|
1
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Calabrese M, Saporita I, Turco F,
Gillessen S, Castro E, Vogl UM, Di Stefano RF, Carfì FM, Poletto S,
Farinea G, et al: Synthetic lethality by co-inhibition of androgen
receptor and polyadenosine diphosphate-ribose in metastatic
prostate cancer. Int J Mol Sci. 25:782023. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yamada Y and Beltran H: The treatment
landscape of metastatic prostate cancer. Cancer Lett. 519:20–29.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Armstrong AJ, Szmulewitz RZ, Petrylak DP,
Holzbeierlein J, Villers A, Azad A, Alcaraz A, Alekseev B, Iguchi
T, Shore ND, et al: ARCHES: A randomized, phase III study of
androgen deprivation therapy with enzalutamide or placebo in men
with metastatic hormone-sensitive prostate cancer. J Clin Oncol.
37:2974–2986. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chi KN, Agarwal N, Bjartell A, Chung BH,
de Santana Gomes AJ, Given R, Soto ÁJ, Merseburger AS, Özgüroğlu M,
Uemura H, et al: Apalutamide for metastatic, castration-sensitive
prostate cancer. N Engl J Med. 381:13–24. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Davis ID, Martin AJ, Stockler MR, Begbie
S, Chi KN, Chowdhury S, Coskinas X, Frydenberg M, Hague WE, Horvath
LG, et al: Enzalutamide with standard first-line therapy in
metastatic prostate cancer. N Engl J Med. 381:121–131. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fizazi K, Tran N, Fein L, Matsubara N,
Rodriguez-Antolin A, Alekseev BY, Özgüroğlu M, Ye D, Feyerabend S,
Protheroe A, et al: Abiraterone plus prednisone in metastatic,
castration-sensitive prostate cancer. N Engl J Med. 377:352–360.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sweeney CJ, Chen YH, Carducci M, Liu G,
Jarrard DF, Eisenberger M, Wong YN, Hahn N, Kohli M, Cooney MM, et
al: Chemohormonal therapy in metastatic hormone-sensitive prostate
cancer. N Engl J Med. 373:737–746. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gillessen S, Armstrong A, Attard G, Beer
TM, Beltran H, Bjartell A, Bossi A, Briganti A, Bristow RG, Bulbul
M, et al: Management of patients with advanced prostate cancer:
Report from the advanced prostate cancer consensus conference 2021.
Eur Urol. 82:115–141. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Verry C, Vincendeau S, Massetti M,
Blachier M, Vimont A, Bazil ML, Bernardini P, Pettré S and Timsit
MO: Pattern of clinical progression until metastatic
castration-resistant prostate cancer: An epidemiological study from
the European prostate cancer registry. Target Oncol. 17:441–451.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tilki D, van den Bergh RCN, Briers E, Van
den Broeck T, Brunckhorst O, Darraugh J, Eberli D, De Meerleer G,
De Santis M, Farolfi A, et al: EAU-EANM-ESTRO-ESUR-ISUP-SIOG
guidelines on prostate cancer. Part II-2024 update: Treatment of
relapsing and metastatic prostate cancer. Eur Urol. 86:164–182.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
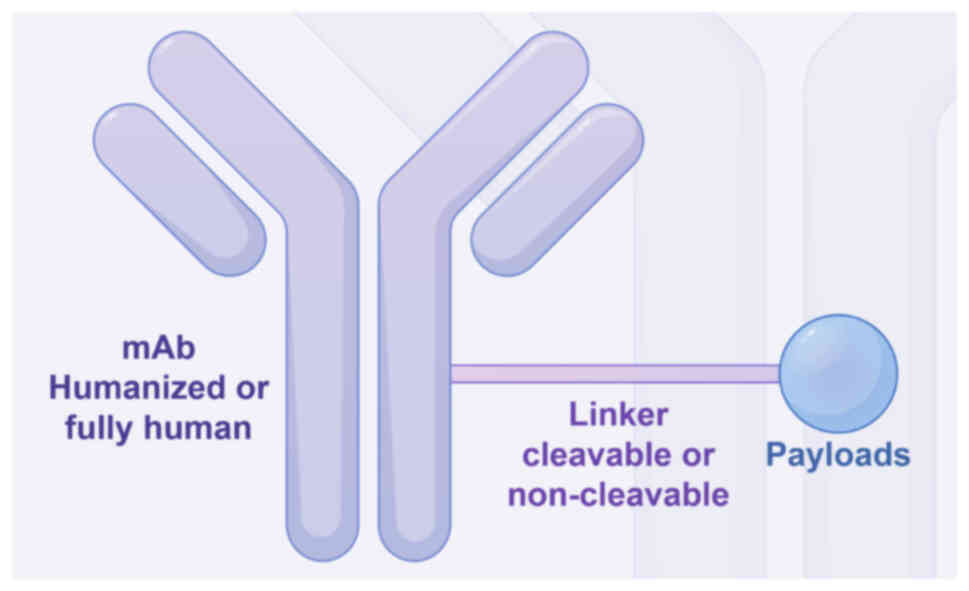
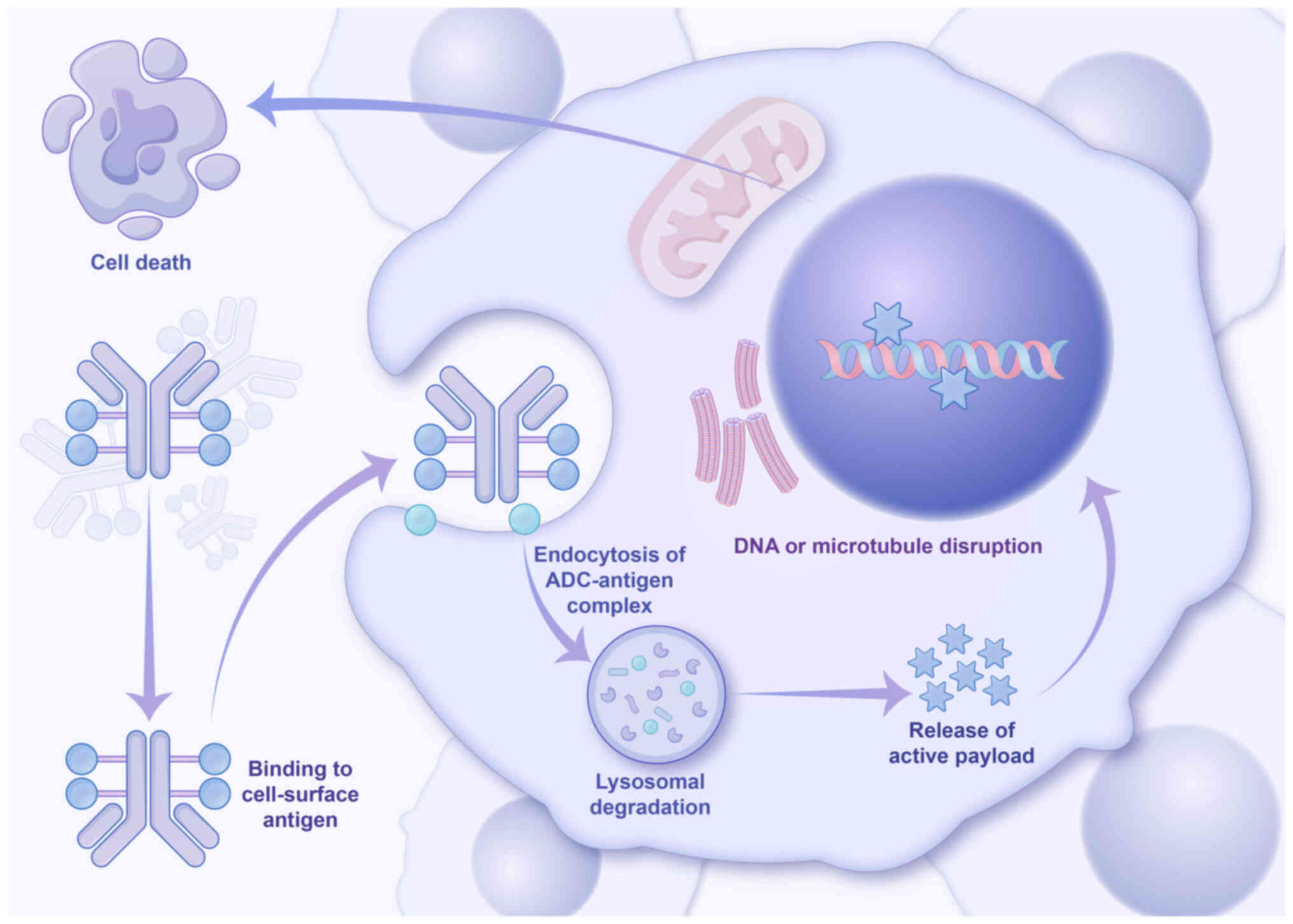
Dumontet C, Reichert JM, Senter PD,
Lambert JM and Beck A: Antibody-drug conjugates come of age in
oncology. Nat Rev Drug Discov. 22:641–661. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Drago JZ, Modi S and Chandarlapaty S:
Unlocking the potential of antibody-drug conjugates for cancer
therapy. Nat Rev Clin Oncol. 18:327–344. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Beck A, Goetsch L, Dumontet C and Corvaia
N: Strategies and challenges for the next generation of
antibody-drug conjugates. Nat Rev Drug Discov. 16:315–337. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Trail PA, Dubowchik GM and Lowinger TB:
Antibody drug conjugates for treatment of breast cancer: Novel
targets and diverse approaches in ADC design. Pharmacol Ther.
181:126–142. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Modi S, Saura C, Yamashita T, Park YH, Kim
SB, Tamura K, Andre F, Iwata H, Ito Y, Tsurutani J, et al:
Trastuzumab deruxtecan in previously treated HER2-positive breast
cancer. N Engl J Med. 382:610–621. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Modi S, Jacot W, Yamashita T, Sohn J,
Vidal M, Tokunaga E, Tsurutani J, Ueno NT, Prat A, Chae YS, et al:
Trastuzumab deruxtecan in previously treated HER2-low advanced
breast cancer. N Engl J Med. 387:9–20. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bardia A, Hurvitz SA, Tolaney SM, Loirat
D, Punie K, Oliveira M, Brufsky A, Sardesai SD, Kalinsky K, Zelnak
AB, et al: Sacituzumab govitecan in metastatic triple-negative
breast cancer. N Engl J Med. 384:1529–1541. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
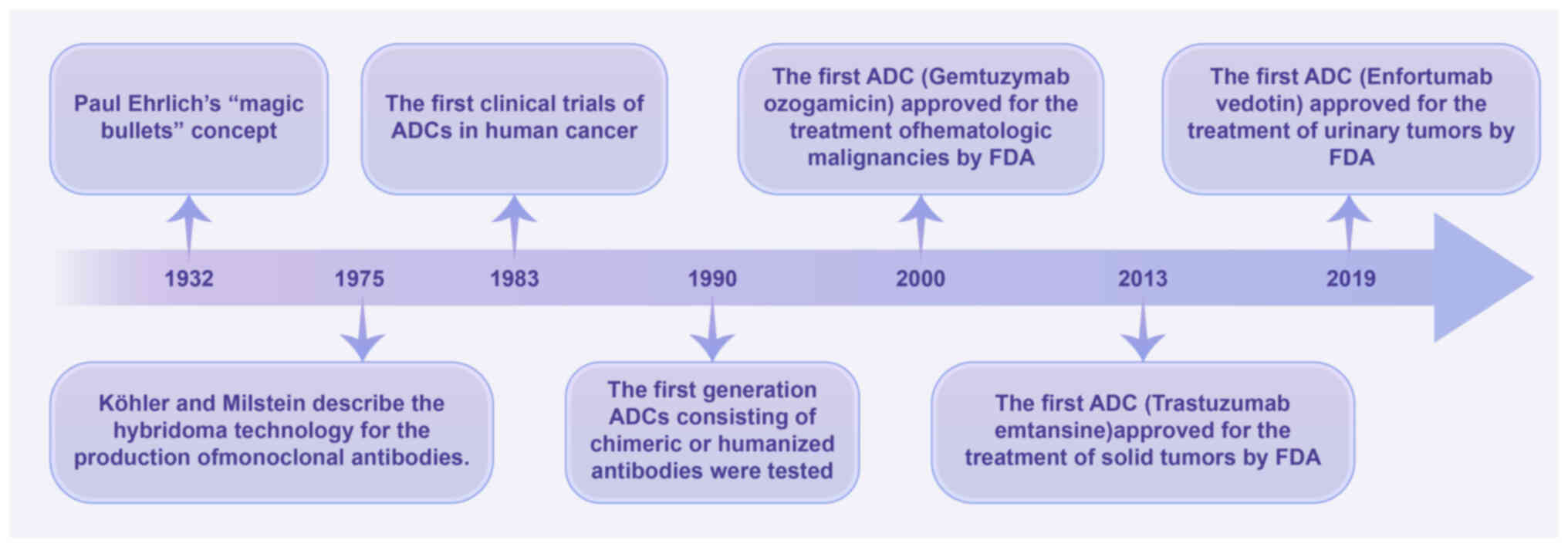
Strebhardt K and Ullrich A: Paul Ehrlich's
magic bullet concept: 100 years of progress. Nat Rev Cancer.
8:473–480. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kohler G and Milstein C: Continuous
cultures of fused cells secreting antibody of predefined
specificity. Nature. 256:495–497. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Carter P: Improving the efficacy of
antibody-based cancer therapies. Nat Rev Cancer. 1:118–129. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schrama D, Reisfeld RA and Becker JC:
Antibody targeted drugs as cancer therapeutics. Nat Rev Drug
Discov. 5:147–159. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sievers EL: Efficacy and safety of
gemtuzumab ozogamicin in patients with CD33-positive acute myeloid
leukaemia in first relapse. Expert Opin Biol Ther. 1:893–901. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Amiri-Kordestani L, Blumenthal GM, Xu QC,
Zhang L, Tang SW, Ha L, Weinberg WC, Chi B, Candau-Chacon R, Hughes
P, et al: FDA approval: Ado-trastuzumab emtansine for the treatment
of patients with HER2-positive metastatic breast cancer. Clin
Cancer Res. 20:4436–4441. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ghose A, Lapitan P, Apte V, Ghosh A,
Kandala A, Basu S, Parkes J, Shinde SD, Boussios S, Sharma A, et
al: Antibody drug conjugates in urological cancers: A review of the
current landscape. Curr Oncol Rep. 26:633–646. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chang E, Weinstock C, Zhang L, Charlab R,
Dorff SE, Gong Y, Hsu V, Li F, Ricks TK, Song P, et al: FDA
approval summary: Enfortumab vedotin for locally advanced or
metastatic urothelial carcinoma. Clin Cancer Res. 27:922–927. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li K, Xie G, Deng X, Zhang Y, Jia Z and
Huang Z: Antibody-drug conjugates in urinary tumors: Clinical
application, challenge, and perspectives. Front Oncol.
13:12597842023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tsuchikama K and An Z: Antibody-drug
conjugates: Recent advances in conjugation and linker chemistries.
Protein Cell. 9:33–46. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Giugliano F, Corti C, Tarantino P,
Michelini F and Curigliano G: Bystander effect of antibody-drug
conjugates: Fact or fiction? Curr Oncol Rep. 24:809–817. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Khera E, Dong S, Huang H, de Bever L, van
Delft FL and Thurber GM: Cellular-Resolution imaging of bystander
payload tissue penetration from antibody-drug conjugates. Mol
Cancer Ther. 21:310–321. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Staudacher AH and Brown MP: Antibody drug
conjugates and bystander killing: Is antigen-dependent
internalisation required? Br J Cancer. 117:1736–1742. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mjaess G, Aoun F, Rassy E, Diamand R,
Albisinni S and Roumeguere T: Antibody-drug conjugates in prostate
cancer: Where are we? Clin Genitourin Cancer. 21:171–174. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Trail PA, King HD and Dubowchik GM:
Monoclonal antibody drug immunoconjugates for targeted treatment of
cancer. Cancer Immunol Immunother. 52:328–337. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li Y, Cozzi PJ and Russell PJ: Promising
tumor-associated antigens for future prostate cancer therapy. Med
Res Rev. 30:67–101. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jin Y, Schladetsch MA, Huang X, Balunas MJ
and Wiemer AJ: Stepping forward in antibody-drug conjugate
development. Pharmacol Ther. 229:1079172022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Conilh L, Sadilkova L, Viricel W and
Dumontet C: Payload diversification: A key step in the development
of antibody-drug conjugates. J Hematol Oncol. 16:32023. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Su Z, Xiao D, Xie F, Liu L, Wang Y, Fan S,
Zhou X and Li S: Antibody-drug conjugates: Recent advances in
linker chemistry. Acta Pharm Sin B. 11:3889–3907. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Baah S, Laws M and Rahman KM:
Antibody-drug conjugates-a tutorial review. Molecules. 26:29432021.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fu Z, Li S, Han S, Shi C and Zhang Y:
Antibody drug conjugate: The ‘biological missile’ for targeted
cancer therapy. Signal Transduct Target Ther. 7:932022. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Samantasinghar A, Sunildutt NP, Ahmed F,
Soomro AM, Salih ARC, Parihar P, Memon FH, Kim KH, Kang IS and Choi
KH: A comprehensive review of key factors affecting the efficacy of
antibody drug conjugate. Biomed Pharmacother. 161:1144082023.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Diamantis N and Banerji U: Antibody-drug
conjugates-an emerging class of cancer treatment. Br J Cancer.
114:362–367. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kaur R, Kaur G, Gill RK, Soni R and
Bariwal J: Recent developments in tubulin polymerization
inhibitors: An overview. Eur J Med Chem. 87:89–124. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cheung-Ong K, Giaever G and Nislow C:
DNA-damaging agents in cancer chemotherapy: Serendipity and
chemical biology. Chem Biol. 20:648–659. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ackerman SE, Pearson CI, Gregorio JD,
Gonzalez JC, Kenkel JA, Hartmann FJ, Luo A, Ho PY, LeBlanc H, Blum
LK, et al: Immune-stimulating antibody conjugates elicit robust
myeloid activation and durable antitumor immunity. Nat Cancer.
2:18–33. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Rahbar K, Afshar-Oromieh A, Jadvar H and
Ahmadzadehfar H: PSMA theranostics: Current status and future
directions. Mol Imaging. 17:15360121187760682018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sun M, Niaz MJ, Niaz MO and Tagawa ST:
Prostate-Specific membrane antigen (PSMA)-targeted radionuclide
therapies for prostate cancer. Curr Oncol Rep. 23:592021.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Milowsky MI, Galsky MD, Morris MJ, Crona
DJ, George DJ, Dreicer R, Tse K, Petruck J, Webb IJ, Bander NH, et
al: Phase 1/2 multiple ascending dose trial of the
prostate-specific membrane antigen-targeted antibody drug conjugate
MLN2704 in metastatic castration-resistant prostate cancer. Urol
Oncol. 34:530 e515–530 e521. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Henry MD, Wen S, Silva MD, Chandra S,
Milton M and Worland PJ: A prostate-specific membrane
antigen-targeted monoclonal antibody-chemotherapeutic conjugate
designed for the treatment of prostate cancer. Cancer Res.
64:7995–8001. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Galsky MD, Eisenberger M, Moore-Cooper S,
Kelly WK, Slovin SF, DeLaCruz A, Lee Y, Webb IJ and Scher HI: Phase
I trial of the prostate-specific membrane antigen-directed
immunoconjugate MLN2704 in patients with progressive metastatic
castration-resistant prostate cancer. J Clin Oncol. 26:2147–2154.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ma D, Hopf CE, Malewicz AD, Donovan GP,
Senter PD, Goeckeler WF, Maddon PJ and Olson WC: Potent antitumor
activity of an auristatin-conjugated, fully human monoclonal
antibody to prostate-specific membrane antigen. Clin Cancer Res.
12:2591–2596. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Petrylak DP, Kantoff P, Vogelzang NJ, Mega
A, Fleming MT, Stephenson JJ Jr, Frank R, Shore ND, Dreicer R,
McClay EF, et al: Phase 1 study of PSMA ADC, an antibody-drug
conjugate targeting prostate-specific membrane antigen, in
chemotherapy-refractory prostate cancer. Prostate. 79:604–613.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Petrylak DP, Vogelzang NJ, Chatta K,
Fleming MT, Smith DC, Appleman LJ, Hussain A, Modiano M, Singh P,
Tagawa ST, et al: PSMA ADC monotherapy in patients with progressive
metastatic castration-resistant prostate cancer following
abiraterone and/or enzalutamide: Efficacy and safety in open-label
single-arm phase 2 study. Prostate. 80:99–108. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Cho S, Zammarchi F, Williams DG, Havenith
CEG, Monks NR, Tyrer P, D'Hooge F, Fleming R, Vashisht K, Dimasi N,
et al: Antitumor activity of MEDI3726 (ADCT-401), a
pyrrolobenzodiazepine antibody-drug conjugate targeting PSMA, in
preclinical models of prostate cancer. Mol Cancer Ther.
17:2176–2186. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
de Bono JS, Fleming MT, Wang JS, Cathomas
R, Miralles MS, Bothos J, Hinrichs MJ, Zhang Q, He P, Williams M,
et al: Phase I study of MEDI3726: A prostate-specific membrane
antigen-targeted antibody-drug conjugate, in patients with mCRPC
after failure of abiraterone or enzalutamide. Clin Cancer Res.
27:3602–3609. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Shen J, Pachynski R, Nordquist LT, Adra N,
Bilen MA, Aggarwal R, Reichert Z, Schweizer M, Iravani A, Aung S,
et al: 1804P APEX-01: First-in-human phase I/II study of ARX517 an
anti-prostate-specific membrane antigen (PSMA) antibody-drug
conjugate (ADC) in patients (pts) with metastatic
castration-resistant prostate cancer (mCRPC). Ann Oncol.
34:S974–S975. 2023. View Article : Google Scholar
|
|
56
|
Gomes IM, Maia CJ and Santos CR: STEAP
proteins: From structure to applications in cancer therapy. Mol
Cancer Res. 10:573–587. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Rocha SM, Nascimento D, Coelho RS, Cardoso
AM, Passarinha LA, Socorro S and Maia CJ: STEAP1 knockdown
decreases the sensitivity of prostate cancer cells to paclitaxel,
docetaxel and cabazitaxel. Int J Mol Sci. 24:66432023. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Danila DC, Szmulewitz RZ, Vaishampayan U,
Higano CS, Baron AD, Gilbert HN, Brunstein F, Milojic-Blair M, Wang
B, Kabbarah O, et al: Phase I study of DSTP3086S, an antibody-drug
conjugate targeting six-transmembrane epithelial antigen of
prostate 1, in metastatic castration-resistant prostate cancer. J
Clin Oncol. 37:3518–3527. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Doronina SO, Toki BE, Torgov MY,
Mendelsohn BA, Cerveny CG, Chace DF, DeBlanc RL, Gearing RP, Bovee
TD, Siegall CB, et al: Development of potent monoclonal antibody
auristatin conjugates for cancer therapy. Nat Biotechnol.
21:778–784. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
60
|
Trerotola M, Ganguly KK, Fazli L, Fedele
C, Lu H, Dutta A, Liu Q, De Angelis T, Riddell LW, Riobo NA, et al:
Trop-2 is up-regulated in invasive prostate cancer and displaces
FAK from focal contacts. Oncotarget. 6:14318–14328. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Sperger JM, Helzer KT, Stahlfeld CN, Jiang
D, Singh A, Kaufmann KR, Niles DJ, Heninger E, Rydzewski NR, Wang
L, et al: Expression and therapeutic targeting of TROP-2 in
treatment-resistant prostate cancer. Clin Cancer Res. 29:2324–2335.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Starodub AN, Ocean AJ, Shah MA, Guarino
MJ, Picozzi VJ Jr, Vahdat LT, Thomas SS, Govindan SV, Maliakal PP,
Wegener WA, et al: First-in-Human trial of a novel anti-trop-2
antibody-sn-38 conjugate, sacituzumab govitecan, for the treatment
of diverse metastatic solid tumors. Clin Cancer Res. 21:3870–3878.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Lang J, Tagawa ST, Slovin S, Emamekhoo H,
Rathkopf D, Abida W, Autio K, Xiao H, Molina AM, Eickhoff J, et al:
1406P Interim results of a phase II trial of sacituzumab govitecan
(SG) in patients (Pts) with metastatic castration resistant
prostate cancer (mCRPC) progressing on androgen receptor signaling
inhibitors (ARSI). Ann Oncol. 33:S11882022. View Article : Google Scholar
|
|
64
|
Corti C, Antonarelli G, Valenza C, Nicolò
E, Rugo H, Cortés J, Harbeck N, Carey LA, Criscitiello C and
Curigliano G: Histology-agnostic approvals for antibody-drug
conjugates in solid tumours: Is the time ripe? Eur J Cancer.
171:25–42. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Elvington M, Liszewski MK and Atkinson JP:
CD46 and oncologic interactions: Friendly fire against cancer.
Antibodies (Basel). 9:592020. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Su Y, Liu Y, Behrens CR, Bidlingmaier S,
Lee NK, Aggarwal R, Sherbenou DW, Burlingame AL, Hann BC, Simko JP,
et al: Targeting CD46 for both adenocarcinoma and neuroendocrine
prostate cancer. JCI Insight. 3:e1214972018. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Aggarwal RR, Vuky J, VanderWeele DJ,
Rettig M, Heath EI, Beer TM, Huang J, Pawlowska N, Sinit R, Abbey
J, et al: Phase 1a/1b study of FOR46, an antibody drug conjugate
(ADC), targeting CD46 in metastatic castration-resistant prostate
cancer (mCRPC). J Clin Oncol. 40:3001. 2022. View Article : Google Scholar
|
|
68
|
Zang X and Allison JP: The B7 family and
cancer therapy: Costimulation and coinhibition. Clin Cancer Res.
13:5271–5279. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Bonk S, Tasdelen P, Kluth M, Hube-Magg C,
Makrypidi-Fraune G, Möller K, Höflmayer D, Rico SD, Büscheck F,
Minner S, et al: High B7-H3 expression is linked to increased risk
of prostate cancer progression. Pathol Int. 70:733–742. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Mendes AA, Lu J, Kaur HB, Zheng SL, Xu J,
Hicks J, Weiner AB, Schaeffer EM, Ross AE, Balk SP, et al:
Association of B7-H3 expression with racial ancestry, immune cell
density, and androgen receptor activation in prostate cancer.
Cancer. 128:2269–2280. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Guo C, Figueiredo I, Gurel B, Neeb A, Seed
G, Crespo M, Carreira S, Rekowski J, Buroni L, Welti J, et al:
B7-H3 as a therapeutic target in advanced prostate Cancer. Eur
Urol. 83:224–238. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Scribner JA, Brown JG, Son T, Chiechi M,
Li P, Sharma S, Li H, De Costa A, Li Y, Chen Y, et al: Preclinical
development of MGC018, a duocarmycin-based antibody-drug conjugate
targeting B7-H3 for solid cancer. Mol Cancer Ther. 19:2235–2244.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Shenderov E, Mallesara GHG, Wysocki PJ, Xu
W, Ramlau R, Weickhardt AJ, Zolnierek J, Spira A, Joshua AM,
Powderly J, et al: 620P MGC018, an anti-B7-H3 antibody-drug
conjugate (ADC), in patients with advanced solid tumors:
Preliminary results of phase I cohort expansion. Ann Oncol.
32:S657–S659. 2021. View Article : Google Scholar
|
|
74
|
Belluomini L, Sposito M, Avancini A,
Insolda J, Milella M, Rossi A and Pilotto S: Unlocking new horizons
in small-cell lung cancer treatment: The onset of antibody-drug
conjugates. Cancers (Basel). 15:53682023. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Breij EC, de Goeij BE, Verploegen S,
Schuurhuis DH, Amirkhosravi A, Francis J, Miller VB, Houtkamp M,
Bleeker WK, Satijn D and Parren PW: An antibody-drug conjugate that
targets tissue factor exhibits potent therapeutic activity against
a broad range of solid tumors. Cancer Res. 74:1214–1226. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Chu AJ: Tissue factor, blood coagulation,
and beyond: An overview. Int J Inflam. 2011:3672842011.PubMed/NCBI
|
|
77
|
Versteeg HH: Tissue factor: Old and new
links with cancer biology. Semin Thromb Hemost. 41:747–755. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Markham A: Tisotumab vedotin: First
approval. Drugs. 81:2141–2147. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
de Bono JS, Concin N, Hong DS,
Thistlethwaite FC, Machiels JP, Arkenau HT, Plummer R, Jones RH,
Nielsen D, Windfeld K, et al: Tisotumab vedotin in patients with
advanced or metastatic solid tumours (InnovaTV 201): A
first-in-human, multicentre, phase 1–2 trial. Lancet Oncol.
20:383–393. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Corti C, Bielo LB, Schianca AC, Salimbeni
BT, Criscitiello C and Curigliano G: Future potential targets of
antibody-drug conjugates in breast cancer. Breast. 69:312–322.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Saunders LR, Bankovich AJ, Anderson WC,
Aujay MA, Bheddah S, Black K, Desai R, Escarpe PA, Hampl J, Laysang
A, et al: A DLL3-targeted antibody-drug conjugate eradicates
high-grade pulmonary neuroendocrine tumor-initiating cells in vivo.
Sci Transl Med. 7:302ra1362015. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Puca L, Gavyert K, Sailer V, Conteduca V,
Dardenne E, Sigouros M, Isse K, Kearney M, Vosoughi A, Fernandez L,
et al: Delta-like protein 3 expression and therapeutic targeting in
neuroendocrine prostate cancer. Sci Transl Med. 11:eaav08912019.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Rudin CM, Pietanza MC, Bauer TM, Ready N,
Morgensztern D, Glisson BS, Byers LA, Johnson ML, Burris HA III,
Robert F, et al: Rovalpituzumab tesirine, a DLL3-targeted
antibody-drug conjugate, in recurrent small-cell lung cancer: A
first-in-human, first-in-class, open-label, phase 1 study. Lancet
Oncol. 18:42–51. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Mansfield AS, Hong DS, Hann CL, Farago AF,
Beltran H, Waqar SN, Hendifar AE, Anthony LB, Taylor MH, Bryce AH,
et al: A phase I/II study of rovalpituzumab tesirine in delta-like
3-expressing advanced solid tumors. NPJ Precis Oncol. 5:742021.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Fuentes-Antras J, Genta S, Vijenthira A
and Siu LL: Antibody-drug conjugates: In search of partners of
choice. Trends Cancer. 9:339–354. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Lohiya V, Aragon-Ching JB and Sonpavde G:
Role of chemotherapy and mechanisms of resistance to chemotherapy
in metastatic castration-resistant prostate cancer. Clin Med
Insights Oncol. 10:57–66. 2016.PubMed/NCBI
|
|
88
|
de Bono JS, Oudard S, Ozguroglu M, Hansen
S, Machiels JP, Kocak I, Gravis G, Bodrogi I, Mackenzie MJ, Shen L,
et al: Prednisone plus cabazitaxel or mitoxantrone for metastatic
castration-resistant prostate cancer progressing after docetaxel
treatment: A randomised open-label trial. Lancet. 376:1147–1154.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Tannock IF, de Wit R, Berry WR, Horti J,
Pluzanska A, Chi KN, Oudard S, Théodore C, James ND, Turesson I, et
al: Docetaxel plus prednisone or mitoxantrone plus prednisone for
advanced prostate cancer. N Engl J Med. 351:1502–1512. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
de Goeij BE, Satijn D, Freitag CM,
Wubbolts R, Bleeker WK, Khasanov A, Zhu T, Chen G, Miao D, van
Berkel PH and Parren PW: High turnover of tissue factor enables
efficient intracellular delivery of antibody-drug conjugates. Mol
Cancer Ther. 14:1130–1140. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Thurber GM, Schmidt MM and Wittrup KD:
Antibody tumor penetration: Transport opposed by systemic and
antigen-mediated clearance. Adv Drug Deliv Rev. 60:1421–1434. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Ruan DY, Wu HX, Meng Q and Xu RH:
Development of antibody-drug conjugates in cancer: Overview and
prospects. Cancer Commun (Lond). 44:3–22. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Autio KA, Boni V, Humphrey RW and Naing A:
Probody therapeutics: An emerging class of therapies designed to
enhance on-target effects with reduced off-tumor toxicity for use
in immuno-oncology. Clin Cancer Res. 26:984–989. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Andreev J, Thambi N, Bay AE, Delfino F,
Martin J, Kelly MP, Kirshner JR, Rafique A, Kunz A, Nittoli T, et
al: Bispecific antibodies and antibody-drug Conjugates (ADCs)
bridging HER2 and prolactin receptor improve efficacy of HER2 ADCs.
Mol Cancer Ther. 16:681–693. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Tang F, Yang Y, Tang Y, Tang S, Yang L,
Sun B, Jiang B, Dong J, Liu H, Huang M, et al: One-pot
N-glycosylation remodeling of IgG with non-natural
sialylglycopeptides enables glycosite-specific and dual-payload
antibody-drug conjugates. Org Biomol Chem. 14:9501–9518. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Colombo R and Rich JR: The therapeutic
window of antibody drug conjugates: A dogma in need of revision.
Cancer Cell. 40:1255–1263. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Tarantino P, Ricciuti B, Pradhan SM and
Tolaney SM: Optimizing the safety of antibody-drug conjugates for
patients with solid tumours. Nat Rev Clin Oncol. 20:558–576. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Zhu Y, Liu K, Wang K and Zhu H:
Treatment-related adverse events of antibody-drug conjugates in
clinical trials: A systematic review and meta-analysis. Cancer.
129:283–295. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Donaghy H: Effects of antibody, drug and
linker on the preclinical and clinical toxicities of antibody-drug
conjugates. MAbs. 8:659–671. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Tumey LN and Han S: ADME considerations
for the development of biopharmaceutical conjugates using cleavable
linkers. Curr Top Med Chem. 17:3444–3462. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Mahalingaiah PK, Ciurlionis R, Durbin KR,
Yeager RL, Philip BK, Bawa B, Mantena SR, Enright BP, Liguori MJ
and Van Vleet TR: Potential mechanisms of target-independent uptake
and toxicity of antibody-drug conjugates. Pharmacol Ther.
200:110–125. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Chen YF, Xu YY, Shao ZM and Yu KD:
Resistance to antibody-drug conjugates in breast cancer: Mechanisms
and solutions. Cancer Commun (Lond). 43:297–337. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Piombino C, Tonni E, Oltrecolli M, Pirola
M, Pipitone S, Baldessari C, Dominici M, Sabbatini R and Vitale MG:
Immunotherapy in urothelial cancer: Current status and future
directions. Expert Rev Anticancer Ther. 23:1141–1155. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
O'Malley DM, Matulonis UA, Birrer MJ,
Castro CM, Gilbert L, Vergote I, Martin LP, Mantia-Smaldone GM,
Martin AG, Bratos R, et al: Phase Ib study of mirvetuximab
soravtansine, a folate receptor alpha (FRalpha)-targeting
antibody-drug conjugate (ADC), in combination with bevacizumab in
patients with platinum-resistant ovarian cancer. Gynecol Oncol.
157:379–385. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Uliano J, Nicolo E, Corvaja C, Salimbeni
BT, Trapani D and Curigliano G: Combination immunotherapy
strategies for triple-negative breast cancer: Current progress and
barriers within the pharmacological landscape. Expert Rev Clin
Pharmacol. 15:1399–1413. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Thana M and Wood L: Immune checkpoint
inhibitors in genitourinary malignancies. Curr Oncol. 27:S69–S77.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Bakhtiar R: Antibody drug conjugates.
Biotechnol Lett. 38:1655–1664. 2016. View Article : Google Scholar : PubMed/NCBI
|