Introduction
Breast cancer is the most frequent malignant tumor
and the leading cause of high mortality induced by cancer in women
worldwide (1,2). In 2009, it was reported that this
disease affects 10–12% of the female population each year and
causes half a million deaths worldwide (3). Despite recent advances in diagnosis
and treatment, tumor invasiveness and cell migration capability
remain the crucial factors affecting patient survival (4–6).
Epithelial-mesenchymal transition (EMT) is the process by which
epithelial cells lose cell adhesion, enhance tumor migration and
cell invasion ability, and acquire mesenchymal characteristics
(7,8). It is associated with basal-like breast
tumors, producing cells with stem-like properties, and allowing
cancer cells to spread and metastasize (9). Reducing the metastasis and invasion
ability of breast cancer cells is crucial to improve the prognosis
and reduce the mortality of patients with breast cancer. However,
the molecular mechanism of cell migration and invasion in breast
cancer remains to be further explored.
Lysosomal protein transmembrane 5 (LAPTM5), also
known as CD40-ligand-activated specific transcript 6 (CLAST6), is a
lysosomal membrane protein that has been identified as a key role
in the diagnosis and prognosis of human cancer (10). For testicular germ cell tumor
(TGCT), LAPTM5 was identified as a potential biomarker (11). Chen et al (12) demonstrated that LAPTM5 was highly
expressed in bladder cancer tissue, and the decrease in LAPTM5
suppressed the proliferation and viability of bladder cancer cells.
Moreover, LAPTM5 was found to be closely associated with poor
prognosis of patients with clear cell renal cell carcinoma (CCRCC)
and was identified as a potential therapeutic target (13). Additionally, LAPTM5 expression was
analyzed by using the Genotype-Tissue Expression (GTEx) and The
Cancer Genome Atlas (TCGA) datasets, and it was found that LAPTM5
was upregulated in breast tumor tissues. Therefore, it was
hypothesized that LAPTM5 may be a potential molecular therapeutic
target to improve the accuracy of breast cancer diagnosis. Thus,
the role of LAPTM5 in breast cancer needs to be explored.
Forkhead box protein 3 (FOXP3) is a member of the
FOX protein family (14), which
participates in the regulation of the occurrence and development of
various human tumors (15). It has
been reported that FOXP3 inhibits tumorigenesis and metastasis
through binding to the promoter of certain genes (16–18).
FOXP3, as a transcription factor necessary to regulate the
differentiation and function of T cells, affects the development
and function of T cells, thereby affecting the proliferation of
tumors. Ladoire et al (19)
revealed that breast cancer patients with high FOXP3 expression
have a lower tumor grade and a better prognosis. However, studies
on FOXP3 in breast cancer cells are limited. According to database
predictions, FOXP3 is expected to bind the LAPTM5 promoter. The
present study aimed to explore the association between FOXP3 and
LAPTM5, and the mechanism by which LAPTM5 may affect breast
cancer.
The Wnt/β-catenin signaling pathway is ubiquitous in
organisms, and it is an important signaling pathway that regulates
cell proliferation and differentiation (20,21).
It participates in the tumor microenvironment and contributes to
the initiation and progression of various human cancer types
(22–24). β-catenin is a key factor of the Wnt
signaling pathway, that could promote the transcription of target
genes regulated by Wnt/β-catenin (25). In breast cancer, Wnt/β-catenin is
the main signaling pathway that induces the EMT of cancer cells,
and has been identified as an important mediator of cell metastasis
and a marker of poor prognosis (9,26).
Therefore, it is necessary to verify whether this signaling pathway
mediates the effect of LAPTM5 on breast cancer.
In the present study, bioinformatic analysis was
used to predict the potential function of LAPTM5 on breast cancer.
LAPTM5 was found to be overexpressed in clinical specimens of
patients with breast cancer. In vitro experiments verified
that LAPTM5 could promote breast cancer cell proliferation,
migration and invasion as well as EMT through positive regulation
of β-catenin. Moreover, LAPTM5 was demonstrated to promote tumor
malignant phenotypes in vivo, and it was suppressed by
transcriptional regulation of FOXP3. Taken together, the present
findings revealed the function and molecular mechanism of LAPTM5 in
breast cancer.
Materials and methods
Bioinformatics analysis
The expression of LAPTM5 in breast cancer tissues
and the association between LAPTM5 and various clinical indicators
were analyzed using Breast Cancer Gene-Expression Miner
(bcGenExMiner v4.8; http://bcgenex.centregauducheau.fr/BC-GEM) (27). The association between patient
overall survival and LAPTM5 was evaluated with the Kaplan-Meier
plotter (http://kmplot.com/analysis/index.php?p=background)
(28). The log-rank test was
performed at the same time.
Tissue collection
The breast cancer samples and para-carcinoma tissues
were obtained from 40 patients (24–82 years old) who had been
diagnosed at the Shengjing Hospital of China Medical University
from March to May in 2019. The samples were collected by biopsy and
stored at −80°C until total RNA was extracted. The collection and
analysis of patient samples were approved (approval no. 2019PS339K)
by the ethics committee of Shengjing Hospital of China Medical
University (Shenyang, China). Written informed consent was provided
by all patients.
Cell culture and transfection
Human normal breast epithelial cells (MCF-10A) and
breast cancer cell lines (MDA-MB-453, MDA-MB-231 and MCF-7) were
purchased from iCell Bioscience Inc., while the breast cancer cell
lines SK-BR-3 and T-47D were purchased from Procell Life Science
and Technology Co., Ltd. All cell lines were cultured at 37°C in a
5% CO2 incubator. The MCF-10A cell line was cultured in
its specific medium (iCell Bioscience Inc.), while MDA-MB-453 and
MDA-MB-231 cells were cultured in L-15 medium (Procell Life Science
and Technology Co., Ltd.) with 10% fetal bovine serum (FBS)
(Zhejiang Tianhang Biotechnology Co., Ltd.). SK-BR-3 cells were
cultured in McCoy's 5A medium (Procell Life Science and Technology
Co., Ltd.) containing 10% FBS. T-47D cells were cultured in
RPMI-1640 medium (Beijing Solarbio Science and Technology Co.,
Ltd.), while MCF-7 cells were cultured in MEM (Beijing Solarbio
Science and Technology Co., Ltd.), both containing 10% FBS.
MDA-MB-231 and T-47D cells were confirmed to be free from
mycoplasma and were verified by STR profiling.
Cells (4×105 cells per well) were
dispensed into six-well plates and incubated in an incubator at
37°C. After the confluency of cells reached 60%, T-47D cells were
transfected with 2.5 µg OE-Vector (pcDNA3.1, Chongqing Unibio
Biological Technology Co., Ltd.) or OE-LAPTM5, and MDA-MB-231 cells
were transfected with 2.5 µg sh-NC (5′-TTCTCCGAACGTGTCACGT-3′),
sh-LAPTM5-1 (sh-1: 5′-GGTGCTACAGATTGATCAA-3′) or sh-LAPTM5-2 (sh-2:
5′-GCGTCTTGTTGTTCATCGA-3′) using Lipofectamine™ 3000 (Invitrogen;
Thermo Fisher Scientific, Inc.). After 48 h, MDA-MB-231 medium was
changed to complete medium containing 400 µg/ml G418 (Shanghai
Aladdin Biochemical Technology Co., Ltd.), and T-47D medium was
replaced with complete medium containing 450 µg/ml G418. Medium of
the two cell lines was changed every 2 days. After one week, the
medium was changed to complete medium and cultured for almost 10
days. Multiple monoclonal cell clusters in the medium were
continued to subculture to obtain stably transfected cell
lines.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from cells with TRIpure
reagent (BioTeke Corporation), and cDNA was synthesized using a kit
named Super M-MLV reverse transcriptase (BioTeke Corporation) and
RNase inhibitor (BioTeke Corporation) according to the
manufacturer's instructions. Next, cDNA was mixed with SYBR Green
(Sigma-Aldrich; Merck KGaA), primers, and 2X Power Taq PCR Master
Mix (BioTeke Corporation), and the mixture was subjected to RT-qPCR
in an Exicycler™ 96 Real-Time Quantitative Thermal Block (Bioneer
Corporation) according to the manufacturer's instructions. The mRNA
expression levels of the target genes were calculated via the
2−ΔΔCq method (29)
using β-actin as the control. The primers were synthesized by
GenScript and the sequences were as follows: LAPTM5 forward,
5′-AGCGTCTTGTTGTTCATCG-3′ and reverse, 5′-GCAGGCACAGGAGATAGTC-3′;
β-catenin forward, 5′-CAAGTGGGTGGTATAGAGG-3′ and reverse,
5′-GGATGGTGGGTGTAAGAG-3′; FOXP3 forward, 5′-TGACCAAGGCTTCATCTGTG-3′
and reverse, 5′-GAGGAACTCTGGGAATGTGC-3′; and β-actin forward,
5′-GGCACCCAGCACAATGAA-3′ and reverse, 5′-TAGAAGCATTTGCGGTGG-3′. The
thermocycling conditions were as follows: 94°C for 5 min; 40 cycles
of 15 sec at 94°C, 25 sec at 60°C, 30 sec at 72°C; 72°C for 5.5
min; 40°C for 2.5 min; 60°C to 94°C, 1.0°C/1 sec; 25°C for 1–2
min.
Western blotting
Whole protein extracts were prepared using cell
lysis buffer (Beyotime Institute of Biotechnology) with
phenylmethanesulfonylfluoride (Beyotime Institute of Biotechnology)
at a final concentration of 1 mM. The BCA Protein Assay kit (cat.
no. P0011; Beyotime Institute of Biotechnology) was used to detect
the concentration of total proteins according to the manufacturer's
instructions. Total proteins were separated by SDS-PAGE and
electro-transferred onto polyvinylidene fluoride membranes
(MilliporeSigma). The concentration of stacking gel was 5%.
Different concentrations of separating gel were used to detect
different proteins. Proteins, including LAPTM5, FOXP3, CyclinD1,
CyclinA, CDK2, CDK4, MMP2, MMP9 and β-actin, were detected under
the 12% separating gel. E-cadherin, N-cadherin and β-catenin were
examined using 8% separating gel. Histone H3 was evaluated in 14%
separating gel. After being blocked with 5% non-fat milk for 1 h at
room temperature, the membranes were probed with primary antibodies
overnight at 4°C. β-actin was used as a loading control, and its
loaded amount was consistent with the detected proteins. Next,
membranes were incubated with HRP-conjugated IgG antibodies for 45
min at 37°C. The secondary antibodies were incubated with proteins
loaded at the aforementioned amount after treatment with primary
antibodies. Therefore, protein loaded amount of secondary
antibodies was also consistent with the detected proteins. Enhanced
chemiluminescence (ECL) (Beyotime Institute of Biotechnology) was
used to visualize the immunoreactive proteins. Details of the
aforementioned antibodies are listed in Table SI.
Cell counting kit-8 (CCK-8) assay
Stably transfected cells were plated into 96-well
plates (3×103 cells per well). After cell culture for 0,
24, 48, 72 and 96 h, 10 µl CCK-8 solution (Beyotime Institute of
Biotechnology) was added to each well, and the cells were incubated
for 2 h at 37°C. When exploring the effect of β-catenin on
LAPTM5-induced cell proliferation, CCK-8 solution was added to the
wells after cell culture for 48 h. The absorbance of the plate at
450 nm was measured with a microplate reader (BioTek Instruments,
Inc.).
Flow cytometric analysis
Cells were cultured in 6-well plates until they
reached 90%. A total of 5×105 cells was collected, and
70% pre-cooled ethanol was added and fixed for 12 h at 4°C. The
samples were processed with a Cell Cycle Analysis kit (cat. no.
C1052; Beyotime Institute of Biotechnology) according to the
manufacturer's instructions. Next, a flow cytometer (ACEA
Biosciences Inc.) was used to detect the cell cycle. The software
used for flow cytometric analysis was NovoExpress (version: 1.4.1;
Agilent Technologies Inc.).
Wound healing assay
Cells (4×105 cells per well) were seeded
in six-well plates. When cells reached 100%, medium was then
changed to serum-free medium containing 1 µg/ml mitomycin C
(Sigma-Aldrich; Merck KGaA) for 1-h treatment. Next, a sterile
200-µl pipette tip was used to create a straight wound. The same
location on each well was recorded with a microscope
(magnification, ×100) (Olympus Corporation). The cell migration
distance was calculated after 24 h. The migration ratio was
calculated using the following formula: Migration rate=(0 h width
of wound −24 h width of wound)/0 h width of wound ×100%.
Transwell assay
Transwell chamber was placed in a 24-well plate and
coated with 40 µl diluted Matrigel (Corning, Inc.), which was
solidified in an incubator at 37°C for 2 h. Cells were collected
and diluted to form a cell suspension. A total of 200 µl this cell
suspension (1.5×104 cells per well) was added to the
upper chamber containing Matrigel. The pore size of the Transwell
chamber inserts was 8.0 µm. In the lower chamber, 800 µl medium
containing 10% FBS was added as a chemoattractant. After cell
culture for 24 h, 4% paraformaldehyde was used to fix the cells for
20 min at room temperature. Next, the cells were stained with 0.5%
crystal violet for 5 min. An inverted microscope (magnification,
×200) was used to observe invasive cells into the lower chamber.
Each sample was randomly selected 5 fields of view to count the
number of cells.
Immunofluorescence (IF) analysis
Cells (8×104 cells per well) were planted
in 24-well plates. When cells reached 70%, they were fixed in 4%
paraformaldehyde for 15 min at room temperature and incubated in
0.1% Triton X-100 for 30 min at room temperature. After blocking
with 1% bovine serum albumin (cat. no. A602440-0050; Sangon Biotech
Co., Ltd.) for 15 min at room temperature, the cells were incubated
with a β-catenin antibody (1:200; cat. no. ab32572; Abcam)
overnight at 4°C. The bound antibody was visualized using an Alexa
Fluor™ 555-labeled goat anti-rabbit IgG antibody (1:200; cat. no.
A27039; Invitrogen; Thermo Fisher Scientific, Inc.). Then, the cell
nuclei were counterstained with 5 µg/ml
4′-6-diamidino-2-phenylindole (cat. no. D106471-5 mg; Shanghai
Aladdin Biochemical Technology Co., Ltd.) for 5 min at room
temperature. Images were captured using a fluorescence microscope
(magnification, ×400).
Xenograft mouse model
To evaluate tumorigenicity in vivo, a total
of 24 6-week-old BALB/c female nude mice (18±1 g) were divided into
4 groups (n=6 per group). The mice could eat and drink freely, and
were raised in an environment of temperature 22±1°C, humidity
45–55%, and 12 h light/dark cycle every day. Cells transfected with
different vectors were collected and prepared into cell
resuspension solution respectively. Cells (1×107) were
subcutaneously injected into the dorsal side of the right armpit of
these mice. The tumor volume was measured every 3 days and was
calculated by the following formula: Volume=1/2 × length ×
width2. At the end of the experiment, mice were treated
by 5% isoflurane for induction of anesthesia followed by performing
cervical dislocation. Tumors were removed and images were captured.
A portion of the tumor tissue was fixed with 4% paraformaldehyde at
room temperature for more than 24–48 h for subsequent experiments.
All experiments involving animals were approved (approval no.
2019PS339K) by the animal ethics committee of Shengjing Hospital of
China Medical University (Shenyang, China).
Immunohistochemistry (IHC)
Tumor tissue sections were prepared and subjected to
IHC analysis. Anti-KI67 antibody (1:100; cat. no. 27309-1-AP;
ProteinTech Group, Inc.) and anti-LAPTM5 (1:100; cat. no.
bs-17100R; BIOSS) antibody were used as primary antibodies, which
were added to cover the tissue sections at 4°C overnight.
HRP-conjugated goat anti-rabbit IgG (1:200; cat. no. 31460; Thermo
Fisher Scientific, Inc.) was used as a secondary antibody, which
was incubated with tissue sections at 37°C for 60 min. Next, the
sections were treated with a 3,3′-diaminobenzidine (DAB) substrate
kit (cat. no. DA1010; Beijing Solarbio Science and Technology Co.,
Ltd.) and stained with hematoxylin (cat. no. H8070; Beijing
Solarbio Science and Technology Co., Ltd.) according to the
manufacturer's instructions. The stained results were observed and
images were captured under a fluorescence microscope
(magnification, ×400) (Olympus Corporation).
Dual-luciferase reporter assay
Breast cancer MDA-MB-231 cells (8×104
cells per well) were seeded in 24-well plates. After cells reached
70%, the cells were co-transfected with pGL3-basic (General
Biosystems Co., Ltd.) or pGL3-LAPTM5 promoter sequence and pcDNA3.1
(Chongqing Unibio Biological Technology Co., Ltd.) or
pcDNA3.1-FOXP3. A Renilla luciferase plasmid was transfected
together with a constructed plasmid for normalizing the efficiency
of transfection. After 48 h, the luciferase activity was measured
with a Dual-Luciferase Reporter Gene Assay kit (Nanjing KeyGen
Biotech Co., Ltd.).
Chromatin immunoprecipitation (ChIP)
assay
A ChIP assay was performed with a Cell ChIP kit
(Wanleibio Co., Ltd.) according to the manufacturer's instructions.
Briefly, MDA-MB-231 cells were fixed in 1% paraformaldehyde at room
temperature for 10 min and sonicated. Next, the cell lysates were
incubated with anti-FOXP3 antibody (undiluted, cat. no. PA1-806;
Thermo Fisher Scientific, Inc.) or negative control IgG (undiluted)
at 4°C overnight, then mixed with 60 µl protein A beads at 4°C. The
immunoprecipitated complex was washed from beads through
centrifuging at 625 × g for 1 min at 4°C and the supernatant was
collected. The target DNA fragments were then amplified by
polymerase chain reaction (PCR), analyzed by 2% agarose gel
electrophoresis and visualized using Gold View nuclear staining
dye. The PCR reaction system contained 2 µl immunoprecipitated
DNAs, 1 µl forward primers, 1 µl reverse primers, and 10 µl 2X Taq
PCR Master-mix (BioTeke Corporation), as well as sterile ultra-pure
water to adjust total volume to 20 µl. The primer sequences were
listed in Table SII. The PCR
amplifications were performed under the following conditions: 95°C
for 5 min; 40 cycles of 20 sec at 95°C, 20 sec at 50°C, 30 sec at
72°C; 25°C for 5 min.
Statistical analysis
Statistical analysis was performed using Prism 7
software (GraphPad Software, Inc.). According to the bc-GenExMiner
online tool, differences among the groups of Fig. 1A were analyzed using ANOVA followed
by Dunnett-Tukey-Kramer's test. Difference of LAPTM5 mRNA
expression between tumor tissues and para-tumor tissues in Fig. 1B was assessed by paired Student's
t-test. The results of tumor volume in in vivo experiments
(Fig. 5A and B) were analyzed by
unpaired Student's t-test. Difference among more than two groups
was examined by one-way analysis of variance (ANOVA) followed by
the Tukey's multiple comparisons test. All data are presented as
the mean ± SD. The in vitro experiments were performed at
least in triplicate, and the in vivo experiments were
performed in six replicates. P<0.05 was considered to indicate a
statistically significant difference.
Results
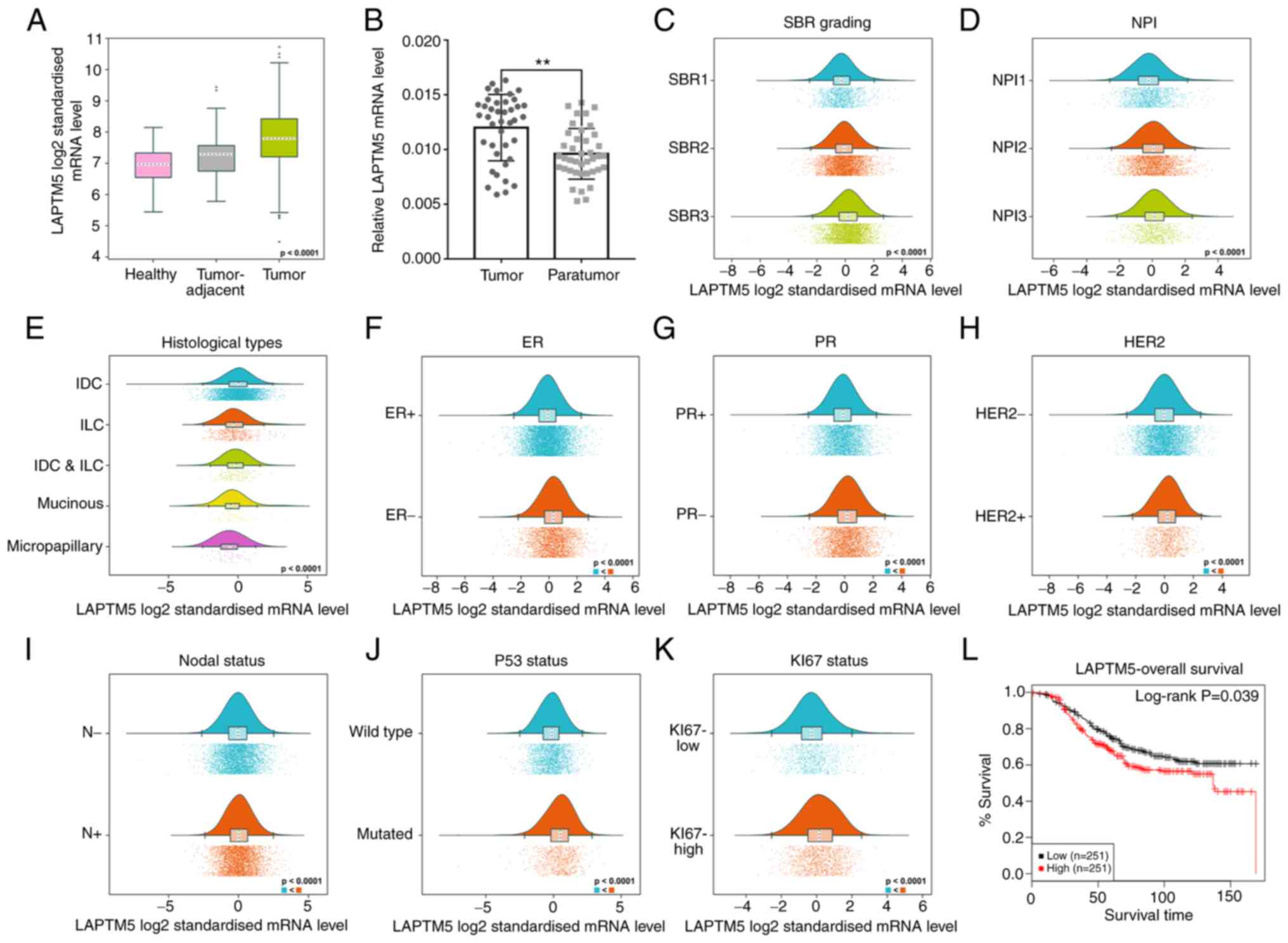
High LAPTM5 level is associated with
poor prognosis of patients with breast cancer
To determine whether LAPTM5 regulates the
development of breast cancer, LAPTM5 expression in the GTEx and
TCGA datasets was analyzed using the bc-GenExMiner online tool.
LAPTM5 mRNA expression in tumor tissue was significantly higher
than that in adjacent tissue and healthy tissue (Fig. 1A). LAPTM5 mRNA expression was
examined in 40 pairs of breast cancer and para-carcinoma tissue
samples, and it was found to be significantly overexpressed in the
breast cancer cases (tumor vs. para-tumor, 0.0120±0.0030 vs.
0.0096±0.0023, P<0.01, Fig.
1B).
Next, the association between LAPTM5 and clinical
indicators was estimated by bc-GenExMiner. The
Scarff-Bloom-Richardson (SBR) grading as a histological grade is
useful for the clinical diagnosis and prognosis of breast cancer
(30). Breast cancer patients with
higher SBR grade were found to express higher LAPTM5 mRNA levels
(Fig. 1C). Patients with increased
levels of LAPTM5 exhibited a worse Nottingham prognostic index
(NPI) (Fig. 1D). Additionally,
LAPTM5 was highly expressed in the infiltrating ductal carcinoma
and lowly expressed in micropapillary carcinoma (Fig. 1E). Furthermore, LAPTM5 expression
was positively associated with human epidermal growth factor
receptor-2 status, and negatively associated with estrogen receptor
(ER) and progesterone receptor (PR) status (Fig. 1F-H). Moreover, patients with
positive nodal status (N+) displayed increased levels of
LAPTM5 (Fig. 1I). Furthermore,
LAPTM5 expression in patients with mutated P53 was significantly
upregulated compared with that of patients with wild type P53,
indicating that LAPTM5 possibly enhanced the development of breast
cancer (Fig. 1J). Patients with
high expression of KI67 tended to express higher LAPTM5 (Fig. 1K). Survival analysis confirmed that
the increase in LAPTM5 was significantly associated with shorter
overall survival in patients with breast cancer (Fig. 1L). In summary, these data revealed
that LAPTM5 possibly promoted the progression of breast cancer.
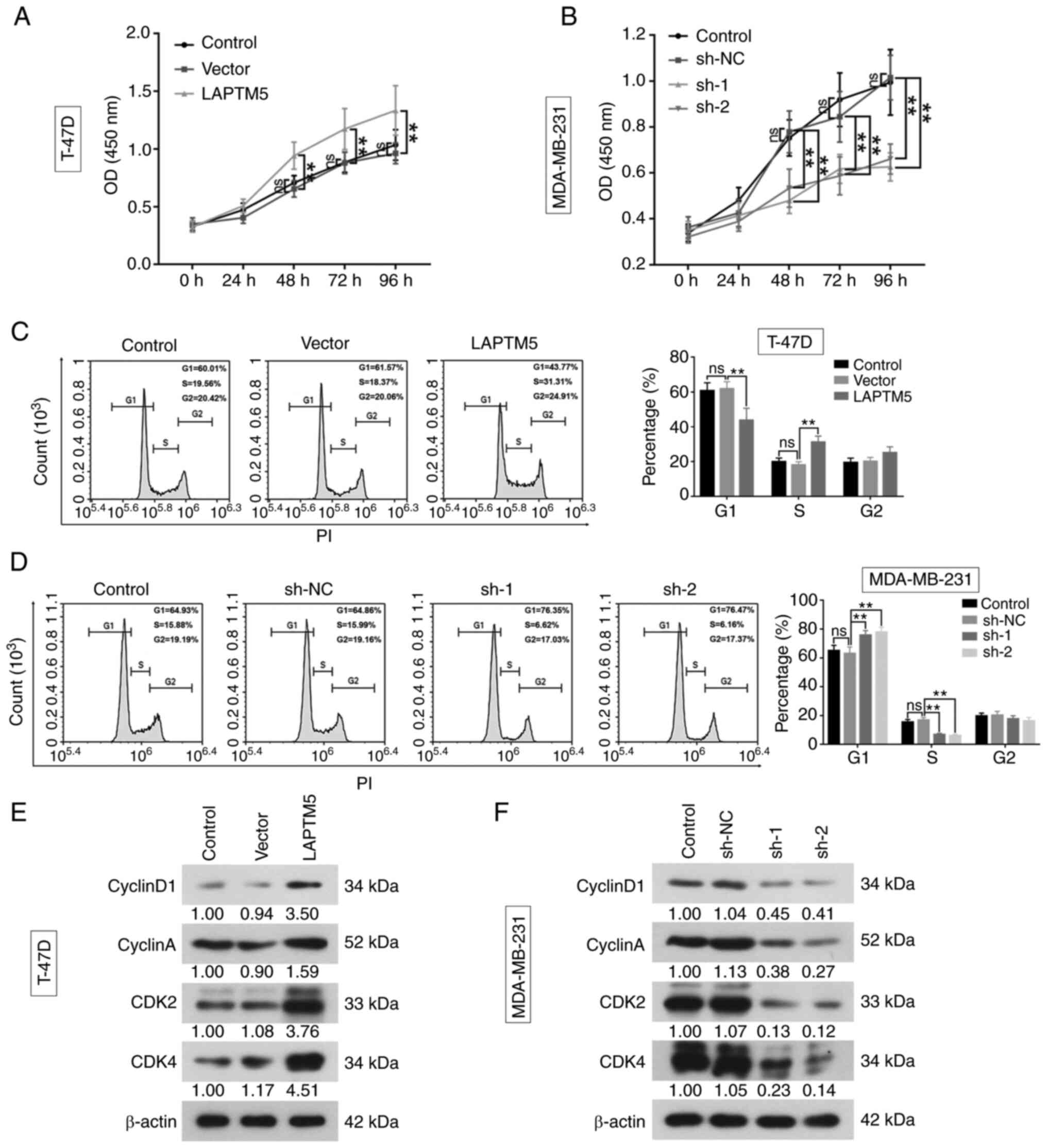
LAPTM5 promotes breast cancer cell
proliferation
To further investigate the role of LAPTM5 in breast
cancer, the protein expression of LAPTM5 was examined in five
breast cancer cell lines and one normal breast epithelial cell line
by western blotting (Fig. S1A).
LAPTM5 mRNA expression was successfully upregulated in the T-47D
cell line (Vector vs. LAPTM5, 1.0240±0.1160 vs. 7.2330±0.7315,
P<0.01) and was inhibited in the MDA-MB-231 cell line (sh-NC vs.
sh-1 vs. sh-2, 0.9489±0.1413 vs. 0.1996±0.0321 vs. 0.2653±0.0311,
P<0.01), which was consistent with the changes of protein
expression in the two cell lines (Fig.
S1B and C). The results of CCK-8 assay demonstrated that the
cell viability of T-47D cells was significantly enhanced by LAPTM5
overexpression (Fig. 2A). The
optical density (OD) of LAPTM5 overexpression group was higher than
that of vector group at 48 h (LAPTM5 vs. vector, 0.942±0.117 vs.
0.654±0.071, P<0.01), 72 h (LAPTM5 vs. vector, 1.173±0.178 vs.
0.879±0.094, P<0.01) and 96 h (LAPTM5 vs. vector, 1.334±0.215
vs. 0.962±0.090, P<0.01). In the MDA-MB-231 cells, the OD level
was significantly decreased at 48 h (sh-NC vs. sh-1 vs. sh-2,
0.779±0.091 vs. 0.480±0.057 vs. 0.534±0.083, P<0.01), 72 h
(sh-NC vs. sh-1 vs. sh-2, 0.854±0.110 vs. 0.618±0.063 vs.
0.587±0.083, P<0.01), and 96 h (sh-NC vs. sh-1 vs. sh-2,
1.015±0.097 vs. 0.627±0.061 vs. 0.661±0.066, P<0.01), indicating
cell viability was remarkably suppressed by silencing LAPTM5
(Fig. 2B).
The flow cytometric results showed that the
percentage of cells in the G1 phase was reduced (Vector vs. LAPTM5,
61.8±4.1 vs. 43.7±6.9, P<0.01) and increased in the S phase
(Vector vs. LAPTM5, 18.1±1.9 vs. 31.2±3.5, P<0.01) by
overexpression of LAPTM5 (Fig. 2C).
Meanwhile, when LAPTM5 was silenced, cells in G1 phase were
upregulated (sh-NC vs. sh-1 vs. sh-2, 63.0±4.6 vs. 75.7±3.2 vs.
77.8±3.4, P<0.01) and them in S phase were decreased (sh-NC vs.
sh-1 vs. sh-2, 16.9±1.8 vs. 6.7±0.9 vs. 6.0±0.9, P<0.01,
Fig. 2D). Further, the western blot
results showed that LAPTM5 overexpression increased the expression
of CyclinD1, CyclinA, cyclin-dependent kinase (CDK) 2 and CDK4 in
breast cancer cells, while silencing LAPTM5 resulted in the
opposite effects (Fig. 2E and F).
The aforementioned results indicated that LAPTM5 may be a tumor
promoter in breast cancer.
LAPTM5 promotes breast cancer cell
migration, invasion and EMT
To further confirm the effects of LAPTM5 on breast
cancer metastasis and invasiveness, wound-healing, Transwell and
western blot assays were performed. The relative cell migration
ratio was significantly increased by LAPTM5 overexpression (Vector
vs. LAPTM5, 51.1±7.1 vs. 80.4±6.9, P<0.01, Fig. 3A), and was suppressed by sh-1 (sh-NC
vs. sh-1, 63.6±8.5 vs. 40.6±5.2, P<0.05) or sh-2 (sh-NC vs.
sh-2, 63.6±8.5 vs. 38.1±5.4, P<0.01, Fig. 3B). In addition, the number of
invasive cells was significantly increased when LAPTM5 was
overexpressed (Vector vs. LAPTM5, 104.7±12.3 vs. 253.2±45.5,
P<0.01, Fig. 3C), while opposite
trends were observed after downregulation of LAPTM5 (sh-NC vs. sh-1
vs. sh-2, 107.5±12.8 vs. 55.9±4.9 vs. 59.7±7.9, P<0.01, Fig. 3D). The western blot results showed
that the expression of matrix metallopeptidase (MMP) 2 and MMP9 was
upregulated with overexpression of LAPTM5, and downregulated with
depleting LAPTM5 (Fig. 3E). When
LAPTM5 was overexpressed, the expression of E-cadherin was reduced,
while N-cadherin was upregulated. When LAPTM5 was silenced, the
opposite effects were observed (Fig.
3F). Previous studies reported that E-cadherin was a tumor
suppressor that functioned as an inhibitor of cell metastasis
(31), while N-cadherin could
induce or enhance the metastatic capacity of invading carcinoma
cells (32).
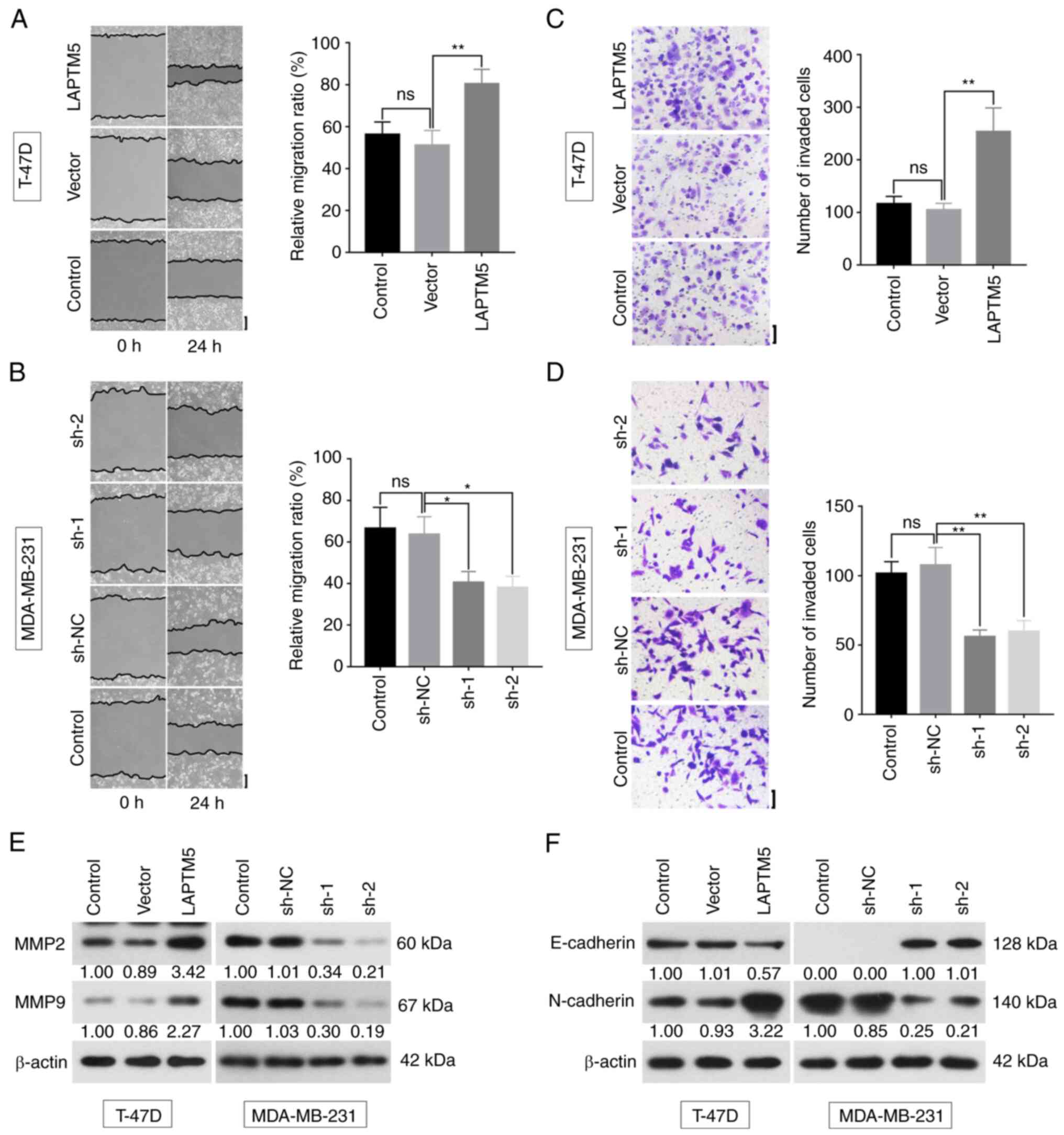 | Figure 3.LAPTM5 promotes migration and
invasion of breast cancer. (A and B) Cell metastatic ability was
evaluated by wound healing assay at 0 and 24 h. The relative
migration ratio was statistically analyzed. Scale bar, 200 µm. (C
and D) Cell invasive ability was detected by Transwell assay.
Number of invasive cells was statistically analyzed. Scale bar, 100
µm. (E and F) Protein expression of MMP2, MMP9, E-cadherin, and
N-cadherin was examined by western blotting. Data originated from
three independent experiments and are presented as the mean ± SD.
*P<0.05 and **P<0.01. LAPTM5, lysosomal protein transmembrane
5; MMP, matrix metallopeptidase; ns, not significant; sh-, short
hairpin; NC, negative control. |
These results indicated that LAPTM5 promoted the
process of cell migration, invasion and EMT.
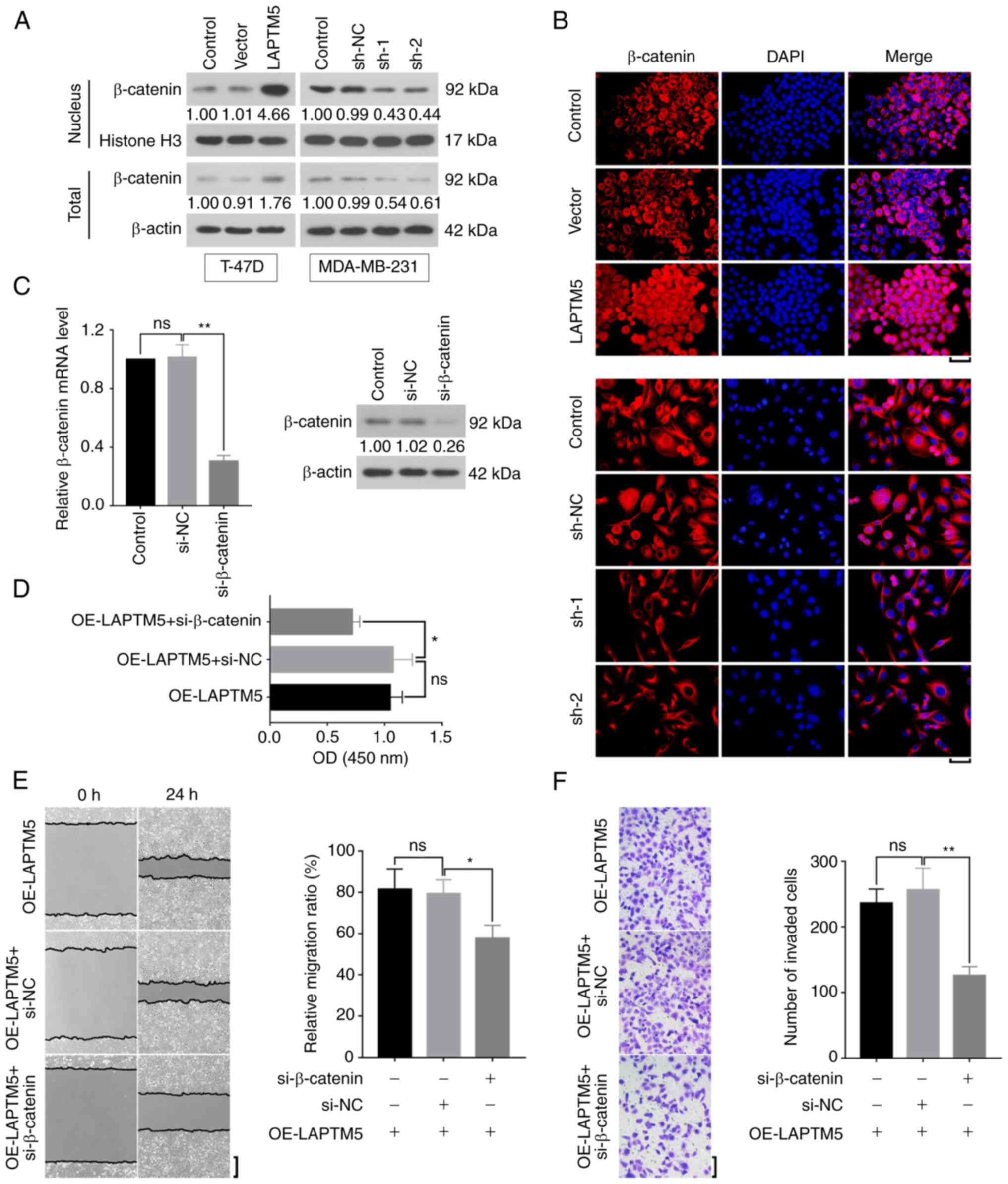
LAPTM5 regulates the proliferation and
migration of breast cancer cells by activation of the Wnt/β-catenin
signaling pathway
To explore the mechanism of LAPTM5 in breast cancer,
activated β-catenin expression was detected by western blotting.
The nuclear β-catenin was upregulated by LAPTM5 overexpression and
downregulated by silencing of LAPTM5 (Fig. 4A). Total β-catenin protein level was
consistent with that in the nucleus. IF displayed that β-catenin
expression was obviously increased in the nucleus when LAPTM5 was
overexpressed, while, when LAPTM5 was downregulated, the level of
β-catenin in the nucleus was decreased (Fig. 4B). Additionally, the mRNA and
protein levels of β-catenin were successfully reduced by
si-β-catenin in the T-47D cells (si-NC vs. si-β-catenin,
1.0140±0.0864 vs. 0.3042±0.0384, P<0.01, Fig. 4C). Furthermore, the results of CCK-8
assay indicated that si-β-catenin inhibited LAPTM5-induced cell
proliferation (OE-LAPTM5 + si-NC vs. OE-LAPTM5 + si-β-catenin,
1.0730±0.1675 vs. 0.7153±0.0670, P<0.05, Fig. 4D). Wound healing assay demonstrated
that downregulation of β-catenin suppressed the migration of
LAPTM5-overexpressed breast cancer cells (OE-LAPTM5 + si-NC vs.
OE-LAPTM5 + si-β-catenin, 79.2±6.8 vs. 57.7±6.4, P<0.05,
Fig. 4E). The results of Transwell
assay showed the same effects of β-catenin on cell invasion
(OE-LAPTM5 + si-NC vs. OE-LAPTM5 + si-β-catenin, 255.9±33.6 vs.
125.7±13.7, P<0.01, Fig. 4E).
Collectively, it was revealed that LAPTM5 promoted the cell
malignant phenotypes of breast cancer through activating the
Wnt/β-catenin signaling pathway.
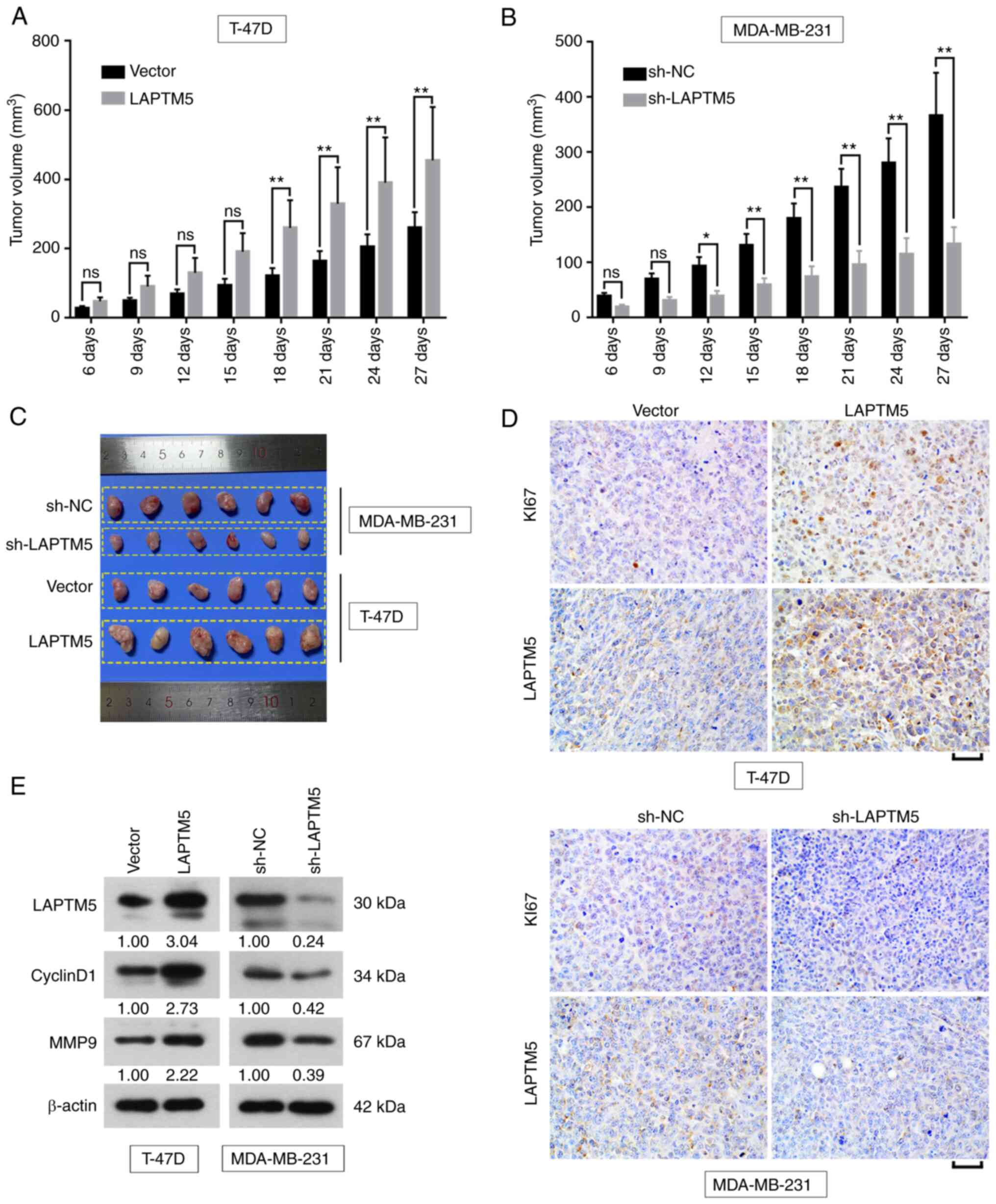
LAPTM5 promotes breast cancer
tumorigenesis in vivo
Based on the in vitro findings, the effects
of LAPTM5 on breast cancer were further investigated in
vivo. After injection of T-47D cells to mice, tumor volume was
significantly increased by LAPTM5 overexpression at day 18 (Vector
vs. LAPTM5, 120.7±22.6 vs. 259.6±80.0, P<0.01), day 21 (Vector
vs. LAPTM5, 163.8±28.7 vs. 329.1±106.0, P<0.01), day 24 (Vector
vs. LAPTM5, 205.1±36.0 vs. 390.2±130.8, P<0.01) and day 27
(Vector vs. LAPTM5, 260.0±44.8 vs. 454.7±154.0, P<0.01, Fig. 5A). When LAPTM5 was knocked down
in vivo, the size of xenograft was reduced at day 12 (sh-NC
vs. sh-LAPTM5, 92.9±16.5 vs. 38.8±9.1, P<0.05), day 15 (sh-NC
vs. sh-LAPTM5, 130.5±20.8 vs. 59.0±11.9, P<0.01), day 18 (sh-NC
vs. sh-LAPTM5, 179.5±27.0 vs. 73.7±19.1, P<0.01), day 21 (sh-NC
vs. sh-LAPTM5, 236.5±33.2 vs. 95.4±25.0, P<0.01), day 24 (sh-NC
vs. sh-LAPTM5, 280.5±44.3 vs. 114.6±29.0, P<0.01) and day 27
(sh-NC vs. sh-LAPTM5, 366.1±78.0 vs. 133.1±30.8, P<0.01,
Fig. 5B). The image of tumors at
day 27 was presented in Fig. 5C.
Furthermore, IHC results exhibited that KI67 was remarkably
increased by LAPTM5 overexpression in the tumor, while, the
expression of KI67 was decreased by suppressing LAPTM5 (Fig. 5D). The protein expression of
CyclinD1 and MMP9 was obviously enhanced by upregulation of LAPTM5,
and was reduced by the downregulation of LAPTM5 (Fig. 5E). The aforementioned findings
illustrated that LAPTM5 functioned as a promoter of tumor growth
in vivo.
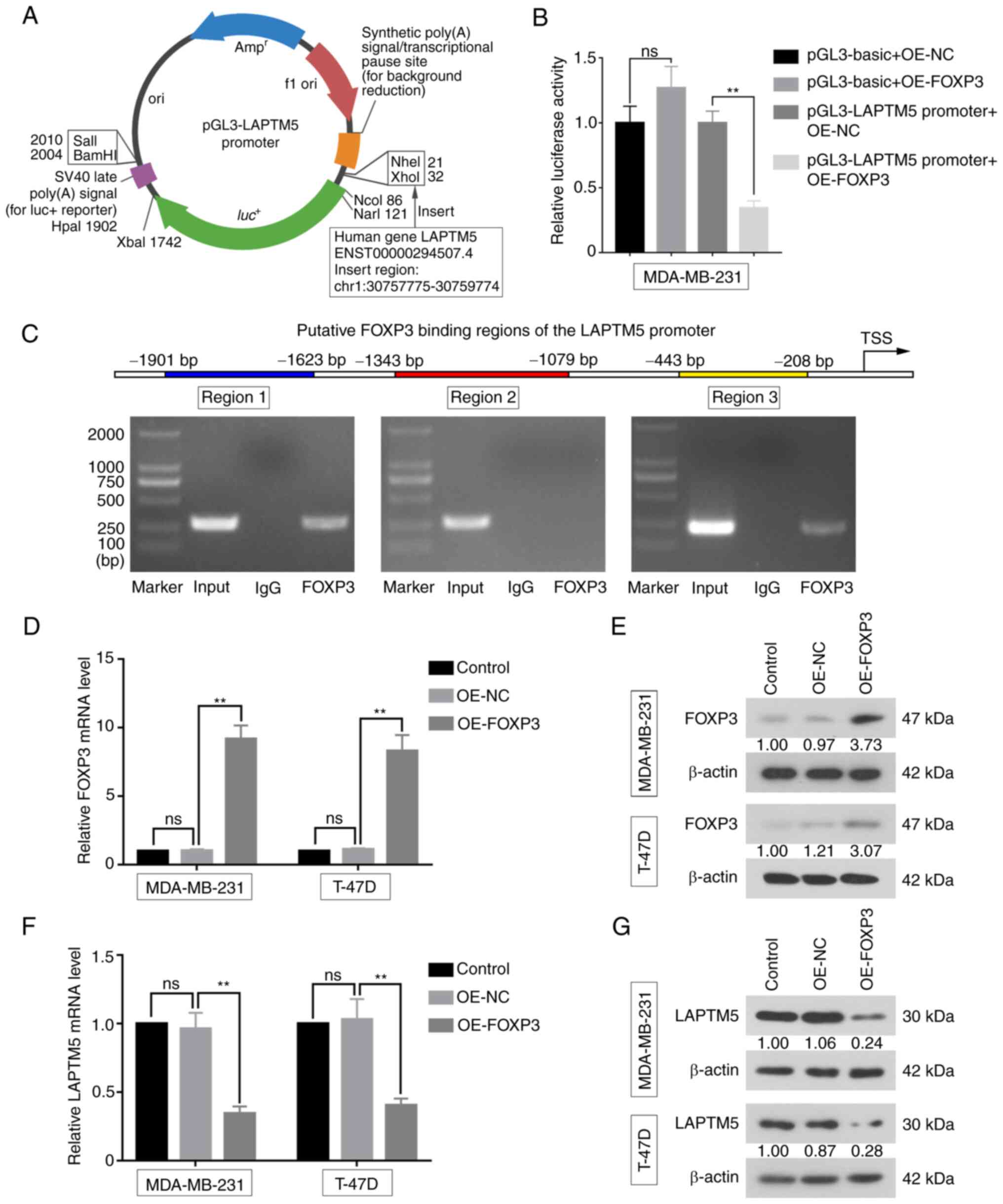
FOXP3 binds to the LAPTM5 promoter
region and suppresses its expression
The present study investigated whether LAPTM5 was
regulated by FOXP3 via the dual-luciferase reporter assay. The
LAPTM5 promoter region was first inserted into the pGL3-basic
luciferase reporter vector (Fig.
6A). Compared with the OE-NC group, relative luciferase
activity of MDA-MB-231 cells co-transfected with OE-FOXP3 and
pGL3-LAPTM5 promoter was significantly decreased (pGL3-LAPTM5
promoter + OE-NC vs. pGL3-LAPTM5 promoter + OE-FOXP3, 1.0000±0.0900
vs. 0.3433±0.0551, P<0.01), indicating that FOXP3 could bind to
the LAPTM5 promoter (Fig. 6B).
Additionally, ChIP assays were performed to evaluate whether FOXP3
directly binds to the LAPTM5 promoter. The primers on region 1 and
region 3 of the LAPTM5 promoter generated positive products
(Fig. 6C), suggesting that FOXP3
could directly bind to these two regions of the LAPTM5 promoter.
Furthermore, the mRNA expression of FOXP3 was significantly
upregulated in MDA-MB-231 (OE-NC vs. OE-FOXP3, 1.0350±0.0905 vs.
9.1760±0.9873, P<0.01) and T-47D cells (OE-NC vs. OE-FOXP3,
1.1220±0.0901 vs. 8.3090±1.1550, P<0.01), and protein expression
displayed the same trends (Fig. 6D and
E). As expected, the relative mRNA expression of LAPTM5 was
downregulated with FOXP3 overexpression in MDA-MB-231 (OE-NC vs.
OE-FOXP3, 0.9627±0.1170 vs. 0.3445±0.0506, P<0.01) and T-47D
cells (OE-NC vs. OE-FOXP3, 1.0300±0.1510 vs. 0.4045±0.0485,
P<0.01, Fig. 6F). Meanwhile,
LAPTM5 protein expression was suppressed in the FOXP3-overexpressed
cells (Fig. 6G). These findings
demonstrated that FOXP3 could bind to the LAPTM5 promoter and act
as a transcriptional suppressor of LAPTM5 in breast cancer
cells.
Discussion
The relapse and metastasis of breast cancer spread
to distant sites remain the leading causes of morbidity and
mortality associated with this disease (9). To optimize the diagnostic methods and
reduce the mortality of patients with breast cancer, it is urgent
to identify molecular targets that participate in cancer cell
metastasis and invasion. The present study found that the
expression of LAPTM5 was significantly higher in breast cancer
tissues than that in para cancer tissues. The results are
consistent with those from previous studies on human tumors, such
as bladder cancer (BCa). Microarray analysis was used to determine
that LAPTM5 was upregulated in BCa tissues at both mRNA and protein
levels (12). Besides, LAPTM5 was
abnormally highly expressed in TGCT, and the prognosis of the
LAPTM5 high-expression group was significantly worse than that of
the low-expression group (11). In
addition, LAPTM5 was highly expressed in CCRCC, which was
significantly associated with the survival prognosis of CCRCC
(13). In the present study, the
association between clinical indicators and LAPTM5 expression was
analyzed. It displayed that LAPTM5 was significantly
positive-associated with SBR grading and NPI, which were the major
tumor grading methods of breast cancer in clinical judgement. High
expression of LAPTM5 was also indicated to associate with worse
overall survival. Based on these results, the status of LAPTM5
expression may be a potential indicator for tumor stage of breast
cancer, which was worth evaluating in clinical applications.
Moreover, a previous study found that LAPTM 4 beta (LAPTM4B), which
belongs to the same lysosomal membrane protein as LAPTM5, was
upregulated in breast cancer, and its high expression was
positively related to TNM stage and lymph node metastasis (33). Recently, Meng et al (34) found that LAPTM5 regulated the
development and spinal metastasis of ER-positive breast cancer
through the glutamine-dependent mTOR signaling, while the present
study demonstrated that LAPTM5, which is affected by the
transcription factor FOXP3, could contribute to the tumor
progression of breast cancer via the Wnt/β-catenin signaling
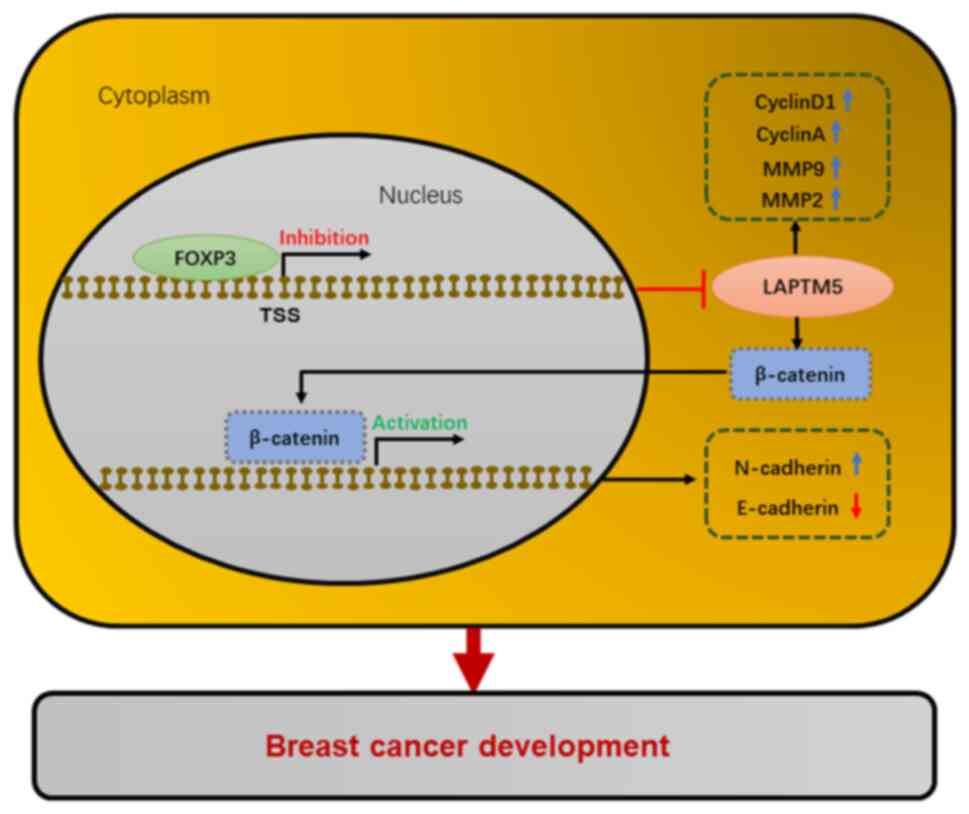
pathway (Fig. 7).
The experiments in vitro and in vivo
identified that the upregulation of LAPTM5 increased the number of
cells in S phase and enhanced the expression of proliferation
marker genes. CyclinD1 facilitates the transition from G1 to the S
phase of the cell cycle by activating CDK4 or CDK6 (35). Meanwhile, CyclinA and CDK2 drive S
phase progression and DNA synthesis (36). When LAPTM5 was overexpressed, these
proteins expressed higher levels, and cells were enriched in the S
phase, which is the main stage of DNA synthesis. By contrast,
downregulation of LAPTM5 suppressed the expression of CyclinD1,
CyclinA, CDK2 and CDK4, and decreased the number of S phase cells.
Overall, these results suggested that LAPTM5 promoted cell
proliferation. Moreover, it was found that MMP2, MMP9 and
N-cadherin were increased with upregulation of LAPTM5, while they
were decreased when LAPTM5 was downregulated. The expression of
E-cadherin led to opposite expression patterns of the
aforementioned proteins, which are closely associated with cell
invasion. Based on these results, LAPTM5 was verified to promote
EMT progress. Importantly, β-catenin was upregulated with the
overexpression of LAPTM5, while downregulation of LAPTM5 produced
the opposite effects. The Wnt/β-catenin signaling pathway plays an
important role in cell development and differentiation, and is
closely associated with different types of diseases and human
cancer (37). β-catenin initiates
the downstream transcription of genes such as CyclinD1 and MMPs
(38), and is an essential binding
partner of various cadherins, such as E-cadherin in adhesion
junctions (39). Furthermore, the
Wnt/β-catenin signaling pathway plays a crucial role in regulating
EMT (40–42). All the aforementioned evidence
demonstrated that LAPTM5 affected the development of breast cancer
through activating the Wnt/β-catenin signaling pathway.
Numerous studies have revealed that transcription
factors participate in cancer pathogenesis through the activation
or inactivation of different genes. As a transcription factor,
FOXP3 is well-known to play a regulatory role in human cancer.
Concerning the mechanisms by which FOXP3 expression inhibits cancer
metastasis, previous studies have been published in different types
of tumors (16,43,44).
Such as in ovarian cancer, cell metastasis and invasion were
suppressed by FOXP3, and the expression of MMP-2 was reduced at the
same time. Furthermore, previous studies indicated that FOXP3 could
block the initiation of tumors, such as prostate cancer (45), as well as breast cancer (17,46).
FOXP3 was identified to suppress the migration, proliferation and
invasion of tumor cells (47). In
addition, FOXP3 has been reported to inhibit breast cancer
tumorigenesis, metastasis and invasion by regulating the expression
of C-X-C Motif Chemokine Receptor 4, SATB homeobox 1, and S-phase
kinase associated protein 2 (46,48,49).
The association between FOXP3 and LAPTM5 was predicted, and certain
binding sites were found in the LAPTM5 promoter region. Western
blot results showed that the expression of LAPTM5 was negatively
regulated by FOXP3. In addition, luciferase reporter and ChIP
assays indicated FOXP3 surely interacted with LAPTM5. FOXP3 was
found to affect the expression of LAPTM5 by binding its promoter
region. Therefore, the results of the present study suggested that
FOXP3 may inhibit development of breast cancer through regulating
the expression of LAPTM5 and the Wnt/β-catenin signaling
pathway.
It is well known that estrogen and progesterone are
crucial factors involved in breast cancer (50,51).
Hormonotherapy is an effective treatment therapy for patients with
hormone-positive breast cancer. Among all subtypes of breast
cancer, triple-negative breast cancer is a specific subtype, which
has high invasiveness and poor prognosis (52,53).
LAPTM5 was expressed at higher levels in ER- or PR-breast cancer
samples, which indicated that breast cancer patients with high
LAPTM5 level may experience greater treatment difficulties, and
that the expression of LAPTM5 may be restricted by hormones. The
present study did not explore the role of LAPTM5 in triple negative
breast cancer cell lines, which is a limitation. Therefore, it is
planned to investigate the mechanism by which hormones affect the
expression of LAPTM5, and how to improve clinical hormone therapy
based on this mechanism.
Experimental cancers may bring pain or suffering to
tumor-bearing animals. Therefore, the associations of experimental
animals suggest implementing humane endpoints in cancer research.
The present study was carried out in accordance with the
regulations of Chinese Association for Laboratory Animal
Science-Laboratory animal-Guidelines for euthanasia (2017), which
specified the humanitarian endpoint on subcutaneous xenograft
tumors: i) Tumor exceeds 10% of the host's original body weight;
ii) Weight loss reached 20–25% of the original body weight. In the
present study, the maximum weight loss rate of tumor-bearing mice
was 4.79%, and the maximum tumor volume was 506.47 mm3.
The tumor weight was not measured in the present study, but it was
estimated via weighing the tumors with similar volume in other
studies (experiment in progress; Chi et al, unpublished
data), and the results showed that tumor volume at 500±5
mm3 weighs 0.5±0.05 g, which is ~2–3% of the host's body
weight. Thus, the mice in the present study did not reach the
humanitarian endpoint specified in the aforementioned literature.
In addition, inquiries regarding suggestions on the humane
endpoints were also addressed to certain international ethical
organizations, including UK Co-ordinating Committee on Cancer
Research (UKCCCR Guidelines for the Welfare of Animals in
Experimental Neoplasia, 1997) and National Institutes of Health
(Institutional Animal Care and Use Committee Guidebook, 2002). It
was stipulated that when tumor exceeds 10% of normal body weight or
results in rapid body weight loss of 20%, the humanitarian endpoint
for execution of experimental mice is reached. Therefore, it can be
clearly observed that the experimental mice in the present study,
whether at home or abroad, were far from meeting the requirement of
humane endpoint. Certainly, the authors also agree that it is very
important to weigh the tumors, and this issue shall be addressed in
the future study.
In addition, the goal of the authors is to
investigate the pathogenesis of breast cancer and investigate for
potential therapeutic molecules. The LAPTM5 was initially displayed
as CLAST6, which was found from high-throughput sequencing results
in the authors' research group (Chi et al, unpublished
data). The roles of the other abnormally expressed genes in breast
cancer shall be investigated in further research.
In summary, the present study provided evidence that
LAPTM5 may exert its biological functions of enhancing cell
proliferation, migration, invasion and EMT of breast cancer cells
via the activation of the Wnt/β-catenin signaling pathway, and
demonstrated that LAPTM5 was negatively regulated by the
transcription factor FOXP3.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SH performed the main experiments and drafted the
manuscript. XJ analyzed the data and prepared the figures. TH
participated in cell culture and infection. FC designed the
research and revised the manuscript. SH, XJ, TH and FC confirm the
authenticity of all the raw data. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
Human studies were performed in accordance with the
Declaration of Helsinki and were approved (approval no. 2019PS339K)
by the ethics committee of Shengjing Hospital of China Medical
University. Written informed consent was provided by all patients.
The animal experiment protocol complied with the international
guidelines and was approved (approval no. 2019PS339K) by the animal
ethics committee of Shengjing Hospital of China Medical University
(Shenyang, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jalkh N, Nassar-Slaba J, Chouery E, Salem
N, Uhrchammer N, Golmard L, Stoppa-Lyonnet D, Bignon YJ and
Mégarbané A: Prevalance of BRCA1 and BRCA2 mutations in familial
breast cancer patients in Lebanon. Hered Cancer Clin Pract.
10:72012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Coughlin SS and Ekwueme DU: Breast cancer
as a global health concern. Cancer Epidemiol. 33:315–318. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Benson JR, Jatoi I, Keisch M, Esteva FJ,
Makris A and Jordan VC: Early breast cancer. Lancet. 373:1463–1479.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lv W, Chen N, Lin Y, Ma H, Ruan Y, Li Z,
Li X, Pan X and Tian X: Macrophage migration inhibitory factor
promotes breast cancer metastasis via activation of HMGB1/TLR4/NF
kappa B axis. Cancer Lett. 375:245–255. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ghislain I, Zikos E, Coens C, Quinten C,
Balta V, Tryfonidis K, Piccart M, Zardavas D, Nagele E,
Bjelic-Radisic V, et al: Health-related quality of life in locally
advanced and metastatic breast cancer: Methodological and clinical
issues in randomised controlled trials. Lancet Oncol. 17:e294–e304.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Luo J, Yao JF, Deng XF, Zheng XD, Jia M,
Wang YQ, Huang Y and Zhu JH: 14, 15-EET induces breast cancer cell
EMT and cisplatin resistance by up-regulating integrin αvβ3 and
activating FAK/PI3K/AKT signaling. J Exp Clin Cancer Res.
37:232018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sun L, Yao Y, Liu B, Lin Z, Lin L, Yang M,
Zhang W, Chen W, Pan C, Liu Q, et al: MiR-200b and miR-15b regulate
chemotherapy-induced epithelial-mesenchymal transition in human
tongue cancer cells by targeting BMI1. Oncogene. 31:432–445. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan
A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al: The
epithelial-mesenchymal transition generates cells with properties
of stem cells. Cell. 133:704–715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
DiMeo TA, Anderson K, Phadke P, Fan C,
Perou CM, Naber S and Kuperwasser C: A novel lung metastasis
signature links Wnt signaling with cancer cell self-renewal and
epithelial-mesenchymal transition in basal-like breast cancer.
Cancer Res. 69:5364–5373. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nuylan M, Kawano T, Inazawa J and Inoue J:
Down-regulation of LAPTM5 in human cancer cells. Oncotarget.
7:28320–28328. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li X, Su Y, Zhang J, Zhu Y, Xu Y and Wu G:
LAPTM5 plays a Key role in the diagnosis and prognosis of
testicular germ cell tumors. Int J Genomics. 2021:88164562021.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen L, Wang G, Luo Y, Wang Y, Xie C,
Jiang W, Xiao Y, Qian G and Wang X: Downregulation of LAPTM5
suppresses cell proliferation and viability inducing cell cycle
arrest at G0/G1 phase of bladder cancer cells. Int J Oncol.
50:263–271. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sui Y, Lu K and Fu L: Prediction and
analysis of novel key genes ITGAX, LAPTM5, SERPINE1 in clear cell
renal cell carcinoma through bioinformatics analysis. PeerJ.
9:e112722021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Brunkow ME, Jeffery EW, Hjerrild KA,
Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF
and Ramsdell F: Disruption of a new forkhead/winged-helix protein,
scurfin, results in the fatal lymphoproliferative disorder of the
scurfy mouse. Nat Genet. 27:68–73. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Szylberg Ł, Karbownik D and Marszałek A:
The role of FOXP3 in human cancers. Anticancer Res. 36:3789–3794.
2016.PubMed/NCBI
|
|
16
|
Zhang C, Xu Y, Hao Q, Wang S, Li H, Li J,
Gao Y, Li M, Li W, Xue X, et al: FOXP3 suppresses breast cancer
metastasis through downregulation of CD44. Int J Cancer.
137:1279–1290. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zuo T, Wang L, Morrison C, Chang X, Zhang
H, Li W, Liu Y, Wang Y, Liu X, Chan MWY, et al: FOXP3 is an
X-linked breast cancer suppressor gene and an important repressor
of the HER-2/ErbB2 oncogene. Cell. 129:1275–1286. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Douglass S, Ali S, Meeson AP, Browell D
and Kirby JA: The role of FOXP3 in the development and metastatic
spread of breast cancer. Cancer Metastasis Rev. 31:843–854. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ladoire S, Mignot G, Dalban C, Chevriaux
A, Arnould L, Rébé C, Apetoh L, Boidot R, Penault-Llorca F,
Fumoleau P, et al: FOXP3 expression in cancer cells and
anthracyclines efficacy in patients with primary breast cancer
treated with adjuvant chemotherapy in the phase III UNICANCER-PACS
01 trial. Ann Oncol. 23:2552–2561. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bahrami A, Hasanzadeh M, ShahidSales S,
Yousefi Z, Kadkhodayan S, Farazestanian M, Joudi Mashhad M, Gharib
M, Mahdi Hassanian S and Avan A: Clinical significance and
prognosis value of Wnt signaling pathway in cervical cancer. J Cell
Biochem. 118:3028–3033. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu J and Wang Y: Long non-coding RNA
KCNQ1OT1 facilitates the progression of cervical cancer and tumor
growth through modulating miR-296-5p/HYOU1 axis. Bioengineered.
12:8753–8767. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Clevers H and Nusse R: Wnt/β-catenin
signaling and disease. Cell. 149:1192–1205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kypta RM and Waxman J: Wnt/β-catenin
signalling in prostate cancer. Nat Rev Urol. 9:418–428. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang Z, Li B, Zhou L, Yu S, Su Z, Song J,
Sun Q, Sha O, Wang X, Jiang W, et al: Prodigiosin inhibits
Wnt/β-catenin signaling and exerts anticancer activity in breast
cancer cells. Proc Natl Acad Sci USA. 113:13150–13155. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li Y, Jin K, van Pelt GW, van Dam H, Yu X,
Mesker WE, Ten Dijke P, Zhou F and Zhang L: c-Myb enhances breast
cancer invasion and metastasis through the Wnt/β-catenin/Axin2
pathway. Cancer Res. 76:3364–3375. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dey N, Barwick BG, Moreno CS,
Ordanic-Kodani M, Chen Z, Oprea-Ilies G, Tang W, Catzavelos C,
Kerstann KF, Sledge GW Jr, et al: Wnt signaling in triple negative
breast cancer is associated with metastasis. BMC Cancer.
13:5372013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jézéquel P, Gouraud W, Ben Azzouz F,
Guérin-Charbonnel C, Juin PP, Lasla H and Campone M: bc-GenExMiner
4.5: New mining module computes breast cancer differential gene
expression analyses. Database (Oxford). 2021:baab0072021.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lánczky A and Győrffy B: Web-based
survival analysis tool tailored for medical research (KMplot):
Development and implementation. J Med Internet Res. 23:e276332021.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Amat S, Penault-Llorca F, Cure H, Le
Bouedëc G, Achard JL, Van Praagh I, Feillel V, Mouret-Reynier MA,
Dauplat J and Chollet P: Scarff-bloom-richardson (SBR) grading: A
pleiotropic marker of chemosensitivity in invasive ductal breast
carcinomas treated by neoadjuvant chemotherapy. Int J Oncol.
20:791–796. 2002.PubMed/NCBI
|
|
31
|
Corso G, Figueiredo J, De Angelis SP,
Corso F, Girardi A, Pereira J, Seruca R, Bonanni B, Carneiro P,
Pravettoni G, et al: E-cadherin deregulation in breast cancer. J
Cell Mol Med. 24:5930–5936. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Thiery JP, Acloque H, Huang RYJ and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xiao M, Jia S, Wang H, Wang J, Huang Y and
Li Z: Overexpression of LAPTM4B: An independent prognostic marker
in breast cancer. J Cancer Res Clin Oncol. 139:661–667. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Meng Q, Zhou L, Liang H, Hu A, Zhou H,
Zhou J, Zhou X, Lin H, Li X, Jiang L and Dong J: Spine-specific
downregulation of LAPTM5 expression promotes the progression and
spinal metastasis of estrogen receptor-positive breast cancer by
activating glutamine-dependent mTOR signaling. Int J Oncol.
60:472022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hosooka T and Ogawa W: A novel role for
the cell cycle regulatory complex cyclin D1-CDK4 in
gluconeogenesis. J Diabetes Investig. 7:27–28. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Malumbres M and Barbacid M: Mammalian
cyclin-dependent kinases. Trends Biochem Sci. 30:630–641. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Nishisho I, Nakamura Y, Miyoshi Y, Miki Y,
Ando H, Horii A, Koyama K, Utsunomiya J, Baba S and Hedge P:
Mutations of chromosome 5q21 genes in FAP and colorectal cancer
patients. Science. 253:665–669. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yang M, Wang M, Li X, Xie Y, Xia X, Tian
J, Zhang K and Tang A: Wnt signaling in cervical cancer? J Cancer.
9:1277–1286. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Peifer M, McCrea PD, Green KJ, Wieschaus E
and Gumbiner BM: The vertebrate adhesive junction proteins
beta-catenin and plakoglobin and the Drosophila segment polarity
gene armadillo form a multigene family with similar properties. J
Cell Biol. 118:681–691. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gao Y and Chen Z, Wang R, Tan X, Huang C,
Chen G and Chen Z: LXRα promotes the differentiation of human
gastric cancer cells through inactivation of Wnt/β-catenin
signaling. J Cancer. 10:156–167. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yeh Y, Guo Q, Connelly Z, Cheng S, Yang S,
Prieto-Dominguez N and Yu X: Wnt/beta-catenin signaling and
prostate cancer therapy resistance. Adv Exp Med Biol. 1210:351–378.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Peng N, Zhang Z, Wang Y, Yang M, Fan J,
Wang Q, Deng L, Chen D, Cai Y, Li Q, et al: Down-regulated
LINC00115 inhibits prostate cancer cell proliferation and invasion
via targeting miR-212-5p/FZD5/Wnt/β-catenin axis. J Cell Mol Med.
25:10627–10637. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gong Z, Jia H, Yu J, Liu Y, Ren J, Yang S,
Hu B, Liu L, Lai PBS and Chen GG: Nuclear FOXP3 inhibits tumor
growth and induced apoptosis in hepatocellular carcinoma by
targeting c-Myc. Oncogenesis. 9:972020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Liu C, Han J, Li X, Huang T, Gao Y, Wang
B, Zhang K, Wang S, Zhang W, Li W, et al: FOXP3 inhibits the
metastasis of breast cancer by downregulating the expression of
MTA1. Front Oncol. 11:6561902021. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wang L, Liu R, Li W, Chen C, Katoh H, Chen
GY, McNally B, Lin L, Zhou P, Zuo T, et al: Somatic single hits
inactivate the X-linked tumor suppressor FOXP3 in the prostate.
Cancer Cell. 16:336–346. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zuo T, Liu R, Zhang H, Chang X and Liu Y,
Wang L, Zheng P and Liu Y: FOXP3 is a novel transcriptional
repressor for the breast cancer oncogene SKP2. J Clin Invest.
117:3765–3773. 2007.PubMed/NCBI
|
|
47
|
Huang Z, Liu F, Wang W, Ouyang S, Sang T,
Huang Z, Liao L and Wu J: Deregulation of circ_003912 contributes
to pathogenesis of erosive oral lichen planus by via sponging
microRNA-123, −647 and −31 and upregulating FOXP3. Mol Med.
27:1322021. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Douglass S, Meeson AP, Overbeck-Zubrzycka
D, Brain JG, Bennett MR, Lamb CA, Lennard TW, Browell D, Ali S and
Kirby JA: Breast cancer metastasis: Demonstration that FOXP3
regulates CXCR4 expression and the response to CXCL12. J Pathol.
234:74–85. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
McInnes N, Sadlon TJ, Brown CY, Pederson
S, Beyer M, Schultze JL, McColl S, Goodall GJ and Barry SC: FOXP3
and FOXP3-regulated microRNAs suppress SATB1 in breast cancer
cells. Oncogene. 31:1045–1054. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Hilton HN, Clarke CL and Graham JD:
Estrogen and progesterone signalling in the normal breast and its
implications for cancer development. Mol Cell Endocrinol. 466:2–14.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Russo J and Russo IH: Development of the
human breast. Maturitas. 49:2–15. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yin L, Duan JJ, Bian XW and Yu SC:
Triple-negative breast cancer molecular subtyping and treatment
progress. Breast Cancer Res. 22:612020. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Borri F and Granaglia A: Pathology of
triple negative breast cancer. Semin Cancer Biol. 72:136–145. 2021.
View Article : Google Scholar : PubMed/NCBI
|