Introduction
S100P is a member of the S100 protein family, first
purified and characterized from the placenta by Becker et al
(1) in 1992, with the ‘P’ denoting
its placental origin. Notably, S100P is exclusively found in the
genomes of vertebrate species (2).
Among vertebrates, the S100 protein family represents the largest
group of innate immune proteins containing EF-hand motifs (3,4), where
the two alpha helices in these motifs provide calcium
(Ca2+) binding sites (5). Consequently, S100P participates in
mediating Ca2+-dependent signaling pathways (6). Uniquely, in humans, the S100P gene is
located on chromosome 4 (4p16). Although S100P is present in many
mammals, its expression is not universally widespread (7). This phenomenon may be due to
incomplete genome sequencing or the loss of corresponding genomic
sequences during the process of speciation (8). Research indicates that S100P is
related to human evolution; for instance, Zhu et al
(9) observed the expression of
S100P protein during hormonal rhythmic fluctuations in the uterine
wall, suggesting its potential involvement in embryo implantation
and development, as well as its functional roles in various adult
human tissues. Numerous studies have demonstrated that S100P is
functionally related to the carcinogenic processes of several
cancers, including lung cancer, pancreatic cancer and breast
cancer. This underscores its significant potential as a tumor
biomarker and therapeutic target (10,11).
Although the specific mechanisms remain elusive in numerous areas,
this review offers new insights and strategies for targeted
therapy. It establishes a theoretical foundation for a deeper
understanding of S100P and its applications in personalized cancer
treatment, highlighting its significant clinical implications.
Information regarding the literature search pertinent to this
review is provided in the supplementary information file.
Additionally, a detailed literature search flowchart is available
in Fig. S1.
S100P-related biomarkers
Biomarkers serve as indicators of abnormal signals
across multiple biological levels, including molecular, cellular
and organismal, which emerge due to the influence of environmental
pollutants prior to the onset of significant damage to the
organism. The specific expression of S100P in cancer indicates its
potential as a histological marker for pan-cancer, with elevated
levels typically correlating with poor prognosis, as illustrated in
Table I. Research has shown that
S100P is highly expressed in various malignancies, such as lung
cancer (12), pancreatic cancer
(13,14), liver cancer (15,16)
and colorectal cancer (CRC) (17).
For instance, in intrahepatic cholangiocarcinoma, S100P serves as a
highly sensitive diagnostic marker for differentiating its various
pathological subtypes (16,18). Furthermore, S100P can function as a
screening marker for early biliary tract lesions during bile
cytology examinations (19).
 | Table I.Expression and significance of S100P
in different types of tumors. |
Table I.
Expression and significance of S100P
in different types of tumors.
| Cancer type | S100P
overexpression | Clinical
correlation | (Refs.) |
|---|
| Non-small cell lung
cancer | mRNA and
protein | OS and PFS | (12,84) |
| Colorectal
cancer | mRNA and
protein | EFS | (17,40) |
| Hepatocellular
carcinoma | mRNA and
protein | OS and RFS | (21,85) |
| Intrahepatic
cholangiocarcinoma | mRNA and
protein | OS and PFS | (15,86) |
| Lung
adenocarcinoma | mRNA and
protein | OS and PFI | (51,75,76) |
| Gastric cancer | mRNA and
protein | OS and PFS | (52) |
| Gallbladder
cancer | mRNA and
protein | OS | (87,88) |
| Breast cancer | mRNA and
protein | RFS | (89–91) |
| Ovarian cancer | mRNA and
protein | OS | (92,93) |
Given the high postoperative recurrence rates
observed in most cancer patients, effective predictive factors
remain elusive. Research conducted by Ji et al (20) identified S100P as a promising
biomarker for predicting the risk of postoperative recurrence in
CRC during follow-up. Similarly, Hwang et al (21) demonstrated that S100P functions as a
postoperative predictive marker for hepatocellular carcinoma (HCC)
within the Asian population. The detection of S100P in body fluids,
such as blood or cerebrospinal fluid, is clinically more feasible
than in tissue samples. For instance, in HCC characterized by
portal vein tumor thrombus and microvascular invasion, serum S100P
levels measured via ELISA are regarded as a robust biomarker for
this condition (22). Currently,
there is a notable absence of effective immunohistochemical (IHC)
markers for the diagnosis of pancreatic tumors. Research has
indicated that employing S100P classification in conjunction with
peptide nucleic acid (PNA)-mediated real-time polymerase chain
reaction (PCR) clamp techniques (i.e., PNA clamp PCR) can
significantly improve the accuracy of diagnosing histologically
challenging cases (23).
Upstream regulators of S100P
In the genomes of eukaryotes, the majority of
transcriptional genes are composed of non-coding RNAs (ncRNAs). The
present study focuses on the regulatory ncRNAs associated with
S100P, which include long ncRNAs (lncRNAs), circular RNAs
(circRNAs) and microRNAs (miRNAs). ncRNAs are crucial for
regulating gene expression and maintaining cellular homeostasis
(24,25), and their roles in cancer regulation
are receiving increasing attention (26). For instance, in pancreatic cancer,
Jiang et al (27)
demonstrated that both transient and stable transfection of miR-495
led to a reduction in the mRNA and protein expression levels of
S100P in pancreatic cancer cell lines (SW1990 and BxPC-3).
Experiments involving the overexpression and knockdown of miR-495
confirmed its role in suppressing tumor growth and invasion in a
manner dependent on S100P (27).
Similarly, another study revealed that miR-671 was sequestered by
circ_0092314, which alleviated the suppressive effect of miR-671 on
S100P, thereby activating the AKT pathway, as discussed in the
earlier section on epithelial-mesenchymal transition (EMT)
(28).
In addition to their roles in metastasis-related
cellular functions (28,29), miRNAs are also implicated in the
development of resistance to conventional chemotherapy, which
represents another detrimental characteristic of cancer cells.
Gemcitabine, a deoxycytidine derivative, is a standard
chemotherapeutic agent used in the treatment of pancreatic cancer
(30). Specifically, miR-365
targets the 3′untranslated regions of src homology 2
domain-containing transforming protein 1 (SHC1) and BAX mRNA,
resulting in decreased expression of these genes, while
simultaneously increasing the expression of the oncogenic factor
S100P (29). SHC1 is localized to
the mitochondrial intermembrane space and plays a role in the
production of reactive oxygen species, which can induce apoptosis
(31). Additionally, BAX enhances
the sensitivity of ovarian cancer cells to cisplatin (32). These findings suggest that
downregulation of SHC1 and BAX may contribute to the development of
gemcitabine resistance (29).
Although the authors did not perform any follow-up studies to
elucidate the interaction mechanisms among S100P, SHC1 and BAX,
prior research indicates that this could represent a promising
mechanism of action. Similarly, in breast cancer, researchers
hypothesized that lncRNAs associated with trastuzumab resistance at
the transcriptional level are closely linked to S100P, although no
specific in-depth studies have been conducted (33). In both breast and lung cancers,
lncRNA non-coding RNA activated by DNA damage (NORAD) is
transcriptionally suppressed via the Hippo pathway, and the
associated transcriptional co-activator with PDZ-binding motif-TEA
domain transcription factors complex, along with the nucleosome
remodeling and deacetylase complex, is also found to be
downregulated. LncRNA NORAD acts as a decoy to sequester S100P,
thereby inhibiting metastasis in these cancers. In cancerous
tissues, the suppression of its repetitive sequences results in an
increase in S100P expression (34).
Metastasis
Tumor metastasis is one of the leading causes of
mortality among cancer patients. EMT is a crucial regulatory
program in cancer development. Through this process, epithelial
cells lose their intercellular connections and polarity, resulting
in the loss of epithelial characteristics and the acquisition of
mesenchymal traits that confer invasive and migratory capabilities
(35). EMT is characterized by
alterations in epithelial markers, such as E-cadherin, and
mesenchymal markers, including vimentin and fibronectin (36). Furthermore, there is an increasingly
recognized association between EMT and drug resistance across
various cancers (36–38). It has been indicated that S100P
plays a significant role in EMT-related processes and is critical
for invasion and metastasis in various tumors (28,39).
In pancreatic adenocarcinoma, circ_0092314
sequesters miR-671, resulting in an increase in S100P, which
promotes EMT and cellular invasion. The inhibitory effect of
miR-671 on S100P is primarily manifested through reduced cell
invasion and spheroid formation, alongside the upregulation of
E-cadherin and downregulation of vimentin (28). In CRC, Zuo et al (39) identified S100P as a crucial
initiating target that interacts with thioredoxin-1, promoting EMT
and enhancing cellular invasion and metastasis. Shen et al
(40,41) validated in murine models that S100P
facilitates EMT in colon adenocarcinoma via the advanced
glycosylation end-product receptors (RAGE) signaling pathway. Of
note, Hsu et al (42)
demonstrated that in lung cancer, RAGE does not mediate
S100P-induced cancer progression; instead, S100P binds to integrin
α7, subsequently mediating cellular invasion and metastasis by
activating the focal adhesion kinase (FAK)/AKT/zinc finger e-box
binding homeobox 1 signaling axis and promoting the EMT process.
Regarding drug resistance, Hamada et al (29) discovered that miR-365, associated
with EMT, upregulates the expression of the oncogenic molecule
S100P, thereby increasing the resistance of pancreatic cancer cells
to gemcitabine through the downregulation of SHC1 and BAX; however,
further specific studies on the interaction between these factors
were not conducted. These findings suggest a close relationship
between the S100P-regulated EMT process and drug resistance,
indicating significant potential for targeting S100P in therapeutic
strategies against resistance in future research.
S100P and signaling pathways
S100P is a tumor-promoting factor that primarily
regulates the activity of multiple targets within signaling
pathways through various modes of interaction, while simultaneously
coordinating other target proteins within multiprotein complexes.
Additionally, S100P modulates proliferation, apoptosis, invasion,
migration and drug resistance in cancer cells (Fig. 1). Furthermore, angiogenesis, the
cell cycle and drug resistance in tumors are associated with S100P
(Fig. 2). Of note, in certain
contexts, S100P can also function as a tumor suppressor in specific
cancer types, such as gastric and lung cancers.
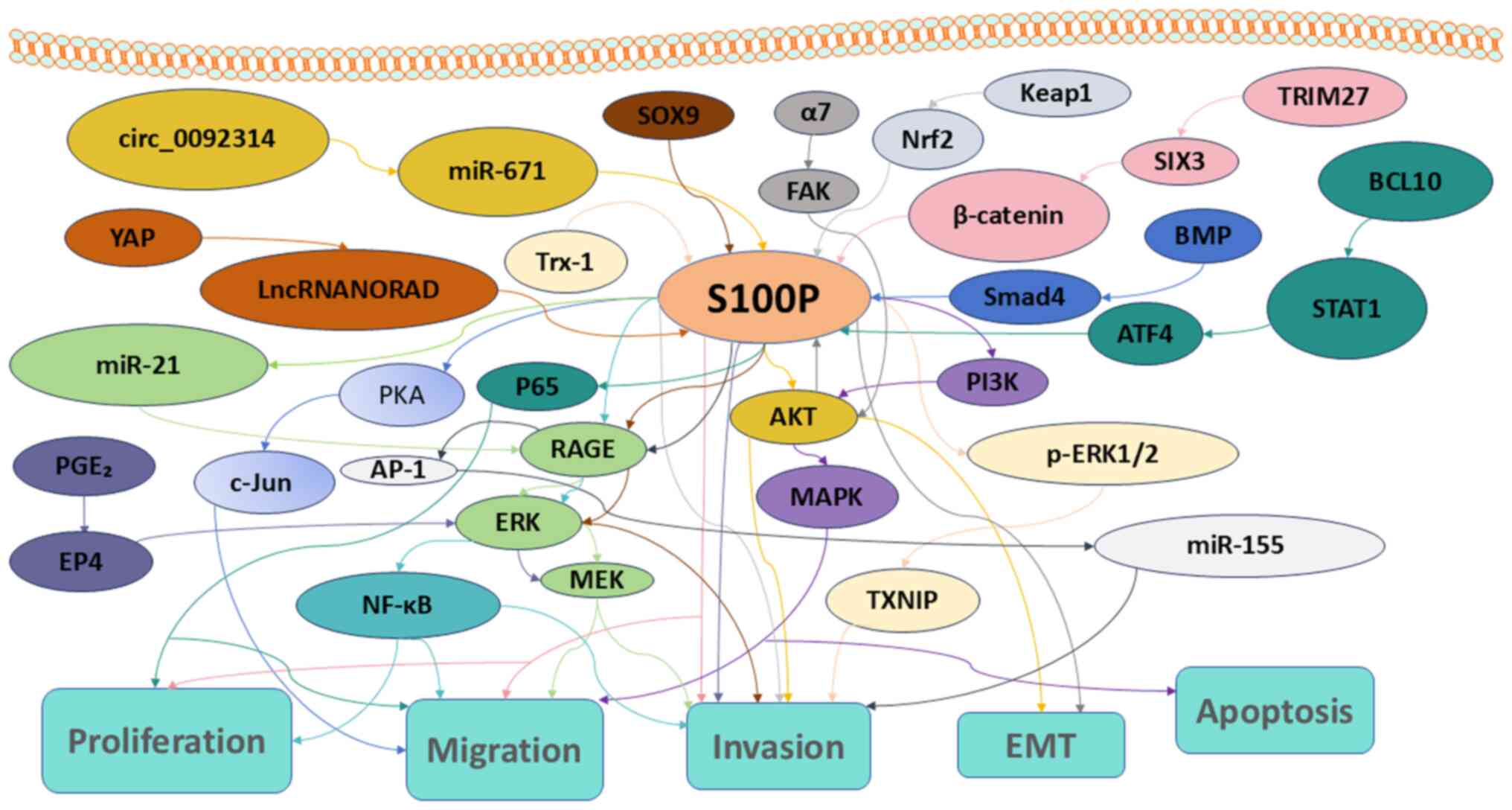 | Figure 1.S100P signaling pathways associated
with proliferation, apoptosis, migration, invasion and EMT in
tumors. EMT, epithelial to mesenchymal transition; miR, microRNA.
YAP, yes-associated protein; lncRNA NORAD, long non-coding RNA
activated by DNA damage; PGE2, prostaglandin
E2; EP4, E-type prostanoid receptor 4; Trx-1,
thioredoxin-1; PKA, protein kinase A; c-Jun, cellular Jun
proto-oncogene; AP-1, activator protein 1; NF-κΒ, nuclear factor
κ-light-chain-enhancer of activated B cells,; SOX9, SRY-Box
transcription factor 9; RAGE, receptor for advanced glycation
end-products; ERK, extracellular signal-regulated kinase; MEK, MAPK
kinase; FAK, focal adhesion kinase; MAPK, mitogen-activated protein
kinase; AKT, protein kinase B; TXNIP, thioredoxin interacting
protein; Keap1, kelch-like ECH associated protein-1; TRIM27,
tripartite motif 27; SIX3, sine oculis homeobox homolog 3; BMP,
bone morphogenetic protein; Smad4, mothers against decapentaplegic
homolog 4; PI3K, phosphoinositide 3-kinase; BCL10, B-cell lymphoma
10; STAT1, signal transducer and activator of transcription 1;
ATF4, activating transcription factor 4. |
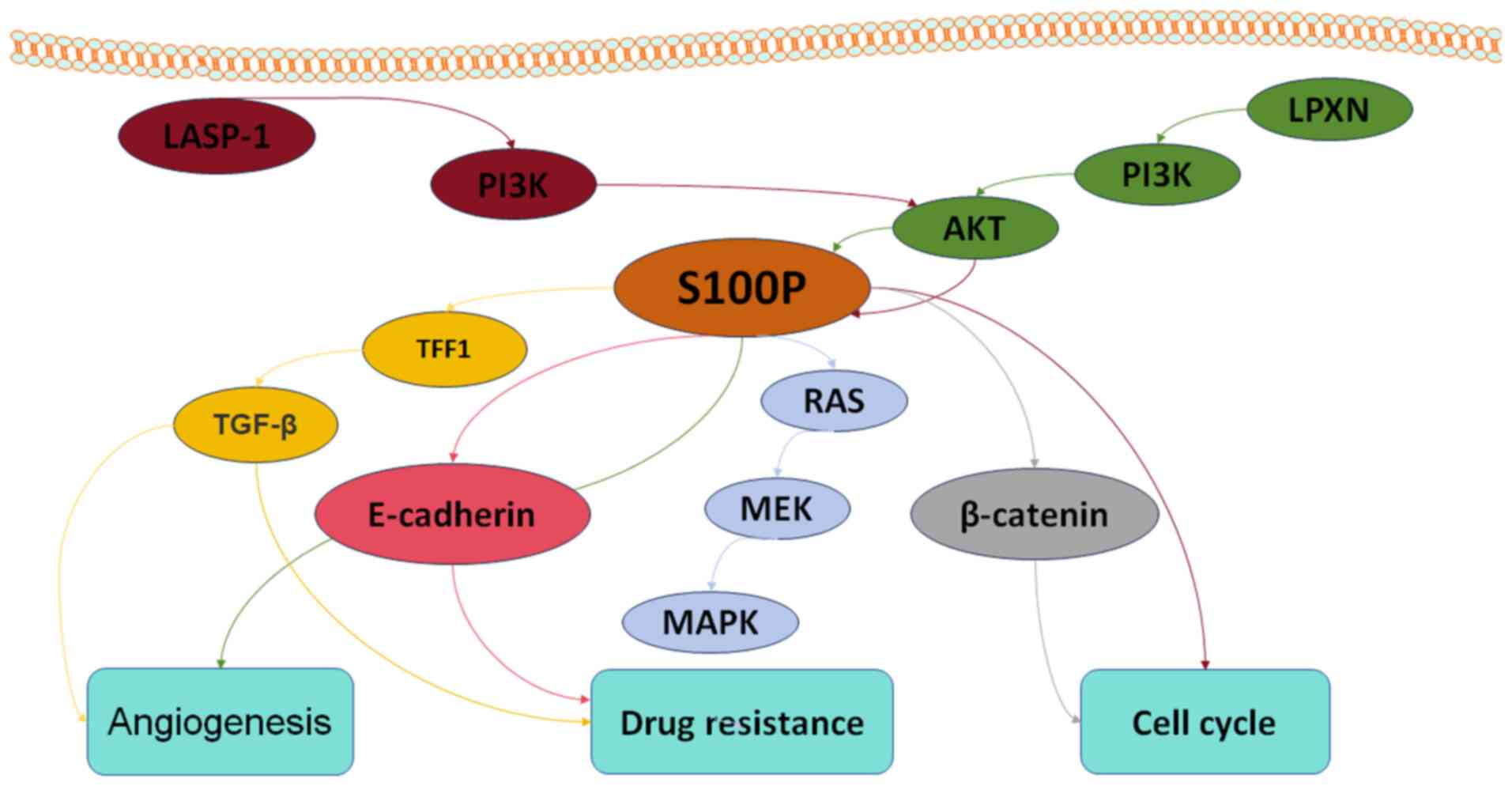 | Figure 2.S100P signaling pathways associated
with angiogenesis, cell cycle and drug resistance in tumors.
LASP-1, LIM and SH3 domain protein 1; PI3K, phosphoinositide
3-kinase; LPXN, leupaxin; AKT, protein kinase B; TFF1, trefoil
factor 1; TGF-β, transforming growth factor-β; RAS, rat sarcoma
viral oncogene homolog; MEK, MAPK kinase; MAPK, mitogen-activated
protein kinase. |
The critical regulatory functions of S100P encompass
a complex network of interactions, including protein-protein and
protein-nucleic acid interactions, which are mediated by signaling
pathways and ncRNAs. Consequently, a review of the S100P-related
signaling pathways in cancer was provided below to establish a
foundation for further clinical translation, as detailed in
Table II.
 | Table II.Signaling pathway in which S100P
participates. |
Table II.
Signaling pathway in which S100P
participates.
| Cancer type | Signaling
pathway | Role in cancer | Tumor
suppressor/promoter role | (Refs.) |
|---|
| Breast cancer |
S100P/RAS/MEK/MAPK | Drug
resistance | Promotion | (33) |
| Lung cancer and
breast cancer | YAP/LncRNA
NORAD/S100P | Migration,
invasion | Suppression | (34) |
| Lung cancer |
α7/FAK/AKT/S100P | Migration,
invasion, EMT | Promotion | (42) |
| Non-small cell lung
cancer |
S100P/TFF1/TGFb | Proliferation,
angiogenesis, drug resistance | Promotion | (12) |
|
|
TRIM27/SIX3/Wnt/β-catenin/S100P | Proliferation,
migration, invasion | Promotion | (49) |
|
|
Keap1/Nrf2/S100P | Migration,
invasion | Suppression | (50) |
| Lung
adenocarcinoma |
S100P/PKA/c-Jun | Migration,
polarization | Promotion | (51) |
| Pancreatic
cancer |
circ_0092314/miR-671/S100P/AKT | EMT, proliferation,
invasion | Promotion | (28) |
|
|
BMP/Smad4/S100P | Migration | Promotion | (95) |
| Bladder cancer |
LPXN/PI3K/AKT/S100P | Proliferation,
invasion, angiogenesis | Promotion | (43) |
| Gallbladder
cancer |
LASP-1/PI3K/AKT/S100P | Proliferation,
migration, cell cycle | Promotion | (44) |
| Endometrial
cancer |
S100P/PI3K-AKT/MAPK | Proliferation,
migration, invasion, apoptosis | Promotion | (45) |
|
|
S100P/β-catenin | Proliferation, cell
cycle | Promotion | (94) |
| Colorectal
cancer |
SOX9/S100P/RAGE/ERK | EMT, invasion,
migration | Promotion | (41) |
|
|
S100P/RAGE/ERK1/2/NFkappaB | Proliferation,
migration | Promotion | (46) |
|
|
S100P/RAGE/AP-1/microRNA-155 | Invasion,
migration | Promotion | (47) |
|
|
S100P/RAGE/microRNA-21/ERK/MEK | Invasion,
migration | Promotion | (48) |
|
|
Trx-1/S100P/p-ERK1/2/TXNIP | Invasion,
migration | Promotion | (54) |
|
|
PGE2/EP4/ERK/MEK/S100P | Invasion, colony
formation | Promotion | (55) |
| Gastric cancer |
S100P/E-cadherin | Apoptosis, Drug
resistance |
Suppression/promotion | (52) |
| Oral cancer |
BCL10/STAT1/ATF4/S100P/P65 | Proliferation,
migration, invasion | Promotion | (53) |
S100P and the PI3K/AKT pathway
The PI3K-Akt signaling pathway represents a crucial
signaling network within cells, activated by a variety of cellular
stimuli or toxic injuries. When growth factors bind to receptor
tyrosine kinases or G protein-coupled receptors on the cell
membrane, they initiate the activation of class Ia and Ib PI3K
isoforms, respectively. These activated PI3Ks catalyze the
phosphorylation of phosphatidylinositol at the cell membrane,
resulting in the production of the second messenger
phosphatidylinositol-3,4,5-trisphosphate (PIP3). Subsequently, PIP3
functions as a pivotal molecule in the activation of Akt (protein
kinase B). The PI3K-Akt signaling pathway ensures that cells can
appropriately respond to external stimuli or damage, thereby
maintaining normal cellular function and homeostasis.
In bladder cancer and gallbladder cancer, S100P has
been confirmed as a target of the PI3K/AKT pathway, promoting
cancer cell invasion and metastasis (43,44).
For instance, Hou et al (43) demonstrated that Leupaxin upregulates
S100P via the PI3K/AKT pathway in bladder cancer. In addition, Li
et al (44) reported that in
gallbladder cancer, LIM and SH3 domain protein 1 downregulates
S100P through the PI3K/AKT pathway, leading to cell cycle arrest.
Of note, in endometrial cancer, S100P enhances the growth and
metastasis of cancer cells by regulating the PI3K/AKT signaling
pathway and can serve as a marker to differentiate between the
squamous cell carcinoma and adenosquamous cell carcinoma subtypes
of endometrial cancer (45).
Knockdown of miR-671 results in the overexpression
of S100P, which enhances the signaling expression of the AKT
pathway, ultimately promoting the EMT process in pancreatic cancer
(28). Similarly, S100P facilitates
EMT, migration and invasion of CRC cells by upregulating S100A4,
thereby activating the AKT signaling pathway (39). In lung cancer, integrin α7 serves as
a binding receptor for S100P, which activates the FAK/AKT axis and
promotes EMT. This further demonstrates the role of the AKT
signaling pathway in tumor invasion and metastasis (42). Numerous studies have suggested that
the activation of S100P within the AKT pathway may contribute to
its carcinogenic effects.
S100P and RAGE/ERK pathways
RAGE activates various signaling pathways, including
MAPK, ERK, PI3K and NF-κB, which regulate critical cellular
processes such as the cell cycle, gene expression, chronic
inflammation and extracellular matrix synthesis. In this context,
the role of S100P as an upstream regulator of the RAGE/ERK pathway
in modulating the phenotypic functions of cancer cells may be
emphasized. The first study, published by Fuentes et al
(46) in 2007, focused on colon
cancer and revealed that, while RAGE was present in both normal and
malignant tissues, S100P expression was restricted to malignant
specimens. The introduction of exogenous S100P to SW480 cells
resulted in the stimulation of ERK1/2 phosphorylation and NF-κB
activity, thereby promoting biological processes such as
proliferation and migration. Co-immunoprecipitation studies
confirmed an interaction between S100P and RAGE; however, the
precise mechanism underlying this interaction remains to be
elucidated (46). Following this,
numerous researchers have further explored this relationship. For
instance, Onyeagucha et al (47) demonstrated that S100P activates
RAGE, which in turn influences the expression of miRNA-155 through
the activator protein-1 (AP-1) in colon cancer cells. Conversely,
the inhibition of MAPK kinase (MEK) using dominant-negative c-Jun
(TAM67) or through genetic suppression of c-Jun activation
attenuated AP-1 activity, leading to a decrease in miR-155
induction by S100P (47).
Furthermore, they discovered that S100P enhanced miR-21 expression
via the RAGE pathway, with ERK/MEK also playing a regulatory role
(48). Additionally, S100P was
shown to stimulate the gene promoters of these two miRNAs,
enriching c-Fos and AP-1 family members (47,48).
Similarly, Shen et al (41)
investigated the regulation of SOX9 on S100P and the RAGE/ERK
pathway, further substantiating the role of S100P in promoting
tumor growth and invasion within this signaling context in colon
cancer.
In addition to the two highlighted pathways
mentioned above, S100P plays a significant role in various other
signaling pathways. In lung cancer, S100P, in conjunction with
trefoil factor 1 (TFF1), enhances the activity of the TGFβ
signaling pathway, thereby promoting the proliferation and
angiogenesis of non-small cell lung cancer (NSCLC) through its
interaction with spread through air spaces (combined aerospatial
diffusion), a recognized aggressive mode in lung cancer (12). Furthermore, in NSCLC, tripartite
motif containing 27 inhibits the downregulation of sine oculis
homeobox homolog 3 in the β-catenin signaling pathway, leading to
an increase in S100P expression, which facilitates tumor migration
and invasion (49). Notably,
overexpression of Keap1 and the knockdown of Nrf2 both aim to
decrease S100P expression independently; additionally, Keap1
inhibits Nrf2 expression, which further suppresses the migration
and invasion of NSCLC (50). In
lung adenocarcinoma, S100P enhances the secretion of chemokines and
polarizing factors in tumor-associated macrophages (TAM) by
activating the PKA/c-Jun pathway, thereby promoting tumor growth
(51). In the context of lung
cancer, lncRNA NORAD inhibits the Hippo/ yes-associated protein
(YAP) pathway, leading to a reduction in S100P functional
expression, which in turn suppresses both the growth and metastasis
of lung cancer (34). Regarding
gastric cancer, researchers have found that the effect of S100P on
the growth and apoptosis of gastric cancer cells is contingent upon
the expression of E-cadherin in tumor cells. Specifically, S100P
promotes the growth of cancer cells in patients with
E-cadherin-negative tumors while inhibiting the expression of
E-cadherin in tumor subpopulations, thus affecting the biological
behavior of these cells (52). In
oral cancer, Wu et al (53)
demonstrated that B-cell lymphoma/leukemia 10 enhances the
progression of oral cancer by upregulating S100P through the
STAT1/activating transcription factor 4 axis, while simultaneously
reversing P65 activation. In CRC, Trx-1 activates S100P gene
transcription; concurrently, S100P promotes Trx-1 expression and
nuclear localization by upregulating phosphorylated ERK1/2 and
downregulating thioredoxin-interacting protein expression,
facilitating CRC cell invasion and metastasis (54). Additionally, another study indicated
that increased prostaglandin E2/E-type prostanoid
receptor 4 expression of S100P correlates with elevated ERK levels
(55). In breast cancer, Merry
et al (33) found that
epigenomic changes at the enhancer level drive S100P upregulation,
activating RAS/MEK/MAPK pathways to compensate for trastuzumab's
inhibition of human EGFR2. LncRNA NORAD is repressed by the YAP
pathway and suppresses lung and breast cancer metastasis by
sequestering S100P (34).
Targeting S100P in cancer therapy
The high degree of tumor heterogeneity and the
complexity of the tumor microenvironment (TME) significantly
influence the occurrence, development and prognosis of cancer
therapies. Consequently, this section summarizes the latest
advancements in S100P therapy.
Granulocyte-macrophage colony-stimulating factor
(GM-CSF) is a pleiotropic myelogenic growth factor and
pro-inflammatory cytokine that has clinical applications across
various indications, making it a promising target for cancer
treatment. Vaccines that incorporate GM-CSF in their therapy may
stimulate effective anti-tumor responses by promoting the
differentiation and activation of dendritic cells (56). One study confirmed that the ‘hinge’
region of S100P and the F89 residues are involved in GM-CSF
recognition. When the same concentration of GM-CSF and S100P was
introduced to THP-1 cells, their combined effect resulted in a
significant reduction in cell viability. In addition, the study
predicted that GM-CSF binding should inhibit S100P from interacting
with RAGE (57), which aligns with
the above-mentioned descriptions in the signaling pathway section.
Under physiological conditions in vitro, S100P interacts
specifically with GM-CSF and several other four-helical cytokines
(58). The similar effects of S100P
binding to another four-helical cytokine, IFN-β, on MCF-7 breast
cancer cell viability (59,60) suggest substantial potential for
GM-CSF therapies targeting S100P. Currently, GM-CSF is utilized
either alone or in combination with chemotherapy, and monoclonal
antibody/cancer vaccines are undergoing clinical trials against
various cancers (56). Building on
previous studies regarding the relationship between IFN-β and S100P
(59,60), researchers have targeted S100P in
prostate cancer treatment by modulating the interferon pathway.
This study analyzed differential gene expression in humans and
dogs, revealing that S100P and interferon-induced transmembrane
protein-like 1 exhibited increased transcript abundance in both
human and canine prostate cancer. Furthermore, it was found that
members of the S100P co-expressed gene community were enriched in
the interferon pathway (61).
Probe technology serves as a crucial instrument for
both detecting tumor lesions and targeting single gene therapies.
In 2020, Sun et al (62)
developed a novel DNA aptamer, AptS100P-1, specifically targeting
the S100P protein in CRC. This aptamer exhibits high specificity
and affinity, effectively binding to S100P to inhibit tumor growth
(62). Although ELISA, IHC and mass
spectrometry are widely recognized diagnostic methods for protein
biomarkers, they often involve complex procedures that are
unsuitable for large-scale sample analyses (63,64).
Surface-enhanced Raman spectroscopy, a vibrational fingerprint
spectroscopy technique, is renowned for its exceptional sensitivity
and accuracy. Recent studies indicate that scholars have employed
this technology in conjunction with molecular probe techniques to
detect biomarkers such as S100P, facilitating the early detection
and assessment of CRC (65,66).
Targeted therapy operates at the molecular level,
focusing on specific carcinogenic targets. It utilizes drugs or
other interventions to disrupt, inhibit and prevent the occurrence,
growth and spread of tumors. In a recent study by Ahmed et
al (67), a small-molecule
therapy for pancreatic ductal adenocarcinoma was developed. Their
approach successfully downregulated the quadruplex expression of
S100P using a tetrad sequence designed to target discrete ‘signals’
within S100P. Furthermore, the combination of the naphthalene
diimide compound QN-302 with the S100P promoter G-quadruplex
significantly reduced tumor proliferation. Although still under
development, QN-302 has been granted orphan drug status by the US
Food and Drug Administration for the treatment of pancreatic cancer
(67).
Additionally, the results of current clinical trials
and the breakthrough progress of S100P were summarized based on
data from PubMed. Due to the absence of reliable markers for the
early diagnosis of pancreatic cancer, the sensitivity and
specificity of routine serum CA19-9 are notably low. Furthermore,
imaging and minimally invasive tests do not serve as universal
screening tools. Consequently, researchers in the US and Japan have
made significant advancements in the investigation of S100P in
duodenal pancreatic juice as a standard for the early diagnosis of
pancreatic cancer (ClinicalTrials.gov ID, NCT01699698).
In breast cancer research, an ongoing clinical
diagnostic study has identified S100P as one of the diagnostic
criteria. Compared to traditional mammography, Visualized Tissue
Metabolism is a functional imaging method that displays metabolic
intensity in real time using colors from the visible spectrum. This
technique can detect metabolic changes prior to anatomical
transformation. It offers advantages such as the absence of
radiation, the need for contrast agents, pain or physical contact,
and it can be utilized without restrictions on exposure time.
Although the study has not yet yielded successful outcomes, it
indicates significant potential for detecting S100P before the
formation of a lump or tumor (ClinicalTrials.gov ID, NCT06045572).
In addition to the aforementioned S100P therapies,
which are still in the development stage, the current studies on
the prognostic modeling of S100P following clinical treatment were
also analyzed. In lung cancer, the combination of S100P and TFF1
has demonstrated a poor prognostic effect in two clinical trial
cohorts [EGFR-tyrosine kinase inhibitor (TKI) therapy and
immunotherapy], adversely affecting patient survival (12). Another study elucidates a novel
prognostic model incorporating S100P and the TME, which similarly
indicates a poor prognosis in the context of immunotherapy
(68–70). Researchers have also integrated
S100P into the prognostic models of TME and TAM (71,72).
Yu et al (73) proposed the
inclusion of drug sensitivity in the risk prediction model,
highlighting that S100P is highly expressed in high-risk patients
as a gene associated with drug resistance. Zhou et al
(74) identified specific drug
resistance genes, focusing on the S100P-based model following
gefitinib and erlotinib treatment targeting EGFR-TKIs. They found
that high-risk patients exhibited a low immune infiltration score,
which holds significant potential for predicting drug responses
(e.g., Docetaxel and Sorafenib) (74). The study of tertiary lymphoid
structures (TLS) plays a crucial role in enhancing therapeutic
efficacy and in predicting and evaluating the effectiveness of
immunotherapy drugs. Consequently, some scholars have developed a
nomogram predictive scoring model based on TLS, elucidating the
relationship between S100P, drug sensitivity, tumor mutation burden
and cancer stem cells (75). In the
context of oxidative regulation, a prognostic model based on
oxidative stress factors has demonstrated that S100P correlates
with poor prognosis (76). Li et
al (77) characterized the
interplay between immunity and hypoxia within the TME as a
prognostic model, revealing that S100P also indicates a poor
prognosis. Autophagy serves as a key regulator of programmed cell
death; thus, researchers have integrated autophagy and immune
subtypes into a two-dimensional index to construct a prognostic
model, wherein the high-risk group expressing S100P showed
sensitivity to autophagy inhibition (78). In cervical cancer (CC), the
nicotinamide adenine dinucleotide (NAD+) metabolizing-related gene
S100P is thought to contribute to cancer pathogenesis and can
predict survival and prognosis in CC. Targeting NAD+ is viewed as a
promising therapeutic approach in oncology (79). In CRC, researchers have established
prognostic models incorporating urea cycle genes and
immune-infiltrating cells, substantiating that S100P is a reliable
independent risk predictor (80).
Given the high recurrence risk associated with cancer, addressing
the reduction of recurrence probability post-treatment is a
critical concern. The expression model of S100P associated with
small extracellular vesicles can effectively predict the risk of
CRC recurrence (20). Furthermore,
another study highlighted the role of S100P in predicting early
recurrence following HCC resection (21).
Limitations of S100P
While the significance of S100P in pan-cancer
regarding its biological functions and mechanisms has been
highlighted in the present review, its role in melanoma remains
underexplored. S100P is notably expressed in both primary and
metastatic melanoma (81,82); however, one study indicates that its
expression level is lower in metastatic melanoma compared to normal
tissues (82). This observation
suggests that S100P may have a negative role in more aggressive,
dedifferentiated melanoma. Furthermore, S100P lacks specificity in
epidermal melanocytes. In normal skin tissue, S100P is highly
expressed in the inner layer of the thoracic duct and in the ducts
of eccrine sweat glands; however, melanocytes exhibit strong
immunoreactivity to S100A4 (83).
Other contents of S100P
In contrast to the interrelated research content
discussed previously, this section presents relatively independent
studies on S100P regarding clinical prognosis and signaling
pathways. In small cell lung cancer, S100P is highly expressed and
negatively correlates with overall survival (OS) and
progression-free survival (PFS) (12,84).
In hepatocellular carcinoma, S100P is also highly expressed and
negatively correlates with OS and recurrence-free survival (RFS)
(21,85). In intrahepatic cholangiocarcinoma,
elevated S100P levels are associated with OS and PFS (15,86).
In gallbladder cancer, increased S100P indicates a reduction in OS
(87,88). In breast cancer, elevated S100P has
been shown to negatively correlate with RFS (89–91).
In ovarian cancer, an increase in S100P has been found to correlate
with a decrease in OS (92,93), as detailed in Table I. Regarding signaling pathways, in
addition to the PI3K/AKT and RAGE/ERK pathways discussed earlier,
S100P plays significant roles in other pathways as well. For
example, in endometrial cancer, S100P promotes cancer cell
proliferation by regulating β-catenin (94). In pancreatic cancer, S100P
facilitates cancer cell migration through the BMP/Smad4/S100P axis
(95), as detailed in Table II.
Conclusion
In this review, the potential of the S100P gene as a
biomarker was thoroughly explored, highlighting its critical role
in cancer metastasis and the associated regulatory mechanisms.
S100P is not only upregulated in various tumor types but also
closely linked to tumor aggressiveness and metastatic potential
(Fig. 3). The upstream regulatory
factors of S100P were analyzed and several signaling pathways were
identified, including PI3K/AKT and RAGE/ERK, which underscore its
multifaceted roles in tumor cell biology, such as promoting cell
proliferation, migration and anti-apoptotic processes. This
analysis offers a new perspective on understanding the functions of
S100P within the TME.
These findings provide new insights into the
mechanisms by which S100P is involved in cancer metastasis and
establish a foundation for future targeted therapeutic strategies.
Targeting S100P presents promising prospects and could yield new
treatment options for cancer patients. Furthermore, future research
should utilize genomic and proteomic approaches to further explore
the functional differences of S100P across various tumor types and
its potential for clinical application, with the aim of enhancing
cancer patient prognoses. The conclusions drawn from this review
offer a novel perspective on the role of S100P in cancer research
and clinical practice, and more in-depth findings in future studies
may be anticipated.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This work was supported by the Natural Science Foundation of
Liaoning Province (grant no. 2023-MSLH-355), the Natural Science
Foundation of Liaoning Province (grant no. 2023JH2/101700095), the
Medical Scientific Research Foundation of Guangdong Province (grant
no. A2022522) and Research Incubation Program of the Stomatological
Hospital of Southern Medical University (grant no. PY2021027).
Availability of data and materials
Not applicable.
Authors' contributions
All authors contributed to the study conception and
design. Data collection and analysis were performed by XW, DZ, EZ
and YG. The first draft of the manuscript was written by XW and DZ.
FC, YX and JL wrote some of the content and revised the paper. ZZ
and XL approved the final version and contributed to the conception
of the paper. All authors commented on previous versions of the
manuscript. All authors read and approved the final manuscript.
Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Becker T, Gerke V, Kube E and Weber K:
S100P, a novel Ca(2+)-binding protein from human placenta. cDNA
cloning, recombinant protein expression and Ca2+ binding
properties. Eur J Biochem. 207:541–547. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zimmer DB, Eubanks JO, Ramakrishnan D and
Criscitiello MF: Evolution of the S100 family of calcium sensor
proteins. Cell Calcium. 53:170–179. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gonzalez LL, Garrie K and Turner MD: Role
of S100 proteins in health and disease. Biochim Biophys Acta Mol
Cell Res. 1867:1186772020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sapkota D, Costea DE, Blø M, Bruland O,
Lorens JB, Vasstrand EN and Ibrahim SO: S100A14 inhibits
proliferation of oral carcinoma derived cells through G1-arrest.
Oral Oncol. 48:219–225. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang H, Wang G, Ding Y, Wang Z,
Barraclough R, Rudland PS, Fernig DG and Rao Z: The crystal
structure at 2A resolution of the Ca2+ -binding protein S100P. J
Mol Biol. 325:785–779. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Donato R: S100: A multigenic family of
calcium-modulated proteins of the EF-hand type with intracellular
and extracellular functional roles. Int J Biochem Cell Biol.
33:637–668. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shang X, Cheng H and Zhou R: Chromosomal
mapping, differential origin and evolution of the S100 gene family.
Genet Sel Evol. 40:449–464. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Prica F, Radon T, Cheng Y and
Crnogorac-Jurcevic T: The life and works of S100P-from conception
to cancer. Am J Cancer Res. 6:562–576. 2016.PubMed/NCBI
|
|
9
|
Zhu HY, Tong XM, Lin XN, Jiang LY, Wang JX
and Zhang SY: Expression and distribution of Calcium-Binding
protein S100P in human placenta during pregnancy. Int J Fertil
Steril. 8:445–452. 2015.PubMed/NCBI
|
|
10
|
Arumugam T and Logsdon CD: S100P: A novel
therapeutic target for cancer. Amino Acids. 41:893–899. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gibadulinova A, Tothova V, Pastorek J and
Pastorekova S: Transcriptional regulation and functional
implication of S100P in cancer. Amino Acids. 41:885–892. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fan G, Xie T, Yang M, Li L, Tang L, Han X
and Shi Y: Spatial analyses revealed S100P + TFF1 + tumor cells in
spread through air spaces samples correlated with undesirable
therapy response in non-small cell lung cancer. J Transl Med.
22:9172024. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hao W, Zhang Y, Dou J, Cui P and Zhu J:
S100P as a potential biomarker for immunosuppressive
microenvironment in pancreatic cancer: A bioinformatics analysis
and in vitro study. BMC Cancer. 23:9972023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Srivastava K, Lines KE, Jach D and
Crnogorac-Jurcevic T: S100PBP is regulated by mutated KRAS and
plays a tumour suppressor role in pancreatic cancer. Oncogene.
42:3422–3434. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xuan Z, Liu L, Zhang G, Zheng X, Jiang J,
Wang K and Huang P: Novel cell subtypes of SPP1 + S100P+,
MS4A1-SPP1 + S100P+ were key subpopulations in intrahepatic
cholangiocarcinoma. Biochim Biophys Acta Gen Subj. 1867:1304202023.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yoshizawa T, Uehara T, Iwaya M, Nakajima
T, Shimizu A, Kubota K, Notake T, Kitagawa N, Masuo H, Sakai H, et
al: An immunohistochemical analysis of osteopontin and S100
Calcium-binding protein P is useful for subclassifying Large- and
Small-duct type intrahepatic cholangiocarcinomas. Am J Surg Pathol.
48:751–760. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Schmid F, Dahlmann M, Röhrich H, Kobelt D,
Hoffmann J, Burock S, Walther W and Stein U: Calcium-binding
protein S100P is a new target gene of MACC1, drives colorectal
cancer metastasis and serves as a prognostic biomarker. Br J
Cancer. 127:675–685. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang XY, Zhu WW, Wang Z, Huang JB, Wang
SH, Bai FM, Li TE, Zhu Y, Zhao J, Yang X, et al: Driver mutations
of intrahepatic cholangiocarcinoma shape clinically relevant
genomic clusters with distinct molecular features and therapeutic
vulnerabilities. Theranostics. 12:260–276. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sano N, Tabata K, Oda T, Yanagita M,
Suzuki T, Komatsubara T, Kawata H and Fukushima N: Bile cytology
diagnosis in challenging cases: Validation of diagnostic bile
cytology criteria and extensive study for immunocytochemical
markers. Diagn Cytopathol. 50:123–132. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ji L, Fu J, Hao J, Ji Y, Wang H, Wang Z,
Wang P and Xiao H: Proteomics analysis of tissue small
extracellular vesicles reveals protein panels for the reoccurrence
prediction of colorectal cancer. J Proteomics. 249:1043472021.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hwang HS, An J, Kang HJ, Oh B, Oh YJ, Oh
JH, Kim W, Sung CO, Shim JH and Yu E: Prognostic molecular indices
of resectable hepatocellular carcinoma: Implications of S100P for
early recurrence. Ann Surg Oncol. 28:6466–6478. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Qi LN, Ma L, Wu FX, Chen YY, Xing WT,
Jiang ZJ, Zhong JH, Chen ZS, Gong WF, Ye JZ, et al: S100P as a
novel biomarker of microvascular invasion and portal vein tumor
thrombus in hepatocellular carcinoma. Hepatol Int. 15:114–126.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim BH, Kwon M, Lee D, Park SW and Shin E:
K-ras mutation detected by peptide nucleic acid-clamping polymerase
chain reaction, Ki-67, S100P, and SMAD4 expression can improve the
diagnostic accuracy of inconclusive pancreatic EUS-FNB specimens.
Pancreatology. 24:584–591. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hadjicharalambous MR and Lindsay MA: Long
Non-coding RNAs and the innate immune response. Noncoding RNA.
5:342019.PubMed/NCBI
|
|
25
|
Ghafouri-Fard S, Majidpoor J, Shoorei H,
Hussen BM, Hadayat Jamal H, Baniahmad A, Taheri M and Mokhtari M:
The interaction between Non-Coding RNAs and calcium binding
proteins. Front Oncol. 12:8483762022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lobera ES, Varela MA, Jimenez RL and
Moreno RB: miRNA as biomarker in lung cancer. Mol Biol Rep.
50:9521–9527. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jiang PF, Zhang XJ, Song CY, Zhang YX and
Wu Y: S100P acts as a target of miR-495 in pancreatic cancer
through bioinformatics analysis and experimental verification.
Kaohsiung J Med Sci. 37:562–571. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shen Q, Zheng G, Zhou Y, Tong J, Xu S, Gao
H, Zhang X and Fu Q: CircRNA circ_0092314 induces
Epithelial-mesenchymal transition of pancreatic cancer cells via
elevating the expression of S100P by sponging miR-671. Front Oncol.
11:6754422021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hamada S, Masamune A, Miura S, Satoh K and
Shimosegawa T: MiR-365 induces gemcitabine resistance in pancreatic
cancer cells by targeting the adaptor protein SHC1 and
pro-apoptotic regulator BAX. Cell Signal. 26:179–185. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hidalgo M: Pancreatic cancer. N Engl J
Med. 362:1605–1617. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Su KG, Savino C, Marracci G, Chaudhary P,
Yu X, Morris B, Galipeau D, Giorgio M, Forte M and Bourdette D:
Genetic inactivation of the p66 isoform of ShcA is neuroprotective
in a murine model of multiple sclerosis. Eur J Neurosci.
35:562–571. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lin C, Zhao XY, Li L, Liu HY, Cao K, Wan
Y, Liu XY, Nie CL, Liu L, Tong AP, et al: NOXA-induced alterations
in the Bax/Smac axis enhance sensitivity of ovarian cancer cells to
cisplatin. PLoS One. 7:e367222012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Merry CR, McMahon S, Forrest ME, Bartels
CF, Saiakhova A, Bartel CA, Scacheri PC, Thompson CL, Jackson MW,
Harris LN and Khalil AM: Transcriptome-wide identification of mRNAs
and lincRNAs associated with trastuzumab-resistance in
HER2-positive breast cancer. Oncotarget. 7:53230–53244. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tan BS, Yang MC, Singh S, Chou YC, Chen
HY, Wang MY, Wang YC and Chen RH: LncRNA NORAD is repressed by the
YAP pathway and suppresses lung and breast cancer metastasis by
sequestering S100P. Oncogene. 38:5612–5626. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Williams ED, Gao D, Redfern A and Thompson
EW: Controversies around Epithelial-mesenchymal plasticity in
cancer metastasis. Nat Rev Cancer. 19:716–732. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Smith BN and Bhowmick NA: Role of EMT in
metastasis and therapy resistance. J Clin Med. 5:172016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Brabletz T, Kalluri R, Nieto MA and
Weinberg RA: EMT in cancer. Nat Rev Cancer. 18:128–134. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ribatti D, Tamma R and Annese T:
Epithelial-mesenchymal transition in cancer: A historical overview.
Transl Oncol. 13:1007732020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zuo Z, Zhang P, Lin F, Shang W, Bi R, Lu
F, Wu J and Jiang L: Interplay between Trx-1 and S100P promotes
colorectal cancer cell epithelial-mesenchymal transition by
up-regulating S100A4 through AKT activation. J Cell Mol Med.
22:2430–2441. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Shen ZY, Fang Y, Zhen L, Zhu XJ, Chen H,
Liu H, Jiang B, Li GX and Deng HJ: Analysis of the predictive
efficiency of S100P on adverse prognosis and the pathogenesis of
S100P-mediated invasion and metastasis of colon adenocarcinoma.
Cancer Genet. 209:143–153. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Shen Z, Deng H, Fang Y, Zhu X, Ye GT, Yan
L, Liu H and Li G: Identification of the interplay between SOX9 and
S100P in the metastasis and invasion of colon carcinoma.
Oncotarget. 6:20672–20684. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hsu YL, Hung JY, Liang YY, Lin YS, Tsai
MJ, Chou SH, Lu CY and Kuo PL: S100P interacts with integrin α7 and
increases cancer cell migration and invasion in lung cancer.
Oncotarget. 6:29585–29598. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hou T, Zhou L, Wang L, Kazobinka G, Chen
Y, Zhang X and Chen Z: Leupaxin promotes bladder cancer
proliferation, metastasis, and angiogenesis through the PI3K/AKT
pathway. Cell Physiol Biochem. 47:2250–2260. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Li Z, Chen Y, Wang X, Zhang H, Zhang Y,
Gao Y, Weng M, Wang L, Liang H, Li M, et al: LASP-1 induces
proliferation, metastasis and cell cycle arrest at the G2/M phase
in gallbladder cancer by down-regulating S100P via the PI3K/AKT
pathway. Cancer Lett. 372:239–250. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Jiang H, Hu H, Lin F, Lim YP, Hua Y, Tong
X and Zhang S: S100P is overexpressed in squamous cell and
adenosquamous carcinoma subtypes of endometrial cancer and promotes
cancer cell proliferation and invasion. Cancer Invest. 34:477–488.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Fuentes MK, Nigavekar SS, Arumugam T,
Logsdon CD, Schmidt AM, Park JC and Huang EH: RAGE activation by
S100P in colon cancer stimulates growth, migration, and cell
signaling pathways. Dis Colon Rectum. 50:1230–1240. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Onyeagucha BC, Mercado-Pimentel ME,
Hutchison J, Flemington EK and Nelson MA: S100P/RAGE signaling
regulates microRNA-155 expression via AP-1 activation in colon
cancer. Exp Cell Res. 319:2081–2090. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Mercado-Pimentel ME, Onyeagucha BC, Li Q,
Pimentel AC, Jandova J and Nelson MA: The S100P/RAGE signaling
pathway regulates expression of microRNA-21 in colon cancer cells.
FEBS Lett. 589:2388–2393. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Liu S, Tian Y, Zheng Y, Cheng Y, Zhang D,
Jiang J and Li S: TRIM27 acts as an oncogene and regulates cell
proliferation and metastasis in non-small cell lung cancer through
SIX3-β-catenin signaling. Aging (Albany NY). 12:25564–25580. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chien MH, Lee WJ, Hsieh FK, Li CF, Cheng
TY, Wang MY, Chen JS, Chow JM, Jan YH, Hsiao M, et al: Keap1-Nrf2
interaction suppresses cell motility in lung adenocarcinomas by
targeting the S100P protein. Clin Cancer Res. 21:4719–4732. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Gao L, Bai Y, Zhou J, Liang C, Dong Y, Han
T, Liu Y, Guo J, Wu J and Hu D: S100P facilitates LUAD progression
via PKA/c-Jun-mediated tumor-associated macrophage recruitment and
polarization. Cell Signal. 120:1111792024. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Carneiro P, Moreira AM, Figueiredo J,
Barros R, Oliveira P, Fernandes MS, Ferro A, Almeida R, Oliveira C,
Carneiro F, et al: S100P is a molecular determinant of E-cadherin
function in gastric cancer. Cell Commun Signal. 17:1552019.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wu TS, Tan CT, Chang CC, Lin BR, Lai WT,
Chen ST, Kuo MY, Rau CL, Jaw FS and Chang HH: B-cell
lymphoma/leukemia 10 promotes oral cancer progression through
STAT1/ATF4/S100P signaling pathway. Oncogene. 34:1207–1219. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Lin F, Zhang P, Zuo Z, Wang F, Bi R, Shang
W, Wu A, Ye J, Li S, Sun X, et al: Thioredoxin-1 promotes
colorectal cancer invasion and metastasis through crosstalk with
S100P. Cancer Lett. 401:1–10. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Chandramouli A, Mercado-Pimentel ME,
Hutchinson A, Gibadulinová A, Olson ER, Dickinson S, Shañas R,
Davenport J, Owens J, Bhattacharyya AK, et al: The induction of
S100p expression by the Prostaglandin E2
(PGE2)/EP4 receptor signaling pathway in colon cancer
cells. Cancer Biol Ther. 10:1056–1066. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Kumar A, Taghi Khani A, Sanchez Ortiz A
and Swaminathan S: GM-CSF: A Double-edged sword in cancer
immunotherapy. Front Immunol. 13:9012772022. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Kazakov AS, Rastrygina VA, Vologzhannikova
AA, Zemskova MY, Bobrova LA, Deryusheva EI, Permyakova ME, Sokolov
AS, Litus EA, Shevelyova MP, et al: Recognition of
granulocyte-macrophage colony-stimulating factor by specific S100
proteins. Cell Calcium. 119:1028692024. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Kazakov AS, Deryusheva EI, Permyakova ME,
Sokolov AS, Rastrygina VA, Uversky VN, Permyakov EA and Permyakov
SE: Calcium-Bound S100P protein is a promiscuous binding partner of
the Four-helical cytokines. Int J Mol Sci. 23:120002022. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Kazakov AS, Mayorov SA, Deryusheva EI,
Avkhacheva NV, Denessiouk KA, Denesyuk AI, Rastrygina VA, Permyakov
EA and Permyakov SE: Highly specific interaction of monomeric S100P
protein with interferon beta. Int J Biol Macromol. 143:633–639.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Kazakov AS, Sofin AD, Avkhacheva NV,
Deryusheva EI, Rastrygina VA, Permyakova ME, Uversky VN, Permyakov
EA and Permyakov SE: Interferon-β activity is affected by S100B
protein. Int J Mol Sci. 23:19972022. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Angel MR, Séguin B, Löhr CV, Beer TM,
Feliciano J, Ramsey SA and Thomas GV: Comparative transcriptomes of
canine and human prostate cancers identify mediators of castration
resistance. Vet Comp Oncol. 22:629–640. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Sun W, Luo L, Fang D, Tang T, Ni W, Dai B,
Sun H and Jiang L: A Novel DNA aptamer targeting S100P induces
antitumor effects in colorectal cancer cells. Nucleic Acid Ther.
30:402–413. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Nedelkov D: Mass spectrometry-based
immunoassays for the next phase of clinical applications. Expert
Rev Proteomics. 3:631–640. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Hussaini HM, Seo B and Rich AM:
Immunohistochemistry and Immunofluorescence. Methods Mol Biol.
2588:439–450. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Chen F, Huang Y, Liu Y, Zhuang Y, Cao X
and Qin X: SERS analysis platform based on aptamer
Recognition-release strategy for efficient and sensitive diagnosis
of colorectal precancerous lesions. Int J Nanomedicine.
19:10009–10021. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Cao X, Liu Z, Qin X, Gu Y, Huang Y, Qian
Y, Wang Z, Li H, Zhu Q and Wei W: LoC-SERS platform for rapid and
sensitive detection of colorectal cancer protein biomarkers.
Talanta. 270:1255632024. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Ahmed AA, Greenhalf W, Palmer DH, Williams
N, Worthington J, Arshad T, Haider S, Alexandrou E, Guneri D,
Waller ZAE and Neidle S: The potent G-Quadruplex-binding compound
QN-302 downregulates S100P gene expression in cells and in an in
vivo model of pancreatic cancer. Molecules. 28:24522023. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Davoodi-Moghaddam Z, Jafari-Raddani F,
Kordasti S and Bashash D: Identification of an immune-related genes
signature in lung adenocarcinoma to predict survival and response
to immune checkpoint inhibitors. J Egypt Natl Canc Inst. 36:302024.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Xu J, Zhang Y, Li M, Shao Z, Dong Y, Li Q,
Bai H, Duan J, Zhong J, Wan R, et al: A single-cell characterised
signature integrating heterogeneity and microenvironment of lung
adenocarcinoma for prognostic stratification. EBioMedicine.
102:1050922024. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Shu J, Jiang J and Zhao G: Identification
of novel gene signature for lung adenocarcinoma by machine learning
to predict immunotherapy and prognosis. Front Immunol.
14:11778472023. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Sun QY, Zhou Y, Du LJ, Zhang MK, Wang JL,
Ren YY and Liu F: Analysis between macrophage-related genes with
prognosis and tumor microenvironment in non-small cell lung cancer.
Yi Chuan. 45:684–699. 2023.PubMed/NCBI
|
|
72
|
Wu J, Zhou J, Xu Q, Foley R, Guo J, Zhang
X, Tian C, Mu M, Xing Y, Liu Y, et al: Identification of key genes
driving tumor associated macrophage migration and polarization
based on immune fingerprints of lung adenocarcinoma. Front Cell Dev
Biol. 9:7518002021. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Yu H, Zhang W, Xu XR and Chen S: Drug
resistance related genes in lung adenocarcinoma predict patient
prognosis and influence the tumor microenvironment. Sci Rep.
13:96822023. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Zhou E, Wu F, Guo M, Yin Z, Li Y, Li M,
Xia H, Deng J, Yang G and Jin Y: Identification of a novel gene
signature of lung adenocarcinoma based on epidermal growth factor
receptor-tyrosine kinase inhibitor resistance. Front Oncol.
12:10082832022. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Wu S, Pan J, Pan Q, Zeng L, Liang R and Li
Y: Multi-omics profiling and experimental verification of tertiary
lymphoid structure-related genes: Molecular subgroups, immune
infiltration, and prognostic implications in lung adenocarcinoma.
Front Immunol. 15:14532202024. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Yu Y, Liu M, Wang Z, Liu Y, Yao M, Wang L
and Zhong L: Identification of oxidative stress signatures of lung
adenocarcinoma and prediction of patient prognosis or treatment
response with single-cell RNA sequencing and bulk RNA sequencing
data. Int Immunopharmacol. 137:1124952024. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Li Y, Huang H, Jiang M, Yu N, Ye X, Huang
Z and Chen L: Identification and validation of a hypoxia-immune
signature for overall survival prediction in lung adenocarcinoma.
Front Genet. 13:9752792022. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Li Q, Xie D, Yao L, Qiu H, You P, Deng J,
Li C, Zhan W, Weng M, Wu S, et al: Combining autophagy and immune
characterizations to predict prognosis and therapeutic response in
lung adenocarcinoma. Front Immunol. 13:9443782022. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Chen A, Jing W, Qiu J and Zhang R:
Prediction of cervical cancer outcome by identifying and validating
a NAD+ Metabolism-derived gene signature. J Pers Med. 12:20312022.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Guo H, Wang Y, Gou L and Wang X, Tang Y
and Wang X: A novel prognostic model based on urea cycle-related
gene signature for colorectal cancer. Front Surg. 9:10276552022.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Zhu L, Ito T, Nakahara T, Nagae K, Fuyuno
Y, Nakao M, Akahoshi M, Nakagawa R, Tu Y, Uchi H and Furue M:
Upregulation of S100P, receptor for advanced glycation end products
and ezrin in malignant melanoma. J Dermatol. 40:973–979. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Xiong TF, Pan FQ and Li D: Expression and
clinical significance of S100 family genes in patients with
melanoma. Melanoma Res. 29:23–29. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Zhu L, Okano S, Takahara M, Chiba T, Tu Y,
Oda Y and Furue M: Expression of S100 protein family members in
normal skin and sweat gland tumors. J Dermatol Sci. 70:211–219.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Li Q, Wang T, Tang Y, Zou X, Shen Z, Tang
Z, Zhou Y and Shi J: A novel prognostic signature based on
smoking-associated genes for predicting prognosis and immune
microenvironment in NSCLC smokers. Cancer Cell Int. 24:1712024.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Wan R, Tan Z, Qian H, Li P, Zhang J, Zhu
X, Xie P and Ren L: Prognostic value of S100 family mRNA expression
in hepatocellular carcinoma. Turk J Gastroenterol. 35:316–334.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Li Z, Huang N, Du Q, Huang W, Wang B, Wang
B, Shen G, Zhang H, Shi S and Wang L: Role of immunophenotypic
characterisation in prognostic subtyping of intrahepatic
cholangiocarcinoma. Pathology. 55:979–988. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Rawal N, Hariprasad G, Bandyopadhyay S,
Ranjan Dash N, Kumar S, Das P, Dey S, Ahmad Khan M, Ranjan A,
Chopra A, et al: Molecular biomarkers involved in the progression
of gallbladder inflammatory lesions to invasive cancer: A proteomic
approach. Biomol Biomed. 25:115–143. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Mathai AM, Alexander J, Huang HY, Li CF,
Jeng YM, Fung KM, Harris WP, Swanson PE, Truong C and Yeh MM: S100P
as a marker for poor survival and advanced stage in gallbladder
carcinoma. Ann Diagn Pathol. 52:1517362021. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Tian Z, Tang J, Liao X, Yang Q, Wu Y and
Wu G: An immune-related prognostic signature for predicting breast
cancer recurrence. Cancer Med. 9:7672–7685. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Mirza Z, Ansari MS, Iqbal MS, Ahmad N,
Alganmi N, Banjar H, Al-Qahtani MH and Karim S: Identification of
novel diagnostic and prognostic gene signature biomarkers for
breast cancer using artificial intelligence and machine learning
assisted transcriptomics analysis. Cancers (Basel). 15:32372023.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Peng C, Chen H, Wallwiener M, Modugno C,
Cuk K, Madhavan D, Trumpp A, Heil J, Marmé F, Nees J, et al: Plasma
S100P level as a novel prognostic marker of metastatic breast
cancer. Breast Cancer Res Treat. 157:329–338. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Wang X, Tian T, Li X, Zhao M, Lou Y, Qian
J, Liu Z, Chen H and Cui Z: High expression of S100P is associated
with unfavorable prognosis and tumor progression in patients with
epithelial ovarian cancer. Am J Cancer Res. 5:2409–2421.
2015.PubMed/NCBI
|
|
93
|
Umezaki Y, Ito M, Nakashima M, Mihara Y,
Naruke Y, Kurohama H, Yatsunami N and Yasuhi I: S100P is a useful
marker for differentiation of ovarian mucinous tumors. Eur J
Gynaecol Oncol. 36:138–141. 2015.PubMed/NCBI
|
|
94
|
Guo L, Chen S, Jiang H, Huang J, Jin W and
Yao S: The expression of S100P increases and promotes cellular
proliferation by increasing nuclear translocation of β-catenin in
endometrial cancer. Int J Clin Exp Pathol. 7:2102–2112.
2014.PubMed/NCBI
|
|
95
|
Hamada S, Satoh K, Hirota M, Fujibuchi W,
Kanno A, Umino J, Ito H, Satoh A, Kikuta K, Kume K, et al:
Expression of the calcium-binding protein S100P is regulated by
bone morphogenetic protein in pancreatic duct epithelial cell
lines. Cancer Sci. 100:103–110. 2009. View Article : Google Scholar : PubMed/NCBI
|