Pancreatic ductal adenocarcinoma (PDAC), a highly
invasive tumor that arises from the epithelial cells lining the
pancreatic duct and constitutes ~80–90% of total pancreatic cancer
(PC) cases (1,2). According to the GLOBOCAN 2022 data,
there were a total of 510,566 newly diagnosed cases of PC worldwide
in 2022, positioning it as the twelfth most prevalent malignancy
globally (3). Due to its insidious
onset, diagnosing early stages of PDAC presents challenges. Most
patients with this condition typically experience upper abdominal
discomfort or exhibit initial symptoms such as dull pain and
swelling.
Currently, the diagnosis of PDAC primarily relies on
imaging examinations, complemented by the detection of serum
biomarker carbohydrate antigen 19-9 (CA19-9) (4). The clinical diagnosis of PDAC often
occurs at an advanced or even metastatic stage, primarily due to
its low specificity and aggressive progression (5). The lack of efficacious targeted drugs
further contributes to the suboptimal clinical diagnosis and
prognosis of PDAC. Therefore, it is imperative to enhance early
diagnostic and therapeutic approaches in order to improve the
overall prognosis of PDAC (6). The
activation of proto-oncogenes has been increasingly found to induce
cancer cells to release a higher quantity of exosomes compared with
healthy cells. This phenomenon significantly impacts the
progression of PDAC through its transmission in both the tumor
microenvironment (TME) and the entire body (7,8).
Therefore, exosomes could be considered as promising diagnostic
biomarkers for tumors, including PDAC (9–11).
Studies have explored the utility of exosomal
markers such as B7-H4, Plectin-1, SATB2, Glypican-1 and microRNAs
(miRNAs or miRs) in diagnosing PDAC and other related neoplasms
(11–14). These exosomal markers have
demonstrated potential in distinguishing between different types of
PC, aiding in the accurate identification of malignant pancreatic
intraductal papillary mucinous neoplasms and cholangiocarcinoma
(15). Despite the challenges in
validation, the use of exosomes as non-invasive diagnostic
biomarkers is essential for the early detection of PDAC, a disease
often diagnosed late due to its asymptomatic nature (16,17).
The ability of exosomes to transfer chemical agents
has garnered significant interest across various fields. A study
conducted by Zheng et al (18) demonstrated the protective effects of
transplantation of mesenchymal stem cell-derived exosomes against
Dox-induced cardiomyopathy. Chen et al (19) proposed that plant exosome-like
nanovesicles (PENVs) and artificial PENV-derived nano-vectors hold
immense potential for efficient delivery of therapeutic small RNA
in mammalian systems, thereby showcasing promising prospects for
future clinical applications. A previous study by Tamura et
al (20) on the Myristoylated
Alanine-Rich C-kinase Substrate (MARCKS)-ED-photodoxaz system
presents a novel and promising approach for cancer treatment
through targeted manipulation of exosomes and utilization of
photochemistry. Kreger et al (21) reported that enrichment of Survivin
in breast cancer (BRCA) cell-derived exosomes treated with
Paclitaxel (PTX) promotes cellular survival and enhances resistance
to chemotherapy. Additionally, engineered exosomes have been found
to possess the ability to directly target cancer cells by
transporting gemcitabine (GEM), PTX and other chemical agents
(22). Overall, the literature
suggests that exosomes possess the capability to transfer various
molecules between cells, highlighting their potential in fields
such as immunology, imaging and targeted therapy (23).
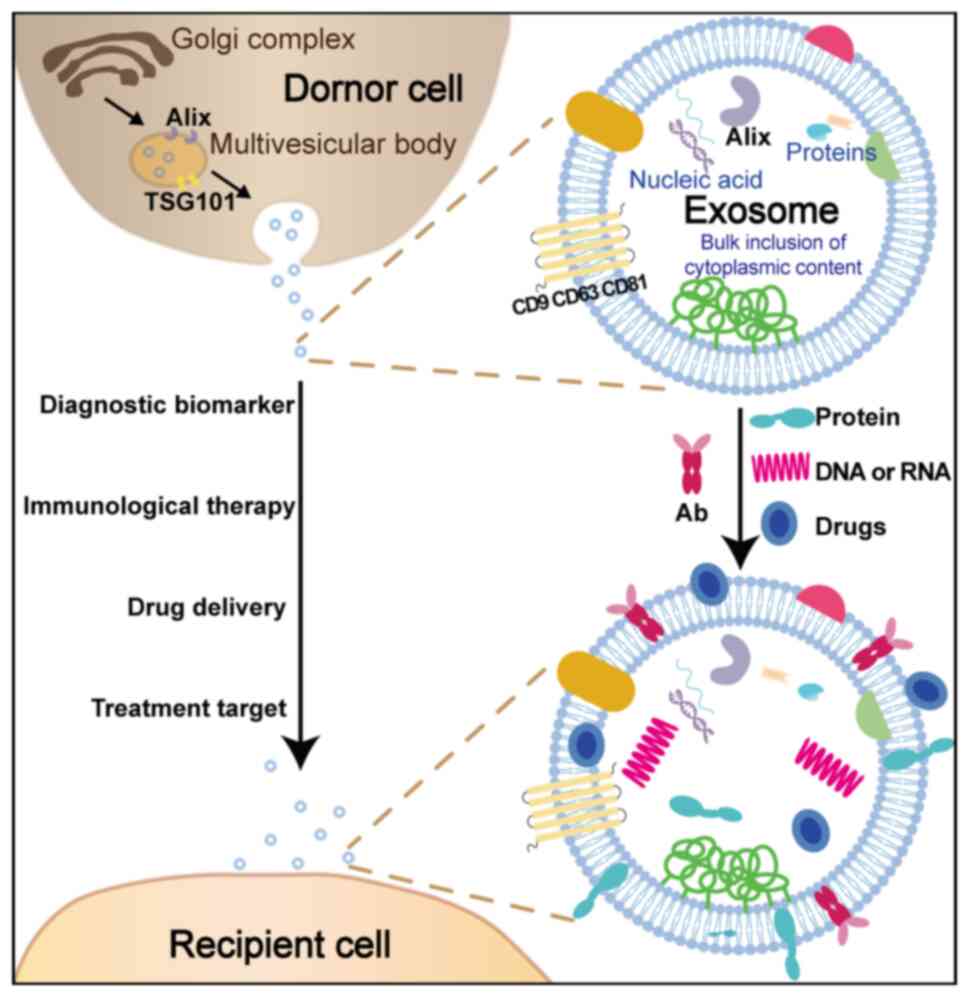
Extracellular vesicles (EVs) are a type of
cell-secreted vesicles with a membrane structure, which can be
categorized into three main groups based on their size, biological
characteristics and formation process: Exosomes, microvesicles and
apoptotic bodies (37,38). Exosomes typically refer to
microvesicles with a diameter ranging from 30 to 150 nm that are
formed through the fusion of multivesicular bodies (MVBs) with the
cell membrane. In general, exosome biogenesis occurs through
endosomal secretion in the early stages, facilitated by cytoplasmic
membrane invagination within endocytic vesicles and subsequent
multiplication during small body engulfment due to gradual lipid
membrane fusion, resulting in the formation of early endosomes.
During the process of endosome maturation, intracellular materials
continue to be internalized by the cell membrane, leading to the
formation of multiple luminal vesicles (ILVs), which eventually
transform into MVBs. Finally, MVBs give rise to exosomes through
their fusion with the plasma membrane and subsequent exocytosis of
ILVs towards the extracellular space (39–41).
The secretion and release of exosomes is a precisely
regulated process, with the involvement of various proteins such as
Alix and TSG101 in exosome formation (42). Additionally, due to their role in
signal transmission, the surface of exosomes typically contains
four transmembrane proteins, including CD9, CD63 and CD81 (43). In addition to specific proteins, the
phospholipid bilayer membrane of exosomes primarily comprises
lipids and phospholipids, which are abundant in substances such as
cholesterol, ceramide and sphingomyelin (44). The interaction between exosomes and
cell surface receptors typically occurs through transmembrane
proteins or lipid ligands, facilitating the delivery of exosomes
and their internal proteins or nucleic acids to recipient cells via
endocytosis (45). Therefore,
exosomes play a crucial role in intercellular communication and are
indispensable for the development and progression of diseases
(23,46). Moreover, exosomes are abundantly
present in various body fluids (47,48).
It has been confirmed that all cells have the ability to secrete
exosomes (49). Cancer cells in
patients with PDAC can transmit bioactive molecules through
exosomes, thereby disrupting the gene expression signals of
neighboring stroma or epithelium and other healthy cells. Compared
with individuals without cancer, cancer cells in patients with PDAC
have the ability to produce a significant quantity of diverse
exosomes. Once identified, isolated and characterized using
conventional methods, these exosomes can serve as potential
biomarkers (50,51).
Recent advancements in exosome research have
diversified the techniques available for isolation and
purification, each with distinct advantages and limitations
influencing their suitability for clinical applications. The
conventional method for exosome purification is differential
centrifugation, which has been considered the ‘gold standard’.
However, Lobb et al (52)
demonstrated that a combination of ultrafiltration and size
exclusion chromatography (SEC) can achieve comparable particle
purity to density gradient purification and efficiently isolate a
large quantity of exosomes from both cell culture media and human
plasma. Tang et al (53)
reported that ultracentrifugation exhibits the lowest yield and
recovery but offers the highest protein purity compared with the
other two exosome isolation methods (ExoQuick and Total Exosome
Isolation Reagent). Vaswani et al (54) developed a robust protocol combining
ultracentrifugation and SEC for isolating exosomes from human and
bovine milk. Additionally, Tayebi et al (55) introduced an innovative approach in
exosome research by integrating affinity-based methods with passive
microfluidic particle trapping techniques. They utilized
streptavidin-coated microbeads and biotinylated antibodies for
targeted exosome capture, thereby enhancing isolation specificity.
This method significantly reduces background noise during
fluorescence-based quantification, though its dependency on
specific antigen-antibody interactions may limit broader
applicability. The diversity of these methodologies illustrates the
ongoing evolution of exosome isolation and purification techniques,
each contributing uniquely to the field's understanding and
potential clinical applications.
TME plays a pivotal role in the progression of
cancer and the failure of therapy (56). Tumor-associated macrophages (TAMs)
are recruited to the TME and have been demonstrated to exert
influence on cancer progression (57). Exosomes, nano-sized bio-vesicles
released into bodily fluids, have been implicated in shaping the
TME and contributing to cancer advancement (58). Macrophage-derived exosomal
miR-501-3p has been shown to promote PDAC progression (59). A previous study has demonstrated the
presence of glypican-1 (GPC1) positive exosomes in the serum of
patients diagnosed with PDAC, exhibiting remarkable specificity and
sensitivity. Moreover, these exosomes have shown potential in
distinguishing individuals with benign pancreatic diseases or
healthy subjects from those afflicted by both early and late-stage
PDAC (22). Furthermore, TAMs are
resident innate immune cells within the TME that actively
contribute to tumor development and progression (60). The metabolic regulation governing
heterogeneity among TAMs and their modulation by the TME remain
areas necessitating further investigation (61). Overall, exosomes derived from
macrophages play a significant role in shaping PDAC advancement as
well as influencing dynamics within the TME itself (57,59).
Extensive prior research has demonstrated that macrophages and
fibroblasts within the TME participate in various stages of tumor
development through diverse pathways (62). By engaging in bidirectional
signaling with tumor cells and other cells via cancer-associated
fibroblast (CAF)-derived cytokines, chemokines, growth factors and
exosomes within the TME, CAFs not only promote tumor proliferation
but also induce immune evasion of cancer cells. Exosomes derived
from macrophages play a crucial role in the progression of PDAC and
contribute to the dynamics of the TME by promoting M2 macrophage
polarization. Exosomes originating from lung tumor cells,
hepatocellular carcinoma (HCC) cells and Schistosoma
japonicum adult worms have demonstrated their ability to
modulate transcriptional and bioenergetic profiles of macrophages,
inducing their polarization towards an M2 phenotype (63,64).
These findings provide compelling evidence for a novel function
played by exosomes in driving M2 macrophage polarization, thereby
offering promising therapeutic targets for immunotherapy against
lung cancer, HCC and PDAC (65,66).
These findings underscore both the potential of targeting exosomes
and TAMs for cancer therapy as well as emphasize the importance of
comprehending their roles in driving cancer progression (67,68).
Furthermore, exosomes have the ability to transport
and deliver diverse biological substances (69,70),
thereby specifically targeting cancer cells or genes implicated in
PDAC development. Consequently, engineered exosomes carrying
specific targeted genes can be harnessed for the treatment of PDAC,
thus playing a pivotal role in its therapeutic approach (71).
Exosomes have emerged as a promising non-viral
delivery system for CRISPR/Cas9 gene editing in PDAC. McAndrews
et al (72) demonstrated the
ability of exosomes to encapsulate CRISPR/Cas9 plasmid DNA and
efficiently deliver it to recipient cancer cells, thereby inducing
targeted gene deletion. This approach has been shown to effectively
suppress proliferation and inhibit tumor growth in preclinical
models of PDAC. The objective of Su et al (73) was to elucidate the mechanisms
underlying exosome-mediated intercellular communication between PC
(Panc-1) cells and macrophages (J771.A1) using a Transwell
co-culture system. Based on these findings, targeted genetic
therapies aimed at selectively manipulating the content of tumor
cell-derived exosomes, exhibiting significant potential for cancer
therapy.
Exosomal heat shock protein 60 plays a pivotal role
in intercellular communication, particularly in the progression of
cancer (74). It holds potential
clinical applications as a biomarker for diagnostics, prognostic
assessment, and monitoring disease progression and treatment
response in cancer. Moreover, heat shock protein 70 is upregulated
in PDAC cells and inhibits caspase-dependent apoptosis, thereby
facilitating cell survival (75).
Heat Shock Factor 1, a protein that governs the heat shock response
pathway, represents a promising target for drug intervention in
cancer and proteinopathy with significant implications for
therapeutic strategies and prognoses (76).
Exosomal nanoparticles secreted by human pancreatic
tumor cell lines exert an inhibitory effect on tumor cell
proliferation through the mitochondria-dependent apoptotic pathway,
mediated by the activation of phosphatase and tensin homolog
deleted on chromosome 10 (PTEN) and glycogen synthase kinase-3β
(GSK-3β) (79). The interaction
between exosomal nanoparticles and cells is considered to involve
membrane lipid rafts. This interaction leads to a downregulation in
the expression of hairy and enhancer-of-split homolog-1 (Hes-1), a
key intranuclear target of the Notch-1 signaling pathway, as well
as induction of apoptosis following G0/G1 phase cell cycle arrest
(80,81). Unexpectedly, blocking presenilin
results in PTEN and GSK-3β activation. Conversely, inhibition of
either PTEN or GSK-3β increases Hes-1 expression and partially
counteracts the proliferation inhibition induced by exosomal
nanoparticles, highlighting reciprocal regulations between Notch
signaling and PTEN/GSK-3β (82).
The present review focuses on the latest
advancements concerning exosomes in early screening and treatment
of PDAC. The aforementioned exosomes-related content was summarized
to explain the roles of exosomes (Fig.
1).
A wide range of studies have consistently
demonstrated that exosomes participate in cellular processes such
as cell migration, invasion, immune regulation, angiogenesis and
cancer cell metastasis (83–85).
During PDAC progression, malignant tumor cells release exosomes
that exhibit a dual function. They not only facilitate cancer
growth but also stimulate fibroblasts within the TME, resulting in
alterations in immune cell subtypes among host cells (86,87).
Consequently, this process hampers effective targeting of
immunocytes towards cancer cells while simultaneously upregulating
immunocyte apoptosis' levels.
On the one hand, exosomes that promote the
progression of PDAC may serve as specific diagnostic markers and
therapeutic targets. Jin et al (77) discovered that exosomes derived from
PC-1.0 (a highly malignant pancreatic cell line) cells can be
internalized by and enhance the proliferation, migration and
invasion abilities of PC-1 (a moderately malignant PC line) cells.
Wang et al (88) found that
hypoxic exosomes derived from PC cells activate macrophages to
adopt the M2 phenotype in a HIF1α or HIF2α-dependent manner,
thereby facilitating the migration, invasion and EMT of PC cells.
Chiba et al (89)
demonstrated through in vitro analyses that exosomes
released from PC (PK-45H) cells induce various gene expressions in
human umbilical vein endothelial cells (89). These findings suggest that exosomes
released from PC cells may serve as novel promoters of
angiogenesis. Furthermore, exosomes play a crucial role in the TME
and mediate communication between PDAC cells and matrix components
such as pancreatic stellate cells, regulating the progression of
PDAC (90). Zhou et al
(91) confirmed that exosomes
derived from PDAC cells can stimulate lymphangiogenesis both in
vitro and in vivo. This mechanism is related to
downregulation of ABHD11 antisense RNA 1 expression in lymphatic
endothelial cells and enhancement of their proliferative capacity,
migratory potential and tube formation ability (91).
On the other hand, exosomes that inhibit the
progression of PDAC could also be utilized as carriers for drug
delivery. For instance, exosomal miR-485-3p derived from pancreatic
ductal epithelial cells suppresses PDAC metastasis by targeting
p21-activated kinase-1 (92). The
miR let-7b-5p is highly enriched in exosomes derived from NK cells
and actively contributes to their antitumor effects against PDAC
cells (93). In addition, exosomal
miR-3607-3p derived from natural killer cells inhibits the
progression of PDAC by targeting IL-26 (94). The downregulation of lncRNA SBF2-AS1
in M2 macrophage-derived exosomes leads to an increase in
miR-122-5p, which restricts X-linked inhibitor of apoptosis protein
and limits the development of PDAC (95). The suppression of pancreatic ductal
cell carcinoma is achieved by human umbilical cord mesenchymal stem
cell-derived exosomes carrying hsa-miRNA-128-3p, which exert their
inhibitory effects on Galectin-3 (96). The specific mechanism underlying the
dual nature of exosomes remains to be elucidated, potentially
attributed to their diverse origins.
The early stage of PDAC, characterized by a high
degree of malignancy, often presents without evident clinical
manifestations. As the disease progresses, patients commonly
experience symptoms such as unexplained weight loss, tenderness in
the upper abdomen and jaundice. Consequently, clinicians typically
rely on these symptomatic presentations for diagnosing PDAC
(97,98).
In clinical practice, following the assessment of
genetic history and conducting a physical examination, clinicians
commonly utilize blood tumor markers for the diagnosis of PDAC.
Prominent diagnostic markers include CA19-9, carcinoembryonic
antigen (CEA) and CA125. Among these, CA19-9 exhibits a specificity
ranging from 80–90% in patients with PDAC and is considered the
most sensitive tumor marker for diagnosing PDAC (99,100).
However, based on scientific research, it has been observed that
the CA19-9 biomarker may produce false positive outcomes in
conditions such as cholangitis, inflammation and biliary tract
obstruction, thereby compromising its accuracy in indicating the
presence of a tumor (101).
Moreover, ~15–25% of patients with early-stage PDAC exhibit CA19-9
levels below the clinical threshold of 37 U/ml (102), while Lewis antigen-negative
individuals within the general population (5–10%) do not produce
CA19-9 at all (102). Furthermore,
achieving early detection and prompt treatment through these
aforementioned methods remains challenging due to the relatively
concealed pathogenesis of most cases (103). According to research findings, a
significant proportion of patients receive their diagnosis when the
disease has already progressed to either the intermediate or
advanced stages. Consequently, there is a risk of overlooking
potential opportunities for administering optimal treatment by
relying solely on conventional diagnostic methods (104). Therefore, in accordance with the
recommendations outlined in the latest literature, comprehensive
diagnosis of PDAC includes utilization of the CA19-9 marker
alongside histopathology, imaging techniques and other biomarkers
(105,106). Recent studies have indicated that
PDAC occurrence involves multitude cytokines, including miRNA,
mRNA, DNA and exosomes (107,108). Given their detectability during
PDAC development, exosomes hold great potential as diagnostic
biomarkers for this disease (109).
The levels of exosomes were quantified in patients
with PDAC, and the results revealed a statistically significant
elevation compared with that observed in healthy controls.
Additionally, there was a positive correlation between exosome
levels and elevated serum CEA concentrations. Further analysis
revealed a significant association between higher exosome levels,
lower tumor differentiation and shorter overall survival (110). Exosome levels in PDAC vary across
different stages and are correlated with disease severity (111). Significant variations in exosomal
miR-10b levels among different stages of PDAC have been previously
demonstrated (112). Furthermore,
exosome-mediated communication contributes to the development of
liver metastases in PDAC. However, comprehensive demonstration and
validation are still required to establish the diagnostic validity
and accuracy of exosomes for PDAC (113).
Simultaneously, bioactive molecules carried by PDAC
promote cell migration and invasion (45,114);
upon absorption by cancer cells, the physiological state of these
cells undergoes changes leading to metastasis and spread to other
parts of the body (115).
Therefore, different types of bioactive molecules carried by
exosomes including mRNA, miRNA, lncRNA, circRNA, DNA and protein
can partially reflect the extent of tumor lesions in patients with
PDAC; thus they can serve as biomarkers for early liquid biopsy
screening for PDAC (33,116–118).
The application of serum exosome diagnosis has been
suggested by numerous studies for early detection of PDAC (57,88,119).
Among them, miRNA, a key regulator of post-transcriptional gene
expression, is one of the most prevalent RNA species encapsulated
within exosomes. Marin et al (120) highlighted that the levels of
miR-125b-3p, miR-122-5p and miR-205-5p in serum exosomes from
patients with PDAC were significantly elevated compared with those
in the control group. Wang et al (121) conducted a study where they
collected blood samples from patients with PDAC, chronic
pancreatitis, and a healthy control group. Interestingly, it was
identified that the level of miR-19b in patients with PDAC was
significantly higher compared with both patients with chronic
pancreatitis and the control group. Furthermore, when compared with
the detection of the biomarker CA19-9, miR-19b exhibited superior
diagnostic accuracy [area under the curve (AUC): 0.942 vs. 0.813,
P=0.0054] (121).
In addition to their direct application in biomarker
diagnosis, the integration of exosomes with existing indicators in
clinical diagnosis and treatment of PDAC may significantly enhance
the precision of medical interventions. Nakamura et al
(122) demonstrated that by
combining miR-21 and miR-155 analysis from pancreatic juice with
pancreatic juice cytology, the diagnostic accuracy increased from
83, 89 and 74% for individual markers to a combined accuracy of 91%
(122). The combination of miR-21,
miR-210 and CA19-9 by Wu et al (110) was also found to enhance the
diagnostic accuracy from 83–85 to 90%. However, the rapid
progression of PDAC necessitates invasive surgical procedures
combined with biomarkers for disease staging, thereby impeding
timely and effective assessment of patient progress (110).
lncRNA is a type of non-coding RNA that plays a
crucial role in maintaining the stable biological function of PDAC
cells through its interactions with miRNA, mRNA, DNA, or protein
molecules. Yu et al (125)
conducted an analysis on the differential expression of lncRNAs
between patients with PDAC and healthy individuals and observed a
significantly elevated level of exosome LINC00623 in patients with
PDAC compared with healthy individuals. Specifically, the
expression level of exosome LINC00623 in patients with PDAC was
found to be ~3.7-fold higher than in healthy controls, with a 95%
confidence interval of 2.8 to 4.5 fold increase (P<0.01)
(125). In vitro
experiments also demonstrated that exosome LINC00623 can bind to
N-acetyltransferase 10 and maintain the stability of oncogenic mRNA
through ac4C acetylation, indicating that lncRNA may serve as a
potential biomarker for PDAC analysis.
The presence of circRNA, along with miRNAs and
lncRNAs, has also been detected in small EVs known as exosomes.
Both circRNA and exosomes have recently demonstrated significant
roles in various types of tumors. For instance, circ-KIAA1244
released in plasma exosomes has been identified as a potential
marker for gastric cancer detection, with a sensitivity of 77.42%
and a specificity of 68.00%. Similarly, hsa_circ_0004771 has been
used to distinguish colorectal cancer, with an AUC of 0.88 (95% CI,
0.815–0.940), a sensitivity of 80.91%, and a specificity of 82.86%.
Additionally, circ_0070396 combined with alpha-fetoprotein
demonstrated 81.98% sensitivity and 100% specificity in the
diagnosis of hepatocellular carcinoma (126).
By investigating the presence and expression levels
of serum exosomal circRNAs, it may be possible to distinguish
patients with cancer from healthy individuals in the future,
thereby identifying novel potential exosome-based cancer
biomarkers.
Exosomes originate from viable cancer cells and may
reflect a distinct biology compared with circulating cell-free DNA
(cfDNA) released from dying tissues. Yadav et al (127) provided an in-depth analysis of the
current status of liquid biopsy in PDAC as both diagnostic and
therapeutic tools, while also discussing future research
perspectives focusing on circulating tumor cells, circulating tumor
DNA and exosomes. The study conducted by Allenson et al
(128) compares exosome-derived
DNA with cfDNA in liquid biopsies of patients diagnosed with PDAC,
revealing a high prevalence of mutant Kirsten rat sarcoma viral
oncogene homologue (KRAS) in circulating exosome-derived DNA from
patients with early-stage PDAC, with KRAS mutations detected in
66.7% of localized PDAC cases, compared with 45.5% detected through
cfDNA. Furthermore, utilizing targeted sequencing, Mizukami et
al (129) conducted an
analysis on the coding regions of 27 cancer-predisposing genes in a
cohort consisting of 1,005 patients with PC and 23,705 controls in
Japan. Their findings revealed a significant association between
pathogenic mutations in BRCA genes and the incidence of PDAC,
particularly with regards to BRCA1 and BRCA2 (129). Although BRCA-mutant PDAC
constitutes a minority of PDAC cases, recent evidence suggests that
BRCA gene testing is indispensable for individuals with a familial
history of PDAC, which demonstrated potential in enhancing
diagnostic accuracy (130). Future
investigations are warranted to explore the detection of minute
fragments of BRCA mutations within exosomal DNA, as this approach
exhibits potential as an innovative diagnostic technique. The
current diagnostic paradigm for PDAC diagnosis, however, exhibits
low diagnostic accuracy in this manner. Although there are few
studies on this topic, it could be interesting for future
investigations.
Exosomal proteins, selectively packaged and released
by cancer cells, significantly contribute to the communication
between tumor cells and their microenvironment. Moreover, specific
protein cargoes within exosomes have been implicated in PDAC
progression as key regulators in signaling pathways associated with
tumor growth, invasion and metastasis. Understanding the dynamic
interplay between exosomal proteins and the complex network of
molecular events in PDAC is crucial for unraveling the intricacies
of this disease and holds promise for developing targeted
therapeutic strategies.
Studies have emphasized the potential of GPC1
enriched exosomes for early detection of PDAC (14,131).
These exosomes demonstrate superior sensitivity and specificity in
distinguishing between PDAC and chronic pancreatitis compared with
CA19-9 alone. Non-invasive blood tests can capture these exosomes,
presenting a novel approach for early detection of PDAC. Moreover,
exosome-based diagnostics overcome genetic variability issues such
as Lewis antigen negativity affecting CA19-9 expression and
secretion, thereby providing a more comprehensive and precise
biomarker system.
In a pivotal study, GPC1-positive circulating
exosomes demonstrated an impressive sensitivity of 98.3% and
specificity of 86.2%, with a remarkable area under the receiver
operating characteristic curve (AUROC) of 0.96, significantly
outperforming CA19-9 which exhibited a sensitivity of 78.3% and
specificity of 65.5% with an AUROC of 0.82 (P<0.0001) (131). Furthermore, the combination of
CA19-9 with the miR-3940-5p/miR-8069 ratio in urine exosomes
enhanced diagnostic accuracy, achieving a sensitivity of 93.0% and
positive predictive value of 78.4%; when both tests yielded
positive results, it resulted in a perfect positive predictive
value of 100% (132). These
findings underscore the potential utility of exosomal biomarkers
not only in surpassing conventional biomarkers in terms of
sensitivity and specificity but also in facilitating non-invasive
diagnostic protocols.
Numerous studies have reported the potential of
exosomal proteins, including CD44 variant isoform 6/complement C1q
binding protein, Claudin-4, epithelial cell adhesion molecule,
CD151, lectin galactoside-binding soluble 3 binding protein,
histone cluster 2 H2B family member E, histone cluster 2 H2B family
member F and others as promising biomarkers for early detection of
PDAC (78,133). Additionally, certain exosomal
proteins such as New York esophageal squamous cell carcinoma-1 and
melanoma antigen gene-A4 remain unidentified but their presence in
soft tissue sarcomas through immunostaining techniques suggests
their prospective significance in PDAC development and warrants
consideration for future screening approaches (134,135).
Exosomes can be isolated from the pancreatic duct
fluid and utilized for the diagnosis of patients PDAC (22). Moreover, exosomal proteins have the
potential to serve as diagnostic markers for patients with PDAC
(32). Exosomes provide a selective
enrichment method for cancer-specific material from the diverse
pool of circulating non-neoplastic tissue-derived nucleic acids.
Additionally, exosomes represent a distinct source of tumor DNA
that may complement other sources in liquid biopsy analysis
(136). However, it remains
unclear which specific types or stages of PDAC can be accurately
distinguished using exosomes, which could be an area for future
research direction (137,138).
The optimal treatment for PDAC is surgical
intervention; however, a significant proportion of patients with
PDAC (~80%) fail to meet the necessary criteria for surgery,
necessitating reliance on conservative pharmacotherapy instead
(141,142). Currently, primary treatment
options for PDAC are GEM in combination with albumin-bound PTX,
GnP, FOLFIRINOX [a regimen comprising 5-fluorouracil (5-FU),
leucovorin, irinotecan and oxaliplatin], and modified FOLFIRINOX
(mFOLFIRINOX). The Phase III trial demonstrated that the
combination of nanoparticle albumin-bound PTX and GEM significantly
enhanced overall survival, progression-free survival, and response
rate in patients with PDAC. However, it was associated with an
increased occurrence of peripheral neuropathy and myelosuppression
(143). Conroy et al
(144) revealed that FOLFIRINOX
exhibited a significant survival benefit compared with GEM, albeit
with an increased incidence of adverse effects. Furthermore,
another study highlighted that the administration of mFOLFIRINOX,
as opposed to FOLFIRINOX, may lead to a reduced frequency of Grade
3 or 4 non-hematological adverse events while maintaining a
comparable response rate (145).
In the NCCN 2023 guidelines for PDAC, the
aforementioned traditional therapies remain commonly used, while
new immunotherapy and targeted therapy options have been proposed,
including the KRAS inhibitor sotorasib and BRCA inhibitor olaparib
(146–148). Although not included in the list
of recommended therapies, exosome therapy has shown promise in
prolonging survival through its effects on immune response,
angiogenesis, drug resistance of tumor cells and other
mechanisms.
In PDAC, mutations in KRAS are frequently observed
and have been shown to promote aggressive phenotypes (149,150). In the CodeBreaK 100 trial
conducted by Strickler et al (147), the administration of KRAS G12D
inhibitor sotorasib primarily resulted in mild adverse reactions
among heavily pretreated populations, with safety outcomes
consistent with previous reports from the same trial. KRAS
mutations play a crucial role in promoting lymphangiogenesis
through exosomes in PC (151).
Chang et al (152)
presented a comprehensive investigation into the role of exosomes
in facilitating cell survival driven by KRAS mutations, revealing
that KRAS-mutant cells secrete exosomes enriched with the
anti-apoptotic protein Survivin. This enrichment not only enhances
the survival of neighboring cells but also contributes to the
overall therapeutic resistance observed in KRAS-mutant tumors.
Their findings suggest that these exosomes act as mediators of
intercellular communication, promoting a supportive
microenvironment for tumor growth and survival. Furthermore,
combining exosomes with other therapies can modulate the
pathological progression of PDAC and improve treatment
effectiveness. The combination of nano-liposomal irinotecan with FU
and folinic acid significantly enhances survival and quality of
life in patients with metastatic PDAC post-GEM therapy,
highlighting its efficacy and favorable safety profile (153–155).
Several studies have demonstrated that exosomes
derived from various cell sources, including immune cells and
mesenchymal stem cells, are capable of carrying and delivering a
diverse range of biological substances. Additionally, they can
specifically target PDAC cells or participate in the progression of
PDAC. This phenomenon significantly influences the advancement and
invasiveness of PDAC, underscoring its crucial role in the
treatment of this disease. Exosomes originating from immune cells
such as dendritic cells, T cells and B cells primarily serve vital
functions in facilitating the transfer of proteins, nucleic acids
and lipids between cells while contributing to intercellular
communication and immune regulation (156,157). Meanwhile, exosomes have been
demonstrated to possess unique bioactive molecules such as major
histocompatibility complex (MHC) and costimulatory molecules, which
play a crucial role in mediating immune responses against cancer
(158). Current studies have
demonstrated that immune exosomes primarily impact the progression
of PDAC by modulating immune responses within the TME. In addition
to being secreted by PDAC cells, a majority of existing research
(159,160) indicates that exosomes are commonly
released by mesenchymal stem cells, which possess convenient
accessibility and exhibit both immunogenic and immunomodulatory
properties (161). Moreover,
several studies have demonstrated the active migration of
mesenchymal stem cells to sites of inflammation and their ability
to modulate immune responses. Furthermore, it has been observed
that paracrine exosomes predominantly convey biological information
rather than direct cell contact (162,163). For example, umbilical cord stem
cells possess robust potential in regulating tissue differentiation
and regeneration due to their strong stem cell capacity (164). The exosomes can additionally
regulate the TME by exerting regulatory control over angiogenesis
and proliferation of PDAC cells within the TME (165). Adipose mesenchymal stem cells are
more likely to play a pivotal role in immunomodulation and
anti-inflammatory processes, as their exosomes have the ability to
regulate the expression of interleukin 6, interleukin 10, and
tumour necrosis factor alpha, thereby participating in immune
modulation and inflammation regulation in vivo (166,167). For instance, Liu et al
(168) demonstrated that exosomes
derived from adipose-derived mesenchymal stem cells can mitigate
oxidative stress, inflammation and infiltration of microglial cells
through the activation of the silent information regulator sirtuin
1 pathway. Therefore, exosomes derived from diverse cellular
origins exert a regulatory influence on the progression of PDAC by
modulating angiogenesis and mitigating the inflammatory
response.
Exosomes derived from antigen-presenting cells,
such as dendritic cells and macrophages, play a pivotal role in
transporting and presenting functional MHC peptide complexes to
modulate antigen-specific T cell responses. This underscores their
protective function in attenuating inflammation or enhancing immune
responses (169). In the context
of cancer vaccine development, computational structural modeling
has been employed to predict immunogenic neoepitopes for cancer
vaccines, with a specific focus on targeting neoantigens predicted
using MHC binding algorithms (170).
Tumor cells typically require the production of
angiogenic growth factors to establish their vascular network,
among which cytokines such as vascular endothelial-derived growth
factor (VEGF), and roundabout guidance receptor 1 (ROBO1) play
crucial roles in the process of angiogenesis within PDAC (171–174). Lee et al (175) and Pakravan et al (176) reported that exosomes derived from
mesenchymal stromal cells (MSCs) can selectively target tumor cells
by utilizing miR-16 or miR-100, thereby modulating the mechanistic
target of rapamycin/hypoxia inducible factor 1-alpha signaling
pathway to suppress intracellular VEGF expression and consequently
attenuate the angiogenic potential of tumor cells.
In addition to MSCs, exosomes derived from PDAC
cells may also possess corresponding anti-angiogenic mechanisms.
Research has demonstrated that miR-29b originating from PDAC cells
can downregulate VEGF expression and diminish the migration rate
and angiogenesis capability of vascular endothelial cells,
primarily by suppressing ROBO1 and SLIT-ROBO Rho GTPase-activating
protein 2, thereby attenuating the angiogenesis induced by PDAC
cells (173).
The TME is a complex and diverse ecosystem
comprising cancer cells, fibroblasts, adipocytes, endothelial cells
and mesenchymal stem cells (177).
The exosomes can also be derived from CAFs and other stromal cells
within the TME (62). CAFs, a
heterogeneous stromal cell population with diverse cellular
origins, phenotypes and functions, represent one of the significant
sources of exosomes within the TME (178,179). For example, CAFs can utilize the
miR-135b5p/Forkhead box transcription factor O1 axis to promote the
formation of new blood vessels for cancer cells (180). Additionally, CAFs have the ability
to induce expression of lnc HOTAIR, which facilitates tumor
metastasis by promoting EMT (181,182). Moreover, exosomes derived from
CAFs enhance tumor drug resistance and self-renewal capabilities of
cancer stem cells by providing them with mitochondrial genomes that
improve oxidative phosphorylation and mitochondrial metabolism
(183). These exosomes also
contain essential metabolites such as amino acids, lipids and
tricarboxylic acid cycle intermediates that are utilized by cancer
cells during periods of nutritional deficiency or stress.
Furthermore, CAFs contribute to tumor resistance against anticancer
treatments through their secretion of multiple exosomes (184). For instance, these exosomes can
activate retinoic acid inducible gene 1 protein signaling in cancer
cells or stimulate NOTCH3 signaling on cancer cells via Jagged 1
present on CAFs. The collaboration between these pathways
ultimately leads to enhanced resistance against radiation therapy
and chemotherapy (185). By
targeting the aforementioned mechanisms, pharmaceutical
interventions can be developed for the treatment of PDAC.
Specialized adipocytes known as cancer-associated
adipocytes (CAAs) play a significant role in various tumor types,
including BRCA, ovarian cancer, PDAC, kidney, gastric and colon
cancers (186). These CAAs
interact with tumor cells, leading to alterations in their
properties and functions. The impact of specific miRNAs, such as
miR-144, miR-126 and miR-155, present in exosomes on tumor growth,
has been observed to be mediated through their targeted influence
on adipocytes (187). Moreover, in
the presence of tumor cells, adipocytes upregulate exosomal
miRNA-155 levels to attract macrophages and facilitate their
differentiation into TAMs that support tumor growth (188–190). Additionally, CAA secretion of
visfatin has been shown to induce M2 phenotype polarization in
macrophages that promotes malignancy and enhances glycolysis.
The exosomes can also originate from normal
fibroblast-like mesenchymal cells and bear distinct markers of PDAC
tumor cells (191,192). Therefore, exosomes within the TME
can originate from a diverse range of cellular sources, extending
beyond MSCs and PDAC cells, including CAFs, CAA and
tumor-associated endothelial cells (193). Exosomes from these sources have
been shown to be related to cancer metabolism and drug resistance
in pan-cancer studies, and it may be possible to diagnose PDAC
cells drug resistance through them, but the specific mechanism has
not been clearly studied (10,194).
The progression of PDAC is intricately linked to
the migration, invasion and metastasis of PDAC cells (195). A disintegrin and metalloproteinase
domain-containing 9 protein is a relevant protein implicated in the
advancement, metastasis and unfavorable prognosis of tumors
(196). Shang et al
(197) discovered that miR-1231
was commonly employed as one of the diagnostic markers for
early-stage PDAC progression in previous studies. However, their
animal experiments revealed that MSC exosomes carrying miR-1231
exerted a negative regulatory effect on the migration, invasion and
adhesion of PDAC cells, thereby effectively inhibiting PDAC
activity (197). The study
conducted by Yao et al (198) proposed that circular RNA present
in mesenchymal stem cell exosomes may play a pivotal role in the
progression of PDAC. Through comprehensive transcriptome gene
sequencing and heat map analysis, circ-0030167 was found to
specifically target the expression of WNT inhibitory factor-1 in
PDAC cells by interacting with miR-338-5p, thereby effectively
suppressing the aberrant activation of the Wnt signaling pathway.
Consequently, this regulatory mechanism exerts inhibitory effects
on both proliferation and migration processes in PDAC cells.
Once tumor cells develop drug resistance, the
effectiveness of chemotherapy drugs will be significantly
diminished. Preliminary experiments have indicated that the drug
resistance in tumor cells is associated with the activation of
calmodulin-dependent kinases/Raf/MEK/ERK signaling pathway, and
in vivo exosomes derived from MSCs may play a crucial role
in the mechanism underlying tumor cell dormancy (199–201). The findings of Ono et al
(202) demonstrated that MSCs
exosomes transferred miR-23b to tumor cells, thereby inducing tumor
dormancy through the inhibition of MARCKS expression. These results
are consistent with those reported by Yang et al (203). They suggested that in vivo,
MSCs could induce tumor cell quiescence and enhance drug resistance
through dormancy, highlighting the significance of exosomes as
crucial targets for reducing tumor drug resistance. Expanding on
this hypothesis, Fu et al (204 and Fan et al
(205) conducted in vitro
experiments to validate the substantial impact of exosome
miR-520-5p and Ephrin type-A receptor 2 (EphA2) on GEM resistance
and transfer of drug resistance in PDAC cells. However, ongoing
exosome research focused on exosomes aimed at mitigating drug
resistance in PDAC necessitates further elucidation of the
influence of drug resistance and its generation mechanism.
The precise targeting capabilities of exosomes
suggest potential for personalized treatment strategies in PDAC.
For instance, an optimized approach to exploit variable biomarker
expressions is being developed in PDAC using a specialized
formulation of exosomes loaded with erastin, which selectively
targets triple-negative BRCA cells by leveraging the overexpression
of folate receptors (206,207).
Exosomes derived from cancer cells have been
employed not only for efficient drug delivery but also as
nanoanalytical contrast agents, particularly in the diagnosis and
monitoring of PDAC. Comparative investigations conducted on other
gastrointestinal malignancies reveal a significant underutilization
of such diagnostic applications in gastric and colon cancers, where
research predominantly focuses on fundamental cellular interactions
and molecular cargo analysis (208).
Furthermore, the novel use of milk-derived exosomes
for the oral delivery of hydrophobic drugs represents a significant
breakthrough with potential applications in gastrointestinal cancer
treatments (209). In PDAC, where
invasive interventions are prevalent, the development of oral
exosome-based therapies could profoundly transform treatment
strategies by offering less invasive and more patient-centric
alternatives.
While significant progress has been made in exosome
research for PDAC, there is a pressing need for more extensive
investigations into diagnostic and therapeutic applications in
other gastrointestinal cancers. The distinct challenges posed by
PDAC, characterized by its aggressive nature and often late-stage
diagnosis, necessitate tailored approaches that may not be directly
applicable to less aggressive or differently behaving tumors.
Nevertheless, the fundamental principles derived from exosome
research can inform broader strategies in gastrointestinal
oncology, fostering interdisciplinary innovations and potentially
identifying universal or cancer-specific therapeutic targets.
To enrich the discourse on the role of exosomes in
the prognosis of PDAC, a comprehensive compilation about various
exosomal markers and their associated biological processes is
presented in Table II. For
instance, exosomes derived from PC cells exhibit markers such as
c-Met and PD-L1, which are implicated in promoting invasive
expansion and immune evasion, thereby adversely affecting prognosis
(210). Additionally, exosomes
from malignant ascites of patients with PC, characterized by the
presence of CD133 and other cancer stem cell markers, contribute to
the establishment of a TME that facilitates tumor growth,
metastasis and angiogenesis (211). These processes further induce
immune suppression and drug resistance, ultimately impacting
patient outcomes.
In the context of blood plasma, exosomal miR-451a
has been identified as a significant independent factor for overall
survival and disease-free survival in PDAC, regulating key
biological processes such as proliferation, invasion and metastasis
(212). Similarly, EphA2 found in
serum exosomes correlates positively with tumor stage and reduced
survival rates, highlighting its role in cancer cell proliferation
and invasion (213).
Moreover, the upregulation of miR-23b-3p in serum
exosomes from patients with PDAC promotes cell proliferation,
migration and invasion, demonstrating its association with CA19-9
levels, a common tumor marker (214). The involvement of miR-3607-3p,
enriched in exosomes from natural killer cells, suggests a
potential prognostic marker, as low levels of this miRNA are linked
to poor prognosis in PDAC (94).
Exosomal miR-222 has been shown to enhance cell
proliferation and invasion by downregulating p27 through the
miR-222/PPP2R2A/AKT pathway, indicating its relevance in PC
incidence and prognosis (215).
Additionally, miR-106b and miR-146a contribute to GEM resistance in
cancer cells, with miR-146a promoting epithelial cell proliferation
and survival upon transfer to recipient cells (216,217).
Finally, the long-term exposure of PDAC cells to
GEM leads to the upregulation of miR-155, which promotes
anti-apoptosis and exosome secretion, thereby facilitating
chemoresistance. This miRNA is also transferred to other PDAC
cells, inducing functional changes that may impact treatment
outcomes (218). MiR-212-3p in
PDAC exosomes has been shown to suppress immune responses by
impairing CD4+ T cell activation, fostering an
immunotolerant microenvironment (219). Furthermore, miR-501-3p, derived
from M2 macrophage exosomes, inhibits transforming growth factor
beta receptor 3, disrupting the TGF-β signaling pathway and
promoting a pro-TME that facilitates PDAC development (220).
To help with a more comprehensive understanding,
exosomal markers in the treatment of PDAC have also been summarized
in Table III. Notably, exosomes
derived from bone marrow mesenchymal stem cells have been shown to
effectively deliver chemotherapeutic agents such as oxaliplatin and
siRNA to tumors, thereby enhancing antitumor immunity and
prolonging the circulation of therapeutic cargo (221). Furthermore, fibroblast-derived
exosomes have been implicated in promoting PC cell survival through
the release of mRNA and miRNA, with the inhibition of exosome
secretion via GW4869 leading to a reversal of drug resistance and
suppression of tumor growth (217).
Additionally, miR-1231 from bone marrow mesenchymal
stem cells has demonstrated a suppressive impact on PC cell
proliferation, migration, invasion and adhesion (197). Autologous exosomes secreted by PC
cells have been utilized for targeted delivery of GEM, highlighting
the potential of exosomes as vehicles for chemotherapeutic agents
(222). The engineering of
exosomes to deliver RNA specifically targeting oncogenic KRASG12D
has also shown promise in achieving targeted inhibition of PC cells
driven by this mutation (223).
Moreover, the combination of tumor-exosome-loaded
dendritic cells with cytotoxic drugs has been reported to enhance T
cell recovery and improve survival outcomes (224). Chlorin e6-loaded tumor-derived
re-assembled exosomes have enabled the integration of photodynamic
therapy and immune therapy, resulting in the generation of reactive
oxygen species and enhanced cytokine release (225). Ongoing research into exosomal
proteins from PANC-1 cells aims to assess their potential in
activating dendritic cells and cytokine-induced killer cells,
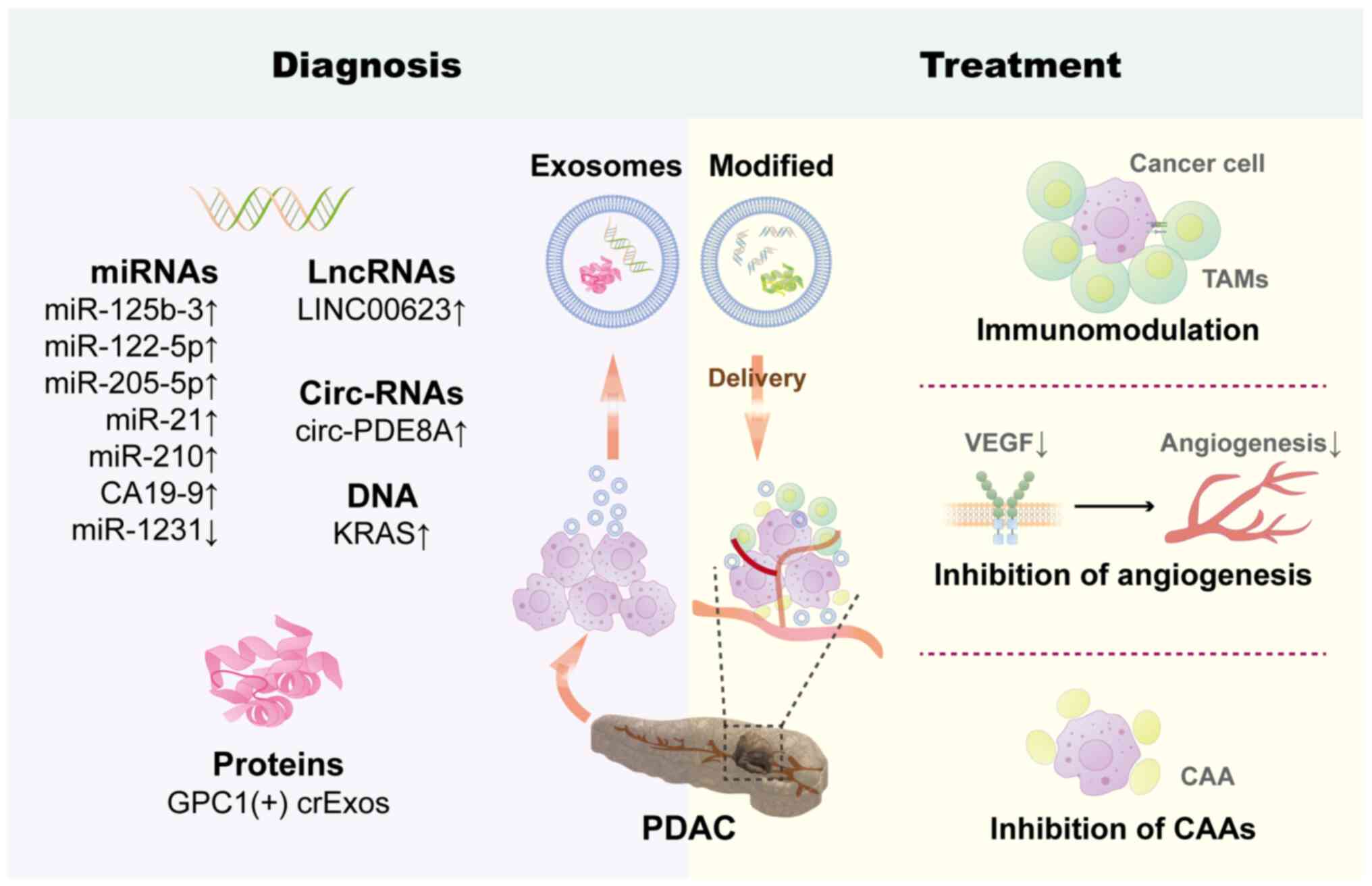
further exploring agonists for their activation (226). Finally, the application of
exosomes in the treatment and diagnosis of PDAC was summarized, as
depicted in Fig. 2.
The exosomes, serving as endogenous carriers of
molecular information between cells, possess the characteristics of
small size and excellent biocompatibility (227). Exosomes can serve as a versatile
drug carrier capable of encapsulating diverse compounds, including
small molecule chemical drugs, proteins and nucleic acids (228,229). The use of exosomes as drug
carriers is advantageous due to their ability to preserve the
biological activity of drugs within the membrane, while also
avoiding any immune response in vivo. This renders them more
suitable for delivery compared with traditional nano-delivery
carriers such as liposomes, thus making it a prominent area of
research in tumor therapy (230,231).
The low immunogenicity and high tissue permeability
of exosomes confer significant advantages for the delivery of small
molecule drugs, such as siRNA and miRNA. Previous studies have
demonstrated that exosomes derived from 293 cells, when loaded with
exogenous siRNA, can effectively inhibit tumor cell growth by
silencing the human epidermal growth factor receptor 2 gene
(232). The study conducted by Zuo
et al (233) employed
ultrasound for the transfection of miR-34a into exosomes. The
findings demonstrated that ExomiR-34a effectively traversed the
cell membrane and downregulated the expression of its target gene
BCL-2, thereby inducing apoptosis in PDAC cells. Moreover, it
significantly impeded tumor proliferation in vivo (233). The miRNA-145-5p functions as a
potent tumor suppressor, exhibiting a significant correlation with
the extent of macrophage infiltration. Ding et al (234) proposed that its dysregulation is
closely associated with aberrant proliferation and invasion of PDAC
cells. By constructing vectors for engineering MSC exosomes,
exogenous miR-145-5p was utilized in animal experiments pertaining
to the progression of PDAC. The findings demonstrated that exosomes
containing miR-145-5p significantly impeded the in vivo
growth of PDAC cells by inducing cell cycle arrest and augmenting
the apoptosis rate of these cells.
Chemical drugs often induce a variety of allergic
or complication reactions due to their low targeting efficacy
(235). However, MSC exosomes
possess inherent tumor-targeting properties derived from their
parental cells, and the abundance of proteins on their membrane
provides numerous targeting ligands and chemical modification sites
for engineering exosomes specifically designed to target tumor
cells (236). Therefore, exosomes
derived from MSCs present a promising solution to overcome this
challenge. Pascucci et al (237) conducted experiments to investigate
the therapeutic effects of MSC exosomes loaded with PTX, which were
generated through co-culture method. The results demonstrated that
PTX-loaded exosomes exhibited a significant inhibitory effect on
the proliferation activity of PDAC cells compared with PTX alone.
Furthermore, in line with this hypothesis, several studies have
also confirmed the efficacy of MSC exosomes loaded with GEM, a
standard chemotherapy drug for PDAC. In vitro experiments
have revealed a certain degree of growth inhibition in PDAC cells,
and from a pharmacological perspective, local administration of MSC
exosomes loaded with GEM can significantly enhance drug
concentration. Consequently, it is possible to design a more
effective targeting strategy for PDAC lesion areas (238). The effect of GEM-loaded MSCs
exosomes on systemic toxicity and efficacy was validated by Li
et al (222) through the
establishment of a PDAC animal model (222). The results demonstrated that
EXO-GEM induced minimal harm to mice compared with direct injection
of GEM, while significantly suppressing tumor proliferation without
any indications of tumor recurrence in mice. Considering the common
clinical application of combined therapies involving GEM and PTX,
Zhou et al (160)
constructed MSCs exosomes loaded with both GEM and PTX for PDAC
treatment. Compared with the chemo-drug group, the MSCs
exosomes-chemo-drug group exhibited greater persistence and
targeting, resulting in higher overall survival rates compared with
other control groups.
It is noteworthy that the clearance of exosomes by
pancreatic and immune cells can exert an influence on the TME and
thereby impact the progression or inhibition of PDAC. This aspect
holds particular significance in cases where exosomes are employed
as drug delivery vehicles. Kamerkar et al (223) have reported that the presence of
CD47 on exosomes hampers their clearance, rendering them more adept
at evading phagocytic elimination compared with liposomes. This
evasion mechanism is partly mediated by cluster of differentiation
47-signal-regulatory protein alpha interactions on exosomes, which
aid in circumventing host immune surveillance signals known as
‘don't eat me’ signals (223). The
presence of plasma membrane-like phospholipids and
membrane-anchored proteins in exosomes may contribute to their
reduced clearance from the circulation (239–241). Although CD47 does not play a
significant role in facilitating the entry of exosomes into
pancreatic cells, the enhanced macropinocytosis observed in
KRAS-mutant cancer cells promotes their uptake of exosomes
(242,243). Further investigation is warranted
to explore whether exosomes can evade lysosome-dependent
degradation of their cargo by utilizing macropinocytosis as an
entry mechanism.
Intriguingly, the conventional notion of the
pancreas as a sterile environment has been recently challenged by
suggesting the presence of bacteria capable of secreting exosomes
(245). These bacterial exosomes
have emerged as potential contributors to the dynamics of PDAC,
exerting influence on tumor progression and drug resistance
(246). Despite their significant
implications, this microbial aspect remains understudied in
relation to PDAC, highlighting a critical gap in current research.
Exploring the intricate interplay between bacterial exosomes and
pancreatic tumors opens up new avenues for comprehending the
multifaceted nature of this malignancy.
From a clinical and anatomical perspective, there
is no direct physical connection between the pancreas and the gut
microbiota; therefore, the pancreas is considered to be a sterile
tissue (247,248). However, microorganisms can migrate
to the pancreas through the bile duct in the digestive tract
(249,250). Numerous studies have demonstrated
a robust correlation between the composition of oral,
gastrointestinal, fecal and organ-specific (pancreatic) microbiota
and PDAC (251). The development
of periodontal disease in the oral cavity has been associated with
several key pathogenic bacteria, including Porphyromonas
gingivalis, Fusobacterium, Streptococcus mitis and Neisseria
elongata (252). The secretion
of peptidyl-arginine deiminase by Porphyromonas gingivalis
is hypothesized to induce mutations in the KRAS and tumor
suppressor p53 genes, thereby degrading arginine metabolism
(253). The oral administration of
fluorescently-labeled Enterococcus faecalis to wild type
mice in an experiment resulted in the observation of fluorescence
in the pancreas, indicating bacterial migration from the
gastrointestinal tract to the pancreas (254). The ability of bacteria to transfer
intracellular materials outside the body via exosomes suggests that
targeting bacterial exosomes could be a potential therapeutic
approach for treating PDAC (255).
The journey from laboratory discovery to clinical
application is always challenging, and this holds true for
PDAC-specific exosomes as well. In this arduous process, the
initial crucial step involves isolating high-quality PDAC-specific
exosomes. Recent advancements in microfluidic technologies have
facilitated the efficient capture of exosomes from small blood
volumes using highly specific antibodies (34). Although this method has the
potential to enable early screening using simple fingertip samples
from patients, there are significant challenges in scaling up the
technology for clinical use due to its complexity, costliness and
requirement for specialized equipment (34).
The second challenge lies in the safety of modified
exosomes. Numerous modified exosomes have been utilized for
targeted delivery of therapeutic agents such as small molecule
drugs, nucleic acids and proteins to cancer cells. A comprehensive
assessment of the ethical and legal implications associated with
these innovative treatments is imperative to ensure patient safety
under appropriate therapeutic conditions. For instance, early
clinical trials have demonstrated the potential efficacy of
mesenchymal stem cell-derived exosomes loaded with therapeutic
siRNA targeting the mutant KRAS-G12D gene for treating metastatic
PDAC. However, concerns exist regarding the inadvertent promotion
of tumor growth and metastasis by these vesicles (34).
Moreover, there are certain limitations associated
with engineering exosomes. Modifications inevitably affect the
natural properties and delivery efficacy of exosomes, thus
achieving a balance between harnessing their complete therapeutic
potential while preserving inherent functionalities remains a key
challenge. Additionally, production and purification processes
require high efficiency and reproducibility to ensure a pure
population of exosomes. However, conventional methods such as
differential centrifugation often yield preparations contaminated
with protein aggregates and other cellular debris (256). This highlights the necessity of
developing advanced and scalable purification techniques that
comply with clinical standards. Continuous innovation in exosome
isolation and purification technologies, along with comprehensive
clinical trials to evaluate the safety and efficacy of
exosome-based interventions, is crucial for addressing these
challenges and limitations.
In addition to PC, gastrointestinal cancers such as
gastric, colorectal and liver cancers have also emerged as
significant areas of interest in exosomal research. Numerous
miRNAs, lncRNAs, circRNAs and proteins have been identified as
potential diagnostic and prognostic biomarkers or therapeutic
targets for gastroenterological malignancies (257–259). Due to its high global mortality
rate, PC has garnered extensive attention from researchers aiming
to develop diverse engineered exosomes that could facilitate the
exploration of novel opportunities for generating clinical-grade
exosomal technologies and drugs.
Therefore, it is imperative to overcome the
challenges and limitations in order to fully exploit the potential
of exosomes in PDAC diagnosis and treatment. This will facilitate
more efficacious disease management and establish a precedent for
broader application of exosomal therapies in the field of
oncology.
PDAC is the most prevalent and lethal disease among
solid malignant tumors. Conventional tumor drugs used for treatment
often lead to drug resistance without any targeted alternatives
available, resulting in a challenging situation for PDAC diagnosis
and treatment. In addition to replacing existing biomarkers for
PDAC diagnosis, exosomes can enhance the accuracy rate by combining
detection methods or serving as an auxiliary tool alongside
existing techniques such as endoscopy, ultrasound detection and
imaging. Moreover, exosomes can differentiate between different
stages of PDAC progression based on their content, potentially
playing a broader role in clinical diagnosis and treatment.
Additionally, due to their intercellular communication capabilities
along with their safety profile and targeting abilities, exosomes
hold potential as a novel therapeutic option. Their unique lipid
structure also enables them to act as efficient drug delivery
vehicles that prolong drug action time within the body while
reducing toxicity levels when treating PDAC and other cancers.
However, current diagnostic and therapeutic applications involving
exosomes are still limited to preclinical experimental stages;
thus, further comprehensive clinical research studies and trials
are warranted. It is anticipated that exosomes will become an
effective tool or method for precise diagnosis and treatment of
PDAC in the future.
Not applicable.
The present study was supported by the National Undergraduate
Training Programs for Innovation and Entrepreneurship (grant no.
202411065052), the Shandong undergraduate training programs for
innovation and entrepreneurship (grant nos. S202311065054 and
S202411065020) and the Qingdao Medical and Health Research Guidance
Project (grant nos. 2022-WJZD108 and 2023-WJZD106).
Not applicable.
Each author has made substantial contributions to
the conception of the study. XCL, XZW, GB, XXL, ZRG, ZSZ, TYH, JPZ,
HJZ, XYL and ZJL drafted the manuscript. FFG, LLD and WHZ
substantively revised the manuscript. All authors read and approved
the final version of the manuscript. Data authentication is not
applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Cancer Genome Atlas Research Network.
Electronic address, . simpleAndrew_aguirre@dfci.harvard.edu;
Cancer Genome Atlas Research Network. Integrated Genomic
Characterization of Pancreatic Ductal Adenocarcinoma. Cancer Cell.
32:185–203.e113. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Klein AP: Pancreatic cancer epidemiology:
Understanding the role of lifestyle and inherited risk factors. Nat
Rev Gastroenterol Hepatol. 18:493–502. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Luo G, Jin K, Deng S, Cheng H, Fan Z, Gong
Y, Qian Y, Huang Q, Ni Q, Liu C and Yu X: Roles of CA19-9 in
pancreatic cancer: Biomarker, predictor and promoter. Biochim
Biophys Acta Rev Cancer. 1875:1884092021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Carmicheal J, Patel A, Dalal V, Atri P,
Dhaliwal AS, Wittel UA, Malafa MP, Talmon G, Swanson BJ, Singh S,
et al: Elevating pancreatic cystic lesion stratification: Current
and future pancreatic cancer biomarker(s). Biochim Biophys Acta Rev
Cancer. 1873:1883182020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bestari MB, Joewono IR and Syam AF: A
quest for survival: a review of the early biomarkers of pancreatic
cancer and the most effective approaches at present. Biomolecules
14: 364. (doi: 10.3390/biom14030364). 2024. View Article : Google Scholar
|
|
7
|
Yang J and Xu R: Early screening and
diagnosis strategies of pancreatic cancer: A comprehensive review.
Cancer Commun (Lond). 41:1257–1274. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jia Y, Chen Y, Wang Q, Jayasinghe U, Luo
X, Wei Q, Wang J, Xiong H, Chen C, Xu B, et al: Exosome: Emerging
biomarker in breast cancer. Oncotarget. 8:41717–41733. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Han QF, Li WJ, Hu KS, Gao J, Zhai WL, Yang
JH and Zhang SJ: Exosome biogenesis: Machinery, regulation, and
therapeutic implications in cancer. Mol Cancer. 21:2072022.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Costa-Silva B, Aiello NM, Ocean AJ, Singh
S, Zhang H, Thakur BK, Becker A, Hoshino A, Mark MT, Molina H, et
al: Pancreatic cancer exosomes initiate pre-metastatic niche
formation in the liver. Nat Cell Biol. 17:816–826. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Awadallah NS, Shroyer KR, Langer DA,
Torkko KC, Chen YK, Bentz JS, Papkoff J, Liu W, Nash SR and Shah
RJ: Detection of B7-H4 and p53 in pancreatic cancer: Potential role
as a cytological diagnostic adjunct. Pancreas. 36:200–206. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bausch D, Mino-Kenudson M, Fernández-Del
Castillo C, Warshaw AL, Kelly KA and Thayer SP: Plectin-1 is a
biomarker of malignant pancreatic intraductal papillary mucinous
neoplasms. J Gastrointest Surg. 13:1948–1954. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yu W, Ma Y, Shankar S and Srivastava RK:
Role of SATB2 in human pancreatic cancer: Implications in
transformation and a promising biomarker. Oncotarget.
7:57783–57797. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou CY, Dong YP, Sun X, Sui X, Zhu H,
Zhao YQ, Zhang YY, Mason C, Zhu Q and Han SX: High levels of serum
glypican-1 indicate poor prognosis in pancreatic ductal
adenocarcinoma. Cancer Med. 7:5525–5533. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Padden J, Ahrens M, Kälsch J, Bertram S,
Megger DA, Bracht T, Eisenacher M, Kocabayoglu P, Meyer HE, Sipos
B, et al: Immunohistochemical markers distinguishing
cholangiocellular carcinoma (CCC) from pancreatic ductal
adenocarcinoma (PDAC) discovered by proteomic analysis of
microdissected cells. Mol Cell Proteomics. 15:1072–1082. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Herreros-Villanueva M and Bujanda L:
Non-invasive biomarkers in pancreatic cancer diagnosis: What we
need versus what we have. Ann Transl Med. 4:1342016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Brezgyte G, Shah V, Jach D and
Crnogorac-Jurcevic T: Non-invasive biomarkers for earlier detection
of pancreatic Cancer-A comprehensive review. Cancers (Basel).
13:2021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zheng H, Liang X, Liu B, Huang X, Shen Y,
Lin F, Chen J, Gao X, He H, Li W, et al: Exosomal miR-9-5p derived
from iPSC-MSCs ameliorates doxorubicin-induced cardiomyopathy by
inhibiting cardiomyocyte senescence. J Nanobiotechnology.
22:1952024. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen YX and Cai Q: Plant Exosome-like
nanovesicles and their role in the innovative delivery of RNA
therapeutics. Biomedicines. 11:18062023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tamura R, Balabanova A, Frakes SA,
Bargmann A, Grimm J, Koch TH and Yin H: Photoactivatable prodrug of
doxazolidine targeting exosomes. J Med Chem. 62:1959–1970. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kreger BT, Johansen ER, Cerione RA and
Antonyak MA: The enrichment of survivin in exosomes from breast
cancer cells treated with paclitaxel promotes cell survival and
chemoresistance. Cancers. 8:1112016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Melo SA, Luecke LB, Kahlert C, Fernandez
AF, Gammon ST, Kaye J, LeBleu VS, Mittendorf EA, Weitz J, Rahbari
N, et al: Glypican-1 identifies cancer exosomes and detects early
pancreatic cancer. Nature. 523:177–182. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao X, Wu D, Ma X, Wang J, Hou W and
Zhang W: Exosomes as drug carriers for cancer therapy and
challenges regarding exosome uptake. Biomed Pharmacother.
128:1102372020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhou X, Yan Y, Shen Y, Xu M and Xu W:
Exosomes: Emerging insights into the progression of pancreatic
cancer. Int J Biol Sci. 20:4098–4113. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Qin C, Li T, Lin C, Zhao B, Li Z, Zhao Y
and Wang W: The systematic role of pancreatic cancer exosomes:
Distant communication, liquid biopsy and future therapy. Cancer
Cell Int. 24:2642024. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen Y, Kleeff J and Sunami Y: Pancreatic
cancer cell- and cancer-associated fibroblast-derived exosomes in
disease progression, metastasis, and therapy. Discov Oncol.
15:2532024. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhou Y, Feng J, Wang Q, Zhao Y, Ding H,
Jiang K, Ji H, Tang Z and Dai R: Knowledge mapping and research
trends of exosomes in pancreatic cancer: A bibliometric analysis
and review (2013–2023). Front Oncol. 14:13624362024. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Trifylli EM, Kriebardis AG, Koustas E,
Papadopoulos N, Fortis SP, Tzounakas VL, Anastasiadi AT, Sarantis
P, Vasileiadi S, Tsagarakis A, et al: A current synopsis of the
emerging role of extracellular vesicles and Micro-RNAs in
pancreatic cancer: A Forward-Looking plan for diagnosis and
treatment. Int J Mol Sci. 25:34062024. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sha G, Zhang W, Jiang Z, Zhao Q, Wang D
and Tang D: Exosomal non-coding RNA: A new frontier in diagnosing
and treating pancreatic cancer: A review. Int J Biol Macromol.
263:1301492024. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Papadakos SP, Dedes N, Pergaris A, Gazouli
M and Theocharis S: Exosomes in the Treatment of Pancreatic Cancer:
A moonshot to PDAC treatment? Int J Mol Sci. 23:36202022.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ariston Gabriel AN, Wang F, Jiao Q, Yvette
U, Yang X, Al-Ameri SA, Du L, Wang YS and Wang C: The involvement
of exosomes in the diagnosis and treatment of pancreatic cancer.
Mol Cancer. 19:1322020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fang X, Lan H, Jin K and Qian J:
Pancreatic cancer and exosomes: Role in progression, diagnosis,
monitoring, and treatment. Front Oncol. 13:11495512023. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bunduc S, Gede N, Váncsa S, Lillik V, Kiss
S, Juhász MF, Erőss B, Szakács Z, Gheorghe C, Mikó A and Hegyi P:
Exosomes as prognostic biomarkers in pancreatic ductal
adenocarcinoma-a systematic review and meta-analysis. Transl Res.
244:126–136. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xu B, Chen Y, Peng M, Zheng JH and Zuo C:
Exploring the potential of exosomes in diagnosis and drug delivery
for pancreatic ductal adenocarcinoma. Int J Cancer. 152:110–122.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hsu SK, Jadhao M, Liao WT, Chang WT, Lin
IL and Chiu CC: The role of exosomes in pancreatic ductal
adenocarcinoma progression and their potential as biomarkers.
Cancers (Basel). 15:17762023. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Han L, Zhao Z, Yang K, Xin M, Zhou L, Chen
S, Zhou S, Tang Z, Ji H and Dai R: Application of exosomes in the
diagnosis and treatment of pancreatic diseases. Stem Cell Res Ther.
13:1532022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Doyle LM and Wang MZ: Overview of
extracellular vesicles, their origin, composition, purpose, and
methods for exosome isolation and analysis. Cells. 8:7272019.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wortzel I, Dror S, Kenific CM and Lyden D:
Exosome-mediated metastasis: Communication from a distance. Dev
Cell. 49:347–360. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
He C, Zheng S, Luo Y and Wang B: Exosome
theranostics: Biology and translational medicine. Theranostics.
8:237–255. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Duan SL, Fu WJ, Jiang YK, Peng LS, Ousmane
D, Zhang ZJ and Wang JP: Emerging role of exosome-derived
non-coding RNAs in tumor-associated angiogenesis of tumor
microenvironment. Front Mol Biosci. 10:12201932023. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kalluri R and LeBleu VS: The biology,
function, and biomedical applications of exosomes. Science.
367:eaau69772020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Pegtel DM and Gould SJ: Exosomes. Annu Rev
Biochem. 88:487–514. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Dai X, Ye Y and He F: Emerging innovations
on exosome-based onco-therapeutics. Front Immunol. 13:8652452022.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Skotland T, Hessvik NP, Sandvig K and
Llorente A: Exosomal lipid composition and the role of ether lipids
and phosphoinositides in exosome biology. J Lipid Res. 60:9–18.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Mashouri L, Yousefi H, Aref AR, Ahadi AM,
Molaei F and Alahari SK: Exosomes: Composition, biogenesis, and
mechanisms in cancer metastasis and drug resistance. Mol Cancer.
18:752019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
He C, Li L, Wang L, Meng W, Hao Y and Zhu
G: Exosome-mediated cellular crosstalk within the tumor
microenvironment upon irradiation. Cancer Biol Med. 18:21–33. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Peng H, Ji W, Zhao R, Yang J, Lu Z, Li Y
and Zhang X: Exosome: A significant nano-scale drug delivery
carrier. J Mater Chem B. 8:7591–7608. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Im H, Shao H, Weissleder R, Castro CM and
Lee H: Nano-plasmonic exosome diagnostics. Expert Rev Mol Diagn.
15:725–733. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hamzah RN, Alghazali KM, Biris AS and
Griffin RJ: Exosome traceability and cell source dependence on
composition and cell-cell cross talk. Int J Mol Sci. 22:53462021.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kawamura S, Iinuma H, Wada K, Takahashi K,
Minezaki S, Kainuma M, Shibuya M, Miura F and Sano K:
Exosome-encapsulated microRNA-4525, microRNA-451a and microRNA-21
in portal vein blood is a high-sensitive liquid biomarker for the
selection of high-risk pancreatic ductal adenocarcinoma patients. J
Hepatobiliary Pancreat Sci. 26:63–72. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Xu L, Wu LF and Deng FY: Exosome: An
emerging source of biomarkers for human diseases. Curr Mol Med.
19:387–394. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lobb RJ, Becker M, Wen SW, Wong CS,
Wiegmans AP, Leimgruber A and Möller A: Optimized exosome isolation
protocol for cell culture supernatant and human plasma. J Extracell
Vesicles. 4:270312015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Tang YT, Huang YY, Zheng L, Qin SH, Xu XP,
An TX, Xu Y, Wu YS, Hu XM, Ping BH and Wang Q: Comparison of
isolation methods of exosomes and exosomal RNA from cell culture
medium and serum. Int J Mol Med. 40:834–844. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Vaswani K, Mitchell MD, Holland OJ, Qin
Koh Y, Hill RJ, Harb T, Davies PSW and Peiris H: A Method for the
isolation of exosomes from human and bovine milk. J Nutr Metab.
2019:57647402019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Tayebi M, Zhou Y, Tripathi P,
Chandramohanadas R and Ai Y: Exosome purification and analysis
using a facile microfluidic hydrodynamic trapping device. Anal
Chem. 92:10733–10742. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Gonzalez H, Hagerling C and Werb Z: Roles
of the immune system in cancer: From tumor initiation to metastatic
progression. Genes Dev. 32:1267–1284. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
He Z, Wang J, Zhu C, Xu J, Chen P, Jiang
X, Chen Y, Jiang J and Sun C: Exosome-derived FGD5-AS1 promotes
tumor-associated macrophage M2 polarization-mediated pancreatic
cancer cell proliferation and metastasis. Cancer Lett.
548:2157512022. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zhang Y, Liu Y, Liu H and Tang WH:
Exosomes: Biogenesis, biologic function and clinical potential.
Cell Biosci. 9:192019. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Wang S, Zheng Y, Yang F, Zhu L, Zhu XQ,
Wang ZF, Wu XL, Zhou CH, Yan JY, Hu BY, et al: The molecular
biology of pancreatic adenocarcinoma: Translational challenges and
clinical perspectives. Signal Transduct Target Ther. 6:2492021.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Kumari N and Choi SH: Tumor-associated
macrophages in cancer: Recent advancements in cancer
nanoimmunotherapies. J Exp Clin Cancer Res. 41:682022. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Qian Y, Yin Y, Zheng X, Liu Z and Wang X:
Metabolic regulation of tumor-associated macrophage heterogeneity:
Insights into the tumor microenvironment and immunotherapeutic
opportunities. Biomark Res. 12:12024. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Mao X, Xu J, Wang W, Liang C, Hua J, Liu
J, Zhang B, Meng Q, Yu X and Shi S: Crosstalk between
cancer-associated fibroblasts and immune cells in the tumor
microenvironment: New findings and future perspectives. Mol Cancer.
20:1312021. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Pritchard A, Tousif S, Wang Y, Hough K,
Khan S, Strenkowski J, Chacko BK, Darley-Usmar VM and Deshane JS:
Lung tumor cell-derived exosomes promote M2 macrophage
polarization. Cells. 9:13032020. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Pan Y, Tang H, Li Q, Chen G and Li D:
Exosomes and their roles in the chemoresistance of pancreatic
cancer. Cancer Med. 11:4979–4988. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Papadakos SP, Machairas N, Stergiou IE,
Arvanitakis K, Germanidis G, Frampton AE and Theocharis S:
Unveiling the Yin-Yang balance of M1 and M2 macrophages in
hepatocellular carcinoma: Role of exosomes in tumor
microenvironment and immune modulation. Cells. 12:20362023.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Wang L, Li Z, Shen J, Liu Z, Liang J, Wu
X, Sun X and Wu Z: Exosome-like vesicles derived by Schistosoma
japonicum adult worms mediates M1 type immune-activity of
macrophage. Parasitol Res. 114:1865–1873. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Chehelgerdi M, Chehelgerdi M, Allela OQB,
Pecho RDC, Jayasankar N, Rao DP, Thamaraikani T, Vasanthan M,
Viktor P, Lakshmaiya N, et al: Progressing nanotechnology to
improve targeted cancer treatment: Overcoming hurdles in its
clinical implementation. Mol Cancer. 22:1692023. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Cui X, Fu Q, Wang X, Xia P, Cui X, Bai X
and Lu Z: Molecular mechanisms and clinical applications of
exosomes in prostate cancer. Biomark Res. 10:562022. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Zhang J, Li S, Li L, Guo C, Yao J and Mi
S: Exosome and exosomal microRNA: Trafficking, sorting, and
function. Genomics Proteomics Bioinformatics. 13:17–24. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Milane L, Singh A, Mattheolabakis G,
Suresh M and Amiji MM: Exosome mediated communication within the
tumor microenvironment. J Control Release. 219:278–294. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Khan MI and Alsayed R: Exosome-mediated
response to cancer therapy: Modulation of Epigenetic Machinery. Int
J Mol Sci. 23:62222022. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
McAndrews KM, Xiao F, Chronopoulos A,
LeBleu VS, Kugeratski FG and Kalluri R: Exosome-mediated delivery
of CRISPR/Cas9 for targeting of oncogenic Kras(G12D) in pancreatic
cancer. Life Sci Alliance. 4:e2020008752021. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Su MJ, Aldawsari H and Amiji M: Pancreatic
cancer cell exosome-mediated macrophage reprogramming and the role
of MicroRNAs 155 and 125b2 transfection using nanoparticle delivery
systems. Sci Rep. 6:301102016. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Caruso Bavisotto C, Cappello F, Macario
AJL, Conway de Macario E, Logozzi M, Fais S and Campanella C:
Exosomal HSP60: A potentially useful biomarker for diagnosis,
assessing prognosis, and monitoring response to treatment. Expert
Rev Mol Diagn. 17:815–822. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Dudeja V, Mujumdar N, Phillips P, Chugh R,
Borja-Cacho D, Dawra RK, Vickers SM and Saluja AK: Heat shock
protein 70 inhibits apoptosis in cancer cells through simultaneous
and independent mechanisms. Gastroenterology. 136:1772–1782. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Shaashua L, Ben-Shmuel A, Pevsner-Fischer
M, Friedman G, Levi-Galibov O, Nandakumar S, Barki D, Nevo R, Brown
LE, Zhang W, et al: BRCA mutational status shapes the stromal
microenvironment of pancreatic cancer linking clusterin expression
in cancer associated fibroblasts with HSF1 signaling. Nat Commun.
13:65132022. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Jin H, Liu P, Wu Y, Meng X, Wu M, Han J
and Tan X: Exosomal zinc transporter ZIP4 promotes cancer growth
and is a novel diagnostic biomarker for pancreatic cancer. Cancer
Sci. 109:2946–2956. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Castillo J, Bernard V, San Lucas FA,
Allenson K, Capello M, Kim DU, Gascoyne P, Mulu FC, Stephens BM,
Huang J, et al: Surfaceome profiling enables isolation of
cancer-specific exosomal cargo in liquid biopsies from pancreatic
cancer patients. Ann Oncol. 29:223–229. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Zhang R, Loganathan S, Humphreys I and
Srivastava SK: Benzyl isothiocyanate-induced DNA damage causes G2/M
cell cycle arrest and apoptosis in human pancreatic cancer cells. J
Nutr. 136:2728–2734. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Ristorcelli E, Beraud E, Mathieu S,
Lombardo D and Verine A: Essential role of Notch signaling in
apoptosis of human pancreatic tumoral cells mediated by exosomal
nanoparticles. Int J Cancer. 125:1016–1026. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Huang J, Chen P, Liu K, Liu J, Zhou B, Wu
R, Peng Q, Liu ZX, Li C, Kroemer G, et al: CDK1/2/5 inhibition
overcomes IFNG-mediated adaptive immune resistance in pancreatic
cancer. Gut. 70:890–899. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Hayes TK, Neel NF, Hu C, Gautam P, Chenard
M, Long B, Aziz M, Kassner M, Bryant KL, Pierobon M, et al:
Long-term ERK inhibition in KRAS-mutant pancreatic cancer is
associated with MYC degradation and Senescence-like growth
suppression. Cancer Cell. 29:75–89. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Li B, Cao Y, Sun M and Feng H: Expression,
regulation, and function of exosome-derived miRNAs in cancer
progression and therapy. FASEB J. 35:e219162021. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Sun W, Ren Y, Lu Z and Zhao X: The
potential roles of exosomes in pancreatic cancer initiation and
metastasis. Mol Cancer. 19:1352020. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Zhou B, Xu JW, Cheng YG, Gao JY, Hu SY,
Wang L and Zhan HX: Early detection of pancreatic cancer: Where are
we now and where are we going? Int J Cancer. 141:231–241. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Zhao C, Gao F, Weng S and Liu Q:
Pancreatic cancer and associated exosomes. Cancer Biomark.
20:357–367. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Yan Y, Fu G and Ming L: Role of exosomes
in pancreatic cancer. Oncol Lett. 15:7479–7488. 2018.PubMed/NCBI
|
|
88
|
Wang X, Luo G, Zhang K, Cao J, Huang C,
Jiang T, Liu B, Su L and Qiu Z: Hypoxic tumor-derived exosomal
miR-301a mediates M2 macrophage polarization via PTEN/PI3Kγ to
promote pancreatic cancer metastasis. Cancer Res. 78:4586–4598.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Chiba M, Kubota S, Sato K and Monzen S:
Exosomes released from pancreatic cancer cells enhance angiogenic
activities via dynamin-dependent endocytosis in endothelial cells
in vitro. Sci Rep. 8:119722018. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Li J, Li Z, Jiang P, Peng M, Zhang X, Chen
K, Liu H, Bi H, Liu X and Li X: Circular RNA IARS (circ-IARS)
secreted by pancreatic cancer cells and located within exosomes
regulates endothelial monolayer permeability to promote tumor
metastasis. J Exp Clin Cancer Res. 37:1772018. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Zhou X, Zhong F, Yan Y, Wu S, Wang H, Liu
J, Li F, Cui D and Xu M: Pancreatic cancer cell-derived exosomes
promote lymphangiogenesis by downregulating ABHD11-AS1 expression.
Cancers (Basel). 14:46122022. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Li M, Zhou J, Zhang Z, Li J, Wang F, Ma L,
Tian X, Mao Z and Yang Y: Exosomal miR-485-3p derived from
pancreatic ductal epithelial cells inhibits pancreatic cancer
metastasis through targeting PAK1. Chin Med J. 135:2326–2337. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Di Pace AL, Pelosi A, Fiore PF, Tumino N,
Besi F, Quatrini L, Santopolo S, Vacca P and Moretta L: MicroRNA
analysis of Natural Killer cell-derived exosomes: The microRNA
let-7b-5p is enriched in exosomes and participates in their
anti-tumor effects against pancreatic cancer cells. Oncoimmunology.
12:22210812023. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Sun H, Shi K, Qi K, Kong H, Zhang J, Dai
S, Ye W, Deng T, He Q and Zhou M: Natural killer cell-derived
exosomal miR-3607-3p inhibits pancreatic cancer progression by
targeting IL-26. Front Immunol. 10:28192019. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Yin Z, Zhou Y, Ma T, Chen S, Shi N, Zou Y,
Hou B and Zhang C: Down-regulated lncRNA SBF2-AS1 in M2
macrophage-derived exosomes elevates miR-122-5p to restrict XIAP,
thereby limiting pancreatic cancer development. J Cell Mol Med.
24:5028–5038. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Xie X, Ji J, Chen X, Xu W, Chen H, Zhu S,
Wu J, Wu Y, Sun Y, Sai W, et al: Human umbilical cord mesenchymal
stem cell-derived exosomes carrying hsa-miRNA-128-3p suppress
pancreatic ductal cell carcinoma by inhibiting Galectin-3. Clin
Transl Oncol. 24:517–531. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Torphy RJ, Fujiwara Y and Schulick RD:
Pancreatic cancer treatment: Better, but a long way to go. Surg
Today. 50:1117–1125. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Zhao Z and Liu W: Pancreatic cancer: A
review of risk factors, diagnosis, and treatment. Technol Cancer
Res Treat. 19:15330338209621172020. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Cai J, Chen H, Lu M, Zhang Y, Lu B, You L,
Zhang T, Dai M and Zhao Y: Advances in the epidemiology of
pancreatic cancer: Trends, risk factors, screening, and prognosis.
Cancer Lett. 520:1–11. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Loveday BPT, Lipton L and Thomson BN:
Pancreatic cancer: An update on diagnosis and management. Aust J
Gen Pract. 48:826–831. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Scarà S, Bottoni P and Scatena R: CA 19-9:
Biochemical and clinical aspects. Adv Exp Med Biol. 867:247–260.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Nakamura K, Zhu Z, Roy S, Jun E, Han H,
Munoz RM, Nishiwada S, Sharma G, Cridebring D, Zenhausern F, et al:
An Exosome-based transcriptomic signature for noninvasive, early
detection of patients with pancreatic ductal adenocarcinoma: A
multicenter cohort study. Gastroenterology. 163:1252–1266.e2. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Tempero MA, Malafa MP, Al-Hawary M,
Behrman SW, Benson AB, Cardin DB, Chiorean EG, Chung V, Czito B,
Del Chiaro M, et al: Pancreatic adenocarcinoma, version 2.2021,
NCCN clinical practice guidelines in oncology. J Natl Compr Canc
Netw. 19:439–457. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Vincent A, Herman J, Schulick R, Hruban RH
and Goggins M: Pancreatic cancer. Lancet. 378:607–620. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Hu ZI and O'Reilly EM: Therapeutic
developments in pancreatic cancer. Nat Rev Gastroenterol Hepatol.
21:7–24. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Lupo F, Pezzini F, Pasini D, Fiorini E,
Adamo A, Veghini L, Bevere M, Frusteri C, Delfino P, D'agosto S, et
al: Axon guidance cue SEMA3A promotes the aggressive phenotype of
basal-like PDAC. Gut. 73:1321–1335. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Reese M and Dhayat SA: Small extracellular
vesicle non-coding RNAs in pancreatic cancer: molecular mechanisms
and clinical implications. J Hematol Oncol. 14:1412021. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Ran Z, Wu S, Ma Z, Chen X, Liu J and Yang
J: Advances in exosome biomarkers for cervical cancer. Cancer Med.
11:4966–4978. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Yuan Y, Li H, Pu W, Chen L, Guo D, Jiang
H, He B, Qin S, Wang K, Li N, et al: Cancer metabolism and tumor
microenvironment: Fostering each other? Sci China Life Sci.
65:236–279. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Wu L, Zhou WB, Zhou J, Wei Y, Wang HM, Liu
XD, Chen XC, Wang W, Ye L, Yao LC, et al: Circulating exosomal
microRNAs as novel potential detection biomarkers in pancreatic
cancer. Oncol Lett. 20:1432–1440. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Condrat CE, Thompson DC, Barbu MG, Bugnar
OL, Boboc A, Cretoiu D, Suciu N, Cretoiu SM and Voinea SC: miRNAs
as biomarkers in disease: Latest findings regarding their role in
diagnosis and prognosis. Cells. 9:2762020. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Ouyang H, Gore J, Deitz S and Korc M:
microRNA-10b enhances pancreatic cancer cell invasion by
suppressing TIP30 expression and promoting EGF and TGF-β actions.
Oncogene. 33:4664–4674. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Yu D, Li Y, Wang M, Gu J, Xu W, Cai H,
Fang X and Zhang X: Exosomes as a new frontier of cancer liquid
biopsy. Mol Cancer. 21:562022. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
He C, Li L, Wang L, Meng W, Hao Y and Zhu
G: Exosome-mediated cellular crosstalk within the tumor
microenvironment upon irradiation. Cancer Biol Med. 18:21–33. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Jiang Z, Wang H, Mou Y, Li L and Jin W:
Functions and clinical applications of exosomes in pancreatic
cancer. Mol Biol Rep. 49:11037–11048. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Ashrafizadeh M, Rabiee N, Kumar AP, Sethi
G, Zarrabi A and Wang Y: Long noncoding RNAs (lncRNAs) in
pancreatic cancer progression. Drug Discov Today. 27:2181–2198.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Kato S and Honda K: Use of biomarkers and
imaging for early detection of pancreatic cancer. Cancers (Basel).
12:19652020. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Zhao X, Ren Y and Lu Z: Potential
diagnostic and therapeutic roles of exosomes in pancreatic cancer.
Biochim Biophys Acta Rev Cancer. 1874:1884142020. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Frampton AE, Prado MM, López-Jiménez E,
Fajardo-Puerta AB, Jawad ZAR, Lawton P, Giovannetti E, Habib NA,
Castellano L, Stebbing J, et al: Glypican-1 is enriched in
circulating-exosomes in pancreatic cancer and correlates with tumor
burden. Oncotarget. 9:19006–19013. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Marin AM, Mattar SB, Amatuzzi RF, Chammas
R, Uno M, Zanette DL and Aoki MN: Plasma exosome-derived microRNAs
as potential diagnostic and prognostic biomarkers in brazilian
pancreatic cancer patients. Biomolecules. 12:7692022. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Wang L, Wu J, Ye N, Li F, Zhan H, Chen S
and Xu J: Plasma-derived exosome MiR-19b acts as a diagnostic
marker for pancreatic cancer. Front Oncol. 11:7391112021.
View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Nakamura S, Sadakari Y, Ohtsuka T, Okayama
T, Nakashima Y, Gotoh Y, Saeki K, Mori Y, Nakata K, Miyasaka Y, et
al: Pancreatic Juice exosomal MicroRNAs as biomarkers for detection
of pancreatic ductal adenocarcinoma. Ann Surg Oncol. 26:2104–2111.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Chen SL, Ma M, Yan L, Xiong SH, Liu Z, Li
S, Liu T, Shang S, Zhang YY, Zeng H, et al: Clinical significance
of exosomal miR-1231 in pancreatic cancer]. Zhonghua Zhong Liu Za
Zhi. 41:46–49. 2019.(In Chinese). PubMed/NCBI
|
|
124
|
Corrigendum to Serum exosomal miR-451a
acts as a candidate marker for pancreatic cancer. Int J Biol
Markers. 37:2242022. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Yu S, Li Y, Liao Z, Wang Z, Wang Z, Li Y,
Qian L, Zhao J, Zong H, Kang B, et al: Plasma extracellular vesicle
long RNA profiling identifies a diagnostic signature for the
detection of pancreatic ductal adenocarcinoma. Gut. 69:540–550.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Hong L, Xu L, Jin L, Xu K, Tang W, Zhu Y,
Qiu X and Wang J: Exosomal circular RNA hsa_circ_0006220, and
hsa_circ_0001666 as biomarkers in the diagnosis of pancreatic
cancer. J Clin Lab Anal. 36:e244472022. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Yadav DK, Bai X, Yadav RK, Singh A, Li G,
Ma T, Chen W and Liang T: Liquid biopsy in pancreatic cancer: The
beginning of a new era. Oncotarget. 9:26900–26933. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Allenson K, Castillo J, San Lucas FA,
Scelo G, Kim DU, Bernard V, Davis G, Kumar T, Katz M, Overman MJ,
et al: High prevalence of mutant KRAS in circulating
exosome-derived DNA from early-stage pancreatic cancer patients.
Ann Oncol. 28:741–747. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Mizukami K, Iwasaki Y, Kawakami E, Hirata
M, Kamatani Y, Matsuda K, Endo M, Sugano K, Yoshida T, Murakami Y,
et al: Genetic characterization of pancreatic cancer patients and
prediction of carrier status of germline pathogenic variants in
cancer-predisposing genes. EBioMedicine. 60:1030332020. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Lai E, Ziranu P, Spanu D, Dubois M, Pretta
A, Tolu S, Camera S, Liscia N, Mariani S, Persano M, et al:
BRCA-mutant pancreatic ductal adenocarcinoma. Br J Cancer.
125:1321–1332. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Moutinho-Ribeiro P, Adem B, Batista I,
Silva M, Silva S, Ruivo CF, Morais R, Peixoto A, Coelho R,
Costa-Moreira P, et al: Exosomal glypican-1 discriminates
pancreatic ductal adenocarcinoma from chronic pancreatitis. Dig
Liver Dis. 54:871–877. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Yoshizawa N, Sugimoto K, Tameda M, Inagaki
Y, Ikejiri M, Inoue H, Usui M, Ito M and Takei Y:
miR-3940-5p/miR-8069 ratio in urine exosomes is a novel diagnostic
biomarker for pancreatic ductal adenocarcinoma. Oncol Lett.
19:2677–2684. 2020.PubMed/NCBI
|
|
133
|
Xie Z, Gao Y, Ho C, Li L, Jin C, Wang X,
Zou C, Mao Y, Wang X, Li Q, et al: Exosome-delivered CD44v6/C1QBP
complex drives pancreatic cancer liver metastasis by promoting
fibrotic liver microenvironment. Gut. 71:568–579. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Ishihara M, Kageyama S, Miyahara Y,
Ishikawa T, Ueda S, Soga N, Naota H, Mukai K, Harada N, Ikeda H and
Shiku H: MAGE-A4, NY-ESO-1 and SAGE mRNA expression rates and
co-expression relationships in solid tumours. BMC Cancer.
20:6062020. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Hashimoto K, Nishimura S, Ito T and Akagi
M: Clinicopathological assessment of cancer/testis antigens
NY-ESO-1 and MAGE-A4 in highly aggressive soft tissue sarcomas.
Diagnostics (Basel). 12:7332022. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Wu H, Chen X, Ji J, Zhou R, Liu J, Ni W,
Qu L, Ni H, Ni R, Bao B and Xiao M: Progress of exosomes in the
diagnosis and treatment of pancreatic cancer. Gene Test Mol
Biomarkers. 23:215–222. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Xu M, Hu W, Liu Z, Xia J, Chen S, Wang PG
and Yang S: Glycoproteomic bioanalysis of exosomes by LC-MS for
early diagnosis of pancreatic cancer. Bioanalysis. 13:861–864.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Zhao Y, Tang J, Jiang K, Liu SY, Aicher A
and Heeschen C: Liquid biopsy in pancreatic cancer-Current
perspective and future outlook. Biochim Biophys Acta Rev Cancer.
1878:1888682023. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Raufi AG, May MS, Hadfield MJ, Seyhan AA
and El-Deiry WS: Advances in Liquid Biopsy Technology and
Implications for Pancreatic Cancer. Int J Mol Sci. 24:42382023.
View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Haeberle L, Schramm M, Goering W, Frohn L,
Driescher C, Hartwig W, Preissinger-Heinzel HK, Beyna T, Neuhaus H,
Fuchs K, et al: Molecular analysis of cyst fluids improves the
diagnostic accuracy of pre-operative assessment of pancreatic
cystic lesions. Sci Rep. 11:29012021. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Karunakaran M and Barreto SG: Surgery for
pancreatic cancer: Current controversies and challenges. Future
Oncol. 17:5135–5162. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Strobel O and Neoptolemos J: Optimizing
the outcomes of pancreatic cancer surgery. Nat Rev Clin Oncol.
16:11–26. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Von Hoff DD, Ervin T, Arena FP, Chiorean
EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, et
al: Increased survival in pancreatic cancer with nab-paclitaxel
plus gemcitabine. N Engl J Med. 369:1691–1703. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Conroy T, Desseigne F, Ychou M, Bouché O,
Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de
la Fouchardière C, et al: FOLFIRINOX versus gemcitabine for
metastatic pancreatic cancer. N Engl J Med. 364:1817–1825. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Yoshida K, Iwashita T, Uemura S, Maruta A,
Okuno M, Ando N, Iwata K, Kawaguchi J, Mukai T and Shimizu M: A
multicenter prospective phase II study of first-line modified
FOLFIRINOX for unresectable advanced pancreatic cancer. Oncotarget.
8:111346–111355. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Pajewska M, Partyka O, Czerw A, Deptała A,
Cipora E, Gąska I, Wojtaszek M, Sygit K, Sygit M, Krzych-Fałta E,
et al: Management of metastatic pancreatic Cancer-comparison of
global guidelines over the last 5 years. Cancers (Basel).
15:44002023. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Strickler JH, Satake H, George TJ, Yaeger
R, Hollebecque A, Garrido-Laguna I, Schuler M, Burns TF, Coveler
AL, Falchook GS, et al: Sotorasib in KRAS p.G12C-mutated advanced
pancreatic cancer. N Engl J Med. 388:33–43. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Golan T, Hammel P, Reni M, Van Cutsem E,
Macarulla T, Hall MJ, Park JO, Hochhauser D, Arnold D, Oh DY, et
al: Maintenance Olaparib for Germline BRCA-mutated metastatic
pancreatic cancer. N Engl J Med. 381:317–327. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Ijichi H, Chytil A, Gorska AE, Aakre ME,
Fujitani Y, Fujitani S, Wright CV and Moses HL: Aggressive
pancreatic ductal adenocarcinoma in mice caused by
pancreas-specific blockade of transforming growth factor-beta
signaling in cooperation with active Kras expression. Genes Dev.
20:3147–3160. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Wang Z, Ali S, Banerjee S, Bao B, Li Y,
Azmi AS, Korc M and Sarkar FH: Activated K-Ras and INK4a/Arf
deficiency promote aggressiveness of pancreatic cancer by induction
of EMT consistent with cancer stem cell phenotype. J Cell Physiol.
228:556–562. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Pirlog R and Calin GA: KRAS mutations as
essential promoters of lymphangiogenesis via extracellular vesicles
in pancreatic cancer. J Clin Invest. 132:e1614542022. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Chang WH, Nguyen TT, Hsu CH, Bryant KL,
Kim HJ, Ying H, Erickson JW, Der CJ, Cerione RA and Antonyak MA:
KRAS-dependent cancer cells promote survival by producing exosomes
enriched in Survivin. Cancer Lett. 517:66–77. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Wang-Gillam A, Li CP, Bodoky G, Dean A,
Shan YS, Jameson G, Macarulla T, Lee KH, Cunningham D, Blanc JF, et
al: Nanoliposomal irinotecan with fluorouracil and folinic acid in
metastatic pancreatic cancer after previous gemcitabine-based
therapy (NAPOLI-1): A global, randomised, open-label, phase 3
trial. Lancet. 387:545–557. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Ahmad SA, Duong M, Sohal DPS, Gandhi NS,
Beg MS, Wang-Gillam A, Wade JL III, Chiorean EG, Guthrie KA, Lowy
AM, et al: Surgical outcome results from SWOG S1505: A randomized
clinical trial of mFOLFIRINOX versus Gemcitabine/Nab-paclitaxel for
perioperative treatment of resectable pancreatic ductal
adenocarcinoma. Ann Surg. 272:481–486. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Kunzmann V, Siveke JT, Algül H, Goekkurt
E, Siegler G, Martens U, Waldschmidt D, Pelzer U, Fuchs M, Kullmann
F, et al: Nab-paclitaxel plus gemcitabine versus nab-paclitaxel
plus gemcitabine followed by FOLFIRINOX induction chemotherapy in
locally advanced pancreatic cancer (NEOLAP-AIO-PAK-0113): A
multicentre, randomised, phase 2 trial. Lancet Gastroenterol
Hepatol. 6:128–138. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Pitt JM, André F, Amigorena S, Soria JC,
Eggermont A, Kroemer G and Zitvogel L: Dendritic cell-derived
exosomes for cancer therapy. J Clin Invest. 126:1224–1232. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Veerman RE, Güçlüler Akpinar G, Eldh M and
Gabrielsson S: Immune Cell-derived extracellular vesicles-functions
and therapeutic applications. Trends Mol Med. 25:382–394. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Zhao Y, Liu T and Zhou M:
Immune-cell-derived exosomes for cancer therapy. Mol Pharm.
19:3042–3056. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Zhao LX, Zhang K, Shen BB and Li JN:
Mesenchymal stem cell-derived exosomes for gastrointestinal cancer.
World J Gastrointest Oncol. 13:1981–1996. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Zhou Y, Zhou W, Chen X, Wang Q, Li C, Chen
Q, Zhang Y, Lu Y, Ding X and Jiang C: Bone marrow mesenchymal stem
cells-derived exosomes for penetrating and targeted chemotherapy of
pancreatic cancer. Acta Pharma Sin B. 10:1563–1575. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Zhang L, Xiang J, Zhang F, Liu L and Hu C:
MSCs can be a double-edged sword in tumorigenesis. Front Oncol.
12:10479072022. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Whiteside TL: Exosome and mesenchymal stem
cell cross-talk in the tumor microenvironment. Semin Immunol.
35:69–79. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Ha DH, Kim HK, Lee J, Kwon HH, Park GH,
Yang SH, Jung JY, Choi H, Lee JH, Sung S, et al: Mesenchymal
Stem/stromal Cell-derived exosomes for immunomodulatory
therapeutics and skin regeneration. Cells. 9:11572020. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Fang S, Xu C, Zhang Y, Xue C, Yang C, Bi
H, Qian X, Wu M, Ji K, Zhao Y, et al: Umbilical Cord-derived
mesenchymal stem cell-derived exosomal MicroRNAs suppress
myofibroblast differentiation by inhibiting the transforming growth
factor-β/SMAD2 pathway during wound healing. Stem Cells Transl Med.
5:1425–1439. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Zhang Y, Hao Z, Wang P, Xia Y, Wu J, Xia
D, Fang S and Xu S: Exosomes from human umbilical cord mesenchymal
stem cells enhance fracture healing through HIF-1α-mediated
promotion of angiogenesis in a rat model of stabilized fracture.
Cell Prolif. 52:e125702019. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Eldaly AS, Mashaly SM, Fouda E, Emam OS,
Aglan A, Abuasbeh J, Khurana A, Hamdar H and Fath AR: Systemic
anti-inflammatory effects of mesenchymal stem cells in burn: A
systematic review of animal studies. J Clin Transl Res. 8:276–291.
2022.PubMed/NCBI
|
|
167
|
Xu X, Yin F, Guo M, Gan G, Lin G, Wen C,
Wang J, Song P, Wang J, Qi ZQ and Zhong CQ: Quantitative proteomic
analysis of exosomes from umbilical cord mesenchymal stem cells and
rat bone marrow stem cells. Proteomics. 23:e22002042023. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Liu M, Yang Y, Zhao B, Yang Y, Wang J,
Shen K, Yang X, Hu D, Zheng G and Han J: Exosomes derived from
Adipose-derived mesenchymal stem cells ameliorate Radiation-induced
brain injury by activating the SIRT1 pathway. Front Cell Dev Biol.
9:6937822021. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Shenoda BB and Ajit SK: Modulation of
immune responses by exosomes derived from Antigen-presenting cells.
Clin Med Insights Pathol. 9 (Suppl 1):S1–S8. 2016.PubMed/NCBI
|
|
170
|
Zaidi N, Soban M, Chen F, Kinkead H,
Mathew J, Yarchoan M, Armstrong TD, Haider S and Jaffee EM: Role of
in silico structural modeling in predicting immunogenic neoepitopes
for cancer vaccine development. JCI Insight. 5:e1369912020.
View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Yadav SS and Narayan G: Role of ROBO4
signalling in developmental and pathological angiogenesis. Biomed
Res Int. 2014:6830252014. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Xu Y, Li WL, Fu L, Gu F and Ma YJ:
Slit2/Robo1 signaling in glioma migration and invasion. Neurosci
Bull. 26:474–478. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Wang L, Yang L, Zhuang T and Shi X:
Tumor-derived exosomal miR-29b reduces angiogenesis in pancreatic
cancer by silencing ROBO1 and SRGAP2. J Immunol Res.
2022:47693852022. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Sun X, Zhang Y, Li B and Yang H: MTA1
promotes the invasion and migration of pancreatic cancer cells
potentially through the HIF-α/VEGF pathway. J Recept Signal
Transduct Res. 38:352–358. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Lee JK, Park SR, Jung BK, Jeon YK, Lee YS,
Kim MK, Kim YG, Jang JY and Kim CW: Exosomes derived from
mesenchymal stem cells suppress angiogenesis by down-regulating
VEGF expression in breast cancer cells. PLoS One. 8:e842562013.
View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Pakravan K, Babashah S, Sadeghizadeh M,
Mowla SJ, Mossahebi-Mohammadi M, Ataei F, Dana N and Javan M:
MicroRNA-100 shuttled by mesenchymal stem cell-derived exosomes
suppresses in vitro angiogenesis through modulating the
mTOR/HIF-1α/VEGF signaling axis in breast cancer cells. Cell Oncol
(Dordr). 40:457–470. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Yang E, Wang X, Gong Z, Yu M, Wu H and
Zhang D: Exosome-mediated metabolic reprogramming: The emerging
role in tumor microenvironment remodeling and its influence on
cancer progression. Signal Transduct Target Ther. 5:2422020.
View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Ye M, Huang X, Wu Q and Liu F: Senescent
stromal cells in the tumor microenvironment: Victims or
accomplices? Cancers (Basel). 15:19272023. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
Zhao Y, Shen M, Wu L, Yang H, Yao Y, Yang
Q, Du J, Liu L, Li Y and Bai Y: Stromal cells in the tumor
microenvironment: Accomplices of tumor progression? Cell Death Dis.
14:5872023. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Dai X, Xie Y and Dong M: Cancer-associated
fibroblasts derived extracellular vesicles promote angiogenesis of
colorectal adenocarcinoma cells through miR-135b-5p/FOXO1 axis.
Cancer Biol Ther. 23:76–88. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Qin Y, Liu X, Pan L, Zhou R and Zhang X:
Long noncoding RNA MIR155HG facilitates pancreatic cancer
progression through negative regulation of miR-802. J Cell Biochem.
120:17926–17934. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Ren Y, Jia HH, Xu YQ, Zhou X, Zhao XH,
Wang YF, Song X, Zhu ZY, Sun T, Dou Y, et al: Paracrine and
epigenetic control of CAF-induced metastasis: The role of HOTAIR
stimulated by TGF-ß1 secretion. Mol Cancer. 17:52018. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Sansone P, Savini C, Kurelac I, Chang Q,
Amato LB, Strillacci A, Stepanova A, Iommarini L, Mastroleo C, Daly
L, et al: Packaging and transfer of mitochondrial DNA via exosomes
regulate escape from dormancy in hormonal therapy-resistant breast
cancer. Proc Natl Acad Sci USA. 114:E9066–E9075. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Achreja A, Zhao H, Yang L, Yun TH, Marini
J and Nagrath D: Exo-MFA-A 13C metabolic flux analysis framework to
dissect tumor microenvironment-secreted exosome contributions
towards cancer cell metabolism. Metab Eng. 43:156–172. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Boelens MC, Wu TJ, Nabet BY, Xu B, Qiu Y,
Yoon T, Azzam DJ, Twyman-Saint Victor C, Wiemann BZ, Ishwaran H, et
al: Exosome transfer from stromal to breast cancer cells regulates
therapy resistance pathways. Cell. 159:499–513. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Duong MN, Geneste A, Fallone F, Li X,
Dumontet C and Muller C: The fat and the bad: Mature adipocytes,
key actors in tumor progression and resistance. Oncotarget.
8:57622–57641. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Frisbie L, Buckanovich RJ and Coffman L:
Carcinoma-associated mesenchymal stem/stromal cells: Architects of
the pro-tumorigenic tumor microenvironment. Stem Cells. 40:705–715.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Habanjar O, Diab-Assaf M, Caldefie-Chezet
F and Delort L: The impact of obesity, adipose tissue, and tumor
microenvironment on macrophage polarization and metastasis. Biology
(Basel). 11:3392022.PubMed/NCBI
|
|
189
|
Li B, Liu S, Yang Q, Li Z, Li J, Wu J, Sun
S, Xu Z, Sun S and Wu Q: Macrophages in tumor-associated adipose
microenvironment accelerate tumor progression. Adv Biol (Weinh).
7:e22001612023. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Wen D, Liang T, Chen G, Li H, Wang Z, Wang
J, Fu R, Han X, Ci T, Zhang Y, et al: Adipocytes encapsulating
telratolimod recruit and polarize Tumor-Associated macrophages for
cancer immunotherapy. Adv Sci (Weinh). 10:e22060012023. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Masuda T, Fukuda A, Yamakawa G, Omatsu M,
Namikawa M, Sono M, Fukunaga Y, Nagao M, Araki O, Yoshikawa T, et
al: Pancreatic RECK inactivation promotes cancer formation,
epithelial-mesenchymal transition, and metastasis. J Clin Invest.
133:e1618472023. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Träger MM and Dhayat SA: Epigenetics of
epithelial-to-mesenchymal transition in pancreatic carcinoma. Int J
Cancer. 141:24–32. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Iwamoto C, Ohuchida K, Shinkawa T, Okuda
S, Otsubo Y, Okumura T, Sagara A, Koikawa K, Ando Y, Shindo K, et
al: Bone marrow-derived macrophages converted into
cancer-associated fibroblast-like cells promote pancreatic cancer
progression. Cancer Lett. 512:15–27. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Padoan A, Plebani M and Basso D:
Inflammation and pancreatic cancer: Focus on metabolism, cytokines,
and immunity. Int J Mol Sci. 20:6762019. View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Malinova A, Veghini L, Real FX and Corbo
V: Cell lineage infidelity in PDAC progression and therapy
resistance. Front Cell Dev Biol. 9:7952512021. View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Chou CW, Huang YK, Kuo TT, Liu JP and Sher
YP: An overview of ADAM9: Structure, Activation, and regulation in
human diseases. Int J Mol Sci. 21:77902020. View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Shang S, Wang J, Chen S, Tian R, Zeng H,
Wang L, Xia M, Zhu H and Zuo C: Exosomal miRNA-1231 derived from
bone marrow mesenchymal stem cells inhibits the activity of
pancreatic cancer. Cancer Med. 8:7728–7740. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Yao X, Mao Y, Wu D, Zhu Y, Lu J, Huang Y,
Guo Y, Wang Z, Zhu S, Li X and Lu Y: Exosomal circ_0030167 derived
from BM-MSCs inhibits the invasion, migration, proliferation and
stemness of pancreatic cancer cells by sponging miR-338-5p and
targeting the Wif1/Wnt8/β-catenin axis. Cancer Lett. 512:38–50.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Ji R, Zhang B, Zhang X, Xue J, Yuan X, Yan
Y, Wang M, Zhu W, Qian H, Xu W, et al: Exosomes derived from human
mesenchymal stem cells confer drug resistance in gastric cancer.
Cell Cycle. 14:2473–2483. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Gomis RR and Gawrzak S: Tumor cell
dormancy. Mol Oncol. 11:62–78. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Haider MT, Smit DJ and Taipaleenmäki H:
The Endosteal niche in breast cancer bone metastasis. Front Oncol.
10:3352020. View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Ono M, Kosaka N, Tominaga N, Yoshioka Y,
Takeshita F, Takahashi RU, Yoshida M, Tsuda H, Tamura K and Ochiya
T: Exosomes from bone marrow mesenchymal stem cells contain a
microRNA that promotes dormancy in metastatic breast cancer cells.
Sci Signal. 7:ra632014. View Article : Google Scholar : PubMed/NCBI
|
|
203
|
Yang Y, Bucan V, Baehre H, von der Ohe J,
Otte A and Hass R: Acquisition of new tumor cell properties by
MSC-derived exosomes. Int J Oncol. 47:244–252. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Fu R, Shao Q, Yang B, Chen Y, Ye Q, Chen X
and Zhu J: MiR-520a-5p/PPP5C regulation pattern is identified as
the key to gemcitabine resistance in pancreatic cancer. Front
Oncol. 12:9034842022. View Article : Google Scholar : PubMed/NCBI
|
|
205
|
Fan J, Wei Q, Koay EJ, Liu Y, Ning B,
Bernard PW, Zhang N, Han H, Katz MH, Zhao Z and Hu Y:
Chemoresistance transmission via exosome-Mediated EphA2 transfer in
pancreatic cancer. Theranostics. 8:5986–5994. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
206
|
Huang L, Rong Y, Tang X, Yi K, Qi P, Hou
J, Liu W, He Y, Gao X, Yuan C and Wang F: Engineered exosomes as an
in situ DC-primed vaccine to boost antitumor immunity in breast
cancer. Mol Cancer. 21:452022. View Article : Google Scholar : PubMed/NCBI
|
|
207
|
Tang W, Xia M, Liao Y, Fang Y, Wen G and
Zhong J: Exosomes in triple negative breast cancer: From bench to
bedside. Cancer Lett. 527:1–9. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
208
|
Scavo MP, Depalo N, Tutino V, De Nunzio V,
Ingrosso C, Rizzi F, Notarnicola M, Curri ML and Giannelli G:
Exosomes for diagnosis and therapy in gastrointestinal cancers. Int
J Mol Sci. 21:3672020. View Article : Google Scholar : PubMed/NCBI
|
|
209
|
Agrawal AK, Aqil F, Jeyabalan J, Spencer
WA, Beck J, Gachuki BW, Alhakeem SS, Oben K, Munagala R, Bondada S
and Gupta RC: Milk-derived exosomes for oral delivery of
paclitaxel. Nanomedicine. 13:1627–1636. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
210
|
Lux A, Kahlert C, Grützmann R and Pilarsky
C: c-Met and PD-L1 on circulating exosomes as diagnostic and
prognostic markers for pancreatic cancer. Int J Mol Sci.
20:33052019. View Article : Google Scholar : PubMed/NCBI
|
|
211
|
Sakaue T, Koga H, Iwamoto H, Nakamura T,
Ikezono Y, Abe M, Wada F, Masuda A, Tanaka T, Fukahori M, et al:
Glycosylation of ascites-derived exosomal CD133: A potential
prognostic biomarker in patients with advanced pancreatic cancer.
Med Mol Morphol. 52:198–208. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
212
|
Takahasi K, Iinuma H, Wada K, Minezaki S,
Kawamura S, Kainuma M, Ikeda Y, Shibuya M, Miura F and Sano K:
Usefulness of exosome-encapsulated microRNA-451a as a minimally
invasive biomarker for prediction of recurrence and prognosis in
pancreatic ductal adenocarcinoma. J Hepatobiliary Pancreat Sci.
25:155–161. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
213
|
Wei Q, Li Z, Feng H and Ren L: Serum
exosomal EphA2 is a prognostic biomarker in patients with
pancreatic cancer. Cancer Manag Res. 13:3675–3683. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
214
|
Chen D, Wu X, Xia M, Wu F, Ding J, Jiao Y,
Zhan Q and An F: Upregulated exosomic miR-23b-3p plays regulatory
roles in the progression of pancreatic cancer. Oncol Rep.
38:2182–2188. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
215
|
Li Z, Tao Y, Wang X, Jiang P, Li J, Peng
M, Zhang X, Chen K, Liu H, Zhen P, et al: Tumor-secreted exosomal
miR-222 promotes tumor progression via regulating P27 expression
and Re-localization in pancreatic cancer. Cell Physiol Biochem.
51:610–629. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
216
|
Fang Y, Zhou W, Rong Y, Kuang T, Xu X, Wu
W, Wang D and Lou W: Exosomal miRNA-106b from cancer-associated
fibroblast promotes gemcitabine resistance in pancreatic cancer.
Exp Cell Res. 383:1115432019. View Article : Google Scholar : PubMed/NCBI
|
|
217
|
Richards KE, Zeleniak AE, Fishel ML, Wu J,
Littlepage LE and Hill R: Cancer-associated fibroblast exosomes
regulate survival and proliferation of pancreatic cancer cells.
Oncogene. 36:1770–1778. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
218
|
Mikamori M, Yamada D, Eguchi H, Hasegawa
S, Kishimoto T, Tomimaru Y, Asaoka T, Noda T, Wada H, Kawamoto K,
et al: MicroRNA-155 controls exosome synthesis and promotes
gemcitabine resistance in pancreatic ductal adenocarcinoma. Sci
Rep. 7:423392017. View Article : Google Scholar : PubMed/NCBI
|
|
219
|
Batista IA and Melo SA: Exosomes and the
future of immunotherapy in pancreatic cancer. Int J Mol Sci.
20:5672019. View Article : Google Scholar : PubMed/NCBI
|
|
220
|
Yin Z, Ma T, Huang B, Lin L, Zhou Y, Yan
J, Zou Y and Chen S: Macrophage-derived exosomal microRNA-501-3p
promotes progression of pancreatic ductal adenocarcinoma through
the TGFBR3-mediated TGF-β signaling pathway. J Exp Clin Cancer Res.
38:3102019. View Article : Google Scholar : PubMed/NCBI
|
|
221
|
Zhou W, Zhou Y, Chen X, Ning T, Chen H,
Guo Q, Zhang Y, Liu P, Zhang Y, Li C, et al: Pancreatic
cancer-targeting exosomes for enhancing immunotherapy and
reprogra-mming tumor microenvironment. Biomaterials.
268:1205462021. View Article : Google Scholar : PubMed/NCBI
|
|
222
|
Li YJ, Wu JY, Wang JM, Hu XB, Cai JX and
Xiang DX: Gemcitabine loaded autologous exosomes for effective and
safe chemotherapy of pancreatic cancer. Acta Biomater. 101:519–530.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
223
|
Kamerkar S, LeBleu VS, Sugimoto H, Yang S,
Ruivo CF, Melo SA, Lee JJ and Kalluri R: Exosomes facilitate
therapeutic targeting of oncogenic KRAS in pancreatic cancer.
Nature. 546:498–503. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
224
|
Xiao L, Erb U, Zhao K, Hackert T and
Zöller M: Efficacy of vaccination with tumor-exosome loaded
dendritic cells combined with cytotoxic drug treatment in
pancreatic cancer. Oncoimmunology. 6:e13190442017. View Article : Google Scholar : PubMed/NCBI
|
|
225
|
Jang Y and Kim H, Yoon S, Lee H, Hwang J,
Jung J, Chang JH, Choi J and Kim H: Exosome-based photoacoustic
imaging guided photodynamic and immunotherapy for the treatment of
pancreatic cancer. J Control Release. 330:293–304. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
226
|
Que RS, Lin C, Ding GP, Wu ZR and Cao LP:
Increasing the immune activity of exosomes: The effect of
miRNA-depleted exosome proteins on activating dendritic
cell/cytokine-induced killer cells against pancreatic cancer. J
Zhejiang Univ Sci B. 17:352–360. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
227
|
Duan L, Xu L, Xu X, Qin Z, Zhou X, Xiao Y,
Liang Y and Xia J: Exosome-mediated delivery of gene vectors for
gene therapy. Nanoscale. 13:1387–1397. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
228
|
Moon B and Chang S: Exosome as a delivery
vehicle for cancer therapy. Cells. 11:3162022. View Article : Google Scholar : PubMed/NCBI
|
|
229
|
Arrighetti N, Corbo C, Evangelopoulos M,
Pastò A, Zuco V and Tasciotti E: Exosome-like nanovectors for drug
delivery in cancer. Curr Med Chem. 26:6132–6148. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
230
|
Liang Y, Duan L, Lu J and Xia J:
Engineering exosomes for targeted drug delivery. Theranostics.
11:3183–3195. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
231
|
Guimarães D, Cavaco-Paulo A and Nogueira
E: Design of liposomes as drug delivery system for therapeutic
applications. Int J Pharm. 601:1205712021. View Article : Google Scholar : PubMed/NCBI
|
|
232
|
Lamichhane TN, Jeyaram A, Patel DB,
Parajuli B, Livingston NK, Arumugasaamy N, Schardt JS and Jay SM:
Oncogene knockdown via active loading of small RNAs into
extracellular vesicles by sonication. Cell Mol Bioeng. 9:315–324.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
233
|
Zuo L, Tao H, Xu H, Li C, Qiao G, Guo M,
Cao S, Liu M and Lin X: Exosomes-coated miR-34a displays potent
antitumor activity in pancreatic cancer both in vitro and in vivo.
Drug Des Devel Ther. 14:3495–3507. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
234
|
Ding Y, Cao F, Sun H, Wang Y, Liu S, Wu Y,
Cui Q, Mei W and Li F: Exosomes derived from human umbilical cord
mesenchymal stromal cells deliver exogenous miR-145-5p to inhibit
pancreatic ductal adenocarcinoma progression. Cancer Lett.
442:351–361. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
235
|
Duan H, Liu Y, Gao Z and Huang W: Recent
advances in drug delivery systems for targeting cancer stem cells.
Acta Pharm Sin B. 11:55–70. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
236
|
Guo G, Tan Z, Liu Y, Shi F and She J: The
therapeutic potential of stem cell-derived exosomes in the
ulcerative colitis and colorectal cancer. Stem Cell Res Ther.
13:1382022. View Article : Google Scholar : PubMed/NCBI
|
|
237
|
Pascucci L, Coccè V, Bonomi A, Ami D,
Ceccarelli P, Ciusani E, Viganò L, Locatelli A, Sisto F, Doglia SM,
et al: Paclitaxel is incorporated by mesenchymal stromal cells and
released in exosomes that inhibit in vitro tumor growth: A new
approach for drug delivery. J Control Release. 192:262–270. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
238
|
Bonomi A, Sordi V, Dugnani E, Ceserani V,
Dossena M, Coccè V, Cavicchini L, Ciusani E, Bondiolotti G, Piovani
G, et al: Gemcitabine-releasing mesenchymal stromal cells inhibit
in vitro proliferation of human pancreatic carcinoma cells.
Cytotherapy. 17:1687–1695. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
239
|
van der Meel R, Fens MH, Vader P, van
Solinge WW, Eniola-Adefeso O and Schiffelers RM: Extracellular
vesicles as drug delivery systems: Lessons from the liposome field.
J Control Release. 195:72–85. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
240
|
Clayton A, Harris CL, Court J, Mason MD
and Morgan BP: Antigen-presenting cell exosomes are protected from
complement-mediated lysis by expression of CD55 and CD59. Eur J
Immunol. 33:522–531. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
241
|
Gomes-da-Silva LC, Fonseca NA, Moura V,
Pedroso de Lima MC, Simões S and Moreira JN: Lipid-based
nanoparticles for siRNA delivery in cancer therapy: Paradigms and
challenges. Acc Chem Res. 45:1163–1171. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
242
|
Simões S, Filipe A, Faneca H, Mano M,
Penacho N, Düzgünes N and de Lima MP: Cationic liposomes for gene
delivery. Expert Opin Drug Deliv. 2:237–254. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
243
|
Willingham SB, Volkmer JP, Gentles AJ,
Sahoo D, Dalerba P, Mitra SS, Wang J, Contreras-Trujillo H, Martin
R, Cohen JD, et al: The CD47-signal regulatory protein alpha
(SIRPa) interaction is a therapeutic target for human solid tumors.
Proc Natl Acad Sci USA. 109:6662–6667. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
244
|
Mendt M, Kamerkar S, Sugimoto H, McAndrews
KM, Wu CC, Gagea M, Yang S, Blanko EVR, Peng Q, Ma X, et al:
Generation and testing of clinical-grade exosomes for pancreatic
cancer. JCI insight. 3:e992632018. View Article : Google Scholar : PubMed/NCBI
|
|
245
|
Bardol T, Dujon AM, Taly V, Dunyach-Remy
C, Lavigne JP, Costa-Silva B, Kurma K, Eslami-S Z, Cayrefourcq L,
Canivet C, et al: Early detection of pancreatic cancer by liquid
biopsy ‘PANLIPSY’: A french nation-wide study project. BMC Cancer.
24:7092024. View Article : Google Scholar : PubMed/NCBI
|
|
246
|
Wei MY, Shi S, Liang C, Meng QC, Hua J,
Zhang YY, Liu J, Zhang B, Xu J and Yu XJ: The microbiota and
microbiome in pancreatic cancer: More influential than expected.
Mol Cancer. 18:972019. View Article : Google Scholar : PubMed/NCBI
|
|
247
|
Riquelme E, Zhang Y, Zhang L, Montiel M,
Zoltan M, Dong W, Quesada P, Sahin I, Chandra V, San Lucas A, et
al: Tumor microbiome diversity and composition influence pancreatic
cancer outcomes. Cell. 178:795–806.e12. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
248
|
Zhang X, Liu Q, Liao Q and Zhao Y:
Pancreatic cancer, gut microbiota, and therapeutic efficacy. J
Cancer. 11:2749–2758. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
249
|
Jiang Z, Mou Y, Wang H, Li L, Jin T, Wang
H, Liu M and Jin W: Causal effect between gut microbiota and
pancreatic cancer: A two-sample Mendelian randomization study. BMC
Cancer. 23:10912023. View Article : Google Scholar : PubMed/NCBI
|
|
250
|
Kartal E, Schmidt TSB, Molina-Montes E,
Rodríguez-Perales S, Wirbel J, Maistrenko OM, Akanni WA, Alashkar
Alhamwe B, Alves RJ, Carrato A, et al: A faecal microbiota
signature with high specificity for pancreatic cancer. Gut.
71:1359–1372. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
251
|
McAllister F, Khan MAW, Helmink B and
Wargo JA: The Tumor microbiome in pancreatic cancer: Bacteria and
beyond. Cancer Cell. 36:577–579. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
252
|
Kabwe M, Dashper S and Tucci J: The
Microbiome in pancreatic cancer-implications for diagnosis and
precision bacteriophage therapy for this low survival disease.
Front Cell Infect Microbiol. 12:8712932022. View Article : Google Scholar : PubMed/NCBI
|
|
253
|
Chow YC, Yam HC, Gunasekaran B, Lai WY, Wo
WY, Agarwal T, Ong YY, Cheong SL and Tan SA: Implications of
Porphyromonas gingivalis peptidyl arginine deiminase and
gingipain R in human health and diseases. Front Cell Infect
Microbiol. 12:9876832022. View Article : Google Scholar : PubMed/NCBI
|
|
254
|
Maekawa T, Fukaya R, Takamatsu S, Itoyama
S, Fukuoka T, Yamada M, Hata T, Nagaoka S, Kawamoto K, Eguchi H, et
al: Possible involvement of Enterococcus infection in the
pathogenesis of chronic pancreatitis and cancer. Biochem Biophys
Res Commun. 506:962–969. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
255
|
Zhou X, Xie F, Wang L, Zhang L, Zhang S,
Fang M and Zhou F: The function and clinical application of
extracellular vesicles in innate immune regulation. Cell Mol
Immunol. 17:323–334. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
256
|
Lai RC, Yeo RW, Tan KH and Lim SK:
Exosomes for drug delivery-a novel application for the mesenchymal
stem cell. Biotechnol Adv. 31:543–551. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
257
|
Otsuka M and Kotani A: Recent advances in
extracellular vesicles in gastrointestinal cancer and lymphoma.
Cancer Sci. 114:2230–2237. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
258
|
Vosough P, Khatami SH, Hashemloo A,
Tajbakhsh A, Karimi-Fard F, Taghvimi S, Taheri-Anganeh M, Soltani
Fard E, Savardashtaki A and Movahedpour A: Exosomal lncRNAs in
gastrointestinal cancer. Clin Chim Acta. 540:1172162023. View Article : Google Scholar : PubMed/NCBI
|
|
259
|
Tang XH, Guo T, Gao XY, Wu XL, Xing XF, Ji
JF and Li ZY: Exosome-derived noncoding RNAs in gastric cancer:
Functions and clinical applications. Mol Cancer. 20:992021.
View Article : Google Scholar : PubMed/NCBI
|