Cancer is a primary contributor to global mortality
and existing cancer treatment methods generally have restricted
effectiveness along with substantial side effects. Hence, safer and
more efficient cancer treatment techniques need to be developed.
Several studies have highlighted the relationship between
ferroptosis and the progression of various diseases, particularly
its role in cancer therapy (1,2).
Stockwell et al (2) proposed
that triggering ferroptosis is an effective strategy in cancer
treatment, particularly for treating mesenchymal and metastatic
cancers, which are generally resistant to conventional therapeutic
techniques. In 2012, Dixon et al (3) coined the term ‘ferroptosis’, which is
an iron-dependent mechanism of cell death caused by lipid peroxide
overload in the cell membrane.
The mechanism of cell death due to ferroptosis
differs significantly from the mechanism of cell death caused by
necrosis, autophagy and apoptosis in terms of cellular morphology,
genetics and biology. For instance, in terms of differences in
morphological characteristics, cells undergoing ferroptosis lack
the typical apoptotic characteristics but instead display shrunken
mitochondria and a decrease in the number of mitochondrial cristae.
The accumulation of lethal lipid peroxides is an important
characteristic of ferroptosis, involving a conflict between systems
that promote and inhibit ferroptosis. Ferroptosis occurs when the
pro-ferroptotic mechanisms driving cellular processes exceed the
antioxidant buffering capacity provided by the ferroptosis defense
systems (4–7). Inducing ferroptosis is a promising
cancer treatment strategy. Further studies in this area can lead to
the development of new and effective cancer treatment techniques.
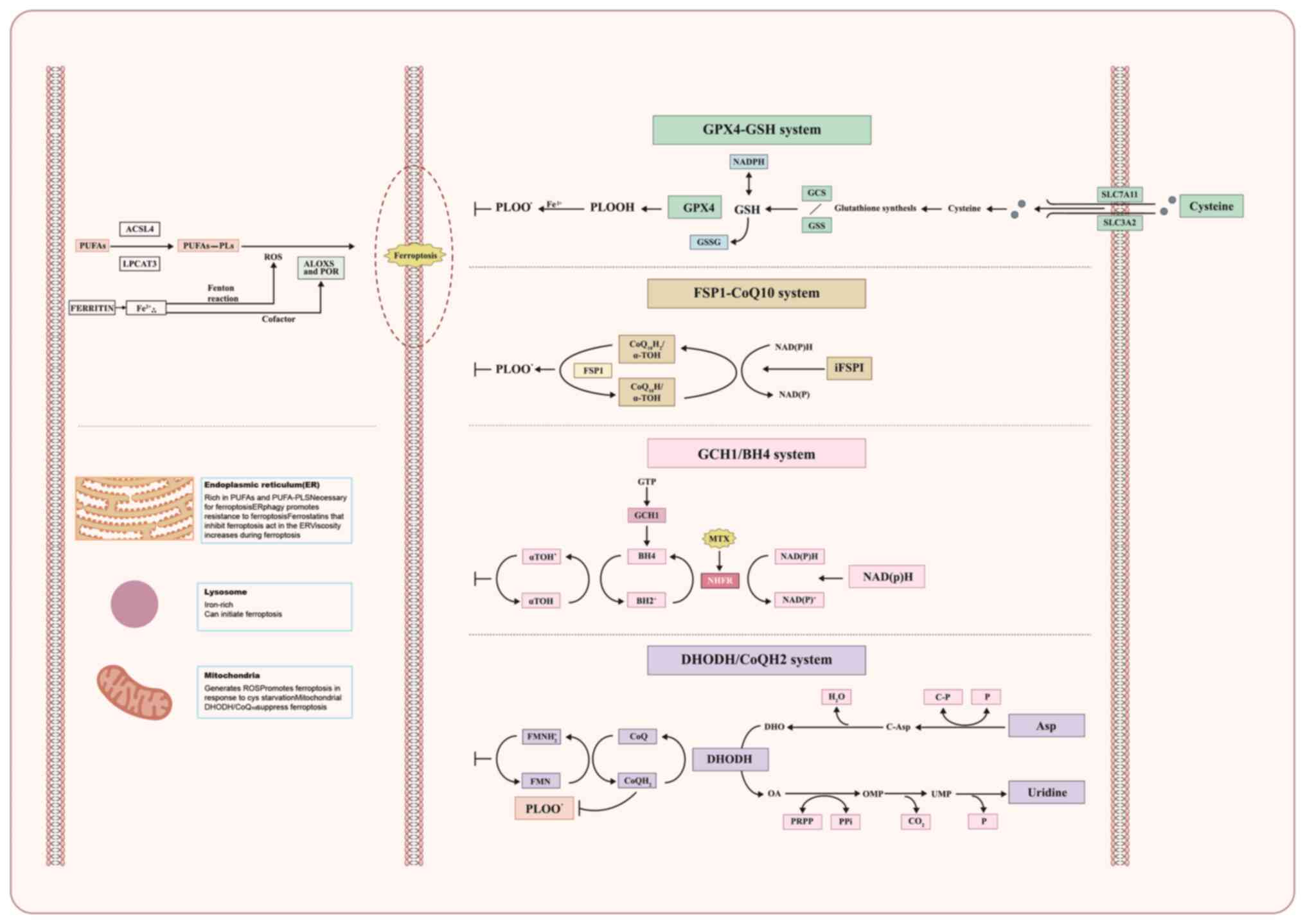
The driving and inhibitory factors of ferroptosis determine whether
ferroptosis will occur. Ferroptosis inducers (FIN) can promote the
driving factors of ferroptosis or weaken the inhibitory factors to
induce ferroptosis. The mechanisms that drive and inhibit
ferroptosis are shown in Fig.
1.
Lipid peroxidation in the cell membrane is a key
step leading to ferroptosis. In the upstream of lipid peroxidation,
the key enzymes responsible for membrane phospholipid synthesis are
long-chain acyl-CoA synthase 4 (ACSL4) and lysolecithin
acetyltransferase 3 (LPCAT3). ACSL4 catalyzes the combination of
acetyl coenzyme A with arachidonic acid (AA) and adrenal acid in
polyunsaturated fatty acids (PUFAs) to form long-chain
polyunsaturated fatty acyl-coenzyme A. Subsequently, LPCAT3
combines this compound with lysophosphatidylethanolamine to form
PUFA-containing phospholipid (PUFA-PL). Due to the presence of
diallyl in PUFA-PL, which can easily undergo peroxidation, the
peroxidation of PUFA-PLs occurs non-enzymatically via the Fenton
reaction catalyzed by ferrous ions (8,9).
Hydroxyl radicals serve as catalysts for the peroxidation of
different biological macromolecules in cells, including PUFAs. The
phospholipid bilayer has an important role in various cellular
functions. Phospholipids consist of hydrophilic phosphoglycerol and
hydrophobic PUFA chains. Peroxidation of these PUFA chains results
in the disruption of the cellular phospholipid bilayer membrane
structure, which increases membrane permeability and ultimately
results in cell death (10,11).
The high level of expression of ACSL4 is considered
to be a marker of ferroptosis. ACSL4 regulates ferroptosis through
a signaling pathway. It may be more similar to caspase-3, the
executor of apoptosis, than a housekeeping protein (12). Artemisinin (ART) and
dihydroartemisinin (DHA) can affect this target (13).
Iron has a key role in the development of
ferroptosis. Unstable intracellular iron (Fe2+) can
generate a substantial amount of reactive oxygen species (ROS)
through the Fenton reaction, providing enough raw material for
lipid peroxidation. Iron is also a cofactor of lipid peroxidase
enzymes [AA lipoxygenases (ALOXs) and cytochrome P450
oxidoreductase] and can determine their activity (2,3).
Accumulation of excess iron may induce ferroptosis in individuals
with cancer (14–16). Transferrin (TF) receptor 1 (TFR1) in
the membrane is responsible for transporting Fe3+ into
cells and can be used as a biomarker for ferroptosis (17). Divalent metal transporter 1 (DMT1)
facilitates the conversion of Fe3+ to Fe2+.
Excess iron is stored within ferritin, which consists of two parts:
Ferritin light chain and ferritin heavy chain 1 (FTH1) (18,19).
Thus, the abundance of ferritin, particularly the presence of FTH1,
plays a key role in suppressing ferroptosis (20). In addition, intracellular iron is
predominantly sequestered within ferritin in an inert form. The
autophagic breakdown of ferritin releases stored iron into the
labile iron pool (LIP). Inhibiting nuclear receptor coactivator 4
(NCOA4)-mediated ferritinophagy decreases the level of the LIP;
thus mitigating ferroptosis (21).
In contrast, enhancing ferritinophagy by inhibiting cytosolic
glutamate oxaloacetate transaminase 1 increases the LIP, thus
facilitating ferroptosis. Furthermore, the redox state of iron
plays an important role in ferroptosis: Fe2+ promotes
ferroptosis, while Fe3+ remains inert and is stored
within ferritin (22). The
application of desferriamine or silencing of TFRs can reverse
ferroptosis induced by erastin, a classical ferroptosis inducer.
Studies have shown that ART compounds (ARTCs) and β-elemene also
act on iron metabolism (23–25).
GPX4 is an essential enzyme that catalyzes the
breakdown of lipid peroxides. GPX4 acts as an important suppressor
of ferroptosis. GPX4 relies on the cofactor GSH to convert harmful
lipid peroxides into harmless lipid alcohols, resulting in the
oxidation of glutathione into oxidized GSH (GSSG) (26). GSH synthesis requires cysteine as
the reactant, which is transported into the cell by the Gys/Glu
reverse transporter (xCT system) located outside the cell. The xCT
system consists of the solute carrier family 7 member 11 (SLC7A11)
and SLC3A2 subunits; SLC7A11 is overexpressed in ~70% of human
tumor cells (3).
The SLC7A11-GSH-GPX4 pathway plays a key role in
protecting against ferroptosis. However, certain cancer cell lines
are resistant to ferroptosis even in the absence of functional
GPX4, which indicates that alternative defense mechanisms against
ferroptosis are in place (27). In
addition, GPX4 is necessary for the proper functioning of various
peripheral tissues, including kidney tubular cells and specific
neuronal subgroups in mice (28).
Thus, targeting GPX4 may lead to significant side effects unless
therapeutic interventions can be used to precisely target tumor
cells (29). Limiting the
availability of cysteine/cysteine in cells by inhibiting the xCT
system is a highly promising approach, particularly because
knocking out the SLC7A11 gene in mice does not lead to any serious
health problems (30). In addition,
an inverse relationship was found between the level of expression
of SLC3A2 and/or SLC7A11 and the clinical prognosis of individuals
with melanoma and glioma. This observation reinforces the efficacy
of targeting this pathway (31,32).
Numerous natural products that can induce ferroptosis were found to
affect this pathway. However, strong inhibition of GPX4 may be
life-threatening (29).
Tumor drug resistance arises due to different
mechanisms; among them, a significant contributor is the
disturbance of the redox equilibrium. Tumor cells promote their
resistance to oxidative stress by decreasing the production of ROS
levels, imparting acquired drug resistance. The KEAP1-NRF2 pathway
serves as a crucial antioxidant defense mechanism. A study found
that this pathway negatively affects ferroptosis regulation
(33).
The protective role of NRF2 differs based on the
cellular and tissue environment. For instance, in pancreatic cancer
cells, NRF2 hinders ferroptosis by stimulating microsomal
glutathione S-transferase 1 (34),
which in turn inhibits ALOX5. By contrast, in hepatocarcinoma
cells, NRF2 promotes resistance to ferroptosis by controlling
ferritin levels (35). In addition,
PUFA biosynthesis can regulate sensitivity to ferroptosis and
energy stress-induced activation of AMPK can limit the biosynthesis
of PUFA by regulating acetyl-CoA carboxylase. Hence, AMPK can
inhibit lipid peroxidation and ferroptosis (22). Numerous natural products promote
ferroptosis by inhibiting the Keap1-Nrf2 pathway or the AMPK
pathway.
A study found that ferroptosis suppressor protein 1
(FSP1) plays a key role in protecting against ferroptosis (36). FSP1 is located on the plasma
membrane and functions as an NAD(P)H-dependent oxidoreductase. It
can convert ubiquinone (CoQH) to reduced ubiquinone
(CoQH2) (37). Besides
transferring electrons in the mitochondria, CoQH2 can
capture lipid peroxidation free radicals; thus, inhibiting lipid
peroxidation and ferroptosis. The FSP1-CoQH2 system
protects cells from undergoing ferroptosis.
When GPX4 is deactivated, the flow through
dihydroorotate dehydrogenase (DHODH) increases substantially,
leading to an increase in the production of CoQH2. This
increase in CoQH2 counteracts lipid peroxidation and
protects against ferroptosis in the mitochondria (38). Therefore, the DHODH-CoQH2
system can be considered to act as a second defense system against
ferroptosis.
A study reported that GTP cyclohydrolase 1
suppresses ferroptosis by producing BH4 as a radical-trapping
antioxidant, as well as via GCH1-mediated production of
CoQH2 and phospholipids containing two PUFA tails
(39). Therefore, the GCH1-BH4
system is another ferroptosis defense system.
Most studies on the induction of ferroptosis by
natural products have focused on the driving factors of
ferroptosis, the SLC7A11-GSH-GPX4 pathway and the p62-Keap1-Nrf2
pathway. Only a few studies have investigated other pathways, such
as the PI3K-AKT mTOR pathway and the Hippo signaling pathway. A
study found that overactive mutations related to PI3K-AKT-mTOR
signaling can induce cancer cells and protect them from oxidative
stress and ferroptosis through sterol receptor element binding
protein-1/stearoyl-CoA desaturase 1-mediated adipogenesis (40). Another study found that the Hippo
pathway and ferroptosis share upstream regulatory factors in
mechanical transduction and matrix hardness, such as piezo type
mechanosensitive ion channel component 1, integrin and transient
receptor potential vanilloid 1. Therefore, mechanical signal
transduction can affect the disease by serving as an upstream
regulatory factor for the Hippo pathway and ferroptosis (41).
Natural ferroptosis inducers are compounds isolated
and extracted from animals or plants that have the ability to
induce ferroptosis. These include monomeric compounds extracted
from Traditional Chinese Medicine, such as artesunate. Synthetic
ferroptosis inducers refer to artificially synthesized compounds,
such as erastin. Several studies have found that natural substances
such as ARTs, tanshinones, isothiocyanate (ITC) and gallic acid,
among others, can trigger ferroptosis (42). These natural products act on
different targets of the ferroptosis removal pathway, inducing
ferroptosis in tumor cells or synergistically increasing the
anti-tumor effects of numerous drugs through the ferroptosis
pathway.
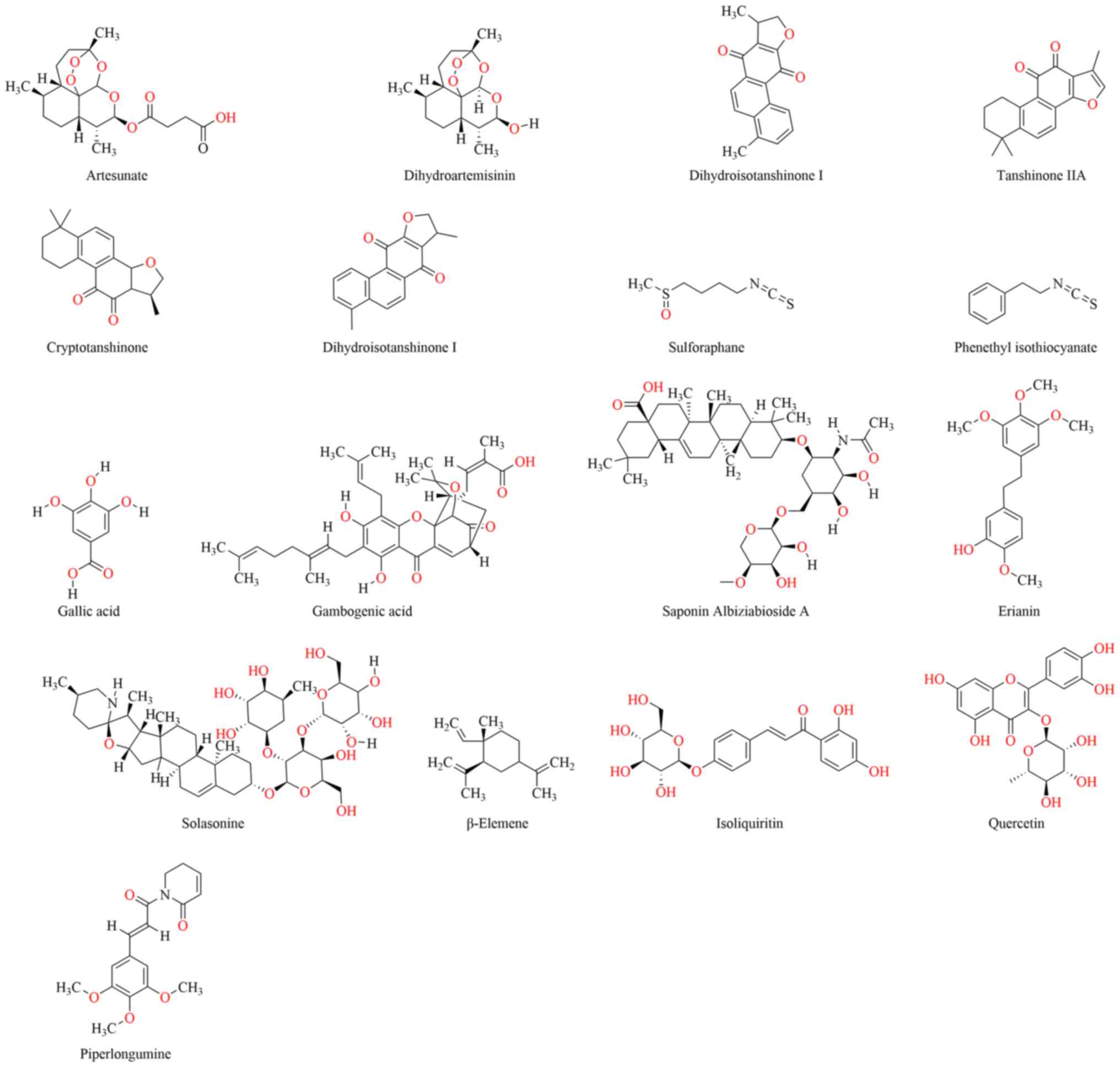
In the present review, studies were systematically
collected and categorized in relation to the role of natural
products in inducing ferroptosis for cancer therapy. Their chemical
structures are presented in Fig. 2.
The study focused on describing their effects and mechanisms. To
better understand the natural products that induce ferroptosis, the
stimulating and inhibitory factors of ferroptosis were summarized
and the key pathways and targets involved were described. After
classifying the natural products, the roles of natural ferroptosis
inducers in cancer treatment were discussed from various aspects,
including their effects and mechanisms, synergistic effects and
structural improvements. This review may act as a framework for
future studies. The mechanism of drug action, synergistic effects
and structural improvements are summarized in Table I.
In 2012, a novel form of iron-dependent cell death
termed ferroptosis was described and ARTCs were found to be closely
associated with the characteristics of ferroptosis (100). Research on the treatment of cancer
by ARTCs through ferroptosis was performed by several groups. The
discovery of ferroptosis also partially explained the mechanism
underlying the inhibitory effects of ARTCs on tumor growth. ATS and
DHA are often used as ferroptosis inducers to study glioma, ovarian
cancer, leukemia, pancreatic cancer, neuroma and gastrointestinal
tumors in vivo and in vitro. Methods such as the MTT,
Cell Counting Kit-8 and lactate dehydrogenase (LDH) assays are used
to detect cell death. The levels of iron, GSH and malondialdehyde
(MDA) are determined. Ferritin, ferritin heavy chain, nuclear
activator 4, FTH1 SLC3A2, heme oxygenase, GPX4, TFR and ferroportin
1 (FPN1) are also detected (44,101–103).
ATS is a clinically versatile ARTC used for treating
mild-to-severe malaria infection (104). Therefore, it is a promising
compound for application in anti-tumor clinical treatment. Several
clinical studies have investigated ATS, mainly focusing on
colorectal cancer and metastatic breast cancer. Among the five
projects that were initiated, three have been completed (105). Compared to ATS, DHA can be further
improved due to its structural characteristics, such as poor water
solubility, instability and rapid clearance (106).
The dysregulation of iron metabolism contributes
significantly to ferroptosis. Tumor cells usually have higher iron
levels compared to normal cells, which highlights the significance
of targeting iron metabolism for treating and preventing tumors
(107,108). Studies have shown that the primary
mechanism underlying the ferroptosis-inducing effect of ARTCs is
the enhancement of ferritin degradation by lysosomes. This
increases the level of free iron, which can ultimately induce
ferroptosis by activating the Fenton reaction and peroxides
(24,25). Both deferoxamine and ferrostatin-1
can inhibit ferroptosis by decreasing free iron levels through
their iron-scavenging properties (61,109).
ARTCs can counteract the effects of these compounds, indicating
that ARTCs can trigger ferroptosis by affecting iron metabolism
(108). Certain studies have shown
that administering ARTCs stimulates lysosomal activity and
facilitates the degradation of ferritin, which can increase
lysosomal iron levels; thus, ARTCs can impart cytotoxic effects on
cancer cells (100,110). Chen et al (24) found that DHA and erastin can induce
ferroptosis by regulating iron metabolism, but the increase in
lysosomal degradation of ferritin and dysregulation of iron in
cells induced by DHA is not related to autophagy. A study also
found that ARTCs showed negligible toxic effects on cells in which
NCOA4 was knocked down (110).
NCOA4 is a cargo receptor that mediates the delivery and
degradation of ferritin in lysosomes (18,111,112). Studies have found that DHA induces
autophagy, thus initiating ferroptosis. For instance, DHA-induced
autophagy, through the activation of the AMPK/mTOR/p70S6 k pathway,
can increase the size of the LIP and promote lipid peroxidation,
ultimately resulting in ferroptosis (62). In addition, ARTCs can also induce
ferroptosis by reducing the level of expression of GPX4. DHA can
significantly reduce the expression of GPX4, leading to ferroptosis
(51), but it does not affect the
level of expression of ACSL4 and xCT (52). DHA and sorafenib share a similar
mechanism and can decrease the levels of GPX4 and GSH (53). In multidrug-resistant leukemia
cells, DHA decreases the viability of multi-drug resistant K562
cells and enhances their sensitivity to adriamycin ADM by promoting
ferroptosis induced by the downregulation of GSH levels and the
expression of GPX4, iron regulatory protein 2 and FTH (54). However, GSH levels may fall and the
expression of GPX4 may decrease due to the secondary effects of the
Fenton reaction induced by iron ions after ARTCs cause an imbalance
in iron metabolism. ARTCs contain an endoperoxide structure. The
internal peroxide structure of these compounds can react with
Fe2+ ions to produce ROS, thus damaging cells (22,113–115). Before ferroptosis was discovered,
researchers speculated that this may be the reason underlying the
inhibitory effects of ARTCs on tumors. However, the relationship
between ART-induced ferroptosis and its ability to stimulate ROS
production has remained to be demonstrated. The initial stage in
ART-induced ferroptosis may involve the production of ROS by ARTCs
and Fe2+. A study found that DHA-induced ferroptosis in
acute myeloid leukemia is associated with iron metabolism and
metallothionein (55).
Certain studies have shown that ARTCs can disrupt
the equilibrium of intracellular iron metabolism by facilitating
the degradation of ferritin via lysosomes and autophagy. This
process leads to the release of ferrous ions, a notable increase in
ROS levels and an accumulation of lipid peroxides. This disruption
promotes ferroptosis, along with a decrease in the level of
expression of GPX4 and depletion of GSH. ARTCs can not only induce
ferroptosis but also induce apoptosis and damage DNA. These
mechanisms and their relationships need to be further investigated,
particularly the relationship between ROS production and various
mechanisms. In addition, the administration of ARTCs leads to
alterations in several proteins involved in the regulation of iron
metabolism. These proteins, including lactoferrin, TFR1 and TFR2,
TF transporter protein and ceruloplasmin, can serve as tumor
markers (117). ARTCs acting on
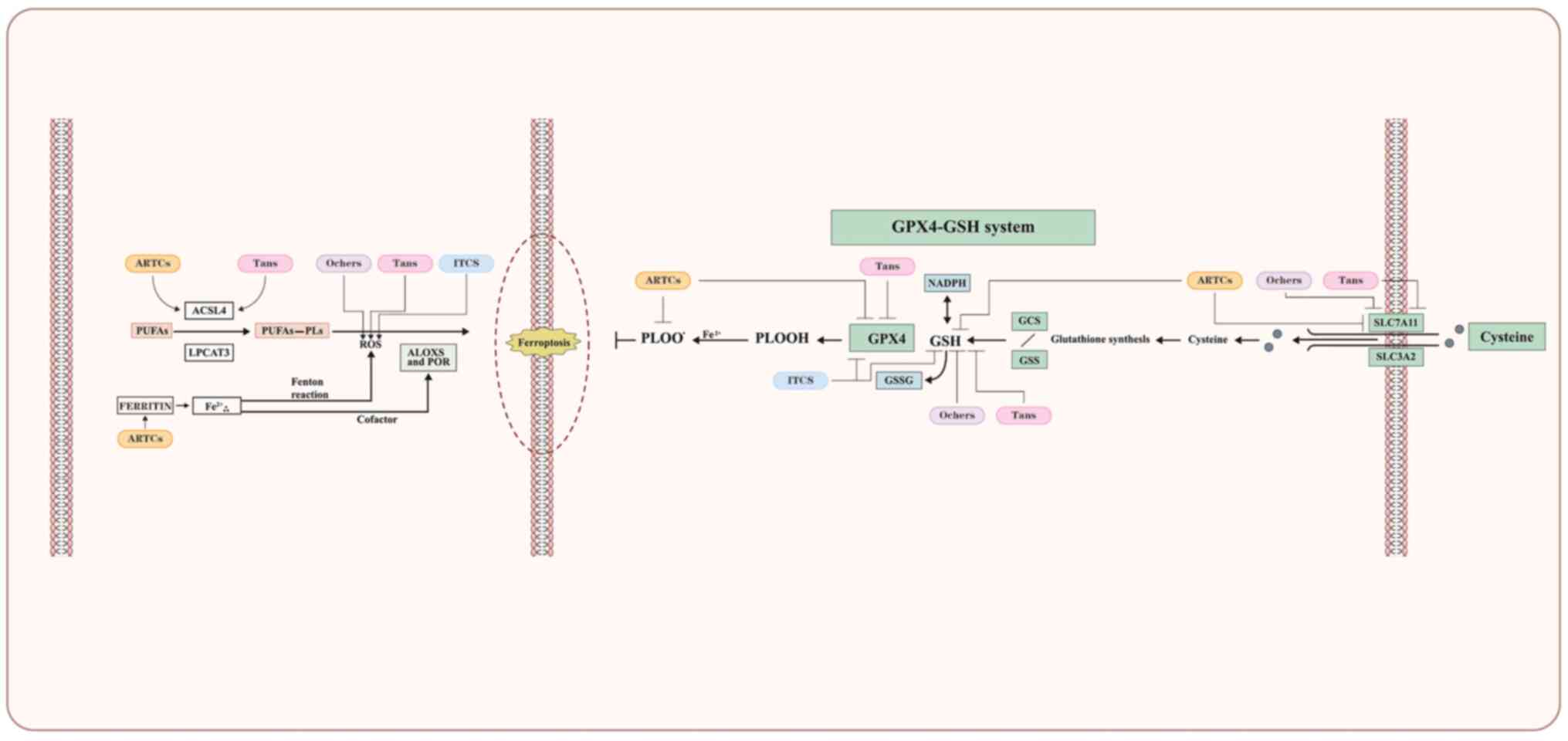
targets of ferroptosis are shown in Fig. 3.
The challenges of severe side effects and acquired
drug resistance in tumor cells have hindered clinical treatment.
Hence, treatment strategies that are more effective and less toxic
need to be developed. Several studies have investigated the
synergistic effects of ARTCs and the reversal of drug resistance.
Synergistic studies combine first-line clinical antitumor drugs
with ARTCs, which often serve as adjuvants. This method can be
further studied as a cancer treatment strategy.
Although sorafenib is used as a first-line targeted
treatment drug for liver cancer, it faces problems related to drug
resistance. The activation of lysosomes by ATS, combined with the
pro-oxidative effects of sorafenib, synergistically promotes
several sequential reactions. These reactions involve the
activation of lysosomal cathepsin B/L, degradation of ferritin,
lipid peroxidation, and finally, induction of ferroptosis. Hence,
ATS can also be used to enhance the sensitivity of sorafenib in the
treatment of hepatocellular carcinoma (HCC) (84). In another study, the simultaneous
administration of DHA and Sora synergistically inhibited HepG2 and
SW480 cells. This combination induced ferroptosis by increasing the
levels of lipid ROS, LIP and MDA, while simultaneously decreasing
the level of GSH in HepG2 cells (53). Following the combined administration
of DHA and DDP, the experimental results showed that they
synergistically inhibited the proliferation of pancreatic ductal
adenocarcinoma (PDAC) cells and induced DNA damage. A further study
found that ferroptosis contributed to the cytotoxic effects on PDAC
cells after DHA and DDP were administered simultaneously. The
treatment also led to the accumulation of large amounts of free
iron and unrestricted lipid peroxidation (63). The study found that DHA and
cisplatin can induce DNA damage and iron-dependent cell death, both
of which can inhibit tumor cell proliferation. However, which
mechanism plays a more significant role in the suppression of tumor
growth remains elusive, and thus, further studies are needed to
address this question.
Certain studies have used DHA in combination with
doxorubicin, RSL3 and erastin, respectively, and found that ARTCs
can have a sensitization role by regulating iron metabolism and
inducing ferroptosis (24). The
inhibition of some related pathways or proteins may also affect
ferroptosis. For instance, a study suggested that combining ATS
application with glucose-regulated protein 78 inhibition may be a
novel technique for effectively killing KRAS mutant PDAC cells
(44). A study found that ATF3 may
sensitize gastric cancer (GC) cells to cisplatin by inducing
ferroptosis via the inhibition of Nrf2/Keap1/xCT signaling
(46). Another study reported that
activation of the p62-Keap1-NRF2 pathway can protect HCC cells
against ferroptosis (35).
Ferroptosis involves oxidation reactions. As a protective factor
against oxidation and reduction, Keap1-NRF2 is an important target
for ferroptosis. Inhibition of NRF2 can reverse drug resistance.
ATS can trigger the activation of the Nrf2-antioxidant response
element pathway in head and neck cancer (HNC) cells, imparting
resistance against ferroptosis. Silencing Keap1, a negative
regulator of Nrf2, reduced the sensitivity of HNC cells to ATS.
Genetic silencing of Nrf2 or treatment with trigonelline was found
to reverse the ferroptosis resistance observed in Keap1-silenced
and cisplatin-resistant HNC cells to ATS in vitro and in
vivo (46). Another study found
that ARTCs strongly inhibited the growth and proliferation of
docetaxel-resistant prostate carcinoma (PCa) cells through
mechanisms involving ferroptosis and apoptosis, whereas normal
cells remained unaffected (47).
The increase in oxidative damage and the impairment of antioxidant
defense mechanisms strongly influence DHA-induced ferroptosis, thus
contributing to the sensitivity of multidrug-resistant leukemia
cells to DHA (54). The activation
of the apoptosis cascade is the primary mechanism for eliminating
cancer cells. However, in numerous types of cancer cell, the
apoptotic pathway is frequently obstructed, leading to the
development of strong resistance to drugs (118). Ferroptosis induction may reverse
this condition.
Overall, the induction of ferroptosis by ARTCs can
specifically inhibit tumor cells and exhibit multi-target activity.
Thus, ARTCs are promising agents for administering combination
therapy and reversing drug resistance. However, further studies are
needed to confirm these speculations. of notes, in certain studies
on the reversal of drug resistance by ATS, the occurrence of
ferroptosis was not reported. For instance, the sensitization
effect of ATS mainly depends on cell cycle arrest and mitochondrial
dysfunction in bladder cancer (119). In addition, inhibition of the
Nrf2-ARE pathway may be an important way for ARTCs to reverse drug
resistance (46).
Delivery systems and modifications of the structures
of compounds are important in improving the pharmacological
activity of compounds. As DHA has poor water solubility and a short
half-life in vivo, most improvements in ARTCs are mainly
related to DHA. Improvements in drug formulations involving DHA
have enhanced its efficacy. Researchers have developed advanced
techniques, such as polymeric nanoparticles, liposomes and
metal-organic frameworks to optimize the therapeutic potential of
DHA; these techniques can be used as a single-drug treatment
strategy or as a part of multidrug therapy (120).
Iron plays an important role in ARTC-induced
ferroptosis in cancer cells. Although certain researchers argue
that not all methods of adding iron can promote ferroptosis induced
by ARTCs, as iron can act as a cofactor for enzymes involved in
cellular proliferation and unwanted negative effects cannot be
excluded, which may promote tumor growth rather than inhibiting it
(121,122). However, adding iron to the
nanoreactor of DHA can strongly promote ferroptosis. Using
chemodynamic therapy as an example, which relies on intracellular
iron ions and H2O2, certain researchers
developed and characterized the DHA@ iron-based metal-organic
framework (MIL-101) nanoreactor. This nanoreactor degraded in the
acidic tumor microenvironment, releasing DHA and iron ions. In
subsequent experiments, DHA@MIL-101 significantly increased
intracellular iron ions due to the disintegration of the
nanoreactor and the recruitment of DHA, eventually triggering ROS
production. Simultaneously, ROS production induced ferroptosis by
depleting GSH, inactivating GPX4 and leading to the accumulation of
lipid peroxide (64). Another study
found that DHA can induce ferroptosis in an immunogenic manner.
Furthermore, the in vivo antitumor efficacy of DHA was
maximized by co-delivering a cholesterol derivative of DHA and
pyropheophorbide-iron (PyroFe) in Zn-pyrophosphate @DHA/Pyro-Fe
core-shell nanoparticles (65).
The use of compounds with similar effects but
different mechanisms by synthesizing chimeric compounds is also a
promising strategy that may be assessed for cancer treatment.
Salinomycin can eliminate cancer cells in the mesenchymal state by
sequestering iron within lysosomes, capitalizing on the iron
dependence of this cell state. In a study, a series of structurally
complex small-molecule chimers combining salinomycin derivatives
and iron-reactive DHA were synthesized with chemoselectivity and
stereoselectivity. These chimers accumulated in lysosomes and
interacted with iron to release bioactive species. As a result,
they induced ferroptosis in drug-tolerant pancreatic cancer cells
and biopsy-derived organoids of PDAC (59). In a study, two ATS-conjugated
phosphorescent rhenium (I) complexes were synthesized and the
complexes exhibited high cytotoxicity against cancer cell lines.
They also induced apoptosis and ferroptosis in HeLa cells (48).
To summarize, the chimers involved in ARTCs and new
drug delivery systems involving iron may be important development
directions in the future. Delivery systems can increase the
targeting and solubility of drugs. Studies on synergistic reactions
and reversal of drug resistance can serve as its research
foundation. In addition, enhancing water solubility can also
improve drug efficacy.
Tanshinone compounds can inhibit various tumor
types, including breast cancer, GC, acute myeloid leukemia, lung
cancer, osteosarcoma, liver cancer, glioma and oral squamous cell
carcinoma (141–148). In 2019, a study showed that DT can
arrest the proliferation of breast cancer cells, including MCF-7
and MDA-MB-231 cells. The researchers also found that DT triggered
apoptosis and ferroptosis in these breast cancer cells,
simultaneously suppressing the expression of the GPX4 protein
(66). Wu et al (71) found that DT can induce ferroptosis
and apoptosis in lung cancer cells. Several studies have also found
that Tan IIA can induce ferroptosis in GC cells and inhibit the
stemness of GC cells (68,69). Another study found that DHTI can
inhibit the proliferation of human glioma cells, including U251 and
U87 cells, by activating ferroptosis (67). In addition, CPT can induce
ferroptosis in lung cancer cells, including the A549 and NCI-H520
NSCLC cell lines. Another study found that only 20% of cell death
in cryptotanshinone is caused by caspase, while others may be
caused by ferroptosis (70).
In a study, after ferroptosis was induced by
tanshinone compounds in tumor cells, a series of substances related
to ferroptosis were altered, including SLC7A11, ACSL-4, ROS,
ferroportin, GPX4 and the GSH/GSSG ratio. These results suggested
that tanshinone-induced ferroptosis may be related to these
substances.
The primary mechanism that prevents cells from
undergoing ferroptosis is the SLC7A11-GSH-GPX4 pathway. Studies
have shown that Tan IIA can trigger ferroptosis by preventing the
decrease in SLC7A11 levels, a process mediated by p53, in BGC-823
and NCI-H87 GC cells (68). P53 can
be recruited to the SLC7A11 promoter region to block the
transcription of SLC7A11 and induce ROS-mediated ferroptosis via a
decrease in GSH production caused by the downregulation of xCT
(149). In addition, Tan IIA can
increase lipid peroxidation and upregulate the level of expression
of prostaglandin-endoperoxide synthase 2 and CHAC1. Another study
on GC found that Tan IIA can inhibit GC stem cells through
SLC7A11-dependent ferroptosis (69). These results suggested that SLC7A11
may be an important target for Tan IIA-induced ferroptosis.
However, a study showed that DT can inhibit the expression of GPX4
and subsequently induce ferroptosis through lipid peroxidation
in vitro and in vivo (71). Of note, some researchers reported
that DHTI can not only reduce the expression of GPX4 but also
enhance the expression of ACSL-4, thus inducing ferroptosis in
human U251 and U87 glioma cells (67). ACSL4 has a key role in fatty acid
metabolism. It also activates long-chain unsaturated fatty acids,
thus facilitating their involvement in the synthesis of membrane
phospholipids and initiation of cell ferroptosis (150). Ferroptosis is associated with
tumor growth inhibition and is a promising strategy for treating
tumors. ACSL4 may be used as a biomarker for predicting the
susceptibility of cells to ferroptosis (151). DHTI can also increase the levels
of LDH and MDA in human glioma cells and decrease the GSH/GSSG
ratio. A study showed that a high level of expression of the
ferroptosis gene FTH1 in head and neck squamous cell carcinoma
(HNSCC), and Tan IIA considerably inhibited HNSCC, partly through
the suppression of FTH1 (152).
Another study found that tanshinone functions as a coenzyme for
NAD(P)H:quinone oxidoreductase 1 (NQO1), which detoxifies lipid
peroxyl radicals and inhibits ferroptosis in vitro and in
vivo. The researchers found a gain of function of NQO1 induced
by tanshinone, which is a novel mechanism for inhibiting
ferroptosis (153).
To summarize, some studies have reported that
SLC7A11 inhibition may strongly influence ferroptosis induced by
tanshinone compounds. In addition, GPX4 inhibition, which
upregulates the expression of ACSL4, may also contribute to
ferroptosis. The induction of NQO1 by tanshinone also needs to be
further investigated. Researchers have reported that the molecular
actions of tanshinone compounds primarily focus on apoptosis and
cell-cycle modulation, which may represent only a part of their
effects. Thus, further studies are needed to determine whether they
interact with other proteins (154). Tans acting on targets of
ferroptosis are presented in Fig.
3.
Studies on the combination of tanshinone compounds
with other drugs to induce ferroptosis are limited. However,
several studies have investigated the synergistic effects and
reversal of drug resistance of combination therapy. For instance,
in studies on breast cancer, CPT and monomethylarsonous acid were
found to promote the apoptosis of MCF-7 breast cancer cells
(155). In addition, the
combination of Tan IIA and paclitaxel can increase the sensitivity
to chemotherapy (156), while the
combination of Tan IIA and doxorubicin can enhance anti-tumor
effects, reverse drug resistance and reduce adverse reactions
(157,158). The effects of combination therapy
primarily include increasing the levels of Bax, Bak and Caspase-9,
decreasing the migration of β-catenin to the nucleus, reducing the
activity of microtubule-associated proteins and impeding the
phosphatase and tensin homolog/Akt pathway. CPT and DHT are highly
efficacious in reversing P-glycoprotein-mediated multidrug
resistance in colon cancer cells (159). Tanshinones can also increase the
sensitivity of apoptosis-resistant colon cancer cells through
autophagic cell death and p53-independent cytotoxicity (160).
To summarize, although numerous studies have used
tanshinones for combination therapy, none have reported whether the
combination therapy can induce ferroptosis to treat cancer. Thus,
further research is needed to determine whether the combination of
tanshinone compounds and other anti-tumor drugs can induce
ferroptosis.
Acetyltanshinone IIA (ATA) is a derivative of Tan
IIA and is highly soluble in water. It has high pro-apoptotic
effects on different cancer cell lines. Administering ATA
stimulates Bax relocation, cytochrome c discharge, caspase-3
activation and apoptosis; it also arrests the growth of xenografted
tumors (161). ATA triggers
oxidation and endoplasmic reticulum stress and activates the
expression of AMPK (162). These
characteristics may be related to ferroptosis, but further research
is necessary to confirm this relationship. TA12 is another
derivative of Tan IIA, which can also activate ROS production and
damage DNA, leading to cell-cycle arrest in the S phase (163).
Nanotechnology has received significant attention in
recent years, as it can effectively overcome the gastrointestinal
barrier. A study showed that poly-N-(2-hydroxypropyl)
methacrylamide-coated wheat germ agglutinin-modified lipid-polymer
hybrid nanoparticles, carrying both CPT and silibinin, were more
toxic to 4T1 cells and prevented their migration and invasion more
effectively compared to the corresponding effects found in the
cells treated with CPT alone (164).
To summarize, modified tanshinone compounds can
increase anti-tumor effects by significantly increasing ROS and
triggering oxidation. However, the relationship between these
compounds and ferroptosis remains largely elusive and further study
is required.
Cruciferous vegetables contain ~100 ITCs, which can
have significant roles in fighting cancer (165). ITCs have a sulfur-containing
functional group with N=C=S bonds (166). The commonly used ITC-containing
compounds include phenethyl ITC (PEITC) from gluconasturtin in
watercress and wasabi, and sulforaphane (SFN) from glucoraphanin in
broccoli, cauliflower, brassicas and kale (167). Studies have suggested that
phenylethyl ITC and sulforaphane have strong anticancer properties
and exert their effects through the ferroptosis pathway (72,168).
In 1994, researchers found that organic ITCs, such
as α-naphthyl ITC, can block chemical carcinogenesis in
experimental tumorigenesis. Since then, studies have found that
PEITC and SFN can inhibit osteosarcoma, lung cancer, colon cancer,
oral squamous carcinoma and PCa (169–173). Another study found that ITCs can
exert their anti-tumor activity through various mechanisms,
including the regulation of phase I and phase II metabolic enzymes
in the human body, inhibition of cell growth by causing cell-cycle
arrest, induction of cell death, and prevention of metastasis and
angiogenesis (174).
Unlike PEITC, SFN can inhibit SCLC. SFN induces
cell death in SCLC cells by inducing ferroptosis and inhibiting the
mRNA and protein expression of SLC7A11. Therefore, SFN may provide
new treatment options for SCLC (74). A study found that SFN can trigger
apoptosis and ferroptosis in acute myeloid leukemia cells in a
dose-dependent manner. Lower doses stimulate caspase-dependent
apoptosis, while higher doses activate ferroptosis, indicated by a
decrease in intracellular GSH levels and GPX4 protein expression
levels (168).
A study on PEITC found that PEITC can induce
ferroptosis through its effect on iron metabolism and the
generation of ROS. Regarding its effect on iron metabolism, PEITC
can upregulate TFR1 and downregulate FTH1, FPN and DMT1, leading to
an increase in labile iron. In addition, PEITC can induce GSH
depletion to stimulate ROS production and lipid peroxidation
(73,171). Furthermore, ITCs can decrease cell
survival by increasing ROS production (174).
SFN triggers ferroptosis, indicated by a decrease
in intracellular GSH levels, suppression of the expression of GPX4
protein and lipid peroxidation. SFN inhibiting system xCT activity
or directly depleting GSH levels, and its inhibitory effect on GPX4
(168). In another study,
SFN-induced cell death in SCLC cells was found to be facilitated by
ferroptosis and the suppression of the mRNA and protein expression
of SLC7A11 (74).
To summarize, PEITC and SFN induce ferroptosis
through different mechanisms. PEITC primarily induces ferroptosis
by generating ROS, which disrupts iron metabolism. However, SFN
mainly induces ferroptosis by modulating the xCT system. The
mechanisms of ITCs acting on targets of ferroptosis are illustrated
in Fig. 3.
In a study, a hybrid androgen receptor (AR)
antagonist containing ITC was designed and synthesized to treat
castration-resistant PCa (CRPC). This strategy was combined with
the administration of the GSH synthesis inhibitor buthionine
sulfoximine to effectively downregulate AR/AR splice variants and
induce the removal of iron from CRPC cells. The results showed that
the drug combination significantly increased lipid peroxidation and
cell viability was effectively rescued by iron chelators,
antioxidants or heme oxygenase-1 inhibitors, which indicated that
ferroptosis was induced (76).
To summarize, the combination of ITCs can stimulate
the induction of iron deficiency anemia. Various researchers have
conducted experiments associated with combination therapy and
obtained promising results.
Besides the above-mentioned compounds, numerous
other natural products are known to induce ferroptosis, but reports
on these products are limited. Thus, they were discussed in this
chapter to help researchers obtain key information. Others acting
on targets of ferroptosis are presented in Fig. 3.
GA is present in various commonly consumed foods,
including edible herbs and vegetables (175). Numerous studies have shown the
inhibitory effects of gallic acid on different types of cancer,
including but not limited to lung cancer (176), PCa (177), skin cancer (178), leukemia (179), lymphoma (180), cervical cancer and breast cancer
(181). GA is assumed to stimulate
cancer cells to undergo apoptosis. This hypothesis is supported by
various markers, including mitochondrial fragmentation, the release
of cytochrome c from mitochondria into the cytosol, nuclear
condensation, DNA damage and activation of caspase-3 (175). Research conducted on various tumor
cell lines, including HeLa, H446 and SH-SY5Y, using GA showed that
it can inhibit tumor-cell proliferation. This effect is attributed
to the mechanisms involving apoptosis, ferroptosis and necrosis
(182). In addition, the mixed
lineage kinase domain-like protein inhibitor necrosulfonamide can
increase the sensitivity of cancer cells to GA; thus exerting a
synergistic effect (77). Another
study showed that the anti-tumor effect of gallic acid is enhanced
under low-intensity laser irradiation. This enhancement occurs
through the production of ROS, induction of cell apoptosis and
promotion of ferroptosis (78).
GNA is a primary bioactive component obtained from
Gamboge, which is the dried resin of Garcinia hanburyi Hook.
f. (182). GNA has various
antitumor activities in vitro and in vivo (183). It can inhibit lung cancer
(184), nasopharyngeal carcinoma
(185) and melanoma (186). Its mechanism of action involves
abnormal autophagy, which mediates cell apoptosis through the AKT
signaling pathway and ferroptosis induced by oxidative stress
(186). A study found that GNA
triggers ferroptosis in TGFβ1-stimulated melanoma cells by
modulating the p53/SLC7A11/GPX4 signaling pathway (187).
PL is a potent alkaloid with excellent biological
activity. It is primarily derived from the long pepper plant
(Piper longum L.). Several studies conducted in vitro
and in vivo have illustrated the targeted initiation of
apoptosis or programmed cell death by PL in different types of
cancer cells, including pancreatic cancer (188), breast cancer (189) and leukemia (190). PL has no anti-proliferative
effects on non-transformed cells, which reflects its specificity
for cancer cells. Furthermore, a study found that PL triggers
cancer cell death, partly by inducing ferroptosis. CN-A and PL, in
combination, synergistically trigger the death of pancreatic cancer
cells; in this process, CN-A increases the ROS levels stimulated by
PL (79).
Alb A is a natural oleanane triterpenoid saponin.
It was found to induce apoptosis in malignant melanoma cells
through the mitochondria-mediated caspase cascade (191). A study found that structural
modifications of Alb A can improve its anti-tumor efficacy by
inhibiting the expression of GPX4 and increasing lipid
peroxidation, thus inducing ferroptosis in HCT116 cells (80). In another study, a combination
treatment with Alb A and pyruvate dehydrogenase kinase inhibitors
also led to the induction of ferroptosis (192).
A study reported the anti-tumor properties of
erianin, a natural compound extracted from Dendrobium
chrysotoxum Lindl., in various types of cancer (193). In a groundbreaking study, erianin
was found to induce ferroptotic cell death in lung cancer cells,
accompanied by the accumulation of ROS, lipid peroxidation and
depletion of GSH. The study found that Ca2+/CaM
signaling acts as a key mediator of erianin-triggered ferroptosis.
Inhibiting this signaling pathway rescued cell death induced by
erianin treatment, highlighting its role in the suppression of
ferroptosis (81). Another study on
erianin also revealed its ability to inhibit the growth of bladder
cancer cells by inducing ferroptosis via Nrf2 inactivation
(82).
Studies have investigated the therapeutic effects
of solasonine, a steroidal alkaloid extracted from the natural herb
Solanum nigrum L. It can inhibit the transcription factor
AP-2 alpha/Otubain 1/SLC7A11 axis, thus activating ferroptosis and
suppressing the progression of pancreatic cancer cells (195). Another study showed that
solasonine induces ferroptosis in HCC cells by disrupting the GSH
redox system via GPX4 (196).
A study found that β-elemene increases the
responsiveness of EGFR-mutant NSCLC to erlotinib by eliciting
ferroptosis. This mechanism involves upregulation of the long
non-coding RNA H19 (83). Another
study found that the combination treatment of β-elemene and
cetuximab was effective in KRAS mutant colorectal cancer cells.
This combination treatment induced ferroptosis and inhibited
epithelial-mesenchymal transition, thus increasing sensitivity
(23).
Isoliquiritin (Iso)-induced ferroptosis. Iso is a
flavonoid glycoside derived from Glycyrrhiza uralensis. A
study showed that isoliquiritin exerts its effects by modulating
ferroptosis by inhibiting NF-κB signaling. Iso can also alleviate
doxorubicin resistance in breast cancer (84).
Quercetin is a flavonoid derived from plants and is
abundant in various fruits and vegetables, including grapes,
apples, onions and green leafy vegetables. It not only has
antioxidant, anti-inflammatory, anti-fibrotic and antiviral effects
but also has high efficacy in suppressing the proliferation of
different types of cancer (197).
Two studies have found that quercetin facilitates the degradation
of lysosome-dependent ferritin, leading to the liberation of free
iron, thus initiating ferroptosis (85,86).
Certain researchers argue that ferroptosis needs to
be more clearly distinguished from necrosis (22). Natural products have varied
structures and different mechanisms of action to induce
ferroptosis. The development and research of natural products to
induce ferroptosis can provide novel and deeper insight into
ferroptosis. For instance, the mechanisms by which ARTCs, such as
tanshinone and ITCs, induce ferroptosis are diverse. Studying the
role of these compounds in inducing ferroptosis may help address
some of the unresolved questions. Numerous studies have
investigated the role of ARTCs in ferroptosis. These compounds have
clear effects and great potential as promising inducers of
ferroptosis for development and utilization. However, the
relationship between their role in inducing ferroptosis and their
anti-tumor effects needs to be elucidated. Studies investigating
the mechanism of action of ferroptosis inducers prioritized the key
drivers and the GPX4 pathway, whereas other ferroptosis defense
mechanisms discussed in this review were paid lesser attention to.
Thus, further research is needed to understand these aspects.
Combination treatment is generally performed to
reduce toxicity and drug resistance. Numerous studies have assessed
the combined application of natural products with
ferroptosis-inducing effects and first-line anti-tumor drugs, such
as the combined administration of ARTCs with sorafenib and
cisplatin. Further research on such combination treatment
strategies is expected to increase. Studies on the combination of
ferroptosis inducers with first-line anti-tumor drugs may become
more prevalent. The combination of drugs with multiple mechanisms
of action may act synergistically and reduce drug toxicity.
Ferroptosis inducers promote ferroptosis by reversing resistance
and allowing first-line anti-tumor drugs to continue exerting their
effects.
The structures of natural products related to
ferroptosis induction are rich and diverse. Thus, they can be used
as a resource pool for comprehensive research on drugs. These
structures provide valuable and novel information on ferroptosis
and drug development. Improving the structure of these products to
enhance their ability to induce ferroptosis can help develop more
effective drugs. Researchers believe that compounds with an inner
peroxide structure, including ARTCs, have strong ferroptosis
inducing ability. They can individually or synergistically induce
ferroptosis. Based on this finding, structural improvements may be
implemented as an effective strategy to develop and screen potent
ferroptosis-inducing compounds.
Not applicable.
Funding: This work was supported by the National Natural Science
Foundation of China (grant nos. 81960739 and 82360797), the Joint
Application and Basic Research Foundation of Kunming Medical
University & Science and Technology Department of Yunnan
Province of China (grant no. 202401AY070001-209), the Research
Project on Undergraduate Educational and Teaching Reforms in Yunnan
Province (grant no. JG2023001) and the Foundation of the Department
of Education of Yunnan Province of China (grant no. 2024J0150).
Not applicable.
XN: Conceptualization, writing-original draft,
supervision, writing-review & editing. LL: Writing-original
draft. DML and JQH: Literature review. LZ and YPZ:
Conceptualization, supervision, writing-review & editing. All
authors have read and approved the final version of the manuscript.
Data authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Ge C, Zhang S, Mu H, Zheng S, Tan Z, Huang
X, Xu C, Zou J, Zhu Y, Feng D and Aa J: Emerging mechanisms and
disease implications of ferroptosis: Potential applications of
natural products. Front Cell Dev Biol. 9:7749572022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stockwell BR, Jiang X and Gu W: Emerging
mechanisms and disesase relevance of ferroptosis. Trends Cell Biol.
30:478–490. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta
R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS,
et al: Ferroptosis: An iron-dependent form of nonapoptotic cell
death. Cell. 149:1060–1072. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Doll S, Freitas FP, Shah R, Aldrovandi M,
da Silva MC, Ingold I, Goya Grocin A, Xavier da Silva TN, Panzilius
E, Scheel CH, et al: FSP1 is a glutathione-independent ferroptosis
suppressor. Nature. 575:693–698. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ingold I, Berndt C, Schmitt S, Doll S,
Poschmann G, Buday K, Roveri A, Peng X, Porto Freitas F, Seibt T,
et al: Selenium utilization by GPX4 is required to prevent
hydroperoxide-induced ferroptosis. Cell. 172:409–422.e21. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kraft VAN, Bezjian CT, Pfeiffer S,
Ringelstetter L, Müller C, Zandkarimi F, Merl-Pham J, Bao X,
Anastasov N, Kössl J, et al: GTP cyclohydrolase
1/tetrahydrobiopterin counteract ferroptosis through lipid
remodeling. ACS Cent Sci. 6:41–53. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang WS, SriRamaratnam R, Welsch ME,
Shimada K, Skouta R, Viswanathan VS, Cheah JH, Clemons PA, Shamji
AF, Clish CB, et al: Regulation of ferroptotic cancer cell death by
GPX4. Cell. 156:317–331. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Doll S, Proneth B, Tyurina YY, Panzilius
E, Kobayashi S, Ingold I, Irmler M, Beckers J, Aichler M, Walch A,
et al: ACSL4 dictates ferroptosis sensitivity by shaping cellular
lipid composition. Nat Chem Biol. 13:91–98. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kagan VE, Mao G, Qu F, Angeli JP, Doll S,
Croix CS, Dar HH, Liu B, Tyurin VA, Ritov VB, et al: Oxidized
arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem
Biol. 13:81–90. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li L, Sun S, Tan L, Wang Y, Wang L, Zhang
Z and Zhang L: Polystyrene nanoparticles reduced ROS and inhibited
ferroptosis by triggering lysosome stress and TFEB nucleus
translocation in a size-dependent manner. Nano Lett. 19:7781–7792.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Park E and Chung SW: ROS-mediated
autophagy increases intracellular iron levels and ferroptosis by
ferritin and transferrin receptor regulation. Cell Death Dis.
10:8222019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu J, Minikes AM, Gao M, Bian H, Li Y,
Stockwell BR, Chen ZN and Jiang X: Intercellular interaction
dictates cancer cell ferroptosis via NF2-YAP signalling. Nature.
572:402–406. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liang L, Liu Y, Wu X and Chen Y:
Artesunate induces ferroptosis by inhibiting the nuclear
localization of SREBP2 in myeloma cells. Int J Med Sci.
20:1535–1550. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu H, Gao L, Xie T, Li J, Zhai T and Xu
Y: Identification and validation of a prognostic signature for
prostate cancer based on ferroptosis-related genes. Front Oncol.
11:6233132021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ma J, Hu X, Yao Y, Wu L, Sheng C, Chen K
and Liu B: Characterization of two ferroptosis subtypes with
distinct immune infiltration and gender difference in gastric
cancer. Front Nutr. 8:7561932021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tang B, Zhu J, Li J, Fan K, Gao Y, Cheng
S, Kong C, Zheng L, Wu F, Weng Q, et al: The ferroptosis and
iron-metabolism signature robustly predicts clinical diagnosis,
prognosis and immune microenvironment for hepatocellular carcinoma.
Cell Commun Signal. 18:1742020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Feng H, Schorpp K, Jin J, Yozwiak CE,
Hoffstrom BG, Decker AM, Rajbhandari P, Stokes ME, Bender HG, Csuka
JM, et al: Transferrin receptor is a specific ferroptosis marker.
Cell Rep. 30:3411–3423.e7. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hou W, Xie Y, Song X, Sun X, Lotze MT, Zeh
HJ III, Kang R and Tang D: Autophagy promotes ferroptosis by
degradation of ferritin. Autophagy. 12:1425–1428. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yanatori I and Kishi F: DMT1 and iron
transport. Free Radic Biol Med. 133:55–63. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mumbauer S, Pascual J, Kolotuev I and
Hamaratoglu F: Ferritin heavy chain protects the developing wing
from reactive oxygen species and ferroptosis. PLoS Genet.
15:e10083962019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gao M, Monian P, Pan Q, Zhang W, Xiang J
and Jiang X: Ferroptosis is an autophagic cell death process. Cell
Res. 26:1021–1032. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Stockwell BR: Ferroptosis turns 10:
Emerging mechanisms, physiological functions, and therapeutic
applications. Cell. 185:2401–2421. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen P, Li X, Zhang R, Liu S, Xiang Y,
Zhang M, Chen X, Pan T, Yan L, Feng J, et al: Combinative treatment
of β-elemene and cetuximab is sensitive to KRAS mutant colorectal
cancer cells by inducing ferroptosis and inhibiting
epithelial-mesenchymal transformation. Theranostics. 10:5107–5119.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen GQ, Benthani FA, Wu J, Liang D, Bian
ZX and Jiang X: Artemisinin compounds sensitize cancer cells to
ferroptosis by regulating iron homeostasis. Cell Death Differ.
27:242–254. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hu Y, Guo N, Yang T, Yan J, Wang W and Li
X: The potential mechanisms by which artemisinin and its
derivatives induce ferroptosis in the treatment of cancer. Oxid Med
Cell Longev. 2022:14581432022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang WS, Kim KJ, Gaschler MM, Patel M,
Shchepinov MS and Stockwell BR: Peroxidation of polyunsaturated
fatty acids by lipoxygenases drives ferroptosis. Proc Natl Acad Sci
USA. 113:E4966–E4975. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Viswanathan VS, Ryan MJ, Dhruv HD, Gill S,
Eichhoff OM, Seashore-Ludlow B, Kaffenberger SD, Eaton JK, Shimada
K, Aguirre AJ, et al: Dependency of a therapy-resistant state of
cancer cells on a lipid peroxidase pathway. Nature. 547:453–457.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Conrad M and Pratt DA: The chemical basis
of ferroptosis. Nat Chem Biol. 15:1137–1147. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jiang X, Stockwell BR and Conrad M:
Ferroptosis: Mechanisms, biology and role in disease. Nat Rev Mol
Cell Biol. 22:266–282. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sato H, Shiiya A, Kimata M, Maebara K,
Tamba M, Sakakura Y, Makino N, Sugiyama F, Yagami K, Moriguchi T,
et al: Redox imbalance in cystine/glutamate transporter-deficient
mice. J Biol Chem. 280:37423–37429. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Robert SM, Buckingham SC, Campbell SL,
Robel S, Holt KT, Ogunrinu-Babarinde T, Warren PP, White DM, Reid
MA, Eschbacher JM, et al: SLC7A11 expression is associated with
seizures and predicts poor survival in patients with malignant
glioma. Sci Transl Med. 7:289ra862015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang W, Green M, Choi JE, Gijón M, Kennedy
PD, Johnson JK, Liao P, Lang X, Kryczek I, Sell A, et al:
CD8+ T cells regulate tumour ferroptosis during cancer
immunotherapy. Nature. 569:270–274. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nie Z, Chen M, Gao Y, Huang D, Cao H, Peng
Y, Guo N, Wang F and Zhang S: Ferroptosis and tumor drug
resistance: Current status and major challenges. Front Pharmacol.
13:8793172022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kuang F, Liu J, Xie Y, Tang D and Kang R:
MGST1 is a redox-sensitive repressor of ferroptosis in pancreatic
cancer cells. Cell Chem Biol. 28:765–775.e5. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sun X, Ou Z, Chen R, Niu X, Chen D, Kang R
and Tang D: Activation of the p62-Keap1-NRF2 pathway protects
against ferroptosis in hepatocellular carcinoma cells. Hepatology.
63:173–184. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bersuker K, Hendricks JM, Li Z, Magtanong
L, Ford B, Tang PH, Roberts MA, Tong B, Maimone TJ, Zoncu R, et al:
The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit
ferroptosis. Nature. 575:688–692. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Stefely JA and Pagliarini DJ: Biochemistry
of mitochondrial coenzyme Q biosynthesis. Trends Biochem Sci.
42:824–843. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mao C, Liu X, Zhang Y, Lei G, Yan Y, Lee
H, Koppula P, Wu S, Zhuang L, Fang B, et al: DHODH-mediated
ferroptosis defence is a targetable vulnerability in cancer.
Nature. 593:586–590. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lei G, Zhuang L and Gan B: Targeting
ferroptosis as a vulnerability in cancer. Nat Rev Cancer.
22:381–396. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Manogaran P, Beeraka NM, Paulraj RS,
Sathiyachandran P and Thammaiappa M: Impediment of cancer by
dietary plant-derived alkaloids through oxidative stress:
Implications of PI3K/AKT pathway in apoptosis, autophagy, and
ferroptosis. Curr Top Med Chem. 23:860–877. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Deng H, Jia Q, Ming X, Sun Y, Lu Y, Liu L
and Zhou J: Hippo pathway in intestinal diseases: Focusing on
ferroptosis. Front Cell Dev Biol. 11:12916862023. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wu Z, Zhong M, Liu Y, Xiong Y, Gao Z, Ma
J, Zhuang G and Hong X: Application of natural products for
inducing ferroptosis in tumor cells. Biotechnol Appl Biochem.
69:190–197. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang N, Zeng GZ, Yin JL and Bian ZX:
Artesunate activates the ATF4-CHOP-CHAC1 pathway and affects
ferroptosis in Burkitt's lymphoma. Biochem Biophys Res Commun.
519:533–539. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang K, Zhang Z, Wang M, Cao X, Qi J, Wang
D, Gong A and Zhu H: Role of GRP78 inhibiting artesunate-induced
ferroptosis in KRAS mutant pancreatic cancer cells. Drug Des Devel
Ther. 13:2135–2144. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Li ZJ, Dai HQ, Huang XW, Feng J, Deng JH,
Wang ZX, Yang XM, Liu YJ, Wu Y, Chen PH, et al: Artesunate
synergizes with sorafenib to induce ferroptosis in hepatocellular
carcinoma. Acta Pharmacol Sin. 42:301–310. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Roh JL, Kim EH, Jang H and Shin D: Nrf2
inhibition reverses the resistance of cisplatin-resistant head and
neck cancer cells to artesunate-induced ferroptosis. Redox Biol.
11:254–262. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Vakhrusheva O, Erb HHH, Bräunig V,
Markowitsch SD, Schupp P, Baer PC, Slade KS, Thomas A, Tsaur I,
Puhr M, et al: Artesunate inhibits the growth behavior of
docetaxel-resistant prostate cancer cells. Front Oncol.
12:7892842022. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ye RR, Chen BC, Lu JJ, Ma XR and Li RT:
Phosphorescent rhenium(I) complexes conjugated with artesunate:
Mitochondrial targeting and apoptosis-ferroptosis dual induction. J
Inorg Biochem. 223:1115372021. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chen W, Xie L, Lv C, Song E, Zhu X and
Song Y: Transferrin-targeted cascade nanoplatform for inhibiting
transcription factor nuclear factor erythroid 2-related factor 2
and enhancing ferroptosis anticancer therapy. ACS Appl Mater
Interfaces. 15:28879–28890. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Huang QF, Li YH, Huang ZJ, Jun M, Wang W,
Chen XL and Wang GH: Artesunate carriers induced ferroptosis to
overcome biological barriers for anti-cancer. Eur J Pharm Biopharm.
190:284–293. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Han N, Li LG, Peng XC, Ma QL, Yang ZY,
Wang XY, Li J, Li QR, Yu TT, Xu HZ, et al: Ferroptosis triggered by
dihydroartemisinin facilitates chlorin e6 induced photodynamic
therapy against lung cancerthrough inhibiting GPX4 and enhancing
ROS. Eur J Pharmacol. 919:1747972022. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yi R, Wang H, Deng C, Wang X, Yao L, Niu
W, Fei M and Zhaba W: Dihydroartemisinin initiates ferroptosis in
glioblastoma through GPX4 inhibition. Biosci Rep.
40:BSR201933142020. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Cui Z, Wang H, Li S, Qin T, Shi H, Ma J,
Li L, Yu G, Jiang T and Li C: Dihydroartemisinin enhances the
inhibitory effect of sorafenib on HepG2 cells by inducing
ferroptosis and inhibiting energy metabolism. J Pharmacol Sci.
148:73–85. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zhang X, Ai Z, Zhang Z, Dong R, Wang L,
Jin S and Wei H: Dihydroartemisinin triggers ferroptosis in
multidrug-resistant leukemia cells. DNA Cell Biol. 41:705–715.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Grignano E, Cantero-Aguilar L, Tuerdi Z,
Chabane T, Vazquez R, Johnson N, Zerbit J, Decroocq J, Birsen R,
Fontenay M, et al: Dihydroartemisinin-induced ferroptosis in acute
myeloid leukemia: Links to iron metabolism and metallothionein.
Cell Death Discov. 9:972023. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Wang Y, Chen F, Zhou H, Huang L, Ye J, Liu
X, Sheng W, Gao W, Yu H and Wang F: Redox dyshomeostasis with dual
stimuli-activatable dihydroartemisinin nanoparticles to potentiate
ferroptotic therapy of pancreatic cancer. Small Methods.
7:e22008882023. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Shi H, Xiong L, Yan G, Du S, Liu J and Shi
Y: Susceptibility of cervical cancer to dihydroartemisinin-induced
ferritinophagy-dependent ferroptosis. Front Mol Biosci.
10:11560622023. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Lai X, Shi Y and Zhou M:
Dihydroartemisinin enhances gefitinib cytotoxicity against lung
adenocarcinoma cells by inducing ROS-dependent apoptosis and
ferroptosis. Kaohsiung J Med Sci. 39:699–709. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Antoszczak M, Müller S, Cañeque T,
Colombeau L, Dusetti N, Santofimia-Castaño P, Gaillet C, Puisieux
A, Iovanna JL and Rodriguez R: Iron-sensitive prodrugs that trigger
active ferroptosis in drug-tolerant pancreatic cancer cells. J Am
Chem Soc. 144:11536–11545. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Fan S, Yang Q, Song Q, Hong M, Liu X, Chen
H, Wang J, Li C and Cheng S: Multi-pathway inducing ferroptosis by
MnO2-based nanodrugs for targeted cancer therapy. Chem Commun
(Camb). 58:6486–6489. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Lin R, Zhang Z, Chen L, Zhou Y, Zou P,
Feng C, Wang L and Liang G: Dihydroartemisinin (DHA) induces
ferroptosis and causes cell cycle arrest in head and neck carcinoma
cells. Cancer Lett. 381:165–175. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Du J, Wang T, Li Y, Zhou Y, Wang X, Yu X,
Ren X, An Y, Wu Y, Sun W, et al: DHA inhibits proliferation and
induces ferroptosis of leukemia cells through autophagy dependent
degradation of ferritin. Free Radic Biol Med. 131:356–369. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Du J, Wang X, Li Y, Ren X, Zhou Y, Hu W,
Zhou C, Jing Q, Yang C, Wang L, et al: DHA exhibits synergistic
therapeutic efficacy with cisplatin to induce ferroptosis in
pancreatic ductal adenocarcinoma via modulation of iron metabolism.
Cell Death Dis. 12:7052021. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Yang XX, Xu X, Wang MF, Xu HZ, Peng XC,
Han N, Yu TT, Li LG, Li QR, Chen X, et al: A nanoreactor boosts
chemodynamic therapy and ferroptosis for synergistic cancer therapy
using molecular amplifier dihydroartemisinin. J Nanobiotechnology.
20:2302022. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Han W, Duan X, Ni K, Li Y, Chan C and Lin
W: Co-delivery of dihydroartemisinin and pyropheophorbide-iron
elicits ferroptosis to potentiate cancer immunotherapy.
Biomaterials. 280:1213152022. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Lin YS, Shen YC, Wu CY, Tsai YY, Yang YH,
Lin YY, Kuan FC, Lu CN, Chang GH, Tsai MS, et al: Danshen improves
survival of patients with breast cancer and dihydroisotanshinone I
induces ferroptosis and apoptosis of breast cancer cells. Front
Pharmacol. 10:12262019. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Tan S, Hou X and Mei L: Dihydrotanshinone
I inhibits human glioma cell proliferation via the activation of
ferroptosis. Oncol Lett. 20:1222020. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Guan Z, Chen J, Li X and Dong N:
Tanshinone IIA induces ferroptosis in gastric cancer cells through
p53-mediated SLC7A11 down-regulation. Biosci Rep.
40:BSR202018072020. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Ni H, Ruan G, Sun C, Yang X, Miao Z, Li J,
Chen Y, Qin H, Liu Y, Zheng L, et al: Tanshinone IIA inhibits
gastric cancer cell stemness through inducing ferroptosis. Environ
Toxicol. 37:192–200. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Li X, Li W, Yang P, Zhou H, Zhang W and Ma
L: Anticancer effects of cryptotanshinone against lung cancer cells
through ferroptosis. Arab J Chem. 14:1031772021. View Article : Google Scholar
|
|
71
|
Wu CY, Yang YH, Lin YS, Chang GH, Tsai MS,
Hsu CM, Yeh RA, Shu LH, Cheng YC and Liu HT: Dihydroisotanshinone I
induced ferroptosis and apoptosis of lung cancer cells. Biomed
Pharmacother. 139:1115852021. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Zheng K, Dong Y, Yang R, Liang Y, Wu H and
He Z: Regulation of ferroptosis by bioactive phytochemicals:
Implications for medical nutritional therapy. Pharmacol Res.
168:1055802021. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Lv H, Zhen C, Liu J and Shang P: PEITC
triggers multiple forms of cell death by GSH-iron-ROS regulation in
K7M2 murine osteosarcoma cells. Acta Pharmacol Sin. 41:1119–1132.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Iida Y, Okamoto-Κatsuyama M, Maruoka S,
Mizumura K, Shimizu T, Shikano S, Hikichi M, Takahashi M, Tsuya K,
Okamoto S, et al: Effective ferroptotic small-cell lung cancer cell
death from SLC7A11 inhibition by sulforaphane. Oncol Lett.
21:712021. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Kasukabe T, Honma Y, Okabe-Kado J, Higuchi
Y, Kato N and Kumakura S: Combined treatment with cotylenin A and
phenethyl isothiocyanate induces strong antitumor activity mainly
through the induction of ferroptotic cell death in human pancreatic
cancer cells. Oncol Rep. 36:968–976. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Qin Z, Ou S, Xu L, Sorensen K, Zhang Y, Hu
DP, Yang Z, Hu WY, Chen F and Prins GS: Design and synthesis of
isothiocyanate-containing hybrid androgen receptor (AR) antagonist
to downregulate AR and induce ferroptosis in GSH-deficient prostate
cancer cells. Chem Biol Drug Des. 97:1059–1078. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Tang HM and Cheung PCK: Gallic acid
triggers iron-dependent cell death with apoptotic, ferroptotic, and
necroptotic features. Toxins (Basel). 11:4922019. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Khorsandi K, Kianmehr Z, Hosseinmardi Z
and Hosseinzadeh R: Anti-cancer effect of gallic acid in presence
of low level laser irradiation: ROS production and induction of
apoptosis and ferroptosis. Cancer Cell Int. 20:182020. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Yamaguchi Y, Kasukabe T and Kumakura S:
Piperlongumine rapidly induces the death of human pancreatic cancer
cells mainly through the induction of ferroptosis. Int J Oncol.
52:1011–1022. 2018.PubMed/NCBI
|
|
80
|
Wei G, Sun J, Hou Z, Luan W, Wang S, Cui
S, Cheng M and Liu Y: Novel antitumor compound optimized from
natural saponin Albiziabioside A induced caspase-dependent
apoptosis and ferroptosis as a p53 activator through the
mitochondrial pathway. Eur J Med Chem. 157:759–772. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Liang X, Hu C, Han M, Yan L, Sun Y, Liu S,
Xiang Y, Zhang M, Pan T, Chen X, et al: Erianin, a novel dibenzyl
compound in Dendrobium extract, inhibits lung cancer cell
growth and migration via calcium/calmodulin-dependent ferroptosis.
Signal Transduct Target Ther. 5:512020. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Xiang Y, Chen X, Wang W, Zhai L, Sun X,
Feng J, Duan T, Zhang M, Pan T, Yan L, et al: Natural product
erianin inhibits bladder cancer cell growth by inducing ferroptosis
via NRF2 inactivation. Front Pharmacol. 12:7755062021. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Xu C, Jiang ZB, Shao L, Zhao ZM, Fan XX,
Sui X, Yu LL, Wang XR, Zhang RN, Wang WJ, et al: β-Elemene enhances
erlotinib sensitivity through induction of ferroptosis by
upregulating lncRNA H19 in EGFR-mutant non-small cell lung cancer.
Pharmacol Res. 191:1067392023. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Wang J, Li Y, Zhang J and Luo C:
Isoliquiritin modulates ferroptosis via NF-κB signaling inhibition
and alleviates doxorubicin resistance in breast cancer.
Immunopharmacol Immunotoxicol. 45:443–454. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
An S and Hu M: Quercetin promotes TFEB
nuclear translocation and activates lysosomal degradation of
ferritin to induce ferroptosis in breast cancer cells. Comput
Intell Neurosci. 2022:52992182022. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Zeng YY, Luo YB, Ju XD, Wu Y, Shi H, Chen
Y, Lu G, Shen HM, Lu GD and Zhou J: Quercetin induces
p53-independent cancer cell death through lysosome activation by
the transcription factor EB and reactive oxygen species-dependent
ferroptosis. Br J Pharmacol. 178:1133–1148. 2021. View Article : Google Scholar
|
|
87
|
Kannan R, Kumar K, Sahal D, Kukreti S and
Chauhan VS: Reaction of artemisinin with haemoglobin: Implications
for antimalarial activity. Biochem J. 385:409–418. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Beekman A, Wierenga P, Woerdenbag H, Van
Uden W, Pras N, Konings AW, el-Feraly FS, Galal AM and Wikström HV:
Artemisinin-derived sesquiterpene lactones as potential antitumour
compounds: Cytotoxic action against bone marrow and tumour cells.
Planta Med. 64:615–619. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Zheng GQ: Cytotoxic Terpenoids and
Flavonoids from Artemisia annua. Planta Med. 60:54–57. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Zhao Y, Jiang W, Li B, Yao Q, Dong J, Cen
Y, Pan X, Li J, Zheng J, Pang X and Zhou H: Artesunate enhances
radiosensitivity of human non-small cell lung cancer A549 cells via
increasing NO production to induce cell cycle arrest at G2/M phase.
Int Immunopharmacol. 11:2039–2046. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Dell'Eva R, Pfeffer U, Vené R, Anfosso L,
Forlani A, Albini A and Efferth T: Inhibition of angiogenesis in
vivo and growth of Kaposi's sarcoma xenograft tumors by the
anti-malarial artesunate. Biochem Pharmacol. 68:2359–2366. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Rasheed SAK, Efferth T, Asangani IA and
Allgayer H: First evidence that the antimalarial drug artesunate
inhibits invasion and in vivo metastasis in lung cancer by
targeting essential extracellular proteases. Int J Cancer.
127:1475–1485. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Wang B, Hou D, Liu Q, Wu T, Guo H, Zhang
X, Zou Y, Liu Z, Liu J, Wei J, et al: Artesunate sensitizes ovarian
cancer cells to cisplatin by downregulating RAD51. Cancer Biol
Ther. 16:1548–1556. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Zhou C, Pan W, Wang XP and Chen TS:
Artesunate induces apoptosis via a Bak-mediated caspase-independent
intrinsic pathway in human lung adenocarcinoma cells. J Cell
Physiol. 227:3778–3786. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Efferth T, Dunstan H, Sauerbrey A, Miyachi
H and Chitambar CR: The anti-malarial artesunate is also active
against cancer. Int J Oncol. 18:767–773. 2001.PubMed/NCBI
|
|
96
|
Disbrow GL, Baege AC, Kierpiec KA, Yuan H,
Centeno JA, Thibodeaux CA, Hartmann D and Schlegel R:
Dihydroartemisinin Is cytotoxic to papillomavirus-expressing
epithelial cells in vitro and In vivo. Cancer Res. 65:10854–10861.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Kelter G, Steinbach D, Konkimalla VB,
Tahara T, Taketani S, Fiebig HH and Efferth T: Role of transferrin
receptor and the ABC transporters ABCB6 and ABCB7 for resistance
and differentiation of tumor cells towards artesunate. PLoS One.
2:e7982007. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Lai H, Nakase I, Lacoste E, Singh NP and
Sasaki T: Artemisinin-transferrin conjugate retards growth of
breast tumors in the rat. Anticancer Res. 29:3807–3810.
2009.PubMed/NCBI
|
|
99
|
Mercer AE, Maggs JL, Sun XM, Cohen GM,
Chadwick J, O'Neill PM and Park BK: Evidence for the involvement of
carbon-centered radicals in the induction of apoptotic cell death
by artemisinin compounds. J Biol Chem. 282:9372–9382. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Eling N, Reuter L, Hazin J, Hamacher-Brady
A and Brady NR: Identification of artesunate as a specific
activator of ferroptosis in pancreatic cancer cells. Oncoscience.
2:517–532. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Yan G, Dawood M, Böckers M, Klauck SM,
Fottner C, Weber MM and Efferth T: Multiple modes of cell death in
neuroendocrine tumors induced by artesunate. Phytomedicine.
79:1533322020. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Song Q, Peng S, Che F and Zhu X:
Artesunate induces ferroptosis via modulation of p38 and ERK
signaling pathway in glioblastoma cells. J Pharmacol Sci.
148:300–306. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Koike T, Takenaka M, Suzuki N, Ueda Y,
Mori M, Hirayama T, Nagasawa H and Morishige KI: Intracellular
ferritin heavy chain plays the key role in artesunate-induced
ferroptosis in ovarian serous carcinoma cells. J Clin Biochem Nutr.
71:34–40. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Morris CA, Duparc S, Borghini-Fuhrer I,
Jung D, Shin CS and Fleckenstein L: Review of the clinical
pharmacokinetics of artesunate and its active metabolite
dihydroartemisinin following intravenous, intramuscular, oral or
rectal administration. Malar J. 10:2632011. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Valashedi MR, Nikoo A, Najafi-Ghalehlou N,
Tomita K, Kuwahara Y, Sato T, Roushandeh AM and Roudkenar MH:
Pharmacological targeting of ferroptosis in cancer treatment. Curr
Cancer Drug Targets. 22:108–125. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Li Y, Shi N, Zhang W, Zhang H, Song Y, Zhu
W and Feng X: Supramolecular hybrids of carbon dots and
dihydroartemisinin for enhanced anticancer activity and mechanism
analysis. J Mater Chem B. 8:9777–9784. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Shterman N, Kupfer B and Moroz C:
Comparison of transferrin receptors, iron content and isoferritin
profile in normal and malignant human breast cell lines.
Pathobiology. 59:19–25. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Torti SV and Torti FM: Iron and cancer:
More ore to be mined. Nat Rev Cancer. 13:342–355. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Cao JY and Dixon SJ: Mechanisms of
ferroptosis. Cell Mol Life Sci. 73:2195–2209. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Yang ND, Tan SH, Ng S, Shi Y, Zhou J, Tan
KS, Wong WS and Shen HM: Artesunate induces cell death in human
cancer cells via enhancing lysosomal function and lysosomal
degradation of ferritin. J Biol Chem. 289:33425–33441. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Dowdle WE, Nyfeler B, Nagel J, Elling RA,
Liu S, Triantafellow E, Menon S, Wang Z, Honda A, Pardee G, et al:
Selective VPS34 inhibitor blocks autophagy and uncovers a role for
NCOA4 in ferritin degradation and iron homeostasis in vivo. Nat
Cell Biol. 16:1069–1079. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Mancias JD, Wang X, Gygi SP, Harper JW and
Kimmelman AC: Quantitative proteomics identifies NCOA4 as the cargo
receptor mediating ferritinophagy. Nature. 509:105–109. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Abrams RP, Carroll WL and Woerpel KA:
Five-membered ring peroxide selectively initiates ferroptosis in
cancer cells. ACS Chem Biol. 11:1305–1312. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Efferth T and Oesch F: Oxidative stress
response of tumor cells: Microarray-based comparison between
artemisinins and anthracyclines. Biochem Pharmacol. 68:3–10. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Horwedel C, Tsogoeva SB, Wei S and Efferth
T: Cytotoxicity of artesunic acid homo- and heterodimer molecules
toward sensitive and multidrug-resistant CCRF-CEM leukemia cells. J
Med Chem. 53:4842–4848. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Lee YS, Lee DH, Choudry HA, Bartlett DL
and Lee YJ: Ferroptosis-induced endoplasmic reticulum stress:
Crosstalk between ferroptosis and apoptosis. Mol Cancer Res.
16:1073–1076. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Ooko E, Saeed ME, Kadioglu O, Sarvi S,
Colak M, Elmasaoudi K, Janah R, Greten HJ and Efferth T:
Artemisinin derivatives induce iron-dependent cell death
(ferroptosis) in tumor cells. Phytomedicine. 22:1045–1054. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Amable L: Cisplatin resistance and
opportunities for precision medicine. Pharmacol Res. 106:27–36.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Zhao F, Vakhrusheva O, Markowitsch SD,
Slade KS, Tsaur I, Cinatl J Jr, Michaelis M, Efferth T, Haferkamp A
and Juengel E: Artesunate impairs growth in cisplatin-resistant
bladder cancer cells by cell cycle arrest, apoptosis and autophagy
induction. Cells. 9:26432020. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Wong KH, Yang D, Chen S, He C and Chen M:
Development of nanoscale drug delivery systems of
dihydroartemisinin for cancer therapy: A review. Asian J Pharm Sci.
17:475–490. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Efferth T: From ancient herb to modern
drug: Artemisia annua and artemisinin for cancer therapy.
Semin Cancer Biol. 46:65–83. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Zhang C and Zhang F: Iron homeostasis and
tumorigenesis: Molecular mechanisms and therapeutic opportunities.
Protein Cell. 6:88–100. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Xu M, Zha H, Han R, Cheng Y, Chen J, Yue
L, Wang R and Zheng Y: Cyclodextrin-derived ROS-generating
nanomedicine with pH-modulated degradability to enhance tumor
ferroptosis therapy and chemotherapy. Small. 18:e22003302022.
View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Huang D, Xu D, Chen W, Wu R, Wen Y, Liu A,
Lin L, Lin X and Wang X: Fe-MnO2 nanosheets loading
dihydroartemisinin for ferroptosis and immunotherapy. Biomed
Pharmacother. 161:1144312023. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Li L, Yang X, Xu H, Yu TT, Li QR, Hu J,
Peng XC, Han N, Xu X, Chen NN, et al: A dihydroartemisinin-loaded
nanoreactor motivates anti-cancer immunotherapy by synergy-induced
ferroptosis to activate Cgas/STING for reprogramming of macrophage.
Adv Healthc Mater. 12:e23015612023. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Liang J, Li L, Tian H, Wang Z, Liu G, Duan
X, Guo M, Liu J, Zhang W, Nice EC, et al: Drug repurposing-based
brain-targeting self-assembly nanoplatform using enhanced
ferroptosis against glioblastoma. Small. 19:e23030732023.
View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Zhang X, Liu H, Li N, Li J, Wang M and Ren
X: A (Traditional Chinese Medicine) TCM-inspired doxorubicin
resistance reversing strategy: Preparation, characterization, and
application of a co-loaded pH-sensitive liposome. AAPS
PharmSciTech. 24:1812023. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Zheng Y, Zheng J, Du M, Yang Y, Li X, Chen
H and Gao Y: An iron-containing ferritin-based nanosensitizer for
synergistic ferroptosis/sono-photodynamic cancer therapy. J Mater
Chem B. 11:4958–4971. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Jiang Z, Gao W and Huang L: Tanshinones,
critical pharmacological components in Salvia miltiorrhiza.
Front Pharmacol. 10:2022019. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Pang H, Wu L, Tang Y, Zhou G, Qu C and
Duan J: Chemical analysis of the herbal medicine salviae
miltiorrhizae radix et Rhizoma (Danshen). Molecules. 21:512016.
View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Huang X, Jin L, Deng H, Wu D, Shen QK,
Quan ZS, Zhang CH and Guo HY: Research and development of natural
product tanshinone I: Pharmacology, total synthesis, and structure
modifications. Front Pharmacol. 13:9204112022. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Li W, Huang T, Xu S, Che B, Yu Y, Zhang W
and Tang K: Molecular mechanism of tanshinone against prostate
cancer. Molecules. 27:55942022. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Dong Y, Morris-Natschke SL and Lee KH:
Biosynthesis, total syntheses, and antitumor activity of
tanshinones and their analogs as potential therapeutic agents. Nat
Prod Rep. 28:529–542. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Fu L, Han B, Zhou Y, Ren J, Cao W, Patel
G, Kai G and Zhang J: The anticancer properties of tanshinones and
the pharmacological effects of their active ingredients. Front
Pharmacol. 11:1932020. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Yang H, Gao Y, Fan X, Liu X, Peng L and Ci
X: Oridonin sensitizes cisplatin-induced apoptosis via
AMPK/Akt/mTOR-dependent autophagosome accumulation in A549 cells.
Front Oncol. 9:7692019. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Yang W, Zhao J, Wang Y, Xu H, Wu Z, Hu Y,
Jiang K, Shen P, Ma C, Guan Z, et al: In vivo inhibitory activity
of andrographolide derivative ADN-9 against liver cancer and its
mechanisms involved in inhibition of tumor angiogenesis. Toxicol
Appl Pharmacol. 327:1–12. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Yu J, Wang X, Li Y and Tang B: Tanshinone
IIA suppresses gastric cancer cell proliferation and migration by
downregulation of FOXM1. Oncol Rep. 37:1394–1400. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Zhang Y, Wei R, Zhu X, Cai L, Jin W and Hu
H: Tanshinone IIA induces apoptosis and inhibits the proliferation,
migration, and invasion of the osteosarcoma MG-63 cell line in
vitro. Anticancer Drugs. 23:212–219. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Zhou M, Zhou G, Hu S and Zhang L:
Tanshinone IIA suppress the proliferation of HNE-1 nasopharyngeal
carcinoma an in vitro study. Saudi J Biol Sci. 25:267–272. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Wu WL, Chang WL and Chen CF: Cytotoxic
activities of tanshinones against human carcinoma cell lines. Am J
Chin Med. 19:207–216. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Cao Y, Huang B and Gao C: Salvia
miltiorrhiza extract dihydrotanshinone induces apoptosis and
inhibits proliferation of glioma cells. Bosn J of Basic Med Sci.
17:235–240. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Li Q, Hu K, Tang S, Xu LF and Luo YC:
Anti-tumor activity of tanshinone IIA in combined with
cyclophosphamide against Lewis mice with lung cancer. Asian Pac J
Trop Med. 9:1084–1088. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Lv C, Zeng HW, Wang JX, Yuan X, Zhang C,
Fang T, Yang PM, Wu T, Zhou YD, Nagle DG and Zhang WD: The
antitumor natural product tanshinone IIA inhibits protein kinase C
and acts synergistically with 17-AAG. Cell Death Dis. 9:1652018.
View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Ma K, Zhang C, Huang MY, Guo YX and Hu GQ:
Crosstalk between beclin-1-dependent autophagy and
caspase-dependent apoptosis induced by tanshinone IIA in human
osteosarcoma MG-63 cells. Oncol Rep. 36:1807–1818. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Qiu W, Sun B, He F and Zhang Y:
MTA-induced notch activation enhances the proliferation of human
dental pulp cells by inhibiting autophagic flux. Int Endod J. 50
(Suppl 2):e52–e62. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Su CC, Chien SY, Kuo SJ, Chen YL, Cheng CY
and Chen DR: Tanshinone IIA inhibits human breast cancer MDA-MB-231
cells by decreasing LC3-II, Erb-B2 and NF-κBp65. Mol Med Rep.
5:1019–1022. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Zhang K, Li J, Meng W, Xing H and Yang Y:
Tanshinone IIA inhibits acute promyelocytic leukemia cell
proliferation and induces their apoptosis in vivo. Blood Cells Mol
Dis. 56:46–52. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Zhang Y, Guo S, Fang J, Peng B, Zhang Y
and Cao T: Tanshinone IIA inhibits cell proliferation and tumor
growth by downregulating STAT3 in human gastric cancer. Exp Ther
Med. 16:2931–2937. 2018.PubMed/NCBI
|
|
149
|
Jiang L, Kon N, Li T, Wang SJ, Su T,
Hibshoosh H, Baer R and Gu W: Ferroptosis as a p53-mediated
activity during tumour suppression. Nature. 520:57–62. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Radif Y, Ndiaye H, Kalantzi V, Jacobs R,
Hall A, Minogue S and Waugh MG: The endogenous subcellular
localisations of the long chain fatty acid-activating enzymes ACSL3
and ACSL4 in sarcoma and breast cancer cells. Mol Cell Biochem.
448:275–286. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Yuan H, Li X, Zhang X, Kang R and Tang D:
Identification of ACSL4 as a biomarker and contributor of
ferroptosis. Biochem Biophys Res Commun. 478:1338–1343. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Mao W, Ding J, Li Y, Huang R and Wang B:
Inhibition of cell survival and invasion by Tanshinone IIA via
FTH1: A key therapeutic target and biomarker in head and neck
squamous cell carcinoma. Exp Ther Med. 24:5212022. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Wang TX, Duan KL, Huang ZX, Xue ZA, Liang
JY, Dang Y, Zhang A, Xiong Y, Ding C, Guan KL and Yuan HX:
Tanshinone functions as a coenzyme that confers gain of function of
NQO1 to suppress ferroptosis. Life Sci Alliance. 6:e2022016672022.
View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Zhao H, Han B, Li X, Sun C, Zhai Y, Li M,
Jiang M, Zhang W, Liang Y and Kai G: Salvia miltiorrhiza in
breast cancer treatment: A review of its phytochemistry,
derivatives, nanoparticles, and potential mechanisms. Front
Pharmacol. 13:8720852022. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Zhang YF, Zhang M, Huang XL, Fu YJ, Jiang
YH, Bao LL, Maimaitiyiming Y, Zhang GJ, Wang QQ and Naranmandura H:
The combination of arsenic and cryptotanshinone induces apoptosis
through induction of endoplasmic reticulum stress-reactive oxygen
species in breast cancer cells. Metallomics. 7:165–173. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Lin H, Zheng L, Li S, Xie B, Cui B, Xia A,
Lin Z and Zhou P: Cytotoxicity of tanshinone IIA combined with
Taxol on drug-resist breast cancer cells MCF-7 through inhibition
of Tau. Phytother Res. 32:667–671. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Li K and Lai H: TanshinoneIIA enhances the
chemosensitivity of breast cancer cells to doxorubicin through
down-regulating the expression of MDR-related ABC transporters.
Biomed Pharmacother. 96:371–377. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Li S, Wu C, Fan C, Zhang P, Yu G and Li K:
Tanshinone II A improves the chemosensitivity of breast cancer
cells to doxorubicin by inhibiting β-catenin nuclear translocation.
J Biochem Mol Toxicol. 35:e226202021. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Hu T, To KKW, Wang L, Zhang L, Lu L, Shen
J, Chan RL, Li M, Yeung JH and Cho CH: Reversal of P-glycoprotein
(P-gp) mediated multidrug resistance in colon cancer cells by
cryptotanshinone and dihydrotanshinone of Salvia
miltiorrhiza. Phytomedicine. 21:1264–1272. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Hu T, Wang L, Zhang L, Lu L, Shen J, Chan
RL, Li M, Wu WK, To KK and Cho CH: Sensitivity of
apoptosis-resistant colon cancer cells to tanshinones is mediated
by autophagic cell death and p53-independent cytotoxicity.
Phytomedicine. 22:536–544. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Tian HL, Yu T, Xu NN, Feng C, Zhou LY, Luo
HW, Chang DC, Le XF and Luo KQ: A novel compound modified from
tanshinone inhibits tumor growth in vivo via activation of the
intrinsic apoptotic pathway. Cancer Lett. 297:18–30. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Guerram M, Jiang ZZ, Yousef BA, Hamdi AM,
Hassan HM, Yuan ZQ, Luo HW, Zhu X and Zhang LY: The potential
utility of acetyltanshinone IIA in the treatment of
HER2-overexpressed breast cancer: Induction of cancer cell death by
targeting apoptotic and metabolic signaling pathways. Oncotarget.
6:21865–21877. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Wu Q, Zheng K, Huang X, Li L and Mei W:
Tanshinone-IIA-based analogues of imidazole alkaloid Act as potent
inhibitors to block breast cancer invasion and metastasis in vivo.
J Med Chem. 61:10488–10501. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Liu Y, Xie X, Hou X, Shen J, Shi J, Chen
H, He Y, Wang Z and Feng N: Functional oral nanoparticles for
delivering silibinin and cryptotanshinone against breast cancer
lung metastasis. J Nanobiotechnology. 18:832020. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Ngo SNT and Williams DB: Protective effect
of isothiocyanates from cruciferous vegetables on breast cancer:
Epidemiological and preclinical perspectives. Anticancer Agents Med
Chem. 21:1413–1430. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Fimognari C, Turrini E, Ferruzzi L, Lenzi
M and Hrelia P: Natural isothiocyanates: Genotoxic potential versus
chemoprevention. Mutat Res. 750:107–131. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Palliyaguru DL, Yuan JM, Kensler TW and
Fahey JW: Isothiocyanates: Translating the power of plants to
people. Mol Nutr Food Res. 62:17009652018. View Article : Google Scholar
|
|
168
|
Greco G, Schnekenburger M, Catanzaro E,
Turrini E, Ferrini F, Sestili P, Diederich M and Fimognari C:
Discovery of sulforaphane as an inducer of ferroptosis in U-937
leukemia cells: Expanding its anticancer potential. Cancers
(Basel). 14:762021. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Chen PY, Lin KC, Lin JP, Tang NY, Yang JS,
Lu KW and Chung JG: Phenethyl isothiocyanate (PEITC) inhibits the
growth of human oral squamous carcinoma HSC-3 Cells through
G(0)/G(1) phase arrest and mitochondria-mediated apoptotic cell
death. Evid Based Complement Alternat Med. 2012:7183202012.
View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Hwang ES and Lee HJ: Effects of
phenylethyl isothiocyanate and its metabolite on cell-cycle arrest
and apoptosis in LNCaP human prostate cancer cells. Int J Food Sci
Nutr. 61:324–336. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Lv H, Zhen C, Liu J and Shang P:
β-Phenethyl isothiocyanate induces cell death in human osteosarcoma
through altering iron metabolism, disturbing the redox balance, and
activating the MAPK signaling pathway. Oxid Med Cell Longev.
2020:50219832020. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Pappa G, Lichtenberg M, Iori R, Barillari
J, Bartsch H and Gerhäuser C: Comparison of growth inhibition
profiles and mechanisms of apoptosis induction in human colon
cancer cell lines by isothiocyanates and indoles from Brassicaceae.
Mutat Res. 599:76–87. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Wu CL, Huang AC, Yang JS, Liao CL, Lu HF,
Chou ST, Ma CY, Hsia TC, Ko YC and Chung JG: Benzyl isothiocyanate
(BITC) and phenethyl isothiocyanate (PEITC)-mediated generation of
reactive oxygen species causes cell cycle arrest and induces
apoptosis via activation of caspase-3, mitochondria dysfunction and
nitric oxide (NO) in human osteogenic sarcoma U-2 OS cells. J
Orthop Res. 29:1199–1209. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Mitsiogianni M, Koutsidis G, Mavroudis N,
Trafalis DT, Botaitis S, Franco R, Zoumpourlis V, Amery T, Galanis
A, Pappa A and Panayiotidis MI: The role of isothiocyanates as
cancer chemo-preventive, chemo-therapeutic and anti-melanoma
agents. Antioxidants (Basel). 8:1062019. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Subramanian AP, John AA, Vellayappan MV,
Balaji A, Jaganathan SK, Supriyanto E and Yusof M: Gallic acid:
Prospects and molecular mechanisms of its anticancer activity. RSC
Adv. 5:35608–35621. 2015. View Article : Google Scholar
|
|
176
|
You BR and Park WH: Gallic acid-induced
lung cancer cell death is related to glutathione depletion as well
as reactive oxygen species increase. Toxicol In Vitro.
24:1356–1362. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Liu KC, Ho HC, Huang AC, Ji BC, Lin HY,
Chueh FS, Yang JS, Lu CC, Chiang JH, Meng M and Chung JG: Gallic
acid provokes DNA damage and suppresses DNA repair gene expression
in human prostate cancer PC-3 cells. Environ Toxicol. 28:579–587.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Subramanian V, Venkatesan B, Tumala A and
Vellaichamy E: Topical application of Gallic acid suppresses the
7,12-DMBA/Croton oil induced two-step skin carcinogenesis by
modulating anti-oxidants and MMP-2/MMP-9 in Swiss albino mice. Food
Chem Toxicol. 66:44–55. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Teng CLJ, Han SM, Wu WC, Hsueh CM, Tsai
JR, Hwang WL and Hsu SL: Mechanistic aspects of lauryl
gallate-induced differentiation and apoptosis in human acute
myeloid leukemia cells. Food Chem Toxicol. 71:197–206. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Kim NS, Jeong SI, Hwang BS, Lee YE, Kang
SH, Lee HC and Oh CH: Gallic acid inhibits cell viability and
induces apoptosis in human monocytic cell line U937. J Med Food.
14:240–246. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Zhao B and Hu M: Gallic acid reduces cell
viability, proliferation, invasion and angiogenesis in human
cervical cancer cells. Oncol Lett. 6:1749–1755. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Asano J, Chiba K, Tada M and Yoshii T:
Cytotoxic xanthones from Garcinia hanburyi. Phytochemistry.
41:815–820. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Su J, Xu T, Jiang G, Hou M, Liang M, Cheng
H and Li Q: Gambogenic acid triggers apoptosis in human
nasopharyngeal carcinoma CNE-2Z cells by activating
volume-sensitive outwardly rectifying chloride channel.
Fitoterapia. 133:150–158. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Mei W, Dong C, Hui C, Bin L, Fenggen Y,
Jingjing S, Cheng P, Meiling S, Yawen H, Xiaoshan W, et al:
Gambogenic acid kills lung cancer cells through aberrant autophagy.
PLoS One. 9:e836042014. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Yan F, Wang M, Chen H, Su J, Wang X, Wang
F, Xia L and Li Q: Gambogenic acid mediated apoptosis through the
mitochondrial oxidative stress and inactivation of Akt signaling
pathway in human nasopharyngeal carcinoma CNE-1 cells. Eur J
Pharmacol. 652:23–32. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Wang M, Li S, Wang Y, Cheng H, Su J and Li
Q: Gambogenic acid induces ferroptosis in melanoma cells undergoing
epithelial-to-mesenchymal transition. Toxicol Appl Pharmacol.
401:1151102020. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Wang M, Cheng H, Wu H, Liu C, Li S, Li B,
Su J, Luo S and Li Q: Gambogenic acid antagonizes the expression
and effects of long non-coding RNA NEAT1 and triggers autophagy and
ferroptosis in melanoma. Biomed Pharmacother. 154:1136362022.
View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Dhillon H, Mamidi S, McClean P and Reindl
KM: Transcriptome analysis of piperlongumine-treated human
pancreatic cancer cells reveals involvement of oxidative stress and
endoplasmic reticulum stress pathways. J Med Food. 19:578–585.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Jin HO, Park JA, Kim HA, Chang YH, Hong
YJ, Park IC and Lee JK: Piperlongumine downregulates the expression
of HER family in breast cancer cells. Biochem Biophys Res Commun.
486:1083–1089. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Alpay M, Yurdakok-Dikmen B, Kismali G and
Sel T: Antileukemic effects of piperlongumine and alpha lipoic acid
combination on Jurkat, MEC1 and NB4 cells in vitro. J Can Res Ther.
12:556–560. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Wei G, Wang S, Cui S, Guo J, Liu Y, Liu Y
and Cheng M: Synthesis and evaluation of the anticancer activity of
albiziabioside A and its analogues as apoptosis inducers against
human melanoma cells. Org Biomol Chem. 12:5928–5935. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Wei G, Sun J, Luan W, Hou Z, Wang S, Cui
S, Cheng M and Liu Y: Natural product albiziabioside A conjugated
with pyruvate dehydrogenase kinase inhibitor dichloroacetate to
induce apoptosis-ferroptosis-M2-TAMs polarization for combined
cancer therapy. J Med Chem. 62:8760–8772. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Wang H, Zhang T, Sun W, Wang Z, Zuo D,
Zhou Z, Li S, Xu J, Yin F, Hua Y and Cai Z: Erianin induces
G2/M-phase arrest, apoptosis, and autophagy via the ROS/JNK
signaling pathway in human osteosarcoma cells in vitro and in vivo.
Cell Death Dis. 7:e22472016. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Xie Y, Zhou X, Li J, Yao XC, Liu WL, Kang
FH, Zou ZX, Xu KP, Xu PS and Tan GS: Identification of a new
natural biflavonoids against breast cancer cells induced
ferroptosis via the mitochondrial pathway. Bioorg Chem.
109:1047442021. View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Liang X, Hu C, Han M, Liu C, Sun X, Yu K,
Gu H and Zhang J: Solasonine inhibits pancreatic cancer progression
with involvement of ferroptosis induction. Front Oncol.
12:8347292022. View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Jin M, Shi C, Li T, Wu Y, Hu C and Huang
G: Solasonine promotes ferroptosis of hepatoma carcinoma cells via
glutathione peroxidase 4-induced destruction of the glutathione
redox system. Biomed Pharmacother. 129:1102822020. View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Kim DH, Khan H, Ullah H, Hassan STS,
Šmejkal K, Efferth T, Mahomoodally MF, Xu S, Habtemariam S, Filosa
R, et al: MicroRNA targeting by quercetin in cancer treatment and
chemoprotection. Pharmacol Res. 147:1043462019. View Article : Google Scholar : PubMed/NCBI
|