Introduction
MicroRNAs (miRNAs/miRs) are a class of noncoding
small RNAs ~22 nucleotides in length. It has been reported that one
miRNA can target dozens of mRNAs by recognizing and binding to
complementary sites in the 3′-untranslated regions (3′-UTRs)
(1). Thus, the prediction of its
impacts in different contexts is difficult (2). The potential target genes of miRNAs
can be predicted using bioinformatics tools, such as TargetScan
(3), RNA22 (4), miRDB (5), STarMir (6) and DIANA-microT-CDS (7); regular updating of these tools is
important to ensure accurate results. A number of the tools are
updated regularly, with TargetScan and miRDB being the ones most
frequently updated (8). Moreover,
it has been shown that miRNA-binding sites within coding sequences
(CDSs) can also participate in the control of gene expression
(9). Among the aforementioned
tools, RNA22, miRDB and STarMir can identify miRNA targets in CDSs,
5′UTRs and 3′UTRs.
miRNAs are dysregulated in various types of human
cancer, and are considered to function as oncogenes or tumor
suppressors, depending on their target genes. Because of their
broad regulatory activity, miRNAs are involved in numerous cellular
processes, including proliferation (10), apoptosis (11) and metastasis (12). Moreover, miRNA expression is
regulated by various factors, such as long noncoding RNAs (lncRNAs)
and circular RNAs (circRNAs). lncRNAs are RNAs that do not encode
proteins and are >200 nt in length (13). lncRNAs regulate gene expression at
different levels through various mechanisms, such as genomic
imprinting, chromatin remodeling, cell cycle regulation and
transcriptional regulation (14).
circRNAs are a unique class of RNA with a closed-loop structure
that can modulate linear RNA transcription, downstream gene
expression and protein production (15). Competitive endogenous RNA is a
mechanism that can explain the regulatory interactions among
diverse RNAs. The theoretical core of this hypothesis is that some
non-coding RNAs, such as lncRNAs, pseudogenes and circRNAs, can
competitively bind to the same miRNA through a miRNA response
element in order to modulate the expression levels of each other
(16).
Among the numerous miRNAs, miR-370 has attracted
great attention. The miR-370 family is located at chromosome
14q32.31 and includes two main members of the human genome,
miR-370-5p and miR-370-3p. At present, most research has focused on
miR-370-3p, with only a few studies reporting on miR-370-5p
(12,17). In general, miR-370 inhibits gene
expression by specifically binding to the 3′-UTR of downstream
target mRNAs, and can function as an oncogene or tumor suppressor
to regulate the occurrence and development of cancer (18). In addition, it has been shown that
miR-370 may act as small activator RNA for P21 in lung cancer
(17). Similarly, accumulating
evidence has indicated that miRNAs can activate gene expression by
targeting promoters (19,20). The potential mechanism underlying
miRNA-mediated gene activation is unclear. miR-370 has been
reported to affect numerous biological behaviors, such as cell
proliferation (21), apoptosis
(22), migration (23), invasion (24), as well as cell cycle progression
(25) and cell stemness (26). Furthermore, miR-370 may affect the
response of cancer cells to anticancer therapy (27).
The present review systematically summarizes the
expression and function of miR-370 in cancer, and predicts its
potential as a novel biomarker, as well as a diagnostic and
prognostic indicator.
The aberrant expression of miR-370 in
cancer
The dysregulation of miR-370 is closely associated
with the pathogenesis and progression of numerous types of cancer.
Existing studies have reported that miR-370 is aberrantly expressed
in various types of cancer, with both upregulation and
downregulation reported (Table I).
Among them, miR-370 has been shown to be downregulated in nine
types of cancer, including oral squamous cell carcinoma (OSCC)
(28), laryngeal squamous cell
carcinoma (LSCC) (29), thyroid
cancer (23,30), esophageal squamous cell carcinoma
(ESCC) (31), hepatocellular
carcinoma (HCC) (32,33), colon cancer (22), ovarian cancer (34), cervical cancer (21) and osteosarcoma (35). Conversely, miR-370 has been reported
to be upregulated in melanoma (36).
 | Table I.Expression patterns and target genes
of microRNA-370 in various types of cancer. |
Table I.
Expression patterns and target genes
of microRNA-370 in various types of cancer.
| Cancer type | Expression | Function | Target gene | (Refs.) |
|---|
| OSCC | Downregulation | Inhibits
proliferation, migration and invasion | IRS-1 | (28) |
| LSCC | Downregulation | Inhibits
proliferation | FoxM1 | (29) |
| Thyroid cancer | Downregulation | Inhibits
proliferation, migration and invasion; induces apoptosis | FZD8/LMO4/MYH9 | (23,30,75,77) |
| NSCLC | / | Inhibits
proliferation, cell cycle, migration and invasion | p21/TRIM44 | (17,81) |
| Breast cancer | Downregulation | Inhibits
proliferation and invasion | LUC7L3 | (12) |
|
| Upregulation | Promotes
proliferation migration, and invasion | FBLN5/ FGF14 | (26,39) |
| ESCC | Downregulation | Inhibits
proliferation and invasion; induces apoptosis | PIM1/Wnt7a | (31,70) |
| Gastric cancer | Downregulation | Inhibits
proliferation, migration and invasion | EGFR | (42) |
|
| Upregulation | Promotes
proliferation, cell cycle, migration and invasion |
FoxO1/UQCRC2/TGFβ-RII | (24,40,41) |
| CRC | Downregulation | Inhibits
proliferation, migration and invasion; induces apoptosis |
β-catenin/MDM4/EZH1 | (22,84,86,88) |
| HCC | Downregulation | Inhibits
proliferation; induces apoptosis | FoxO3a/ISG15 | (32,33) |
| Ovarian cancer | Downregulation | Inhibits
proliferation, cell cycle and invasion |
CDK6/FoxM1/RAB17 | (25,34,61) |
| Cervical
cancer | Downregulation | Inhibits
proliferation and migration; induces apoptosis | RAF1 | (21) |
| Osteosarcoma | Downregulation | Inhibits
proliferation, migration and invasion | RUVBL1/PIM1 | (35,69) |
| Melanoma | Upregulation | Promotes
proliferation, migration and invasion; inhibits apoptosis | PDHB | (36) |
| Prostate
cancer | Upregulation | Promotes
proliferation and cell cycle | FoxO1 | (44) |
|
| Downregulation | Inhibits
proliferation, migration and invasion | DDX3X | (43) |
| Bladder cancer | / | Inhibits
proliferation, cell cycle migration and invasion; induces
apoptosis | Wnt7a/SLD5 | (51,63) |
| AML | Downregulation | Inhibits
proliferation, migration and invasion; induces apoptosis | MAPK1 | (38,53) |
|
| Upregulation | Promotes
proliferation | NF1 | (37) |
Inconsistent results have been reported regarding
miR-370 expression in four types of cancer, including acute myeloid
leukemia (AML), breast cancer, gastric cancer and prostate cancer.
Notably, overexpression of miR-370 has been reported in AML
(37); however, it has also been
demonstrated that miR-370 is significantly decreased in the
peripheral blood of pediatric patients with AML (38). These two conflicting results may be
due to the effect of age on the results or may be because miR-370
expression differs in tissue and peripheral blood. In addition, in
breast cancer, one study reported downregulation of miR-370
expression (12), whereas two other
studies have shown upregulation of miR-370 expression (26,39).
In gastric cancer, three studies have indicated that miR-370 is
upregulated (24,40,41),
whereas one study reported that miR-370 is downregulated (42). In prostate cancer, inconsistent
results regarding miR-370 expression have also been observed
(43,44). Therefore, in most cancer types,
miR-370 acts as a tumor suppressor; however, in some cancer types,
it may promote tumor progression. In addition, there are
inconsistent results regarding miR-370 expression in four types of
cancer. The underlying mechanisms of these differences remain
unclear and may be related to tumor heterogeneity.
The biological role of miR-370 in
cancer
miR-370 and different signaling
pathways
The role of miR-370 in signaling pathways associated
with cancer occurrence and development has been extensively
explored. Numerous studies have shown that miR-370 can influence
cancer development and progression by participating in the
Wnt/β-catenin, MAPK, NF-κB and PI3K/Akt signaling pathways,
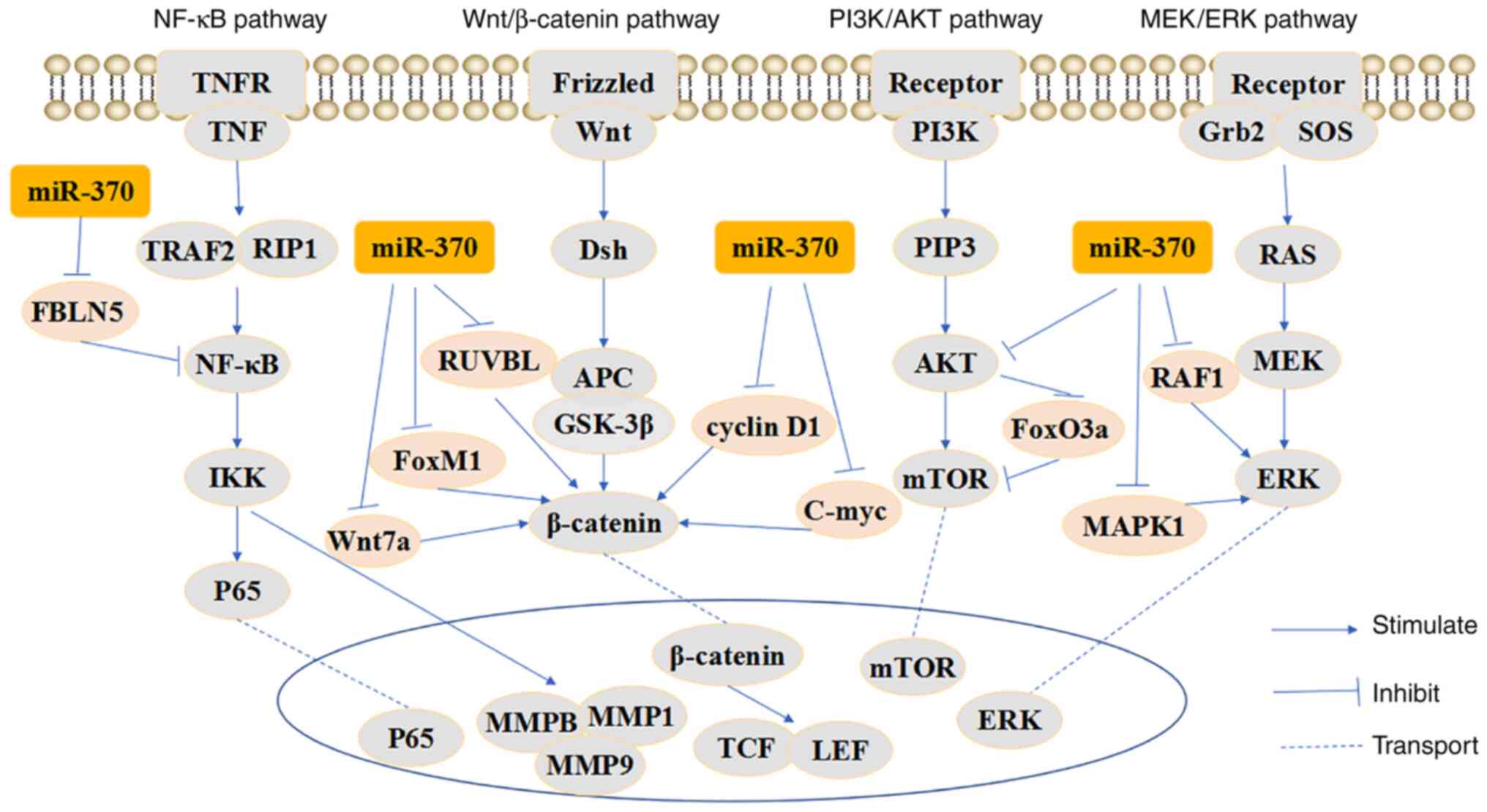
alongside others (Fig. 1).
The Wnt signaling pathway
Aberrant activation of the Wnt signaling pathway can
facilitate the progression of multiple types of cancer (45). Core elements of the Wnt signaling
pathway comprise the extracellular factor Wnt and the transmembrane
receptor β-catenin (46). It has
been reported that RuvB-like ATPase 1 (RUVBL1) can positively
regulate Wnt/β-catenin signaling (47). miR-370-3p can directly target RUVBL
to inhibit the interaction between RUVBL1 and β-catenin/LEF1
complex, thereby inhibiting Wnt/β-catenin signaling and further
inhibiting osteosarcoma progression (35). Another study reported that DNA
methylation-mediated reduction of miR-370 leads to upregulation of
forkhead box M1 (FoxM1), thus activating the Wnt/β-catenin
signaling pathway and promoting cell proliferation in osteosarcoma
(48). Wnt family member 7A (Wnt7a)
is a Wnt ligand that activates the classical Wnt/β-catenin
signaling pathway to promote cancer progression (49). Wnt7a activates the Wnt signaling
pathway, stimulates the invasion of bladder cancer cells and is
inhibited by miR-370-3p (50).
Furthermore, miR-370-3p has been reported to inhibit Wnt7a protein
expression and Wnt/β-catenin signaling, further suppressing bladder
cancer proliferation (51). In
response to miR-370-3p overexpression, cyclin D1 and C-myc have
been shown to be decreased in thyroid cancer, which is related to
the Wnt signaling pathway (30).
These results suggested that miR-370 may act as an oncosuppressor
in the Wnt signaling pathway by regulating a range of targets
associated with this pathway.
The MEK/ERK signaling pathway
The activity of the MAPK/ERK kinase (MEK)/ERK
signaling pathway is crucial for promoting cancer progression. The
serine/threonine kinase RAF1 serves a vital role in activating the
MEK1/2 dual-specificity protein kinases, which then activate ERK1/2
(52). miR-370-3p can downregulate
RAF1 expression, thereby inhibiting the MEK/ERK pathway in cervical
cancer (21). In AML, miR-370-3p
has been reported to inhibit MAPK1 expression, in turn suppressing
ERK signaling, which further inhibits AML progression (53). These results indicated that miR-370
may function as a tumor suppressor in the MEK/ERK pathway by
regulating different target genes.
The PI3K/AKT signaling pathway
The PI3K/Akt signaling pathway plays an essential
role in the occurrence and development of tumors. The PI3K/AKT
signaling pathway phosphorylates PI3K and AKT proteins, thus
facilitating tumor cell proliferation and malignancy, and
suppressing tumor cell apoptosis (54). miR-370 has been reported to inhibit
cell proliferation in liver cancer by suppressing AKT, which in
turn inhibits forkhead box O3a (FoxO3a) (32).
The NF-κB signaling pathway
Emerging research has suggested that dysregulated
NF-κB pathway activity contributes to a variety of diseases,
including cancer (55). The NF-κB
signaling pathway affects cancer cell proliferation, apoptosis and
metastasis, thereby influencing tumor progression (56). It has been reported that fibulin-5
(FBLN5) acts as a tumor suppressor to suppress cancer tumorigenesis
and progression (57). miR-370-3p
can promote breast cancer progression by directly targeting FBLN5
and activating the NF-κB signaling pathway (26). This result suggested that miR-370
may act as an oncogene, which is contradictory to the usual
situation and may be related to tumor heterogeneity.
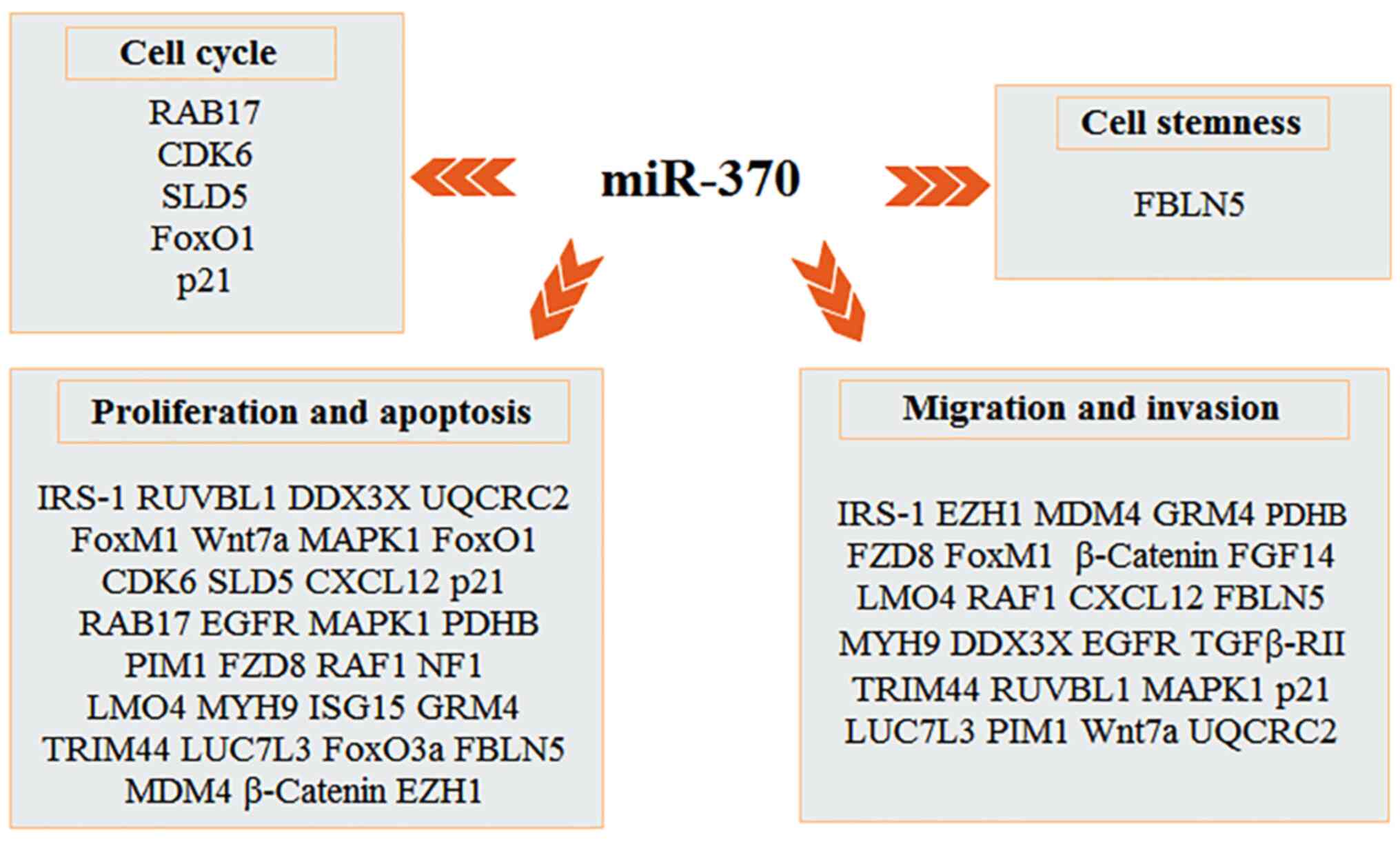
miR-370 and the cell cycle
The cell cycle is a complex process involving a
number of regulatory proteins that guide the cell through a
particular sequence of events, ultimately leading to mitosis and
the creation of two daughter cells (58). The cell cycle is affected by various
factors, such as cyclin D1, cyclin-dependent kinases (CDKs) and CDK
inhibitors (59). A number of
studies have confirmed that miR-370 may affect cell cycle
progression by targeting different genes (Fig. 2). RAB proteins can modulate
intracellular transport, and abnormal expression of RABs has been
found in a variety of diseases, including cancer (60). Overexpressed RAB17 in ovarian cancer
has been shown to promote the cell cycle, decreasing the number of
G1 phase cells and increasing the number of S phase
cells (61). Notably, RAB17 is a
direct target of miR-370-3p. Furthermore, miR-370-3p arrests the
cell cycle and promotes apoptosis of ovarian cancer cell through
inhibiting CDK6 (25). SLD5, a
component of the GINS complex, is crucial for DNA replication
(62). SLD5 has been reported to
accelerate the cell cycle of bladder cancer cells and to be
negatively regulated by miR-370 (63). Furthermore, miR-370 can induce
non-small cell lung cancer (NSCLC) cell cycle arrest, through
inhibiting the cyclin D1-CDK4/CDK6 pathway by activating p21
(17). It is proposed in this
research that miR-370 may upregulate p21 gene expression in lung
cancer through binding the p21 promoter; however, the mechanism
involved is unknown.
In contrast to the aforementioned findings, a small
number of studies have shown that miR-370 promotes cell cycle
progression. Forkhead box transcription factor O1 (FoxO1), a member
of the FoxO family of transcription factors, is crucial for
modulating cytokine and chemokine secretion (64). FoxO1 acts as a tumor suppressor and
has been demonstrated to be involved in a variety of critical cell
functions, such as cell proliferation, cell cycle, apoptosis and
angiogenesis (65). In prostate
cancer, miR-370 can accelerate the G1/S cell cycle
transition by inhibiting FoxO1, which increases the expression of
p21Cip1 and p27Kip1 (44). Besides,
similar findings have been observed in gastric cancer (40). In summary, miR-370 acts as a
promoter or inhibitor in the cell cycle process, depending on the
different targets.
miR-370 and cell proliferation and
apoptosis
miR-370 has been reported to be involved in cell
proliferation and apoptosis through directly regulating various
targets (Fig. 2). FoxM1, a critical
member of the FOX transcription factor family, regulates
proliferation and invasion in a number of cancer types (66,67).
miR-370 has been shown to function as an inhibitor in LSCC
(29) and ovarian cancer (34) that regulates cell proliferation by
decreasing FoxM1. PIM1, which is one of the Pim family kinases, has
a critical role in the progression of human malignancies (68). miR-370 acts as a tumor suppressor in
ESCC to suppress cell proliferation and induce cell apoptosis via
downregulating PIM1 (31).
Furthermore, miR-370 regulates osteosarcoma cell proliferation
through downregulating PIM1 (69).
Wnt7a can facilitate cell proliferation and decrease apoptosis, and
is targeted by miR-370-3p in cisplatin-resistant esophageal cancer
(70) and bladder cancer (51). These results suggested that miR-370
may regulate the same target to affect cell proliferation and
apoptosis in various types of cancer.
Insulin receptor substrate-1 (IRS-1) is the
substrate of insulin-like growth factor receptor, which activates
AKT/mTOR signaling in malignant tumors (71). miR-370 can act as a suppressor to
reduce the anchorage-independent growth of OSCC cells by
downregulating IRS-1 (28).
Frizzled class receptor 8 (FZD8), which is one of the Frizzled
family of serpentine proteins, promotes the progression of various
types of cancer by activating the Wnt pathway (72). In addition, FZD8 promotes the
proliferation of thyroid cancer cells and is decreased by
miR-370-3p (30). LIM domain-only 4
(LMO4) is a member of the LIM-only family of proteins and has been
demonstrated to act as an oncogene in different types of cancer
(73,74). miR-370-3p has been shown to function
as a tumor suppressor to inhibit thyroid cancer cell proliferation
and promote apoptosis through suppressing LMO4 (75). Myosin heavy chain 9 (MYH9) serves as
an oncogene in multiple types of cancer, and MYH9 inhibition
markedly inhibits the malignant phenotypes and chemoresistance of
cancer cells (76). Notably,
miR-370-3p can inhibit cell proliferation and induce cell apoptosis
by decreasing MYH9 in 131I-resistant differentiated thyroid
carcinoma cells (77).
In NSCLC, miR-370 can inhibit cell proliferation and
colony formation by upregulating p21 (17). Furthermore, the knockdown of miR-370
can partially restore the inhibitory effect of silenced KCNK15-AS1
on lung cancer cell proliferation (78). Tripartite motif-containing 44
(TRIM44) is an atypical TRIM-family protein and emerging studies
have demonstrated that TRIM44 stimulates cancer progression,
including lung cancer (79,80). miR-370-3p acts as a tumor suppressor
in NSCLC to inhibit cell proliferation by downregulating TRIM44
(81). In breast cancer, miR-370-5p
suppresses cell proliferation through targeting LUC7-like 3
pre-mRNA splicing factor (LUC7L3), which is negatively regulated by
miR-370-5p (12).
It has previously been reported that EGFR serves an
important role in the pathogenesis of human malignancy (82). In gastric cancer, inhibition of EGFR
can suppress cell proliferation, and miR-370 works as a potential
regulator of EGFR (32,83). In colon cancer, miR-370-3p can
restrain cell proliferation by targeting β-catenin (84). Mouse double minute 4 (MDM4) has been
validated as an oncogene that can negatively regulate
transcriptional activity of the tumor suppressor p53 (85). miR-370 functions as a suppressor to
inhibit cell proliferation and promote apoptosis by targeting MDM4
in colorectal cancer (CRC) (22,86).
Both zeste homologue 1 (EZH1), and its homolog EZH2, act as histone
H3 lysine 27 (H3K27) methyltransferases that regulate target gene
transcription. H3K27 has been reported to be involved in the
development and progression of various types of cancer (87). Knockdown of miR-370-5p can
facilitate the proliferative ability of CRC by targeting EZH1
(88).
FoxO3a is a member of a subfamily of forkhead
transcription factors that had a crucial role in carcinogenesis
(89). miR-370 works as a tumor
suppressor to inhibit the proliferation of human liver cancer cells
by inhibiting AKT, which further suppresses FoxO3a (32). The expression of IFN-stimulated gene
15 (ISG15) has been shown to be associated with the differentiation
grade, migration and survival in patients with HCC (90). Furthermore, miR-370 regulates the
IFN-α-induced apoptosis of HCC cells through downregulating ISG15
(33).
In ovarian cancer, miR-370-3p restrains cell
proliferation and promotes apoptosis by targeting CDK6 (25). Furthermore, miR-370-3p can suppress
colony formation of ovarian cancer cells via targeting RAB17
(61). miR-370-3p also suppresses
the proliferation of cervical cancer cells by downregulating RAF1
(21). DDX3X, one of the DEAD-box
helicase family members, has a key role in almost all stages of RNA
metabolism and is involved in the progression of multiple diseases,
such as viral infection and cancer (91). miR-370-3p overexpression can inhibit
cell proliferation by directly targeting DDX3X in prostate cancer
(43). In addition, it has been
reported that miR-370 inhibits bladder cancer cell proliferation by
downregulating SLD5 (63).
MAPK1 has previously been reported to regulate cell
differentiation, proliferation and migration in various types of
cancer (92,93). miR-370-3p inhibits the proliferation
and promotes the apoptosis of AML cells by targeting MAPK1
(53). CXCL12, a member of the
chemokine family, serves a critical role in tumor development
(94). CXCL12 overexpression
facilitates melanoma cell proliferation and is regulated by
miR-370-3p (95). Moreover,
miR-370-3p targets RUVBL1 directly to suppress the proliferation of
osteosarcoma cells (35).
Contrary to the aforementioned findings, existing
studies have shown that miR-370 can also promote proliferation in
several types of cancer. Ubiquinol-cytochrome c reductase core
protein 2 (UQCRC2) is a crucial mitochondrial respiratory complex
III subunit, which has been confirmed to be involved in various
types of cancer as either an oncogene or a tumor suppressor gene
(96). Enhanced miR-370 has been
reported to facilitate gastric cancer cell proliferation through
suppressing UQCRC2 (24). In
gastric cancer (40) and prostate
cancer (44), FoxO1 inhibits cell
proliferation and is negatively regulated by miR-370. Furthermore,
miR-370-3p can promote prostate cancer cell proliferation by
downregulating P21 (97). Pyruvate
dehydrogenase B (PDHB) is a mitochondrial enzyme that catalyzes the
conversion of glucose-derived pyruvate to acetyl-CoA (98). Upregulation of miR-370 can
facilitate melanoma cell proliferation and can inhibit apoptosis
through targeting PDHB (36). A
previous study revealed that microdeletions of NF1 are common
events, resulting in decreased NF1 expression in AML (99). miR-370 has been shown to enhance AML
cell proliferation potential by downregulating NF1, which is
identified as a tumor suppressor (37). Glutamate metabotropic receptor 4
(GRM4), which is a member of the GRM protein family, has been
reported to be involved in adaptive immunity reactions in cancer.
Notably, GRM4 significantly inhibits breast cancer cell
proliferation under the direct regulation of miR-370-3p (100). Moreover, FBLN5 inhibits the
proliferation of breast cancer cells and is negatively regulated by
miR-370-3p (26). Overall, miR-370
may have a critical role in cell proliferation in multiple types of
cancer through regulating different targets. The conflicting
results of miR-370 in different cancer types may suggest it has a
context-dependent role.
miR-370 and cell migration and
invasion
Metastasis is the process by which cancer cells
spread from the tumor and acquire a more mesenchymal phenotype
through epithelial-mesenchymal transition (EMT). When cancer cells
migrate from the tumor they can invade the healthy stroma to
produce new tumors (101). miR-370
is deemed to be a significant miRNA related to tumor cell migration
and invasion (Fig. 2). In OSCC,
miR-370 can regulate IRS-1 expression and restrain the tumor
phenotype (28). In thyroid cancer,
miR-370-3p has been shown to suppress cell migration and invasion
by targeting FZD8 (30), LMO4
(75) and MYH9 (77). In addition, miR-370-3p suppresses
NSCLC cell migration and invasion through regulating TRIM44
(81) and p21 (17). It has also been reported that
miR-370-5p directly targets LUC7L3 to inhibit cell invasion in
breast cancer (12). In ESCC,
miR-370-3p suppresses cell invasion by downregulating Wnt7a
(70). In bladder cancer,
miR-370-3p directly targets Wnt7a and suppresses the Wnt signaling
pathway, thereby inhibiting cell invasion (50). Overexpressed miR-370 has been shown
to suppress gastric cancer cell migration by targeting EGFR
(42). In colon cancer, enforced
miR-370-3p can inhibit EMT through modulating β-catenin (84). miR-370 can also downregulate MDM4
and inhibit the metastatic ability of CRC cells (86). EZH1 has been shown to facilitate CRC
cell invasion and has been identified as a target of miR-370-5p
(88). Overexpressed miR-370 can
suppress ovarian cancer cell invasion by downregulating FoxM1
(34). In cervical cancer,
miR-370-3p inhibits cell migratory abilities by regulating RAF1
expression (21). Enforced
expression of miR-370-3p can suppress prostate cancer cell
migration, as well as invasion, and can lead to downregulation of
the DDX3X protein (43). miR-370-3p
may also suppress cell EMT by directly downregulating RUVBL1 in
osteosarcoma (35). Overexpression
of miR-370 inhibits osteosarcoma cell migration capacity by
targeting PIM1, which is negatively regulated by miR-370 (69). By directly binding to the 3′-UTR of
MAPK1, miR-370-3p has been reported to decrease its expression, and
to inhibit the migration and invasion of AML cells (53). Elevated miR-370-3p may also inhibit
the migration and invasion of melanoma cells by downregulating
CXCL12 expression (95).
Several studies have shown that miR-370 can promote
cell migration and invasion in breast cancer, stomach cancer and
melanoma. Fibroblast growth factor 14 (FGF14) belongs to the FGF
family and a recent study suggested that FGF14 functions as a tumor
suppressor in colorectal cancer (102). FGF14 overexpression can decrease
breast cancer cell migration and invasion, is negatively regulated
by miR-370-3p (39). miR-370-3p
facilitates breast cancer cell metastasis through suppressing FBLN5
and by activating the NF-κB signaling pathway (26). GRM4 may act as a potential tumor
suppressor to inhibit breast cancer cell migration and invasion, is
modulated by miR-370-3p (100).
Blocking transforming growth factor-β (TGFβ) signaling in a gastric
cancer model has been reported to promote tumor growth, and
resistance to a TGFβ inhibitor appears to be a critical event in
the occurrence of gastric cancer (103). miR-370 can increase the metastatic
ability of gastric cancer cells by downregulation of TGFβ-RII
(41). In addition, miR-370 may
modulate EMT signaling pathways to facilitate tumor metastasis by
targeting UQCRC2 in gastric cancer (24). Overexpressed miR-370 can also
enhance melanoma cell invasion and glycolysis by downregulating
PDHB (36). In most tumors, miR-370
inhibits cell metastasis; however, in some tumors, miR-370 both
inhibits and promotes cell migration and invasion. The mechanisms
underlying these effects are unknown and may be associated with
tumor heterogeneity.
Stemness
Cancer stem cells (CSCs) are a small subpopulation
of tumor cells with self-renewal, differentiation and tumorigenic
properties (104). CSCs have been
identified as one of the key factors contributing to tumor
recurrence and drug resistance (105). NF-κB-mediated signaling pathways
have been confirmed to be implicated in the maintenance of CSC
characteristics associated with tumor progression (106). miR-370-3p has been shown to reduce
FBLN5 expression and activate the NF-κB signaling pathway to
stimulate breast cancer cell stemness (26).
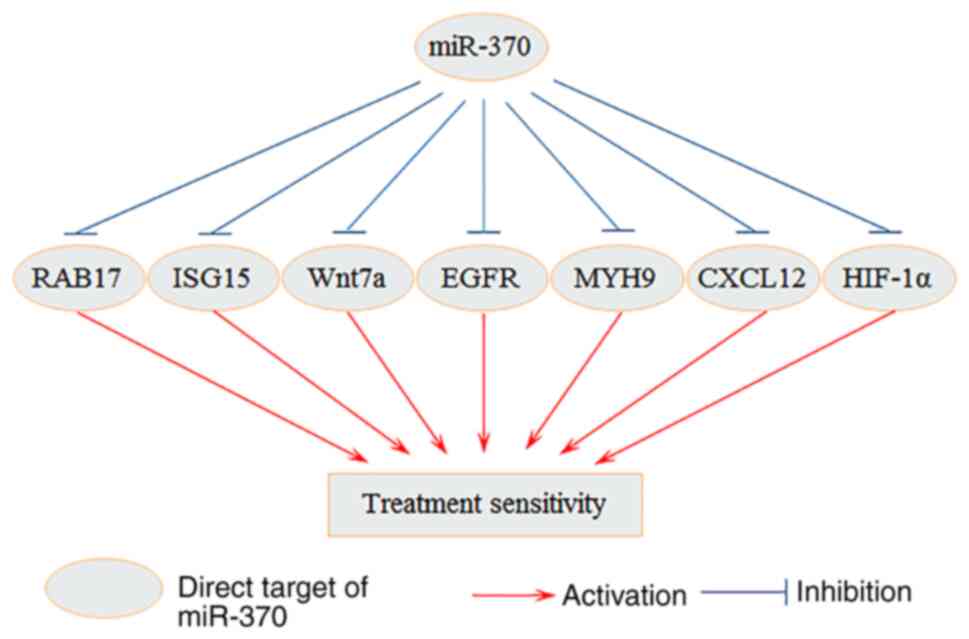
MiR-370 and cancer treatment
Since miR-370 is involved in different signaling
pathways, it has been considered a promising target for the
development of anticancer treatments (Fig. 3). A previous study reported that
RAB17 can affect the paclitaxel resistance of ovarian cancer cells
through the CDK6/RB signaling pathway, is negatively regulated by
miR-370-3p (61). Targeting of
ISG15 by miR-370 can also regulate the sensitivity of HCC cells to
IFN-α treatment (33). Upregulated
Wnt7a expression has been reported to promote cisplatin resistance
in esophageal cancer and to be negatively regulated by miR-370-3p
(70). Furthermore, miR-370
enhances the radiosensitivity of NSCLC cells by downregulating EGFR
and hypoxia-inducible factor 1α (107). miR-370-3p inhibits 131I resistance
in thyroid cancer cells through downregulating MYH9 expression
(77). Furthermore, it has been
reported that miR-370-3p facilitates the therapeutic effect of
anti-PD-1 treatment in melanoma through downregulating the CXCL12
(95). These results indicated that
it may be promising to use miR-370-targeted therapy in combination
with other existing anticancer therapies to improve efficacy.
The regulation of miR-370 in cancer
Previous studies have identified multiple factors
involved in the regulation of miR-370, such as lncRNAs, circRNAs,
methyltransferases and other factors (Table II).
 | Table II.Regulatory factors regulate the
expression of microRNA-370 in various cancers. |
Table II.
Regulatory factors regulate the
expression of microRNA-370 in various cancers.
| A, lncRNA |
|---|
|
|---|
| First author,
year | Factor | Cancer type | (Refs.) |
|---|
| Li, 2019 | lncRNA TUG1 | AML | (53) |
| Peng, 2019 | lncRNA
KCNK15-AS1 | Lung cancer | (78) |
| Li, 2021 | lncRNA CASC9 | Gastric cancer | (83) |
| Wang, 2019 | lncRNA
LINC00511 | Ovarian cancer | (108) |
| Wang, 2021 | lncRNA SNHG15 | Ovarian cancer | (25) |
| Zhang, 2020 | lncRNA BCAR4 | Bladder cancer | (51) |
| Pan, 2021 | lncRNA HCG18 | Prostate
cancer | (43) |
| Zhang, 2021 | lncRNA SNHG3 | CRC | (88) |
| Jin, 2020 | lncRNA
FGF14-AS2 | Breast cancer | (39) |
|
| B,
circRNA |
|
| First author,
year | Factor | Cancer
type | (Refs.) |
|
| Chen, 2018; Chen,
2021 | circNEK6 | Thyroid
carcinoma | (30,77) |
| Liu, 2020 | circ_0058124 | Thyroid
carcinoma | (75) |
| Xiong, 2021 | circUBAP2 | Thyroid
carcinoma | (23) |
| Wang, 2022 | circFBXW8 | NSCLC | (81) |
| Zou, 2020 | circ_001275 | ESCC | (70) |
| Mo, 2022 | circCCDC66 | CRC | (86) |
| Guo, 2020 | circ_0000714 | Ovarian cancer | (61) |
| Chen, 2018 | circ_0061140 | Ovarian cancer | (34) |
| Wu, 2019 | circAGFG1 | Cervical
cancer | (21) |
| Endo, 2020 | circITGA7 | Prostate
cancer | (109) |
| Fang, 2020 | circITGA7 | Osteosarcoma | (69) |
| Chen, 2019 | circMYO10 | Osteosarcoma | (35) |
| Wei, 2020 | circ_0020710 | Melanoma | (95) |
|
| C,
Others |
|
| First author,
year | Factor | Cancer
type | (Refs.) |
|
| Yamane, 2016 | DNMT1 | Bladder cancer | (63) |
| Pan, 2016 | DNMT1 | HCC | (111) |
| Zhang, 2017 | DNMT1 | Osteosarcoma | (48) |
| Han, 2016 |
Alpinumisoflavone | ESCC | (31) |
lncRNAs
lncRNAs are RNA molecules known to have gene
regulatory functions similar to those of miRNAs. lncRNAs contain
miRNA-binding sites and also act as miRNA sponges, which can lead
to the reduced bioavailability of mature miRNAs. lncRNA TUG1 has
been reported to modulate cell proliferation and migration in AML
through modulating the miR-370-3p/MAPK1/ERK signaling pathway
(53). Knockdown of KCNK15-AS1 can
suppress lung cancer cell proliferation by upregulating miR-370
(78). Furthermore, lncRNA CASC9
acts as an oncogene to promote the progression of gastric cancer by
modulating the miR-370/EGFR axis (83). Through downregulating miR-370-5p,
LINC00511 promotes the proliferation and invasion of estrogen
receptor 1-expressing ovarian cancer cells (108). Similarly, lncRNA SNHG15
facilitates ovarian cancer progression through regulating CDK6 by
sponging miR-370-3p (25). lncRNA
BCAR4 affects the cell proliferation and apoptosis of bladder
cancer by sponging miR-370-3p (51). In addition, through sponging
miR-370-3p, lncRNA HCG18 can promote the proliferation and
migration of prostate cancer cells by upregulating DDX3X (43). lncRNA SNHG3 enhances the
proliferative and invasive ability of CRC through modulating the
miR-370-5p/EZH1 axis (88). In
addition, lncRNA FGF14-AS2 suppresses breast cancer cell migration
and invasion through targeting FGF14 via acting as a sponge of
miR-370-3p (39).
circRNAs
circRNAs can also function as miRNA sponges, and
they carry several miRNA-binding sites. Multiple studies have shown
that circRNAs are involved in miR-370 regulation. In thyroid
carcinoma, circNEK6 (30,77), circ_0058124 (75), and circUBAP2 (23) have been identified to regulate
miR-370 expression via direct sponging and ultimately promote
cancer progression by modulating the expression of targets of
miR-370. Furthermore, circFBXW8 promotes NSCLC progression by
elevating TRIM44, through acting as a sponge of miR-370-3p
(81). circRNA_001275 elevates
Wnt7a expression through sponging miR-370-3p to promote esophageal
cancer progression and cisplatin resistance (70). In CRC, circCCDC66 facilitates cancer
progression through regulating the miR-370/MDM4 axis (86). In addition, circ_0000714 acts as a
sponge of miR-370-3p and influences cell paclitaxel resistance and
the cell cycle in ovarian cancer cells (61). circ_0061140 promotes cell
proliferation and migration in ovarian cancer by inhibiting FoxM1
through sponging miR-370 (34).
Moreover, circAGFG1 regulates cervical cancer cell proliferative
and migratory abilities via the miR-370-3p/RAF1 axis (21). circITGA7 can serve as a sponge for
miR-370-3p to inhibit prostate cancer cell proliferation (109). Furthermore, circITGA7 (69) and circMYO10 (35) function as sponges for miR-370-3p to
promote cancer progression in osteosarcoma. circ_0020710 has been
reported to act as an oncogene in melanoma cells to promote tumor
progression through regulating the miR-370-3p/CXCL12 axis (95).
DNA methylation and other factors
DNA methyltransferase 1 (DNMT1) is essential for
maintaining DNA methylation patterns during cell replication and
has been demonstrated to participate in the regulation of miRNAs in
cancer cells (110). IL-6-induced
overexpression of DNMT1 inhibits miR-370, leading to upregulated
SLD5, which promotes cell proliferation of bladder cancer (63). A previous study suggested that
physcion (an active ingredient in the medicinal plant Radix et
Rhizoma Rhei) can induce HCC cell apoptosis through upregulating
miR-370 by regulating the AMPK/Sp1/DNMT1 signaling pathway
(111). Osteosarcoma cells treated
with the DNA methylation inhibitor, 5-AZA-2′-deoxycytidine, exhibit
elevated miR-370 expression, which regulates osteosarcoma cell
proliferation. This result indicates that DNA methylation is
critical for miR-370 expression in osteosarcoma (48). Furthermore, the potential antitumor
effects of alpinumisoflavone (AIF) have been demonstrated (112). AIF inhibits tumor growth via
inducing cell apoptosis through modulating miR-370/PIM1 signaling
in ESCC (31).
The diagnostic and prognostic value of
miR-370 in cancer
The diagnostic value of miR-370 in
cancer
Emerging research has demonstrated the potential of
miR-370 as a diagnostic biomarker for different types of cancer,
such as AML, breast cancer and gastric carcinoma (Table III). In particular, miR-370 is
downregulated in the peripheral blood of pediatric patients with
AML, with a sensitivity of 96.9% and specificity of 93.3%. In this
previous study, the receiver operating characteristic (ROC) curve
had an area under the curve (AUC) of 0.966 for miR-370 (38). In another study, serum miR-370
expression was shown to be markedly reduced in patients with AML,
and high sensitivity and specificity were detected in AML serum
samples (91.46 and 100.00%), with an AUC of the ROC of 0.909
(113). It has been shown that
miR-370-3p is upregulated in the serum and exosome specimens of
patients with breast cancer, with a sensitivity of 59.26% and
specificity of 74.07%. The AUC of the ROC curve for miR-370-3p was
0.6735. Serum exosome miR-370 levels were also correlated with
breast cancer tumor size, lymph node metastasis and TNM stage
(26). This result was higher than
other markers, such as carcinoembryonic antigen and neuron-specific
enolase (sensitivity, 0.48 and 0.39, respectively) (114). Further studies are recommended to
verify the value of miR-370 in diagnosis because of its relatively
high specificity and sensitivity compared with other markers. In
addition, miR-370 expression is markedly higher in the plasma of
patients with gastric carcinoma compared with that in healthy
controls. Patients with gastric carcinoma with more aggressive or
advanced tumors have been shown to have higher miR-370 plasma
levels (41). These results
suggested that the role of miR-370 as a diagnostic biomarker is
accompanied by its role as a biomarker of prognosis.
 | Table III.Diagnostic and prognostic value of
microRNA-370 in various types of cancer. |
Table III.
Diagnostic and prognostic value of
microRNA-370 in various types of cancer.
| Cancer type | Expression | Diagnostic or
prognostic value | (Refs.) |
|---|
| AML | Downregulation | Diagnostic
biomarker and associated with poor OS | (38,113) |
| Breast cancer | Upregulation | Diagnostic
biomarker and associated with tumor size, lymph node metastasis and
TNM stage | (26) |
| Gastric cancer | Upregulation | Diagnostic
biomarker, and associated with more advanced lymph node metastasis
and higher clinical stage | (41) |
| ESCC | Downregulation | Associated with TNM
stage and pathological grade | (31) |
| HCC | Downregulation | Associated with
tumor lymph node metastasis and vascular invasion, and poor OS | (115) |
The prognostic value of miR-370 in
cancer
Various studies have also shown that miR-370 is
significantly associated with the prognosis of patients with cancer
(Table III). miR-370 is decreased
in ESCC, and miR-370 expression is negatively associated with ESCC
clinicopathological characteristics, including TNM stage and
pathologic grade (31). In gastric
carcinoma, miR-370 has been shown to be upregulated. Compared with
in the control tissues, the higher levels of miR-370 expression in
gastric carcinoma tissues were revealed to be correlated with more
advanced lymph node metastasis and higher clinical stage (41). It has also been reported that low
miR-370 expression is significantly correlated with tumor lymph
node metastasis and vascular invasion in patients with HCC.
Furthermore, a low level of miR-370 is associated with poor
prognosis in patients with HCC and miR-370 can serve as an
independent prognostic marker (115).
Conclusion
miR-370 is widely involved in the occurrence and
development of various types of cancer and is abnormally expressed
in cancer. Notably, miR-370 affects cancer progression by
regulating the Wnt/β-catenin, MAPK, NF-κB and PI3K/Akt signaling
pathways, alongside others. Additionally, miR-370 can participate
in various biological processes by regulating target genes,
including cell proliferation and apoptosis, cell migration and
invasion, cell cycle progression and cell stemness. miR-370 can
also modulate the response of cancer cells to anticancer therapies
by regulating their target genes. Furthermore, miR-370 may serve as
a specific diagnostic and prognostic marker for various types of
cancer. Through the regulation of miR-370, lncRNAs, circRNAs,
methyltransferases and other factors are involved in the
development and progression of cancer.
However, there are still a number of shortcomings
in the studies on miR-370. First of all, available studies have
revealed that miR-370 serves an inhibitory role in various types of
cancer; however, it can act as a tumor promoter in melanoma. In
addition, inconsistent expression levels of miR-370 have been
detected in AML, breast cancer, gastric cancer and prostate cancer.
The mechanisms underlying these differences need to be elucidated.
Secondly, miR-370 is under the regulation of numerous factors, and
the interaction of miR-370 with its targets constitutes its
regulatory network. The complexity of this regulatory network
provides miR-370 with diverse biological functions that depend
specifically on the cellular environment. Future studies need to
focus on the regulatory network of miR-370, which will contribute
to a deeper understanding of the mechanisms underlying the effects
of miR-370 on cancer. Furthermore, in spite of the fact that
miR-370 plays different roles in chemotherapy for different types
of cancer, it is still regarded as a promising treatment target for
cancer therapy. Future research may focus on understanding more
clearly the mechanisms of how miR-370 regulates treatment
resistance in cancer. Finally, numerous studies have confirmed that
miR-370 can efficiently modulate the cellular response to
chemotherapy, thus indicating that the use of miR-370-targeted
therapy in combination with chemotherapy may be promising in
certain types of cancer to improve therapeutic efficacy.
In summary, miR-370 serves a significant role in
the initiation and progression of multiple types of cancer. Hence,
miR-370 may be the focus of future research for the treatment of
cancer. The present review comprises an overview of the research
progress regarding miR-370 in cancer, which may provide more
insight into the molecular mechanisms of miR-370 and its functions
in cancer.
Acknowledgements
Not applicable.
Funding
This research was funded by the National Nature Science
Foundation of China (grant no. 82103032), the Medical Research
Grant of Jiangsu Commission of Health (grant no. M20200100, the
Health Science and Technology Development Fund of Nanjing (grant
no. YKK21224), the Science Foundation of Jiangsu Health Vocational
College (grant no. JKC201948), the Science and Technology
Development Fund of Nanjing Medical University (grant no.
NMUB2019235), and the Research and Development Fund of Kangda
College of Nanjing Medical University (grant nos. KD2020KYJJZD006
and KD2021KYJJZD026).
Availability of data and materials
Not applicable.
Authors' contributions
LY, JinqW and QZ conceived the study. LY and KY
wrote the original draft. JinyW and FW, reviewed and edited the
manuscript, and performed supervision. HW, HY and ZY reviewed and
edited the manuscript. Data authentication is not applicable. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen X, Mangala LS, Rodriguez-Aguayo C,
Kong X, Lopez-Berestein G and Sood AK: RNA interference-based
therapy and its delivery systems. Cancer Metastasis Rev. 1:107–124.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Seitz H: Issues in current microRNA target
identification methods. RNA Biol. 14:831–834. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shi Y, Yang F, Wei S and Xu G:
Identification of key genes affecting results of hyperthermia in
osteosarcoma based on integrative ChIP-Seq/TargetScan analysis. Med
Sci Monit. 23:2042–2048. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Loher P and Rigoutsos I: Interactive
exploration of RNA22 microRNA target predictions. Bioinformatics.
28:3322–3323. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen Y and Wang X: miRDB: An online
database for prediction of functional microRNA targets. Nucleic
Acids Res. 48:D127–D131. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rennie W, Liu C, Carmack CS, Wolenc A,
Kanoria S, Lu J, Long D and Ding Y: STarMir: A web server for
prediction of microRNA binding sites. Nucleic Acids Res.
42:W114–W118. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Akhtar MM, Micolucci L, Islam MS, Olivieri
F and Procopio AD: Bioinformatic tools for microRNA dissection.
Nucleic Acids Res. 44:24–44. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rincón-Riveros A, Morales D, Rodríguez JA,
Villegas VE and López-Kleine L: Bioinformatic tools for the
analysis and prediction of ncRNA interactions. Int J Mol Sci.
22:113972021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Reczko M, Maragkakis M, Alexiou P, Grosse
I and Hatzigeorgiou AG: Functional microRNA targets in protein
coding sequences. Bioinformatics. 6:771–776. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Qin X, Zhang J, Lin Y, Sun XM, Zhang JN
and Cheng ZQ: Identification of MiR-211-5p as a tumor suppressor by
targeting ACSL4 in Hepatocellular Carcinoma. J Transl Med.
18:3262020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dou XQ, Chen XJ, Zhou Q, Wen MX, Zhang SZ
and Zhang SQ: miR-335 modulates Numb alternative splicing via
targeting RBM10 in endometrial cancer. Kaohsiung J Med Sci.
36:171–177. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sang K, Yi T, Huang X, Pan C, Zhou J and
Yu L: MiR-370-5p inhibits the progression of breast cancer via
targeting LUC7L3. J Recept Signal Transduct Res. 41:442–450. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bridges MC, Daulagala AC and Kourtidis A:
LNCcation: lncRNA localization and function. J Cell Biol.
220:e2020090452021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Herman AB, Tsitsipatis D and Gorospe M:
Integrated lncRNA function upon genomic and epigenomic regulation.
Mol Cell. 82:2252–2266. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu P, Mo Y, Peng M, Tang T, Zhong Y, Deng
X, Xiong F, Guo C, Wu X, Li Y, et al: Emerging role of
tumor-related functional peptides encoded by lncRNA and circRNA.
Mol Cancer. 19:222020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tay Y, Rinn J and Pandolfi PP: The
multilayered complexity of ceRNA crosstalk and competition. Nature.
505:344–352. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li C, Ge Q, Liu J, Zhang Q, Wang C, Cui K
and Chen Z: Effects of miR-1236-3p and miR-370-5p on activation of
p21 in various tumors and its inhibition on the growth of lung
cancer cells. Tumour Biol. 39:10104283177108242017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zou Y, Zhong C, Hu Z and Duan S:
MiR-873-5p: A potential molecular marker for cancer diagnosis and
prognosis. Front Oncol. 11:7437012021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang C, Chen Z, Ge Q, Hu J, Li F, Hu J, Xu
H, Ye Z and Li LC: Up-regulation of p21(WAF1/CIP1) by miRNAs and
its implications in bladder cancer cells. FEBS Lett. 588:4654–4664.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li S, Wang C, Yu X, Wu H, Hu J, Wang S and
Ye Z: miR-3619-5p inhibits prostate cancer cell growth by
activating CDKN1A expression. Oncol Rep. 37:241–248. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu F and Zhou J: CircAGFG1 promotes
cervical cancer progression via miR-370-3p/RAF1 signaling. BMC
Cancer. 19:10672019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shen X, Zuo X, Zhang W, Bai Y, Qin X and
Hou N: MiR-370 promotes apoptosis in colon cancer by directly
targeting MDM4. Oncol Lett. 15:1673–1679. 2018.PubMed/NCBI
|
|
23
|
Xiong H, Yu J, Jia G, Su Y, Zhang J, Xu Q
and Sun X: Emerging roles of circUBAP2 targeting miR-370-3p in
proliferation, apoptosis, and invasion of papillary thyroid cancer
cells. Hum Cell. 34:1866–1877. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang DW, Su F, Zhang T, Yang TC, Wang HQ,
Yang LJ, Zhou FF and Feng MH: The miR-370/UQCRC2 axis facilitates
tumorigenesis by regulating epithelial-mesenchymal transition in
Gastric Cancer. J Cancer. 11:5042–5055. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang Y, Ding M, Yuan X, Jiao R, Zhu D,
Huang W, Deng W and Liu Y: lncRNA SNHG15 promotes ovarian cancer
progression through regulated CDK6 via sponging miR-370-3p. Biomed
Res Int. 2021:93945632021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mao J, Wang L, Wu J, Wang Y, Wen H, Zhu X,
Wang B and Yang H: miR-370-3p as a novel biomarker promotes breast
cancer progression by targeting FBLN5. Stem Cells Int.
2021:46498902021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Maftouh M, Avan A, Funel N, Frampton AE,
Fiuji H, Pelliccioni S, Castellano L, Galla V, Peters GJ and
Giovannetti E: miR-211 modulates gemcitabine activity through
downregulation of ribonucleotide reductase and inhibits the
invasive behavior of pancreatic cancer cells. Nucleosides
Nucleotides Nucleic Acids. 33:384–393. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chang KW, Chu TH, Gong NR, Chiang WF, Yang
CC, Liu CJ, Wu CH and Lin SC: miR-370 modulates insulin receptor
substrate-1 expression and inhibits the tumor phenotypes of oral
carcinoma. Oral Dis. 19:611–619. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yungang W, Xiaoyu L, Pang T, Wenming L and
Pan X: miR-370 targeted FoxM1 functions as a tumor suppressor in
laryngeal squamous cell carcinoma (LSCC). Biomed Pharmacother.
68:149–154. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen F, Feng Z, Zhu J, Liu P, Yang C,
Huang R and Deng Z: Emerging roles of circRNA_NEK6 targeting
miR-370-3p in the proliferation and invasion of thyroid cancer via
Wnt signaling pathway. Cancer Biol Ther. 19:1139–1152. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Han Y, Yang X, Zhao N, Peng J, Gao H and
Qiu X: Alpinumisoflavone induces apoptosis in esophageal squamous
cell carcinoma by modulating miR-370/PIM1 signaling. Am J Cancer
Res. 6:2755–2771. 2016.PubMed/NCBI
|
|
32
|
Sun G, Hou YB, Jia HY, Bi XH, Yu L and
Chen DJ: MiR-370 promotes cell death of liver cancer cells by
Akt/FoxO3a signalling pathway. Eur Rev Med Pharmacol Sci.
20:2011–2019. 2016.PubMed/NCBI
|
|
33
|
Liu Z, Ma M, Yan L, Chen S, Li S, Yang D,
Wang X, Xiao H, Deng H, Zhu H, et al: miR-370 regulates ISG15
expression and influences IFN-α sensitivity in hepatocellular
carcinoma cells. Cancer Biomark. 22:453–466. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen Q, Zhang J, He Y and Wang Y:
hsa_circ_0061140 knockdown reverses FOXM1-mediated cell growth and
metastasis in ovarian cancer through miR-370 sponge activity. Mol
Ther Nucleic Acids. 13:55–63. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen J, Liu G, Wu Y, Ma J, Wu H, Xie Z,
Chen S, Yang Y, Wang S, Shen P, et al: CircMYO10 promotes
osteosarcoma progression by regulating miR-370-3p/RUVBL1 axis to
enhance the transcriptional activity of β-catenin/LEF1 complex via
effects on chromatin remodeling. Mol Cancer. 18:1502019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wei S and Ma W: MiR-370 functions as
oncogene in melanoma by direct targeting pyruvate dehydrogenase B.
Biomed Pharmacother. 90:278–286. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
García-Ortí L, Cristóbal I, Cirauqui C,
Guruceaga E, Marcotegui N, Calasanz MJ, Castello-Cros R and Odero
MD: Integration of SNP and mRNA arrays with microRNA profiling
reveals that MiR-370 is upregulated and targets NF1 in acute
myeloid leukemia. PLoS One. 7:e477172012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ali MM, Mohamed RH, Sayed AA, Ahmed S,
Yassin DA and El-Sayed WM: miR-370 is better than miR-375 as a
non-invasive diagnostic biomarker for pediatric acute myeloid
leukemia patients. Cancer Biomark. 34:403–411. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jin Y, Zhang M, Duan R, Yang J, Yang Y,
Wang J, Jiang C, Yao B, Li L, Yuan H, et al: Long noncoding RNA
FGF14-AS2 inhibits breast cancer metastasis by regulating the
miR-370-3p/FGF14 axis. Cell Death Discov. 6:1032020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fan C, Liu S, Zhao Y, Han Y, Yang L, Tao
G, Li Q and Zhang L: Upregulation of miR-370 contributes to the
progression of gastric carcinoma via suppression of FOXO1. Biomed
Pharmacother. 67:521–526. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lo SS, Hung PS, Chen JH, Tu HF, Fang WL,
Chen CY, Chen WT, Gong NR and Wu CW: Overexpression of miR-370 and
downregulation of its novel target TGFβ-RII contribute to the
progression of gastric carcinoma. Oncogene. 31:226–237. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ning T, Zhang H, Wang X, Li S, Zhang L,
Deng T, Zhou L, Liu R, Wang X, Bai M, et al: miR-370 regulates cell
proliferation and migration by targeting EGFR in gastric cancer.
Oncol Rep. 38:384–392. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Pan X, Chen G and Hu W: lncRNA HLA complex
group 18 (HCG18) facilitated cell proliferation, invasion, and
migration of prostate cancer through modulating miR-370-3p/DDX3X
axis. Reprod Sci. 28:3406–3416. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wu Z, Sun H, Zeng W, He J and Mao X:
Upregulation of MircoRNA-370 induces proliferation in human
prostate cancer cells by downregulating the transcription factor
FOXO1. PLoS One. 7:e458252012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Xia S, Ji R and Zhan W: Long noncoding RNA
papillary thyroid carcinoma susceptibility candidate 3 (PTCSC3)
inhibits proliferation and invasion of glioma cells by suppressing
the Wnt/β-catenin signaling pathway. BMC Neurol. 17:302017.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Klaus A and Birchmeier W: Wnt signalling
and its impact on development and cancer. Nat Rev Cancer.
8:387–398. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Bauer A, Chauvet S, Huber O, Usseglio F,
Rothbächer U, Aragnol D, Kemler R and Pradel J: Pontin52 and
reptin52 function as antagonistic regulators of beta-catenin
signalling activity. EMBO J. 19:6121–6130. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhang W, Duan N, Zhang Q, Song T, Li Z,
Zhang C, Chen X and Wang K: DNA methylation mediated
down-regulation of miR-370 regulates cell growth through activation
of the Wnt/β-catenin signaling pathway in human osteosarcoma cells.
Int J Biol Sci. 13:561–573. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Xu X, Zhang M, Xu F and Jiang S: Wnt
signaling in breast cancer: Biological mechanisms, challenges and
opportunities. Mol Cancer. 19:1652020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Huang X, Zhu H, Gao Z, Li J, Zhuang J,
Dong Y, Shen B, Li M, Zhou H, Guo H, et al: Wnt7a activates
canonical Wnt signaling, promotes bladder cancer cell invasion, and
is suppressed by miR-370-3p. J Biol Chem. 293:6693–6706. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhang R, Wang J, Jia E, Zhang J, Liu N and
Chi C: lncRNA BCAR4 sponges miR-370-3p to promote bladder cancer
progression via Wnt signaling. Int J Mol Med. 45:578–588.
2020.PubMed/NCBI
|
|
52
|
Asati V, Mahapatra DK and Bharti SK:
PI3K/Akt/mTOR and Ras/Raf/MEK/ERK signaling pathways inhibitors as
anticancer agents: Structural and pharmacological perspectives. Eur
J Med Chem. 109:314–341. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Li G, Zheng P, Wang H, Ai Y and Mao X:
Long Non-coding RNA TUG1 modulates proliferation, migration, and
invasion of acute myeloid leukemia cells via regulating
miR-370-3p/MAPK1/ERK. Onco Targets Ther. 12:10375–10388. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Aoki M and Fujishita T: Oncogenic roles of
the PI3K/AKT/mTOR axis. Curr Top Microbiol Immunol. 7:153–189.
2017.PubMed/NCBI
|
|
55
|
Poma P: NF-κB and disease. Int J Mol Sci.
21:91812020. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Patel M, Horgan PG, McMillan DC and
Edwards J: NF-κB pathways in the development and progression of
colorectal cancer. Transl Res. 197:43–56. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Li R, Wu H, Jiang H, Wang Q, Dou Z, Ma H,
Yan S, Yuan C, Yang N and Kong B: FBLN5 is targeted by
microRNA-27a-3p and suppresses tumorigenesis and progression in
high-grade serous ovarian carcinoma. Oncol Rep. 44:2143–2151.
2020.PubMed/NCBI
|
|
58
|
Matthews HK, Bertoli C and de Bruin RAM:
Cell cycle control in cancer. Nat Rev Mol Cell Biol. 23:74–88.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Liu J, Peng Y and Wei W: Cell cycle on the
crossroad of tumorigenesis and cancer therapy. Trends Cell Biol.
32:30–44. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Jiang X, Yang L, Gao Q, Liu Y, Feng X, Ye
S and Yang Z: The role of RAB GTPases and its potential in
predicting immunotherapy response and prognosis in colorectal
cancer. Front Genet. 13:8283732022. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Guo M, Li S, Zhao X, Yuan Y, Zhang B and
Guan Y: Knockdown of circular RNA Hsa_circ_0000714 can regulate
RAB17 by sponging miR-370-3p to reduce paclitaxel resistance of
ovarian cancer through CDK6/RB pathway. Onco Targets Ther.
13:13211–13224. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Takayama Y, Kamimura Y, Okawa M, Muramatsu
S, Sugino A and Araki H: GINS, a novel multiprotein complex
required for chromosomal DNA replication in budding yeast. Genes
Dev. 17:1153–1165. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Yamane K, Naito H, Wakabayashi T, Yoshida
H, Muramatsu F, Iba T, Kidoya H and Takakura N: Regulation of SLD5
gene expression by miR-370 during acute growth of cancer cells. Sci
Rep. 6:309412016. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Wang Y, Lyu Z, Qin Y, Wang X, Sun L, Zhang
Y, Gong L, Wu S, Han S, Tang Y, et al: FOXO1 promotes tumor
progression by increased M2 macrophage infiltration in esophageal
squamous cell carcinoma. Theranostics. 10:11535–11548. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Tang Y, Jiang L, Zhao X, Hu D, Zhao G, Luo
S, Du X and Tang W: FOXO1 inhibits prostate cancer cell
proliferation via suppressing E2F1 activated NPRL2 expression. Cell
Biol Int. 45:2510–2520. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Yang H, Wen L, Wen M, Liu T, Zhao L, Wu B,
Yun Y, Liu W, Wang H, Wang Y and Wen N: FoxM1 promotes
epithelial-mesenchymal transition, invasion, and migration of
tongue squamous cell carcinoma cells through a c-Met/AKT-dependent
positive feedback loop. Anticancer Drugs. 29:216–226. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Zhang Y, Ye X, Chen L, Wu Q, Gao Y and Li
Y: PARI functions as a new transcriptional target of FOXM1 involved
in gastric cancer development. Int J Biol Sci. 14:531–541. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Narlik-Grassow M, Blanco-Aparicio C and
Carnero A: The PIM family of serine/threonine kinases in cancer.
Med Res Rev. 34:136–159. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Fang C, Wang X, Guo D, Fang R and Zhu T:
Circular RNA CircITGA7 promotes tumorigenesis of osteosarcoma via
miR-370/PIM1 axis. Comput Math Methods Med. 2020:13675762020.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Zou FW, Yang SZ, Li WY, Liu CY, Liu XH, Hu
CH, Liu ZH and Xu S: circRNA_001275 upregulates Wnt7a expression by
competitively sponging miR-370-3p to promote cisplatin resistance
in esophageal cancer. Int J Oncol. 57:151–160. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Reiss K, Del Valle L, Lassak A and
Trojanek J: Nuclear IRS-1 and cancer. J Cell Physiol. 27:2992–3000.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Li Q, Ye L, Zhang X, Wang M, Lin C, Huang
S, Guo W, Lai Y, Du H, Li J, et al: FZD8, a target of p53, promotes
bone metastasis in prostate cancer by activating canonical
Wnt/β-catenin signaling. Cancer Lett. 402:166–176. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Wang N, Dong Q and Zhou XN: LMO4 promotes
the invasion and proliferation of gastric cancer by activating
PI3K-Akt-mTOR signaling. Am J Transl Res. 11:6534–6543.
2019.PubMed/NCBI
|
|
74
|
Xiong X, Feng Y, Li L, Yao J, Zhou M, Zhao
P, Huang F, Zeng L and Yuan L: Long non-coding RNA SNHG1 promotes
breast cancer progression by regulation of LMO4. Oncol Rep.
43:1503–1515. 2020.PubMed/NCBI
|
|
75
|
Liu L, Yan C, Tao S and Wang H:
Circ_0058124 aggravates the progression of papillary thyroid
carcinoma by activating LMO4 expression via targeting miR-370-3p.
Cancer Manag Res. 12:9459–9470. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Wang B, Qi X, Liu J, Zhou R, Lin C,
Shangguan J, Zhang Z, Zhao L and Li G: MYH9 promotes growth and
metastasis via activation of MAPK/AKT signaling in colorectal
cancer. J Cancer. 10:874–884. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Chen F, Yin S, Feng Z, Liu C, Lv J, Chen
Y, Shen R, Wang J and Deng Z: Knockdown of circ_NEK6 decreased
131I resistance of differentiated thyroid carcinoma via
regulating miR-370-3p/MYH9 axis. Technol Cancer Res Treat.
20:153303382110049502021. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Peng J, Chen XL, Cheng HZ, Xu ZY, Wang H,
Shi ZZ, Liu J, Ning XG and Peng H: Silencing of KCNK15-AS1 inhibits
lung cancer cell proliferation via upregulation of miR-202 and
miR-370. Oncol Lett. 18:5968–5976. 2019.PubMed/NCBI
|
|
79
|
Luo Q, Lin H, Ye X, Huang J, Lu S and Xu
L: Trim44 facilitates the migration and invasion of human lung
cancer cells via the NF-κB signaling pathway. Int J Clin Oncol.
20:508–517. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Ma Q, Huai B, Liu Y, Jia Z and Zhao Q:
Circular RNA circ_0020123 promotes non-small cell lung cancer
progression through miR-384/TRIM44 axis. Cancer Manag Res.
13:75–87. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Wang X, Lv J, He B and Zhou D: CircFBXW8
acts an oncogenic role in the malignant progression of non-small
cell lung carcinoma by miR-370-3p-dependent regulation of TRIM44.
Biochem Genet. 60:1313–1332. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Ciardiello F and Tortora G: EGFR
antagonists in cancer treatment. N Engl J Med. 358:1160–1174. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Li C, Zhang J, Zhou Y and Li B: Long
non-coding RNA CASC9 promotes the progression and development of
gastric cancer via regulating miR-370/EGFR axis. Dig Liver Dis.
53:509–516. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Lin L, Wang D, Qu S, Zhao H and Lin Y:
miR-370-3p alleviates ulcerative Colitis-related colorectal cancer
in mice through inhibiting the inflammatory response and
epithelial-mesenchymal transition. Drug Des Devel Ther.
14:1127–1141. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Wu J, Lu G and Wang X: MDM4 alternative
splicing and implication in MDM4 targeted cancer therapies. Am J
Cancer Res. 11:5864–5880. 2021.PubMed/NCBI
|
|
86
|
Mo Y, Lu Q, Zhang Q, Chen J, Deng Y, Zhang
K, Tao R, Liu W and Wang Y: Circular RNA CCDC66 improves murine
double minute 4 (MDM4) expression through targeting miR-370 in
colorectal cancer. Comput Math Methods Med. 2022:77239952022.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Kusakabe Y, Chiba T, Oshima M, Koide S,
Rizq O, Aoyama K, Ao J, Kaneko T, Kanzaki H, Kanayama K, et al:
EZH1/2 inhibition augments the anti-tumor effects of sorafenib in
hepatocellular carcinoma. Sci Rep. 11:213962021. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Zhang Y, Li L, Lu XK, Yu LB, Meng J and
Liu CY: LncRNA SNHG3 is responsible for the deterioration of
colorectal carcinoma through regulating the miR-370-5p/EZH1 axis.
Eur Rev Med Pharmacol Sci. 25:6131–6137. 2021.PubMed/NCBI
|
|
89
|
Liu Y, Ao X, Ding W, Ponnusamy M, Wu W,
Hao X, Yu W, Wang Y, Li P and Wang J: Critical role of FoxO3a in
carcinogenesis. Mol Cancer. 17:1042018. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Li C, Wang J, Zhang H, Zhu M, Chen F, Hu
Y, Liu H and Zhu H: Interferon-stimulated gene 15 (ISG15) is a
trigger for tumorigenesis and metastasis of hepatocellular
carcinoma. Oncotarget. 5:8429–8441. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Riva V and Maga G: From the magic bullet
to the magic target: Exploiting the diverse roles of DDX3X in viral
infections and tumorigenesis. Future Med Chem. 11:1357–1381. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Xu Y, Zheng Y, Liu H and Li T: Modulation
of IGF2BP1 by long non-coding RNA HCG11 suppresses apoptosis of
hepatocellular carcinoma cells via MAPK signaling transduction. Int
J Oncol. 51:791–800. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Wagner EF and Nebreda AR: Signal
integration by JNK and p38 MAPK pathways in cancer development. Nat
Rev Cancer. 9:537–549. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Yin X, Liu Z, Zhu P, Wang Y, Ren Q, Chen H
and Xu J: CXCL12/CXCR4 promotes proliferation, migration, and
invasion of adamantinomatous craniopharyngiomas via PI3K/AKT signal
pathway. J Cell Biochem. 120:9724–9736. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Wei CY, Zhu MX, Lu NH, Liu JQ, Yang YW,
Zhang Y, Shi YD, Feng ZH, Li JX, Qi FZ and Gu JY: Circular RNA
circ_0020710 drives tumor progression and immune evasion by
regulating the miR-370-3p/CXCL12 axis in melanoma. Mol Cancer.
19:842020. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Shang Y, Zhang F, Li D, Li C, Li H, Jiang
Y and Zhang D: Overexpression of UQCRC2 is correlated with tumor
progression and poor prognosis in colorectal cancer. Pathol Res
Pract. 214:1613–1620. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Luo G, Li G, Wan Z, Zhang Y, Liu D and Guo
Y: circITGA7 Acts as a miR-370-3p sponge to suppress the
proliferation of prostate cancer. J Oncol. 2021:80603892021.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Saunier E, Benelli C and Bortoli S: The
pyruvate dehydrogenase complex in cancer: An old metabolic
gatekeeper regulated by new pathways and pharmacological agents.
Int J Cancer. 138:809–817. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Parkin B, Ouillette P, Wang Y, Liu Y,
Wright W, Roulston D, Purkayastha A, Dressel A, Karp J, Bockenstedt
P, et al: NF1 inactivation in adult acute myelogenous leukemia.
Clin Cancer Res. 16:4135–4147. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Xiao B, Chen D, Zhou Q, Hang J, Zhang W,
Kuang Z, Sun Z and Li L: Glutamate metabotropic receptor 4 (GRM4)
inhibits cell proliferation, migration and invasion in breast
cancer and is regulated by miR-328-3p and miR-370-3p. BMC Cancer.
19:8912019. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Bera K, Kiepas A, Godet I, Li Y, Mehta P,
Ifemembi B, Paul CD, Sen A, Serra SA, Stoletov K, et al:
Extracellular fluid viscosity enhances cell migration and cancer
dissemination. Nature. 611:365–373. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Su T, Huang L, Zhang N, Peng S, Li X, Wei
G, Zhai E, Zeng Z and Xu L: FGF14 Functions as a Tumor Suppressor
through Inhibiting PI3K/AKT/mTOR Pathway in Colorectal Cancer. J
Cancer. 11:819–825. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Komuro A, Yashiro M, Iwata C, Morishita Y,
Johansson E, Matsumoto Y, Watanabe A, Aburatani H, Miyoshi H,
Kiyono K, et al: Diffuse-type gastric carcinoma: Progression,
angiogenesis, and transforming growth factor beta signaling. J Natl
Cancer Inst. 101:592–604. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Nassar D and Blanpain C: Cancer stem
cells: Basic concepts and therapeutic implications. Annu Rev
Pathol. 11:47–76. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Walcher L, Kistenmacher AK, Suo H, Kitte
R, Dluczek S, Strauß A, Blaudszun AR, Yevsa T, Fricke S and
Kossatz-Boehlert U: Cancer stem cells-origins and biomarkers:
Perspectives for targeted personalized therapies. Front Immunol.
11:12802020. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Kaltschmidt C, Banz-Jansen C, Benhidjeb T,
Beshay M, Förster C, Greiner J, Hamelmann E, Jorch N, Mertzlufft F,
Pfitzenmaier J, et al: A role for NF-κB in organ specific cancer
and cancer stem cells. Cancers (Basel). 11:6552019. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Liu AM, Zhu Y, Huang ZW, Lei L, Fu SZ and
Chen Y: Long noncoding RNA FAM201A involves in radioresistance of
non-small-cell lung cancer by enhancing EGFR expression via
miR-370. Eur Rev Med Pharmacol Sci. 23:5802–5814. 2019.PubMed/NCBI
|
|
108
|
Wang K, Zhu G, Bao S and Chen S: Long
non-coding RNA LINC00511 mediates the effects of ESR1 on
proliferation and invasion of ovarian cancer through miR-424-5p and
miR-370-5p. Cancer Manag Res. 11:10807–10819. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Endo M, Tanaka Y, Otsuka M and Minami Y:
E2F1-Ror2 signaling mediates coordinated transcriptional regulation
to promote G1/S phase transition in bFGF-stimulated NIH/3T3
fibroblasts. FASEB J. 34:3413–3428. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Zeng B, Li Z, Chen R, Guo N, Zhou J, Zhou
Q, Lin Q, Cheng D, Liao Q, Zheng L and Gong Y: Epigenetic
regulation of miR-124 by hepatitis C virus core protein promotes
migration and invasion of intrahepatic cholangiocarcinoma cells by
targeting SMYD3. FEBS Lett. 586:3271–3278. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Pan X, Wang H, Tong D, Wang C, Sun L, Zhao
C, Li Y, Zhu L and Wu D: Physcion induces apoptosis in
hepatocellular carcinoma by modulating miR-370. Am J Cancer Res.
6:2919–2931. 2016.PubMed/NCBI
|
|
112
|
Kuete V, Mbaveng AT, Nono EC, Simo CC,
Zeino M, Nkengfack AE and Efferth T: Cytotoxicity of seven
naturally occurring phenolic compounds towards multi-factorial
drug-resistant cancer cells. Phytomedicine. 23:856–863. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Ye LH, Ma X and Xu SC: Expression and
clinical significance of serum MiR-370 and MiR-203 in patients with
acute myeloid leukemia. Zhongguo Shi Yan Xue Ye Xue Za Zhi.
29:445–449. 2021.(In Chinese). PubMed/NCBI
|
|
114
|
Bautista-Sánchez D, Arriaga-Canon C,
Pedroza-Torres A, De La Rosa-Velázquez IA, González-Barrios R,
Contreras-Espinosa L, Montiel-Manríquez R, Castro-Hernández C,
Fragoso-Ontiveros V, Álvarez-Gómez RM and Herrera LA: The Promising
role of miR-21 as a cancer biomarker and its importance in
RNA-based therapeutics. Mol Ther Nucleic Acids. 20:409–420. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Pan XP, Huang LH and Wang X: MiR-370
functions as prognostic marker in patients with hepatocellular
carcinoma. Eur Rev Med Pharmacol Sci. 21:3581–3585. 2017.PubMed/NCBI
|