Introduction
Glioblastoma (GBM) is the most common malignant
tumor of the brain, accounting for >50% of primary intracranial
malignant tumors, and is also regarded as one of the most
intractable early-death solid tumors in neurosurgery due to its
strong aggression, rapid postoperative recurrence and high
mortality (1). At present, GBM
therapy continues to be a challenging medical issue. Multiple drug
resistance and a high recurrence rate are the two main barriers to
effective treatment; GBM therapy resistance has been attributed to
tumor heterogeneity, hypermutation, hypoxia and immune-suppressive
tumor microenvironment (2,3). Despite progress in the main treatment
modalities for GBM, including surgery, radiotherapy and
chemotherapy, the outcome for patients remains almost generally
fatal, with a median survival of <2 years (4,5).
Therefore, it is critically necessary to identify more effective
therapeutic targets and improved therapeutic strategies for the
treatment of GBM.
With the rapid advancement of natural medicines, the
outstanding antitumor activity of traditional Chinese medicine
monomers has steadily drawn more attention in recent years.
Cucurbitacin E (CUE), a highly oxidized tetracyclic triterpene
compound isolated from species of the genus Cucurbita, has
been reported to exert anti-inflammatory and anti-analgesic
properties (6). During the past few
years, numerous studies have demonstrated that CUE has also
anticancer effects, including inhibiting the proliferation of
various cancer types, including gastric, liver, lung and colon
cancers (7,8), as well as the capacity of inducing
apoptosis and G2/M arrest in a number of cancer cells (9,10). CUE
can also disrupt the cytoskeleton of actin and vimentin in prostate
cancer cells, alter the morphology of tumor cells (11,12),
and inhibit angiogenesis in human prostate tumors through the Janus
kinase 2 (JAK2)-signal transducer and activator of transcription 3
(STAT3) signaling pathway mediated by vascular endothelial growth
factor receptor 2 (VEGFR2) (13).
Additionally, it has been revealed that CUE can inhibit
Yes-associated protein signaling pathway and brain metastases of
human non-small cell lung cancer (14), suppress the proliferation and
invasion of osteosarcoma cells through phosphoinositide 3-kinase
(PI3K)/AKT/mammalian target of rapamycin (mTOR) signaling pathway
(15) and induce cellular
senescence in colon cancer via modulating the
miR-371b-5p/transcription factor AP-4 (TFAP4) axis (16). Of note, a previous study has shown
that CUE inhibits the growth of GBM by arresting the cell cycle at
G2/M phase through GADD45γ gene expression and blockade of
cyclinB1/CDC2 complex (17). There
is also evidence suggesting that CUE delays the onset of mitosis in
GBM cells by upregulating GADD45β rather than downregulating the
phosphorylation of extracellular signal-regulated kinase (ERK) and
c-Jun N-terminal kinase (JNK) (18). However, the underlying mechanisms by
which CUE exerts its anticancer effects on GBM remain to be fully
elucidated.
Receptor tyrosine kinases (RTKs), a family of cell
surface receptors, play critical roles in cell proliferation,
survival and migration (19). The
epidermal growth factor receptor (EGFR), which belongs to the ErbB
family of RTKs, is frequently mutated and/or overexpressed in
numerous human cancers (20). Once
activated, EGFR activates numerous downstream signaling pathways,
including mitogen-activated protein kinase (MAPK), PI3K/AKT,
JAK/STAT and protein kinase C (PKC), which are highly associated
with the proliferation, migration, angiogenesis and apoptosis of
tumor cells (21–23). Focal adhesion kinase (FAK) plays an
important role in tumor development. Previous studies have revealed
that FAK activation promotes breast cancer angiogenesis (24). In an FAK-deficient mouse model, the
cell motility is reduced and focal adhesion contact formation is
enhanced (25). In addition,
inhibition of the FAK and EGFR signaling pathways synergistically
promotes apoptosis in breast cancer cells (26).
Protein kinase B (AKT), a serine/threonine kinase,
is dysregulated in human cancers and plays a crucial role in tumor
growth (27). Once activated, it
transmits signals to numerous downstream effectors, including
glycogen synthase kinase-3β (GSK3β) and forkhead box protein O
(FOXO) (27). Collected evidence
suggests that the FAK/AKT/GSK3β pathway is essential for the
proliferation, invasion and metastasis of numerous cancers,
including GBM (28–32). Furthermore, it has been identified
that dysregulation of the cell cycle checkpoint proteins, including
cyclinB1 and cyclinD1, contributes to the uncontrolled cellular
growth and tumorigenesis (33). For
example, cyclinB1 and cyclinD1 are overexpressed in numerous
cancers which are involved in neogenesis and progression (34,35).
However, it is not clear whether these signaling cascades are
involved in the antitumor effect of CUE.
In the present study, it was aimed to demonstrate
the effects of CUE on the proliferation of GBM cells and reveal the
possible underlying molecular mechanisms.
Materials and methods
Antibodies and reagents
CUE was purchased from MedChemExpress. Antibodies
for western blot analysis, including phospho-FAK (Tyr397) (1:2,000;
cat. no. 8556S), FAK (1:2,000; cat. no. 3285S), phospho-AKT
(Ser473) (1:2,000; cat. no. 4058S), AKT (1:2,000; cat. no. 4691S),
phospho-GSK3β (1:2,000; cat. no. 9336S), GSK3β (1:2,000; cat. no.
9315S; all from Cell Signaling Technology, Inc.), cyclinB1
(1:2,000; cat. no. ab181593), cylinD1 (1:10,000; cat. no.
ab134175), cyclinA2 (1:2,000; cat. no. ab181591), cyclinE1
(1:5,000; cat. no. ab133266; all from Abcam), Proliferating Cell
Nuclear Antigen (PCNA; 1:2,000; cat. no. 10205-2-AP; Proteintech
Group, Inc.), Glyceraldehyde-3-phosphate dehydrogenase (GAPDH;
monoclonal, rabbit anti-mouse; 1:5,000; cat. no. KC-5G4; Zhejiang
Kangchen Biotech, Co., Ltd.), goat anti-rabbit IgG HRP-linked
antibody (1:5,000; cat. no. 31460) and goat anti-mouse IgG
HRP-linked antibody (1:5,000; cat. no. 31431; both from Thermo
Fisher Scientific, Inc.). PF-562271 (FAK selective inhibitor, 10
µM) (cat. no. HY-10459) was purchased from MedChemExpress. EGF
(cat. no. AF-100-15; 20 ng/ml) was purchased from PeproTech, Inc.
Reagents used for cell culture including Dulbecco's modified
Eagle's medium (DMEM), fetal bovine serum (FBS) and trypsin-EDTA
solution were purchased from Gibco; Thermo Fisher Scientific,
Inc.
Cell culture
Human GBM cell lines U87-MG (derived from GBM of
unknown origin; American Type Culture Collection no. HTB-14; cat.
no. TCHu 138) and U251-MG (cat. no. TCHu 58) were purchased from
the Cell Bank of the Chinese Academy of Sciences (Shanghai, China)
in 2018. Authentication testing of U87-MG and U251-MG cell lines
was performed by Shanghai Biowing Applied Biotechnology Co. Ltd.
via STR profiling. STR profiles match the standards recommended for
U87-MG and U251-MG cell lines authentication. U87-MG and U251-MG
cells were cultured in DMEM containing 4.5 g/l glucose supplemented
with 10% FBS. Cells were maintained at 37°C in an incubator with 5%
CO2.
Cell viability
A total of 2.0×103 cells/well were seeded
onto 96-well plates and incubated at 37°C in an incubator with 5%
CO2. After adherence, the cells were incubated with
different concentration of CUE (0, 0.01, 0.025, 0.25, 2.5 and 25
µM) for 24 h. Then, 10 µl Cell Counting Kit-8 (CCK-8; cat. no.
CK04; Dojindo Laboratories, Inc.) reagent was used to detect the
cell viability, and the optical density (OD) at 450 nm was
measured. Cell survival was expressed as fold of the control group.
The optimal treatment concentration was 2.5 µM and thus was
selected for the following experiments.
Western blot analysis
Cells were cultured overnight at 37°C in an
incubator. After treatment, the cells were washed with PBS, and
cell lysis buffer (cat. no. R0010; Beijing Solarbio Science &
Technology Co., Ltd.) was added at 4°C for 25 min. The lysate was
centrifuged at 12,000 × g at 4°C for 15 min. The protein
concentration was measured by bicinchoninic acid protein assay. The
equivalent amounts of protein (20 µg) were loaded into 10% SDS-PAGE
gel (30% acrylamide) and separated at 110 V voltage. The proteins
in the gel were transferred to a PVDF membrane and blocked in 5%
skim milk for 2 h at room temperature. Subsequently, the membrane
was incubated with different primary antibodies including
phospho-FAK, FAK, phospho-AKT, AKT, phospho-GSK3β, GSK3β, cyclinB1,
cylinD1, cyclinA2, cyclinE1 and GAPDH overnight at 4°C and then
incubated with HRP-conjugated anti-rabbit IgG secondary antibody
(1:5,000) or HRP-conjugated anti-mouse IgG secondary antibody
(1:5,000) for 90 min at room temperature. Immunoreactive bands were
visualized by enhanced chemiluminescence (Pierce; Thermo Fisher
Scientific, Inc.) and semi-quantified using ImageJ software
(version 1.47t; National Institutes of Health).
Immunofluorescence confocal
microscopy
A total of 6.0×104 cells/well were seeded
onto 12-well plates and treated with 2.5 µM CUE for 24 h. After
washed with pre-cooled PBS, the cells were fixed with 4%
paraformaldehyde for 15 min followed by permeabilization with 0.2%
Triton X-100 in PBS for 10 min. Subsequently, the cells were
blocked in PBST (0.1% Tween-20) containing 1% bovine serum albumin
(cat. no. A8020; Beijing Solarbio Science & Technology Co.,
Ltd.) for 40 min. After washing with pre-cooled PBS, the cells were
incubated with anti-human rabbit Ki67 antibody (1:1,000; cat. no.
27309-1-AP; Proteintech Group, Inc.) overnight at 4°C in a wet box.
Next, the cells were then washed with pre-cooled PBS and incubated
with anti-rabbit Alexa Fluor® 594 secondary antibody
[(1:500; cat. no. Ab150080; Abcam), Excitation wavelength: 590 nm;
Emission wavelength: 617 nm] for 1 h. The nucleus was stained with
DAPI (cat. no. AR1176, no dilution; Boster Biological Technology
Co., Ltd.), and the fluorescence signal was detected under an
inverted fluorescent microscope.
Statistical analysis
Data are presented as the mean ± SEM of at least
three independent experiments and were analysed via GraphPad Prism
7.0 (Dotmatics). Unpaired Student's t-test (two groups) or
one/two-way ANOVA with Bonferroni's multiple comparison tests (more
than two groups) were used for comparisons. P<0.05 was
considered to indicate a statistically significant difference.
Results
CUE inhibits the proliferation of GBM
cells
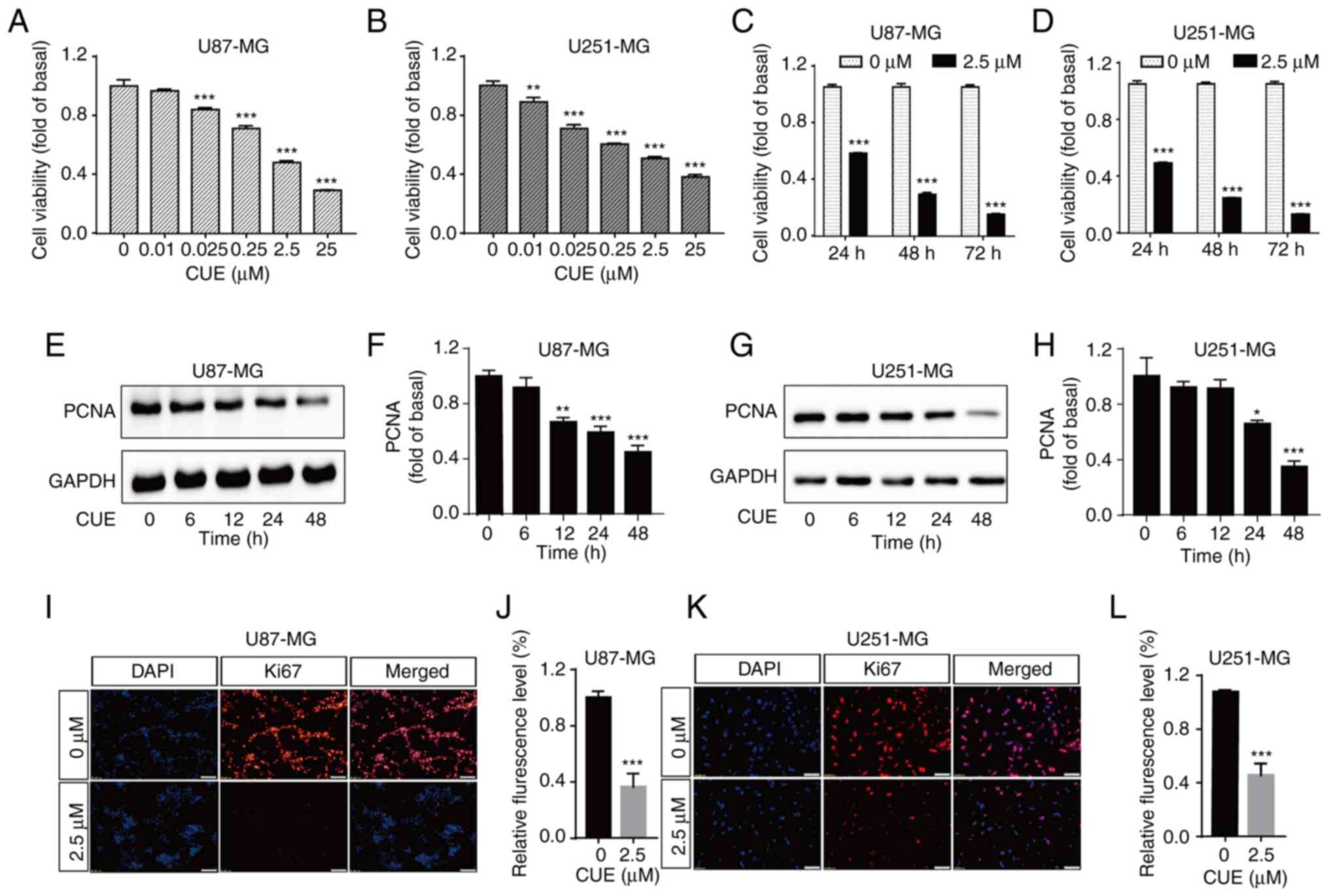
The effect of CUE on the growth of GBM cells was
first examined using CCK-8 assay. Compared with the control group,
CUE significantly inhibited the proliferation of U87-MG and U251-MG
cells in a dose-dependent manner (Fig.
1A and B). The half maximal inhibitory concentration was ~2.5
µM. To further detect the influence of CUE on cell viability at
different treatment times, the cells were exposed to 2.5 µM CUE for
24, 48 or 72 h, respectively. The results demonstrated that CUE
significantly inhibited the proliferation of GBM cells in a
time-dependent manner (Fig. 1C and
D).
To further confirm the effect of CUE on the
proliferation of GBM cells, the expression of PCNA and Ki67, which
are important indicators of tumor cells proliferation, was then
detected. The cells were treated with 2.5 µM CUE, and then the
expression of Ki67 and PCNA were measured by immunofluorescence and
western blot assays, respectively. It was observed that the
expression of PCNA was significantly downregulated in CUE-treated
cells as compared with the control group (Fig. 1E-H). In addition, red fluorescence
intensity of the experimental group was significantly decreased,
indicating that CUE-treated cells had less ki67 expressed than
untreated cells (Fig. 1I-L). Taken
together, these results suggested that CUE significantly inhibits
the proliferation of GBM cells (Fig.
1E-L).
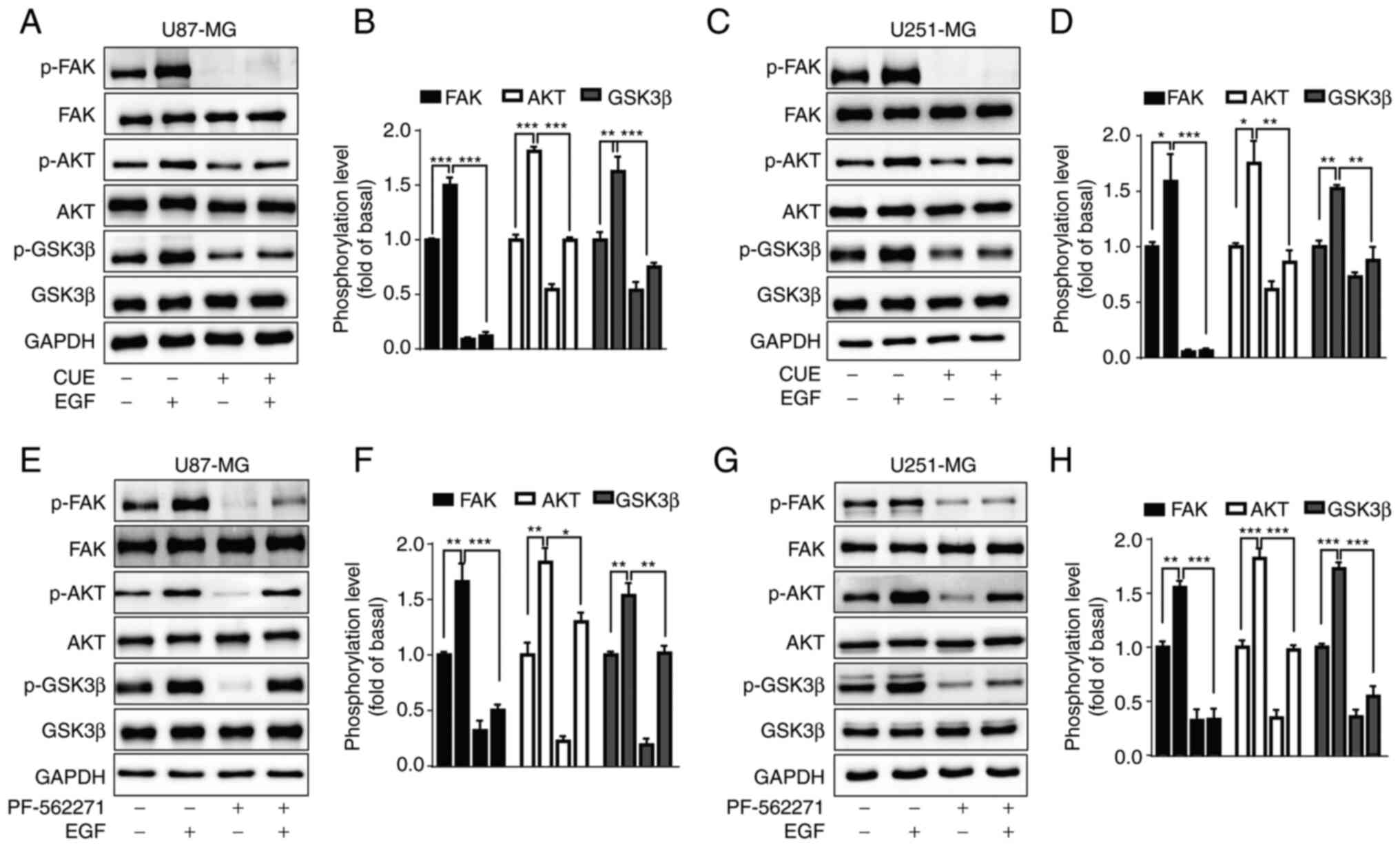
CUE downregulates the phosphorylation
level of FAK, AKT and GSK3β in GBM cells
FAK is a tyrosine kinase, which is closely related
to the occurrence and development of tumors. Numerous studies have
shown that downregulating the expression of FAK greatly inhibits
the proliferation, migration and invasion of tumor cells (32,36,37).
To evaluate whether the FAK-mediated signal transduction pathways,
including AKT and GSK3β, were engaged in the antitumor effect of
CUE, the phosphorylation level of FAK, AKT and GSK3β was measured
in GBM cells following 2.5 µM CUE treatment at various time-points
(0, 0.5, 1, 2, 8 and 24 h). The results revealed that CUE
significantly decreased the phosphorylation level of FAK, AKT and
GSK3β in U87-MG cells (Fig. 2A-D).
Similar results were observed in U251-MG cells (Fig. 2E-H). These results suggested that
CUE may inhibit the proliferation of GBM cells through
FAK/AKT/GSK3β signaling pathway.
CUE blocks EGF-induced FAK, AKT and
GSK3β phosphorylation in GBM cells
It has been reported that EGF upregulates the
phosphorylation level of FAK, AKT and GSK3β in GBM cells (38–40).
The effects of CUE on EGF-induced FAK, AKT and GSK3β
phosphorylation were then explored. It was demonstrated that EGF
(20 ng/ml) significantly increased the phosphorylation of FAK, AKT
and GSK3β in both GBM cell lines, which were significantly blocked
by CUE pre-treatment (Fig. 3A-D).
In order to confirm whether AKT and GSK3β were in the downstream of
FAK, the impact of PF-562271 (10 µM), a selective inhibitor of FAK,
was examined on EGF-induced AKT and GSK3β phosphorylation in GBM
cells. The results showed that PF-562271 significantly inhibited
EGF-induced AKT and GSK3β phosphorylation (Fig. 3E-H), suggesting that AKT and GSK3β
were downstream of FAK.
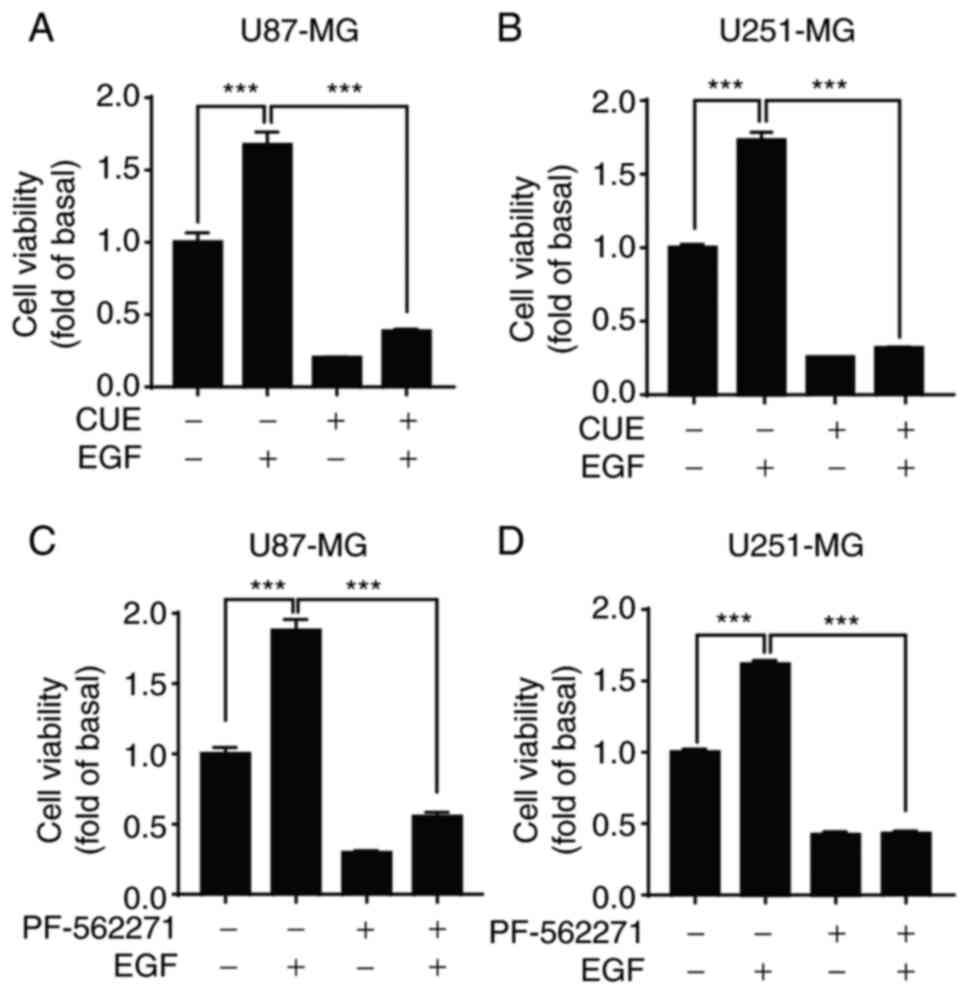
CUE inhibits the proliferation of GBM
cells through the EGF-mediated FAK/AKT/GSK3β signaling pathway
After confirming that CUE inhibited the
proliferation of GBM cells and blocked EGF-induced FAK/AKT/GSK3β
phosphorylation, it was then investigated whether CUE inhibits the
proliferation of GBM cells through the FAK/AKT/GSK3β signaling
pathway. To this end, the effect of CUE on EGF-induced cell
proliferation was first explored. It was found that CUE (2.5 µM)
significantly inhibited the proliferation of EGF-induced GBM cells
after treating the cells with it, independently of EGF (20 ng/ml)
being present or absent (Fig. 4A and
B). Similarly, FAK-specific inhibitor PF-562271 (10 µM) also
significantly inhibited EGF-induced proliferation of GBM cells
(Fig. 4C and D). Collectively,
these results indicated that FAK/AKT/GSK3β signaling pathway is
involved in the anti-proliferative effect of CUE in GBM cells.
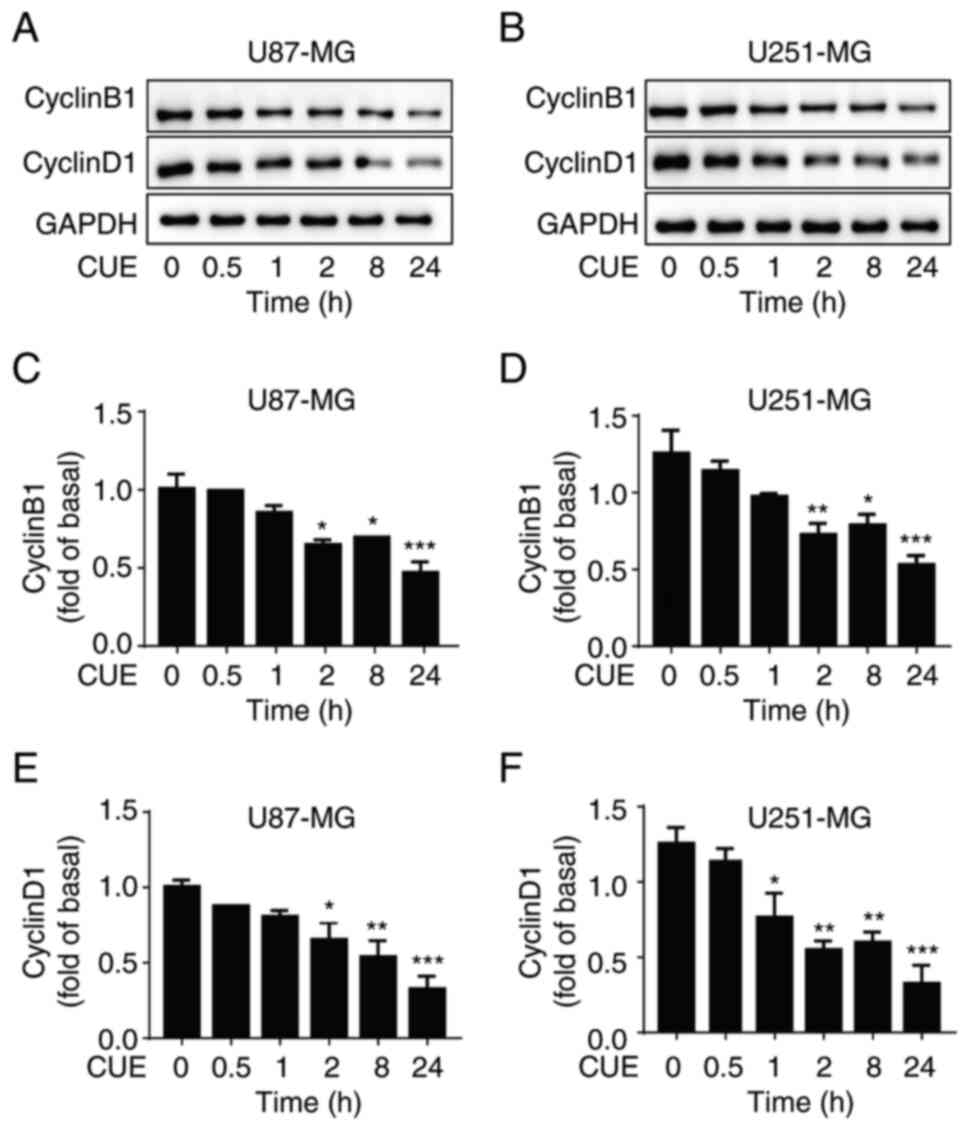
CUE inhibits the expression of
cyclinB1 and cyclinD1 in GBM cells
The effects of CUE on the expression of cyclinB1 and
cyclinD1, two cyclins which play crucial roles in the proliferation
of tumor cells, were also evaluated. In GBM cells treated with 2.5
µM CUE, cyclinB1 and cyclinD1 expression significantly decreased as
compared with the control group (Fig.
5A-F). Notably, in contrast to cyclinB1 and cyclinD1, CUE had
no discernible effect on cyclinA2 and cyclinE1 expression (Fig. S1).
Discussion
In the present study, the effect of CUE on GBM cell
proliferation and its underlying molecular mechanisms were
investigated. The results demonstrated that CUE reduced the
expression of cyclinB1 and cyclinD1, downregulated the
FAK/AKT/GSK3β signaling pathway and consequently inhibited the
proliferation of GBM cells.
Traditional Chinese medicine offers a number of drug
candidates for cancer treatment. CUE, a member of the
Cucurbitaceae family, inhibits the growth of multiple
cancers (7,8). Previous studies have stated that CUE
inhibited breast tumor metastasis (41) and induced autophagy of cancer cells
by decreasing mTORC1 signaling and increasing Adenosine
5′-monophosphate (AMP)-activated protein kinase (AMPK) activity
(42). In addition to its
anticancer properties, CUE also improved liver fibrosis and
inhibited the production of inflammatory factors (43,44).
However, the effect of CUE on GBM is rarely reported except in two
previous studies showing that CUE could induce mitosis delay in GBM
cells by upregulating GADD45β and inhibit GBM growth via arresting
the cell cycle at G2/M phase (17,18).
In the present study, it was found that CUE significantly decreased
the cell viability of GBM cell lines U87-MG and U251-MG in a
dose-dependent and time-dependent manner. This finding was further
confirmed by measuring the expression of Ki67 and PCNA, which are
nuclear antigens linked to dividing cells, and both can be used to
assess the level of cell proliferation (45). Using an immunofluorescence assay, it
was identified that the amount and intensity of Ki67 fluorescence
in cells treated with CUE were significantly reduced. Furthermore,
it was revealed by western blot analysis that CUE suppressed PCNA
expression. These findings indicated that CUE significantly
inhibits the proliferation of U87-MG and U251-MG cells.
FAK, which is overexpressed and phosphorylated in
various advanced solid tumors, is essential for tumor growth,
proliferation and metastasis (46,47).
Thus, FAK has become a potential target for cancer therapy
(48). AKT is one of the
best-characterized kinases known to regulate multiple cellular
functions through phosphorylation of various substrates. Previous
studies have demonstrated its critical role in the survival and
death of cancer cells (49). As a
downstream target of AKT, GSK3β signaling pathway is one of the
crucial signal transduction pathways implicated in the development
of numerous cancers (50). Although
it has been established that AKT and GSK3β can be downstream
signaling molecules of FAK, it is unknown if the FAK/AKT/GSK3β
signaling pathway contributes to the GBM development process. In
the present study, it was identified that CUE significantly
downregulated the phosphorylation of FAK, AKT and GSK3β in GBM
cells, but the exact molecular mechanism of how CUE affects the
phosphorylation of FAK, AKT and GSK3β remains to be further
studied. Notably, it was also found that CUE significantly blocked
EGF-induced phosphorylation of FAK, AKT and GSK3β. Considering that
numerous evidences have indicated that EGFR is frequently
overexpressed in human cancers and overactivation of EGFR signaling
cascades are highly associated with the occurrence and development
of tumors (51,52), the aforementioned finding further
confirmed the potential value of CUE in tumor therapy. Notably, in
the present study, it was not explored which enzymes phosphorylate
the FAK/AKT/GSK3β. It is worth noting that previous studies
reported that FAK is downstream of EGFR. Once activated, EGFR
transmits signals to the downstream Src/FAK pathway and the
phosphorylation of the Src/FAK complex can initiate the activation
of the MAPK or PI3K/AKT pathway (53). Furthermore, it has been reported
that Src-3Δ4 mediates the interaction of EGFR with FAK and leads to
EGF-induced FAK phosphorylation (54). Such evidence suggests that EGFR and
Src-3Δ4 may be promising candidate enzymes that phosphorylate the
FAK/AKT/GSK3β.
Another important finding in the present study was
that CUE reduced the expression of cyclinB1 and cyclinD1 in GBM
cells. Both cyclinB1 and cyclinD1 are important cell cycle-driven
proteins. CyclinB1 regulates the G2/M phase transition of the cell
cycle (33), while cyclinD1
regulates the G1/S phase transition of the cell cycle (55). Numerous studies have demonstrated
that downregulation of cyclinB1 or cyclinD1 would lead to mitotic
block and would inhibit the proliferation of numerous tumor cells
(56–59). However, whether the reduced
expression of cyclinB1 or cyclinD1 is related to the
antiproliferation effect of CUE, and whether FAK/AKT/GSK3β pathway
participated in this process remains to be further
investigated.
While the current study revealed that CUE inhibited
the proliferation of U87-MG and U251-MG cells by modulating the
FAK/AKT/GSK3β signaling pathway, certain important questions remain
to be answered. For example, in the present study, the antitumor
effect of CUE on cultured cell lines was only evaluated; thus, it
would be interesting to further validate the antitumor effect of
CUE on the growth of GBM using tumor xenograft animal models, and
then isolate the tumor tissues to detect the phosphorylation of
FAK, AKT and GSK3β to further elucidate the molecular mechanisms.
In addition, whether CUE has any effect on GBM migration, invasion
and apoptosis, and whether the FAK/AKT/GSK3β signaling pathway is
involved in these processes remains unknown. Answering these
important questions will help us fully understand the antitumor
effect of CUE.
In conclusion, the present study demonstrated that
CUE exerts a distinct antitumor effect on GBM cells. CUE may
inhibit the proliferation of GBM cells through the FAK/AKT/GSK3β
signaling pathway. The present finding provides a promising basis
for the development of effective new drugs for GBM therapy.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science
Foundation of China (grant no. 32160184), the Scientific Project of
Jiangxi (grant no. 20181BAB215018) and the Department of Public
Health of Jiangxi (grant no. 20185226).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
PH and WC designed the study. WC conducted the
majority of the experiments. FL, XL and LL contributed to the data
collection and statistical analysis. WTC performed parts of the
western blot experiments. TZ and YL performed immunofluorescence
experiments. LN and YZ analysed the western blot data. WC and PH
wrote the manuscript. All authors read and approved the final
manuscript. PH and WC confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Perus LJM and Walsh LA: Microenvironmental
heterogeneity in brain malignancies. Front Immunol. 10:22942019.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Goenka A, Tiek D, Song X, Huang T, Hu B
and Cheng SY: The many facets of therapy resistance and tumor
recurrence in glioblastoma. Cells. 10:4842021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Guo G, Sun Y, Hong R, Xiong J, Lu Y, Liu
Y, Lu J, Zhang Z, Guo C, Nan Y and Huang Q: IKBKE enhances
TMZ-chemoresistance through upregulation of MGMT expression in
glioblastoma. Clin Transl Oncol. 22:1252–1262. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jackson CM, Choi J and Lim M: Mechanisms
of immunotherapy resistance: Lessons from glioblastoma. Nat
Immunol. 20:1100–1109. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rong L, Li N and Zhang Z: Emerging
therapies for glioblastoma: Current state and future directions. J
Exp Clin Cancer Res. 41:1422022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Alghasham AA: Cucurbitacins-a promising
target for cancer therapy. Int J Health Sci (Qassim). 7:77–89.
2013.PubMed/NCBI
|
|
7
|
Si W, Lyu J, Liu Z, Wang C, Huang J, Jiang
L and Ma T: Cucurbitacin E inhibits cellular proliferation and
enhances the chemo-response in gastric cancer by suppressing AKt
activation. J Cancer. 10:5843–5851. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Feng H, Zang L, Zhao ZX and Kan QC:
Cucurbitacin-E inhibits multiple cancer cells proliferation through
attenuation of Wnt/β-catenin signaling. Cancer Biother Radiopharm.
29:210–214. 2014.PubMed/NCBI
|
|
9
|
He X, Gao Q, Qiang Y, Guo W and Ma Y:
Cucurbitacin E induces apoptosis of human prostate cancer cells via
cofilin-1 and mTORC1. Oncol Lett. 13:4905–4910. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kong Y, Chen J, Zhou Z, Xia H, Qiu MH and
Chen C: Cucurbitacin E induces cell cycle G2/M phase arrest and
apoptosis in triple negative breast cancer. PLoS One.
9:e1037602014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Duncan KL, Duncan MD, Alley MC and
Sausville EA: Cucurbitacin E-induced disruption of the actin and
vimentin cytoskeleton in prostate carcinoma cells. Biochem
Pharmacol. 52:1553–1560. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Momma K, Masuzawa Y, Nakai N, Chujo M,
Murakami A, Kioka N, Kiyama Y, Akita T and Nagao M: Direct
interaction of cucurbitacin E isolated from Alsomitra macrocarpa to
actin filament. Cytotechnology. 56:33–39. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dong Y, Lu B, Zhang X, Zhang J, Lai L, Li
D, Wu Y, Song Y, Luo J, Pang X, et al: Cucurbitacin E, a
tetracyclic triterpenes compound from Chinese medicine, inhibits
tumor angiogenesis through VEGFR2-mediated Jak2-STAT3 signaling
pathway. Carcinogenesis. 31:2097–2104. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hsu PC, Tian B, Yang YL, Wang YC, Liu S,
Urisman A, Yang CT, Xu Z, Jablons DM and You L: Cucurbitacin E
inhibits the Yes-associated protein signaling pathway and
suppresses brain metastasis of human non-small cell lung cancer in
a murine model. Oncol Rep. 42:697–707. 2019.PubMed/NCBI
|
|
15
|
Wang Y, Xu S, Wu Y and Zhang J:
Cucurbitacin E inhibits osteosarcoma cells proliferation and
invasion through attenuation of PI3K/AKT/mTOR signalling pathway.
Biosci Rep. 36:e004052016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang P, Lian Q, Fu R, Ding GB, Amin S and
Li Z and Li Z: Cucurbitacin E triggers cellular senescence in colon
cancer cells via regulating the miR-371b-5p/TFAP4 signaling
pathway. J Agric Food Chem. 70:2936–2947. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hsu YC, Chen MJ and Huang TY: Inducement
of mitosis delay by cucurbitacin E, a novel tetracyclic triterpene
from climbing stem of Cucumis melo L., through GADD45γ in
human brain malignant glioma (GBM) 8401 cells. Cell Death Dis.
5:e10872014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cheng AC, Hsu YC and Tsai CC: The effects
of cucurbitacin E on GADD45β-trigger G2/M arrest and
JNK-independent pathway in brain cancer cells. J Cell Mol Med.
23:3512–3519. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lemmon MA and Schlessinger J: Cell
signaling by receptor tyrosine kinases. Cell. 141:1117–1134. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sigismund S, Avanzato D and Lanzetti L:
Emerging functions of the EGFR in cancer. Mol Oncol. 12:3–20. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rajaram P, Chandra P, Ticku S, Pallavi BK,
Rudresh KB and Mansabdar P: Epidermal growth factor receptor: Role
in human cancer. Indian J Dent Res. 28:687–694. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lui VW, Thomas SM, Zhang Q, Wentzel AL,
Siegfried JM, Li JY and Grandis JR: Mitogenic effects of
gastrin-releasing peptide in head and neck squamous cancer cells
are mediated by activation of the epidermal growth factor receptor.
Oncogene. 22:6183–6193. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mishra R, Hanker AB and Garrett JT:
Genomic alterations of ERBB receptors in cancer: Clinical
implications. Oncotarget. 8:114371–114392. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mitra SK, Mikolon D, Molina JE, Hsia DA,
Hanson DA, Chi A, Lim ST, Bernard-Trifilo JA, Ilic D, Stupack DG,
et al: Intrinsic FAK activity and Y925 phosphorylation facilitate
an angiogenic switch in tumors. Oncogene. 25:5969–5984. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ilić D, Furuta Y, Kanazawa S, Takeda N,
Sobue K, Nakatsuji N, Nomura S, Fujimoto J, Okada M and Yamamoto T:
Reduced cell motility and enhanced focal adhesion contact formation
in cells from FAK-deficient mice. Nature. 377:539–544. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Golubovskaya V, Beviglia L, Xu LH, Earp HS
III, Craven R and Cance W: Dual inhibition of focal adhesion kinase
and epidermal growth factor receptor pathways cooperatively induces
death receptor-mediated apoptosis in human breast cancer cells. J
Biol Chem. 277:38978–38987. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Revathidevi S and Munirajan AK: Akt in
cancer: Mediator and more. Semin Cancer Biol. 59:80–91. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xie Y, Du J, Liu Z, Zhang D, Yao X and
Yang Y: MiR-6875-3p promotes the proliferation, invasion and
metastasis of hepatocellular carcinoma via BTG2/FAK/Akt pathway. J
Exp Clin Cancer Res. 38:72019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen X, Guo ZQ, Cao D, Chen Y and Chen J:
MYC-mediated upregulation of PNO1 promotes glioma tumorigenesis by
activating THBS1/FAK/Akt signaling. Cell Death Dis. 12:2442021.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fan Z, Xu Q, Wang C, Lin X, Zhang Q and Wu
N: A tropomyosin-like meretrix meretrix linnaeus polypeptide
inhibits the proliferation and metastasis of glioma cells via
microtubule polymerization and FAK/Akt/MMPs signaling. Int J Biol
Macromol. 145:154–164. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang JF, Chen YY, Zhang SW, Zhao K, Qiu Y,
Wang Y, Wang JC, Yu Z, Li BP, Wang Z and Chen JQ: ITGA5 promotes
tumor progression through the activation of the FAK/AKT signaling
pathway in human gastric cancer. Oxid Med Cell Longev.
2022:86113062022.PubMed/NCBI
|
|
32
|
Benelli R, Monteghirfo S, Venè R, Tosetti
F and Ferrari N: The chemopreventive retinoid 4HPR impairs prostate
cancer cell migration and invasion by interfering with
FAK/AKT/GSK3beta pathway and beta-catenin stability. Mol Cancer.
9:1422010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xie X, Lin W, Zheng W, Chen T, Yang H, Sun
L, Huang F, Wang Z, Lin H, Chen L, et al: Downregulation of
G2/mitotic-specific cyclinB1 triggers autophagy via
AMPK-ULK1-dependent signal pathway in nasopharyngeal carcinoma
cells. Cell Death Dis. 10:942019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yuan J, Yan R, Krämer A, Eckerdt F, Roller
M, Kaufmann M and Strebhardt K: Cyclin B1 depletion inhibits
proliferation and induces apoptosis in human tumor cells. Oncogene.
23:5843–5852. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Qi Y, Wang D, Huang W, Wang B, Huang D,
Xiong F, Chen X and Chen Y: CyclinD1 inhibits dicer and crucial
miRNA expression by chromatin modification to promote the
progression of intrahepatic cholangiocarcinoma. J Exp Clin Cancer
Res. 38:4132019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bian ZQ, Luo Y, Guo F, Huang YZ, Zhong M
and Cao H: Overexpressed ACP5 has prognostic value in colorectal
cancer and promotes cell proliferation and tumorigenesis via
FAK/PI3K/AKT signaling pathway. Am J Cancer Res. 9:22–35.
2019.PubMed/NCBI
|
|
37
|
Zhang B, Ma X, Li Y, Li S and Cheng J:
Pleuromutilin inhibits proliferation and migration of A2780 and
Caov-3 ovarian carcinoma cells and growth of mouse A2780 tumor
xenografts by down-regulation of pFAK2. Med Sci Monit.
26:e9204072020.PubMed/NCBI
|
|
38
|
Nuñez RE, del Valle MM, Ortiz K, Almodovar
L and Kucheryavykh L: Microglial cytokines induce invasiveness and
proliferation of human glioblastoma through Pyk2 and FAK
activation. Cancers (Basel). 13:61602021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Toyama M, Hamaoka Y and Katoh H: EphA3 is
up-regulated by epidermal growth factor and promotes formation of
glioblastoma cell aggregates. Biochem Biophys Res Commun.
508:715–721. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zou Q, Hou Y, Shen F and Wang Y: Polarized
regulation of glycogen synthase kinase-3β is important for glioma
cell invasion. PLoS One. 8:e818142013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang T, Li J, Dong Y, Zhai D, Lai L, Dai
F, Deng H, Chen Y, Liu M and Yi Z: Cucurbitacin E inhibits breast
tumor metastasis by suppressing cell migration and invasion. Breast
Cancer Res Treat. 135:445–458. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zha QB, Zhang XY, Lin QR, Xu LH, Zhao GX,
Pan H, Zhou D, Ouyang DY, Liu ZH and He XH: Cucurbitacin E induces
autophagy via downregulating mTORC1 signaling and upregulating AMPK
activity. PLoS One. 10:e01243552015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wu YL, Zhang YJ, Yao YL, Li ZM, Han X,
Lian LH, Zhao YQ and Nan JX: Cucurbitacin E ameliorates hepatic
fibrosis in vivo and in vitro through activation of AMPK and
blocking mTOR-dependent signaling pathway. Toxicol Lett.
258:147–158. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Jia Q, Cheng W, Yue Y, Hu Y, Zhang J, Pan
X, Xu Z and Zhang P: Cucurbitacin E inhibits TNF-α-induced
inflammatory cytokine production in human synoviocyte MH7A cells
via suppression of PI3K/Akt/NF-κB pathways. Int Immunopharmacol.
29:884–890. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Juríková M, Danihel Ľ, Polák Š and Varga
I: Ki67, PCNA, and MCM proteins: Markers of proliferation in the
diagnosis of breast cancer. Acta Histochem. 118:544–552. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhang J and Hochwald SN: The role of FAK
in tumor metabolism and therapy. Pharmacol Ther. 142:154–163. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sulzmaier FJ, Jean C and Schlaepfer DD:
FAK in cancer: Mechanistic findings and clinical applications. Nat
Rev Cancer. 14:598–610. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yoon H, Dehart JP, Murphy JM and Lim STS:
Understanding the roles of FAK in cancer: Inhibitors, genetic
models, and new insights. J Histochem Cytochem. 63:114–128. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Sharma AK, Kline CL, Berg A, Amin S and
Irby RB: The Akt inhibitor ISC-4 activates prostate apoptosis
response protein-4 and reduces colon tumor growth in a nude mouse
model. Clin Cancer Res. 17:4474–4483. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Gao F, Huang W, Zhang Y, Tang S, Zheng L,
Ma F, Wang Y, Tang H and Li X: Hes1 promotes cell proliferation and
migration by activating Bmi-1 and PTEN/Akt/GSK3β pathway in human
colon cancer. Oncotarget. 6:38667–38680. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Liu X, Wang P, Zhang C and Ma Z: Epidermal
growth factor receptor (EGFR): A rising star in the era of
precision medicine of lung cancer. Oncotarget. 8:50209–50220. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yarden Y: The EGFR family and its ligands
in human cancer. Signalling mechanisms and therapeutic
opportunities. Eur J Cancer. 37 (Suppl 4):S3–S8. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Laurent-Puig P, Lievre A and Blons H:
Mutations and response to epidermal growth factor receptor
inhibitors. Clin Cancer Res. 15:1133–1139. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Long W, Yi P, Amazit L, LaMarca HL,
Ashcroft F, Kumar R, Mancini MA, Tsai SY, Tsai MJ and O'Malley BW:
SRC-3Delta4 mediates the interaction of EGFR with FAK to promote
cell migration. Mol Cell. 37:321–332. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Li M, Zheng W and Wang C: CyclinD1
promotes lymph node metastasis by inducing lymphangiogenesis in
human ovarian carcinoma. Int J Clin Exp Pathol. 11:3726–3731.
2018.PubMed/NCBI
|
|
56
|
Tang Y, Xie M, Jiang N, Huang F, Zhang X,
Li R, Lu J, Liao S and Liu Y: Icarisid II inhibits the
proliferation of human osteosarcoma cells by inducing apoptosis and
cell cycle arrest. Tumour Biol. 39:10104283177057452017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Wei Y, Huang C, Wu H and Huang J: Estrogen
receptor beta (ERβ) mediated-cyclinD1 degradation via autophagy
plays an anti-proliferation role in colon cells. Int J Biol Sci.
15:942–952. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Rattanaburee T, Tipmanee V, Tedasen A,
Thongpanchang T and Graidist P: Inhibition of CSF1R and AKT by
(±)-kusunokinin hinders breast cancer cell proliferation. Biomed
Pharmacother. 129:1103612020. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Hu Y, Cheng Y, Jiang X, Zhang Y, Wang H,
Ren H, Xu Y, Jiang J, Wang Q, Su H, et al: PCGF3 promotes the
proliferation and migration of non-small cell lung cancer cells via
the PI3K/AKT signaling pathway. Exp Cell Res. 400:1124962021.
View Article : Google Scholar : PubMed/NCBI
|