Introduction
Tumor heterogeneity is a key characteristic of
cancer and the presence of cancer stem cells (CSCs) has become
increasingly associated with treatment failure and tumor
progression/relapse (1,2). CSCs are operationally defined as a
subset of cancer cells that i) is capable of self-renewal, ii) has
tumor-initiating ability and iii) is resistant to ionizing
radiation and chemotherapy (3).
CSCs can be identified by various markers. CSCs from human breast
tumors, characterized as having a
CD24−/low/CD44+ phenotype, were first
identified for their tumorigenic potential in xeno-transplanted
immune-deficient mice (4).
Additional markers are associated with CSC characteristics and high
aldehyde dehydrogenase activity (ALDH+), a marker of
normal and malignant human mammary stem cells, is a predictor of
poor clinical outcome in breast cancer (5). The subpopulations detected by these
markers only partially overlap, indicating that different lineages
of CSCs may coexist within the same tumor (6).
In addition, evidence connects
epithelial-mesenchymal transition (EMT) with the acquisition of
stem cell properties (7). Many
dysregulated pathways in breast CSCs [Notch, hedgehog, Wingless
(Wnt), transforming growth factor β (TGFβ) and NFκB], via
activation of mesenchymal transcription factors (Twist, Snail and
Zeb), induce EMT (8). Most
mesenchymal (M) cells generated by EMT acquire a
CD24−/low/CD44+ pattern (7,9,10).
On the other hand, studies have shown the existence of epithelial
(E)-like breast CSCs with a CD24+/CD44low
phenotype and high ALDH activity (5,11).
Finally, several transition states occurring during EMT have been
identified and a hybrid E/M phenotype is associated with increased
tumor stemness (12,13). In breast cancer, CSCs are endowed
with plasticity that enables them to reverse transition between E
and M states and increased metastatic or tumorigenic potential and
poor clinical prognosis have been associated with CSCs in a hybrid
E/M state (12,14).
By definition, breast CSCs are relatively resistant
to traditional cancer therapies, including chemotherapy and
radiation therapy (15) and this
resistance has been observed for
CD24−/low/CD44+ M CSCs but also for
ALDH+ E CSCs (16–19).
Different mechanisms have been proposed to explain the genotoxic
stress survival of CSCs (20),
including cell quiescence, increased ability to repair DNA damage,
increased antiapoptotic defense, dysregulation of autophagy,
metabolic changes and resistance to reactive oxygen species (ROS);
many of these pathways are mediated by redox imbalance (21).
Our previous study showed that high-dose irradiation
(IR) of breast cancer cell lines leads to transitory selection of a
CD24−/low subpopulation independently of CD44 expression
(22) and that loss of CD24
expression promotes radiation resistance association with decreased
ROS levels (23). Lower intrinsic
levels of ROS, associated with radioresistance, are also
characteristic of CD24−/low/CD44+ M breast
CSCs (10,16,24).
CD24 is a small cell surface protein molecule
frequently overexpressed in human cancer, especially breast cancer
(25,26). CD24 is hypothesized to function as
an adhesion molecule but due to variable glycosylation pattern it
acts as a versatile ligand with diverse physiological functions
(26), making its mechanisms
complex to understand. Despite CD24 being a marker strongly
associated with EMT in breast cancer, its biological functions and
role in cancer progression and treatment resistance remain poorly
documented. Moreover, clinical studies on the association between
CD24 expression and breast tumor progression are conflicting,
especially due to the poor specificity of CD24 antibodies used in
these studies (27,28).
ROS mediate the effects of anticancer drugs as well
as ionizing radiation (29–32),
and our previous study observed an association between CD24
expression and ROS levels (10);
therefore it was hypothesized CD24 may serve a role in the radio-
and chemo-resistance of breast cancer cells.
The present study used the non-tumorigenic HMLE cell
model developed by Mani in R. Weinberg's team (7) to study EMT in breast cancer cells,
with the main advantage to study E and M cells in the same genetic
background (CD24−/low/CD44+ M-HMLE cells are
obtained after induction of EMT by TGFβ in parental
CD24+/CD44low E-HMLE). Here, we investigated
if CD24 could be defined as an actor of both radiation- and
chemo-resistance of breast cancer cells.
Materials and methods
Cell culture
All cell lines were cultivated in Heraeus Thermo
Scientific BBD6220 incubator at 37°C in a humidified atmosphere of
5% CO2 and 95% air. Presence of mycoplasma was regularly
tested with the MycoAlert™ Mycoplasma detection kit from Lonza
Group, Ltd. Tumor breast cancer cell lines T47D and MCF7 were from
the American Type Culture Collection and Human Mammary Epithelial
HMLE cell line was kindly provided by Professor Robert A. Weinberg
(Whitehead Institute). HMLE cells come from Human Mammary
Epithelial Cells (HMECs) immortalized by human telomerase reverse
transcriptase (hTERT) and transformed by large T antigen (SV40)
(33). E and M HMLE phenotypes
were previously characterized by Mani in R. Weinberg's team
(7) and our group (10). All cell lines were grown in
adherent conditions in cell culture media supplemented with 10%
(v/v) heat-inactivated fetal bovine serum (Sigma-Aldrich; Merck
KGaA) and 1 mM antibiotic-antimycotic (Invitrogen; Thermo Fisher
Scientific, Inc.) For T47D and MCF7, Dulbecco's Modified Eagle's
Medium (DMEM), high glucose, GlutaMAX™ Supplement, pyruvate (Gibco;
Thermo Fisher Scientific, Inc.) was used. HMLE cells were cultured
in DMEM/F-12 Nutrient Mixture, GlutaMAX Supplement (Gibco; Thermo
Fisher Scientific, Inc.) with 10 ng/ml human epidermal growth
factor, 0.5 µg/ml hydrocortisone and 10 µg/ml insulin (all
Sigma-Aldrich; Merck KGaA). Medium for transfected cell lines was
supplemented with 0.4 puromycin and 16.0 µg/ml blasticidin (both
Gibco; Thermo Fisher Scientific, Inc.) during the transfectant
selection phase and 0.2 puromycin and 8.0 µg/ml blasticidin for
routine cell culture. Mesenchymal M HMLE cells were induced and
FACS-sorted after a 10 day treatment with 2.5 ng/ml recombinant
TGFβ1 (Thermo Fisher Scientific, Inc.). Cell proliferation and
survival analysis were performed in ≥3 experiments, by scoring
cells with a TC20 automated cell counter (Bio-Rad Laboratories,
Inc.). Mammosphere formation assay was performed as described by
Lombardo et al (34)
(Appendix S1).
Chemicals, reagents and
antibodies
5-Fluorouracil (5FU), cisplatin and paclitaxel
(Taxol) were from Sigma-Aldrich (Merck KGaA). 5FU and paclitaxel
were diluted in DMSO to concentration stock of 400 and 1 mM,
respectively. Cisplatin was diluted with sodium chloride to a
concentration stock of 1 mM. Each drug was used at different
concentrations depending on the experiments. Antibodies against
CD24 (clone ML5, Cat no: 555428), CD44 (clone no. G44-26, Cat. no:
555478) and isotypic controls were from BD Biosciences.
Plasmids and transfection
To stably knock down expression of CD24 in MCF-7,
T47D and HMLE cells, replicative short hairpin (shRNA)-expressing
vectors [puromycin-resistant pEBV-small interfering (si)RNA] that
impose a strong and stable gene silencing in human cells, even
after several months in culture, were used (plasmid backbone:
pEBVsiRNA, patent n°WO2006085016, CEA). siRNA design and cloning in
pEBVsiRNA vectors and establishment of stable knockdown and control
clones were performed as previously described (35). To design shRNA sequences, DSIR
program developed by Vert et al (36) was used. Forward shCD24 sequence
(pBD2506 plasmid):
5′-GATCCCGCCAAGAAACGTCTTCTAAATTCAAGAGATTTAGAAGACGTTTCTTGGTTTTTTGGAAA-3′;
Reverse shCD24 sequence:
5′-AGCTTTTCCAAAAAACCAAGAAACGTCTTCTAAATCTCTTGAATTTAGAAGACGTTTCTTGGCGG-3′.
Control cells carried the pBD650 plasmid which expressed an
inefficient shRNA validated on more than 200 genes. Forward shCTL
sequence:
5′-GATCCCGAATTGCGGCGAGCAGTAATTCAAGAGATTACCTGCTCGCCGCCAATTCTTTTTGGAAA-3′;
Reverse shCTL sequence:
5′-AGCTTTTCCAAAAAAGTCAAGAAGCATTAGAAGATCTCTTGAATCTTCTAATGCTTCTTGACGG-3′.
To express CD24 into HMLE cells already depleted for
CD24, open reading frame (ORF) of human CD24 was amplified from an
IMAGE clone (ID_5591617; Thermo Fisher Scientific, Inc.) with the
following primers: 5′-ATGGGCAGAGCAATGGTGGCCAGGCTC-3′ and
5′-TTAAGAGTAGAGATGCAGAAGAGAGAG-3′ and introduced into a
blasticidin-resistant pEBV plasmid downstream of a CAG promoter
(pBD2915; CEA).
After one day of seeding (density: 50%) into 6-well
plate; MCF-7, T47D and HMLE cells were transfected with
jetPRIME® reagent (Polyplus-transfection SA) according
to the manufacturer's recommendations. Following 24 h incubation,
cells were trypsinized and seeded (density: 25%) in culture medium
supplemented with puromycin alone or with blasticidin. Experiments
were performed either on the whole transfected population or on
selected clones.
IR
For all experiments except clonogenic assay, cells
were plated at least 24 h prior to IR. On day 0, γ-IR of cells were
performed on a GSR D1 irradiator (Gamma Medical Service GmbH;
Appendix S1). Studied cells were
irradiated at 2, 4, 6 and 10 Gy and control cells were submitted to
sham IR.
Aldefluor assay
The Aldefluor kit (Stemcell Technologies, Inc.) was
used to analyze the population with high ALDH enzymatic activity
according to the manufacturer's intructions. HMLE cells were
incubated in the Aldefluor assay buffer containing ALDH substrate
(BODIPY aminoacetaldehyde (BAAA); 1 µmol/l/1×106 cells)
and incubated for 40 min at 37°C. As negative control, for each
sample of cells, an aliquot was treated with 50 mmol/l
diethylaminobenzaldehyde, a specific ALDH inhibitor.
Clonogenic assay
Sub-confluent HMLE cells were trypsinized using
TrypLE express solution (Thermo Fisher Scientific, Inc.). Live
cells were counted using an automated cell counter (TC20; Bio-Rad
Laboratories, Inc.) and trypan blue exclusion. In the IR group,
cells were immediately irradiated in suspension to generate a dose
curve of 0, 2, 4 and 6 Gy and colony-forming assay was performed
immediately following IR by plating cells in 60-mm Petri dishes, in
triplicate.
In the drug treatment group, following
trypsinization and counting, cells were plated in 60-mm diameter
Petri dishes, in triplicate. At 6 h after plating, drug treatment
(4 to 16 µM exposition to 5FU, 2 to 6 µM exposition to Cisplatin
and 1 to 3 nM exposition to Paclitaxel) was performed for three
days, then cells were washed (PBS) and incubated for 7 days with
fresh medium.
Number of cells seeded increased with radiation dose
or drug concentration but was identical for each cell line tested.
After 7 days, cells were fixed at room temperature for 30 min in 4%
paraformaldehyde, washed (PBS) and stained at room temperature for
at least 2 h in methylene blue/30% methanol. Colonies containing
>50 cells were manually counted. The surviving fraction at each
radiation dose was normalized to that of the non-irradiated sample
and points were fitted using an exponential tendency curve. At
least three independent experiments were performed.
Cell staining
CSC marker labeling and analysis were performed as
described by Bensimon et al (22,23).
Intracellular concentrations of ROS prooxidants were determined
using dihydroethidium (DHE) probe (Invitrogen; Thermo Fisher
Scientific, Inc.). Adherent HMLE cells were incubated with 10 µM
DHE for 30 min at 37°C, then washed with PBS, trypsinized and
immediately analyzed by flow cytometry. Intracellular
concentrations of mitochondrial ROS were determined using MitoSOX™
Red Mitochondrial Superoxide Indicator (Invitrogen; Thermo Fisher
Scientific, Inc.). Adherent cells were incubated with 5 µM MitoSOX
Red for 10 min at 37°C, washed with PBS, trypsinized and
immediately analyzed by flow cytometry. Mitochondrial mass was
analyzed using MitoTracker™ Green (MTG) probe (Invitrogen; Thermo
Fisher Scientific, Inc.). Following soft trypsinization, cells were
loaded with 200 nM MTG and incubated for 20 min at 37°C, then
immediately analyzed by flow cytometry. Mitochondrial membrane
potential was quantified using tetramethylrhodamine, ethyl ester,
perchlorate (TMRE) probe (Invitrogen; Thermo Fisher Scientific,
Inc.). Following soft trypsinization, cells were loaded with 10 nM
TMRE and incubated for 20 min at 37°C, then immediately analyzed by
flow cytometry.
Flow cytometry
Cells were analyzed on a SORP LSR-II analyzer
(configuration: 488, 561, 405, 355 and 635 nm; BD Biosciences) or
BD FACSCalibur (configuration: 488 and 635 nm; BD Biosciences).
Data were analyzed with FlowJo v10.7.1 (Tree Star). Cells were
sorted on a BD Influx sorter (BD Biosciences; configuration: 488,
561, 405, 355 and 635 nm).
RNA extraction and reverse
transcription-quantitative (RT-q)PCR
Total RNA was extracted from frozen MCF-7, T47D and
HMLE cell pellets (−80°C) with Total RNA purification kit (Norgen
Biotek Corp.), according to the manufacturer's instructions. DNAse
treatment was performed using TURBO DNA-free kit (Invitrogen;
Thermo Fisher Scientific, Inc.), according to the manufacturer's
instructions. cDNA synthesis was performed with the SuperScript™
VILO™ cDNA Synthesis kit (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's instructions. RT-qPCR was
performed with ABI Prism 7300 detection apparatus (Applied
Biosystems; Thermo Fisher Scientific, Inc.) using Taqman Universal
Master Mix according to the manufacturer's recommendations. Cq
value was determined with Sequence Detection System software. All
primers were from Applied Biosystems (Thermo Fisher Scientific,
Inc.; Appendix S1). Levels of
gene expression were determined using GENORM software and
normalized using GAPDH and RPLPO.
Cell migration analysis
Cells were plated on 24-well glass bottom dishes at
4,000 cells/well. Live-cell imaging was performed using Plan APO
20× DIC objective (Numerical aperture: 0.7) on a Nikon A1R confocal
laser scanning microscope system attached to an inverted ECLIPSE Ti
(Nikon Corporation) thermostated at 37°C under a 5% CO2
atmosphere. Mosaic images were recorded every 15 min for 5 h.
Tracking of individual cells was performed using the MtrackJ plugin
in ImageJ software (37). Dynamic
parameters such as migration velocity and mean square displacement
(MSD) were calculated with the open-source computer program DiPer
(38).
Statistical analysis
All statistical tests were performed using GraphPad
Prism 8 (GraphPad Software, Inc.). ANOVA or Kruskal-Wallis tests
followed by a suitable multiple comparisons correction test and
Mann-Whitney tests were used. Each test used is specified in the
legend of the figures. Data are presented as the mean ± SD of
independent experiments (minimum three, depending of the
experiments). P<0.05 was considered to indicate a statistically
significant difference.
Results
Low CD24 expression defines a radio-
and chemo-resistant subpopulation of breast cancer cells
To investigate the role of CD24 in resistance to
γ-IR and widely used anticancer drugs, the present study
investigated CD24 expression in epithelial HMLE cells following
high dose IR and treatment with high concentrations of three drugs
with different mechanisms of action: 5FU, cisplatin and paclitaxel.
CD24 expression was analyzed by flow cytometry and
CD24−/low subpopulation was defined as the 10% of cells
expressing the lowest fluorescence in untreated cells.
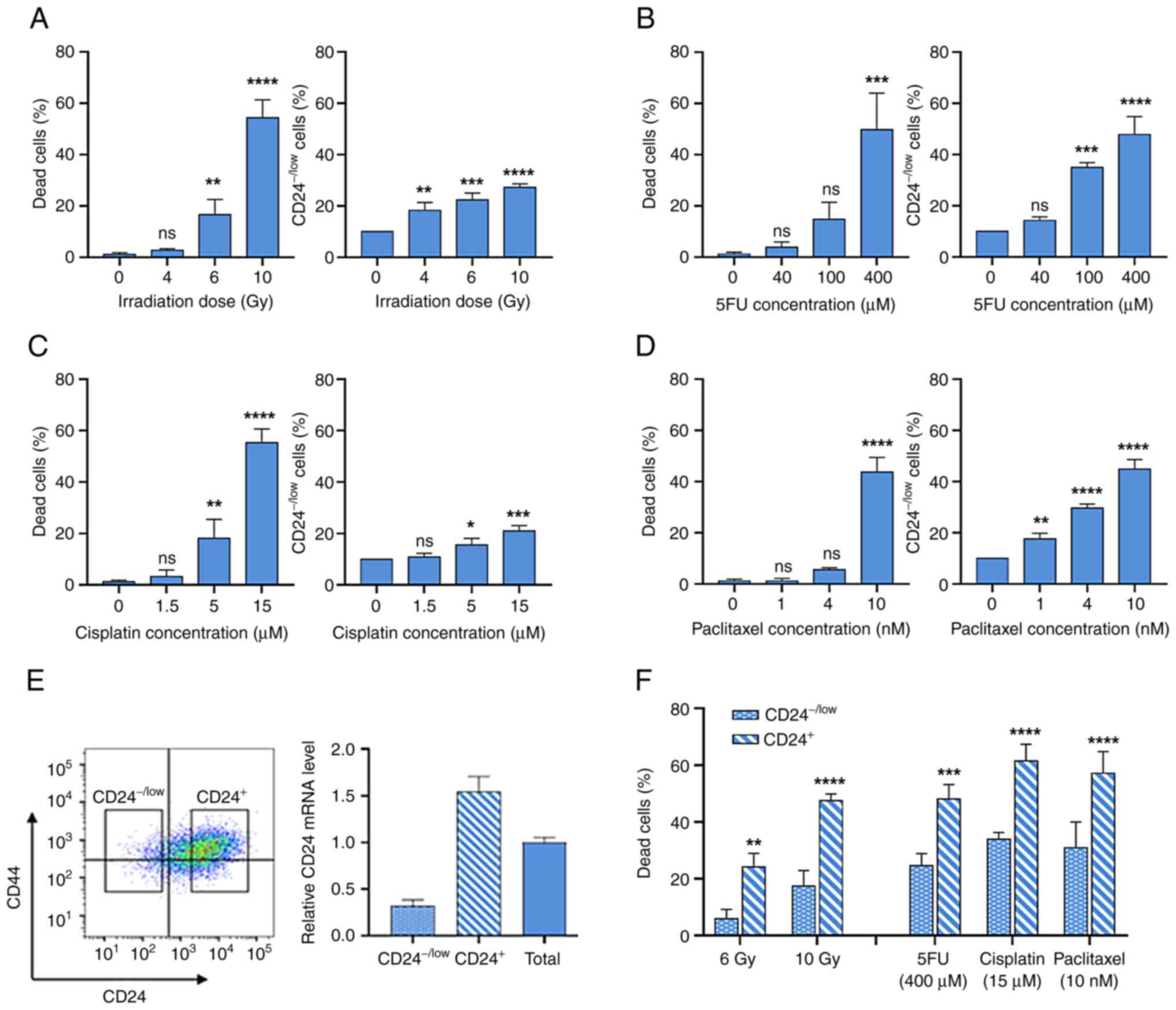
Following IR, dose-dependent cell death was
observed, which is a delayed apoptotic process (10) (Fig.
1A). IR selectively enriches the CD24−/low cells
subpopulation (Fig. 1A).
Similarly, chronic exposure to increased concentrations of
anticancer drugs led to dose-dependent cell death (Fig. 1B-D). For the three drugs used, a
clear increase in the percentage of CD24−/low cells was
observed following chronic exposure. It was next investigated
whether CD24−/low cells were more radio- and
chemo-resistant than CD24+ cells. These populations were
isolated by flow cytometry (Fig.
1E) and studied separately. Membrane expression levels of CD24
observed by FACS were associated with CD24 mRNA expression in
CD24−/low and CD24+ cells (Fig. 1E). Following 6 and 10 Gy IR, death
rate was significantly lower in CD24−/low cells than in
CD24+ cells (Fig. 1F).
In the same way, following treatment with high doses of anticancer
drugs, death rate was significantly decreased in
CD24−/low cells compared with CD24+ cells.
Therefore, CD24−/low cells exhibited an enhanced ability
to survive following IR and chronic exposure to chemotherapeutic
agents. Long-term culture of sorted CD24−/low HMLE cells
showed the appearance of CD24+ cells after six days and
after 21 days, the heterogeneity of CD24 levels in parental
population was observed (Fig.
S1).
Taken together, these results indicated that the
CD24−/low cell subpopulation was more radio- and
chemo-resistant than CD24+ cells. Hence, IR or drug
exposure led to transient enrichment of the CD24−/low
cell subpopulation in the whole cell culture.
Loss of CD24 expression does not
modify overall E features of HMLE cells but induces hybrid E/M
state
To determine whether CD24 mediated radio- and
chemo-resistance of breast cancer cells, E HMLE cells were
transfected with p-EBV-plasmid expressing siRNA against CD24
(E_CD24−). As control, the parental
CD24+/CD44low HMLE cells were transfected
with p-EBV vector expressing inefficient shRNA sequence (E_vec).
Flow cytometry confirmed the loss of surface expression of CD24 in
E_CD24− cells compared with E cells (Fig. 2A). A purified population of M HMLE
cells was obtained following FACS analysis of
CD24−/low/CD44+ cells induced by prolonged
exposure to TGFβ1 (10). To
discard potential off-target effects of the siRNA,
CD24-complemented E cells (E_CD24−c) were generated by
transfecting E_CD24− cells with a p-EBV-plasmid coding
for CD24 ORF. High levels of CD24 were observed at the membrane of
E_CD24−c cells (Fig.
2A). Modulation of CD24 expression levels was further validated
by RT-qPCR analysis, showing a strong downregulation in
E_CD24− cells compared with E or E_vec cells (Fig. S2).
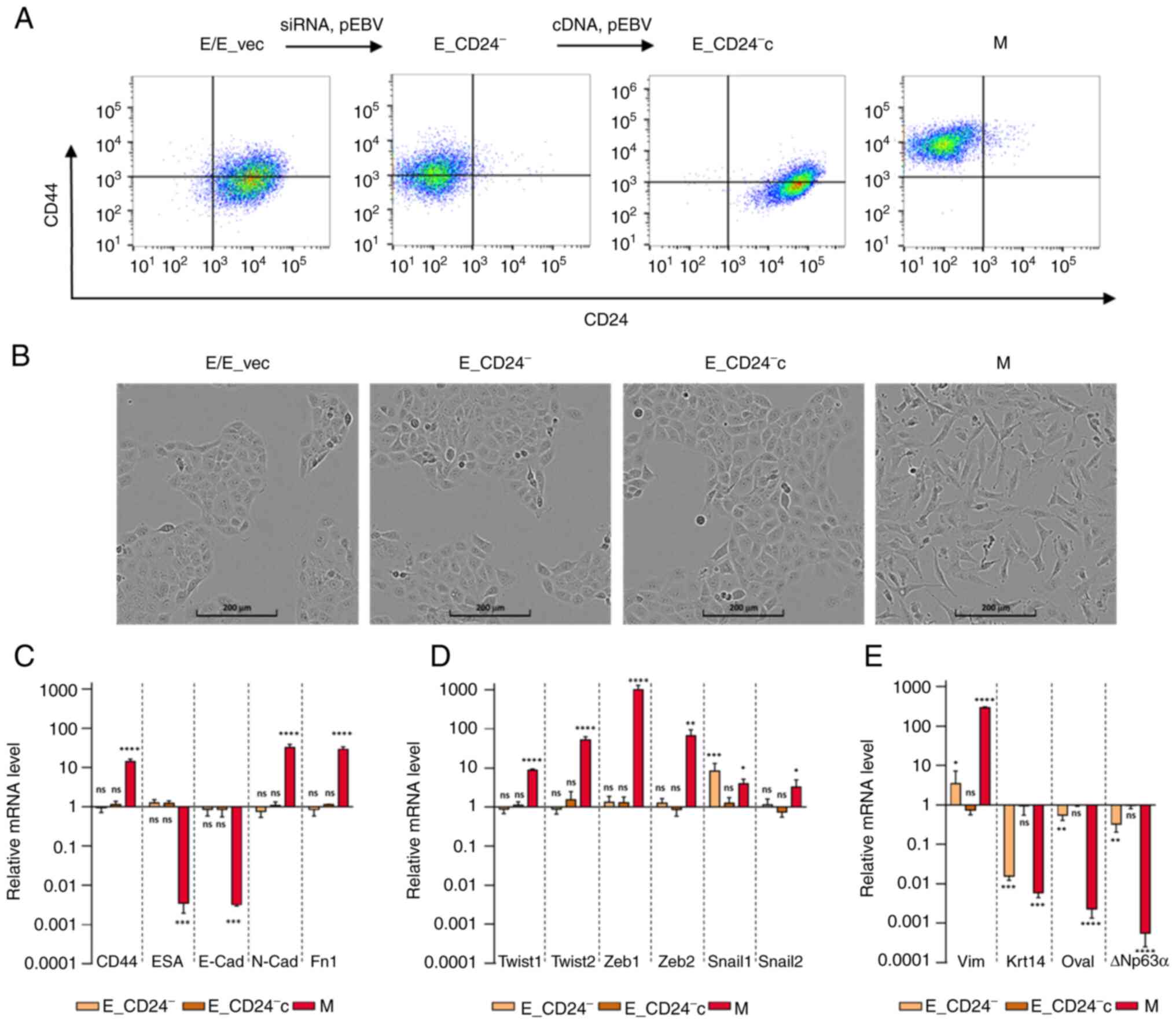 | Figure 2.Characterization of E, M and
E-transfected HMLE cells. (A) Flow cytometry characterization
following CD24/CD44 labelling of parental E cells, E cells
transfected with control p-EBV vector (E_vec) and p-EBV-plasmid
expressing a CD24 small interfering RNA (E_CD24−),
E_CD24− cells transfected with p-EBV-plasmid expressing
CD24 mRNA (E_CD24−c) and M cells obtained following FACS
analysis of CD24−/low/CD44+ cells induced by
prolonged exposure to TGFβ1. (B) Phase-contrast images of E, E_vec,
E_CD24−, E_CD24−c and M cells. Analysis by
reverse transcription-quantitative PCR of relative expression of
mRNAs encoding (C) EMT-associated factors (CD44, ESA, E-cad, N-cad
and Fn1), (D) primary transcription factors of EMT (Twist-1,
Twist-2, Snail-1, Snail-2, Zeb-1 and Zeb-2) and (E) other factors
involved in EMT (Vim, Krt14, DTP63a and OVOL2) in E_CD24-, E_CD24-c
and M compared with E cells. Results were normalized to expression
levels in E cells. Data are presented as the mean ± SD of 3
independent experiments. Significant differences (compared with E
cells) were analyzed by one-way ANOVA followed by Dunnett's
multiple comparisons correction test. *P<0.05, **P<0.01,
***P<0.001 and ****P<0.0001. ns, not significant; E,
epithelial; M, mesenchymal; EMT, E-M transition; ESA, epithelial
cell adhesion molecule; Cad, cadherin; Fn, fibronectin; Zeb, zinc
finger E-box binding homeobox; Vim, vimentin; Krt, keratin; OVOL2,
ovo-like zinc finger 2. |
The effect of CD24 expression on cellular phenotypes
classically associated with EMT in breast cancer was assessed.
E_CD24− cells kept the cuboidal-like E morphology of the
parental E cells and formed cobblestone cell islands (Fig. 2B). This cobblestone morphology was
different from that observed for fibroblast-like M cells. To
confirm that silencing of CD24 did not modify E characteristics of
transfected E HMLE cells, the expression of EMT-associated genes
was measured by RT-qPCR (Fig. 2C).
The results were normalized to expression levels in E cells. ESA
(Ep-CAM) and E-cadherin, detectable at low levels in M cells
compared with E cells were not affected by altered expression of
CD24. In the same way, CD44, N-cadherin and fibronectin mRNAs
expression levels were markedly increased in M cells but not in
transfected E cells. The expression of mRNAs encoding the primary
transcription factors driving EMT was also studied [Twist-1,
Twist-2, Snail-1, Snail-2, zinc finger E-box binding homeobox
(Zeb)-1 and Zeb-2; Fig. 2D].
Expression of these mRNAs was increased in M cells but not in E
transfected cells with the exception of Snail-1, which was
upregulated when CD24 expression was decreased. However, expression
of other markers suggested that E_CD24− cells were not
in a completely E state (Fig. 2E).
Keratin 14, a classical E marker in breast cancer (39), was downregulated in
E_CD24− and M cells compared with E cells. Vimentin was
weakly upregulated and E transcription factors ovo-like zinc finger
(OVOL)2 and ΔNp63α, the predominant p63 isoform in mammary E cells
(40) were weakly expressed in
E_CD24− cells. Because recent studies have suggested
that NRF2 activates partial EMT and is maximally present in a
hybrid E/M phenotype (41,42), NRF2 expression was investigated.
Upregulation of NRF2 was observed in M cells but not in E_CD24-
cells (data not shown). Therefore, in the present model,
overexpression of NRF2 was not associated with hybrid E/M
state.
To ensure these results were not specific to the
HMLE cells model, the T47D epithelial breast cell line was
transfected with the p-EBV-plasmid expressing CD24 siRNA to obtain
a low CD24 expression (T47D_CD24− cells).
T47D_CD24− cells kept the cuboidal-like E morphology of
parental cells and formed cobblestone cell islands, but
upregulation of Vimentin and downregulation of DNp63a expression
was also observed, indicating that cells were not in a completely E
state (Fig. S3).
Therefore, these results indicated that loss of CD24
expression does not modify E characteristics of breast E cells but
induces an intermediate hybrid E/M state.
Stemness properties and migration
potential are associated with hybrid E/M phenotype of
E_CD24− HMLE cells
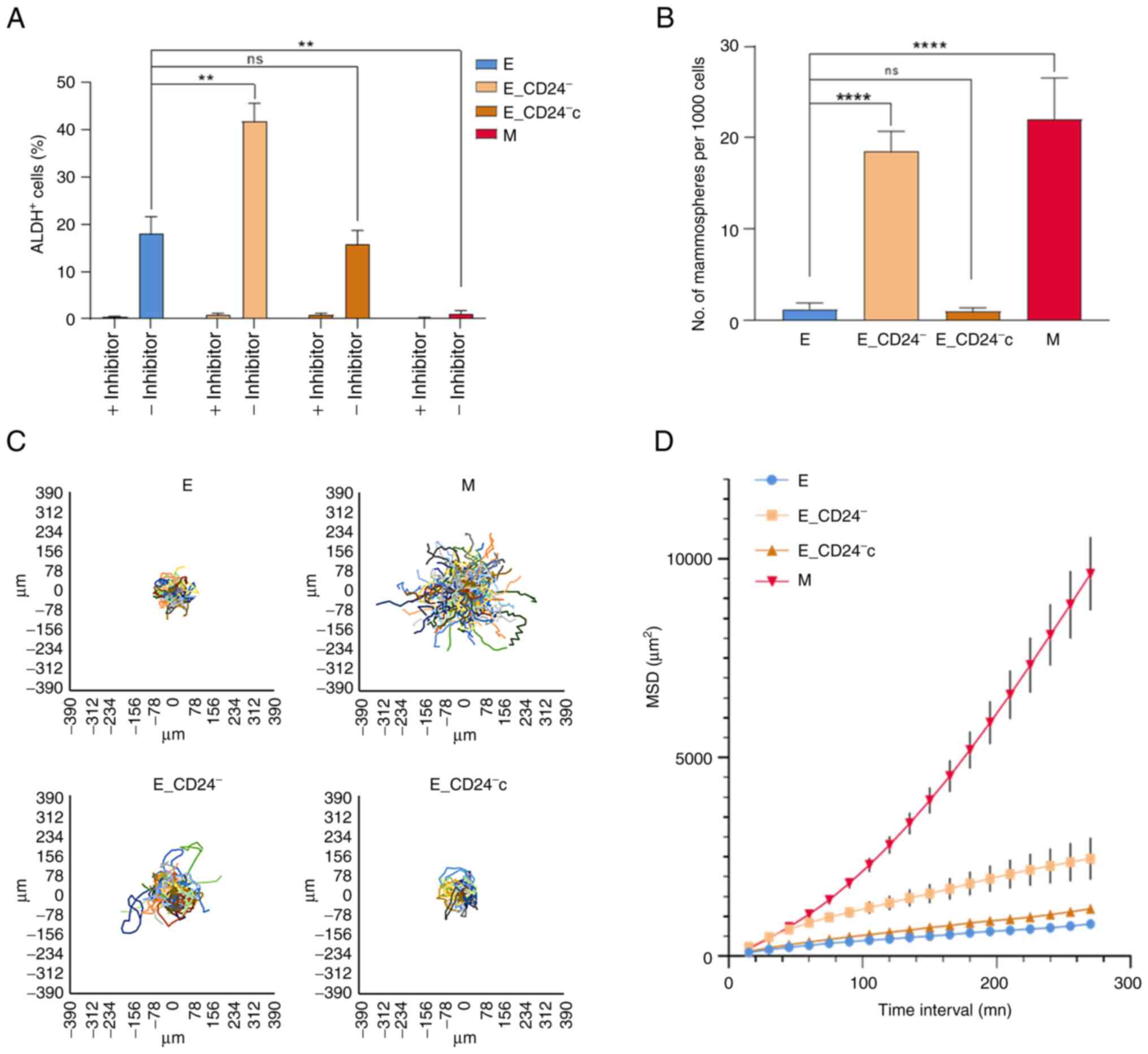
Stemness properties acquisition during EMT is
associated with the appearance of cells in an intermediate state,
often characterized as E-like CSCs displaying increased ALDH1
activity (11). The present study
investigated whether ALDH1 activity was associated with CD24
expression in HMLE cells (Figs. 3A
and S4). A total of 15–20% of
parental E cells were ALDH+; this subpopulation reached
40% in E_CD24− cells and re-expression of CD24
(E_CD24−c cells) abolished this increase.
ALDH+ subpopulation was absent in
CD24−/low/CD44+ M cells.
Another feature of breast CSCs is their potential to
form spheres (mammospheres) (7).
There was a significant increase in mammosphere formation
efficiency (MFE) of CD24−/low/CD44+ M cells
compared with E cells (Fig. 3B).
The MFE was also significantly increased in E_CD24−
cells compared with parental E cells and re-expression of CD24
(E_CD24−c cells) abolished this increase.
CSCs, as well as hybrid and M tumor cells, are
associated with motility and tumor propagating characteristics
(12); therefore, migration
potential of HMLE cells was investigated. MSD analysis indicated
that M cells exhibited a markedly higher migratory potential than E
cells (Fig. 3C and D). In the same
way, E_CD24− cells presented significantly increased
migratory potential than E cells; this increase was abolished
following re-expression of CD24 (E_CD24−c cells).
Altogether, these results indicated that
E_CD24− cells in a hybrid E/M state were associated with
the acquisition of stemness properties. Thus, CD24 knockdown may
influence stemness properties, leading to ALDH+ E-like
CSCs rather than CD24−/low/CD44+ M-like
CSCs.
Loss of CD24 expression alone promotes
radio- and chemo-resistance of E HMLE cells
To determine effects of CD24 on radiation and drug
sensitivity, HMLE cells were irradiated at 10 Gy or treated with
high concentration 5FU, cisplatin and paclitaxel. Delayed cell
death was observed between E and M cells 6 days after IR (Fig. 4A). In CD24-/low/CD44+ M cells, cell
death was significantly delayed and remained lower than in E (or
E_vec) cells. Cell death rate after IR was significantly decreased
in E_CD24− cells compared with parental E cells and
re-expression of CD24 (E_CD24−c cells) abolished this
resistance, indicating that CD24 controls radiation response. In
the same way, loss of CD24 expression induced resistance to chronic
exposure of anticancer drugs, similar to that observed in M cells
(Fig. 4B). When CD24 was
re-expressed, E_CD24−c cells became drug sensitive, cell
death was restored to a similar level as that in parental cells.
These data indicated that E_CD24− cells exhibited an
enhanced ability to survive following IR and chronic treatment with
anticancer drugs.
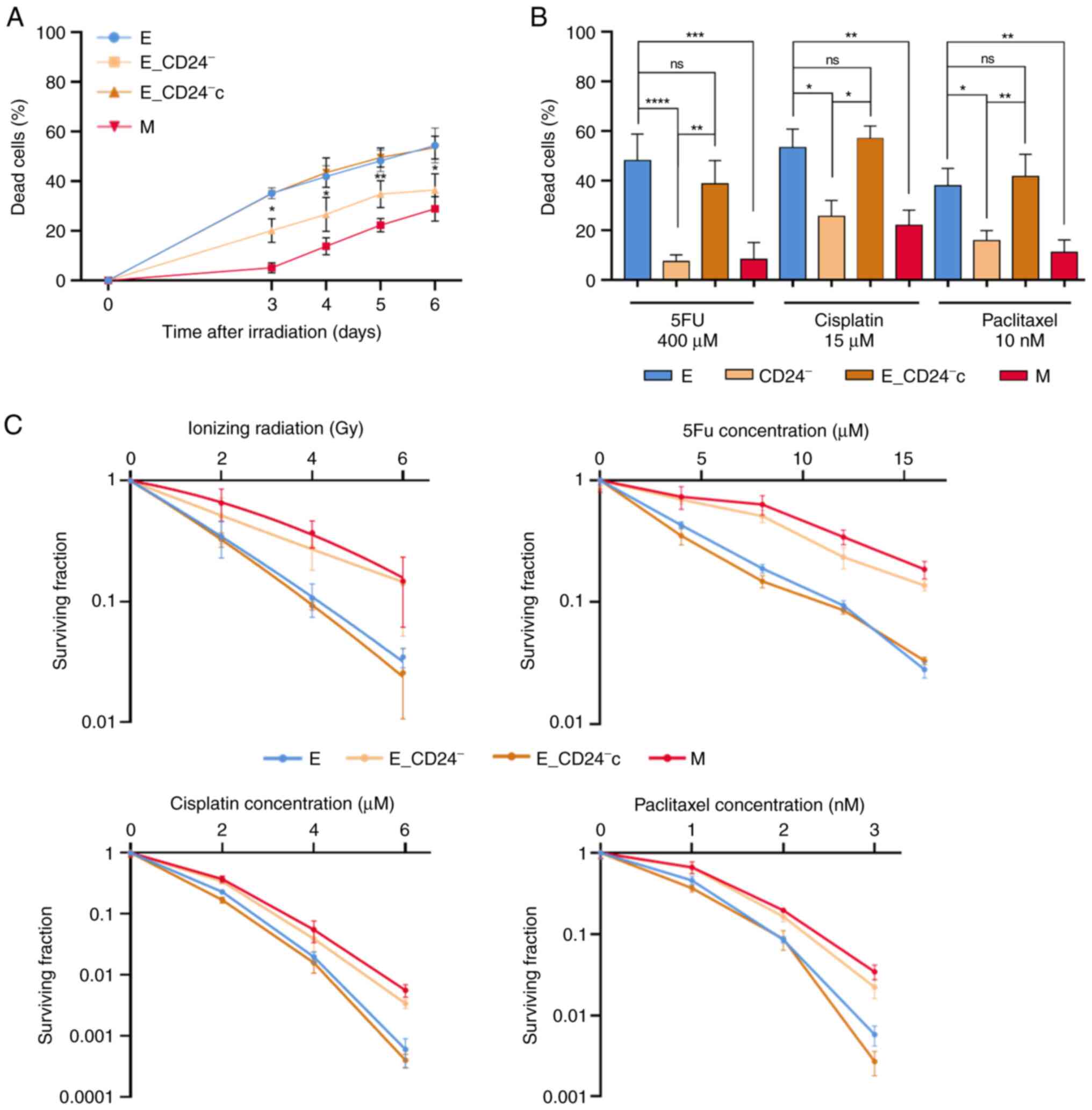 | Figure 4.Decreased CD24 expression enhances
radio- and chemo-resistance in HMLE.E cells. (A) Time course of
death of 10 Gy-irradiated cells. Data are presented as the mean ±
SD of 3 independent experiments. (B) Percentage of dead cells
following three day exposure to 400 µM 5FU, 15 µM cisplatin and 10
nM paclitaxel. Data are presented as the mean ± SD of 4–12
independent experiments. Significant differences (compared with E
cells) were analyzed by one-way ANOVA with Kruskal-Wallis followed
by Dunn's multiple comparisons correction test. *P<0.05,
**P<0.01, ***P<0.001, ****P<0.0001. (C) Clonogenic cell
survival curves following 2–6 Gy irradiation, 4–16 µM 5FU, 2–6 µM
cisplatin and 1–3 nM paclitaxel treatment. ns, non-significant;
5FU, 5-fluorouracil; E, epithelial; M, mesenchymal. |
To ensure that these results were not specific to
the HMLE cell model, E breast cell lines MCF7 and T47D were also
transfected with the p-EBV-plasmid expressing a CD24 siRNA to
obtain two lines with a low CD24 expression (MCF7_CD24−
and T47D_CD24− cells). Forced extinction of CD24
expression alone promoted resistance of MCF7_CD24− and
T47D_CD24− cells against chronic exposure to high dose
5FU and cisplatin (Fig. S5).
It was next investigated whether differences in cell
death rate following IR or drug treatment between CD24−
and CD24+ cells had an impact on long-term survival, as
measured by clonogenic assay. Following IR (Fig. 4C), surviving fractions at 2, 4 and
6 Gy were significantly higher in E_CD24− than in
control E/E_vec cells and similar to those observed in M cells.
E_CD24−c cells exhibited decreased of cloning
efficiency, similar to that in parental E cells. Following drug
treatment, similar results were observed: Surviving fractions for
all doses of drug tested were higher in E_CD24− and M
cells than in parental E and E_CD24− cells. These
results indicate that E_CD24− and M cells exhibited
greater clonogenic capacity than CD24+ E cells.
Taken together, these data indicated that CD24
controlled the response to radiation and chemotherapeutic
drugs.
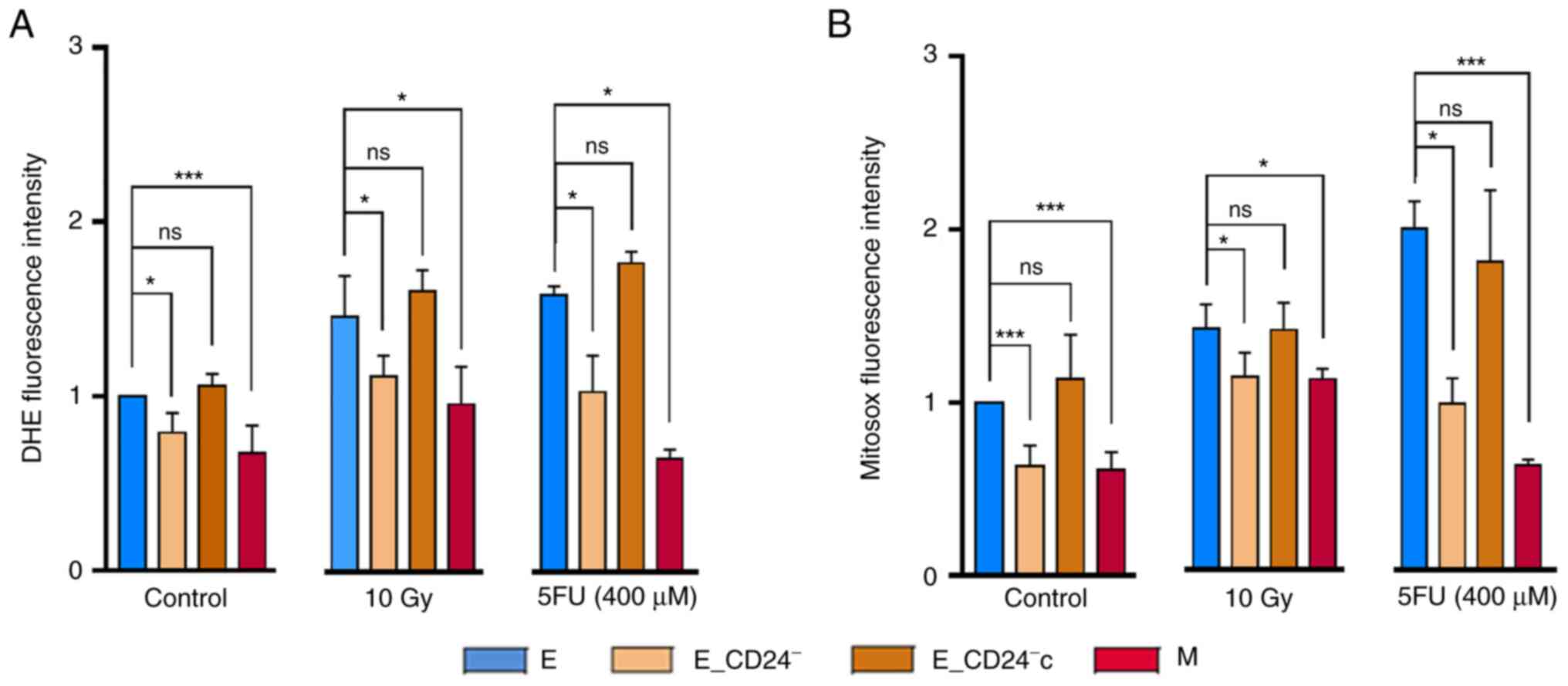
Decreased CD24 expression decreases
intracellular ROS concentration
As ROS production is reported to be an essential
inducer of apoptosis (43), it was
investigated whether changes in ROS levels were associated with
radio- and chemo-sensitivity in HMLE cells. First, the
intracellular concentrations of ROS were measured using DHE
staining. Flow cytometry analysis indicated that M cells contained
significantly lower concentrations of ROS than E/E_vec cells
(Fig. 5A). E_CD24−
cells also displayed lower DHE staining than E control cells and
re-expression of CD24 abolished this decreased staining. Because
mitochondria are the primary source of ROS in cancer cells, a
similar experiment was performed using Mitosox-Red, a selective
probe for mitochondrial superoxide (Fig. 5B). Same results were observed: a
lower mitochondrial ROS level in M cells than in their parental
counterparts, a decrease of mitochondrial ROS in CD24 knockdown
cells and a return to the level observed in E cells when CD24 was
re-expressed.
The effect of CD24 expression on ROS levels 3 days
following IR or 5FU treatment was investigated. ROS levels
increased in all cell lines tested (Fig. 5A and B), but a lower concentration
of ROS was maintained in E_CD24− and M cells compared
with parental E or E_CD24−c cells. Similar results were
obtained with both DHE and Mitosox-Red.
Taken together, these data indicated that CD24
downregulation led to decreased basal levels of total and
mitochondrial ROS. Following IR or drug treatment, there was an
increase in ROS levels but these remained lower in
E_CD24− cells than in parental E cells, consistent with
the rate of cell death observed in different cell lines. Therefore,
CD24 expression may affect radio- and chemo-resistance by
controlling ROS levels.
CD24 controls ROS levels via
regulation of mitochondrial function independently of antioxidant
activity
Lower ROS levels are commonly ascribed to the CSC
phenotype in breast tumors and are associated with enhanced ROS
scavengers and/or decreased mitochondrial mass (44). First, it was determined whether ROS
modulation was associated with differential regulation of oxidative
stress-associated genes. Our previous study showed that HMLE.M
cells exhibit higher antioxidant activity than HMLE.E cells and
characterized genes involved in ROS metabolism (10). Thus, RT-qPCR was performed to
assess expression of four key genes in this model: Superoxide
dismutase 2, heme oxygenase 1, glutathione-sulfide reductase and
thioredoxin reductase 1 (Fig. 6A).
The expression of all these genes was increased in M cells, but not
modified in transfected E cells. Lower ROS levels observed in
E_CD24− cells were not associated with high
intracellular levels of radical scavengers.
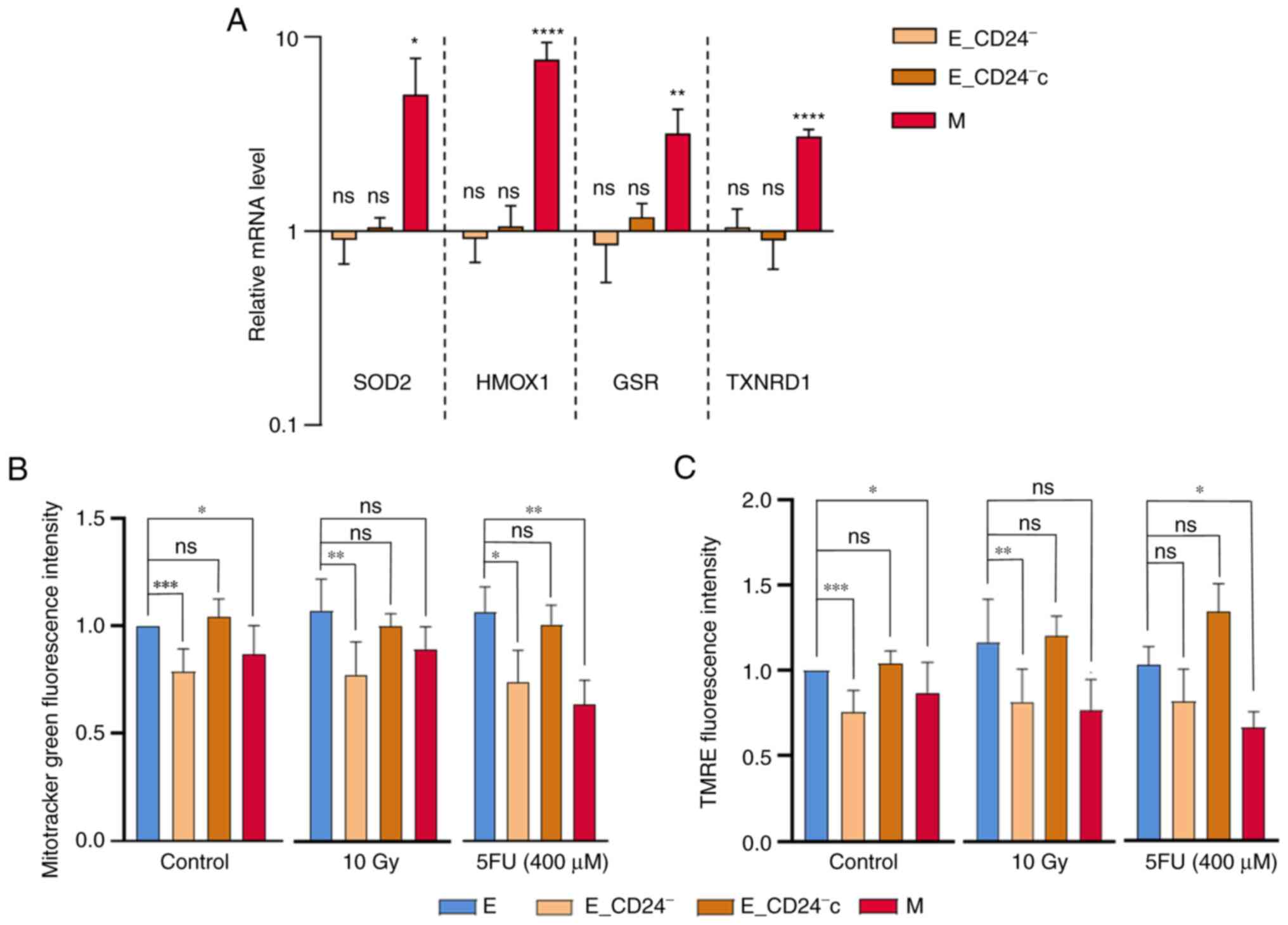 | Figure 6.CD24 downregulation has no impact on
ROS scavengers but decreases mitochondrial mass and membrane
potential. (A) Analysis by reverse transcription-quantitative PCR
of the relative expression of mRNAs encoding stress-associated
factors involved in ROS metabolism in E_CD24-, E_CD24-c and M
compared with E cells. For each gene, expression in E cells was
normalized to 1 and ratio of relative mRNA level of E to E_CD24-,
E_CD24-c and M cells is presented. Data are presented as the mean ±
SD of 3 independent experiments. Significant differences (compared
with E cells) were analyzed by one-way ANOVA followed by Dunnett's
multiple comparisons correction test. *P<0.05, **P<0.01,
***P<0.001 and ****P<0.0001. ns: not significant.
Mitochondrial (B) mass was assessed by Mitotracker Green probe and
(C) membrane potential was assessed using TMRE staining.
Mitochondrial mass and membrane potential were studied in untreated
cells and at three days following 10 Gy irradiation and exposure to
400 µM 5FU. Data are presented as the mean ± SD of 4–10 independent
experiments. Significant differences were analyzed by one-way ANOVA
with Kruskal-Wallis followed by Dunn's multiple comparisons
correction test. *P<0.05, **P<0.01, ***P<0.001. ns,
non-significant. SOD, superoxide dismutase 2; HMOX1, heme oxygenase
1; GSR, glutathione-sulfide reductase; TXNDR1, thioredoxin
reductase 1; TMRE, tetramethylrhodamine, ethyl ester; E,
epithelial; M, mesenchymal; 5FU, 5-fluorouracil; ROS, reactive
oxygen species. |
It was next studied if modulation of CD24 expression
affects mitochondrial function. Mitochondrial mass was analyzed
using MTG, a mitochondrial selective probe, and mitochondrial
membrane potential was quantified using TMRE staining. Flow
cytometry analysis indicated that mitochondrial mass was
significantly lower in M and E_CD24− cells than in
parental E and E_CD24−c cells (Fig. 6B). In the same way, mitochondrial
membrane potential decreased in M and in E_CD24− cells
compared with E cells, and a return to the level observed in E
cells was showed in E_CD24−c cells (Fig. 6C). The TMRE/MTG ratio was similar
for all the cell lines analyzed (Fig.
S6), indicating that mitochondrial membrane potential observed
in the cell lines was associated with mitochondrial mass. Then,
similar experiments were performed following IR or drug treatment
(Fig. 6B and C). Mitochondrial
mass and membrane potential were lower in E_CD24−cells
(with the exception of TMRE staining following 5FU treatment: a
small but not significant decrease is observed) compared with
parental E cells and E_CD24−c cells.
Taken together, these data indicated that lower ROS
levels observed in CD24−/low cells were associated with
lower mitochondrial membrane potential and mass independently of
intrinsic antioxidant activity. Markedly decreased mitochondrial
ROS production may be a key event in radio- and chemo-resistance
controlled by CD24 expression.
Discussion
The present data demonstrated that intrinsic radio-
and chemo-resistance of breast cancer cells was directly associated
with membrane expression level of CD24 and that CD24 may be
considered not only as a marker but also as a mediator of this
resistance. In our model, as for the mesenchymal phenotype
(45), the CD24−/low
phenotype of epithelial HMLE cells is reversible (46,47).
CD24 expression exhibits dynamic regulation (48) as its expression is reversible and
under epigenetic control. Expression of CD24 was heterogenous in
the HMLE cell model and is frequently observed in breast cancer
cell lines and tumor tissue (49).
Radio- and chemo-resistance of breast cancer cells
has been highlighted using IR or different classes of
chemotherapeutic drugs (50). The
present study used high doses of IR (4–10 Gy) and chronic exposure
with high concentration of drugs from three categories of antitumor
agents: 5FU, an antimetabolite, cisplatin, an alkylating agent, and
paclitaxel, a mitotic inhibitor. In parental HMLE.E cells
dose-dependent death was observed following IR and drug treatment;
this cell death is primarily associated with late apoptosis
(9,51–54).
Cell death was concomitant with a clear increase in percentage of
CD24−/low surviving cells. This enrichment in
CD24−/low cells may be the consequence of selection
(22) and associated with
induction of an active EMT program (55). CD24−/low cells exhibited
greater ability to survive than CD24+ cells following IR
or antitumor drug treatment. Altogether, the present results
demonstrated an association between CD24−/low expression
and treatment resistance, independent of the mechanism of
CD24−/low cell enrichment.
Failure of conventional treatments is commonly
associated with CSC survival (15), suggesting that E_CD24−
cells have acquired stemness properties. In the past 20 years,
numerous studies have associated loss of CD24 expression with EMT.
The widely used CD24−/low/CD44+ marker allows
characterization of M cells enriched in CSC properties compared
with CD24+/CD44low E cells (4,7,10).
However, in the present study, E_CD24− cells retained
the primary characteristics of E cells: Cuboidal-like E morphology
and formation of cobblestone cell islands. Furthermore, the
expression of the primary EMT-associated genes (ESA, E-cadherin,
CD44, N-cadherin and fibronectin) and 5 out of 6 transcription
factors driving EMT (Twist-1, Twist-2, Snail-2, Zeb-1 and Zeb-2)
were not modified. Therefore, treatment resistance in
E_CD24− cells was not associated with acquisition of M
characteristics.
The development of biomarkers to identify breast
CSCs demonstrated that cells with high ALDH activity also exhibit
stemness properties; radio- and chemo-resistance have been also
associated with ALDH+ breast cancer cells (15–18).
Loss of CD24 expression may promote stemness characteristics,
allowing genotoxic stress survival of E_CD24− cells and
radio- and chemo-resistance of E_CD24− cells are
associated with ALDH activity. Moreover, the association between
stem cell features and E_CD24− cells was supported by
the increased potential to form mammospheres and migratory
potential observed for these cells.
The hypothesis that only ‘full’ EMT is associated
with increased stemness was challenged by later studies
demonstrating the existence of E-like breast CSCs (11,13,14).
These E CSCs with a CD24+/CD44low phenotype
are characterized by high ALDH activity (11). E-M plasticity is a spectrum of
transitory cell states, and tumor cells with the highest stem cell
capabilities reside in a hybrid E/M state, associated with
increased tumor propagating potential (13,14,39,56).
In the present study, when overall E
characteristics of HMLE cells were maintained, deregulation of
other genes associated with transition states occurring during EMT
was also observed: Snail-1, Vimentin, Keratin 14, ΔNp63α and OVOL2.
The strong downregulation of Keratin 14 expression (E marker) and
the low but significant overexpression of Vimentin (M marker)
suggested that E_CD24− cells acquired few M
characteristics. Among the primary EMT transcription factors, only
Snail1 was strongly overexpressed in E_CD24− cells.
Snail1 expression has been implicated in reprogramming somatic
cells to pluripotency (57,58)
and Snail1 maintains stem-like properties, chemoresistance and ALDH
activity in mouse breast cancer cells (59). Kroger et al (14) showed that both in vitro and
in vivo, breast cancer cells reside stably and with low
plasticity in a highly tumorigenic hybrid E/M state, which is
driven primarily by Snail1 and canonical Wnt signaling. The E
transcription factors ΔNp63α and OVOL2 were weakly downregulated in
E_CD24− cells but at a lower level than in M cells.
Suppression of ΔNp63α has been implicated in EMT induction in
mammary E cells (40). OVOL2 is a
key regulator of E-M plasticity as well as CSCs (13). Expression of OVOL2 induces
mesenchymal-to-epithelial transition (MET), antagonizes TGF-β
signaling and has been implicated in inducing mammary E cells to
enter an intermediate E/M state (60–62).
Moreover, OVOL2 and ΔNp63α may serve a key role in partial
retention of E traits associated with collective migration by
clusters of circulating tumor cells and high tumor-initiation
potential (63,64). NRF2 signaling may be involved in
CSC-like properties of several types of cancer cell (65). High NRF2 levels activate partial
EMT (41), mediate cancer stem
cell-like properties of ALDH+ cells (42) and contribute to radio-resistance
(18). In the present model, NRF2
overexpression was associated with M phenotype but not intermediate
E/M state, indicating that NRF2 pathway was not modulated by CD24
expression and was not involved in radio- and chemo-resistance of
E_CD24− cells.
E_CD24− cells exhibit significantly
increased migratory potential compared to E cells, which is
strongly associated with the increased tumor propagating potential
observed in breast cancer cells in a hybrid E/M state (12). Altogether, our results reinforced
the hypothesis that the hybrid E/M state of E_CD24−
cells leads to acquisition of stemness and maintenance of stem cell
properties is independent of phenotypic plasticity (66,67).
The present results are in agreement with the cooperation
metastasis model proposed by Grosse-Wilde et al (68), where metastasis-initiating cells
are in a mixed E/M state and may originate from cells in the E
state.
The model used in the present study has the
advantage of using cells with the same genetic background and
plasticity between E and M allows the characterization of the
alterations induced by regulation of CD24 expression in E cells and
a fine mapping in the E/M hybrid state of E_CD24− cells.
This model not only allows generalizability of our previous
observations (23) but facilitates
understanding of the sequence of events and consequences of
downregulation of CD24.
The intrinsic radio- and chemo-resistance of CSCs
is associated with many mechanisms, often mediated by redox
imbalance and ROS control (21).
Moreover, ROS production is reported to be an essential regulator
or inducer of apoptosis in cancer cells and increased intracellular
ROS levels mediate cell death induced by ionizing radiation
(29) as well as anticancer drugs
(30–32). Increased ROS scavengers are
associated with lower ROS levels observed in CSCs (10,24),
but few papers also report that CSCs that have undergone EMT show
decreased mitochondrial mass and membrane potential, consume less
oxygen per cell and produce markedly lower levels of ROS (69,70).
The present results demonstrated that CD24 downregulation led to
decreased basal levels of total and mitochondrial ROS via
regulation of mitochondrial function. An association between
mitochondrial suppression and metabolic reprogramming has been
proposed and lower mitochondrial levels and activity reflect the
metabolic switch that has been reported during EMT (71,72).
Moreover, Snail may serve a central role in this process (13,57–59).
Altogether, the present results showed an association between CD24
expression and therapeutic resistance. In our study, we observe
that in breast cancer cell culture, CD24 expression is
heterogeneous and CD24−/low subpopulation is selected
following radiation and drug treatment. Artificial modulation of
CD24 expression shows that radio- and chemo-sensitivity are
directly controlled by CD24, and that loss of CD24 expression in E
HMLE cells increase the presence of ALDH+ E-like CSCs,
in a hybrid E/M state. In CD24−/low/CD44+
CSCs, the resistance properties are strongly associated to a low
ROS level, but for the two subtypes of CSCs the mechanisms leading
to decrease ROS level are different. For ALDH+
E_CD24− cells, we observed decreased mitochondrial mass
and membrane potential, while for
CD24−/low/CD44+ M cells, decrease ROS level
is under the control of both enhanced ROS scavenger and decrease of
mitochondrial mass and membrane potential. These results are
summarized in a model in Fig.
7.
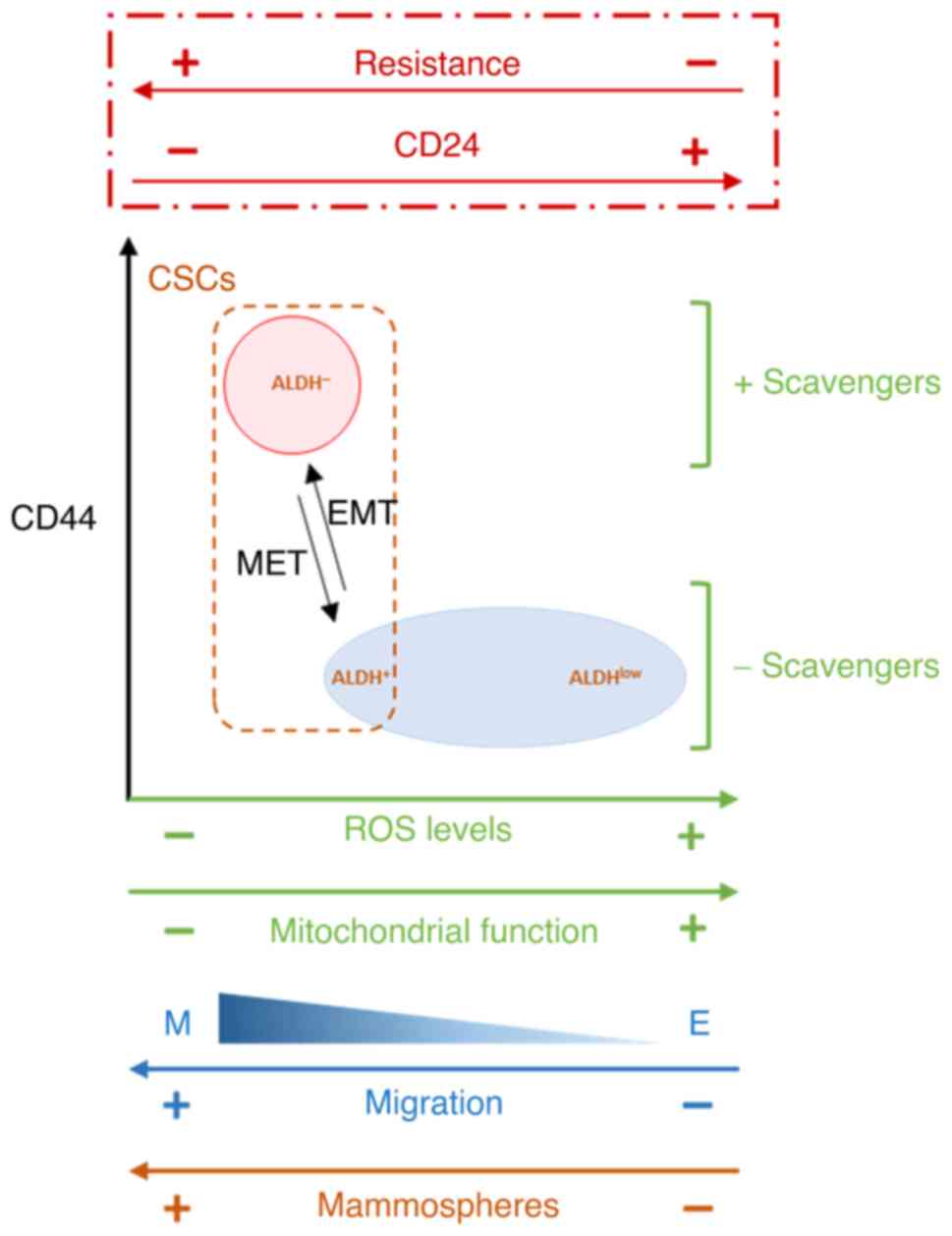 | Figure 7.Proposed model of the association
between CD24 expression and EMT, stemness properties, ROS levels
and scavengers and mitochondrial function. In HMLE cells cultures,
CD24 expression is heterogeneous and CD24−/low
subpopulation is selected following radiation and drug treatment.
Artificial modulation of CD24 expression shows that radio- and
chemo-sensitivity are directly controlled by CD24, and that loss of
CD24 expression in E HMLE cells increase the presence of
ALDH+ E-like CSCs, in a hybrid E/M state, in association
with decreased mitochondrial mass and membrane potential. EMT,
epithelial-mesenchymal transition; ROS, reactive oxygen species;
CSC, cancer stem cell; ALDH, aldehyde dehydrogenase; MET,
mesenchymal-to-epithelial transition. |
In the past decade, few papers have studied the
role of CD24 in human tumors; when different functions of CD24 have
been proposed, its role in cancer progression and treatment
resistance remain poorly documented and the signaling downstream of
CD24 has not been clearly elucidated (26,73).
The intrinsic chemo-resistance of CD24−/low cells may
depend on the type of drug. Deng et al (74) reported that cells with
CD24-knockdown are more sensitive to docetaxel, while
CD24-overexpressing cells are more sensitive to doxorubicin in
triple-negative breast cancer. Therefore, CD24 may be a promising
biomarker candidate to guide chemotherapy. Finally, the
heterogeneity of breast cancer and molecular subtypes should be
considered (75). In breast
tumors, high CD24 expression is frequently associated with a
terminally differentiated, luminal phenotype, while most basal-like
tumors are classified as CD24−/low. In parallel, studies
have shown that CD44/CD24 and ALDH1 are expressed differentially in
different subtypes of breast cancer (49). ALDH+ cells are more
common in HER2-overexpressing and basal/E breast cancer, while
CD24−/low/CD44+ phenotype is associated with
basal-like breast cancer (49,76).
Moreover, only a fraction of CD24−/low/CD44+
breast cancer cells are ALDH+, these cells being more
tumorigenic in athymic mice (5,11).
In conclusion, the present study suggested that
CD24 expression may be a key factor in transition between different
subtypes of breast CSCs and that loss of CD24 expression was
associated with de-differentiation. De-differentiation is
associated with invasion potential and metastasis and promotes
resistance to a wide spectrum of chemotherapy drugs and radiation
exposure (77).
Supplementary Material
Supporting Data
Acknowledgements
We would like to thank Professor Robert A. Weinberg
(Whitehead Institute, Cambridge, MA, USA), for the donation of HMLE
cells.
Funding
The present study was supported by Electricité de France and by
the Transverse Division n. 4 (Radiobiology) of the French
Alternative Energies and Atomic Energy Commission (Segment n. 4
Radiobiologie).
Availability of data and materials
The datasets used and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
IB, AC, FB, DB and JL designed the study and wrote
the manuscript. IB, CL, ND, TK, EB, DB CS and JL performed the
experiments. DB, TK and FB contributed reagents/analytic tools. IB,
SC, AC and JL analyzed data. IB, EB, FB, AC and JL edited the
paper. EB and AC confirm the authenticity of all the raw data. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Marusyk A, Almendro V and Polyak K:
Intra-tumour heterogeneity: A looking glass for cancer? Nat Rev
Cancer. 12:323–334. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pece S, Tosoni D, Confalonieri S, Mazzarol
G, Vecchi M, Ronzoni S, Bernard L, Viale G, Pelicci PG and Di Fiore
PP: Biological and molecular heterogeneity of breast cancers
correlates with their cancer stem cell content. Cell. 140:62–73.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Batlle E and Clevers H: Cancer stem cells
revisited. Nat Med. 23:1124–1134. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ginestier C, Hur MH, Charafe-Jauffret E,
Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG,
Liu S, et al: ALDH1 is a marker of normal and malignant human
mammary stem cells and a predictor of poor clinical outcome. Cell
Stem Cell. 1:555–567. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hwang-Verslues WW, Kuo WH, Chang PH, Pan
CC, Wang HH, Tsai ST, Jeng YM, Shew JY, Kung JT, Chen CH, et al:
Multiple lineages of human breast cancer stem/progenitor cells
identified by profiling with stem cell markers. PLoS One.
4:e83772009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan
A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al: The
epithelial-mesenchymal transition generates cells with properties
of stem cells. Cell. 133:704–715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang R, Tu J and Liu S: Novel molecular
regulators of breast cancer stem cell plasticity and heterogeneity.
Semin Cancer Biol. 82:11–25. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Morel AP, Lièvre M, Thomas C, Hinkal G,
Ansieau S and Puisieux A: Generation of breast cancer stem cells
through epithelial-mesenchymal transition. PLoS One. 3:e28882008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Konge J, Leteurtre F, Goislard M, Biard D,
Morel-Altmeyer S, Vaurijoux A, Gruel G, Chevillard S and Lebeau J:
Breast cancer stem cell-like cells generated during TGFβ-induced
EMT are radioresistant. Oncotarget. 9:23519–23531. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu S, Cong Y, Wang D, Sun Y, Deng L, Liu
Y, Martin-Trevino R, Shang L, McDermott SP, Landis MD, et al:
Breast cancer stem cells transition between epithelial and
mesenchymal states reflective of their normal counterparts. Stem
Cell Reports. 2:78–91. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pastushenko I and Blanpain C: EMT
transition states during tumor progression and metastasis. Trends
Cell Biol. 29:212–226. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pasani S, Sahoo S and Jolly MK: Hybrid E/M
phenotype(s) and stemness: A mechanistic connection embedded in
network topology. J Clin Med. 10:602020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kröger C, Afeyan A, Mraz J, Eaton EN,
Reinhardt F, Khodor YL, Thiru P, Bierie B, Ye X, Burge CB and
Weinberg RA: Acquisition of a hybrid E/M state is essential for
tumorigenicity of basal breast cancer cells. Proc Natl Acad Sci
USA. 116:7353–7362. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Luo M, Brooks M and Wicha MS:
Epithelial-mesenchymal plasticity of breast cancer stem cells:
Implications for metastasis and therapeutic resistance. Curr Pharm
Des. 21:1301–1310. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Phillips TM, McBride WH and Pajonk F: The
response of CD24(−/low)/CD44+ breast cancer-initiating cells to
radiation. J Natl Cancer Inst. 98:1777–1785. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li X, Lewis MT, Huang J, Gutierrez C,
Osborne CK, Wu MF, Hilsenbeck SG, Pavlick A, Zhang X, Chamness GC,
et al: Intrinsic resistance of tumorigenic breast cancer cells to
chemotherapy. J Natl Cancer Inst. 100:672–679. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kamble D, Mahajan M, Dhat R and Sitasawad
S: Keap1-Nrf2 pathway regulates ALDH and contributes to
radioresistance in breast cancer stem cells. Cells. 10:832021.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tanei T, Morimoto K, Shimazu K, Kim SJ,
Tanji Y, Taguchi T, Tamaki Y and Noguchi S: Association of breast
cancer stem cells identified by aldehyde dehydrogenase 1 expression
with resistance to sequential Paclitaxel and epirubicin-base
chemotherapy for breast cancers. Clin Cancer Res. 15:4234–4241.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Palomeras S, Ruiz-Martínez S and Puig T:
Targeting breast cancer stem cells to overcome treatment
resistance. Molecules. 23:21932018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
García-Heredia JM and Carnero A: Role of
mitochondria in cancer stem cell resistance. Cells. 9:16932020.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bensimon J, Altmeyer-Morel S, Benjelloun
H, Chevillard S and Lebeau J: CD24(−/low) stem-like breast cancer
marker defines the radiation-resistant cells involved in
memorization and transmission of radiation-induced genomic
instability. Oncogene. 32:251–258. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bensimon J, Biard D, Paget V, Goislard M,
Morel-Altmeyer S, Konge J, Chevillard S and Lebeau J: Forced
extinction of CD24 stem-like breast cancer marker alone promotes
radiation resistance through the control of oxidative stress. Mol
Carcinog. 55:245–254. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Diehn M, Cho RW, Lobo NA, Kalisky T, Dorie
MJ, Kulp AN, Qian D, Lam JS, Ailles LE, Wong M, et al: Association
of reactive oxygen species levels and radioresistance in cancer
stem cells. Nature. 458:780–783. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee JH, Kim SH, Lee ES and Kim YS: CD24
overexpression in cancer development and progression: A
meta-analysis. Oncol Rep. 22:1149–1156. 2009.PubMed/NCBI
|
|
26
|
Altevogt P, Sammar M, Hüser L and
Kristiansen G: Novel insights into the function of CD24: A driving
force in cancer. Int J Cancer. 148:546–559. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kristiansen G, Machado E, Bretz N, Rupp C,
Winzer KJ, König AK, Moldenhauer G, Marmé F, Costa J and Altevogt
P: Molecular and clinical dissection of CD24 antibody specificity
by a comprehensive comparative analysis. Lab Invest. 90:1102–1116.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Weber E, Lehmann HP, Beck-Sickinger AG,
Wawrzynczak EJ, Waibel R, Folkers G and Stahel RA: Antibodies to
the protein core of the small cell lung cancer workshop antigen
cluster-w4 and to the leucocyte workshop antigen CD24 recognize the
same short protein sequence leucine-alanine-proline. Clin Exp
Immunol. 93:279–285. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Riley PA: Free radicals in biology:
Oxidative stress and the effects of ionizing radiation. Int J
Radiat Biol. 65:27–33. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mohiuddin M and Kasahara K: Cisplatin
activates the growth inhibitory signaling pathways by enhancing the
production of reactive oxygen species in non-small cell lung cancer
carrying an EGFR exon 19 deletion. Cancer Genomics Proteomics. 18
(3 Suppl):S471–S486. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mosca L, Ilari A, Fazi F, Assaraf YG and
Colotti G: Taxanes in cancer treatment: Activity, chemoresistance
and its overcoming. Drug Resist Updat. 54:1007422021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pan X, Zhang X, Sun H, Zhang J, Yan M and
Zhang H: Autophagy inhibition promotes 5-fluorouraci-induced
apoptosis by stimulating ROS formation in human non-small cell lung
cancer A549 cells. PLoS One. 8:e566792013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Elenbaas B, Spirio L, Koerner F, Fleming
MD, Zimonjic DB, Donaher JL, Popescu NC, Hahn WC and Weinberg RA:
Human breast cancer cells generated by oncogenic transformation of
primary mammary epithelial cells. Genes Dev. 15:50–65. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lombardo Y, de Giorgio A, Coombes CR,
Stebbing J and Castellano L: Mammosphere formation assay from human
breast cancer tissues and cell lines. J Vis Exp.
526712015.PubMed/NCBI
|
|
35
|
Biard DS, Despras E, Sarasin A and Angulo
JF: Development of new EBV-based vectors for stable expression of
small interfering RNA to mimick human syndromes: Application to NER
gene silencing. Mol Cancer Res. 3:519–529. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vert JP, Foveau N, Lajaunie C and
Vandenbrouck Y: An accurate and interpretable model for siRNA
efficacy prediction. BMC Bioinformatics. 7:5202006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Meijering E, Dzyubachyk O and Smal I:
Methods for cell and particle tracking. Methods Enzymol.
504:183–200. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gorelik R and Gautreau A: Quantitative and
unbiased analysis of directional persistence in cell migration. Nat
Protoc. 9:1931–1943. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pastushenko I, Brisebarre A, Sifrim A,
Fioramonti M, Revenco T, Boumahdi S, Van Keymeulen A, Brown D,
Moers V, Lemaire S, et al: Identification of the tumour transition
states occurring during EMT. Nature. 556:463–468. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yoh KE, Regunath K, Guzman A, Lee SM,
Pfister NT, Akanni O, Kaufman LJ, Prives C and Prywes R: Repression
of p63 and induction of EMT by mutant Ras in mammary epithelial
cells. Proc Natl Acad Sci USA. 113:E6107–E6116. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bocci F, Tripathi SC, Vilchez Mercedes SA,
George JT, Casabar JP, Wong PK, Hanash SM, Levine H, Onuchic JN and
Jolly MK: NRF2 activates a partial epithelial-mesenchymal
transition and is maximally present in a hybrid
epithelial/mesenchymal phenotype. Integr Biol (Camb). 11:251–263.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kim D, Choi B, Ryoo I and Kwak MK: High
NRF2 level mediates cancer stem cell-like properties of aldehyde
dehydrogenase (ALDH)-high ovarian cancer cells: Inhibitory role of
all-trans retinoic acid in ALDH/NRF2 signaling. Cell Death Dis.
9:8962018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tang JY, Ou-Yang F, Hou MF, Huang HW, Wang
HR, Li KT, Fayyaz S, Shu CW and Chang HW: Oxidative
stress-modulating drugs have preferential anticancer
effects-involving the regulation of apoptosis, DNA damage,
endoplasmic reticulum stress, autophagy, metabolism, and migration.
Semin Cancer Biol. 58:109–117. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Peiris-Pagès M, Martinez-Outschoorn UE,
Pestell RG, Sotgia F and Lisanti MP: Cancer stem cell metabolism.
Breast Cancer Res. 18:552016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bhatia S, Monkman J, Blick T, Pinto C,
Waltham M, Nagaraj SH and Thompson EW: Interrogation of phenotypic
plasticity between epithelial and mesenchymal states in breast
cancer. J Clin Med. 8:8932019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gupta PB, Fillmore CM, Jiang G, Shapira
SD, Tao K, Kuperwasser C and Lander ES: Stochastic state
transitions give rise to phenotypic equilibrium in populations of
cancer cells. Cell. 146:633–644. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ruscetti M, Dadashian EL, Guo W,
Mulholland DJ, Park JW, Tran LM, Kobayashi N, Bianchi-Frias D, Xing
Y, Nelson PS and Wu H: HDAC inhibition impedes
epithelial-mesenchymal plasticity and suppresses metastatic,
castration-resistant prostate cancer. Oncogene. 35:3781–3795. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Meyer MJ, Fleming JM, Ali MA, Pesesky MW,
Ginsburg E and Vonderhaar BK: Dynamic regulation of CD24 and the
invasive, CD44posCD24neg phenotype in breast cancer cell lines.
Breast Cancer Res. 11:R822009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ricardo S, Vieira AF, Gerhard R, Leitão D,
Pinto R, Cameselle-Teijeiro JF, Milanezi F, Schmitt F and Paredes
J: Breast cancer stem cell markers CD44, CD24 and ALDH1: Expression
distribution within intrinsic molecular subtype. J Clin Pathol.
64:937–946. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Xia F and Powell SN: The molecular basis
of radiosensitivity and chemosensitivity in the treatment of breast
cancer. Semin Radiat Oncol. 12:296–304. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Luce A, Courtin A, Levalois C,
Altmeyer-Morel S, Romeo PH, Chevillard S and Lebeau J: Death
receptor pathways mediate targeted and non-targeted effects of
ionizing radiations in breast cancer cells. Carcinogenesis.
30:432–439. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Longley DB, Harkin DP and Johnston PG:
5-fluorouracil: Mechanisms of action and clinical strategies. Nat
Rev Cancer. 3:330–338. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Tchounwou PB, Dasari S, Noubissi FK, Ray P
and Kumar S: Advances in our understanding of the molecular
mechanisms of action of cisplatin in cancer therapy. J Exp
Pharmacol. 13:303–328. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Abu Samaan TM, Samec M, Liskova A, Kubatka
P and Büsselberg D: Paclitaxel's mechanistic and clinical effects
on breast cancer. Biomolecules. 9:7892019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Pinto CA, Widodo E, Waltham M and Thompson
EW: Breast cancer stem cells and epithelial mesenchymal
plasticity-implications for chemoresistance. Cancer Lett.
341:56–62. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Bierie B, Pierce SE, Kroeger C, Stover DG,
Pattabiraman DR, Thiru P, Liu Donaher J, Reinhardt F, Chaffer CL,
Keckesova Z and Weinberg RA: Integrin-β4 identifies cancer stem
cell-enriched populations of partially mesenchymal carcinoma cells.
Proc Natl Acad Sci USA. 114:E2337–E2346. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Unternaehrer JJ, Zhao R, Kim K, Cesana M,
Powers JT, Ratanasirintrawoot S, Onder T, Shibue T, Weinberg RA and
Daley GQ: The epithelial-mesenchymal transition factor SNAIL
paradoxically enhances reprogramming. Stem Cell Reports. 3:691–698.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Gingold JA, Fidalgo M, Guallar D, Lau Z,
Sun Z, Zhou H, Faiola F, Huang X, Lee DF, Waghray A, et al: A
genome-wide RNAi screen identifies opposing functions of Snai1 and
Snai2 on the Nanog dependency in reprogramming. Mol Cell.
56:140–152. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Ma SY, Park JH, Jung H, Ha SM, Kim Y, Park
DH, Lee DH, Lee S, Chu IH, Jung SY, et al: Snail maintains
metastatic potential, cancer stem-like properties, and
chemoresistance in mesenchymal mouse breast cancer TUBO-P2J cells.
Oncol Rep. 38:1867–1876. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Roca H, Hernandez J, Weidner S, McEachin
RC, Fuller D, Sud S, Schumann T, Wilkinson JE, Zaslavsky A, Li H,
et al: Transcription factors OVOL1 and OVOL2 induce the mesenchymal
to epithelial transition in human cancer. PLoS One. 8:e767732013.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Wu RS, Hong JJ, Wu JF, Yan S, Wu D, Liu N,
Liu QF, Wu QW, Xie YY, Liu YJ, et al: OVOL2 antagonizes TGF-β
signaling to regulate epithelial to mesenchymal transition during
mammary tumor metastasis. Oncotarget. 8:39401–39416. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Hong T, Watanabe K, Ta CH,
Villarreal-Ponce A, Nie Q and Dai X: An Ovol2-Zeb1 mutual
inhibitory circuit governs bidirectional and multi-step transition
between epithelial and mesenchymal states. PLoS Comput Biol.
11:e10045692015. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Westcott JM, Camacho S, Nasir A, Huysman
ME, Rahhal R, Dang TT, Riegel AT, Brekken RA and Pearson GW:
ΔNp63-regulated epithelial-to-mesenchymal transition state
heterogeneity confers a leader-follower relationship that drives
collective invasion. Cancer Res. 80:3933–3944. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Jolly MK, Boareto M, Debeb BG, Aceto N,
Farach-Carson MC, Woodward WA and Levine H: Inflammatory breast
cancer: A model for investigating cluster-based dissemination. NPJ
Breast Cancer. 3:1–8. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Ryoo I, Lee S and Kwak MK: Redox
modulating NRF2: A potential mediator of cancer stem cell
resistance. Oxid Med Cell Longev. 2016:24281532016. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Jia D, Tan Y, Liu H, Ooi S, Li L, Wright
K, Bennett S, Addison CL and Wang L: Cardamonin reduces
chemotherapy-enriched breast cancer stem-like cells in vitro and in
vivo. Oncotarget. 7:771–785. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Vipparthi K, Hari K, Chakraborty P, Ghosh
S, Patel AK, Ghosh A, Biswas NK, Sharan R, Arun P, Jolly MK and
Singh S: Emergence of hybrid states of stem-like cancer cells
correlates with poor prognosis in oral cancer. iScience.
25:1043172022. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Grosse-Wilde A, Kuestner RE, Skelton SM,
MacIntosh E, d'Hérouël AF, Ertaylan G, Del Sol A, Skupin A and
Huang S: Loss of inter-cellular cooperation by complete
epithelial-mesenchymal transition supports favorable outcomes in
basal breast cancer patients. Oncotarget. 9:20018–20033. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Gammon L, Biddle A, Heywood HK,
Johannessen AC and Mackenzie IC: Sub-sets of cancer stem cells
differ intrinsically in their patterns of oxygen metabolism. PLoS
One. 8:e624932013. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Sciacovelli M and Frezza C: Metabolic
reprogramming and epithelial-to-mesenchymal transition in cancer.
FEBS J. 284:3132–3144. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Lee SY, Ju MK, Jeon HM, Lee YJ, Kim CH,
Park HG, Han SI and Kang HS: Reactive oxygen species induce
epithelial-mesenchymal transition, glycolytic switch, and
mitochondrial repression through the Dlx-2/Snail signaling pathways
in MCF-7 cells. Mol Med Rep. 20:2339–2346. 2019.PubMed/NCBI
|
|
72
|
Jia D, Park JH, Kaur H, Jung KH, Yang S,
Tripathi S, Galbraith M, Deng Y, Jolly MK, Kaipparettu BA, et al:
Towards decoding the coupled decision-making of metabolism and
epithelial-to-mesenchymal transition in cancer. Br J Cancer.
124:1902–1911. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Fang X, Zheng P, Tang J and Liu Y: CD24:
From A to Z. Cell Mol Immunol. 7:100–103. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Deng X, Apple S, Zhao H, Song J, Lee M,
Luo W, Wu X, Chung D, Pietras RJ and Chang HR: CD24 expression and
differential resistance to chemotherapy in triple-negative breast
cancer. Oncotarget. 8:38294–38308. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Shen Y, Schmidt BUS, Kubitschke H,
Morawetz EW, Wolf B, Käs JA and Losert W: Detecting heterogeneity
in and between breast cancer cell lines. Cancer Converg. 4:12020.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Li W, Ma H, Zhang J, Zhu L, Wang C and
Yang Y: Unraveling the roles of CD44/CD24 and ALDH1 as cancer stem
cell markers in tumorigenesis and metastasis. Sci Rep. 7:138562017.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Gupta PB, Pastushenko I, Skibinski A,
Blanpain C and Kuperwasser C: Phenotypic plasticity: Driver of
cancer initiation, progression, and therapy resistance. Cell Stem
Cell. 24:65–78. 2019. View Article : Google Scholar : PubMed/NCBI
|