Introduction
Esophageal cancer is a common malignant digestive
tumor, ranking only second to gastric cancer in incidence (1). Globally, ~450,000 people are affected
by esophageal cancer each year and this incidence is growing in
2018 (2). Esophageal squamous cell
carcinoma (ESCC) is a major histopathological subtype of esophageal
cancer with high prevalence in China (2). Even though the clinical diagnosis and
therapeutic strategies have improved over the past two decades, the
5-year survival rate of ESCC patients remains unfavorable.
Therefore, novel therapeutic modalities are urgently needed to
improve prognosis of ESCC.
Substance P (SP) is a member of the tachykinin
family, and is an extensively distributed neurotransmitter. After
specifically binding to the neurokinin-1 receptor (NK1R), SP
performs multiple functions in cells. Importantly, the potential
mitogenic role of SP has been found in several different cancer
types (3–5). NK1R has 2 subtypes, full length-NK1R
(fl-NK1R) and truncated-NK1RT (tr-NK1R). The fl-NK1R has 407 amino
acids, while the tr-NK1R subtype only has 311 amino acids, with
this deficiency located at the C-terminus (6). Tr-Nk1R can also bind to G proteins but
is less efficient than fl-NK1R in internalization and
desensitization (7). Tr-NK1R is
highly overexpressed in hepatoblastoma, whereas fl-NK1R is
expressed in negligible quantities (8). The SP/NK1R complex is an essential
component of cancer cells and tumor microenvironment, and plays an
oncogenic role in cell proliferation, migration and angiogenesis in
hepatoblastoma and gallbladder cancer (8,9).
Therefore, the application of the NK1R antagonist as an innovative
anticancer drug warrants further study.
NK1R antagonists can be divided into peptide and
non-peptide types. Aprepitant is a member of the non-peptide group
that can cross the blood-brain barrier. It is mainly used to
prevent acute and delayed nausea and vomiting during the initial
and succeeding cycles treatment of antitumor chemotherapy (10,11).
Importantly, only mild, transient, and tolerable side effects and
no significant toxic side effects have been observed thus far for
this drug, even in high doses (12).
The present study aimed to investigate the functions
and therapeutic potential of the r-NK1R complex in human ESCC
progression. It was hypothesized that aprepitant could inhibit the
progression of ESCC by competitively binding tr-NK1R with SP.
Towards this goal, SP and tr-NK1R expression was detected in ESCC
cell lines and specimens, and the functions and molecular
mechanisms of the NK1R antagonist aprepitant in ESCC were
investigated.
Materials and methods
Patient's samples
ESCC tissue and corresponding normal esophageal
tissue samples were collected from 25 patients (17 men and 8 women;
age range, 50–72 years) with ESCC who underwent esophagectomy
surgery in the Fourth Hospital of Hebei Medical University
(Shijiazhuang, China) between January 2019 and April 2019. In
addition, 84 samples (61 men and 23 women; age range, 48–80 years)
of ESCC tissues were also collected from patients with ESCC who
underwent esophagectomy surgery in the Fourth Hospital of Hebei
Medical University between October 2016 and January 2017. All
patients did not undergo preoperative adjuvant chemotherapy and
radiotherapy. However, patients received radiotherapy or
chemotherapy postoperatively, which may have had an impact on
survival.
The present study was approved (approval no.
2020KY227) by the Medical Ethics Committee of the Fourth Hospital
of Hebei Medical University (Shijiazhuang, China) and was conducted
according to the tents of the Helsinki Declaration (seventh
revision, 2013). Written informed consent was provided by all
patients.
Cell culture
The human ESCC cell lines TE1, KYSE-150, and
KYSE-170 were cultured in Dulbecco's Modified Eagle Medium (DMEM
Gibco; Thermo Fisher Scientific, Inc.) containing 10% fetal calf
serum and 1% penicillin/streptomycin. Cells were cultured at 37°C
in a water-saturated atmosphere of 5% CO2 in air. Human
fibroblasts (Human fetal lung fibroblast, HLF1, cat. no. CL-0106)
were purchased from Procell Life Science & Technology Co.,
Ltd., and cultured in Ham's F-12K (Gibco; Thermo Fisher Scientific,
Inc.) containing 10% fetal calf serum and 1%
penicillin/streptomycin.
Drugs
The PI3K inhibitor, Pictilisib (cat. no. HY-50094)
and AKT inhibitor, Capivasertib (cat. no. HY-15431) were purchased
from MedChemExpress.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the cells or tissues by
using a TRIzol® solution (Promega Corporation). Reverse
transcription reactions were performed with reverse transcriptase
(Go Script Reverse Transcription; Promega Corporation). RT-qPCR was
conducted by using an SYBR Green PCR Kit (Promega Corporation) with
a real-time PCR System (ABI 7500). The thermocycling conditions of
qPCR were as follows: Initial denaturation, 70°C for 5 min;
annealing, 25°C for 5 min; extension, 42°C for 60 min; and
denaturation, 70°C for 15 min. The gene-specific RT-qPCR primers
were as follows: fl-NK1R (specific primers were designed for
>NM_001058.4; forward, 5′-GTTCCGTCTGGGCTTCAA-3′ and reverse,
5′-CCAGGCGGCTGACTTTGT-3′); tr-NK1R (specific primers were designed
for >NM_015727.3; forward, 5′-GGGCCACAAGACCATCTACA-3′ and
reverse, 5′-AAGTTAGCTGCAGTCCCCAC-3′); Arg-1 forward,
5′-GCAAGGTGATGGAAGAA-3′ and reverse, 5′-CTGGTGTGAAAGATGGGT-3′;
CD206 forward, 5′-CGTGTGCACCTACCTCAAGA-3′ and reverse,
5′-AAGGACAGACCAGTACAATTCAGT-3′; IL-10 forward,
5′-GGAGAACCTGAAGACCCT-3′ and reverse, 5′-GGCTTTGTAGATGCCTTTC-3′;
CCL22 forward, 5′-GCCTACTCTGATGACCGTGG-3′ and reverse,
5′-AGAGAGTTGGCACAGGCTTC-3′; Class A macrophage scavenger receptor
(SR) forward, 5′-GCAGGGCCCTCTTAAGATCA-3′ and reverse,
5′-AACACGGGAACCAAAGTCAT-3′; and GAPDH forward,
5′-AGAAGGCTGGGGCTCATTTG-3′ and reverse,
5′-GCAGGAGGCATTGCTGATGAT-3′. RNA expression levels were normalized
to GAPDH expression levels. Relative expression levels were
calculated using the 2−∆∆Cq method (13).
Western blot analysis
The total protein was prepared by using RIPA buffer
(Beyotime Institute of Biotechnology). Protein concentrations were
detected by using a BCA protein assay kit (Thermo Fisher
Scientific, Inc.). Protein (40 µg/lane) was separated by
electrophoresis on 6–15% SDS-polyacrylamide gels and transferred
onto polyvinylidene fluoride membranes. Membranes were cut and
incubated at 37°C in 5% BSA (Beijing Solarbio Science &
Technology Co., Ltd.) for 1 h, and then incubated at 4°C overnight
with primary antibodies at a 1:1,000 dilution. The primary
antibodies against cleaved Caspase-3 (cat. no. 19677-1-AP), cleaved
poly (ADP-ribose) polymerase (PARP, cat. no. 13371-1-AP) and
β-actin (cat. no. 20536-1-AP) were purchased from Proteintech
Group, Inc. Meanwhile, PI3k-p110α (cat. no. 4255), total AKT (cat.
no. 9272), phospho-AKT (cat. no. 4060), total mTOR (cat. no. 2983),
phospho-mTOR (cat. no. 5536), total 4EBP1 (cat. no. 9644),
phospho-4EBP1 (cat. no. 9451), total p70S6K (cat. no. 2708) and
phospho-p70S6K (cat. no. 9234) were purchased from Cell Signaling
Technology, Inc. Membranes were then washed with tris-buffered
saline Tween (1% Tween-20) and incubated with secondary
HRP-conjugated antibodies (1:10,000) for 2 h at room temperature.
The secondary antibodies were purchased from Proteintech Group, Inc
(cat. no. PR30012 and PR30012). The antibody was detected by
enhanced chemiluminescence reaction, visualization was performed
using an ECL kit (Thermo Fisher Scientific, Inc.). ImageJ software
(version 1.8.0_172; National Institutes of Health) was used to
analyze the gray value of the western blot.
To distinguish between the tr-NK1R and fl-NK1R on
western blot analysis, two specific antibodies were used to detect
the C- and N-terminus: one antibody bound to an epitope at the
N-terminus (cat. no. NB300-119; Novus Biologicals, LLC), and one
antibody bound to an epitope at the C-terminus (cat. no. S8305;
Sigma-Aldrich; Merck KGaA).
Cell Counting Kit-8 (CCK-8) assay
Cell proliferation was evaluated by using the Cell
Counting Kit-8 (Dojindo Molecular Technologies, Inc.). First, the
cell suspension was inoculated (100 µl/well) in a 96-well plate,
and the plate was incubated in a humidified incubator. Second, 10
µl of the CCK-8 solution was added to each well of the plate, and
the plate was incubated at 37°C for 1.5 h in the incubator.
Finally, the absorbance was measured at 450 nm by using a
microplate reader.
Transwell migration and invasion
assays
Transwell migration assay was performed by using
chambers (24-well insert, Corning, Inc.) with 8-µm pore size
polycarbonate filters coated without Matrigel (Beyotime Institute
of Biotechnology) on the upper side. Meanwhile, the invasion assay
was conducted by using the chambers with Matrigel on the upper
side. Transwell membranes were precoated with Matrigel for 1 h at
37°C. The chambers were placed into a 24-well plate, and the lower
chamber was filled with DMEM containing 20% fetal bovine serum
(FBS, Gibco; Thermo Fisher Scientific, Inc.). The ESCC cell
suspension was inoculated (3×104/well) in the upper
chamber with and without aprepitant, and the lower chamber was
filled with DMEM containing 20% FBS. The 24-well plate was then
incubated at 37°C for 24 h. The cells that passed through the
filter and attached to the lower compartment of the filter were
identified by crystal violet staining. The number of transmembrane
cells in different groups was counted using a light microscope.
Wound healing experiments
The cells (1×106) were inoculated in a
six-well plate. After 12 h, when the cell fusion rate reached ~90%,
the cells were lined with a pipette tip at the same angle and
force. To ensure the detection in the same location, the back of
the six-well plate was marked. The medium was replaced with
serum-free medium with different concentrations of aprepitant.
Phase-contrast images were recorded randomly with an inverted light
microscope at the time of wounding and after 12 and 24 h. The
experiment was repeated 3 times to ensure the accuracy of the
results.
Analysis of apoptosis
Apoptosis was assessed by TUNEL staining. Briefly,
after treatment with the different concentrations of aprepitant,
the cells (3×103) were fixed in 4% paraformaldehyde for
30 min. This was followed by incubation with 0.1% Triton X-100 for
15 min. Then, 50 µl of TUNEL incubation buffer was added to every
well for 2 h. The number of apoptotic cells was recorded by
fluorescence microscopy. The number of apoptotic cells was
determined from five randomly selected fields. Apoptosis was also
monitored with Annexin V-FITC/PI staining using Cell Apoptosis kit
with Annexin V-FITC and PI (cat. no. 40302ES20; Shanghai Yeasen
Biotechnology, Co., Ltd.). According to the instructions, 5 µl of
Annexin V-FITC and 10 µl of PI staining solution were added to the
cells and gently mixed, and incubated for 10–15 min at room
temperature and protected from light. Fluorescence microscopy was
used to observe and capture images. The Annexin V-FITC fluorescence
signal is green and the PI fluorescence signal is red.
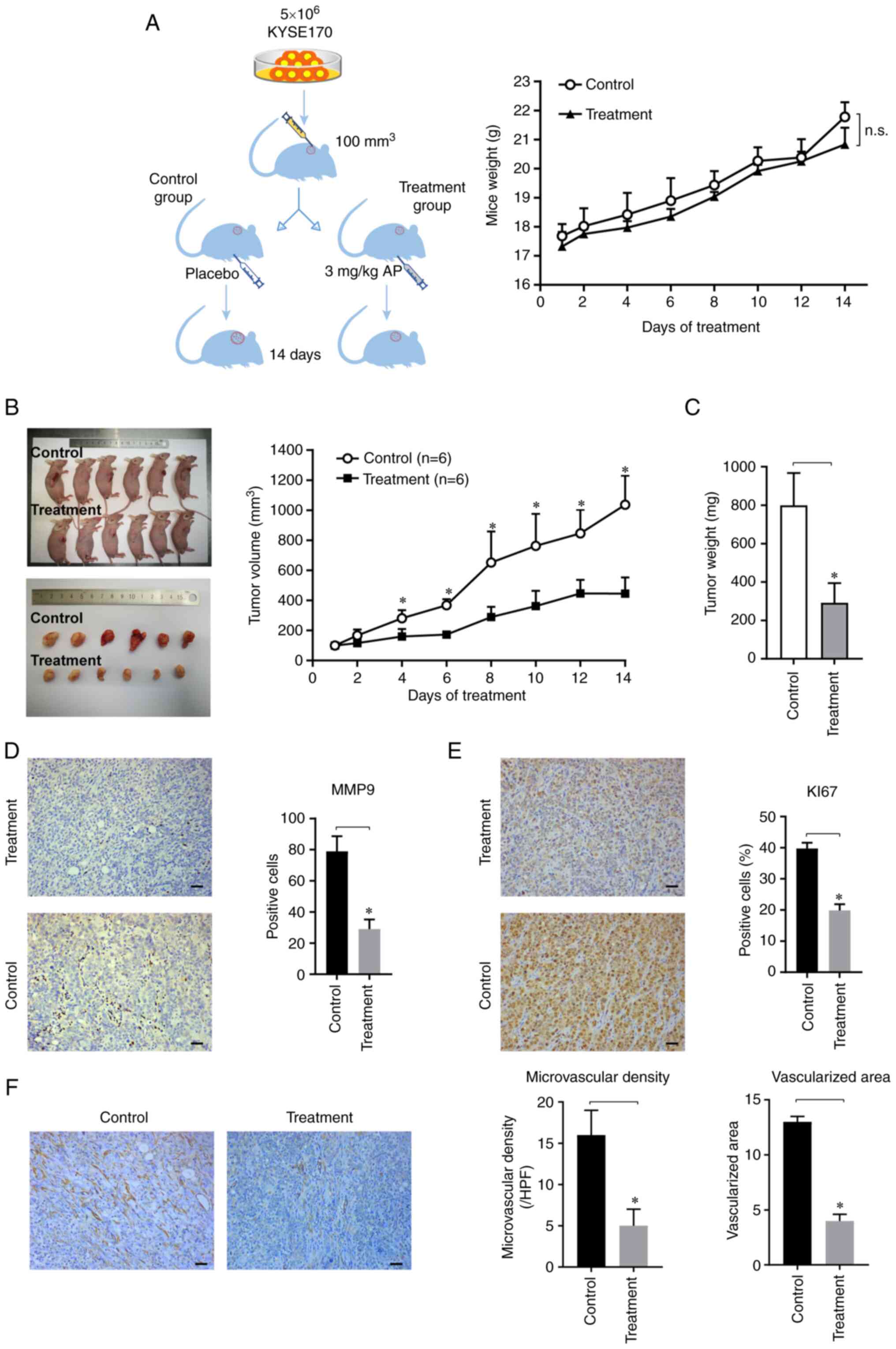
Xenograft experiments
A total of 10 four-week old male BALB/c
immunocompromised mice weighing about 20 g were obtained from the
Vital River Laboratory Animal Technology Co. Ltd., Beijing. The
animals were housed at 22–25°C, 40–60% humidity, 12/12-h dark/light
cycles, and free access to food and water. First, 5×106
KYSE-170 cells in 200 µl phosphate-buffered saline (PBS) were
injected under the skin of the right flank. Mice were weighed, and
the tumor was measured every other day. When the tumor volume
reached 100 mm3, the mice were randomly divided into two
groups. The treatment group was treated daily with an 0.3 mg/kg
aprepitant by intraperitoneal injection every other day. The
control group received 200 µl of solvent. After 2 weeks of
administration, the mice were sacrificed (Tail vein injection of
pentobarbital sodium, 200 mg/kg), and the tumor volume and weight
were measured. Tumor volume was calculated using the following
formula: mm3=0.5 × length × width2. A tissue
section was fixed at 4°C for 16 h with 4% formaldehyde for
immunohistochemical analyses, and another section was used to
extract RNA for molecular analyses.
All animal experiments were performed at the Animal
Laboratory Center of the Fourth Hospital of Hebei Medical
University. All animal experiments were approved (approval no.
20190008) by the Animal Care Committee of the Fourth Hospital of
Hebei Medical University (Shijiazhuang, China).
Immunohistochemistry
Paraffin-embedded slides (5 µM) were deparaffinized
in xylene, rehydrated using a decreasing alcohol gradient and
washed with 1X PBS three times for 5 min. The sections were then
heated in a microwave oven for 5 min in 10 mmol/l Na-citrate buffer
(pH 6.0) for antigen retrieval and washed again with 1X PBS. The
sections were immersed in 0.3% hydrogen peroxide in methanol for 20
min to suppress endogenous peroxidase activity. After further
washing with 1X PBS, the sections were incubated in 10% normal goat
serum (Proteintech Group, Inc.) at room temperature in a humidified
chamber for 30 min to prevent non-specific immunoglobulin binding.
The sections were then treated with the 1:100-diluted antibodies at
4°C overnight. The primary antibodies against CD68 (cat. no.
66231-2-Ig), CD163 (cat. no. 16646-1-AP), Ki67 (cat. no.
27309-1-AP), MMP-9 (cat. no. 10375-2-AP) and CD31 (cat. no.
11265-1-AP) were purchased from Proteintech Group, Inc. Normal IgG
(cat. no. ab172730; Abcam) instead of the primary antibody served
as the negative control. A streptavidin-biotinylated HRP-based
detection system was used to reveal specific binding. The sections
were counterstained with hematoxylin for light microscopic review
and evaluation. The expression was ranked on the sum of intensity
and area from 0 to 7: 0–2, negative expression; 3–7, positive
expression (3–4, weak positive expression; 5–7, strong positive
expression). Staining intensity was graded as follows: 0, no
staining; 1, mild staining; 2, moderate staining; and 3, intense
staining. The staining area was scored as follows: 0, no staining;
1, 1–25% area; 2, 26–50% area; 3, 51–75% area; and 4, 76–100%
area.
Immunofluorescence
The steps before blocking were the same as those
aforementioned in the Immunohistochemistry section. The sections
were incubated in 10% BSA at 37°C in a humidified chamber for 30
min to block non-specific immunoglobulin binding. The sections were
then treated with the 1:100-diluted antibodies SP (cat. no. S1542;
MilliporeSigma) and CD163 (cat. no. ab156769; Abcam) at 4°C
overnight. Fluorescein (FITC)-conjugated Affinipure Goat Anti-Mouse
IgG (H+L) (cat. no. SA00003-1) and Rhodamine (TRITC)-conjugated
Goat Anti-Rabbit IgG (H+L) (cat. no. SA00007-2) were used to detect
different fluorescence signal. Cell nuclei were stained with 0.2 mg
DAPI/ml PBS for 10 min. The sections were sealed by
anti-fluorescence quenching sealed tablets after PBS three times
washing. Then images were captured using a fluorescent
microscope.
Enzyme-linked immunosorbent assay
(ELISA)
Cell supernatants were collected for ELISA. ELISA
assays were performed in 96-well ELISA plates using an SP ELISA kit
(cat. no. ab133029; Abcam), according to the manufacturer's
instructions.
Small interfering RNA (siRNA)
transfection
TE1, KYSE-150 and KYSE-170 cells were transfected
with a double siRNA (Guangzhou RiboBio Co., Ltd.) with Hi-perfect
Transfection Reagent (Qiagen GmbH) according to the manufacturer's
instructions. The final concentration of siRNA was 50 nM. After 48
h of transfection, the knockdown gene effect was measured using
western blot analysis and RT-qPCR. Specific siRNAs were designed
for fl-NK1R derived from transcript >NM_001058.4 and tr-NK1R
derived from transcript >NM_015727.3. The sequences were as
follows: fl-NK1R sense, 5′-CCACCAUCUCCACAGUGGU-3′ and antisense,
5′-ACCACUGUGGAGAUGGUGG-3′; tr-NK1R sense, 5′-ACCCAGCUGUGAGACAAGA-3′
and antisense, 5′-UCUUGUCUCACAGCUGGGU-3′; and si-NC sense,
5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense,
5′-ACGUGACACGUUCGGAGAATT-3′.
Statistical analysis
Data were presented as the mean ± standard deviation
(SD) and compared using paired Student's t-test and Mann-Whitney U
test. The chi-square test was used to analyze the association
between protein expression and clinicopathological parameters.
Kaplan-Meier method was used for survival analysis and comparison
among groups was conducted using the log-rank test. All statistical
analyses were performed using SPSS 22.0 software (IBM Corp.).
P<0.05 was considered to indicate a statistically significant
difference.
Results
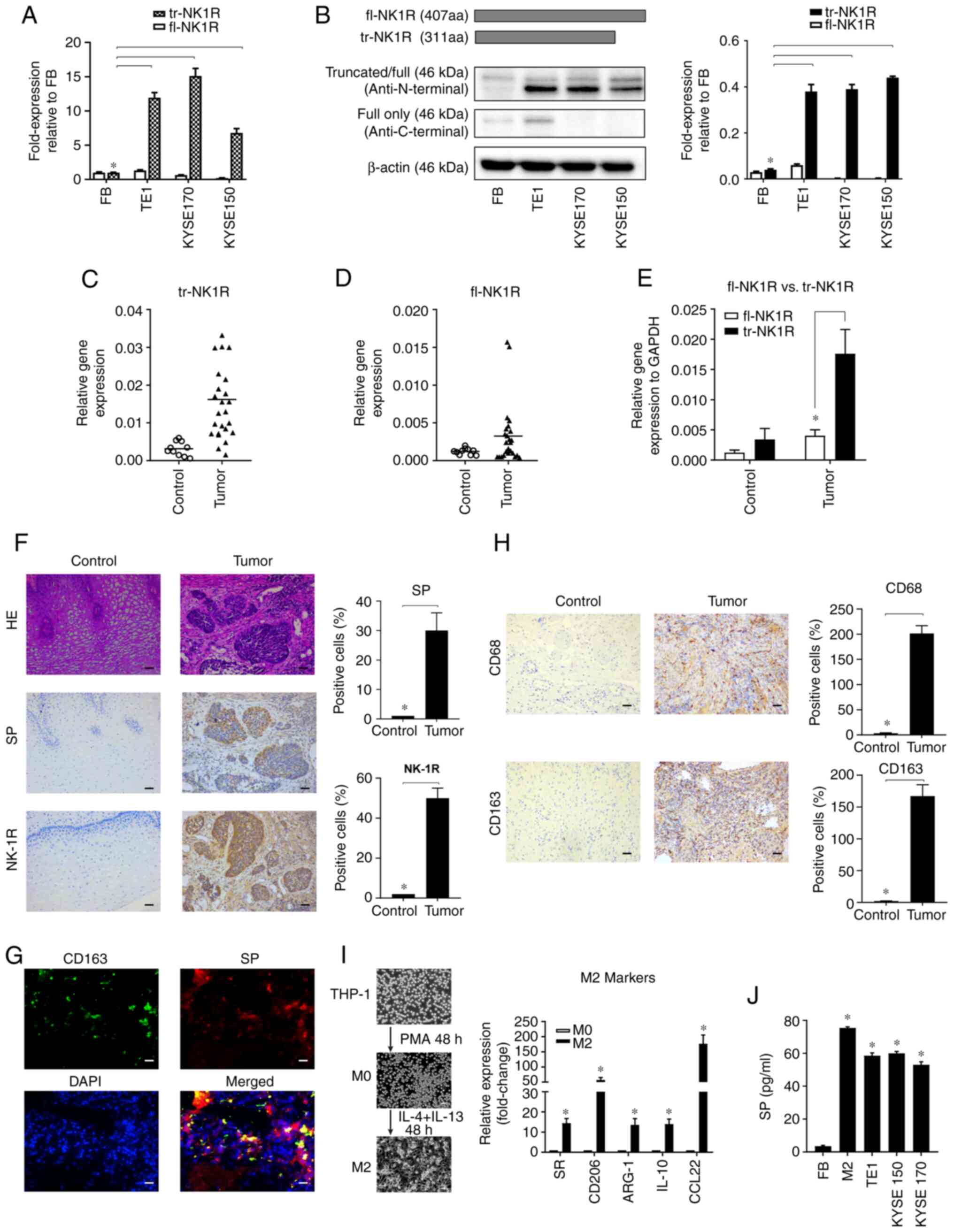
tr-NK1R is highly expressed in human
ESCC specimens
To detect the expression of NK1R in ESCC cell lines,
two sets of primers to detect two types of NK-1 receptors in ESCC
cells were first designed: fl-NK1R and tr-NK-1R. The results showed
downregulated fl-NK1R expressed in ESCC cells with human
fibroblasts as the negative control (Fig. 1A). By contrast, tr-NK-1R expression
was higher in ESCC cells than in human fibroblasts. For protein
analysis, because there was no antibody specifically manufactured
for tr-NK-1R commercially available, the C-terminus and N-terminus
of the NK1R protein was detected to distinguish the expression of
these two subtypes. The antibody used for the N-terminus can detect
both fl-NK1R and tr-NK-1R, but antibody for the C-terminus can only
detect fl-NK1R expression. As demonstrated in Fig. 1B, only tr-NK-1R was upregulated at
the protein level in ESCC cell lines, consistent with the mRNA
expression.
To further investigate the role of the SP/NK1R
system in ESCC, SP and NK1R expression was detected in 25 pairs of
ESCC and normal esophageal tissues. At the mRNA level, tr-NK1R
overexpression was found in 19 samples (Fig. 1C) and fl-NK1R was found in only 4
samples (Fig. 1D). Overall, tr-NK1R
expression was significantly higher in ESCC tissues (Fig. 1E). At the protein level, both NK1R
and SP expression were increased in the ESCC tissue compared with
normal esophageal tissue (Fig. 1F).
Collectively, these results revealed that tr-NK1R is highly
expressed in human ESCC lines and specimens.
In normal tissues, SP is mainly expressed in human
immune cells, including monocytes, macrophages, lymphocytes,
microglia, dendritic cells and bone marrow stem cells (14). Therefore, SP expression was also
detected in immune cells of ESCC tissues using immunofluorescence.
SP was also highly expressed in M2 polarized macrophages labeled
with CD163 (Fig. 1G). As the most
abundant the immune cells in the tumor microenvironment, M2
polarized macrophages play crucial roles in tumor progression
(14). The infiltration of M2
polarized macrophages labeled with CD68 and CD163 was increased in
ESCC tissues (Fig. 1H).
Furthermore, when human myeloid leukemia mononuclear THP-1 cells
were polarized into M2 macrophages with IL-4 and IL-13, SP was also
significantly increased to the similar level of ESCC cells
(Fig. 1I and J). This suggested
that ESCC cells and M2 macrophages are two important sources of SP
in ESCC tissues.
NK1R antagonist aprepitant inhibits
SP-induced proliferation of human ESCC cell lines
TE1, KYSE-150 and KYSE-170 cells were stimulated
with increasing concentrations of SP, and cell growth of the cells
after 48 h was observed. Cell growth was most pronounced at
concentrations of 10−7 M (Fig. 2A). When the NK1R antagonist
aprepitant was added to ESCC cell lines, CCK-8 proliferation assay
showed significant growth inhibition (Fig. 2B). However, compared with ESCC cell
lines, fibroblasts that expressed less NK1R, were less sensitive to
this treatment. These results suggested that human fibroblast cells
are resistant to the proliferation inhibition effect of aprepitant
due to the lower expression of tr-NK1R compared with ESCC cell
lines. However, SP blockage of the anti-SP antibody significantly
reduced the growth of these 3 cell strains (Fig. 2C), suggesting that ESCC cells can
promote their growth by autocrine secretion of SP. However, after
treatment with a sublethal concentration of aprepitant, the
addition of SP reversed the anti-proliferation effect of aprepitant
(Fig. 2D). In summary, ESCC cell
lines expressed higher tr-NK1R and were more sensitive to treatment
than human fibroblasts. This result clearly indicated that the
treatment effect of aprepitant in ESCC cells is specifically
triggered via the SP/NK1R complex, and not via drug toxicity.
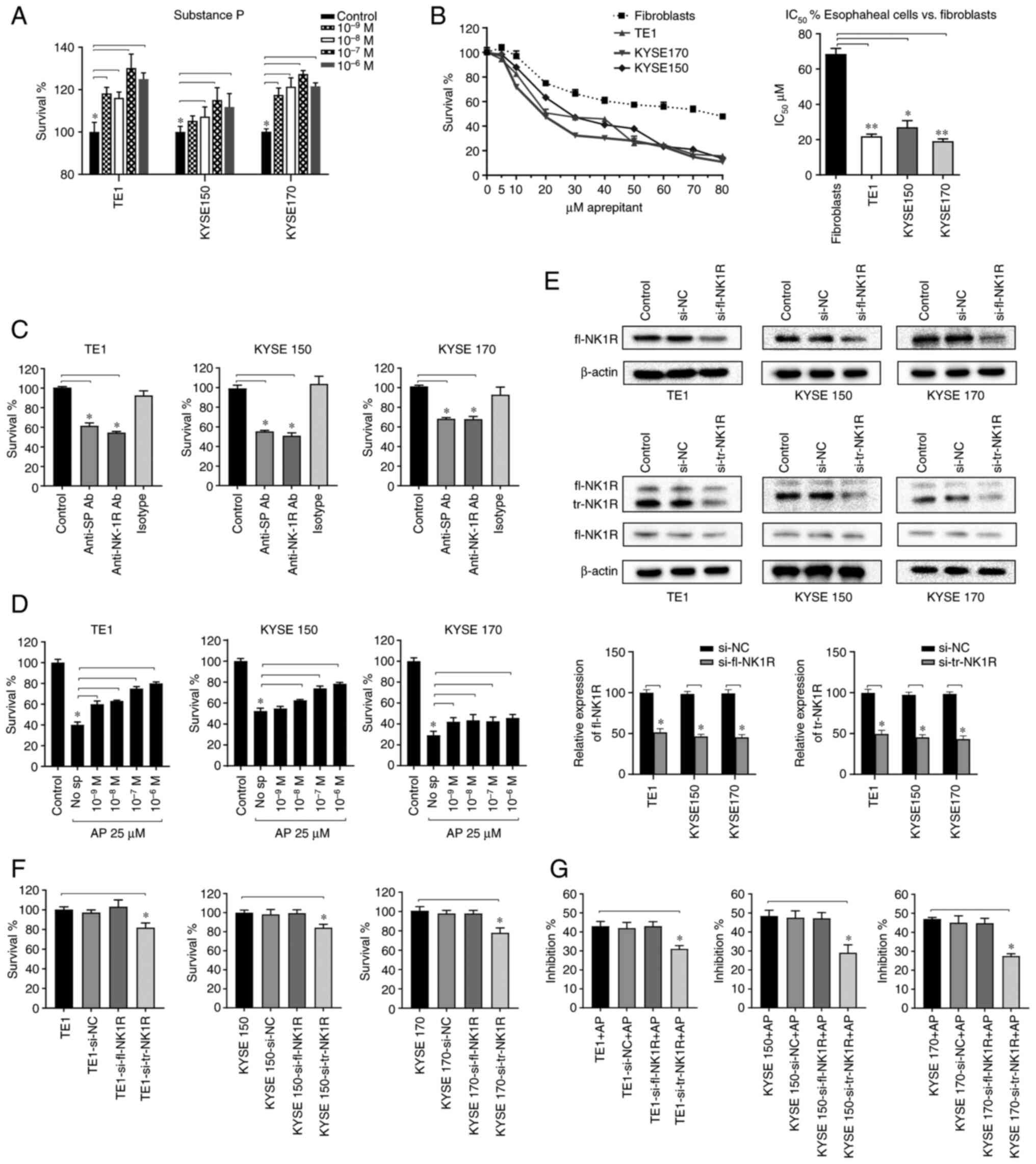 | Figure 2.AP inhibits the SP-induced
proliferation of human ESCC cell lines. (A) TE1, KYSE-150, and
KYSE-170 cells were stimulated with different concentrations of SP
to clarify its mitogenic potential in ESCC cells. (B) CCK-8 assays
determining cell survival after treatment with aprepitant for 48 h
are shown for the cell lines TE1, KYSE-150 and KYSE-170, and for
human fibroblasts. Based on these data, IC50 (µM) was
calculated and compared with fibroblasts for statistical analysis.
(C) TE1, KYSE-150 and KYSE-170 cells were treated with anti-SP,
anti-NK1R and isotype antibodies at a final concentration of 1:100.
The effects were analyzed with CCK-8 proliferation assays. (D) All
three ESCC cell lines were treated with different concentrations of
SP and 25 µM aprepitant, and survival effects were detected by
CCK-8 assay. (E) The knockdown efficiency of fl-NK1R and tr-NK1R
detected by western blot analysis in TE1, KYSE-150 and KYSE-170
cells. (F) The proliferation of TE1, KYSE-150 and KYSE170 cells,
respectively, after fl-NK1R and tr-NK1R were knocked down. (G)
After fl-NK1R and tr-NK1R were knocked down respectively in TE1,
KYSE-150 and KYSE-170 cells, IC50 of aprepitant was
applied to observe the inhibitory rate. *P<0.05 and **P<0.01.
AP, aprepitant; SP, substance P; ESCC, esophageal squamous cell
carcinoma; CCK-8, Cell Counting Kit-8; IC50, half
maximal inhibitory concentration; NK1R, neurokinin-1 receptor; fl,
full length; tr, truncated; si-, small interfering; NC, negative
control. |
To further explore the role of different subtypes of
NK1R, fl-NK1R and tr-NK1R levels were decreased by transfection
with specific siRNA in TE1, KYSE-150 and KYSE-170 cells. Si-tr-NK1R
downregulated both tr-NK1R and fl-NK1R. However, tr-NK1R decreased
significantly, while fl-NK1R decreased slightly, and tr-NK1R was
substantially more expressed than fl-NK1R in ESCC. It is considered
that si-tr-NK1R still acts mainly on tr-NK1R, and the western blot
results supported this (Fig. 2E).
Compared with the untreated ESCC cells, downregulated tr-NK1R
expression suppressed proliferation, while downregulated of fl-NK1R
expression did not alter cell proliferation (Fig. 2F). When sublethal dose of aprepitant
was applied to transfected ESCC cells, downregulated tr-NK1R
expression attenuated the inhibition rate of aprepitant in all
three ESCC cell lines (Fig. 2G).
These results suggested that tr-NK1R, not fl-NK1R, plays a major
role in ESCC proliferation. When tr-NK1R was downregulated, the
effect of aprepitant was weakened, indicating that aprepitant
mainly played an anti-proliferative role by binding to tr-NK1R.
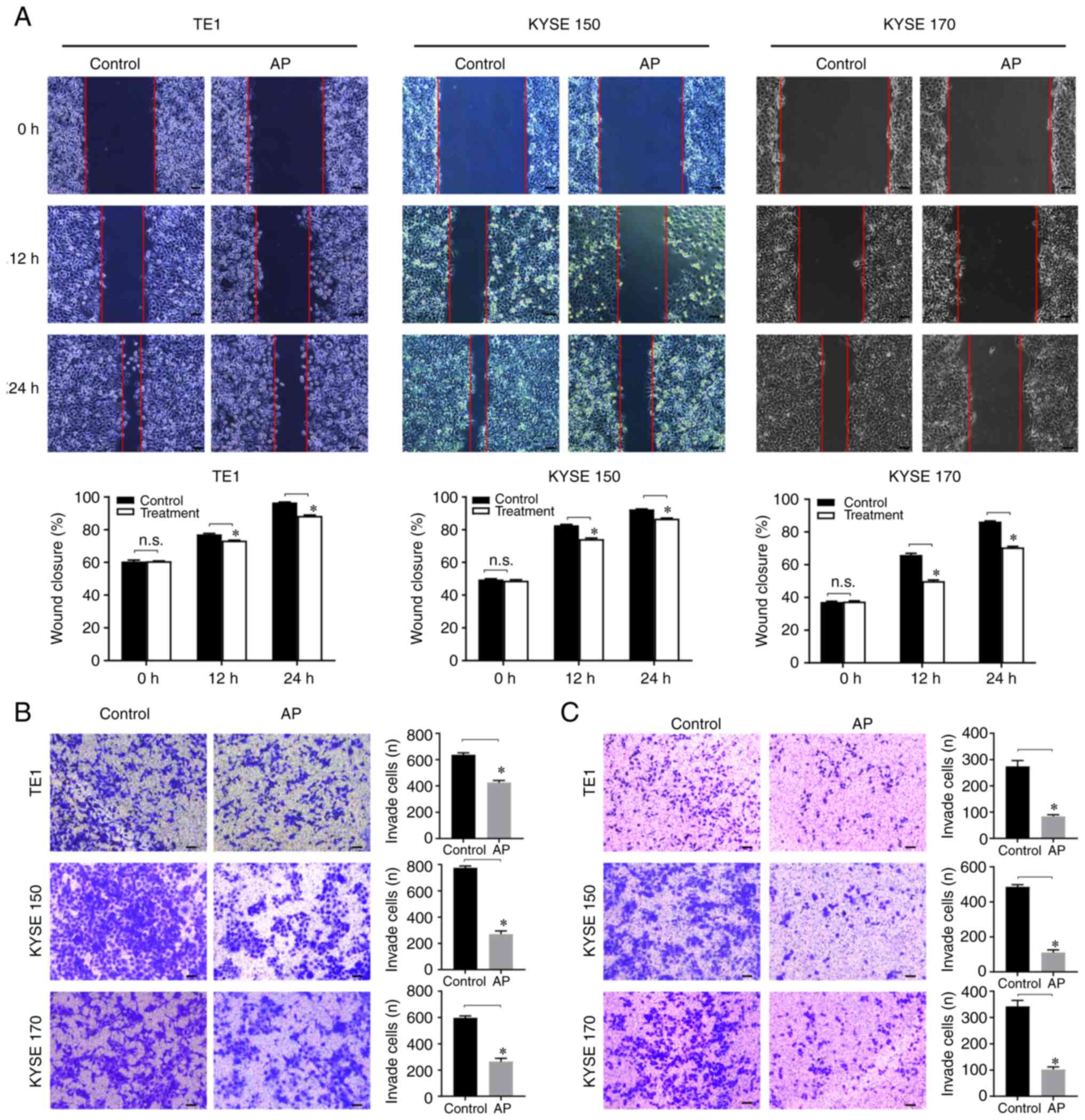
Aprepitant inhibits migration and
invasion in human ESCC cells
To clarify the effect of aprepitant on cell
migration and invasion, wound healing and Transwell assays were
used to detect the migration and invasion of ESCC cell lines in
vitro. In the wound healing assay, the scratch healing rate
significantly declined in the aprepitant group (Fig. 3A), indicating that aprepitant
inhibited the migration in ESCC cells. In the Transwell migration
assay, the number of cells passing through the basement membrane of
the chamber was observed under an inverted microscope. Compared
with the control group, the aprepitant treatment group showed a
significantly lower number of migratory cells in all three
esophageal cancer cell lines (Fig.
3B). For the invasion assay, the number of cells that
transferred to the Matrigel and reached the lower surface was
significantly reduced after treatment with aprepitant (Fig. 3C).
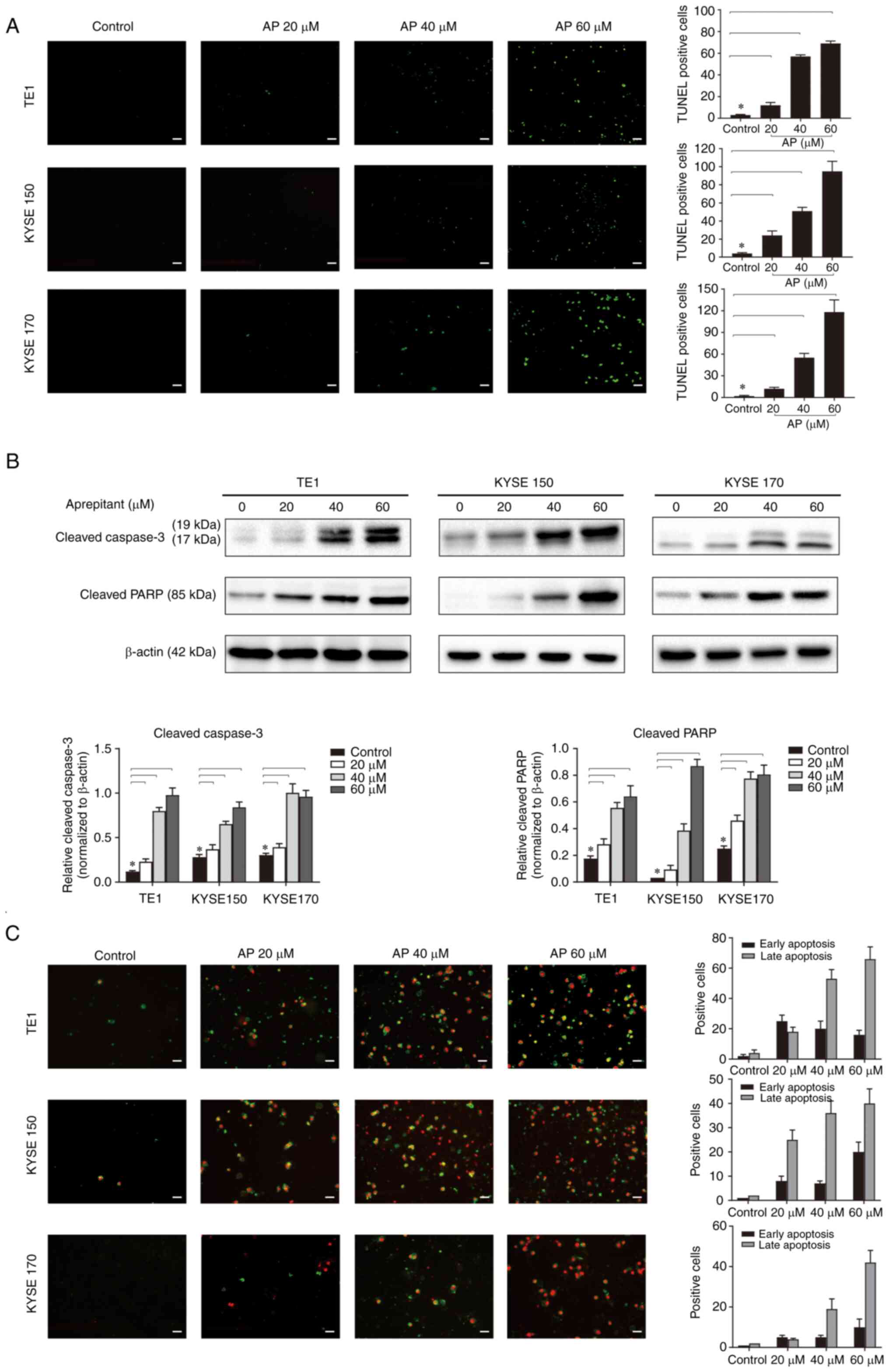
Aprepitant induces apoptosis in human
ESCC cells
Blocking NK1R has been reported to induce apoptosis
in vitro and in vivo via increase of mitochondrial
reactive oxygen species (15).
Therefore, it was detected whether aprepitant could affect the
apoptosis in ESCC cell lines. TUNEL staining results revealed that
aprepitant induced ESCC cell apoptosis in a dose-dependent manner
after 24-h treatment (Fig. 4A). To
analyze apoptosis in more detail, apoptotic markers for poly
(ADP-ribose) polymerase (PARP) and caspase-3 were analyzed at the
protein level by western blotting. Compared with the negative
control, both the cleaved PARP and the cleaved caspase-3 were
increased in a dose-dependent manner after aprepitant treatment for
24 h (Fig. 4B). The changes in
apoptotic markers showed that the late apoptotic mechanism was
activated. Additionally, these cells were stained with anti-Annexin
V-FITC antibody and propidium iodide to assess apoptosis by
fluorescence microscopy (Fig. 4C),
which showed a dose-dependent increase of late apoptotic cells.
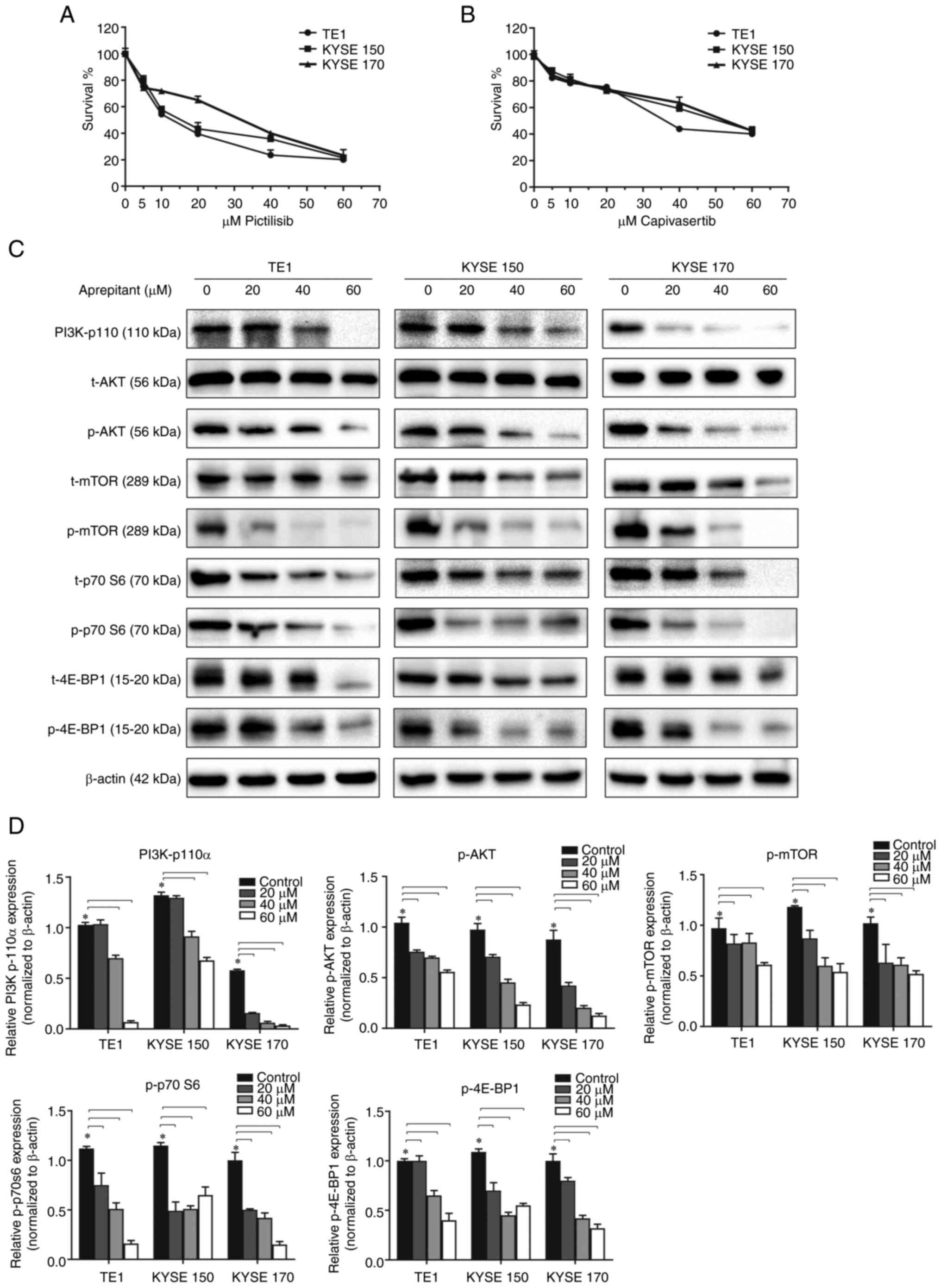
Aprepitant affects ESCC cells function
by downregulating PI3K/AKT/mTOR signaling pathways
When PI3K and AKT inhibitors (Pictilisib and
Capivasertib, respectively) were applied to ESCC cells, cell
proliferation was significantly reduced (Fig. 5A and B), suggesting that the
PI3K/AKT signaling pathway was involved in the development of ESCC.
Previous studies reported new evidence of a positive
cross-relationship between NK1R overexpression and
PI3K/AKT-mediated cell proliferation (16). However, this cross-relationship in
ESCC remains unclear. To investigate the PI3K/AKT signaling
pathways when NK1R was antagonized, ESCC was treated with gradually
increasing doses of aprepitant for 24 h. Western blot analysis for
PI3K, AKT, mTOR, 4E-BP1, and p70S6K was then performed in their
total and phosphorylated form. A robust decrease was observed in
PI3K-p110α. Although both the total and phosphorylated AKT mTOR,
4E-BP1, and p70S6K forms were downregulated, the phosphorylated
form was more significantly decreased. This resulted in a sharply
decreased phosphorylation to total protein ratios. The result of
western blotting indicated a strong downregulation of the
PI3K/AKT/mTOR signaling pathway at the protein level by NK1R
inhibition with aprepitant (Fig. 5C and
D).
Aprepitant inhibits tumor progression
in ESCC xenograft mice
To investigate the effect of aprepitant on ESCC
progression in vivo, human KYSE-170 cells were
subcutaneously implanted in the right flank of nude mice, and
aprepitant was intraperitoneally injected when the tumor volume
reached 100 mm3. There was no significant difference in
weight or health status between the two groups throughout the whole
treatment period (Fig. 6A). The
results revealed that the tumor volume in nude mice was
significantly reduced in the aprepitant-treated group on day 4
(Fig. 6B). After 14 days of
treatment, tumor weight was significantly decreased in the
aprepitant-treated group (Fig. 6C).
None of the treated animals showed adverse effects, and there was
no significant difference in morphology and structure of the
important organs between the two groups (Fig. S1). Immunohistochemical staining
revealed a high expression of total NK1R in both the control and
treatment groups, and there was no significant difference in
staining between these two groups (Fig. S2A). In addition, there was no
difference in the expression of SP between these two groups
(Fig. S2B).
To detect tumor-associated migration, matrix
metalloproteinase 9 (MMP-9) was measured using immunohistochemical
evaluation. The results showed that the number of positive cells
were significantly reduced in the experimental group (Fig. 6D). Ki-67 staining in tumor cells
exhibited a significantly decreased proliferation rate in the
treatment group (Fig. 6E).
Angiogenesis in vivo was further investigated by
immunohistochemical analysis with CD31. The results revealed that
both the microvascular density and the vascularized area were
significantly reduced in the treatment group (Fig. 6F). These data suggested that
aprepitant inhibited tumor progression in ESCC xenograft mice.
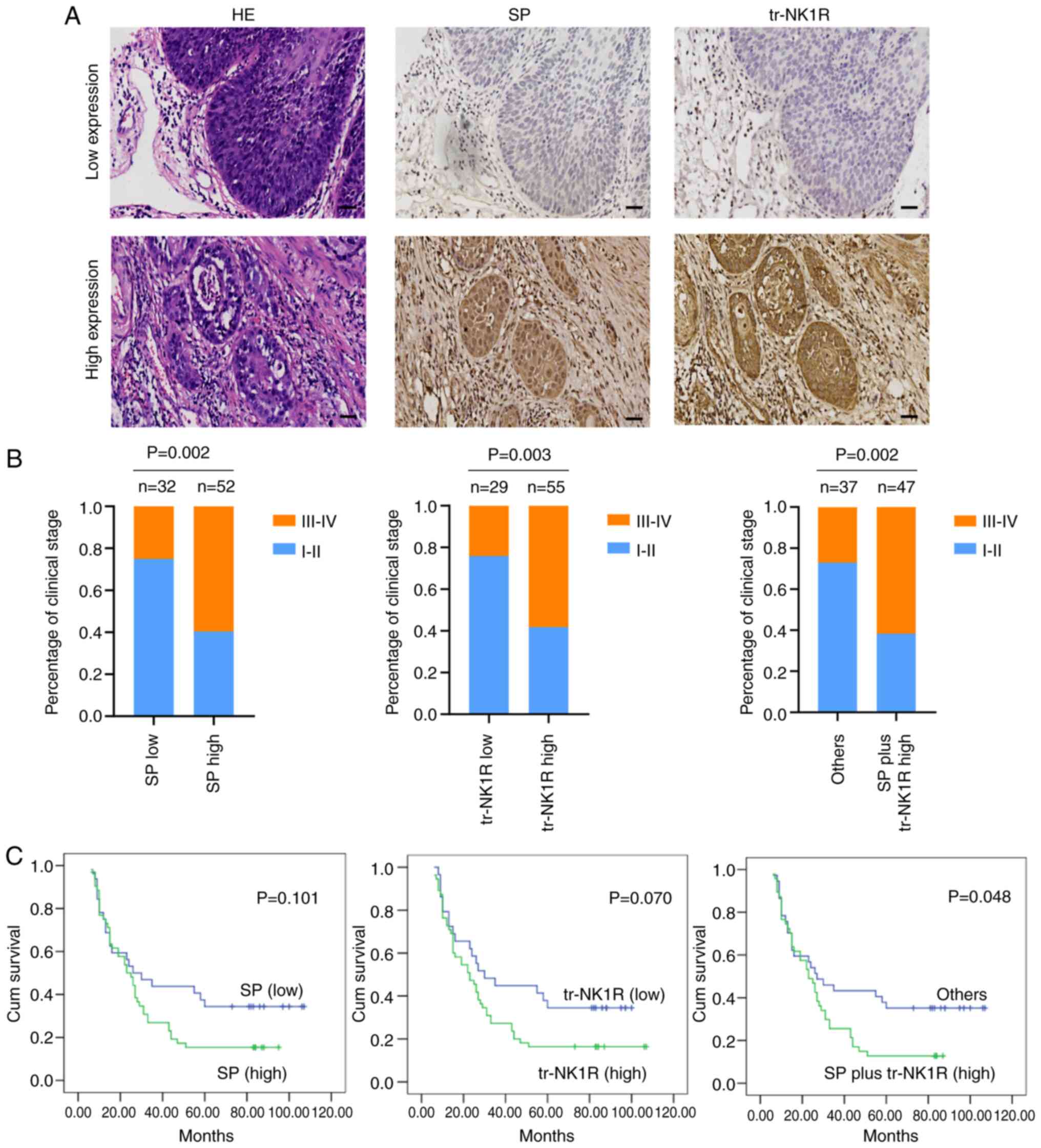
High expression of SP plus tr-NK1R
indicates poor prognosis in ESCC patients
To further explore the clinical significance of SP
and tr-NK1R in ESCC progression, SP and tr-NK1R expression were
analyzed using serial sections in 84 samples of ESCC tissues
(Fig. 7A). High expression of SP
and tr-NK1R were correlated with poor clinical stage (Fig. 7B). The results demonstrated that
high expression levels of SP plus tr-NK1R indicate poor prognosis
of ESCC, but high expression of SP or tr-NK1R alone did not affect
the outcome of patients with ESCC (Fig.
7C).
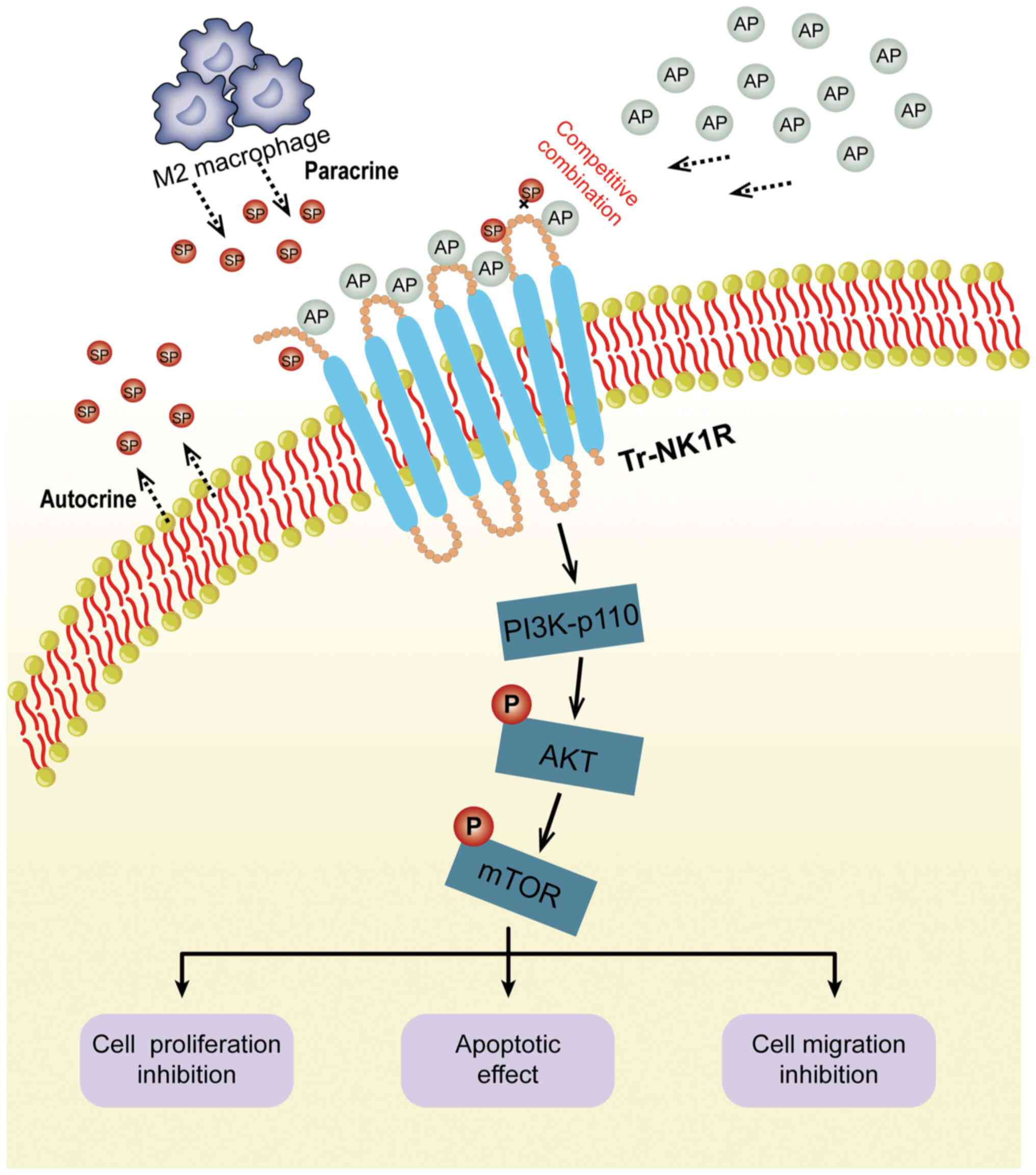
Discussion
The SP/NK1R complex is involved in various types of
cancer (9,17,18).
However, there are only few studies on the expression and function
of the SP/NK1R complex in ESCC. The present study found that
tr-NK1R is overexpressed in ESCC cell lines and tissues. In ESCC
tissues, SP mainly originates from cancer cells and M2 macrophages.
Blocking NK1R with its antagonist aprepitant induced tumor
suppression (Fig. 8), both in
vitro and in vivo. Furthermore, it was identified that
high expression levels of SP plus tr-NK1R indicate poor prognosis
of ESCC patients without aprepitant therapy. Collectively, these
results indicated that the SP/NK1R complex may be a novel
therapeutic target for patients with ESCC. To the best of our
knowledge, the present study is the first to report tr-NK1R
overexpression in ESCC cells.
SP, a member of the tachykinin family, is widely
distributed in nerve fibers (19).
It performs a series of biological functions after specific binding
to NK1R. Previous studies have shown that SP plays an essential
role in tumor development. Cancer cells can secrete SP to promote
growth, invasion, migration, angiogenesis and inhibit apoptosis
(20–22). Cancer cells can secrete SP and
promote their growth. Mohammadi et al (23) reported that SP could accelerate the
progression of human ESCC growth via MMP-2, MMP-9, VEGF-A and
VEGFR1 overexpression. Specific SP monoclonal antibody treatment
was also found to impair cell proliferation and increase cell
apoptosis in breast cancer cell lines (24). The current study found a
dose-dependent proliferation of ESCC cells under SP treatment,
whereas cell proliferation was inhibited after anti-SP and
anti-NK1R antibodies were added. In addition, ESCC cell supernatant
was detected by ELISA, and the results revealed the presence of SP.
Collectively, these findings support that ESCC cells promote their
own growth by auto-stimulatory SP production.
The tumor microenvironment is formed by cancer cells
and extracellular matrix, which affects tumor growth, drug
resistance and metastasis (25). As
a vital component of the tumor microenvironment, macrophages have
been reported to produce SP under inflammatory conditions (26). The results of the present study
revealed that in ESCC tissues, most infiltrating macrophages were
M2 type macrophages, and these M2 macrophages could produce SP.
The results of the present study present a novel
promising antitumor method via antagonism of NK1R. To the best of
our knowledge, this is the first study to identify that tr-NK1R was
overexpressed in ESCC cell lines, and proliferation, invasion and
migration were significantly inhibited after blockage of tr-NK1R.
Further, SP can induce ESCC proliferation, and a certain amount of
SP can reverse the inhibitory effect of aprepitant. Overall, it was
observed that SP and its receptor antagonist aprepitant
competitively interact with NK1R, and the combination is
reversible.
Aprepitant is a novel and promising compound that is
currently approved by the Food and Drug Administration for
preventing nausea and vomiting caused by oral chemotherapy
(27). Based on the positive
results in earlier controlled studies of aprepitant, clinicians
have also used it as a treatment for major depressive disorder,
pain and migraine (28). As
numerous patients with cancer experience cancer pain and
post-chemotherapy nausea, it can be assumed that blocking NK1R can
suppress the effect of SP, making aprepitant particularly useful
for tumor treatment (29). In
addition, aprepitant may have a therapeutic antitumor response
effect and could alleviate certain of the adverse symptoms of
cancer and its therapy (30). In
the present study, wound healing and Transwell assays showed a
significantly decreased migration and invasion abilities after
aprepitant administration. Furthermore, aprepitant induced
apoptosis in human ESCC cells. Javid et al (19) also reported that aprepitant promoted
caspase-dependent apoptotic cell death and G2/M arrest in cancer
stem-like ESCC spheres. Aprepitant appears to be a promising
treatment modality for ESCC as a single agent or in conjunction
with other chemotherapeutic drugs.
The current study investigated changes in signaling
pathways after treatment with aprepitant in ESCC cells. After NK1R
was antagonized by aprepitant, the phosphorylation levels of both
AKT and mTOR were significantly decreased. To the best of our
knowledge, these changes have not been described in ESCC cell lines
thus far. PI3K-p110α, p-AKT and p-mTOR were inhibited in a
dose-dependent manner. Ge et al (15) reported that the NK1R antagonists
aprepitant and SR140333 could induce apoptosis in myeloid leukemia
through oxidative stress. They also examined the PI3K/AKT/mTOR
signaling pathways and found that inhibition of these pathways no
noticeable effect on the proliferation of myeloid leukemia cells.
By contrast, the current study revealed that the P13K/AKT/mTOR
signaling pathway was significantly inhibited after treatment with
the NK1R antagonist aprepitant for 48 h, which may be due to the
different sources of tumor tissue and the longer duration of
treatment. To further explore the role of aprepitant in the
PI3K/AKT/mTOR pathway, the PI3K inhibitor Pictilisib was used to
block the effects of aprepitant. It was found that PI3Kp110,
phosphorylated AKT and mTOR were decreased after using Pictilisib.
However, aprepitant also exerts an inhibiting effect on ESCC
progression by inhibiting the PI3K/AKT/mTOR signaling pathway.
Currently, the PI3K/AKT/mTOR signaling pathway inhibitors cannot
not be used to block aprepitant to validate the critical role of
this pathway. Therefore, the PI3K/AKT/mTOR signaling pathway
activator could be included in the study to further the effect of
aprepitant in the PI3K/AKT/mTOR signaling pathway. In future
studies, the critical role of the aprepitant in this pathway will
be verified by investigating whether the PI3K/AKT/mTOR signaling
pathway activators can block the action of aprepitant. The present
study provided a new idea for studying the mechanism of NK1R
antagonists and pointed out a new direction for researching the
role of NK1R antagonists in ESCC.
The current evaluation of tumorigenesis in nude mice
demonstrated that aprepitant has a prominent antitumor effect,
consistent with previous findings (8,24,31).
Bigioni et al (32) reported
that NK1R targeting in breast carcinoma cell lines in xenografted
mice had a similar in vivo effect. Another study described a
therapeutic effect in hepatoblastoma cells of xenografted mice
(8). Unlike the aforementioned two
studies that used an intravenous or oral NK1R antagonist, the NK1R
antagonist was administered peritoneally. Therapeutic outcomes were
achieved in all administration methods. Experiments with higher
doses of 40 mg/kg/day orally, which is markedly higher than the
intraperitoneal dose and also showed a significant therapeutic
effect. In clinical samples, the high expression levels of SP plus
tr-NK1R indicated poor prognosis of ESCC, but high expression of SP
or tr-NK1R alone had no prognostic impact.
Although the current study revealed certain effects
of the SP/NK1R complex, tr-NK1R was overexpressed in ESCC cell
lines, whereas fl-NK1R was expressed at extremely low levels. This
expression pattern of two different splice variants at the mRNA
level was in accordance with that found at the cellular level.
Further studies are needed to clarify why T tr-NK1R but not fl-NK1R
is overexpressed in ESCC.
The SP/NK1R complex is expressed in various types of
cancer, but the expression of its NK1R splicing variant has not
been reported in ESCC. The present observations indicated that only
tr-NK1R is highly expressed in ESCC cell lines, and this provides
relevant evidence for targeted treatment in ESCC. Importantly, it
was found that human ESCC cells overexpress NK1R, particularly the
truncated type, and its antagonist aprepitant had significant
inhibitory effects both in vivo and in vitro. These
results support the SP/NK1R complex as a new therapeutic target in
human ESCC. There are certain limitations to the present study;
further studies are needed to explore the usefulness of NK1R
antagonists as an anticancer strategy against ESCC.
In conclusion, in ESCC tissues, SP is mainly derived
from ESCC cells and M2 macrophages. The NK1R antagonist aprepitant
inhibited the SP-induced proliferation, migration and invasion, and
induced the apoptosis of human ESCC cells through downregulating
the PI3K/AKT/mTOR signaling pathways. Furthermore, aprepitant
inhibited tumor progression in ESCC xenograft mice. In human ESCC
tissues, high expression of SP plus tr-NK1R indicated poor
prognosis, suggesting the strong potential of aprepitant as an
anticancer treatment modality in ESCC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Financial Supporting
Program of Hebei [grant nos. (2014)1257 and (2016)361006].
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YZ, MS and BS conceived and designed the study. YZ
wrote the manuscript. YZ, JL and YW performed experiments. FL and
LG. contributed to data interpretation and statistical analysis.
All the authors reviewed the manuscript. YZ and MS confirm the
authenticity of all the raw data. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved (approval no.
2020KY227) by the Medical Ethics Committee of the Fourth Hospital
of Hebei Medical University (Shijiazhuang, China) and was conducted
according to the tents of the Helsinki Declaration. Written
informed consent was provided by all patients. All animal
experiments were approved (approval no. 20190008) by the Animal
Care Committee of the Fourth Hospital of Hebei Medical University
(Shijiazhuang, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2022. CA Cancer J Clin. 72:7–33. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Abnet CC, Arnold M and Wei WQ:
Epidemiology of esophageal squamous cell carcinoma.
Gastroenterology. 154:360–373. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Coveñas R and Muñoz M: Cancer progression
and substance P. Histol Histopathol. 29:881–890. 2014.PubMed/NCBI
|
|
4
|
González-Moles MÁ, Ramos-García P and
Esteban F: Significance of the overexpression of substance P and
its receptor NK-1R in head and neck carcinogenesis: A systematic
review and meta-analysis. Cancers (Basel). 13:13492021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Patel HJ, Ramkissoon SH, Patel PS and
Rameshwar P: Transformation of breast cells by truncated
neurokinin-1 receptor is secondary to activation by
preprotachykinin-A peptides. Proc Natl Acad Sci USA.
102:17436–17441. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gillespie E, Leeman SE, Watts LA, Coukos
JA, O'Brien MJ, Cerda SR, Farraye FA, Stucchi AF and Becker JM:
Truncated neurokinin-1 receptor is increased in colonic epithelial
cells from patients with colitis-associated cancer. Proc Natl Acad
Sci USA. 108:17420–17425. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ramkissoon SH, Patel PS, Taborga M and
Rameshwar P: Nuclear factor-kappaB is central to the expression of
truncated neurokinin-1 receptor in breast cancer: Implication for
breast cancer cell quiescence within bone marrow stroma. Cancer
Res. 67:1653–1659. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Berger M, Neth O, Ilmer M, Garnier A,
Salinas-Martín MV, de Agustín Asencio JC, von Schweinitz D, Kappler
R and Muñoz M: Hepatoblastoma cells express truncated neurokinin-1
receptor and can be growth inhibited by aprepitant in vitro and in
vivo. J Hepatol. 60:985–994. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Deng XT, Tang SM, Wu PY, Li QP, Ge XX, Xu
BM, Wang HS and Miao L: SP/NK-1R promotes gallbladder cancer cell
proliferation and migration. J Cell Mol Med. 23:7961–7973. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Inoue T, Kimura M, Uchida J, Nishino K,
Kumagai T, Taniguchi J and Imamura F: Aprepitant for the treatment
of breakthrough chemotherapy-induced nausea and vomiting in
patients receiving moderately emetogenic chemotherapy. Int J Clin
Oncol. 22:600–604. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yahata H, Kobayashi H, Sonoda K, Shimokawa
M, Ohgami T, Saito T, Ogawa S, Sakai K, Ichinoe A, Ueoka Y, et al:
Efficacy of aprepitant for the prevention of chemotherapy-induced
nausea and vomiting with a moderately emetogenic chemotherapy
regimen: A multicenter, placebo-controlled, double-blind,
randomized study in patients with gynecologic cancer receiving
paclitaxel and carboplatin. Int J Clin Oncol. 21:491–497. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Muñoz M and Coveñas R: Neurokinin-1
receptor antagonists as antitumor drugs in gastrointestinal cancer:
A new approach. Saudi J Gastroenterol. 22:260–268. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Douglas SD and Leeman SE: Neurokinin-1
receptor: Functional significance in the immune system in reference
to selected infections and inflammation. Ann N Y Acad Sci.
1217:83–95. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ge C, Huang H, Huang F, Yang T, Zhang T,
Wu H, Zhou H, Chen Q, Shi Y, Sun Y, et al: Neurokinin-1 receptor is
an effective target for treating leukemia by inducing oxidative
stress through mitochondrial calcium overload. Proc Natl Acad Sci
USA. 116:19635–19645. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chanmee T, Ontong P, Konno K and Itano N:
Tumor-associated macrophages as major players in the tumor
microenvironment. Cancers (Basel). 6:1670–1690. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhou Y, Zhao L, Xiong T, Chen X, Zhang Y,
Yu M, Yang J and Yao Z: Roles of full-length and truncated
neurokinin-1 receptors on tumor progression and distant metastasis
in human breast cancer. Breast Cancer Res Treat. 140:49–61. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lewis KM, Harford-Wright E, Vink R and
Ghabriel MN: NK1 receptor antagonists and dexamethasone as
anticancer agents in vitro and in a model of brain tumours
secondary to breast cancer. Anticancer Drugs. 24:344–354. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Javid H, Mohammadi F, Zahiri E and Hashemy
SI: The emerging role of substance P/neurokinin-1 receptor
signaling pathways in growth and development of tumor cells. J
Physiol Biochem. 75:415–421. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Garcia-Recio S, Fuster G,
Fernandez-Nogueira P, Pastor-Arroyo EM, Park SY, Mayordomo C,
Ametller E, Mancino M, Gonzalez-Farre X, Russnes HG, et al:
Substance P autocrine signaling contributes to persistent HER2
activation that drives malignant progression and drug resistance in
breast cancer. Cancer Res. 73:6424–6434. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ma J, Yuan S, Cheng J, Kang S, Zhao W and
Zhang J: Substance P promotes the progression of endometrial
adenocarcinoma. Int J Gynecol Cancer. 26:845–850. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Esteban F, Muñoz M, González-Moles MA and
Rosso M: A role for substance P in cancer promotion and
progression: A mechanism to counteract intracellular death signals
following oncogene activation or DNA damage. Cancer Metastasis Rev.
25:137–145. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mohammadi F, Javid H, Afshari AR, Mashkani
B and Hashemy SI: Substance P accelerates the progression of human
esophageal squamous cell carcinoma via MMP-2, MMP-9, VEGF-A, and
VEGFR1 overexpression. Mol Biol Rep. 47:4263–4272. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mayordomo C, García-Recio S, Ametller E,
Fernández-Nogueira P, Pastor-Arroyo EM, Vinyals L, Casas I, Gascón
P and Almendro V: Targeting of substance P induces cancer cell
death and decreases the steady state of EGFR and Her2. J Cell
Physiol. 227:1358–1366. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cui Y, Zhang S, Hu X and Gao F:
Tumor-associated fibroblasts derived exosomes induce the
proliferation and cisplatin resistance in esophageal squamous cell
carcinoma cells through RIG-I/IFN-β signaling. Bioengineered.
13:12462–12474. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ho WZ, Lai JP, Zhu XH, Uvaydova M and
Douglas SD: Human monocytes and macrophages express substance P and
neurokinin-1 receptor. J Immunol. 159:5654–5660. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dupuis LL, Lingertat-Walsh K and Walker
SE: Stability of an extemporaneous oral liquid aprepitant
formulation. Support Care Cancer. 17:701–706. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Munoz M, Covenas R, Esteban F and Redondo
M: The substance P/NK-1 receptor system: NK-1 receptor antagonists
as anti-cancer drugs. J Biosci. 40:441–463. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rapoport BL, Jordan K, Boice JA, Taylor A,
Brown C, Hardwick JS, Carides A, Webb T and Schmoll HJ: Aprepitant
for the prevention of chemotherapy-induced nausea and vomiting
associated with a broad range of moderately emetogenic
chemotherapies and tumor types: A randomized, double-blind study.
Support Care Cancer. 18:423–431. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Muñoz M and Rosso M: The NK-1 receptor
antagonist aprepitant as a broad spectrum antitumor drug. Invest
New Drugs. 28:187–193. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Palma C, Bigioni M, Irrissuto C, Nardelli
F, Maggi CA and Manzini S: Anti-tumour activity of tachykinin NK1
receptor antagonists on human glioma U373 MG xenograft. Br J
Cancer. 82:480–487. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bigioni M, Benzo A, Irrissuto C, Maggi CA
and Goso C: Role of NK-1 and NK-2 tachykinin receptor antagonism on
the growth of human breast carcinoma cell line MDA-MB-231.
Anticancer Drugs. 16:1083–1089. 2005. View Article : Google Scholar : PubMed/NCBI
|