Introduction
Lymphoma, a malignant hematological tumor that
originates from the lymph nodes or other lymphoid tissues, is
mainly divided into Hodgkin lymphoma and non-Hodgkin lymphoma (NHL)
subgroups. Collectively, B-cell lymphoma is the most important
subgroup of NHL, which causes significant clinical challenges
because of heterogeneity and frequent recurrence (1). The programmed cell death protein 1
(PD-1) receptor and its ligand, PD-L1, play a pivotal role in the
tumor immune escape mechanism. Immune checkpoint therapy targeting
PD-1 and PD-L1 has received regulatory approval for the treatment
of specific malignancies, including hematological malignancies
(2). PD-1 blockade has demonstrated
notable efficacy in recovering T-cell activation in various
malignancies, including melanoma, gastric carcinoma, lung cancer
and Hodgkin lymphoma (3–9). This immunotherapeutic approach
capitalizes on the body's own immune system to combat cancer cells;
however, most patients with B-cell lymphoma have no response to
PD-1 blockade therapy. The reasons for the poor clinical efficacy
of PD-1 blockade in B-cell lymphoma remain indistinct; therefore,
drug screening that improves the response to PD-1 blocking
therapies is a challenge for B-cell lymphoma.
Histone acetylation is regulated by the homeostasis
of histone acetyltransferases and histone deacetylases (HDACs).
HDAC expression is frequently dysregulated in numerous types of
cancer, including B-cell lymphoma (10–12).
HDACs are categorized into four classes based on their structure,
mechanism and cellular localization (13): Class I (HDAC1, HDAC2, HDAC3, HDAC8),
Class IIa (HDAC4, HDAC5, HDAC7, HDAC9), Class IIb (HDAC6, HDAC10)
and Class IV (HDAC11). Altered HDAC activity is associated with
neurodegenerative disorders, genetic diseases and cancer (14–16).
In cancer, HDAC overexpression is correlated with poor outcomes,
and can contribute to the development and progression of
hematological malignancies, such as acute lymphoblastic leukemia,
acute myeloid leukemia and chronic myeloid leukemia (13). A total of four HDAC inhibitors
(HDACis), belinostat, vorinostat, panobinostat and romidepsin, have
been applied in hematological cancers (17,18).
Previous studies have illustrated that HDACis can heighten tumor
immunogenicity (19,20). In recent years, the pursuit of novel
HDACis has been unwavering, leading to significant progress. Wu
et al (21) examined
chidamide, an innovative HDACi, to ascertain its therapeutic
efficacy in diffuse large B-cell lymphoma (DLBCL), offering a
scientific basis for targeting HDACs in such patients.
Bioinformatics analyses coupled with experimental data have
revealed notably elevated expression levels of several HDACs
(HDAC1, 2, 3, 4, 6, 7, 8 and 9) in DLBCL lymph node samples
relative to whole blood cell controls. Furthermore, the mutation
frequency of HDACs in DLBCL tissues has been shown to be amplified.
Targeting HDACs with selective inhibitors, such as chidamide,
presents a prospective therapeutic strategy for patients with DLBCL
(21). HDACi drugs are a novel
class of antitumor drugs that regulate gene expression and cellular
function by inhibiting the activity of HDACis (22). Although no HDACi drugs are currently
available specifically for the treatment of B-cell lymphoma in the
clinic, available research has suggested that this class of drugs
shows promising efficacy and potential in the treatment of other
types of tumors. In 2006, the first HDACi was approved by the Food
and Drug Administration (FDA) for the treatment of cutaneous T-cell
lymphoma, namely SAHA (vorinostat) (23). After SAHA, three other HDACis were
approved by the FDA (24). Studying
HDACi drugs for B-cell lymphoma treatment is significant as it
allows initial exploration of their mechanism, assessing their
therapeutic potential and safety. Understanding their impact on
tumor cell processes, such as proliferation, differentiation and
apoptosis, provides a scientific basis for new therapeutic
strategies.
Based on the current research, the following
hypothesis was proposed: The synergistic effects of HDACis with
PD-1 blockade therapies may improve efficacy in B-cell lymphoma.
Romidepsin is an oral selective HDACi that primarily inhibits HDAC1
and HDAC2 activity. It is widely used to treat specific types of
lymphoma and other blood cancers, such as cutaneous T-cell lymphoma
and peripheral T-cell lymphoma (25). BMS-1 is a monoclonal antibody that
targets the PD-1 receptor, blocking its interaction with PD-L1 and
PD-L2; this disruption renews the T cell-mediated immune response,
particularly against tumor cells expressing PD-L1 (26). However, their role in B-cell
lymphoma cells is not yet fully understood. Therefore, the present
study investigated the combined therapeutic potential of romidepsin
and BMS-1 in B-cell lymphoma and its preliminary mechanism of
action through in vivo experiments, aiming to improve the
treatment of this type of lymphoma, and to provide a theoretical
basis and a new therapeutic strategy for future clinical
application.
Materials and methods
Cell culture
Mouse B-cell lymphoma cell lines are often
considered a valid model to study human lymphomas because they have
similar biological properties. As there are currently no HDACi
drugs specifically approved for the treatment of B-cell lymphomas,
the present study aimed to use this relatively simple and
controlled model for initial testing to explore new drug
candidates. The mouse B-cell lymphoma A20 cell line (cat. no.
TIB-208) was obtained from the American Type Culture Collection,
and was cultured in RPMI-1640 medium (Gibco; Thermo Fisher
Scientific, Inc.; cat. no. 11875093) supplemented with 10% fetal
bovine serum (Gibco; Thermo Fisher Scientific, Inc.; cat. no.
10099141C) and penicillin/streptomycin (Biosharp Life Sciences;
cat. no. BL505A) in a humidified incubator containing 5%
CO2 at 37°C.
MTT assay
To analyze the effects of individual inhibitors and
their combination on the proliferation of A20 cells, the MTT assay
was employed. Briefly, A20 cells were seeded into 96-well plates at
a suitable density of 5×103 cells/well, along with
appropriate controls. Different concentrations of romidepsin (0, 1,
2, 5, 10 and 20 µM) or BMS-1 (0, 2.5, 5, 7.5, 10 and 15 nM)
(purchased from Shanghai Aladdin Biochemical Technology Co., Ltd.)
were added for treatment, with at least three replicates for each
concentration. After incubation at 37°C for 48 h, 50 µl/well MTT (1
mg/ml; Sigma Aldrich; Merck KGaA) was added. Following a 3-h
incubation, DMSO (150 µl/well; Beyotime Institute of Biotechnology)
was added and the absorbance was measured at 570 nm using a
solution mixture. Dose-response curves were plotted based on the
results. IC50 values were calculated using GraphPad
Prism.
Isograft mouse model
A total of 32 Balb/c mice (female; age, 4 weeks;
weight, 20 g), which are widely used in animal experiments in
immunology and physiology, were purchased from Charles River
Bio-company, and were adaptively maintained in the indicated
environment (pathogen-free, 12-h light/dark cycle, 22°C). Mouse
B-cell lymphoma A20 isografts were established by the intradermal
injection of logarithmic growth phase A20 cells (1×107
cells/ml, 200 µl, 2×106/per mice) into the right flank.
A total of 1 week after the injection, by which time the mice were
tumor-bound, the mice were randomly divided into four groups
(n=8/group), and were administered saline (control), BMS-1 (500
µg/ml; 100 µl/each), romidepsin (1 mg/kg body weight), or a
combination of both drugs daily for 19 days. Tumor size was
measured using a micrometer caliper. Tumor volume (V,
mm3) was calculated every 2 days using the following
formula: V=(a × b2)/2, where a refers to the longest
diameter and b to the shortest diameter. When the volume of the
tumor reached 1,500 mm3, the mice were euthanized by
cervical dislocation. Once it was confirmed that the mice showed no
signs of life (that is, there was no chest movement, and the
eyelids were pale with no visual response), tumors were collected
to measure the volume. Data are expressed as the mean ± SEM. In
addition, the body weight of each mouse was measured to evaluate
the toxicity of the treatment on the mice. Before sacrifice,
peripheral blood from each mouse was collected to obtain serum and
to detect IFN-γ levels using an ELISA kit (cat. no. MM-0182M1;
http://www.mmbio.cn/goods.php?id=989), according to
the manufacturer's instructions. In addition, the collected tumor
tissues were fixed with formalin and embedded in paraffin. All
animal studies were approved by the Ethics Committee of Hangzhou
Medical College (approval no. 2021-246; Hangzhou, China), and were
conducted according to the AAALAC and the IACUC guidelines.
Immunofluorescence analysis of mouse
tissue
Mouse tissues (5 µm) were deparaffinized by xylene
(Sinopharm Chemical Reagent Co., Ltd.; cat. no. 100234192) and
rehydrated. Slices were placed in anhydrous ethanol (Sinopharm
Chemical Reagent Co., Ltd.; cat. no. 100092680) for 5 min (repeated
3 times). Endogenous peroxidase activity was blocked using 3%
hydrogen peroxide in methanol. Heat-induced antigen retrieval was
carried out for all sections by incubating them in a steamer with
0.01 M citrate buffer (pH 6.0) at 95°C for 30 min. Secondly,
CD3-FITC + CD4-PE antibodies and CD3-FITC + CD8-PE antibodies (all
obtained from Abcam; CD3 antibody, cat. no. ab33429; 1:100; CD4
antibody, cat. no. ab288724; 1:100; and CD8 antibody, cat. no.
ab316778; 1:200) were diluted with BSA (cat. no. A8020; Beijing
Solarbio Science & Technology Co., Ltd.) to a concentration of
1:100, and were used to incubate the sections at 4°C overnight.
Finally, 10 µg/ml DAPI (Beyotime Institute of Biotechnology) was
applied for 5 min and the sections were visualized under a
fluorescence microscope (Olympus Corporation). The number of
positive cells was examined using ImageJ software (National
Institutes of Health).
TUNEL assay for cell apoptosis
After deparaffinization, tumor tissue sections were
subjected to TUNEL staining assay for apoptotic cell detection.
Briefly, after gently washing with phosphate-buffered saline, the
sections were incubated in 70% ethanol for 30 min at 4°C. The
subsequent analysis was performed using a commercially available
TUNEL assay kit (cat. no. ab206386; Abcam), according to the
manufacturer's protocol. The data were further visualized under a
fluorescence microscope (Olympus Corporation) and were analyzed
using ImageJ software.
Statistical analysis
All data are expressed as the mean ± SEM. All
statistical analyses were performed using GraphPad Prism 5.0
(GraphPad Software, Inc.; Dotmatics) and SPSS 13.0 (SPSS, Inc.)
software packages. Statistical significance was determined using
two-way ANOVA or one-way ANOVA for multiple groups, and Tukey's
Honestly Significant Difference was used for the post-hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Combined use of romidepsin and BMS-1
produces a synergistic effect in inhibiting lymphoma growth
To investigate the effects of a HDACi (romidepsin)
and PD-1 blockade (BMS-1) on lymphoma, the mouse B-cell lymphoma
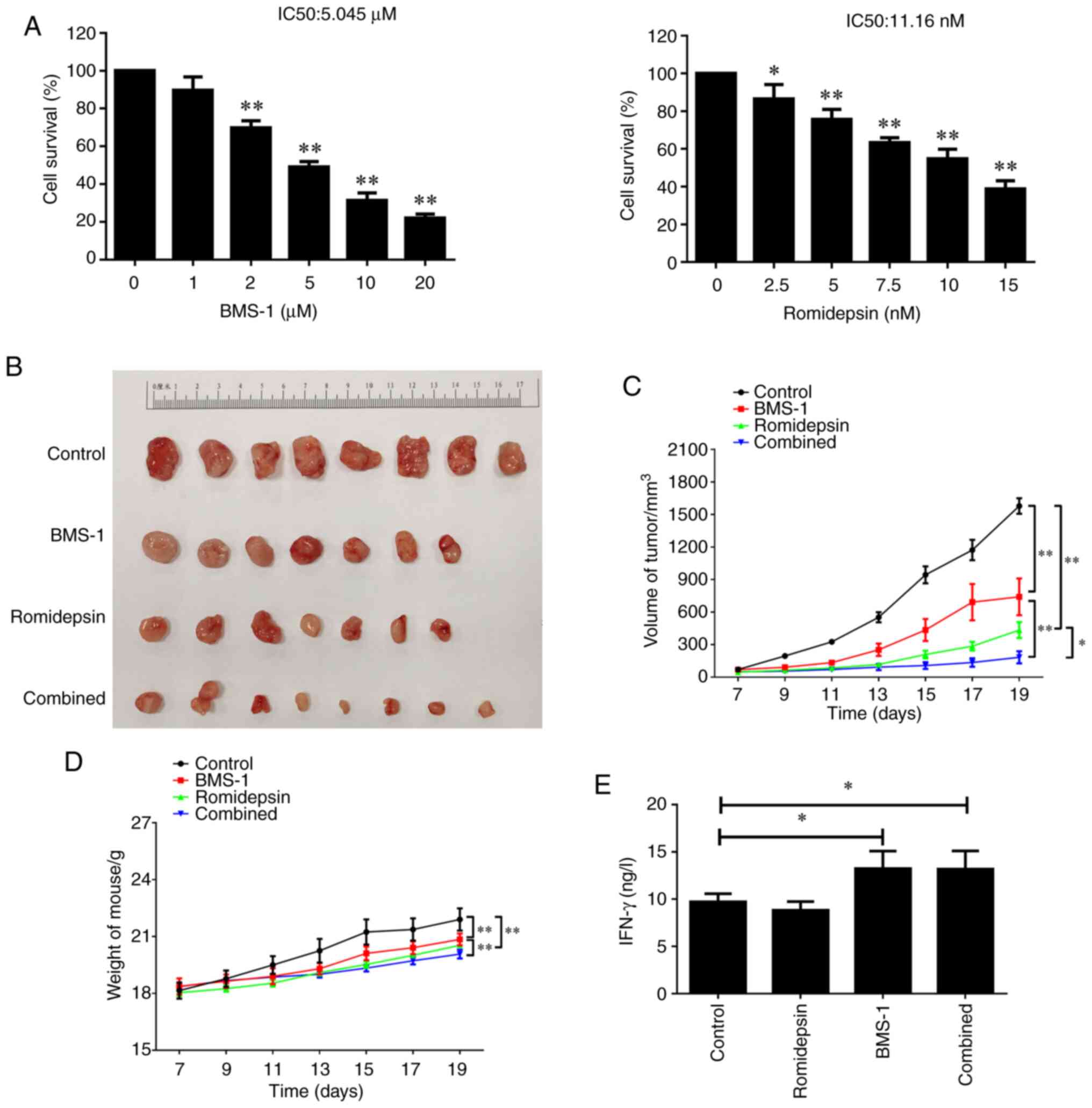
A20 isografts were established. First, the MTT assay confirmed that
both single-drug treatments impacted the proliferative capacity of
A20 cells in a time- and dose-dependent manner. The IC50
values for BMS-1 and romidepsin were 5.045 µM and 11.16 nM,
respectively (Fig. 1A). In the
preliminary experiment, the inhibitory effect of the two inhibitors
on tumor growth was time- and dose-dependent (Fig. S1). As revealed in Fig. 1B and C, both romidepsin and BMS-1,
when administered individually, could exert a certain impact on
tumor growth and tumor volume compared with the control group.
Furthermore, the combination of romidepsin and PD-1 blockade more
significantly reduced tumor growth and tumor volume than that in
the groups treated with romidepsin and PD-1 blockade alone, with
more than a 5-fold decrease in their tumor volume after 19 days
compared with in the control group. In addition, the body weights
of the combination group were lighter than those in the other
groups as time progressed (Fig.
1D). Furthermore, the tissues treated with BMS-1 or in
combination with romidepsin significantly elevated the levels of
IFN-γ, a T-cell biomarker (27),
while romidepsin alone had no significant effect on serum IFN-γ
levels detected by ELISA at the end of the experiment (Fig. 1E). The results suggested that
romidepsin and BMS-1 treatment synergistically inhibited the growth
of A20 isograft lymphomas, which might be associated with the
action of romidepsin on the activation of T-cell infiltration.
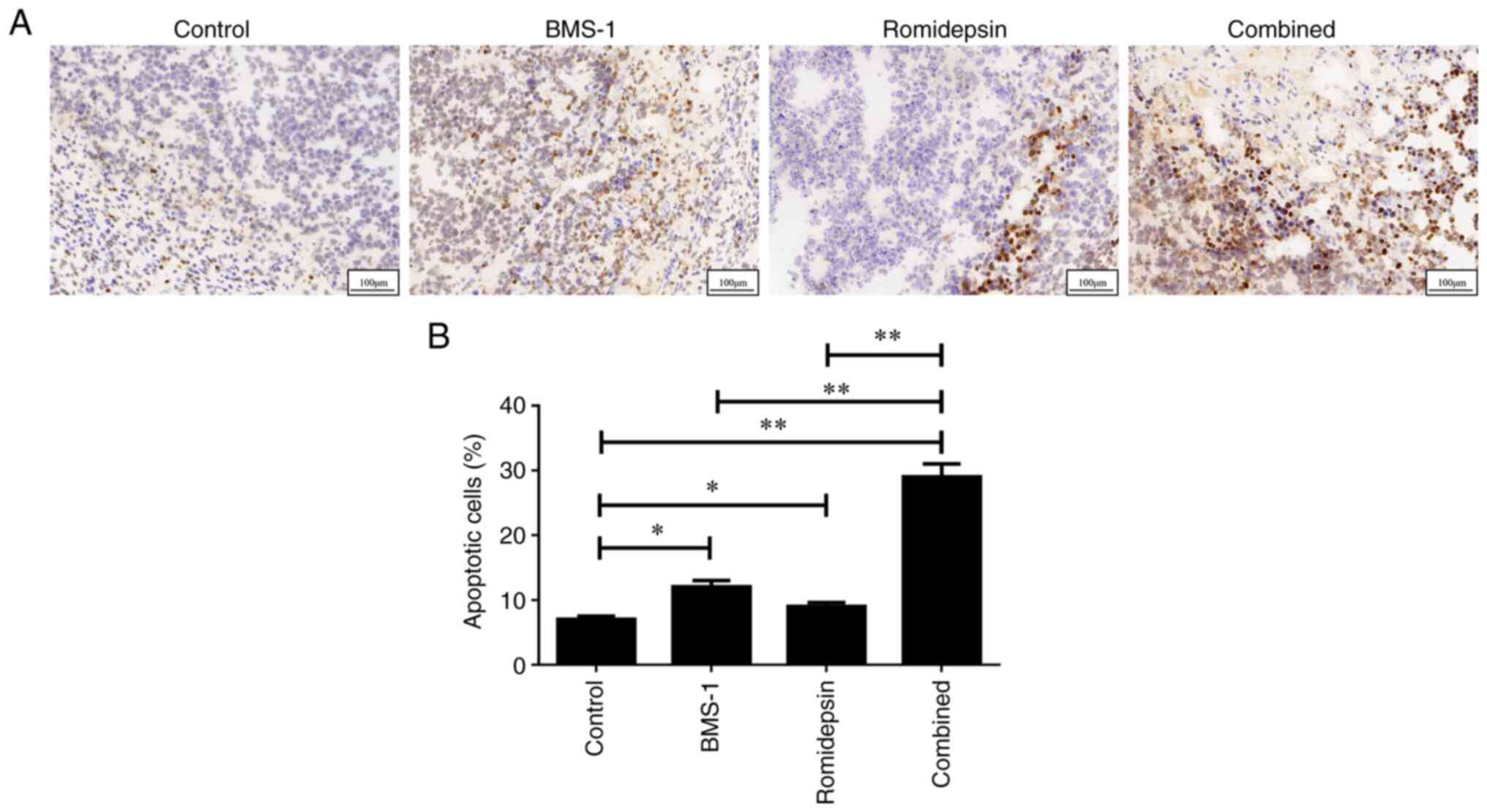
Combination treatment of romidepsin
and BMS-1 increases apoptosis in A20-derived lymphoma
To determine whether apoptosis was required for the
efficacy of the combination treatment, the TUNEL assay was
performed to detect apoptosis in A20 lymphoma mouse tissues. As
shown in Fig. 2A and B, the groups
treated with romidepsin and BMS-1 independently exhibited a
significantly higher induction of apoptosis compared with the
control group. Furthermore, the combination of romidepsin and BMS-1
resulted in a significantly greater enhancement of tumor apoptosis
than when either romidepsin or BMS-1 was used alone. These data
indicated that romidepsin and BMS-1 synergistically triggered
apoptosis in murine B-cell lymphoma.
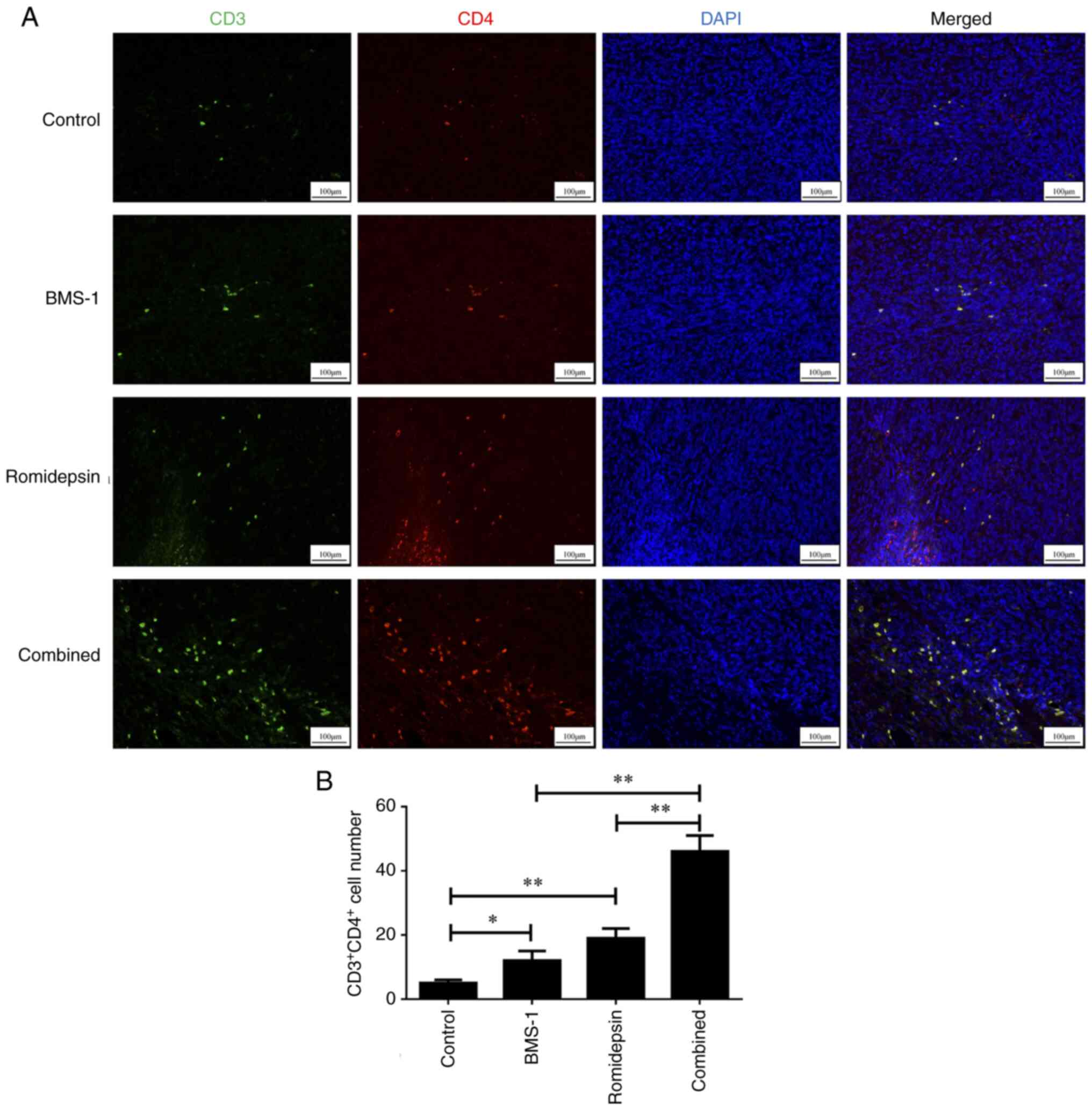
Combination treatment of romidepsin
and BMS-1 activates the CD4+ and CD8+
tumor-infiltrating lymphocytes (TILs) in A20-derived lymphoma
To determine whether CD4+ and
CD8+ TILs were involved in the efficacy of combination
treatment, an immunofluorescence assay was performed to assess the
A20 lymphoma mouse tissues. As demonstrated in Fig. 3A and B, the groups treated only with
romidepsin or BMS-1 showed a significant increase in
CD4+ TILs compared with those in the control group. In
addition, the combination of romidepsin and BMS-1 led to a
significantly greater activation of CD4+ TILs compared
with the groups treated with romidepsin or BMS-1 alone.
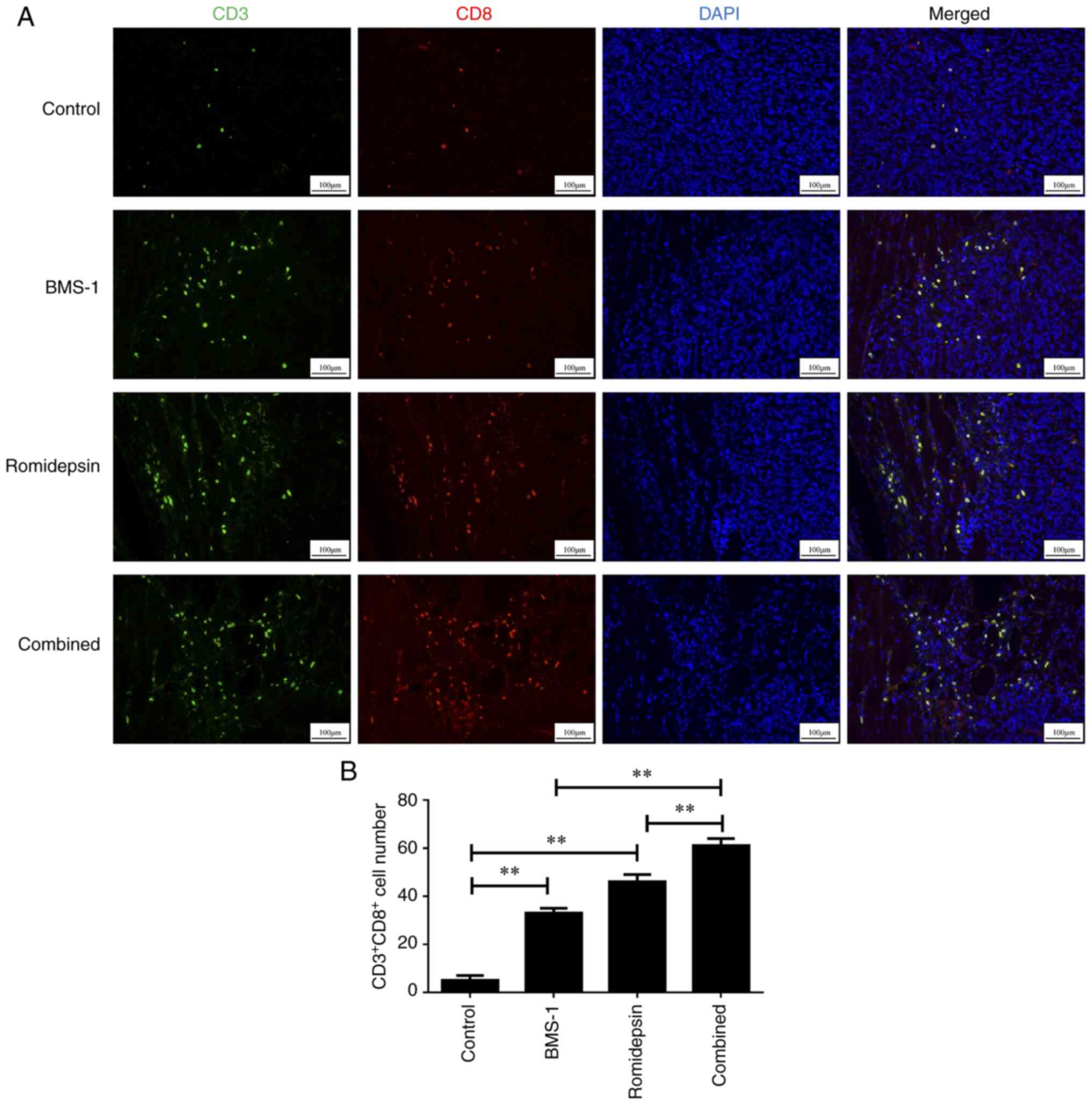
Additionally, as revealed in Fig. 4A
and B, the groups treated with romidepsin and BMS-1 alone
exhibited increased activation of CD8+ TILs compared
with that in the control group. Furthermore, the combination of
romidepsin and BMS-1 more significantly activated CD8+
TILs than that in the groups treated with romidepsin and BMS-1
alone. These data suggested that romidepsin and BMS-1 combination
accelerated CD4+ and CD8+ TILs
activation.
Discussion
Patients with relapsed/refractory B-cell lymphoma
frequently have a poor prognosis (28); therefore, it is essential to explore
combination therapeutic strategies for B-cell lymphoma. One
mechanism facilitating PD-1 blockade resistance may be reduction of
MHC class I and II expression in cancer (29). MHC molecules present peptides to
CD4+ and CD8+ T cells; thus, MHC is
indispensable for the identification of cancer cells by
antigen-specific CD4+ and CD8+ T cells.
Furthermore, HDACis can upregulate the expression of chemokines
that attract T cells to the tumor microenvironment (TME). By
enhancing the migration of T cells into the TME, HDACis can
synergistically enhance the efficacy of PD-1 blockade, ensuring a
sufficient number of activated T cells are available to interact
with the tumor following inhibition of the PD-1 pathway (30,31).
In the present study, using an intradermal mouse tumor model, it
was found that HDACi and PD1-blockade treatment synergistically
activated CD4+ and CD8+ TILs.
Studies have demonstrated that a portion of HDAC
family members are abnormally expressed in tumors, such as
pancreatic cancer, breast cancer, lung cancer and ovarian cancer
(32–35). It has been reported that several
HDACis exert potential roles in anticancer immunity (36). For instance, the antitumor effects
of vorinostat are associated with the immune system (37,38).
Inhibitors have also shown promising anticancer outcomes when used
in combination with traditional chemotherapy drugs in tumors
(39,40); however, their role in B-cell
lymphoma cells is not yet fully understood.
The present study examined the therapeutic efficacy
of a combination of a HDACi (romidepsin) and PD-L1 inhibitor
(BMS-1) in the treatment of an A20-induced B-cell lymphoma model.
The findings revealed that both romidepsin and BMS-1 independently
inhibited tumor growth in a dose- and time-dependent manner, and
induced the apoptosis of tumor cells. Furthermore, when used in
combination, there was a significant reduction in tumor growth and
increase in apoptosis, suggesting a synergistic effect. On the one
hand, romidepsin works by inhibiting HDACs, which leads to
hyperacetylation of histones and modulation of gene expression;
this results in cell cycle arrest and the induction of tumor cell
apoptosis (41). However,
romidepsin not only affects gene expression and survival of tumor
cells, but also regulates the immune system. Its ability to enhance
the immune response in the TME may be related to increased
expression of tumor-associated antigens and the modulation of
immune checkpoint molecules. This dual action makes romidepsin a
promising agent in anticancer therapy and immunotherapy (2). On the other hand, BMS-1 functions as a
PD-L1 inhibitor, blocking its interaction with the PD-1 receptor on
T cells. This interference relieves the immune suppression imposed
on T cells by tumor cells expressing PD-L1, thereby enhancing the
immune response against the tumor (26). This is consistent with the present
findings, which demonstrated that the individual administration of
romidepsin and BMS-1 could stimulate CD4+ and
CD8+ TILs in an intradermal mouse tumor model, but that
combining these treatments was more effective.
While the present study shows promising synergy
between romidepsin and BMS-1 in B-cell lymphoma treatment,
providing insights into their combined use in the clinic, several
limitations must be acknowledged. Firstly, the present study noted
an increase in IFN-γ levels only upon romidepsin administration;
however, the combination of romidepsin and BMS-1 did not
substantially enhance IFN-γ production, which may be due to the
regulation of IFN-γ production by multiple cytokines and signaling
pathways, as well as the existence of complex feedback mechanisms
in the immune system. This will be the direction of our future
research. Secondly, the research focused solely on A20 lymphoma
cells, leaving other B-cell lymphoma subtypes unexplored; varying
responses across subtypes could impact treatment generalizability.
Finally, the longer-term effects and analyses of tumors observed at
various time points after treatment and at different doses of this
combination have not been explored, requiring extensive clinical
trials to confirm chronic outcomes and safety. In conclusion, the
present study highlighted the synergistic effects of a HDACi and
PD-1 blockade on anticancer response, and recommended this
combination therapeutic strategy for the treatment of B-cell
lymphoma.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural Science
Foundation of China (grant no. 82174018), the Zhejiang Medical and
Health Science and Technology Project (grant nos. 2022KY470 and
2021KY009) and the Zhejiang TCM Science and Technology Project
(grant no. 2022ZB005).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
HJ and YG designed the study. TW and XY performed
experiments and analyzed the data. TW wrote the draft of the
manuscript. TW, XY, HJ and YG confirm the authenticity of all the
raw data. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
All animal studies were performed in compliance with
the regulations and guidelines of Zhejiang Hospital animal care and
conducted according to the AAALAC and the IACUC guidelines. The
present study was approved by the Ethics Committee of Hangzhou
Medical College (approval no. 2021-246; Hangzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Al-Hamadani M, Habermann TM, Cerhan JR,
Macon WR, Maurer MJ and Go RS: Non-Hodgkin lymphoma subtype
distribution, geodemographic patterns, and survival in the US: A
longitudinal analysis of the National Cancer Data Base from 1998 to
2011. Am J Hematol. 90:790–795. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ansell SM, Lesokhin AM, Borrello I,
Halwani A, Scott EC, Gutierrez M, Schuster SJ, Millenson MM, Cattry
D, Freeman GJ, et al: PD-1 blockade with nivolumab in relapsed or
refractory Hodgkin's lymphoma. N Engl J Med. 372:311–319. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fourcade J, Sun Z, Benallaoua M, Guillaume
P, Luescher IF, Sander C, Kirkwood JM, Kuchroo V and Zarour HM:
Upregulation of Tim-3 and PD-1 expression is associated with tumor
antigen-specific CD8+ T cell dysfunction in melanoma patients. J
Exp Med. 207:2175–2186. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shaw G, Cavalcante L, Giles FJ and Taylor
A: Elraglusib (9-ING-41), a selective small-molecule inhibitor of
glycogen synthase kinase-3 beta, reduces expression of immune
checkpoint molecules PD-1, TIGIT and LAG-3 and enhances CD8(+) T
cell cytolytic killing of melanoma cells. J Hematol Oncol.
15:1342022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xu C, Xie X, Kang N and Jiang H:
Neoadjuvant PD-1 inhibitor and apatinib combined with S-1 plus
oxaliplatin for locally advanced gastric cancer patients: A
multicentered, prospective, cohort study. J Cancer Res Clin Oncol.
149:4091–4099. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Liu J, Wei L, Hu N, Wang D, Ni J, Zhang S,
Liu H, Lv T, Yin J, Ye M and Song Y: FBW7-mediated ubiquitination
and destruction of PD-1 protein primes sensitivity to anti-PD-1
immunotherapy in non-small cell lung cancer. J Immunother Cancer.
10:e0051162022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nagai Y, Sata M, Ohta H, Onuki T, Saito T,
Uchiyama A, Kurosaki A, Yoshizumi N, Takigami A, Nakazawa S, et al:
Herpes zoster in patients with lung cancer treated with PD-1/PD-L1
antibodies. Immunotherapy. 14:1211–1217. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jimenez O, Mangiaterra T, Colli S, Garcia
Lombardi M, Preciado MV, De Matteo E and Chabay P: PD-1 and LAG-3
expression in EBV-associated pediatric Hodgkin lymphoma has
influence on survival. Front Oncol. 12:9572082022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rossi C and Casasnovas RO: PD-1 inhibitors
in patients with Hodgkin lymphoma. Eur J Cancer. 164:114–116. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zain J and O'Connor OA: Targeting histone
deacetyalses in the treatment of B- and T-cell malignancies. Invest
New Drugs. 28 Suppl 1 (Suppl 1):S58–S78. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shin DY, Kim A, Kang HJ, Park S, Kim DW
and Lee SS: Histone deacetylase inhibitor romidepsin induces
efficient tumor cell lysis via selective down-regulation of LMP1
and c-myc expression in EBV-positive diffuse large B-cell lymphoma.
Cancer Lett. 364:89–97. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yu H, Mi L, Zhang W, Ye Y, Li M, Hu D, Cao
J, Wang D, Wang X, Ding N, et al: Ibrutinib combined with low-dose
histone deacetylases inhibitor chidamide synergistically enhances
the anti-tumor effect in B-cell lymphoma. Hematol Oncol.
40:894–905. 2022. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang P, Wang Z and Liu J: Role of HDACs in
normal and malignant hematopoiesis. Mol Cancer. 19:52020.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chuang DM, Leng Y, Marinova Z, Kim HJ and
Chiu CT: Multiple roles of HDAC inhibition in neurodegenerative
conditions. Trends Neurosci. 32:591–601. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Consalvi S, Saccone V, Giordani L, Minetti
G, Mozzetta C and Puri PL: Histone deacetylase inhibitors in the
treatment of muscular dystrophies: Epigenetic drugs for genetic
diseases. Mol Med. 17:457–465. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang FF, Hu T, Liu JQ, Yu XQ and Ma LY:
Histone deacetylases (HDACs) as the promising immunotherapeutic
targets for hematologic cancer treatment. Eur J Med Chem. 245((Pt
2)): 1149202023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Marks PA: The clinical development of
histone deacetylase inhibitors as targeted anticancer drugs. Expert
Opin Investig Drugs. 19:1049–1066. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang L, Wu Z, Xia Y, Lu X, Li J, Fan L,
Qiao C, Qiu H, Gu D, Xu W, et al: Single-cell profiling-guided
combination therapy of c-Fos and histone deacetylase inhibitors in
diffuse large B-cell lymphoma. Clin Transl Med. 12:e7982022.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Llopiz D, Ruiz M, Villanueva L, Iglesias
T, Silva L, Egea J, Lasarte JJ, Pivette P, Trochon-Joseph V,
Vasseur B, et al: Enhanced anti-tumor efficacy of checkpoint
inhibitors in combination with the histone deacetylase inhibitor
Belinostat in a murine hepatocellular carcinoma model. Cancer
Immunol Immunother. 68:379–393. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Burke B, Eden C, Perez C, Belshoff A, Hart
S, Plaza-Rojas L, Delos Reyes M, Prajapati K, Voelkel-Johnson C,
Henry E, et al: Inhibition of histone deacetylase (HDAC) enhances
checkpoint blockade efficacy by rendering bladder cancer cells
visible for T cell-mediated destruction. Front Oncol. 10:6992020.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu C, Song Q, Gao S and Wu S: Targeting
HDACs for diffuse large B-cell lymphoma therapy. Sci Rep.
14:2892024. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
McClure JJ, Li X and Chou CJ: Advances and
challenges of HDAC inhibitors in cancer therapeutics. Adv Cancer
Res. 138:183–211. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mann BS, Johnson JR, Cohen MH, Justice R
and Pazdur R: FDA approval summary: Vorinostat for treatment of
advanced primary cutaneous T-cell lymphoma. Oncologist.
12:1247–1252. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Squarzoni A, Scuteri A and Cavaletti G:
HDACi: The columbus' egg in improving cancer treatment and reducing
neurotoxicity? Cancers (Basel). 14:52512022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bachy E, Camus V, Thieblemont C, Sibon D,
Casasnovas RO, Ysebaert L, Damaj G, Guidez S, Pica GM, Kim WS, et
al: Romidepsin Plus CHOP Versus CHOP in patients with previously
untreated peripheral T-Cell Lymphoma: Results of the Ro-CHOP phase
III study (Conducted by LYSA). J Clin Oncol. 40:242–251. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tsuruta A, Shiiba Y, Matsunaga N, Fujimoto
M, Yoshida Y, Koyanagi S and Ohdo S: Diurnal Expression of PD-1 on
tumor-associated macrophages underlies the dosing time-dependent
antitumor effects of the PD-1/PD-L1 Inhibitor BMS-1 in B16/BL6
Melanoma-Bearing Mice. Mol Cancer Res. 20:972–982. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Boulch M, Cazaux M, Cuffel A, Guerin MV,
Garcia Z, Alonso R, Lemaître F, Beer A, Corre B, Menger L, et al:
Tumor-intrinsic sensitivity to the pro-apoptotic effects of IFN-γ
is a major determinant of CD4(+) CAR T-cell antitumor activity. Nat
Cancer. 4:968–983. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Moleti ML, Testi AM and Foa R: Treatment
of relapsed/refractory paediatric aggressive B-cell non-Hodgkin
lymphoma. Br J Haematol. 189:826–843. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ren D, Hua Y, Yu B, Ye X, He Z, Li C, Wang
J, Mo Y, Wei X, Chen Y, et al: Predictive biomarkers and mechanisms
underlying resistance to PD1/PD-L1 blockade cancer immunotherapy.
Mol Cancer. 19:192020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Beg AA and Gray JE: HDAC inhibitors with
PD-1 blockade: A promising strategy for treatment of multiple
cancer types? Epigenomics. 8:1015–1017. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Que Y, Zhang XL, Liu ZX, Zhao JJ, Pan QZ,
Wen XZ, Xiao W, Xu BS, Hong DC, Guo TH, et al: Frequent
amplification of HDAC genes and efficacy of HDAC inhibitor
chidamide and PD-1 blockade combination in soft tissue sarcoma. J
Immunother Cancer. 9:e0016962021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Knoche SM, Brumfield GL, Goetz BT, Sliker
BH, Larson AC, Olson MT, Poelaert BJ, Bavari A, Yan Y, Black JD and
Solheim JC: The histone deacetylase inhibitor M344 as a
multifaceted therapy for pancreatic cancer. PLoS One.
17:e02735182022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rahbari R, Rahimi K, Rasmi Y,
Khadem-Ansari MH and Abdi M: miR-589-5p inhibits cell proliferation
by targeting histone deacetylase 3 in triple negative breast
cancer. Arch Med Res. 53:483–491. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
You Q, Wang J, Yu Y, Li F, Meng L, Chen M,
Yang Q, Xu Z, Sun J, Zhuo W and Chen Z: The histone deacetylase
SIRT6 promotes glycolysis through the HIF-1α/HK2 signaling axis and
induces erlotinib resistance in non-small cell lung cancer.
Apoptosis. 27:883–898. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Pramanik SD, Kumar Halder A, Mukherjee U,
Kumar D, Dey YN and R M: Potential of histone deacetylase
inhibitors in the control and regulation of prostate, breast and
ovarian cancer. Front Chem. 10:9482172022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hull EE, Montgomery MR and Leyva KJ: HDAC
inhibitors as epigenetic regulators of the immune system: Impacts
on cancer therapy and inflammatory diseases. Biomed Res Int.
2016:87972062016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
West AC, Mattarollo SR, Shortt J, Cluse
LA, Christiansen AJ, Smyth MJ and Johnstone RW: An intact immune
system is required for the anticancer activities of histone
deacetylase inhibitors. Cancer Res. 73:7265–7276. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Surolia I and Bates SE: Entinostat finds a
path: A new study elucidates effects of the histone deacetylase
inhibitor on the immune system. Cancer. 124:4597–4600. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lu F, Hou L, Wang S, Yu Y, Zhang Y, Sun L,
Wang C, Ma Z and Yang F: Lysosome activable polymeric vorinostat
encapsulating PD-L1KD for a combination of HDACi and immunotherapy.
Drug Deliv. 28:963–972. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wu J, Nie J, Luan Y and Ding Y: Hybrid
histone deacetylase inhibitor: An effective strategy for cancer
therapy. Curr Med Chem. 30:2267–2311. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
El Omari N, Lee LH, Bakrim S, Makeen HA,
Alhazmi HA, Mohan S, Khalid A, Ming LC and Bouyahya A: Molecular
mechanistic pathways underlying the anticancer therapeutic
efficiency of romidepsin. Biomed Pharmacother. 164:1147742023.
View Article : Google Scholar : PubMed/NCBI
|