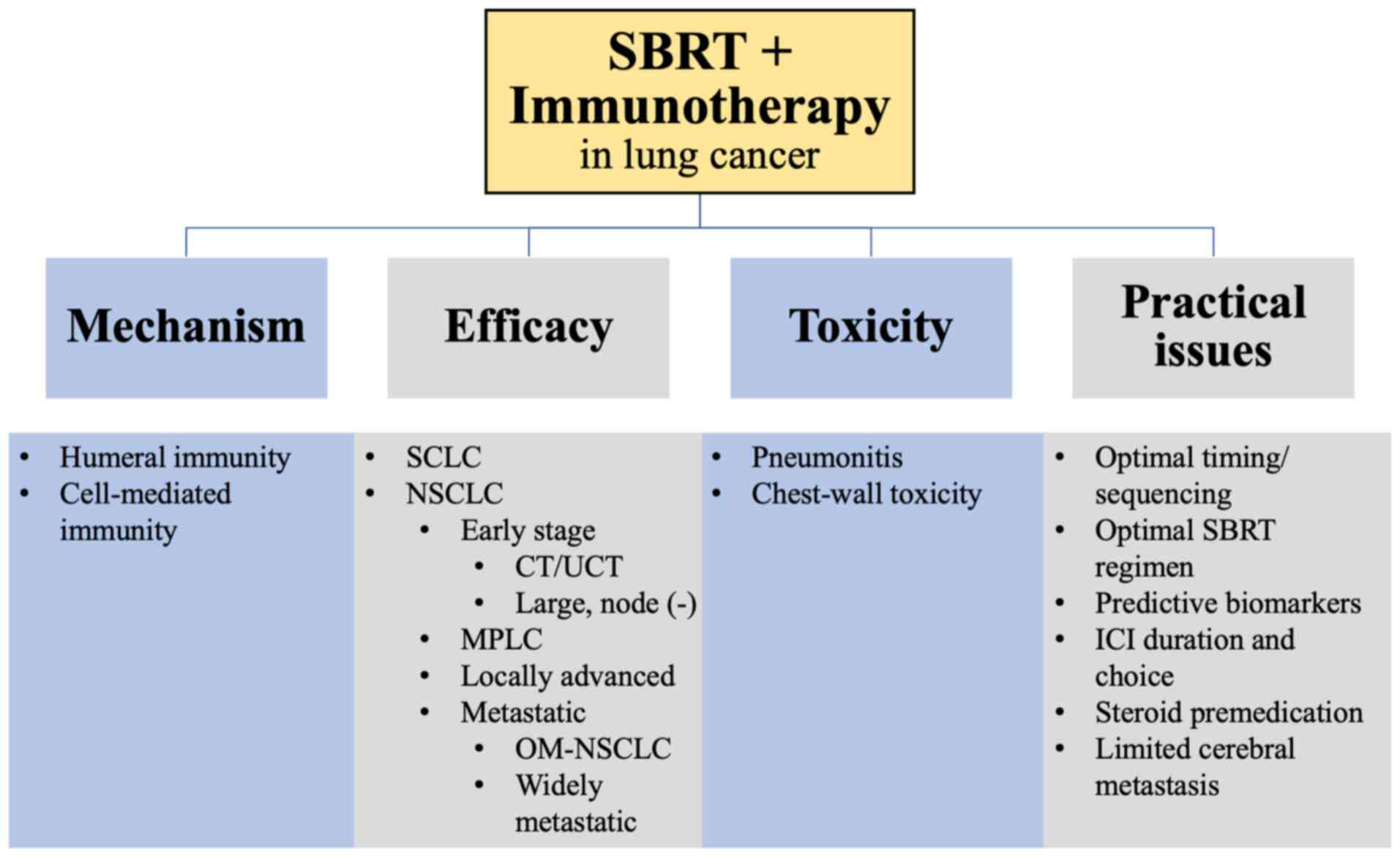
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xiao Y and Zhuang H: Effect of
stereotactic radiotherapy on immune microenvironment of lung
cancer. Front Immunol. 13:10258722022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Billing DL and Rimner A: Results of
radiation therapy as local ablative therapy for oligometastatic
non-small cell lung cancer. Cancers (Basel). 13:57732021.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chakraborty M, Abrams SI, Camphausen K,
Liu K, Scott T, Coleman CN and Hodge JW: Irradiation of tumor cells
up-regulates Fas and enhances CTL lytic activity and CTL adoptive
immunotherapy. J Immunol. 170:6338–6347. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Garnett CT, Palena C, Chakraborty M, Tsang
KY, Schlom J and Hodge JW: Sublethal irradiation of human tumor
cells modulates phenotype resulting in enhanced killing by
cytotoxic T lymphocytes. Cancer Res. 64:7985–7994. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lugade AA, Moran JP, Gerber SA, Rose RC,
Frelinger JG and Lord EM: Local radiation therapy of B16 melanoma
tumors increases the generation of tumor antigen-specific effector
cells that traffic to the tumor. J Immunol. 174:7516–7523. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Reits EA, Hodge JW, Herberts CA, Groothuis
TA, Chakraborty M, Wansley EK, Camphausen K, Luiten RM, de Ru AH,
Neijssen J, et al: Radiation modulates the peptide repertoire,
enhances MHC class I expression, and induces successful antitumor
immunotherapy. J Exp Med. 203:1259–1271. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schaue D, Ratikan JA, Iwamoto KS and
McBride WH: Maximizing tumor immunity with fractionated radiation.
Int J Radiat Oncol Biol Phys. 83:1306–1310. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Formenti SC, Rudqvist NP, Golden E, Cooper
B, Wennerberg E, Lhuillier C, Vanpouille-Box C, Friedman K, Ferrari
de Andrade L, Wucherpfennig KW, et al: Radiotherapy induces
responses of lung cancer to CTLA-4 blockade. Nat Med. 24:1845–1851.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Navarro-Martín A, Galiana IL, Berenguer
Frances MA, Cacicedo J, Cañas Cortés R, Comas Anton S, Padrones
Sánchez S, Bolívar Cuevas S, Parry R and Guedea Edo F: Preliminary
study of the effect of stereotactic body radiotherapy (SBRT) on the
immune system in lung cancer patients unfit for surgery:
Immunophenotyping analysis. Int J Mol Sci. 19:39632018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou P, Chen D, Zhu B, Chen W, Xie Q, Wang
Y, Tan Q, Yuan B, Zuo X, Huang C, et al: Stereotactic body
radiotherapy is effective in modifying the tumor genome and tumor
immune microenvironment in non-small cell lung cancer or lung
metastatic carcinoma. Front Immunol. 11:5942122021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang T, Yu H, Ni C, Zhang T, Liu L, Lv Q,
Zhang Z, Wang Z, Wu D, Wu P, et al: Hypofractionated stereotactic
radiation therapy activates the peripheral immune response in
operable stage I non-small-cell lung cancer. Sci Rep. 7:48662017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lockney NA, Zhang M, Morris CG, Nichols
RC, Okunieff P, Swarts S, Zhang Z, Zhang B, Zhang A and Hoppe BS:
Radiation-induced tumor immunity in patients with non-small cell
lung cancer. Thorac Cancer. 10:1605–1611. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lei QQ, Sui JD, Jin F, Luo HL, Shan JJ,
Tang L, Wang Y and Wu YZ: Impact of high-dose rate radiotherapy on
B and natural killer (NK) cell polarization in peripheral blood
mononuclear cells (PBMCs) via inducing non-small cell lung cancer
(NSCLC)-derived exosomes. Transl Cancer Res. 10:3538–3547. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen Y, Gao M, Huang Z, Yu J and Meng X:
SBRT combined with PD-1/PD-L1 inhibitors in NSCLC treatment: A
focus on the mechanisms, advances, and future challenges. J Hematol
Oncol. 13:1052020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Breen WG, Leventakos K, Dong H and Merrell
KW: Radiation and immunotherapy: Emerging mechanisms of synergy. J
Thorac Dis. 12:7011–7023. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Heinzerling JH, Mileham KF and Simone CB
II: The utilization of immunotherapy with radiation therapy in lung
cancer: A narrative review. Transl Cancer Res. 10:2596–2608. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
He J and Hu Q: Progress in the clinical
application of immune checkpoint inhibitors in small cell lung
cancer. Front Immunol. 14:11265822023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Luna J, Zafra J, Areses Manrique MC,
Rodríguez A, Sotoca A, Fírvida JL, Chicas-Sett R, Mielgo X, Reyes
JCT and Couñago F: New challenges in the combination of
radiotherapy and immunotherapy in non-small cell lung cancer. World
J Clin Oncol. 12:983–999. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schlick B, Shields MD, Marin-Acevedo JA,
Patel I and Pellini B: Immune checkpoint inhibitors and
chemoradiation for limited-stage small cell lung cancer. Curr Treat
Options Oncol. 23:1104–1120. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Azghadi S and Daly ME: Radiation and
immunotherapy combinations in non-small cell lung cancer. Cancer
Treat Res Commun. 26:1002982021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chicas-Sett R, Zafra-Martin J,
Morales-Orue I, Castilla-Martinez J, Berenguer-Frances MA,
Gonzalez-Rodriguez E, Rodriguez-Abreu D and Couñago F:
Immunoradiotherapy as an effective therapeutic strategy in lung
cancer: From palliative care to curative intent. Cancers (Basel).
12:21782020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Oweida A, Hararah MK, Phan A, Binder D,
Bhatia S, Lennon S, Bukkapatnam S, Van Court B, Uyanga N, Darragh
L, et al: Resistance to radiotherapy and PD-L1 blockade is mediated
by TIM-3 upregulation and regulatory T-cell infiltration. Clin
Cancer Res. 24:5368–5380. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Della Corte CM, Ciaramella V, Ramkumar K,
Vicidomini G, Fiorelli A, Nardone V, Cappabianca S, Cozzolino I,
Zito Marino F, Di Guida G, et al: Triple blockade of Ido-1, PD-L1
and MEK as a potential therapeutic strategy in NSCLC. J Transl Med.
20:5412022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Han MG, Wee CW, Kang MH, Kim MJ, Jeon SH
and Kim IA: Combination of OX40 co-stimulation, radiotherapy, and
PD-1 inhibition in a syngeneic murine triple-negative breast cancer
model. Cancers (Basel). 14:26922022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pieper AA, Zangl LM, Speigelman DV, Feils
AS, Hoefges A, Jagodinsky JC, Felder MA, Tsarovsky NW, Arthur IS,
Brown RJ, et al: Radiation augments the local anti-tumor effect of
in situ vaccine with CpG-oligodeoxynucleotides and anti-OX40 in
immunologically cold tumor models. Front Immunol. 12:7638882021.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Giaccone G, Bazhenova LA, Nemunaitis J,
Tan M, Juhász E, Ramlau R, van den Heuvel MM, Lal R, Kloecker GH,
Eaton KD, et al: A phase III study of belagenpumatucel-L, an
allogeneic tumour cell vaccine, as maintenance therapy for
non-small cell lung cancer. Eur J Cancer. 51:2321–2329. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
O'Brien MER, Anderson H, Kaukel E, O'Byrne
K, Pawlicki M, Von Pawel J and Reck M; SR-ON-12 Study Group, :
SRL172 (killed Mycobacterium vaccae) in addition to standard
chemotherapy improves quality of life without affecting survival,
in patients with advanced non-small-cell lung cancer: Phase III
results. Ann Oncol. 15:906–914. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhou L, Wang XL, Deng QL, Du YQ and Zhao
NQ: The efficacy and safety of immunotherapy in patients with
advanced NSCLC: A systematic review and meta-analysis. Sci Rep.
6:320202016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xiong W, Zhao Y, Du H and Guo X: Current
status of immune checkpoint inhibitor immunotherapy for lung
cancer. Front Oncol. 11:7043362021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schild SE, Wang X, Bestvina CM, Williams
T, Masters G, Singh AK, Stinchcombe TE, Salama JK, Wolf S, Zemla T,
et al: Alliance A082002-a randomized phase II/III trial of modern
immunotherapy-based systemic therapy with or without SBRT for
PD-L1-negative, advanced non-small cell lung cancer. Clin Lung
Cancer. 23:e317–e320. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Butts C, Socinski MA, Mitchell PL,
Thatcher N, Havel L, Krzakowski M, Nawrocki S, Ciuleanu TE, Bosquée
L, Trigo JM, et al: Tecemotide (L-BLP25) versus placebo after
chemoradiotherapy for stage III non-small-cell lung cancer (START):
A randomised, double-blind, phase 3 trial. Lancet Oncol. 15:59–68.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kumar SS, Higgins KA and McGarry RC:
Emerging therapies for stage III non-small cell lung cancer:
stereotactic body radiation therapy and immunotherapy. Front Oncol.
7:1972017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Modi C, Berim L, Isserow L, Malhotra J,
Patel M, Langenfeld J, Aisner J, Almeldin D and Jabbour SK:
Combining radiation therapy and immunotherapy for lung cancers: A
narrative review. Shanghai Chest. 5:102021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shaverdian N, Lisberg AE, Bornazyan K,
Veruttipong D, Goldman JW, Formenti SC, Garon EB and Lee P:
Previous radiotherapy and the clinical activity and toxicity of
pembrolizumab in the treatment of non-small-cell lung cancer: A
secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol.
18:895–903. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Thompson M and Rosenzweig KE: The evolving
toxicity profile of SBRT for lung cancer. Transl Lung Cancer Res.
8:48–57. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Murray L, Karakaya E, Hinsley S, Naisbitt
M, Lilley J, Snee M, Clarke K, Musunuru HB, Ramasamy S, Turner R
and Franks K: Lung stereotactic ablative radiotherapy (SABR):
Dosimetric considerations for chest wall toxicity. Br J Radiol.
89:201506282016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Welsh J, Thomas J, Shah D, Allen PK, Wei
X, Mitchell K, Gao S, Balter P, Komaki R and Chang JY: Obesity
increases the risk of chest wall pain from thoracic stereotactic
body radiation therapy. Int J Radiat Oncol Biol Phys. 81:91–96.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Owen D and Sio TT: Stereotactic body
radiotherapy (SBRT) for central and ultracentral node-negative lung
tumors. J Thorac Dis. 12:7024–7031. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Schneider BJ, Daly ME, Kennedy EB,
Antonoff MB, Broderick S, Feldman J, Jolly S, Meyers B, Rocco G,
Rusthoven C, et al: Stereotactic body radiotherapy for early-stage
non-small-cell lung cancer: American society of clinical oncology
endorsement of the American society for radiation oncology
evidence-based guideline. J Clin Oncol. 36:710–719. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Naidoo J, Wang X, Woo KM, Iyriboz T,
Halpenny D, Cunningham J, Chaft JE, Segal NH, Callahan MK, Lesokhin
AM, et al: Pneumonitis in patients treated with anti-programmed
death-1/programmed death ligand 1 therapy. J Clin Oncol.
35:709–717. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tian S, Switchenko JM, Buchwald ZS, Patel
PR, Shelton JW, Kahn SE, Pillai RN, Steuer CE, Owonikoko TK, Behera
M, et al: Lung stereotactic body radiation therapy and concurrent
immunotherapy: A multicenter safety and toxicity analysis. Int J
Radiat Oncol Biol Phys. 108:304–313. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Korpics MC, Katipally RR, Partouche J,
Cutright D, Pointer KB, Bestvina CM, Luke JJ, Pitroda SP, Dignam
JJ, Chmura SJ and Juloori A: Predictors of pneumonitis in combined
thoracic stereotactic body radiation therapy and immunotherapy. Int
J Radiat Oncol Biol Phys. 114:645–654. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Simone CB II, Bogart JA, Cabrera AR, Daly
ME, DeNunzio NJ, Detterbeck F, Faivre-Finn C, Gatschet N, Gore E,
Jabbour SK, et al: Radiation therapy for small cell lung cancer: An
ASTRO clinical practice guideline. Pract Radiat Oncol. 10:158–173.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Amin MB, Greene FL, Edge SB, Compton CC,
Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR and
Winchester DP: The eighth edition AJCC cancer staging manual:
Continuing to build a bridge from a population-based to a more
‘personalized’ approach to cancer staging. CA Cancer J Clin.
67:93–99. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Stahel RA, Ginsberg R, Havemann K, Hirsch
FR, Ihde DC, Jassem J, Karrer K, Maurer LH, Osterlind K and van
Houtte P: Staging and prognostic factors in small cell lung cancer:
A consensus report. Lung Cancer. 5:119–126. 1989. View Article : Google Scholar
|
|
47
|
Jones GS, Elimian K, Baldwin DR, Hubbard
RB and McKeever TM: A systematic review of survival following
anti-cancer treatment for small cell lung cancer. Lung Cancer.
141:44–55. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Verma V, Simone CB II, Allen PK, Gajjar
SR, Shah C, Zhen W, Harkenrider MM, Hallemeier CL, Jabbour SK,
Matthiesen CL, et al: Multi-institutional experience of
stereotactic ablative radiation therapy for stage I small cell lung
cancer. Int J Radiat Oncol Biol Phys. 97:362–371. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Pakkala S, Higgins K, Chen Z, Sica G,
Steuer C, Zhang C, Zhang G, Wang S, Hossain MS, Nazha B, et al:
Durvalumab and tremelimumab with or without stereotactic body
radiation therapy in relapsed small cell lung cancer: A randomized
phase II study. J Immunother Cancer. 8:e0013022020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Luke JJ, Lemons JM, Karrison TG, Pitroda
SP, Melotek JM, Zha Y, Al-Hallaq HA, Arina A, Khodarev NN, Janisch
L, et al: Safety and clinical activity of pembrolizumab and
multisite stereotactic body radiotherapy in patients with advanced
solid tumors. J Clin Oncol. 36:1611–1618. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Theelen WSEM, Peulen HMU, Lalezari F, van
der Noort V, de Vries JF, Aerts JGJV, Dumoulin DW, Bahce I,
Niemeijer AN, de Langen AJ, et al: Effect of pembrolizumab after
stereotactic body radiotherapy vs pembrolizumab alone on tumor
response in patients with advanced non-small cell lung cancer:
Results of the PEMBRO-RT phase 2 randomized clinical trial. JAMA
Oncol. 5:1276–1282. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Chen D, Patel RR, Verma V, Ramapriyan R,
Barsoumian HB, Cortez MA and Welsh JW: Interaction between
lymphopenia, radiotherapy technique, dosimetry, and survival
outcomes in lung cancer patients receiving combined immunotherapy
and radiotherapy. Radiother Oncol. 150:114–120. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Larkin J, Chiarion-Sileni V, Gonzalez R,
Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M,
Rutkowski P, et al: Combined nivolumab and ipilimumab or
monotherapy in untreated melanoma. N Engl J Med. 373:23–34. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Huang YS, Li Z, Xiao ZF, Li D and Liu WY:
Case report: Radiotherapy plus pneumococcal conjugate vaccine
stimulates abscopal immune response in a patient with ALK+ NSCLC.
Front Immunol. 13:9502522022. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
de Sousa LG, McGrail DJ, Li K,
Marques-Piubelli ML, Gonzalez C, Dai H, Ferri-Borgogno S, Godoy M,
Burks J, Lin SY, et al: Spontaneous tumor regression following
COVID-19 vaccination. J Immunother Cancer. 10:e0043712022.
View Article : Google Scholar
|
|
56
|
Papachristofilou A, Hipp MM, Klinkhardt U,
Früh M, Sebastian M, Weiss C, Pless M, Cathomas R, Hilbe W, Pall G,
et al: Phase Ib evaluation of a self-adjuvanted protamine
formulated mRNA-based active cancer immunotherapy, BI1361849
(CV9202), combined with local radiation treatment in patients with
stage IV non-small cell lung cancer. J Immunother Cancer. 7:382019.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Román-Jobacho A, Hernández-Miguel M,
García-Anaya MJ, Gómez-Millán J, Medina-Carmona JA and Otero-Romero
A: Oligometastatic non-small cell lung cancer: Current management.
J Clin Transl Res. 7:311–319. 2021.PubMed/NCBI
|
|
58
|
Al-Shafa F, Arifin AJ, Rodrigues GB, Palma
DA and Louie AV: A Review of ongoing trials of stereotactic
ablative radiotherapy for oligometastatic cancers: Where will the
evidence lead? Front Oncol. 9:5432019. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Milano MT, Biswas T, Simone CB II and Lo
SS: Oligometastases: History of a hypothesis. Ann Palliat Med.
10:5923–5930. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Bates JE and Milano MT: Prognostic
significance of sites of extrathoracic metastasis in patients with
non-small cell lung cancer. J Thorac Dis. 9:1903–1910. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Hendriks LE, Derks JL, Postmus PE, Damhuis
RA, Houben RM, Troost EG, Hochstenbag MM, Smit EF and Dingemans AM:
Single organ metastatic disease and local disease status,
prognostic factors for overall survival in stage IV non-small cell
lung cancer: Results from a population-based study. Eur J Cancer.
51:2534–2544. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Gomez DR, Tang C, Zhang J, Blumenschein GR
Jr, Hernandez M, Lee JJ, Ye R, Palma DA, Louie AV, Camidge DR, et
al: Local consolidative therapy vs maintenance therapy or
observation for patients with oligometastatic non-small-cell lung
cancer: Long-term results of a multi-institutional, phase II,
randomized study. J Clin Oncol. 37:1558–1565. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Iyengar P, Wardak Z, Gerber DE, Tumati V,
Ahn C, Hughes RS, Dowell JE, Cheedella N, Nedzi L, Westover KD, et
al: Consolidative radiotherapy for limited metastatic
non-small-cell lung cancer: A phase 2 randomized clinical trial.
JAMA Oncol. 4:e1735012018. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Brandão M, Durieux V and Berghmans T:
Current and future research efforts in oligometastatic non-small
cell lung cancer-a systematic review. Transl Lung Cancer Res.
10:3473–3485. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Bauml JM, Mick R, Ciunci C, Aggarwal C,
Davis C, Evans T, Deshpande C, Miller L, Patel P, Alley E, et al:
Pembrolizumab after completion of locally ablative therapy for
oligometastatic non-small cell lung cancer: A phase 2 trial. JAMA
Oncol. 5:1283–1290. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Watanabe T, Firat E, Scholber J, Gaedicke
S, Heinrich C, Luo R, Ehrat N, Multhoff G, Schmitt-Graeff A, Grosu
AL, et al: Deep abscopal response to radiotherapy and anti-PD-1 in
an oligometastatic melanoma patient with unfavorable pretreatment
immune signature. Cancer Immunol Immunother. 69:1823–1832. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Griswold CR, Kerrigan K and Patel SB:
Combination of local ablative therapy and continuation of immune
checkpoint inhibitor (ICI) therapy provides durable treatment
response past oligometastatic progression in NSCLC: A case report.
Case Rep Oncol. 12:866–871. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Cho BC, Abreu DR, Hussein M, Cobo M, Patel
AJ, Secen N, Lee KH, Massuti B, Hiret S, Yang JCH, et al:
Tiragolumab plus atezolizumab versus placebo plus atezolizumab as a
first-line treatment for PD-L1-selected non-small-cell lung cancer
(CITYSCAPE): Primary and follow-up analyses of a randomised,
double-blind, phase 2 study. Lancet Oncol. 23:781–792. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Lieverse RIY, Van Limbergen EJ, Oberije
CJG, Troost EGC, Hadrup SR, Dingemans AC, Hendriks LEL, Eckert F,
Hiley C, Dooms C, et al: Stereotactic ablative body radiotherapy
(SABR) combined with immunotherapy (L19-IL2) versus standard of
care in stage IV NSCLC patients, ImmunoSABR: A multicentre,
randomised controlled open-label phase II trial. BMC Cancer.
20:5572020. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Van Limbergen EJ, Hoeben A, Lieverse RIY,
Houben R, Overhof C, Postma A, Zindler J, Verhelst F, Dubois LJ, De
Ruysscher D, et al: Toxicity of L19-interleukin 2 combined with
stereotactic body radiation therapy: A phase 1 study. Int J Radiat
Oncol Biol Phys. 109:1421–1430. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Liu Y, Jiang S, Lin Y, Yu H, Yu L and
Zhang X: Research landscape and trends of lung cancer radiotherapy:
A bibliometric analysis. Front Oncol. 12:10665572022. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Antonia SJ, Villegas A, Daniel D, Vicente
D, Murakami S, Hui R, Yokoi T, Chiappori A, Lee KH, de Wit M, et
al: Durvalumab after chemoradiotherapy in stage III non-small-cell
lung cancer. N Engl J Med. 377:1919–1929. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Feddock J, Arnold SM, Shelton BJ, Sinha P,
Conrad G, Chen L, Rinehart J and McGarry RC: Stereotactic body
radiation therapy can be used safely to boost residual disease in
locally advanced non-small cell lung cancer: A prospective study.
Int J Radiat Oncol Biol Phys. 85:1325–1331. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Wang Z, Qiang Y, Shen Q, Zhu XX and Song
Y: Neoadjuvant programmed cell death protein 1 blockade combined
with stereotactic body radiation therapy for stage III(N2)
non-small cell lung cancer: A case series. Front Oncol.
12:7792512022. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Luna J, Sotoca A, Fernández P, Miralles C
and Rodríguez A: Recent advances in early stage lung cancer. J Clin
Transl Res. 7:163–174. 2021.PubMed/NCBI
|
|
76
|
Ball D, Mai GT, Vinod S, Babington S,
Ruben J, Kron T, Chesson B, Herschtal A, Vanevski M, Rezo A, et al:
Stereotactic ablative radiotherapy versus standard radiotherapy in
stage 1 non-small-cell lung cancer (TROG 09.02 CHISEL): A phase 3,
open-label, randomised controlled trial. Lancet Oncol. 20:494–503.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Daly ME, Redman M, Simone CB, Monjazeb AM,
Bauman JR, Hesketh P, Feliciano J, Kashani R, Steuer C, Ganti AK,
et al: SWOG/NRG S1914: A randomized phase III trial of
induction/consolidation atezolizumab + SBRT vs SBRT alone in high
risk, early-stage NSCLC (NCT#04214262). Int J Radiat Oncol Biol
Phys. 114:e4142022. View Article : Google Scholar
|
|
78
|
Hallqvist A, Koyi H, de Petris L, Lindberg
K, Farooqi S, Helland Å, Wikström A, Johansson M, Planck M,
Lindberg L, et al: 63MO Safety analysis of durvalumab following
stereotactic body radiotherapy (SBRT) in early-stage non-small cell
lung cancer (NSCLC) patients: A first report of a randomized phase
II trial (ASTEROID). J Thorac Oncol. 16 (Suppl):S729–S730. 2021.
View Article : Google Scholar
|
|
79
|
Bezjak A, Paulus R, Gaspar LE, Timmerman
RD, Straube WL, Ryan WF, Garces YI, Pu AT, Singh AK, Videtic GM, et
al: Safety and efficacy of a five-fraction stereotactic body
radiotherapy schedule for centrally located non-small-cell lung
cancer: NRG oncology/RTOG 0813 trial. J Clin Oncol. 37:1316–1325.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Sun B, Brooks ED, Komaki RU, Liao Z, Jeter
MD, McAleer MF, Allen PK, Balter PA, Welsh JD, O'Reilly MS, et al:
7-year follow-up after stereotactic ablative radiotherapy for
patients with stage I non-small cell lung cancer: Results of a
phase 2 clinical trial. Cancer. 123:3031–3039. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Verma V and Simone CB II: Approaches to
stereotactic body radiation therapy for large (≥5 centimeter)
non-small cell lung cancer. Transl Lung Cancer Res. 8:70–77. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Ichinose Y, Yano T, Yokoyama H, Inoue T,
Asoh H and Katsuda Y: The correlation between tumor size and
lymphatic vessel invasion in resected peripheral stage I
non-small-cell lung cancer. A potential risk of limited resection.
J Thorac Cardiovasc Surg. 108:684–686. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Strauss GM, Herndon JE II, Maddaus MA,
Johnstone DW, Johnson EA, Harpole DH, Gillenwater HH, Watson DM,
Sugarbaker DJ, Schilsky RL, et al: Adjuvant paclitaxel plus
carboplatin compared with observation in stage IB non-small-cell
lung cancer: CALGB 9633 with the cancer and leukemia group B,
radiation therapy oncology group, and north central cancer
treatment group study groups. J Clin Oncol. 26:5043–5051. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Pignon JP, Tribodet H, Scagliotti GV,
Douillard JY, Shepherd FA, Stephens RJ, Dunant A, Torri V, Rosell
R, Seymour L, et al: Lung adjuvant cisplatin evaluation: A pooled
analysis by the LACE collaborative group. J Clin Oncol.
26:3552–3559. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
National Comprehensive Cancer Network, .
Non-small cell lung cancer. (version 2.2024). 2024.
|
|
86
|
Brahmer J, Reckamp KL, Baas P, Crinò L,
Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE,
Holgado E, et al: Nivolumab versus docetaxel in advanced
squamous-cell non-small-cell lung cancer. N Engl J Med.
373:123–135. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Zhao L, Liu C, Xie G, Wu F and Hu C:
Multiple primary lung cancers: A new challenge in the era of
precision medicine. Cancer Manag Res. 12:10361–10374. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Boyle JM, Tandberg DJ, Chino JP, D'Amico
TA, Ready NE and Kelsey CR: Smoking history predicts for increased
risk of second primary lung cancer: A comprehensive analysis.
Cancer. 121:598–604. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Dovedi SJ, Adlard AL, Lipowska-Bhalla G,
McKenna C, Jones S, Cheadle EJ, Stratford IJ, Poon E, Morrow M,
Stewart R, et al: Acquired resistance to fractionated radiotherapy
can be overcome by concurrent PD-L1 blockade. Cancer Res.
74:5458–5468. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Woody S, Hegde A, Arastu H, Peach MS,
Sharma N, Walker P and Ju AW: Survival is worse in patients
completing immunotherapy prior to SBRT/SRS compared to those
receiving it concurrently or after. Front Oncol. 12:7853502022.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Bestvina CM, Pointer KB, Karrison T,
Al-Hallaq H, Hoffman PC, Jelinek MJ, Juloori A, Melotek JM, Murgu
S, Partouche J, et al: A phase 1 trial of concurrent or sequential
ipilimumab, nivolumab, and stereotactic body radiotherapy in
patients with stage IV NSCLC study. J Thorac Oncol. 17:130–140.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Welsh JW, Tang C, de Groot P, Naing A,
Hess KR, Heymach JV, Papadimitrakopoulou VA, Cushman TR, Subbiah V,
Chang JY, et al: Phase II trial of ipilimumab with stereotactic
radiation therapy for metastatic disease: Outcomes, toxicities, and
low-dose radiation-related abscopal responses. Cancer Immunol Res.
7:1903–1909. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Windisch P and Guckenberger M: Randomized
phase II trial reporting overall survival advantage by adding local
consolidative therapy to systemic therapy for oligometastatic
non-small cell lung cancer: Another step forward on the long road
of evidence-based medicine for oligometastatic disease. J Thorac
Dis. 11 (Suppl 15):S1869–S1873. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Mitchell P, Thatcher N, Socinski MA,
Wasilewska-Tesluk E, Horwood K, Szczesna A, Martín C, Ragulin Y,
Zukin M, Helwig C, et al: Tecemotide in unresectable stage III
non-small-cell lung cancer in the phase III START study: updated
overall survival and biomarker analyses. Ann Oncol. 26:1134–1142.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Patel JD, Lee JW, Carbone DP, Wagner H,
Shanker A, de Aquino MTP, Horn L, Johnson ML, Gerber DE, Liu JJ, et
al: P Phase II study of immunotherapy with tecemotide and
bevacizumab after chemoradiation in patients with unresectable
stage III non-squamous non-small-cell lung cancer (NS-NSCLC): A
trial of the ECOG-ACRIN cancer research group (E6508). Clin Lung
Cancer. 21:520–526. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Alite F, Shaikh PM and Mahadevan A:
Influence of dexamethasone premedication on acute lung toxicity in
lung SBRT. Front Oncol. 12:8375772022. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Arbour KC, Mezquita L, Long N, Rizvi H,
Auclin E, Ni A, Martínez-Bernal G, Ferrara R, Lai WV, Hendriks LEL,
et al: Impact of baseline steroids on efficacy of programmed cell
death-1 and programmed death-ligand 1 blockade in patients with
non-small-cell lung cancer. J Clin Oncol. 36:2872–2878. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Im SJ, Hashimoto M, Gerner MY, Lee J,
Kissick HT, Burger MC, Shan Q, Hale JS, Lee J, Nasti TH, et al:
Defining CD8+ T cells that provide the proliferative burst after
PD-1 therapy. Nature. 537:417–421. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Floudas CS, Brar G, Mabry-Hrones D, Duffy
AG, Wood B, Levy E, Krishnasamy V, Fioravanti S, Bonilla CM, Walker
M, et al: A pilot study of the PD-1 targeting agent AMP-224 used
with low-dose cyclophosphamide and stereotactic body radiation
therapy in patients with metastatic colorectal cancer. Clin
Colorectal Cancer. 18:e349–e360. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Nikitas J, Roach M, Robinson C, Bradley J,
Huang J, Perkins S, Tsien C and Abraham C: Treatment of
oligometastatic lung cancer with brain metastases using
stereotactic radiosurgery (SRS) and stereotactic body radiation
therapy (SBRT). Clin Transl Radiat Oncol. 21:32–35. 2019.PubMed/NCBI
|
|
101
|
Rodríguez Plá M, Dualde Beltrán D and
Ferrer Albiach E: Immune checkpoints inhibitors and SRS/SBRT
synergy in metastatic non-small-cell lung cancer and melanoma: A
systematic review. Int J Mol Sci. 22:116212021. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Shields MD, Chen K, Dutcher G, Patel I and
Pellini B: Making the rounds: Exploring the role of circulating
tumor DNA (ctDNA) in non-small cell lung cancer. Int J Mol Sci.
23:90062022. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Spaas M, Sundahl N, Hulstaert E, Kruse V,
Rottey S, De Maeseneer D, Surmont V, Meireson A, Brochez L,
Reynders D, et al: Checkpoint inhibition in combination with an
immunoboost of external beam radiotherapy in solid tumors (CHEERS):
Study protocol for a phase 2, open-label, randomized controlled
trial. BMC Cancer. 21:5142021. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Ni J, Yang L, Zhu H, Chu M, Zhang C, Zhao
W, Yang M, Xu X, Zheng E, Jiang X, et al: A patient with metastatic
non-small cell lung cancer who received pembrolizumab monotherapy
after stereotactic body radiotherapy had progression-free survival
of nearly 5 years: A case report. Ann Palliat Med. 10:4999–5009.
2021. View Article : Google Scholar : PubMed/NCBI
|