Introduction
Pancreatic cancer (PC), usually occurring as
pancreatic ductal adenocarcinoma, is a common malignant digestive
tumor, which is characterized by silent onset, rapid progress and a
poor prognosis (1,2). The onset of secondary diabetes caused
by PC, which is termed as PC-associated diabetes (PCAD), occurs
almost 10-13 months prior to the diagnosis of PC (3–5),
indicating that PCAD may be used as an early clinical manifestation
for PC screening. Therefore, it is noteworthy to explore the
pathogenesis and search for specific biomarkers of PCAD.
Vanin-1 (VNN1) is anchored to the cellular membrane
with pantetheinase activity, which hydrolyzes pantetheine to
produce cysteamine (6–8). VNN1 can promote oxidative stress and
the inflammatory response (9).
Additionally, VNN1 has been confirmed to be overexpressed in cancer
tissues of patients with PCAD and can be used as a blood biomarker
for the discrimination of PCAD from type 2 diabetes (10). A previous study by the authors also
demonstrated that VNN1 was overexpressed in neoplastic cells in the
majority of cases of PCAD (11).
Furthermore, the authors previously co-cultured VNN1-overexpressing
human PC cell lines with insulinoma cell lines (INS-1 and β-TC-6),
and examined the effects of the overexpression of VNN1 on
insulinoma cells and explored the underlying mechanisms (11). It was demonstrated that the
extracellular cysteamine concentration of VNN1-overexpressing PC
cells was markedly increased, which aggravated oxidative stress in
paraneoplastic insulinoma cells by upregulating reactive oxygen
species (ROS) levels and downregulating the peroxisome
proliferator-activated receptor γ (PPARγ)/glutathione (GSH)
concentrations, and ultimately, the viability and function of
insulinoma cells were impaired more significantly (11). Therefore, VNN1 may participate in
the pathogenesis of PCAD and may be used as a specific biomarker of
PCAD for the early diagnosis of PC.
Exosomes (Exos) are encapsulated by a bilayer lipid
membrane and contain a variety of bioactive molecules, such as DNA,
RNA, protein, and can be used as the intercellular conveyance
medium of molecules and signal pathways (12). PC cells secrete a large amount of
Exos into the surrounding microenvironment, which causes
paraneoplastic β-cell dysfunction (13). Moreover, PC cell-derived Exos
(PC-Exos) can impair the functions of skeletal muscle cells,
adipocytes and intestinal mucosal cells, leading to decreased
insulin secretion and insulin resistance (14–17).
In the present study, mouse primary islets were used
for more accurately researching the effects of VNN1-overexpressing
PC cells on paraneoplastic β-cells. The results revealed that
VNN1-overexpressing PC cells could also inhibit the viability and
function of islets by increasing oxidative stress. In addition, for
the first time, to the best of our knowledge, it was found that
VNN1-overexpressing PC-Exos could induce β-cell dedifferentiation,
which further reduced the insulin secretion of islets. Thus, the
present study also aimed to explore the potential underlying
mechanisms. Furthermore, islets were transplanted under the kidney
capsule of diabetic mice in order to observe the effects of islets
co-cultured with VNN1-overexpressing PC cells on blood glucose
regulation in vivo.
Materials and methods
Cell culture and islet isolation
The human PC cell lines, PANC-1 (cat. no. TCHu98)
and CFPAC-1 (cat. no. TCHu112), were purchased from The Cell Bank
of Type Culture Collection of the Chinese Academy of Sciences and
cultured in RPMI-1640 medium (cat. no. 11875119; Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum
(FBS; cat. no. 12484028; Gibco; Thermo Fisher Scientific, Inc.) and
100 IU/ml penicillin and streptomycin (cat. no. 450-201-EL; Wisent,
Inc.), at 37°C in a humid atmosphere (5% CO2 and 95%
air). Pancreatic islets were isolated from C57BL/6J (B6) mice as
previously described (18). All
animal experiments in the present study were approved by the Animal
Ethics Committee of The First Affiliated Hospital of Zhengzhou
University, Zhengzhou, China (Ethics no. 2018-03-003) and conducted
in accordance with the approved guidelines. A total of 150 B6 mice
(female; 4-6 weeks old; weighing 18-20 g; Jackson Laboratory) were
used for islet isolation. These B6 mice were housed in a specific
pathogen-free (SPF) environment under a 12-h light/dark cycle with
ad libitum access to food and water at 24°C and 55%
humidity. The B6 mice were sacrificed by cervical dislocation
following anesthesia by isoflurane inhalation (2–6% for induction;
1–3% for maintenance), and 2.5 ml collagenase (type V, 2.0 mg/ml;
cat. no. 40511ES60; Yeasen Biotechnology Co., Ltd.) was injected
into the common bile ducts of these mice, and the distended
pancreas was then harvested and incubated in a shaking water bath
at 37°C for 15 min. Purified islets were obtained by Ficoll (Ficoll
PM400; cat. no. F4375; Sigma-Aldrich Trading Co., Ltd.) gradient
centrifugation at 3,000 × g for 20 min at 4°C and cultured in HAM's
F10 medium (cat. no. 11550043; Gibco; Thermo Fisher Scientific,
Inc.). One islet equivalent (IEQ) was calculated as previously
described (19).
Lentiviral vector transfection
A full-length human VNN1 cDNA was cloned into a
lentiviral vector (Shanghai GeneChem Co., Ltd.) for constitutive
gene expression. The lentiviral vector was co-transfected with
packaging vectors (Shanghai GeneChem Co., Ltd.) into 293T cells
(cat. no. GNHu17; from The Cell Bank of Type Culture Collection of
the Chinese Academy of Sciences). The PANC-1 and CFPAC-1 cells were
infected with empty or VNN1-expressing lentiviruses. The PANC-1 and
CFPAC-1 cells with a stable overexpression of VNN1 were termed as
PV and CV, and the PANC-1 and CFPAC-1 cells transfected with the
empty vector were referred to as PE and CE.
Establishment of co-culture
system
The lower and upper compartments of Transwell
chambers (6-well; cat. no. 3412; Corning, Inc.) were separated by
polyvinylpyrrolidone-free polycarbonate filters (pore size, 0.4
µm). Each lower compartment was seeded with islets (200
islets/well), and the upper compartment was loaded with PC cells
(2×105 cells/well).
Cell viability assay
PC cell viability was determined using MTT assay
(cat. no. CT01-5; MilliporeSigma). Cells seeded in a 96-well plate
were treated with MTT for 4 h, and after removing the supernatant,
DMSO (cat. no. 94563; MilliporeSigma) was added to each well. After
shaking the plate, an ELISA reader (BioTek Instruments, Inc.) was
used to measure the optical density value at 570 nm. Islets were
pre-treated with 10 µM GSH (cat. no. G4251; MilliporeSigma) or 10
µM thiazolidinedione (TZD; cat. no. 375004; MilliporeSigma) for 2 h
at 37°C. Islets not pre-treated with GSH or TZD were used as
controls. Following gentle agitation for 5 min in calcium-free
medium containing Trypsin/EDTA (cat. no. T4049; MilliporeSigma) at
in a water bath at 37°C, islets were dissociated into single cells,
and single cells were then cultured in HAM's F10 medium (cat. no.
11550043; Gibco; Thermo Fisher Scientific, Inc.); single cells were
stained with 0.4% Trypan blue (cat. no. T6146; MilliporeSigma) for
3 min at room temperature and cell viability was determined by
counting the live and dead cells using a hemocytometer (cat. no.
MDH-2N1; MilliporeSigma) under a light microscope (Olympus IX-71;
Olympus Corporation).
Isolation and characterization of
Exos
After the PC cells grew to 80% confluency, the
supernatants were replaced by medium with exosome-free FBS
(differential centrifugation at 100,000 × g for 16 h at 4°C).
Supernatants were collected following culture for 48 h, and Exos
were then isolated from the supernatants using differential
centrifugation as previously described (14). A transmission electron microscope
(Philips Healthcares) and Zetasizer Nano ZS (Malvern Instruments,
Ltd.) were used to characterize the Exos. Exos isolated from the
PANC-1, PE and PV cells were termed as PANC1-Exos, PE-Exos and
PV-Exos, respectively.
Western blot analysis
Western blot analysis procedures were performed in
accordance with standard protocols (14). Total protein was extracted from PC
cells, islets and Exos using RIPA lysis buffer (cat. no. R0278;
MilliporeSigma) supplemented with a protease inhibitor cocktail
(cat. no. P8340; MilliporeSigma). Nuclear and cytoplasmic proteins
were extracted using NE-PER Nuclear and Cytoplasmic Extraction
Reagents (cat. no. 78833; Thermo Fisher Scientific, Inc.) Protein
concentrations were quantified using the BCA Protein Quantification
kit (cat. no. 23225; Thermo Fisher Scientific, Inc.). The protein
lysates (30 µg/lane) were separated by 10% SDS-polyacrylamide gels
and transferred to polyvinylidene difluoride membranes (cat. no.
ISEQ00010; MilliporeSigma). The membranes were blocked with 5%
skimmed milk for 2 h at room temperature and incubated with
antibody dilutions overnight at 4°C using the following specific
primary antibodies: VNN1 (1:1,000; cat. no. ab205912; Abcam),
β-actin (1:1,000; cat. no. ab8227; Abcam), PPARγ (1:1,000; cat. no.
ab209350; Abcam), tumor susceptibility gene 101 (TSG101; 1:1,000;
cat. no. ab133586; Abcam), Alix (1:1,000; cat. no. ab275337;
Abcam), C-myc (1:2,000; cat. no. ab185656; Abcam), pancreatic and
duodenal homeobox 1 (Pdx1; 1:3,000; cat. no. ab47267; Abcam),
neurogenic differentiation 1 (NeuroD1; 1:1,000; cat. no. ab109224;
Abcam), glyceraldehyde 3-phosphate dehydrogenase (GAPDH; 1:2,500;
cat. no. ab9485; Abcam), Lamin B (1:1,000; cat. no. ab16048;
Abcam), Forkhead box protein O1 (FoxO1; 1:1,000; cat. no. ab52857;
Abcam), cleaved caspase-3 (1:1,000; cat. no. 9664; Cell Signaling
Technology, Inc.), cleaved caspase-9 (1:1,000; cat. no. 9509; Cell
Signaling Technology, Inc.), MAF BZIP transcription factor A (Mafa;
1:1,000; cat. no. 79737; Cell Signaling Technology, Inc.),
AMP-activated protein kinase (AMPK; 1:1,000; cat. no. 2532; Cell
Signaling Technology, Inc.), phosphorylated (p-)AMPK (1:1,000; cat.
no. 2535; Cell Signaling Technology, Inc.), tubulin (1:1,000; cat.
no. 2146; Cell Signaling Technology, Inc.) and sirtuin 1 (Sirt1;
1:1,000; cat. no. 2028; Cell Signaling Technology, Inc.). The
membranes were then incubated with anti-rabbit IgG (HRP-linked)
secondary antibody (1:1,000; cat. no. 7074; Cell Signaling
Technology, Inc.) for 1 h at room temperature. The protein bands
were visualized using EZ-ECL (cat. no. 20-500-120; Biological
Industries, Inc.). The bands were quantitated using ImageJ software
(version 1.8.0; National Institutes of Health).
Insulin secretion assay
Following co-culture with PC cells or PC-Exos for 24
h, the islets were incubated in Krebs-Ringer bicarbonate buffer
(KRBB) for 45 min. Subsequently, the medium was removed, and the
islets were incubated in KRBB containing either 5.6 or 16.7 mM
glucose for 30 min. The supernatants were collected following
centrifugation at 1,000 × g for 5 min at 4°C, and the islets were
then dissociated into single cells and incubated overnight in
acidified ethanol at 4°C. The supernatants were then collected
following centrifugation at 3,000 × g for 5 min at 4°C. The insulin
contents in all the supernatants were analyzed using the Insulin
Radioimmunoassay kit (cat. no. S10930046; Beijing North Institute
of Biotechnology Co., Ltd.). Total cell protein was determined
using BCA assay for normalizing the insulin content
measurements.
Determination of the cysteamine
content using high- performance liquid chromatography (HPLC)
The cysteamine contents in the conditioned media of
PC cells and lysates of islets were determined using HPLC. The
detailed procedures were performed according to standard protocols,
as described in a previous study by the authors (11). The HPLC system was constructed using
an ESA-model 542 pump and an ESA Coulochem III coulometric detector
(Waters Corporation). The analysis voltage, current and output
voltage of detector 1 (E1) were set at −150 mV, 10 nA and −1.00 V,
respectively; the analysis voltage and current of detector 2 (E2)
were set at +200 mV and 1 µA, respectively. The chromatographic
column (Hypersil BDS C18 column, 250×4.6 mm I.D., 5 µM; Dalian
Elite Analytical Instruments Co., Ltd.) was rinsed using a mixture
of ultrapure water and acetonitrile overnight to remove salts or
other impurities. The pH of the mobile phase (50 mM
NaH2PO4, 0.05 mM octane sulfonic acid, 1%
acetonitrile and 0.5% N,N-dimethylformamide) was adjusted to 2.52,
and the mobile phase was then filtered using nylon filters with a
pore size of 0.22 µm (MilliporeSigma). A 100 µl conditioned medium
or islets lysate was mixed with an equal volume of perchloric acid,
and the supernatants were then collected following centrifugation
at 13,000 × g for 20 min at 4°C, the supernatants were transferred
to Amicon Ultra-0.5 3K centrifugal filters (cat. no. UFC5003BK;
MilliporeSigma) and centrifuged at 8,000 × g for 20 min at 4°C. The
solutions in the lower tubes were used as samples to be tested. The
flow rate of the mobile phase was 0.6 ml/min. A 20 µl standard
sample of cysteamine (cat. no. 30070; MilliporeSigma) or the sample
to be tested were loaded into the autosampler (Waters Corporation),
respectively. EZStart software (version 7.2; Scientific Systems
Inc.) was used to analyze chromatograms.
Measurement of reactive oxygen species
(ROS) generation
Following co-culture with PC cells, the islets were
dissociated into single cells and treated with 10 µM DCF-DA
(ROS-specific fluorescent probe; cat. no. 35845; MilliporeSigma)
for 30 min at 37°C; the cells were then resuspended in ice-cold PBS
for analysis using flow cytometry (FCM; FACSCanto™ II;
BD Biosciences). The data were analyzed using FlowJo software
(version 7.6; FlowJo LLC).
Detection of GSH concentration
Following co-culture with PC cells, the islets were
lysed with 10 mM HCl, then mixed with 5% sulfosalicylic acid. The
supernatants were collected following centrifugation at 8,000 × g
for 10 min at 4°C and GSH in the supernatants was detected using a
Total Glutathione Quantification kit (cat. no. T419; Dojindo
Laboratories, Inc.).
Co-immunoprecipitation (Co-IP)
Following pre-treatment with PC-Exos, non-denaturing
protein lysates of islets were prepared in Nonidet P40 (NP-40)
lysis buffer (50 mM Tris-HCl, pH 7.5, 1% NP-40, 100 mM NaCl)
supplemented with a complete protease inhibitor cocktail (cat. no.
11697498001; Roche Co., Ltd.). Protein lysates were incubated with
Sirt1 primary antibody (1:50; cat. no. 2028; Cell Signaling
Technology, Inc.) and negative control IgG (1:50; cat. no. 2729;
Cell Signaling Technology, Inc.) at 4°C for 12 h, and
immunocomplexes were incubated with the Protein A/G Beads (cat. no.
LSKMAGAG; MilliporeSigma) for 2 h at 4°C. The beads were washed
three times with lysis buffer and boiled for 5 min at 100°C, and
immunocomplexes were then separated by 10% SDS-PAGE and examined
using western blot analysis. For detection of FoxO1 acetylation,
islet lysates were incubated with acetylated-lysine antibody
(1:100; cat. no. 9441; Cell Signaling Technology, Inc.) for 12 h at
4°C, and the immunocomplexes were then examined using western blot
analysis following incubation with FoxO1 antibody (1:1,000; cat.
no. ab52857; Abcam) overnight at 4°C.
Islet transplantation
As aforementioned, all animal experiments in the
present study were approved by the Animal Ethics Committee of The
First Affiliated Hospital of Zhengzhou University. A total of 40 B6
mice (female; 6 weeks old) were used as possible recipients for
islet transplantation. These B6 mice were housed at 5 mice per cage
under SPF conditions (temperature, 24°C; humidity, 55%; 12-h
light/dark cycle; free access to food and water). The B6 mice were
rendered diabetic by an intraperitoneal injection of streptozotocin
(180 mg/kg; cat. no. S0130; MilliporeSigma) 4-5 days prior to islet
transplantation and the blood glucose levels of these mice were
monitored using blood samples obtained from the tail vein. When two
consecutive blood glucose levels were >18 mM, the B6 mice were
confirmed as diabetic. A total of 30 B6 diabetic mice, which were
active and had a good appetite, were selected as recipients and
randomly divided into six groups (5 mice in each group). The other
10 B6 mice were euthanized by cervical dislocation following
anesthesia by isoflurane inhalation (2–6% for induction; 1–3% for
maintenance) 1 week later. The recipient mice were anaesthetized
with isoflurane inhalation (2–6% for induction; 1–3% for
maintenance). A 0.5-cm left subcostal incision was made to expose
the left kidney. Subsequently, 200 or 400 IEQ islets treated or
untreated with PC cells were transplanted under the left kidney
capsule using a micromanipulator syringe. After the surgery,
incisions were applied with 5% lidocaine cream (cat. no. H20063466;
Beijing Ziguang Pharmaceutical Co., Ltd.) to alleviate the
post-operative pain and the mice were placed in a warm environment
until they were fully awake and had recovered from the anesthesia.
The blood glucose levels of the recipients were monitored once a
week. Blood glucose levels <10 mM was considered as
normoglycemia. At the 14th week following islet transplantation,
all recipient mice underwent a survival nephrectomy of the
graft-bearing kidney using the same anesthesia and post-operative
care methods as described above, and the blood glucose levels of
these mice were then monitored. If the blood glucose levels
increased significantly, this indicated that the normoglycemic
levels of recipients were regulated by the transplanted islets
under the kidney capsule.
When reaching one of the humane endpoints (>20%
body weight loss, inability to eat, signs of immobility, abnormal
posture, post-operative infection, post-operative hemorrhaging),
the mice were euthanized. None of the mice reached the humane
endpoints and died during the experimental procedures. Animal
health and behavior were monitored daily. All recipient mice were
euthanized by cervical dislocation with prior anesthesia by
isoflurane inhalation (2–6% for induction; 1–3% for maintenance) at
the 15th week following islet transplantation. Death was verified
by the observation of pupil dilation, and by the cessation of
respiration and heartbeat for 10 min.
Immunohistochemistry
The left kidneys of the recipients were resected at
the 14th week following islet transplantation and fixed in zinc
formalin fixative (cat. no. 3261477; MilliporeSigma) overnight at
4°C, and then washed three times with 70% ethanol and embedded in
paraffin. After the islets were incubated with PC-Exos for 24 h at
37°C, the islets were fixed in zinc formalin fixative overnight at
4°C and embedded in 1% agarose gel. The agarose gel-embedded tissue
sections were incubated with VNN1 primary antibody (1:100; cat. no.
ab205912; Abcam) overnight at 4°C, and the paraffin-embedded tissue
sections were incubated with insulin primary antibody (1:100; cat.
no. 4590; Cell Signaling Technology, Inc.) overnight at 4°C. Then
biotin-labeled secondary antibody and streptavidin-peroxidase
complex working solution (cat. no. SA1028; Boster Biological
Technology Co., Ltd.) were incubated with the tissue sections for 1
h at 37°C and a DAB kit (cat. no. AR1027; Boster Biological
Technology Co., Ltd.) was used to produce a brown positive
reaction. Finally, All sections were stained with hematoxylin (cat.
no. C0105S-1; Beyotime Institute of Biotechnology) for 2 min at
room temperature and eosin (cat. no. C0105S-2; Beyotime Institute
of Biotechnology) for 30 sec at room temperature. Scans were
performed and images were captured using a light microscope
(Olympus IX-71; Olympus Corporation).
Statistical analysis
Statistical analysis was performed using SPSS
software (version 19.0; IBM Corp.). Data are expressed as the mean
± standard deviation (SD). One-way analysis of variance (ANOVA)
with a Tukey's post hoc test was used for multiple-group
comparisons. P<0.05 was considered to indicate a statistically
significant difference. Data visualization was performed using
GraphPad Prism software (version 5.0; GraphPad Software, Inc.).
Results
VNN1 overexpressed in PC cells
inhibits the viability and further impairs the functions of
paraneoplastic islets
The PANC-1 and CFPAC-1 cells were transfected with
VNN1 vector (PV and CV, respectively) or empty vector (PE and CE,
respectively), and the transfection efficiency was verified using
western blot analysis (Fig. 1A).
VNN1 overexpression had no significant effect on the proliferation
of the PANC-1 and CFPAC-1 cells (Fig.
S1). Primary islets were isolated from the pancreases of B6
mice and cultured in vitro (Fig.
1B). The islets were then co-cultured with PC cells for 24 h
using Transwell chambers (Fig. 1C).
As shown in Fig. 1D, the survival
rate of the islets was decreased significantly in the PV or CV
co-culture group compared with the control groups. Furthermore, the
levels of pro-apoptotic proteins (cleaved caspase-3/9) in the PV or
CV co-cultured islets were increased significantly (Fig. 1E). Compared with the untreated
group, the basal insulin secretion of islets markedly decreased in
all co-culture groups, and the decrease was more pronounced in the
PV co-culture group (Fig. 1F, left
panel). Following low-glucose (5.6 mM) or high-glucose (16.7 mM)
stimulation, the insulin secretion markedly increased in the PANC-1
and PE co-culture groups, while the insulin secretion exhibited no
response in the PV co-culture group (Fig. 1F, left panel). Similar results were
obtained for the CFPAC-1, CE and CV co-culture groups (Fig. 1F, right panel).
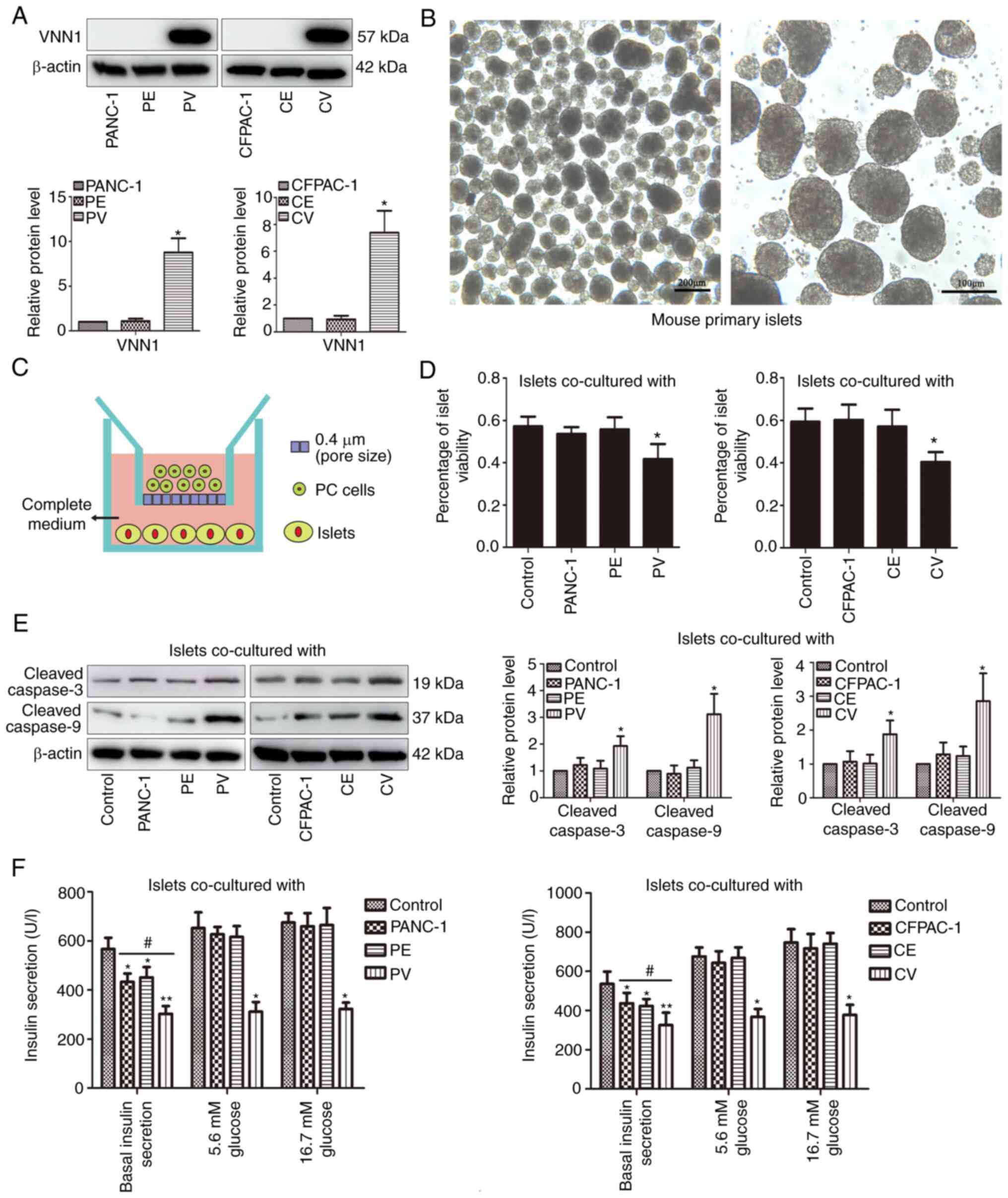 | Figure 1.VNN1-overexpressing PC cells inhibit
the viability and function of islets. (A) PANC-1 and CFPAC-1 cells
were transfected with empty vector or VNN1 vector, and VNN1
expression in cell lysates was examined using western blot
analysis. β-actin was used as the loading control. (B) Primary
islets from B6 mice were cultured in vitro. (C) Co-culture
system of islets with PC cells was constructed using Transwell
chambers. (D) Following co-culture with PC cells for 24 h, islets
were dissociated into single cells and cell viability was
determined using Trypan blue staining. (E) Following co-culture
with PC cells, cleaved caspase-3/9 expression levels in islets were
examined using western blot analysis. β-actin was used as the
loading control. (F) Following co-culture with PC cells, the
insulin secretion of islets was determined using radioimmunoassay.
Data are presented as the mean ± SD (n=3). Data were analyzed using
one-way ANOVA followed by Tukey's post-hoc test. *P<0.05 and
**P<0.01 compared with the PANC-1, CFPAC-1 or control group.
#P<0.05 compared with the PANC-1, PE, CFPAC-1 or CE
groups. VNN1, Vanin-1; PC, pancreatic cancer; PV, PANC-1 cells with
the stable overexpression of VNN1; PE, PANC-1 cells transfected
with empty vector; CV, CFPAC-1 cells with the stable overexpression
of VNN1; CE, CFPAC-1 cells transfected with empty vector. |
Inhibition of VNN1-mediated oxidative
stress improves the viability and functions of paraneoplastic
islets
The HPLC method was used (Fig. S2) to observe the cysteamine
concentrations in the conditioned media of PV and CV cells; the
cysteamine concentrations were increased significantly in the PV
and CV cells compared with the control cells (Fig. 2A). Correspondingly, the cysteamine
and ROS contents in the islets co-cultured with PV and CV cells
were also markedly increased (Fig. 2B
and C). In addition, the GSH and PPARγ expression levels in the
islets were inhibited in the PV and CV co-culture groups (Fig. 2D and E). Following islet
pre-incubation with 10 µM GSH or 10 µM thiazolidinedione (TZD,
PPARγ agonist) for 2 h, the decreased survival rate and increased
expression levels of pro-apoptotic proteins in the islets were
reversed in the PV co-culture group (Fig. 2F and G). Moreover, following islet
pre-treatment with GSH or TZD for 2 h, the basal insulin secretion
was increased significantly and the insulin secretion was also
markedly elevated following low- or high-glucose stimulation in the
PV co-culture group (Fig. 2H).
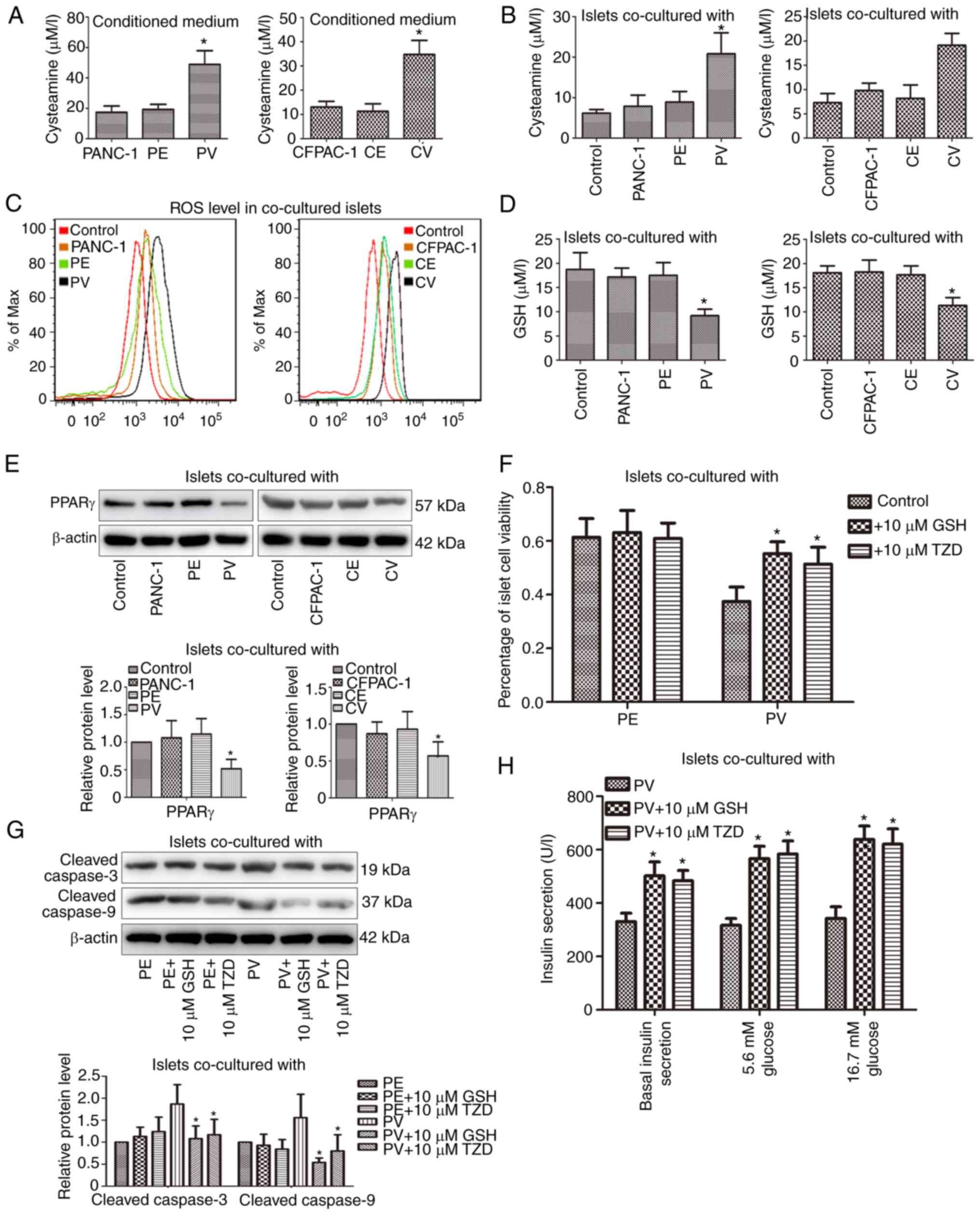 | Figure 2.The viability and function of
paraneoplastic islets are improved after suppressing VNN1-induced
oxidative stress by GSH or TZD. (A and B) The extracellular and
intracellular cysteamine concentrations were detected using
high-performance liquid chromatography. (C) ROS contents in islets
were analyzed using flow cytometry. (D) GSH concentrations in
islets were detected using spectrophotometry. (E) PPARγ expression
in islets was examined using western blot analysis. β-actin was
used as the loading control. (F) Following islet pre-treatment with
GSH or TZD, islet viability was determined using Trypan blue
staining. (G) Following islet pre-treatment with GSH or TZD,
cleaved caspase-3/9 levels in islets were examined using western
blot analysis. β-actin was used as the loading control. (H)
Following islet pre-treatment with GSH or TZD, the insulin
secretion was determined using radioimmunoassay. Data are presented
as the mean ± SD (n=3). Data were analyzed using one-way ANOVA
followed by Tukey's post-hoc test. *P<0.05 compared with the
PANC-1, CFPAC-1, PV or control group. ROS, reactive oxygen species;
PPARγ, peroxisome proliferator activated receptor gamma; GSH,
glutathione; TZD, thiazolidinedione; PV, PANC-1 cells with the
stable overexpression of VNN1; PE, PANC-1 cells transfected with
empty vector; CV, CFPAC-1 cells with the stable overexpression of
VNN1; CE, CFPAC-1 cells transfected with empty vector; VNN1,
Vanin-1. |
Exos derived from PV cells further
suppress the insulin secretion of paraneoplastic islets
As was demonstrated, the cysteamine concentration in
the conditioned medium of PV cells was higher than that of the
control cells (Fig. 3A). In order
to ensure that the conditioned media of PE and PV cells contain the
same content of cysteamine, the difference value (D-value) of
cysteamine content between the two types of conditioned media was
calculated, and complete medium (CM) containing the standard sample
of cysteamine (the amount was equal to the D-value) was mixed with
conditioned medium of PE cells (PE + cysteamine), and the equal
volume of CM without cysteamine was mixed with conditioned medium
of PE cells (PE + CM) or PV cells (PV + CM), respectively. As shown
in Fig. 3B, the cysteamine
concentration in the conditioned medium of the PE + cysteamine
group was equal to that of the PV + CM group. Following islet
incubation with the different conditioned media for 24 h, the
cysteamine content in the islets exhibited no marked differences
between the PE + cysteamine and PV + CM groups (Fig. 3C). Although the insulin secretion of
islets was inhibited in both the PE + cysteamine and PV + CM
groups, the inhibitory effect was more prominent in the PV + CM
group (Fig. 3D), which indicated
that other unknown substances released by VNN1-overexpressing PC
cells may also aggravate islet dysfunction, apart from
cysteamine.
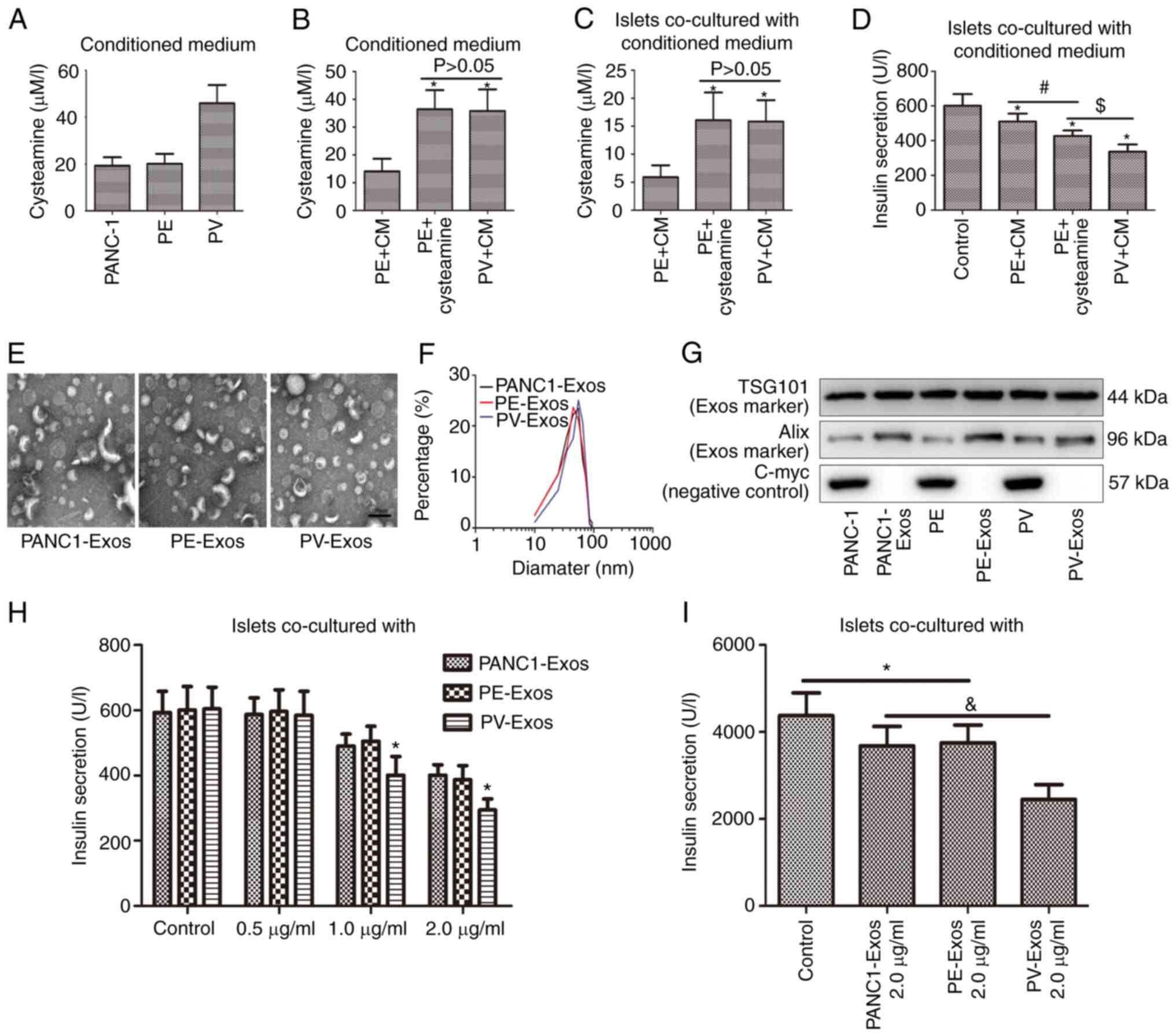 | Figure 3.Islet dysfunction was exacerbated by
Exos extracted from VNN1-overexpressing PC cells. (A and B)
Cysteamine contents in conditioned media were determined using
high-performance liquid chromatography. After the islets
wereco-cultured with different conditioned media, (C) the
cysteamine contents in islets were detected using high-performance
liquid chromatography and (D) insulin secretion was determined
using radioimmunoassay. (E) Transmission microcopy images of PC-
Exos. (F) Size distributions of PC-Exos. (G) Exo markers (TSG101
and Alix) and negative marker (C-myc) in PC-Exos were determined
using western blot analysis. TSG101 and Alix were used as the
positive loading controls, and C-myc was used as the negative
loading control. (H) Following treatment with different PC-Exos,
the insulin secretion of islets was determined using
radioimmunoassay. (I) Following treatment with different PC-Exos,
the insulin content in islets was also determined using
radioimmunoassay. Data are presented as the mean ± SD (n=3). Data
were analyzed using one-way ANOVA followed by Tukey's post-hoc
test. *P<0.05 compared with the PANC-1, PE + CM, PANC1-Exos or
control group. #P<0.05 compared with the PE + CM
group. $P<0.05 compared with the PE + cysteamine
group. &P<0.05 compared with the PANC1-Exos or
PE-Exos group. CM, complete medium; Exos, exosomes; VNN1, Vanin-1;
PC, pancreatic cancer; PV, PANC-1 cells with the stable
overexpression of VNN1; TSG101, tumor susceptibility gene 101; PE,
PANC-1 cells transfected with empty vector. |
Transmission electron microscopy images revealed
that the morphologies of extracellular vesicles (EVs) extracted
from PC cells were diverse (Fig.
3E), and the particle sizes of the EVs ranged from 30 to 80 nm
(Fig. 3F). All EVs contained
Exo-specific proteins (TSG101 and Alix), apart from C-myc, which
was confirmed not to be expressed in Exos (Fig. 3G). Therefore, the extracted EVs were
Exos. The islets were incubated with types of Exos (PANC1-Exos,
PE-Exos and PV-Exos) for 24 h. Compared with the untreated group,
the insulin secretion of islets in the PC-Exo-treated groups were
markedly decreased as the concentration of Exos increased, and the
decrease was particularly evident in the PV-Exo-treated group
(Fig. 3H). After the islets were
incubated with 2 µg/ml of PC-Exos for 24 h, the insulin content in
the islets decreased in every PC-Exo-treated group, and the
decrease was particularly pronounced in the PV-Exo-treated group
(Fig. 3I).
VNN1 can be transferred into
paraneoplastic islets through PC-Exos and induces β-cell
dedifferentiation
As shown in Fig. 4A,
VNN1 expression in PV-Exos was higher than that in PANC1-Exos and
PE-Exos. After the islets were incubated with PC-Exos (2 µg/ml) for
24 h, the VNN1 content in the PV-Exo-treated islets was also higher
than that in the PANC1-Exos and PE-Exo-treated islets (Fig. 4B and C). These results suggested
that VNN1 could be transferred into islets via PC-Exos. Notably,
there was no marked difference in the cysteamine content among
these three PC-Exo-treated islets (Fig.
4D). The islets were incubated with various concentrations of
PV-Exos for 24 h, and no marked changes were observed in the
cysteamine content of islets with the increased concentration of
PV-Exos (Fig. 4E). These results
demonstrated that the further inhibition of islet function by
PV-Exos was not dependent on cysteamine-mediated oxidative stress.
Therefore, the present study continued to explore the mechanisms of
the PV-Exo-induced aggravation of islet dysfunction. Compared with
the PANC1-Exos and PE-Exos, the PV-Exos significantly decreased the
expression of β-cell differentiation markers (Pdx1, Mafa and
NeuroD1) in the islets (Fig. 4F),
which indicated that PV-Exos induced β-cell dedifferentiation.
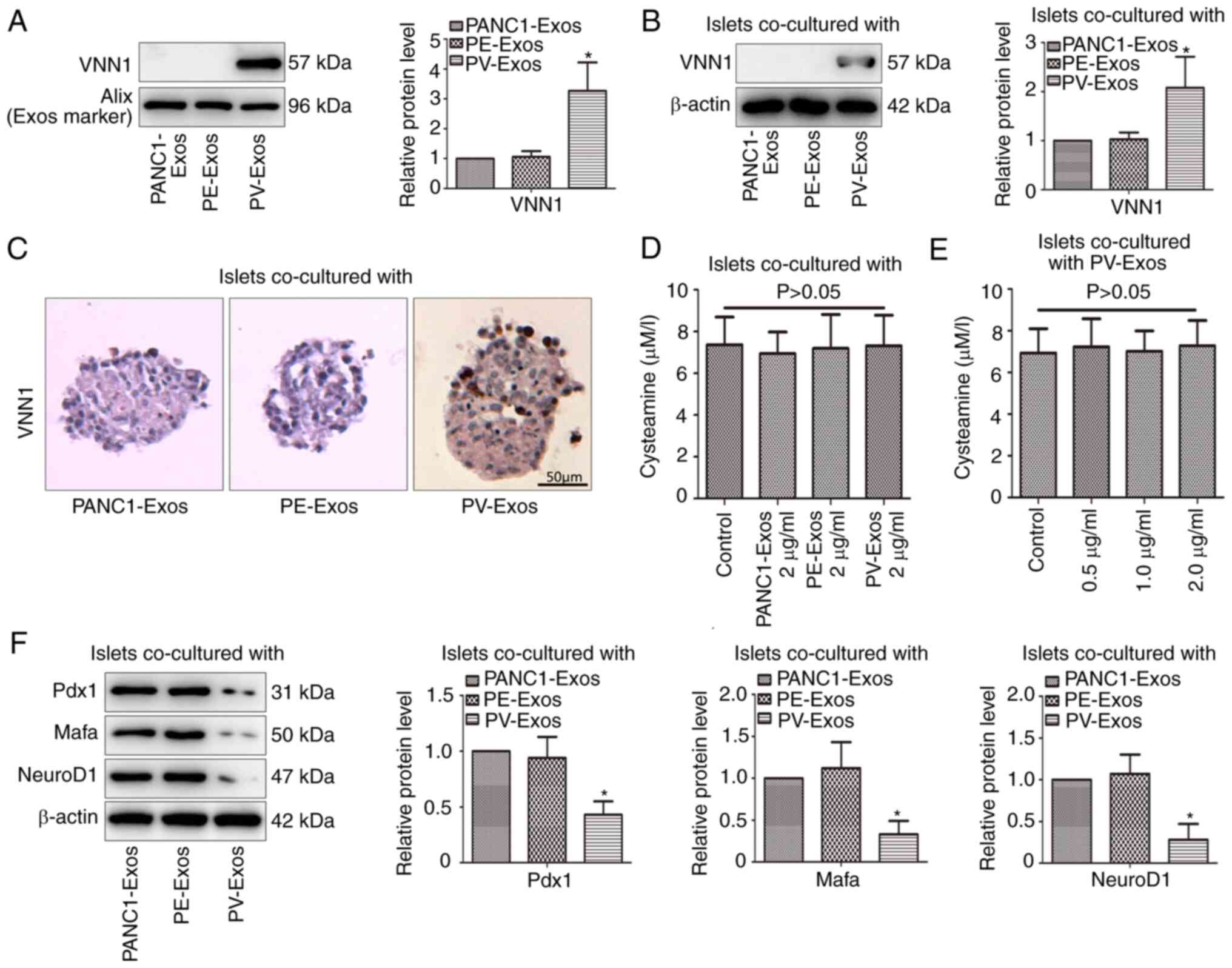 | Figure 4.Exos derived from VNN1-overexpressing
PC cells induce β-cell dedifferentiation. (A) VNN1 in PC-Exos was
determined using western blot analysis. As an Exo marker, Alix was
used as the loading control. (B) Following treatment with PC-Exos,
VNN1 expression in islets was determined using western blot
analysis. β-actin was used as the loading control. Following
co-culture with PC-Exos, (C) VNN1 expression in islets was analyzed
using immunohistochemistry, and (D) cysteamine in islets was
determined using high-performance liquid chromatography. (E)
Following incubation with various concentrations of PV-Exos,
cysteamine in islets was determined using high-performance liquid
chromatography. (F) Following co-culture with PC-Exos, β-cell
differentiation markers (Pdx1, Mafa and NeuroD1) in islets were
examined using western blot analysis. β-actin was used as the
loading control. Data are presented as the mean ± SD (n=3). Data
were analyzed using one-way ANOVA followed by Tukey's post-hoc
test. *P<0.05 compared with the PANC1-Exos group. VNN1, Vanin-1;
Exos, exosomes; Pdx1, pancreatic and duodenal homeobox 1; Mafa, MAF
BZIP transcription factor A; NeuroD1, neurogenic differentiation 1;
PC, pancreatic cancer; PV, PANC-1 cells with the stable
overexpression of VNN1; PE, PANC-1 cells transfected with empty
vector. |
VNN1 in PC-Exos induce β-cell
dedifferentiation via the inhibition of the AMPK/GAPDH/Sirt1/FoxO1
signaling pathway
The present study attempted to investigate the
mechanisms of PV-Exo-induced β-cell dedifferentiation. It was found
that VNN1 overexpression inhibited the phosphorylation of AMPK in
PANC-1 cells, and the expression of p-AMPK in the PV-Exo-treated
islets was lower than that in the PANC1-Exos and PE-Exo-treated
islets (Fig. 5A), suggesting that
VNN1 transferred by PC-Exos inhibited the phosphorylation of AMPK
in paraneoplastic islets. The present study further examined the
effects of PV-Exos on downstream cytokines of the AMPK signaling
pathway in islets. Compared with the control groups, the content of
GAPDH was decreased in the nucleus of PV-Exo-treated islets
(Fig. 5B). A Co-IP assay was also
performed to confirm that Sirt1 could bind with GAPDH and FoxO1 in
PC-Exo-treated islets, while the bindings were inhibited in
PV-Exo-treated islets (Fig. 5C). In
addition, Co-IP assay revealed that lysine of FoxO1 was markedly
acetylated in the PV-Exo-treated islets (Fig. 5D), indicating that PV-Exos
suppressed FoxO1 deacetylation in islets. As FoxO1 functions as a
downstream protein of the AMPK/GAPDH/Sirt1 signaling pathway and
FoxO1 deacetylation induces β-cell differentiation, these results
suggested that VNN1 in PC-Exos inhibited the AMPK/GAPDH/Sirt1/FoxO1
signaling pathway to induce β-cell dedifferentiation.
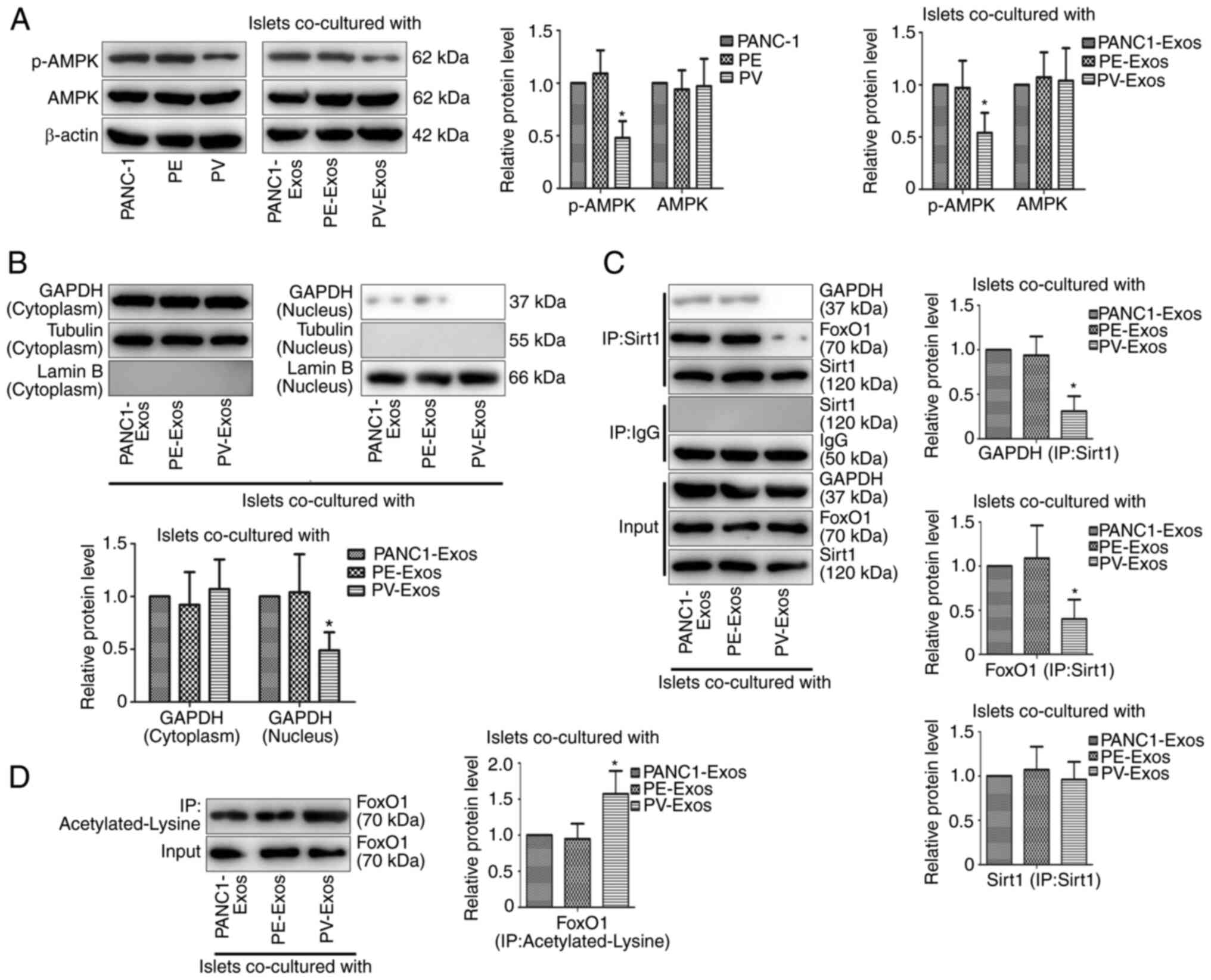 | Figure 5.VNN1 in PC-Exos inhibits the
AMPK/GAPDH/Sirt1/FoxO1 signaling pathway in islets. (A, left panel)
p-AMPK levels in PC cells were determined using western blot
analysis. β-actin was used as the loading control. (A, right panel)
Following treatment with PC-Exos, p-AMPK levels in islets were
examined using western blot analysis. β-actin was used as the
loading control. (B) Following treatment with PC-Exos,
nuclear-localized GAPDH in islets were examined using western blot
analysis. Tubulin and Lamin B were used as the loading controls.
(C) Following co-culture with PC-Exos, the interaction of GAPDH or
FoxO1 with Sirt1 in islets was detected using
co-immunoprecipitation. GAPDH, FoxO1 and Sirt1 were used as the
loading controls. (D) Following co-culture with PC-Exos,
acetylated-lysine of FoxO1 in islets was detected using
co-immunoprecipitation. FoxO1 was used as the loading control. Data
are presented as the mean ± SD (n=3). Data were analyzed using
one-way ANOVA followed by Tukey's post-hoc test. *P<0.05
compared with the PANC-1 or PANC1-Exos group. p-, phosphorylated;
Exos, exosomes; VNN1, Vanin-1; AMPK, AMP-activated protein kinase;
PC, pancreatic cancer; PV, PANC-1 cells with the stable
overexpression of VNN1; PE, PANC-1 cells transfected with empty
vector. |
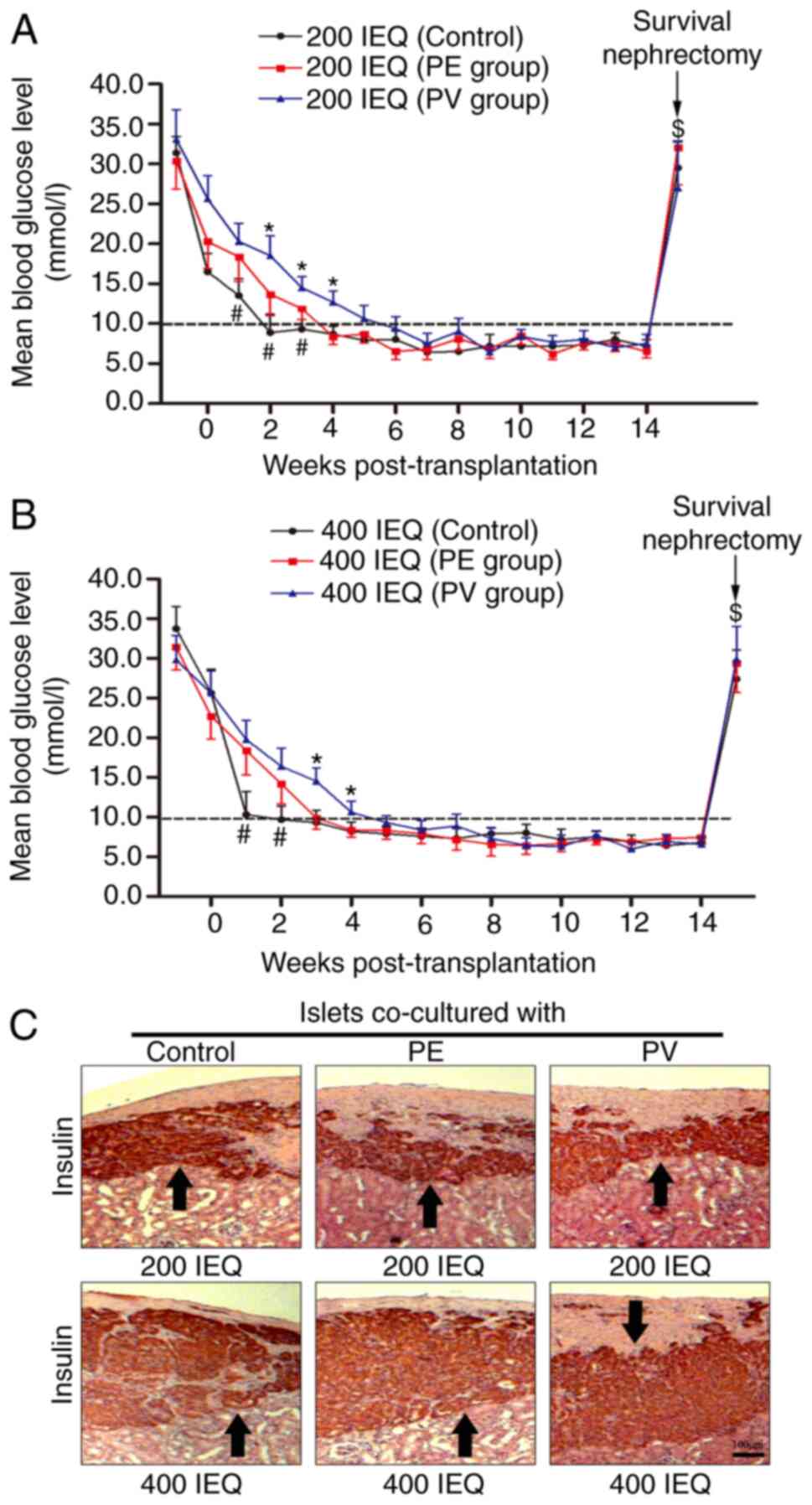
Co-culture with PV cells aggravates
the dysfunction of islets transplanted under the kidney capsule of
diabetic mice
Islets were co-cultured with PE and PV cells for 24
h, and these islets and untreated islets were then transplanted
under the kidney capsule of B6 diabetic mice. As shown in Fig. 6A, the mice that received 200 IEQ
untreated islets achieved normoglycemia at the 2nd week
post-transplantation, and the mice that received the same amount of
PE or PV cell-treated islets achieved normoglycemia at the 4th or
6th week post-transplantation, respectively. The fasting blood
glucose (FBG) levels of the untreated group were lower at the 1st
to 3rd weeks post-transplantation compared to the PE and PV groups.
The FBG levels of the PV group were higher at the 2nd to 4th weeks
post-transplantation compared to the PE group. Similar results were
observed for the mice which received 400 IEQ islets. As shown in
Fig. 6B, mice in the untreated, PE
and PV groups achieved normoglycemia at the 2nd, 3rd and 5th week
post-transplantation, respectively. The FBG levels in the untreated
group were lower at the 1st to 2nd weeks post-transplantation
compared to the PE and PV groups. The FBG levels in the PV group
were higher at the 3rd to 4th weeks post-transplantation compared
to the PE group. Following the excision of kidney containing the
transplanted islets at the 14th week, the FBG levels of all mice
rebounded significantly (Fig. 6A and
B), indicating that the transplanted islets were responsible
for maintaining normoglycemia prior to nephrectomy. The excised
kidneys were used for immunohistochemical staining, and the islets
stained positive for insulin could be observed under the kidney
capsule (Fig. 6C).
Discussion
VNN1 can regulate the oxidative stress response via
multiple pathways (9,20–22).
In addition, oxidative stress can impair the activity and function
of β-cells by damaging molecules or organelles, which leads to the
onset and development of diabetes (23–26).
In a previous study, the authors found that the high expression of
VNN1 in PC cells aggravated the oxidative stress of paraneoplastic
insulinoma cell lines by paracrine cysteamine, thereby inhibiting
the activity and function of insulinoma cells (11). Cell lines derived from tumor cells
differ from normal cells in terms of gene expression, metabolic
pathways, growth patterns, etc. Therefore, primary mouse islets
were used in the present study in order to obtain more accurate
research results. As the paracrine effect of PC cells is likely to
induce islet dysfunction (27–29),
the primary islets were co-cultured with PC cells in vitro
to simulate the paracrine effect of PC cells on paraneoplastic
islets in vivo in the present study. Consistent with the
findings of the previous study by the authors (11), the present study also found that
VNN1-overexpressing PC cells increased the ROS levels, and
decreased the GSH and PPARγ concentrations in paraneoplastic islets
by secreting cysteamine, and subsequently aggravated oxidative
stress in islets, ultimately inhibiting the viability and insulin
secretion of islets (Fig. S3).
In the present study, it was found that other
substances secreted by VNN1-overexpressing PC cells also further
inhibited the function of paraneoplastic islets in addition to
cysteamine. Some studies have indicated that PC-Exos may induce the
occurrence of PC-DM. Javeed et al (13) demonstrated that adrenomedullin in
PC-Exos inhibited the insulin secretion of β-cells by inducing
endoplasmic reticulum stress. Moreover, adrenomedullin in PC-Exos
also leads to lipolysis, which produces free fatty acids to impair
insulin secretion and induce insulin resistance (15,17).
Wang et al (14) found that
microRNAs (miRNAs/miRs; e.g., miR-883b-5p, etc.) in PC-Exos
triggered the insulin resistance of skeletal muscle cells. Zhang
et al (16) observed that
miRNAs (miR-6796-3p, etc.) in PC-Exos decreased the expression of
incretins in enteroendocrine cells, thereby inhibiting insulin
secretion and reducing insulin sensitivity. The present study found
that PC-Exos (1 or 2 µg/ml) inhibited the insulin secretion of
islets, and the inhibitory effect of VNN1-overexpressing PC-Exos
was more prominent. In addition, it was found that VNN1 could be
transferred into paraneoplastic islets via PC-Exos. However, there
was no significant difference in the cysteamine content in islets
following incubation with PC-Exos (≤2 µg/ml), regardless of whether
VNN1 was overexpressed in PC cells. This indicated that the further
inhibition of islet function by VNN1-overexpressing PC-Exos was not
dependent on cysteamine-mediated oxidative stress.
Previous studies have suggested that β-cell
apoptosis caused by oxidative stress is the primary etiology of
diabetes which results in the decrease in the number and
dysfunction of β-cells (30,31).
However, other researchers have found that the decrease in the
number and dysfunction of β-cells is not proportional to the degree
of β-cell apoptosis, indicating that other mechanisms may also
inhibit the activity and function of β-cells besides apoptosis
(32). Cell dedifferentiation
refers to the transformation of functionally mature cells into more
primitive precursor cells after losing their specific phenotype and
function. Recent research has reported that dedifferentiated
β-cells lose their ability to secrete insulin and transform into
endocrine precursor cells with pluripotent differentiation
potential, resulting in the insufficiency in quantity and function
of β-cells (33). Therefore, β-cell
dedifferentiation may be another key mechanism in the pathogenesis
of diabetes.
Chang et al (34) found that cytoplasmic GAPDH can be
phosphorylated by activated AMPK, which causes GAPDH to
redistribute into the nucleus, and nuclear-localized GAPDH then
interacts directly with Sirt1 and disassociates Sirt1 from its
inhibitor, deleted in breast cancer 1 (DBC1), thereby causing Sirt1
to be activated. Nakae et al (35) observed that activated Sirt1 could
bind directly with FoxO1 and induce the deacetylation of FoxO1,
thereby increasing the transcriptional activity of FoxO1. Some
studies have confirmed that the increased transcriptional activity
of FoxO1 can promote the expression of β-cell differentiation
markers, such as Mafa and NeuroD1, while the decreased
transcriptional activity of FoxO1 induces β-cell dedifferentiation
(36–38). The present study demonstrated that
VNN1-overexpressing PC-Exos inhibited the expression of β-cell
differentiation markers (Pdx1, Mafa and NeuroD1) in islets. It was
also found that VNN1 inhibited the phosphorylation of AMPK in
islets via PC-Exos. The present study observed that the GAPDH
content was decreased in the nucleus of islets following incubation
with VNN1-overexpressing PC-Exos, suggesting that VNN1 inhibited
the redistribution of GAPDH from the cytoplasm to the nucleus in
islets. Moreover, the present study demonstrated that
VNN1-overexpressing PC-Exos inhibited the binding of Sirt1 with
GAPDH and FoxO1 in islets. As previously demonstrated, three lysine
sites (Lys242, Lys245 and Lys262) of FoxO1 can be acetylated by
lysine acetylation transferases, resulting in the acetylation of
FoxO1 (39). The present study
found that the acetylated lysine level of FoxO1 in islets was
upregulated following incubation with VNN1-overexpressing PC-Exos,
suggesting that VNN1 in PC-Exos may promote the acetylation of
FoxO1. In other words, VNN1 inhibited FoxO1 deacetylation in
islets.
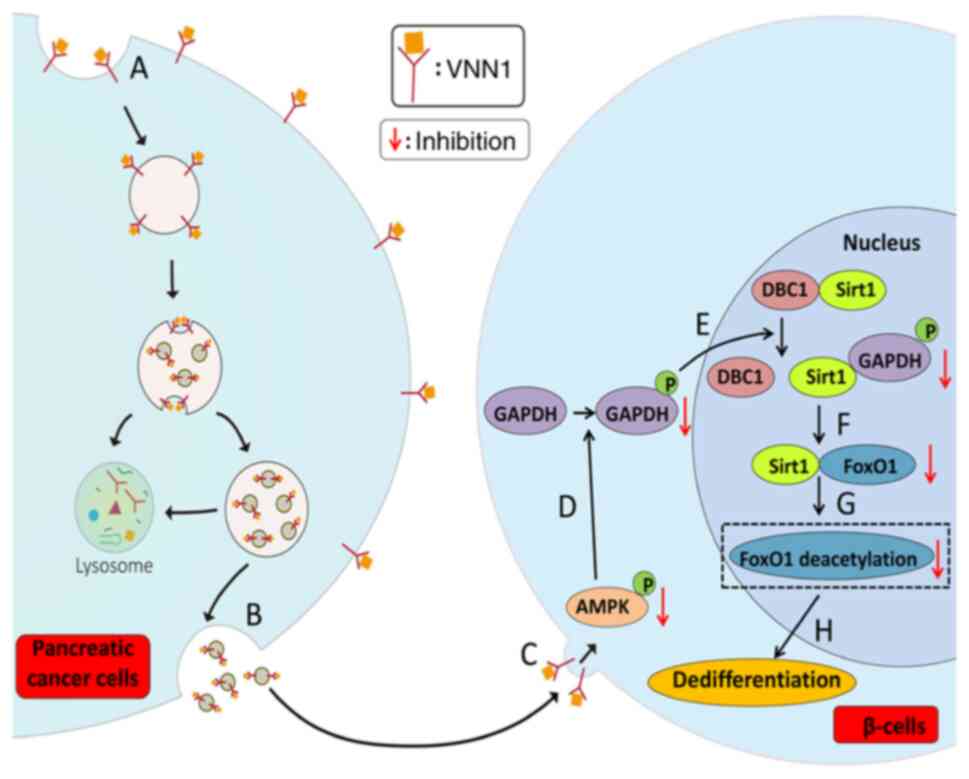
As summarized in Fig.
7, the possible mechanisms responsible for the
dedifferentiation of paraneoplastic β-cells by VNN1-overexpressing
PC-Exos are the following: i) VNN1 located in the PC cell membrane
is acquired and enriched by PC-Exos; ii) PC-Exos are captured by
paraneoplastic β-cells, and enriched VNN1 is transferred into
β-cells; iii) VNN1 inhibits the phosphorylation of AMPK, thereby
blocking the phosphorylation of GAPDH; iv) GAPDH is prevented from
moving into the nucleus and binding with Sirt1, thereby limiting
the disassociation of Sirt1 from its inhibitor, DBC1, resulting in
a decrease in Sirt1 activity; v) inactivated Sirt1 cannot interact
with FoxO1 and thus FoxO1 cannot be deacetylated, causing a
decrease in the transcriptional activity of FoxO1; vi) β-cell
dedifferentiation is initiated thereof.
A previous study reported that the blood glucose
levels of SCID mice were evidently increased after a continuous
intraperitoneal injection of conditioned medium of PC cells for 10
days (29). In addition, in another
study, the blood glucose levels of athymic nude mice were
significantly increased after an injection of PC cells
subcutaneously and orthotopically at 4 weeks (40). However, these studies have not
confirmed which cells are targeted by PC cells to cause
hyperglycemia in vivo. In vitro experiments have
demonstrated that the secretions of PC cells can impair the
functions of β-cells, adipocytes, hepatocytes, skeletal muscle
cells and enteroendocrine cells, all of which participate in the
regulation of blood glucose in vivo (13–17,41).
In the present study, to investigate the effects of islets
following co-culture with PC cells on blood glucose regulation
in vivo and to exclude the effects of the PC cell-induced
dysfunction of other target cells on blood glucose regulation, B6
diabetic mice with islets transplanted under the kidney capsule
were used. It was found that mice transplanted with 200 or 400 IEQ
untreated islets achieved normoglycemia earlier than the mice
transplanted with islets co-cultured with PC cells, while mice
transplanted with islets treated with VNN1-overexpressing PC cells
achieved normoglycemia at the latest stage. These results indicate
that the secretions of PC cells can impair the functions of islets
in vivo, and the secretions of VNN1-overexpressing PC cells
aggravate islet dysfuntion. However, whether transplanted islets
were co-cultured with PC cells or not, all mice achieved
normoglycemia eventually, which may be caused by the following two
reasons: i) The co-culture time of islets with PC cells was brief,
and the secretions of PC cells could not function continuously on
transplanted islets; ii) the quantity of transplanted islets was
sufficient.
Previous reports have demonstrated that VNN1
overexpression in colorectal or adrenocortical cancer promotes
tumor progression and is associated with a poor prognosis (42–44).
However, the effects of VNN1 on PC progression remain unknown. In
the present study, it was found that VNN1 inhibited the
phosphorylation of AMPK in PC cells. As activated AMPK can suppress
the invasion and migration of PC cells (45,46),
it was hypothesized that VNN1 may promote PC progression by
inhibiting AMPK signaling pathway. Therefore, it may be noteworthy
to investigate the role of VNN1 in PC progression and develop
potential VNN1-targeted therapies for PC in the future.
In conclusion, the present study re-affirmed that
VNN1 in PC cells aggravated oxidative stress in paraneoplastic
islets by secreting cysteamine, thereby inhibiting the activity and
function of islets. In addition, it was found that VNN1 was
transferred into islets via PC-Exos and inhibited the
AMPK/GAPDH/Sirt1/FoxO1 signaling pathway, resulting in β-cell
dedifferentiation. Furthermore, a renal subcapsular islet
transplantation model was used to demonstrate that the secretions
of VNN1-overexpressing PC cells aggravated islet dysfunction in
vivo. Therefore, the present study further explored the
mechanisms of VNN1-induced PCAD and provide new evidence that VNN1
can be used as a specific biomarker for early PCAD screening.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the National
Natural Science Foundation of China (nos. 81301889 and 81700682),
the Natural Science Foundation of Zhejiang Province (no.
LY19H160042) and the Youth Innovation Fund Project of the First
Affiliated Hospital of Zhengzhou University (no. 215709).
Availability of data and materials
The datasets used and/or analyzed during this study
are available from the corresponding author on reasonable
request.
Authors' contributions
WQ and MK designed the study. WQ, MK and CL
performed the experiments. WQ, MK, CL, WZ and QG analyzed the
statistical data, and assembled and installed the figures. WQ and
MK supervised the study and confirm the authenticity of all the raw
data. WQ wrote the manuscript. All authors have read and approved
the final version of the manuscript.
Ethics approval and consent to
participate
The animal experiments were approved by the Ethics
Committee of The First Affiliated Hospital of Zhengzhou University
(Ethics no. 2018-03-003; Zhengzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
VNN1
|
Vanin-1
|
|
PCAD
|
pancreatic cancer-associated
diabetes
|
|
Exos
|
exosomes
|
|
ROS
|
reactive oxygen species
|
|
PPARγ
|
peroxisome proliferator-activated
receptor γ
|
|
GSH
|
glutathione
|
|
AMPK
|
AMP-activated protein kinase
|
|
GAPDH
|
glyceraldehyde 3-phosphate
dehydrogenase
|
|
Sirt1
|
sirtuin 1
|
|
FoxO1
|
Forkhead box protein O1
|
References
|
1
|
Cai J, Chen H, Lu M, Zhang Y, Lu B, You L,
Zhang T, Dai M and Zhao Y: Advances in the epidemiology of
pancreatic cancer: Trends, risk factors, screening, and prognosis.
Cancer Lett. 520:1–11. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sinha V, Shinde S, Saxena S, Thakur S,
Walia T, Dixit V, Tiwari AK, Vishvakarma NK, Dwivedi M and Shukla
D: A comprehensive review of diagnostic and therapeutic strategies
for the management of pancreatic cancer. Crit Rev Oncog.
25:381–404. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pannala R, Basu A, Petersen GM and Chari
ST: New-onset diabetes: A potential clue to the early diagnosis of
pancreatic cancer. Lancet Oncol. 10:88–95. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pelaez-Luna M, Takahashi N, Fletcher JG
and Chari ST: Resectability of presymptomatic pancreatic cancer and
its relationship to onset of diabetes: A retrospective review of CT
scans and fasting glucose values prior to diagnosis. Am J
Gastroenterol. 102:2157–2163. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Boursi B, Finkelman B, Giantonio BJ,
Haynes K, Rustgi AK, Rhim AD, Mamtani R and Yang YX: A clinical
prediction model to assess risk for pancreatic cancer among
patients with new-onset diabetes. Gastroenterology. 152:840–850.e3.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Aurrand-Lions M, Galland F, Bazin H,
Zakharyev VM, Imhof BA and Naquet P: Vanin-1, a novel GPI-linked
perivascular molecule involved in thymus homing. Immunity.
5:391–405. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Martin F, Malergue F, Pitari G, Philippe
JM, Philips S, Chabret C, Granjeaud S, Mattei MG, Mungall AJ,
Naquet P, et al: Vanin genes are clustered (human 6q22-24 and mouse
10A2B1) and encode isoforms of pantetheinase ectoenzymes.
Immunogenetics. 53:296–306. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pitari G, Malergue F, Martin F, Philippe
JM, Massucci MT, Chabret C, Maras B, Duprè S, Naquet P and Galland
F: Pantetheinase activity of membrane-bound Vanin-1: Lack of free
cysteamine in tissues of Vanin-1 deficient mice. FEBS Lett.
483:149–154. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dammanahalli KJ, Stevens S and Terkeltaub
R: Vanin-1 pantetheinase drives smooth muscle cell activation in
post-arterial injury neointimal hyperplasia. PLoS One.
7:e391062012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang H, Dong X, Kang MX, Xu B, Chen Y,
Zhang B, Chen J, Xie QP and Wu YL: Novel blood biomarkers of
pancreatic cancer-associated diabetes mellitus identified by
peripheral blood-based gene expression profiles. Am J
Gastroenterol. 105:1661–1669. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kang M, Qin W, Buya M, Dong X, Zheng W, Lu
W, Chen J, Guo Q and Wu Y: VNN1, a potential biomarker for
pancreatic cancer-associated new-onset diabetes, aggravates
paraneoplastic islet dysfunction by increasing oxidative stress.
Cancer Lett. 373:241–250. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Milane L, Singh A, Mattheolabakis G,
Suresh M and Amiji MM: Exosome mediated communication within the
tumor microenvironment. J Control Release. 219:278–294. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Javeed N, Sagar G, Dutta SK, Smyrk TC, Lau
JS, Bhattacharya S, Truty M, Petersen GM, Kaufman RJ, Chari ST and
Mukhopadhyay D: Pancreatic cancer-derived exosomes cause
paraneoplastic β-cell dysfunction. Clin Cancer Res. 21:1722–1733.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang L, Zhang B, Zheng W, Kang M, Chen Q,
Qin W, Li C, Zhang Y, Shao Y and Wu Y: Exosomes derived from
pancreatic cancer cells induce insulin resistance in C2C12 myotube
cells through the PI3K/Akt/FoxO1 pathway. Sci Rep. 7:53842017.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sagar G, Sah RP, Javeed N, Dutta SK, Smyrk
TC, Lau JS, Giorgadze N, Tchkonia T, Kirkland JL, Chari ST and
Mukhopadhyay D: Pathogenesis of pancreatic cancer exosome-induced
lipolysis in adipose tissue. Gut. 65:1165–1174. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang Y, Huang S, Li P, Chen Q, Li Y, Zhou
Y, Wang L, Kang M, Zhang B, Yang B, et al: Pancreatic
cancer-derived exosomes suppress the production of GIP and GLP-1
from STC-1cells in vitro by down-regulating the PCSK1/3. Cancer
Lett. 431:190–200. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sah RP, Nagpal SJ, Mukhopadhyay D and
Chari ST: New insights into pancreatic cancer-induced
paraneoplastic diabetes. Nat Rev Gastroenterol Hepatol. 10:423–433.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cai H, Yang B, Xu Z, Zhang B, Xu B, Li X,
Wu P, Chen K, Rajotte RV, Wu Y and Rayat GR: Cyanidin-3-O-glucoside
enhanced the function of syngeneic mouse islets transplanted under
the kidney capsule or into the portal vein. Transplantation.
99:508–514. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rayat GR, Gazda LS, Hawthorne WJ, Hering
BJ, Hosking P, Matsumoto S and Rajotte RV: First update of the
international xenotransplantation association consensus statement
on conditions for undertaking clinical trials of porcine islet
products in type 1 diabetes-chapter 3: Porcine islet product
manufacturing and release testing criteria. Xenotransplantation.
23:38–45. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Berruyer C, Martin FM, Castellano R,
Macone A, Malergue F, Garrido-Urbani S, Millet V, Imbert J, Duprè
S, Pitari G, et al: Vanin-1-/- mice exhibit a glutathione-mediated
tissue resistance to oxidative stress. Mol Cell Biol. 24:7214–7224.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang B, Lo C, Shen L, Sood R, Jones C,
Cusmano-Ozog K, Park-Snyder S, Wong W, Jeng M, Cowan T, et al: The
role of vanin-1 and oxidative stress-related pathways in
distinguishing acute and chronic pediatric ITP. Blood.
117:4569–4579. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Berruyer C, Pouyet L, Millet V, Martin FM,
LeGoffic A, Canonici A, Garcia S, Bagnis C, Naquet P and Galland F:
Vanin-1 licenses inflammatory mediator production by gut epithelial
cells and controls colitis by antagonizing peroxisome
proliferator-activated receptor gamma activity. J Exp Med.
203:2817–2827. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ryu S, Ornoy A, Samuni A, Zangen S and
Kohen R: Oxidative stress in Cohen diabetic rat model by
high-sucrose, low-copper diet: Inducing pancreatic damage and
diabetes. Metabolism. 57:1253–1261. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Robertson RP, Harmon J, Tran PO, Tanaka Y
and Takahashi H: Glucose toxicity in beta-cells: Type 2 diabetes,
good radicals gone bad, and the glutathione connection. Diabetes.
52:581–587. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Piro S, Anello M, Di Pietro C, Lizzio MN,
Patanè G, Rabuazzo AM, Vigneri R, Purrello M and Purrello F:
Chronic exposure to free fatty acids or high glucose induces
apoptosis in rat pancreatic islets: Possible role of oxidative
stress. Metabolism. 51:1340–1347. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang M, Veeraperumal S, Zhong S and Cheong
KL: Fucoidan-derived functional oligosaccharides: Recent
developments, preparation, and potential applications. Foods.
12:8782023. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pezzilli R and Pagano N: Is diabetes
mellitus a risk factor for pancreatic cancer? World J
Gastroenterol. 19:4861–4866. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang F, Larsson J, Adrian TE, Gasslander T
and Permert J: In vitro influences between pancreatic
adenocarcinoma cells and pancreatic islets. J Surg Res. 79:13–19.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Basso D, Brigato L, Veronesi A, Panozzo
MP, Amadori A and Plebani M: The pancreatic cancer cell line MIA
PaCa2 produces one or more factors able to induce hyperglycemia in
SCID mice. Anticancer Res. 15:2585–2588. 1995.PubMed/NCBI
|
|
30
|
Bonnefont-Rousselot D, Bastard JP, Jaudon
MC and Delattre J: Consequences of the diabetic status on the
oxidant/antioxidant balance. Diabetes Metab. 26:163–176.
2000.PubMed/NCBI
|
|
31
|
Kaneto H, Katakami N, Kawamori D,
Miyatsuka T, Sakamoto K, Matsuoka TA, Matsuhisa M and Yamasaki Y:
Involvement of oxidative stress in the pathogenesis of diabetes.
Antioxid Redox Signal. 9:355–366. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Butler PC, Meier JJ, Butler AE and Bhushan
A: The replication of beta cells in normal physiology, in disease
and for therapy. Nat Clin Pract Endocrinol Metab. 3:758–768. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bensellam M, Jonas JC and Laybutt DR:
Mechanisms of β-cell dedifferentiation in diabetes: Recent findings
and future research directions. J Endocrinol. 236:R109–R143. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chang C, Su H, Zhang D, Wang Y, Shen Q,
Liu B, Huang R, Zhou T, Peng C, Wong CC, et al: AMPK-dependent
phosphorylation of GAPDH triggers Sirt1 activation and is necessary
for autophagy upon glucose starvation. Mol Cell. 60:930–940. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nakae J, Cao Y, Daitoku H, Fukamizu A,
Ogawa W, Yano Y and Hayashi Y: The LXXLL motif of murine forkhead
transcription factor FoxO1 mediates Sirt1-dependent transcriptional
activity. J Clin Invest. 116:2473–2483. 2006.PubMed/NCBI
|
|
36
|
Buteau J and Accili D: Regulation of
pancreatic beta-cell function by the forkhead protein FoxO1.
Diabetes Obes Metab. 9 Suppl 2:140–146. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kitamura YI, Kitamura T, Kruse JP, Raum
JC, Stein R, Gu W and Accili D: FoxO1 protects against pancreatic
beta cell failure through NeuroD and MafA induction. Cell Metab.
2:153–163. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Talchai C, Xuan S, Lin HV, Sussel L and
Accili D: Pancreatic β cell dedifferentiation as a mechanism of
diabetic β cell failure. Cell. 150:1223–1234. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Matsuzaki H, Daitoku H, Hatta M, Aoyama H,
Yoshimochi K and Fukamizu A: Acetylation of Foxo1 alters its
DNA-binding ability and sensitivity to phosphorylation. Proc Natl
Acad Sci USA. 102:11278–11283. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Aggarwal G, Ramachandran V, Javeed N,
Arumugam T, Dutta S, Klee GG, Klee EW, Smyrk TC, Bamlet W, Han JJ,
et al: Adrenomedullin is up-regulated in patients with pancreatic
cancer and causes insulin resistance in β cells and mice.
Gastroenterology. 143:1510–1517.e1. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Valerio A, Basso D, Brigato L, Ceolotto G,
Baldo G, Tiengo A and Plebani M: Glucose metabolic alterations in
isolated and perfused rat hepatocytes induced by pancreatic cancer
conditioned medium: A low molecular weight factor possibly
involved. Biochem Biophys Res Commun. 257:622–628. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chai CY, Zhang Y, Song J, Lin SC, Sun S
and Chang IW: VNN1 overexpression is associated with poor response
to preoperative chemoradiotherapy and adverse prognosis in patients
with rectal cancers. Am J Transl Res. 8:4455–4463. 2016.PubMed/NCBI
|
|
43
|
Zhang L, Li L, Gao G, Wei G, Zheng Y, Wang
C, Gao N, Zhao Y, Deng J, Chen H, et al: Elevation of GPRC5A
expression in colorectal cancer promotes tumor progression through
VNN-1 induced oxidative stress. Int J Cancer. 140:2734–2747. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Latre de Late P, El Wakil A, Jarjat M, de
Krijger RR, Heckert LL, Naquet P and Lalli E: Vanin-1 inactivation
antagonizes the development of adrenocortical neoplasia in Sf-1
transgenic mice. Endocrinology. 155:2349–2354. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wang C, Huang B, Sun L, Wang X, Zhou B,
Tang H and Geng W: MK8722, an AMPK activator, inhibiting carcinoma
proliferation, invasion and migration in human pancreatic cancer
cells. Biomed Pharmacother. 144:1123252021. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Park TH and Kim HS: Eupatilin suppresses
pancreatic cancer cells via glucose uptake inhibition, AMPK
activation, and cell cycle arrest. Anticancer Res. 42:483–491.
2022. View Article : Google Scholar : PubMed/NCBI
|