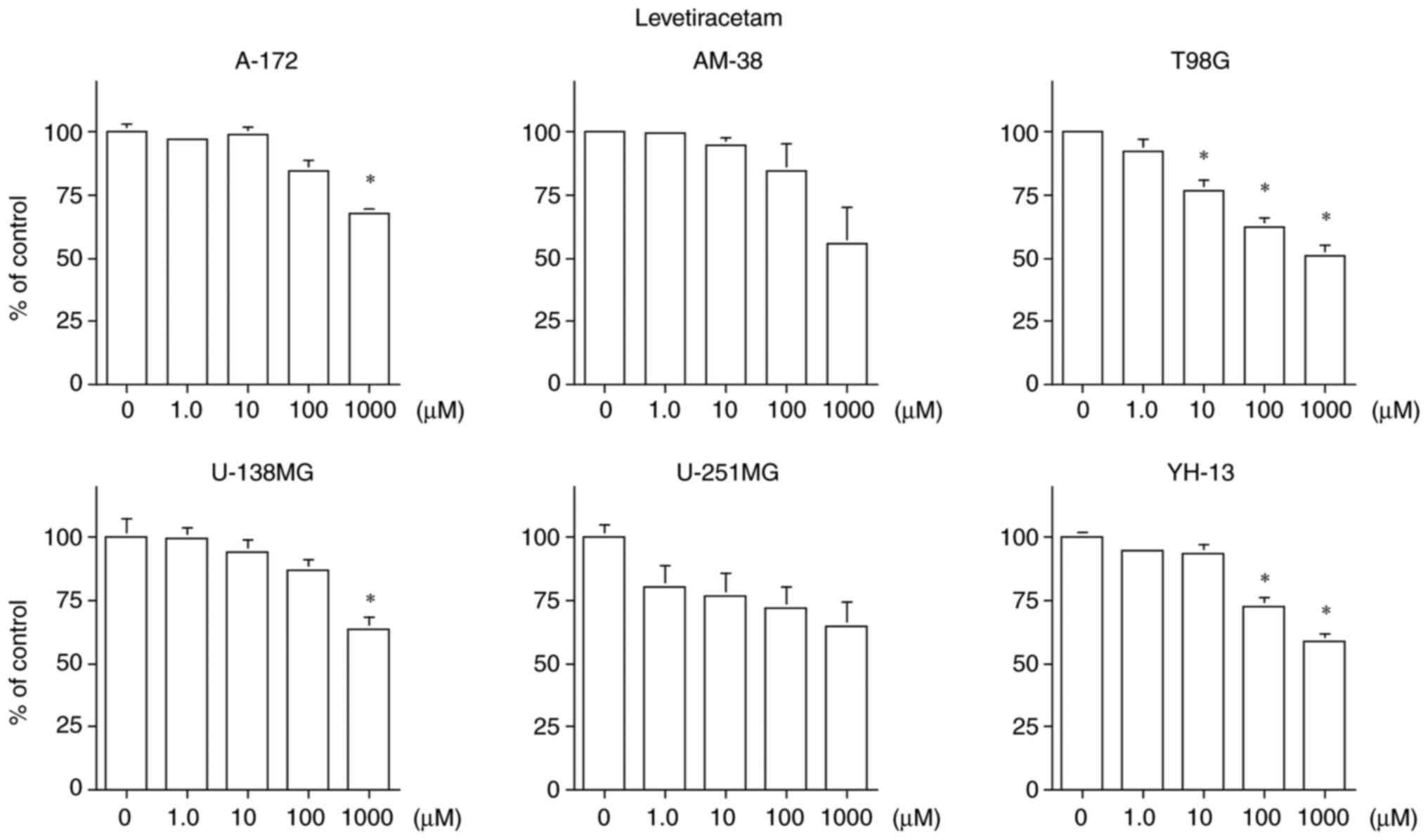
LEV inhibited cell proliferation of the A-172, T98G,
U-138MG and YH-13 cell lines, but had no statistically significant
effect on the AM-38 and U-251MG cell lines. Cell proliferation
inhibition was suppressed only at 1,000 µM in the A-172 and U-138MG
cell lines, which is higher than the maximum therapeutic blood
concentration of 270 µM for LEV. Cell proliferation was dependent
on the concentration of LEV in the T98G and YH-13 cell lines
(Fig. 4). The IC50 of
each cell line exceeded the therapeutic blood concentration of 70
to 270 µM as an antiepileptic drug.
Cell proliferation inhibition by
combined TMZ and antiepileptic drug
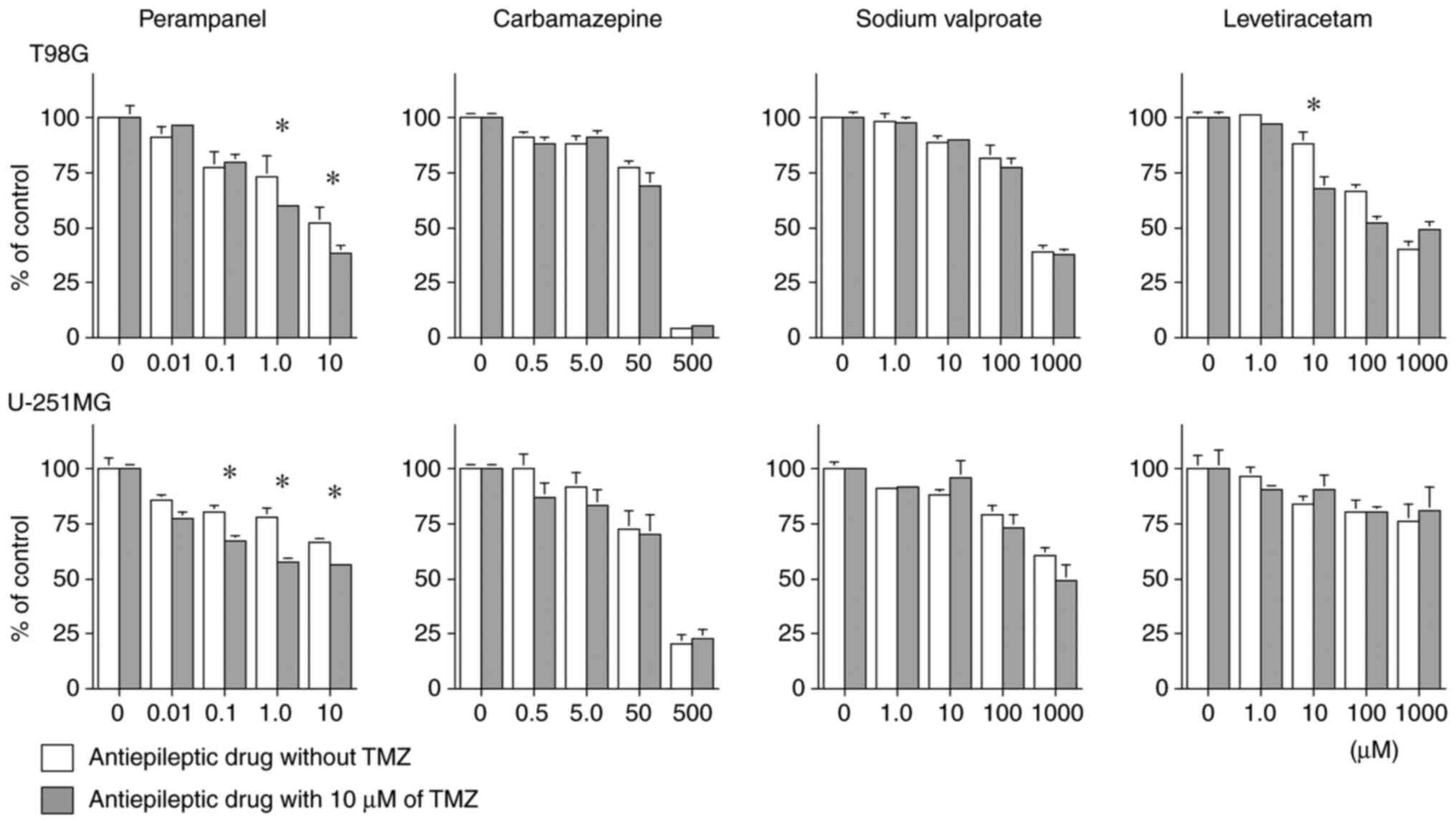
As presented in Fig.
5, perampanel in combination with 10 µM of TMZ produced a
significantly enhanced cell proliferation inhibition compared with
only TMZ from the concentration of 1.0 µM in T98G (1.0 µM of
perampanel without TMZ vs. with TMZ: 73.08±9.77 vs. 59.89±1.75%; 10
µM of perampanel without TMZ vs. with TMZ: 51.92±7.76 vs.
38.55±3.57% compared to the control; P<0.05), and from the
concentration of 0.1 µM in U-251MG cells (0.1 µM of perampanel
without TMZ vs. with TMZ: 80.58±2.56 vs. 66.92±2.56%; 1.0 µM of
perampanel without TMZ vs. with TMZ: 78.21±4.13 vs. 57.58±2.06%;
and 10 µM of perampanel without TMZ vs. with TMZ: 66.50±1.97 vs.
56.6±1.59% compared to the control; P<0.05). These results, for
perampanel in combination with TMZ, were partially reported (but
not in detail) in the previous study by our group (13). Other than perampanel, the
combination of 10 µM of LEV and 10 µM of TMZ had enhanced cell
proliferation inhibition in the T98G cell line compared to only TMZ
(10 µM of LEV without TMZ vs. with TMZ: 88.08±4.33 vs. 67.78±5.23%
compared to the control, P<0.05).
Effect of antiepileptic drugs on cell
migration and invasion
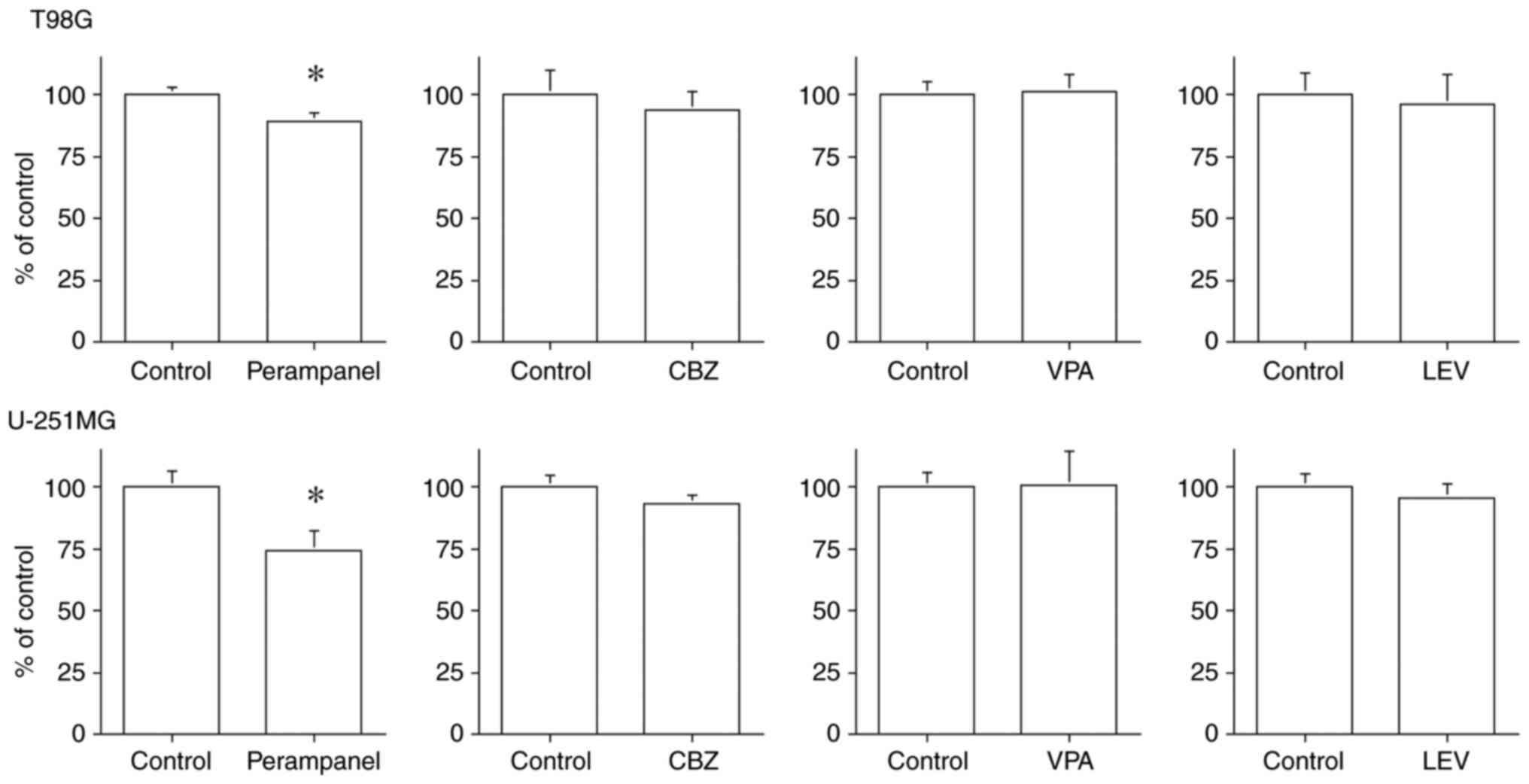
Fig. 6 demonstrates
a significant suppression of cell migration ability in the
perampanel-treated group (T98G: 89.11±3.29% and U-251MG:
74.17±7.98% compared to the control; P<0.05), but none in the
other antiepileptic drug-treated groups.
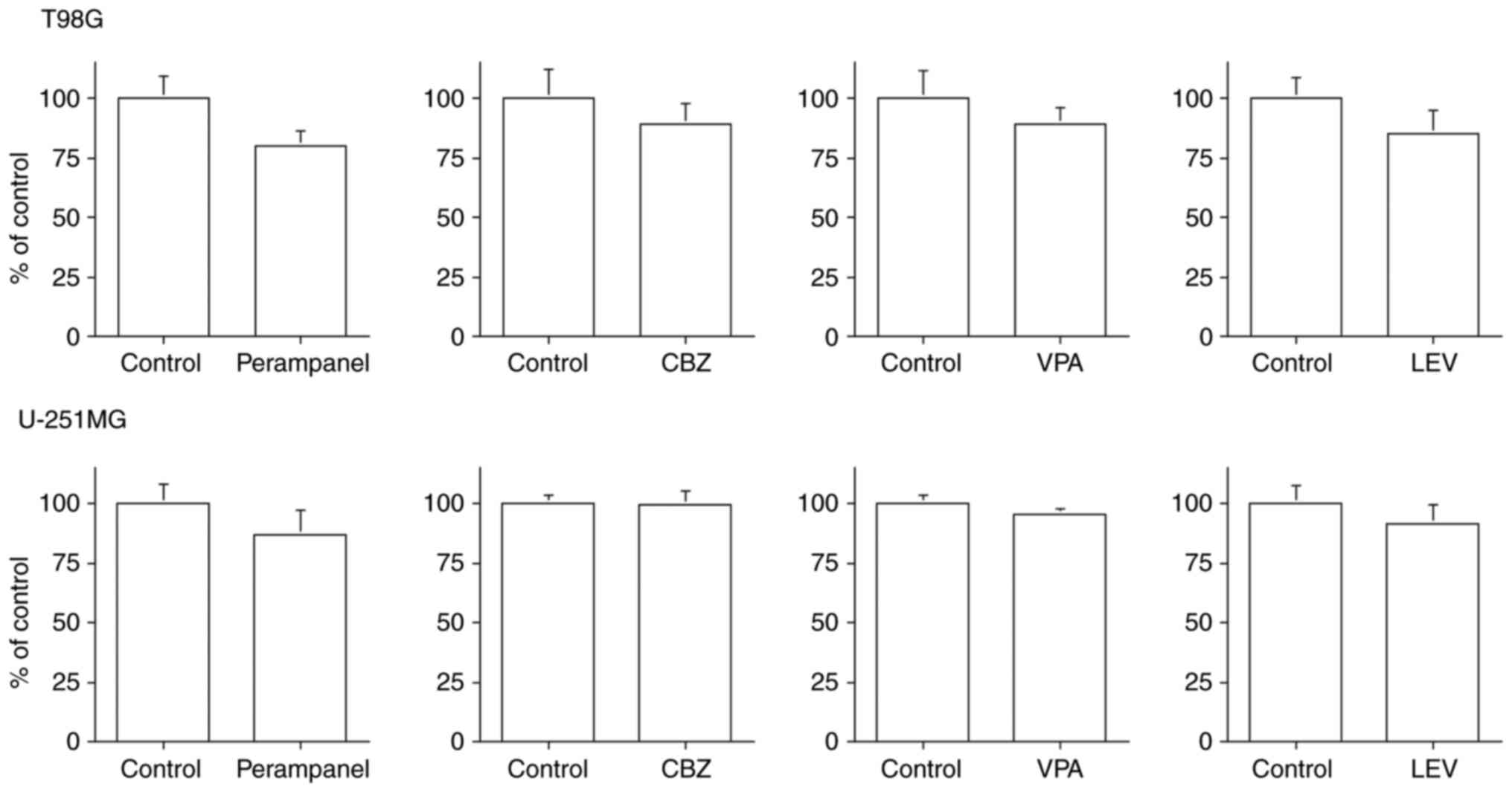
All four antiepileptic drugs had a tendency to
suppress cell invasion, but no significant difference was observed
in the T98G cell line (Fig. 7).
Perampanel and LEV tended to suppress cell invasion compared with
the other antiepileptic drugs, but no significant difference was
observed in the U-251MG cell line.
Perampanel suppresses EMT of malignant
glioma cells
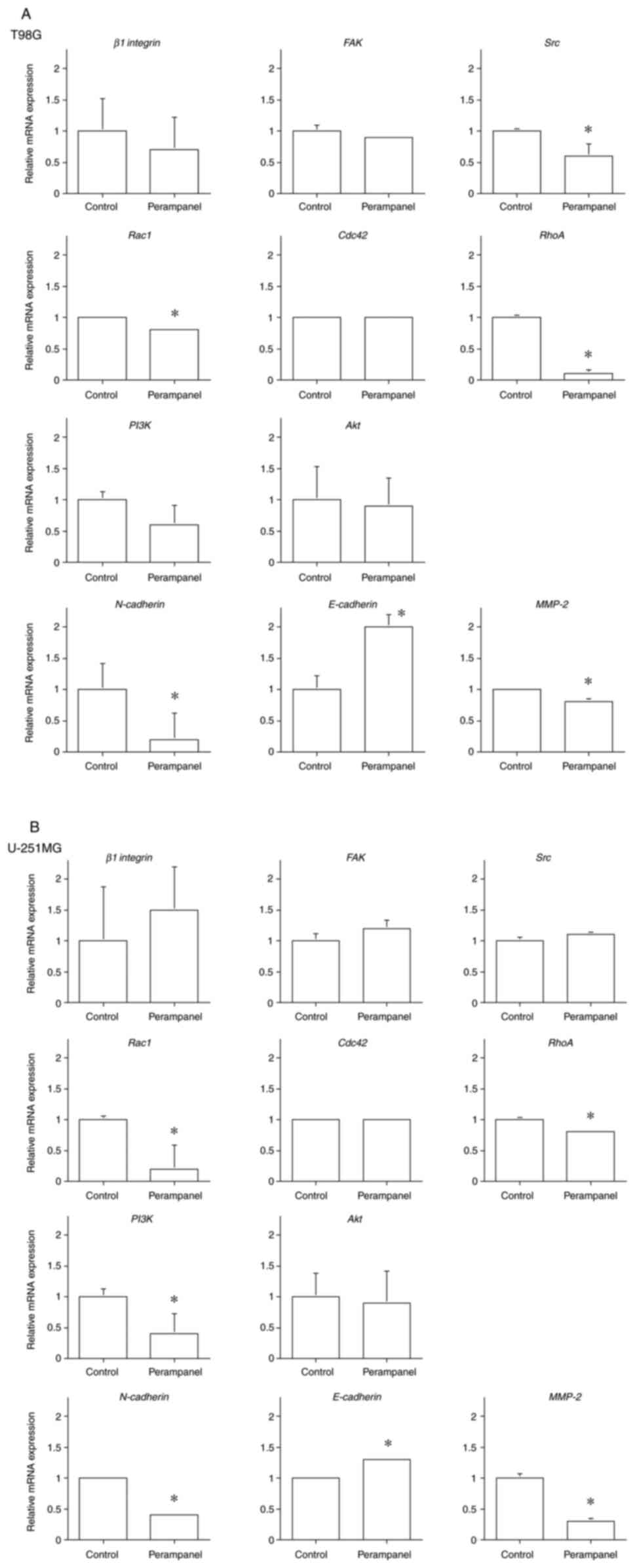
No significant change was observed in the expression
of β1 integrin after treatment with perampanel in both T98G
and U-251MG cell lines, but downstream of the FAK/Src pathway, the
expression of Src was decreased only in the T98G cell line
(0.60±0.19 compared to the control; P<0.05). Further downstream,
the expression of Rac1 and RhoA, which reconstruct
the cytoskeleton that enhance cell motility, was reduced in both
T98G (Rac1: 0.80±0.03 and RhoA: 0.10±0.06 compared to
the control; P<0.05) and U-251MG cells (Rac1: 0.20±0.39
and RhoA: 0.80±0.03 compared to the control; P<0.05), but
Cdc42 expression was unchanged in both cell lines (Fig. 8).
PI3K/Akt is another pathway that induces EMT, and a
significant decrease in PI3K expression was observed in the
U-251MG cell line after treatment with perampanel (0.40±0.33
compared to the control; P<0.05), but the decrease was not
significant in the T98G cell line. The expression of Akt
exhibited no change in both cell lines compared with the control
group. However, the expression of the mesenchymal marker
N-cadherin, which promotes cell migration and infiltration,
was decreased in both cell lines (T98G: 0.20±0.42 and U-251MG:
0.40±0.03 compared to the control; P<0.05). On the other hand,
the expression of the epithelial marker E-cadherin, which
strengthens cell-cell adhesion and reduces cell motility, was
increased in both cell lines (T98G: 2.00±0.20 and U-251MG:
1.30±0.03 compared to the control; P<0.05). Furthermore, the
expression of MMP-2, a proteolytic enzyme, was reduced in
both cell lines (T98G: 0.80±0.05 and U-251MG: 0.30±0.05 compared to
the control; P<0.05) (Fig.
8).
Discussion
The previous study by our group on cell
proliferation inhibitory effects using six malignant glioma cell
lines indicated perampanel has a concentration-dependent inhibitory
effect on proliferation in all cell lines (13). Although perampanel has been
reported to have antitumor effects, the amount of perampanel used
in those studies was much higher than the blood concentration for
its clinical use as an antiepileptic drug (10,11).
However, in the present study, much like our previous study,
perampanel demonstrated significant inhibition of cell
proliferation in most cell lines at the therapeutic blood
concentration level as an antiepileptic agent. By contrast, CBZ, a
voltage-gated Na+ channel inhibitor, had a cell growth
inhibitory effect only on U-138MG cells. Furthermore, the
IC50 of CBZ for U-138MG was <50 µM and the
therapeutic blood concentration range of CBZ as an antiepileptic
drug is 17–50 µM (24). VPA is a
gamma-aminobutyric acid (GABA) metabolism inhibitor and the
antiepileptic action is mainly due to the increased concentration
of GABA, an inhibitory neurotransmitter, which binds to the
receptor and promotes the influx of Cl−, causing the
suppression of nerve excitement (27). Therapeutic concentrations of VPA
had no inhibitory effect on cell proliferation in U-138MG cells and
almost none in A172 cells. However, AM-38, T98G, U-251MG and YH-13
cells demonstrated an inhibitory effect of 50% or more at a maximum
therapeutic blood concentration of 600 µM of VPA. LEV acts as an
antiepileptic drug by binding to the synaptic vesicle protein SV2A
and reducing the release of synaptic vesicles (27). LEV inhibited cell proliferation at
a concentration of 100 µM (blood concentration range as
anticonvulsant, 70–270 µM) in only T98G and YH-13 cells, and no
cell line exhibited a cell proliferation inhibitory effect of 50%
or more at the therapeutic range of the blood concentration as an
antiepileptic drug. A significant cell growth inhibitory effect of
perampanel was observed within each concentration as anticonvulsant
in all 6 cell lines, while half of the cell lines were sensitive to
CBZ, four to VPA and two to LEV. In addition, the IC50
values of certain cells for penampanel, CBZ and VPA were within the
concentrations of the drugs used as anticonvulsants. CBZ only
exerted a specific cell growth inhibitory effect on U-138MG. Only
perampanel and VPA were observed to have antiproliferative effects
on malignant glioma cells. In the present study, the mechanism of
action of the cell growth inhibitory effect of each anticonvulsant
was not examined in detail. The previous study by our group
reported that the mechanism of the antitumor effect of perampanel
was not cell cycle-related, but was associated with induction of
apoptosis (13), which is somewhat
consistent with previous findings (10,11).
However, VPA has numerous reported effects on tumor cells,
including cell proliferation inhibition and apoptosis induction
associated with histone deacetylase and cell cycle inhibition
through glycogen synthase kinase 3β inhibition (28,29).
In the present study, the antitumor effect of the
antiepileptic drugs combined with 10 µM of TMZ, a therapeutic level
of a standard chemotherapeutic drug for glioblastoma, was
investigated using the T98G and U-251MG cell lines, which tended to
be sensitive to the antiepileptic drugs at therapeutic levels. It
was investigated which anticonvulsants elicit further antitumor
effects when used with TMZ. Perampanel produced a significant
inhibition of cell growth from 1.0 µM in both cell lines, and LEV
also demonstrated a significant inhibition of cell growth at 10 µM
in the T98G cell line. These antiepileptic drugs may have further
antitumor effects in combination with TMZ. Synergistic effects
between perampanel and TMZ have also been reported by another study
(11). On the other hand, the
expression of MGMT is strongly associated with susceptibility to
TMZ and 45–75% of malignant gliomas express MGMT (30). T98G cells express MGMT and are
resistant to TMZ treatment, whereas U-251MG cells do not express
MGMT (22). The present study did
not further elucidate the mechanisms of the cell growth inhibitory
effect of perampanel in combination with TMZ on T98G and U-251MG
cells, including how perampanel alters MGMT implicated in TMZ
resistance. However, it was previously reported that LEV suppresses
the expression of MGMT and enhances the effect of TMZ by activating
the apoptotic pathway (31). The
present study indicated that the combination of LEV and TMZ
enhanced the inhibition of cell proliferation in T98G cells.
Therefore, LEV may increase TMZ sensitivity of certain glioma
cells, which may be due to a variety of mechanisms, including an
inhibitory effect on MGMT.
Only perampanel significantly suppressed cell
migration and perampanel tended to suppress cell invasion more than
the other antiepileptic drugs. Thus, further experiments (mRNA
expression of EMT-related molecules) were subsequently conducted
for only perampanel. The present results indicated that perampanel
downregulated the Rac1 and RhoA, as well as the β1
integrin/FAK/Src pathways; furthermore, it upregulated the
E-cadherin and downregulated the N-cadherin and
PI3K/Akt pathways. These effects may inhibit changes in cell
morphology or reduce cell motility and increase intercell adhesion.
The present study suggested that perampanel suppressed the EMT and
inhibited cell migration. Further studies, including western blot
analysis, such as phosphorylation (activation) of Akt, are
required, since protein analysis is critical. Such studies would
make the present results more conclusive.
Various antiepileptic drugs are clinically used to
treat and control symptomatic epilepsy caused by malignant glioma.
Controlling epileptic seizures is important for maintaining the
patients' quality of life and it is vital to continue the treatment
for brain tumors. However, which antiepileptic drug has the best
seizure-suppressing effect remains to be determined. A systematic
review comparing the effects of antiepileptic drugs on patients
with grade 2–4 gliomas reported that the 12-month seizure-free rate
was 43% for CBZ, 37% for VPA and 74% for LEV (32). Perampanel monotherapy data were not
included, but combination therapy with another anticonvulsant
achieved a 45% 12-month seizure-free rate. The treatment failure
rate for epilepsy in 12 months was 0% for perampanel, 26% for CBZ,
21% for VPA and 24% for LEV. The present study indicated that
perampanel and LEV had a high seizure-control effect on brain
tumor-related epilepsy, but perampanel has a low risk of side
effects and is generally more effective (32). Therefore, perampanel may be
selected as a higher-priority therapeutic drug, for both
antiepileptic and antitumor effects.
As a limitation of the present study, DMSO was used
as the vehicle for the anticonvulsants perampanel and CBZ. DMSO
concentrations of <10% are considered to be of low toxicity
(33). The concentrations of DMSO
in the 10 µM perampanel and 500 µM CBZ (highest concentration of
DMSO for each drug) groups were 0.1 and 0.5% DMSO in the present
study, respectively. Therefore, no DMSO vehicle control was
included in the present study to check for solvent toxicity.
In conclusion, the present study on the four common
antiepileptic drugs, perampanel, CBZ, VPA and LEV, indicated that
perampanel not only suppresses cell proliferation but also enhances
the cell proliferation inhibitory effect when used in combination
with TMZ in certain malignant glioma cell lines. In addition,
perampanel had an antitumor effect that inhibited cell migration.
Therefore, perampanel may be more beneficial in terms of antitumor
efficacy than other antiepileptic drugs in the treatment of
malignant glioma.
Acknowledgements
Certain parts of this study are included in the
Japanese-language PhD thesis of the author CY at Nihon University
School of Medicine (Tokyo, Japan).
Funding
This work was supported in part by a Grant-in-Aid for Scientific
Research from the Japan Society for the Promotion of Science (grant
no. 19K09491).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CY, JT and ES developed the experimental design,
performed most of the experiments and analysis, and drafted the
manuscript. YH, YO, SYo, SYa, KS, HH and YK were involved in the
conception and design of the study, performed parts of the
experiments, analyzed the data and contributed to the writing of
the manuscript. AY contributed to the experimental design, analyzed
the data and was involved in writing the manuscript. CY, JT, ES, YH
and AY confirm the authenticity of all raw data. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent to participate
Not applicable.
Competing interests
AY has received research funds for other research
projects from Medtronic Japan Co., Ltd. and Eisai Co., Ltd., Tokyo,
Japan. He has also, in accordance with the rules, reported their
competing interests (including a small amount of research funds for
other research projects) to his main academic society, the Japan
Neurosurgical Society. The other authors have no competing
interests to declare.
References
|
1
|
No authors listed, . Brain tumor registry
of Japan (2005–2008). Neurol Med Chir (Tokyo). 57 (Suppl
1):S9–S102. 2017. View Article : Google Scholar
|
|
2
|
Knudsen-Baas KM, Engeland A, Gilhus NE,
Storstein AM and Owe JF: Does the choice of antiepileptic drug
affect survival in glioblastoma patients? J Neurooncol.
129:461–469. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Weller M, Gorlia T, Cairncross JG, van den
Bent MJ, Mason W, Belanger K, Brandes AA, Bogdahn U, Macdonald DR,
Forsyth P, et al: Prolonged survival with valproic acid use in the
EORTC/NCIC temozolomide trial for glioblastoma. Neurology.
77:1156–1164. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Barker CA, Bishop AJ, Chang M, Beal K and
Chan TA: Valproic acid use during radiation therapy for
glioblastoma associated with improved survival. Int J Radiat Oncol
Biol Phys. 86:504–509. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jabbarli R, Ahmadipour Y, Rauschenbach L,
Santos AN, Darkwah Oppong M, Pierscianek D, Quesada CM, Kebir S,
Dammann P, Guberina N, et al: How about levetiracetam in
glioblastoma? An institutional experience and meta-analysis.
Cancers (Basel). 13:37702021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Happold C, Gorlia T, Chinot O, Gilbert MR,
Nabors LB, Wick W, Pugh SL, Hegi M, Cloughesy T, Roth P, et al:
Does valproic acid or levetiracetam improve survival in
glioblastoma? A pooled analysis of prospective clinical trials in
newly diagnosed glioblastoma. J Clin Oncol. 34:731–739. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
French JA, Krauss GL, Biton V, Squillacote
D, Yang H, Laurenza A, Kumar D and Rogawski MA: Adjunctive
perampanel for refractory partial-onset seizures: Randomized phase
III study 304. Neurology. 79:589–596. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Grossman SA, Ye X, Chamberlain M,
Mikkelsen T, Batchelor T, Desideri S, Piantadosi S, Fisher J and
Fine HA: Talampanel with standard radiation and temozolomide in
patients with newly diagnosed glioblastoma: A multicenter phase II
trial. J Clin Oncol. 27:4155–4161. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Iwamoto FM, Kreisl TN, Kim L, Duic JP,
Butman JA, Albert PS and Fine HA: Phase 2 trial of talampanel, a
glutamate receptor inhibitor, for adults with recurrent malignant
gliomas. Cancer. 116:1776–1782. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lange F, Weßlau K, Porath K, Hörnschemeyer
J, Bergner C, Krause BJ, Mullins CS, Linnebacher M, Köhling R and
Kirschstein T: AMPA receptor antagonist perampanel affects
glioblastoma cell growth and glutamate release in vitro. PLoS One.
14:e02116442019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Salmaggi A, Corno C, Maschio M, Donzelli
S, D'Urso A, Perego P and Ciusani E: Synergistic effect of
perampanel and temozolomide in human glioma cell lines. J Pers Med.
11:3902021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Izumoto S, Miyauchi M, Tasaki T, Okuda T,
Nakagawa N, Nakano N, Kato A and Fujita M: Seizures and tumor
progression in glioma patients with uncontrollable epilepsy treated
with perampanel. Anticancer Res. 38:4361–4366. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tatsuoka J, Sano E, Hanashima Y, Yagi C,
Yamamuro S, Sumi K, Hara H, Takada K, Kanemaru K, Komine-Aizawa S,
et al: Anti-tumor effects of perampanel in malignant glioma cells.
Oncol Lett. 24:4212022. View Article : Google Scholar
|
|
14
|
Lefranc F, Le Rhun E, Kiss R and Weller M:
Glioblastoma quo vadis: Will migration and invasiveness reemerge as
therapeutic targets? Cancer Treat Rev. 68:145–154. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Corsi L, Mescola A and Alessandrini A:
Glutamate receptors and glioblastoma multiforme: An old ‘route’ for
new perspectives. Int J Mol Sci. 20:17962019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vollmann-Zwerenz A, Leidgens V, Feliciello
G, Klein CA and Hau P: Tumor cell invasion in glioblastoma. Int J
Mol Sci. 21:19322020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Piao Y, Lu L and de Groot J: AMPA
receptors promote perivascular glioma invasion via beta1
integrin-dependent adhesion to the extracellular matrix. Neuro
Oncol. 11:260–273. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhong J, Paul A, Kellie SJ and O'Neill GM:
Mesenchymal migration as a therapeutic target in glioblastoma. J
Oncol. 2010:4301422010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Catalano M, D'Alessandro G, Lepore F,
Corazzari M, Caldarola S, Valacca C, Faienza F, Esposito V,
Limatola C, Cecconi F and Di Bartolomeo S: Autophagy induction
impairs migration and invasion by reversing EMT in glioblastoma
cells. Mol Oncol. 9:1612–1625. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhu X, Hu B, Hu M, Qian D and Wang B:
Human cytomegalovirus infection enhances invasiveness and migration
of glioblastoma cells by epithelial-to-mesenchymal transition. Int
J Clin Exp Pathol. 13:2637–2647. 2020.PubMed/NCBI
|
|
21
|
Cuddapah VA, Robel S, Watkins S and
Sontheimer H: A neurocentric perspective on glioma invasion. Nat
Rev Neurosci. 15:455–465. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yoshino A, Ogino A, Yachi K, Ohta T,
Fukushima T, Watanabe T, Katayama Y, Okamoto Y, Naruse N, Sano E
and Tsumoto K: Gene expression profiling predicts response to
temozolomide in malignant gliomas. Int J Oncol. 36:1367–1377. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wischhusen J, Naumann U, Ohgaki H,
Rastinejad F and Weller M: CP-31398, a novel p53-stabilizing agent,
induces p53-dependent and p53-independent glioma cell death.
Oncogene. 22:8233–8245. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Japanese Society of Neurology, . CQ12-2:
Serum concentration monitoring is useful for which drugs? Clinical
practice guidelines for epilepsy 2018. Igaku-Shoin Ltd; Tokyo: pp.
103–105. 2018, Available at:. https://neurology-jp.org/guidelinem/epilepsy/documents/guideline2018.pdfpp.
123–125. https://www.neurology-jp.org/guidelinem/tenkan_2018.htmlin
Japanese.
|
|
25
|
Ostermann S, Csajka C, Buclin T, Leyvraz
S, Lejeune F, Decosterd LA and Stupp R: Plasma and cerebrospinal
fluid population pharmacokinetics of temozolomide in malignant
glioma patients. Clin Cancer Res. 10:3728–3736. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Brodie MJ, Covanis A, Gil-Nagel A, Lerche
H, Perucca E, Sills GJ and White HS: Antiepileptic drug therapy:
Does mechanism of action matter? Epilepsy Behav. 21:331–341. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang C, Liu S, Yuan X, Hu Z, Li H, Wu M,
Yuan J, Zhao Z, Su J, Wang X, et al: Valproic acid promotes human
glioma U87 cells apoptosis and inhibits glycogen synthase kinase-3β
through ERK/Akt signaling. Cell Physiol Biochem. 39:2173–2185.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Han W and Guan W: Valproic acid: A
promising therapeutic agent in glioma treatment. Front Oncol.
11:6873622021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bello MJ, Alonso ME, Amiñoso C, Anselmo
NP, Arjona D, Gonzalez-Gomez P, Lopez-Marin I, de Campos JM,
Gutierrez M, Isla A, et al: Hypermethylation of the DNA repair gene
MGMT: Association with TP53 G:C to A:T transitions in a series of
469 nervous system tumors. Mutat Res. 554:23–32. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Scicchitano BM, Sorrentino S, Proietti G,
Lama G, Dobrowolny G, Catizone A, Binda E, Larocca LM and Sica G:
Levetiracetam enhances the temozolomide effect on glioblastoma stem
cell proliferation and apoptosis. Cancer Cell Int. 18:1362018.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
de Bruin ME, van der Meer PB, Dirven L,
Taphoorn MJB and Koekkoek JAF: Efficacy of antiepileptic drugs in
glioma patients with epilepsy: A systematic review. Neurooncol
Pract. 8:501–517. 2021.PubMed/NCBI
|
|
33
|
Galvao J, Davis B, Tilley M, Normando E,
Duchen MR and Cordeiro MF: Unexpected low-dose toxicity of the
universal solvent DMSO. FASEB J. 28:1317–1330. 2014. View Article : Google Scholar : PubMed/NCBI
|