Introduction
Renal cell carcinoma (RCC), also known as renal
adenocarcinoma, is a type of kidney cancer that arises from the
renal tubule cells, representing 2–3% of all adult cancers
(1). Each year, physicians identify
an estimated 210,000 new instances of kidney cancer worldwide,
making it the seventh most prevalent cancer in men and the ninth
most common cancer in women (2).
The United States, northern Europe, Canada, Australia, and New
Zealand have the highest rates of kidney cancer according to the
National Cancer Institute. Thailand, the Philippines, and China
have the lowest incidences (3).
Clear cell RCC (ccRCC) accounts for 70–80% of RCC cases, while the
other subtypes are primarily composed of chromophobe tumors (3–5%)
and papillary tumors (10–15%) (4).
Furthermore, ~4% of RCCs are hereditary and 96% are sporadic
(5). Cancers are characterized by
disordered metabolism (6). Tumor
growth is facilitated by certain metabolic processes that are
necessary for cell transformation or other related biological
processes (7). Cancer cells can
modify their metabolic systems in various ways to meet their needs
for energy and biosynthesis. The first strategy involves increasing
the intake and utilization of nutrients and substrates, mainly
glucose since the glycolytic rate of some types of cancer cells is
~30 times higher than that of normal cells (8). The second approach is to use metabolic
pathways that are advantageous to biosynthesis when breaking down
nutrients (9). The Warburg effect,
which refers to the observation that cancer cells tend to use
anaerobic glycolysis to utilize glucose even in aerobic
environments, is one of the most important examples (10). The third strategy involves the
aberrant activation of biosynthetic pathways. Tumor cells actively
engage in pathways related to fatty acid production and
desaturation to meet the increased demands for their cell membranes
and signaling molecules (11,12).
Reprogrammed metabolic processes are crucial for the proliferation
of cancer cells and are also becoming recognized as a critical
factor in determining the outcome of the individual cells (13). Kidney cancer or renal cancer is a
prime example of metabolic reprogramming among cancer types due to
its distinct reliance on altered metabolic pathways to support
tumor growth and adaption (14,15).
Researchers have linked numerous altered, inactive, or
hyperactivated genes in RCC to the control of several metabolic
processes, including glutamine metabolism, glycolysis, and the
tricarboxylic acid (TCA) cycle (16,17),
ATP synthesis, and the regulation of pathways crucial for the
balance of redox and hypoxia reactions. Kidney cancer can also be
characterized by its metabolic alterations, as the disease involves
marked changes in metabolic pathways that support tumor growth and
survival (17,18).
Therapeutic approaches for renal cancer are often
hindered by the resistance to multiple drugs exhibited by tumor
cells, which is a major cause of chemotherapy failure (19). This resistance arises from metabolic
and cellular physiological responses triggered by the tumor,
including the evasion of drug-induced apoptosis, activation of
detoxification pathways, reduction in drug uptake and activation of
DNA repair mechanisms (20). In
this context, the utilization of flavonoids in a clinical trial has
been instrumental in suppressing resistance mechanisms and inducing
reprogramming of cancer cells, and thus, is of utmost importance in
the search for novel genotoxic therapeutic approaches against
tumors (21). These natural
products have facilitated the development of more effective
strategic combinations with fewer side effects for the treatment of
renal cancer and have enhanced the understanding of metabolic
reprogramming, cancer cell defense and resistance mechanisms
(22). The alteration of gene
expression patterns in ccRCC cells is linked to genetic and
epigenetic events (23). Flavonoids
have gained prominence in anticancer pharmaceutical studies since
they inhibit glycolysis and oxidative phosphorylation (OXPHOS), and
modulate key enzymes such as hexokinase (HK) and pyruvate kinase
(24,25). A summary of the metabolic
reprogramming altered by flavonoids in ccRCC is shown in Fig. 1. Flavonoids possess potent
epigenetic properties that regulate DNA methylation, histone
modification, and microRNAs in the context of cancer therapy.
Specifically, flavonoids exhibit the ability to modulate crucial
metabolic pathways by targeting tumor suppressor genes and pivotal
catalytic enzymes in cancer cells (24,26).
Their efficacy has been demonstrated when combined with
chemotherapy drugs or other natural compounds, thus driving
extensive research efforts and the development of novel therapeutic
strategies for cancer treatment (27).
Anticancer drugs targeting metabolic
reprogramming
The field of cancer biology has observed a gradual
increase in knowledge and comprehension of the numerous targets of
metabolic reprogramming as advancements in research occur. These
advancements have facilitated the development of customized
pharmacological therapies for various components of metabolism
(28). Medicines targeting
pertinent metabolic pathways may inhibit the growth and
proliferation of RCC cells (29).
Scientists categorize drugs according to their target metabolic
pathways, which include glucose metabolism, glycolysis,
tricarboxylic acid cycle, oxidative phosphorylation, pentose
phosphate pathway, lactate metabolism, lipid metabolism, and amino
acid metabolism. Table SI
summarizes these metabolic pathways, detailing the drugs targeting
each pathway, the models in which they have been tested, and their
current phase of research. While the majority of anticancer
medications are still in the preclinical stage, a few have already
exhibited significant promise in cancer treatment and have advanced
to phase IV studies or clinical trials (30). For example, curcumin derived from
Curcuma longa has been investigated for its potential to
target metabolic reprogramming in cancer cells, including
modulation of glycolysis and oxidative stress pathways (31). Resveratrol has shown potential in
targeting metabolic pathways such as glucose metabolism and
mitochondrial function in cancer cells (32). Regarding treatment options for
recurrent colorectal cancer, tumor drug-induced cell drug
resistance is considered to be the primary factor for the
ineffectiveness of chemotherapy. This occurs because the
drug-induced apoptosis decreases drug absorption and activates DNA
repair mechanisms (33). Natural
molecules have several advantages over synthetic medications or
chemicals, including reasonable safety, minimal side effects, and
multistep targeting (34–36). Numerous natural substances have been
employed as preventative and therapeutic measures against various
illnesses, including cancer (37–39).
Some molecules from food and beverages, traditional Chinese
medicines, and medicinal plants have an impact on the initiation,
growth and metastasis of human cancers (40). For example, curcumin has been shown
to inhibit cancer cell proliferation, induce apoptosis and reduce
metastasis in various cancer types through multiple mechanisms,
including modulation of signaling pathways and reduction of
inflammation (41). Berberine has
shown potential in inhibiting cancer cell growth, and modulating
multiple signaling pathways involved in cancer progression
(42). Epigallocatechin-3-gallate
(EGCG) has demonstrated anticancer effects by inducing apoptosis
and blocking angiogenesis (43).
Although numerous secondary metabolites, such as flavonoids from
medicinal plants, have been utilized for a long time, it is still
unclear which molecular mechanism underlies their tumor-suppressive
actions and which anticancer properties they have (44).
Role of flavonoids in RCC metabolic
reprogramming
There are >10,000 known subtypes of
bioflavonoids, making them a diverse class of natural compounds.
The most prevalent phenolic compounds in the human diet are
flavonoids, mostly in cereals. Dried food, nuts, seeds, fruits,
vegetables, cereal-like foods, green tea and wine are everyday
dietary items that are frequently consumed and can be sources of
bioactive compounds, including flavonoids. These compounds have
been studied for their potential health benefits, including their
anticancer properties. These everyday dietary items are readily
available and can be incorporated into a regular diet. Their
consumption may contribute to overall health and potentially offer
protective effects against cancer through their bioactive compounds
(24). Depending on the degree of
unsaturation, oxidation of the C ring, and chemical structure,
flavonoids can be further classified into six subgroups. These
subcategories include isoflavones or chalcones, anthocyanins,
flavones, flavonols and flavanones or catechin. Each flavonoid is
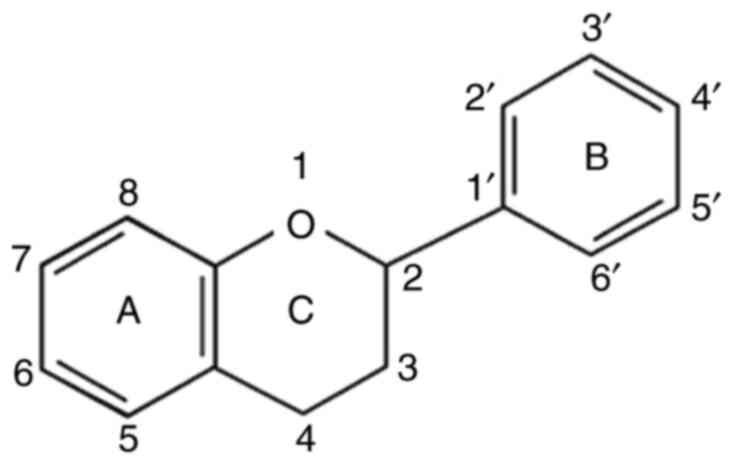
composed of two benzene rings joined by a heterocyclic pyran ring
(2-phenyl-1,4-benzopyran) and has 15 carbons in its chemical
structure (C6-C3-C6) (Fig. 2)
(45,46). Flavonoids exhibit numerous
biological properties, including antiviral (47), antifungal (48), antibacterial (49), antioxidant (50), anti-inflammatory (51), antidiabetic (52), antimutagenic (53), anti-obesity (54), cardioprotective (55) and anticancer (24) activities. Moderate levels of
reactive oxygen species (ROS), generated through mitochondrial
activity, function as redox signaling molecules that regulate
growth, differentiation, and cell proliferation pathways. However,
excessive ROS can be detrimental to cancer cells, leading to cell
death. As a result, tumor cells develop adaptive detoxification
mechanisms to counteract high levels of ROS (56). The elevation of ROS can induce
apoptosis in cancer cells, and therapeutic strategies that modulate
ROS levels have exhibited efficacy as anticancer drugs (57). Flavonoids exhibit dual activity:
Antioxidant effects in non-tumor cells and pro-oxidant effects in
cancer cells. In non-tumor cells, flavonoids exert antioxidant
effects, reducing oxidative stress by scavenging ROS. Conversely,
in cancer cells, flavonoids induce oxidative stress, increasing ROS
levels and thereby inhibiting cell proliferation signaling and
metabolic reprogramming of cancer cells, suppressing
pro-inflammatory cytokines, and promoting apoptosis, necrosis, and
autophagy (58). The ability of
flavonoids to scavenge ROS is attributed to the presence of a
number of phenolic hydroxyl groups in their molecular structure,
which facilitate electron exchange and stable compound formation
through substitution reactions with free radicals. Therefore,
flavonoids with a higher number of hydroxyl groups exhibit greater
antioxidant and pro-oxidant capacities (59,60). A
study has demonstrated that ovarian cancer cells treated with
flavonoids, such as apigenin, luteolin and myricetin, exhibit a
dose-dependent increase in intracellular ROS levels compared with
untreated control cells. This ROS elevation triggers activation of
the intrinsic apoptotic pathway, leading to cell cycle arrest and
inhibition of invasion (61).
Similarly, quercetin has been reported to induce cancer cell death
by positively modulating ROS levels (62).
ccRCC undergoes metabolic reprogramming to sustain
excessive cell proliferation, directly influencing the maintenance
and aggressiveness of neoplastic cells (63). For instance, glutathione (GSH)
metabolism has been extensively investigated in tumor progression
and as a targeted therapeutic strategy for cancer (64,65).
The upregulation of GSH levels is closely associated with cellular
detoxification mechanisms, providing certain types of cancer,
including breast cancer, in which elevated GSH levels have been
linked to resistance against chemotherapy drugs such as doxorubicin
and cisplatin, and non-small cell lung cancer, in which high levels
of GSH contribute to resistance against various chemotherapeutic
agents such as cisplatin and paclitaxel, with an advantage by
eliminating and detoxifying specific chemotherapeutic agents, thus
conferring therapeutic resistance (66,67).
Additionally, elevated levels of GSH contribute to tumor
development and increase the likelihood of metastasis. Depletion of
GSH levels can induce various types of cell death, including
apoptosis, necroptosis, ferroptosis, and autophagy (68). This serves as the foundation for
studies investigating the suppression of GSH levels as a
chemo-sensitization approach in cancer therapies, rendering tumor
cells more susceptible to the cytotoxic and cytoprotective effects
of antineoplastic agents (69,70).
In this context, tangeretin has been shown to prevent GSH depletion
in cells exposed to tert-butyl hydroperoxide (71). The anticancer properties of
flavonoids have been extensively studied, revealing that they
mediate antitumor effects through multiple mechanisms. These
include promoting autophagy and apoptosis, inhibiting tumor
invasion, growth and angiogenesis, as well as modulating ROS levels
in tumor cells (24). Additionally,
flavonoids can inhibit carcinogens and regulate pro-inflammatory
pathways, further contributing to their potential as therapeutic
agents (51).
Flavonoids, a group of naturally occurring
polyphenolic compounds, exhibit anticancer properties by modulating
various key processes involved in carcinogenesis (72). These processes include apoptosis,
proliferation, angiogenesis and metastatic progression, which are
often driven by dysregulation of tumor suppressor genes and
activation of oncogenes (73).
Quercetin and genistein have been demonstrated to induce apoptosis
in cancer cells through the activation of intrinsic and extrinsic
apoptotic pathways. They enhance the expression of pro-apoptotic
proteins (Bax and p53) and inhibit anti-apoptotic proteins (Bcl-2)
(74). EGCG and lutein inhibit
cyclin-dependent kinases (CDKs) and upregulate CDK inhibitors,
including p21 and p27, leading to cycle arrest (75,76).
Apigenin and kaempferol have been reported to inhibit angiogenesis
by downregulating VEGF and its receptors, thereby reducing the
supply of nutrients to tumors (77,78).
Naringenin and hesperidin have been demonstrated to suppress
epithelial-mesenchymal transition markers, including vimentin and
N-cadherin, and reduce the activity of MMPs, limiting cancer cell
invasion and migration (79). A
study has demonstrated that flavonoids exert anticancer effects by
regulating cascades that influence the metabolic reprogramming of
various pathways, including lipid metabolism, amino acid metabolism
and ketogenesis, in both in vitro and in vivo
experiments (80), and they
represent a viable strategy to inhibit key stages in the
development of cancer (81).
Table SII summarizes the findings
of research studies investigating the influence of flavonoids on
critical metabolic pathway components across various cancer types,
with distinctions made where the studies focus specifically on
RCC.
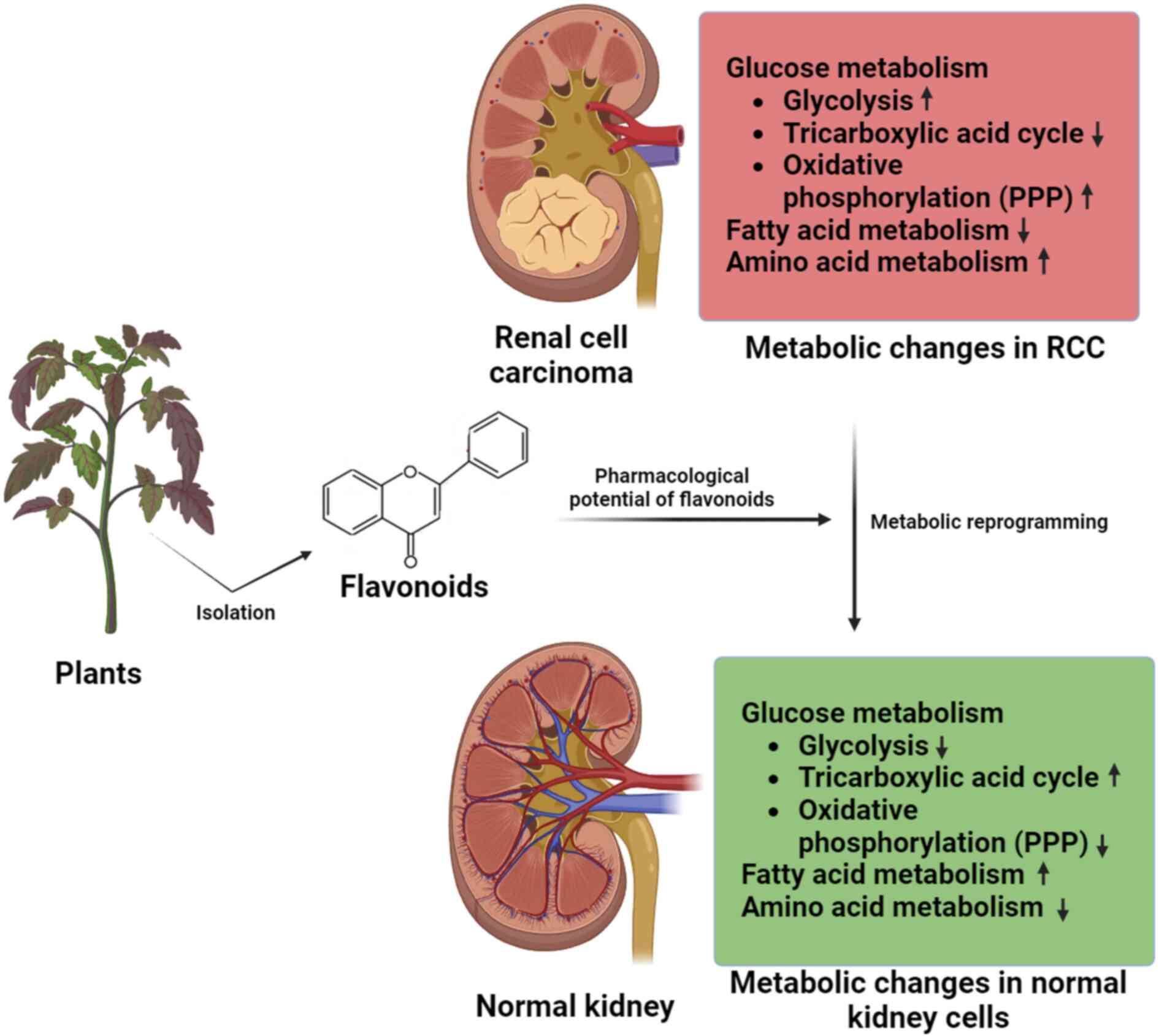
Glucose metabolism in RCC
The fundamental and significant source of energy in
the biological system is glucose. In RCC cells, glucose deprivation
causes oxidative stress and cellular cytotoxicity (82). Several mechanisms, including glucose
absorption, glycolysis, glycogenolysis, gluconeogenesis, lactate
reabsorption, and lactate excretion, maintain glucose homeostasis
(83) (Fig. 3).
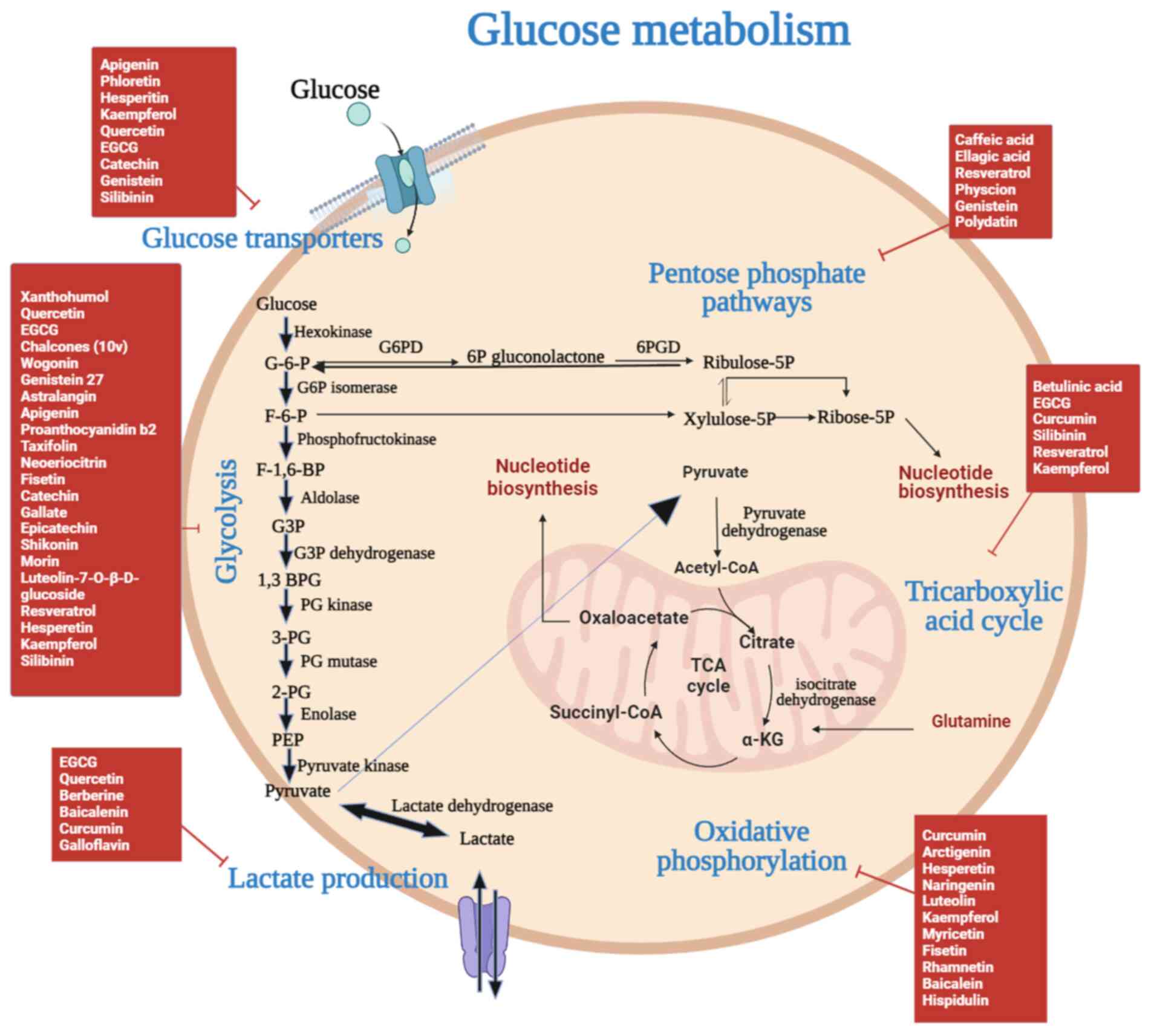 | Figure 3.Flavonoids pharmacologically target
glucose metabolism in RCC. Glycolysis and the PPP in the cytoplasm,
as well as the TCA in the mitochondria, make up glucose metabolism.
RCC is associated with increased levels of glycolysis and
activation of the PPP. After being transported into the cells by
glucose transporters, glucose proceeds through glycolysis to
produce pyruvate, which is then converted to ATP via the TCA cycle.
The majority of pyruvate in cancer cells enters lactic acid
fermentation to produce ATP quickly, whereas the flow of pyruvate
into the TCA cycle decreases. Various flavonoids control the
metabolism of glucose. α-KG, α-ketoglutaric acid; 1,3 BPG,
1,3-bisphosphoglyceric acid; 2-PG, 2-phosphoglyceric acid; 3-PG,
3-phosphoglyceric acid; 6PGD, 6-phosphogluconate dehydrogenase;
EGCG, epigallocatechin-3-gallate; F-1,6-BP, fructose
1,6-bisphosphate; F-6-P, fructose 6-phosphate; G-6-P, glucose
6-phosphate; G3P, glycerol-3-phosphate; G6PD, glucose-6-phosphate
dehydrogenase; PEP, phosphoenol pyruvate; PPP, pentose phosphate
pathway; RCC, renal cell carcinoma; TCA, tricarboxylic acid
cycle. |
Transmembrane glucose transport
Renal carcinoma cells need more glucose than normal
cells to function as an energy source and as a resource for the
production of various chemicals (84). Several cancer cell types exhibit
upregulated expression levels of glucose transporters (GLUTs),
particularly GLUT1, to maintain steady glucose uptake. Therefore,
targeting GLUTs using various natural substances, such as
flavonoids, is an optimal strategy for RCC treatment. Catechins
from green tea exhibit inhibitory action against GLUT1 in RCC
(85). Other polyphenols, such as
epicatechin gallate (ECG) and EGCG, inhibit GLUT1 by directly
binding to the transporter (86),
which can change how it recognizes its substrates competitively or
non-competitively. The connections of the extracellular side of the
transporter with ECG and EGCG can competitively prevent glucose
from binding to GLUT1 (87).
Studies have demonstrated that quercetin exerts non-competitive
inhibition by binding to GLUT1. Kinetic analysis has also
demonstrated that flavonoids can decrease the Michaelis constant
and maximum velocity values of GLUT1 (87,88).
Genistein, an iso-flavonoid compound, exerts a preventive effect
against prostate and breast cancer by blocking GLUT1 activity and
acting as a competitive inhibitor (89). Due to its potent activities,
including antioxidant, chemopreventive and anticancer activities,
resveratrol might directly inhibit GLUT1 by non-competitively
binding to its internal domains, decreasing uptake of glucose in
human leukemia cells (90).
Flavonoids, natural substances in plants with anticancer and
antioxidant properties, might affect GLUTs in RCC (91,92).
Flavonoids may affect how RCC cells utilize glucose, interfering
with their energy metabolism and preventing the formation of tumors
(24). More research is required to
fully comprehend the mechanism and therapeutic implications of
flavonoids in the metabolic reprogramming of RCC. Research on the
possible therapeutic effect of flavonoids in targeting glucose
metabolism in renal cancer is ongoing.
Glycolysis
In normal cells, glycolysis converts the majority of
glucose to pyruvate. The mitochondria undergo this process to
participate in the TCA cycle (83).
Through OXPHOS, pyruvate causes the synthesis of ATP. Renal cancer
cells use the enzyme lactate dehydrogenase (LDH)A to ferment lactic
acid to convert pyruvate into lactate (93). This mechanism produces less energy
compared with OXPHOS (94). To
compensate for this lower energy yield, renal cancer cells require
a higher rate of glucose consumption. A complex web of processes,
including glucose absorption, glycolysis, glycogenolysis,
gluconeogenesis, glucose reabsorption, and glucose excretion,
manages glucose homeostasis, which is influenced by kidney function
(83). ccRCC cells exhibit
increased glycolysis, suppressed pyruvate dehydrogenase (PDH) flux,
and decreased TCA cycle activity compared with other tumor cells. A
substantial difference is observed when comparing the rate of
glycolysis in ccRCC cells with that in adjacent kidney cells
(94). Renal cancer cells have been
found to exhibit the conventional Warburg effect, which is
characterized by increased cellular expression of all
glycolysis-related enzymes (93).
Fructose-1,6-bisphosphate, the rate-limiting enzyme, is also
recognized as a tumor suppressor in RCC tumors (94). Various biological effects, including
antioxidative, antiangiogenic and general antitumor effects, have
been attributed to certain phytochemicals (24,47–49).
Flavonoids target the modulation of certain glycolysis-related
enzyme activities (24); therefore,
flavonoids offer a promising therapeutic strategy for
cancer-related studies. A common dietary flavonoid, apigenin,
inhibits multiple biochemical pathways involved in the formation of
tumors and has anticancer properties. Apigenin inhibits glycolysis
by regulating pyruvate kinase M2 (PKM2) activity in HCT116 colon
cancer cells (95). Inhibition by
apigenin can result in the maintenance of a low PKM2/pyruvate
kinase 1 ratio (96).
Proanthocyanidin B2 influences PKM2 activity in hepatocellular
carcinoma (97). The first
irreversible stage in glycolysis is the phosphorylation of hexoses,
carried out by the enzyme HK (98).
Since cancer cells mostly exhibit upregulated expression levels of
HK, HK may be a promising molecular target for flavonoid-based
treatment (99). Xanthohumol is a
flavonoid that affects colon cancer by inhibiting HK2, a crucial
enzyme involved in glycolysis (98). In hepatocellular carcinoma,
quercetin inhibits Akt/mTOR signaling and decreases the activity of
HK2 (99). Both in vitro and
in vivo, the synthetic flavonoids Gl-v9 and 10v reduce HK2
expression (100,101). Morin, a flavonoid, may prevent LDH
from acting enzymatically in RCC (102). In addition, to efficiently
inhibiting the growth and proliferation of several cancer cell
lines, quercetin also reduces the levels of enzymes linked to
glycolysis, such as LDH and LDHA (103). The flavonoid EGCG decreases the
activity of LDH, LDHA and phosphofructokinase in in vitro
analysis (86,104,105). Quercetin also downregulates the
enzymatic activity of aldolase, GAPDH and α-enolase (106). Overall, researchers are currently
studying how flavonoids affect glycolysis in the metabolic
reprogramming of RCC. Although the preclinical results are
encouraging, more thorough research, including clinical trials, is
required to determine whether flavonoids can be used to treat RCC
by modulating glycolytic pathways.
TCA cycle
Nephrological disorders, such as type 2 diabetes,
chronic kidney disease, and kidney damage, can disrupt the TCA
cycle by impairing mitochondrial function and reducing levels of
key TCA cycle intermediates leading to altered energy metabolism
(107,108). The enzyme PDH restores the
metabolic flux to the TCA cycle in renal cancer cells, which is
often disrupted by downregulation of pathways such as glycolysis,
lipid metabolism, and amino acid metabolism (93). These enzymes catalyze the
biochemical reactions that generate end products, which either
enter the TCA cycle directly or are converted into intermediates
that feed into the cycle (94). PDH
catalyzes the conversion of pyruvate into acetyl-CoA (109,110). Different flavonoids, such as
betulinic acid, can inhibit the activity of PDH in cancer cells, as
demonstrated in an in vitro study (111). Typically, RCC cells divert glucose
for aerobic glycolysis breakdown from the TCA cycle. Thus,
glutamine and fatty acids are needed by kidney cancer cells to
power the TCA cycle (111,112). Further study is required to fully
establish the direct actions of flavonoids against the TCA cycle in
the metabolic reprogramming of RCC. Future research is needed to
fully understand how flavonoids affect RCC metabolism, particularly
given the complex interactions suggested by their potential
influence on mitochondrial function, redox balance, and enzyme
activity.
OXPHOS
Normal kidney cells filter blood and reabsorb
nutrients, which are processes that are heavily dependent on ATP.
These cells have high OXPHOS activity, which is supported by the
electron transport chain (ETC) (94). In renal cancer cells, decreasing the
activity of the TCA cycle also results in diminished ETC activity
(94). A study has measured the
activity of OXPHOS and the ETC in RCC tissues (93). From the less aggressive to the most
aggressive type of RCC, there is an increase in mitochondrial
damage. Marked downregulation of OXPHOS complexes, which impairs
the overall OXPHOS process, has been observed. This impairment is
linked to dysfunction in the ETC, which is responsible for
generating the proton gradient required for ATP synthesis (113). Different phytochemicals present in
various medicinal plants control the activity of different
complexes. Curcumin is a flavonoid that suppresses the activity of
ATP synthase (113–116). A study examined the role of
metabolic reprogramming in breast cancer, specifically focusing on
the targeting of the ATP synthase complex (117). The flavonoid arctigenin also
inhibits mitochondrial complexes II and IV, selectively killing
only OXPHPOS-dependent pancreatic cancer cells (116). Some flavonoids, including
luteolin, myricetin, fisetin, rhammetin, and baicalein, directly
inhibit complex I activity by lowering H2O2
production in rat heart mitochondria (118). Other flavonoids, such as
hispidulin and eupafolin, inhibit complex III by lowering
H2O2 production (118). Flavonoids may influence
mitochondrial processes that could modify cellular energy
metabolism, thereby potentially affecting OXPHOS in the metabolic
reprogramming of RCC. The therapeutic implications of flavonoids in
targeting OXPHOS in RCC require additional investigation, including
clinical studies.
Kidney cancer cells, particularly ccRCC cells,
exhibit distinct metabolic reprogramming that supports their rapid
proliferation and survival under hypoxic conditions. This
reprogramming often involves a shift from OXPHOS to glycolysis,
even in the presence of oxygen (91). Hypoxia-inducible factor 1 (HIF-1)
serves a central role in this metabolic adaptation. HIF-1 is a
transcription factor that is stabilized and activated under hypoxic
conditions, leading to the upregulation of genes involved in
glycolysis, angiogenesis, and cell survival (91,119).
Quercetin, resveratrol, and EGCG have been shown to decrease HIF-1α
protein levels by promoting its degradation and inhibiting its
synthesis (91,120). This effect occurs through the
inhibition of the PI3K/Akt/mTOR signaling pathway, which serves a
crucial role in the translation and stabilization of HIF-1α
(121). Kaempferol influences
mitochondrial function and OXPHOS, providing an additional layer of
metabolic regulation by activating the AMP-activated protein kinase
pathway. This shifts the metabolic balance towards OXPHOS, reducing
the reliance on glycolysis and inhibiting cancer cell proliferation
(73). Luteolin promotes
mitochondrial apoptosis by increasing the production of ROS and
disrupting the mitochondrial membrane potential. This leads to the
activation of caspase-dependent apoptotic pathways, thereby
reducing the survival of cancer cells (122).
Pentose phosphate pathway (PPP)
Glycolysis is diverted from glucose 6-phosphate
(G-6-P) to fructose 6-phosphate by the PPP. In the context of
cellular metabolism, this process provides crucial components for
nucleotide synthesis. Specifically, it generates five-carbon sugars
(ribose and deoxyribose) and reduces equivalents in the form of
NADPH. The five-carbon sugars are essential for the backbone
structure of nucleotides, which are the building blocks of DNA and
RNA. NADPH is a key reducing agent that supplies the necessary
electrons for various biochemical reactions, including those
involved in the synthesis of nucleotides. This synthesis is vital
for cell proliferation and repair, making these components critical
for maintaining cellular function and integrity (123). The PPP in renal cancer cells acts
as a defense against high levels of oxidative stress. This pathway
influences kidney diseases by altering key metabolic processes
involved in disease progression. For example, in diabetic kidney
disease, the pathway can affect the accumulation of advanced
glycation end-products and oxidative stress, which are critical in
the development of diabetic nephropathy. By modulating these
factors, the pathway may help mitigate inflammation, fibrosis, and
oxidative damage associated with kidney injury. In cases of kidney
damage, the pathway modulation of oxidative stress and cellular
repair mechanisms can impact the extent of tissue damage and repair
processes, potentially slowing disease progression and improving
renal function (12,93). According to a previous study, RCCs
may rewire their metabolism to control glucose flow into the PPP
(124). G-6-P enters the PPP
pathway, which produces precursors for lipids, nucleotides, and
NADPH. These molecules provide cells with the energy and substrates
required for the creation of macromolecules, which promotes the
growth of tumors (125). Two steps
in the PPP are often reprogrammed in cancer. First, the oxidative
phase, an irreversible step that involves the enzyme G6PD, which
catalyzes the conversion of G6P to 6-phosphoglucose lactone. The
step is considered rate-limiting and is crucial for generating
NADPH, which is essential for counteracting oxidative stress.
Second, the non-oxidative phase, the subsequent step involving the
enzyme ribulose-5-phosphate epimerase, which converts
ribulose-5-phosphate into xylulose-5-phosphate. This step is
important for the generation of nucleotides and amino acids, which
are often upregulated in cancer cells to support rapid cell
proliferation (126).
Renal cancer cells express glucose-6-phosphate
dehydrogenase (G6PD), the first and rate-limiting enzyme of the
PPP, at high levels (127).
Similar to G6PD, other oxidative branch NADPH-generating enzymes,
such as 6-phosphogluconate dehydrogenase (6PGD), regulate PPP flux
in renal cancer cells (93).
Different phytochemicals, including caffeic acid, ellagic acid and
physcion, directly downregulate the activity of G6PD and 6PGD in
different types of cancer, including lung cancer, leukemia, and
breast cancer (124,125,128). Limited research has been performed
on the precise impact of flavonoids on the PPP in the metabolic
reprogramming of RCC. Flavonoids may indirectly impact the PPP. The
PPP is essential to sustain redox equilibrium and supply the
building blocks for nucleotide synthesis (129,130). Although further research is
required to understand the precise mechanism and clinical
implications, the aforementioned information suggests that
flavonoids may impact the course of RCC by modulating the PPP.
Fatty acid metabolism in RCC
One of the most prominent metabolic abnormalities
found in cancer cells is defective lipid metabolism, which serves a
major role in the development and metastasis of cancer cells
(131). Dysregulated lipid de
novo synthesis was observed in tumor cells in the 1950s, and
this was found to be a critical metabolic state for cancer cells
(132). Obesity is frequently
linked to RCC, and patients with ccRCC have been found to exhibit
greater levels of cholesterol ester accumulation in their kidneys
(93). An outline of the metabolic
pathways for fatty acids in ccRCC and how flavonoids alter them is
shown in Fig. 4. Metabolomics
research has revealed that ccRCC cells exhibit higher levels of
fatty acyl-carnitines and carnitine than control cells (16). These differences were shown to be
closely linked to the clinical features of patients with kidney
cancer. Furthermore, RCC cells have a markedly compromised
β-oxidation pathway, which may cause a greater accumulation of
fatty acyl-carnitines (133).
Since the formation of lipid droplets in the cytoplasm produces the
characteristic clear cell phenotype, ccRCC is considered to be
characterized by these droplets (134). Additionally, the accumulation of
lipid droplets around the endoplasmic reticulum (ER) supports the
ER integrity in ccRCC cells (135). It has been demonstrated that,
compared with normal kidney cells, ccRCC cells exhibit
downregulation of fatty acid oxidation enzymes, such as acetyl CoA
carboxylase (ACC), fatty acid synthase (FAS), and stearoyl CoA
desaturase (SCD) (136). NADPH
oxidation is catalyzed by the β-ketoacyl reductase (KR) and enoyl
reductase domains of the multifunctional enzyme FAS (93). EGCG can inhibit the enzymatic
activity of FAS in vitro by competing with NADPH to bind the
KR domain (135,137). Emodin flavonoids can also inhibit
FAS activity in breast, liver, prostate, leukemia, and colon cancer
(133). SCD1 is the enzyme that is
responsible for lipid storage, which is highly expressed in ccRCC
and serves an important role in growth and proliferation (138). Flavonoids, such as betulinic acid
and platyphylloside, directly inhibit SCD1 in colon cancer
(139). Flavonoids reduce the
activity of SCD1, which subsequently inhibits RCC growth. FAS is
also associated with RCC tumor growth aggressiveness and poor
patient survival (140). The
activity of FAS can be directly affected by flavonoids such as
kaempferol, luteolin, morin, platyphylloside, quercetin, and
resveratrol (131,133,135,137,141,142). HIFs are essential for the
proliferation of RCC cells (91).
Flavonoids such as oroxylin A, resveratrol,
methylalpinumisoflavone, and EGCG can also regulate the activity of
HIFs, which has been primarily observed in patients with breast
cancer (133,143,144). Malonyl CoA is produced by ACC, a
rate-limiting enzyme for fatty acid synthesis (145). Soraphen A is a polyketide that
inhibits the activity of ACC1, which is upregulated in ccRCC
(139). Additionally, quercetin
reduces the production of triacylglycerol and fatty acids in rat
hepatocytes and inhibits ACC without having any detectable
influence on FAS (146). One of
the distinctive features of kidney cancer is the reprogramming of
the glycerophospholipids and arachidonic acid metabolism (147). The primary constituents of cell
membranes are glycerophospholipids, which are also the sources of
triacylglycerol, lysophosphatidic acid (LPA), and phosphatidic
acid, which are the building blocks of lipid storage. Autotaxin,
the enzyme responsible for producing LPA, is highly expressed in
the endothelial cells surrounding the tumor, and its activity can
be inhibited by several polyphenols (148). Arachidonic acid, a crucial
compound in RCC derived from membrane phospholipids, is synthesized
through pathways involving inflammatory enzymes such as
cyclooxygenase (COX)-1, COX-2, and lipoxygenases (149). Different flavonoids have a
potential effect on these inflammatory enzymes and have exhibited
inhibitory effects on them. Quercetin, EGCG, and resveratrol have
been reported to inhibit the activity of COX-1 and COX-2 in
enzymatic assays (82,135,150). Compared with normal kidney cells,
RCC cells express more of these enzymes (82). Numerous investigations have
demonstrated that elevated COX-2 levels are linked to tumor size,
stage, and grade in RCC. These findings imply that COX-2 may be a
target in ccRCC (151,152). Sterol regulatory element binding
protein-1 (SREBP-1) is a key regulator of lipid metabolism.
Specifically, flavonoids may inhibit SREBP-1 activity, thereby
reducing the expression of genes involved in lipogenesis and
contributing to the reprogramming of metabolic pathways in ccRCC
(153). Recognized antioxidants
and anti-inflammatory flavonoids may affect important facets of RCC
fatty acid metabolism. According to some research, flavonoids may
alter lipid metabolism-related enzymes and pathways, which may have
an impact on lipid synthesis, storage, and utilization in RCC
cells. Targeting fatty acid metabolism with flavonoids offers a
promising approach to understanding and creating treatment plans
for RCC; however, further research is required to fully evaluate
their effectiveness.
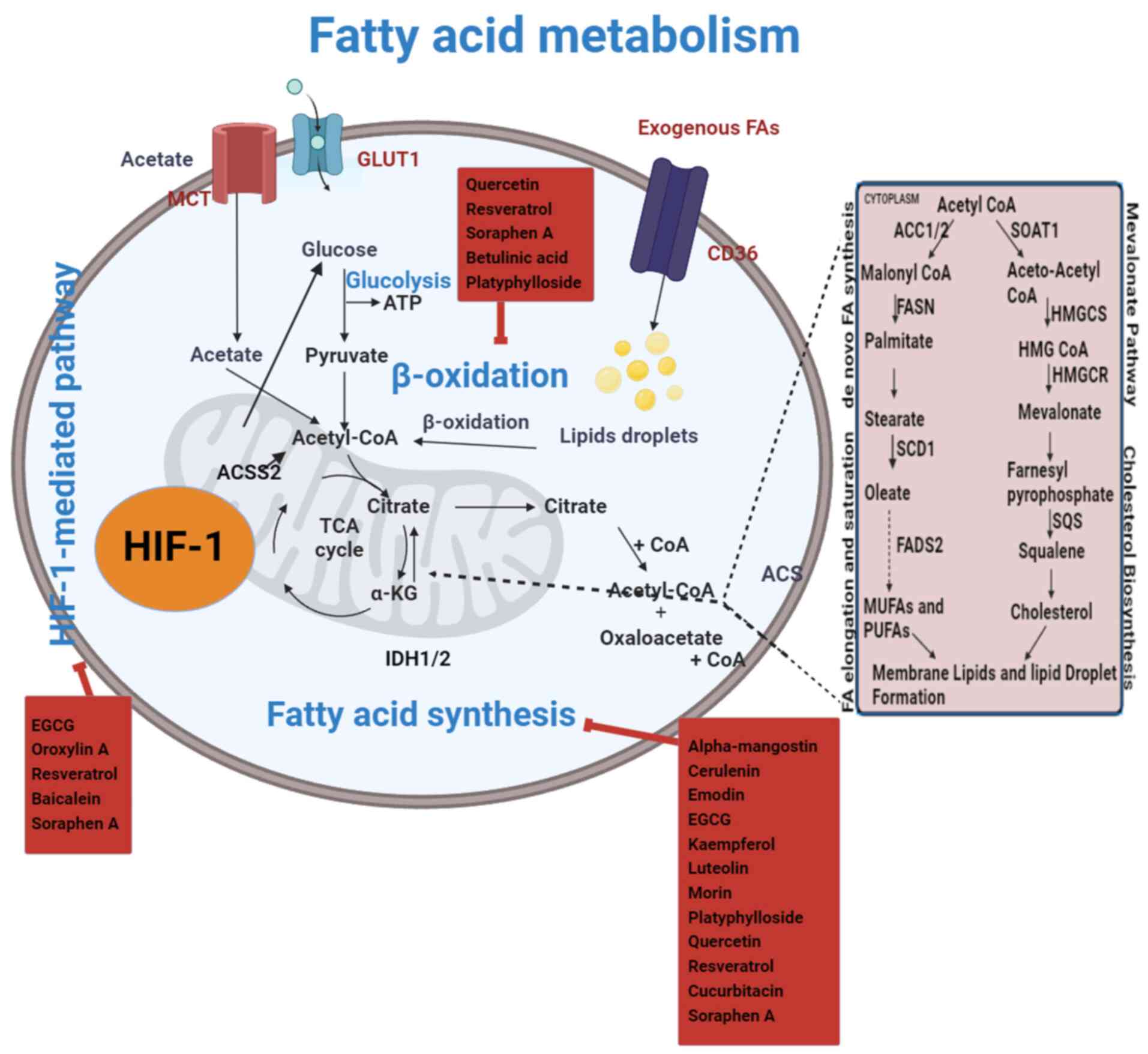 | Figure 4.Flavonoids pharmacologically target
FA metabolism in RCC. The metabolic pathways for FAs include
synthesis, degradation and utilization processes. In RCC, these
pathways are altered. Specifically, the balance is shifted towards
increased lipid production, which surpasses lipid degradation. In
RCC, exogenous FAs (FAs from external sources) contribute to the
increased lipid pool, further exacerbating the imbalance between
lipid synthesis and degradation. The uptake of these FAs supports
enhanced lipid production, which is critical for tumor cell
proliferation and metabolic reprogramming. Lipid production
surpasses lipid degradation in RCC. The lipid β-oxidation pathway
is downregulated in RCC, leading to reduced fatty acid breakdown.
Consequently, acetyl-CoA levels are maintained, which continues to
support the TCA cycle. This metabolic shift allows cancer cells to
utilize acetyl-CoA for energy production and biosynthesis despite
the decreased activity of lipid β-oxidation. However, RCC is
associated with elevated levels of carnitine, FA synthesis,
phospholipid synthesis and cholesterol production. By focusing on
metabolism enzymes, flavonoids inhibit the FA production pathway
and are considered to be a possible inhibitor for the metabolic
reprogramming of RCC. α-KG, α-ketoglutaric acid; ACC, acetyl CoA
carboxylase; ACS, acyl CoA synthetase; ACSS2, acyl CoA synthetase
short-chain family member 2; EGCG, epigallocatechin-3-gallate; FA,
fatty acid; FADS2, fatty acid desaturase-2; FAS, fatty acid
synthase; GLUT1, glucose transporter 1; HIF-1, hypoxia-inducible
factor 1; IDH1/2, isocitrate dehydrogenase; HMG,
hydroxymethylglutaryl; HMGCR, anti-3-hydroxy-3-methylglutaryl-CoA
reductase; HMGCS, hydroxymethylglutaryl CoA synthase; MCT,
monocarboxylate transporter; MUFAs, monounsaturated fatty acids;
PUFAs, polyunsaturated fatty acids; RCC, renal cell carcinoma;
SCD1, stearoyl CoA desaturase-1; SOAT1, sterol O-acyltransferase 1;
SQS, squalene synthase; TCA, tricarboxylic acid. |
Amino acid metabolism in RCC
In the context of cancer cell metabolic
reprogramming, amino acid metabolism is increasingly recognized for
its critical role. Amino acids serve not only as substrates for
protein synthesis but also act as essential mediators of redox
homeostasis and support various biosynthetic pathways that are
upregulated in kidney cells (154)
(Fig. 5). Their significance is
underscored by the rapid proliferation of renal cancer cells, which
depend heavily on amino acids as both metabolites and metabolic
regulators to promote growth (94).
This underscores the potential of targeting amino acid metabolic
pathways as a strategy to manage and inhibit cancer cell
proliferation effectively in different ways mentioned below
(155).
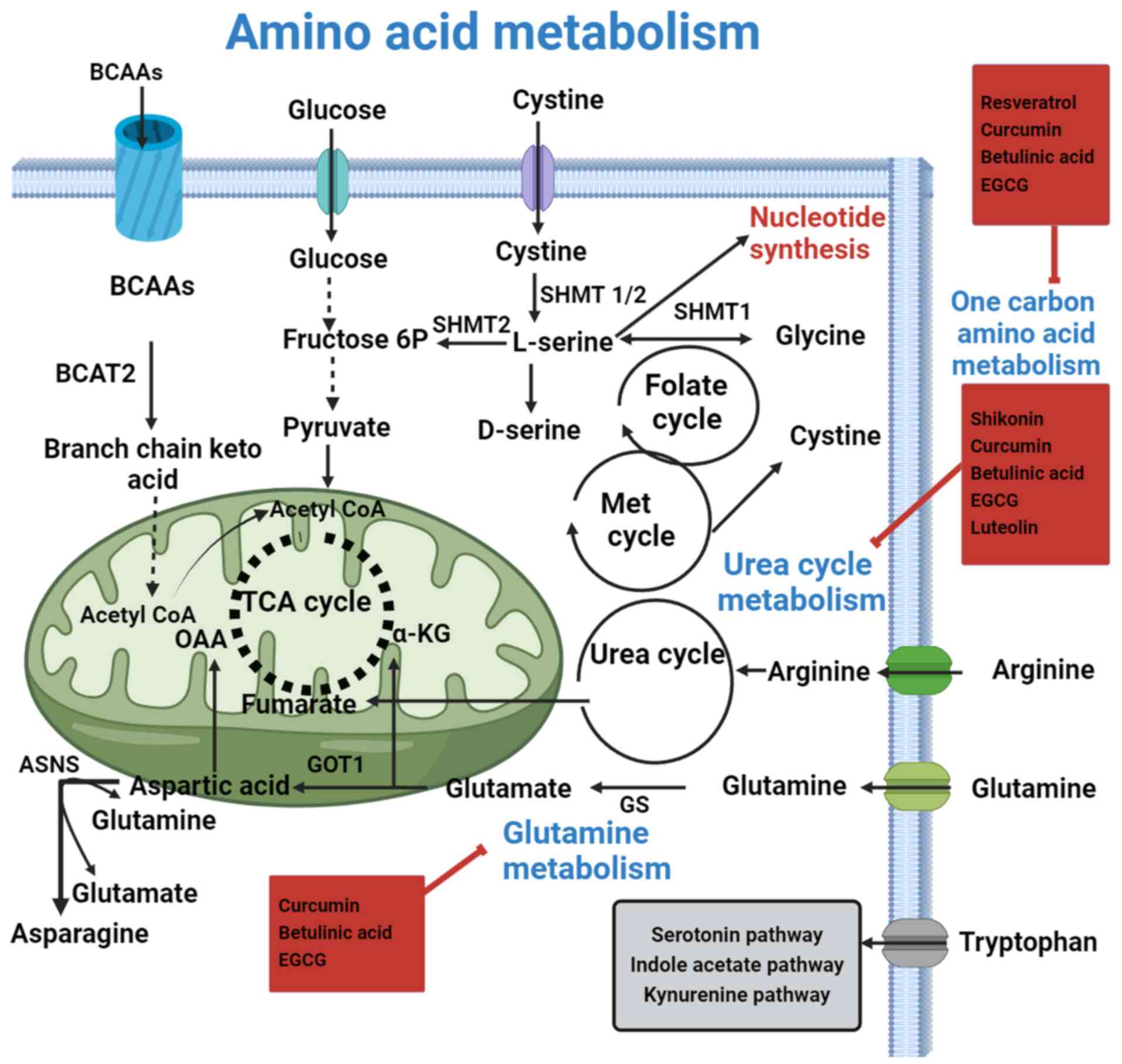 | Figure 5.Flavonoids pharmacologically target
AA metabolism in RCC. AAs provide metabolic intermediates, such as
acetyl CoA, that enable energy generation and lipogenesis, which
are necessary for a cell to grow and develop. Both essential and
non-essential AAs promote altered metabolism by acting as energy
sources. Finding strong and specific inhibitors, as well as
practical methods for metabolic reprogramming of RCC might
potentially be accomplished by focusing on AA metabolism. To
effectively exert their anticancer effects, flavonoids target
various metabolic pathways, including amino acid metabolism. They
inhibit glutamine metabolism, contributing to their anticancer
effects by reducing available glutamine levels. This metabolic
adjustment helps counteract oxidative stress and supports cancer
cell survival and proliferation. α-KG, α-ketoglutaric acid; AAs,
amino acids; ASNS, asparagine synthetase; BCAA, branched-chain
amino acid (valine, leucine, isoleucine); BCAT, branched-chain
amino acid transaminase; EGCG, epigallocatechin-3-gallate; GOT1,
aspartate transaminase; GS, glutamine synthetase; Met, methionine;
OAA, oxaloacetic acid; RCC, renal cell carcinoma; SHMT, serine
hydroxymethyltransferase; TCA, tricarboxylic acid. |
Glutamine metabolism
Glutamine is one of the primary nutrients that
cancer cells use to preserve their biomass and bioenergetics.
Furthermore, it serves as a component in the synthesis of lipids
and proteins. In the renal cortex, glutamine is employed to keep
the pH of the urinary system stable (156). Compared with that in normal kidney
tissues, the use of glutamine in ccRCC is increased, and the
GSH/GSH disulfide balance is strictly regulated (156). Increased levels of glutamine are
linked to elevated levels of free fatty acids in RCC (93,156).
Furthermore, in rapidly proliferating renal cancer cells, one of
the predominant metabolic pathways is the reductive carboxylation
of glutamine (156). In transgenic
mouse models of human RCC, there was an increase in the amounts of
glutamate and α-ketoglutarate, alongside upregulation of
glutaminase (GLS), which serves a crucial role in glutamine
metabolism and supports the metabolic needs of rapidly
proliferating cancer cells (156).
Based on assessment of the literature, it has been established that
HIF expression serves a crucial role in triggering the reductive
carboxylation of α-ketoglutarate in RCC cells. Additionally, the
reversal of isocitrate dehydrogenase flux to the reductive
carboxylation of glutamine to citrate has been predicted (93,156).
GSH peroxidase 1 (GPX1) expression is upregulated in ccRCC cells.
The activity of GLS and GPX1 can be controlled by the flavonoids
present in different medicinal plants (93). Curcumin is a flavonoid that
downregulates the activity of GLS in RCC (93). GLS is an important enzyme that
converts glutamine into glutamate in the metabolic reprogramming of
RCC, which can be directly inhibited by betulinic acid (150,157).
Serine and glycine metabolism
Serine can be obtained through extracellular
absorption, with a portion of it derived from glucose metabolism
(155). Serine and glycine are two
amino acids that are connected during biosynthesis, and function as
vital precursors for the formation of proteins, lipids and nucleic
acid building blocks, all of which are essential for the growth of
cancer (155). Under the catalysis
of serine hydroxy methyltransferases, serine, whether synthesized
de novo from 3-phosphoglycerate or imported from external
sources, can be further converted into glycine (158). Threonine dehydrogenase and glycine
C-acetyltransferase may also convert threonine into glycine
(159). The creation of
macromolecules, including lipids, proteins and nucleic acid,
requires methyl groups for one-carbon metabolism, which glycine
subsequently supplies (160).
Serine is also involved in DNA methylation, an important step for
the metabolic reprogramming of cancerous cells (161). Various synthetic drugs are
currently in clinical trials targeting different metabolic pathways
to reprogram renal cancer cells (159). However, synthetic drugs often come
with side effects, which makes natural alternatives an appealing
option (40). Plant-derived
secondary metabolites, such as flavonoids, including quercetin,
apigenin, morin and resveratrol, have shown efficacy in
reprogramming cancer cells with potentially fewer side effects
(162).
Arginine metabolism
Numerous solid tumor cells quickly succumb to
growth media without arginine, which is an important amino acid
(163). Additionally, it
participates in several crucial cellular metabolic processes,
including the urea cycle, the manufacture of nitric oxide, proline
and glutamate, as well as nucleotide biosynthesis (16). Arginine can also be depleted by
catalytically converting arginine to citrulline using the pegylated
version of arginine deaminase (163). In addition to catalyzing the
synthesis of argininosuccinate from citrulline and aspartic acid,
argininosuccinate synthetase (ASS) is an enzyme that limits the
pace at which arginine may be synthesized entirely from the
beginning (163). Renal and
parenchymal cells, which can recycle citrulline back to arginine,
are examples of cells with normal expression levels of ASS1
(16). However, cells lacking ASS1,
such as ccRCC cells, are unable to convert citrulline into arginine
(16). Other enzymes, such as
argininosuccinate lyase, catalyze the conversion of
argininosuccinate to arginine and fumaric acid, which in turn
connects arginine metabolism to the TCA cycle-generated energy
metabolism of glucose (163).
According to the literature, certain human malignancies, such as
hepatocellular carcinoma and malignant melanoma, lack ASS, making
them vulnerable to arginine deprivation treatment since they are
unable to synthesize arginine (164). The activity of these enzymes may
be influenced by the flavonoids present in different medicinal
plants (165). During oncogenesis,
cells often become dependent on external supplies of arginine due
to the loss or absence of the enzyme ASS1. While ASS1 is normally
expressed in proximal tubule cells, it is absent or not
significantly expressed in ccRCC (16,163–165). In arginine metabolism, the
activity of arginase and nitric oxide synthase serves vital roles
in cancer cell proliferation and immune response (165). Researchers have investigated
flavonoids such as quercetin and genistein for their potential
effects on nitric oxide production, which is intricately linked to
arginine metabolism (123,166). The specific impact of flavonoids
on RCC and arginine metabolism requires further research.
Tryptophan
There are three main downstream pathways of
tryptophan. The serotonin, indole acetate and kynurenine pathways
are significant in understanding its metabolism (15,94).
Indoleamine 2,3-deoxygenase (IDO) catabolizes the majority of
tryptophan. Through the kynurenine pathways, it serves as a
rate-limiting enzyme in this reaction. Tryptophan levels in ccRCC
are lower compared with those in normal kidney cells, indicating
higher consumption (167). ccRCC
tissues exhibit elevated amounts of quinolinate and kynurenine
(168). On the other hand, there
is a decrease in the amounts of enzymes that support the kynurenine
pathways, such as aldehyde dehydrogenase 2, monoamine oxidase and
DOPA decarboxylase, in RCC, which is considered to be related to
the serotonin and indole acetate pathways (169). These findings suggest the
reduction of tryptophan/kynurenine through IDO enrichment in RCC
(170). The approval of PD-1
inhibitors such as nivolumab as second-line treatments for RCC
represents an advancement in immunotherapy. These inhibitors work
by blocking the PD-1 pathways, which normally help to dampen the
immune response and prevent autoimmunity. By inhibiting PD-1, these
drugs enhance the activity of cytotoxic T cells against cancer
cells, leading to increased tumor cell death. In addition to PD-1
inhibitors, adjuvant therapies that stimulate cytotoxic T-cell
activity are employed to further boost the immune response
(171). Tryptophan catabolism by
IDO and other enzymes can suppress T-cell activity and contribute
to immune evasion by tumor (172).
These therapies help to optimize the effectiveness of PD-1
inhibitors by promoting a more robust and sustained immune attack
on RCC cells (173). The activity
of enzymatic pathways can be influenced by flavonoids such as
morin, quercetin, EGCG, resveratrol and betulinic acid because
tryptophan metabolism is involved in various cellular processes,
including immune modulation and the production of metabolites, such
as serotonin and kynurenine (174).
Toxicity associated with flavonoids
While it is commonly considered that using plant
secondary metabolites as an alternative medication is safe or does
not cause side effects (175),
literature has also demonstrated that extended exposure to large
dosages of some flavonoids can be potentially harmful (176). One flavonoid with anticancer
properties is resveratrol. A study conducted in vivo
suggested that consuming it at a higher dosage (3,000 mg/kg body
weight) may be harmful to the kidneys (177). Turmeric, a commonly used
traditional medicine in the Indian subcontinent, contains a complex
mixture of chemical compounds. Among these, there are numerous
substances that can be categorized based on their biological
effects. Specifically, turmeric has been associated with 136
mutagenic, 153 carcinogenic, and 64 hepatotoxic compounds. Among
the active ingredients with anticancer potential is curcumin, which
exhibits dose-dependent hepatotoxicity (178). Overdosing and continuous usage of
curcumin in rats produces ROS and proinflammatory cytokines. It
also gradually reduces antioxidants, such as superoxide dismutase
and glutathione S-transferase, which leads to liver damage
(179). Genistein neutralizes the
protective effect of letrozole, an aromatase inhibitor, against
estrogen-dependent breast cancer (180). Although EGCG has numerous health
advantages, research has demonstrated that a high dosage of the
compound in mice can cause hepatotoxicity, which has been linked to
the suppression of antioxidant enzymes (181). Both patients and doctors should be
aware of the potential side effects of herbal medicines, as well as
how they may interact with other prescription medications. Dietary
supplements should be used cautiously, considering both the
positive and negative consequences. Before being approved for use
in the treatment of any disease, herbal drugs must undergo quality
and pharmacological evaluations for toxicity.
Dietary bioactive compounds as
chemopreventive agents
Considering the global increase in cancer cases,
mortality, and treatment limits, cancer prevention must continue to
be a focus for improved cancer management (182). Lifestyle changes can postpone the
beginning of cancer, with nutrition serving a notable role
(183). Additionally, consuming a
variety of plant-based foods may help prevent cancer and aid in its
treatment (182). Numerous
plant-based bioactive compounds, including EGCG from green tea,
lycopene from tomatoes, apigenin from parsley, curcumin from
turmeric, resveratrol from grapes, genistein from soybeans, and
gingerol from ginger, have anti-cancer properties and can be used
as an easily accessible and affordable cancer prevention strategy
(183,184). The American Cancer Society advises
individuals, including patients with cancer, to include a variety
of plant-based foods in their diet, as this can contribute to
overall health and may lower the risk of developing various types
of cancer. These include garlic, oranges, green tea, cereals,
beans, soy-based food, peas, and other fruits and vegetables
(185). The Food and Agriculture
Organization and the World Health Organization jointly organized
conferences in Japan in 2004 and 2021 titled ‘Fruits and Vegetables
for Health’, which advocated for consuming 400 g of fruits and
vegetables daily to lower the risk of various illnesses, including
cancer (186).
Combination therapy with bioactive compounds
against cancer
Numerous bioactive compounds such as resveratrol,
quercetin and curcumin may have distal mechanisms of action to
produce anticancer effects (187).
Their combined utilization suggests promising treatment techniques.
Numerous investigations have demonstrated that the combinations of
chemicals, such as co-treatments with emodin and curcumin, might
have a synergistic impact in preventing the proliferation and
invasion of breast cancer cells (188–190). Additionally, the antitumor
activities of paclitaxel against lung cancer are synergistically
improved by emodin in vivo and in vitro (191). Cerulenin and emodin are inhibitors
of FAS and have cumulative effects on FAS inhibition in colon
cancer cells (133). As a result,
combining two or more chemicals can result in stronger anticancer
effects. However, this depends on the specific genes or metabolic
pathways of each molecule and has to be validated by
experiments.
Summary
The metabolic reprogramming observed in RCC
exhibits heterogeneity, demonstrating varying metabolic preferences
and patterns among different cancer types. Unlike molecular
inhibitors and traditional chemotherapeutics, which mainly target a
single metabolic pathway, flavonoids exhibit a unique mechanism of
action by impacting several metabolic pathways to achieve their
antitumor effects. The molecular mechanisms by which flavonoids
control the reprogrammed metabolic pathways in RCC are described in
the present review, with particular attention paid to how they
target necessary metabolic rate-limiting enzymes such as HK2, FAS,
LDHA and SREBP-1. Most flavonoids affect the metabolic pathways of
glucose and fats, but some also affect the metabolic pathways of
amino acids in RCC. For example, genistein inhibits both the intake
of glucose and the process of glycolysis.
By interfering with metabolic pathways, some
flavonoids not only directly lower RCC cell viability but also
improve the antitumor effectiveness of traditional
chemotherapeutics. For instance, shikonin activates the
mitochondria to cause intracellular oxidative stress and inhibits
the glycolytic process by lowering PKM2 activity. In contrast to
traditional cancer treatments, such as radiation, chemotherapy, and
surgery, which focus solely on the illness, the use of flavonoids
is intended to enhance the defenses of the body against cancer by
promoting the mobilization and regulation of all physiological
systems. This holistic approach underscores the potential of
flavonoids in directly impacting tumor metabolism but also
synergistically enhancing the effectiveness of existing cancer
treatments.
Flavonoid therapeutic approaches mainly target
enhancing blood flow, supporting overall health, and strengthening
the defenses of the body against disease. These approaches aim to
provide systemic detoxification, reduce inflammation, and alleviate
pain (192). For example, EGCG has
the potential to improve the quality of life for patients with
cancer by both preventing the growth of cancer and relieving the
neuropathic pain caused by paclitaxel (193). Flavonoids exhibit marked
advantages in impeding the growth and proliferation of cancer cells
in vivo by modulating metabolic pathways. Several studies
have validated the inhibitory effects of natural constituents in
animal models (194,195); however, it is imperative to note
that the quality of some studies requires further systematic
investigation. Additionally, it is important to dispel the
misconception that flavonoids are inherently gentler or less toxic
than synthetic chemical drugs. While flavonoids are natural
compounds with potential therapeutic benefits, they can still have
adverse effects and interactions, and their safety profile must be
carefully evaluated in clinical contexts, just as with synthetic
drugs. Certain flavonoids exhibit hepatotoxicity or nephrotoxicity,
potentially leading to irreversible impairments in patients
(196).
According to the traditional theory of
compatibility, toxic herbal medicines, including some flavonoids,
could be combined with other appropriate conventional Chinese
medicines to mitigate potential toxicity risks (178). Challenges persist in standardizing
the production and quality control for flavonoids. Furthermore,
evaluating the efficacy of flavonoids in interfering with
reprogrammed cell metabolism in RCC necessitates randomized
controlled clinical trials. Addressing these aspects is essential
to advance the understanding of the therapeutic potential of
flavonoids, and ensure their safe and effective application in the
context of RCC treatment.
Flavonoids represent a promising class of compounds
for the treatment of RCC due to their ability to target multiple
metabolic pathways with lower toxicity compared with conventional
chemotherapeutics (197). Future
research should focus on exploring the synergistic effects of
flavonoids with other therapeutic agents. Understanding the
long-term outcomes of flavonoid-based interactions with specific
metabolic targets is crucial for evaluating their efficacy and
safety in cancer treatment. Comprehensive studies are needed to
assess how these interactions affect metabolic pathways over
extended periods and determine their potential impact on overall
patient health and treatment outcomes. These studies could pave the
way for the development of more effective and personalized
treatment strategies for RCC.
Conclusion
Flavonoids hold significant promise as anticancer
agents due to their ability to modulate various pathways involved
in cancer metabolism. Their natural origin and multifunctional
properties make them appealing alternatives to synthetic drugs.
Flavonoids exhibit lower toxicity and fewer side effects compared
with conventional chemotherapy agents, enhancing patient compliance
and quality of life (197). This
makes them preferable to other anticancer drugs targeting metabolic
reprogramming. Furthermore, the ability of flavonoids to
selectively inhibit cancer cell proliferation, induce apoptosis,
and prevent metastasis underscores their potential as more
effective anticancer agents compared with other phytochemicals and
synthetic drugs (197).
Because of the extensive reprogramming of metabolic
pathways, extracellular stress and the immune system, renal cancer
is frequently recognized as a metabolic disorder. By improving
tumor imaging and identifying novel therapeutic targets, all of
these programmed metabolic pathways may be used to create a more
successful RCC treatment. By controlling the metabolic
reprogramming of cancer cells, flavonoids have effective inhibitory
effects on tumor cells and may also improve the sensitization of
cancer cells to chemotherapy treatments. Due to
poly-pharmacological actions, flavonoids may be able to reduce
cancer pain and enhance the quality of life of patients with
cancer. Flavonoids counteract metabolic reprogramming in RCC
through diverse mechanisms, including inhibition of key metabolic
pathways, modulation of glucose transporters and reduction of
oxidative stress. By targeting multiple signaling pathways and
metabolic processes, flavonoids may disrupt the survival advantage
conferred by the altered metabolism of cancer cells.
Additionally, their low toxicity profile and
accessibility from natural sources make them attractive drug
candidates for further clinical investigation. Integrating
flavonoid-based interventions into comprehensive therapeutic
strategies may offer novel avenues to improve the prognosis and
treatment outcomes of individuals with RCC. Continued research in
this field is essential to advance the understanding of the
molecular mechanisms involved and ultimately translate these
findings into effective clinical applications.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science
Foundation of China (grant nos. 82460510, 82203565, 82103388, and
31960145), Yunnan Province Applied Research Funds (grant nos.
202201AY070001-011, 202201AY070001-043 and 202201AS070077), and
Science and Technology Innovation Team of Tumor Metabolism
Research, Kunming Medical University (grant no. CXTD202102).
Availability of data and materials
Not applicable.
Authors' contributions
QZ and ZY conceived the study, supervised the
research and contributed to the critical revision of the
manuscript. AS conceived the study, collected data, wrote the
original draft, and reviewed and edited the manuscript. WL, BS and
YZ provided resources, contributed to drafting the manuscript,
prepared the tables and figures, and reviewed and edited the
manuscript. XL, YS, JX and KC provided resources, contributed to
the critical revision of the manuscript, and reviewed and edited
the manuscript. Data authentication is not applicable. All authors
have read and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
RCC
|
renal cell carcinoma
|
|
ccRCC
|
clear cell renal cell carcinoma
|
|
GLUT
|
glucose transporter
|
|
ECG
|
epicatechin gallate
|
|
EGCG
|
epigallocatechin-3-gallate
|
|
TCA
|
tricarboxylic acid
|
|
LDH
|
lactate dehydrogenase
|
|
LDHA
|
LDH A
|
|
PKM2
|
pyruvate kinase M2
|
|
HK
|
hexokinase
|
|
PDH
|
pyruvate dehydrogenase
|
|
ETC
|
electron transport chain
|
|
PPP
|
pentose phosphate pathway
|
|
6PGD
|
6-phosphogluconate dehydrogenase
|
|
ACC
|
acetyl CoA carboxylase
|
|
FAS
|
fatty acid synthase
|
|
SCD
|
stearoyl CoA desaturase
|
|
LPA
|
lysophosphatidic acid
|
|
COX
|
cyclooxygenase
|
|
GLS
|
glutaminase
|
|
GPX1
|
glutathione peroxidase 1
|
References
|
1
|
Rini BI, Campbell SC and Escudier B: Renal
cell carcinoma. Lancet. 373:1119–1132. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Bray F, Pisani P and Parkin D:
Globocan 2002: Cancer incidence, mortality and prevalence
worldwide. IARC Cancerbase. 52004.
|
|
3
|
Parkin DM and Bray F: International
patterns of cancer incidence and mortality. Cancer Epidemiol
Prevention. 101–138. 2006. View Article : Google Scholar
|
|
4
|
Mohammadian M, Pakzad R, Towhidi F,
Makhsosi BR, Ahmadi A and Salehiniya H: Incidence and mortality of
kidney cancer and its relationship with HDI (Human Development
Index) in the world in 2012. Clujul Med. 90:2862017.PubMed/NCBI
|
|
5
|
Lobo J, Ohashi R, Amin MB, Berney DM,
Compérat EM, Cree IA, Gill AJ, Hartmann A, Menon S, Netto GJ, et
al: Who 2022 landscape of papillary and chromophobe renal cell
carcinoma. Histopathology. 81:426–438. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hoerner CR, Miao SY, Hsieh JJ and Fan AC:
Targeting metabolic pathways in kidney cancer: Rationale and
therapeutic opportunities. Cancer J. 26:407–418. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Barron CC, Bilan PJ, Tsakiridis T and
Tsiani E: Facilitative glucose transporters: implications for
cancer detection, prognosis and treatment. Metabolism. 65:124–139.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Furuta E, Okuda H, Kobayashi A and Watabe
K: Metabolic genes in cancer: Their roles in tumor progression and
clinical implications. Biochim Biophys Acta. 1805:141–152.
2010.PubMed/NCBI
|
|
9
|
Menendez JA and Lupu R: Fatty acid
synthase (FASN) as a therapeutic target in breast cancer. Expert
Opin Ther Targets. 21:1001–1016. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vander Heiden MG, Cantley LC and Thompson
CB: Understanding the warburg effect: The metabolic requirements of
cell proliferation. Science. 324:1029–1033. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
DeBerardinis RJ, Lum JJ, Hatzivassiliou G
and Thompson CB: The biology of cancer: Metabolic reprogramming
fuels cell growth and proliferation. Cell Metab. 7:11–20. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cai L and Tu BP: Driving the cell cycle
through metabolism. Annu Rev Cell Dev Biol. 28:59–87. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rathmell WK, Rathmell JC and Linehan WM:
Metabolic pathways in kidney cancer: Current therapies and future
directions. J Clin Oncol. JCO2018792309. 2018.doi:
10.1200/JCO.2018.79.2309 (Epub ahead of print). View Article : Google Scholar
|
|
14
|
Weiss RH: Metabolomics and metabolic
reprogramming in kidney cancer. Semin Nephrol. 38:175–182. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wettersten HI: Reprogramming of metabolism
in kidney cancer. Semin Nephrol. 40:2–13. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wettersten HI, Aboud OA, Lara PN Jr and
Weiss RH: Metabolic reprogramming in clear cell renal cell
carcinoma. Nat Rev Nephrol. 13:410–419. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Linehan WM, Srinivasan R and Schmidt LS:
The genetic basis of kidney cancer: A metabolic disease. Nat Rev
Urol. 7:277–285. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
De Marinis F, Rinaldi M, Ardizzoni A,
Bruzzi P, Pennucci MC, Portalone L, D'Aprile M, Ripanti P, Romano
F, Belli M, et al: The role of vindesine and lonidamine in the
treatment of elderly patients with advanced non-small cell lung
cancer: A phase III randomized FONICAP trial. Italian Lung Cancer
Task Force. Tumori. 85:177–182. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Acharya N and Singh KP: Recent advances in
the molecular basis of chemotherapy resistance and potential
application of epigenetic therapeutics in chemorefractory renal
cell carcinoma. WIREs Mech Dis. 14:e15752022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hussain SA, Sulaiman AA, Balch C, Chauhan
H, Alhadidi QM and Tiwari AK: Natural polyphenols in cancer
chemoresistance. Nutr Cancer. 68:879–891. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
de Luna FCF, Ferreira WAS, Casseb SMM and
de Oliveira EHC: Anticancer potential of flavonoids: An overview
with an emphasis on tangeretin. Pharmaceuticals (Basel).
16:12292023. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kumar A and Jaitak V: Natural products as
multidrug resistance modulators in cancer. Eur J Med Chem.
176:268–291. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chrun ES, Modolo F and Daniel FI: Histone
modifications: A review about the presence of this epigenetic
phenomenon in carcinogenesis. Pathol Res Pract. 213:1329–1339.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kopustinskiene DM, Jakstas V, Savickas A
and Bernatoniene J: Flavonoids as anticancer agents. Nutrients.
12:4572020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sun L, Zhang H and Gao P: Metabolic
reprogramming and epigenetic modifications on the path to cancer.
Protein Cell. 13:877–919. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Slika H, Mansour H, Wehbe N, Nasser SA,
Iratni R, Nasrallah G, Shaito A, Ghaddar T, Kobeissy F and Eid AH:
Therapeutic potential of flavonoids in cancer: ROS-mediated
mechanisms. Biomed Pharmacother. 146:1124422022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Garcia-Oliveira P, Otero P, Pereira AG,
Chamorro F, Carpena M, Echave J, Fraga-Corral M, Simal-Gandara J
and Prieto MA: Status and challenges of Plant-anticancer compounds
in cancer treatment. Pharmaceuticals (Basel). 14:1572021.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu Y: Inhibitors of basal glucose
transport and their anticancer activities and mechanism. Ohio
University; 2012
|
|
29
|
Chan DA, Sutphin PD, Nguyen P, Turcotte S,
Lai EW, Banh A, Reynolds GE, Chi JT, Wu J, Solow-Cordero DE, et al:
Targeting GLUT1 and the Warburg effect in renal cell carcinoma by
chemical synthetic lethality. Sci Transl Med. 3:94ra702011.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kodama M and Nakayama KI: A second
warburg-like effect in cancer metabolism: The metabolic shift of
glutamine-derived nitrogen: A shift in glutamine-derived nitrogen
metabolism from glutaminolysis to de novo nucleotide biosynthesis
contributes to malignant evolution of cancer. Bioessays.
42:20001692020. View Article : Google Scholar
|
|
31
|
Sharma RA, Gescher AJ and Steward WP:
Curcumin: The story so far. Eur J Cancer. 41:1955–1968. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pavan AR, Silva GD, Jornada DH, Chiba DE,
Fernandes GF, Man Chin C and Dos Santos JL: Unraveling the
anticancer effect of curcumin and resveratrol. Nutrients.
8:6282016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Nong S, Han X, Xiang Y, Qian Y, Wei Y,
Zhang T, Tian K, Shen K, Yang J and Ma X: Metabolic reprogramming
in cancer: Mechanisms and therapeutics. MedComm (2020). 4:e2182023.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Alamgir A and Alamgir A: Drugs: Their
natural, synthetic, and biosynthetic sources. Therapeutic Use of
Medicinal Plants and Their Extracts: Volume 1 = Pharmacognosy.
105–123. 2017. View Article : Google Scholar
|
|
35
|
Yuan H, Ma Q, Ye L and Piao G: The
traditional medicine and modern medicine from natural products.
Molecules. 21:5592016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Atanasov AG, Zotchev SB, Dirsch VM;
International Natural Product Sciences Taskforce, ; Supuran CT:
Natural products in drug discovery: Advances and opportunities. Nat
Rev Drug Discov. 20:200–216. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fabiani R: Antitumoral properties of
natural products. Molecules. 25:6502020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lichota A and Gwozdzinski K: Anticancer
activity of natural compounds from plant and marine environment.
Int J Mol Sci. 19:35332018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Naeem A, Hu P, Yang M, Zhang J, Liu Y, Zhu
W and Zheng Q: Natural products as anticancer agents: Current
status and future perspectives. Molecules. 27:83672022. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Karimi A, Majlesi M and Rafieian-Kopaei M:
Herbal versus synthetic drugs; beliefs and facts. J
Nephropharmacol. 4:27–30. 2015.PubMed/NCBI
|
|
41
|
Anand P, Kunnumakkara AB, Newman RA and
Aggarwal BB: Bioavailability of curcumin: Problems and promises.
Mol Pharm. 4:807–818. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jiang X, Jiang Z, Jiang M and Sun Y:
Berberine as a potential agent for the treatment of colorectal
cancer. Front Med (Lausanne). 9:8869962022. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yang CS and Wang X: Green tea and cancer
prevention. Nutr Cancer. 62:931–937. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Bosetti C, Rossi M, McLaughlin JK, Negri
E, Talamini R, Lagiou P, Montella M, Ramazzotti V, Franceschi S and
LaVecchia C: Flavonoids and the risk of renal cell carcinoma.
Cancer Epidemiol Biomarkers Prev. 16:98–101. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Silva A, Silva V, Igrejas G, Aires A,
Falco V, Valentão P and Poeta P: Phenolic compounds classification
and their distribution in winemaking by-products. Eur Food Res
Technol. 249:207–239. 2023. View Article : Google Scholar
|
|
46
|
Razi SM and Rashidinejad A: Bioactive
compounds: Chemistry, structure, and functionality. In: Spray
drying encapsulation of bioactive materials; CRC Press: pp. 1–46.
2021
|
|
47
|
Badshah SL, Faisal S, Muhammad A, Poulson
BG, Emwas AH and Jaremko M: Antiviral activities of flavonoids.
Biomed Pharmacother. 140:1115962021. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Al Aboody MS and Mickymaray S: Anti-fungal
efficacy and mechanisms of flavonoids. Antibiotics (Basel).
9:452020. View Article : Google Scholar
|
|
49
|
Xie Y, Yang W, Tang F, Chen X and Ren L:
Antibacterial activities of flavonoids: Structure-activity
relationship and mechanism. Curr Med Chem. 22:132–149. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Heim KE, Tagliaferro AR and Bobilya DJ:
Flavonoid antioxidants: Chemistry, metabolism and
structure-activity relationships. J Nutr Biochem. 13:572–584. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Rathee P, Chaudhary H, Rathee S, Rathee D,
Kumar V and Kohli K: Mechanism of action of flavonoids as
anti-inflammatory agents: A review. Inflamm Allergy Drug Targets.
8:229–235. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Al-Ishaq RK, Abotaleb M, Kubatka P, Kajo K
and Büsselberg D: Flavonoids and their Anti-diabetic effects:
Cellular mechanisms and effects to improve blood sugar levels.
Biomolecules. 9:4302019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Snijman PW, Swanevelder S, Joubert E,
Green IR and Gelderblom WC: The antimutagenic activity of the major
flavonoids of rooibos (Aspalathus linearis): Some dose-response
effects on mutagen activation-flavonoid interactions. Mutat Res.
631:111–123. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Oliveira AKS, de Oliveira E Silva AM,
Pereira RO, Santos AS, Barbosa Junior EV, Bezerra MT, Barreto RSS,
Quintans-Junior LJ and Quintans JSS: Anti-obesity properties and
mechanism of action of flavonoids: A review. Crit Rev Food Sci
Nutr. 62:7827–7848. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Luo Y, Shang P and Li D: Luteolin: A
flavonoid that has multiple Cardio-protective effects and its
molecular mechanisms. Front Pharmacol. 8:6922017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Trachootham D, Alexandre J and Huang P:
Targeting cancer cells by ROS-mediated mechanisms: A radical
therapeutic approach? Nat Rev Drug Discov. 8:579–591. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Gorrini C, Harris IS and Mak TW:
Modulation of oxidative stress as an anticancer strategy. Nat Rev
Drug Discov. 12:931–947. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Huang MZ and Li JY: Physiological
regulation of reactive oxygen species in organisms based on their
physicochemical properties. Acta Physiol (Oxf). 228:e133512020.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Cao G, Sofic E and Prior RL: Antioxidant
and prooxidant behavior of flavonoids: Structure-activity
relationships. Free Radic Biol Med. 22:749–760. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Havsteen BH: The biochemistry and medical
significance of the flavonoids. Pharmacol Ther. 96:67–202. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Tavsan Z and Kayali HA: Flavonoids showed
anticancer effects on the ovarian cancer cells: Involvement of
reactive oxygen species, apoptosis, cell cycle and invasion. Biomed
Pharmacother. 116:1090042019. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Biswas P, Dey D, Biswas PK, Rahaman TI,
Saha S, Parvez A, Khan DA, Lily NJ, Saha K, Sohel M, et al: A
comprehensive analysis and Anti-cancer activities of quercetin in
ROS-mediated cancer and cancer stem cells. Int J Mol Sci.
23:117462022. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Reyes-Farias M and Carrasco-Pozo C: The
anti-cancer effect of quercetin: Molecular implications in cancer
metabolism. Int J Mol Sci. 20:31772019. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Bansal A and Simon MC: Glutathione
metabolism in cancer progression and treatment resistance. J Cell
Biol. 217:22912018. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Desideri E, Ciccarone F and Ciriolo MR:
Targeting glutathione metabolism: Partner in crime in anticancer
therapy. Nutrients. 11:19262019. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Bansal A and Simon MC: Glutathione
metabolism in cancer progression and treatment resistance. J Cell
Biol. 217:2291–2298. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Yoo D, Jung E, Noh J, Hyun H, Seon S, Hong
S, Kim D and Lee D: Glutathione-depleting Pro-oxidant as a
selective anticancer therapeutic agent. ACS Omega. 4:10070–10077.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Traverso N, Ricciarelli R, Nitti M,
Marengo B, Furfaro AL, Pronzato MA, Marinari UM and Domenicotti C:
Role of glutathione in cancer progression and chemoresistance. Oxid
Med Cell Longev. 2013:9729132013. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Wu JH and Batist G: Glutathione and
glutathione analogues; therapeutic potentials. Biochim Biophys
Acta. 1830:3350–3353. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Sobhakumari A: Dual role of oxidative
stress in head and neck cancer chemotherapy: Cytotoxicity and
pro-survival autophagy. The University of Iowa; 2013, View Article : Google Scholar
|
|
71
|
Liang F, Fang Y, Cao W, Zhang Z, Pan S and
Xu X: Attenuation of tert-Butyl Hydroperoxide (t-BHP)-induced
oxidative damage in HepG2 cells by tangeretin: Relevance of the
Nrf2-ARE and MAPK signaling pathways. J Agric Food Chem.
66:6317–6325. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Fatima N, Baqri SSR, Bhattacharya A, Koney
NK-K, Husain K, Abbas A and Ansari RA: Role of flavonoids as
epigenetic modulators in cancer prevention and therapy. Front
Genet. 12:7587332021. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Ponte LGS, Pavan ICB, Mancini MCS, da
Silva LGS, Morelli AP, Severino MB, Bezerra RMN and Simabuco FM:
The hallmarks of flavonoids in cancer. Molecules. 26:20292021.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Seo HS, Ku JM, Choi HS, Choi YK, Woo JK,
Kim M, Kim I, Na CH, Hur H, Jang BH, et al: Quercetin induces
Caspase-dependent extrinsic apoptosis through inhibition of signal
transducer and activator of transcription 3 signaling in
HER2-overexpressing BT-474 breast cancer cells. Oncol Rep.
36:31–42. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Akhtar MF, Saleem A, Rasul A, Baig MM,
Bin-Jumah M and Daim MM: Anticancer natural medicines: An overview
of cell signaling and other targets of anticancer phytochemicals.
Eur J Pharmacol. 888:1734882020. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Hung PF, Wu BT, Chen HC, Chen YH, Chen CL,
Wu MH, Liu HC, Lee MJ and Kao YH: Antimitogenic effect of green tea
(−)-epigallocatechin gallate on 3T3-L1 preadipocytes depends on the
ERK and Cdk2 pathways. Am J Physiol Cell Physiol. 288:C1094–C108.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Han DW, Lee MH, Kim HH, Hyon SH and Park
JC: Epigallocatechin-3-gallate regulates cell growth, cell cycle
and phosphorylated nuclear factor-κB in human dermal fibroblasts.
Acta Pharmacol Sin. 32:637–646. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Shih LJ, Hsu PC, Chuu CP, Shui HA, Yeh CC,
Chen YC and Kao YH: Epigallocatechin-3-gallate synergistically
enhanced arecoline-induced cytotoxicity by redirecting cycle arrest
to apoptosis. Curr Issues Mol Biol. 46:1516–1529. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Zhang J, Hu Z, Horta CA and Yang J:
Regulation of epithelial-mesenchymal transition by tumor
microenvironmental signals and its implication in cancer
therapeutics. Semin Cancer Biol. 88:46–66. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Mendes LF, Gaspar VM, Conde TA, Mano JF
and Duarte IF: Flavonoid-mediated immunomodulation of human
macrophages involves key metabolites and metabolic pathways. Sci
Rep. 9:149062019. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Usuwanthim K, Wisitpongpun P and
Luetragoon T: Molecular identification of phytochemical for
anticancer treatment. Anticancer Agents Med Chem. 20:651–666. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Hay N: Reprogramming glucose metabolism in
cancer: Can it be exploited for cancer therapy? Nat Rev Cancer.
16:635–649. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Triplitt CL: Understanding the kidneys'
role in blood glucose regulation. Am J Manag Care. 18 (1
Suppl):S11–S16. 2012.PubMed/NCBI
|
|
84
|
Boroughs LK and DeBerardinis RJ: Metabolic
pathways promoting cancer cell survival and growth. Nat Cell Biol.
17:351–359. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Morani F, Phadngam S, Follo C, Titone R,
Aimaretti G, Galetto A, Alabiso O and Isidoro C: PTEN regulates
plasma membrane expression of glucose transporter 1 and glucose
uptake in thyroid cancer cells. J Mol Endocrinol. 53:247–258. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Wei R, Mao L, Xu P, Zheng X, Hackman RM,
Mackenzie GG and Wang Y: Suppressing glucose metabolism with
epigallocatechin-3-gallate (EGCG) reduces breast cancer cell growth
in preclinical models. Food Funct. 9:5682–5696. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Moreira L, Araújo I, Costa T,
Correia-Branco A, Faria A, Martel F and Keating E: Quercetin and
epigallocatechin gallate inhibit glucose uptake and metabolism by
breast cancer cells by an estrogen receptor-independent mechanism.
Exp Cell Res. 319:1784–1795. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Prpa E: A mechanistic investigation into
the acute effects of apple polyphenols on carbohydrate digestion
and absorption. King's College London; 2021
|
|
89
|
Pérez A, Ojeda P, Ojeda L, Salas Mn,
Rivas CI, Vera JC and Reyes AM: Hexose transporter GLUT1 harbors
several distinct regulatory binding sites for flavones and
tyrphostins. Biochemistry. 50:8834–8845. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Patra S, Pradhan B, Nayak R, Behera C,
Rout L, Jena M, Efferth T and Bhutia SK: Chemotherapeutic efficacy
of curcumin and resveratrol against cancer: Chemoprevention,
chemoprotection, drug synergism and clinical pharmacokinetics.
Semin Cancer Biol. 73:310–320. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Samec M, Liskova A, Koklesova L, Mersakova
S, Strnadel J, Kajo K, Pec M, Zhai K, Smejkal K, Mirzaei S, et al:
Flavonoids targeting HIF-1: Implications on cancer metabolism.
Cancers. 13:1302021. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Zambrano A, Molt M, Uribe E and Salas M:
Glut 1 in cancer cells and the inhibitory action of resveratrol as
a potential therapeutic strategy. Int J Mol Sci. 20:33742019.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Hakimi AA, Reznik E, Lee CH, Creighton CJ,
Brannon AR, Luna A, Aksoy BA, Liu EM, Shen R, Lee W, et al: An
integrated metabolic atlas of clear cell renal cell carcinoma.
Cancer Cell. 29:104–116. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Chakraborty S, Balan M, Sabarwal A,
Choueiri TK and Pal S: Metabolic reprogramming in renal cancer:
Events of a metabolic disease. Biochim Biophys Acta Rev Cancer.
1876:1885592021. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Shan S, Shi J, Yang P, Jia B, Wu H, Zhang
X and Li Z: Apigenin restrains colon cancer cell proliferation via
targeted blocking of pyruvate kinase M2-dependent glycolysis. J
Agric Food Chem. 65:8136–8144. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Monteiro F and Shetty SS: Natural
antioxidants as inhibitors of pyruvate kinase M2 in warburg
phenotypes. J Herbal Med. 42:1007502023. View Article : Google Scholar
|
|
97
|
Feng J, Wu L, Ji J, Chen K, Yu Q, Zhang J,
Chen J, Mao Y, Wang F, Dai W, et al: PKM2 is the target of
proanthocyanidin B2 during the inhibition of hepatocellular
carcinoma. J Exp Clin Cancer Res. 38:2042019. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Liu W, Li W, Liu H and Yu X: Xanthohumol
inhibits colorectal cancer cells via downregulation of hexokinases
II-mediated glycolysis. Int J Biol Sci. 15:24972019. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Wu H, Pan L, Gao C, Xu H, Li Y, Zhang L,
Ma L, Meng L, Sun X and Qin H: Quercetin inhibits the proliferation
of Glycolysis-Addicted HCC cells by reducing hexokinase 2 and
Akt-mTOR pathway. Molecules. 24:19932019. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Deng X, Liu R, Li J, Li Z, Liu J, Xiong R,
Lei X, Zheng X, Xie Z and Tang G: Design, synthesis, and
preliminary biological evaluation of 3′,4′,5′-trimethoxy flavonoid
salicylate derivatives as potential anti-tumor agents. N J
Chemistry. 43:1874–1884. 2019. View Article : Google Scholar
|
|
101
|
Guo Y, Wei L, Zhou Y, Lu N, Tang X, Li Z
and Wang X: Flavonoid Gl-V9 induces apoptosis and inhibits
glycolysis of breast cancer via disrupting GSK-3β-modulated
mitochondrial binding of HKII. Free Radic Biol Med. 146:119–129.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Mazlaghaninia M, Atri MS and Seyedalipour
B: Scopoletin and morin inhibit lactate dehydrogenase enzyme
activity, which is critical for cancer metabolism. Hormozgan Med J.
23:e882692019. View Article : Google Scholar
|
|
103
|
Jia L, Huang S, Yin X, Zan Y, Guo Y and
Han L: Quercetin suppresses the mobility of breast cancer by
suppressing glycolysis through akt-mtor pathway mediated autophagy
induction. Life Sci. 208:123–130. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Bader A, Tuccinardi T, Granchi C,
Martinelli A, Macchia M, Minutolo F, De Tommasi N and Braca A:
Phenylpropanoids and flavonoids from phlomis kurdica as inhibitors
of human lactate dehydrogenase. Phytochemistry. 116:262–268. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Li S, Wu L, Feng J, Li J, Liu T, Zhang R,
Xu S, Cheng K, Zhou Y, Zhou S, et al: In vitro and in vivo study of
epigallocatechin-3-gallate-induced apoptosis in aerobic glycolytic
hepatocellular carcinoma cells involving inhibition of
phosphofructokinase activity. Sci Rep. 6:284792016. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Dihal AA, van der Woude H, Hendriksen PJ,
Charif H, Dekker LJ, IJsselstijn L, de Boer VC, Alink GM, Burgers
PC, Rietjens IM, et al: Transcriptome and proteome profiling of
colon mucosa from quercetin fed F344 rats point to tumor preventive
mechanisms, increased mitochondrial fatty acid degradation and
decreased glycolysis. Proteomics. 8:45–61. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Jiménez-Uribe AP, Hernández-Cruz EY,
Ramírez-Magaña KJ and Pedraza-Chaverri J: Involvement of
tricarboxylic acid cycle metabolites in kidney diseases.
Biomolecules. 11:12592021. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Liu JJ, Liu S, Gurung RL, Ching J, Kovalik
J-P, Tan TY and Lim SC: Urine tricarboxylic acid cycle metabolites
predict progressive chronic kidney disease in type 2 diabetes. J
Clin Endocrinol Metab. 103:4357–4364. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Saunier E, Benelli C and Bortoli S: The
pyruvate dehydrogenase complex in cancer: An old metabolic
gatekeeper regulated by new pathways and pharmacological agents.
Int J Cancer. 138:809–817. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Blouin JM, Penot G, Collinet M, Nacfer M,
Forest C, Laurent-Puig P, Coumoul X, Barouki R, Benelli C and
Bortoli S: Butyrate elicits a metabolic switch in human colon
cancer cells by targeting the pyruvate dehydrogenase complex. Int J
Cancer. 128:2591–2601. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Coricovac D, Dehelean CA, Pinzaru I, Mioc
A, Aburel OM, Macasoi I, Draghici GA, Petean C, Soica C, Boruga M,
et al: Assessment of betulinic acid cytotoxicity and mitochondrial
metabolism impairment in a human melanoma cell line. Int J Mol Sci.
22:48702021. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Peeters TH, Lenting K, Breukels V, van
Lith SA, van den Heuvel CN, Molenaar R, van Rooij A, Wevers R, Span
PN, Heerschap A and Leenders WPJ: Isocitrate dehydrogenase
1-mutated cancers are sensitive to the green tea polyphenol
epigallocatechin-3-gallate. Cancer Metab. 7:42019. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Bianchi G, Ravera S, Traverso C, Amaro A,
Piaggio F, Emionite L, Bachetti T, Pfeffer U and Raffaghello L:
Curcumin induces a fatal energetic impairment in tumor cells in
vitro and in vivo by inhibiting ATP-synthase activity.
Carcinogenesis. 39:1141–1150. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Patel K, Singh GK and Patel DK: A review
on pharmacological and analytical aspects of naringenin. Chi J
Integr Med. 24:551–560. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Abotaleb M, Samuel SM, Varghese E,
Varghese S, Kubatka P, Liskova A and Büsselberg D: Flavonoids in
cancer and apoptosis. Cancers (Basel). 11:282018. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Brecht K, Riebel V, Couttet P, Paech F,
Wolf A, Chibout SD, Pognan F, Krähenbühl S and Uteng M: Mechanistic
insights into selective killing of oxphos-dependent cancer cells by
arctigenin. Toxicol In Vitro. 40:55–65. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Wang T, Ma F and Qian HL: Defueling the
cancer: ATP synthase as an emerging target in cancer therapy. Mol
Ther Oncolytics. 23:82–95. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Kicinska A and Jarmuszkiewicz W:
Flavonoids and mitochondria: Activation of cytoprotective pathways?
Molecules. 25:30602020. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Zheng KY, Choi RC, Cheung AW, Guo AJ, Bi
CW, Zhu KY, Fu Q, Du Y, Zhang WL, Zhan JY, et al: Flavonoids from
radix astragali induce the expression of erythropoietin in cultured
cells: A signaling mediated via the accumulation of
hypoxia-inducible factor-1α. J Agric Food Chem. 59:1697–1704. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Wang R, Zhou S and Li S: Cancer
therapeutic agents targeting hypoxia-inducible factor-1. Curr Med
Chem. 18:3168–3189. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Roy M and Datta A: Cancer genetics and
therapeutics: Focus on phytochemicals. Springer Nature; 2019,
View Article : Google Scholar
|
|
122
|
Kittiratphatthana N, Kukongviriyapan V,
Prawan A and Senggunprai L: Luteolin induces cholangiocarcinoma
cell apoptosis through the mitochondrial-dependent pathway mediated
by reactive oxygen species. J Pharmacy Pharmacol. 68:1184–1192.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Nenkov M, Ma Y, Gaßler N and Chen Y:
Metabolic reprogramming of colorectal cancer cells and the
microenvironment: Implication for therapy. Int J Mol Sci.
22:62622021. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Adem S, Comakli V, Kuzu M and Demirdag R:
Investigation of the effects of some phenolic compounds on the
activities of glucose-6-phosphate dehydrogenase and
6-phosphogluconate dehydrogenase from human erythrocytes. J Biochem
Mol Toxicol. 28:510–514. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Gomez LS, Zancan P, Marcondes MC,
Ramos-Santos L, Meyer-Fernandes JR, Sola-Penna M and Da Silva D:
Resveratrol decreases breast cancer cell viability and glucose
metabolism by inhibiting 6-phosphofructo-1-kinase. Biochimie.
95:1336–1343. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Jiang P, Du W and Wu M: Regulation of the
pentose phosphate pathway in cancer. Protein Cell. 5:592–602. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Zhang C, Zhang Z, Zhu Y and Qin S:
Glucose-6-phosphate dehydrogenase: A biomarker and potential
therapeutic target for cancer. Anticancer Agents Med Chem.
14:280–289. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Lin R, Elf S, Shan C, Kang HB, Ji Q, Zhou
L, Hitosugi T, Zhang L, Zhang S, Seo JH, et al: 6-phosphogluconate
dehydrogenase links oxidative PPP, lipogenesis and tumour growth by
inhibiting LKB1-AMPK signalling. Nat Cell Biol. 17:1484–1496. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Kerimi A and Williamson G: Differential
impact of flavonoids on redox modulation, bioenergetics, and cell
signaling in normal and tumor cells: A comprehensive review.
Antioxid Redox Signal. 29:1633–1659. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Mazzio EA, Close F and Soliman KF: The
biochemical and cellular basis for nutraceutical strategies to
attenuate neurodegeneration in parkinson's disease. Int J Mol Sci.
12:506–569. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Li P, Tian W and Ma X: Alpha-mangostin
inhibits intracellular fatty acid synthase and induces apoptosis in
breast cancer cells. Mol Cancer. 13:1382014. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Sciacovelli M, Gaude E, Hilvo M and Frezza
C: The metabolic alterations of cancer cells. Methods Enzymol.
542:1–23. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Lee KH, Lee MS, Cha EY, Sul JY, Lee JS,
Kim JS, Park JB and Kim JY: Inhibitory effect of emodin on fatty
acid synthase, colon cancer proliferation and apoptosis. Mol Med
Rep. 15:2163–2173. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Sainero-Alcolado L, Garde-Lapido E,
Snaebjörnsson MT, Schoch S, Stevens I, Ruiz-Pérez MV, Dyrager C,
Pelechano V, Axelson H, Schulze A and Arsenian-Henriksson M:
Targeting myc induces lipid droplet accumulation by upregulation of
hilpda in clear cell renal cell carcinoma. Proc Natl Acad Sci USA.
121:e23104791212024. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Wang X and Tian W: Green tea
epigallocatechin gallate: A natural inhibitor of Fatty-acid
synthase. Biochem Biophys Res Commun. 288:1200–1206. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Tan SK, Hougen HY, Merchan JR, Gonzalgo ML
and Welford SM: Fatty acid metabolism reprogramming in ccRCC:
Mechanisms and potential targets. Nat Rev Urol. 20:48–60. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Huang CH, Tsai SJ, Wang YJ, Pan MH, Kao JY
and Way TD: EGCG inhibits protein synthesis, lipogenesis, and cell
cycle progression through activation of AMPK in p53 positive and
negative human hepatoma cells. Mol Nutr Food Res. 53:1156–1165.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Qi X, Li Q, Che X, Wang Q and Wu G: The
uniqueness of clear cell renal cell carcinoma: Summary of the
process and abnormality of glucose metabolism and lipid metabolism
in ccRCC. Front Oncol. 11:7277782021. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Potze L, Di Franco S, Grandela C,
Pras-Raves ML, Picavet DI, van Veen HA, van Lenthe H, Mullauer FB,
van der Wel NN, Luyf A, et al: Betulinic acid induces a novel cell
death pathway that depends on cardiolipin modification. Oncogene.
35:427–437. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Horiguchi A, Asano T, Asano T, Ito K,
Sumitomo M and Hayakawa M: Fatty acid synthase over expression is
an indicator of tumor aggressiveness and poor prognosis in renal
cell carcinoma. J Urol. 180:1137–1140. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Funabashi H, Kawaguchi A, Tomoda H, Omura
S, Okuda S and Iwasaki S: Binding site of cerulenin in fatty acid
synthetase. J Biochem. 105:751–755. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Li BH and Tian WX: Inhibitory effects of
flavonoids on animal fatty acid synthase. J Biochem. 135:85–91.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Jung KH, Lee JH, Quach CHT, Paik JY, Oh H,
Park JW, Lee EJ, Moon SH and Lee KH: Resveratrol suppresses cancer
cell glucose uptake by targeting reactive oxygen species-mediated
Hypoxia-inducible factor-1α activation. J Nucl Med. 54:2161–2167.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Chen F, Zhuang M, Zhong C, Peng J, Wang X,
Li J, Chen Z and Huang Y: Baicalein reverses hypoxia-induced 5-FU
resistance in gastric cancer AGS cells through suppression of
glycolysis and the PTEN/Akt/HIF-1α signaling pathway. Oncol Rep.
33:457–463. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Liu D, Xiao Y, Evans BS and Zhang F:
Negative feedback regulation of fatty acid production based on a
Malonyl-coA Sensor-actuator. ACS Synth Biol. 4:132–140. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Vahlensieck H, Pridzun L, Reichenbach H
and Hinnen A: Identification of the yeast ACC1 gene product
(acetyl-CoA carboxylase) as the target of the polyketide fungicide
soraphen A. Curr Genet. 25:95–100. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Kandori S, Kojima T, Matsuoka T, Yoshino
T, Sugiyama A, Nakamura E, Shimazui T, Funakoshi Y, Kanaho Y and
Nishiyama H: Phospholipase D2 promotes disease progression of renal
cell carcinoma through the induction of angiogenin. Cancer Sci.
109:1865–1875. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Daurkin I, Eruslanov E, Stoffs T, Perrin
GQ, Algood C, Gilbert SM, Rosser CJ, Su LM, Vieweg J and Kusmartsev
S: Tumor-associated macrophages mediate immunosuppression in the
renal cancer microenvironment by activating the 15-lipoxygenase-2
pathway. Cancer Res. 71:6400–6409. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Wu J, Zhang Y, Frilot N, Kim JI, Kim WJ
and Daaka Y: Prostaglandin E2 regulates renal cell carcinoma
invasion through the EP4 Receptor-Rap GTPase signal transduction
pathway. J Biol Chem. 286:33954–33962. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Fan WH, Wang FC, Jin Z, Zhu L and Zhang
JX: Curcumin synergizes with cisplatin to inhibit colon cancer
through targeting the MicroRNA-137-Glutaminase axis. Curr Med Sci.
42:108–117. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Hassanein M, Hoeksema MD, Shiota M, Qian
J, Harris BK, Chen H, Clark JE, Alborn WE, Eisenberg R and Massion
PP: SLC1A5 mediates glutamine transport required for lung cancer
cell growth and survival. Clin Cancer Res. 19:560–570. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Tuna B, Yorukoglu K, Gurel D, Mungan U and
Kirkali Z: Significance of COX-2 expression in human renal cell
carcinoma. Urology. 64:1116–1120. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Galleano M, Calabro V, Prince PD, Litterio
MC, Piotrkowski B, Vazquez-Prieto MA, Miatello RM, Oteiza PI and
Fraga CG: Flavonoids and metabolic syndrome. Ann N Y Acad Sci.
1259:87–94. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Brusselmans K, Vrolix R, Verhoeven G and
Swinnen JV: Induction of cancer cell apoptosis by flavonoids is
associated with their ability to inhibit fatty acid synthase
activity. J Biol Chem. 280:5636–5645. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Maddocks OD, Berkers CR, Mason SM, Zheng
L, Blyth K, Gottlieb E and Vousden KH: Serine starvation induces
stress and p53-dependent metabolic remodelling in cancer cells.
Nature. 493:542–546. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Metallo CM, Gameiro PA, Bell EL, Mattaini
KR, Yang J, Hiller K, Jewell CM, Johnson ZR, Irvine DJ, Guarente L,
et al: Reductive glutamine metabolism by IDH1 mediates lipogenesis
under hypoxia. Nature. 481:380–384. 2012. View Article : Google Scholar
|
|
157
|
Wang G, Wang YZ, Yu Y, Yin PH and Xu K:
The antitumor activity of betulinic Acid-loaded nanoliposomes
against colorectal cancer in vitro and in vivo via glycolytic and
glutaminolytic pathways. J Biomed Nanotechnol. 16:235–251. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Newman AC, Labuschagne CF, Vousden KH and
Maddocks OD: Use of 13C315N1-Serine or 13C515N1-Methionine for
studying methylation dynamics in cancer cell metabolism and
epigenetics. Methods Mol Biol. 1928:55–67. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Pan S, Fan M, Liu Z, Li X and Wang H:
Serine, glycine and One-carbon metabolism in cancer (Review). Int J
Oncol. 58:158–170. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Papalazarou V, Newman AC, Huerta-Uribe A,
Legrave NM, Falcone M, Zhang T, McGarry L, Athineos D, Shanks E,
Blyth K, et al: Phenotypic profiling of solute carriers
characterizes serine transport in cancer. Nat Metab. 5:2148–2168.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Amelio I, Cutruzzolá F, Antonov A,
Agostini M and Melino G: Serine and glycine metabolism in cancer.
Trends Biochem Sci. 39:191–198. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Samec M, Mazurakova A, Lucansky V,
Koklesova L, Pecova R, Pec M, Golubnitschaja O, Al-Ishaq RK,
Caprnda M, Gaspar L, et al: Flavonoids attenuate cancer metabolism
by modulating lipid metabolism, amino acids, ketone bodies and
redox state mediated by Nrf2. Eur J Pharmacol. 949:1756552023.
View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Rabinovich S, Adler L, Yizhak K, Sarver A,
Silberman A, Agron S, Stettner N, Sun Q, Brandis A, Helbling D, et
al: Diversion of aspartate in ASS1-deficient tumours fosters de
novo pyrimidine synthesis. Nature. 527:379–383. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Bowles TL, Kim R, Galante J, Parsons CM,
Virudachalam S, Kung HJ and Bold RJ: Pancreatic cancer cell lines
deficient in argininosuccinate synthetase are sensitive to arginine
deprivation by arginine deiminase. Int J Cancer. 123:1950–1955.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Ensor CM, Holtsberg FW, Bomalaski JS and
Clark MA: Pegylated arginine deiminase (ADI-SS PEG20,000 mw)
inhibits human melanomas and hepatocellular carcinomas in vitro and
in vivo. Cancer Res. 62:5443–5450. 2002.PubMed/NCBI
|
|
166
|
Pournourmohammadi S, Grimaldi M, Stridh
MH, Lavallard V, Waagepetersen HS, Wollheim CB and Maechler P:
Epigallocatechin-3-gallate (EGCG) activates AMPK through the
inhibition of glutamate dehydrogenase in muscle and pancreatic
ß-cells: A potential beneficial effect in the pre-diabetic state?
Int J Biochem Cell Biol. 88:220–225. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Wettersten HI, Hakimi AA, Morin D, Bianchi
C, Johnstone ME, Donohoe DR, Trott JF, Aboud OA, Stirdivant S, Neri
B, et al: Grade-dependent metabolic reprogramming in kidney cancer
revealed by combined proteomics and metabolomics analysis. Cancer
Res. 75:2541–2552. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Hornigold N, Dunn KR, Craven RA, Zougman
A, Trainor S, Shreeve R, Brown J, Sewell H, Shires M, Knowles M, et
al: Dysregulation at multiple points of the kynurenine pathway is a
ubiquitous feature of renal cancer: Implications for tumour immune
evasion. Br J Cancer. 123:137–147. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Riesenberg R, Weiler C, Spring O, Eder M,
Buchner A, Popp T, Castro M, Kammerer R, Takikawa O, Hatz RA, et
al: Expression of indoleamine 2,3-dioxygenase in tumor endothelial
cells correlates with Long-term survival of patients with renal
cell carcinoma. Clin Cancer Res. 13:6993–7002. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Trott JF, Kim J, Aboud OA, Wettersten H,
Stewart B, Berryhill G, Uzal F, Hovey RC, Chen CH, Anderson K, et
al: Inhibiting tryptophan metabolism enhances interferon therapy in
kidney cancer. Oncotarget. 7:66540–66557. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Beckermann KE, Johnson DB and Sosman JA:
PD-1/PD-l1 blockade in renal cell cancer. Expert Rev Clin Immunol.
13:77–84. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Munn DH, Shafizadeh E, Attwood JT,
Bondarev I, Pashine A and Mellor AL: Inhibition of T cell
proliferation by macrophage tryptophan catabolism. J Exp Med.
189:1363–1372. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Jochems C, Fantini M, Fernando RI, Kwilas
AR, Donahue RN, Lepone LM, Grenga I, Kim YS, Brechbiel MW, Gulley
JL, et al: The IDO1 selective inhibitor epacadostat enhances
dendritic cell immunogenicity and lytic ability of tumor
Antigen-specific T cells. Oncotarget. 7:37762–37772. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Naoi M, Wu Y, Shamoto-Nagai M and Maruyama
W: Mitochondria in neuroprotection by phytochemicals: Bioactive
polyphenols modulate mitochondrial apoptosis system, function and
structure. Int J Mol Sci. 20:24512019. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Abdel-Aziz SM, Aeron A and Kahil TA:
Health benefits and possible risks of herbal medicine. Microbes
Food Health. 97–116. 2016. View Article : Google Scholar
|
|
176
|
Ojo O, Ajuwape A, Otesile E, Owoade A,
Oyekunle M and Adetosoye A: Potentially zoonotic shiga
Toxin-producing escherichia coli serogroups in the faeces and meat
of Food-producing animals in ibadan, Nigeria. Int J Food Microbiol.
142:214–221. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Crowell JA, Korytko PJ, Morrissey RL,
Booth TD and Levine BS: Resveratrol-associated renal toxicity.
Toxicol Sci. 82:614–619. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Balaji S and Chempakam B: Toxicity
prediction of compounds from turmeric (Curcuma longa L). Food Chem
Toxicol. 48:2951–2959. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Qiu P, Man S, Li J, Liu J, Zhang L, Yu P
and Gao W: Overdose intake of curcumin initiates the unbalanced
state of bodies. J Agric Food Chem. 64:2765–2771. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
van Duursen MB, Nijmeijer S, De Morree E,
de Jong PC and van den Berg M: Genistein induces breast
Cancer-associated aromatase and stimulates Estrogen-dependent tumor
cell growth in in vitro breast cancer model. Toxicology. 289:67–73.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Wang D, Wang Y, Wan X, Yang CS and Zhang
J: Green tea polyphenol (−)-epigallocatechin-3-gallate triggered
hepatotoxicity in mice: Responses of major antioxidant enzymes and
the Nrf2 rescue pathway. Toxicol Appl Pharmacol. 283:65–74. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
182
|
McCullough ML and Giovannucci EL: Diet and
cancer prevention. Oncogene. 23:6349–6364. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Gonzalez CA: Nutrition and cancer: The
current epidemiological evidence. Br J Nutr. 96 (Suppl 1):S42–S45.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Surh YJ: Cancer chemoprevention with
dietary phytochemicals. Nat Rev Cancer. 3:768–780. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Shree TJ, Poompavai S, Begum S, Gowrisree
V, Hemalatha S, Sieni E and Sundararajan R: Cancer-fighting
phytochemicals: Another look. J Nanomed Biother Discov.
9:1622019.
|
|
186
|
Tohill BC and Joint F: Dietary intake of
fruit and vegetables and management of body weight (electronic
resource). World Health Organization; 2005
|
|
187
|
Chen H and Liu RH: Potential mechanisms of
action of dietary phytochemicals for cancer prevention by targeting
cellular signaling transduction pathways. J Agric Food Chem.
66:3260–3276. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Guo J, Li W, Shi H, Xie X, Li L, Tang H,
Wu M, Kong Y, Yang L, Gao J, et al: Synergistic effects of curcumin
with emodin against the proliferation and invasion of breast cancer
cells through upregulation of mir-34a. Mol Cell Biochem.
382:103–111. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Islam MR, Rahman MM, Dhar PS, Nowrin FT,
Sultana N, Akter M, Rauf A, Khalil AA, Gianoncelli A and Ribaudo G:
The role of natural and Semi-synthetic compounds in ovarian cancer:
Updates on mechanisms of action, current trends and perspectives.
Molecules. 28:20702023. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Hemaiswarya S, Prabhakar PK and Doble M:
Synergistic herb interactions with anticancer drugs. In: Herb-drug
combinations: A new complementary therapeutic strategy; Springer:
pp. 145–173. 2022
|
|
191
|
Chen S, Zhang Z and Zhang J: Emodin
enhances antitumor effect of paclitaxel on human non-small-cell
lung cancer cells in vitro and in vivo. Drug Des Devel Ther.
1145–1153. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Bai L, Li X, He L, Zheng Y, Lu H and Li J,
Zhong L, Tong R, Jiang Z, Shi J and Li J: Antidiabetic potential of
flavonoids from traditional chinese medicine: A review. Am J Chin
Med. 47:933–957. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Wu BY, Liu CT, Su YL, Chen SY, Chen YH and
Tsai MY: A review of complementary therapies with medicinal plants
for Chemotherapy-induced peripheral neuropathy. Complement Ther
Med. 42:226–232. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Zhang ZJ: Therapeutic effects of herbal
extracts and constituents in animal models of psychiatric
disorders. Life Sci. 75:1659–1699. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Mahmoud NN, Carothers AM, Grunberger D,
Bilinski RT, Churchill MR, Martucci C, Newmark HL and Bertagnolli
MM: Plant phenolics decrease intestinal tumors in an animal model
of familial adenomatous polyposis. Carcinogenesis. 21:921–927.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Galati G and O'brien PJ: Potential
toxicity of flavonoids and other dietary phenolics: Significance
for their chemopreventive and anticancer properties. Free Radic
Biol Med. 37:287–303. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Siddiqi A, Rani M, Bansal P and Rizvi MMA:
Renal cell carcinoma management: A step to Nano-chemoprevention.
Life Sci. 308:1209222022. View Article : Google Scholar : PubMed/NCBI
|