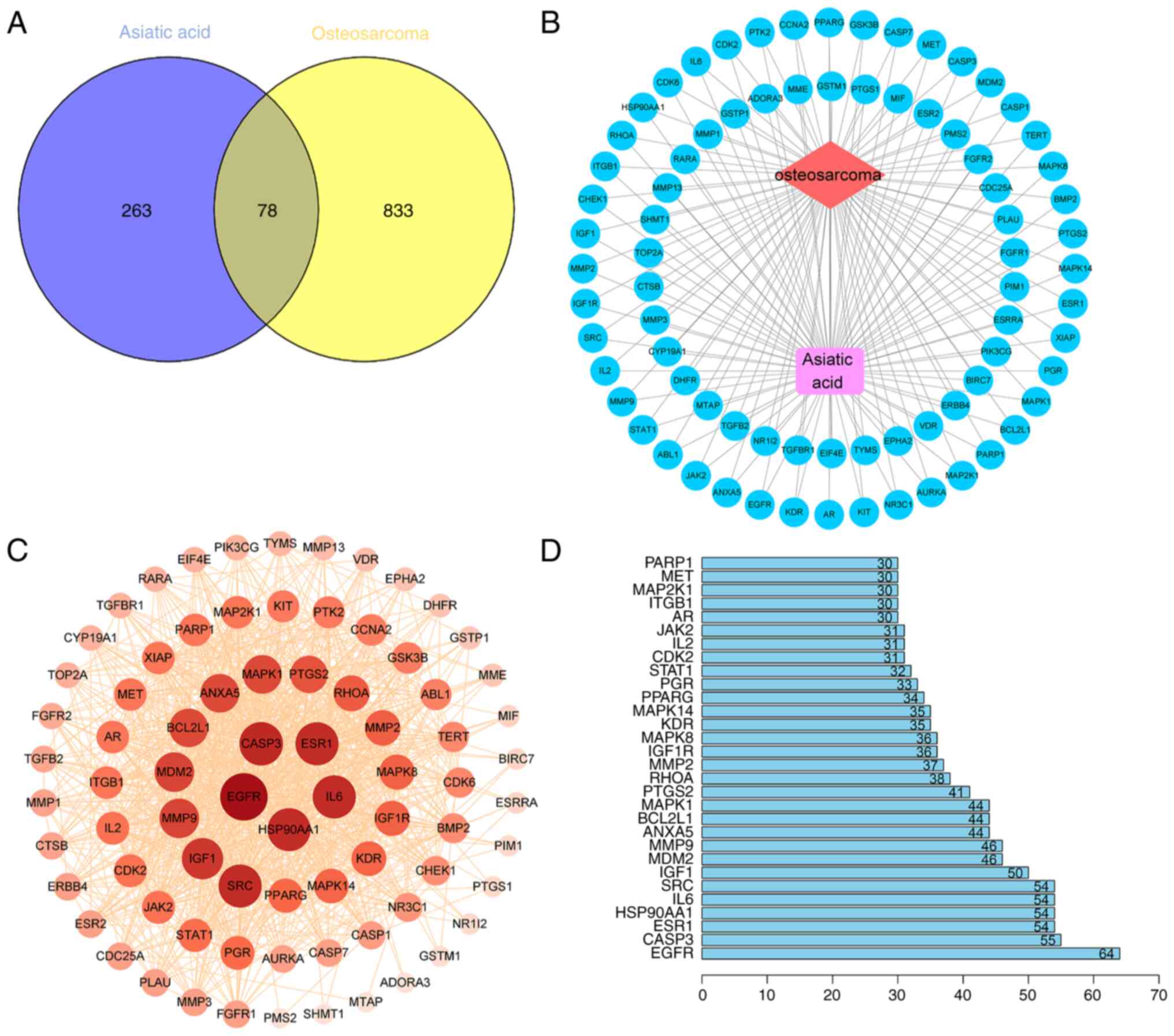
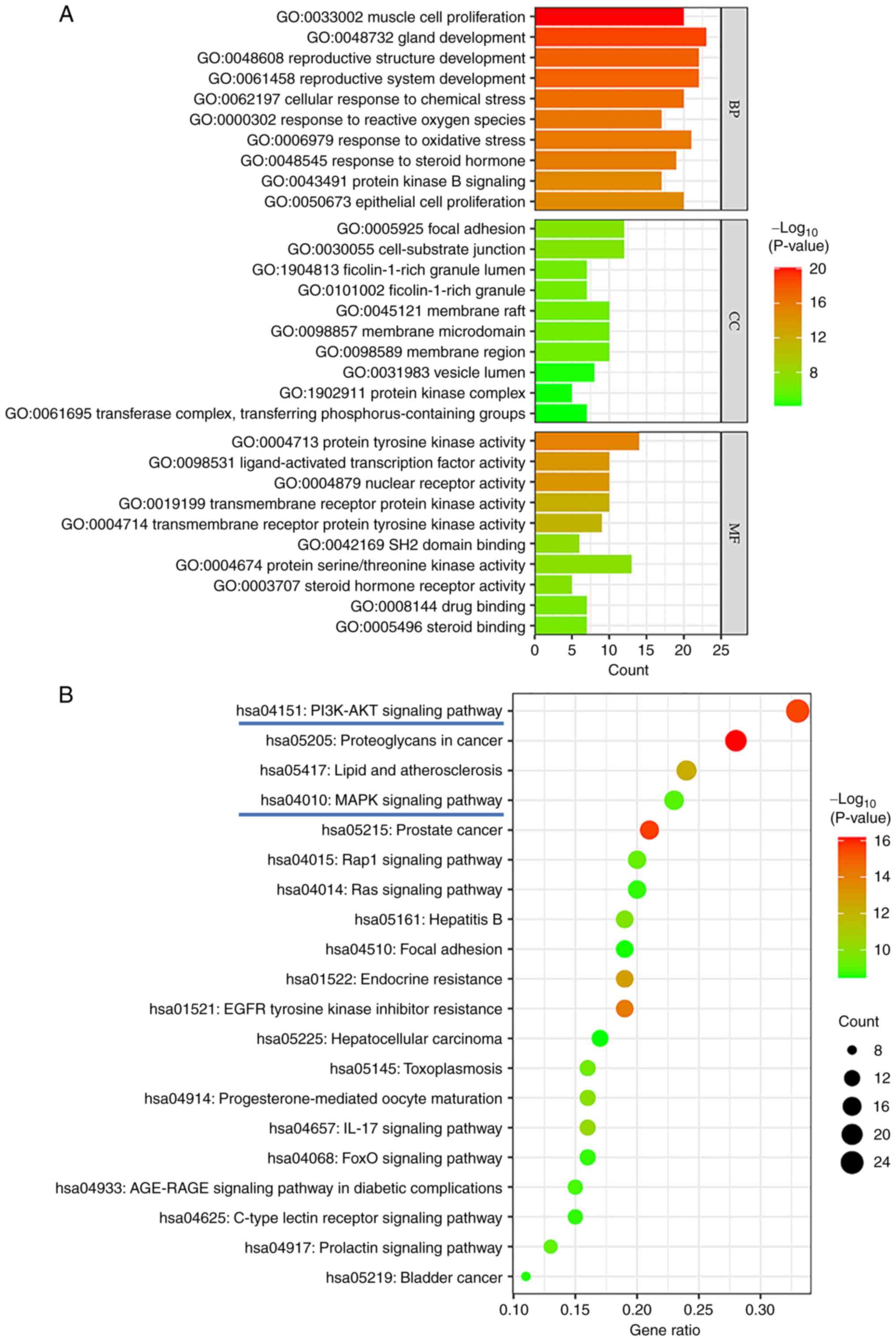
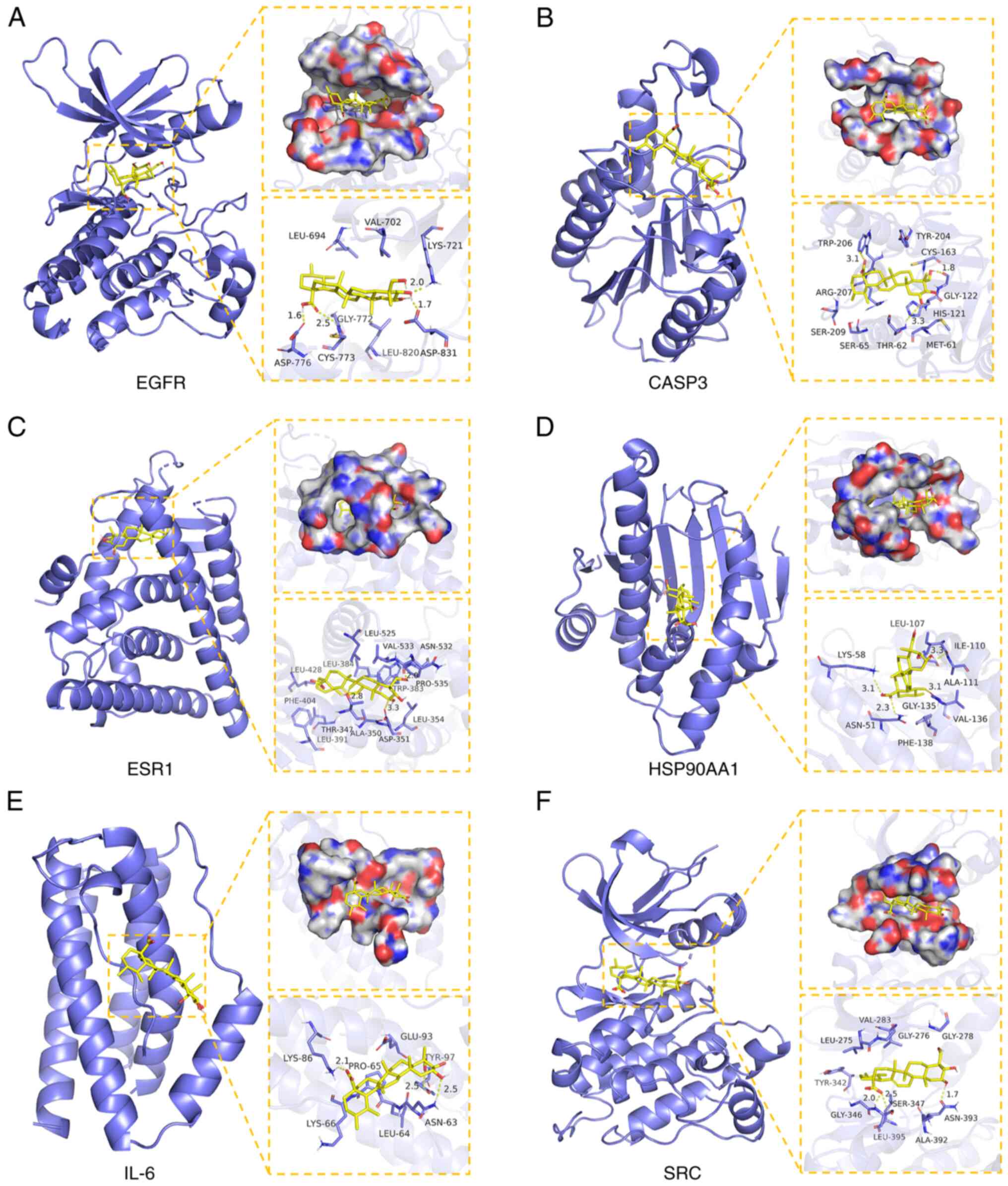
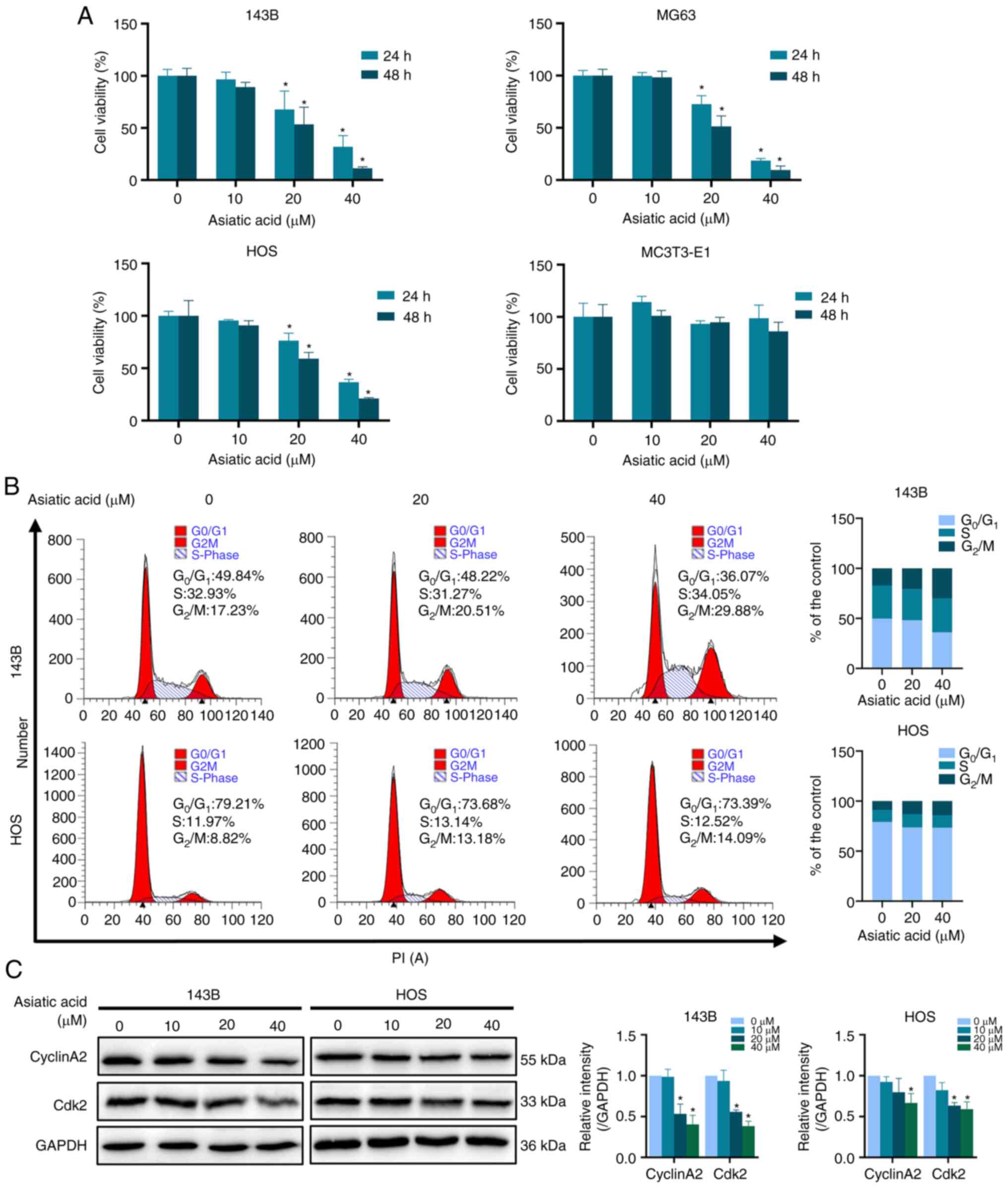
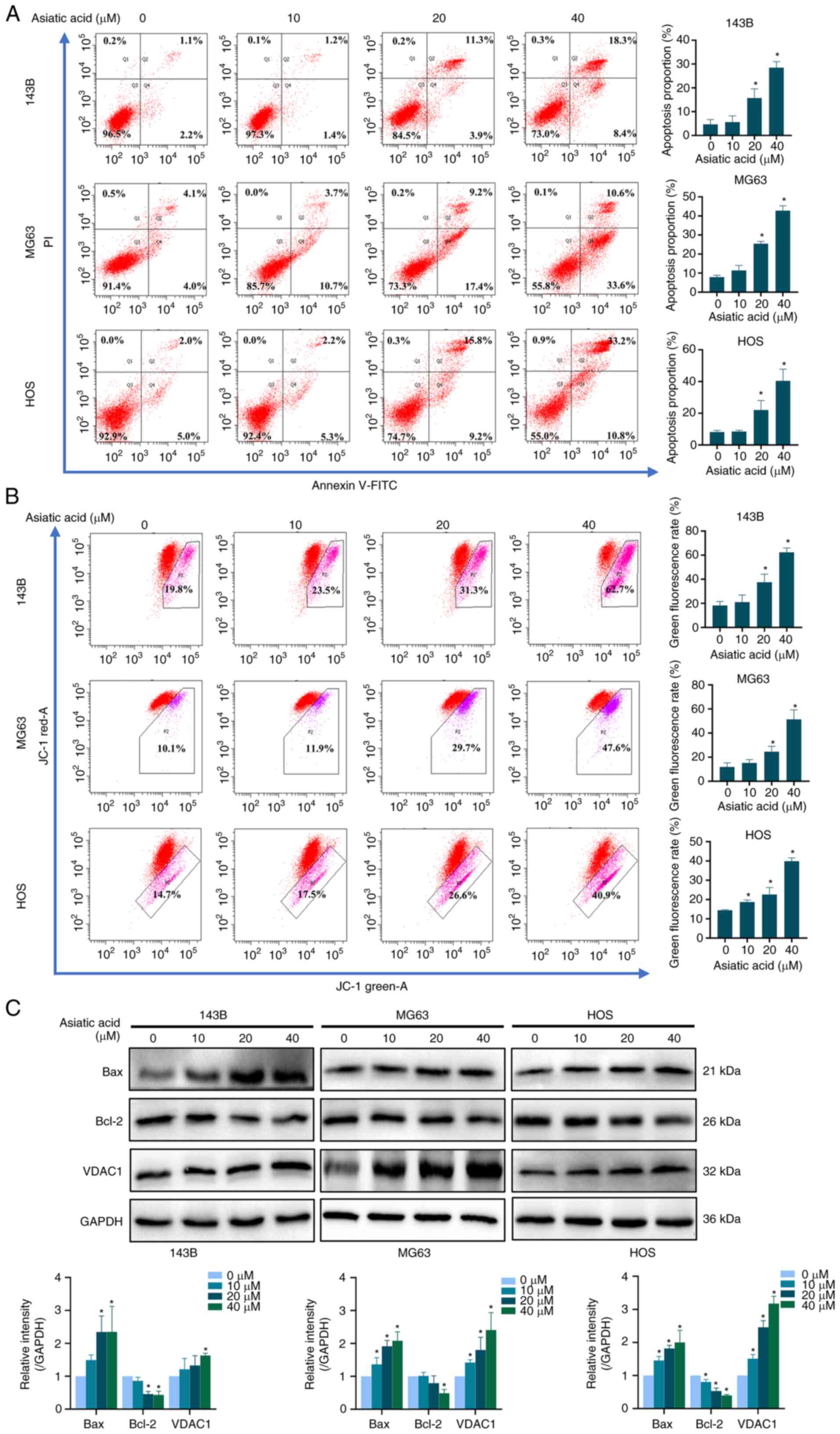
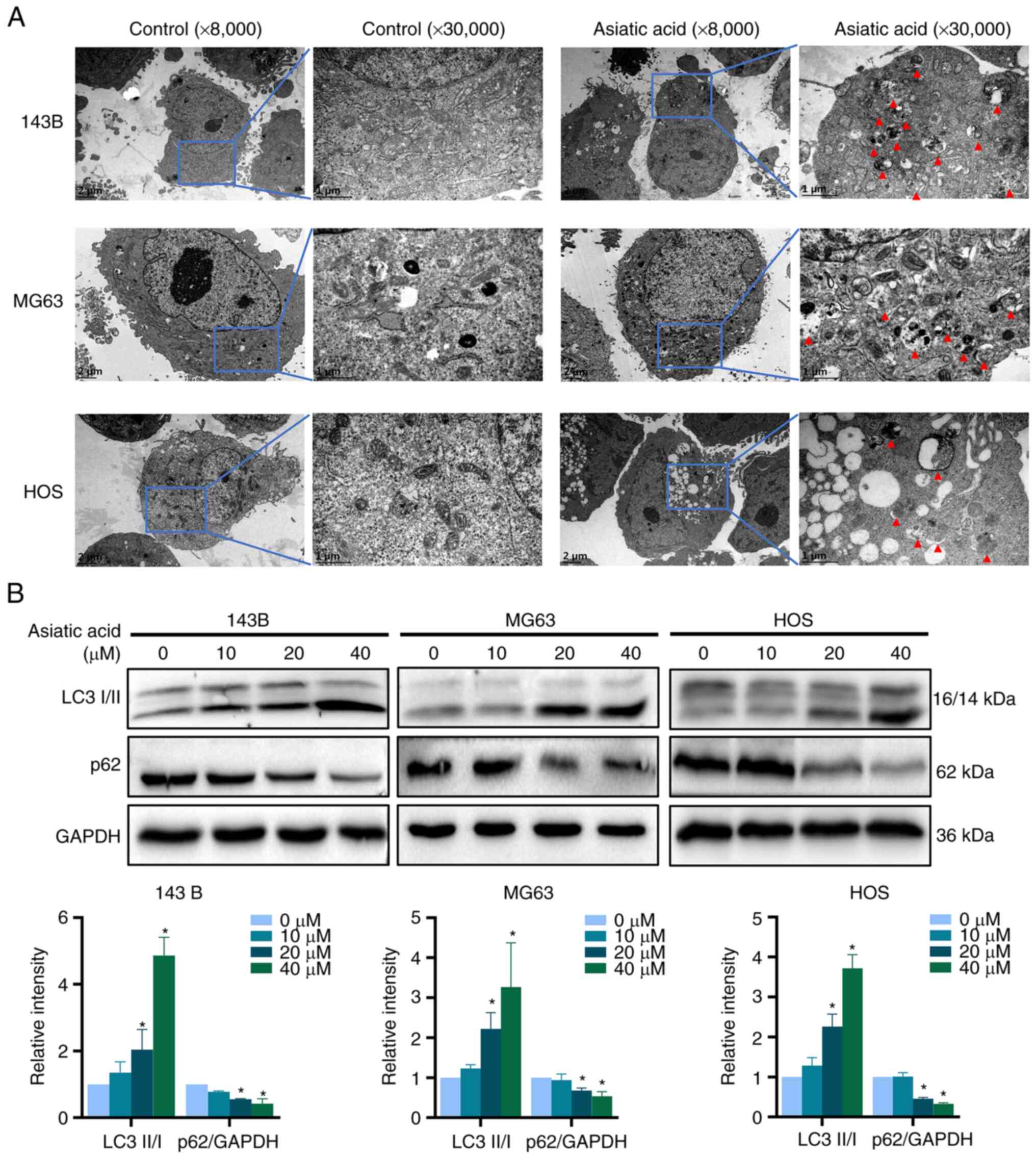
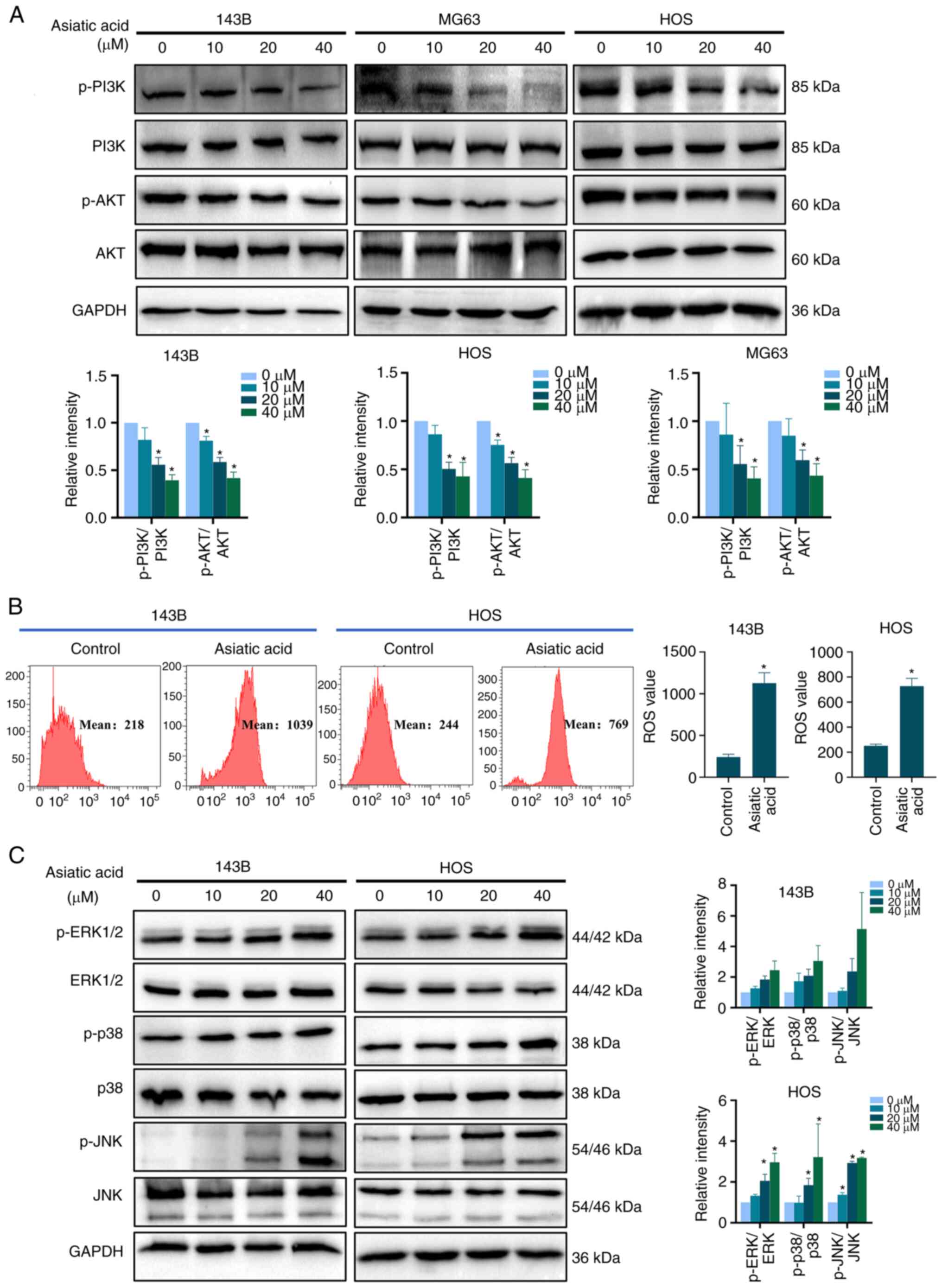
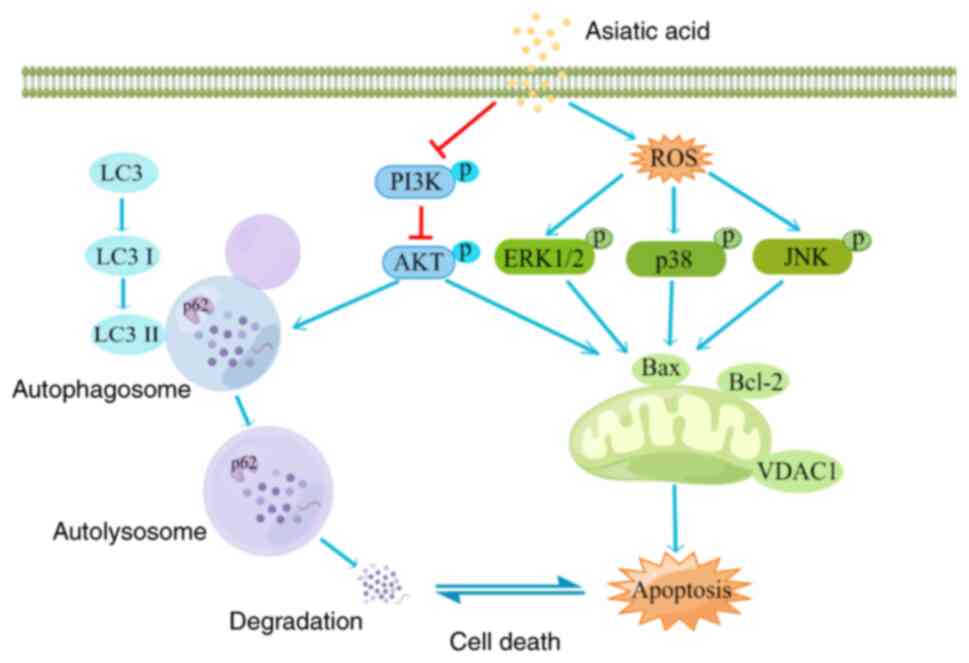
|
1
|
Karadurmus N, Sahin U, Bahadir Basgoz B
and Demirer T: Is there a role of high dose chemotherapy and
autologous stem cell transplantation in the treatment of Ewing's
sarcoma and osteosarcomas? J BUON. 23:1235–1241. 2018.PubMed/NCBI
|
|
2
|
Zhu T, Han J, Yang L, Cai Z, Sun W, Hua Y
and Xu J: Immune microenvironment in osteosarcoma: Components,
therapeutic strategies and clinical applications. Front Immunol.
13:9075502022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zheng C, Tang F, Min L, Hornicek F, Duan Z
and Tu C: PTEN in osteosarcoma: Recent advances and the therapeutic
potential. Biochim Biophys Acta Rev Cancer. 1874:1884052020.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cortini M, Avnet S and Baldini N:
Mesenchymal stroma: Role in osteosarcoma progression. Cancer Lett.
405:90–99. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mutsaers A and Walkley C: Cells of origin
in osteosarcoma: Mesenchymal stem cells or osteoblast committed
cells? Bone. 62:56–63. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cascini C and Chiodoni C: The immune
landscape of osteosarcoma: Implications for prognosis and treatment
response. Cells. 10:16682021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ghafouri-Fard S, Shirvani-Farsani Z,
Hussen B and Taheri M: The critical roles of lncRNAs in the
development of osteosarcoma. Biomed Pharmacother. 135:1112172021.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Osborne T and Khanna C: A review of the
association between osteosarcoma metastasis and protein
translation. J Comp Pathol. 146:132–142. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen C, Xie L, Ren T, Huang Y, Xu J and
Guo W: Immunotherapy for osteosarcoma: Fundamental mechanism,
rationale, and recent breakthroughs. Cancer Lett. 500:1–10. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Prudowsky Z and Yustein J: Recent insights
into therapy resistance in osteosarcoma. Cancers (Basel).
13:832020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Berner K, Johannesen TB, Berner A,
Haugland HK, Bjerkehagen B, Bøhler PJ and Bruland ØS: Time-trends
on incidence and survival in a nationwide and unselected cohort of
patients with skeletal osteosarcoma. Acta Oncol. 54:25–33. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Grinberg S, Posta A, Weber K and Wilson R:
Limb salvage and reconstruction options in osteosarcoma. Adv Exp
Med Biol. 1257:13–29. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bielack S, Jürgens H, Jundt G, Kevric M,
Kühne T, Reichardt P, Zoubek A, Werner M, Winkelmann W and Kotz R:
Osteosarcoma: The COSS experience. Cancer Treat Res. 152:289–308.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bielack S, Kempf-Bielack B, Delling G,
Exner GU, Flege S, Helmke K, Kotz R, Salzer-Kuntschik M, Werner M,
Winkelmann W, et al: Prognostic factors in high-grade osteosarcoma
of the extremities or trunk: An analysis of 1,702 patients treated
on neoadjuvant cooperative osteosarcoma study group protocols. J
Clin Oncol. 20:776–790. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kager L, Zoubek A, Kastner U,
Kempf-Bielack B, Potratz J, Kotz R, Exner GU, Franzius C, Lang S,
Maas R, et al: Skip metastases in osteosarcoma: Experience of the
cooperative osteosarcoma study group. J Clin Oncol. 24:1535–1541.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang Y, Lou Y, Wang J, Yu C and Shen W:
Research status and molecular mechanism of the traditional chinese
medicine and antitumor therapy combined strategy based on tumor
microenvironment. Front Immunol. 11:6097052020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mioc M, Milan A, Malița D, Mioc A, Prodea
A, Racoviceanu R, Ghiulai R, Cristea A, Căruntu F and Șoica C:
Recent advances regarding the molecular mechanisms of triterpenic
acids: A review (Part I). Int J Mol Sci. 23:77402022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hong G, Zhou L, Han X, Sun P, Chen Z, He
W, Tickner J, Chen L, Shi X and Xu J: Asiatic acid inhibits
OVX-induced osteoporosis and osteoclastogenesis regulating
RANKL-mediated NF-κb and Nfatc1 signaling pathways. Front
Pharmacol. 11:3312020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sycz Z, Tichaczek-Goska D and Wojnicz D:
Anti-planktonic and Anti-biofilm properties of pentacyclic
triterpenes-asiatic acid and ursolic acid as promising
antibacterial future pharmaceuticals. Biomolecules. 12:982022.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Songvut P, Chariyavilaskul P, Tantisira M
and Khemawoot P: Safety and pharmacokinetics of standardized
extract of centella asiatica (ECa 233) Capsules in healthy thai
volunteers: A phase 1 clinical study. Planta Med. 85:483–490. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Palmer N and Kaldis P: Less-well known
functions of cyclin/CDK complexes. Semin Cell Dev Biol. 107:54–62.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jirawatnotai S, Dalton S and
Wattanapanitch M: Role of cyclins and cyclin-dependent kinases in
pluripotent stem cells and their potential as a therapeutic target.
Semin Cell Dev Biol. 107:63–71. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu W, Jin W, Zhu S, Chen Y and Liu B:
Targeting regulated cell death (RCD) with small-molecule compounds
in cancer therapy: A revisited review of apoptosis,
autophagy-dependent cell death and necroptosis. Drug Discov Today.
27:612–625. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Morana O, Wood W and Gregory C: The
apoptosis paradox in cancer. Int J Mol Sci. 23:13282022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Carneiro B and El-Deiry WS: Targeting
apoptosis in cancer therapy. Nat Rev Clin Oncol. 17:395–417. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fulda S: Targeting extrinsic apoptosis in
cancer: Challenges and opportunities. Semin Cell Dev Biol.
39:20–25. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tan Y, Zhang X, Zhang S, Zhu T, Garg M,
Lobie PE and Pandey V: Mitochondria: The metabolic switch of
cellular oncogenic transformation. Biochim Biophys Acta Rev Cancer.
1876:1885342021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Noguchi M, Hirata N, Tanaka T, Suizu F,
Nakajima H and Chiorini J: Autophagy as a modulator of cell death
machinery. Cell Death Dis. 11:5172020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gerada C and Ryan K: Autophagy, the innate
immune response and cancer. Mol Oncol. 14:1913–1929. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Braicu C, Zanoaga O, Zimta AA, Tigu AB,
Kilpatrick KL, Bishayee A, Nabavi SM and Berindan-Neagoe I: Natural
compounds modulate the crosstalk between apoptosis- and
autophagy-regulated signaling pathways: Controlling the
uncontrolled expansion of tumor cells. Semin Cancer Biol.
80:218–236. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
du Plessis M, Davis T, Loos B, Pretorius
E, de Villiers W and Engelbrecht A: Molecular regulation of
autophagy in a pro-inflammatory tumour microenvironment: New
insight into the role of serum amyloid A. Cytokine Growth Factor
Rev. 59:71–83. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jing Y, Liang W, Liu J, Zhang L, Wei J,
Yang J, Zhang Y and Huang Z: Autophagy-mediating microRNAs in
cancer chemoresistance. Cell Biol Toxicol. 36:517–536. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Miller D and Thorburn A: Autophagy and
organelle homeostasis in cancer. Dev Cell. 56:906–918. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ning B, Liu Y, Huang T and Wei Y:
Autophagy and its role in osteosarcoma. Cancer Med. 12:5676–5687.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Das C, Banerjee I and Mandal M:
Pro-survival autophagy: An emerging candidate of tumor progression
through maintaining hallmarks of cancer. Semin Cancer Biol.
66:59–74. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Long M and McWilliams T: Monitoring
autophagy in cancer: From bench to bedside. Semin Cancer Biol.
66:12–21. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gupta R, Ambasta R and Pravir K: Autophagy
and apoptosis cascade: Which is more prominent in neuronal death?
Cell Mol Life Sci. 78:8001–8047. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu K, Ren T, Huang Y, Sun K, Bao X, Wang
S, Zheng B and Guo W: Apatinib promotes autophagy and apoptosis
through VEGFR2/STAT3/BCL-2 signaling in osteosarcoma. Cell Death
Dis. 8:e30152017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hao DC and Xiao P: Network pharmacology: A
Rosetta stone for traditional Chinese medicine. Drug Dev Res.
75:299–312. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Stanzione F, Giangreco I and Cole J: Use
of molecular docking computational tools in drug discovery. Prog
Med Chem. 60:273–343. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bock FJ and Tait SWG: Mitochondria as
multifaceted regulators of cell death. Nat Rev Mol Cell Biol.
21:85–100. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
42. Mariño G, Niso-Santano M, Baehrecke E
and Kroemer G: Self-consumption: The interplay of autophagy and
apoptosis. Nat Rev Mol Cell Biol. 15:81–94. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Su Z, Yang Z, Xu Y, Chen Y and Yu Q:
Apoptosis, autophagy, necroptosis, and cancer metastasis. Mol
Cancer. 14:482015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ritter J and Bielack S: Osteosarcoma. Ann
Oncol. 21 (Suppl 7):vii320–vii325. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
A sleeping beauty screen highlights cancer
drivers in osteosarcoma. Cancer Discov. 5:6902015. View Article : Google Scholar
|
|
46
|
Spalato M and Italiano A: The safety of
current pharmacotherapeutic strategies for osteosarcoma. Expert
Opin Drug Safety. 20:427–438. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sevelda F, Mayr L, Kubista B, Lötsch D,
van Schoonhoven S, Windhager R, Pirker C, Micksche M and Berger W:
EGFR is not a major driver for osteosarcoma cell growth in vitro
but contributes to starvation and chemotherapy resistance. J Exp
Clin Cancer Res. 34:1342015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kato S, Lippman S, Flaherty K and Kurzrock
R: The conundrum of genetic ‘Drivers’ in benign conditions. J Natl
Cancer Inst. 108:djw0362016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wang W, Zhao HF, Yao TF and Gong H:
Advanced development of ErbB family-targeted therapies in
osteosarcoma treatment. Invest New Drugs. 37:175–183. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wan Z, Huang S, Mo F, Yao Y, Liu G, Han Z,
Chen M and Zhiyun L: CSN5 controls the growth of osteosarcoma via
modulating the EGFR/PI3K/Akt axis. Exp Cell Res. 384:1116462019.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Kersting C, Gebert C, Agelopoulos K,
Schmidt H, van Diest PJ, Juergens H, Winkelmann W, Kevric M,
Gosheger G, Brandt B, et al: Epidermal growth factor receptor
expression in high-grade osteosarcomas is associated with a good
clinical outcome. Clin Cancer Res. 13:2998–3005. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wang SL, Zhong GX, Wang XW, Yu FQ, Weng
DF, Wang XX and Lin JH: Prognostic significance of the expression
of HER family members in primary osteosarcoma. Oncol Lett.
16:2185–2194. 2018.PubMed/NCBI
|
|
53
|
Yadav P, Yadav R, Jain S and Vaidya A:
Caspase-3: A primary target for natural and synthetic compounds for
cancer therapy. Chem Biol Drug Des. 98:144–165. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wang J, Chen C, Chen C, Wu P and Chen WM:
Suppression of estrogen receptor alpha inhibits cell proliferation,
differentiation and enhances the chemosensitivity of P53-positive
U2OS osteosarcoma cell. Int J Mol Sci. 22:112382021. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Taipale M, Jarosz D and Lindquist S: HSP90
at the hub of protein homeostasis: Emerging mechanistic insights.
Nat Rev Mol Cell Biol. 11:515–528. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhang M, Peng Y, Yang Z, Zhang H, Xu C,
Liu L, Zhao Q, Wu J, Wang H and Liu J: DAB2IP down-regulates
HSP90AA1 to inhibit the malignant biological behaviors of
colorectal cancer. BMC Cancer. 22:5612022. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Chu S, Liu Y, Zhang L, Liu B and Li L, Shi
JZ and Li L: Regulation of survival and chemoresistance by HSP90AA1
in ovarian cancer SKOV3 cells. Mol Biol Rep. 40:1–6. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Xiao X, Wang W, Li Y, Yang D, Li X, Shen
C, Liu Y, Ke X, Guo S and Guo Z: HSP90AA1-mediated autophagy
promotes drug resistance in osteosarcoma. J Exp Clin Cancer Res.
37:2012018. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Szulc-Kielbik I, Kielbik M, Nowak M and
Klink M: The implication of IL-6 in the invasiveness and
chemoresistance of ovarian cancer cells. Systematic review of its
potential role as a biomarker in ovarian cancer patients. Biochim
Biophys Acta Rev Cancer. 1876:1886392021. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Tzeng HE, Tsai CH, Chang ZL, Su CM, Wang
SW, Hwang WL and Tang CH: Interleukin-6 induces vascular
endothelial growth factor expression and promotes angiogenesis
through apoptosis signal-regulating kinase 1 in human osteosarcoma.
Biochem Pharmacol. 85:531–540. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Itoh H, Kadomatsu T, Tanoue H, Yugami M,
Miyata K, Endo M, Morinaga J, Kobayashi E, Miyamoto T, Kurahashi R,
et al: TET2-dependent IL-6 induction mediated by the tumor
microenvironment promotes tumor metastasis in osteosarcoma.
Oncogene. 37:2903–2920. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Zhang C, Ma K and Li WY: IL-6 promotes
cancer stemness and oncogenicity in U2OS and MG-63 Osteosarcoma
Cells by Upregulating the OPN-STAT3 pathway. J Cancer.
10:6511–6525. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Parkin A, Man J, Timpson P and Pajic M:
Targeting the complexity of Src signalling in the tumour
microenvironment of pancreatic cancer: From mechanism to therapy.
FEBS J. 286:3510–3539. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Yang Z, Xie J, Fang J, Lv M, Yang M, Deng
Z, Xie Y and Cai L: Nigericin exerts anticancer effects through
inhibition of the SRC/STAT3/BCL-2 in osteosarcoma. Biochem
Pharmacol. 198:1149382022. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Urciuoli E, Coletta I, Rizzuto E, De Vito
R, Petrini S, D'Oria V, Pezzullo M, Milano GM, Cozza R, Locatelli F
and Peruzzi B: Src nuclear localization and its prognostic
relevance in human osteosarcoma. J Cell Physiol. 233:1658–1670.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Zhao G, Gao Z, Zhang Q, Tang XF, Lv YF,
Zhang ZS, Zhang Y, Tan QL, Peng DB, Jiang DM and Guo QN: TSSC3
promotes autophagy via inactivating the Src-mediated PI3K/Akt/mTOR
pathway to suppress tumorigenesis and metastasis in osteosarcoma,
and predicts a favorable prognosis. J Exp Clin Cancer Res.
37:1882018. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Klein MJ: Cyclin-dependent kinase
inhibition: An opportunity to target protein-protein interactions.
Adv Protein Chem Struct Biol. 121:115–141. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Zou T and Lin Z: The involvement of
ubiquitination machinery in cell cycle regulation and cancer
progression. Int J Mol Sci. 22:57542021. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Han C, Wang Z, Chen S, Li L, Xu Y, Kang W,
Wei C, Ma H, Wang M and Jin X: Berbamine suppresses the progression
of bladder cancer by modulating the ROS/NF-κ B axis. Oxid Med Cell
Longev. 2021:88517632021. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Fischer M and Müller GJ: Cell cycle
transcription control: DREAM/MuvB and RB-E2F complexes. Crit Rev
Biochem Mol Biol. 52:638–662. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Zhang C, Huang C, Yang P, Li C and Li M:
Eldecalcitol induces apoptosis and autophagy in human osteosarcoma
MG-63 cells by accumulating ROS to suppress the PI3K/Akt/mTOR
signaling pathway. Cell Signal. 78:1098412021. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Wirries A, Jabari S, Jansen EP, Roth S,
Figueroa-Juárez E, Wissniowski TT, Neureiter D, Klieser E, Lechler
P, Ruchholtz S, et al: Panobinostat mediated cell death: A novel
therapeutic approach for osteosarcoma. Oncotarget. 9:32997–33010.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Mickymaray S, Alfaiz FA, Paramasivam A,
Veeraraghavan VP, Periadurai ND, Surapaneni KM and Niu G:
Rhaponticin suppresses osteosarcoma through the inhibition of
PI3K-Akt-mTOR pathway. Saudi J Biol Sci. 28:3641–3649. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Tung FI, Chen LC, Wang YC, Chen MH, Shueng
PW and Liu TY: Using a hybrid radioenhancer to discover tumor
cell-targeted treatment for osteosarcoma: An in vitro study. Curr
Med Chem. 28:3877–3889. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Burke PJ: Mitochondria, bioenergetics and
apoptosis in cancer. Trends Cancer. 3:857–870. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Praharaj PP, Naik PP, Panigrahi DP, Bhol
CS, Mahapatra KK, Patra S, Sethi G and Bhutia SK: Intricate role of
mitochondrial lipid in mitophagy and mitochondrial apoptosis: Its
implication in cancer therapeutics. Cell Mol Life Sci.
76:1641–1652. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Gibson CJ and Davids MS: BCL-2 antagonism
to target the intrinsic mitochondrial pathway of apoptosis. Clin
Cancer Res. 21:5021–5029. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Shoshan-Barmatz V, Shteinfer-Kuzmine A and
Verma A: VDAC1 at the intersection of cell metabolism, apoptosis,
and diseases. Biomolecules. 10:14852020. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Shoshan-Barmatz V, Krelin Y and Chen Q:
VDAC1 as a player in mitochondria-mediated apoptosis and target for
modulating apoptosis. Curr Med Chem. 24:4435–4446. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Shoshan-Barmatz V, De S and Meir A: The
mitochondrial voltage-dependent anion channel 1, Ca2+ transport,
apoptosis, and their regulation. Front Oncol. 7:602017. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Shoshan-Barmatz V, Krelin Y,
Shteinfer-Kuzmine A and Arif T: Voltage-dependent anion channel 1
as an emerging drug target for novel anti-cancer therapeutics.
Front Oncol. 7:1542017. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Xia H, Green D and Zou W: Autophagy in
tumour immunity and therapy. Nat Rev Cancer. 21:281–297. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Levy J, Towers C and Thorburn A: Targeting
autophagy in cancer. Nat Rev Cancer. 17:528–542. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Jacquet M, Guittaut M, Fraichard A and
Despouy G: The functions of Atg8-family proteins in autophagy and
cancer: Linked or unrelated? Autophagy. 17:599–611. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Heckmann BL and Green DR: LC3-associated
phagocytosis at a glance. J Cell Sci. 132:2019. View Article : Google Scholar
|
|
86
|
Moscat J, Karin M and Diaz-Meco MT: p62 in
cancer: Signaling adaptor beyond autophagy. Cell. 167:606–609.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
He Y, Sun MM, Zhang GG, Yang J, Chen KS,
Xu WW and Li B: Targeting PI3K/Akt signal transduction for cancer
therapy. Signal Transduct Target Ther. 6:4252021. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Zhang J, Yu X, Yan Y, Wang C and Wang WJ:
PI3K/Akt signaling in osteosarcoma. Clin Chim Acta. 444:182–192.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Liu M, Liu F, Li YJ, Yin JN, Gao YL, Wang
XY, Yang C, Liu JG and Li HJ: Ginsenoside Rg5 inhibits human
osteosarcoma cell proliferation and induces cell apoptosis through
PI3K/Akt/mTORC1-Related LC3 autophagy pathway. Oxid Med Cell
Longev. 2021:50403262021.PubMed/NCBI
|
|
90
|
Angulo P, Kaushik G, Subramaniam D,
Dandawate P, Neville K, Chastain K and Anant S: Natural compounds
targeting major cell signaling pathways: A novel paradigm for
osteosarcoma therapy. J Hematol Oncol. 10:102017. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Khezri MR, Jafari R, Yousefi K and
Zolbanin NM: The PI3K/AKT signaling pathway in cancer: Molecular
mechanisms and possible therapeutic interventions. Exp Mol Pathol.
127:1047872022. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Rezatabar S, Karimian A, Rameshknia V,
Parsian H, Majidinia M, Kopi TA, Bishayee A, Sadeghinia A, Yousefi
M, Monirialamdari M and Yousefi B: RAS/MAPK signaling functions in
oxidative stress, DNA damage response and cancer progression. J
Cell Physiol. 234:14951–14965. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Wagner E and Nebreda AR: Signal
integration by JNK and p38 MAPK pathways in cancer development. Nat
Rev Cancer. 9:537–549. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Ding S, Pang ZY, Chen XM, Li Z, Liu XX,
Zhai QL, Huang JM and Ruan ZY: Urolithin a attenuates IL-1β-induced
inflammatory responses and cartilage degradation via inhibiting the
MAPK/NF-κB signaling pathways in rat articular chondrocytes. J
Inflamm (Lond). 17:132020. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Lv H, Zhen C, Liu J and Shang PJ:
β-Phenethyl isothiocyanate induces cell death in human osteosarcoma
through altering iron metabolism, disturbing the redox balance, and
activating the MAPK signaling pathway. Oxid Med Cell Longev.
2020:50219832020. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Zhu J, Yu W, Liu B, Wang Y, Shao J, Wang
J, Xia K, Liang C, Fang W, Zhou C and Tao H: Escin induces
caspase-dependent apoptosis and autophagy through the ROS/p38 MAPK
signalling pathway in human osteosarcoma cells in vitro and in
vivo. Cell Death Dis. 8:e31132017. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Chen X, Han D, Liu T, Huang C, Hu Z, Tan X
and Wu S: Asiatic acid improves high-fat-diet-induced osteoporosis
in mice via regulating SIRT1/FOXO1 signaling and inhibiting
oxidative stress. Histol Histopathol. 37:769–777. 2022.PubMed/NCBI
|
|
98
|
Huang X, Zuo L, Lv Y, Chen C, Yang Y, Xin
H, Li Y and Qian Y: Asiatic acid attenuates myocardial
ischemia/reperfusion injury via Akt/GSK-3β/HIF-1α signaling in rat
H9c2 cardiomyocytes. Molecules. 21:12482016. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Qi Z, Ci X, Huang J, Liu Q, Yu Q, Zhou J
and Deng X: Asiatic acid enhances Nrf2 signaling to protect HepG2
cells from oxidative damage through Akt and ERK activation. Biomed
Pharmacother. 88:252–259. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Xu Y, Yao J, Zou C, Zhang H, Zhang S, Liu
J, Ma G, Jiang P and Zhang W: Asiatic acid protects against hepatic
ischemia/reperfusion injury by inactivation of Kupffer cells via
PPARγ/NLRP3 inflammasome signaling pathway. Oncotarget.
8:86339–86355. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Dutta S, Chakraborty P, Basak S, Ghosh S,
Ghosh N, Chatterjee S, Dewanjee S and Sil PC: Synthesis,
characterization, and evaluation of in vitro cytotoxicity and in
vivo antitumor activity of asiatic acid-loaded poly
lactic-co-glycolic acid nanoparticles: A strategy of treating
breast cancer. Life Sci. 307:1208762022. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Wu T, Geng J, Guo W, Gao J and Zhu XJ:
Asiatic acid inhibits lung cancer cell growth in vitro and in vivo
by destroying mitochondria. Acta Pharm Sin B. 7:65–72. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Park B, Bosire K, Lee E, Lee Y and Kim JJ:
Asiatic acid induces apoptosis in SK-MEL-2 human melanoma cells.
Cancer Lett. 218:81–90. 2005. View Article : Google Scholar : PubMed/NCBI
|