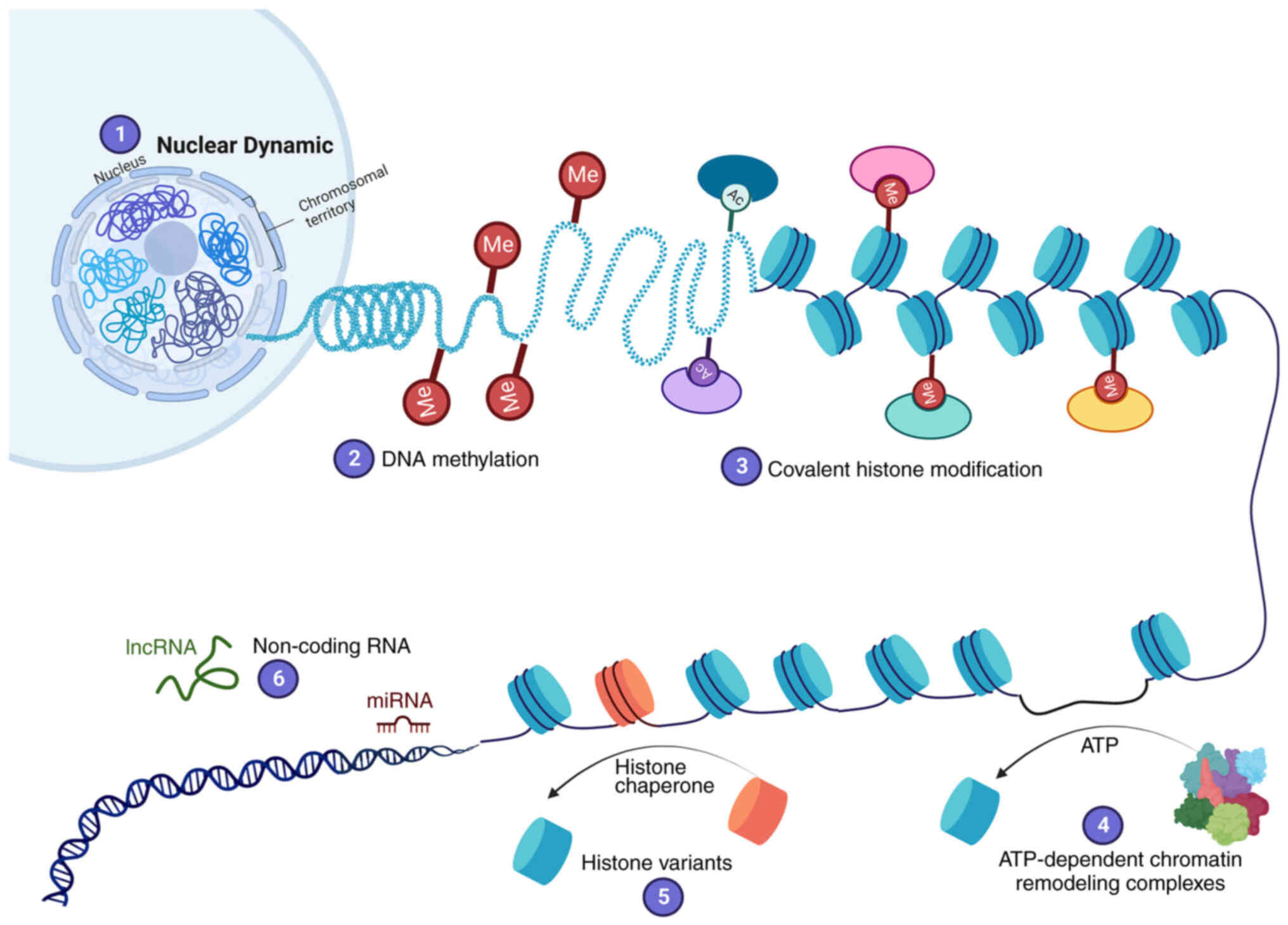
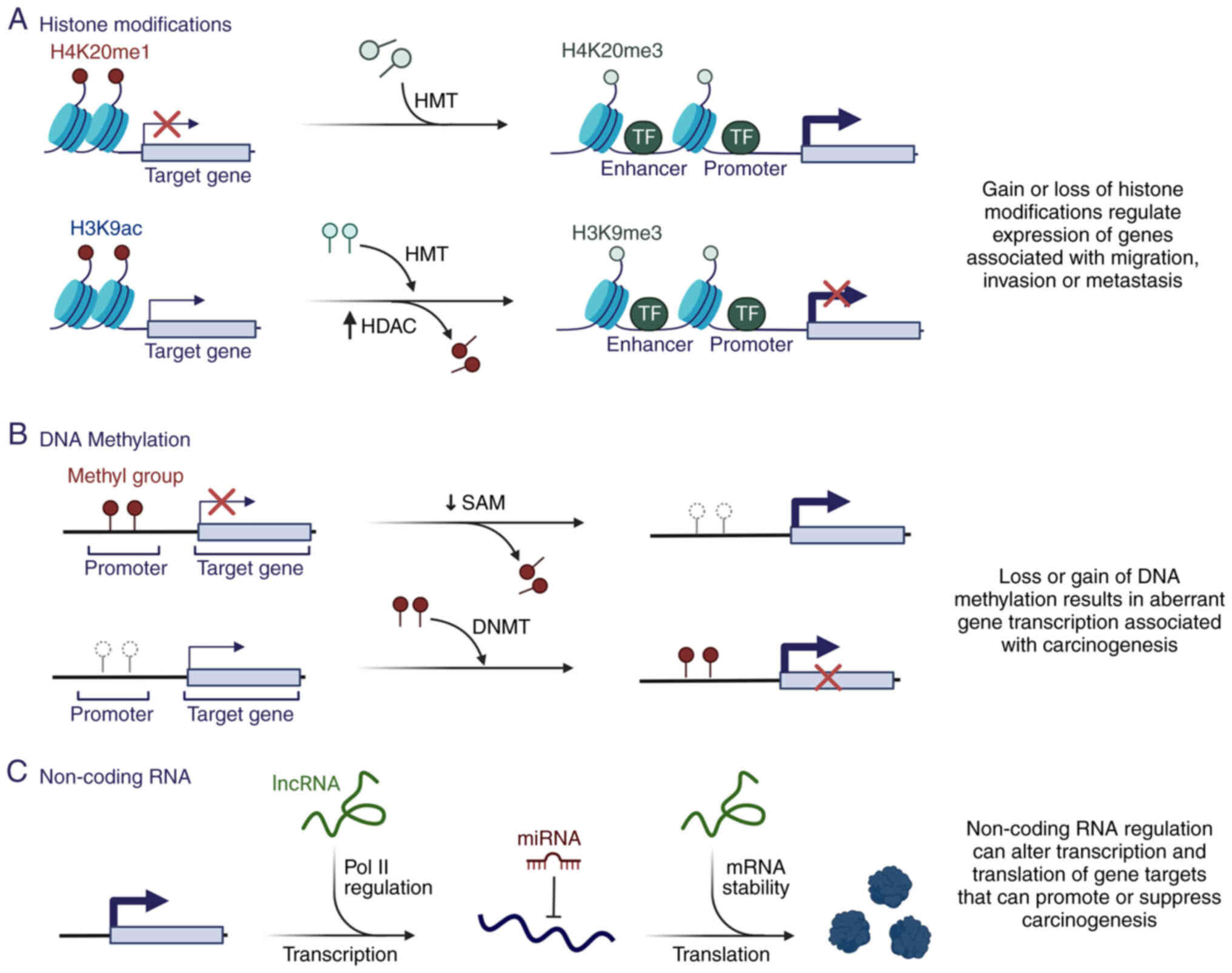
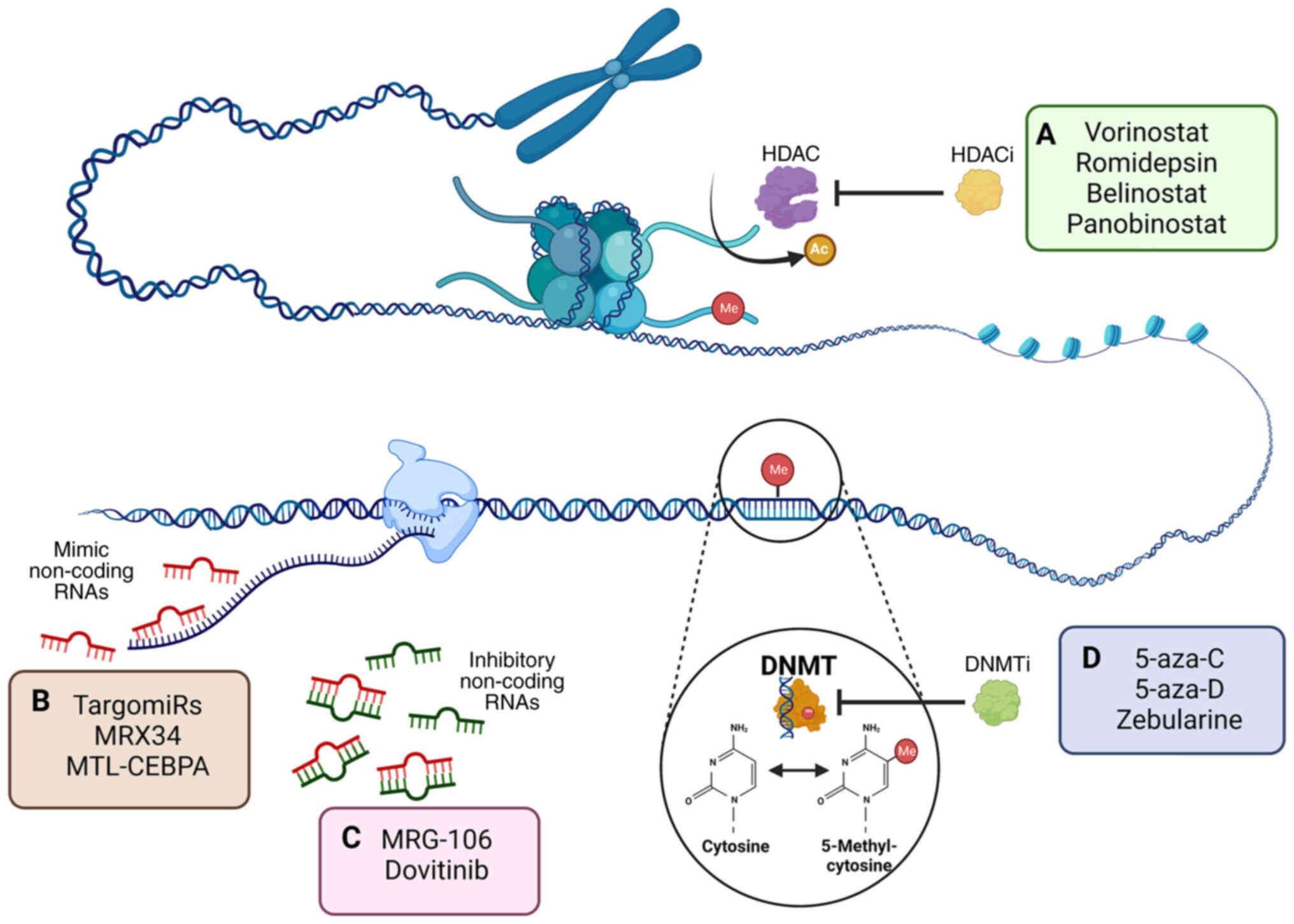
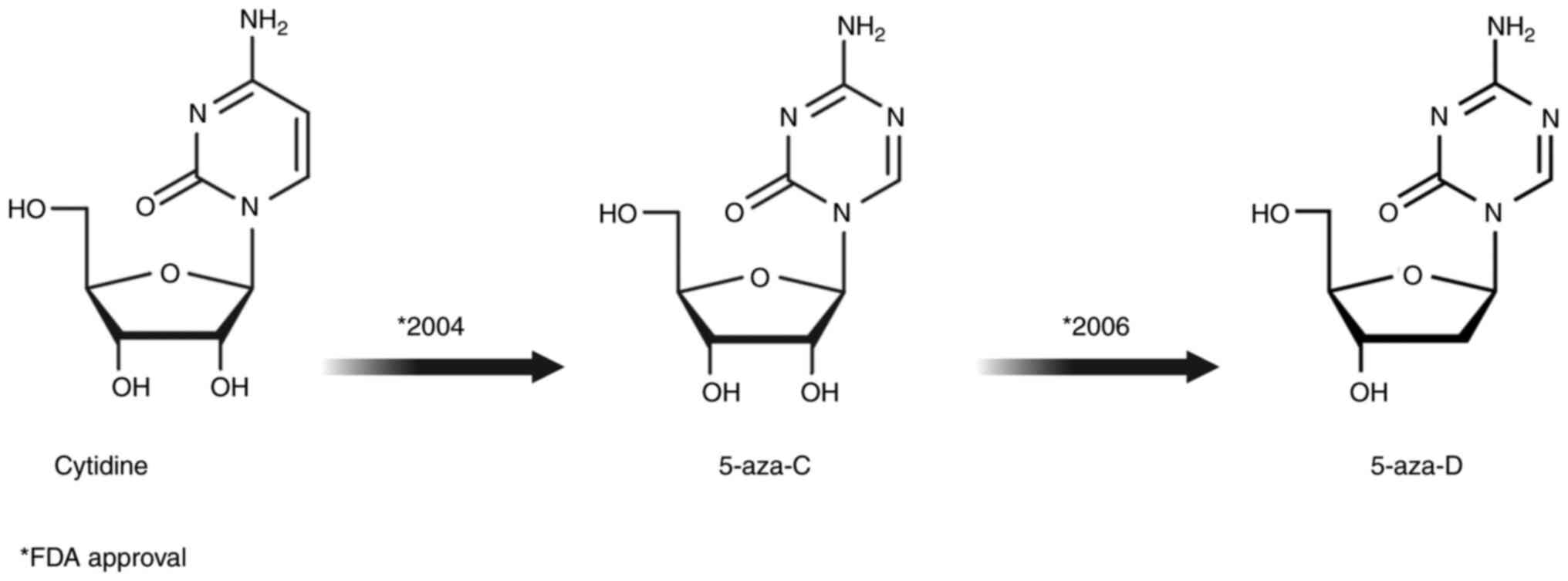
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mantovani F, Collavin L and Del Sal G:
Mutant p53 as a guardian of the cancer cell. Cell Death Differ.
26:199–212. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Deans C and Maggert KA: What do you mean,
‘epigenetic’? Genetics. 199:887–896. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bashyam MD, Animireddy S, Bala P, Naz A
and George SA: The Yin and Yang of cancer genes. Gene. 704:121–133.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen P, Li W and Li G: Structures and
functions of chromatin fibers. Annu Rev Biophys. 50:95–116. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ferreira HJ and Esteller M: Non-coding
RNAs, epigenetics, and cancer: Tying it all together. Cancer
Metastasis Rev. 37:55–73. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang L, Lu Q and Chang C: Epigenetics in
health and disease. Adv Exp Med Biol. 1253:3–55. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lu Y, Chan YT, Tan HY, Li S, Wang N and
Feng Y: Epigenetic regulation in human cancer: The potential role
of epi-drug in cancer therapy. Mol Cancer. 19:792020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ding X, He M, Chan AWH, Song QX, Sze SC,
Chen H, Man MKH, Man K, Chan SL, Lai PBS, et al: Genomic and
epigenomic features of primary and recurrent hepatocellular
carcinomas. Gastroenterology. 157:1630–1645.e6. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Malouf GG, Taube JH, Lu Y, Roysarkar T,
Panjarian S, Estecio MR, Jelinek J, Yamazaki J, Raynal NJ, Long H,
et al: Architecture of epigenetic reprogramming following
Twist1-mediated epithelial-mesenchymal transition. Genome Biol.
14:R1442013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Miranda Furtado CL, Dos Santos Luciano MC,
Silva Santos RD, Furtado GP, Moraes MO and Pessoa C: Epidrugs:
targeting epigenetic marks in cancer treatment. Epigenetics.
14:1164–1176. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lyko F: The DNA methyltransferase family:
a versatile toolkit for epigenetic regulation. Nat Rev Genet.
19:81–92. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ren W, Gao L and Song J: Structural basis
of DNMT1 and DNMT3A-mediated DNA methylation. Genes (Basel).
9:6202018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen Z and Zhang Y: Role of mammalian DNA
methyltransferases in development. Annu Rev Biochem. 89:135–158.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Smith ZD and Meissner A: DNA methylation:
Roles in mammalian development. Nat Rev Genet. 14:204–220. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang ZM, Lu R, Wang P, Yu Y, Chen D, Gao
L, Liu S, Ji D, Rothbart SB, Wang Y, et al: Structural basis for
DNMT3A-mediated de novo DNA methylation. Nature. 554:387–391. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Veland N, Lu Y, Hardikar S, Gaddis S, Zeng
Y, Liu B, Estecio MR, Takata Y, Lin K, Tomida MW, et al: DNMT3L
facilitates DNA methylation partly by maintaining DNMT3A stability
in mouse embryonic stem cells. Nucleic Acids Res. 47:152–167. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Loaeza-Loaeza J, Beltran AS and
Hernández-Sotelo D: DNMTs and impact of CpG content, transcription
factors, consensus motifs, lncRNAs, and histone marks on DNA
methylation. Genes (Basel). 11:13362020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Onodera A, González-Avalos E, Lio CWJ,
Georges RO, Bellacosa A, Nakayama T and Rao A: Roles of TET and TDG
in DNA demethylation in proliferating and non-proliferating immune
cells. Genome Biol. 22:1862021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rasmussen KD and Helin K: Role of TET
enzymes in DNA methylation, development, and cancer. Genes Dev.
30:733–750. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sabino JC, de Almeida MR, Abreu PL,
Ferreira AM, Caldas P, Domingues MM, Santos NC, Azzalin CM, Grosso
AR and de Almeida SF: Epigenetic reprogramming by TET enzymes
impacts co-transcriptional R-loops. Elife. 11:e694762022.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li C, Fan Y, Li G, Xu X, Duan J, Li R,
Kang X, Ma X, Chen X, Ke Y, et al: DNA methylation reprogramming of
functional elements during mammalian embryonic development. Cell
Discov. 4:412018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dossin F, Pinheiro I, Żylicz JJ, Roensch
J, Collombet S, Le Saux A, Chelmicki T, Attia M, Kapoor V, Zhan Y,
et al: SPEN integrates transcriptional and epigenetic control of
X-inactivation. Nature. 578:455–460. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tucci V, Isles AR, Kelsey G and
Ferguson-Smith AC; Erice Imprinting Group, : Genomic imprinting and
physiological processes in mammals. Cell. 176:952–965. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang M, Wu J, Zhong W, Zhao Z and He W:
DNA-methylation-induced silencing of DIO3OS drives non-small cell
lung cancer progression via activating hnRNPK-MYC-CDC25A axis. Mol
Ther Oncolytics. 23:205–219. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kennedy EM, Goehring GN, Nichols MH,
Robins C, Mehta D, Klengel T, Eskin E, Smith AK and Conneely KN: An
integrated-omics analysis of the epigenetic landscape of gene
expression in human blood cells. BMC Genomics. 19:4762018.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kanwal R, Gupta K and Gupta S: Cancer
epigenetics: An introduction. Methods Mol Biol. 1238:3–25. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Klutstein M, Nejman D, Greenfield R and
Cedar H: DNA methylation in cancer and aging. Cancer Res.
76:3446–3450. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sheaffer KL, Elliott EN and Kaestner KH:
DNA hypomethylation contributes to genomic instability and
intestinal cancer initiation. Cancer Prev Res (Phila). 9:534–546.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hatziapostolou M and Iliopoulos D:
Epigenetic aberrations during oncogenesis. Cell Mol Life Sci.
68:1681–1702. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Guerrero-Preston R, Michailidi C,
Marchionni L, Pickering CR, Frederick MJ, Myers JN,
Yegnasubramanian S, Hadar T, Noordhuis MG, Zizkova V, et al: Key
tumor suppressor genes inactivated by ‘greater promoter’
methylation and somatic mutations in head and neck cancer.
Epigenetics. 9:1031–1046. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Faam B, Ghaffari MA, Khorsandi L, Ghadiri
AA, Totonchi M, Amouzegar A, Fanaei SA, Azizi F, Shahbazian HB and
Hashemi Tabar M: RAP1GAP functions as a tumor suppressor gene and
is regulated by DNA methylation in differentiated thyroid cancer.
Cytogenet Genome Res. 161:227–235. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chantre-Justino M, Gonçalves da Veiga
Pires I, Cardoso Figueiredo M, Dos Santos Moreira A, Alves G and
Faria Ornellas MH: Genetic and methylation status of CDKN2A
(p14ARF/p16INK4A) and TP53 genes in recurrent
respiratory papillomatosis. Hum Pathol. 119:94–104. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Han B, Yang X, Zhang P, Zhang Y, Tu Y, He
Z, Li Y, Yuan J, Dong Y, Hosseini DK, et al: DNA methylation
biomarkers for nasopharyngeal carcinoma. PLoS One. 15:e02305242020.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hoang NM and Rui L: DNA methyltransferases
in hematological malignancies. J Genet Genomics. 47:361–372. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Steele N, Finn P, Brown R and Plumb JA:
Combined inhibition of DNA methylation and histone acetylation
enhances gene re-expression and drug sensitivity in vivo. Br J
Cancer. 100:758–763. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bao Y, Oguz G, Lee WC, Lee PL, Ghosh K, Li
J, Wang P, Lobie PE, Ehmsen S, Ditzel HJ, et al: EZH2-mediated PP2A
inactivation confers resistance to HER2-targeted breast cancer
therapy. Nat Commun. 11:58782020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Vijayaraghavalu S and Labhasetwar V:
Nanogel-mediated delivery of a cocktail of epigenetic drugs plus
doxorubicin overcomes drug resistance in breast cancer cells. Drug
Deliv Transl Res. 8:1289–1299. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhou Z, Li HQ and Liu F: DNA
methyltransferase inhibitors and their therapeutic potential. Curr
Top Med Chem. 18:2448–2457. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kedhari Sundaram M, Hussain A, Haque S,
Raina R and Afroze N: Quercetin modifies 5′CpG promoter methylation
and reactivates various tumor suppressor genes by modulating
epigenetic marks in human cervical cancer cells. J Cell Biochem.
120:18357–18369. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sanaei M, Kavoosi F and Behjoo H: Effect
of valproic acid and zebularine on SOCS-1 and SOCS-3 gene
expression in colon carcinoma SW48 cell line. Exp Oncol.
42:183–187. 2020.PubMed/NCBI
|
|
42
|
Capdevila J, Arqués O, Hernández Mora JR,
Matito J, Caratù G, Mancuso FM, Landolfi S, Barriuso J,
Jimenez-Fonseca P, Lopez Lopez C, et al: Epigenetic EGFR gene
repression confers sensitivity to therapeutic BRAFV600E blockade in
colon neuroendocrine carcinomas. Clin Cancer Res. 26:902–909. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Marques-Magalhães Â, Graça I, Henrique R
and Jerónimo C: Targeting DNA methyltranferases in urological
tumors. Front Pharmacol. 9:3662018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Xylinas E, Hassler MR, Zhuang D,
Krzywinski M, Erdem Z, Robinson BD, Elemento O, Clozel T and
Shariat SF: An epigenomic approach to improving response to
neoadjuvant cisplatin chemotherapy in bladder cancer. Biomolecules.
6:372016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Stresemann C and Lyko F: Modes of action
of the DNA methyltransferase inhibitors azacytidine and decitabine.
Int J Cancer. 123:8–13. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chakrawarti L, Agrawal R, Dang S, Gupta S
and Gabrani R: Therapeutic effects of EGCG: A patent review. Expert
Opin Ther Pat. 26:907–916. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Beisler JA: Isolation, characterization,
and properties of a labile hydrolysis product of the antitumor
nucleoside, 5-azacytidine. J Med Chem. 21:204–208. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Rahman MF, Raj R and Govindarajan R:
Identification of structural and molecular features involved in the
transport of 3′-Deoxy-nucleoside analogs by human equilibrative
nucleoside transporter 3. Drug Metab Dispos. 46:600–609. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Momparler RL and Derse D: Kinetics of
phosphorylation of 5-aza-2′-deoxyycytidine by deoxycytidine kinase.
Biochem Pharmacol. 28:1443–1444. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Santi DV, Garrett CE and Barr PJ: On the
mechanism of inhibition of DNA-cytosine methyltransferases by
cytosine analogs. Cell. 33:9–10. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Seelan RS, Mukhopadhyay P, Pisano MM and
Greene RM: Effects of 5-Aza-2′-deoxycytidine (decitabine) on gene
expression. Drug Metab Rev. 50:193–207. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zheng Z, Li L, Liu X, Wang D, Tu B, Wang
L, Wang H and Zhu WG: 5-Aza-2′-deoxycytidine reactivates gene
expression via degradation of pRb pocket proteins. FASEB J.
26:449–459. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Sorm F, Pískala A, Cihák A and Veselý J:
5-Azacytidine, a new, highly effective cancerostatic. Experientia.
20:202–203. 1964. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Sorm F and Vesely J: The activity of a new
antimetabolite, 5-azacytidine, against lymphoid leukaemia in AK
mice. Neoplasma. 11:123–130. 1964.PubMed/NCBI
|
|
55
|
Case DC Jr: 5-azacytidine in refractory
acute leukemia. Oncology. 39:218–221. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Tanaka K, Appella E and Jay G:
Developmental activation of the H-2K gene is correlated with an
increase in DNA methylation. Cell. 35:457–465. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Vogler WR, Miller DS and Keller JW:
5-Azacytidine (NSC 102816): A new drug for the treatment of
myeloblastic leukemia. Blood. 48:331–337. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Kaminskas E, Farrell A, Abraham S, Baird
A, Hsieh LS, Lee SL, Leighton JK, Patel H, Rahman A, Sridhara R, et
al: Approval summary: Azacitidine for treatment of myelodysplastic
syndrome subtypes. Clin Cancer Res. 11:3604–3608. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Ganesan A, Arimondo PB, Rots MG, Jeronimo
C and Berdasco M: The timeline of epigenetic drug discovery: From
reality to dreams. Clin Epigenetics. 11:1742019. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Duchmann M and Itzykson R: Clinical update
on hypomethylating agents. Int J Hematol. 110:161–169. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Schaefer M, Hagemann S, Hanna K and Lyko
F: Azacytidine inhibits RNA methylation at DNMT2 target sites in
human cancer cell lines. Cancer Res. 69:8127–8132. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Hollenbach PW, Nguyen AN, Brady H,
Williams M, Ning Y, Richard N, Krushel L, Aukerman SL, Heise C and
MacBeth KJ: A comparison of azacitidine and decitabine activities
in acute myeloid leukemia cell lines. PLoS One. 5:e90012010.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Venturelli S, Berger A, Weiland T, Essmann
F, Waibel M, Nuebling T, Häcker S, Schenk M, Schulze-Osthoff K,
Salih HR, et al: Differential induction of apoptosis and senescence
by the DNA methyltransferase inhibitors 5-azacytidine and
5-aza-2′-deoxycytidine in solid tumor cells. Mol Cancer Ther.
12:2226–2236. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Jin S, Cojocari D, Purkal JJ, Popovic R,
Talaty NN, Xiao Y, Solomon LR, Boghaert ER, Leverson JD and
Phillips DC: 5-Azacitidine induces NOXA to prime AML cells for
venetoclax-mediated apoptosis. Clin Cancer Res. 26:3371–3383. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Fabre C, Grosjean J, Tailler M, Boehrer S,
Adès L, Perfettini JL, de Botton S, Fenaux P and Kroemer G: A novel
effect of DNA methyltransferase and histone deacetylase inhibitors:
NFkappaB inhibition in malignant myeloblasts. Cell Cycle.
7:2139–2145. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Carbajo-García MC, Corachán A,
Segura-Benitez M, Monleón J, Escrig J, Faus A, Pellicer A, Cervelló
I and Ferrero H: 5-aza-2′-deoxycitidine inhibits cell
proliferation, extracellular matrix formation and Wnt/β-catenin
pathway in human uterine leiomyomas. Reprod Biol Endocrinol.
19:1062021. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Linnekamp JF, Kandimalla R, Fessler E, de
Jong JH, Rodermond HM, van Bochove GGW, The FO, Punt CJA, Bemelman
WA, van de Ven AWH, et al: Pre-operative decitabine in colon cancer
patients: Analyses on WNT target methylation and expression.
Cancers (Basel). 13:23572021. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Santini V: How I treat MDS after
hypomethylating agent failure. Blood. 133:521–529. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Mabaera R, Greene MR, Richardson CA,
Conine SJ, Kozul CD and Lowrey CH: Neither DNA hypomethylation nor
changes in the kinetics of erythroid differentiation explain
5-azacytidine's ability to induce human fetal hemoglobin. Blood.
111:411–420. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Susanto JM, Colvin EK, Pinese M, Chang DK,
Pajic M, Mawson A, Caldon CE, Musgrove EA, Henshall SM, Sutherland
RL, et al: The epigenetic agents suberoylanilide hydroxamic acid
and 5-AZA-2′ deoxycytidine decrease cell proliferation, induce cell
death and delay the growth of MiaPaCa2 pancreatic cancer cells in
vivo. Int J Oncol. 46:2223–2230. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Evans IC, Barnes JL, Garner IM, Pearce DR,
Maher TM, Shiwen X, Renzoni EA, Wells AU, Denton CP, Laurent GJ, et
al: Epigenetic regulation of cyclooxygenase-2 by methylation of
c8orf4 in pulmonary fibrosis. Clin Sci (Lond). 130:575–586. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Allis CD and Jenuwein T: The molecular
hallmarks of epigenetic control. Nat Rev Genet. 17:487–500. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Meng CF, Zhu XJ, Peng G and Dai DQ:
Promoter histone H3 lysine 9 di-methylation is associated with DNA
methylation and aberrant expression of p16 in gastric cancer cells.
Oncol Rep. 22:1221–1227. 2009.PubMed/NCBI
|
|
74
|
Lee SH, Kim J, Kim WH and Lee YM: Hypoxic
silencing of tumor suppressor RUNX3 by histone modification in
gastric cancer cells. Oncogene. 28:184–194. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Nguyen CT, Weisenberger DJ, Velicescu M,
Gonzales FA, Lin JCY, Liang G and Jones PA: Histone H3-lysine 9
methylation is associated with aberrant gene silencing in cancer
cells and is rapidly reversed by 5-aza-2′-deoxycytidine. Cancer
Res. 62:6456–6461. 2002.PubMed/NCBI
|
|
76
|
Griffiths EA and Gore SD: Epigenetic
therapies in MDS and AML. Adv Exp Med Biol. 754:253–283. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Nguyen AN, Hollenbach PW, Richard N,
Luna-Moran A, Brady H, Heise C and MacBeth KJ: Azacitidine and
decitabine have different mechanisms of action in non-small cell
lung cancer cell lines. Lung Cancer (Auckl). 1:119–140.
2010.PubMed/NCBI
|
|
78
|
Home-ClinicalTrials.govNovember 22–2022https://clinicaltrials.gov/
|
|
79
|
Hurd PJ, Whitmarsh AJ, Baldwin GS, Kelly
SM, Waltho JP, Price NC, Connolly BA and Hornby DP: Mechanism-based
inhibition of C5-cytosine DNA methyltransferases by 2-H
pyrimidinone. J Mol Biol. 286:389–401. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Yoo CB, Cheng JC and Jones PA: Zebularine:
A new drug for epigenetic therapy. Biochem Soc Trans. 32:910–912.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Ferguson LR, Tatham AL, Lin Z and Denny
WA: Epigenetic regulation of gene expression as an anticancer drug
target. Curr Cancer Drug Targets. 11:199–212. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Dueñas-Gonzalez A, Coronel J, Cetina L,
González-Fierro A, Chavez-Blanco A and Taja-Chayeb L:
Hydralazine-valproate: A repositioned drug combination for the
epigenetic therapy of cancer. Expert Opin Drug Metab Toxicol.
10:1433–1444. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Singh N, Dueñas-González A, Lyko F and
Medina-Franco JL: Molecular modeling and molecular dynamics studies
of hydralazine with human DNA methyltransferase 1. ChemMedChem.
4:792–799. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Daher-Reyes GS, Merchan BM and Yee KWL:
Guadecitabine (SGI-110): An investigational drug for the treatment
of myelodysplastic syndrome and acute myeloid leukemia. Expert Opin
Investig Drugs. 28:835–849. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Salvador LA and Luesch H: Discovery and
mechanism of natural products as modulators of histone acetylation.
Curr Drug Targets. 13:1029–1047. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Zhang Y, Wang X, Han L, Zhou Y and Sun S:
Green tea polyphenol EGCG reverse cisplatin resistance of A549/DDP
cell line through candidate genes demethylation. Biomed
Pharmacother. 69:285–290. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Alaskhar Alhamwe B, Khalaila R, Wolf J,
von Bülow V, Harb H, Alhamdan F, Hii CS, Prescott SL, Ferrante A,
Renz H, et al: Histone modifications and their role in epigenetics
of atopy and allergic diseases. Allergy Asthma Clin Immunol.
14:392018. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Lawrence M, Daujat S and Schneider R:
Lateral thinking: How histone modifications regulate gene
expression. Trends Genet. 32:42–56. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Lakshmaiah KC, Jacob LA, Aparna S,
Lokanatha D and Saldanha SC: Epigenetic therapy of cancer with
histone deacetylase inhibitors. J Cancer Res Ther. 10:469–478.
2014.PubMed/NCBI
|
|
90
|
Albaugh BN, Arnold KM and Denu JM:
KAT(ching) metabolism by the tail: Insight into the links between
lysine acetyltransferases and metabolism. Chembiochem. 12:290–298.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Yang J, Song C and Zhan X: The role of
protein acetylation in carcinogenesis and targeted drug discovery.
Front Endocrinol (Lausanne). 13:9723122022. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Cohen I, Poręba E, Kamieniarz K and
Schneider R: Histone modifiers in cancer: Friends or foes? Genes
Cancer. 2:631–647. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Fan P, Zhao J, Meng Z, Wu H, Wang B, Wu H
and Jin X: Overexpressed histone acetyltransferase 1 regulates
cancer immunity by increasing programmed death-ligand 1 expression
in pancreatic cancer. J Exp Clin Cancer Res. 38:472019. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Sun XJ, Man N, Tan Y, Nimer SD and Wang L:
The role of histone acetyltransferases in normal and malignant
hematopoiesis. Front Oncol. 5:1082015. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Wang P, Wang Z and Liu J: Role of HDACs in
normal and malignant hematopoiesis. Mol Cancer. 19:52020.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Carraway HE, Malkaram SA, Cen Y, Shatnawi
A, Fan J, Ali HEA, Abd Elmageed ZY, Buttolph T, Denvir J, Primerano
DA and Fandy TE: Activation of SIRT6 by DNA hypomethylating agents
and clinical consequences on combination therapy in leukemia. Sci
Rep. 10:103252020. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Hu XT, Xing W, Zhao RS, Tan Y, Wu XF, Ao
LQ, Li Z, Yao MW, Yuan M, Guo W, et al: HDAC2 inhibits EMT-mediated
cancer metastasis by downregulating the long noncoding RNA H19 in
colorectal cancer. J Exp Clin Cancer Res. 39:2702020. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Körholz K, Ridinger J, Krunic D, Najafi S,
Gerloff XF, Frese K, Meder B, Peterziel H, Vega-Rubin-de-Celis S,
Witt O and Oehme I: Broad-spectrum HDAC inhibitors promote
autophagy through FOXO transcription factors in neuroblastoma.
Cells. 10:10012021. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Chun P: Histone deacetylase inhibitors in
hematological malignancies and solid tumors. Arch Pharm Res.
38:933–949. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Jęśko H, Wencel P, Strosznajder RP and
Strosznajder JB: Sirtuins and their roles in brain aging and
neurodegenerative disorders. Neurochem Res. 42:876–890. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Willis-Martinez D, Richards HW, Timchenko
NA and Medrano EE: Role of HDAC1 in senescence, aging, and cancer.
Exp Gerontol. 45:279–285. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Glozak MA and Seto E: Histone deacetylases
and cancer. Oncogene. 26:5420–5432. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Audia JE and Campbell RM: Histone
modifications and cancer. Cold Spring Harb Perspect Biol.
8:a0195212016. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Bergamin E, Sarvan S, Malette J, Eram MS,
Yeung S, Mongeon V, Joshi M, Brunzelle JS, Michaels SD, Blais A, et
al: Molecular basis for the methylation specificity of ATXR5 for
histone H3. Nucleic Acids Res. 45:6375–6387. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Mohan M, Herz HM and Shilatifard A:
SnapShot: Histone lysine methylase complexes. Cell. 149:498–498.e1.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
From the American Association of
Neurological Surgeons (AANS), American Society of Neuroradiology
(ASNR), Cardiovascular and Interventional Radiology Society of
Europe (CIRSE), Canadian Interventional Radiology Association
(CIRA), Congress of Neurological Surgeons (CNS), European Society
of Minimally Invasive Neurological Therapy (ESMINT), European
Society of Neuroradiology (ESNR), European Stroke Organization
(ESO), Society for Cardiovascular Angiography and Interventions
(SCAI), Society of Interventional Radiology (SIR), ; et al:
Multisociety consensus quality improvement revised consensus
statement for endovascular therapy of acute ischemic stroke. Int J
Stroke. 13:612–632. 2018.PubMed/NCBI
|
|
107
|
McGrath J and Trojer P: Targeting histone
lysine methylation in cancer. Pharmacol Ther. 150:1–22. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Wan YCE, Liu J and Chan KM: Histone H3
mutations in cancer. Curr Pharmacol Rep. 4:292–300. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Salz T, Deng C, Pampo C, Siemann D, Qiu Y,
Brown K and Huang S: Histone methyltransferase hSETD1A is a novel
regulator of metastasis in breast cancer. Mol Cancer Res.
13:461–469. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Behjati S, Tarpey PS, Presneau N, Scheipl
S, Pillay N, Van Loo P, Wedge DC, Cooke SL, Gundem G, Davies H, et
al: Distinct H3F3A and H3F3B driver mutations define
chondroblastoma and giant cell tumor of bone. Nat Genet.
45:1479–1482. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Papillon-Cavanagh S, Lu C, Gayden T,
Mikael LG, Bechet D, Karamboulas C, Ailles L, Karamchandani J,
Marchione DM, Garcia BA, et al: Impaired H3K36 methylation defines
a subset of head and neck squamous cell carcinomas. Nat Genet.
49:180–185. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Yang T, Yang Y and Wang Y: Predictive
biomarkers and potential drug combinations of epi-drugs in cancer
therapy. Clin Epigenetics. 13:1132021. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Bates SE: Epigenetic therapies for cancer.
N Engl J Med. 383:650–663. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Yaseen A, Chen S, Hock S, Rosato R, Dent
P, Dai Y and Grant S: Resveratrol sensitizes acute myelogenous
leukemia cells to histone deacetylase inhibitors through reactive
oxygen species-mediated activation of the extrinsic apoptotic
pathway. Mol Pharmacol. 82:1030–1041. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Bubna AK: Vorinostat-an overview. Indian J
Dermatol. 60:4192015. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Richon VM: Cancer biology: Mechanism of
antitumour action of vorinostat (suberoylanilide hydroxamic acid),
a novel histone deacetylase inhibitor. Br J Cancer. 95 (Suppl
1):S2–S6. 2006. View Article : Google Scholar
|
|
117
|
Bolden JE, Peart MJ and Johnstone RW:
Anticancer activities of histone deacetylase inhibitors. Nat Rev
Drug Discov. 5:769–784. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Siegel D, Hussein M, Belani C, Robert F,
Galanis E, Richon VM, Garcia-Vargas J, Sanz-Rodriguez C and Rizvi
S: Vorinostat in solid and hematologic malignancies. J Hematol
Oncol. 2:312009. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Nakajima H, Kim YB, Terano H, Yoshida M
and Horinouchi S: FR901228, a potent antitumor antibiotic, is a
novel histone deacetylase inhibitor. Exp Cell Res. 241:126–133.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
VanderMolen KM, McCulloch W, Pearce CJ and
Oberlies NH: Romidepsin (Istodax, NSC 630176, FR901228, FK228,
depsipeptide): A natural product recently approved for cutaneous
T-cell lymphoma. J Antibiot (Tokyo). 64:525–531. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Marshall JL, Rizvi N, Kauh J, Dahut W,
Figuera M, Kang MH, Figg WD, Wainer I, Chaissang C, Li MZ and
Hawkins MJ: A phase I trial of depsipeptide (FR901228) in patients
with advanced cancer. J Exp Ther Oncol. 2:325–332. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Petrich A and Nabhan C: Use of class I
histone deacetylase inhibitor romidepsin in combination regimens.
Leuk Lymphoma. 57:1755–1765. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
O'Connor OA, Horwitz S, Masszi T, Van Hoof
A, Brown P, Doorduijn J, Hess G, Jurczak W, Knoblauch P, Chawla S,
et al: Belinostat in patients with relapsed or refractory
peripheral T-cell lymphoma: Results of the pivotal phase II BELIEF
(CLN-19) study. J Clin Oncol. 33:2492–2499. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Poole RM: Belinostat: First global
approval. Drugs. 74:1543–1554. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Autin P, Blanquart C and Fradin D:
Epigenetic drugs for cancer and microRNAs: A focus on histone
deacetylase inhibitors. Cancers (Basel). 11:15302019. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Spratlin JL, Pitts TM, Kulikowski GN,
Morelli MP, Tentler JJ, Serkova NJ and Eckhardt SG: Synergistic
activity of histone deacetylase and proteasome inhibition against
pancreatic and hepatocellular cancer cell lines. Anticancer Res.
31:1093–1103. 2011.PubMed/NCBI
|
|
127
|
Lee MJ, Kim YS, Kummar S, Giaccone G and
Trepel JB: Histone deacetylase inhibitors in cancer therapy. Curr
Opin Oncol. 20:639–649. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Gibney ER and Nolan CM: Epigenetics and
gene expression. Heredity (Edinb). 105:4–13. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
He J, Xie Q, Xu H, Li J and Li Y: Circular
RNAs and cancer. Cancer Lett. 396:138–144. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Khanna A and Stamm S: Regulation of
alternative splicing by short non-coding nuclear RNAs. RNA Biol.
7:480–485. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Kwek KY, Murphy S, Furger A, Thomas B,
O'Gorman W, Kimura H, Proudfoot NJ and Akoulitchev A: U1 snRNA
associates with TFIIH and regulates transcriptional initiation. Nat
Struct Mol Biol. 9:800–805. 2002.PubMed/NCBI
|
|
132
|
Eilebrecht S, Brysbaert G, Wegert T,
Urlaub H, Benecke BJ and Benecke A: 7SK small nuclear RNA directly
affects HMGA1 function in transcription regulation. Nucleic Acids
Res. 39:2057–2072. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Szymanski M, Erdmann VA and Barciszewski
J: Noncoding RNAs database (ncRNAdb). Nucleic Acids Res.
35:(Database Issue). D162–D164. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Chu CY and Rana TM: Small RNAs: Regulators
and guardians of the genome. J Cell Physiol. 213:412–419. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Abi A, Farahani N, Molavi G and Gheibi
Hayat SM: Circular RNAs: Epigenetic regulators in cancerous and
noncancerous skin diseases. Cancer Gene Ther. 27:280–293. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Quinn JJ and Chang HY: Unique features of
long non-coding RNA biogenesis and function. Nat Rev Genet.
17:47–62. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Ding X, Zhang S, Li X, Feng C, Huang Q,
Wang S, Wang S, Xia W, Yang F, Yin R, et al: Profiling expression
of coding genes, long noncoding RNA, and circular RNA in lung
adenocarcinoma by ribosomal RNA-depleted RNA sequencing. FEBS Open
Bio. 8:544–555. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Lam JKW, Chow MYT, Zhang Y and Leung SWS:
siRNA versus miRNA as therapeutics for gene silencing. Mol Ther
Nucleic Acids. 4:e2522015. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Meister G and Tuschl T: Mechanisms of gene
silencing by double-stranded RNA. Nature. 431:343–349. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Kim VN: MicroRNA biogenesis: Coordinated
cropping and dicing. Nat Rev Mol Cell Biol. 6:376–385. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Lee Y, Kim M, Han J, Yeom KH, Lee S, Baek
SH and Kim VN: MicroRNA genes are transcribed by RNA polymerase II.
EMBO J. 23:4051–4060. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Tomari Y and Zamore PD: Perspective:
Machines for RNAi. Genes Dev. 19:517–529. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Paturi S and Deshmukh MV: A glimpse of
‘dicer biology’ through the structural and functional perspective.
Front Mol Biosci. 8:6436572021. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Alagia A, Jorge AF, Aviñó A, Cova TFGG,
Crehuet R, Grijalvo S, Pais AACC and Eritja R: Exploring
PAZ/3′-overhang interaction to improve siRNA specificity. A
combined experimental and modeling study. Chem Sci. 9:2074–2086.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Wu L, Fan J and Belasco JG: MicroRNAs
direct rapid deadenylation of mRNA. Proc Natl Acad Sci USA.
103:4034–4039. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Chen L, Dahlstrom JE, Lee SH and Rangasamy
D: Naturally occurring endo-siRNA silences LINE-1 retrotransposons
in human cells through DNA methylation. Epigenetics. 7:758–771.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Guo B, Li D, Du L and Zhu X: piRNAs:
Biogenesis and their potential roles in cancer. Cancer Metastasis
Rev. 39:567–575. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Sparmann A: piRNAs-guardians of the
germline. Nat Res. 2019.
|
|
149
|
Iwasaki YW, Siomi MC and Siomi H:
PIWI-interacting RNA: Its biogenesis and functions. Annu Rev
Biochem. 84:405–433. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Caramuta S, Egyházi S, Rodolfo M, Witten
D, Hansson J, Larsson C and Lui WO: MicroRNA expression profiles
associated with mutational status and survival in malignant
melanoma. J Invest Dermatol. 130:2062–2070. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Chi J, Ballabio E, Chen XH, Kušec R,
Taylor S, Hay D, Tramonti D, Saunders NJ, Littlewood T, Pezzella F,
et al: MicroRNA expression in multiple myeloma is associated with
genetic subtype, isotype and survival. Biol Direct. 6:232011.
View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Garzon R, Garofalo M, Martelli MP,
Briesewitz R, Wang L, Fernandez-Cymering C, Volinia S, Liu CG,
Schnittger S, Haferlach T, et al: Distinctive microRNA signature of
acute myeloid leukemia bearing cytoplasmic mutated nucleophosmin.
Proc Natl Acad Sci USA. 105:3945–3950. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Iorio MV, Ferracin M, Liu CG, Veronese A,
Spizzo R, Sabbioni S, Magri E, Pedriali M, Fabbri M, Campiglio M,
et al: MicroRNA gene expression deregulation in human breast
cancer. Cancer Res. 65:7065–7070. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Calin GA, Cimmino A, Fabbri M, Ferracin M,
Wojcik SE, Shimizu M, Taccioli C, Zanesi N, Garzon R, Aqeilan RI,
et al: MiR-15a and miR-16-1 cluster functions in human leukemia.
Proc Natl Acad Sci USA. 105:5166–5171. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Cimmino A, Calin GA, Fabbri M, Iorio MV,
Ferracin M, Shimizu M, Wojcik SE, Aqeilan RI, Zupo S, Dono M, et
al: miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc Natl
Acad Sci USA. 102:13944–13949. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Mogilyansky E and Rigoutsos I: The
miR-17/92 cluster: A comprehensive update on its genomics,
genetics, functions and increasingly important and numerous roles
in health and disease. Cell Death Differ. 20:1603–1614. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Petitjean A, Mathe E, Kato S, Ishioka C,
Tavtigian SV, Hainaut P and Olivier M: Impact of mutant p53
functional properties on TP53 mutation patterns and tumor
phenotype: Lessons from recent developments in the IARC TP53
database. Hum Mutat. 28:622–629. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Liao JM, Cao B, Zhou X and Lu H: New
insights into p53 functions through its target microRNAs. J Mol
Cell Biol. 6:206–213. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Madrigal T, Hernández-Monge J, Herrera LA,
González-De la Rosa CH, Domínguez-Gómez G, Candelaria M,
Luna-Maldonado F, Calderón González KG and Díaz-Chávez J:
Regulation of miRNAs expression by mutant p53 gain of function in
cancer. Front Cell Dev Biol. 9:6957232021. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Tornesello ML, Annunziata C, Tornesello
AL, Buonaguro L and Buonaguro FM: Human oncoviruses and p53 tumor
suppressor pathway deregulation at the origin of human cancers.
Cancers (Basel). 10:2132018. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Hassler MR, Turanov AA, Alterman JF,
Haraszti RA, Coles AH, Osborn MF, Echeverria D, Nikan M, Salomon
WE, Roux L, et al: Comparison of partially and fully
chemically-modified siRNA in conjugate-mediated delivery in vivo.
Nucleic Acids Res. 46:2185–2196. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Statello L, Guo CJ, Chen LL and Huarte M:
Gene regulation by long non-coding RNAs and its biological
functions. Nat Rev Mol Cell Biol. 22:96–118. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Gao N, Li Y, Li J, Gao Z, Yang Z, Li Y,
Liu H and Fan T: Long non-coding RNAs: The regulatory mechanisms,
research strategies, and future directions in cancers. Front Oncol.
10:5988172020. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Fang Y and Fullwood MJ: Roles, functions,
and mechanisms of long non-coding RNAs in cancer. Genomics
Proteomics Bioinformatics. 14:42–54. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Mathy NW and Chen XM: Long non-coding RNAs
(lncRNAs) and their transcriptional control of inflammatory
responses. J Biol Chem. 292:12375–12382. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Wang KC and Chang HY: Molecular mechanisms
of long noncoding RNAs. Mol Cell. 43:904–914. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Amirinejad R, Rezaei M and
Shirvani-Farsani Z: An update on long intergenic noncoding RNA p21:
A regulatory molecule with various significant functions in cancer.
Cell Biosci. 10:822020. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Hall JR, Messenger ZJ, Tam HW, Phillips
SL, Recio L and Smart RC: Long noncoding RNA lincRNA-p21 is the
major mediator of UVB-induced and p53-dependent apoptosis in
keratinocytes. Cell Death Dis. 6:e17002015. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Huarte M, Guttman M, Feldser D, Garber M,
Koziol MJ, Kenzelmann-Broz D, Khalil AM, Zuk O, Amit I, Rabani M,
et al: A large intergenic noncoding RNA induced by p53 mediates
global gene repression in the p53 response. Cell. 142:409–419.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Dai X, Kaushik AC and Zhang J: The
emerging role of major regulatory RNAs in cancer control. Front
Oncol. 9:9202019. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Fox AH, Nakagawa S, Hirose T and Bond CS:
Paraspeckles: Where long noncoding RNA meets phase separation.
Trends Biochem Sci. 43:124–135. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Sun TT, He J, Liang Q, Ren LL, Yan TT, Yu
TC, Tang JY, Bao YJ, Hu Y, Lin Y, et al: LncRNA GClnc1 promotes
gastric carcinogenesis and may Act as a modular scaffold of WDR5
and KAT2A complexes to specify the histone modification pattern.
Cancer Discov. 6:784–801. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Wang J, Zhu S, Meng N, He Y, Lu R and Yan
GR: ncRNA-encoded peptides or proteins and cancer. Mol Ther.
27:1718–1725. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Xing J, Liu H, Jiang W and Wang L:
LncRNA-encoded peptide: Functions and predicting methods. Front
Oncol. 10:6222942021. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Kong S, Tao M, Shen X and Ju S:
Translatable circRNAs and lncRNAs: Driving mechanisms and functions
of their translation products. Cancer Lett. 483:59–65. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Huang JZ, Chen M, Chen D, Gao XC, Zhu S,
Huang H, Hu M, Zhu H and Yan GR: A peptide encoded by a putative
lncRNA HOXB-AS3 suppresses colon cancer growth. Mol Cell.
68:171–184.e6. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Chen Y, Long W, Yang L, Zhao Y, Wu X, Li
M, Du F, Chen Y, Yang Z, Wen Q, et al: Functional peptides encoded
by long non-coding RNAs in gastrointestinal cancer. Front Oncol.
11:7773742021. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Chakraborty S, Andrieux G, Hasan AMM,
Ahmed M, Hosen MI, Rahman T, Hossain MA and Boerries M: Harnessing
the tissue and plasma lncRNA-peptidome to discover peptide-based
cancer biomarkers. Sci Rep. 9:123222019. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Welden JR and Stamm S: Pre-mRNA structures
forming circular RNAs. Biochim Biophys Acta Gene Regul Mech.
1862:1944102019. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Eger N, Schoppe L, Schuster S, Laufs U and
Boeckel JN: Circular RNA splicing. Xiao J: Circular RNAs. Advances
in Experimental Medicine and Biology. 1087. Springer; Singapore:
pp. 41–52. 2018, View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Nisar S, Bhat AA, Singh M, Karedath T,
Rizwan A, Hashem S, Bagga P, Reddy R, Jamal F, Uddin S, et al:
Insights into the role of CircRNAs: Biogenesis, characterization,
functional, and clinical impact in human malignancies. Front Cell
Dev Biol. 9:6172812021. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Zhao J, Tao Y, Zhou Y, Qin N, Chen C, Tian
D and Xu L: MicroRNA-7: A promising new target in cancer therapy.
Cancer Cell Int. 15:1032015. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Liu T, Liu S, Xu Y, Shu R, Wang F, Chen C,
Zeng Y and Luo H: Circular RNA-ZFR inhibited cell proliferation and
promoted apoptosis in gastric cancer by sponging miR-130a/miR-107
and modulating PTEN. Cancer Res Treat. 50:1396–1417. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Yu CY and Kuo HC: The emerging roles and
functions of circular RNAs and their generation. J Biomed Sci.
26:292019. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Jiang MP, Xu WX, Hou JC, Xu Q, Wang DD and
Tang JH: The emerging role of the interactions between circular
RNAs and RNA-binding proteins in common human cancers. J Cancer.
12:5206–5219. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Huang A, Zheng H, Wu Z, Chen M and Huang
Y: Circular RNA-protein interactions: Functions, mechanisms, and
identification. Theranostics. 10:3503–3517. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Du WW, Yang W, Li X, Awan FM, Yang Z, Fang
L, Lyu J, Li F, Peng C, Krylov SN, et al: A circular RNA circ-DNMT1
enhances breast cancer progression by activating autophagy.
Oncogene. 37:5829–5842. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Chen S, Thorne RF, Zhang XD, Wu M and Liu
L: Non-coding RNAs, guardians of the p53 galaxy. Semin Cancer Biol.
75:72–83. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Du WW, Yang W, Liu E, Yang Z, Dhaliwal P
and Yang BB: Foxo3 circular RNA retards cell cycle progression via
forming ternary complexes with p21 and CDK2. Nucleic Acids Res.
44:2846–2858. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Wang C, Tao W, Ni S and Chen Q: Circular
RNA circ-Foxo3 induced cell apoptosis in urothelial carcinoma via
interaction with miR-191-5p. Onco Targets Ther. 12:8085–8094. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Yang Y, Gao X, Zhang M, Yan S, Sun C, Xiao
F, Huang N, Yang X, Zhao K, Zhou H, et al: Novel role of FBXW7
circular RNA in repressing glioma tumorigenesis. J Natl Cancer
Inst. 110:304–315. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Park JW, Lagniton PNP, Liu Y and Xu RH:
mRNA vaccines for COVID-19: What, why and how. Int J Biol Sci.
17:1446–1460. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Damase TR, Sukhovershin R, Boada C,
Taraballi F, Pettigrew RI and Cooke JP: The limitless future of RNA
therapeutics. Front Bioeng Biotechnol. 9:6281372021. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Qian Y, Shi L and Luo Z: Long non-coding
RNAs in cancer: Implications for diagnosis, prognosis, and therapy.
Front Med (Lausanne). 7:6123932020. View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Kristensen LS, Hansen TB, Venø MT and
Kjems J: Circular RNAs in cancer: Opportunities and challenges in
the field. Oncogene. 37:555–565. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Ling H: Non-coding RNAs: Therapeutic
strategies and delivery systems. Adv Exp Med Biol. 937:229–237.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Weinberg MS and Morris KV: Transcriptional
gene silencing in humans. Nucleic Acids Res. 44:6505–6517. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Kotowska-Zimmer A, Pewinska M and
Olejniczak M: Artificial miRNAs as therapeutic tools: Challenges
and opportunities. Wiley Interdiscip Rev RNA. 12:e16402021.
View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Turner AMW and Morris KV: Controlling
transcription with noncoding RNAs in mammalian cells.
Biotechniques. 48:9–16. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Yoon S and Rossi JJ: Therapeutic potential
of small activating RNAs (saRNAs) in human cancers. Curr Pharm
Biotechnol. 19:604–610. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Scoles DR, Minikel EV and Pulst SM:
Antisense oligonucleotides: A primer. Neurol Genet. 5:e3232019.
View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Raghavendra P and Pullaiah T: RNA-based
applications in diagnostic and therapeutics for cancer. Advances in
Cell and Molecular Diagnostics. Elsevier; pp. 33–55. 2018,
View Article : Google Scholar
|
|
203
|
Ratti M, Lampis A, Ghidini M, Salati M,
Mirchev MB, Valeri N and Hahne JC: MicroRNAs (miRNAs) and long
non-coding RNAs (lncRNAs) as new tools for cancer therapy: First
steps from bench to bedside. Targ Oncol. 15:261–278. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Vicentini C, Galuppini F, Corbo V and
Fassan M: Current role of non-coding RNAs in the clinical setting.
Noncoding RNA Res. 4:82–85. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
205
|
Giudice V, Mensitieri F, Izzo V,
Filippelli A and Selleri C: Aptamers and antisense oligonucleotides
for diagnosis and treatment of hematological diseases. Int J Mol
Sci. 21:32522020. View Article : Google Scholar : PubMed/NCBI
|
|
206
|
Maruyama R and Yokota T: Knocking down
long noncoding RNAs using antisense oligonucleotide gapmers.
Methods Mol Biol. 2176:49–56. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
207
|
Bhan A, Hussain I, Ansari KI, Bobzean SAM,
Perrotti LI and Mandal SS: Bisphenol-A and diethylstilbestrol
exposure induces the expression of breast cancer associated long
noncoding RNA HOTAIR in vitro and in vivo. J Steroid Biochem Mol
Biol. 141:160–170. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
208
|
Simonson B and Das S: MicroRNA
therapeutics: The next magic bullet? Mini Rev Med Chem. 15:467–474.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
209
|
Zhong Y, Du Y, Yang X, Mo Y, Fan C, Xiong
F, Ren D, Ye X, Li C, Wang Y, et al: Circular RNAs function as
ceRNAs to regulate and control human cancer progression. Mol
Cancer. 17:792018. View Article : Google Scholar : PubMed/NCBI
|
|
210
|
Ebert MS, Neilson JR and Sharp PA:
MicroRNA sponges: Competitive inhibitors of small RNAs in mammalian
cells. Nat Methods. 4:721–726. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
211
|
Banks IR, Zhang Y, Wiggins BE, Heck GR and
Ivashuta S: RNA decoys: An emerging component of plant regulatory
networks? Plant Signal Behav. 7:1188–1193. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
212
|
Ebert MS and Sharp PA: MicroRNA sponges:
Progress and possibilities. RNA. 16:2043–2050. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
213
|
Bernardo BC, Ooi JY, Lin RC and McMullen
JR: miRNA therapeutics: A new class of drugs with potential
therapeutic applications in the heart. Future Med Chem.
7:1771–1792. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
214
|
Yoo J, Hajjar R and Jeong D: Generation of
efficient miRNA inhibitors using tough decoy constructs. Ishikawa
K: Cardiac Gene Therapy. Methods in Molecular Biology. 1521. Humana
Press; New York, NY: pp. 41–53. 2017, View Article : Google Scholar : PubMed/NCBI
|
|
215
|
Feng R, Patil S, Zhao X, Miao Z and Qian
A: RNA therapeutics-research and clinical advancements. Front Mol
Biosci. 8:7107382021. View Article : Google Scholar : PubMed/NCBI
|
|
216
|
Aimo A, Castiglione V, Rapezzi C, Franzini
M, Panichella G, Vergaro G, Gillmore J, Fontana M, Passino C and
Emdin M: RNA-targeting and gene editing therapies for transthyretin
amyloidosis. Nat Rev Cardiol. 19:655–667. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
217
|
Dever DP, Bak RO, Reinisch A, Camarena J,
Washington G, Nicolas CE, Pavel-Dinu M, Saxena N, Wilkens AB,
Mantri S, et al: CRISPR/Cas9 β-globin gene targeting in human
haematopoietic stem cells. Nature. 539:384–389. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
218
|
Long C, Amoasii L, Mireault AA, McAnally
JR, Li H, Sanchez-Ortiz E, Bhattacharyya S, Shelton JM, Bassel-Duby
R and Olson EN: Postnatal genome editing partially restores
dystrophin expression in a mouse model of muscular dystrophy.
Science. 351:400–403. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
219
|
Yin H, Xue W, Chen S, Bogorad RL,
Benedetti E, Grompe M, Koteliansky V, Sharp PA, Jacks T and
Anderson DG: Genome editing with Cas9 in adult mice corrects a
disease mutation and phenotype. Nat Biotechnol. 32:551–553. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
220
|
Lee SWL, Paoletti C, Campisi M, Osaki T,
Adriani G, Kamm RD, Mattu C and Chiono V: MicroRNA delivery through
nanoparticles. J Control Release. 313:80–95. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
221
|
Kawakami S and Hashida M: Targeted
delivery systems of small interfering RNA by systemic
administration. Drug Metab Pharmacokinet. 22:142–151. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
222
|
Soengas MS, Capodieci P, Polsky D, Mora J,
Esteller M, Opitz-Araya X, McCombie R, Herman JG, Gerald WL,
Lazebnik YA, et al: Inactivation of the apoptosis effector Apaf-1
in malignant melanoma. Nature. 409:207–211. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
223
|
Mann BS, Johnson JR, Cohen MH, Justice R
and Pazdur R: FDA approval summary: Vorinostat for treatment of
advanced primary cutaneous T-cell lymphoma. Oncologist.
12:1247–1252. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
224
|
Grant C, Rahman F, Piekarz R, Peer C, Frye
R, Robey RW, Gardner ER, Figg WD and Bates SE: Romidepsin: A new
therapy for cutaneous T-cell lymphoma and a potential therapy for
solid tumors. Expert Rev Anticancer Ther. 10:997–1008. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
225
|
Moore D: Panobinostat (Farydak): A novel
option for the treatment of relapsed or relapsed and refractory
multiple myeloma. P T. 41:296–300. 2016.PubMed/NCBI
|
|
226
|
Gilles ME and Slack FJ: Let-7 microRNA as
a potential therapeutic target with implications for immunotherapy.
Expert Opin Ther Targets. 22:929–939. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
227
|
Segal M, Biscans A, Gilles ME,
Anastasiadou E, De Luca R, Lim J, Khvorova A and Slack FJ:
Hydrophobically modified let-7b miRNA enhances biodistribution to
NSCLC and downregulates HMGA2 in vivo. Mol Ther Nucleic Acids.
19:267–277. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
228
|
Wu L, Wang Q, Yao J, Jiang H, Xiao C and
Wu F: MicroRNA let-7g and let-7i inhibit hepatoma cell growth
concurrently via downregulation of the anti-apoptotic protein
B-cell lymphoma-extra large. Oncol Lett. 9:213–218. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
229
|
Pan X, Wang G and Wang B: MicroRNA-1182
and let-7a exert synergistic inhibition on invasion, migration and
autophagy of cholangiocarcinoma cells through down-regulation of
NUAK1. Cancer Cell Int. 21:1612021. View Article : Google Scholar : PubMed/NCBI
|
|
230
|
Li M, Dou J, Pan M, Xu H and Xu Z:
MicroRNA-7 agomir is a potential bioactive material for breast
cancer therapy by inhibiting breast cancer stem cell
tumorigenicity. Mater Express. 11:824–831. 2021. View Article : Google Scholar
|
|
231
|
Hong DS, Kang YK, Borad M, Sachdev J,
Ejadi S, Lim HY, Brenner AJ, Park K, Lee JL, Kim TY, et al: Phase 1
study of MRX34, a liposomal miR-34a mimic, in patients with
advanced solid tumours. Br J Cancer. 122:1630–1637. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
232
|
van Zandwijk N, Pavlakis N, Kao SC, Linton
A, Boyer MJ, Clarke S, Huynh Y, Chrzanowska A, Fulham MJ, Bailey
DL, et al: Safety and activity of microRNA-loaded minicells in
patients with recurrent malignant pleural mesothelioma: A
first-in-man, phase 1, open-label, dose-escalation study. Lancet
Oncol. 18:1386–1396. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
233
|
Winata P, Williams M, McGowan E, Nassif N,
van Zandwijk N and Reid G: The analysis of novel microRNA mimic
sequences in cancer cells reveals lack of specificity in stem-loop
RT-qPCR-based microRNA detection. BMC Res Notes. 10:6002017.
View Article : Google Scholar : PubMed/NCBI
|
|
234
|
Filippova EA, Fridman MV, Burdennyy AM,
Loginov VI, Pronina IV, Lukina SS, Dmitriev AA and Braga EA: Long
Noncoding RNA GAS5 in breast cancer: Epigenetic mechanisms and
biological functions. Int J Mol Sci. 22:68102021. View Article : Google Scholar : PubMed/NCBI
|
|
235
|
Williams GT and Pickard MR: Long
non-coding RNAs: New opportunities and old challenges in cancer
therapy. Transl Cancer Res. 5 (Suppl 3):S564–S565. 2016. View Article : Google Scholar
|
|
236
|
Liu W, Zhan J, Zhong R, Li R, Sheng X, Xu
M, Lu Z and Zhang S: Upregulation of long noncoding RNA_GAS5
suppresses cell proliferation and metastasis in laryngeal cancer
via regulating PI3K/AKT/mTOR signaling pathway. Technol Cancer Res
Treat. 20:15330338219900742021. View Article : Google Scholar : PubMed/NCBI
|
|
237
|
Smaldone MC and Davies BJ: BC-819, a
plasmid comprising the H19 gene regulatory sequences and diphtheria
toxin A, for the potential targeted therapy of cancers. Curr Opin
Mol Ther. 12:607–616. 2010.PubMed/NCBI
|
|
238
|
Zhao X, Reebye V, Hitchen P, Fan J, Jiang
H, Sætrom P, Rossi J, Habib NA and Huang KW: Mechanisms involved in
the activation of C/EBPα by small activating RNA in hepatocellular
carcinoma. Oncogene. 38:3446–3457. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
239
|
Reebye V, Sætrom P, Mintz PJ, Huang KW,
Swiderski P, Peng L, Liu C, Liu X, Lindkaer-Jensen S, Zacharoulis
D, et al: Novel RNA oligonucleotide improves liver function and
inhibits liver carcinogenesis in vivo. Hepatology. 59:216–227.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
240
|
Seto AG, Beatty X, Lynch JM, Hermreck M,
Tetzlaff M, Duvic M and Jackson AL: Cobomarsen, an oligonucleotide
inhibitor of miR-155, co-ordinately regulates multiple survival
pathways to reduce cellular proliferation and survival in cutaneous
T-cell lymphoma. Br J Haematol. 183:428–444. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
241
|
Ijaz M, Wang F, Shahbaz M, Jiang W, Fathy
AH and Nesa EU: The role of Grb2 in cancer and peptides as Grb2
antagonists. Protein Pept Lett. 24:1084–1095. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
242
|
Ohanian M, Tari Ashizawa A, Garcia-Manero
G, Pemmaraju N, Kadia T, Jabbour E, Ravandi F, Borthakur G,
Andreeff M, Konopleva M, et al: Liposomal Grb2 antisense
oligodeoxynucleotide (BP1001) in patients with refractory or
relapsed haematological malignancies: A single-centre, open-label,
dose-escalation, phase 1/1b trial. Lancet Haematol. 5:e136–e146.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
243
|
Golan T, Khvalevsky EZ, Hubert A, Gabai
RM, Hen N, Segal A, Domb A, Harari G, David EB, Raskin S, et al:
RNAi therapy targeting KRAS in combination with chemotherapy for
locally advanced pancreatic cancer patients. Oncotarget.
6:24560–24570. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
244
|
Timar J and Kashofer K: Molecular
epidemiology and diagnostics of KRAS mutations in human cancer.
Cancer Metastasis Rev. 39:1029–1038. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
245
|
Zhang P, Liu X, Abegg D, Tanaka T, Tong Y,
Benhamou RI, Baisden J, Crynen G, Meyer SM, Cameron MD, et al:
Repro-gramming of protein-targeted small-molecule medicines to RNA
by ribonuclease recruitment. J Am Chem Soc. 143:13044–13055. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
246
|
Shortridge MD, Chaubey B, Zhang HJ,
Pavelitz T, Olsen GL, Calin GA and Varani G: Drug-like small
molecules that inhibit expression of the oncogenic microRNA-21.
bioRxiv. doi:. https://doi.org/10.1101/2022.04.30.490150
|
|
247
|
Donlic A, Morgan BS, Xu JL, Liu A, Roble C
Jr and Hargrove AE: Discovery of small molecule ligands for MALAT1
by tuning an RNA-binding scaffold. Angew Chem Int Ed Engl.
57:13242–13247. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
248
|
Goyal B, Yadav SRM, Awasthee N, Gupta S,
Kunnumakkara AB and Gupta SC: Diagnostic, prognostic, and
therapeutic significance of long non-coding RNA MALAT1 in cancer.
Biochim Biophys Acta Rev Cancer. 1875:1885022021. View Article : Google Scholar : PubMed/NCBI
|