Introduction
Lung cancer (LC) was the most frequently diagnosed
cancer and was responsible for the largest proportion of all
cancer-related deaths worldwide in 2022 (1–3). Lung
adenocarcinoma (LUAD) is the most common type of LC, comprising
almost one-half of all LC cases globally (4–6). LUAD
is typically diagnosed at an advanced stage and is highly resistant
to conventional radiotherapy and chemotherapy (4,7,8).
Therefore, it is important to search for potential therapeutic
targets.
Ubiquitination is a highly conserved
post-translational modification in eukaryotes that is catalyzed by
an enzymatic cascade involving the sequential action of E1, E2 and
E3 enzymes (9). In previous
decades, the role of E3 ubiquitin ligase in regulating numerous
cellular processes has attracted increasing interest due to its
essential function in determining the specificity and fate of
target proteins. Ring finger protein 125 (RNF125; also termed
TRAC-1) functions as a ubiquitin E3 ligase in lymphoid tissues that
positively regulates T cell activation (10,11).
Previous studies have shown that RNF125 can suppress the
progression of several cancers, including hepatocellular carcinoma,
head and neck squamous cell carcinoma and melanoma (12–15).
However, the functional role of RNF125 in LUAD is largely
unknown.
Programmed cell death ligand 1 (PD-L1) is a
transmembrane protein that is regarded as a co-suppressor of the
immune response (16). PD-L1 also
serves a pro-oncogenic role in various malignancies, including LC,
by attenuating the host immune response to tumor cells (17). Knockdown of GFAT1, a positive
regulator upstream of PD-L1, has been shown to significantly
enhance T cell activation and NK cell killing of LC cells (18). A previous study showed that high
PD-L1 expression was associated with poor prognosis in patients
with LUAD and correlated with immune-related pathways (19). A previous study reported that RNF125
promoted K48-linked polyubiquitination of PD-L1 and mediated its
degradation (20). Therefore, it
could be hypothesized that RNF125 enhances tumor immunity and
reduces PD-L1 expression levels in LUAD.
Muscleblind-like 1 (MBNL1), an RNA-binding protein
(RBP), increases the stability of downstream gene transcripts by
binding to their 3′ untranslated region (UTR), resulting in the
upregulation of gene expression levels (21,22). A
number of studies have shown that MBNL1 inhibits cancer cell
proliferation, migration and invasion, and suppresses tumor
progression in breast cancer, gastric cancer and glioblastoma
(21,23,24).
Additionally, MBNL1 expression is reduced in LC tissues and is
associated with poor prognosis in patients (23). TIMER2.0 database (http://timer.cistrome.org/) analysis revealed that
MBNL1 was positively correlated with RNF125 expression levels in
LUAD tissues (25). Meanwhile, the
RBPDB database (http://rbpdb.ccbr.utoronto.ca/) predicted that the
RNF125 transcript 3′UTR has putative MBNL1-binding sites (26). Whether MBNL1 upregulates RNF125
expression levels by increasing the stability of RNF125 transcripts
needs to be further clarified.
The present study aimed to investigate the role of
RNF125 in LUAD progression, focusing on its effects on tumor
growth, chemosensitivity and antitumor immunity, as well as its
upstream and downstream molecular mechanisms.
Materials and methods
Bioinformatics
Gene Expression Omnibus Series datasets GSE75037
(https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=gse75037)
(27), GSE31210 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE31210)
(28,29) and GSE116959 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE116959)
(30) containing LUAD and
corresponding non-cancer samples were retrieved from the National
Institutes of Health Gene Expression Omnibus dataset database
(https://www.ncbi.nlm.nih.gov/geo/).
Differentially expressed genes (DEGs) were identified using the R
package ‘limma’ (R version 4.3.0, limma version 3.58.1) under the
threshold of absolute value of log2 fold change ≥1 and adjusted
P<0.01. The protein expression of RNF125 in LUAD tissues was
evaluated using the Human Protein Atlas database (https://www.proteinatlas.org/) (31). Pan-cancer analysis of RNF125 was
performed using the TNMplot platform (https://tnmplot.com/) (32). RNF125 expression in different LUAD
tumor stages was analyzed using the Tumor-Immune System
Interactions Database web portal (http://cis.hku.hk/TISIDB/) (33). Survival analysis was performed using
the GSE30219 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE30219)
(34) and GSE11969 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE11969)
(35,36) datasets via the PROGgeneV2 web portal
(http://www.compbio.iupui.edu/proggene) (37).
The gene list of RNF125 interactors reported in at
least two studies was downloaded from the BioGRID database
(https://thebiogrid.org/) (38). A protein-protein interaction network
of these RNF125 interactors was constructed using the GeneMANIA
database (https://genemania.org/) (39). Gene Ontology Biological Process and
Reactome pathway enrichment analysis of RNF125 interactors was
performed using the DAVID database (https://davidbioinformatics.nih.gov/) (40). The gene list of RBPs with known pro-
or antitumor functions in LC was downloaded from the GeneCards
database (https://www.genecards.org/) (41). The potential RNF125-binding RBPs
were predicted using two RBP databases: RBPDB (http://rbpdb.ccbr.utoronto.ca/) (26) and RBPmap (http://rbpmap.technion.ac.il/) (42). The gene list of human E3 ubiquitin
ligases was downloaded from the UbiNet 2.0 database (https://awi.cuhk.edu.cn/~ubinet/index.php) (43). Correlation analysis between immune
cell infiltration or RBP expression and RNF125 expression was
performed using the TIMER2.0 database (http://timer.cistrome.org/) (25).
Cell culture
LUAD cell lines (NCI-H1975, NCI-H2228, NCI-H1395,
HCC-827, CALU-3 and NCI-H1437), NK-92 and Jurkat T cells were
purchased from iCell Bioscience, Inc. NCI-H1975 (cat. no.
iCell-h156), NCI-H2228 (cat. no. iCell-h351), NCI-H1395 (cat. no.
iCell-h154), HCC-827 (cat. no. iCell-h068), NCI-H1437 (cat. no.
iCell-h284) and Jurkat T (cat. no. iCell-h117) cells were cultured
in RPMI-1640 medium (Beijing Solarbio Science & Technology Co.,
Ltd.) containing 10% fetal bovine serum (FBS; Zhejiang Tianhang
Biotechnology Co., Ltd.). CALU-3 cells were cultured in MEM medium
(Procell Life Science & Technology Co., Ltd.) containing 10%
FBS. NK-92 (cat. no. iCell-h0388) cells were maintained under
dedicated culture conditions [MEMα (Wuhan Servicebio Technology
Co., Ltd.) containing 12.5% FBS and 12.5% horse serum (Beijing
Solarbio Science & Technology Co., Ltd.)]. Cells were cultured
in a humidified 5% CO2 atmosphere at 37°C. All media
were supplemented with 1% penicillin/streptomycin (cat. no. BL505A;
Biosharp Life Sciences). All the cell lines were authenticated by
short tandem repeats (STR) analysis.
Cell transfection
NCI-H1395 and HCC-827 cells were seeded into a
6-well plate 1 day prior to transfection. The cell transfection
experiment was conducted when cells reached ~70% confluence.
NCI-H1395 and HCC-827 cells were transfected with RNF125
overexpression plasmids or RNF125 small hairpin (sh)RNA or small
interfering (si)RNAs targeting MBNL1 using
Lipofectamine® 3000 reagent (Thermo Fisher Scientific,
Inc.) according to the manufacturer's instructions. For each well,
2.5 µg of plasmids or 75 pmol of siRNA were added to cells for
transfection. Cells were then incubated at 37°C for 48 h, after
which, cells were subjected to subsequent experiments.
pcDNA3.1-EGFP vector and pGCsi-H1-Neo-GFP vector were obtained from
Anhui General Biotech Co., Ltd. and were used for constructing
RNF125 overexpression plasmid and RNF125 shRNA plasmid,
respectively.
Stable cell lines were constructed by antibiotic
selection and used for subsequent experiments. The shRNA and siRNA
sequences used in the present study were as follows: shRNA negative
control (shNC) sense, 5′-TTCTCCGAACGTGTCACGT-3′ and antisense,
5′-ACGTGACACGTTCGGAGAA-3′; sh1-RNF125 sense,
5′-GAATGAAATCAGAGTATAA-3′ and antisense, 5′-TTATACTCTGATTTCATTC-3′;
sh2-RNF125 sense, 5′-GTCAGAAGTACATAGATAA-3′ and antisense,
5′-TTATCTATGTACTTCTGAC-3′; si-NC sense, 5′-UUCUCCGAACGUGUCACGU-3′
and antisense, 5′-ACGUGACACGUUCGGAGAA-3′; si1-MBNL1 sense,
5′-GCCAACCAGAUACCCAUAAUA-3′ and antisense,
5′-UAUUAUGGGUAUCUGGUUGGC-3′; and si2-MBNL1 sense,
5′-GCCUGCUUUGAUUCAUUGAAA-3′ and antisense,
5′-UUUCAAUGAAUCAAAGCAGGC-3′.
Reverse transcription-quantitative PCR
(RT-qPCR)
The extraction of RNA was performed using a standard
phenol/chloroform protocol. NCI-H1395 and HCC-827 cells were lysed
in 1 ml TRIpure Reagent (BioTeke Corporation). After incubation for
5 min at room temperature, 200 µl of chloroform was added, gently
mixed and incubated for 3 min at room temperature. After
centrifugation at 10,000 × g for 10 min at 4°C, the aqueous phase
was transferred to a new tube and an equal volume of isopropanol
was added, mixed and incubated at −20°C overnight. The samples were
centrifuged at 10,000 × g for 10 min at 4°C. The supernatant was
discarded and 1 ml of 75% ethanol was added. After centrifugation
at 3,400 × g for 3 min at 4°C, the supernatant was discarded and
the resulting RNA pellet was dried for 5 min at room temperature,
then dissolved in 30 µl RNase-free ddH2O. The
concentration of RNA in each sample was determined using a
UV-visible NanoDrop2000 spectrophotometer (Thermo Fisher
Scientific, Inc.). The RNA samples were reverse transcribed into
cDNA using random primers, RNase inhibitor (Biosharp Life
Sciences), BeyoRT II M-MLV reverse transcriptase (Beyotime
Institute of Biotechnology) and 5× reaction buffer provided with
the reverse transcriptase. qPCR was performed using a qPCR
instrument (Bioneer Corporation) using 1 µl cDNA template, 0.3 µl
SYBR Green (Beijing Solarbio Science & Technology Co., Ltd.), 1
µl forward and reverse primers [General Biotech (Anhui) Co., Ltd.],
10 µl 2X Taq PCR Mastermix (Beijing Solarbio Science &
Technology Co., Ltd) and ddH2O. The amplification
protocol was 95°C for 5 min; 40 cycles of 95°C for 10 sec, 60°C for
10 sec and 72°C for 15 sec; followed by the melt curve analysis for
verification of primer specificity. The relative expression of
target genes was normalized to β-actin. The data analyses were
performed using the 2−ΔΔCq method (44). The primer sequences were: β-actin
forward (F), 5′-GCACAGAGCCTCGCCTT-3′ and reverse (R),
5′-CCTTGCACATGCCGGAG-3′; MBNL1 F, 5′-AAAACGCAGTTGGAGATAA-3′ and R,
5′-GAGAAACAGGTCCCAGATAG-3′; and RNF125 F, 5′-CTGCCGTTCCTGTATTG-3′
and R, 5′-CACCTTGCTGCTGTCTC-3′.
Western blotting
After extracting the total protein from the cells
using RIPA lysis buffer (Beijing Solarbio Science & Technology
Co., Ltd) containing 1 mM PMSF (Beijing Solarbio Science &
Technology Co., Ltd), a BCA protein assay kit (Beijing Solarbio
Science & Technology Co., Ltd) was used to measure the protein
concentrations. A total of 10–20 µg proteins were loaded per lane,
separated by SDS-PAGE with 5% stacking gel and 8/13% separating gel
and transferred to PVDF membranes (MilliporeSigma). The membranes
were blocked with a Western blocking buffer (cat. no. SW3010;
Beijing Solarbio Science & Technology Co., Ltd) at room
temperature for 1 h, followed by incubation with primary antibodies
against RNF125 (cat. no. DF4024; 1:1,000; Affinity Biosciences),
cleaved poly ADP-ribose polymerase (PARP; cat. no. AF7023; 1:1,000;
Affinity Biosciences), PD-L1 (cat. no. BF8035; 1:1,000; Affinity
Biosciences), MBNL1 (cat. no. A8054; 1:1,000; ABclonal Biotech,
Co., Ltd.) and β-actin (cat. no. 66009-1-Ig; 1:10,000; Proteintech
Group, Inc.) at 4°C overnight. The following day, the membranes
were washed using TBST (0.15% Tween-20) and incubated with
horseradish peroxidase-conjugated secondary antibodies goat
anti-rabbit or anti-mouse IgG (cat nos. SE134 and SE131,
respectively; 1:3,000; Beijing Solarbio Science & Technology
Co., Ltd.) at 37°C for 1 h. The blots were visualized using the ECL
Western Blotting Substrate (Beijing Solarbio Science &
Technology Co., Ltd) and analyzed with the Gel-Pro-Analyzer
software (Media Cybernetics).
Colony formation assay
When the cultured NCI-H1395 and HCC-827 cells
reached ~70% confluence, the cells were then subjected to trypsin
digestion with 0.25% trypsin/0.02% EDTA for 2–5 min at 37°C,
centrifugation at 150 × g for 3 min at 4°C and resuspension. The
cells were seeded in the 6-cm cell culture dishes (300 cells/dish)
and incubated for 2 weeks. Cells were fixed with 4%
paraformaldehyde (Shanghai Aladdin Biochemical Technology Co.,
Ltd.) for 25 min at room temperature and then stained using a
Wright-Giemsa composite stain kit for 5 min at room temperature
(Nanjing Keygen Biotech, Co., Ltd.). After washing, an inverted
phase-contrast microscope (Olympus Corporation) was used for cell
photography and counting to calculate the colony formation
efficiency: Colony formation efficiency (%)=(number of
colonies/number of cells seeded) ×100. Quantification was performed
by manual counting under the microscope. Cell clusters containing
>50 cells were counted as colonies.
Transwell invasion and migration
assays
Transwell chambers (Beijing Landeco Technology Co.,
Ltd.) coated with (for invasion assay) or without (for migration
assay) Matrigel (Corning, Inc.) at 37°C for 2 h were placed into
24-well plates. A total of 800 µl culture medium containing 10% FBS
was added to the lower chamber and 200 µl cell suspension (2,000
cells/well for migration assay and 20,000 cells/well for invasion
assay) in serum-free RPMI-1640 medium was added to the upper
chamber. After 24 h incubation at 37°C, the cells that had migrated
or invaded to the lower surface of the Transwell membrane were
fixed with 4% paraformaldehyde for 20 min at room temperature,
stained with crystal violet staining solution for 5 min at room
temperature and imaged using an inverted light microscope (Olympus
Corporation).
Cisplatin sensitivity assay
NCI-H1395 and HCC-827 cells were treated with
different concentrations of cisplatin (0.0, 0.5, 1.0, 2.0, 5.0,
10.0, 20.0 and 50.0 µM; Dalian Meilun Biology Technology Co., Ltd.)
for 48 h at 37°C. Cisplatin-induced changes in cell viability were
measured with the MTT assay.
MTT assay
NCI-H1395 and HCC-827 Cells were inoculated in
96-well plates at 5×103 cells/well and cultured for 0,
24, 48 and 72 h. Each group was set up with 5 multiple wells. At
the indicated time points, 50 µl MTT staining solution was added to
each well (Nanjing Keygen Biotech, Co., Ltd.) and incubated for 4 h
at 37°C. The supernatant was aspirated and 150 µl DMSO (Nanjing
Keygen Biotech, Co., Ltd.) was added to dissolve the purple-colored
formazan crystals. The optical density was measured at 490 nm using
an Enzyme-labeled Instrument (BioTek; Agilent Technologies,
Inc.).
Caspase-3 activity assay
Caspase-3 activity was measured using the Caspase-3
Activity Assay Kit (cat. no. C1116; Beyotime Institute of
Biotechnology) according to the manufacturer's protocol. The
measurement of caspase-3 activity is based on the ability of
caspase-3 to change Ac-DEVD-pNA into the yellow formazan
product, p-nitroaniline (pNA). NCI-H1395 and HCC-827
cells were lysed with lysis buffer included in the kit in an ice
bath for 15 min and centrifuged at 16,000 × g for 15 min at 4°C.
The supernatant was incubated with Ac-DEVD-pNA (caspase-3
substrate) at 37°C for 2 h. Caspase-3 activity was measured by
spectrophotometric detection of pNA at a wavelength of 405
nm.
Co-immunoprecipitation (Co-IP)
The total protein extraction was performed as
described in the western blotting section. The total protein
concentration was determined using the BCA Protein Assay Kit
(Beyotime Institute of Biotechnology) following the manufacturer's
instructions. Co-IP analysis was performed according to the
manufacturer's instructions for Pierce Co-IP Kit (cat. no. 26149,
Thermo Fisher Scientific, Inc.). For each IP reaction, 2 µg
anti-PD-L1 antibodies (cat. no. BF8035; 1:100; Affinity
Biosciences) or negative control IgG (cat. no. A7016; 1:100;
Beyotime) were crosslinked to 20 µl AminoLink Plus Coupling Resin
slurry. A total of 500 µg lysates were incubated with the
antibody-coupled resin at 4°C overnight. The following day, the
samples were washed using 200 µl IP Lysis/Wash buffer, 200 µl
Modified Dulbecco's PBS and 100 µl Conditioning Buffer included in
the Co-IP kit and the flow-through was discarded. Next, the
immunoprecipitates were washed with 10 µl Elution Buffer included
in the Co-IP kit. Subsequently, 50 µl Elution Buffer was added and
incubated for 5 min at room temperature. Finally, the tube was
briefly centrifuged, and the flow-through was collected. The
protein samples were separated by SDS-PAGE as described in the
western blotting section.
Evaluation of Jurkat T cell
activation
Evaluation of Jurkat T cell activation was performed
as previously described (18).
Jurkat T cells were resuspended in basal culture medium (RPMI-1640
+ 10% FBS) containing 20 ng/ml phorbol 12-myristate 13-acetate and
200 µg/ml ionomycin and co-cultured with LUAD (NCI-H1395 and
HCC-827) cells. The ratio of cancer cells to T cells was 1:4. The
level of IL-2 in the cell culture supernatants was determined using
an ELISA kit (cat. no. EK102; Hangzhou Lianke Biotechnology Co.,
Ltd.) according to the manufacturer's protocol.
NK cell cytotoxicity assay
NK cell cytotoxicity assay was performed as
previously described (17). In
brief, NK-92 cells were co-cultured with LUAD (NCI-H1395 and
HCC-827) cells at different cancer cell to NK cell ratios (1:0,
1:2.5, 1:5 and 1:10) for 4 h. The viability of cancer cells was
detected by MTT assay after refreshing the culture medium to remove
NK cells.
RNA stability analysis
RNA stability analysis was performed as previously
described (45). Cells were
transfected with MBNL1 siRNA or the corresponding control siRNA. A
total of 48 h after cell transfection, the cells were treated with
5 mg/ml actinomycin D for different times at 37°C (0, 1, 2.5 and 5
h). qPCR was performed to detect RNF125 mRNA levels, using 18S rRNA
as the endogenous normalization control as previously described
(46). The primer sequences of 18S
rRNA were: F, 5′-AGCGAAAGCATTTGCCAAGA-3′ and R,
5′-TATGGTCGGAACTACGACGGT-3′. The percentage of remaining RNF125
mRNA relative to 0 h at the indicated time points was
calculated.
RNA immunoprecipitation (RIP)-PCR
RIP-PCR experiments were performed to verify the
binding of the MBNL1 protein to the RNF125 transcript. The cells
were resuspended in RIP lysis buffer (MilliporeSigma). A total of 5
µg anti-MBNL1 antibodies (cat. no. A8054, 1:1,000; ABclonal Biotech
Co., Ltd.) were pre-bound to Protein A/G magnetic beads in
immunoprecipitation buffer and then incubated with cell lysates for
12 h at 4°C. After washing the magnetic beads, the RNA-protein
complex was added to the proteinase K buffer included in the RIP
kit (cat. no. 17-701; MilliporeSigma). The extraction of RNA was
performed using a standard phenol/chloroform protocol as previously
described in the RT-qPCR section of the manuscript. The extracted
RNA was reverse transcribed to cDNA using BeyoRT II M-MLV reverse
transcriptase (Beyotime Institute of Biotechnology) according to
the manufacturer's instructions. The resulting cDNA was used as the
template for PCR. The primer sequences were: F,
5′-AAAAGGGACCACTGAAT-3′ and R, 5′-CACCTACTTGCCTACCA-3′. The
amplification protocol was 95°C for 5 min; 40 cycles of 95°C for 15
sec, 55°C for 25 sec and 72°C for 30 sec; followed by 25°C for 5
min. The PCR product was electrophoresed on a 2% agarose gel.
Electrophoresis images were obtained using a gel imaging analysis
system (Beijing LIUYI Biotechnology Co., Ltd.
Immunohistochemical (IHC) staining and
analysis
A total of 21 pairs of non-cancerous and cancerous
tissues were collected from patients with LUAD [8 men and 13 women;
median age, 64 years (range, 32–74 years)] who underwent surgery at
the Harbin Medical University Cancer Hospital (Harbin, China) in
December 2024. These samples were collected as part of an overall
project on IHC staining and analysis of lung cancer specimens.
Tissue specimens were fixed with 4% paraformaldehyde
for 24 h at 4°C, embedded with paraffin and sliced at a 5-µm
thickness. The sections were then deparaffinized by baking at 64°C
for 2–4 h and xylene and rehydrated by transfer through a
decreasing concentration gradient of ethanol solutions. After
antigen retrieval, the sections were incubated with 3%
H2O2 solution for 15 min at room temperature
to block the endogenous peroxidase activity. Prior to
immunostaining with primary antibodies against RNF125 (cat. no.
13290-1-AP; 1:50; Proteintech Group, Inc.), the sections were
blocked with 1% bovine serum albumin for 15 min at room temperature
(Sangon Biotech Co., Ltd.). After overnight incubation with the
primary antibodies at 4°C, the sections were washed and incubated
with the HRP-conjugated secondary goat anti-rabbit IgG antibodies
(cat. no. 31460; 1:500; Thermo Fisher Scientific, Inc.) at 37°C for
1 h. The IHC signal was developed by 3,3′-diaminobenzidine
substrates. The sections were incubated in hematoxylin for 3 min at
room temperature for counterstaining. After which, the sections
were observed under a light microscope. The signal intensity and
percentage of stained cells were measured as previously described
(47). The staining intensity was
manually scored from 0 (lowest) to 3 (highest). The number of
stained cells was counted using the Image-Pro Plus version 6.0
software (Media Cybernetics). The IHC score was determined by the
percentage of stained cells multiplied by the staining intensity
(IHC score=percentage of stained cells × staining intensity).
Statistical analysis
Data were presented as the mean ± standard deviation
(SD) and analyzed using the GraphPad Prism (version 9; Dotmatics).
Each experiment was repeated in triplicate. The Wilcoxon matched
pairs signed rank test was used to compare the differences between
the IHC staining scores of RNF125. Unpaired student's t-tests were
used to examine the differences between two groups. A one- or
two-way ANOVA with Tukey's multiple comparison test was used to
compare the means of ≥3 groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
RNF125 is downregulated in human LC
and predicts unfavorable survival
To identify the differentially expressed E3
ubiquitin ligases in LUAD, first three GSE cohorts GSE75037
(27), GSE31210 (28,29)
and GSE116959 (30) containing LUAD
and corresponding non-cancer samples were analyzed. After which,
the differentially expressed genes (DEGs; absolute value of log2
fold change ≥1 and adjusted P<0.01) were overlapped with the
gene list of human E3 ubiquitin ligases downloaded from the UbiNet
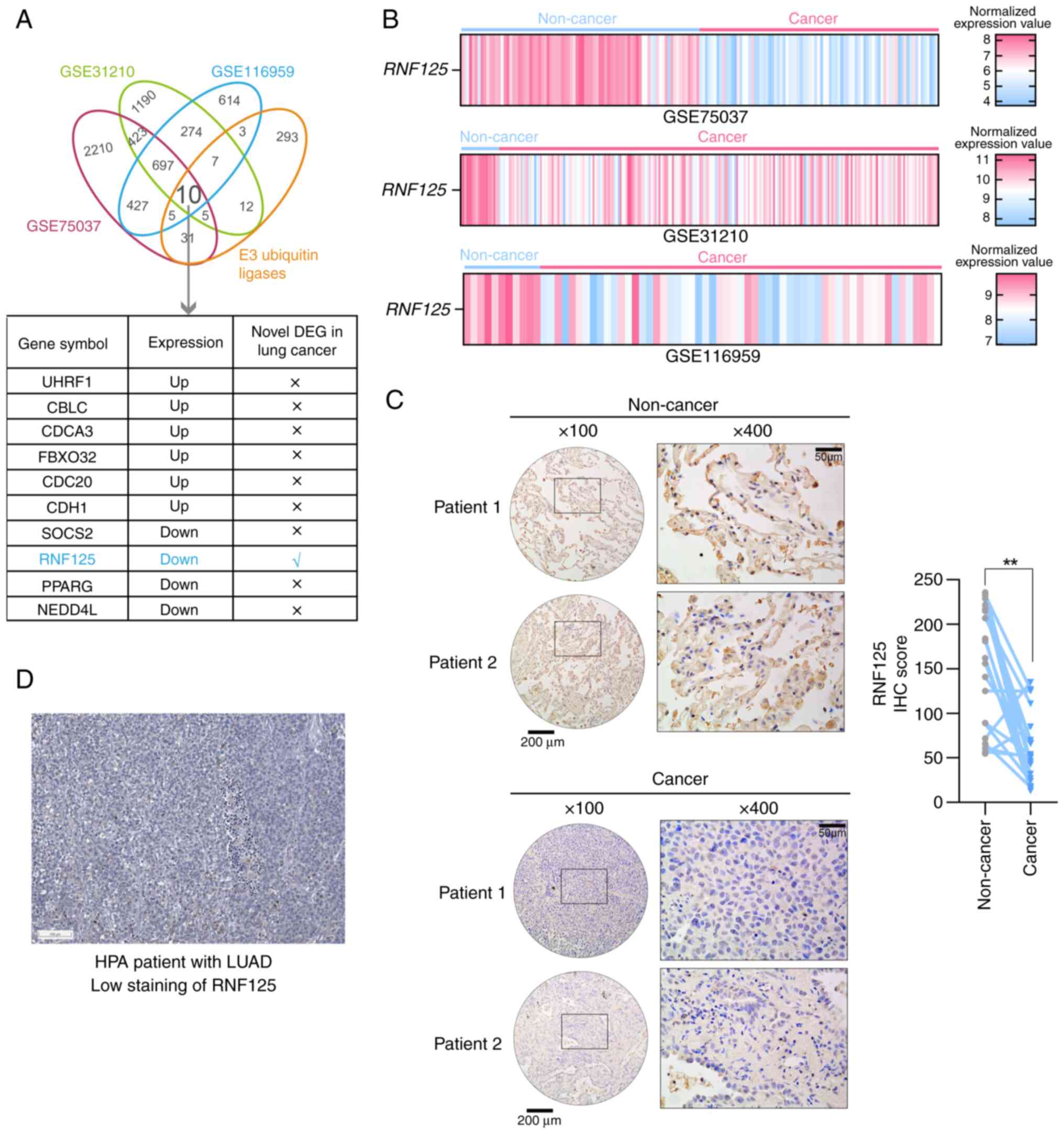
2.0 database (https://awi.cuhk.edu.cn/~ubinet/index.php) (43). As a result, 10 E3 ubiquitin ligases
were found to be differentially expressed in LUAD (Fig. 1A). The functions of most of these
ligases have been previously reported in LC (48–56),
with only RNF125 identified as a novel DEG in LC (Fig. 1A). The expression of RNF125 was
downregulated in LUAD samples compared with non-cancerous samples
(Fig. 1B). Subsequently, IHC
staining was performed for RNF125 in 21 pairs of LUAD tissue and
adjacent non-cancerous tissue. Compared with the non-cancerous
tissues, LUAD tissues had a lower IHC score for RNF125, suggesting
that RNF125 was downregulated in human LUAD tissues (Fig. 1C). Similarly, the Human Protein
Atlas database (https://www.proteinatlas.org/) (31) was explored to assess the protein
expression of RNF125 in LUAD tissues, showing a low staining of
RNF125 in LUAD, which was consistent with the present results
(Fig. 1D).
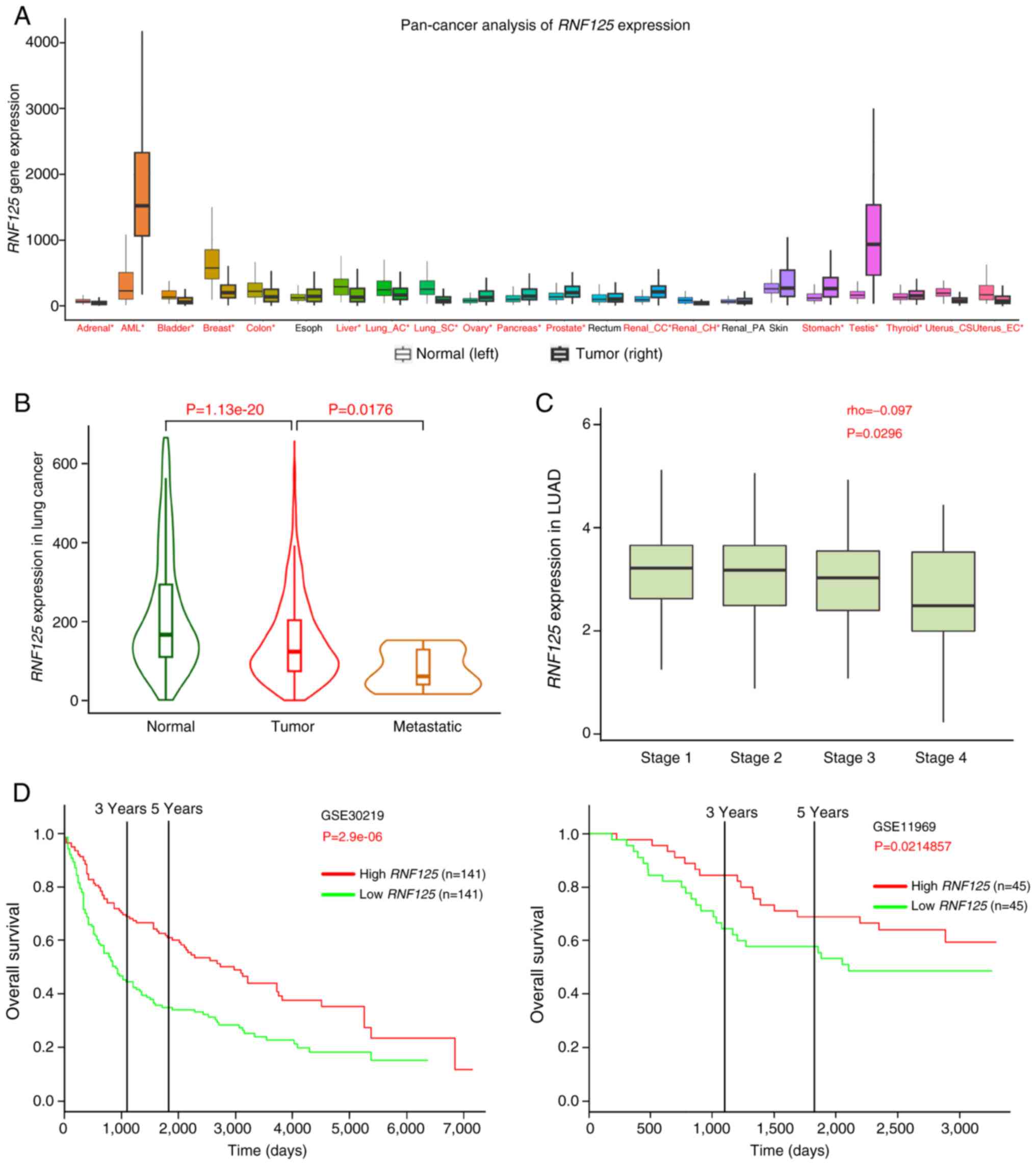
Next, a pan-cancer analysis of RNF125 was performed
using the TNMplot platform and it was demonstrated that RNF125 was
significantly downregulated in several cancers including LC
(Fig. 2A) (32). Moreover, RNF125 expression was
significantly gradually decreased in normal lung tissues, primary
tumor and metastatic LC tissues (Fig.
2B) (32). After which, RNF125
expression was analyzed in different LUAD tumor stages via the
TISIDB web portal (http://cis.hku.hk/TISIDB/) (33). These results showed a weak negative
association between RNF125 expression and LUAD tumor stage
(Fig. 2C). Survival analysis was
performed using two GSE cohorts: GSE30219 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE30219)
(34) and GSE11969 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE11969)
(35,36) via the PROGgeneV2 web portal
(http://www.compbio.iupui.edu/proggene) (37). The results indicated that increased
expression of RNF125 was associated with high overall survival of
patients (Fig. 2D). These data
suggested that RNF125 expression was decreased in LC and that
reduced RNF125 levels predicted unfavorable survival.
RNF125 inhibits proliferation, colony
formation, migration and invasion of LUAD cells
The expression of RNF125 in LUAD cells was detected
by RT-qPCR. NCI-H1975 and NCI-H2228 cells showed the highest levels
of RNF125 expression; CALU-3 and NCI-H1437 cells showed the lowest
levels of RNF125 expression; and NCI-H1395 and HCC-827 cells showed
moderate RNF125 expression levels (Fig.
3A). Since the present study intended to determine the effects
of RNF125 deficiency or overexpression on the same LUAD cell line,
NCI-H1395 and HCC-827 cells with moderate RNF125 expression were
selected to establish RNF125-silenced and RNF125-overexpressing
cell lines for subsequent experiments. To address the role of
RNF125 in LUAD, RNF125 overexpression or stable knockdown LUAD
cells were constructed to evaluate the effect of RNF125 on the
proliferation, colony formation, migration and invasion of LUAD
cells. The results of RT-qPCR and western blotting demonstrated
that RNF125 was effectively overexpressed or knocked down (Fig. 3B and C). Compared with the sh-NC
group, silencing of RNF125 significantly promoted proliferation of
LUAD cells (Fig. 3D). Conversely,
compared with the Vector group, the overexpression of RNF125
significantly inhibited proliferation of LUAD cells (Fig. 3E). Compared with the sh-NC group,
RNF125 silencing significantly promoted colony formation of
NCI-H1395 cells and compared with the Vector group, RNF125
overexpression significantly inhibited colony formation of
NCI-H1395 cells (Fig. 3F) Similar
results were observed in HCC-827 cells (Fig. 3G).
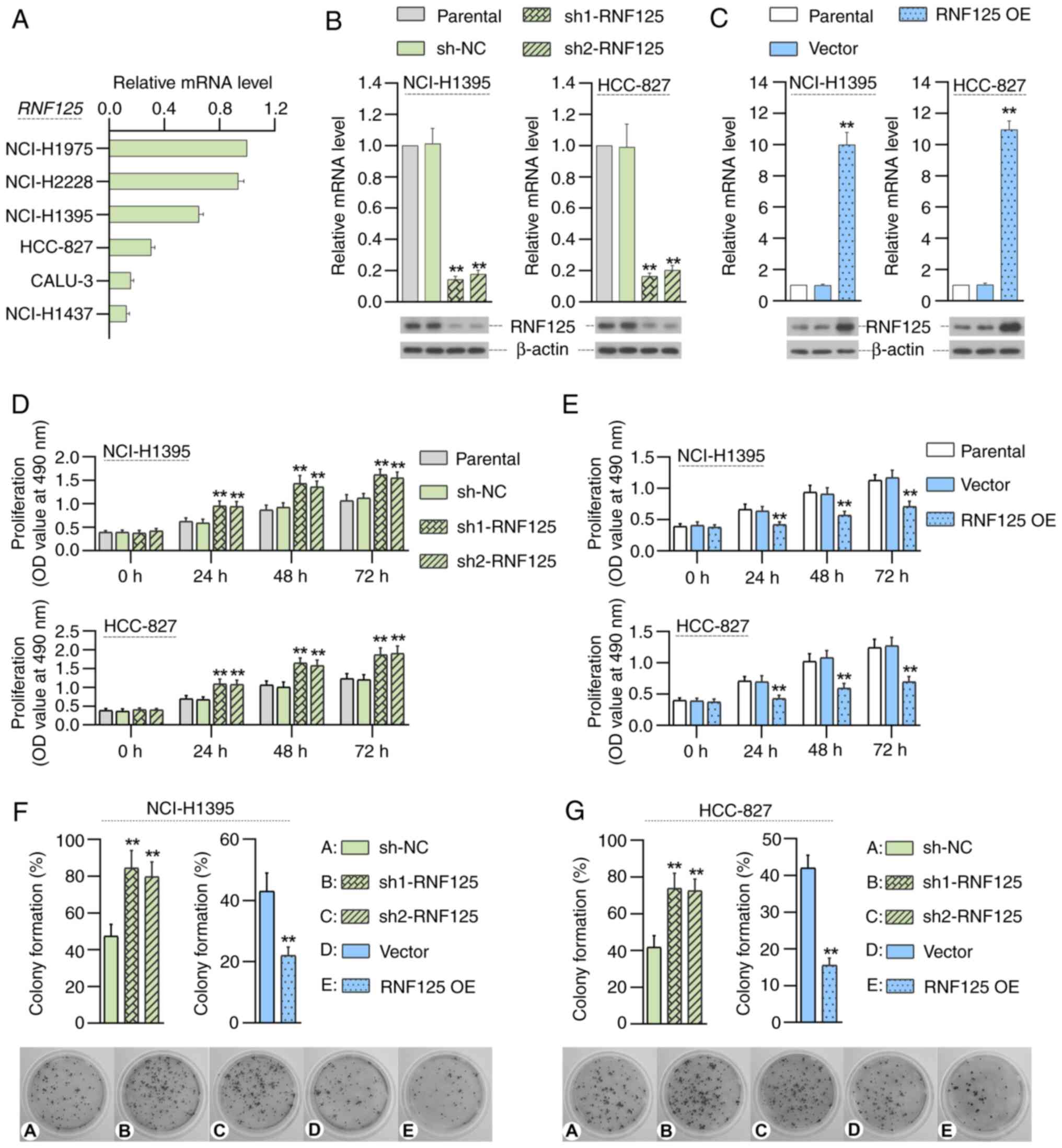 | Figure 3.RNF125 inhibits the proliferation and
colony formation of LUAD cells. (A) RT-qPCR shows RNF125 expression
in six LC cell lines (NCI-H1975, NCI-H2228, NCI-H1395, HCC-827,
CALU-3 and NCI-H1437). RT-qPCR and western blotting demonstrate
plasmid-mediated RNF125 (B) knockdown or (C) overexpression in
NCI-H1395 and HCC-827 cells. MTT assays show the effect of RNF125
(D) knockdown or (E) overexpression on the proliferation of
NCI-H1395 and HCC-827 cells. Colony formation analysis shows the
effect of RNF125 knockdown or overexpression on colony formation of
(F) NCI-H1395 and (G) HCC-827 cells. Data are presented as mean ±
SD. **P<0.01 vs. shNC or vector. LUAD, lung adenocarcinoma;
RT-qPCR, reverse transcription-quantitative PCR; RNF125, ring
finger protein 125; sh, short hairpin RNA; NC, negative control;
OE, overexpression; OD, optical density. |
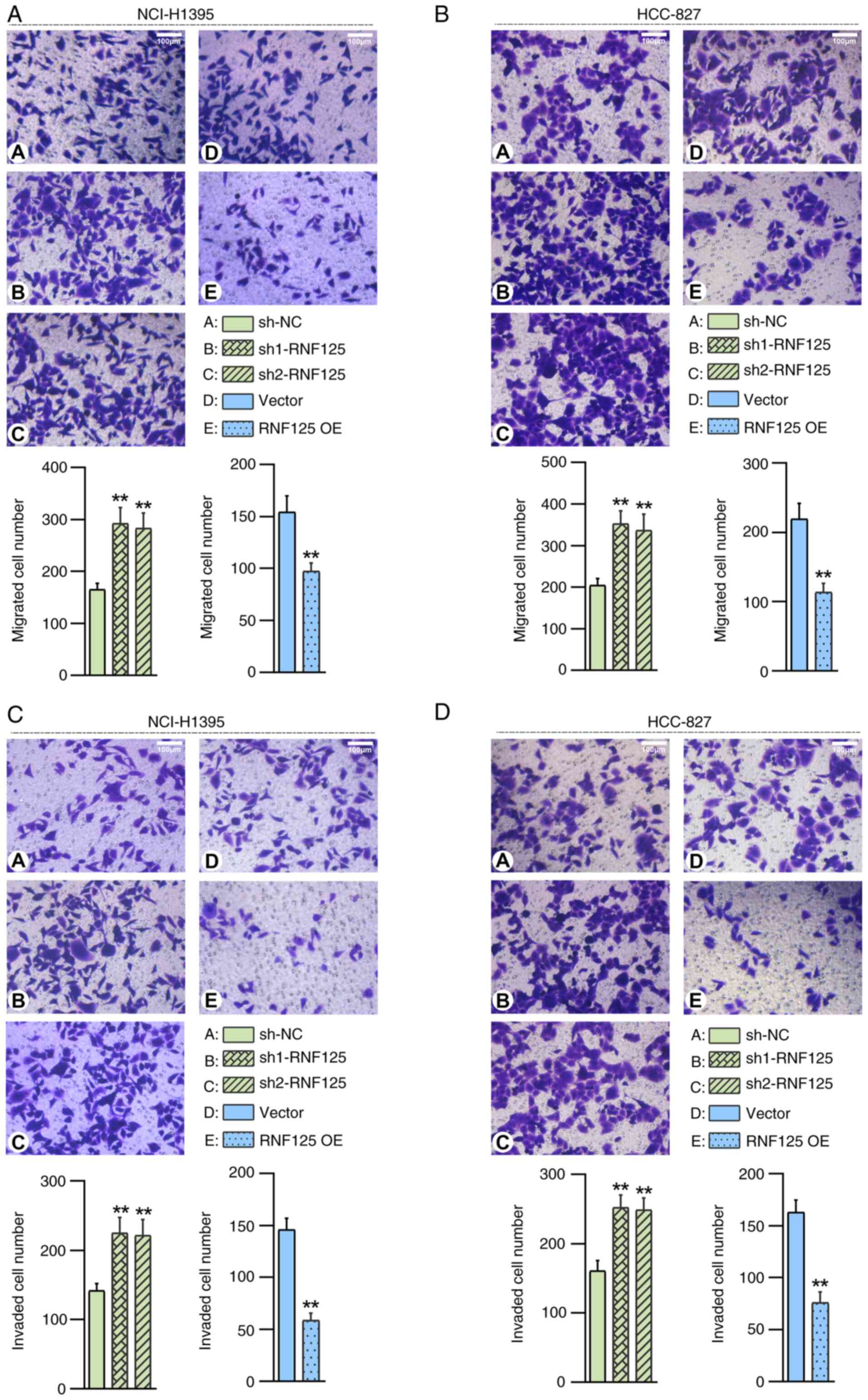
As demonstrated by the Transwell migration assay
results, the cell migration capacity was significantly enhanced in
RNF125-silenced NCI-H1395 cells compared with the sh-NC group and
suppressed in RNF125-overexpressing NCI-H1395 cells compared with
the Vector group (Fig. 4A). Similar
results were observed in HCC-827 cells as well (Fig. 4B). Additionally, the capabilities of
cell invasion were significantly enhanced in RNF125-silenced LUAD
cells compared to the sh-NC group and suppressed in
RNF125-overexpressing LUAD cells compared to the Vector group
(Fig. 4C and D).
Taken together, these data suggested that RNF125
suppressed proliferation, colony formation, migration and invasion
of LUAD cells.
RNF125 enhances the efficacy of
cisplatin in LUAD cells
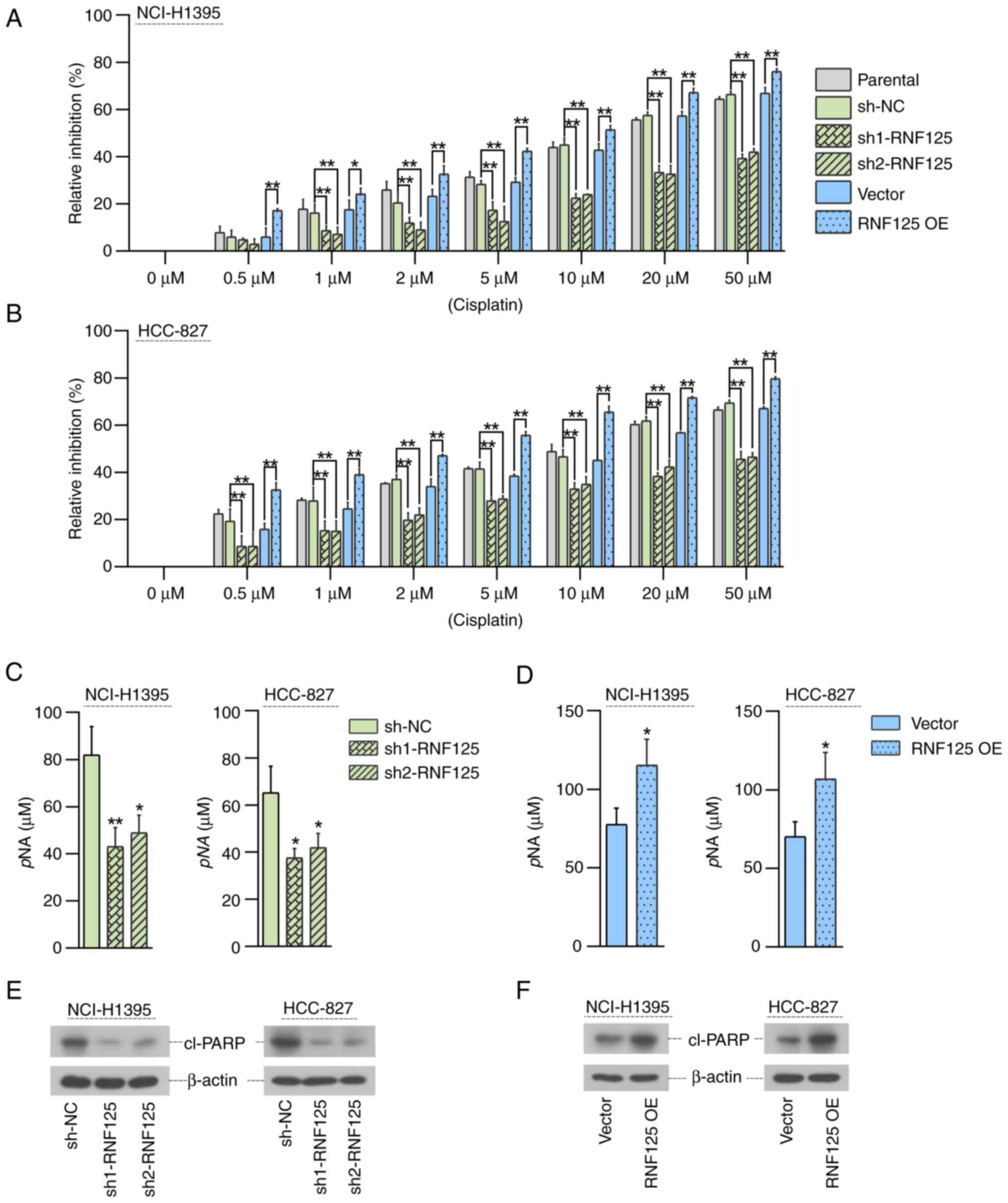
The role of RNF125 in the sensitization of LUAD
cells to cisplatin was investigated. Cells were treated with
different concentrations of cisplatin for 48 h and the MTT assay
was used to detect cell viability to calculate the relative
inhibition rate. After treatment with each indicated concentration
of cisplatin, the inhibitory effect of cisplatin on LUAD cell
viability was significantly decreased in RNF125-silenced cells and
significantly promoted in RNF125-overexpressing cells compared with
the corresponding control groups (sh-NC group or Vector group)
(Fig. 5A and B). Furthermore,
compared with the corresponding control groups (sh-NC group or
Vector group), the caspase-3 levels in cisplatin-treated LUAD cells
were significantly reduced by RNF125 knockdown (Fig. 5C) but increased by RNF125
overexpression (Fig. 5D).
Similarly, compared with the corresponding control groups (sh-NC
group or Vector group), the protein expression levels of cleaved
PARP in cisplatin-treated LUAD cells was significantly reduced by
RNF125 knockdown (Fig. 5E) but
increased by RNF125 overexpression (Fig. 5F). Collectively, these results
showed that RNF125 enhanced the sensitivity of LUAD cells to
cisplatin.
RNF125 knockdown in LUAD cells
increases PD-L1, suppresses T-cell activation and attenuates NK
cell killing of cancer cells
To investigate the downstream molecular mechanism of
RNF125 in LUAD, the RNF125 interactors reported by at least two
studies were downloaded from the BioGRID database (https://thebiogrid.org/) (38). A protein-protein interaction network
of these RNF125 interactors was constructed using the GeneMANIA
database (https://genemania.org/) (39), in which CD274 (also termed PD-L1)
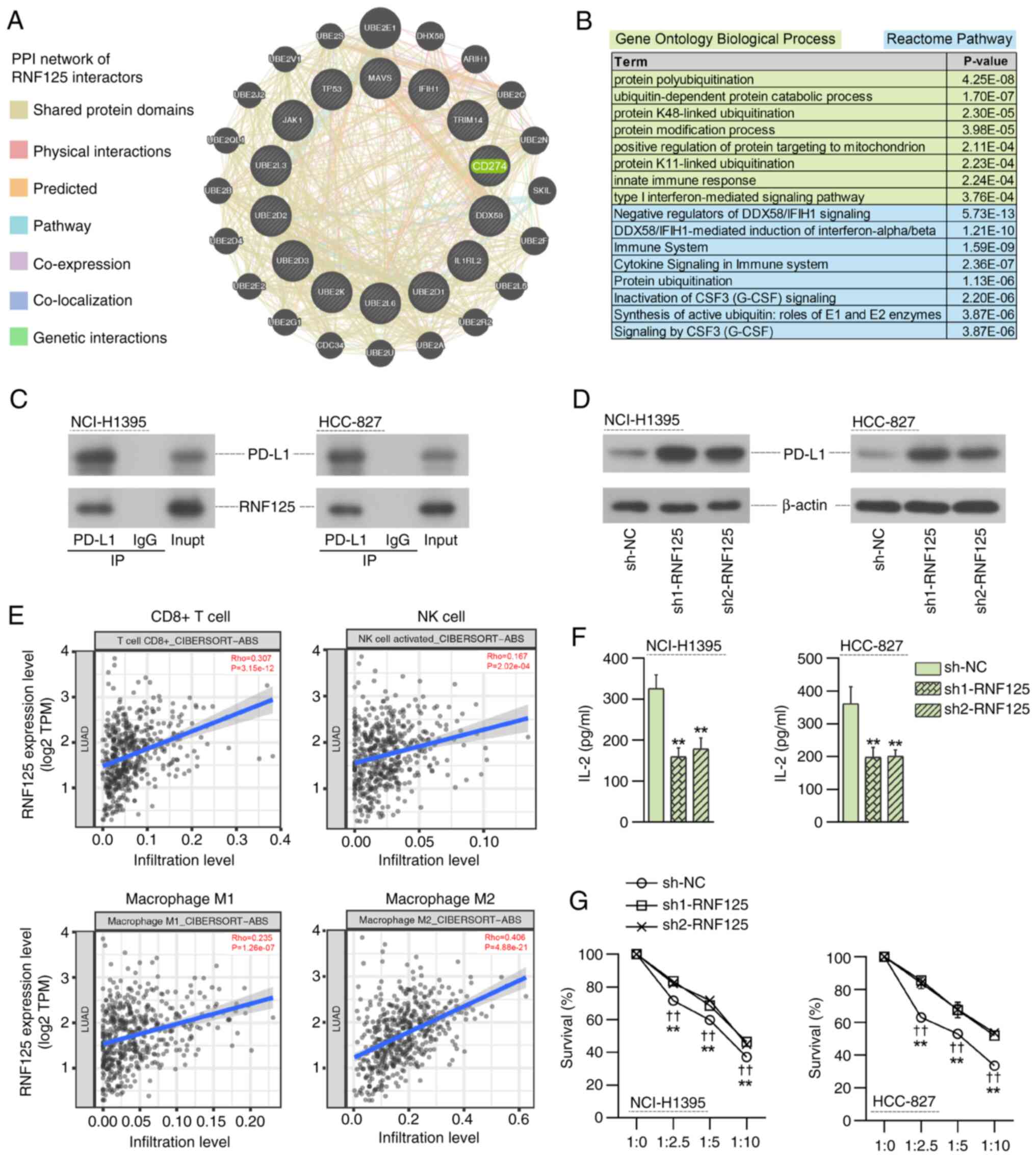
was identified as an important interactor of RNF125 (Fig. 6A). Gene Ontology Biological Process
and Reactome pathway enrichment analysis revealed that these RNF125
interactors were markedly enriched in protein ubiquitination and
immune response-related processes and pathways (Fig. 6B). These findings were consistent
with a previous study showing that RNF125 facilitated PD-L1
ubiquitination and degradation and thereby suppressed immune
evasion in head and neck squamous cell carcinoma (14).
To investigate the effect of RNF125 on PD-L1
expression, Co-IP and western blotting assays were performed. Co-IP
assay results confirmed the interaction between RNF125 and PD-L1
(Fig. 6C). The results of the
western blotting indicated that RNF125 silencing increased the
expression of PD-L1 (Fig. 6D).
The TIMER2.0 database (http://timer.cistrome.org/) (25) was used to investigate the
association between RNF125 expression and immune cell infiltration
in LUAD. A positive correlation was observed between RNF125
expression and CD8+ T cell, NK cell and M1/M2 macrophage
infiltration levels in LUAD, suggesting the potential involvement
of RNF125 in tumor immunity in LUAD (Fig. 6E).
Additionally, the effect of RNF125 knockdown on the
antitumor activity of Jurkat T cells and NK-92 cells was evaluated.
Compared with those co-cultured with sh-NC LUAD cells, the
activated Jurkat T cells co-cultured with RNF125-silenced LUAD
cells released significantly lower IL-2 (Fig. 6F). Similarly, co-culture with NK
cells resulted in the death of sh-NC LUAD cells, which was
significantly suppressed in the LUAD cells with RNF125 knockdown
(Fig. 6G). Taken together, the loss
of RNF125 enhanced the immune escape of LUAD cells.
MBNL1 is an upstream regulator of
RNF125 in LUAD
To investigate the molecular mechanism underlying
RNF125 expression regulation in LUAD, the potential RNF125-binding
RBPs were predicted using two RBP databases RBPDB (http://rbpdb.ccbr.utoronto.ca/) (26) and RBPmap (http://rbpmap.technion.ac.il/) (42). In addition, the RBPs with known
pro-tumor or anti-tumor functions in LC were searched for in the
GeneCards database (https://www.genecards.org/) (41). A total of five RNF125-binding RBPs
were obtained (Fig. 7A).
Furthermore, expression correlation analysis was performed between
RNF125 and these RBPs in LUAD using the TIMER2.0 database and it
was found that MBNL1 had the highest correlation coefficient with
RNF125 in LUAD (Fig. 7B).
Therefore, MBNL1 was selected for further study.
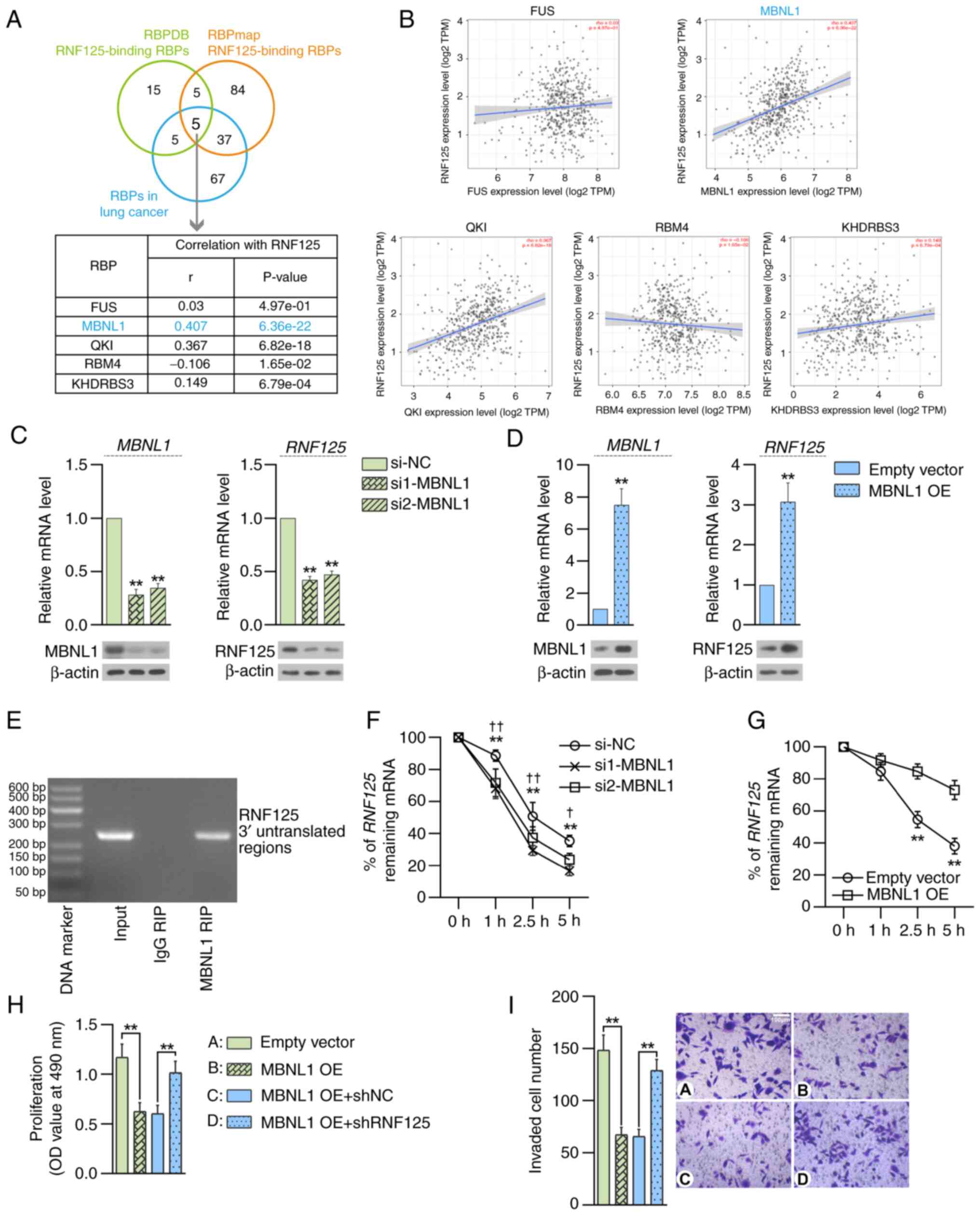 | Figure 7.MBNL1 is an upstream modulator of
RNF125 in LUAD. (A) Potential RNF125-binding RBPs were predicted
using two RBP databases (RBPDB and RBPmap) and overlapped with RBPs
involved in lung cancer progression. (B) Correlation analysis
between RNF125 and the indicated RBPs in LUAD was performed using
the TIMER2.0 portal. RT-qPCR and western blotting show MBNL1 and
RNF125 expression in NCI-H1395 cells 48 h after transfection of (C)
MBNL1-specific siRNAs or (D) plasmids containing MBNL1 coding
sequences (MBNL1 OE). **P<0.01 vs. siNC or empty vector. (E)
RIP-PCR and agarose gel electrophoresis showed that RNF125
transcripts were detected in the MBNL1 antibody RIP products from
NCI-H1395 cells. After treatment with actinomycin D, RT-qPCR
analysis showed the % of remaining RNF125 mRNA relative to 0 h at
the indicated time points in (F) MBNL1-silenced (**P<0.01 vs.
si1-MBNL1; †P<0.05, ††P<0.01 vs.
si2-MBNL1) or (G) MBNL1-overexpressing NCI-H1395 cells (**P<0.01
vs. empty vector). (H) MTT assays determine the viability of
NCI-H1395 cells 48 h after co-transfection of MBNL1 OE and shRNF125
plasmids. (I) Representative images (right) of Transwell assays in
the presence of Matrigel and the mean number of invaded cells
(left) are shown. Scale bar, 100 µm. **P<0.01. Data are
presented as mean ± SD. MBNL1, muscleblind-like 1; RNF125, ring
finger protein 125; LUAD, lung adenocarcinoma; RBP, receptor
binding protein; RT-qPCR, reverse transcription-quantitative PCR;
siRNA, small interfering RNA; OE, overexpression; NC, negative
control; sh, short hairpin RNA; TPM, transcripts per million. |
The effect of MBNL1 on RNF125 expression in LUAD
cells was examined. RT-qPCR and western blotting results suggested
that the expression levels of MBNL1 and RNF125 were significantly
upregulated upon MBNL1 overexpression and significantly
downregulated upon MBNL1-silencing in LUAD cells (Fig. 7C and D). RIP-PCR demonstrated that
MBNL1 protein bound to the 3′UTR of RNF125 transcripts (Fig. 7E). To verify the effect of MBNL1 on
RNF125 transcript stability, LUAD cells were treated with the
transcriptional inhibitor actinomycin D. Downregulation of MBNL1
promoted the decay of RNF125 mRNA, whereas overexpression of MBNL1
suppressed the decay of RNF125 mRNA, as demonstrated by qPCR assay
(Fig. 7F and G). Additionally,
MBNL1 overexpression suppressed the proliferation of LUAD cells
compared with the empty vector control and RNF125 knockdown
significantly eliminated the effect of MBNL1 overexpression
compared with the corresponding control (Fig. 7H). Similarly, MBNL1 overexpression
suppressed the invasion of LUAD cells and RNF125 knockdown
eliminated the effect of MBNL1 overexpression compared with the
corresponding control groups (Fig.
7I). Overall, these results suggested that MBNL1 may be an
upstream regulator of RNF125 in LUAD.
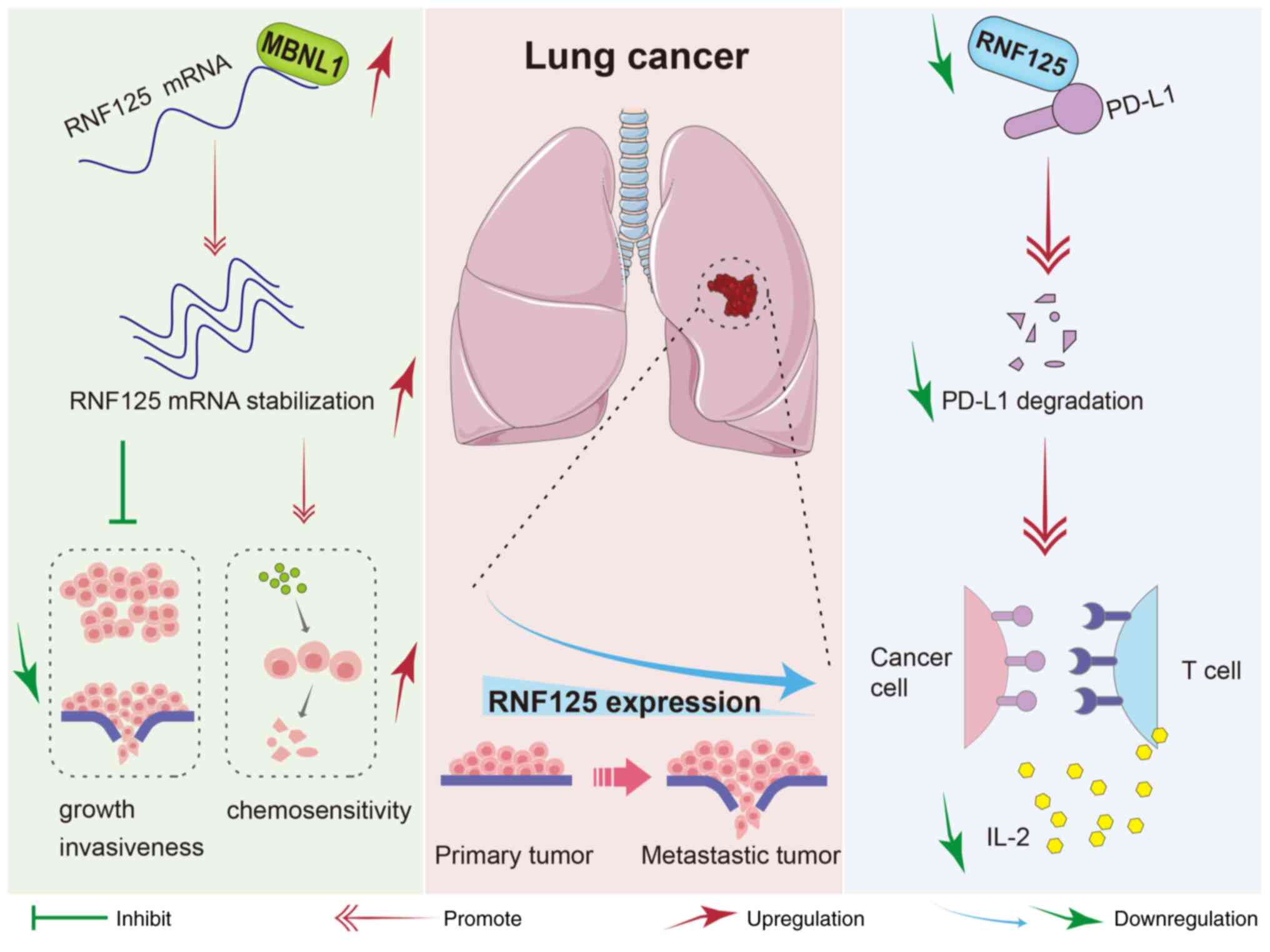
Fig. 8 depicts a
schematic diagram of the function and molecular regulatory
mechanism of RNF125 in LC. In short, RNF125 is downregulated in LC,
and its low expression is associated with advanced-stage disease.
RNF125 inhibits the LUAD cell growth and invasiveness and enhances
the chemosensitivity of LUAD cells to cisplatin. RNF125 acts as an
E3 ubiquitin ligase of PD-L1. Knockdown of RNF125 suppresses PD-L1
degradation, thereby impairing T-cell activation and anti-tumor
cytokine secretion. Mechanistically, the RBP MBNL1 serves as an
upstream regulator of RNF125 by stabilizing RNF125 mRNA.
Discussion
Ubiquitin E3 ligases exert an important role in
eukaryotes by facilitating protein ubiquitination and degradation
(57). RNF125 is a ubiquitin E3
ligase that is aberrantly expressed in several cancers, including
hepatocellular carcinoma, head and neck squamous cell carcinoma and
melanoma, and has been reported to inhibit tumor progression
(12–15). However, its function in LUAD has not
been reported. In the present study, the tumor suppressor role of
RNF125 in LUAD was described and MBNL1 was proposed as a potential
upstream regulator of RNF125 by controlling the stability of RNF125
mRNA.
Analysis based on clinical data showed that the
RNF125 expression level was decreased and associated with
metastatic status, advanced tumor stage and poor overall survival
in LC, indicating a tumor suppressor role of RNF125 in LC. Kodama
et al (11) reported that
RNF125 limited hepatocellular carcinoma progression by inhibiting
cancer cell proliferation. Consistent with the aforementioned
results, the data from the present study showed that the
overexpression of RNF125 inhibited LUAD cell growth. Moreover, it
was demonstrated that RNF125 overexpression also inhibited cell
migration and invasion. These findings suggested that RNF125 serves
an important role in suppressing LUAD progression.
Platinum-based chemotherapy, particularly the use of
cisplatin, is an important treatment for patients with advanced
LUAD (7,58). Yet, the application of this drug is
hampered by the poor response of patients with advanced LUAD to
chemotherapy (59,60). Therefore, it is warranted to develop
innovative and efficient strategies for increasing the sensitivity
of LUAD cells to drug therapy. In the present study, it was
demonstrated that knockdown of RNF125 decreased cisplatin-mediated
cell apoptosis which corresponded with an increase in cell
viability, whereas the overexpression of RNF125 exerted
antithetical effects. In terms of molecular mechanisms, RNF125
knockdown reduced the activity of caspase-3 and the expression
levels of cleaved PARP in cisplatin-treated LUAD cells. Similarly,
downregulated RNF125 has been found to contribute to the resistance
of melanoma to BRAF inhibitors (15). These findings support a potential
role for RNF125 in regulating the sensitivity of tumor cells to
therapeutic agents.
PD-L1 serves a critical role in regulating immune
evasion, particularly in suppressing T cell functions (17,61–64).
Previous research has shown that RNF125 suppresses immune escape by
reducing PD-L1 expression through promoting PD-L1 ubiquitination
and proteasomal degradation (14,20).
In the present study, it was demonstrated that knockdown of RNF125
downregulated PD-L1 in LUAD cells and inhibited T cell activation.
These findings suggest a potential link to RNF125-mediated
modulation of PD-L1 with the previously reported role of RNF125 as
a positive regulator of T cell activation (11). In addition, it was observed that the
knockdown of RNF125 impaired NK cell lysis of LUAD cells. Taken
together, these findings indicate that RNF125 may be an important
regulator of immune evasion of LUAD. In addition, it was also
hypothesized that the role of RNF125 in antitumor immunity might be
associated with macrophages. As shown in the IHC staining of RNF125
in the Human Protein Atlas database (https://www.proteinatlas.org/ENSG00000101695–RNF125/tissue/lung)
(31), medium expression of RNF125
in the normal lung was observed in macrophages, which requires
further investigation into the specific function of RNF125 in
macrophages and immune regulation.
RBP MBNL1 is a class of RNA metabolism regulators
that control pre-mRNA splicing (65). Increasing MBNL1 protein expression
levels in tumors inhibits tumor progression, resulting in notably
prolonged survival of mice bearing human glioma stem cell-derived
orthotopic xenografts (22). A
previous study reported that MBNL1 increased the mRNA stability of
metastasis suppressors debrin like (DBNL) and transforming acidic
coiled-coil containing protein 1 (TACC1) to inhibit the
invasiveness of breast cancer cells by binding to the 3′UTRs of
DBNL and TACC1 mRNA (21). In the
present study, the data showed that RNF125 knockdown abrogated the
inhibitory effects of MBNL1 overexpression on proliferation and
invasion of LUAD cells. Mechanistically, the MBNL1 protein bound to
the 3′UTR of RNF125 transcripts and enhanced its stability, thereby
promoting RNF125 expression. Correlation analysis demonstrated a
positive correlation between the expression levels of RNF125 and
MBNL1 in tumor tissues derived from patients with LUAD. These
results indicated that MBNL1/RNF125 may serve an important role in
regulating LUAD progression.
In summary, the present study demonstrated that
RNF125 served a tumor suppressor role in LUAD. Moreover, MBNL1
augmented RNF125 expression levels through binding to the 3′UTR of
RNF125 transcripts. Collectively, these findings provided potential
novel therapeutic targets for LUAD treatment in the future.
Acknowledgments
Not applicable.
Funding
Funding: Not applicable.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YY and XJ were responsible for designing the
methodology and performing experiments. YY and XK performed data
analysis. XK visualized the data. JB curated the data. JB and BN
were responsible for data validation. YY drafted the manuscript and
SX reviewed and edited the manuscript. ZR contributed to the
conceptualization and supervision of the project. JG contributed to
data curation and project administration. SX conceptualized the
study and provided resources. All authors read and approved the
final version of the manuscript. YY and SX confirm the authenticity
of all the raw data.
Ethics approval and consent to
participate
LUAD samples and paired non-cancerous samples were
obtained in accordance with the protocol approved by the Ethics
Committee of Harbin Medical University Cancer Hospital approval no.
JS2024-30; Harbin, China). Written informed consent to participate
was obtained from each patient or the patient's guardian.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
Co-IP
|
co-immunoprecipitation
|
|
IHC
|
immunohistochemical
|
|
LC
|
lung cancer
|
|
LUAD
|
lung adenocarcinoma
|
|
MBNL1
|
muscleblind-like 1
|
|
pNA
|
p-nitroaniline
|
|
PARP
|
poly ADP-ribose polymerase
|
|
PD-L1
|
programmed cell death ligand 1
|
|
RBP
|
RNA-binding protein
|
|
RIP
|
RNA immunoprecipitation
|
|
RNF125
|
ring finger protein 125
|
References
|
1
|
Jones GS and Baldwin DR: Recent advances
in the management of lung cancer. Clin Med (Lond). 18 (Suppl
2):S41–S46. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nasim F, Sabath BF and Eapen GA: Lung
cancer. Med Clin North Am. 103:463–473. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Denisenko TV, Budkevich IN and Zhivotovsky
B: Cell death-based treatment of lung adenocarcinoma. Cell Death
Dis. 9:1172018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kuhn E, Morbini P, Cancellieri A, Damiani
S, Cavazza A and Comin CE: Adenocarcinoma classification: Patterns
and prognosis. Pathologica. 110:5–11. 2018.PubMed/NCBI
|
|
6
|
Nguyen TT, Lee HS, Burt BM, Wu J, Zhang J,
Amos CI and Cheng C: A lepidic gene signature predicts patient
prognosis and sensitivity to immunotherapy in lung adenocarcinoma.
Genome Med. 14:52022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nooreldeen R and Bach H: Current and
future development in lung cancer diagnosis. Int J Mol Sci.
22:86612021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lahiri A, Maji A, Potdar PD, Singh N,
Parikh P, Bisht B, Mukherjee A and Paul MK: Lung cancer
immunotherapy: Progress, pitfalls, and promises. Mol Cancer.
22:402023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Glickman MH and Ciechanover A: The
ubiquitin-proteasome proteolytic pathway: Destruction for the sake
of construction. Physiol Rev. 82:373–428. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhao H, Li CC, Pardo J, Chu PC, Liao CX,
Huang J, Dong JG, Zhou X, Huang Q, Huang B, et al: A novel E3
ubiquitin ligase TRAC-1 positively regulates T cell activation. J
Immunol. 174:5288–5297. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Giannini AL, Gao Y and Bijlmakers MJ:
T-cell regulator RNF125/TRAC-1 belongs to a novel family of
ubiquitin ligases with zinc fingers and a ubiquitin-binding domain.
Biochem J. 410:101–111. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kodama T, Kodama M, Jenkins NA, Copeland
NG, Chen HJ and Wei Z: Ring finger protein 125 is an
anti-proliferative tumor suppressor in hepatocellular carcinoma.
Cancers (Basel). 14:25892022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Feng Z, Ke S, Wang C, Lu S, Xu Y, Yu H, Li
Z, Yin B, Li X, Hua Y, et al: RNF125 attenuates hepatocellular
carcinoma progression by downregulating SRSF1-ERK pathway.
Oncogene. 42:2017–2030. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jiang C, He L, Xiao S, Wu W, Zhao Q and
Liu F: E3 ubiquitin ligase RNF125 suppresses immune escape in head
and neck squamous cell carcinoma by regulating PD-L1 expression.
Mol Biotechnol. 65:891–903. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim H, Frederick DT, Levesque MP, Cooper
ZA, Feng Y, Krepler C, Brill L, Samuels Y, Hayward NK, Perlina A,
et al: Downregulation of the ubiquitin ligase RNF125 underlies
resistance of melanoma cells to BRAF inhibitors via JAK1
deregulation. Cell Rep. 11:1458–1473. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Han Y, Liu D and Li L: PD-1/PD-L1 pathway:
Current researches in cancer. Am J Cancer Res. 10:727–742.
2020.PubMed/NCBI
|
|
17
|
Cha JH, Chan LC, Li CW, Hsu JL and Hung
MC: Mechanisms controlling PD-L1 expression in cancer. Mol Cell.
76:359–370. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen W, Saxton B, Tessema M and Belinsky
SA: Inhibition of GFAT1 in lung cancer cells destabilizes PD-L1
protein. Carcinogenesis. 42:1171–1178. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ma J, Chi D, Wang Y, Yan Y, Zhao S, Liu H,
Jing J, Pu H and Zhang M: Prognostic value of PD-L1 expression in
resected lung adenocarcinoma and potential molecular mechanisms. J
Cancer. 9:3489–3499. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wei M, Mo Y, Liu J, Zhai J, Li H, Xu Y,
Peng Y, Tang Z, Wei T, Yang X, et al: Ubiquitin ligase RNF125
targets PD-L1 for ubiquitination and degradation. Front Oncol.
12:8356032022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fish L, Pencheva N, Goodarzi H, Tran H,
Yoshida M and Tavazoie SF: Muscleblind-like 1 suppresses breast
cancer metastatic colonization and stabilizes metastasis suppressor
transcripts. Genes Dev. 30:386–398. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang Q, Wu Y, Chen J, Tan F, Mou J, Du Z,
Cai Y, Wang B and Yuan C: The regulatory role of both MBNL1 and
MBNL1-AS1 in several common cancers. Curr Pharm Des. 28:581–585.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ray D, Yun YC, Idris M, Cheng S, Boot A,
Iain TBH, Rozen SG, Tan P and Epstein DM: A tumor-associated
splice-isoform of MAP2K7 drives dedifferentiation in MBNL1-low
cancers via JNK activation. Proc Natl Acad Sci USA.
117:16391–16400. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Voss DM, Sloan A, Spina R, Ames HM and Bar
EE: The alternative splicing factor, MBNL1, inhibits glioblastoma
tumor initiation and progression by reducing hypoxia-induced
stemness. Cancer Res. 80:4681–4692. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li T, Fu J, Zeng Z, Cohen D, Li J, Chen Q,
Li B and Liu XS: TIMER2.0 for analysis of tumor-infiltrating immune
cells. Nucleic Acids Res. 48((W1)): W509–W514. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cook KB, Kazan H, Zuberi K, Morris Q and
Hughes TR: RBPDB: A database of RNA-binding specificities. Nucleic
Acids Res. 39:D301–D308. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Girard L, Rodriguez-Canales J, Behrens C,
Thompson DM, Botros IW, Tang H, Xie Y, Rekhtman N, Travis WD,
Wistuba II, et al: An expression signature as an aid to the
histologic classification of non-small cell lung cancer. Clin
Cancer Res. 22:4880–4889. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Okayama H, Kohno T, Ishii Y, Shimada Y,
Shiraishi K, Iwakawa R, Furuta K, Tsuta K, Shibata T, Yamamoto S,
et al: Identification of genes upregulated in ALK-positive and
EGFR/KRAS/ALK-negative lung adenocarcinomas. Cancer Res.
72:100–111. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yamauchi M, Yamaguchi R, Nakata A, Kohno
T, Nagasaki M, Shimamura T, Imoto S, Saito A, Ueno K, Hatanaka Y,
et al: Epidermal growth factor receptor tyrosine kinase defines
critical prognostic genes of stage I lung adenocarcinoma. PLoS One.
7:e439232012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Leon LM, Gautier M, Allan R, Ilié M,
Nottet N, Pons N, Paquet A, Lebrigand K, Truchi M, Fassy J, et al:
The nuclear hypoxia-regulated NLUCAT1 long non-coding RNA
contributes to an aggressive phenotype in lung adenocarcinoma
through regulation of oxidative stress. Oncogene. 38:7146–7165.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pontén F, Jirström K and Uhlen M: The
human protein atlas-a tool for pathology. J Pathol. 216:387–393.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bartha Á and Győrffy B: TNMplot.com: A web
tool for the comparison of gene expression in normal, tumor and
metastatic tissues. Int J Mol Sci. 22:26222021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ru B, Wong CN, Tong Y, Zhong JY, Zhong
SSW, Wu WC, Chu KC, Wong CY, Lau CY, Chen I, et al: TISIDB: An
integrated repository portal for tumor-immune system interactions.
Bioinformatics. 35:4200–4202. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rousseaux S, Debernardi A, Jacquiau B,
Vitte AL, Vesin A, Nagy-Mignotte H, Moro-Sibilot D, Brichon PY,
Lantuejoul S, Hainaut P, et al: Ectopic activation of germline and
placental genes identifies aggressive metastasis-prone lung
cancers. Sci Transl Med. 5:186ra662013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Takeuchi T, Tomida S, Yatabe Y, Kosaka T,
Osada H, Yanagisawa K, Mitsudomi T and Takahashi T: Expression
profile-defined classification of lung adenocarcinoma shows close
relationship with underlying major genetic changes and
clinicopathologic behaviors. J Clin Oncol. 24:1679–1688. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Matsuyama Y, Suzuki M, Arima C, Huang QM,
Tomida S, Takeuchi T, Sugiyama R, Itoh Y, Yatabe Y, Goto H and
Takahashi T: Proteasomal non-catalytic subunit PSMD2 as a potential
therapeutic target in association with various clinicopathologic
features in lung adenocarcinomas. Mol Carcinog. 50:301–309. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Goswami CP and Nakshatri H: PROGgeneV2:
Enhancements on the existing database. BMC Cancer. 14:9702014.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Stark C, Breitkreutz BJ, Reguly T, Boucher
L, Breitkreutz A and Tyers M: BioGRID: A general repository for
interaction datasets. Nucleic Acids Res. 34((Database issue)):
D535–D539. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Warde-Farley D, Donaldson SL, Comes O,
Zuberi K, Badrawi R, Chao P, Franz M, Grouios C, Kazi F, Lopes CT,
et al: The GeneMANIA prediction server: Biological network
integration for gene prioritization and predicting gene function.
Nucleic Acids Res. 38((Web Server issue)): W214–W220. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sherman BT, Hao M, Qiu J, Jiao X, Baseler
MW, Lane HC, Imamichi T and Chang W: DAVID: A web server for
functional enrichment analysis and functional annotation of gene
lists (2021 update). Nucleic Acids Res. 50((W1)): W216–W221. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Stelzer G, Rosen N, Plaschkes I, Zimmerman
S, Twik M, Fishilevich S, Stein TI, Nudel R, Lieder I, Mazor Y, et
al: The GeneCards suite: From gene data mining to disease genome
sequence analyses. Curr Protoc Bioinformatics. 54:1.30.1–1.30.33.
2016. View
Article : Google Scholar : PubMed/NCBI
|
|
42
|
Paz I, Kosti I, Ares M Jr, Cline M and
Mandel-Gutfreund Y: RBPmap: A web server for mapping binding sites
of RNA-binding proteins. Nucleic Acids Res. 42:W361–W367. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li Z, Chen S, Jhong JH, Pang Y, Huang KY,
Li S and Lee TY: UbiNet 2.0: A verified, classified, annotated and
updated database of E3 ubiquitin ligase-substrate interactions.
Database (Oxford). 8:baab0102021. View Article : Google Scholar
|
|
44
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Amin EM, Liu Y, Deng S, Tan KS, Chudgar N,
Mayo MW, Sanchez-Vega F, Adusumilli PS, Schultz N and Jones DR: The
RNA-editing enzyme ADAR promotes lung adenocarcinoma migration and
invasion by stabilizing FAK. Sci Signal. 10:eaah39412017.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Siang DTC, Lim YC, Kyaw AMM, Win KN, Chia
SY, Degirmenci U, Hu X, Tan BC, Walet ACE, Sun L and Xu D: The
RNA-binding protein HuR is a negative regulator in adipogenesis.
Nat Commun. 11:2132020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Jia Y, Vong JS, Asafova A, Garvalov BK,
Caputo L, Cordero J, Singh A, Boettger T, Günther S, Fink L, et al:
Lamin B1 loss promotes lung cancer development and metastasis by
epigenetic derepression of RET. J Exp Med. 216:1377–1395. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kostyrko K, Román M, Lee AG, Simpson DR,
Dinh PT, Leung SG, Marini KD, Kelly MR, Broyde J, Califano A, et
al: UHRF1 is a mediator of KRAS driven oncogenesis in lung
adenocarcinoma. Nat Commun. 14:39662023. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hong SY, Kao YR, Lee TC and Wu CW:
Upregulation of E3 ubiquitin ligase CBLC enhances EGFR
dysregulation and signaling in lung adenocarcinoma. Cancer Res.
78:4984–4996. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Volonte D, Sedorovitz M and Galbiati F:
Impaired Cdc20 signaling promotes senescence in normal cells and
apoptosis in non-small cell lung cancer cells. J Biol Chem.
298:1024052022. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Cai S, Zhang B, Huang C, Deng Y, Wang C,
Yang Y, Xiang Z, Ni Y, Wang Z, Wang L, et al: CTRP6 protects
against ferroptosis to drive lung cancer progression and metastasis
by destabilizing SOCS2 and augmenting the xCT/GPX4 pathway. Cancer
Lett. 579:2164652023. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhang S, You X, Zheng Y, Shen Y, Xiong X
and Sun Y: The UBE2C/CDH1/DEPTOR axis is an oncogene and tumor
suppressor cascade in lung cancer cells. J Clin Invest.
133:e1624342023. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Hua TNM, Namkung J, Phan ANH, Vo VTA, Kim
MK, Jeong Y and Choi JW: PPARgamma-mediated ALDH1A3 suppression
exerts anti-proliferative effects in lung cancer by inducing lipid
peroxidation. J Recept Signal Transduct Res. 38:191–197. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wu J, Wen T, Marzio A, Song D, Chen S,
Yang C, Zhao F, Zhang B, Zhao G, Ferri A, et al: FBXO32-mediated
degradation of PTEN promotes lung adenocarcinoma progression. Cell
Death Dis. 15:2822024. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kildey K, Gandhi NS, Sahin KB, Shah ET,
Boittier E, Duijf PHG, Molloy C, Burgess JT, Beard S, Bolderson E,
et al: Elevating CDCA3 levels in non-small cell lung cancer
enhances sensitivity to platinum-based chemotherapy. Commun Biol.
4:6382021. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhang R, Zhang W, Zeng Y, Li Y, Zhou J,
Zhang Y, Wang A, Lv Y, Zhu J, Liu Z and Huang JA: The regulation of
CPNE1 ubiquitination by the NEDD4L is involved in the pathogenesis
of non-small cell lung cancer. Cell Death Discov. 7:3362021.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zheng N and Shabek N: Ubiquitin ligases:
Structure, function, and regulation. Annu Rev Biochem. 86:129–157.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Konoshenko M, Lansukhay Y, Krasilnikov S
and Laktionov P: MicroRNAs as predictors of lung-cancer resistance
and sensitivity to cisplatin. Int J Mol Sci. 23:75942022.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Taheri M, Shoorei H, Anamag FT,
Ghafouri-Fard S and Dinger ME: LncRNAs and miRNAs participate in
determination of sensitivity of cancer cells to cisplatin. Exp Mol
Pathol. 123:1046022021. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Lv P, Man S, Xie L, Ma L and Gao W:
Pathogenesis and therapeutic strategy in platinum resistance lung
cancer. Biochim Biophys Acta Rev Cancer. 1876:1885772021.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Gou Q, Dong C, Xu H, Khan B, Jin J, Liu Q,
Shi J and Hou Y: PD-L1 degradation pathway and immunotherapy for
cancer. Cell Death Dis. 11:9552020. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Xiong W, Gao Y, Wei W and Zhang J:
Extracellular and nuclear PD-L1 in modulating cancer immunotherapy.
Trends Cancer. 7:837–846. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Sun C, Mezzadra R and Schumacher TN:
Regulation and function of the PD-L1 checkpoint. Immunity.
48:434–452. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Yu H, Boyle TA, Zhou C, Rimm DL and Hirsch
FR: PD-L1 expression in lung cancer. J Thorac Oncol. 11:964–975.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Hung CS and Lin JC: Alternatively spliced
MBNL1 isoforms exhibit differential influence on enhancing brown
adipogenesis. Biochim Biophys Acta Gene Regul Mech.
1863:1944372020. View Article : Google Scholar : PubMed/NCBI
|