Introduction
Non-small cell lung carcinoma (NSCLC), responsible
for >80% of all lung cancer cases, is the deadliest form of
malignancy worldwide (1). Despite
advancements in therapeutic strategies such as surgical resection,
chemotherapy, radiotherapy, immunotherapy and combination
therapies, each focusing primarily on symptom alleviation, the
5-year overall survival rate of NSCLC remains <20% (2). This is further complicated by the
widespread occurrence of chemoresistance and the increased
metastatic potential of the disease, which undermine the efficacy
of conventional treatments (3).
This challenge is, at least in part, attributable to the
self-renewal properties of cancer stem cells (4), which are resistant to chemotherapy,
rendering traditional treatments less effective. As such, there is
an urgent need for innovative therapeutic approaches targeting
NSCLC cancer stem cells. Research has intensified efforts to
understand self-renewal and tumor growth mechanisms, aiming to
identify novel therapeutic targets or develop innovative curative
interventions for various cancer types, including NSCLC (4–6).
Studies have identified a small subset of tumor cells that, derived
from bulk tumors, possess self-renewal and tumor-initiating
abilities (7–9). These cells are typically enriched
using cell surface markers (CD133, CD44, Sox2 and Oct4) or sphere
formation assays in suspension culture (4,5,8,10,11).
Notably, the sphere culture assay has proven effective in enriching
sphere-forming cells (SFCs) from NSCLC cell lines, and SFCs exhibit
stronger self-renewal potential (12,13).
There is a critical need to develop effective therapeutic
strategies, particularly novel targeted drugs aimed at directly
addressing cancer cell self-renewal and tumor growth in NSCLC.
Genistein, an isoflavone found abundantly in
soybeans and related products, has exhibited anticancer properties
across various malignancies such as retinoblastoma, laryngeal
cancer and colorectal cancer (14–16).
7-difluoromethoxyl-5,4′-di-n-octylgenistein (DFOG), a new genistein
analog synthesized independently by the Department of Pharmacy,
Hunan Normal University (Changsha, China), has been shown to induce
apoptosis in ovarian cancer cells, and to inhibit their
self-renewal and carcinogenesis (17,18).
Despite these promising findings, the exact mechanism through which
DFOG suppresses self-renewal and tumor growth in NSCLC cells
remains to be fully elucidated.
MicroRNAs (miRNAs/miRs) are evolutionarily
conserved, endogenous small noncoding RNAs, typically 19–23
nucleotides in length (19).
Despite lacking protein-coding potential, they regulate a wide
array of genes post-transcriptionally through complementary binding
to mRNAs (20). These molecules are
critical in processes such as apoptosis, differentiation,
proliferation and metabolism, making them central to cancer
development and stemness in cancer cells (21). One such miRNA, miR-152, has been
identified as a tumor suppressor and is linked to malignant
phenotypes of various types of cancer such as colon cancer, breast
cancer, prostate cancer and ovarian cancer (22–25). A
recent study has demonstrated that miR-152-3p is involved in the
self-renewal and tumor growth of non-small cell lung cancer
(26). Additionally, miR-152-3p is
associated with tumor invasion, metastasis, drug resistance and
proliferation (27,28). However, whether DFOG can inhibit the
self-renewal and tumor growth of NSCLC by regulating miR-152-3p
remains to be determined.
Persistent activation of STAT3 impacts gene
regulation, thereby influencing self-renewal, migration and
invasion in cancer cells (29,30).
The miR-152/STAT3 axis is associated with poor prognosis in
epithelial ovarian cancer (31). A
previous study using JSI-124, a specific STAT3 inhibitor, suggested
that suppressing STAT3 activation can diminish stem cell-like
properties in hepatocellular carcinoma cells (32). However, it remains unclear whether
miR-152-3p-mediated STAT3 inactivation can effectively reduce the
self-renewal and tumor growth of SFCs derived from NSCLC.
Therefore, the present study aimed to examine the hypothesis that
reinstating miR-152-3p expression to suppress STAT3 can
synergistically enhance the inhibitory effects of DFOG on
self-renewal and tumor growth in SFCs derived from NSCLC.
Materials and methods
Cells and sphere cultures
The NCI-H460 and NCI-A549 human NSCLC cell lines
were obtained from Shanghai Zhong Qiao Xin Zhou Biotechnology Co.,
Ltd., and Procell Life Science & Technology Co., Ltd.,
respectively, while the BEP2D human bronchial epithelial cell line
was sourced from Otwo Biotech. All cell lines were authenticated
through short tandem repeat profiling and mycoplasma testing. The
cells were cultured in DMEM (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.),
penicillin (100 U/ml) and streptomycin (100 µg/ml). All cells were
maintained in a 5% CO2 incubator at 37°C. The STAT3
inhibitor S3I 201 (97%; cat. no. ab146606; Abcam) was stored at
room temperature, and the cells were treated with S3I 201 (10 µM)
at 37°C for 24 h before subsequent experiments.
To study sphere formation, H460 and A549 cells were
cultured in stem-cell culture medium at a density of 5,000 cells
per well in ultra-low attachment 6-well plates until spheres
containing >20 cells formed (12,13).
Stem-cell culture medium (DMEM/F12; Gibco; Thermo Fisher
Scientific, Inc.), supplemented with 2% B27, 1%
penicillin/streptomycin, basic fibroblast growth factor (20 ng/ml),
epidermal growth factor (20 ng/ml) and insulin (4 µg/ml), was used.
The inhibitory effects of DFOG on sphere formation were assessed by
incubating SFCs derived from H460 and A549 cells with varying
concentrations of DFOG (1, 5 and 10 µM) at 37°C for 72 h.
Subsequently, the cells were reseeded at a density of 1,000 cells
per well in ultra-low attachment 24-well plates and cultured until
spheres reformed in the absence of DFOG. Subsequently, the number
and status of spheres were evaluated manually under a light
microscope (Leica Microsystems GmbH). The sphere formation rate was
calculated using the following formula: Number of spheres/number of
cells seeded ×100%. Experiments were performed in triplicate.
Cell viability assessment
Cell viability was assessed using the Cell Counting
Kit-8 (CCK-8) assay (Dojindo Laboratories, Inc.). Single-cell
suspensions were seeded at 1,000 cells per well in 96-well plates
for 24 h and treated with varying concentrations of DFOG (1, 5 and
10 µM) at 37°C. After 72 h, the cells were incubated with 10 µl
CCK-8 solution per well for 2 h, and the optical density
(OD450) was measured using a microplate reader (BioTek;
Agilent Technologies, Inc.).
Reverse transcription-quantitative PCR
(RT-qPCR)
The Superscript IV RT kit and SYBR Green fluorophore
were purchased from Thermo Fisher Scientific, Inc. The RT reaction
conditions were incubation at 37°C for 5 min, 50°C for 15 min and
75°C for 5 min. Total RNA was extracted from H460 cells, A549 cells
or SFCs (1×105 cells) using TRIzol® reagent
(Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. For RNA extraction from tissue samples, grinding using a
tissue homogenizer (Beyotime Institute of Biotechnology) was first
performed. cDNA synthesis was conducted according to the supplier's
instructions (Thermo Fisher Scientific, Inc.). The
2−ΔΔCq method (33) was
used for qPCR, with U6 as the internal control for miR-152 and
GAPDH as the internal control for STAT3. To identify candidate
miRNAs affected by DFOG treatment, H460 cells, A549 cells or SFCs
were treated with DFOG (5 µM) at 37°C for 24 h, followed by total
RNA extraction and cDNA synthesis. PCR amplification was performed
using specific primers (Table I),
with the following thermocycling conditions: 95°C for 10 min,
followed by 40 cycles of 95°C for 30 sec, 55°C for 30 sec and 70°C
for 30 sec. For miRNA quantification, 2 µg total miRNA was
transcribed and amplified using the All-in-One™ miRNA qRT-PCR
Detection Kit (Applied Biosystems; Thermo Fisher Scientific, Inc.)
and the TaqMan MicroRNA Assay (GeneCopoeia, Inc.), with U6 (Sangon
Biotech Co., Ltd.) as the reference gene. Data analysis was
conducted using the 2−ΔΔCq method. All experiments were
conducted in triplicate independently.
 | Table I.Primer sequences for reverse
transcription-quantitative PCR. |
Table I.
Primer sequences for reverse
transcription-quantitative PCR.
| Gene name | Primer sequence
(5′-3′) |
|---|
| U6 | F:
CTCGCTTCGGCAGCACA |
|
| R:
AACGCTTCACGAATTTGCGT |
| STAT3 | F:
GGGAGAGAGTTACAGGTTGGACAT |
|
| R:
AGACGCCATTACAAGTGCCA |
| GAPDH | F:
CGGAGTCAACGGATTTGGTCGTAT |
|
| R:
ATCCTTCTCCATGGTGGTGAAGAC |
| miR-671-5p | F:
AGGAAGCCCTGGAGGGGC |
|
| R:
CAGTGCAGGGTCCGAGGTAT |
| miR-148a-3p | F:
TCAGTGCACTACAGAACTTTGT |
|
| R:
AGTGCAGGGTCCGAGGTAT |
| miR-340-5p | F:
TTATAAAGCAATGAGACTGATT |
|
| R:
AGTGCAGGGTCCGAGGTATT |
| miR-342-3p | F:
TCTCACACAGAAATCGCACCC |
|
| R:
AGTGCAGGGTCCGAGGTAT |
| miR-34a-5p | F:
TGGCAGTGTCTTAGCTGGTTGT |
|
| R:
AGTGCAGGGTCCGAGGTATT |
| miR-152-3p | F:
TCAGTGCATGACAGAACTTGG |
|
| R:
TGCAGGGTCCGAGGTAT |
Clonogenic assay
For the colony formation assay, a bottom agar layer
was prepared by mixing 1.2% agarose (Invitrogen; Thermo Fisher
Scientific, Inc.) with DMEM in equal proportions, and 500 µl of
this mixture was added to each well of a 24-well plate. The top
agar layer was prepared by mixing H460 cells, A549 cells or SFCs
(1,000 cells) with 0.7% agarose and 500 µl of 20% FBS-supplemented
DMEM. After 12 h of cell seeding, different concentrations of DFOG
(1, 5 and 10 µM) were added according to the needs of each group.
The drug was continuously administered at 37°C until the end of the
experiment. Images of colony formation were captured under a light
microscope (Leica Microsystems GmbH) and colonies were counted
manually. More than 20 cells were defined as a colony. The cells
were incubated for 14 days at 37°C, and colonies were counted to
calculate the colony formation rate per 1,000 cells based on
triplicate experiments.
Immunoblot assay
The RIPA protein extraction kit was purchased from
Thermo Fisher Scientific, Inc. BCA was used to quantitatively
determine the protein concentration. Each lane was loaded with 20
µg of protein. The gel concentration used was 10%. After
electrophoresis, protein was transferred to a PVDF membrane.
Blocking was performed using 5% skimmed milk at 37°C for 1 h.
Membranes were incubated with the primary antibody at 4°C for 6 h,
and membranes were incubated with the horseradish
peroxidase-conjugated IgG secondary antibody (1:1,000 dilution;
cat. no. RGAR011; Proteintech Group, Inc.) at room temperature for
1 h. Antibodies against α-tubulin (1:1,000 dilution; cat. no. 2125;
Cell Signaling Technology, Inc.), STAT3 (1:1,000 dilution; cat. no.
12640; Cell Signaling Technology, Inc.), phosphorylated-STAT3
(p-STAT3; 1:2,000 dilution; cat. no. 9145; Cell Signaling
Technology, Inc.), CD133 (1:1,000 dilution; cat. no. 64326; Cell
Signaling Technology, Inc.), CD44 (1:1,000 dilution; cat. no.
37259; Cell Signaling Technology, Inc.), Oct4 (1:1,000 dilution;
cat. no. 2890; Cell Signaling Technology, Inc.) and Sox2 (1:1,000
dilution; cat. no. 3579; Cell Signaling Technology, Inc.) were used
as previously described (12).
Finally, the chemiluminescent substrate (ECL) was purchased from
Beyotime Institute of Biotechnology, and ImageJ 1.37 (National
Institutes of Health) was used for gray-scale analysis.
miRNA transfection
MicrON™ miR-152-3p mimic
(5′-UCAGUGCAUGACAGAACUUGG-3′) and micrOFF™ miR-152-3p inhibitor
(5′-CCAAGUUCUGUCAUGCACUGA-3′), miR-152-3p mimic negative control
(5′-UUGUACUACACAAAAGUACUG-3′) and miR-152-3p inhibitor negative
control (5′-GGAACUUAGCCACUGUGAAUU-3′), were purchased from
Guangzhou RiboBio Co., Ltd., and transfected into SFCs using
transfection reagent iboFECT™ CP (Guangzhou RiboBio Co., Ltd.) at a
concentration of 50 nM, according to the manufacturer's
instructions. The small RNA complexes were incubated with cells for
2 h before the medium was replaced, then the culture was continued
at 37°C for 48 h, and cells were used for subsequent
experiments.
Luciferase reporter assay
The binding sites of miR-152-3p and STAT3 were
predicted using RNAhybrid (http://bibiserv.techfak.uni-bielefeld.de/rnahybrid).
For the luciferase reporter assays, SFCs were co-transfected with
miR-152-3p or miR-control and the pGL3 luciferase vector (Guangzhou
RiboBio Co., Ltd.) containing the firefly luciferase reporter,
along with the wild-type (WT) or mutant (MUT) 3′-untranslated
region (UTR) sequence of STAT3. After 48 h, luciferase activity was
measured using a luciferase assay kit (Promega Corporation), and
normalized to Renilla luciferase activity in triplicate
experiments.
Plasmid transfection
Transfection was performed using 10 µg nucleic acid
with a concentration of 1 µg/µl at 37°C for 48 h, and subsequent
experiments were performed 48 h after transfection. For STAT3
overexpression, cells were transfected with pcDNA3.1-Control or
pcDNA3.1-STAT3 plasmids obtained from Invitrogen; Thermo Fisher
Scientific, Inc., using Lipofectamine™ 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.) in Opti-MEM (Gibco; Thermo Fisher
Scientific, Inc.), according to the manufacturer's guidelines.
In vivo therapeutic effects in nude
mice
A total of 18 female pathogen-free nude BALB/c mice
(aged 4–5 weeks; weight, 18–22 g) were sourced from GemPharmatech
Co., Ltd., and housed in a specific pathogen-free facility [SYXK
(Xiang) 2020-0012] under a standard 12-h light/12-h dark cycle, at
20–26°C, with an atmospheric pressure of 20–50 Pa and a relative
humidity of 40–70%, and ad libitum access to regular mouse
chow and water. The site of cell injection was the armpit of the
upper limb. When the tumor grew to ~100 cm3, the mouse
was treated with drug treatment for 21 days. The time interval
between the injection of cells and the end of the experiment was 6
weeks, and tumor volume was detected every 2 days until the end of
the experiment. The ethical approval (approval no. D2023045) was
granted by the Ethics Committee of Hunan Normal University
(Changsha, China).
To evaluate the effects of DFOG in the xenograft
mouse model, 1×106 SFCs were suspended in PBS and mixed
with 100% Matrigel at a 1:1 ratio (BD Biosciences). A 100 µl
mixture was subcutaneously injected into each mouse. When the
xenograft volume reached ~100 mm3, mice in the control
group received 200 µl of 2% DMSO every 2 days, while those in the
experimental groups were orally administered DFOG (10 and 50 mg/kg)
for 3 weeks every 2 days. Each group consisted of 6 mice. Tumor
volume was calculated using the following formula: V
(mm3)=(L × W2)/2, where L is the longest
diameter and W is the shortest diameter of the xenograft, measured
using a Vernier caliper. At the end of the experiment,
xenograft-bearing mice were euthanized using CO2
asphyxiation (CO2 replacement rate of 30%), and the
xenografts were collected, weighed, and snap-frozen in liquid
nitrogen, and tumor tissues were fixed in 4% paraformaldehyde for
subsequent H&E staining and immunohistochemistry. Tumor tissues
for qPCR analysis were preserved in RNAlater.
For H&E staining, tumor tissues were fixed in 4%
paraformaldehyde at 4°C for 24 h, and the slice thickness was 4 µm.
Hematoxylin staining was performed for 5 min, followed by eosin
staining for 1 min, and these were performed at 25°C. Staining was
observed under a light microscope (Leica Microsystems GmbH).
Immunohistochemical staining
Tumor tissues were fixed in 4% paraformaldehyde at
4°C for 24 h. Tissue sections (thickness, 4 µm) from
paraffin-embedded and fixed samples were subjected to
deparaffinization in citrate buffer. Sections were heated in an
oven at 90°C for 20 min, followed by a series of ethanol washes
(anhydrous ethanol, 95, 85 and 75% ethanol). Subsequently, the
slices underwent three consecutive washes with PBS. After blocking
endogenous peroxidase activity with 3% H2O2
and nonspecific binding with 5% goat serum (Beyotime Institute of
Biotechnology) for 15 min at 25°C, the sections were incubated
overnight at 4°C with the primary antibody against p-STAT3 (1:200
dilution; cat. no. 9145; Cell Signaling Technology, Inc.). As a
negative control, PBS was used in place of the primary antibody.
The sections were then incubated with horseradish
peroxidase-conjugated anti-rabbit IgG antibodies (1:500 dilution;
cat. no. RGAR011; Proteintech Group, Inc.) for 20 min at 25°C.
Staining was developed using the 3,3′diaminobenzidine substrate
(Fuzhou Maixin Biotechnology Development Co., Ltd.). The results
were observed and images were captured under a light microscope
(Leica Microsystems GmbH). The signal intensity was evaluated as
previously described (34), and
semi-quantitatively analyzed using ImageJ 1.37.
Statistical analysis
All statistical analyses were performed using
GraphPad Prism Software 9 (Dotmatics). Data are presented as the
mean ± SD. Comparisons between groups were performed using one-way
analysis of variance with Tukey's post hoc test or an unpaired
Student's t-test. For in vitro analyses, experiments were
performed in triplicate. P<0.05 was considered to indicate a
statistically significant difference.
Results
DFOG small-scale miRNAs efficacy
screen, and analysis of self-renewal-related stemness of
H460-derived SFCs
The effect of DFOG on cell viability was assessed in
BEP2D, H460 and A549 cell lines using the CCK-8 assay, following
treatment at various concentrations. A reduction in cell viability
was observed in H460 and A549 cells compared with the control group
(0 µM), yielding an IC50 of ~10 µM (Fig. 1A). Noncytotoxic concentrations of
DFOG (1, 5 and 10 µM) were selected for subsequent experiments.
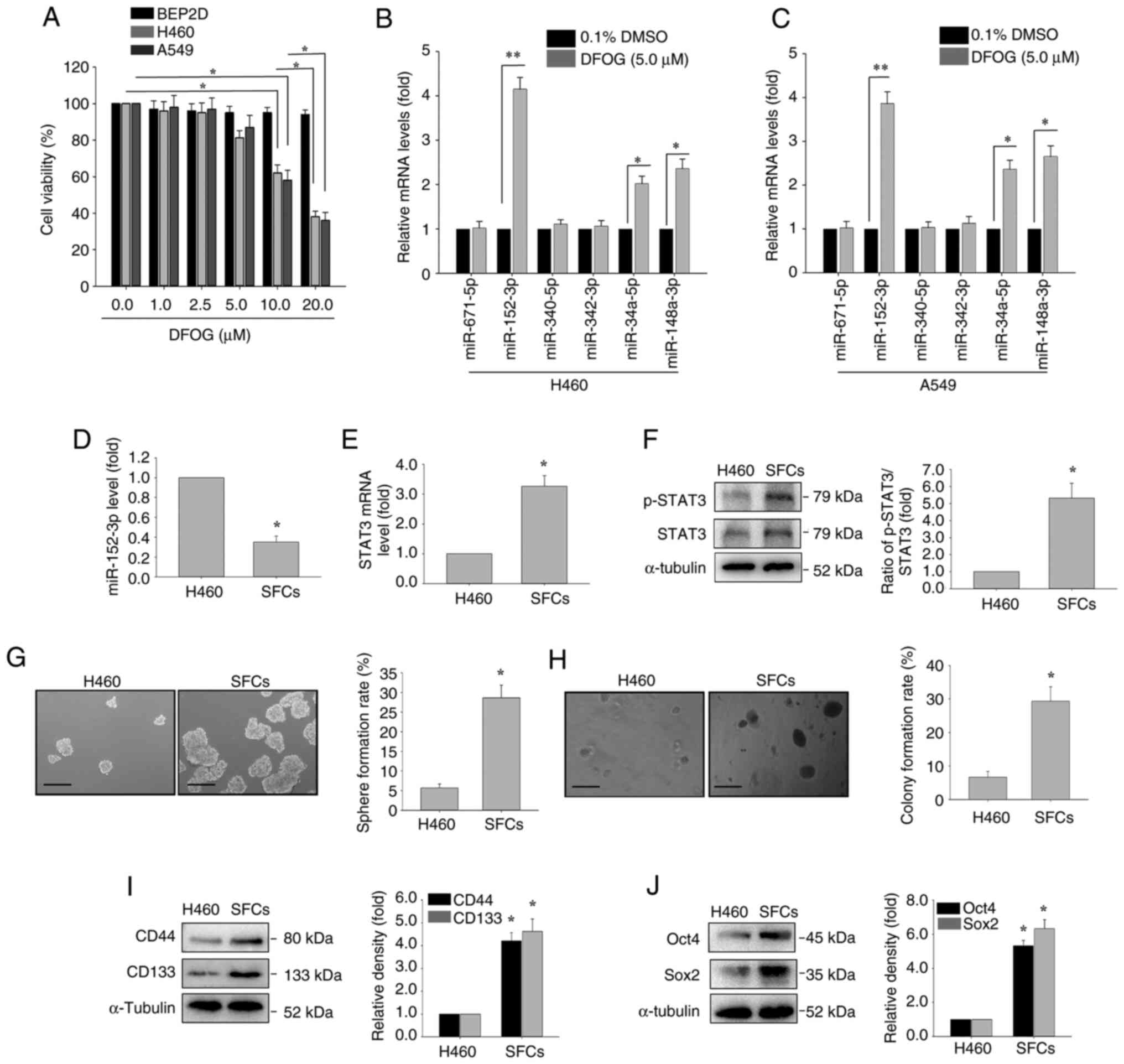 | Figure 1.mRNA expression analysis by RT-qPCR,
and assessment of self-renewal and tumor growth in H460-derived
SFCs. (A) BEP2D, H460 and A549 cells were treated with DFOG (0–20
µM) for 48 h, and cell viability was assessed using a Cell Counting
Kit-8 assay. H460 and A549 cells were treated with DFOG (5 µM) for
24 h. RT-qPCR was used to evaluate the effects of DFOG (5 µM) on
tumor-suppressive miRNAs, including miR-671-5p, miR-148a-3p,
miR-340-5p, miR-342-3p, miR-34a-5p and miR-152-3p in (B) H460 and
(C) A549 cells. (D) Comparison of miR-152-3p expression between
H460 cells and H460-derived SFCs. (E) STAT3 mRNA levels and (F)
p-STAT3 protein levels. Rates of (G) sphere formation and (H)
colony formation (scale bar, 100 µm). Western blot analysis of (I)
CD44 and CD133 expression, as well as (J) Oct4 and Sox2 expression.
*P<0.05, **P<0.01 (n=3). DFOG,
7-difluoromethoxyl-5,4′-di-n-octylygenistein; miR/miRNA, microRNA;
p-, phosphorylated; RT-qPCR, reverse transcription-quantitative
PCR; SFC, sphere-forming cell. |
Natural phytochemicals have been reported to
modulate miRNA-mediated suppression of stemness characteristics in
hepatocellular carcinoma cells (34). Therefore, it was detected whether
tumor suppressor miRNAs in NSCLC cells, including miR-671-5p
(31), miR-148a-3p (35), miR-340-5p (36), miR-342-3p (37), miR-34a-5p (38) and miR-152-3p (39), are regulated by DFOG (5 µM). DFOG
treatment (5 µM) led to significant upregulation of miR-152-3p in
both H460 and A549 cell lines, with the most notable increase among
the tested miRNAs (Fig. 1B and
C).
To examine the role of miR-152-3p and STAT3
expression in self-renewal and tumor growth in NSCLC, miR-152-3p
expression was compared between H460 cells and H460-derived SFCs.
The results revealed lower miR-152-3p levels in SFCs compared with
H460 cells (Fig. 1D). Additionally,
SFCs exhibited elevated STAT3 mRNA expression and p-STAT3 levels
(Fig. 1E and F).
The sphere formation and colony formation were
significantly increased in H460-derived SFCs compared with H460
cells (Fig. 1G and H). Furthermore,
the expression levels of stem cell-associated markers, including
CD133, CD44, Oct4 and Sox2, were elevated in H460-derived SFCs
compared with H460 cells (Fig. 1I and
J).
DFOG inhibits self-renewal-related
stemness possibly by upregulating miR-152-3p and inhibiting p-STAT3
in H460-derived SFCs
DFOG treatment at noncytotoxic concentrations (1, 5
and 10 µM) induced a dose-dependent increase in miR-152-3p
expression in H460-derived SFCs (Fig.
2A). miR-152-3p has been recognized for its role in suppressing
carcinogenesis by inhibiting carcinogenic transcription factors and
signaling pathways in colon cancer, breast cancer, prostate cancer
and ovarian cancer (22–25). Subsequently, the effects of DFOG on
STAT3 mRNA expression and p-STAT3 protein levels were assessed
using RT-qPCR and western blotting. DFOG treatment resulted in a
decrease in STAT3 mRNA expression (Fig.
2B) and a significant reduction in p-STAT3 protein levels
(Fig. 2C).
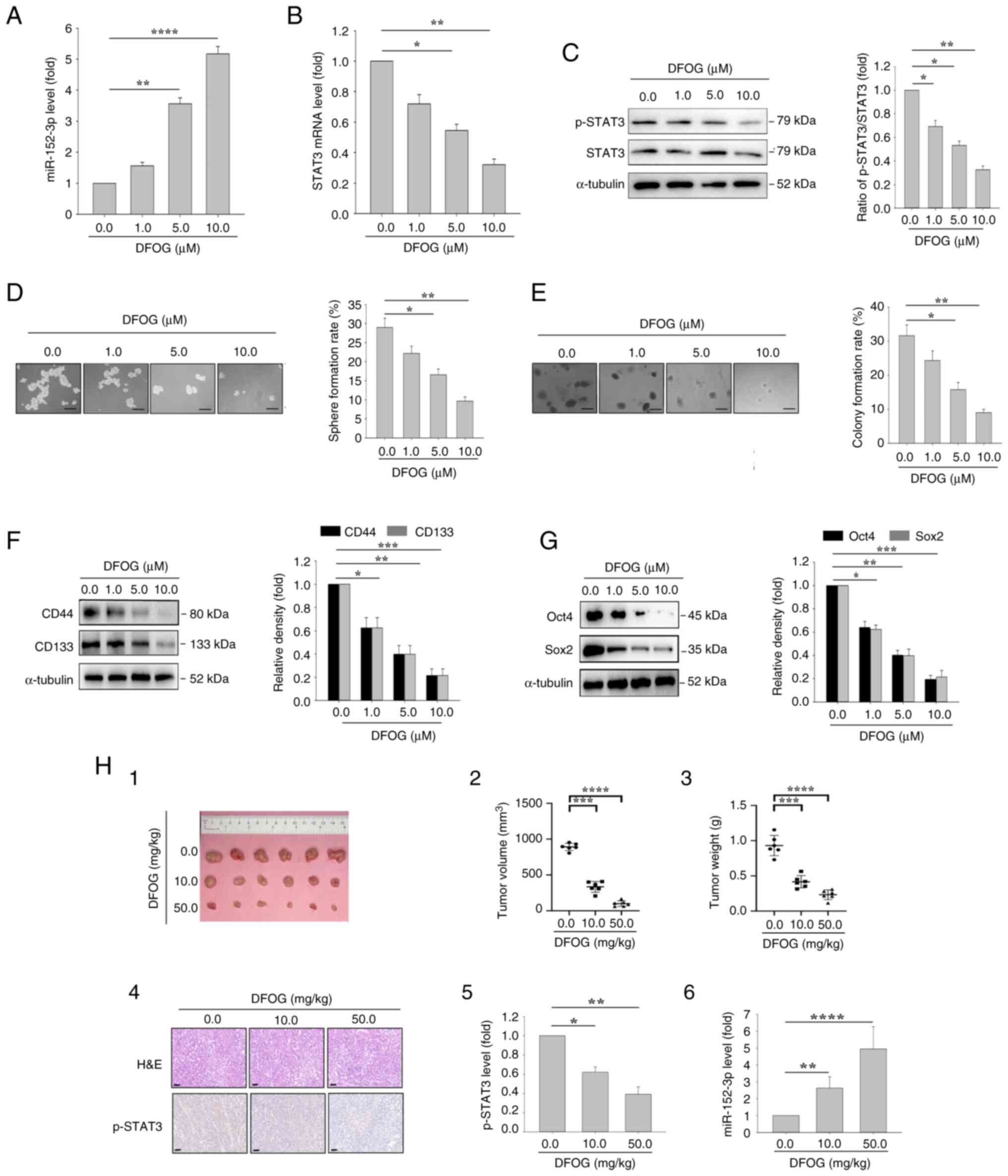 | Figure 2.DFOG induces miR-152-3p expression,
and inhibits self-renewal and tumor growth in H460-derived SFCs. At
the indicated concentrations, DFOG (A) upregulated miR-152-3p
expression, and (B) decreased STAT3 mRNA expression and (C) p-STAT3
protein levels in H460-derived SFCs. (D) Sphere formation and (E)
colony formation were reduced (scale bar, 100 µm). Western blot
analysis showed downregulation of (F) CD44 and CD133 expression, as
well as (G) Oct4 and Sox2 expression. *P<0.05,
**P<0.01,***P<0.001, ****P<0.0001 (n=3). (H) (1) Images of tumor tissue; (2) volume quantification; (3) weight quantification; (4) H&E staining and immunohistochemical
staining using an anti-p-STAT3 antibody (scale bar, 50 µm).
(5) Quantification of p-STAT3
protein levels and (6) miR-152-3p
levels in xenograft tumors of nude mice bearing H460-derived SFCs
treated with DFOG at the indicated doses. *P<0.05, **P<0.01,
***P<0.001, ****P<0.0001. DFOG,
7-difluoromethoxyl-5,4′-di-n-octylygenistein; miR, microRNA; p-,
phosphorylated; SFC, sphere-forming cell. |
To evaluate the effect of DFOG on self-renewal and
tumor growth in NSCLC, sphere formation and colony formation assays
were performed. DFOG treatment led to reduced sphere (Fig. 2D) and colony (Fig. 2E) formation rates in H460-derived
SFCs. Additionally, the protein levels of the stem cell markers
CD133 and CD44 were significantly decreased (Fig. 2F). Consistent with these results,
DFOG treatment substantially decreased the protein levels of Oct4
and Sox2 (Fig. 2G) in H460-derived
SFCs.
For in vivo assessment, DFOG was orally
administered to mice with H460-derived SFC xenograft tumors, with
DMSO administered in the control group. The volumes of xenografts
are shown in Table II and the
maximum diameter is shown in Table
III. As shown in Fig. 2H-1, −2
and −3, DFOG significantly suppressed the growth of xenograft
tumors. Mechanistically, DFOG exerted a dual effect by reducing the
p-STAT3 levels (Fig. 2H-4 and −5)
and enhancing miR-152-3p expression (Fig. 2H-6). These results suggested that
DFOG inhibited the self-renewal and tumor growth of H460-derived
SFCs both in vitro and in vivo, likely through
modulation of miR-152-3p and its target, STAT3.
 | Table II.Maximum volume of the xenograft
(mm3) in each mouse across all experimental groups. |
Table II.
Maximum volume of the xenograft
(mm3) in each mouse across all experimental groups.
| DFOG (0 mg/kg) | DFOG (10
mg/kg) | DFOG (50
mg/kg) |
|---|
| 953.06 | 408.32 | 166.08 |
| 884.01 | 367.26 | 105.36 |
| 877.36 | 316.19 | 115.17 |
| 896.21 | 402.42 | 62.97 |
| 806.09 | 309.19 | 88.68 |
| 931.01 | 207.37 | 60.69 |
 | Table III.Maximum diameter measured of the
xenograft (mm) in each mouse across all experimental groups. |
Table III.
Maximum diameter measured of the
xenograft (mm) in each mouse across all experimental groups.
| DFOG (0 mg/kg) | DFOG (10
mg/kg) | DFOG (50
mg/kg) |
|---|
| 14.56 | 11.23 | 6.32 |
| 13.09 | 10.21 | 5.21 |
| 13.32 | 9.68 | 5.69 |
| 12.25 | 8.99 | 4.62 |
| 14.32 | 8.32 | 5.17 |
| 14.87 | 7.96 | 4.16 |
miR-152-3p mimic increases
DFOG-induced suppression of p-STAT3 and self-renewal
To further elucidate the role of miR-152-3p
regulation in DFOG-mediated suppression of self-renewal,
H460-derived SFCs were transfected with a miR-152-3p mimic. As
shown in Fig. 3A, the miR-152-3p
mimic, in combination with DFOG (5 µM), elevated miR-152-3p
expression. Furthermore, both DFOG (5 µM) and the miR-152-3p mimic
synergistically reduced STAT3 mRNA levels and p-STAT3 protein
levels (Fig. 3B and C). Notably,
miR-152-3p overexpression enhanced the inhibitory effects of DFOG
on self-renewal, leading to a reduction in sphere (Fig. 3D) and colony (Fig. 3E) formation rates compared with the
control group (0 µM). The combined action of miR-152-3p and DFOG
resulted in decreased expression of the stemness markers CD133 and
CD44 (Fig. 3F), as well as the
pluripotent factors Oct4 and Sox2 (Fig.
3G). These results suggested that DFOG elevated miR-152-3p
expression, which in turn suppressed STAT3 transcription and
activity, thereby inhibiting self-renewal in H460-derived SFCs.
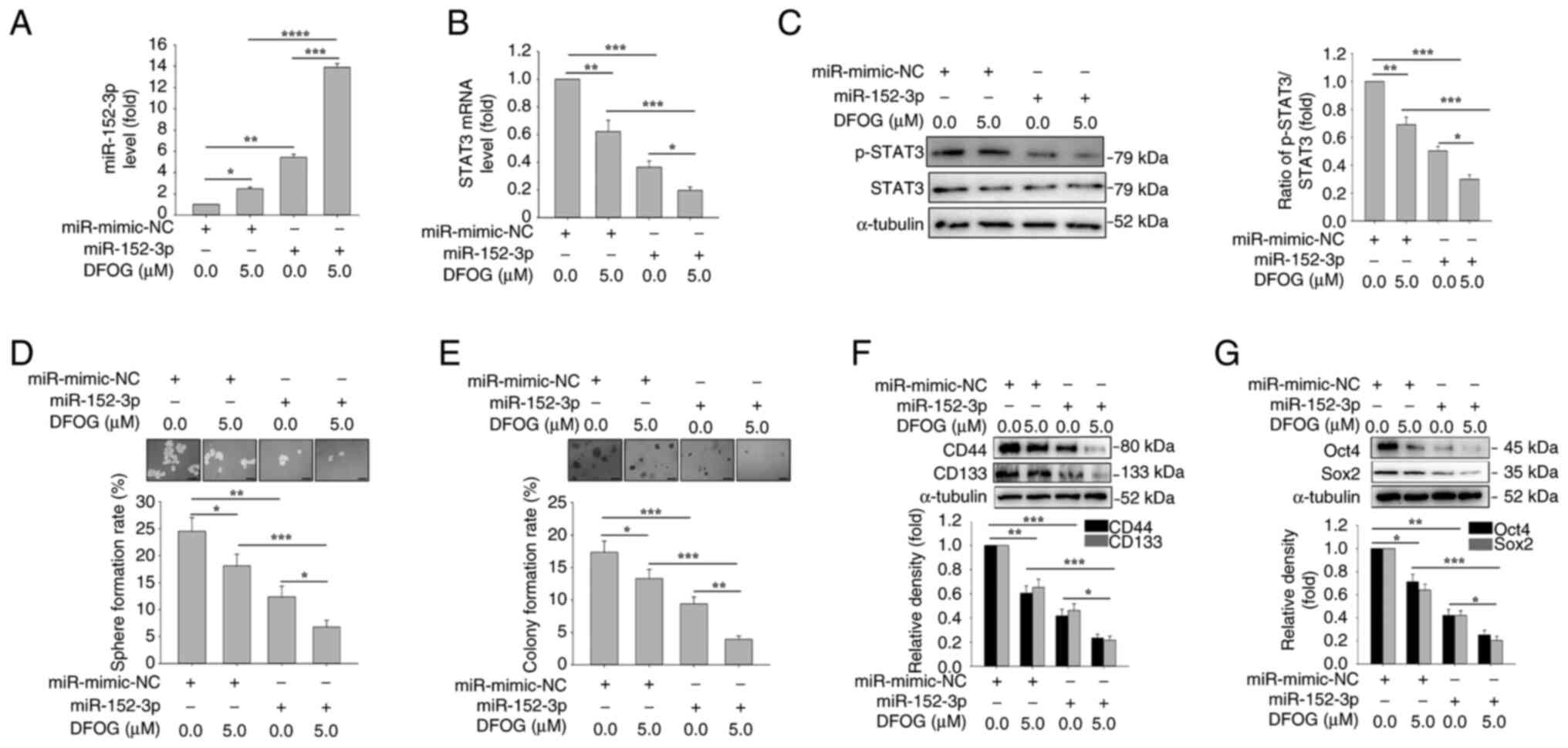 | Figure 3.miR-152-3p mimic enhances
DFOG-induced downregulation of p-STAT3 levels and inhibits
self-renewal in H460-derived SFCs. Expression levels of (A)
miR-152-3p and (B) STAT3 mRNA, and (C) p-STAT3 protein levels. (D)
Spheres and (E) colonies formed were quantified (scale bar, 100
µm). Western blot analysis of (F) CD44 and CD133, as well as (G)
Oct4 and Sox2 expression in H460-derived SFCs transfected with
miR-152-3p mimic and/or treated with DFOG (5 µM). *P<0.05,
**P<0.01, ***P<0.001 and ****P<0.0001 (n=3). DFOG,
7-difluoromethoxyl-5,4′-di-n-octylygenistein; miR, microRNA; NC,
negative control; p-, phosphorylated; SFC, sphere-forming cell. |
miR-152-3p inhibitor antagonizes
DFOG-induced suppression of p-STAT3 expression and
self-renewal
To further validate the role of miR-152-3p
regulation in DFOG-induced suppression of self-renewal,
H460-derived SFCs were transfected with miR-152-3p inhibitor or
miR-inhibitor-NC, followed by treatment with or without DFOG (5
µM). As shown in Fig. 4A,
miR-152-3p inhibitor transfection effectively reversed the
DFOG-induced increase in miR-152-3p expression. Additionally,
miR-152-3p inhibitor counteracted the reduction in STAT3 mRNA
expression and p-STAT3 protein levels induced by DFOG (Fig. 4B and C). Notably, miR-152-3p
inhibitor mitigated the inhibitory effects of DFOG on self-renewal,
as evidenced by an increase in sphere (Fig. 4D) and colony formation rates
(Fig. 4E). miR-152-3p inhibitor
transfection also increased the expression of the
stemness-associated markers CD133 and CD44 (Fig. 4F), and the pluripotent factors Oct4
and Sox2 (Fig. 4G), reversing the
suppressive effect of DFOG. These results suggested that inhibiting
the expression of miR-152-3p could counteract the inhibition of
p-STAT3 activity and self-renewal induced by DFOG treatment in
H460-derived SFCs.
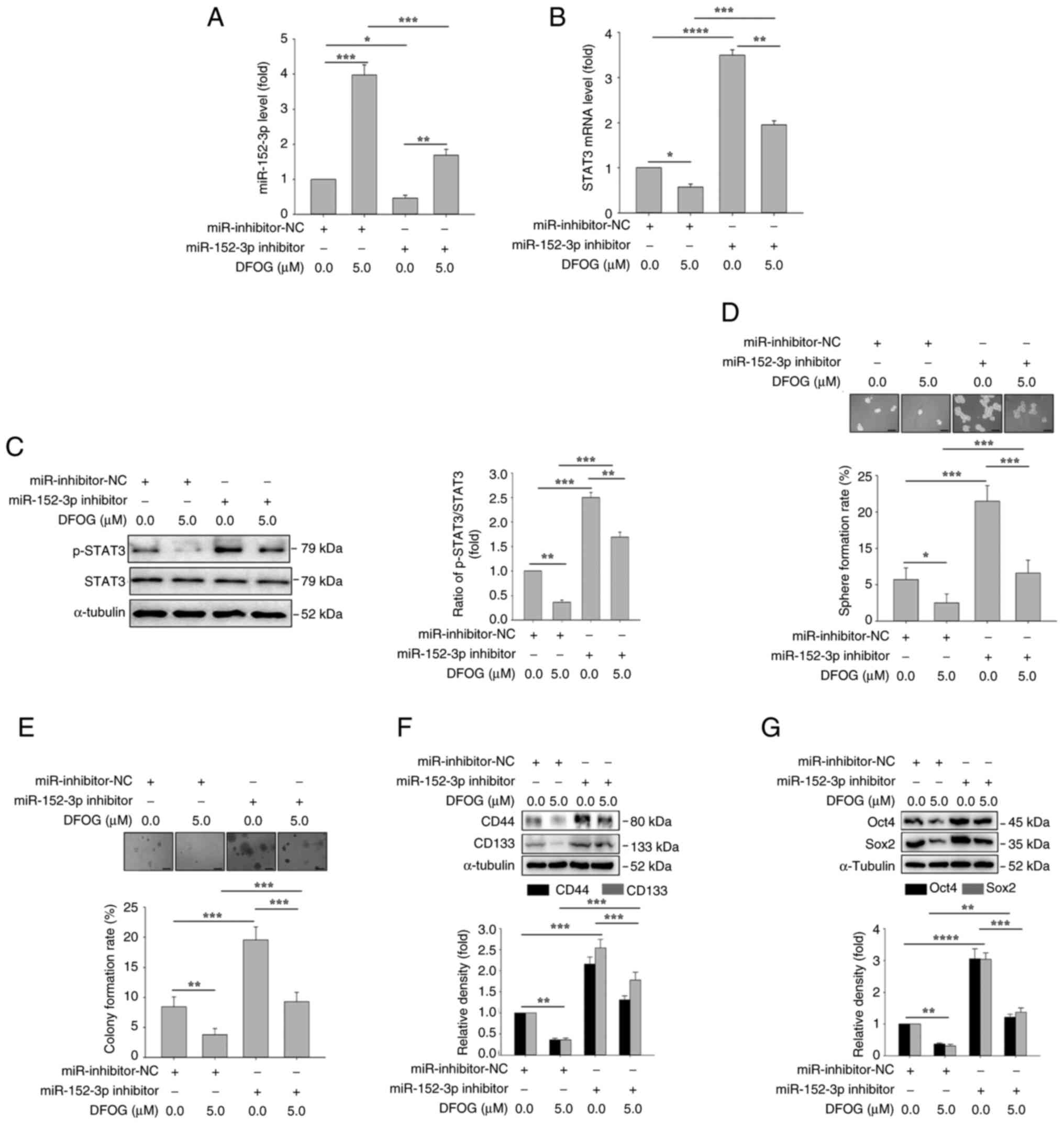 | Figure 4.miR-152-3p inhibitor antagonizes
DFOG-induced suppression of p-STAT3 levels and self-renewal in
H460-derived SFCs. Expression levels of (A) miR-152-3p and (B)
STAT3 mRNA, and (C) p-STAT3 protein levels. (D) Spheres and (E)
colonies formed were quantified (scale bar, 100 µm). Western blot
analysis of (F) CD44 and CD133, as well as (G) Oct4 and Sox2
expression in H460-derived SFCs transfected with miR-152-3p
inhibitor and/or treated with DFOG (5 µM). *P<0.05, **P<0.01,
***P<0.001 and ****P<0.0001 (n=3). DFOG,
7-difluoromethoxyl-5,4′-di-n-octylygenistein; miR, microRNA; NC,
negative control; p-, phosphorylated; SFC, sphere-forming cell. |
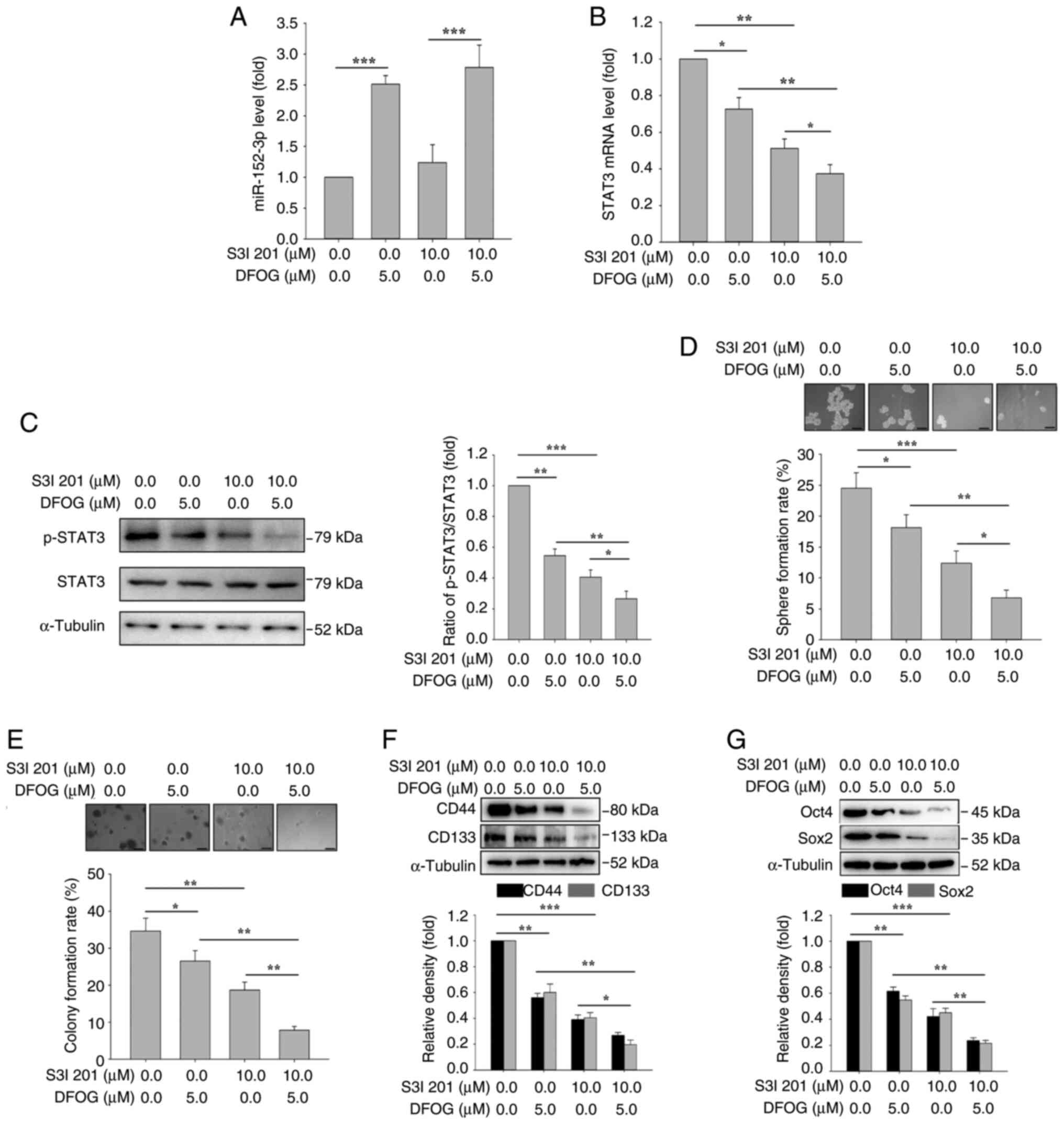
STAT3 inhibitor increases DFOG-induced
self-renewal
To investigate whether the DFOG-induced suppression
of self-renewal was linked to the regulation of STAT3 mRNA
expression and activity, H460-derived SFCs were treated with S3I
201, a specific STAT3 inhibitor. As shown in Fig. 5A, S3I 201 (10 µM) treatment did not
affect the DFOG-induced elevation of miR-152-3p expression.
However, as illustrated in Fig. 5B and
C, S3I 201 (10 µM), in combination with DFOG (5 µM),
effectively decreased STAT3 mRNA expression and p-STAT3 protein
levels. Inhibition of STAT3 activity enhanced the suppressive
effects of DFOG on self-renewal compared with S3I 201 (10 µM) or
DFOG (5 µM) treatment alone, leading to a significant reduction in
sphere (Fig. 5D) and colony
(Fig. 5E) formation rates.
Furthermore, the combination of S3I 201 and DFOG resulted in
decreased protein levels of the stemness markers CD133 and CD44
(Fig. 5F), as well as the
pluripotent factors Oct4 and Sox2 (Fig.
5G). These results highlighted that modulation of STAT3 mRNA
expression and activity by DFOG contributed to the suppression of
self-renewal in H460-derived SFCs.
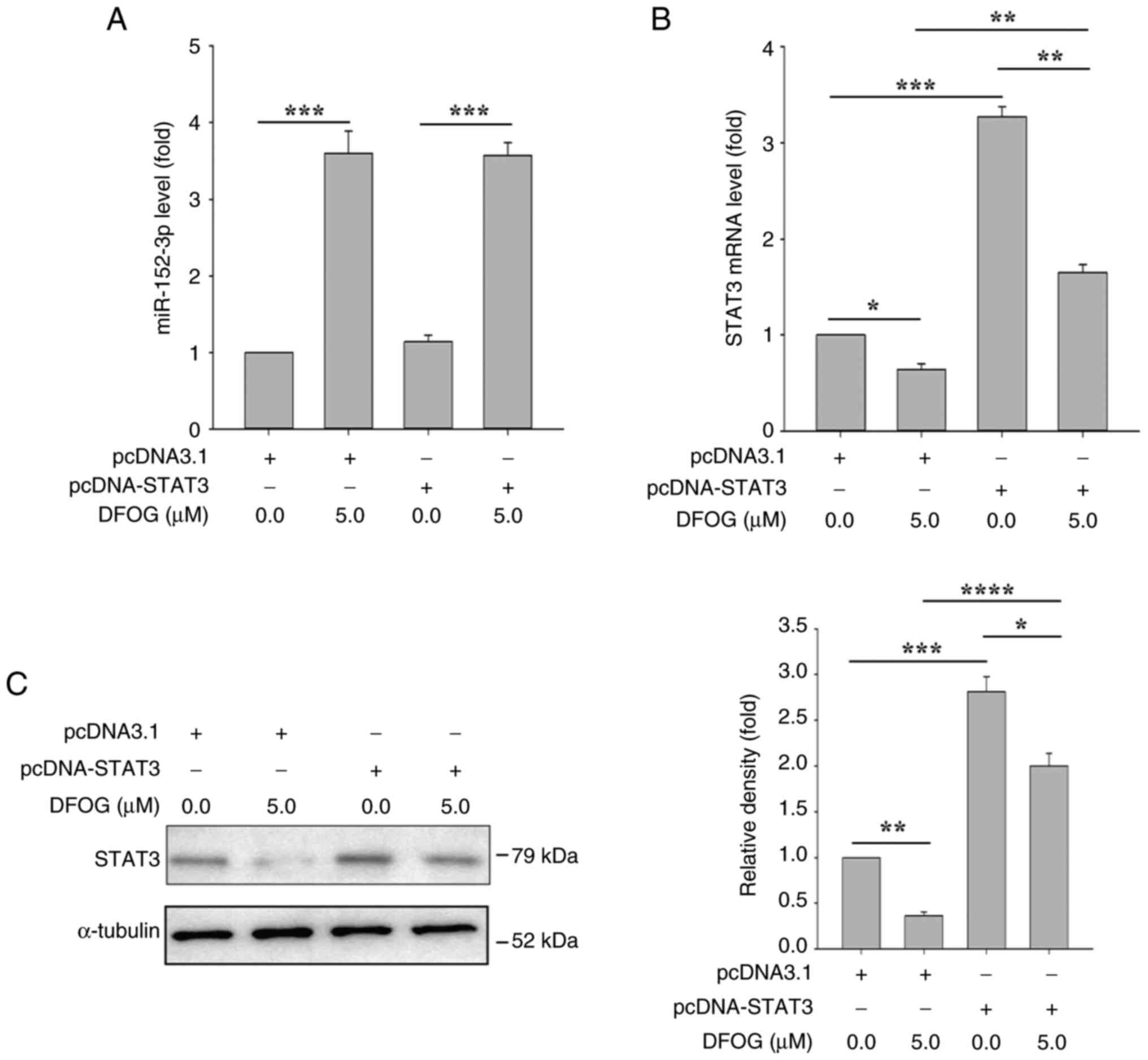
STAT3 overexpression does not
significantly affect miR-152-3p expression
To verify the upstream and downstream relationship
between miR-152-3p and STAT3, H460-derived SFCs were transfected
with STAT3 cDNA and pcDNA3.1, followed by treatment with or without
DFOG (5 µM). As shown in Fig. 6A,
transfection with STAT3 cDNA did not affect the miR-152-3p
expression induced by DFOG. However, STAT3 cDNA transfection
significantly counteracted the reduction in STAT3 mRNA and protein
levels caused by DFOG (Fig. 6B and
C).
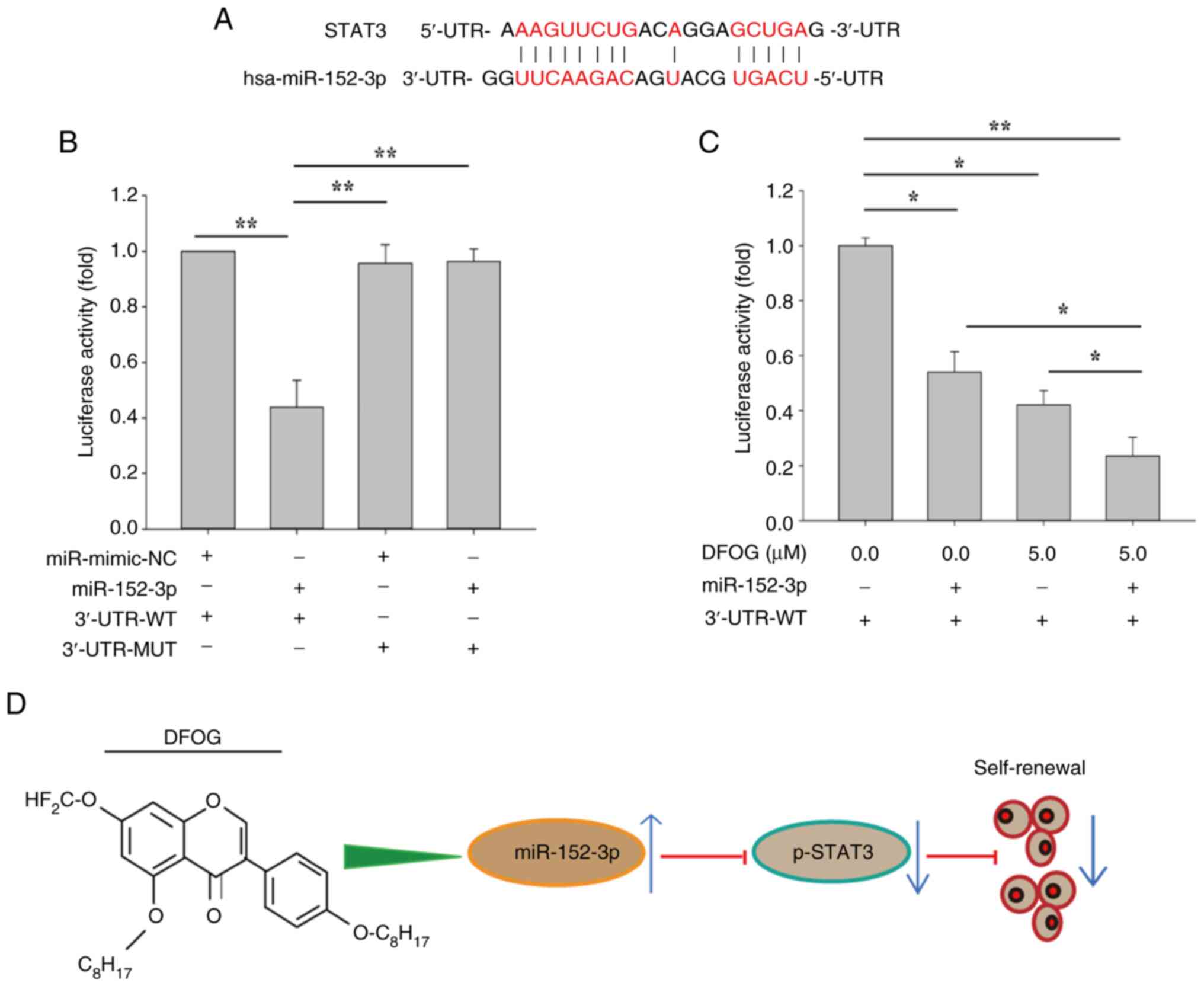
miR-152-3p mimic suppresses the
transcriptional activity of STAT3
To determine whether miR-152-3p directly targets
STAT3, a luciferase reporter assay was conducted in H460-derived
SFCs to identify the specific binding site of the miR-152-3p seed
sequence on the 3′-UTR of STAT3 mRNA. RNAhybrid predicted binding
sites for miR-152-3p and STAT3 (Fig.
7A). As shown in Fig. 7B,
luciferase activity was reduced in cells co-transfected with the
miR-152-3p mimic and STAT3-3′-UTR-WT, while no change in luciferase
activity was observed following co-transfection with
STAT3-3′-UTR-MUT. Additionally, the luciferase activity was further
decreased in NSCLC cells co-transfected with miR-152-3p mimic and
STAT3-3′-UTR-WT after DFOG (5 µM) treatment, compared with cells
treated with miR-152-3p mimic or DFOG alone (Fig. 7C). These results demonstrated that
DFOG inhibited self-renewal by upregulating miR-152-3p, which
directly suppressed STAT3 expression by disrupting its
transcriptional activity (Fig.
7D).
DFOG halts self-renewal in
A549-derived SFCs
The inhibitory effect of DFOG on self-renewal was
also assessed in A549-derived SFCs. Consistently, DFOG
dose-dependently increased miR-152-3p expression, and downregulated
STAT3 mRNA expression and p-STAT3 protein levels in A549 cells
(Fig. 8A-C). Additionally, sphere
formation (Fig. 8D) and colony
formation (Fig. 8E) rates, as well
as the expression of the stem cell markers CD44 and CD133 (Fig. 8F), and the pluripotent factors Oct4
and Sox2 (Fig. 8G), were all
suppressed. These results indicated that DFOG-induced inhibition of
self-renewal in A549-derived SFCs was mediated through the
upregulation of miR-152-3p, and the suppression of STAT3 mRNA and
activity.
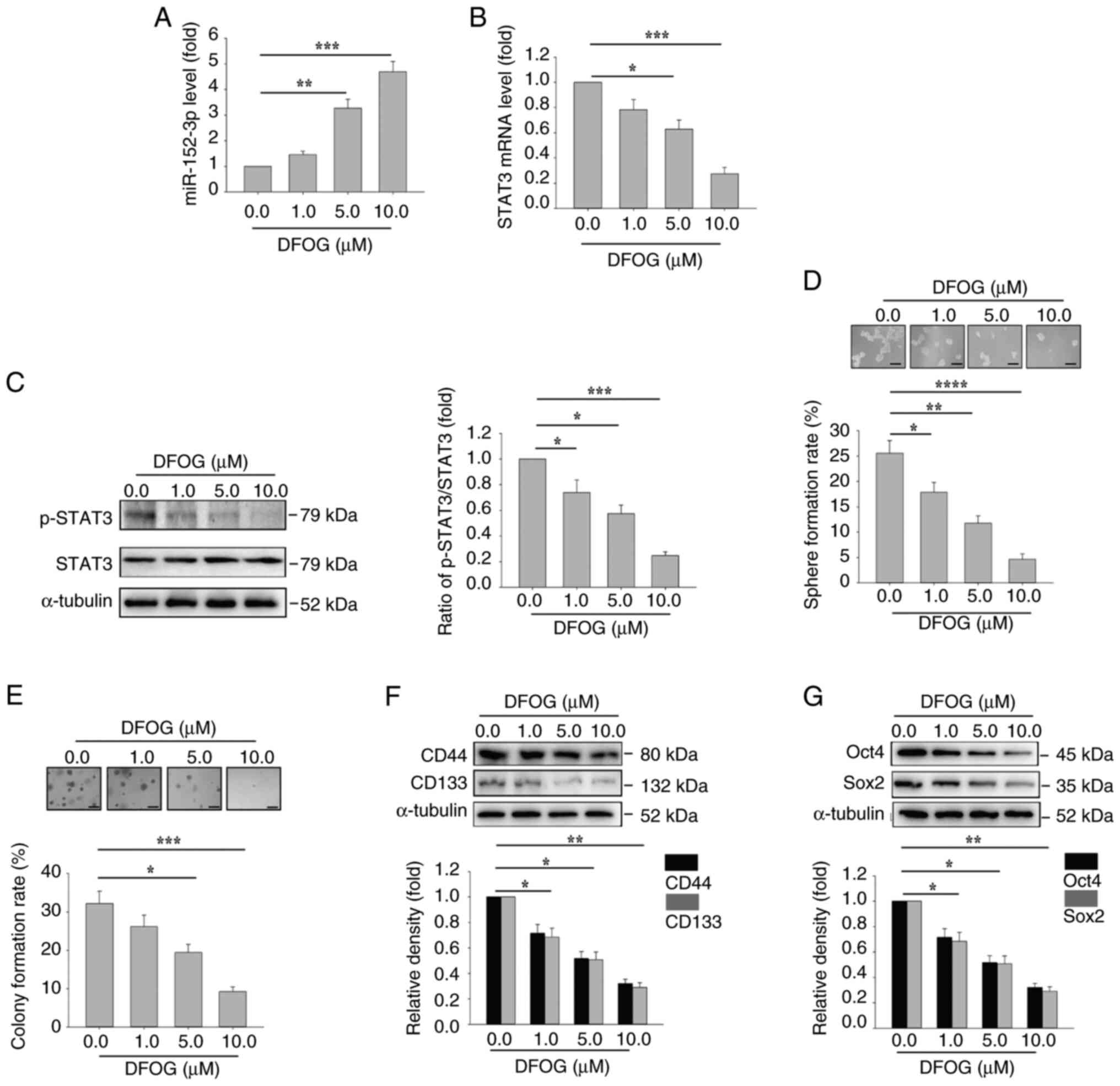 | Figure 8.DFOG induces miR-152-3p expression,
and inhibits STAT3 activation and self-renewal in A549-derived
SFCs. At the indicated concentrations, DFOG (A) upregulated
miR-152-3p expression, and decreased (B) STAT3 mRNA expression and
(C) p-STAT3 protein levels in A549-derived SFCs. (D) Spheres and
(E) colonies formed were quantified (scale bar, 100 µm). Western
blot analysis of (F) CD44 and CD133, as well as (G) Oct4 and Sox2
expression in A549-derived SFCs. *P<0.05, **P<0.01,
***P<0.001 and ****P<0.0001 (n=3). DFOG,
7-difluoromethoxyl-5,4′-di-n-octylygenistein; miR, microRNA; p-,
phosphorylated; SFC, sphere-forming cell. |
Discussion
The present study provided evidence that DFOG
inhibited self-renewal and tumor growth in NSCLC cells by
upregulating miR-152-3p, thereby suppressing the expression and
activity of STAT3. These findings have substantial implications for
the potential use of DFOG as a therapeutic strategy for human
NSCLC, particularly targeting cancer cells with
self-renewal-related stemness properties.
Dysregulated miRNA expression contributes to the
progression of breast cancer, liver cancer and lung cancer
(31,35–37,40,41).
Reduced miR-152-3p expression has been linked to multiple aspects
of malignancy, including progression, proliferation, invasion and
metastasis, in colon cancer, breast cancer, prostate cancer and
ovarian cancer (22–25). However, further investigation is
required, particularly in lung cancer. Our results demonstrated
that DFOG upregulated miR-152-3p, miR-34a-5p and miR-148a-3p, with
miR-152-3p exhibiting the most pronounced increase. To the best of
our knowledge, the present study was the first to report that DFOG
enhanced self-renewal and tumor growth traits in NSCLC cells by
elevating miR-152-3p expression, thus revealing a novel mechanism
through which DFOG exerts its inhibitory effects on self-renewal
and tumor growth. Furthermore, the molecular mechanisms underlying
DFOG-induced suppression of tumor cells involve multiple signaling
pathways, including the inactivation of FoxM1 and NF-κB (17,18).
One study indicated that the expression of miR-152 and STAT3 was
associated with poor prognosis in epithelial ovarian cancer
(31). STAT3, an oncogenic
transcription factor mediating signaling from the cell surface to
the nucleus, is frequently upregulated in various malignancies,
including NSCLC (29,30). Notably, targeting STAT3 has been
shown to reduce tumor stem cell properties (30,41,42).
In the present study, a luciferase reporter assay identified STAT3
as a direct target of miR-152-3p. This finding was further
corroborated by the reduced transcription of STAT3 following
combined treatment with DFOG and a miR-152-3p mimic in H460-derived
SFCs. The present results demonstrated that DFOG-induced
upregulation of miR-152-3p inhibited self-renewal and tumor growth
by downregulating STAT3 expression at the mRNA level and impairing
its activity, as evidenced by decreased p-STAT3 protein levels in
H460-derived SFCs. Overexpression of STAT3 reversed this effect,
highlighting the interconnection among DFOG, miR-152-3p and STAT3.
Collectively, these findings suggested that the inhibitory effects
of DFOG on self-renewal and tumor growth were mediated through
miR-152-3p upregulation and STAT3 downregulation, underscoring its
potential as a preventive and therapeutic agent for NSCLC,
particularly in targeting self-renewing tumor cells.
In conclusion, the present study elucidated the
mechanism by which DFOG targeted STAT3 through miR-152-3p
upregulation, effectively suppressing self-renewal and tumor growth
in NSCLC. Therefore, DFOG may be a promising candidate for novel
preventive and therapeutic interventions for NSCLC in humans.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Science and Technology
Innovation Leading Academics of National High-level Personnel of
Special Support Program from Ministry of Science and Technology,
P.R. China (grant no. GKFZ-2018-29), Department of Science and
Technology of Guizhou Province [grant no. QKHJC-ZK(2022)-YB-666],
and the Natural Science Foundation of Hunan Province (grant no.
2021JJ30462).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
QY, XL and XC conducted the experiments, contributed
to data collection and drafted the manuscript. JX and JZ performed
the data analysis and contributed to the study design. XL, XC and
JX provided resources. JZ and QY confirm the authenticity of all
the raw data. All authors have read and approved the final version
of the manuscript.
Ethics approval and consent to
participate
All animal studies were approved (approval no.
D2023045) by the Ethics Committee of Hunan Normal University
(Changsha, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
DFOG
|
7-difluoromethoxyl-5,4′-di-n-octylygenistein
|
|
NSCLC
|
non-small cell lung carcinoma
|
|
p-STAT3
|
phosphorylated-STAT3
|
References
|
1
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2022. CA Cancer J Clin. 72:7–33. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chansky K, Detterbeck FC, Nicholson AG,
Rusch VW, Vallières E, Groome P, Kennedy C, Krasnik M, Peake M,
Shemanski L, et al: The IASLC lung cancer staging project: External
validation of the revision of the TNM stage groupings in the eighth
edition of the TNM classification of lung cancer. J Thorac Oncol.
12:1109–1121. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang C, Wu Y, Shao J, Liu D and Li W:
Clinicopathological variables influencing overall survival,
recurrence and post-recurrence survival in resected stage I
non-small-cell lung cancer. BMC Cancer. 20:1502020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang X, Chen Y, Wang X, Tian H, Wang Y,
Jin J, Shan Z, Liu Y, Cai Z, Tong X, et al: Stem cell factor SOX2
confers ferroptosis resistance in lung cancer via upregulation of
SLC7A11. Cancer Res. 81:5217–5229. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Clara JA, Monge C, Yang Y and Takebe N:
Targeting signalling pathways and the immune microenvironment of
cancer stem cells-a clinical update. Nat Rev Clin Oncol.
17:204–232. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schaal CM, Bora-Singhal N, Kumar DM and
Chellappan SP: Regulation of Sox2 and stemness by nicotine and
electronic-cigarettes in non-small cell lung cancer. Mol Cancer.
17:1492018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sunayama J, Matsuda K, Sato A, Tachibana
K, Suzuki K, Narita Y, Shibui S, Sakurada K, Kayama T, Tomiyama A
and Kitanaka C: Crosstalk between the PI3K/mTOR and MEK/ERK
pathways involved in the maintenance of self-renewal and
tumorigenicity of glioblastoma stem-like cells. Stem Cells.
28:1930–1939. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nimmakayala RK, Leon F, Rachagani S, Rauth
S, Nallasamy P, Marimuthu S, Shailendra GK, Chhonker YS, Chugh S,
Chirravuri R, et al: Metabolic programming of distinct cancer stem
cells promotes metastasis of pancreatic ductal adenocarcinoma.
Oncogene. 40:215–231. 202 View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sharif T, Martell E, Dai C, Singh SK and
Gujar S: Regulation of the proline regulatory axis and autophagy
modulates stemness in TP73/p73 deficient cancer stem-like cells.
Autophagy. 15:934–936. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kaufhold S, Garbán H and Bonavida B: Yin
Yang 1 is associated with cancer stem cell transcription factors
(SOX2, OCT4, BMI1) and clinical implication. J Exp Clin Cancer Res.
35:842016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Walcher L, Kistenmacher AK, Suo H, Kitte
R, Dluczek S, Strauß A, Blaudszun AR, Yevsa T, Fricke S and
Kossatz-Boehlert U: Cancer stem cells-Origins and biomarkers:
Perspectives for targeted personalized therapies. Front Immunol.
11:12802020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fu Z, Cao X, Yang Y, Song Z, Zhang J and
Wang Z: Upregulation of FoxM1 by MnSOD overexpression contributes
to cancer Stem-like cell characteristics in the lung cancer H460
cell line. Technol Cancer Res Treat. 17:15330338187896352018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu F, Cao X, Liu Z, Guo H, Ren K, Quan M,
Zhou Y, Xiang H and Cao J: Casticin suppresses self-renewal and
invasion of lung cancer stem-like cells from A549 cells through
down-regulation of pAkt. Acta Biochim Biophys Sin (Shanghai).
46:15–21. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wei D, Yang L, Lv B and Chen L: Genistein
suppresses retinoblastoma cell viability and growth and induces
apoptosis by upregulating miR-145 and inhibiting its target ABCE1.
Mol Vis. 23:385–394. 2017.PubMed/NCBI
|
|
15
|
Ma CH, Zhang YX, Tang LH, Yang XJ, Cui WM,
Han CC and Ji WY: MicroRNA-1469, a p53-responsive microRNA promotes
Genistein induced apoptosis by targeting Mcl1 in human laryngeal
cancer cells. Biomed Pharmacother. 106:665–671. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang L, Zhang J, Gong Y and Lv L:
Systematic and experimental investigations of the anti-colorectal
cancer mediated by genistein. Biofactors. 46:974–982. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ning Y, Xu M, Cao X, Chen X and Luo X:
Inactivation of AKT, ERK and NF-κB by genistein derivative,
7-difluoromethoxyl-5,4′-di-n-octylygenistein, reduces ovarian
carcinoma oncogenicity. Oncol Rep. 38:949–958. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xiang HL, Liu F, Quan MF, Cao JG and Lv Y:
7-difluoromethoxyl-5,4′-di-n-octyl genistein inhibits growth of
gastric cancer cells through down-regulating forkhead box M1. World
J Gastroenterol. 18:4618–4626. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ren W, Hou J, Yang C, Wang H, Wu S, Wu Y,
Zhao X and Lu C: Extracellular vesicles secreted by hypoxia
pre-challenged mesenchymal stem cells promote non-small cell lung
cancer cell growth and mobility as well as macrophage M2
polarization via miR-21-5p delivery. J Exp Clin Cancer Res.
38:622019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li P, Xing W, Xu J, Yuan D, Liang G, Liu B
and Ma H: microRNA-301b-3p downregulation underlies a novel
inhibitory role of long non-coding RNA MBNL1-AS1 in non-small cell
lung cancer. Stem Cell Res Ther. 10:1442019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xie C, Zhu J, Jiang Y, Chen J, Wang X,
Geng S, Wu J, Zhong C, Li X and Meng Z: Sulforaphane inhibits the
acquisition of tobacco Smoke-induced lung cancer stem cell-like
properties via the IL-6/ΔNp63α/Notch axis. Theranostics.
9:4827–4840. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lai SW, Chen MY, Bamodu OA, Hsieh MS,
Huang TY, Yeh CT, Lee WH and Cherng YG: Exosomal lncRNA PVT1/VEGFA
axis promotes colon cancer metastasis and stemness by
downregulation of tumor suppressor miR-152-3p. Oxid Med Cell
Longev. 2021:99598072021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jiang CF, Xie YX, Qian YC, Wang M, Liu LZ,
Shu YQ, Bai XM and Jiang BH: TBX15/miR-152/KIF2C pathway regulates
breast cancer doxorubicin resistance via promoting PKM2
ubiquitination. Cancer Cell Int. 21:5422021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Moya L, Meijer J, Schubert S, Matin F and
Batra J: Assessment of miR-98-5p, miR-152-3p, miR-326 and miR-4289
expression as biomarker for prostate cancer diagnosis. Int J Mol
Sci. 20:11542019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lyu M, Li X, Shen Y, Lu J, Zhang L, Zhong
S and Wang J: CircATRNL1 and circZNF608 inhibit ovarian cancer by
sequestering miR-152-5p and encoding protein. Front Genet.
13:7840892022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yuan Q, Wang R, Li X, Sun F, Lin J, Fu Z
and Zhang J: DNMT1/miR-152-3p/SOS1 signaling axis promotes
self-renewal and tumor growth of cancer stem-like cells derived
from non-small cell lung cancer. Clin Epigenetics. 16:552024.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhao L, Wu X, Zhang Z, Fang L, Yang B and
Li Y: ELF1 suppresses autophagy to reduce cisplatin resistance via
the miR-152-3p/NCAM1/ERK axis in lung cancer cells. Cancer Sci.
114:2650–2663. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huang J, Lou J, Liu X and Xie Y: LncRNA
PCGsEM1 contributes to the proliferation, migration and invasion of
Non-small cell lung cancer cells via acting as a sponge for
miR-152-3p. Curr Pharm Des. 27:4663–4670. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Huang R, Wang S, Wang N, Zheng Y, Zhou J,
Yang B, Wang X, Zhang J, Guo L, Wang S, et al: CCL5 derived from
tumor-associated macrophages promotes prostate cancer stem cells
and metastasis via activating β-catenin/STAT3 signaling. Cell Death
Dis. 11:2342020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ouyang S, Li H, Lou L, Huang Q, Zhang Z,
Mo J, Li M, Lu J, Zhu K, Chu Y, et al: Inhibition of
STAT3-ferroptosis negative regulatory axis suppresses tumor growth
and alleviates chemoresistance in gastric cancer. Redox Biol.
52:1023172022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cao Y, Shi H, Ren F, Jia Y and Zhang R:
Long non-coding RNA CCAT1 promotes metastasis and poor prognosis in
epithelial ovarian cancer. Exp Cell Res. 359:185–194. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Al-Harbi B and Aboussekhra A: Cucurbitacin
I (JSI-124)-dependent inhibition of STAT3 permanently suppresses
the pro-carcinogenic effects of active breast cancer-associated
fibroblasts. Mol Carcinog. 60:242–251. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li X, Wang L, Cao X, Zhou L, Xu C, Cui Y,
Qiu Y and Cao J: Casticin inhibits stemness of hepatocellular
carcinoma cells via disrupting the reciprocal negative regulation
between DNMT1 and miR-148a-3p. Toxicol Appl Pharmacol.
396:1149982020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen MJ, Cheng YM, Chen CC, Chen YC and
Shen CJ: MiR-148a and miR-152 reduce tamoxifen resistance in ER+
breast cancer via downregulating ALCAM. Biochem Biophys Res Commun.
483:840–846. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
He WW, Ma HT, Guo X, Wu WM, Gao EJ and
Zhao YH: lncRNA SNHG3 accelerates the proliferation and invasion of
non-small cell lung cancer by downregulating miR-340-5p. J Biol
Regul Homeost Agents. 34:2017–2027. 2020.PubMed/NCBI
|
|
37
|
Chen Z, Ying J, Shang W, Ding D, Guo M and
Wang H: miR-342-3p regulates the proliferation and apoptosis of
NSCLC cells by targeting BCL-2. Technol Cancer Res Treat.
20:153303382110411932021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li YY, Tao YW, Gao S, Li P, Zheng JM,
Zhang SE, Liang J and Zhang Y: Cancer-associated fibroblasts
contribute to oral cancer cells proliferation and metastasis via
Exosome-mediated paracrine miR-34a-5p. EBioMedicine. 36:209–220.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li LW, Xiao HQ, Ma R, Yang M, Li W and Lou
G: miR-152 is involved in the proliferation and metastasis of
ovarian cancer through repression of ERBB3. Int J Mol Med.
41:1529–1535. 2018.PubMed/NCBI
|
|
40
|
Chang DL, Wei W, Yu ZP and Qin CK:
miR-152-5p inhibits proliferation and induces apoptosis of liver
cancer cells by up-regulating FOXO expression. Pharmazie.
72:338–343. 2017.PubMed/NCBI
|
|
41
|
Reichenbach N, Delekate A, Plescher M,
Schmitt F, Krauss S, Blank N, Halle A and Petzold GC: Inhibition of
Stat3-mediated astrogliosis ameliorates pathology in an Alzheimer's
disease model. EMBO Mol Med. 11:e96652019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu YX, Xu BW, Niu XD, Chen YJ, Fu XQ,
Wang XQ, Yin CL, Chou JY, Li JK, Wu JY, et al: Inhibition of
Src/STAT3 signaling-mediated angiogenesis is involved in the
anti-melanoma effects of dioscin. Pharmacol Res. 175:1059832022.
View Article : Google Scholar : PubMed/NCBI
|