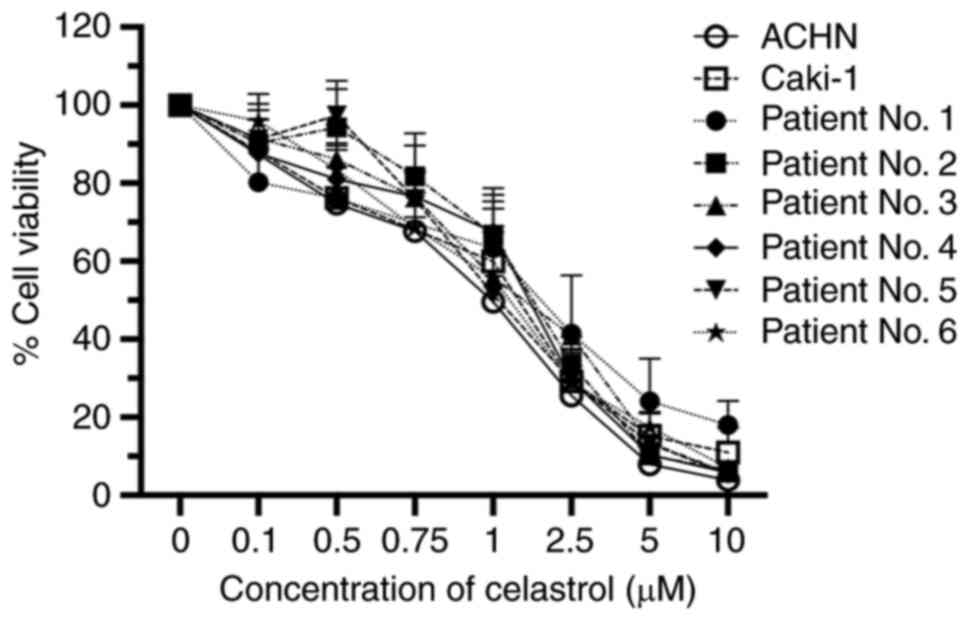
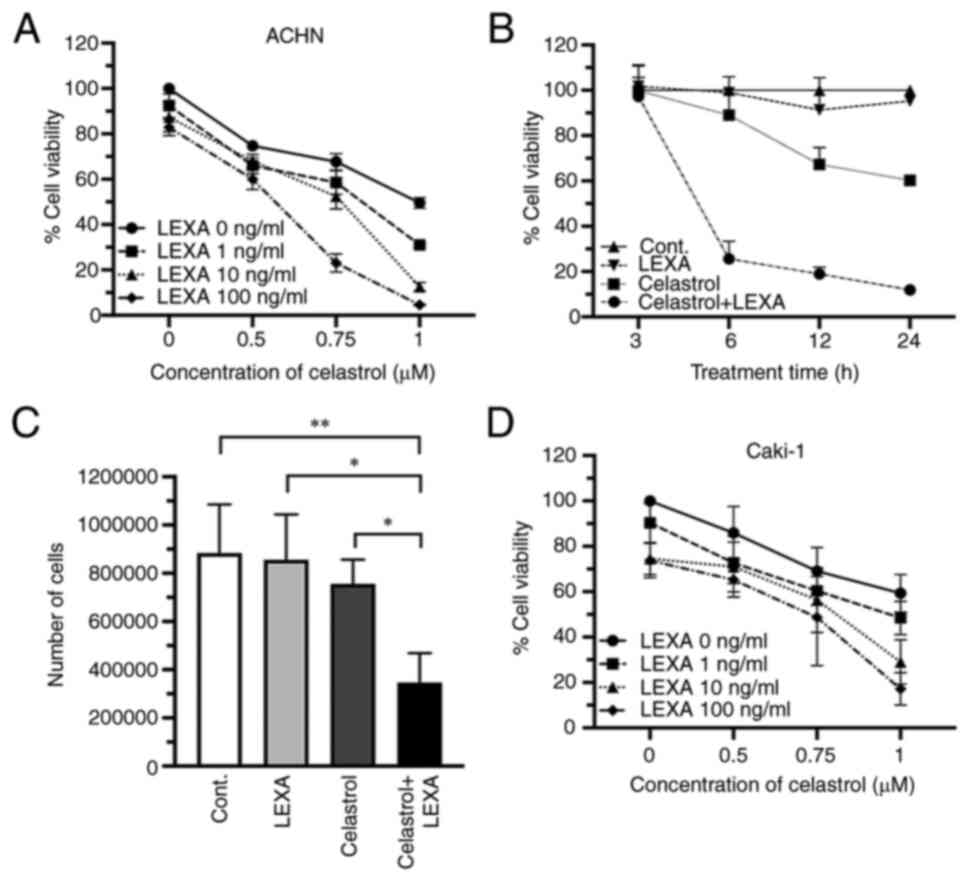
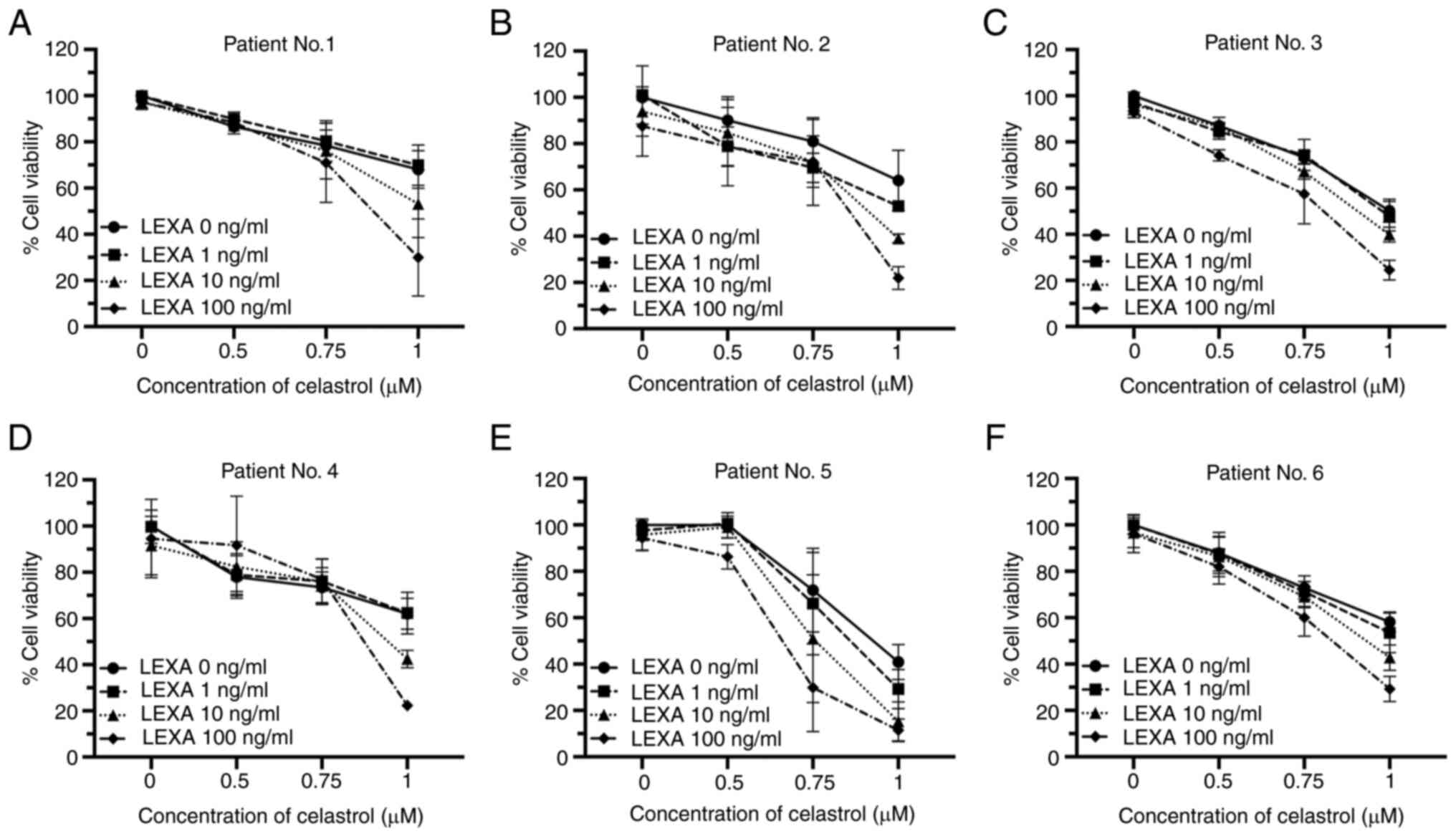
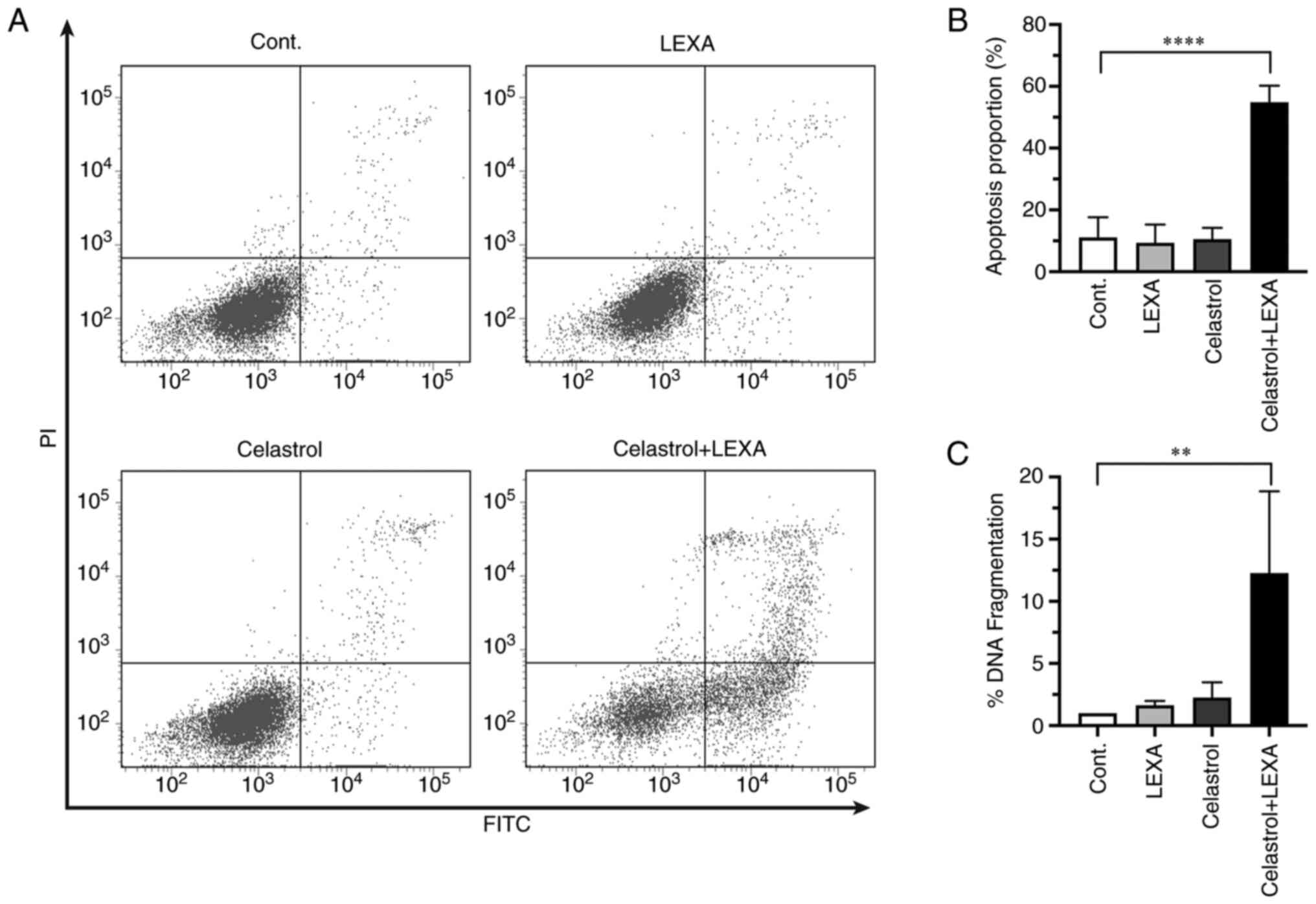
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Albiges L, Oudard S, Negrier S, Caty A,
Gravis G, Joly F, Duclos B, Geoffrois L, Rolland F, Guillot A, et
al: Complete remission with tyrosine kinase inhibitors in renal
cell carcinoma. J Clin Oncol. 30:482–487. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Motzer RJ, Powles T, Burotto M, Escudier
B, Bourlon MT, Shah AY, Suárez C, Hamzaj A, Porta C, Hocking CM, et
al: Nivolumab plus cabozantinib versus sunitinib in first-line
treatment for advanced renal cell carcinoma (CheckMate 9ER):
Long-term follow-up results from an open-label, randomised, phase 3
trial. Lancet Oncol. 23:888–898. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Motzer RJ, Rini BI, McDermott DF, Arén
Frontera O, Hammers HJ, Carducci MA, Salman P, Escudier B,
Beuselinck B, Amin A, et al: Nivolumab plus ipilimumab versus
sunitinib in first-line treatment for advanced renal cell
carcinoma: Extended follow-up of efficacy and safety results from a
randomised, controlled, phase 3 trial. Lancet Oncol. 20:1370–1385.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Powles T, Plimack ER, Soulières D, Waddell
T, Stus V, Gafanov R, Nosov D, Pouliot F, Melichar B, Vynnychenko
I, et al: Pembrolizumab plus axitinib versus sunitinib monotherapy
as first-line treatment of advanced renal cell carcinoma
(KEYNOTE-426): Extended follow-up from a randomised, open-label,
phase 3 trial. Lancet Oncol. 21:1563–1573. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Martins F, Sofiya L, Sykiotis GP, Lamine
F, Maillard M, Fraga M, Shabafrouz K, Ribi C, Cairoli A,
Guex-Crosier Y, et al: Adverse effects of immune-checkpoint
inhibitors: Epidemiology, management and surveillance. Nat Rev Clin
Oncol. 16:563–580. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Walczak H, Miller RE, Ariail K, Gliniak B,
Griffith TS, Kubin M, Chin W, Jones J, Woodward A, Le T, et al:
Tumoricidal activity of tumor necrosis factor-related
apoptosis-inducing ligand in vivo. Nat Med. 5:157–163. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ashkenazi A and Dixit VM: Death receptors:
Signaling and modulation. Science. 281:1305–1308. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chuntharapai A, Dodge K, Grimmer K,
Schroeder K, Marsters SA, Koeppen H, Ashkenazi A and Kim KJ:
Isotype-dependent inhibition of tumor growth in vivo by monoclonal
antibodies to death receptor 4. J Immunol. 166:4891–4898. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ichikawa K, Liu W, Zhao L, Wang Z, Liu D,
Ohtsuka T, Zhang H, Mountz JD, Koopman WJ, Kimberly RP and Zhou T:
Tumoricidal activity of a novel anti-human DR5 monoclonal antibody
without hepatocyte cytotoxicity. Nat Med. 7:954–960. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zeng Y, Wu XX, Fiscella M, Shimada O,
Humphreys R, Albert V and Kakehi Y: Monoclonal antibody to tumor
necrosis factor-related apoptosis-inducing ligand receptor 2
(TRAIL-R2) induces apoptosis in primary renal cell carcinoma cells
in vitro and inhibits tumor growth in vivo. Int J Oncol.
28:421–430. 2006.PubMed/NCBI
|
|
12
|
Shimada O, Wu X, Jin X, Nouh MA, Fiscella
M, Albert V, Matsuda T and Kakehi Y: Human agonistic antibody to
tumor necrosis factor-related apoptosis-inducing ligand receptor 2
induces cytotoxicity and apoptosis in prostate cancer and bladder
cancer cells. Urology. 69:395–401. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Szliszka E, Mazur B, Zydowicz G, Czuba ZP
and Król W: TRAIL-induced apoptosis and expression of death
receptor TRAIL-R1 and TRAIL-R2 in bladder cancer cells. Folia
Histochem Cytobiol. 47:579–585. 2009.PubMed/NCBI
|
|
14
|
Wu XX, Jin XH, Zeng Y, El Hamed AM and
Kakehi Y: Low concentrations of doxorubicin sensitizes human solid
cancer cells to tumor necrosis factor-related apoptosis-inducing
ligand (TRAIL)-receptor (R) 2-mediated apoptosis by inducing
TRAIL-R2 expression. Cancer Sci. 98:1969–1976. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Allison AC, Cacabelos R, Lombardi VR,
Alvarez XA and Vigo C: Celastrol, a potent antioxidant and
anti-inflammatory drug, as a possible treatment for Alzheimer's
disease. Prog Neuropsychopharmacol Biol Psychiatry. 25:1341–1357.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang H, Chen D, Cui QC, Yuan X and Dou QP:
Celastrol, a triterpene extracted from the Chinese ‘Thunder of God
Vine,’ is a potent proteasome inhibitor and suppresses human
prostate cancer growth in nude mice. Cancer Res. 66:4758–4765.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhu Y, Liu X, Zhao P, Zhao H, Gao W and
Wang L: Celastrol suppresses glioma vasculogenic mimicry formation
and angiogenesis by blocking the PI3K/Akt/mTOR signaling pathway.
Front Pharmacol. 11:252020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen G, Zhu X, Li J, Zhang Y, Wang X,
Zhang R, Qin X, Chen X, Wang J, Liao W, et al: Celastrol inhibits
lung cancer growth by triggering histone acetylation and acting
synergically with HDAC inhibitors. Pharmacol Res. 185:1064872022.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ni H, Han Y and Jin X: Celastrol inhibits
colon cancer cell proliferation by downregulating miR-21 and
PI3K/AKT/GSK-3β pathway. Int J Clin Exp Pathol. 12:808–816.
2019.PubMed/NCBI
|
|
20
|
Wang X, Liu Q, Wu S, Xu N, Li H and Feng
A: Identifying the effect of celastrol against ovarian cancer with
network pharmacology and in vitro experiments. Front Pharmacol.
13:7394782022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee JH, Won YS, Park KH, Lee MK, Tachibana
H, Yamada K and Seo KI: Celastrol inhibits growth and induces
apoptotic cell death in melanoma cells via the activation
ROS-dependent mitochondrial pathway and the suppression of PI3K/AKT
signaling. Apoptosis. 17:1275–1286. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li HY, Zhang J, Sun LL, Li BH, Gao HL, Xie
T, Zhang N and Ye ZM: Celastrol induces apoptosis and autophagy via
the ROS/JNK signaling pathway in human osteosarcoma cells: An in
vitro and in vivo study. Cell Death Dis. 6:e16042015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang CJ, Zhu N, Long J, Wu HT, Wang YX,
Liu BY, Liao DF and Qin L: Celastrol induces lipophagy via the
LXRα/ABCA1 pathway in clear cell renal cell carcinoma. Acta
Pharmacol Sin. 42:1472–1485. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu H, Zhao H, Ding C, Jiang D, Zhao Z, Li
Y, Ding X, Gao J, Zhou H, Luo C, et al: Celastrol suppresses
colorectal cancer via covalent targeting peroxiredoxin 1. Signal
Transduct Target Ther. 8:512023. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhu H, Ding WJ, Wu R, Weng QJ, Lou JS, Jin
RJ, Lu W, Yang B and He QJ: Synergistic anti-cancer activity by the
combination of TRAIL/APO-2L and celastrol. Cancer Invest. 28:23–32.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cha Z, Cheng J, Xiang H, Qin J, He Y, Peng
Z, Jia J and Yu H: Celastrol enhances TRAIL-induced apoptosis in
human glioblastoma via the death receptor pathway. Cancer Chemother
Pharmacol. 84:719–728. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen SR, Dai Y, Zhao J, Lin L and Wang Y
and Wang Y: A mechanistic overview of triptolide and celastrol,
natural products from Tripterygium wilfordii Hook F. Front
Pharmacol. 9:1042018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pukac L, Kanakaraj P, Humphreys R,
Alderson R, Bloom M, Sung C, Riccobene T, Johnson R, Fiscella M,
Mahoney A, et al: HGS-ETR1, a fully human TRAIL-receptor 1
monoclonal antibody, induces cell death in multiple tumour types in
vitro and in vivo. Br J Cancer. 92:1430–1441. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Luster TA, Carrell JA, McCormick K, Sun D
and Humphreys R: Mapatumumab and lexatumumab induce apoptosis in
TRAIL-R1 and TRAIL-R2 antibody-resistant NSCLC cell lines when
treated in combination with bortezomib. Mol Cancer Ther. 8:292–302.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Marini P, Denzinger S, Schiller D, Kauder
S, Welz S, Humphreys R, Daniel PT, Jendrossek V, Budach W and Belka
C: Combined treatment of colorectal tumours with agonistic TRAIL
receptor antibodies HGS-ETR1 and HGS-ETR2 and radiotherapy:
Enhanced effects in vitro and dose-dependent growth delay in vivo.
Oncogene. 25:5145–5154. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mizutani Y, Bonavida B, Fukumoto M and
Yoshida O: Enhanced susceptibility of c-myc antisense
oligonucleotide-treated human renal cell carcinoma cells to lysis
by peripheral blood lymphocytes. J Immunother Emphasis Tumor
Immunol. 17:78–87. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bao Y, Wu X, Jin X, Kanematsu A, Nojima M,
Kakehi Y and Yamamoto S: Apigenin inhibits renal cell carcinoma
cell proliferation through G2/M phase cell cycle arrest. Oncol Rep.
47:602022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xu SP, Sun GP, Shen YX, Peng WR, Wang H
and Wei W: Synergistic effect of combining paeonol and cisplatin on
apoptotic induction of human hepatoma cell lines. Acta Pharmacol
Sin. 28:869–878. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wu XX, Kakehi Y, Mizutani Y, Lu J, Terachi
T and Ogawa O: Activation of caspase-3 in renal cell carcinoma
cells by anthracyclines or 5-fluorouracil. Int J Oncol. 19:19–24.
2001.PubMed/NCBI
|
|
35
|
Amantana A, London CA, Iversen PL and Devi
GR: X-linked inhibitor of apoptosis protein inhibition induces
apoptosis and enhances chemotherapy sensitivity in human prostate
cancer cells. Mol Cancer Ther. 3:699–707. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu Q, Hilsenbeck S and Gazitt Y: Arsenic
trioxide-induced apoptosis in myeloma cells: p53-dependent G1 or
G2/M cell cycle arrest, activation of caspase-8 or caspase-9, and
synergy with APO2/TRAIL. Blood. 101:4078–4087. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Galligan L, Longley DB, McEwan M, Wilson
TR, McLaughlin K and Johnston PG: Chemotherapy and TRAIL-mediated
colon cancer cell death: The roles of p53, TRAIL receptors, and
c-FLIP. Mol Cancer Ther. 4:2026–2036. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lacour S, Hammann A, Wotawa A, Corcos L,
Solary E and Dimanche-Boitrel MT: Anticancer agents sensitize tumor
cells to tumor necrosis factor-related apoptosis-inducing
ligand-mediated caspase-8 activation and apoptosis. Cancer Res.
61:1645–1651. 2001.PubMed/NCBI
|
|
39
|
Derosier LC, Vickers SM, Zinn KR, Huang Z,
Wang W, Grizzle WE, Sellers J, Stockard CR Jr, Zhou T, Oliver PG,
et al: TRA-8 anti-DR5 monoclonal antibody and gemcitabine induce
apoptosis and inhibit radiologically validated orthotopic
pancreatic tumor growth. Mol Cancer Ther. 6:3198–3207. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
O'Kane HF, Watson CJ, Johnston SR, Petak
I, Watson RW and Williamson KE: Targeting death receptors in
bladder, prostate and renal cancer. J Urol. 175:432–438. 2006.
View Article : Google Scholar : PubMed/NCBI
|