Introduction
Pancreatic cancer (PC) still has a poor prognosis.
Even with the latest multimodality treatment, the 5-year relative
survival rate is only 11%, which is substantially worse than for
other gastrointestinal cancers (1).
This is partly because PC tends to metastasize to lymph nodes or
distant sites at an early stage due to its high capacity for
invasion and migration. Therefore, to improve the prognosis, it is
important to examine the cause of PC's high malignant
potential.
Previous studies demonstrated that the tumor stromal
components, which are abundant in PC, are involved in the malignant
potential (2,3). In particular, fibroblasts are the main
component of the stromal tissue-referred to as ‘cancer-associated
fibroblasts (CAFs)’-and are reportedly involved in tumor
proliferation, invasion, and drug resistance through interactions
with cancer cells via chemokines, cytokines and growth factors
(4–6). Furthermore, previous studies reported
that CAFs can be classified into subpopulations based on various
functional characteristics, and their heterogeneity may be related
to cancer malignancy (7,8). The heterogeneity of CAFs in PC was
also reported to be closely related to its malignant potential
(9,10), however, it remains unclear how CAFs
affect PC malignancy.
In general, malignancy risk increases with aging
(11,12), and numerous studies have focused on
the relationship between cellular senescence and malignant tumors
(12,13). Cellular senescence is the
irreversible growth arrest of normal cells with proliferative
potential-caused by telomere shortening, oncogene activation, and
unrepairable DNA damage with carcinogenic risk, which can be
induced by oxidative stress (14,15).
This phenomenon was originally considered a mechanism to protect
bodies from the carcinogenic stress that accumulates with aging
(16,17). Furthermore, previous studies
indicated that senescent cells could foster a tumor-promoting
microenvironment by increasing the expressions of
inflammation-related genes, such as inflammatory cytokines and
chemokines, and, i.e., the senescence-associated secretory
phenotype (SASP) (18–20).
Although several studies described the role of SASP
in various type of cancers such as liver, colorectal and prostate
cancer (21–23), it is not well understood how SASP
derived from CAFs may affect the malignancy of PC. In the present
study, the differences in malignant potential of CAFs derived from
several primary PC tumors were investigated and it was clarified
how the senescence of CAFs affected PC cells by examining the
significance of SASP in CAFs.
Materials and methods
Primary culture of CAFs
Tissue samples of human PC were collected with the
approval (approval no. 18138-4) of the Human Ethics Review
Committee of the Graduate School of Medicine of Osaka University
(Osaka, Japan) and the National Institutes of Biomedical
Innovation, Health and Nutrition (approval no. 201-01; Osaka,
Japan). Written informed consent for sample use was obtained from
all patients before surgery. All tissue samples were collected at
Osaka University Hospital (Suita, Japan) from June 2019 to March
2020. Human pancreatic stellate cells, the reported origin cells of
CAFs, were cultured from the tissue samples as previously described
(24,25). Briefly, the cancerous portion was
cut from the tissue samples, and chopped into small pieces. These
tissue pieces were washed with phosphate-buffered saline (PBS)
containing 5% penicillin-streptomycin (Sigma-Aldrich; Merck KGaA),
and then three pieces were sown in each well of a six-well plate
(Corning, Inc.). The samples were cultured in Dulbecco's modified
Eagle's medium (DMEM) containing 20% fetal bovine serum (FBS) for 2
days at 37°C, and thereafter in DMEM containing 10% FBS. Gradual
proliferation of only spindle-shaped cells was observed as
previously reported (Fig. S1),
which was considered to be CAFs based on their cell morphology.
This outgrowth method, which was previously reported by Bachem
et al (24), was already
well-established method for the CAF primary culture. It was
indicated in the aforementioned study that α-smooth muscle actin
(α-SMA), vimentin (VIM), and desmin were expressed in all primary
cultured cells and immunocytochemistry of primary cultured cells
was also performed in previous studies and it has been already
confirmed that primary culture cells were true CAFs and there was
no contamination of cancer cells (24,25).
On the 14th day of culture, these CAFs were cryopreserved for use
in further experiments. In the present study, primary CAFs were
used without immortalization. This is because, to immortalize
primary cultured cells, viral vector or gene induction such as
telomerase reverse transcriptase (TERT) had to be used, but it is
considered that such immortalized cells were very different from
originally primary cultured cells. Therefore, primary cultured
cells were established with great care and cell damage was
minimized by using a programmed freezer when storing cells and
accutase when passaging CAFs. In addition, all assays were
performed using primary culture CAFs within three passages. This is
due to the fact that culture time and passages could cause changes
in cell morphology and gene expression in CAFs.
Primary culture of CAFs was performed using tissue
samples collected from 8 PC patients. The clinical information for
these 8 patients before the initiation of treatment are presented
in Table SI. The median age was 67
years (range 53–77 years), 3 were male, and the median tumor
diameter was 25.1 mm (range 9.5-34.3 mm). Of the 8 patients, 6
underwent preoperative chemotherapy (details presented in Table SII). The mean age of the male and
female groups was 65.6 and 65.7 years, respectively, with no
significant difference (P=0.993). The tumor diameters measured by
contrast-enhanced CT before and after chemotherapy, and the
calculated tumor size ratio (TSR) for each case: TSR=(Tumor size
after neoadjuvant chemotherapy)/(Tumor size before neoadjuvant
chemotherapy) are also included in Table SII. The macroscopic and microscopic
pathology findings for each case are demonstrated in Fig. S2. The following procedure was used
for H&E staining: First, tissues were fixed with neutral
formalin 10% at room temperature for 2 days, embedded in paraffin,
and manually sectioned with a microtome to obtain 3-µm thick
paraffin sections. The sections were dewaxed with xylene and
stained with hematoxylin for 6 min and eosin for 2 min and 30 sec
at room temperature. Tissue-Tek®Eosin and
Tissue-Tek® Mayer hematoxylin for Prisma (both from
Sakura Finetek USA, Inc.) were used as staining solutions, and
staining was performed on a Tissue-Tek®DRS TM 2000
(Sakura Finetek USA, Inc.).
Co-culture assay
The co-culture assay was performed in a non-contact
manner, using a Transwell with 0.4-µm pores (Corning, Inc.). First,
CAFs (1.0×104 per well) were plated on the top layer and
incubated overnight in DMEM containing 10% FBS. Once the CAFs had
settled in the Transwell, PSN-1 cells (a human PC cell line, p53
mutant) were plated at 1.0×105 per well on the lower
layer of a 24-well culture plate (Corning, Inc.). PSN-1 cells were
obtained from the American Type Culture Collection. After an
additional 48 h of co-culture in DMEM containing 0.5% FBS, the
Transwell was removed and PSN-1 cell proliferation was
evaluated.
Proliferation assay
PSN-1 cell proliferation was evaluated by
fluorescence staining using Hoechst 33342 (Thermo Fisher
Scientific, Inc.). Briefly, after removing the Transwell and the
culture medium, diluted Hoechst 33342 was added and allowed to
react for 10 min. Then the PSN-1 cells were washed three times with
PBS, and the fluorescence intensity was measured using the EnSpire
Multimode Plate Reader (PerkinElmer, Inc.). An assay was performed
to compare the effects of CAFs from 8 different patients on the
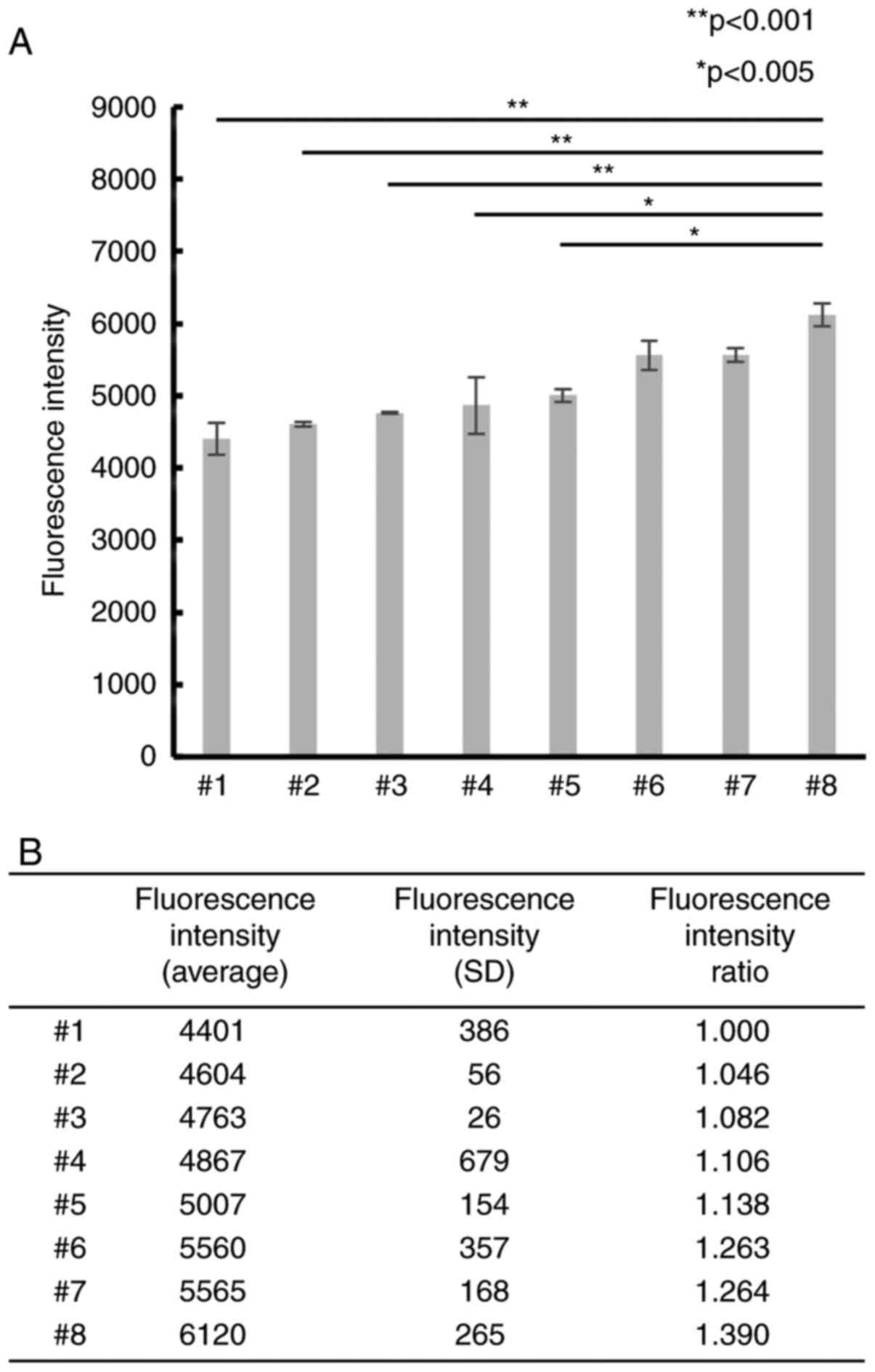
proliferation of PSN-1 cells. The fluorescence intensity ratio
(FIR) was calculated based on the fluorescence intensity of the
canonical CAFs (#1) showing the lowest fluorescence intensity: FIR
(#X)=Fluorescence intensity (#X)/Fluorescence intensity (#1).
Reverse transcription-quantitative
(RT-q) PCR array
RT-qPCR arrays were performed using two kits: QIAGEN
RT2 Profiler™ PCR Array ‘Human Cellular Senescence’
(GeneGlobe ID-PAHS-050Z) and ‘Human Cancer Inflammation and
Immunity Crosstalk’ (GeneGlobe ID-PAHS-181Z) following the
supplier's instructions (http://www.sabiosciences.com). After CAFs were settled
on the plate, they were incubated at 37°C for 1 day under
serum-free conditions, and then incubated at 37°C for 12 h with or
without serum stimulation (2% human AB serum), respectively, before
mRNA extraction was performed. RNA was extracted from CAFs of
1.0×104 cells under both serum unstimulated and serum
stimulated conditions using the RNeasy Micro kit (Qiagen GmbH),
following the supplier's protocol. At the same time, gene
expression of actin alpha 2 (ACTA2: alias α-SMA) and fibroblast
activation protein (FAP) were also examined for CAFs. The following
primers were used for gene expression analysis of human ACTA2 and
FAP: ACTA2 forward, 5′-GTGTTGCCCCTGAAGAGCAT-3′ and reverse,
5′-GCTGGGACATTGAAAGTCTCA-3′; FAP forward,
5′-TCTAAGGAAAGAAAGGTGCCAA-3′ and reverse,
5′-GATCAGTGCGTCCATCATGAAG-3′. Expression level was calculated using
the 2−ΔΔCq method based on the expression level of
GAPDH, the housekeeping gene. Additionally, the results were
analyzed by the 2−ΔΔCq method, using the sample without
serum stimulation as a reference (26). Gene expression levels were evaluated
as the ratio of expression with serum stimulation to expression
without serum stimulation: RNA level (fold change)=(Expression
level with serum stimulation)/(Expression level without serum
stimulation).
Pathway enrichment analysis
Pathway enrichment analysis was performed using two
databases: Kyoto Encyclopedia of Genes and Genomes (KEGG:
http://www.genome.ad.jp/kegg) and
Reactome (https://reactome.org/). Enriched
pathways were identified according to the cut-off value of a false
discovery rate (FDR) <0.05.
Inhibition of p53 expression in
CAFs
First, CAFs were plated and incubated overnight in
DMEM containing 10% FBS. Once the CAFs had settled in the plate,
the medium was changed and CAFs were cultured in DMEM containing
10% FBS for 5 days with the p53 inhibitor pifithrin-alpha
(PFT-alpha) (Sigma-Aldrich; Merck KGaA), or with the same amount of
DMSO as a control. A PFT-alpha concentration of 10 µM was used
based on previous a previous study (27). After 5 days of incubation, the cells
were thoroughly washed and seeded in the top layer of Transwell,
and then co-cultured with PSN-1 cells for 2 days, as in the
aforementioned co-culture assay, to assess proliferation of the
PSN-1 cells. Notably, viability of CAFs at the start of co-culture
was measured using Guava® Muse cell analyzer (Luminex)
as described in the instructions to confirm that no problems with
CAF viability had occurred after 5 days of incubation. This assay
was conducted using CAFs from three patients, #2, #7 and #8. The
experiments of #2, #7, and #8 were performed simultaneously on the
same plate. Data for a control, a sample without CAF is shared.
IL-6 quantification by ELISA
The IL-6 concentration was analyzed in the
co-culture supernatant using an enzyme-linked immunosorbent assay
(ELISA) kit (cat. no 3460-1A-6; Mabtech, Inc.), following the
manufacturer's instructions. Co-culture supernatants were collected
at the end of the 2-day co-culture in the aforementioned
experiment. When performing the analysis, the co-culture
supernatant was diluted 10-fold. This assay was also conducted
using CAFs from three patients, #2, #7 and #8.
Statistical analysis
Numerical data are presented as mean ± standard
deviation. Statistical analysis between two groups was performed
using a two-tailed Student's t-test (unpaired t-test). Statistical
analysis among three groups or more was performed using Dunnett's
t-test, with the test level α=0.05. Correlation analysis was
performed using Pearson's correlation coefficient. P<0.05 was
considered to indicate a statistically significant difference. JMP
software (JMP® Pro 16.2.0, SAS Institute, Inc.) was used
as the software for these analyses.
Results
Differences in CAFs induce differences
in PC cell proliferation
To examine whether differences in CAFs affected PC
cell proliferation, 8 CAF samples were primarily cultured and
co-cultured with PSN-1 cells. The PSN-1 showed different
proliferation ability when cultured with each CAFs from different
patients (Fig. 1). In particular,
the mean fluorescence intensity of #8 was significantly higher than
that of #1. The fluorescence intensity rate (FIR) of #8 was ~1.4
(P<0.001), indicating that differences in CAFs could affect the
proliferation ability of PC cells.
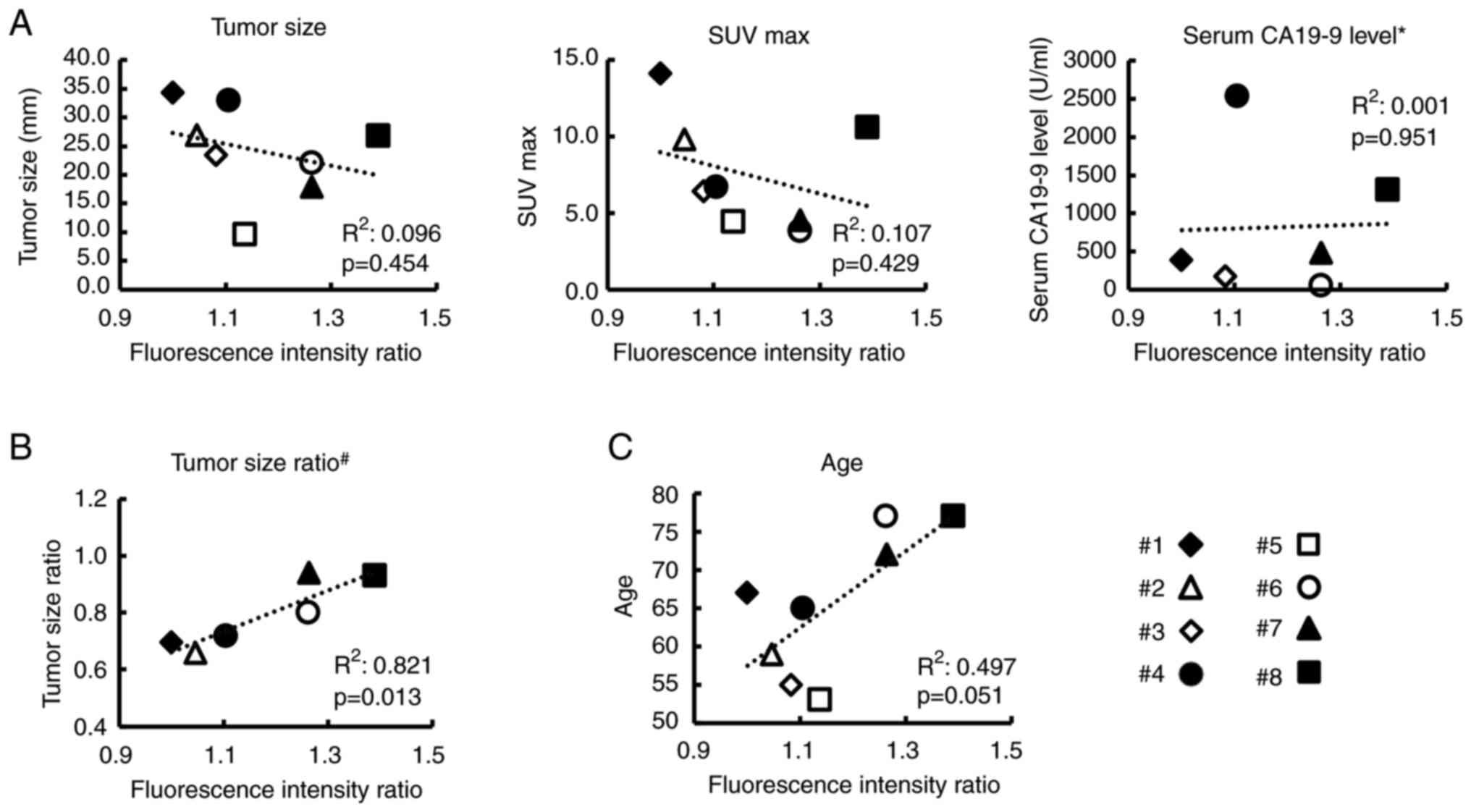
The clinical impact of CAF's malignant
potential
It was examined how the results of co-culture assays
were associated with the original clinical data of patients. The
correlation between FIR in co-culture assays and five clinical data
is demonstrated in Fig. 2.
Unfortunately, tumor size, serum CA19-9 level, and SUV max before
the initiation of treatment were not significantly associated with
the FIR (Fig. 2A) and this may be
partially because sample size (only 8 patients) was too small and
these factors depended on the time of diagnosis. There was also no
significant association between sex and co-culture assays results.
Fluorescence intensity (mean ± SD) in the co-culture experiment was
5,139.4±682.6 for females and 5,063.4±536.8 for males
(P=0.779).
Thus, next we examined the correlation between TSR
and FIR in six patients who received preoperative treatment to
clarify the clinical impact of CAFs on the effect of preoperative
treatment. As revealed in Fig. 2B,
the FIR was significantly correlated with the TSR
(R2=0.821, P=0.013), indicating that the efficacy of
chemotherapy may be determined by the malignant potential of CAFs.
Notably, further examination of the relationship between FIR and
the patients' original clinical data revealed that patient's age
was marginally related to the FIR (P=0.051), as shown in Fig. 2C, raising the suspicion that it was
possible that the senescence of CAFs may be related to its
malignant potential to grow cancer cells.
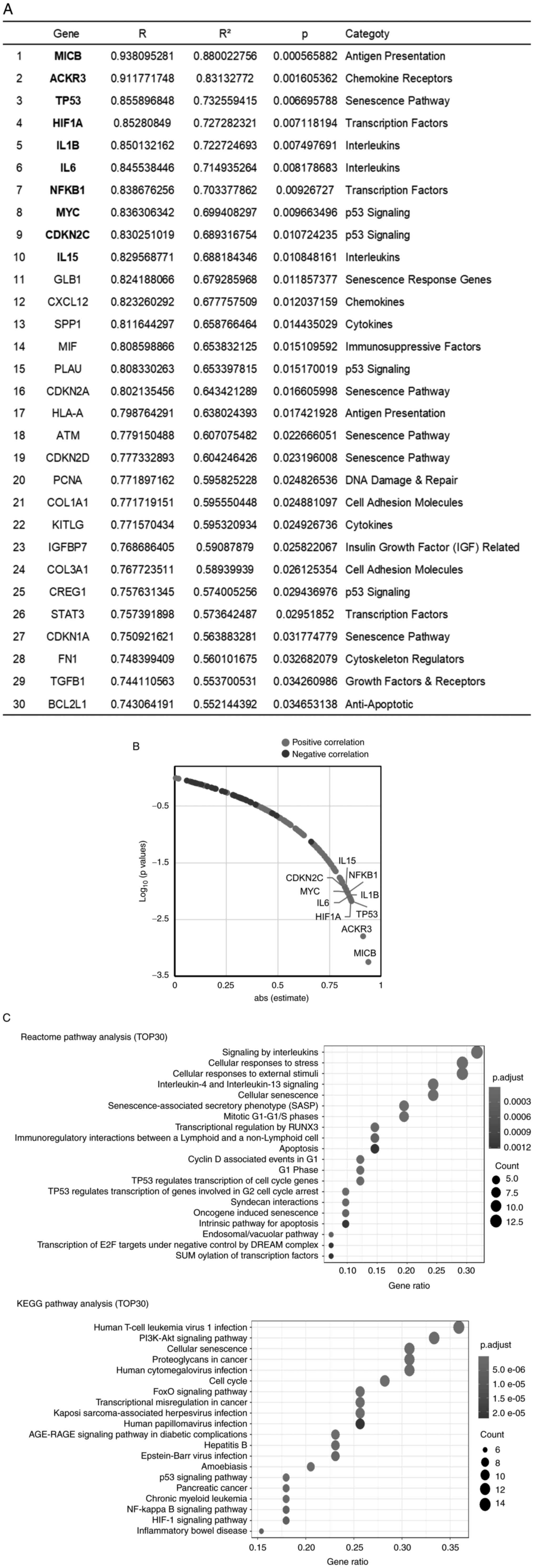
Gene expression profiling related to
cellular senescence and SASP in CAFs
To elucidate the senescence of CAFs really affected
the malignant potential of CAFs, a PCR array was performed among
the 8 CAFs by using the ‘Human Cellular Senescence’ PCR array kit
(GeneGlobe ID-PAHS-050Z QUIAGEN) and ‘Human Cancer Inflammation and
Immunity Crosstalk’ PCR array kit (GeneGlobe ID-PAHS-181Z QUIAGEN).
First, the expression of the CAF markers, ACTA-2 and FAP, was
examined simultaneously with the analysis using these kits.
Fortunately, the genes analyzed in these kits included genes known
to be marker of CAF, VIM. Therefore, the expression of these genes
in 8 primary CAFs was investigated and it was found that the
expression of these 3 genes was positive in all CAFs (Fig. S3). By contrast, the expression of
TERT, which is expressed in 85–90% of cancer tissues and is also
known to be expressed in PC (28,29),
was extremely low, suggesting that the cells in primary culture in
the present study were CAFs without cancer cells. The gene
expression of CAFs in relation to cancer cell growth was
subsequently examined by investigating the relationship between the
data of each gene expression analyzed by RT-qPCR and the results of
the co-culture assay. Unfortunately, each gene expression with and
without serum stimulation did not significantly correlate with the
results of the co-culture assay. Then, the fold-change of RNA level
of each gene was calculated using the 2−ΔΔCq method and
the relationship between fold-change of RNA level and co-culture
assay data was analyzed (Table
SIII and Fig. 3). The top 30
genes that exhibited a significant correlation between the
fold-change of RNA level and FIR are presented in Fig. 3A. Table
SIII shows the results for all 162 genes. The plot of absolute
value of the correlation coefficient versus the P-value for each
gene showed that 38 genes were significantly correlated with the
FIR (Fig. 3B). These 38 genes
included cellular senescence pathway-associated genes [such as
tumor protein p53 (TP53), cyclin-dependent kinase inhibitor 1A
(CDKN1A)/p21 and cyclin-dependent kinase inhibitor 2A (CDKN2A)/p16]
and cytokines and chemokines known as SASP factors (such as IL1B,
IL6, and CXCL12). Next, to clarify which pathway was related to the
malignant potential of CAFs, pathway analysis for the top 30 genes
was performed by using the KEGG and Reactome databases. The
cellular senescence pathway was significantly enriched in both the
KEGG and Reactome databases (Fig.
3C). Furthermore, Reactome pathway enrichment analysis revealed
that the ‘SASP’ pathway showed the 6th strongest relationship with
malignancy-related genes in CAFs.
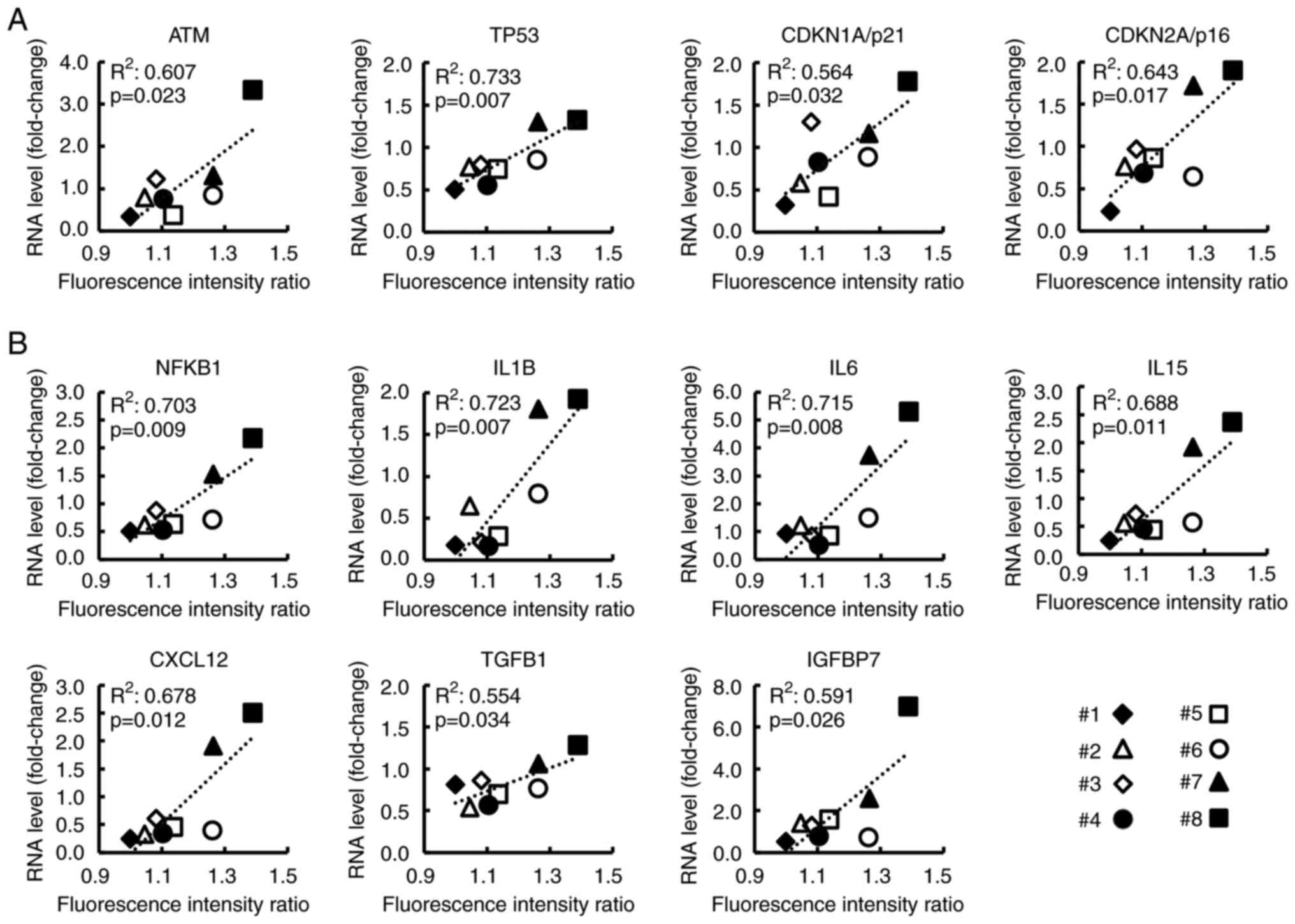
Expression of cellular senescence- and
SASP-related genes in CAFs correlates with clinical data related to
PC malignant potential
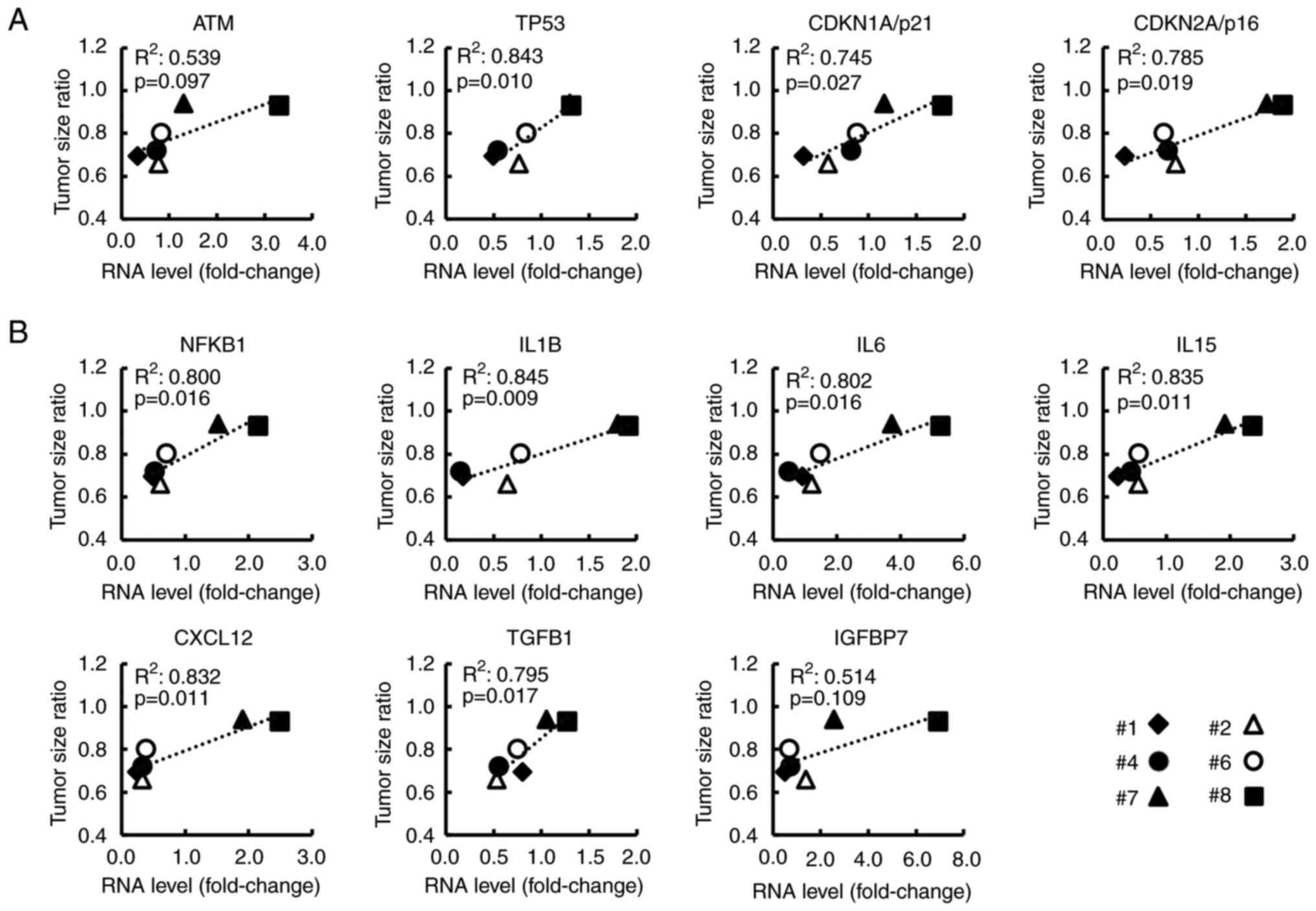
As revealed in Fig.
4, for the 8 CAF samples, the relationship between FIR and the
RNA levels of genes (selected from the top 30 genes) was analyzed.
Ataxia telangiectasia mutated (ATM), TP53, CDKN1A/p21, and
CDKN2A/p16 were related to the p53/p21 pathway or p16/RB pathway,
both of which are known as cellular senescence pathways (30–32)
(Fig. 4A). SASP-related genes, such
as nuclear factor kappa B subunit 1 (NFKB1), IL6, and CXCL12 are
revealed in Fig. 4B. NFKB1, which
is reportedly essential for SASP induction (33,34),
showed a strong correlation with FIR (R2=0.703,
P=0.009). Additionally, the expression of these genes without and
with serum stimulation, respectively, is shown in Fig. S4. Notably, most of these genes were
significantly related to the TSR (Fig.
5A and B) and to the serum CA19-9 level at the time of surgery
which is related to postoperative prognosis (Fig. S5). These findings indicated that
these genes may be associated with the malignant potential of
CAFs.
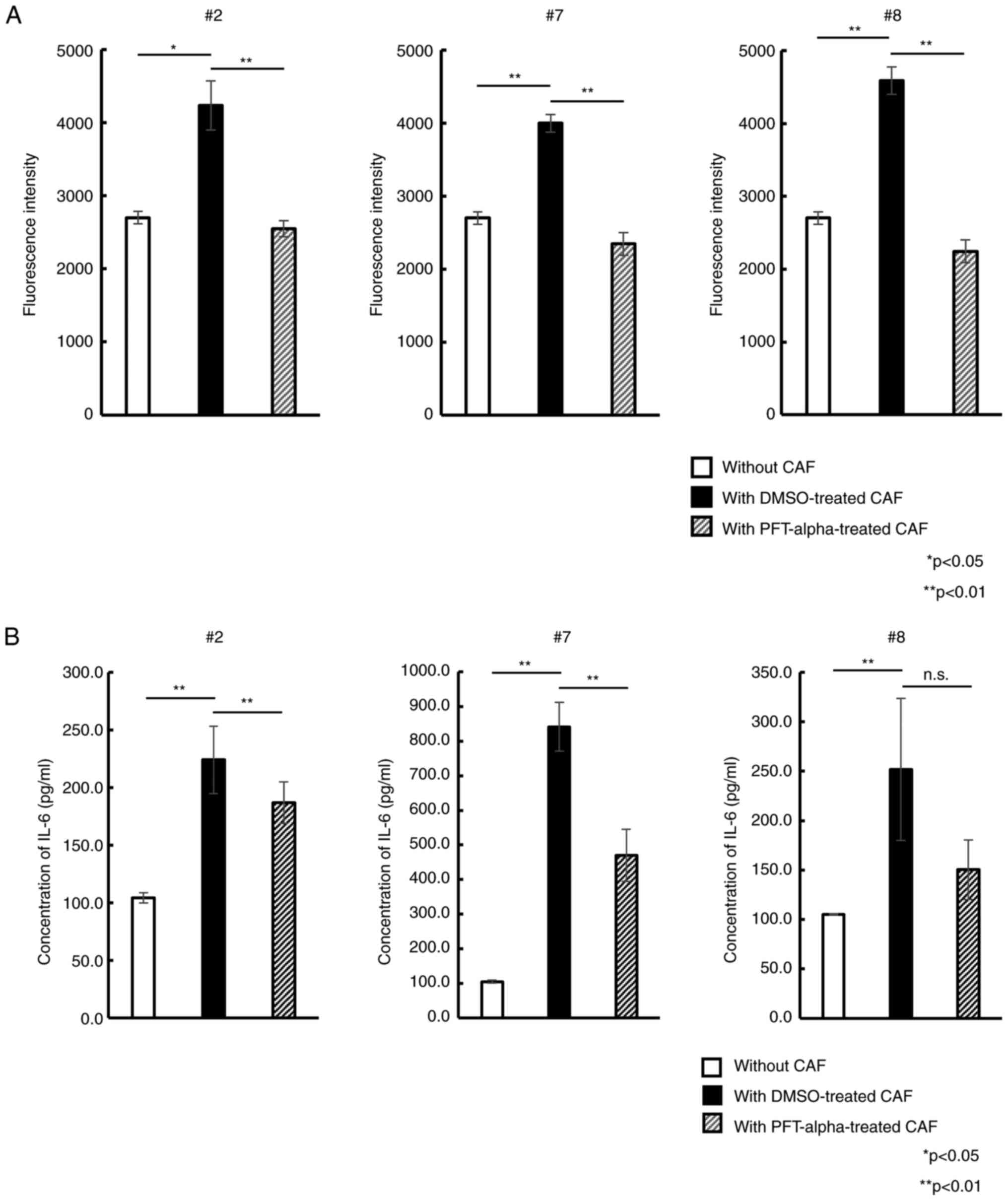
Suppression of p53 expression in CAFs
inhibits PC cell proliferation in co-culture assays
Based on the present results, focus was next
addressed on the p53-mediated cellular senescence of CAFs. It was
examined how CAFs treatment with the p53 inhibitor PFT-alpha
altered the PC cell proliferation in co-culture assays. After 5
days of exposure to PFT-alpha, each CAF samples retained its
spindle-like shape (Fig. S6) and
exhibited no decrease of viability (all >90%). Upon co-culture
of PSN-1 cells with DMSO-treated CAFs, a significant increase of
fluorescence intensity compared with PSN-1 cells cultured alone was
observed. This indicated that DMSO-treated CAFs also enhanced PSN-1
proliferation similar to untreated native CAFs. By contrast, PSN-1
co-cultured with PFT-alpha-treated CAFs showed significantly
reduced fluorescence intensity compared with PSN-1 with
DMSO-treated CAFs (Fig. 6A). This
trend was observed in all 3 different CAFs. To further examine why
the p53 inhibitor affected proliferative potential, the IL-6
concentration in the co-culture supernatant was measured. As
revealed in Fig. 6B, IL-6 level
significantly increased in PSN-1 cells with DMSO-treated CAFs
compared with PSN-1 alone. By contrast, IL-6 level in PSN-1
co-cultured with PFT-alpha-treated CAFs was significantly reduced
almost to the level of PSN-1 alone, indicating that PFT-alpha could
suppress IL-6 secretion from CAFs by inhibiting p53-mediated
cellular senescence, resulting in inhibition of PSN-1
proliferation.
Discussion
In recent years, a deeper understanding of the
interactions between stromal and cancer cells in the senescent
microenvironment has been achieved. Yang et al (35) reported elevated activity of
β-galactosidase in stromal fibroblasts, which resulted in senescent
fibroblasts with high p53 expression and promoting tumor
proliferation and migration potential in colorectal cancer. Yang
et al (36) indicated the
RAS-activated Gro-1 strongly induces senescence of stromal
fibroblasts and those senescent fibroblasts enhance the cancer cell
proliferation in ovarian cancer. In addition to the senescence of
fibroblasts, SASP such as IL-6 and TNF-α induced by senescent CAFs
was also reported to promote cancer cell proliferation and invasion
potential in prostate and breast cancer (37,38).
Nevertheless, the significance of cellular senescence of stromal
cells in tumor microenvironment remains unclear due to the apparent
complexity of the relationship between senescent stroma and cancer
cells. Notably, these interactions seem to differ depending on the
type of cancer, malignant potential of cancer cell itself and
disease stage. Thus, well designed research is considered to be
necessary to elucidate this topic.
In the present study, primary CAFs were examined and
it was found that differences in the expression of cellular
senescence and SASP-related genes in CAFs affected the PC cell
proliferation. The advantage of the present study was that not
primary PC cells but established cancer cell lines were used to
examine the malignant potential of CAFs. Although the gene
expression of CAFs may be influenced by cancer cells, the present
study was able to assess the true malignancy of each CAF by
conducting experiments using primary cultures of CAFs without
cancer cells and unified cancer cell lines. In the current results,
the same cancer cell grew differently by co-culture with different
primary CAFs, indicating directly that different primary CAFs had
different malignant potential. In addition, the malignant potential
of each CAFs was significantly related to original age of the
patient, thus it was considered that the malignant potential could
be relevant to the senescence of CAFs and SASP. Actually, Toste
et al (39) reported that
gemcitabine-treated CAFs showed upregulated SASP cytokines, which
exacerbated PC proliferation, metastasis and resistance to
treatment. The present study also indicated that increased
expression of cellular senescence and SASP-related genes in CAFs
was correlated with tumor cell proliferation and the tumor size
ratio (i.e., chemotherapy resistance). If the effects of CAF
reported in the present study are similarly observed in experiments
using other PC cell lines, it would be more certain that cellular
senescence and SASP in CAFs increase the malignant potential of PC.
It was actually examined whether the same effect could be obtained
by co-culturing CAFs with other PC cell lines such as MiaPaCa-2,
Panc1, TYPK1 and SUIT2. However, in the limited opportunity to use
primary CAFs, it was not possible to obtain the similar results as
satisfactorily as the results with PSN-1. This is possibly due to
the fact that different cell types have somewhat different rates of
proliferation and cell sizes; under the experimental conditions of
the present study, significant differences in the effects of each
CAF as observed with PSN-1 were not observed with the other cell
lines.
Notably, what was important for the cancer cell
proliferation was not the expression levels of senescence and
SASP-related genes in the presence or absence of serum stimulation,
but the increase of the expression ratio of the genes with versus
without serum stimulation. Serum stimulation is known to promote
cell division and cause telomere shortening and replicative
senescence (40,41). Although two types of fibroblast
senescence, replicative senescence and stress-induced premature
senescence, are known, they are considered to be different
phenomena, as reported by Dierick et al (42). Therefore, in the present study,
senescence was not induced by stress factors such as irradiation or
H2O2, but experiments were conducted by
inducing senescence via cell culture in serum-containing medium.
Therefore, the senescence changes were not stress-related but
development-related under serum stimulation. In fact, based on
RT-qPCR analysis, the gene expression of p53/p21-related and
p16/Rb-related cellular senescence pathways including ATM, p53,
p21, and p16/Rb were altered by serum stimulation, suggesting that
cellular senescence is induced by serum stimulation. Therefore, it
may be suggested that CAFs with higher rates of increased
expression of these genes due to replicative senescence more
aggressively promote SASP, which in turn promotes cancer
proliferation. Meanwhile, 6 of the 8 patients in the present study
had received preoperative chemotherapy containing gemcitabine. As
aforementioned, since gemcitabine is reported to induce cellular
senescence in CAFs (38), it was
desirable to study only the sample without preoperative treatment
to exclude this effect. However, neoadjuvant chemotherapy has been
increasingly performed in PC, even when resectable PC, and samples
without preoperative treatment were not readily available. The use
of EUS-FNA samples collected prior to neoadjuvant therapy was also
considered, but the volume of samples was markedly smaller than
that from resected specimens, and it was considered difficult to
culture sufficient quantities of fibroblasts for the experiment. If
more samples without neoadjuvant chemotherapy are collected, the
analysis would reflect in vivo phenomena in an improved
way.
Based on our co-culture assays and PCR array
results, a validation experiment focusing on TP53 was further
conducted, among 11 senescence- and SASP-related genes that are
considered to be related to cancer cell proliferation. Focus was
addressed on TP53 because p53, the nuclear protein encoded by TP53
and one of the best-known tumor suppressor genes, plays a vital
role in the induction of both cellular senescence and SASP.
Additionally, the specific inhibitor of p53, PFT-alpha, reportedly
alters post-translational modification patterns and differentially
inhibits p53 target genes (43),
such that it was relatively easy to optimize the experimental
conditions (27). The results of
the present study indicated that treatment of CAFs with 10 µM PFT-α
attenuated the ability of CAFs to promote PC cell proliferation and
this may be related to cellular senescence via the p53/p21 pathway
in CAFs. Notably, in the present experiment, the supernatant medium
containing PFT-alpha was completely washed out before initiating
co-culturing; therefore, there was no direct effect of PFT-alpha on
the cancer cells. Moreover, it was verified that PFT-alpha up to at
least 10 µM did not affect the proliferation ability of cancer
cells. Nevertheless, it remains unclear whether inhibition of the
p53/p21 pathway by PFT-alpha directly affects secretion of the SASP
factor. Further investigations are needed to address this point.
Additionally, it is also understandable that numerous other SASP
besides IL-6 secreted from senescent CAFs were related to cell
proliferation potential of cancer cells and it has to be examined
which cytokine was dominant in future research. Meanwhile, it is
understandable that cellular senescence was induced not only by p53
but by other significant molecules, such as p16, thus experiments
were also conducted using RRD-251, an inhibitor of the RB gene in
the senescence pathway, although this result was not included
because the data are very preliminary. Similar to the experiment
with PFT-α, the proliferation potential of cancer cells was reduced
by co-culture with RRD-251 treated CAFs, in which the senescence of
CAFs was recovered by the inhibition of RB. Unfortunately, due to a
lack of sample stock, these experiments were performed in only two
other CAFs, but it is considered by the authors that it does
provide some support for the importance of the p53/p21 cellular
senescence pathway in CAFs.
The present study revealed that cellular senescence
of CAFs and expression of SASP-related genes are involved in the
malignant potential of PC by analyzing CAFs alone, but other
parameters in addition to the age of patients may also influence
the malignant potential of CAFs, such as the characteristics of the
original cancer, and this requires further investigation. It is
also very important to examine whether this cellular senescence of
CAF is a change unique to CAFs or it also occurs in normal
fibroblasts. The authors are also interested in the differences
between normal fibroblasts and CAFs and primary culture of normal
fibroblasts from normal pancreatic tissue in resected specimens has
been attempted. However, normal fibroblasts were difficult to
culture and the quality of the cells was unfortunately inadequate
for the assay. The results may indicate that CAFs may have been
affected in some way by the original cancer cells, thus in the next
study it will be attempted to perform further assays to determine
the differences between the original fibroblasts and CAFs. It is
also considered that future study is needed to examine the effects
of the cancer microenvironment on CAFs in terms of induction of
cellular senescence.
In summary, the present data provided the first
evidence, to the best of our knowledge, that p53-mediated cellular
senescence and SASP of CAFs affect the malignant potential of PC
cells. Thus, controlling cellular senescence and SASP in CAFs may
be a new strategy for PC treatment.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
The authors would like to thank Eiko Moriishi and
Mami Ikeda (Laboratory of Immunosenescence, Center for Vaccine and
Adjuvant Research, National Institutes of Biomedical Innovation,
Health and Nutrition, Osaka) for sample preservation and data
analyses.
Funding
The present study was supported by the following grants: AMED
(grant nos. 21fk0410040h0001 and 21fk0210057h0003) and JSPS KAKENHI
(grant nos. 20H03728, 19K09171 and 19K18114). The funding sources
had no role in data collection, analysis, or interpretation; or in
the decision to submit the article for publication.
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
MH and HM designed the present study. ST helped to
analyze data. HA, SK, ST, YI, DY, YT, TN, KG, YD, TY and HE helped
to design the study. SK and TY confirm the authenticity of all the
raw data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Tissue samples of human PC were collected with the
approval (approval no. 18138-4) of the Human Ethics Review
Committee of the Graduate School of Medicine of Osaka University
(Osaka, Japan) and the National Institutes of Biomedical
Innovation, Health and Nutrition, (approval no. 201-01; Osaka,
Japan). Written informed consent for sample use was obtained from
all patients before surgery.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ACTA2
|
actin alpha 2
|
|
α-SMA
|
α-smooth muscle actin
|
|
ATM
|
ataxia telangiectasia mutated
|
|
CAF
|
cancer-associated fibroblasts
|
|
CDKN1A
|
cyclin-dependent kinase inhibitor
1A
|
|
CDKN2A
|
cyclin-dependent kinase inhibitor
2A
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
FAP
|
fibroblast activation protein
|
|
FBS
|
fetal bovine serum
|
|
FIR
|
fluorescence intensity ratio
|
|
GADPH
|
glyceraldehyde-3-phosphate
dehydrogenase
|
|
KEGG
|
Kyoto encyclopedia of genes and
genomes
|
|
NFKB1
|
nuclear factor kappa B subunit 1
|
|
PC
|
pancreatic cancer
|
|
PFT
|
pifithrin
|
|
SASP
|
senescence-associated secretory
phenotype
|
|
TERT
|
telomerase reverse transcriptase
|
|
TP53
|
tumor protein p53
|
|
TSR
|
tumor size ratio
|
|
VIM
|
vimentin
|
References
|
1
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2022. CA Cancer J Clin. 72:7–33. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hwang RF, Moore T, Arumugam T,
Ramachandran V, Amos KD, Rivera A, Ji B, Evans DB and Logsdon CD:
Cancer-associated stromal fibroblasts promote pancreatic tumor
progression. Cancer Res. 68:918–926. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Orozco CA, Martinez-Bosch N, Guerrero PE,
Vinaixa J, Dalotto-Moreno T, Iglesias M, Moreno M, Djurec M,
Poirier F, Gabius HJ, et al: Targeting galectin-1 inhibits
pancreatic cancer progression by modulating tumor-stroma crosstalk.
Proc Natl Acad Sci USA. 115:E3769–E3778. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Guan J, Zhang H, Wen Z, Gu Y, Cheng Y, Sun
Y, Zhang T, Jia C, Lu Z and Chen J: Retinoic acid inhibits
pancreatic cancer cell migration and EMT through the downregulation
of IL-6 in cancer associated fibroblast cells. Cancer Lett.
345:132–139. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ireland L, Santos A, Ahmed MS, Rainer C,
Nielsen SR, Quaranta V, Weyer-Czernilofsky U, Engle DD,
Perez-Mancera PA, Coupland SE, et al: Chemoresistance in pancreatic
cancer is driven by stroma-derived insulin-like growth factors.
Cancer Res. 76:6851–6863. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wei L, Ye H, Li G, Lu Y, Zhou Q, Zheng S,
Lin Q, Liu Y, Li Z and Chen R: Cancer-associated fibroblasts
promote progression and gemcitabine resistance via the SDF-1/SATB-1
pathway in pancreatic cancer. Cell Death Dis. 9:10652018.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Su S, Chen J, Yao H, Liu J, Yu S, Lao L,
Wang M, Luo M, Xing Y, Chen F, et al: CD10 + GPR77 +
cancer-associated fibroblasts promote cancer formation and
chemoresistance by sustaining cancer stemness. Cell. 172:841–856.
e162018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pelon F, Bourachot B, Kieffer Y, Magagna
I, Mermet-Meillon F, Bonnet I, Costa A, Givel AM, Attieh Y,
Barbazan J, et al: Cancer-associated fibroblast heterogeneity in
axillary lymph nodes drives metastases in breast cancer through
complementary mechanisms. Nat Commun. 11:4042020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Elyada E, Bolisetty M, Laise P, Flynn WF,
Courtois ET, Burkhart RA, Teinor JA, Belleau P, Biffi G, Lucito MS,
et al: Cross-species single-cell analysis of pancreatic ductal
adenocarcinoma reveals antigen-presenting cancer-associated
fibroblasts. Cancer Discov. 9:1102–1123. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Biffi G, Oni TE, Spielman B, Hao Y, Elyada
E, Park Y, Preall J and Tuveson DA: IL1-induced JAK/STAT signaling
is antagonized by TGFβ to shape CAF heterogeneity in pancreatic
ductal adenocarcinoma. Cancer Discov. 9:282–301. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pilleron S, Sarfati D, Janssen-Heijnen M,
Vignat J, Ferlay J, Bray F and Soerjomataram I: Global cancer
incidence in older adults, 2012 and 2035: A population-based study.
Int J Cancer. 144:49–58. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Balducci L and Ershler WB: Cancer and
ageing: A nexus at several levels. Nat Rev Cancer. 5:655–662. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee S and Schmitt CA: The dynamic nature
of senescence in cancer. Nat Cell Biol. 21:94–101. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Childs BG, Gluscevic M, Baker DJ, Laberge
RM, Marquess D, Dananberg J and van Deursen JM: Senescent cells: An
emerging target for diseases of ageing. Nat Rev Drug Discov.
16:718–735. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Micco RD, Krizhanovsky V, Baker D and di
Fagagna F: Cellular senescence in ageing: From mechanisms to
therapeutic opportunities. Nat Rev Mol Cell Biol. 22:75–95. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ovadya Y and Krizhanovsky V: Strategies
targeting cellular senescence. J Clin Invest. 128:1247–1254. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sun Y, Coppé JP and Lam EWF: Cellular
senescence: The sought or the unwanted? Trends Mol Med. 24:871–885.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kuilman T, Michaloglou C, Vredeveld LCW,
Douma S, van Doorn R, Desmet CJ, Aarden LA, Mooi WJ and Peeper DS:
Oncogene-induced senescence relayed by an interleukin-dependent
inflammatory network. Cell. 133:1019–1031. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Acosta JC, O'Loghlen A, Banito A, Guijarro
MV, Augert A, Raguz S, Fumagalli M, Costa MD, Brown C, Popov N, et
al: Chemokine signaling via the CXCR2 receptor reinforces
senescence. Cell. 133:1006–1018. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Coppé JP, Patil CK, Rodier F, Sun Y, Muñoz
DP, Goldstein J, Nelson PS, Desprez PY and Campisi J:
Senescence-associated secretory phenotypes reveal
cell-nonautonomous functions of oncogenic RAS and the p53 tumor
suppressor. PLoS Biol. 6:e3012008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yoshimoto S, Loo TM, Atarashi K, Kanda H,
Sato S, Oyadomari S, Iwakura Y, Oshima K, Morita H, Hattori M, et
al: Obesity-induced gut microbial metabolite promotes liver cancer
through senescence secretome. Nature. 499:97–101. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Guo Y, Ayers JL, Carter KT, Wang T, Maden
SK, Edmond D, Newcomb P, Li C, Ulrich C, Yu M, et al:
Senescence-associated tissue microenvironment promotes colon cancer
formation through the secretory factor GDF15. Aging Cell.
18:e130132019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Toso A, Revandkar A, Mitri DD, Guccini I,
Proietti M, Sarti M, Pinton S, Zhang J, Kalathur M, Civenni G, et
al: Enhancing chemotherapy efficacy in Pten-deficient prostate
tumors by activating the senescence-associated antitumor immunity.
Cell Rep. 9:75–89. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bachem MG, Schünemann M, Ramadani M, Siech
M, Beger H, Buck A, Zhou S, Schmid-Kotsas A and Adler G: Pancreatic
carcinoma cells induce fibrosis by stimulating proliferation and
matrix synthesis of stellate cells. Gastroenterology. 128:907–921.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mukai Y, Yamada D, Eguchi H, Iwagami Y,
Asaoka T, Noda T, Kawamoto K, Gotoh K, Kobayashi S, Takeda Y, et
al: Vitamin D supplementation is a promising therapy for pancreatic
ductal adenocarcinoma in conjunction with current chemoradiation
therapy. Ann Surg Oncol. 25:1868–1879. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Crochemore C, Fernández-Molina C, Montagne
B, Salles A and Ricchetti M: CSB promoter downregulation via
histone H3 hypoacetylation is an early determinant of replicative
senescence. Nat Commun. 10:55762019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Vasef MA, Ross JS and Cohen MB: Telomerase
activity in human solid tumors. Diagnostic utility and clinical
applications. Am J Clin Pathol. 112:S68–S75. 1999.PubMed/NCBI
|
|
29
|
Uehara H, Nakaizumi A, Tatsuta M, Baba M,
Takenaka A, Uedo N, Sakai N, Yano H, Iishi H, Ohigashi H, et al:
Diagnosis of pancreatic cancer by detecting telomerase activity in
pancreatic juice: Comparison with K-ras mutations. Am J
Gastroenterol. 94:2513–2518. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sharpless NE and Sherr CJ: Forging a
signature of in vivo senescence. Nat Rev Cancer. 15:397–408. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hara E, Smith R, Parry D, Tahara H, Stone
S and Peters G: Regulation of p16CDKN2 expression and its
implications for cell immortalization and senescence. Mol Cell
Biol. 16:859–867. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Alcorta DA, Xiong Y, Phelps D, Hannon G,
Beach D and Barrett JC: Involvement of the cyclin-dependent kinase
inhibitor p16 (INK4a) in replicative senescence of normal human
fibroblasts. Proc Natl Acad Sci USA. 93:13742–13747. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kang C, Xu Q, Martin TD, Li MZ, Demaria M,
Aron L, Lu T, Yankner BA, Campisi J and Elledge SJ: The DNA damage
response induces inflammation and senescence by inhibiting
autophagy of GATA4. Science. 349:aaa56122015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Strzeszewska A, Alster O, Mosieniak G,
Ciolko A and Sikora E: Insight into the role of PIKK family members
and NF-кB in DNAdamage-induced senescence and senescence-associated
secretory phenotype of colon cancer cells. Cell Death Dis.
9:442018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yang M, Jiang Z, Yao G, Wang Z, Sun J, Qin
H and Zhao H: GALC Triggers tumorigenicity of colorectal cancer via
senescent fibroblasts. Front Oncol. 10:3802020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yang G, Rosen DG, Zhang Z, Bast RC Jr,
Mills GB, Colacino JA, Mercado-Uribe I and Liu J: The chemokine
growth-regulated oncogene 1 (Gro-1) links RAS signaling to the
senescence of stromal fibroblasts and ovarian tumorigenesis. Proc
Natl Acad Sci USA. 103:16472–16477. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Taddei ML, Cavallini L, Comito G, Giannoni
E, Folini M, Marini A, Gandellini P, Morandi A, Pintus G,
Raspollini MR, et al: Senescent stroma promotes prostate cancer
progression: The role of miR-210. Mol Oncol. 8:1729–1746. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Toste PA, Nguyen AH, Kadera BE, Duong M,
Wu N, Gawlas I, Tran LM, Bikhchandani M, Li L, Patel SG, et al:
Chemotherapy-induced inflammatory gene signature and
pro-tumorigenic phenotype in pancreatic CAFs via stress-associated
MAPK. Mol Cancer Res. 14:437–447. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Coppé JP, Desprez PY, Krtolica A and
Campisi J: The senescence-associated secretory phenotype: The dark
side of tumor suppression. Annu Rev Pathol. 5:99–118. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Harley CB, Futcher AB and Greider CW:
Telomeres shorten during ageing of human fibroblasts. Nature.
345:458–460. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hernandez-Segura A, Brandenburg S and
Demaria M: Induction and validation of cellular senescence in
primary human cells. J Vis Exp. 20:577822018.PubMed/NCBI
|
|
42
|
Dierick JF, Eliaers F, Remacle J, Raes M,
Fey SJ, Larsen PM and Toussaint O: Stress-induced premature
senescence and replicative senescence are different phenotypes,
proteomic evidence. Biochem Pharmacol. 64:1011–1017. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhu J, Singh M, Selivanova G and Peuget S:
Pifithrin-α alters p53 post-translational modifications pattern and
differentially inhibits p53 target genes. Sci Rep. 10:10492020.
View Article : Google Scholar : PubMed/NCBI
|