Introduction
Drug sensitivity and the severity of side effects
vary from patient to patient; therefore, it is necessary to develop
precision medicine designed to provide the optimal type and amount
of treatment for each patient. The development of precision
medicine based on patient genetic information has markedly improved
therapeutic methods for some types of adult cancer, such as chronic
myelocytic leukemia and breast cancer; however, precision medicine
for pediatric cancer has not been fully developed because of its
rarity and diversity (1).
Immunodeficient mouse models, cell-derived xenograft
(CDX) models and patient-derived xenograft (PDX) models have been
commonly used for cancer drug research; however, these murine
models have some weaknesses: i) Establishment and maintenance of a
PDX model is time-consuming and a high cost is incurred to manage
its quality, ii) experimental procedures should be undertaken to
reduce the number of animals used per study or to refine procedures
to improve animal welfare, and iii) it is impossible to visualize
the site of xenografts (2–5).
The chorioallantoic membrane (CAM) is an
extraembryonic membrane consisting of chorion and allantois in
fertilized chicken eggs. The CAM assay has been widely used to
study angiogenesis, infiltration and metastasis using human-,
rodent- and bird-derived xenografts (6–9). The
CAM assay has the following advantages compared with conventional
PDX models: i) High vascularization and an immature immune system
enable the establishment of time-saving and easy-to-handle PDX
models (3,4,10,11),
ii) chick embryos are not specified as laboratory animals in a
number of countries, which support compliance with the 3R principle
of animal research (replacement, reduction and refinement)
(11–13), iii) the CAM can be visually observed
by peeling the shell membrane, which allows the visualization of
blood vessels and xenografts. The CAM assay is thus considered a
suitable model to evaluate the biological and pharmacological
characteristics of tumor tissues.
Rhabdomyosarcoma (RMS) is the most common type of
highly malignant pediatric soft tissue sarcoma in the United States
(≥50% of pediatric soft tissue sarcomas) (14,15).
The two major subtypes of pediatric RMS, embryonal RMS (ERMS) and
alveolar RMS (ARMS), have some differences in histopathological
features, age of onset, site of primary lesions and expression of
fused genes, which are utilized for the diagnosis and
classification of risk groups (15–19).
The prognosis is less favorable in fusion-positive ARMS (80% of
patients with ARMS) than ERMS (14,17,20).
However, fusion-negative ARMS is genetically and critically similar
to ERMS (21,22).
Progress in multidisciplinary treatment has improved
the 5-year survival rate of low/intermediate-risk patients to
70–90%, but that of high-risk patients remains <30% in the
United States (23–25). While the survival rate has improved,
it remains a serious problem that nonspecific high-dose
chemotherapy and radiotherapy can cause various complications at
later stages of life, including facial deformity, growth hormone
deficiency, fertility disorders and secondary cancer (26–29).
The present study aimed to explore whether the CAM
assay is a novel treatment model that could contribute to precision
medicine for pediatric cancer. As a first step, this study aimed to
establish a protocol for the CAM assay with transplantation of RMS
cells on the CAM and examined how to evaluate the effect of
anticancer drugs.
Materials and methods
Cell culture and reagents
The present study was approved by the Institutional
Gene Recombination Experimentation Committee of the Kyoto
Prefectural University of Medicine (approval no. #2019-35; Kyoto,
Japan). The human ERMS cell line RD was purchased from the Japanese
Collection of Research Bioresources Cell Bank, and the human ARMS
cell line SJ-Rh30 was kindly provided by Dr Peter J. Houghton
(Greehey Children's Cancer Research Institute, University of Texas
Health Science Center, San Antonio, TX, USA) (30). Firefly luciferase-expressing RD and
SJ-Rh30 were established by transducing RediFect Red-Luc-Puromycin
lentiviral particles (PerkinElmer, Inc.) into the cells. Briefly,
RD and SJ-Rh30 cells were plated at 5.0×105 cells/100-mm
dish. After 24 h, hexadimethrine bromide was added (8 mg/ml; cat.
no, H9268; MilliporeSigma), followed by each particle solution
(MOI, 0.5). After another 24 h, media were removed and fresh media
were added. The following day, puromycin was added (5 mg/ml; cat.
no. P8833; MilliporeSigma). Puromycin-resistant clones were
selected with cloning rings at day 14, with continuous puromycin
selection at all times. Cell lines were cultured in Dulbecco's
modified Eagle's medium (4.5 g/l glucose) (Nacalai Tesque, Inc.)
containing 10% fetal bovine serum (Gibco; Thermo Fisher Scientific,
Inc.) and 1 µg/ml puromycin (Thermo Fisher Scientific, Inc.) at
37°C under a 5% CO2 atmosphere in an incubator.
Vincristine (VCR) (cat. no. S1241; Selleck Chemicals) was dissolved
in dimethyl sulfoxide (cat. no. D2950; MilliporeSigma) and stored
as a 1 mM stock solution at −20°C. D-Luciferin potassium salt
(D-luciferin; cat. no. 12507; AAT Bioquest, Inc.) was dissolved in
phosphate-buffered saline (PBS; Nacalai Tesque, Inc.) and stored as
a 15 mg/ml stock solution at −20°C. The cells were screened
periodically for mycoplasma contamination using a Mycoplasma
Detection kit (MycoStrip™; Invivogen).
Chick CAM assay
A total of 350 fertilized eggs from a local
commercial hatchery were incubated in an upright position at 37°C
and 60% humidity. The day the eggs were kept in the incubator was
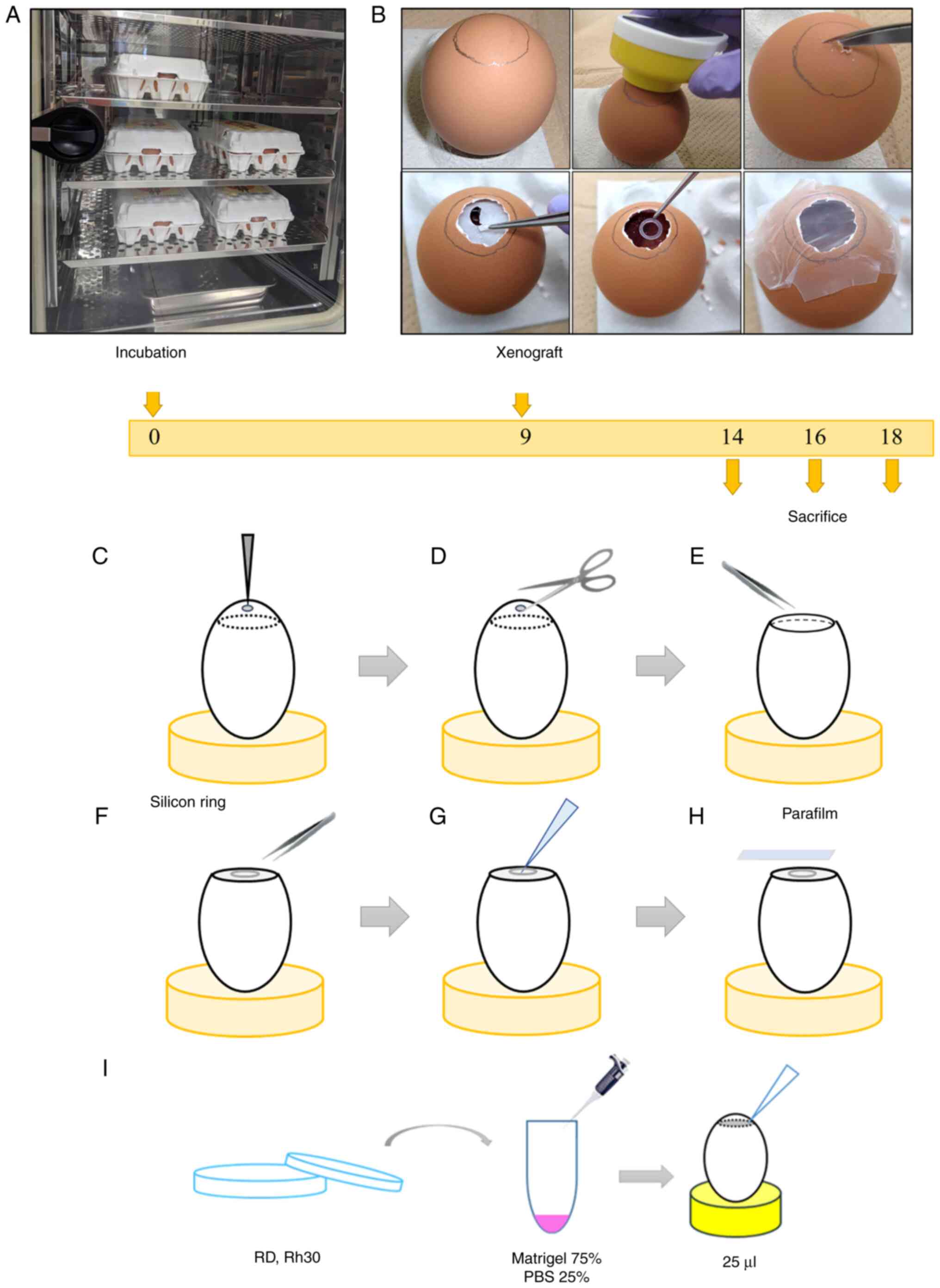
designated as day 0 (Fig. 1A). On
day 9, a small hole was made in the air chamber side of the
eggshell with an egg piercer and surgical scissors after tracing
the edges of the air chamber with a pencil under illumination in a
darkroom. The eggshell membrane was removed with tweezers and a
sterile silicon ring (inner diameter, 5 mm) was placed at the
center of the CAM (Fig. 1B-F).
Tumor cells were detached from culture dishes using
trypsin/ethylenediaminetetraacetic acid and were counted. Cell line
suspensions were resuspended in Matrigel and PBS (3:1 ratio) at
2.0×106 cells/25 µl Matrigel solution and the mixture
was directly pipetted into the center of the ring (Fig. 1G and I). The hole was resealed with
parafilm and the eggs were placed in the incubator (Fig. 1H). Successful development of cell
xenografts was confirmed by visual observation and chemiluminescent
imaging with the G:BOX Chemi XRQ gel doc system (Syngene
International, Ltd.). On days 12, 14, 16 and 18,50 µl D-luciferin
was added to cell xenografts before chemiluminescent imaging
(D-luciferin was thawed and diluted to 1:100 with Dulbecco's
modified Eagle's medium before use). On days 14, 16 and 18, the
xenograft tumors were resected, and the length of three sides was
measured with a vernier caliper and the product of the lengths of
three sides was taken as the volume (length × width × height). For
the control immunostaining samples, the leg muscular tissues from
one chick embryo were also resected on day 16. Chick embryos were
euthanized by an overdose of pentobarbital (100 mg/kg) before
resection of the xenograft tumors. The excised tumors were fixed
with 100% formalin overnight at room temperature and
paraffin-embedded for hematoxylin and eosin (H&E) staining.
Paraffin-embedded sections (4 µm) were deparaffinized in xylene and
rehydrated in ethanol after incubation on a paraffin spreading unit
at 65°C for 15 min. The sections were stained with hematoxylin (6.5
min) and eosin (1 min) at room temperature, and the slides were
observed under a light microscope.
Immunohistochemistry (IHC)
Formalin-fixed paraffin-embedded chick embryo
tissues and CAM assay xenografts were deparaffinized in xylene and
rehydrated in ethanol. IHC was performed using an anti-human
vimentin antibody (1:200; cat. no. ab16700; Abcam). Blocking, HRP
micro-polymer secondary antibody incubation and DAB detection were
performed using a rabbit-specific HRP/DAB Detection IHC Kit (cat.
no. ab236469; Abcam). The sections were blocked with Protein Block
for 10 min at room temperature and incubated with primary antibody
overnight at 4°C. After blocking with 3% hydrogen peroxide for 10
min at room temperature, incubation with secondary antibody was
performed for 20 min at room temperature. DAB detection was
performed for 3 min at room temperature and nuclei were
counterstained with 3% methyl green for 20 min at room temperature
(cat. no. 12001; Muto Pure Chemicals Co. Ltd.). The slides were
observed under a light microscope.
WST-8 cell viability assay
WST-8 colorimetric assays were performed using a
Cell Counting Kit-8 (Dojindo Laboratories, Inc.) according to the
manufacturer's instructions. RD cells were plated in a 96-well
plate at a density of 5.0×103/well in 80 µl culture
media. After 24 h, dimethyl sulfoxide or VCR (1 pM-1 µM) was added
to each well. Cell viability was determined every 24 h after
treatment with VCR by measuring the absorbance at 450 nm using a
microplate reader (Multiscan JX; Sumitomo Pharma Co., Ltd.). The
dose-response curve was generated using ImageJ (National Institutes
of Health; version 1.52a). The mean half-maximal inhibitory
concentration (IC50) was calculated based on the
dose-response curve on day 3.
Statistical analysis
All data are presented by the mean ± standard error.
The statistical significance of differences between samples was
determined using one-way ANOVA and Dunnett's post hoc test.
P<0.05 was considered to indicate a statistically significant
difference. R2 values were calculated using the
least-squares method. All statistical analyses were performed with
EZR (Saitama Medical Center, Jichi Medical University, Saitama,
Japan), which is a graphical user interface for R (The R Foundation
for Statistical Computing; version 1.40) (31).
Results
Establishment of a RSM model using the
CAM assay
As shown in Fig. 1,
day 0 was defined as the day when the commercial fertilized eggs
were kept in the incubator and a hole was made in the eggshell on
day 9. A total of 7 days after transplantation with
firefly-expressing RD and Rh30 cells onto the CAM, both tumors
formed a mass that could be visualized by adding D-luciferin
(Fig. 2A). The xenograft tumors
were resected and their volume was calculated using measurements
obtained with a Vernier caliper on days 14, 16 and 18 (i.e., 5, 7
and 9 days after xenograft generation on the CAM). The RD and Rh30
cell-derived tumors increased temporally and three-dimensionally
over time (Fig. 2B). In addition,
pathological analysis of the tumor tissue on day 16 was performed,
which confirmed that tumor cells were densely aggregated along the
CAM (Fig. 2C, black arrows), with
the invasion of chick blood cells with prominent nuclei (32) (Fig.
2C, white dotted line) suggesting the formation of feeding
vessels from the CAM. Tumor cells were positive for human-specific
vimentin and CAM was negative as confirmed by IHC (Fig. 2D), which indicated the RMS
characteristics of these tumor masses. Notably, human vimentin
antibodies did not cross-react with chicken tissues (Fig. S1; Data
S1), indicating that IHC for human vimentin could discriminate
between the human RMS-derived tissue and chicken muscular
tissues.
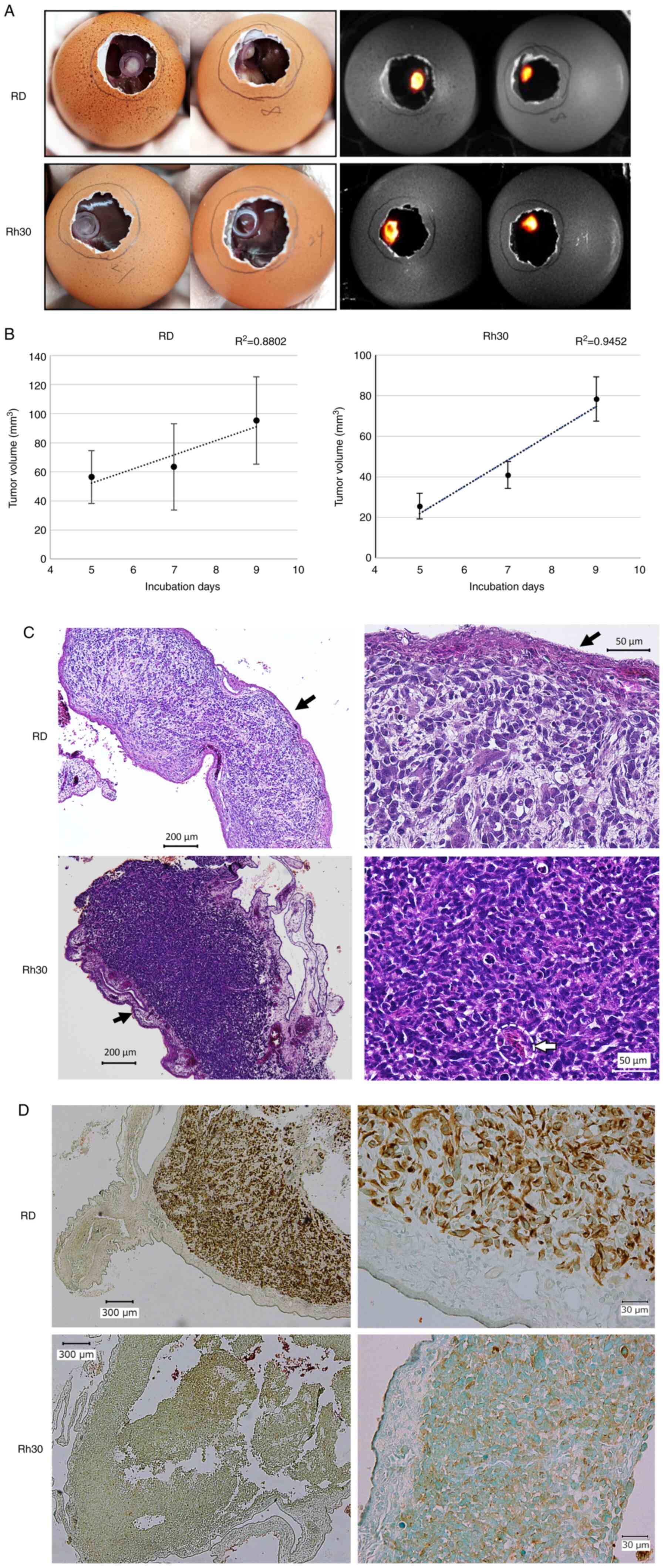 | Figure 2.Establishment of a cell-derived
xenograft model on the CAM using the RMS cell lines, RD and Rh30.
(A) Tumor formed on the CAM on day 16 (7 days after transplantation
of RD or Rh30 cells). Images on the left were captured on a clean
bench, whereas images on the right were observed using the G:BOX
Chemi XRQ gel doc system following the addition of luciferin. (B)
Temporal changes in tumor volume. Tumors were resected on days 14,
16 and 18, and the volume was calculated using Vernier caliper
measurements. (C) Hematoxylin and eosin staining of the resected
tumors on day 16 (left, ×40 magnification; right, ×200
magnification). Accumulation of cells and formation of RMS tissue
along the CAM (black arrow), and infiltration of some chick red
blood cells into the tissue (inside white dotted line) were
observed. (D) Immunohistochemical staining of anti-human vimentin
in the tumor tissue (left, ×40 magnification; right, ×200
magnification). Counterstaining of sections was performed with
methyl green. These results indicated that the resected tumor
consisted of human RMS cells transplanted on day 9. CAM,
chorioallantoic membrane; RMS, rhabdomyosarcoma. |
Utilization of the CAM assay for
anticancer drug screening
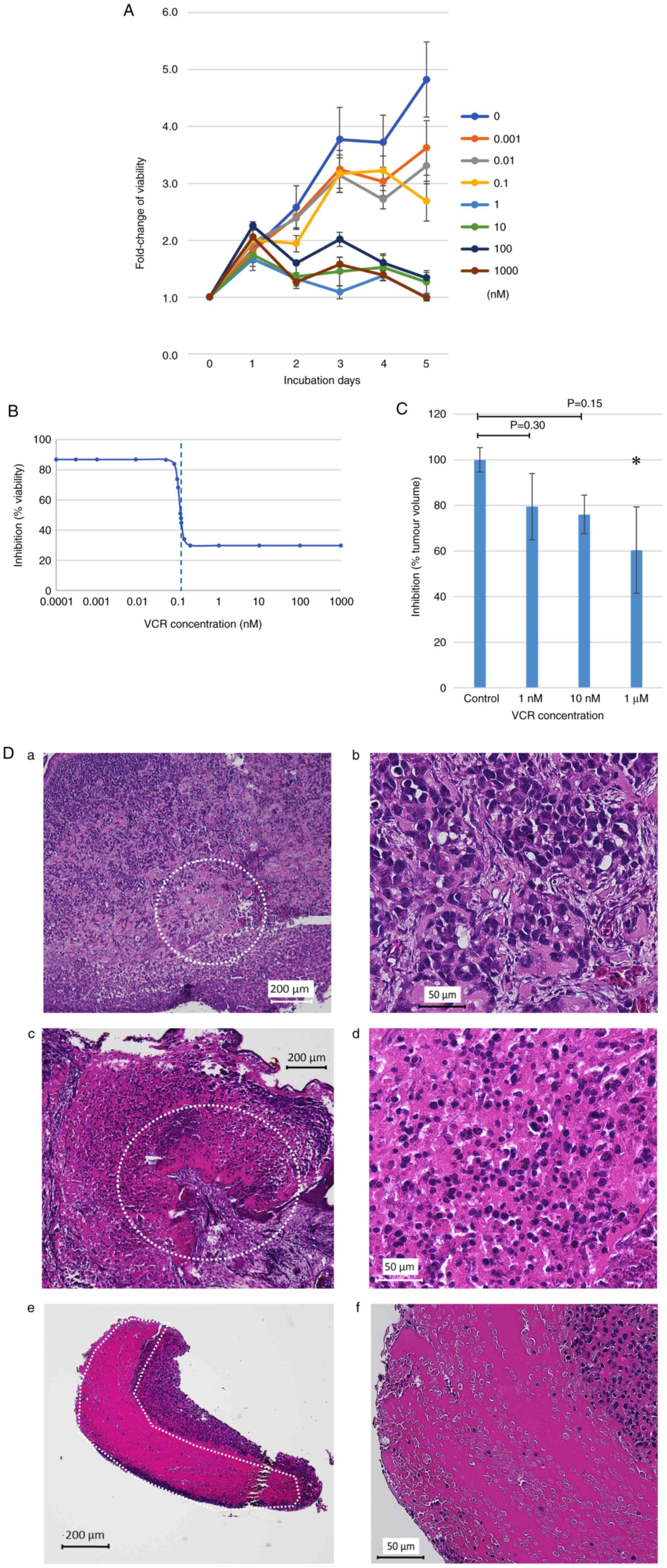
VCR is one of the key chemotherapeutic agents for
RMS and is widely used to treat RMS. For the anticancer drug
screening using the RD-derived tumor established on the CAM, the
present study first evaluated the sensitivity of VCR against RD
cells using the WST-8 cell viability assay (Fig. 3A). The IC50 of VCR was
0.114 nM and a clear decrease in the viability of RD cells was
detected when they were treated with >1 nM VCR (Fig. 3B). Based on these results, the
concentrations of VCR for the treatment of RD-derived tumors on the
CAM were selected. Briefly, the RD cell suspension was grafted on
the CAM on day 9. The implanted eggs were then divided into three
groups, and 1 nM, 10 nM or 1 µM VCR was placed directly on the
RD-derived tumors on day 12 (3 days after xenograft generation).
Tumors were resected on day 16 for further evaluation. It was
observed that the volume of resected tumors decreased in a
concentration-dependent manner (Fig.
3C). Furthermore, it was observed that the range of necrotic
tissue spread in a concentration-dependent manner (Fig. 3D); suggesting that the RD-derived
tissue on the CAM was sensitive to VCR and the findings were
similar to those obtained from the in vitro drug-sensitivity
assay.
Discussion
The present study established a CDX model using a
CAM assay with the human ERMS cell line, RD, and the ARMS cell
line, Rh30. The formation of a three-dimensional tumor mass on the
CAM was confirmed by visual observation and the temporal
multiplication of grafted cells by calculating the volume using a
Vernier caliper. Moreover, pathological assessments confirmed that
transplanted cells gathered and formed tumor tissue along the CAM.
Some chick red blood cells infiltrated the tissue, indicating that
the transplanted cells were nourished by the chick host and that
the CAM assay was helpful in the establishment of CDX models.
Moreover, tumors on the CAM were sensitive to VCR in a
concentration-dependent manner, confirming the utility of the CAM
assay as a therapeutic model both three-dimensionally and
histologically. Therefore, the CAM assay could be useful to
determine the sensitivity of anticancer drugs.
The CAM assay has been widely used in the research
field of oncological morphology; however, the protocol of tumor
engraftment is not standardized. Some researchers have placed the
fertilized eggs horizontally (10,13),
whereas others have created ex ovo xenograft models by
transferring chick embryos onto sterilized trays (12,33).
After trying the former method several times, it was revealed that
the CAM was often damaged during the process of punching holes in
the eggshells. In addition, there was often a considerable
difference between the area of the hole on the eggshell and the
range of motion of the tumor on the CAM, which made it challenging
to observe the tumors on the CAM. The ex ovo method was
previously reported to lower the chick embryo survival ratio
compared with the in ovo method (2). The present study adopted a method of
using fertilized eggs placed vertically due to its simplicity and
stability. The time taken for cell line transplantation and tumor
resection to minimize damage to the CAM and improve the survival
rate of chick embryos was determined by referring to previous
reports (10,13). To help the local formation of
spherical tumors, silicone rings were placed on the CAM and the
cell suspension was added to their center.
Although we initially tried to assess how grafted
RMS cells multiplied on the CAM through fluorescence analysis using
luciferase-transgenic cell lines, we shifted to calculating the
volume of resected tumors with measurements using Vernier calipers
because the results of this assessment were revealed to be
consistent with those of H&E staining. Fluorescence analysis
was limited to two dimensions in the range visible through the hole
in the eggshell, and was easily influenced by tumor crookedness and
movement under the eggshell related to embryo motion.
The CAM assay has a number of advantages over mouse
models. First, it is time- and cost-effective, whereas mouse models
require long observation periods (weeks to months) (16,17,34),
the CAM assay is completed within 10 days after cell xenograft. The
CAM assay can also assess the effectiveness of chemotherapy agents
more rapidly. Second, chick embryos are not regarded as animals in
numerous countries, and CAM assay experiments do not require the
approval of animal experimental ethics committees. Furthermore, the
CAM is not innervated, thus preventing the infliction of pain and
suffering on the chick embryos (10). Third, the CAM can be seen directly,
so that it is possible to not only visualize the sites of tumor
cells or tissues xenografts and drug administration but also to
easily observe the development of tumors on the CAM and therapeutic
efficacy.
In the field of pediatric cancer, the use of
precision medicine has made less progress than in adult cancer, and
a remedy based on the oncogenic background of patients has not yet
been established. Previous studies have applied the CAM assay as an
alternative model for RMS studies (35–38);
however, these studies have not mentioned the application of this
model to precision medicine. In addition, other studies have
investigated how to take advantage of the CAM assay for precision
medicine (3,4), yet these studies have not assessed
RMS. It may be hypothesized that the RMS CDX model described in the
present study using the CAM assay has the potential to substantiate
and develop novel patient-specific therapeutic medicines, and may
become the foundation of precision medicine for RMS and intractable
pediatric cancer.
The present study has some limitations. First, only
one cell line was examined for each ERMS and ARMS; in the future,
we aim to examine other RMS cell lines. Second, the less damaging
method of administering VCR via intravenous injection could not
adequately be examined.
In conclusion, the present study established a CAM
assay protocol using human RMS cell lines and confirmed the
formation of a three-dimensional tumor mass on the CAM. Moreover,
the anticancer efficacy of VCR was demonstrated on established
human RMS CDX models. The CAM assay may therefore be useful as both
a CDX model and a therapeutic model. In the future, we aim to
establish CDX models using other RMS cell lines and PDX models
using tumor tissue resected from mouse CDX models or patients, and
to examine the utility of the models.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Dr Peter J.
Houghton, (Greehey Children's Cancer Research Institute, University
of Texas Health Science Center, San Antonio, TX, USA) for providing
the Rh30 cell line. The authors are also grateful to Dr Satoshi
Miyagaki, Dr Akihiro Nishida and Ms. Mami Kotoura (Department of
Pediatrics, Graduate School of Medical Science, Kyoto Prefectural
University of Medicine, Kyoto, Japan) for teaching us the
experimental techniques.
Funding
This work was supported by a grant from JSPS KAKENHI (grant no.
JP 20K08187).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CS performed the experiments. KK and TI designed
this study and confirmed the authenticity of all the raw data. CS,
KK, HY, MM, SY, KT and HH interpreted and processed the
experimental data, and performed the analysis. TN contributed to
the original technical methods using CAM. CS, KK, HY, MM, SY, KT,
TN, HH and TI discussed the results and contributed to the final
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Faculty of Science
Ethics Committee of the Kyoto Prefectural University of Medicine
(approval no. #2019-35).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ARMS
|
alveolar RMS
|
|
CAM
|
chorioallantoic membrane
|
|
CDX
|
cell line-derived xenograft
|
|
D-luciferin
|
D-luciferin potassium salt
|
|
ERMS
|
embryonal RMS
|
|
H&E
|
hematoxylin and eosin
|
|
IHC
|
immunohistochemistry
|
|
PBS
|
phosphate-buffered saline
|
|
PDX
|
patient-derived xenograft
|
|
RMS
|
rhabdomyosarcoma
|
References
|
1
|
Do K, O'Sullivan Coyne G and Chen AP: An
overview of the NCI precision medicine trials-NCI MATCH and MPACT.
Chin Clin Oncol. 4:312015.PubMed/NCBI
|
|
2
|
Lokman NA, Elder ASF, Ricciardelli C and
Oehler MK: Chick chorioallantoic membrane (CAM) assay as an in vivo
model to study the effect of newly identified molecules on ovarian
cancer invasion and metastasis. Int J Mol Sci. 13:9959–9970. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
DeBord LC, Pathak RR, Villaneuva M, Liu
HC, Harrington DA, Yu W, Lewis MT and Sikora AG: The chick
chorioallantoic membrane (CAM) as a versatile patient-derived
xenograft (PDX) platform for precision medicine and preclinical
research. Am J Cancer Res. 8:1642–1660. 2018.PubMed/NCBI
|
|
4
|
Chu PY, Koh AP, Antony J and Huang RY:
Applications of the chick chorioallantoic membrane as an
alternative model for cancer studies. Cells Tissues Organs.
211:222–237. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Valdes TI, Kreutzer D and Moussy F: The
chick chorioallantoic membrane as a novel in vivo model for the
testing of biomaterials. J Biomed Mater Res. 62:273–282. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ribatti D: Chicken chorioallantoic
membrane angiogenesis model. Methods Mol Biol. 843:47–57. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fiorentzis M, Viestenz A, Siebolts U,
Seitz B, Coupland SE and Heinzelmann J: The potential use of
electrochemotherapy in the treatment of uveal melanoma: In vitro
results in 3D tumor cultures and in vivo results in a chick embryo
model. Cancers (Basel). 11:13442019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Moreno-Jiménez I, Lanham SA, Kanczler JM,
Hulsart-Billstrom G, Evans ND and Oreffo ROC: Remodelling of human
bone on the chorioallantoic membrane of the chicken egg: De novo
bone formation and resorption. J Tissue Eng Regen Med.
12:1877–1890. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rasmussen SV, Berlow NE, Price LH, Mansoor
A, Cairo S, Rugonyi S and Keller C: Preclinical therapeutics ex ovo
quail eggs as a biomimetic automation-ready xenograft platform. Sci
Rep. 11:233022021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kunz P, Schenker A, Sähr H, Lehner B and
Fellenberg J: Optimization of the chicken chorioallantoic membrane
assay as reliable in vivo model for the analysis of osteosarcoma.
PLoS One. 14:e02153122019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ribatti D: The chick embryo
chorioallantoic membrane (CAM) assay. Reprod Toxicol. 70:97–101.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nowak-Sliwinska P, Segura T and
Iruela-Arispe ML: The chicken chorioallantoic membrane model in
biology, medicine and bioengineering. Angiogenesis. 17:779–804.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vu BT, Shahin SA, Croissant J, Fatieiev Y,
Matsumoto K, Le-Hoang Doan T, Yik T, Simargi S, Conteras A, Ratliff
L, et al: Chick chorioallantoic membrane assay as an in vivo model
to study the effect of nanoparticle-based anticancer drugs in
ovarian cancer. Sci Rep. 8:85242018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dasgupta R, Fuchs J and Rodeberg D:
Rhabdomyosarcoma. Semin Pediatr Surg. 25:276–283. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ognjanovic S, Linabery AM, Charbonneau B
and Ross JA: Trends in childhood rhabdomyosarcoma incidence and
survival in the United States, 1975–2005. Cancer. 115:4218–4226.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nakagawa N, Kikuchi K, Yagyu S, Miyachi M,
Iehara T, Tajiri T, Sakai T and Hosoi H: Mutations in the RAS
pathway as potential precision medicine targets in treatment of
rhabdomyosarcoma. Biochem Biophys Res Commun. 512:524–530. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ouchi K, Miyachi M, Yagyu S, Kikuchi K,
Kuwahara Y, Tsuchiya K, Iehara T and Hosoi H: Oncogenic role of
HMGA2 in fusion-negative rhabdomyosarcoma cells. Cancer Cell Int.
20:1922020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gurria JP and Dasgupta R: Rhabdomyosarcoma
and extraosseous ewing sarcoma. Children (Basel).
5:1652018.PubMed/NCBI
|
|
19
|
Malempati S and Hawkins DS:
Rhabdomyosarcoma: Review of the children's oncology group (COG)
soft-tissue sarcoma committee experience and rationale for current
COG studies. Pediatr Blood Cancer. 59:5–10. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Otabe O, Kikuchi K, Tsuchiya K, Katsumi Y,
Yagyu S, Miyachi M, Iehara T and Hosoi H: MET/ERK2 pathway
regulates the motility of human alveolar rhabdomyosarcoma cells.
Oncol Rep. 37:98–104. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Davicioni E, Anderson MJ, Finckenstein FG,
Lynch JC, Qualman SJ, Shimada H, Schofield DE, Buckley JD, Meyer
WH, Sorensen PH and Triche TJ: Molecular classification of
rhabdomyosarcoma-genotypic and phenotypic determinants of
diagnosis: A report from the children's oncology group. Am J
Pathol. 174:550–564. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Williamson D, Missiaglia E, de Reyniès A,
Pierron G, Thuille B, Palenzuela G, Thway K, Orbach D, Laé M,
Fréneaux P, et al: Fusion gene-negative alveolar rhabdomyosarcoma
is clinically and molecularly indistinguishable from embryonal
rhabdomyosarcoma. J Clin Oncol. 28:2151–2158. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Meza JL, Anderson J, Pappo AS and Meyer
WH; Children's Oncology Group, : Analysis of prognostic factors in
patients with nonmetastatic rhabdomyosarcoma treated on intergroup
rhabdomyosarcoma studies III and IV: The children's oncology group.
J Clin Oncol. 24:3844–3851. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Arndt CAS, Stoner JA, Hawkins DS, Rodeberg
DA, Hayes-Jordan AA, Paidas CN, Parham DM, Teot LA, Wharam MD,
Breneman JC, et al: Vincristine, actinomycin, and cyclophosphamide
compared with vincristine, actinomycin, and cyclophosphamide
alternating with vincristine, topotecan, and cyclophosphamide for
intermediate-risk rhabdomyosarcoma: Children's oncology group study
D9803. J Clin Oncol. 27:5182–5188. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Oberlin O, Rey A, Lyden E, Bisogno G,
Stevens MC, Meyer WH, Carli M and Anderson JR: Prognostic factors
in metastatic rhabdomyosarcomas: Results of a pooled analysis from
United States and European cooperative groups. J Clin Oncol.
26:2384–2389. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Arndt CAS, Rose PS, Folpe AL and Laack NN:
Common musculoskeletal tumors of childhood and adolescence. Mayo
Clin Proc. 87:475–487. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lockney NA, Friedman DN, Wexler LH, Sklar
CA, Casey DL and Wolden SL: Late toxicities of intensity-modulated
radiation therapy for head and neck rhabdomyosarcoma. Pediatr Blood
Cancer. 63:1608–1614. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Clement SC, Schoot RA, Slater O, Chisholm
JC, Abela C, Balm AJM, van den Brekel MW, Breunis WB, Chang YC,
Davila Fajardo R, et al: Endocrine disorders among long-term
survivors of childhood head and neck rhabdomyosarcoma. Eur J
Cancer. 54:1–10. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Walterhouse D and Watson A: Optimal
management strategies for rhabdomyosarcoma in children. Paediatr
Drugs. 9:391–400. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Douglass EC, Valentine M, Etcubanas E,
Parham D, Webber BL, Houghton PJ, Houghton JA and Green AA: A
specific chromosomal abnormality in rhabdomyosarcoma. Cytogenet
Cell Genet. 45:148–155. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Schwartz SO and Stansbury F: Significance
of nucleated red blood cells in peripheral blood; analysis of 1,496
cases. J Am Med Assoc. 154:1339–1340. 1954. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ghaffari-Tabrizi-Wizsy N, Passegger CA,
Nebel L, Krismer F, Herzer-Schneidhofer G, Schwach G and Pfragner
R: The avian chorioallantoic membrane as an alternative tool to
study medullary thyroid cancer. Endocr Connect. 8:462–467. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Linardic CM, Downie DL, Qualman S, Bentley
RC and Counter CM: Genetic modeling of human rhabdomyosarcoma.
Cancer Res. 65:4490–4495. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dolgikh N, Hugle M, Vogler M and Fulda S:
NRAS-mutated rhabdomyosarcoma cells are vulnerable to mitochondrial
apoptosis induced by coinhibition of MEK and PI3Kα. Cancer Res.
78:2000–2013. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Heinicke U, Kupka J, Fichter I and Fulda
S: Critical role of mitochondria-mediated apoptosis for
JNJ-26481585-induced antitumor activity in rhabdomyosarcoma.
Oncogene. 35:3729–3741. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Asam C, Buerger K, Felthaus O, Brébant V,
Rachel R, Prantl L, Witzgall R, Haerteis S and Aung T: Subcellular
localization of the chemotherapeutic agent doxorubicin in renal
epithelial cells and in tumor cells using correlative light and
electron microscopy. Clin Hemorheol Microcirc. 73:157–167. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Abraham J, Prajapati SI, Nishijo K,
Schaffer BS, Taniguchi E, Kilcoyne A, McCleish AT, Nelon LD, Giles
FG, Efstratiadis A, et al: Evasion mechanisms to Igf1r inhibition
in rhabdomyosarcoma. Mol Cancer Ther. 10:697–707. 2011. View Article : Google Scholar : PubMed/NCBI
|