Introduction
Pancreatic ductal adenocarcinoma (PDAC) is among the
most aggressive cancers with a 5-year survival rate of 8–10%
(1–3). Hypoxia, a stress condition in which
oxygen levels are insufficient for typical cellular function,
contributes to PDAC pathophysiology and treatment resistance
(4). Protein synthesis in the
endoplasmic reticulum (ER) demands more energy and oxygen compared
with other cellular processes (5,6); as
such hypoxia significantly hinders protein translation and folding
(7). This results in accumulated
misfolded or unfolded proteins activating ER stress sensors, thus
triggering the unfolded protein response (UPR) (Fig. 1) (7,8).
Within the UPR pathway, activating transcription factor 4 (ATF4) is
one of the master regulators that activates transcription of genes
required for amino acid metabolism, synthesis and transport
(7). These genes, among others,
generally alleviate hypoxia-induced ER stress to promote cell
recovery and homeostasis (7,9–11).
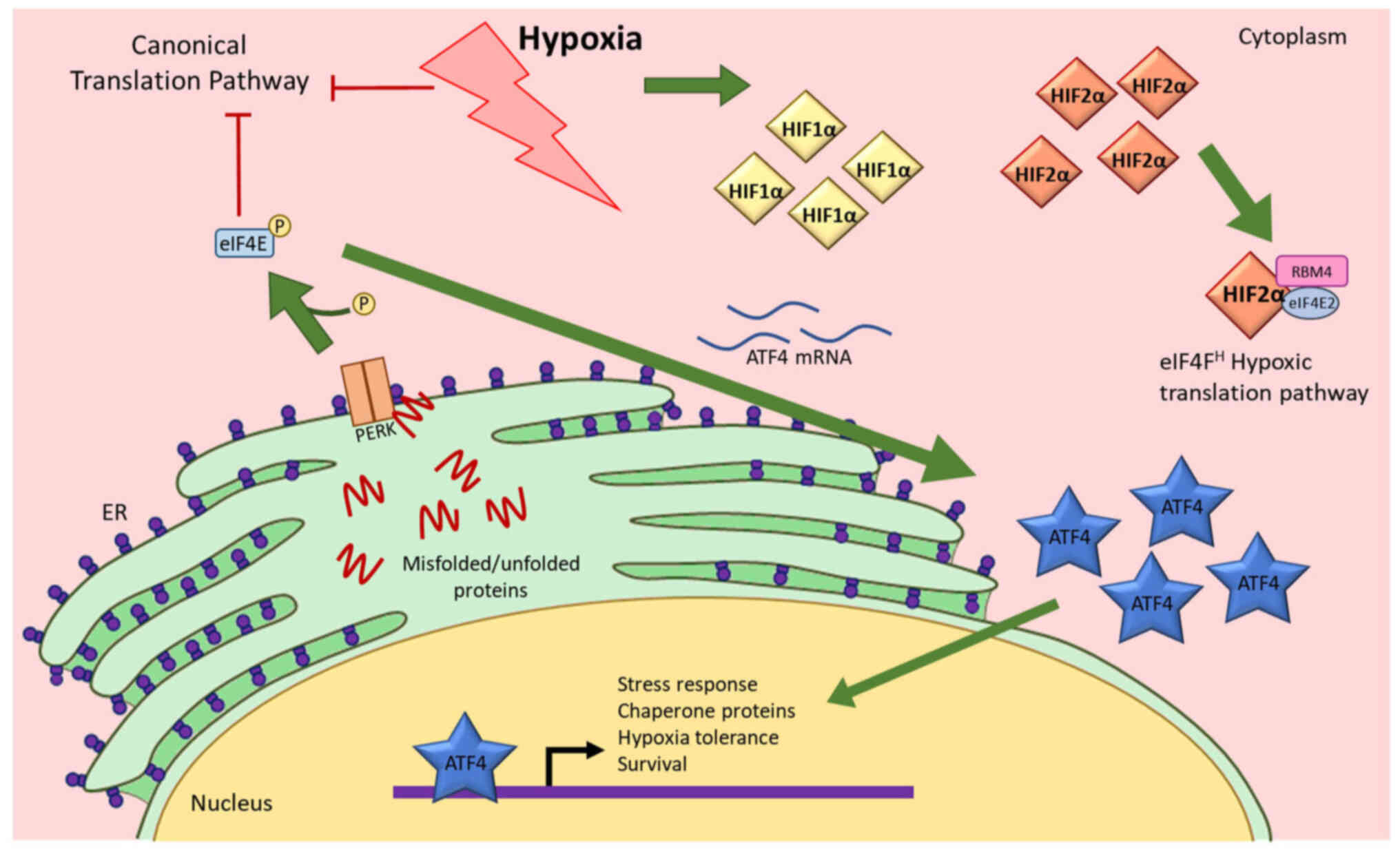 | Figure 1.Hypoxia activates the UPR pathway and
causes ATF4 expression to increase. Hypoxia causes inhibition of
the canonical translation pathway, resulting in misfolded or
unfolded proteins. These peptides activate PERK that consequently
induces ATF4 mRNA translation, resulting in an increase in ATF4
protein expression. ATF4 then translocates to the nucleus to
initiate gene expression changes. Hypoxia also causes increases in
HIF1α and HIF2α. Both HIFs also translocate to the nucleus to
function as transcription factors. HIF2α also functions as a
translation factor that initiates translation when cells are under
hypoxic stress (17). UPR,
unfolded protein response; ATF4, activating transcription factor 4;
eIF4E, eukaryotic initiation factor 4E; ER, endoplasmic reticulum;
HIF1α, hypoxia-inducible factor 1α; HIF2α, hypoxia-inducible factor
2α; PERK, protein kinase R (PKR)-like endoplasmic reticulum kinase;
P, phosphate. |
PDAC exhibits enhanced cellular response to hypoxic
stress and significantly higher ATF4 expression compared with
healthy pancreatic cells (12–14).
Numerous chemotherapeutic drugs are ineffective in PDAC due to ATF4
functions that promote drug resistance in hypoxia (14). ATF4 is unique in the UPR pathway
because it is translated more efficiently in hypoxia compared with
normoxia (7,15). This higher translation efficiency
is orchestrated by upstream open reading frames (uORFs) in the mRNA
transcript that promote translation of a truncated, inactive
protein under normal conditions and initiate translation of
functional ATF4 protein under cell stress conditions, such as
hypoxia. Increased ATF4 protein expression in hypoxia is also
promoted by post-translational modifications that stabilize the
protein (16).
In addition to activating ATF4 in the UPR pathway,
cells also respond to hypoxia by activating hypoxia-inducible
factors (HIFs), a family of proteins that initiate hypoxia-response
pathways (17). HIFs are well
known for their roles as transcription factors in hypoxia because
they activate the transcription of genes that promote cell
adaptation during oxygen deprivation (17). HIF1α and HIF2α subunits are capable
of transcribing genes that play important roles in cancer
progression such as carbonic anhydrase IX (CA9) and octamer-binding
transcription factor 4 (Oct4), among others (18,19).
Independent of its transcriptional functions, HIF2α is also a
translational factor that forms a complex with other proteins to
activate an alternative cap-dependent translation mechanism to
synthesize proteins from select mRNA that are necessary for
response to hypoxia (17,20–22).
In chronic hypoxia, the canonical translation initiation pathway is
inhibited, resulting in significantly decreased global protein
synthesis (20).
Previous studies on ATF4 and HIFs in pancreatic
cancers have been conducted at atmospheric oxygen levels, but not
in prolonged hypoxia of more than 24 h, a more pathophysiologically
relevant condition in which the majority of PDAC cases exist. In
vitro studies performed in normoxia demonstrated that ATF4
inhibition prevents cancer progression and treatment resistance.
However, while ATF4 expression is higher in PDAC cells compared
with healthy pancreatic cells in these studies, the cells used in
normoxic experiments do not express HIFs at levels similar to those
that are observed in patients, making it difficult to identify any
relevant interactions between ATF4 and HIFs. Furthermore, other
studies showed that more severe hypoxia of <1% oxygen levels are
necessary for significant UPR activation (23,24).
In the present study, ATF4 activity was explored in the hypoxia
response pathway mediated by HIFs in conditions of severe, chronic
hypoxia in pancreatic cancer cells.
Materials and methods
Cell culture
Pancreatic epithelial carcinoma cell lines PANC-1
(cat. no. CRL-1469) and MiaPaCa-2 [cat. no. CRL-1420; both from
American Type Culture Collection (ATCC)] were cultured in Advanced
DMEM media supplemented with 10% FBS, 1% GlutaMAX (cat. no.
35050061; Gibco; Thermo Fisher Scientific, Inc.), and 1% penicillin
and streptomycin (cat. no. 15140-163; Gibco; Thermo Fisher
Scientific, Inc.). Healthy skin fibroblasts WS1 cells (cat. no.
CRL-1502; ATCC) were cultured in advanced DMEM supplemented with
15% FBS, penicillin and streptomycin, GlutaMAX, β-mercaptoethanol,
and basic fibroblast growth factor. All cells were cultured under
standard conditions of 37°C with 5% CO2, 21%
O2 (normoxic) or as indicated in hypoxia. All cells were
tested for mycoplasma ~every 3 months and confirmed to be free of
contamination using a PCR mycoplasma detection kit (cat. no.
30-1012K; ATCC).
Hypoxia treatment
For hypoxia treatment, Ruskinn InvivO2
500 hypoxia workstation was used (Baker Ruskinn). Cells were seeded
or plated and incubated under standard conditions in normoxia for
24 h prior to hypoxia exposure. Cells were then placed into a
sterile hypoxia glove box pre-set to either 1% oxygen for healthy
fibroblasts or 0.2% for cancer cell lines, 5% CO2, a
balance of N2 and at 37°C. WS1 fibroblasts were exposed
to 1% oxygen rather than 0.2% oxygen since cancer cells have a
lower threshold for oxygen before hypoxia response pathways are
activated, while normal fibroblasts are less tolerant of hypoxia.
Cells were handled inside of the hypoxia glove box for the duration
of the experiments until they were either fixed or lysed.
Transfection
To assess the effects of gene inhibition on hypoxic
PDAC, PANC-1 cells were transiently transfected using Lipofectamine
RNAiMAX Transfection Reagent (Thermo Fisher Scientific, Inc.) and
pre-designed Silencer Select small interfering (si)RNAs (Thermo
Fisher Scientific, Inc.) at 1 pmol per well: HIF1α (cat. no.
s6539), HIF2α (cat. no. s4698) and ATF4 (cat. no. s1702). The
siRNA-lipid complexes were constructed in reduced serum Opti-MEM
media (cat. no. 31985070; Thermo Fisher Scientific, Inc.) according
to manufacturer protocols, incubated at room temperature for at
least 5 min and were added to each well in either six-, 12- or
96-well plates. The cells were then seeded into the plates in
normal growth media. Lipofectamine RNAiMAX Transfection Reagent
only requires reduced serum media when siRNA-lipid complexes are
made and not when treated to cells in growth media containing
serum. The cells were incubated at 37°C under normoxic conditions
for 24 h before they were placed in the hypoxia chamber or remained
in the normoxic incubator. Knockdown efficacy was determined by
reverse transcription-quantitative (RT-q) PCR.
RNA extraction, quantification and
RT-q PCR
Cells incubated in normoxia were washed with PBS
while hypoxic cells were washed with de-oxygenated PBS. RNA was
extracted using TRIzol® (Thermo Fisher Scientific, Inc.)
and purified by RNeasy column centrifugation kit (Qiagen). Purified
RNA was quantified using Nanodrop spectrophotometer at a wavelength
of 260 nm (Thermo Fisher Scientific, Inc.). cDNA was synthesized
using qScript cDNA synthesis kit (Quantabio) according to the
manufacturer's protocol and qPCR was conducted using Taqman
primer-probes (Thermo Fisher Scientific, Inc.) and Taqman Gene
Expression Master Mix (Applied Biosystems; Thermo Fisher
Scientific, Inc.) according to the manufacturer'σ protocol provided
for the Taqman Master Mix. The Taqman probes used were
Hs00153153_m1 (HIF1α), Hs01026149_m1 (HIF2α), Hs00154208_m1 (CA9),
Hs00999632_g1 (Oct4) and Hs00909569_g1 (ATF4). The RT-qPCR data was
quantified by calculating the 2−ΔΔCq values as defined
by Livak and Schmittgen, 2001.
Protein extraction and western blot
analysis
Normoxic and hypoxic cells were washed in either
oxygenated PBS or de-oxygenated PBS, respectively. Mammalian
Protein Extraction Reagent was supplemented with Halt Protease
Inhibitor cocktail (Thermo Fisher Scientific, Inc.) and added to
the cells for lysis. The cells were frozen for at least 6 h to
promote lysis, and the samples were sonicated. The samples were
centrifuged at 4°C for 10 min at 14,000 × g. The supernatant was
transferred to new tubes and Bicinchoninic Acid (BCA) assay
(Pierce; Thermo Fisher Scientific, Inc.) was conducted and measured
using the EnVision plate reader (PerkinElmer, Inc.) to quantify
protein.
For western blotting, the Criterion Blotter Western
Blot system was used (Bio-Rad Laboratories, Inc.); 30 µg of total
protein in Laemmli sample buffer was boiled for 10 min at 95–100°C.
The samples were loaded into 10% gels (Bio-Rad Laboratories, Inc.)
and electrophoresis commenced at 60–100 V. The protein in the gel
was transferred to PVDF membranes by wet-transfer at 100 V for 30
min. The membranes were blocked in 5% w/v fat-free powdered milk in
1X Tris buffered solution with 0.1% Tween 20 detergent (TBS-T;
Sigma-Aldrich; Merck KGaA) for 1 h at room temperature, probed with
primary antibodies overnight at 4°C in 5% BSA (Sigma-Aldrich; Merck
KGaA) and with secondary antibodies for 1 h at room temperature in
5% BSA. The primary antibodies used were HIF1α (1:1,000; cat. no.
MA1516; Thermo Fisher Scientific, Inc.), HIF2α (1:1,000; cat. no.
ab8365; Abcam), ATF4 (1:1,000; cat. no. 11815S; Cell Signaling
Technology, Inc.) and β-actin (1:1,000; cat. no. sc-47778; Santa
Cruz Biotechnology, Inc.). Horseradish peroxidase-conjugated mouse
IgGκ light chain binding protein (1:1,000; cat. no. sc-516102;
Santa Cruz Biotechnology, Inc.) and mouse anti-rabbit secondary
antibodies (1:1,000; cat. no. sc-2357; Santa Cruz Biotechnology,
Inc.) were used. The blots were visualized by enhanced
chemiluminescence (ECL) incubation and C-DiGit Blot Scanner (LI-COR
Biosciences). The images were quantified by ImageJ software version
1.53e (National Institutes of Health).
Cell viability assay
Cells (2,000/well) were seeded into 96-well plates
and incubated overnight in normoxia under standard culturing
conditions. The plates were placed into hypoxia or maintained at
normoxia. After hypoxic exposure, the cells were removed from the
chamber and reconstituted CellTiterGlo (Promega Corporation)
reagents were added to the plate, following the manufacturer's
protocol. The plate was incubated at room temperature for at least
10 min and the luminescence at 560 nm was measured on the
PerkinElmer Envision plate reader.
Colony formation assay
Cells were seeded into each well (2,000 cells per
well) in six-well plates. The cells were incubated at 37°C for 24 h
and then placed in 0.2% oxygen or maintain in normoxia. The cells
in hypoxia were incubated for 16 or 48 h and then placed in
normoxia for 7–10 days of incubation for colony formation.
Afterwards, the cells were washed with cold PBS, fixed with acetic
acid and methanol fixing solution (1:4 mixture) at room temperature
for at least 15 min until dry and stained at room temperature for
at least 30 min with 0.5% w/v crystal violet (cat. no. C0775-25G;
Sigma Aldrich; Merck KGaA) in 25% ethanol solution. The plates were
then washed, and colonies were counted manually.
Cell migration (scratch) assay
Cells were transiently transfected following the
previously outlined protocol. After siRNA-lipid complexes were
added to 24-well plates, 1×105 PANC-1 cells in growth
media were seeded into each well and incubated at 37°C for 24 h.
Each well was then ‘scratched’ using a P1000 pipette tip down the
middle of each well. Images of all wells were obtained. The plates
were then placed in either 0.2% oxygen or maintained in normoxia
and incubated for either 16 or 48 h. After incubation in normoxia
or hypoxia, images of each well were captured using an inverted
light microscope (Invertoskop 40C; Carl Zeiss AG) and analyzed
using an ImageJ/Fiji plugin tool (25).
Statistical analysis
All experiments were conducted in at least three
replicates. For all experiments consisting of two groups, unpaired
Student's t-test was conducted for statistical significance. For
all experiments consisting of more than two groups, tests for
homogeneity of variance, such as Levene's test, were performed.
One-way analyses of variance (ANOVA) tests were conducted, followed
by the Bonferroni multiple variance post hoc tests. Data were
graphed and analyzed using GraphPad Prism software version 9.3
(GraphPad Software, Inc.). P<0.05 was considered to indicate a
statistically significant difference.
Results
ATF4 expression increases in acute and
chronic hypoxia
ATF4 is over-expressed in PDAC cells compared with
healthy pancreatic cells and is associated with poor prognosis
(26). To first determine ATF4
expression in PDAC cells in hypoxia, PANC-1 and Mia-PaCa2 cells
were cultured in a 0.2% oxygen environment or in a 21% oxygen
environment for 24 h. Hypoxia was defined as 0.2% oxygen in PDAC
cells because while different tissue types activate the
hypoxia-responsive pathways at varying levels of oxygen, pancreatic
cancers have been shown to be severely hypoxic (<1%) with ~0.4%
oxygen in tumors (27–29). It was determined that 0.2% oxygen
was necessary to ensure the PDAC cells adequately activate
hypoxia-response pathways by confirming HIF and ATF4 activity at
0.2% oxygen (Figs. 2 and 3). Furthermore, the UPR pathway activates
under severe hypoxic conditions, which is needed to investigate
ATF4 (23,24).
PANC-1 cells were used to assess ATF4 expression in
acute and chronic hypoxia. Literature values for acute hypoxia
generally do not surpass 24 h, while chronic hypoxia tends to be
more than 24 h. In the present study, acute was defined as 16 h and
chronic as 48 h (26,30,31).
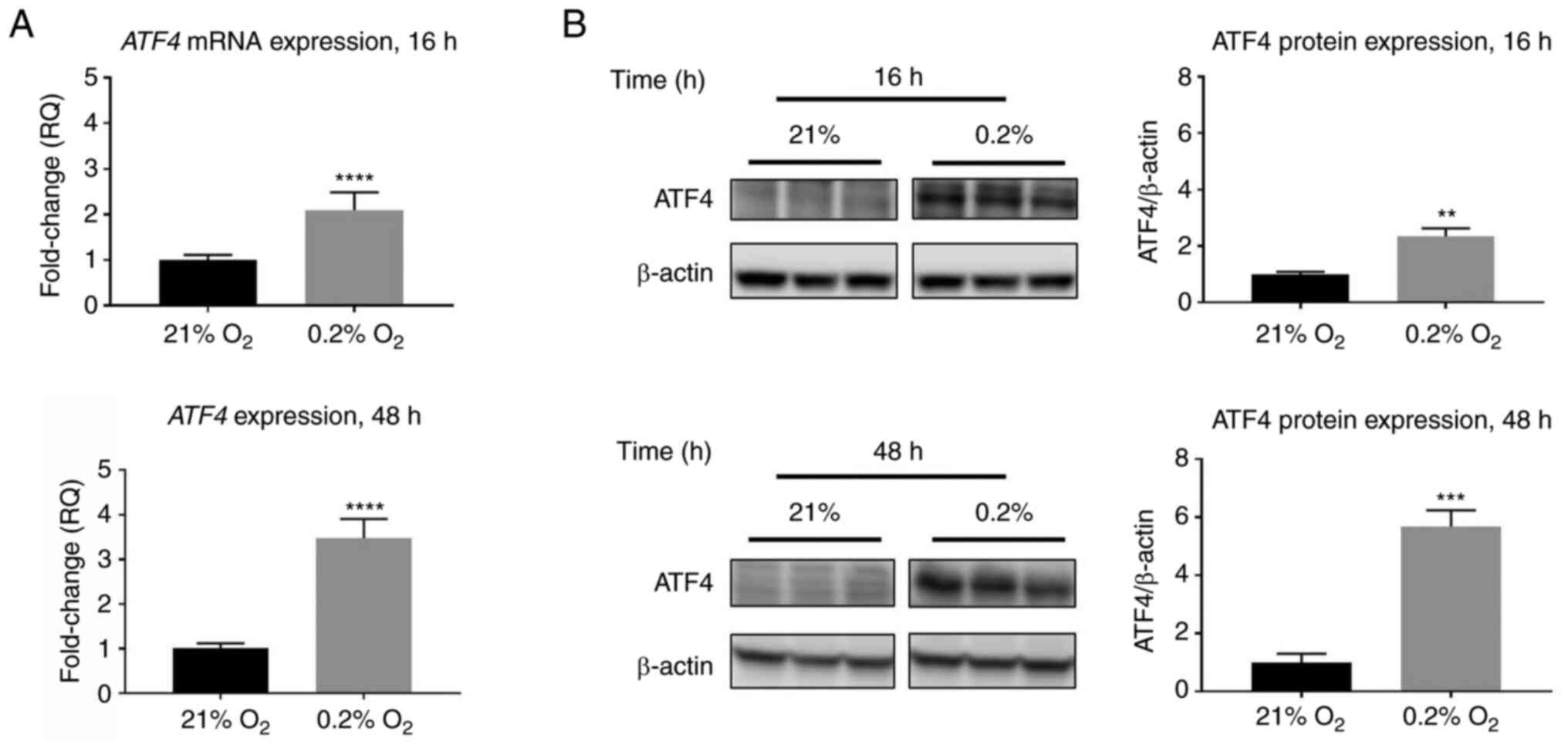
RT-qPCR data revealed that ATF4 mRNA expression increased by
2.1±0.1-fold in acute hypoxia compared with cells exposed to
normoxia (P<0.0001), while ATF4 mRNA levels in chronic hypoxia
increased 3.5±0.4-fold compared with normoxia levels (P<0.0001;
Fig. 2A). Western blot analysis
revealed similar expression patterns in which ATF4 protein levels
increase in hypoxia compared with normoxic levels. Under normoxic
conditions, little to no ATF4 protein expression was detected, but
upon hypoxic exposure, ATF4 expression increased significantly. In
acute hypoxia, the PANC-1 cells exhibited a 2.3±0.1-fold increase
in ATF4 protein expression (P<0.01), while in chronic hypoxia,
ATF4 levels increased by 5.7±0.3-fold compared with normoxia
(P<0.001; Fig. 2B).
HIFs and expression of HIF target
genes vary over time in hypoxia
In addition to ATF4 expression changes, hypoxia
leads to increased HIF1α and HIF2α expression in PDAC (17). However, HIF expression profiles
differ depending on cell type and duration in hypoxia (17). While all HIF levels oscillate over
time, increased HIF1α expression is generally associated with acute
hypoxic exposure whereas increased HIF2α expression is typically
associated with chronic hypoxia (17,32).
HIF expression profiles can vary greatly among different cell
types, and, therefore, HIF expression and activity in PANC-1 cells
were determined in acute and chronic hypoxia. To confirm HIF1α and
HIF2α expression and activity, the cells were incubated in either
normoxic conditions or hypoxia over time. As compared with normoxic
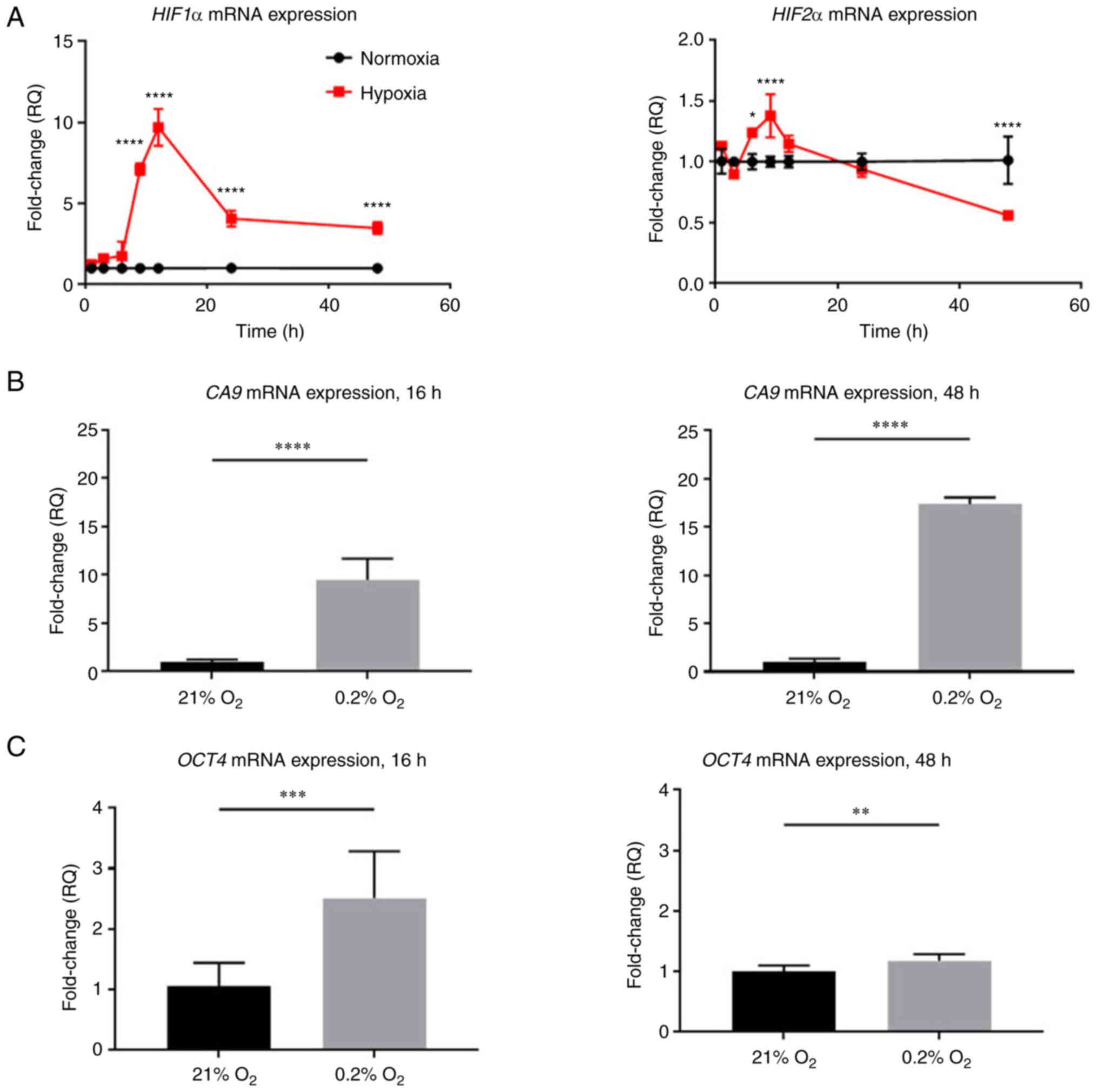
control cells, HIF1α mRNA expression significantly increased up to
9.7±1.1-fold after 12 h of hypoxic exposure, decreased at 24 h
compared with the 12-h timepoint and then ultimately plateaued at a
level 3.5±0.4-fold change higher than cells in normoxia
(P<0.0001). HIF2α mRNA expression levels, while lower than HIF1α
expression levels, increased significantly up to 1.4±0.1-fold
higher than normoxic levels at 9 h (P<0.0001), and then
decreased to 0.6±0.02-fold lower than normoxic levels (P<0.0001;
Fig. 3A).
HIFs are subject to post-translational regulation
and degradation in normoxia, whereas in hypoxia, HIF proteins are
stabilized and accumulate. While HIF mRNA levels may vary over
time, known HIFα target genes were also analyzed to assess HIF
activities in acute and chronic hypoxia. To confirm HIF1α activity,
the mRNA levels of HIF1α-specific transcriptional target gene CA9
were measured (Fig. 3B) (33). CA9 expression increased
9.5±2.2-fold after 16 h of hypoxia (P<0.0001) and continued to
increase up to 17.8±0.7-fold after 48 h of hypoxia compared with
normoxic controls (P<0.0001). To measure HIF2α activity, the
mRNA levels of HIF2α transcriptional target gene Oct were analyzed
(Fig. 3C) (19). Oct4 expression increased
2.5±0.8-fold after 16 h of hypoxia (P<0.001), and in chronic
hypoxia, Oct4 mRNA levels decreased close to normoxic levels with
only a 1.2±0.1-fold decrease compared with normoxic levels
(P<0.01). The increase in CA9 and Oct4 confirmed that HIF1α and
HIF2α are active in acute and chronic hypoxia.
HIF1α and HIF2α knockdowns do not
affect ATF4 mRNA or protein expression in chronic hypoxia
HIF transcriptional and translational functions make
it possible that HIF1α or HIF2α affect ATF4 expression to mediate
the UPR pathway in response to hypoxia. To understand the effects
that HIF1α and HIF2α have on ATF4 in acute and chronic hypoxia in
PDAC cells, siRNA was used to knockdown ATF4, HIF1α or HIF2α for
loss-of-function experiments (Fig.
S1). After 24 h of incubation in normal cell culture
conditions, the cells were either placed in hypoxia or maintained
in normoxia for 16 or 48 h. mRNA and protein expression levels of
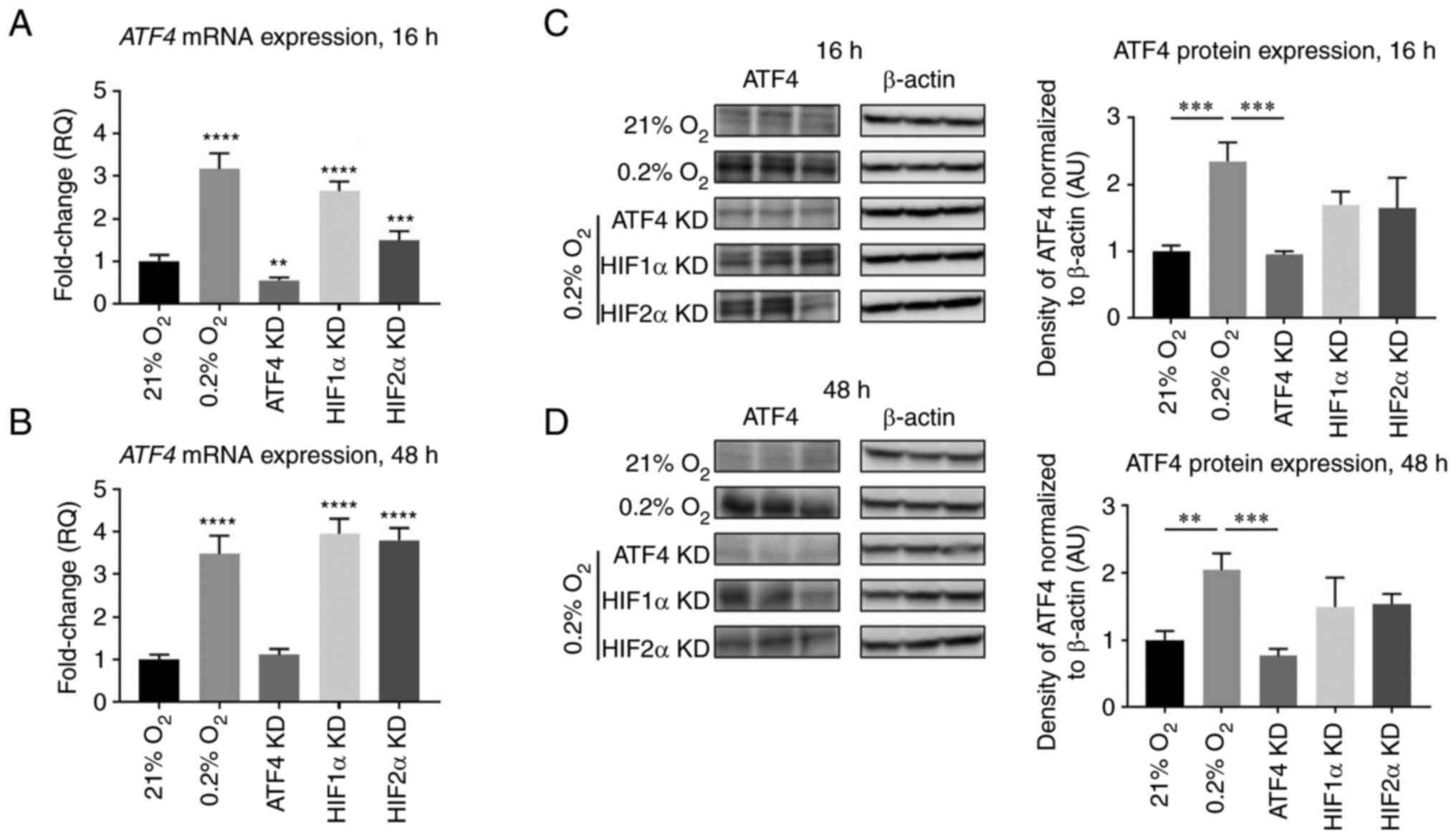
ATF4, HIF1α and HIF2α were determined. In acute hypoxia, knockdown
of either HIF1α or HIF2α caused significant decreases in ATF4 mRNA
levels compared with the hypoxia control (P<0.0001 and
P<0.001, respectively; Fig.
4A). In chronic hypoxia, ATF4 mRNA expression significantly
increased after HIF1α knockdown compared with the hypoxia (0.2%
O2) control group (P<0.05) and was not affected after
HIF2α knockdown compared with the hypoxia (0.2% O2)
control group (Fig. 4B).
The protein expression levels of ATF4 were also
determined after ATF4, HIF1α or HIF2α knockdowns. In normoxia, ATF4
protein expression levels were minimal, and with hypoxic exposure,
ATF4 protein levels increased by 2.3±0.3-fold after 16 h
(P<0.001) and 2.0±0.2-fold after 48 h compared with normoxic
levels, respectively (P<0.05; Fig.
4C and D). ATF4 protein levels significantly decreased after
ATF4 knockdown (P<0.001; Fig. 4C
and D). HIF1α knockdown did not affect ATF4 protein expression
levels in either acute (P>0.05) or chronic hypoxia (P>0.05).
HIF2α knockdown had no effect on ATF4 protein expression in either
acute (P>0.05) or chronic hypoxia (P>0.05).
ATF4 knockdown decreases HIF1α and
increases HIF2α expression in chronic, but not acute, hypoxia
ATF4 is a master regulator within the UPR pathway
when cells are under stress, such as in hypoxia. To understand the
effects that ATF4 have on HIF1α and HIF2α in acute and chronic
hypoxia in PDAC cells, ATF4, HIF1α or HIF2α were knocked down using
siRNA, incubated in normoxia for 24 h and then placed in either
hypoxia or maintained in normoxia for 16 or 48 h. HIF1α and HIF2α
mRNA and protein levels were then determined.
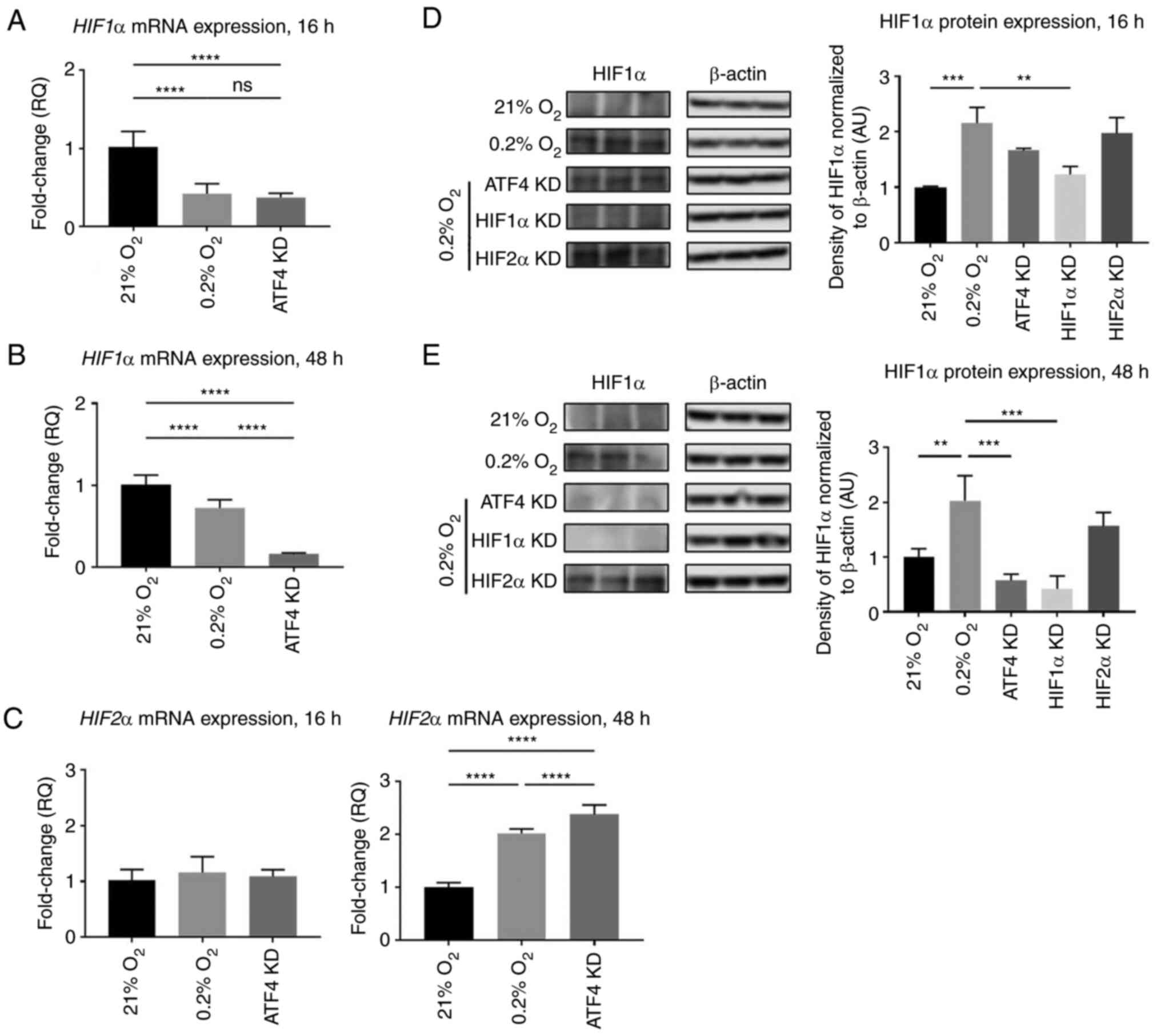
Under acute hypoxia, HIF1α mRNA expression was
0.4±0.1-fold lower in the hypoxic vehicle-treated cells with no
siRNA compared with normoxic cells (P<0.0001; Fig. 5A). There was no significant
difference in HIF1α mRNA expression between the hypoxic
vehicle-treated cells and the ATF4 knockdown cells (P>0.05;
Fig. 5A). HIF1α protein expression
in hypoxia alone was 2.2±0.3-fold higher than in normoxia,
indicating that HIF protein levels do not appear to be solely
determined by the corresponding hypoxic mRNA levels (P<0.001;
Fig. 5D). Upon ATF4 knockdown in
acute hypoxia, HIF1α protein expression was 1.7±0.1-fold higher
compared with the normoxia-treated cells (P<0.05) but not
significantly different compared with the hypoxia treatment cells
(P>0.05; Fig. 5D). HIF1α
knockdown in hypoxia significantly reduced HIF1α protein expression
compared with the hypoxia treatment group with no knockdown
(P<0.01). HIF2α knockdown did not affect HIF1α protein
expression compared with hypoxia treatment alone (P>0.05;
Fig. 5D). ATF4 knockdown had no
effect on HIF2α mRNA expression in acute hypoxia with only a
1.1±0.1-fold increase compared with normoxia treated cells
(P>0.05). However, in chronic hypoxia, HIF2α mRNA expression
levels increased by 2.4±0.2-fold compared with normoxia-treated
cells (P<0.0001; Fig. 5C).
Furthermore, in chronic hypoxia, ATF4 knockdown
significantly decreased HIF1α mRNA levels to 0.15±0.02-fold of the
normoxia-treated and hypoxia-treated cells (P<0.05; Fig. 5B). This expression pattern was also
identified in protein expression where ATF4 knockdown resulted in a
0.4±0.2-fold decrease in HIF1α protein expression levels
(P<0.0001; Fig. 5E). While ATF4
did not affect HIF1α or HIF2α during acute hypoxia, it did affect
HIF1α and HIF2α mRNA and protein expression levels during chronic
hypoxia.
ATF4 knockdown increases cell
migration in chronic but not acute hypoxia
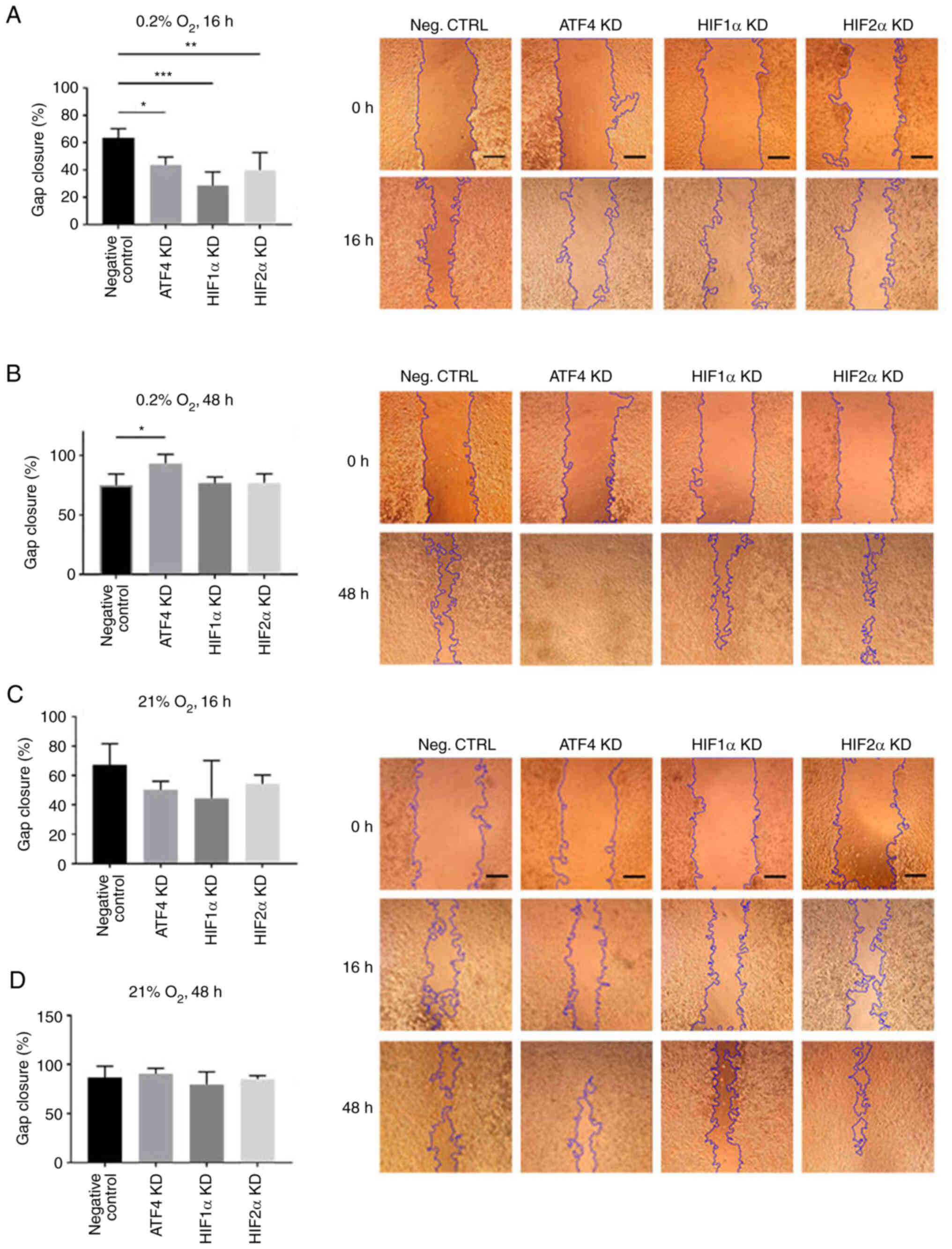
To determine the effect of ATF4 inhibition on cell
migration in acute and chronic hypoxia, a scratch assay was
performed. PANC-1 cells transiently transfected with ATF4, HIF1α or
HIF2α siRNA were seeded and scratched prior to 16 or 48 h of
hypoxic exposure. After 16 h of hypoxic exposure, the negative
control group, PANC-1 cells with no knockdown siRNA, exhibited the
greatest gap closure among the treatment groups with 63.4±6.8% gap
closure (Fig. 6A). In normoxia,
there were no changes in gap closure rates among the treatment
groups, with all treatments after 16 h of normoxic incubation
resulted in at least 45% closure (Fig.
6C) and at least 80% closure after 48 h of incubation (Fig. 6D). Relative to the negative control
group, hypoxia-treated cells without siRNA knockdowns, the ATF4
knockdown cells in acute hypoxia closed 43.7±5.7% of the gap
measured at the initial scratch (P<0.05). The HIF1α and HIF2α
knockdown cells also exhibited a significant decline in cell
migration with 28.60±9.95% (P<0.001) and 39.8±13.0% (P<0.01)
gap closure, respectively (Fig.
6A). In chronic hypoxia, the results demonstrated that HIF1α
and HIF2α knockdown cell migration rates were comparable with those
of the hypoxia-treated cells without siRNA knockdowns. The negative
control cells closed 75.1±9.4% of the gap after 48 h of hypoxia
(Fig. 6B). HIF1α knockdown cells
showed 76.7±5.2% gap closure, and HIF2α knockdown cells exhibited
77.0±7.6% gap closure. The ATF4 knockdown cells, on the other hand,
showed significantly more cell migration with 93.4±7.6% gap closure
(P<0.05) compared with the negative control group and HIF1α or
HIF2α knockdown cells (Fig. 6B).
The scratch assay revealed that ATF4 knockdown inhibits cell
migration in acute hypoxia.
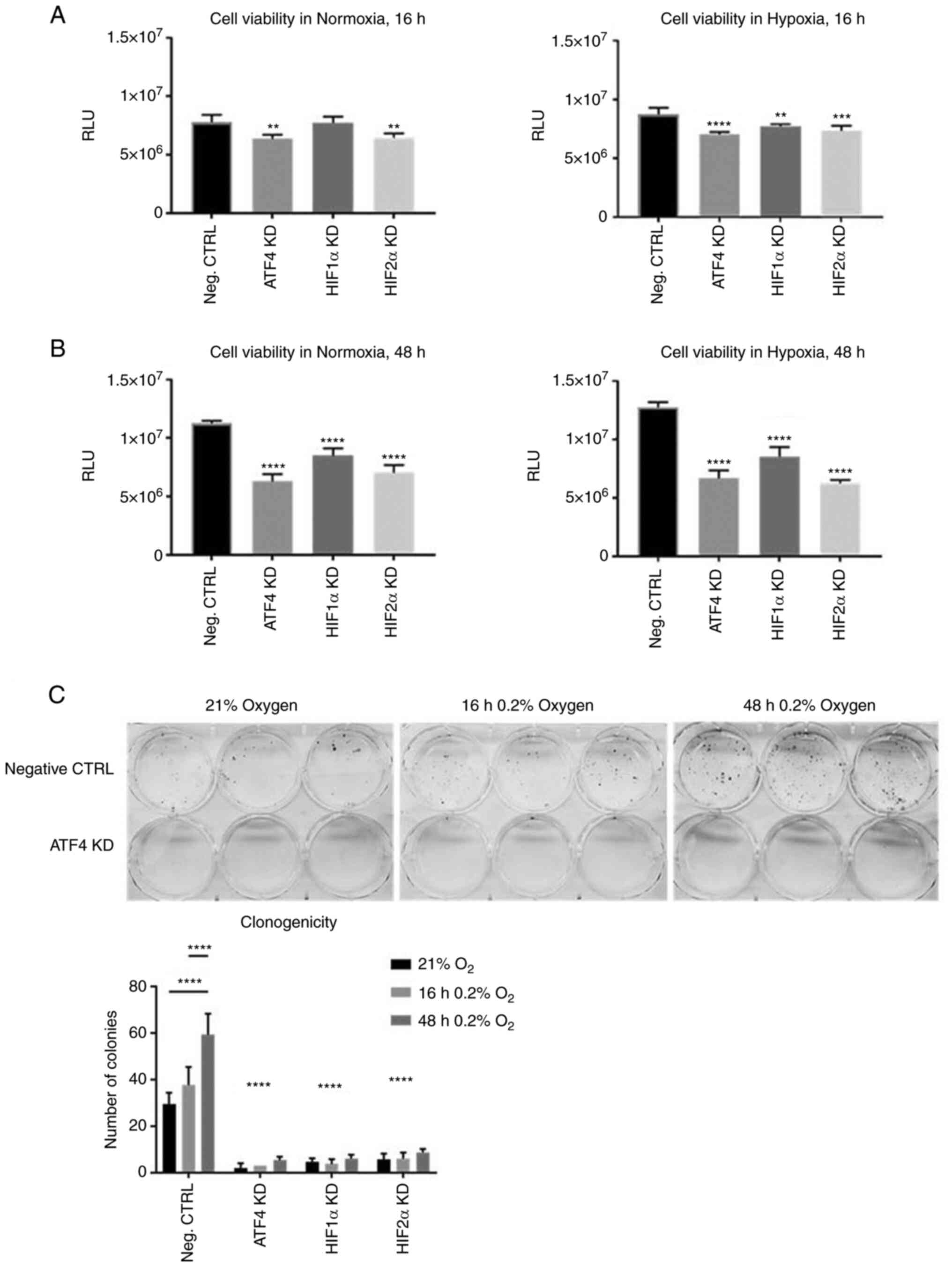
ATF4 inhibition decreases cell
viability and colony formation
Considering that ATF4 knockdown promoted cell
migration in chronic hypoxia, the effects of ATF4 knockdown on cell
viability and clonogenicity were next determined by conducting cell
viability assays and colony formation assays. PANC-1 cells were
transiently transfected and seeded into 96-well plates. The cells
were incubated in normoxia for 24 h, and then placed in either
normoxia or hypoxia for 16 or 48 h. A CellTiterGlo Luminescent
assay was used to measure ATP. After ATF4 knockdown, cells in
normoxia and hypoxia for 16 and 48 h all showed significant
decrease in cell viability compared with the PANC-1 cells with no
knockdowns (P<0.05; Fig. 7A and
B). HIF1α knockdown in normoxia did not affect cell viability
but did in cells in acute hypoxia (Fig. 7A). HIF1α and HIF2α knockdowns
significantly decreased cell viability (P<0.05) in both normoxia
and chronic hypoxia (Fig. 7B) and
there were no significant differences in cell viability between
normoxic and chronically hypoxic cells in each treatment group
(Fig. S2). The colony formation
assays showed significantly less colonies after ATF4 knockdown
(P<0.0001; Fig. 7C), as well as
after HIF1α and HIF2α knockdown (P<0.0001; Figs. 7C and S3). The negative control group cells
that were exposed to chronic hypoxia had significantly more
colonies compared with the acute hypoxia cells and normoxic cells
(P<0.05; Fig. 7C). Overall,
ATF4 inhibition significantly decreases cell viability and colony
formation abilities in PANC-1 cells in both normoxia and chronic
hypoxia (26).
Transforming growth factor beta (TGF-β) has been
shown to be prevalent in tumors and is secreted by
cancer-associated fibroblasts to supplement cancer cells and
promote growth. One proposed pathway that TGF-β promotes cancer
survival and growth is by inducing ATF4 expression and activity
(26). However, the effect that
TGF-β may have on PDAC cells in hypoxia has not yet been studied.
To understand if TGF-β has the same function in hypoxic PDAC,
PANC-1 wells were treated with TGF-β in acute and chronic hypoxia.
Western blot analysis revealed that TGF-β did not induce ATF4
expression in normoxic cells (Fig.
S4A). ATF4 was upregulated in 16 h of hypoxic exposure, but
there was little ATF4 expression detected in cells exposed to
chronic hypoxia, despite TGF-β treatment (Fig. S4A). The colony formation assay
used to assess clonogenicity of the cells revealed that TGF-β did
improve clonogenicity in cells exposed to acute hypoxia, but not
chronic hypoxia, compared with normoxic cells (Fig. S4B). There were minimal colonies
formed in cells with ATF4 knockdown.
HIF1α, HIF2α and ATF4 expression
increases in healthy WS1 skin fibroblast cells but not in Mia-PaCa2
PDAC cell line
Next, it was investigated how ATF4 and HIFs
regulation in PANC-1 PDAC cells compares to another PDAC, MiaPaCa2
and to non-cancerous WS1 fibroblasts. To determine whether ATF4 is
expressed in Mia-PaCa2 cells in 21 and 0.2% oxygen conditions,
cells were incubated in normoxia and hypoxia for 24 h. ATF4 mRNA
levels in Mia-PaCa2 cells increased in hypoxia by 1.1±0.1-fold
(P<0.1) and protein levels in Mia-PaCa2 were not detectable in
either normoxia or hypoxia (Fig. 8A
and B). In PANC-1 cells, however, ATF4 protein expression
increased in hypoxia compared with normoxia (Fig. 8B).
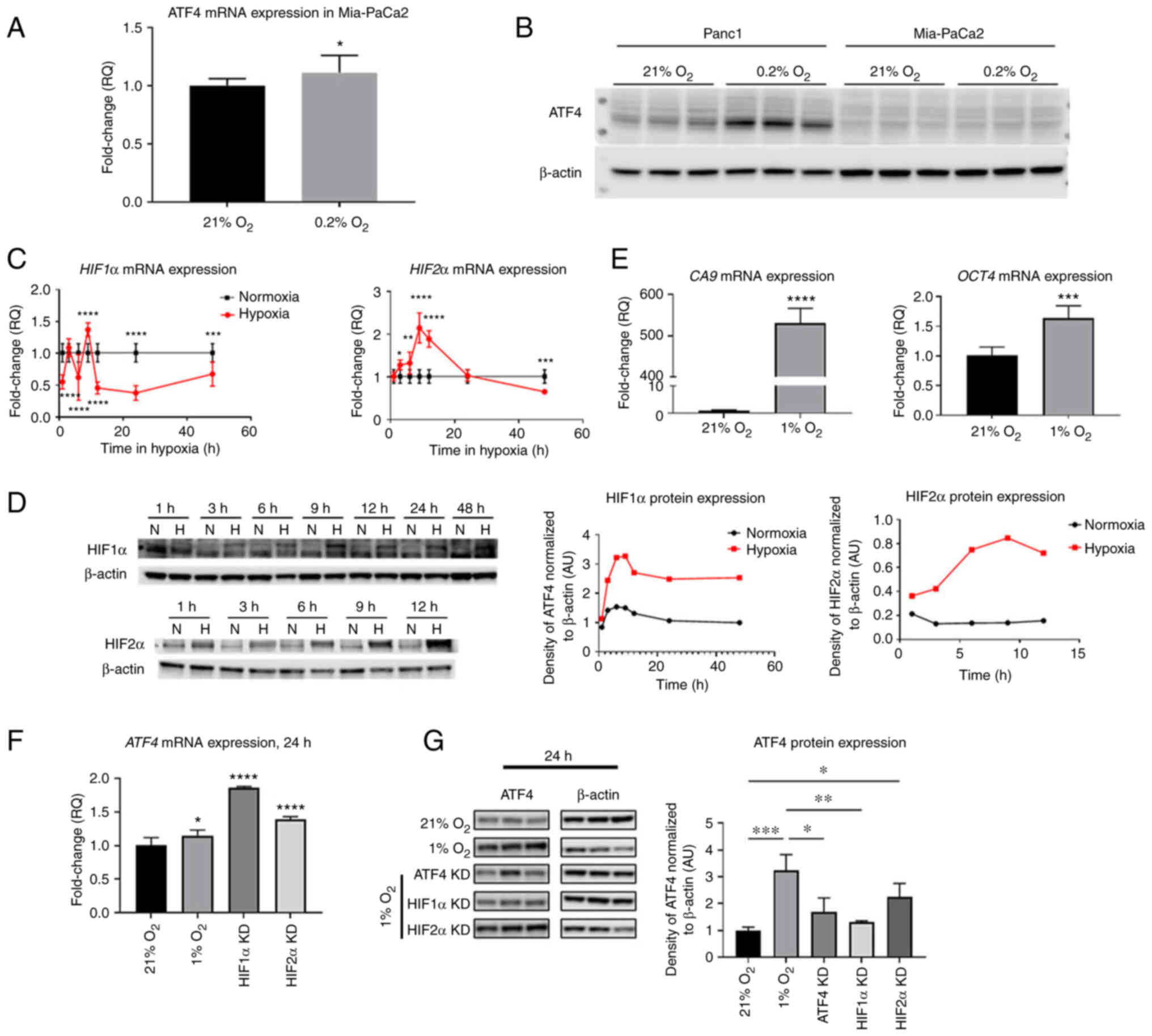 | Figure 8.HIF1α, HIF2α and ATF4 mRNA levels and
HIF target genes change over time in hypoxia in WS1 healthy cells
but ATF4 was not detectable in hypoxic Mia-PaCa2 cells. PANC-1 and
Mia-PaCa2 cells were placed in either normoxia (21% oxygen) or
hypoxia (0.2% oxygen) for 24 h. (A) ATF4 mRNA levels in Mia-PaCa2
cells were determined by RT-qPCR, and (B) ATF4 protein levels were
determined in Mia-PaCa2 cells using western blot analysis. WS1
fibroblast cells were treated with hypoxia (1% oxygen) or normoxia
(21% oxygen) for durations from 1 to 48 h. (C) RT-qPCR and (D)
western blot analyses were conducted to determine HIF1α and HIF2α
mRNA and protein levels, respectively. (E) CA9 and Oct4 mRNA levels
were also determined by RT-qPCR. (F and G) ATF4 mRNA and protein
levels were also measured using (F) RT-qPCR and (G) western blot
analysis, respectively. Fold changes for RT-qPCR are shown relative
to normoxic cells. Error bars represent the mean ± SD for n=9. The
fold changes were analyzed using unpaired Student's t-test.
*P<0.05, **P<0.01, ***P<0.001 and ****P<0.0001. HIF,
hypoxia-inducible factor; ATF4, activating transcription factor 4;
RT-qPCR, reverse transcription-quantitative PCR; CA9, carbonic
anhydrase IX; Oct4, octamer-binding transcription factor 4; KD,
knockdown. |
To determine HIF1α, HIF2α and ATF4 expression in
hypoxia in healthy cells, WS1 skin fibroblast cells were incubated
in normoxia (21% oxygen) or hypoxia (1% oxygen) from 1 to 48 h of
treatment. After 1 h of hypoxic exposure, HIF1α mRNA significantly
decreased 0.6±0.1-fold (P<0.0001). At 3 h, there was no
significant difference of HIF1α mRNA levels in hypoxia compared
with normoxia (1.1±0.1-fold). After 6 h of hypoxic exposure, mRNA
levels decreased 0.6±0.4-fold, and they significantly increased
after 9 h of exposure by 1.4±0.1-fold (P<0.0001). The HIF1α mRNA
levels became significantly lower than normoxic levels from 12 h of
hypoxia by 0.5±0.1-fold (P<0.0001) to 48 h of hypoxia
(0.7±0.2-fold) (P<0.001; Fig.
8C). HIF2α mRNA expression steadily increased from 3 h of
hypoxia and reached a peak at 9 h of hypoxia by 2.1±0.4-fold. The
mRNA levels decreased continually from 12 h of exposure to 48 h, at
which point, the mRNA levels were 0.7±0.1-fold lower than normoxic
levels (Fig. 8C). It was confirmed
that HIF1α and HIF2α protein levels were increased in hypoxia by
western blotting, which demonstrated steady increases of HIF
protein expression in all time points compared with normoxic levels
(Fig. 8D).
The expression of HIF transcriptional target genes
CA9 and Oct4 was also measured. CA9 is a HIF1α-specific target gene
while Oct4 is a HIF2α-specific target gene. After 24 h of hypoxic
exposure, results showed that CA9 mRNA levels significantly
increased by 530.6±33.7-fold compared with normoxia while Oct4 mRNA
levels were significantly higher in hypoxia by 1.6±0.2-fold
compared with normoxic levels (Fig.
8E). ATF4 expression was also analyzed in normoxia, hypoxia and
with HIF1α and HIF2α knocked down. ATF4 mRNA levels were
significantly higher after 24 h in hypoxia than in normoxia by
1.1±0.08-fold (P<0.05; Fig.
8F). Upon HIF1α and HIF2α knockdowns, ATF4 mRNA expression
significantly increased by 1.9±0.01 and 1.4±0.04-fold, respectively
(Fig. 8F). Western blot analysis
revealed that hypoxia significantly increased ATF4 protein
expression in healthy cells by 3.2±0.6-fold compared with normoxia.
While ATF4 mRNA increased upon HIF1α and HIF2α knockdown, ATF4
protein expression significantly decreased upon HIF1α knockdown in
hypoxia (1.3±0.05-fold) compared with cells in hypoxia without
knockdowns (P<0.01; Fig. 8G).
There was no significant difference in cells with HIF2α knockdown
compared with hypoxia-treated cells without knockdowns (Fig. 8G).
Discussion
Pancreatic cancer cells exist in a hypoxic
environment, and yet, most research conducted is performed under
normoxic conditions or in acute hypoxia, which is less
pathophysiologically relevant. Despite the chronic hypoxic
condition observed in PDAC in patients, the function of ATF4 during
chronic hypoxia has remained largely unstudied in pancreatic
cancers. In the present study, evidence was provided that ATF4
regulates HIF1α and HIF2α expression and significantly affects cell
migration, cell viability and colony formation in chronically
hypoxic pancreatic cancer cells.
Loss-of-function experiments were conducted to
ascertain the relationship between ATF4 and HIFs in PANC-1 cells in
chronic hypoxia. HIF1α and HIF2α are regulators of cellular hypoxic
response (17). The two isoforms
have unique roles in mediating cell survival in hypoxia (32). Generally, HIF1α activates genes
that promote changes to cell metabolism while HIF2α activates genes
that promote processes such as stemness and extracellular signaling
and remodeling (31,34,35).
HIF1α and HIF2α are also regulated in a type of ‘switch’ in which
when HIF1α expression decreases or increases, HIF2α expression
tends to change inversely. This ‘HIF switch’ is critical to
regulate the different response pathways as the time in hypoxia
increases (32). The switch is
largely attributed to microRNAs that control HIF1α and HIF2α levels
by preventing HIF mRNA translation, reducing the amount of protein
synthesized (32).
Hypoxia-associated factors (HAFs) also downregulate HIF1α by
inducing ubiquitination and protein degradation while upregulating
HIF2α activation, including in a variety of different cancers such
as pancreatic cancer and renal clear cell carcinoma (31,36).
The present data revealed, for the first time to the best of our
knowledge, that ATF4 functions as a HIF regulator by decreasing
HIF2α and increasing HIF1α expression in chronic hypoxia. ATF4
regulates the HIFs inversely to microRNAs and HAFs that increase
HIF2α and decrease HIF1α in chronic hypoxia (31,37,38).
This may possibly prevent a complete shutdown of HIF1α specific
functions by counteracting the negative regulation of HIF1α by
microRNAs and HAFs. While HIF2α is commonly associated with chronic
hypoxia and HIF1α with acute hypoxia, it was demonstrated that
HIF1α expression remains essential in chronic hypoxia for
maintaining certain cell survival pathways, such as metabolic
reprogramming (31,35,39).
ATF4 may function to promote HIF1α expression to maintain critical
these pathways that are activated by only HIF1α and prevent a
complete inhibition of HIF1α functions and improve hypoxic
tolerance in chronic hypoxia. Whether ATF4 affects HIFs in chronic
hypoxia directly or indirectly remains to be understood. Further
studies are planned to analyze downstream ATF4 transcriptional
target genes to determine potential mechanisms used to affect HIF
expression.
Our data also showed that ATF4 promotes colony
formation and cell viability in chronically hypoxic PDAC cells
(26,40). In chronic hypoxia, ATF4 knockdown
significantly decreased colony formation and cell viability. Hence,
ATF4 acts as a pro-survival factor for PDAC cells under conditions
of chronic hypoxia and may be a viable target for PDAC therapies.
ATF4 has been previously shown to promote colony formation and cell
viability by regulating the Wnt/β-catenin pathway and the
PERK/eIF2α/ATF4 axis in different cancers, including lung cancer
and glioblastoma cells (41,42).
It is plausible that ATF4 affects colony formation and viability of
PDAC cells in chronic hypoxia the same way it does in normoxia.
However, these functions of ATF4 may be more robust in chronic
hypoxia compared with normoxia. Targeting ATF4 may be a therapeutic
strategy for treating hypoxic PDAC which is otherwise difficult to
treat.
ATF4 roles in cell migration in hypoxic PDAC cells
were also determined. ATF4 stimulates cell migration and metastasis
in breast cancer cells, as well as in PDAC (26,40).
However, it was demonstrated that under chronic hypoxia, ATF4
inhibition increased cell migration in PDAC cells, evidence that
rather than promoting cell migration as observed in normoxia, ATF4
negatively regulates cell migration in chronic hypoxia. While it
has been previously revealed in breast cancer that ATF4 stimulates
cell migration, these studies were conducted under normoxic
conditions or in oxygen levels that are likely markedly higher than
in patients (43,44). Previous studies showed that breast
cancers typically exhibit oxygen levels of 0.2% oxygen or less,
similar levels to that of PDAC (29,40,45,46).
Furthermore, these experiments did not exceed 24 h of hypoxic
exposure. There may also be differences in the physiology of the
two cancers that can account for this discrepancy, which remain to
be explored. Another study showed that ATF4 promotes cell migration
in PDAC (26). However, this was
not conducted in hypoxia, acute or chronic, and our data indicated
that ATF4 functions differently in hypoxia than in normoxia. This
suggested that ATF4 functions change or are different depending on
the presence or absence of oxygen and the duration of hypoxia. The
present results not only indicated a negative regulatory role of
ATF4 on cell migration, but they also emphasized the importance of
conducting pancreatic cancer research in pathophysiologically
relevant conditions, particularly chronic hypoxia. The discovery of
the ‘HIF switch’, particularly the dominance of HIF1α in acute
hypoxia and HIF2α in chronic hypoxia, has illuminated the
importance of distinguishing acute and chronic hypoxia in cancers
(31,32,37,47).
However, the same principles should be applied when investigating
other stress response pathways in hypoxia, including ATF4 in the
UPR pathway. As it was revealed, the role of ATF4 in acute and
chronic hypoxia is not the same, indicative of a mechanism that
facilitates this switch in function that is yet to be found.
It is unknown whether ATF4 directly interacts with
HIF1α and HIF2α mRNA or protein to regulate expression or
indirectly by way of another downstream factor that functions as a
mediator between ATF4 and HIFs. It was demonstrated that HIF1α,
HIF2α and ATF4 are all expressed at the protein level in hypoxia in
healthy cells and in PANC-1 cells. Somewhat surprisingly, our data
showed that hypoxia does not induce ATF4 expression in Mia-PaCa2
cells compared with PANC-1 cells which exhibit a robust increase in
ATF4 protein expression. This can be due to several reasons,
including the different phenotypes exhibited by Mia-PaCa2 cells and
PANC-1 cells. For instance, low expression of neural cell adhesion
molecule CD56, a protein found in PANC-1 cells but not Mia-PaCa2
cells, has been revealed to result in low levels of ER stress
markers, including ATF4 (48,49).
The lack of CD56 in Mia-PaCa2 cells may contribute to the low
levels of ATF4, despite hypoxic exposure. Another protein that may
contribute to the low ATF4 levels in Mia-PaCa2 but high induction
in PANC-1 cells is E-cadherin, a cell-to-cell adhesion molecule.
High E-cadherin levels, as observed in Mia-PaCa2 cells but not in
PANC-1 cells, are associated with low expression of ATF4 (43). These types of differing expression
profiles observed in the two cell lines may contribute to the low
ATF4 expression identified in Mia-PaCa2 cells and high levels in
PANC-1 cells. Notably, PANC-1 have been described as more
aggressive and with greater metastasizing potential (48). The ATF4 related mechanism, found
only in the PANC-1 cells and which is notably quite different than
healthy WS-1 cells, is a candidate for mediating such a phenotype
and therefore represents a potential biomarker for metastatic
potential, though further studies are required to explore this
hypothesis.
One major finding from the present study was that in
chronically hypoxic PANC-1 cells, ATF4 was revealed to affect HIF1α
expression while HIFs did not affect ATF4. This, however, was not
observed in healthy cells. As observed in PANC-1 cells, ATF4 mRNA
expression significantly increased when HIF1α was knocked down.
However, in healthy cells, HIF1α knockdown caused ATF4 protein
expression levels to be significantly lower than the hypoxic cells
expressing HIF1α. This result is in contrast to what was observed
in PANC-1 cells, in which HIF1α knockdown did not change ATF4. The
present findings indicated that in healthy cells, HIF1α may
regulate ATF4 in hypoxia, while in PDAC cells, this regulation
pathway is either inhibited or is negated by other mechanisms that
cause ATF4 overexpression (14,26,50).
One such mechanism is the protein CD56 found in PANC-1 cells that
causes high ATF4 expression (48,49).
Since HIF1α has never been shown to affect ATF4 in hypoxia in
healthy cells, the mechanism in which HIF1α may affect ATF4 protein
expression requires further study. It will also be important to
further validate these findings with other methods in in
vitro PDAC models, such as the use of 3D spheroids, and in
in vivo models which replicate in an improved way the
heterogeneity of the tumor microenvironment observed in patients.
This shall be the focus of our future efforts.
In conclusion, it was demonstrated that ATF4 is
upregulated in chronically hypoxic PDAC cells and that HIF1α
expression is dependent on ATF4 while ATF4 negatively regulates
HIF2α in PDAC cells in chronic hypoxia. It was also demonstrated
that while ATF4 may decrease cell migration in chronic hypoxia, its
inhibition is detrimental to clonogenicity and cell viability in
hypoxia, and therefore, is an attractive target for chronically
hypoxic PDAC. The present study has emphasized the importance of
conducting research in pathophysiologically relevant systems
related to hypoxia. These findings elucidated a novel understanding
of PDAC in which hypoxia causes the UPR pathway to regulate the
hypoxia response pathways, possibly contributing to the aggressive
qualities of PDAC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Institutes of
Health (grant nos. R01NS092671 and R01MH110441), the University of
Miami Sylvester Comprehensive Cancer Center Molecular Therapeutics
Shared Resource (MTSR) and the Jay Weiss Institute for Health
Equity.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
NTC and SPB conceptualized the idea and designed
experiments. NTC conducted experiments, procured and analyzed data
and led manuscript writing and organization. ZM and CHC provided
feedback on data interpretation and experimental design. SW was
involved in planning and executing experiments. NTC and SPB confirm
the authenticity of all raw data. All authors contributed to the
analysis of results and writing of the manuscript. All authors read
and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ATF4
|
activating transcription factor 4
|
|
CA9
|
carbonic anhydrase IX
|
|
HAF
|
HIF activating factor
|
|
HIF1α
|
hypoxia inducible factor 1α
|
|
HIF2α
|
hypoxia inducible factor 2α
|
|
Oct4
|
octamer-binding transcription factor
4
|
|
PDAC
|
pancreatic ductal adenocarcinoma
|
|
uORF
|
upstream open reading frame
|
|
UPR
|
unfolded protein response
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hessmann E, Buchholz SM, Demir IE, Singh
SK, Gress TM, Ellenrieder V and Neesse A: Microenvironmental
determinants of pancreatic cancer. Physiol Rev. 100:1707–1751.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Orth M, Metzger P, Gerum S, Mayerle J,
Schneider G, Belka C, Schnurr M and Lauber K: Pancreatic ductal
adenocarcinoma: Biological hallmarks, current status, and future
perspectives of combined modality treatment approaches. Radiat
Oncol. 14:1412019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yuen A and Díaz B: The impact of hypoxia
in pancreatic cancer invasion and metastasis. Hypoxia (Auckl).
2:91–106. 2014.PubMed/NCBI
|
|
5
|
Buttgereit F and Brand MD: A hierarchy of
ATP-consuming processes in mammalian cells. Biochem J. 312:163–167.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fähling M: Surviving hypoxia by modulation
of mRNA translation rate. J Cell Mol Med. 13:2770–2779. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wortel IMN, van der Meer LT, Kilberg MS
and van Leeuwen FN: Surviving stress: Modulation of ATF4-mediated
stress responses in normal and malignant cells. Trends Endocrinol
Metab. 28:794–806. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
B'chir W, Maurin AC, Carraro V, Averous J,
Jousse C, Muranishi Y, Parry L, Stepien G, Fafournoux P and Bruhat
A: The eIF2α/ATF4 pathway is essential for stress-induced autophagy
gene expression. Nucleic Acids Res. 41:7683–7699. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Guo X, Aviles G, Liu Y, Tian R, Unger BA,
Lin YT, Wiita AP, Xu K, Correia MA and Kampmann M: Mitochondrial
stress is relayed to the cytosol by an OMA1-DELE1-HRI pathway.
Nature. 579:427–432. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pathria G, Scott DA, Feng Y, Sang Lee J,
Fujita Y, Zhang G, Sahu AD, Ruppin E, Herlyn M, Osterman AL and
Ronai ZA: Targeting the Warburg effect via LDHA inhibition engages
ATF4 signaling for cancer cell survival. EMBO J. 37:e997352018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fernandez MR and Cleveland JL: ATF4-amino
acid circuits: A recipe for resistance in melanoma. EMBO J.
37:e1006002018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chiou SH, Risca VI, Wang GX, Yang D,
Grüner BM, Kathiria AS, Ma RK, Vaka D, Chu P, Kozak M, et al:
BLIMP1 induces transient metastatic heterogeneity in pancreatic
cancer. Cancer Discov. 7:1184–1199. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mesclon F, Lambert-Langlais S, Carraro V,
Parry L, Hainault I, Jousse C, Maurin AC, Bruhat A, Fafournoux P
and Averous J: Decreased ATF4 expression as a mechanism of acquired
resistance to long-term amino acid limitation in cancer cells.
Oncotarget. 8:27440–27453. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Palam LR, Gore J, Craven KE, Wilson JL and
Korc M: Integrated stress response is critical for gemcitabine
resistance in pancreatic ductal adenocarcinoma. Cell Death Dis.
6:e19132015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ait Ghezala H, Jolles B, Salhi S,
Castrillo K, Carpentier W, Cagnard N, Bruhat A, Fafournoux P and
Jean-Jean O: Translation termination efficiency modulates ATF4
response by regulating ATF4 mRNA translation at 5′ short ORFs.
Nucleic Acids Res. 40:9557–9570. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Blais JD, Filipenko V, Bi M, Harding HP,
Ron D, Koumenis C, Wouters BG and Bell JC: Activating transcription
factor 4 is translationally regulated by hypoxic stress. Mol Cell
Biol. 24:7469–7482. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chee NT, Lohse I and Brothers SP:
mRNA-to-protein translation in hypoxia. Mol Cancer. 18:492019.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Logsdon DP, Shah F, Carta F, Supuran CT,
Kamocka M, Jacobsen MH, Sandusky GE, Kelley MR and Fishel ML:
Blocking HIF signaling via novel inhibitors of CA9 and APE1/Ref-1
dramatically affects pancreatic cancer cell survival. Sci Rep.
8:137592018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang S, Zhao L, Wang J, Chen N, Yan J and
Pan X: HIF-2α and Oct4 have synergistic effects on survival and
myocardial repair of very small embryonic-like mesenchymal stem
cells in infarcted hearts. Cell Death Dis. 8:e25482017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Uniacke J, Holterman CE, Lachance G,
Franovic A, Jacob MD, Fabian MR, Payette J, Holcik M, Pause A and
Lee S: An oxygen-regulated switch in the protein synthesis
machinery. Nature. 486:126–129. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ho JJD, Wang M, Audas TE, Kwon D, Carlsson
SK, Timpano S, Evagelou SL, Brothers S, Gonzalgo ML, Krieger JR, et
al: Systemic reprogramming of translation efficiencies on oxygen
stimulus. Cell Rep. 14:1293–1300. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Uniacke J, Perera JK, Lachance G,
Francisco CB and Lee S: Cancer cells exploit eIF4E2-directed
synthesis of hypoxia response proteins to drive tumor progression.
Cancer Res. 74:1379–1389. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rzymski T, Milani M, Pike L, Buffa F,
Mellor HR, Winchester L, Pires I, Hammond E, Ragoussis I and Harris
AL: Regulation of autophagy by ATF4 in response to severe hypoxia.
Oncogene. 29:4424–4435. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Koumenis C and Wouters BG: ‘Translating’
tumor hypoxia: Unfolded protein response (UPR)-dependent and
UPR-independent pathways. Mol Cancer Res. 4:423–436. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Suarez-Arnedo A, Torres Figueroa F,
Clavijo C, Arbeláez P, Cruz JC and Muñoz-Camargo C: An image J
plugin for the high throughput image analysis of in vitro scratch
wound healing assays. PLoS One. 15:e02325652020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wei L, Lin Q, Lu Y, Li G, Huang L, Fu Z,
Chen R and Zhou Q: Cancer-associated fibroblasts-mediated ATF4
expression promotes malignancy and gemcitabine resistance in
pancreatic cancer via the TGF-β1/SMAD2/3 pathway and ABCC1
transactivation. Cell Death Dis. 12:3342021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Koong AC, Mehta VK, Le QT, Fisher GA,
Terris DJ, Brown JM, Bastidas AJ and Vierra M: Pancreatic tumors
show high levels of hypoxia. Int J Radiat Oncol Biol Phys.
48:919–922. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Graffman S, Björk P, Ederoth P and Ihse I:
Polarographic pO2 measurements of intra-abdominal adenocarcinoma in
connection with intraoperative radiotherapy before and after change
of oxygen concentration of anaesthetic gases. Acta Oncol.
40:105–107. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
McKeown SR: Defining normoxia, physoxia
and hypoxia in tumours-implications for treatment response. Br J
Radiol. 87:201306762014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Saxena K and Jolly MK: Acute vs chronic vs
cyclic hypoxia: Their differential dynamics, molecular mechanisms,
and effects on tumor progression. Biomolecules. 9:3392019.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Koh MY, Lemos R Jr, Liu X and Powis G: The
hypoxia-associated factor switches cells from HIF-1α- to
HIF-2α-dependent signaling promoting stem cell characteristics,
aggressive tumor growth and invasion. Cancer Res. 71:4015–4027.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Koh MY and Powis G: Passing the baton: The
HIF switch. Trends Biochem Sci. 37:364–372. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Onnis B, Rapisarda A and Melillo G:
Development of HIF-1 inhibitors for cancer therapy. J Cell Mol Med.
13:2780–2786. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Downes NL, Laham-Karam N, Kaikkonen MU and
Ylä-Herttuala S: Differential but complementary HIF1α and HIF2α
transcriptional regulation. Mol Ther. 26:1735–1745. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Holmquist-Mengelbier L, Fredlund E,
Löfstedt T, Noguera R, Navarro S, Nilsson H, Pietras A,
Vallon-Christersson J, Borg A, Gradin K, et al: Recruitment of
HIF-1alpha and HIF-2alpha to common target genes is differentially
regulated in neuroblastoma: HIF-2alpha promotes an aggressive
phenotype. Cancer Cell. 10:413–423. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Koh MY, Darnay BG and Powis G:
Hypoxia-associated factor, a novel E3-ubiquitin ligase, binds and
ubiquitinates hypoxia-inducible factor 1alpha, leading to its
oxygen-independent degradation. Mol Cell Biol. 28:7081–7095. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Serocki M, Bartoszewska S,
Janaszak-Jasiecka A, Ochocka RJ, Collawn JF and Bartoszewski R:
miRNAs regulate the HIF switch during hypoxia: A novel therapeutic
target. Angiogenesis. 21:183–202. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lu ZJ, Lu LG, Tao KZ, Chen DF, Xia Q, Weng
JJ, Zhu F, Wang XP and Zheng P: MicroRNA-185 suppresses growth and
invasion of colon cancer cells through inhibition of the
hypoxia-inducible factor-2α pathway in vitro and in
vivo. Mol Med Rep. 10:2401–2408. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Uchida T, Rossignol F, Matthay MA, Mounier
R, Couette S, Clottes E and Clerici C: Prolonged hypoxia
differentially regulates hypoxia-inducible factor (HIF)-1alpha and
HIF-2alpha expression in lung epithelial cells: Implication of
natural antisense HIF-1alpha. J Biol Chem. 279:14871–14878. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nagelkerke A, Bussink J, Mujcic H, Wouters
BG, Lehmann S, Sweep FC and Span PN: Hypoxia stimulates migration
of breast cancer cells via the PERK/ATF4/LAMP3-arm of the unfolded
protein response. Breast Cancer Res. 15:R22013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Du J, Liu H, Mao X, Qin Y and Fan C: ATF4
promotes lung cancer cell proliferation and invasion partially
through regulating Wnt/β-catenin signaling. Int J Med Sci.
18:1442–1448. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Dadey DYA, Kapoor V, Khudanyan A, Thotala
D and Hallahan DE: PERK regulates glioblastoma sensitivity to ER
stress although promoting radiation resistance. Mol Cancer Res.
16:1447–1453. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zeng P, Sun S, Li R, Xiao ZX and Chen H:
HER2 upregulates ATF4 to promote cell migration via activation of
ZEB1 and downregulation of E-cadherin. Int J Mol Sci. 20:22232019.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
González-González A, Muñoz-Muela E,
Marchal JA, Cara FE, Molina MP, Cruz-Lozano M, Jiménez G, Verma A,
Ramírez A, Qian W, et al: Activating transcription factor 4
modulates TGFβ-induced aggressiveness in triple-negative breast
cancer via SMAD2/3/4 and mTORC2 signaling. Clin Cancer Res.
24:5697–5709. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Rundqvist H and Johnson RS: Tumour
oxygenation: Implications for breast cancer prognosis. J Intern
Med. 274:105–112. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Vaupel P, Schlenger K, Knoop C and Höckel
M: Oxygenation of human tumors: Evaluation of tissue oxygen
distribution in breast cancers by computerized O2 tension
measurements. Cancer Res. 51:3316–3322. 1991.PubMed/NCBI
|
|
47
|
Lando D, Peet DJ, Whelan DA, Gorman JJ and
Whitelaw ML: Asparagine hydroxylation of the HIF transactivation
domain a hypoxic switch. Science. 295:858–861. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Gradiz R, Silva HC, Carvalho L, Botelho MF
and Mota-Pinto A: MIA PaCa-2 and PANC-1-pancreas ductal
adenocarcinoma cell lines with neuroendocrine differentiation and
somatostatin receptors. Sci Rep. 6:216482016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yoshida T, Ri M, Kinoshita S, Narita T,
Totani H, Ashour R, Ito A, Kusumoto S, Ishida T, Komatsu H and Iida
S: Low expression of neural cell adhesion molecule, CD56, is
associated with low efficacy of bortezomib plus dexamethasone
therapy in multiple myeloma. PLoS One. 13:e01967802018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Garcia-Carbonero N, Li W, Cabeza-Morales
M, Martinez-Useros J and Garcia-Foncillas J: New hope for
pancreatic ductal adenocarcinoma treatment targeting endoplasmic
reticulum stress response: A systematic review. Int J Mol Sci.
19:24682018. View Article : Google Scholar : PubMed/NCBI
|