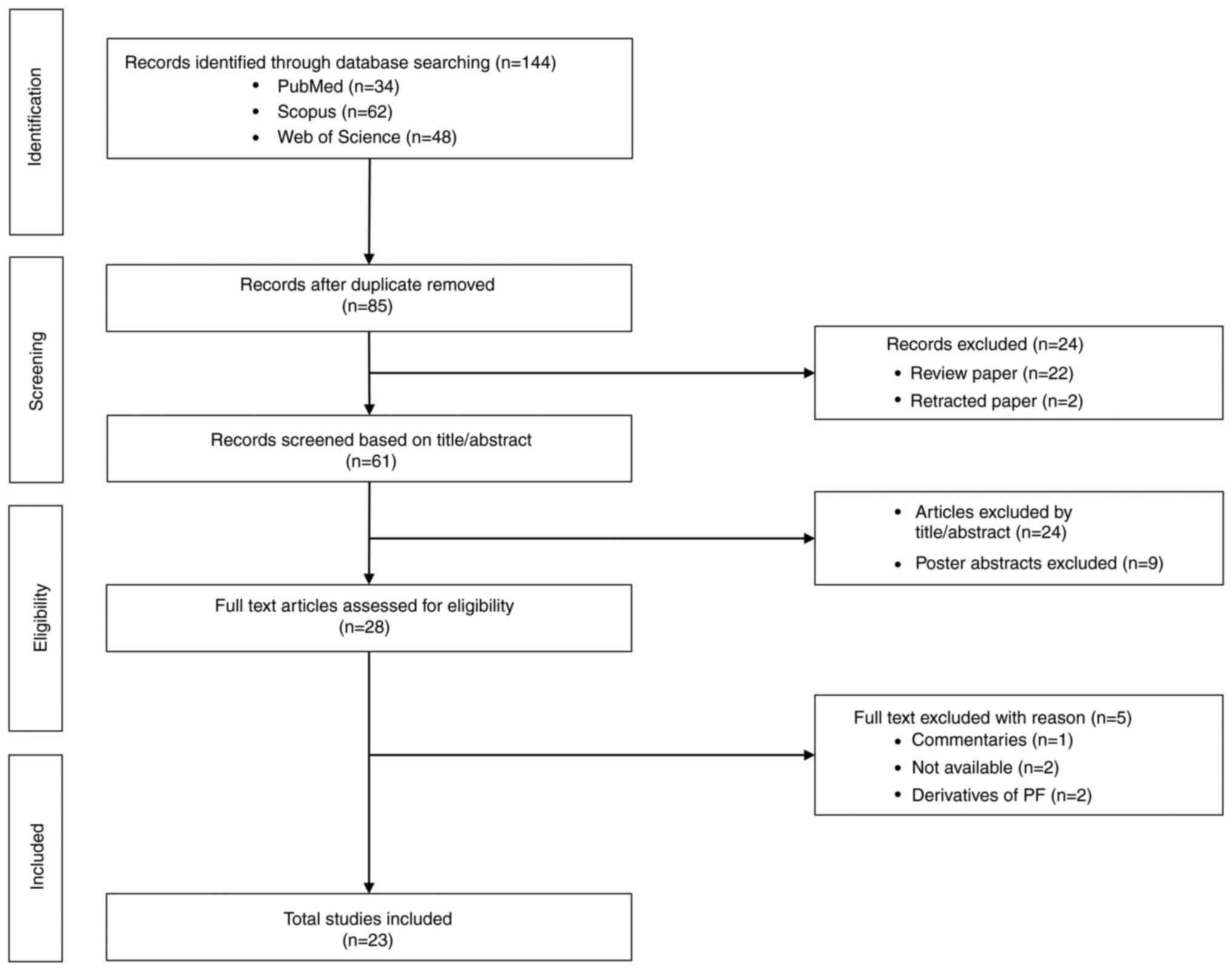
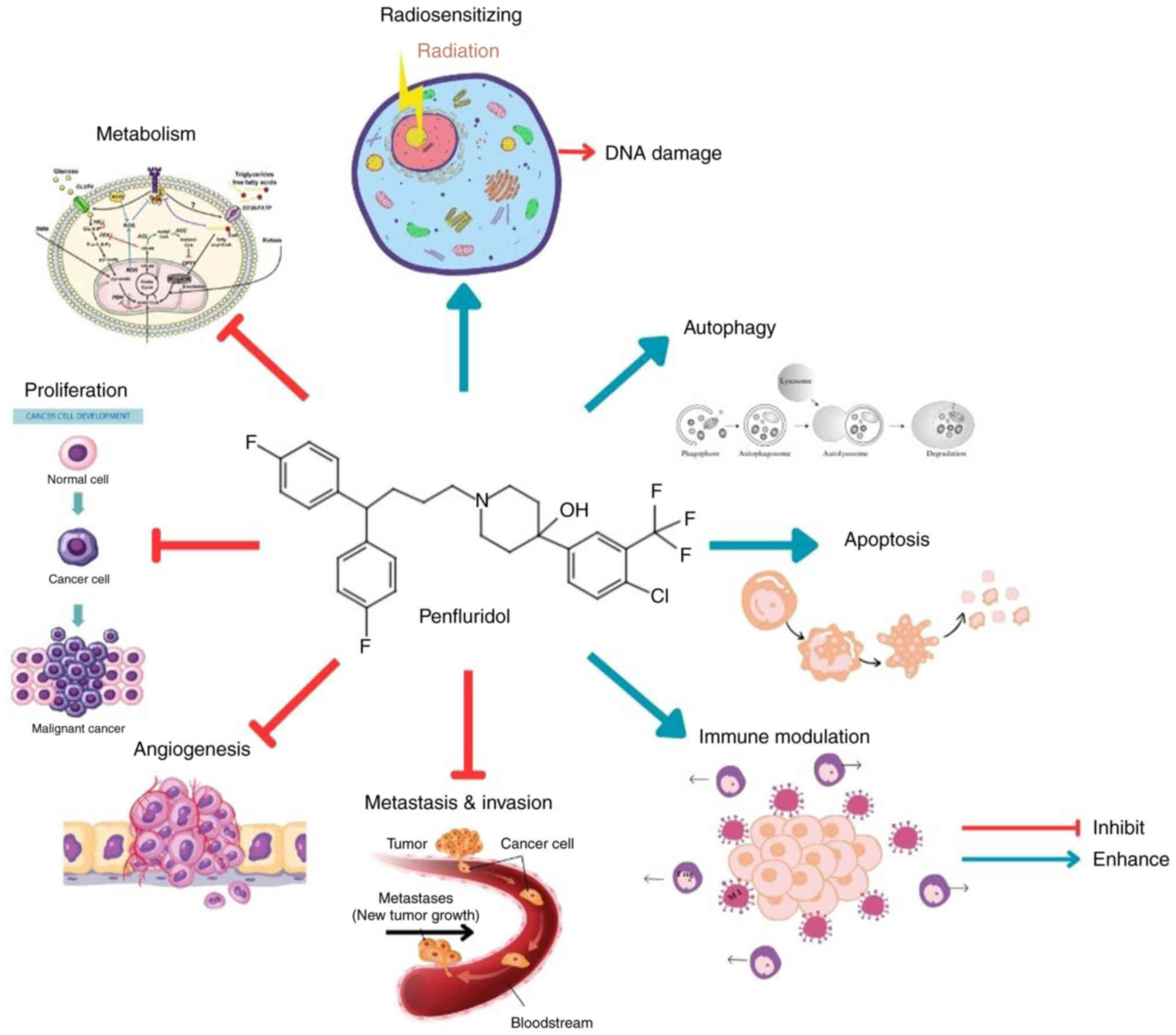
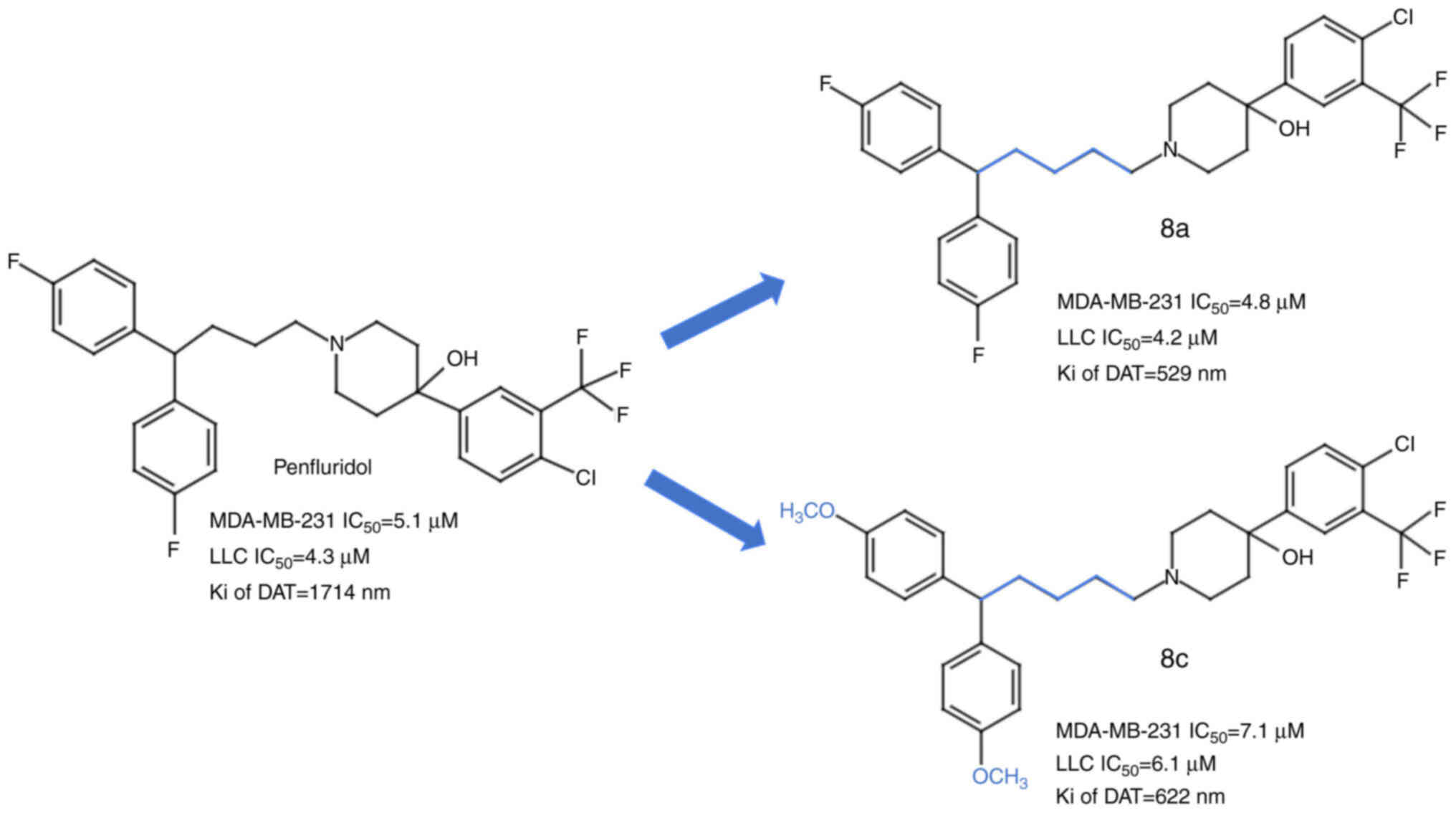
|
1
|
Ferlay J, Colombet M, Soerjomataram I,
Parkin DM, Piñeros M, Znaor A and Bray F: Cancer statistics for the
year 2020: An overview. Int J Cancer. Apr 5–2021.(Epub ahead of
print). View Article : Google Scholar
|
|
2
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
World Health Organization (WHO), . Cancer.
WHO; Geneva: 2022
|
|
4
|
Chen S, Cao Z, Prettner K, Kuhn M, Yang J,
Jiao L, Wang Z, Li W, Geldsetzer P, Bärnighausen T, et al:
Estimates and projections of the global economic cost of 29 cancers
in 204 countries and territories from 2020 to 2050. JAMA Oncol.
9:465–472. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gordon N, Stemmer SM, Greenberg D and
Goldstein DA: Trajectories of injectable cancer drug costs after
launch in the United States. J Clin Oncol. 36:319–325. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hendouei N, Saghafi F, Shadfar F and
Hosseinimehr SJ: Molecular mechanisms of anti-psychotic drugs for
improvement of cancer treatment. Eur J Pharmacol. 856:1724022019.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Qu LG, Brand NR, Chao A and Ilbawi AM:
Interventions addressing barriers to delayed cancer diagnosis in
low- and middle-income countries: A systematic review. Oncologist.
25:e1382–e1395. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Masuda T, Tsuruda Y, Matsumoto Y, Uchida
H, Nakayama KI and Mimori K: Drug repositioning in cancer: The
current situation in Japan. Cancer Sci. 111:1039–1046. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wouters OJ, McKee M and Luyten J:
Estimated Research and Development Investment Needed to Bring a New
Medicine to Market, 2009–2018. JAMA. 323:844–853. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Low ZY, Farouk IA and Lal SK: Drug
Repositioning: New approaches and future prospects for
life-debilitating diseases and the COVID-19 pandemic outbreak.
Viruses. 12:10582020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brown JS: Treatment of cancer with
antipsychotic medications: Pushing the boundaries of schizophrenia
and cancer. Neurosci Biobehav Rev. 141:1048092022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vlachos N, Lampros M, Voulgaris S and
Alexiou GA: Repurposing antipsychotics for cancer treatment.
Biomedicines. 9:17852021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shaw V, Srivastava S and Srivastava SK:
Repurposing antipsychotics of the diphenylbutylpiperidine class for
cancer therapy. Semin Cancer Biol. 68:75–83. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Varalda M, Antona A, Bettio V, Vachamaram
A, Yellenki V, Massarotti A, Baldanzi G and Capello D: Psychotropic
drugs show anticancer activity by disrupting mitochondrial and
lysosomal function. Front Oncol. 10:5621962020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Soares BG and Lima MS: Penfluridol for
schizophrenia. Cochrane Database Syst Rev.
2006:CD0029232006.PubMed/NCBI
|
|
16
|
Chokhawala K and Lee S: Antipsychotic
medications. StatPearls [Internet]. StatPearls Publishing; Treasure
Island, FL: 2023
|
|
17
|
Tricco AC, Lillie E, Zarin W, O'Brien KK,
Colquhoun H, Levac D, Moher D, Peters MDJ, Horsley T, Weeks L, et
al: PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist
and Explanation. Ann Intern Med. 169:467–473. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mak S and Thomas A: Steps for conducting a
scoping review. J Grad Med Educ. 14:565–567. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Arksey H and O'Malley L: Scoping studies:
Towards a methodological framework. Int J Soc Res Methodol.
8:19–32. 2005. View Article : Google Scholar
|
|
20
|
Hedrick E, Li XX and Safe S: Penfluridol
represses integrin expression in breast cancer through induction of
reactive oxygen species and downregulation of Sp transcription
factors. Mol Cancer Ther. 16:205–216. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gupta N, Gupta P and Srivastava S:
Penfluridol overcomes paclitaxel resistance in metastatic breast
cancer. Sci Rep. 9:50662019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ranjan A, Gupta P and Srivastava SK:
Penfluridol: An antipsychotic agent suppresses metastatic tumor
growth in triple-negative breast cancer by inhibiting integrin
signaling axis. Cancer Res. 76:877–890. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Srivastava S, Zahra FT, Gupta N, Tullar
PE, Srivastava SK and Mikelis CM: Low Dose of Penfluridol Inhibits
VEGF-Induced Angiogenesis. Int J Mol Sci. 21:7552020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lai TC, Lee YL, Lee WJ, Hung WY, Cheng GZ,
Chen JQ, Hsiao M, Chien MH and Chang JH: Synergistic tumor
inhibition via energy elimination by repurposing penfluridol and
2-Deoxy-D-Glucose in lung cancer. Cancers (Basel). 14:27502022.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hung WY, Chang JH, Cheng Y, Cheng GZ,
Huang HC, Hsiao M, Chung CL, Lee WJ and Chien MH: Autophagosome
accumulation-mediated ATP energy deprivation induced by penfluridol
triggers nonapoptotic cell death of lung cancer via activating
unfolded protein response. Cell Death Dis. 10:5382019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xue Q, Liu Z, Feng Z, Xu Y, Zuo W, Wang Q,
Gao T, Zeng J, Hu X, Jia F, et al: Penfluridol: An antipsychotic
agent suppresses lung cancer cell growth and metastasis by inducing
G0/G1 arrest and apoptosis. Biomed Pharmacother. 121:1095982020.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hung WY, Lee WJ, Cheng GZ, Tsai CH, Yang
YC, Lai TC, Chen JQ, Chung CL, Chang JH and Chien MH: Blocking
MMP-12-modulated epithelial-mesenchymal transition by repurposing
penfluridol restrains lung adenocarcinoma metastasis via
uPA/uPAR/TGF-β/Akt pathway. Cell Oncol (Dordr). 44:1087–1103. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ranjan A, German N, Mikelis C,
Srivenugopal K and Srivastava SK: Penfluridol induces endoplasmic
reticulum stress leading to autophagy in pancreatic cancer. Tumour
Biol. 39:10104283177055172017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ranjan A and Srivastava SK: Penfluridol
suppresses pancreatic tumor growth by autophagy-mediated apoptosis.
Sci Rep. 6:261652016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dandawate P, Kaushik G, Ghosh C, Standing
D, Ali Sayed AA, Choudhury S, Subramaniam D, Manzardo A, Banerjee
T, Santra S, et al: Diphenylbutylpiperidine Antipsychotic Drugs
Inhibit Prolactin Receptor Signaling to Reduce Growth of Pancreatic
Ductal Adenocarcinoma in Mice. Gastroenterology. 158:1433–1449.e27.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chien W, Sun QY, Lee KL, Ding LW, Wuensche
P, Torres-Fernandez LA, Tan SZ, Tokatly I, Zaiden N, Poellinger L,
et al: Activation of protein phosphatase 2A tumor suppressor as
potential treatment of pancreatic cancer. Mol Oncol. 9:889–905.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ranjan A and Srivastava SK: Penfluridol
suppresses glioblastoma tumor growth by Akt-mediated inhibition of
GLI1. Oncotarget. 8:32960–32976. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim H, Chong K, Ryu BK, Park KJ, Yu MO,
Lee J, Chung S, Choi S, Park MJ, Chung YG and Kang SH: Repurposing
penfluridol in combination with temozolomide for the treatment of
glioblastoma. Cancers (Basel). 11:13102019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ranjan A, Wright S and Srivastava SK:
Immune consequences of penfluridol treatment associated with
inhibition of glioblastoma tumor growth. Oncotarget. 8:47632–47641.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hu J, Cao J, Jin R, Zhang B, Topatana W,
Juengpanich S, Li S, Chen T, Lu Z, Cai X and Chen M: Inhibition of
AMPK/PFKFB3 mediated glycolysis synergizes with penfluridol to
suppress gallbladder cancer growth. Cell Commun Signal. 20:1052022.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
van der Horst G, van de Merbel AF, Ruigrok
E, van der Mark MH, Ploeg E, Appelman L, Tvingsholm S, Jäätelä M,
van Uhm J, Kruithof-de Julio M, et al: Cationic amphiphilic drugs
as potential anticancer therapy for bladder cancer. Mol Oncol.
14:3121–3134. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zheng C, Yu X, Liang Y, Zhu Y, He Y, Liao
L, Wang D, Yang Y, Yin X, Li A, et al: Targeting PFKL with
penfluridol inhibits glycolysis and suppresses esophageal cancer
tumorigenesis in an AMPK/FOXO3a/BIM-dependent manner. Acta Pharm
Sin B. 12:1271–1287. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wu SY, Wen YC, Ku CC, Yang YC, Chow JM,
Yang SF, Lee WJ and Chien MH: Penfluridol triggers cytoprotective
autophagy and cellular apoptosis through ROS induction and
activation of the PP2A-modulated MAPK pathway in acute myeloid
leukemia with different FLT3 statuses. J Biomed Sci. 26:632019.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tung MC, Lin YW, Lee WJ, Wen YC, Liu YC,
Chen JQ, Hsiao M, Yang YC and Chien MH: Targeting DRD2 by the
antipsychotic drug, penfluridol, retards growth of renal cell
carcinoma via inducing stemness inhibition and autophagy-mediated
apoptosis. Cell Death Dis. 13:4002022. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wu LL, Liu YY, Li ZX, Zhao Q, Wang X, Yu
Y, Wang YY, Wang YQ and Luo F: Anti-tumor effects of penfluridol
through dysregulation of cholesterol homeostasis. Asian Pac J
Cancer Prev. 15:489–494. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Du J, Shang J, Chen F, Zhang Y, Yin N, Xie
T, Zhang H, Yu J and Liu F: A CRISPR/Cas9-Based screening for
non-homologous end joining inhibitors reveals ouabain and
penfluridol as radiosensitizers. Mol Cancer Ther. 17:419–431. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Janssen PA, Niemegeers CJ, Schellekens KH,
Lenaerts FM, Verbruggen FJ, Van Nueten JM and Schaper WK: The
pharmacology of penfluridol (R 16341) a new potent and orally
long-acting neuroleptic drug. Eur J Pharmacol. 11:139–154. 1970.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Airoldi L, Marcucci F, Mussini E and
Garattini S: Distribution of penfluridol in rats and mice. Eur J
Pharmacol. 25:291–295. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Andrade C: Psychotropic drugs with long
half-lives: Implications for drug discontinuation, occasional
missed doses, dosing interval, and pregnancy planning. J Clin
Psychiatry. 83:22f145932022. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bhattacharyya R, Bhadra R, Roy U,
Bhattacharyya S, Pal J and Saha SS: Resurgence of penfluridol:
Merits and demerits. East J Psychiatry. 18:23–29. 2015. View Article : Google Scholar
|
|
46
|
Nikvarz N, Vahedian M and Khalili N:
Chlorpromazine versus penfluridol for schizophrenia. Cochrane
database Syst Rev. 9:CD0118312017.PubMed/NCBI
|
|
47
|
Wang RI, Larson C and Treul SJ: Study of
penfluridol and chlorpromazine in the treatment of chronic
schizophrenia. J Clin Pharmacol. 22:236–242. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Andrade C: The practical importance of
half-life in psychopharmacology. J Clin Psychiatry.
83:22f145842022. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Clarke Z: Penfluridol. Elsevier; New York,
NY: pp. 1–4. 2007
|
|
50
|
Enyeart JJ, Biagi BA, Day RN, Sheu SS and
Maurer RA: Blockade of low and high threshold Ca2+ channels by
diphenylbutylpiperidine antipsychotics linked to inhibition of
prolactin gene expression. J Biol Chem. 265:16373–16379. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Cabrera M, Gomez N, Remes Lenicov F,
Echeverría E, Shayo C, Moglioni A, Fernández N and Davio C: G2/M
cell cycle arrest and tumor selective apoptosis of acute leukemia
cells by a promising benzophenone thiosemicarbazone compound. PLoS
One. 10:e01368782015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Abbas T and Dutta A: p21 in cancer:
Intricate networks and multiple activities. Nat Rev Cancer.
9:400–414. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Boudreau RT, Conrad DM and Hoskin DW:
Apoptosis induced by protein phosphatase 2A (PP2A) inhibition in T
leukemia cells is negatively regulated by PP2A-associated p38
mitogen-activated protein kinase. Cell Signal. 19:139–151. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Nakamura H and Takada K: Reactive oxygen
species in cancer: Current findings and future directions. Cancer
Sci. 112:3945–3952. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Yang H, Villani RM, Wang H, Simpson MJ,
Roberts MS, Tang M and Liang X: The role of cellular reactive
oxygen species in cancer chemotherapy. J Exp Clin Cancer Res.
37:2662018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Shah MA and Rogoff HA: Implications of
reactive oxygen species on cancer formation and its treatment.
Semin Oncol. 48:238–245. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Singh R and Manna PP: Reactive oxygen
species in cancer progression and its role in therapeutics. Explor
Med. 3:43–57. 2022. View Article : Google Scholar
|
|
58
|
Perillo B, Di Donato M, Pezone A, Di Zazzo
E, Giovannelli P, Galasso G, Castoria G and Migliaccio A: ROS in
cancer therapy: The bright side of the moon. Exp Mol Med.
52:192–203. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Kim SJ, Kim HS and Seo YR: Understanding
of ROS-Inducing strategy in anticancer therapy. Oxid Med Cell
Longev. 2019:53816922019. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Miller DM, Thomas SD, Islam A, Muench D
and Sedoris K: c-Myc and cancer metabolism. Clin Cancer Res.
18:5546–5553. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Gao FY, Li XT, Xu K, Wang RT and Guan XX:
c-MYC mediates the crosstalk between breast cancer cells and tumor
microenvironment. Cell Commun Signal. 21:282023. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Safe S: Specificity Proteins (Sp) and
Cancer. Int J Mol Sci. 24:51642023. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Vellingiri B, Iyer M, Devi Subramaniam M,
Jayaramayya K, Siama Z, Giridharan B, Narayanasamy A, Abdal Dayem A
and Cho SG: Understanding the role of the transcription factor sp1
in ovarian cancer: From theory to practice. Int J Mol Sci.
21:11532020. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Dufour S, Broders-Bondon F and Bondurand
N: Chapter 13 - β1-Integrin Function and Interplay during Enteric
Nervous System Development. Academic Press; Boston, MA: pp.
153–166. 2015
|
|
65
|
Bergonzini C, Kroese K, Zweemer AJM and
Danen EHJ: Targeting integrins for cancer therapy-disappointments
and opportunities. Front cell Dev Biol. 10:8638502022. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Valdembri D and Serini G: The roles of
integrins in cancer. Fac Rev. 10:452021. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Yousefi H, Vatanmakanian M, Mahdiannasser
M, Mashouri L, Alahari NV, Monjezi MR, Ilbeigi S and Alahari SK:
Understanding the role of integrins in breast cancer invasion,
metastasis, angiogenesis, and drug resistance. Oncogene.
40:1043–1063. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Desgrosellier JS and Cheresh DA: Integrins
in cancer: Biological implications and therapeutic opportunities.
Nat Rev Cancer. 10:9–22. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Kim SY: Cancer energy metabolism: Shutting
power off cancer factory. Biomol Ther (Seoul). 26:39–44. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Chelakkot C, Chelakkot VS, Shin Y and Song
K: Modulating glycolysis to improve cancer therapy. Int J Mol Sci.
24:26062023. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Fadaka A, Ajiboye B, Ojo O, Adewale O,
Olayide I and Emuowhochere R: Biology of glucose metabolization in
cancer cells. J Oncol Sci. 3:45–51. 2017. View Article : Google Scholar
|
|
72
|
Lu J, Chen S, Bai X, Liao M, Qiu Y, Zheng
LL and Yu H: Targeting cholesterol metabolism in Cancer: From
molecular mechanisms to therapeutic implications. Biochem
Pharmacol. 218:1159072023. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Fan YJ and Zong WX: The cellular decision
between apoptosis and autophagy. Chin J Cancer. 32:121–129.
2013.PubMed/NCBI
|
|
74
|
Das S, Shukla N, Singh SS, Kushwaha S and
Shrivastava R: Mechanism of interaction between autophagy and
apoptosis in cancer. Apoptosis. 26:512–533. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Mulcahy Levy JM and Thorburn A: Autophagy
in cancer: Moving from understanding mechanism to improving therapy
responses in patients. Cell Death Differ. 27:843–857. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Koukourakis MI, Kalamida D, Giatromanolaki
A, Zois CE, Sivridis E, Pouliliou S, Mitrakas A, Gatter KC and
Harris AL: Autophagosome Proteins LC3A, LC3B and LC3C have distinct
subcellular distribution kinetics and expression in cancer cell
lines. PLoS One. 10:e01376752015. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Fares J, Fares MY, Khachfe HH, Salhab HA
and Fares Y: Molecular principles of metastasis: A hallmark of
cancer revisited. Signal Transduct Target Ther. 5:282020.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Ribatti D, Tamma R and Annese T:
Epithelial-Mesenchymal transition in cancer: A historical overview.
Transl Oncol. 13:1007732020. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Huang Y, Hong W and Wei X: The molecular
mechanisms and therapeutic strategies of EMT in tumor progression
and metastasis. J Hematol Oncol. 15:1292022. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Liu ZL, Chen HH, Zheng LL, Sun LP and Shi
L: Angiogenic signaling pathways and anti-angiogenic therapy for
cancer. Signal Transduct Target Ther. 8:1982023. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Yang Y and Cao Y: The impact of VEGF on
cancer metastasis and systemic disease. Semin Cancer Biol. 86((Pt
3)): 251–261. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Zhang L, Zhang M, Wang L, Li J, Yang T,
Shao Q, Liang X, Ma M, Zhang N, Jing M, et al: Identification of
CCL4 as an immune-related prognostic biomarker associated with
tumor proliferation and the tumor microenvironment in clear cell
renal cell carcinoma. Front Oncol. 11:6946642021. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Rezayatmand H, Razmkhah M and
Razeghian-Jahromi I: Drug resistance in cancer therapy: The
Pandora's Box of cancer stem cells. Stem Cell Res Ther. 13:1812022.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Zheng HC: The molecular mechanisms of
chemoresistance in cancers. Oncotarget. 8:59950–59964. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Gong L, Zhang Y, Liu C, Zhang M and Han S:
Application of radiosensitizers in cancer radiotherapy. Int J
Nanomedicine. 16:1083–1102. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Ashraf-Uz-Zaman M, Shahi S, Akwii R, Sajib
MS, Farshbaf MJ, Kallem RR, Putnam W, Wang W, Zhang R, Alvina K, et
al: Design, synthesis and structure-activity relationship study of
novel urea compounds as FGFR1 inhibitors to treat metastatic
triple-negative breast cancer. Eur J Med Chem. 209:1128662021.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Ashraf-Uz-Zaman M, Sajib MS, Cucullo L,
Mikelis CM and German NA: Analogs of penfluridol as
chemotherapeutic agents with reduced central nervous system
activity. Bioorg Med Chem Lett. 28:3652–3657. 2018. View Article : Google Scholar : PubMed/NCBI
|