Introduction
Histone deacetylase (HDAC) inhibitors are promising
anticancer agents for various types of cancer. Recently, an
increasing number of structurally diverse HDAC inhibitors,
including suberoylanilide hydroxamic acid (SAHA), trapoxin and
sodium butyrate, have been reported to be valuable anticancer
agents (1–3). HDAC inhibitors belong to a
heterogeneous class of compounds, including derivatives of
short-chain fatty acids, hydroxamic acids, cyclic tetrapeptides and
benzamides (4,5). SAHA is a commonly used potent class I
and II HDAC inhibitor. These HDAC inhibitors regulate the
expression of target genes involved in cancer cell growth and
proliferation (6,7), morphological transformation of
oncogenic cells (8), and inhibition
of cancer cell invasion and migration (9,10). The
potential use of HDAC inhibitors in cancer chemotherapy is
currently being investigated in clinical trials, and well-tolerated
safety profiles of these drugs have been demonstrated in clinical
studies (11).
Breast cancer is the most serious health problem in
women worldwide (12). After
surgery, chemotherapy with anticancer agents is used for breast
cancer treatment. HDAC inhibitors exhibit potent anti-proliferative
effects in vitro and in vivo and interfere with
estrogen signaling, regulating estrogen receptor (ER)α and ERβ
expression and function (13). HDAC
inhibitors induce apoptosis in both ERα-positive and -negative
breast tumor cells (14,15). Failure of breast cancer treatment is
generally attributed to drug resistance and toxicity. Therefore,
effective and safe anticancer agents are required. Medicinal plants
have gained attention as effective and value-added sources of
anticancer agents (6).
Previously, naturally occurring dietary compounds
that inhibit HDAC activity have been shown to exhibit a potent
sensitization effect on cancer cells, rendering them susceptible to
apoptosis by various anticancer drugs (16,17).
In particular, triterpenoids, present in most plants, are mainly
used in the medical field (16,17).
Previously, certain triterpenoids have been reported to have
immunomodulatory and anticancer effects (18). Astilbe chinensis (A.
chinensis) (Maxim.) Franch. & Sav. is a perennial herb of
the family Saxifragaceae that grows in humid areas of
mountains (19). A.
chinensis is known to cure chronic bronchitis, arthralgia,
inflammation-induced pain, headaches and stomachalgia. In addition,
previous studies have reported that triterpenoids isolated from
A. chinensis have in vitro cytotoxic effects on
various cell lines (20,21). However, the detailed anticancer
mechanisms of the isolated compounds remain unknown.
In the present study,
3β,6β-dihydroxyurs-12-en-27-oic acid (ACT-3) was isolated from
A. chinensis and its anticancer effect was investigated in
human breast cancer cells. In the present study, the anticancer
activity and cellular mechanism of ACT-3 was established. The
results suggested that ACT-3 has the potential to replace or be
used in combination with existing anticancer agents for breast
cancer therapy.
Materials and methods
Chemicals and reagents
SAHA was purchased from Sigma-Aldrich; Merck KGaA.
Dulbecco's modified Eagle's medium (DMEM), fetal bovine serum
(FBS), and cell culture supplements were obtained from Gibco;
Thermo Fisher Scientific, Inc. Primary antibodies against
acetyl-Histone H3 (cat. no. 8848), HDAC1 (cat. no. 34589), HDAC3
(cat. no. 3949), B-cell lymphoma-2 (Bcl-2; cat. no. 15071), Bcl-2
associated X protein (Bax; cat no. 5023), β-actin (cat no. 3700),
light chain 3 (LC3; cat no. 3868), beclin-1 (cat. no. 3738), Atg5
(cat. no. 12994), Atg7 (cat. no. 8558), cleaved-poly (ADP-ribose)
polymerase (c-PARP; cat. no. 9541), caspase-3 (cat. no. 9662),
caspase-8 (cat. no. 9746), caspase-9 (cat. no. 9508),
cyclin-dependent kinase 2 (CDK2; cat. no. 2546), CDK4 (cat. no.
12790), p53 (cat. no. 2527), p21 (cat. no. 2947), phosphoinositide
3-kinase (PI3K; cat. no. 4255), phosphorylated (p)-PI3K (cat. no.
4228), Akt (cat. no. 9272), p-Akt (cat. no. 9271), ERK1/2 (cat. no.
9102), p-ERK1/2 (cat. no 9101), mTOR (cat. no. 2972), p-mTOR (cat.
no. 2971) and MMP2 (cat. no. 4022), MMP9 (cat. no. 3852), TIMP1
(cat. no. 8946) and TIMP2 (cat. no. 5738) were purchased from Cell
Signaling Technology, Inc. Primary antibodies against cyclin A
(cat. no. sc-271682), cyclin D1 (cat. no. sc-8396), p27 (cat. no.
sc-53871) and horseradish peroxidase-conjugated secondary
antibodies (anti-mouse IgG; cat. no. sc-516102 and anti-rabbit IgG;
cat. no. sc-2357) were purchased from Santa Cruz Biotechnology,
Inc. Immunoblots for total H1 were performed with an anti-H1
antibody (cat. no. ab17584; Abcam). All drugs were dissolved in
dimethyl sulfoxide (DMSO) and stored at −20°C until use. The final
concentration of DMSO was less than 0.1% (v/v).
Plant material
Aerial parts of A. chinensis were collected
in September 2018 at Hambaek mountain (Gangwon, Republic of Korea).
A voucher specimen was deposited in the School of Pharmacy of
Sungkyunkwan University (SKKU-Ph-18-021).
Extraction and isolation of ACT-3 from
A. chinensis
The dried aerial parts of A. chinensis (300
g) were extracted twice with methanol (MeOH, 5 l) at room
temperature for a day, and once at 60°C for 5 h. The extracts were
then combined and concentrated under reduced pressure at 40°C. MeOH
extract (40.56 g) was suspended in 500 ml distilled water and
partitioned with three organic solvents to give dichloromethane
(CH2Cl2; 8.75 g), ethyl acetate (EtOAc; 5.29
g), n-butanol (n-BuOH; 8.14 g), and water (18.50 g) fractions. The
dichloromethane fraction was subjected to column chromatographic
separation using silica gel with stepwise elution of hexane-EtOAc
(50:1-1:1), hexane-EtOAc-MeOH (10:10:0.5 to 10:10:4) and
CH2Cl2-MeOH (1:1) to yield 13 fractions
(MC-1-MC-13). MC-9 fraction was further chromatographed twice using
silica gel (hexane-EtOAc=7:1 and 5:1) to obtain ACT-3. The chemical
structure of ACT-3 was identified from spectral data obtained NMR
experiments that were performed on Bruker AvanceCore 400
spectrometer and Bruker AVANCE III 700 spectrometer. To determine
the purity of ACT-3, high-performance liquid chromatography (HPLC)
analysis was carried out on a reversed-phase (RP) C18
column and a UV detector using gradient elution. HPLC analysis was
performed using a Knauer HPLC system consisting of a Manager 5000,
two Pumps 1,000, a UV Detector 2500, and a Kinetex (Phenomenex)
5-µm C18 100 Å column (150×4.6 mm). The eluent consisted of (A)
acetonitrile and (B) water. The gradient profile was as follows:
0–3 min, isocratic elution with 43% A in B; 3–20 min, linear change
from 43–80% A in B; 20–30 min, linear change from 80–100% A in B.
The flow rate and column oven temperature were set to 1.0 ml/min
and 40°C, respectively. The UV absorption was measured at a
wavelength of 210 nm.
HDAC enzyme activity assay
To assess the HDAC inhibitory potency of ACT-3,
commercially available HeLa cell nuclear extracts were treated with
ACT-3 (2, 4 and 8 µM). Total HDAC activity was measured using the
SensoLyte 520 fluorometric HDAC Activity Assay kit (AnaSpec),
according to the manufacturer's instructions. Suramin (10 and 20
µM) and SAHA (5 µM) were used as reference compounds. Briefly, HDAC
enzyme was incubated with the vehicle or various concentrations of
suramin and SAHA at 37°C for 30 min in the presence of an HDAC
fluorometric substrate (22). The
HDAC assay developer (which produces a fluorophore in the reaction
mixture) was added, and fluorescence was measured using VICTOR 3
(Perkin Elmer, Inc.) with excitation at 490 nm and emission at 520
nm. The activities were calculated using GraphPad Prism (GraphPad
Software, Inc.).
Cytotoxicity assay
Human breast cancer MCF-7 cells were purchased from
the American Type Culture Collection. MCF-7 cells were cultured in
DMEM supplemented with 10% FBS, streptomycin (100 µg/ml) and
penicillin (100 U/ml). Cells were maintained as monolayers and
incubated at 37°C in a humidified atmosphere containing 5%
CO2. Cytotoxicity was assessed using
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetra-zolium bromide
(MTT; 5 mg/ml; Sigma-Aldrich). Cells were seeded into a 96-well
plate at a density of 7×103 cells/well. After incubation
at 37°C for 24 h, cells were treated with ACT-3 (3.125–50 µM) and
SAHA (5 µM) for 48 h. After incubation, 15 µl of the MTT reagent
was loaded into each well, and the plates were incubated at 37°C
for 4 h in the dark. The supernatant of each well was aspirated and
formazan crystals were dissolved in 100 µl DMSO at 37°C for 15 min
with gentle agitation. A VERSA MAX Microplate Reader (Molecular
Devices Corp.) was used to measure the absorbance of each well at
540 nm wavelength. Three independent experiments were performed for
each condition. The median inhibitory concentration
(IC50) values were calculated from the sigmoidal
concentration-response using the GraphPad Prism software (version
5.0 for Windows) statistical software package (GraphPad Software,
Inc.).
Colony formation assay
The colony formation assay was performed as
previously described (23). Cells
(1,000 cells per well) seeded in a six-well plate were cultured for
10 days and treated with various concentrations of either ACT-3 or
SAHA for 14 days until the appearance of colonies. The culture
medium was replaced with fresh medium every two days. Cells were
fixed with 4% paraformaldehyde at room temperature for 30 min,
followed by staining with 0.05% crystal violet, and incubated at
37°C for 15 min. Colonies containing more than 20 cells were
counted.
Cell cycle analysis
The cells were treated with ACT-3 (2, 4 and 8 µM)
and SAHA (5 µM) for 48 h. Suspended or adhered cells were harvested
separately. The cells (1×106) were washed with 1% bovine
serum albumin (Sigma-Aldrich; Merck KGaA), fixed in chilled 95%
ethanol, and stained with cold propidium iodide (PI) solution (10
µg/ml PI and 100 µg/ml RNase in phosphate-buffered saline (PBS) in
the dark for 30 min at room temperature. Data acquisition and
analysis were performed using a flow cytometry system (BD Accuri
C6; Cytometers, Inc.).
Evaluation of apoptosis
The Annexin V-FITC binding assay was performed using
Annexin V-FITC detection kit I (BD Biosciences), according to the
manufacturer's instructions. The cells were treated with ACT-3 and
SAHA for 48 h. Cells were counted after trypsinization and washed
twice with cold PBS. The cell pellet was resuspended in 100 µl
binding buffer at a density of 1×103 cells/ml and
incubated with 5 µl FITC-conjugated Annexin-V and 5 µl PI for 15
min at room temperature in the dark. A total of 400 µl of 1X
binding buffer were added to each sample tube, and the samples were
immediately analyzed via flow cytometry (Accuri Cytometers,
Inc.).
Western blot analysis
Cells were treated with ACT-3 and SAHA for 48 h,
harvested via trypsinization, and washed twice with cold PBS. For
total protein isolation, the cells were suspended in PRO-PREP
protein extract solution (Intron Biotechnology, Inc.). Protein
concentrations were measured using a protein assay kit (cat. no.
5000002; Bio-Rad Laboratories, Inc.), according to the
manufacturer's instructions. The cell extract with 20 µg protein
was loaded onto 6–15% sodium dodecyl sulfate-polyacrylamide gels.
After electrophoresis, proteins were transferred onto
polyvinylidene difluoride (PVDF) membranes (Millipore Sigma). The
membranes were incubated for 1 h in TNA (10 mM Tris-Cl, pH-7.6, 100
mM NaCl, and 0.5% Tween 20) buffer containing 5% skim milk.
Membranes were then incubated with primary antibodies (1:200 or
1:250) overnight at 4°C. After washing for 1 h with TNA buffer, the
membranes were incubated with horseradish peroxidase-conjugated
anti-mouse (1:10,000) or anti-rabbit antibodies (1:10,000) for 30
min at room temperature. The blots were developed using an enhanced
chemiluminescence (ECL)-plus kit (Amersham; Cytiva). Using the
ImageJ software (1.52v; National Institutes of Health), the band
intensities were densitometrically quantified.
Acridine orange staining
Cells were cultured in cover-glass bottom dish at a
density of 1×105 cells per dish, cultured for 24 h, and
incubated with the indicated drug in DMEM containing 1% FBS for 48
h. The medium was removed, and the cells were stained with acridine
orange (1 µg/ml) at 37°C for 15 min. After removing the staining
solution, PBS was added to the dish and the cells were examined
under a fluorescence microscope at ×600 magnification (FV10i;
Olympus Corporation).
Wound-healing assay
MCF-7 cells (3×104 cells/well) were
seeded into a 24-well plate in media containing 10% FBS and
incubated at 37°C for 24 h. After incubation, scratches were
introduced using a wound maker (Essen Bioscience) and the indicated
concentrations of ACT-3 (2, 4 and 8 µM) or SAHA (1 µM) were used to
treat the cells for seven days. The culture medium was replaced
with fresh culture medium containing ACT-3 or SAHA every two days.
Then, the cells were washed twice with PBS and the wound images
were obtained daily using IncuCyte ZOOM (Sartorius AG), and scratch
density was determined using the IncuCyte software (v2019B;
Satorius AG).
Statistical analysis
Data are expressed as the mean ± SD of at least
three independent experiments. Statistical analysis was performed
using one-way analysis of variance, followed by Bonferroni's
multiple comparison tests. P<0.05 was considered to indicate a
statistically significant difference. All statistical comparisons
were performed using the SigmaPlot software and Statistical Package
for the Social Sciences v.13 (SPSS, Inc.).
Results
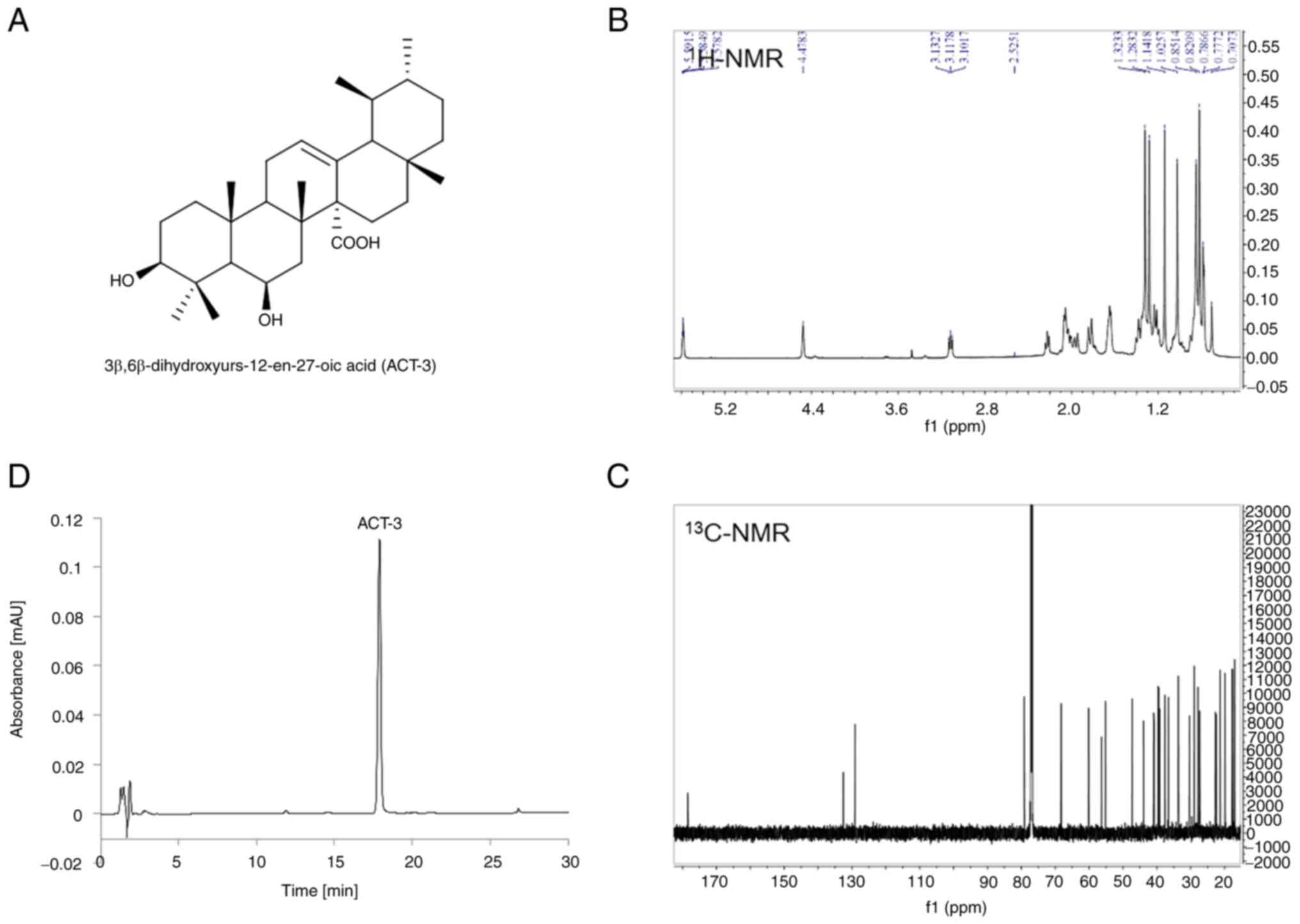
Isolation and characterization of
ACT-3
ACT-3, an ursane-type triterpene, was isolated from
the aerial part of A. chinensis and identified as
3β,6β-dihydroxyurs-12-en-27-oic acid (Fig. 1A) by comparing its spectral data
(Fig. 1B and C) with literature
values (21). The purity of ACT-3
was determined to be 98.3% by HPLC analysis using RP C18
column and UV detector (Fig.
1D).
ACT-3 inhibits the activity and
expression of HDAC in MCF-7 cells
The effect of ACT-3 on total HDAC enzyme activity
was measured. As revealed in Fig.
2A, ACT-3 significantly inhibited the total HDAC enzyme
activity in a concentration-dependent manner. The potency of the
enzyme activity was similar to that of SAHA. In addition, the
effects of ACT-3 and SAHA on the expression levels of acetylated H3
and HDAC1/3 were examined in MCF-7 cells. ACT-3 significantly
increased acetylated H3 expression at sub-micromolar
concentrations. Similar to SAHA, ACT-3 significantly reduced the
expression levels of HDAC1/3 in MCF-7 cells (Fig. 2B and C). The cytotoxicity of ACT-3
on the normal rat tubular epithelial cell line NRK-52E was also
determined. ACT-3 showed a less potent cytotoxicity against normal
cells compared with the MDA-MB-231 cells (Fig. S1).
 | Figure 2.Effect of ACT-3 on HDAC expression
levels in MCF-7 cells. (A) Effects of ACT-3, suramin, and SAHA on
total HDAC enzyme activity. HDAC enzyme activity was measured using
the SensoLyte 520 FRET total HDAC assay kit. (B and C) MCF-7 cells
were treated with ACT-3 and SAHA for 48 h and expression levels of
Ac-H3, HDAC1, HDAC3 and Histone H1 were analyzed via western
blotting. The data are expressed as the mean ± SD of duplicate
experiments. Statistical analysis was performed using one-way
analysis of the variance (ANOVA), followed by Bonferroni's multiple
comparison tests. *P<0.05 and **P<0.01 vs. the control.
ACT-3, 3β,6β-dihydroxyurs-12-en-27-oic acid; HDAC, histone
deacetylase; SAHA, suberoylanilide hydroxamic acid; Ac-H3,
acetylated histone 3. |
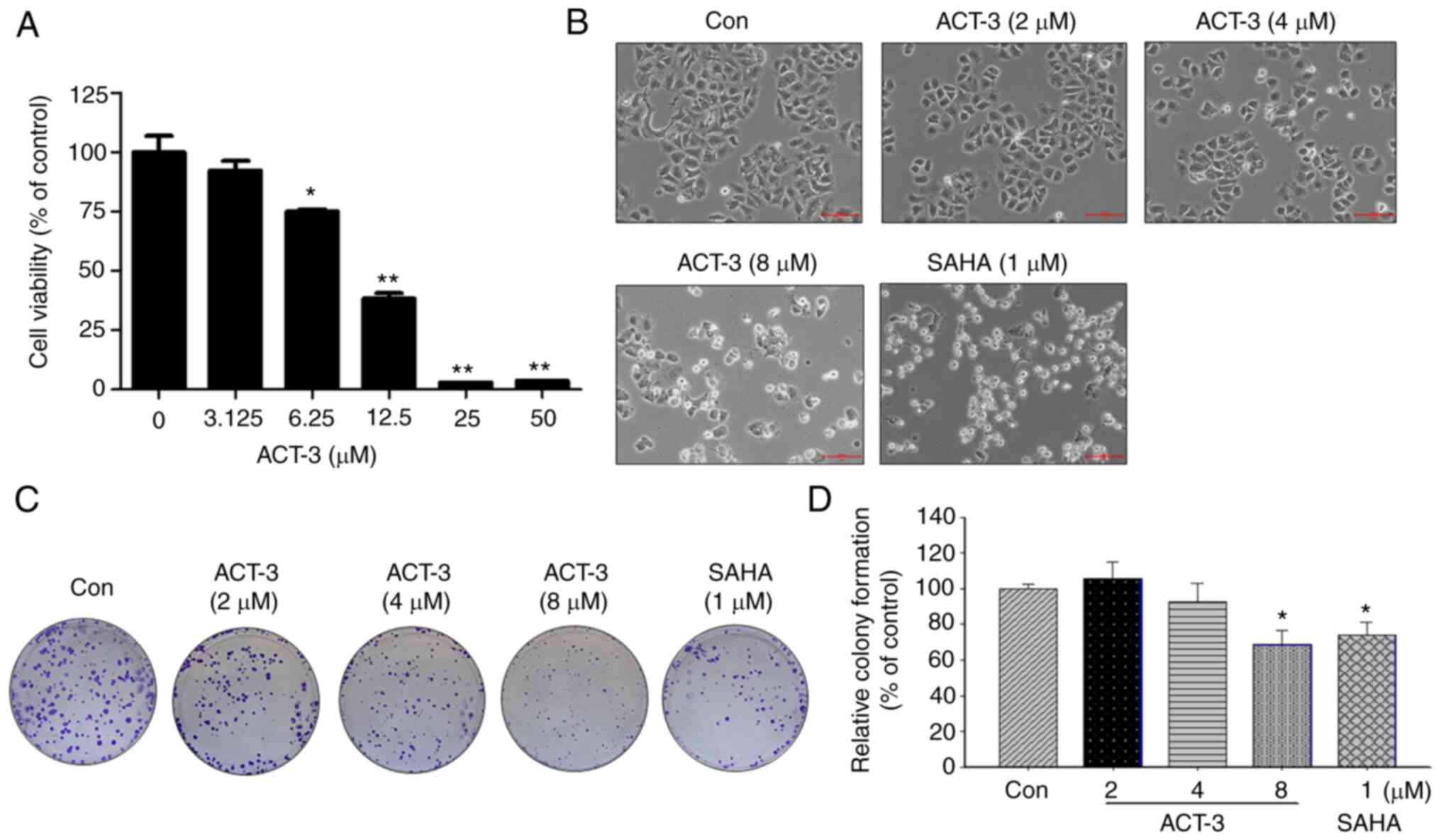
Cytotoxicity of ACT-3 in MCF-7
cells
To investigate the anticancer activity of ACT-3 in
MCF-7 breast cancer cells, cell viability was measured using an MTT
assay. ACT-3 significantly reduced the viability of MCF-7 cells in
a concentration-dependent manner (Fig.
3A). The IC50 value of ACT-3 in MCF-7 cells was 8.25
µM. The morphological change in MCF-7 cells induced by ACT-3
treatment resulted in cell shrinkage and increased suspended cell
population. Similar morphological changes in MCF-7 cells were
observed following SAHA treatment (Fig.
3B). To demonstrate the anticancer effects of ACT-3, a colony
formation assay was performed. As revealed in Fig. 3C and D, colony formation by MCF-7
cells was significantly inhibited following treatment with ACT-3
and SAHA.
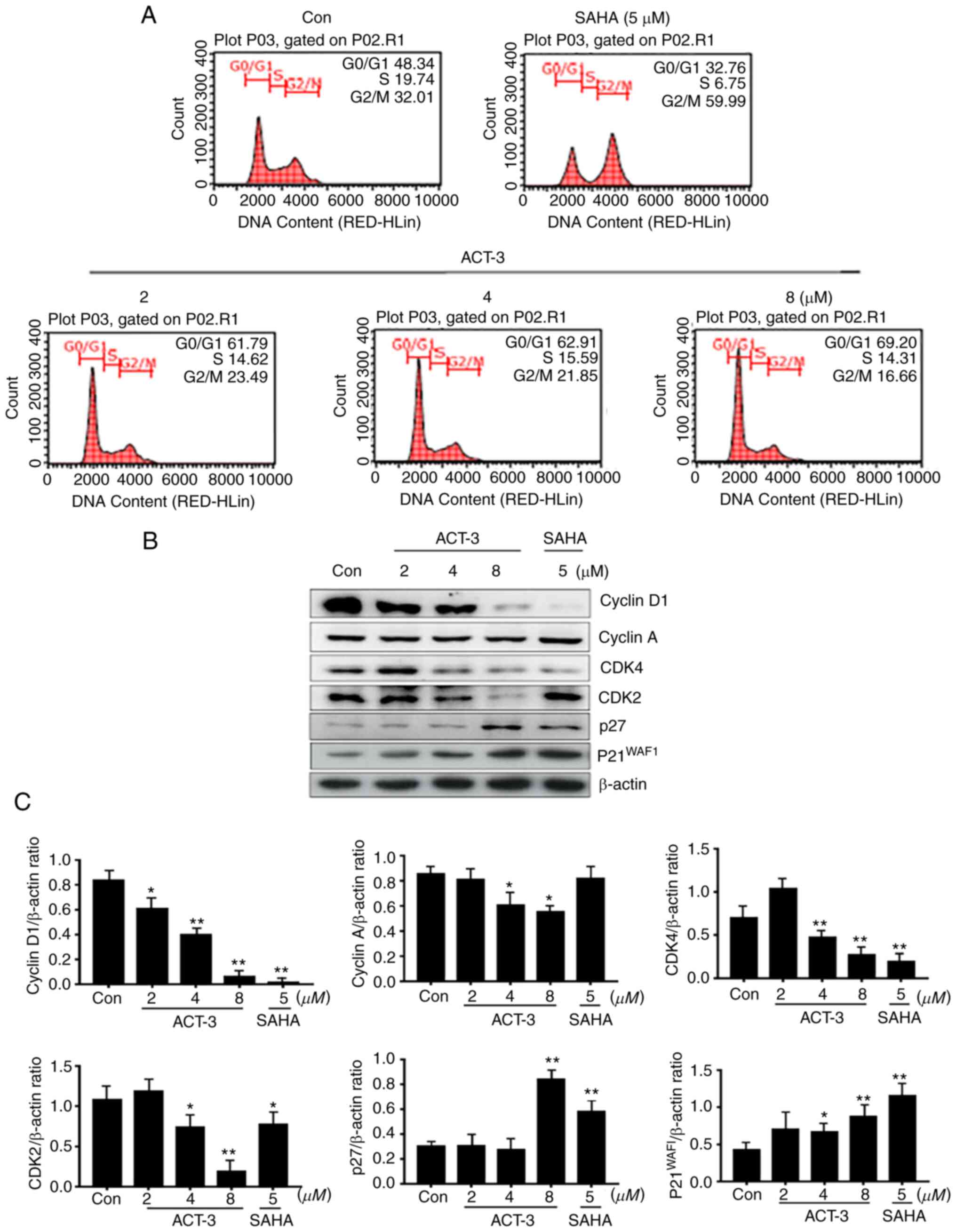
ACT-3 induces cell cycle arrest in
G0/G1 phase in MCF-7 cells
HDAC inhibitors significantly block the
proliferation of various cancer cells via cell cycle arrest at a
specific phase (24,25). To confirm the anticancer effect of
ACT-3, cell cycle was analyzed in MCF-7 cells treated with ACT-3
(2, 4 or 8 µM) and SAHA (5 µM) for 48 h. As demonstrated in
Fig. 4A, ACT-3 induced G0/G1 phase
cell cycle arrest in a concentration-dependent manner. However,
SAHA (5 µM) increased the G2/M phase cell accumulation to 59.99%
compared with that in the control (32.01%). To evaluate the effect
of ACT-3 on the expression levels of cell cycle regulators, the
expression of cyclins and CDK levels were determined. ACT-3
significantly reduced the expression levels of cyclin D1, CDK4 and
CDK2. Furthermore, ACT-3 significantly increased p27 and p21
expression at a high concentration (8 µM) in MCF-7 cells (Fig. 4B and C).
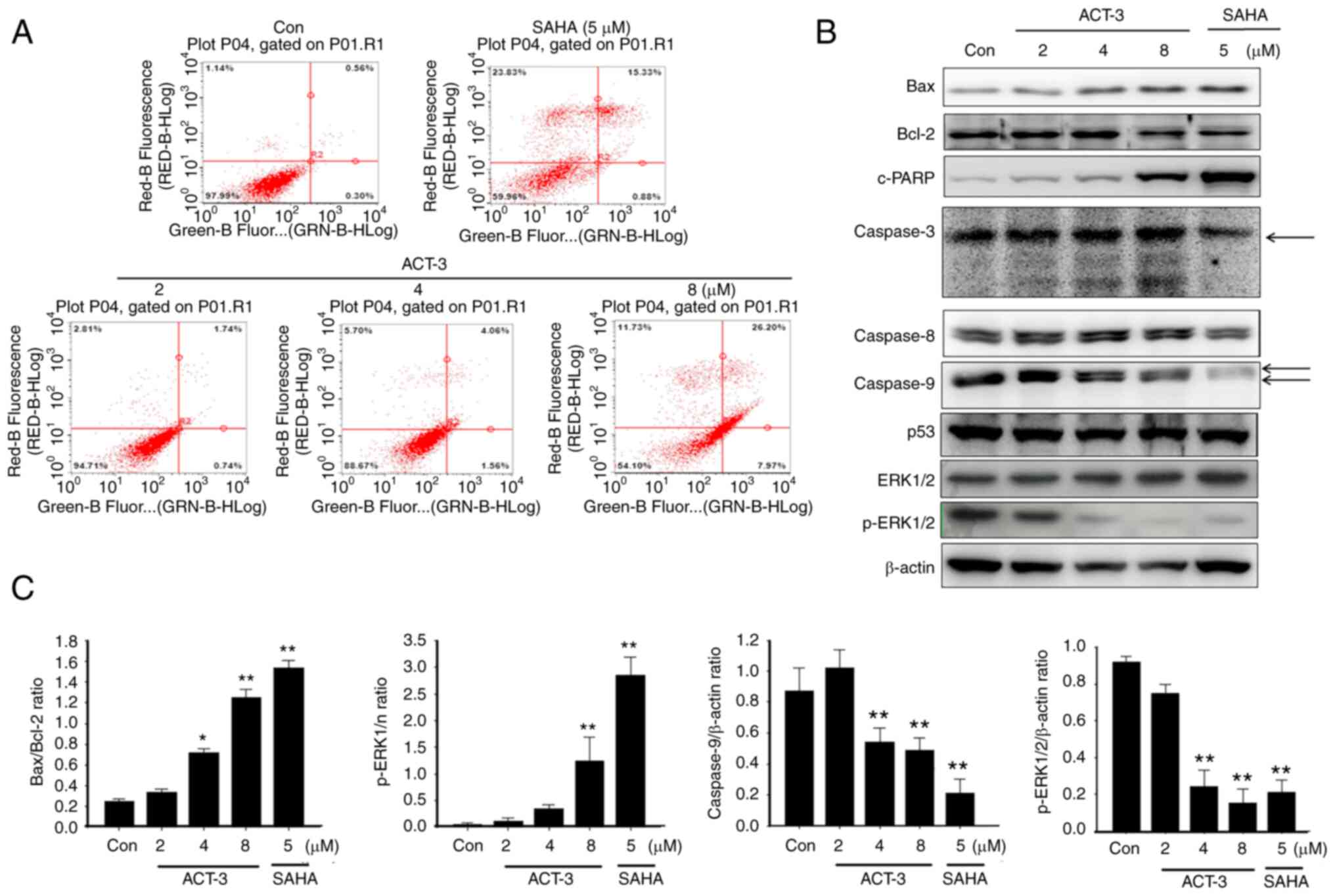
ACT-3 induces apoptotic cell death in
MCF-7 cells
To evaluate the apoptotic cell death in MCF-7 cells
after ACT-3 treatment, annexin V-FITC-conjugated staining and
western blot analysis were performed. Despite the pronounced
concentration-dependent cell death observed in the cytotoxicity
assay, only a small change in apoptotic cell death was detected at
the highest ACT-3 concentration (Fig.
5A). A significant increase in Bax and simultaneous decrease in
Bcl-2 expression levels were observed following ACT-3 treatment
(Fig. 5B and C). Moreover, there
was a significant increase in the expression levels of c-PARP in
MCF-7 cells following ACT-3 (8 µM) and SAHA treatment (Fig. 5B). However, p53 expression was not
changed in MCF-7 cells by ACT-3 and SAHA treatment (Fig. 5B).
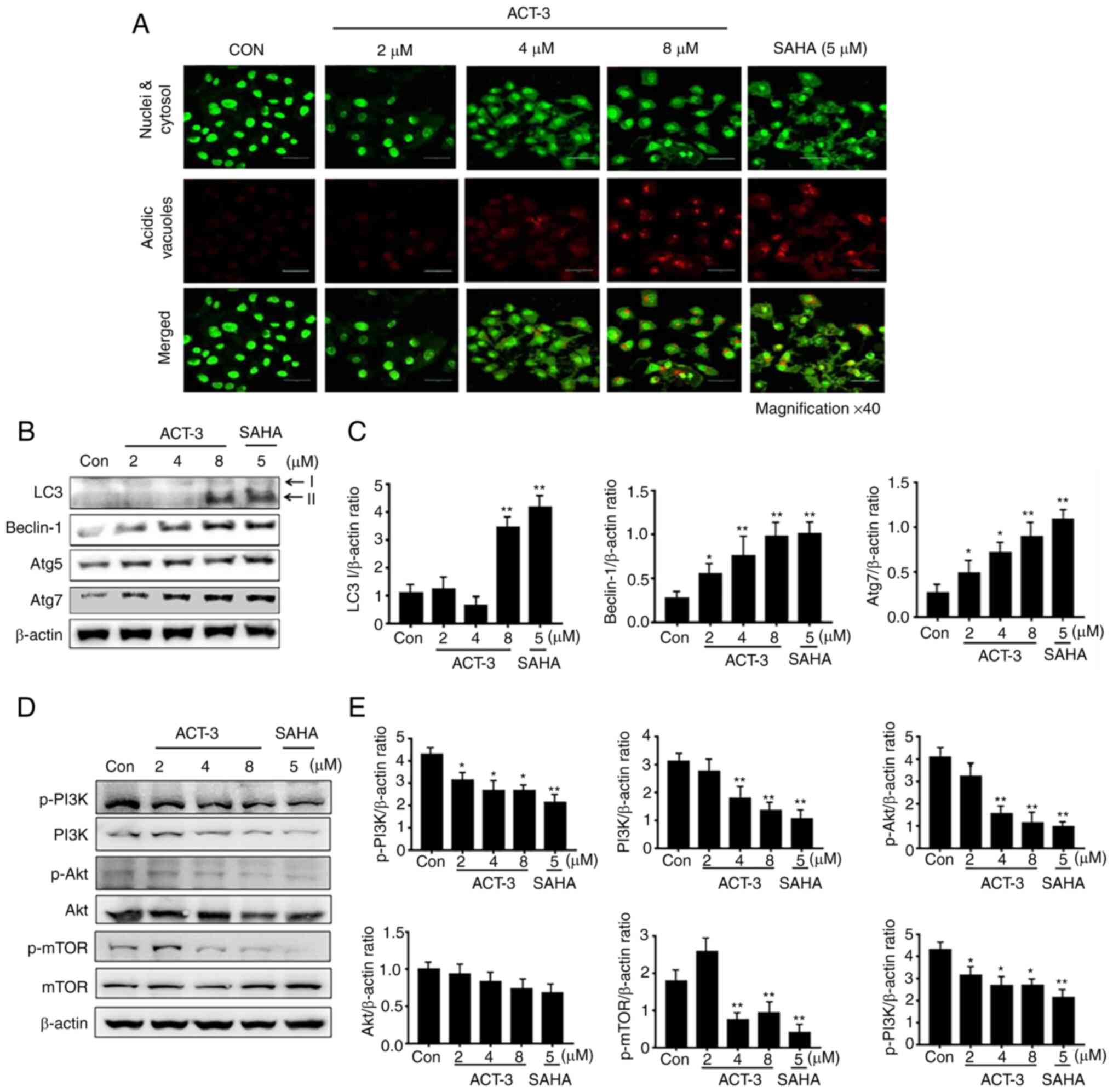
ACT-3 induces autophagic cell death in
MCF-7 cells
To elucidate ACT-mediated autophagic cell death by
ACT-3, firstly acridine orange staining was performed. Acridine
orange is a fluorescent dye that accumulates in acidic cellular
compartments in a pH-dependent manner. Under neutral conditions,
the dye emits a green light. However, within acidic vesicles, the
dye changes to bright red (26).
With acridine orange staining, the control cells and 2-µM ACT-3
showed almost undetectable red fluorescence, indicating the lack of
acidic vacuoles (AVOs). However, accumulation of red fluorescence
was observed with 8 µM ACT-3 and SAHA, indicating the presence of
numerous AVOs. Similarly, AVOs were analyzed with acridine orange
in the merged figures (Fig. 6A). To
evaluate autophagic proteins, their expression levels were
determined using western blotting. The levels of LC3-II, a marker
of autophagosomes, were significantly increased after treatment
with ACT-3 and SAHA-treated cells. The levels of beclin-1 and Atg7
were increased, but Atg5 did not show any changes (Fig. 6B and C). Previous studies have
indicated that a majority of anticancer agents suppress the
PI3K/mTOR/Akt pathway, which is related to cell proliferation
(27). To evaluate the effect of
ACT-3 on the PI3K/mTOR/Akt pathway, protein expression levels were
evaluated using western blotting. The expression of p-PI3K, PI3K,
p-mTOR and p-Akt levels were decreased after ACT-3 treatment in
MCF-7 cells (Fig. 6D and E).
However, the p-PI3K/PI3K ratio remained unchanged. These results
suggested that ACT-3 only downregulates the mTOR/Akt pathway.
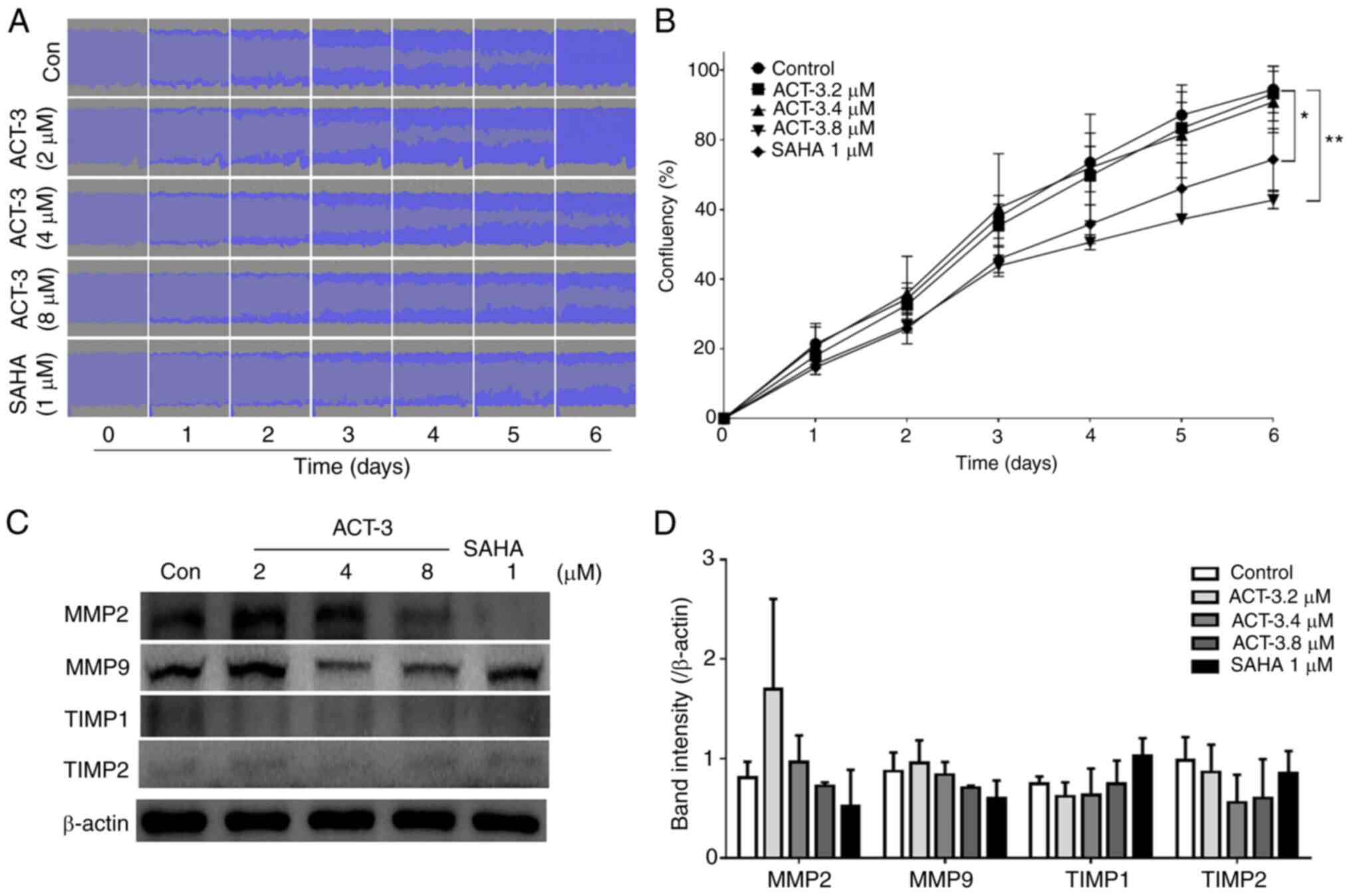
ACT-3 inhibits MCF-7 cell
migration
To investigate the effects of ACT-3 on the
inhibition of MCF-7 cell migration, a wound-healing assay was
performed in response to the mechanical scratch wound in the
absence or presence of ACT-3 and SAHA. Images of scratch areas from
0–6 days are illustrated. The representative control at each time
point is revealed in Fig. 7A,
indicating that the scratch was half-closed within three days and
completely closed after six days. The percentage of open wound area
was determined to quantify the effects of the putative migration
inhibitors (Fig. 7B). The present
data clearly indicated that treatment with ACT-3 significantly
inhibited the cell migration in a concentration-dependent manner.
These results suggested that ACT-3 may be an effective inhibitor of
MCF-7 breast cancer cell migration. Moreover, the levels of matrix
metalloproteinase (MMP)-2 and MMP9 were reduced after treatment
with ACT-3 in a concentration-dependent manner. However, ACT-3 did
not alter the expression levels of tissue inhibitor of
metalloproteinase (TIMP)-1 or TIMP2 (Fig. 7C and D).
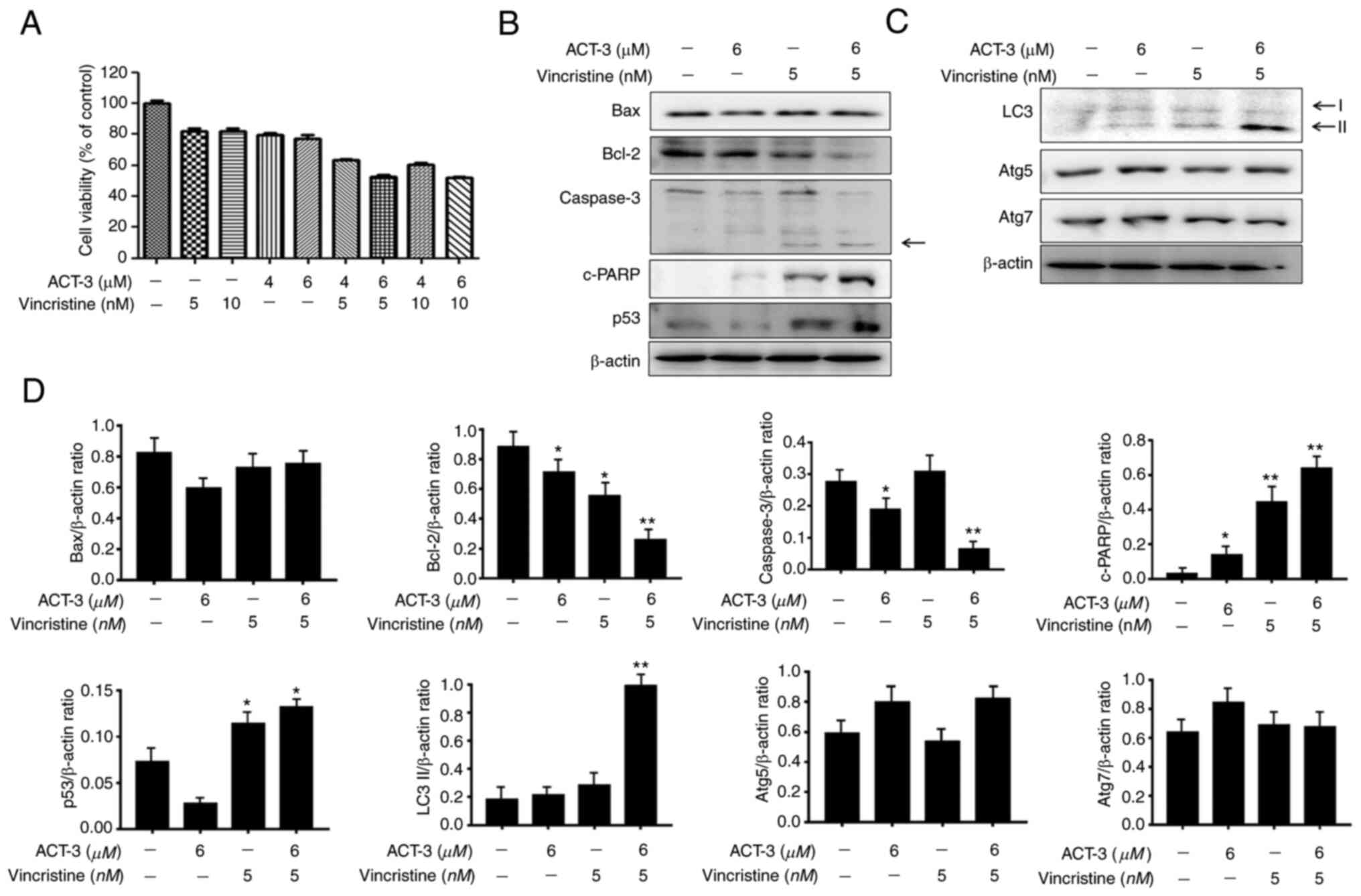
ACT-3 shows synergistic anticancer
activity with microtubule inhibitors in MCF-7 cells
To investigate the combined effect of ACT-3 and
vincristine, the cytotoxicity of MCF-7 cells was measured using the
MTT assay with the indicated concentrations of drugs. ACT-3 showed
potent cytotoxicity at 6 µM, and vincristine at 5 µM. Combination
treatment with ACT-3 (6 µM) and vincristine (5 µM) significantly
increased the cytotoxic activity against MCF-7 cells compared with
that of the single treatment (Fig.
8A). To evaluate the synergism between apoptotic and autophagic
cell death, the expression levels of proteins related to apoptosis
and autophagy were evaluated via western blotting. Bax levels were
almost unchanged, but Bcl-2 levels were significantly reduced by
the combination treatment (Fig. 8B and
D). Caspase 3 levels were also decreased by the combination
treatment, while cleaved caspase-3 levels were increased (Fig. 8B and D). c-PARP and p53 levels were
significantly increased by the combination treatment (Fig. 8B and D). The expression levels of
the autophagy-related proteins, Atg5 and Atg7, were not altered
(Fig. 8C and D). However, LC3-II
protein levels were significantly increased by the combination
treatment (Fig. 8C and D). These
results indicated that the combination therapy with ACT-3 and
vincristine is effective against MCF-7 breast cancer cells.
Discussion
Previously, much attention was paid to the use of
HDAC inhibitors as anticancer agents since they can induce either
apoptosis or autophagic cell death (22,28,29).
In the present study, it was found that ACT-3 induces apoptosis in
MCF-7 cells through cell cycle arrest at the G2/M phase. Similar to
other HDAC inhibitors, ACT-3 effectively blocked the deacetylation
of histone H3 and H4 proteins as well as HDAC1/2 expression, which
influenced the expression of key target genes that regulate
oncogenic pathways and inhibit the expression of cell cycle
regulators.
Previous studies have indicated that natural
products of A. chinensis exhibit hepatoprotective,
anti-obesity, anticancer and anti-inflammatory activities. The
major mechanism of this anti-inflammatory effect is closely
associated with the inhibition of NF-κB activation (30–32).
In addition, the extracts or fractions of A. chinensis
exhibit anticancer activity via the activation of apoptosis by ROS
production (33). However, the
anticancer activity of the major components isolated from A.
chinensis has not been clearly investigated. Thus, the major
components of A. chinensis were isolated using solvent
extraction and HPLC and it was found that the most active component
was triterpenoid, which also exhibited a potent HDAC enzyme
inhibitor. In the present study, ACT-3 showed potent cytotoxicity
against MCF-7 cells and the cytotoxicity results were confirmed by
cell morphological analysis compared with SAHA, a reference HDAC
inhibitor. Previously, a number of studies reported cell shrinkage
and that the cells were smaller than before, the cytoplasm was
dense, and other organelles were packed during early apoptosis
(34,35).
In the present study, a series of experiments were
performed to detect the effects of ACT-3 on cancer cell
proliferation and migration in vitro. The results showed
that ACT-3 exhibited cytotoxicity against MCF-7 cells, with an
IC50 value of 8 µM. The wound-healing assay indicated
that MCF-7 cells showed inhibition of migration after treatment
with ACT-3. Furthermore, subsequent mechanistic investigation
revealed that ACT-3 induced apoptosis and arrested the MCF-7 cell
cycle at the G0/G1 stage to exert cytotoxic effects. The expression
levels of representative proteins associated with apoptosis were
also examined. The proteins of the Bcl-2 family play a vital role
in the mitochondria-mediated apoptosis pathway. In the present
study, the inhibition of Bcl-2 expression and the increase in Bax
protein levels by ACT-3 demonstrated that the compound could
promote apoptosis by targeting the mitochondrial pathway.
Generally, regulation of the cell cycle plays a vital role in cell
growth in a rapidly changing microenvironment (36). During the cell cycle, cyclins and
CDKs bind together and successfully perform their roles to regulate
the cell cycle. After CDKs bind to cyclins, they activate or
inactivate target proteins to enter the next phase of the cell
cycle (37–39). In the case of ACT-3, cyclin D1, cdc2
and CDK2 expression levels were inhibited. Cyclin D1 and cdc2
inhibition may suppress the entry of the G0 phase to the G1 phase
and exit the G2/M phase. Thus, ACT-3 arrested cells that entered
the cell cycle in the G1 phase by inhibiting CDK2. In SAHA, CDK2
was activated. This difference caused the SAHA-treated group to
pass the S phase, and the inhibition of cyclin B1 and cdc2
prevented entry into mitosis, resulting in arrest in the G2/M
phase. To control cell cycle progression, cells generate CDK
inhibitors, including protein/kinase inhibitory protein (CIP/KIP),
p21 and p27. These proteins are primarily activated by p53, which
is triggered by DNA damage (40,41).
In the case of ACT-3, increased protein levels of p53 and p27 have
been observed to regulate the cell cycle.
Numerous anticancer drugs exert their effects
through apoptosis and autophagy after cell growth stops (42). Apoptosis was also analyzed to
elucidate the underlying anticancer mechanism. Apoptotic cells have
several features including DNA breakdown, protein cross-linking and
protein cleavage (43). In the case
of ACT-3, the levels of intrinsic-related caspases 3 and 9 slightly
decreased in a dose-dependent manner. In fact, tumor cells acquire
resistance to apoptosis by overexpression of the anti-apoptotic
protein Bcl-2 or the apoptotic protein Bax. Both Bcl-2 and Bax are
regulated by p53, a tumor suppressor gene (44,45).
p53 induced by DNA damage can maintain the cell cycle. If DNA
damage is irreversible, p53 mediates apoptosis (46). ACT-3 inhibits this evasion mechanism
by increasing the expression level of Bax and decreasing the
expression level of Bcl-2. PARP-1 is a nuclear enzyme that responds
to DNA damage and is essential for the maintenance of gene
integrity. When DNA damage begins, PARP-1 is proteolyzed by
caspases to a DNA-binding domain and formed c-PARP-1 (47). ACT-3 increased PARP-1 activity,
which was confirmed by the increased expression level of
c-PARP.
Autophagy is a self-degradable process that plays a
critical role in balancing cellular environmental homeostasis
(48). Autophagy can be activated
by various stress conditions such as radiotherapy or chemotherapy,
nutrient shortage and cellular damage by intrinsic or extrinsic
stress (49,50). Responding to the various stress
conditions, expression level of autophagy-related proteins
including microtubule-associated protein LC3, beclin-1 and p62 is
increased. LC3 is cleaved by ATG4. LC3 combined with
phosphatidylethanolamine by ATG3/7 induces a change from the
cytosolic form (LC3-I) to the membrane-bound form (LC3-II)
(51). Western blot analysis and
acridine orange staining showed that LC3-II was increased in 8 µM
concentration ACT-3 and SAHA cells. The increased expression of
Beclin-1 also confirmed the activation of autophagy. Autophagy is
affected by various signaling pathways. First, the protein
expression levels of mTOR kinase, which plays a crucial role in
cellular growth and protein synthesis, were investigated. Autophagy
activation is initiated by decreasing the activity of mTOR, which
is regulated by PI3K/Akt, p53, AMP-activated protein kinase (AMPK)
and MAPK/ERK1/2 pathways (52).
Western blot analysis revealed that mTOR signaling was decreased by
the Akt pathway. Owing to the decreased total form of PI3K, the
p-PI3K/PI3K ratio was not significantly decreased. However, the
p-Akt/Akt ratio was decreased. It was concluded that mTOR signaling
was affected by the Akt pathway.
Microtubule inhibitors, traditionally used as
anticancer agents, remain a major therapy for numerous solid
tumors, including numerous types of breast cancers. However, they
have problems of drug resistance, neuropathy, immunosuppression and
poor solubility. Currently, these problems have been overcome by
changing the drug combination with chemotherapeutic drugs to reduce
its toxicity and increase drug potency (53). In particular, the use of vincristine
in cancer patients is dose-limited due to toxicity (54). While HDAC inhibitors are generally
well-tolerated in humans, combining with vincristine may reduce the
risk of toxicity to normal cells. Therefore, vincristine was used
as a combination therapy with ACT-3 to determine its synergetic
efficacy against MCF-7 cells. Cell cytotoxicity of the combination
therapy showed the best effect with ACT-3 and vincristine. In
previous studies, relatively high concentrations (>10 nM) of
microtubule inhibitors have been shown to induce microtubule mass.
However, a relatively low concentration (<10 nM) affects only
the suppression of microtubule dynamics. This means that
combination therapy with ACT-3 could reduce the side effects of
vincristine while maintaining its anticancer effect (54,55).
This synergetic effect could be explained briefly by the
significant increase in p53 expression. As a result,
apoptosis-related proteins, such as Bcl-2 and c-PARP, were also
significantly increased. The autophagosome membrane protein LC3-II
also greatly increased with the combination drug treatment. To
date, numerous breast cancer chemotherapies have been developed;
but alternative new drugs are required.
In conclusion, the present study aimed to determine
the anticancer activity of ACT-3 in MCF-7 breast cancer cells. It
was identified that ACT-3 inhibited cell proliferation and induced
apoptosis and autophagic cell death via Akt/mTOR inhibition in
MCF-7 cells. Moreover, combination therapy with traditional
anticancer agents, including vincristine, showed a synergetic
effect. Therefore, ACT-3 may be used alone or in combination with
other anticancer drugs for the treatment of breast cancer.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Research
Foundation (NRF) of Korea (grant nos. NRF-2019R1A2C2002923,
NRF-2022R1A4A1018930 and 2021R1A2C1014595), funded by the Korean
government.
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
JSL and HSK conceived and designed the experiments.
JSL and SYK performed the experiments. YJ and SYK separated and
isolated the small molecules. JSL and JHK collected the data. JSL
and HSK analyzed and interpreted the data and wrote the manuscript.
ISK, JHK and HSK made critical revisions to the manuscript and
confirm the authenticity of all the raw data. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pojani E and Barlocco D: Romidepsin
(FK228), A histone deacetylase inhibitor and its analogues in
cancer chemotherapy. Curr Med Chem. 28:1290–1303. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
West AC and Johnstone RW: New and emerging
HDAC inhibitors for cancer treatment. J Clin Invest. 124:30–39.
2014. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bass AKA, El-Zoghbi MS, Nageeb EM, Mohamed
MFA, Badr M and Abuo-Rahma GEA: Comprehensive review for anticancer
hybridized multitargeting HDAC inhibitors. Eur J Med Chem.
209:1129042021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sanaei M and Kavoosi F: Histone
deacetylases and histone deacetylase inhibitors: Molecular
mechanisms of action in various cancers. Adv Biomed Res. 8:632019.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ediriweera MK, Tennekoon KH and Samarakoon
SR: Emerging role of histone deacetylase inhibitors as
anti-breast-cancer agents. Drug Discov Today. 24:685–702. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hanikoglu A, Hanikoglu F and Ozben T:
Natural product inhibitors of histone deacetylases as new
anticancer agents. Curr Protein Pept Sci. 19:333–340. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wawruszak A, Kalafut J, Okon E, Czapinski
J, Halasa M, Przybyszewska A, Miziak P, Okla K, Rivero-Muller A and
Stepulak A: Histone deacetylase inhibitors and phenotypical
transformation of cancer cells. Cancers (Basel). 11:1482019.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dawood M, Fleischer E, Klinger A,
Bringmann G, Shan L and Efferth T: Inhibition of cell migration and
induction of apoptosis by a novel class II histone deacetylase
inhibitor, MCC2344. Pharmacol Res. 160:1050762020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Suraweera A, O'Byrne KJ and Richard DJ:
Combination therapy with histone deacetylase inhibitors (HDACi) for
the treatment of cancer: Achieving the full therapeutic potential
of HDACi. Front Oncol. 8:922018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mottamal M, Zheng S, Huang TL and Wang G:
Histone deacetylase inhibitors in clinical studies as templates for
new anticancer agents. Molecules. 20:3898–3941. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen R, Zhang M, Zhou Y, Guo W, Yi M,
Zhang Z, Ding Y and Wang Y: The application of histone deacetylases
inhibitors in glioblastoma. J Exp Clin Cancer Res. 39:1382020.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Momenimovahed Z and Salehiniya H:
Epidemiological characteristics of and risk factors for breast
cancer in the world. Breast Cancer (Dove Med Press). 11:151–164.
2019.PubMed/NCBI
|
|
13
|
Damaskos C, Garmpis N, Valsami S, Kontos
M, Spartalis E, Kalampokas T, Kalampokas E, Athanasiou A, Moris D,
Daskalopoulou A, et al: Histone deacetylase inhibitors: An
attractive therapeutic strategy against breast cancer. Anticancer
Res. 37:35–46. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Naimo GD, Gelsomino L, Catalano S, Mauro L
and Andò S: Interfering role of ERα on adiponectin action in breast
cancer. Front Endocrinol (Lausanne). 11:662020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li J, Zhang T, Yang F, He Y, Dai F, Gao D,
Chen Y, Liu M and Yi Z: Inhibition of breast cancer progression by
a novel histone deacetylase inhibitor, LW479, by down-regulating
EGFR expression. Br J Pharmacol. 172:3817–3830. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Newman DJ and Cragg GM: Natural products
as sources of new drugs over the last 25 years. J Nat Prod.
70:461–477. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Connolly JD and Hill RA: Triterpenoids.
Nat Prod Rep. 24:465–486. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dzubak P, Hajduch M, Vydra D, Hustova A,
Kvasnica M, Biedermann D, Markova L, Urban M and Sarek J:
Pharmacological activities of natural triterpenoids and their
therapeutic implications. Nat Prod Rep. 23:394–411. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Na M, Min BS, An RB, Jin W, Kim YH, Song
KS, Seong YH and Bae K: Effect of the rhizomes of Astilbe
chinensis on UVB-induced inflammatory response. Phytother Res.
18:1000–1004. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun HX, Ye YP and Pan YJ: Cytotoxic
oleanane triterpenoids from the rhizomes of Astilbe
chinensis (Maxim.) Franch. et Savat. J Ethnopharmacol.
90:261–265. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hu JY, Yao Z, Xu YQ, Takaishi Y and Duan
HQ: Triterpenes from Astilbe chinensis. J Asian Nat Prod
Res. 11:236–242. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tae IH, Son JY, Lee SH, Ahn MY, Yoon K,
Yoon S, Moon HR and Kim HS: A new SIRT1 inhibitor, MHY2245, induces
autophagy and inhibits energy metabolism via PKM2/mTOR pathway in
human ovarian cancer cells. Int J Biol Sci. 16:1901–1916. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ding J, Liu J, Zhang Z, Guo J, Cheng M,
Wan Y, Wang R, Fang Y, Guan Z, Jin Y and Xie SS: Design, synthesis
and biological evaluation of coumarin-based N-hydroxycinnamamide
derivatives as novel histone deacetylase inhibitors with anticancer
activities. Bioorg Chem. 101:1040232020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li Y and Seto E: HDACs and HDAC inhibitors
in cancer development and therapy. Cold Spring Harb Perspect Med.
6:a0268312016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li Y, Yuan YY, Meeran SM and Tollefsbol
TO: Synergistic epigenetic reactivation of estrogen receptor-α
(ERα) by combined green tea polyphenol and histone deacetylase
inhibitor in ERα-negative breast cancer cells. Mol Cancer.
9:2742010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Millot C, Millot JM, Morjani H, Desplaces
A and Manfait M: Characterization of acidic vesicles in
multidrug-resistant and sensitive cancer cells by acridine orange
staining and confocal microspectrofluorometry. J Histochem
Cytochem. 45:1255–1264. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dong C, Wu J, Chen Y, Nie J and Chen C:
Activation of PI3K/AKT/mTOR pathway causes drug resistance in
breast cancer. Front Pharmacol. 12:6286902021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cras A, Darsin-Bettinger D, Balitrand N,
Cassinat B, Soulié A, Toubert ME, Delva L and Chomienne C:
Epigenetic patterns of the retinoic acid receptor beta2 promoter in
retinoic acid-resistant thyroid cancer cells. Oncogene.
26:4018–4024. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Godman CA, Joshi R, Tierney BR, Greenspan
E, Rasmussen TP, Wang HW, Shin DG, Rosenberg DW and Giardina C:
HDAC3 impacts multiple oncogenic pathways in colon cancer cells
with effects on Wnt and vitamin D signaling. Cancer Biol Ther.
7:1570–1580. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang XH, Wang Z, Kang BG, Hwang SH, Lee
JY, Lim SS and Huang B: Antiobesity effect of Astilbe
chinensis Franch. et Savet. Extract through regulation of
adipogenesis and AMP-activated protein kinase pathways in 3T3-L1
adipocyte and high-fat diet-induced C57BL/6N obese mice. Evid Based
Complement Alternat Med. 2018:13476122018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sancheti S, Sancheti S, Lee SH, Lee JE and
Seo SY: Screening of Korean medicinal plant extracts for
α-glucosidase inhibitory activities. Iran J Pharm Res. 10:261–264.
2011.PubMed/NCBI
|
|
32
|
Gil TY, Jin BR, Hong CH, Park JH and An
HJ: Astilbe chinensis ethanol extract suppresses
inflammation in macrophages via NF-κB pathway. BMC Complement Med
Ther. 20:3022020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang YB, Ye YP, Wu XD and Sun HX:
Astilbotriterpenic acid induces growth arrest and apoptosis in HeLa
cells through mitochondria-related pathways and reactive oxygen
species (ROS) production. Chem Biodivers. 6:218–230. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Häcker G: The morphology of apoptosis.
Cell Tissue Res. 301:5–17. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zeiss CJ: The apoptosis-necrosis
continuum: Insights from genetically altered mice. Vet Pathol.
40:481–495. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vermeulen K, Van Bockstaele DR and
Berneman ZN: The cell cycle: A review of regulation, deregulation
and therapeutic targets in cancer. Cell Prolif. 36:131–149. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nigg EA: Cyclin-dependent protein kinases:
Key regulators of the eukaryotic cell cycle. Bioessays. 17:471–480.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Malumbres M and Barbacid M: Cell cycle,
CDKs and cancer: A changing paradigm. Nat Rev Cancer. 9:153–166.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Satyanarayana A and Kaldis P: Mammalian
cell-cycle regulation: Several Cdks, numerous cyclins and diverse
compensatory mechanisms. Oncogene. 28:2925–2939. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sauer K and Lehner CF: The role of cyclin
E in the regulation of entry into S phase. Prog Cell Cycle Res.
1:125–139. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Peter M: The regulation of
cyclin-dependent kinase inhibitors (CKIs). Prog Cell Cycle Res.
3:99–108. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hengartner MO: The biochemistry of
apoptosis. Nature. 407:770–776. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Miyashita T, Krajewski S, Krajewska M,
Wang HG, Lin HK, Liebermann DA, Hoffman B and Reed JC: Tumor
suppressor p53 is a regulator of bcl-2 and bax gene expression in
vitro and in vivo. Oncogene. 9:1799–1805. 1994.PubMed/NCBI
|
|
45
|
Vaux DL, Cory S and Adams JM: Bcl-2 gene
promotes haemopoietic cell survival and cooperates with c-myc to
immortalize pre-B cells. Nature. 335:440–442. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Pietenpol JA and Stewart ZA: Cell cycle
checkpoint signaling: Cell cycle arrest versus apoptosis.
Toxicology. 181–182. 475–481. 2002.PubMed/NCBI
|
|
47
|
Beneke R, Geisen C, Zevnik B, Bauch T,
Müller WU, Küpper JH and Möröy T: DNA excision repair and DNA
damage-induced apoptosis are linked to Poly(ADP-ribosyl)ation but
have different requirements for p53. Mol Cell Biol. 20:6695–6703.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Galluzzi L and Green DR:
Autophagy-independent functions of the autophagy machinery. Cell.
177:1682–1699. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Apel A, Herr I, Schwarz H, Rodemann HP and
Mayer A: Blocked autophagy sensitizes resistant carcinoma cells to
radiation therapy. Cancer Res. 68:1485–1494. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Döring T, Zeyen L, Bartusch C and Prange
R: Hepatitis B virus subverts the autophagy elongation complex
Atg5-12/16L1 and does not require Atg8/LC3 lipidation for viral
maturation. J Virol. 92:e01513–e01517. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Li F, Guo H, Yang Y, Feng M, Liu B, Ren X
and Zhou H: Autophagy modulation in bladder cancer development and
treatment (review). Oncol Rep. 42:1647–1655. 2019.PubMed/NCBI
|
|
52
|
Perez EA: Microtubule inhibitors:
Differentiating tubulin-inhibiting agents based on mechanisms of
action, clinical activity, and resistance. Mol Cancer Ther.
8:2086–2095. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Dumontet C and Jordan MA:
Microtubule-binding agents: A dynamic field of cancer therapeutics.
Nat Rev Drug Discov. 9:790–803. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Jordan VC: Chemoprevention of breast
cancer with selective oestrogen-receptor modulators. Nat Rev
Cancer. 7:46–53. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Younis AM, Wu FS and El Shikh HH:
Antimicrobial activity of extracts of the oyster culinary medicinal
mushroom Pleurotus ostreatus (higher basidiomycetes) and
identification of a new antimicrobial compound. Int J Med
Mushrooms. 17:579–590. 2015. View Article : Google Scholar : PubMed/NCBI
|