Introduction
B-ALL is a hematologic malignancy caused by the
proliferation of malignant B lymphoblast clones (1), predominantly affecting children and
adolescents (2), but also observed
in adults (3). Treatment strategies
for B-ALL include chemotherapy (4),
targeted therapy (5) and
immunotherapy (6), which together
improve remission rates and overall outcomes. Although >90% of
patients achieve initial complete response through chemotherapy, up
to 50% of patients with B-ALL experience a relapse, developing
chemotherapy-resistant disease (7).
Immunotherapy has shown promise in reducing relapse by effectively
targeting minimum residual disease, but its efficacy remains
limited due to the intrinsically low immunogenicity of B-ALL cells
(7). Therefore, identifying novel
therapeutic targets to enhance treatment response and improve
long-term survival remains a critical priority in B-ALL
research.
In 2023, Krali et al (8) developed a machine learning algorithm
to patients with subtype pediatric B-ALL based on a molecular
profile, generating the GSE227832 dataset. This dataset includes
gene expression profiles from 315 newly diagnosed cases, 8 relapse
cases, 10 healthy donors, and 2 cases in remission. By analyzing
the GSE227832 dataset in combination with the TARGET-ALL-P3 dataset
[from The Cancer Genome Atlas (TCGA)] including clinical data from
79 patients, three core hub genes closely associated with the
progression of B-ALL were identified: Glucose-dependent
insulinotropic polypeptide receptor (GIPR), hepatocyte growth
factor (HGF) and sorting nexin 10 (SNX10). These genes, all linked
to disease progression, present promising targets for further
investigation into the molecular mechanisms driving B-ALL. In the
present study, a focus was placed on SNX10 due to its unique and
previously unexplored role in hematologic malignancies,
particularly in B-ALL.
SNX10 is a member of the phosphoinositide-binding
protein family and contains a phox homology (PX) domain,
facilitating intracellular protein transport through interaction
with phosphoinositide-enriched membranes (9). Known to be highly expressed in bones
and intestines, SNX10 regulates various physiological processes,
including osteoclast bone resorption (10), gastric acid secretion (11) and glucose metabolism (12). Increasing evidence suggests that
SNX10 also plays complex roles in cancer. For example, SNX10 has
demonstrated tumor-suppressive effects in colorectal cancer
(13), driving inflammation-related
colorectal cancer through a chaperone-mediated autophagy mechanism.
By contrast, elevated SNX10 expression in cervical cancer has been
associated with increased invasion and metastasis (14), and this is associated with a poor
prognosis in patients with glioblastoma (15) suggesting that the role of SNX10 in
cancer is context-dependent. These findings underscore the dual
role that SNX10 may play in cancer biology, likely influenced by
tissue type and cellular context. Despite mounting evidence linking
SNX10 to various aspects of cancer, its role in B-ALL remains
undetermined, to the best of our knowledge. Given the regulatory
functions of SNX10 in intracellular transport and signaling, it is
plausible that it could influence B-ALL pathophysiology by
interacting with key signaling pathways.
The PI3K/Akt signaling pathway is a critical cell
signaling pathway involved in various biological processes such as
cell survival (16), proliferation
(17), differentiation (18), migration (19) and metabolism (20), and is well-established to be
implicated in various hematologic malignancies, including leukemia
(21). Typically activated by
growth factors (22) or signaling
molecules binding to cell membrane receptors, this pathway triggers
a cascade leading to PI3K activation and subsequently Akt
phosphorylation. p-Akt then regulates cell cycle progression
through the mTOR pathway (23) and
inhibits apoptosis by modulating Bcl-2 family proteins (24). Additionally, the
PI3K/Akt/PIKfyve/PtdIns(3,5)P2 signaling pathway is crucial for
viral entry into cells (25), with
PIKfyve, which phosphorylates PI3P to PI(3,5)P2, colocalizing with
Snx10 in early endosomes of osteoclasts to regulate
endosome/lysosome homeostasis (26). However, the potential relationship
between SNX10 and the PI3K/Akt signaling pathway in B-ALL has not
been previously examined.
In the present study, the role of SNX10 in B-ALL was
examined. Using systematic in vitro and in vivo
experiments, it was revealed that SNX10 impacted B-ALL
proliferation, apoptosis and migration by modulating the PI3K/Akt
signaling pathway. These findings provide novel insights into the
involvement of SNX10 in B-ALL progression, addressing previously
unexplored questions regarding its function in leukemia and
highlighting potential therapeutic avenues for the management of
B-ALL.
Materials and methods
Screening of common differentially
expressed genes (DEGs) in B-ALL
The GSE227832 dataset was downloaded from the Gene
Expression Omnibus (GEO) database (https://www.ncbi.nlm.nih.gov/geo/) for differential
analysis. Subtypes of B-ALL with at least 10 samples were selected.
The subtypes included were ALLIUM B-other, BCR:ABL1, BCR:ABL1-like,
DUX4-r, ETV6:RUNX1, ETV6:RUNX1-like, HeH, KMT2A-r, PAX5alt,
TCF3:PBX1, ZNF384-r and iAMP21. The limma package (https://bioconductor.org/packages/release/bioc/html/limma.html)
was used to calibrate and normalize the raw data in R (https://www.r-project.org/), and then DEGs in each
subtype were selected using the criteria |log2FC|<1.5
and P.adjust <0.05, separately. Finally, a basic function in R,
the intersect function was used to find the common DEGs in the 12
different subtypes of B-ALL.
Screening for survival-associated
DEGs
The gene expression profiles and clinical data for
79 patients were retrieved from TCGA (https://portal.gdc.cancer.gov/projects/TARGET-ALL-P3).
Samples with gene expression levels above the mean were categorized
into the high expression group (H), whereas those at or below the
average were categorized into the low expression group (L). To
identify survival-associated DEGs (Survdiffs), a log-rank test from
the survival Package (https://cran.r-project.org/web/packages/survival/index.html)
was performed to compare survival rates between the two groups in
R. Subsequently, the survfit function from the survival package was
used to fit a Kaplan-Meier survival curve. Finally, the survival
time curve analysis was visualized using the ggsurvplot function
from the survminer package (https://cran.r-project.org/web/packages/survminer/index.html).
Identification of core hub genes in
B-ALL
The Least Absolute Shrinkage and Selection Operator
(LASSO) is a linear regression regularization method used for
feature selection and model simplification (27). LASSO shrinks certain regression
coefficients to zero, retaining only variables with non-zero
coefficients within the model. In the present study, common DEGs
and Survdiffs were imported into R. The intersect function was used
to find the intersection of the common DEG sets and Survdiffs sets,
and the ggvenn Package (https://cran.r-project.org/web/packages/ggvenn/index.html)
was used for visualization. LASSO analysis with the glmnet package
(https://cran.r-project.org/web/packages/glmnet/index.html)
was used to refine these intersecting genes and identify the Core
Hub genes. Finally, a Cox proportional hazards regression model was
calculated using the coxph function in the survival package, and
the survminer package was used to summarize and visualize the
results of the survival analysis.
Research population and sample
The subjects recruited for the present included 24
patients with newly diagnosed B-ALL at the Affiliated Hospital of
Guizhou Medical University (Guiyang, China) between May 2023 and
September 2024, and 24 age-matched healthy controls (HC). The age
range of the patients was 1–79 years, a median age of 15 years, and
including 10 males and 14 females. Written informed consent was
obtained from all participants or their legal guardians for those
under the legal age of consent. The study was approved by the
Ethics Committee of the Affiliated Hospital of Guizhou Medical
University (approval no. 2022328). The procedures performed in the
present study strictly adhered to the ethical principles outlined
in the Declaration of Helsinki (28). The patient's clinicopathological
information is shown in Table SI.
Bone marrow mononuclear cells (BMMCs) were isolated from bone
marrow specimens of patients with B-ALL and normal healthy donors
using Ficoll density centrifugation kit, according to the
manufacturer's protocol (Beijing Solarbio Science & Technology
Co., Ltd.). Briefly, an equal volume of PBS was added to the bone
marrow sample, mixed gently, and then carefully layered onto a tube
that contained 3 ml Ficoll separation reagent. The mixture was
centrifuged at 400 × g for 35 min at room temperature, and the
middle white cell layer, which was the mononuclear cell layer, was
gently aspirated.
Cell lines
The human B-ALL Nalm-6 (cat. no. CC-Y1706) and
Rs4;11 (cat. no. CC-Y1741) cells (both from Shanghai EK-Bioscience
Biotechnology Co., Ltd.) were cultured in RPMI-1640 medium
containing 10% FBS (both from Gibco; Thermo Fisher Scientific, Inc)
and 1% penicillin-streptomycin at 37°C in a humidified incubator
supplied with 5% CO2 air. 293T cells (Pricella
Biotechnology Co., Ltd.) were cultured in DMEM medium (Gibco;
Thermo Fisher Scientific, Inc.) supplemented with 10% FBS and 1%
penicillin-streptomycin at 37°C in a humidified incubator supplied
with 5% CO2 air. The identity of these cell lines was
genetically confirmed by short tandem repeat analysis and was
routinely tested for mycoplasma contamination.
Reverse transcriptase-quantitative PCR
(RT-qPCR)
According to the manufacturer's protocol, total RNA
was extracted from BMMCs using the R6834 Total RNA extraction kit
(Omega Bio-Tek, Inc.). A total of 1 µg RNA was reverse transcribed
into cDNA using a PrimeScript RT-PCR kit, according to the
manufacturer's protocol (Shanghai Yeasen Biotechnology Co., Ltd.).
cDNA, SYBR Green PCR Master Mix kit (Shanghai Yeasen Biotechnology
Co., Ltd.), and gene-specific primers for SNX10 were used for qPCR
on a Gentier 96E fully automated medical PCR analysis system (Xi'an
Tianlong Technology Co., Ltd.). All reactions were conducted in a
20 µl reaction volume in triplicate. Primers were synthesized by
Sangon Biotech Co., Ltd. The sequences of the primers were: SNX10
forward, 5′-CACTTTTGCTTTCAGATAGCAGC-3′ and reverse,
5′-ACACACGCCTCAATGTCTTCT-3′, and β-actin forward,
5′-CCTGGCACCCAGCACAAT-3′ and reverse, 5′-GGGCCGGACTCGTCATAC-3′. The
thermocycling conditions were: Initial denaturation for 2 min at
95°C, followed by 40 cycles of 95°C for 10 sec and 60°C for 30 sec.
After the reaction was completed, the relative mRNA expression
levels were normalized to β-actin and calculated using the
2−ΔΔCq method (29).
Western blotting
Following the manufacturer's guidelines, proteins
were extracted using RIPA lysis buffer (Beijing Solarbio Science
& Technology Co., Ltd.) with 1 mmol/l PMSF and 1% Phosphatase
Inhibitor Cocktail (×100) (both from Shanghai Epizyme Biomedical
Technology Co., Ltd.). Protein concentrations were estimated using
an Omni-Easy™ Instant BCA Protein Assay Kit (Shanghai
Epizyme Biomedical Technology Co., Ltd.). Equal quantities of
protein extracts (30 µg) were loaded on 10% SDS-gels, resolved
using SDS-PAGE, and transferred to PVDF membranes (MilliporeSigma).
The membranes were blocked with 5% BSA (Beyotime Institute of
Biotechnology) for 2 h at room temperature, followed by overnight
incubation with primary antibodies at 4°C. The following day,
membranes were washed and incubated with the HRP-conjugated
secondary antibody (1:10,000; cat. no. SA00001-1 or cat. no.
SA00001-2; Proteintech Group, Inc.) at 37°C for 1 h. Membranes were
washed again with TBS-0.1% Tween three times, and signals were
visualized using an enhanced chemiluminescent reagent (Dalian
Meilun Biotechnology Co., Ltd.) and observed using an ECL
chemiluminescence system (Bio-Rad Laboratories, Inc.).
Densitometric analysis was performed using ImageJ (version no.1.53;
National Institutes of Health) and normalized to the loading
control (β-actin). The following antibodies were used: Anti-SNX10
(1:1,000; cat. no. MG928896S), anti-kinase p85-α (1:1,000; cat. no.
A31285), anti-phospho-PI3-kinase p85-α/γ (1:1,000; cat. no. T40116)
all from Abmart Shanghai Co., Ltd.; anti-Akt(pan) 1/2/3 (1:1,000;
cat. no. A75065), anti-P-Akt (1:1,000; cat. no. A52820) from Nature
biosciences Co., Ltd.; and anti-human β-actin (1:10,000; cat. no.
AC026) from ABclonal Biotech Co., Ltd.
Plasmid and lentivirus infection
SNX10 overexpression plasmid (OE), empty vector
plasmid (EV), SNX10 short hairpin plasmid (shSNX10), and the
negative control (shNC), all plasmids labeled with FITC
fluorescence, were purchased from Guangzhou IGE Biotechnology Co.,
Ltd. The SNX10 OE plasmid and shSNX10 sequences are shown in
Table SII. For transfection, 500
µl Opti-MEM medium was added into each of two 1.5 ml EP tubes.
Then, 10 µl Lipo8000™ transfection reagent (Beckman Coulter, Inc.)
and a plasmid mixture (the ratio of target plasmid to packaging
plasmids psPAX2 and pMD2.G was 1.0:0.5:1.0 µg) were added. The
plasmids were mixed separately and left to stand for 5 min.
Following this, the Lipo8000™ transfection reagent was
added to the plasmid mixture, gently mixed, and allowed to sit for
20 min prior to transfection into 293T cells that were cultured in
a 60-mm dish at 80% confluence (30). A total of 6–8 h after transfection,
the medium was replaced, and the viral supernatant was collected
after 24, 48 and 72 h, and filtered through a 0.45-µm filter
membrane. To concentrate every 20 ml of viral supernatant, 5.5 ml
of 44% PEG8000 (Beijing Solarbio Technology Co., Ltd.) and 2 ml (4
mol/l) NaCl were added, mixed, and incubated overnight at 4°C.
After 24 h, the mixture was centrifuged at 3,500 × g for 15 min,
and the pellet was resuspended in 150 µl PBS. A total of 50 µl of
the concentrated virus solution was then added to Nalm-6 or RS4:11
cells (5×106 cells) in the presence of 5 µl (0.8 µg/ml)
polybrene (Beijing Solarbio Science & Technology Co., Ltd.).
The culture medium was replaced 6–8 h after infection, and the
cells were cultured for 7 days. Stable cell lines were selected
using puromycin (Beijing Solarbio Science & Technology Co.,
Ltd.) at concentrations of 1.5 µg/ml for Nalm-6 cells and 2.0 µg/ml
for RS4:11 cells (31). The protein
and mRNA expression levels were assessed using western blotting and
RT-qPCR, respectively.
Pathway inhibition assays
Idelalisib (IDEL, TargetMoi, Cas: 870281-82-6) is a
small molecule inhibitor that targets the PI3K catalytic subunit
p110δ. A 10 mM stock solution was prepared by dissolving it in DMSO
and stored at −20°C. Subsequently, 2 ml of 5×105
transfected cells/ml was seeded into a 6-well plate, and IDEL was
added to achieve a final concentration of 10 µM (32). In the vehicle control group, an
equivalent volume of 0.1% DMSO was used as the solvent control
group. After gently mixing, the cells were incubated for 24 h for
subsequent detection.
Cell proliferation assay
According to the manufacturer's protocol, the
transfected cells were harvested and diluted to a concentration of
3×105 cells/ml. A total of 100 µl (3×104
cells) was added to each well of a 96-well plate, with three
replicates per condition. After 0, 24, 48 and 72 h of incubation,
10 µl Cell Counting Kit-8 solution (Dalian Meilun Biotechnology
Co., Ltd.) was added to each well, followed by an additional 2 h of
incubation. At each time point, the absorbance at 450 nm was
measured using a microplate reader (Thermo Fisher Scientific,
Inc.).
Cell apoptosis assay
According to the manufacturer's guidelines, the
transfected cells were harvested and diluted to a concentration of
1×106 cells/ml with 1X Binding Buffer (Pricella
Biotechnology Co., Ltd.). A total of 100 µl of the cell suspension
(1×105 cells) was transferred to a new tube, and 5 µl
Annexin V-APC and 5 µl Cyanine7/7-AAD (Pricella Biotechnology Co.,
Ltd.) were added. The solution was gently mixed and incubated in
the dark at room temperature for 15 min. Following incubation, 400
µl 1× Binding Buffer was added. Finally, these cells were analyzed
using a flow cytometer (NAVIOS; Beckman Coulter Inc.). The
experimental data were processed using FlowJo software (v10.8.1,
FlowJo LLC).
Cell cycle analysis
According to the manufacturer's guidelines, the
transfected cells were harvested and diluted to a concentration of
1×106 cells/ml. A total of 1 ml of the suspension was
taken, the supernatant was removed by centrifugation at 150 × g for
5 min, and then resuspended in 500 µl pre-cooled 70% ethanol. The
cells were fixed at 4°C overnight and then washed with PBS to
remove the supernatant. The cells were resuspended in a mixture of
500 µl RNase A and PI (in a 1:9 ratio) and incubated in the dark at
37°C for 30 min. Analysis was then performed using a flow cytometer
(NAVIOS; Beckman Coulter Inc.). The experimental data were
processed using ModFit software (v3.1; Verity Software House).
Quantitative proteomics
To extract total protein, 5×106 cells
were lysed with 300 µl lysis buffer (8 M urea, 1 mM PMSF, 2 mM
EDTA), then sonicated on ice for 5 min. The sample was then
centrifuged at 4°C for 10 min at 15,000 × g, and the supernatant
was collected. The protein concentration was determined using a BCA
kit (Beyotime Institute of Biotechnology). A total of 100 µg of the
protein solution was received and the volume was adjusted to 200 µl
with 8 M urea. The solution was reduced with 5 mM DTT at 37°C for
45 min, followed by alkylation with 11 mM iodoacetamide in a dark
room at room temperature for 15 min. Subsequently, 800 µl 25 mM
ammonium bicarbonate solution and 3 µl trypsin (Promega
Corporation) were added, and digestion was performed overnight at
37°C. The pH of the digested peptides was adjusted to 2–3 using 20%
trifluoroacetic acid, and desalination was performed using C18
resin (MilliporeSigma). Finally, the peptide concentration was
determined using the Pierce™ Quantitative Peptide
Detection Reagent and Standard Kit (Thermo Fisher Scientific,
Inc.). Chromatographic separation was performed using the nanoscale
Vanquish Neo system (Thermo Fisher Scientific, Inc.), and the
samples were separated by nanoscale high-performance liquid
chromatography and analyzed using an Orbitrap Astral
high-resolution mass spectrometer (Thermo Fisher Scientific, Inc.)
for data-independent acquisition mass spectrometry analysis.
Finally, Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway
enrichment analysis was performed using the enrichKEGG function and
the KEGG enrichment results were visualized using a barplot in R.
The mass spectrometry proteomics data have been deposited to the
ProteomeXchange Consortium (https://proteomecentral.proteomexchange.org) via the
iProX partner repository (33,34)
with the dataset identifier PXD061830.
Cell-derived xenograft (CDX) mouse
model
A total of 20 female NOD-SCID mice were purchased
from Beijing Vital River Laboratory Animal Technology Co., Ltd.,
aged 8–12 weeks and weighing ~22 g, and were randomly divided into
the SNX10 OE group (n=10) and the EV group (n=10), and were housed
in the SPF laboratory of the Affiliated Hospital of Guizhou Medical
University. When cells reached the logarithmic phase of growth,
they were collected and counted. A total of 5×106
transfected cells in 200 µl PBS were injected through the tail vein
and the mice were monitored for 60 days. The body weight and the
dates of onset and death were recorded. On day 21 post-injection,
five mice were randomly selected from each group, and bone marrow,
peripheral blood, liver, spleen and lymph nodes were collected for
analysis. At the end of the experimental period, mice were
euthanized by cervical dislocation under 2.0–2.5% isoflurane
inhalation anesthesia. Death was confirmed by monitoring of vital
signs to confirm death. The animal experiments were approved by the
Guizhou Medical University Animal Care Committee (approval no.
2402141).
Flow cytometry analysis
On the 21st day post-injection, five mice from each
group were randomly selected for euthanasia, and bone marrow,
peripheral blood, and lymph node tissues were collected to prepare
a single-cell suspension. Following the manufacturer's guidelines,
red blood cells were lysed using a red blood cell lysis buffer
(Gibco; Thermo Fisher Scientific, Inc), and the remaining cells
were resuspended in 100 µl sheath fluid. The cells were then
incubated in the dark at 4°C for 15 min with the addition of 5 µl
anti-human CD19 antibody (cat. no. 4058218; BD Biosciences).
Following the incubation, 400 µl sheath fluid was added. The human
B-ALL cells (human CD19-APC/GFP-FITC) were identified using a flow
cytometer (BD CANTO PLUS; BD Biosciences).
Hematoxylin and eosin (H&E)
staining
On the 21st day of the experiment, the liver and
spleen of the euthanized mice were collected. The weight and volume
of the liver and spleen were recorded. According to the
manufacturer's guidelines, the tissue samples were fixed with 4%
formaldehyde (Beijing Solarbio Science & Technology Co., Ltd.)
for 24 h at room temperature, followed by dehydration, clearing,
and infiltration with paraffin to create paraffin-embedded blocks.
These blocks were then cut into 4-m thick sections and placed on
slides. The slides were immersed in xylene for deparaffinization
twice, each for 5 min. Dehydration was performed using 100, 95, 90
and 80% ethanol solutions (5 min each), followed by a rinse with
water. The sections were stained in hematoxylin solution (Wuhan
Siwega Biotechnology Co., Ltd.) for 15 min at room temperature,
rinsed, and then differentiated in 1% hydrochloric acid alcohol for
1 min, after which they were rinsed with water. The sections were
subsequently immersed in eosin solution (Wuhan Siwega Biotechnology
Co., Ltd.) for 5 min, rinsed, and dehydrated with 80, 90, 95, and
100% ethanol solutions (5 min each). Finally, the sections were
cleared using xylene, immersed in mounting medium, and a coverslip
was placed on top. The tissues were observed under a microscope and
underwent electronic scanning (Pannoramic MIDI, 3DHISTECH
Ltd.).
Statistical analysis
R and GraphPad Prism version 9.0 (GraphPad Software
Inc.; Dotmatics) were used for statistical analyses. An unpaired
Student's t-test was used to compare differences between two
groups. For comparisons between multiple groups, a one-way ANOVA or
two-way ANOVA were used and followed by Tukey's multiple
comparisons test. Survival data were plotted using Kaplan-Meier
survival curves, and a log-rank test was used to compare
differences in survival. P<0.05 was considered to indicate a
statistically significant difference.
Results
GIPR, HGF and SNX10 are core hub genes
in B-ALL
A dataset containing samples of 12 B-ALL subtypes
was downloaded from GEO and analyzed. A total of 1,209 common DEGs
were identified (Table SIII,
Fig. 1A). Concurrently, another
dataset from TCGA was obtained and analysis of this dataset showed
152 survival-associated DEGs (Table
SIV, Fig. 1A). Using a Venn
diagram, the intersection of these DEGs showed nine common genes:
TNS1, SCARF1, NRP1, HGF, DNMT3B, SPI1, GIPR, SNX10 and SYDE2
(Table SV, Fig. 1A). Through LASSO regression
analysis, GIPR (LASSO coefficient=0.2062), HGF (LASSO
coefficient=−0.1533) and SNX10 (LASSO coefficient=0.0945) were
confirmed as hub genes within B-ALL protein network (Fig. 1B and C), indicating their potential
regulatory and functional significance in B-ALL biology.
Furthermore, results from the Cox proportional hazards model
indicated that high expression levels of GIPR (HR=1.88, P=0.049),
SNX10 (HR=1.38, P=0.049), and low expression of HGF (HR=0.77,
P=0.012), were independent adverse prognostic factors for overall
survival (OS) in patients with B-ALL (Fig. 1D). Boxplot analyses indicated that
compared with the HC group, the expression of GIPR and SNX10 was
downregulated in patients with B-ALL, whereas HGF expression was
upregulated (Fig. 1E). Kaplan-Meier
survival curves further supported these findings, showing that
patients with low expression of GIPR and SNX10 had a higher OS
rate, whereas those with high expression levels exhibited a lower
OS rate. Conversely, high expression of HGF is linked to a higher
OS rate, and low expression was correlated with a poorer survival
outcome (Fig. 1F).
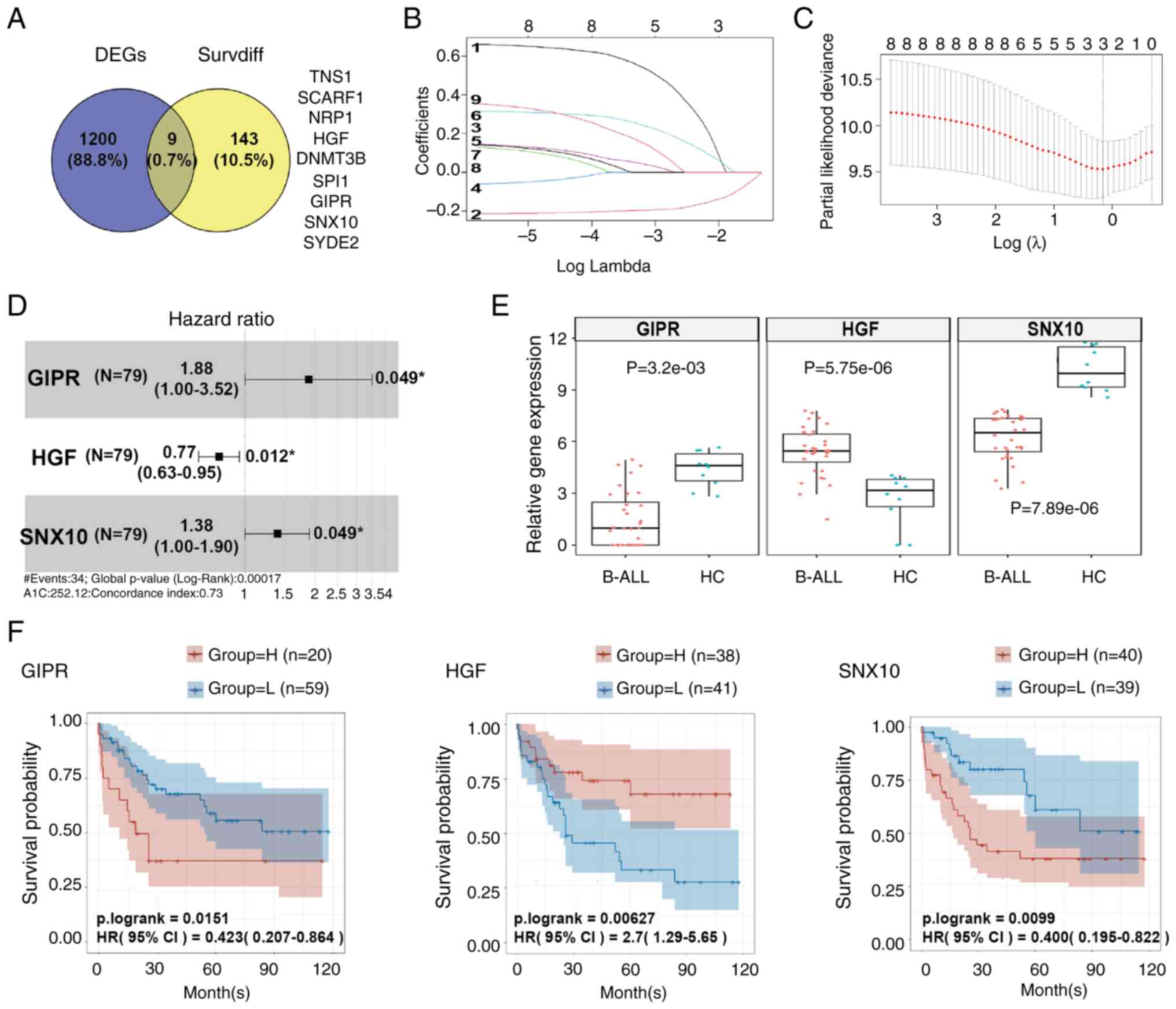 | Figure 1.GIPR, HGF and SNX10 are confirmed as
core hub genes in B-ALL. (A) Venn diagram illustrating the nine
overlapping genes in common DEGs and Survdiffs. (B) LASSO
coefficient path diagram of nine risk factors. (C) Cross-validation
curves of GIPR, HGF and SNX10. (D) Cox proportional hazards model
of GIPR, SNX10 and HGF. (E) Box plots showing the gene expression
levels of GIPR, HGF, and SNX10 in patients with B-ALL (n=32) and
the HC group (n=10). (F) Kaplan-Meier survival analysis curves for
GIPR, HGF and SNX10. B-ALL, B-cell acute lymphoblastic leukemia;
HC, healthy control; GIPR, gastric inhibitory polypeptide receptor;
HGF, hepatocyte growth factor; SNX10, sorting nexin 10; DEG,
differentially expressed gene; Survdiff, survival-associated
differentially expressed gene; LASSO, Least Absolute Shrinkage and
Selection Operator; HC, healthy control. |
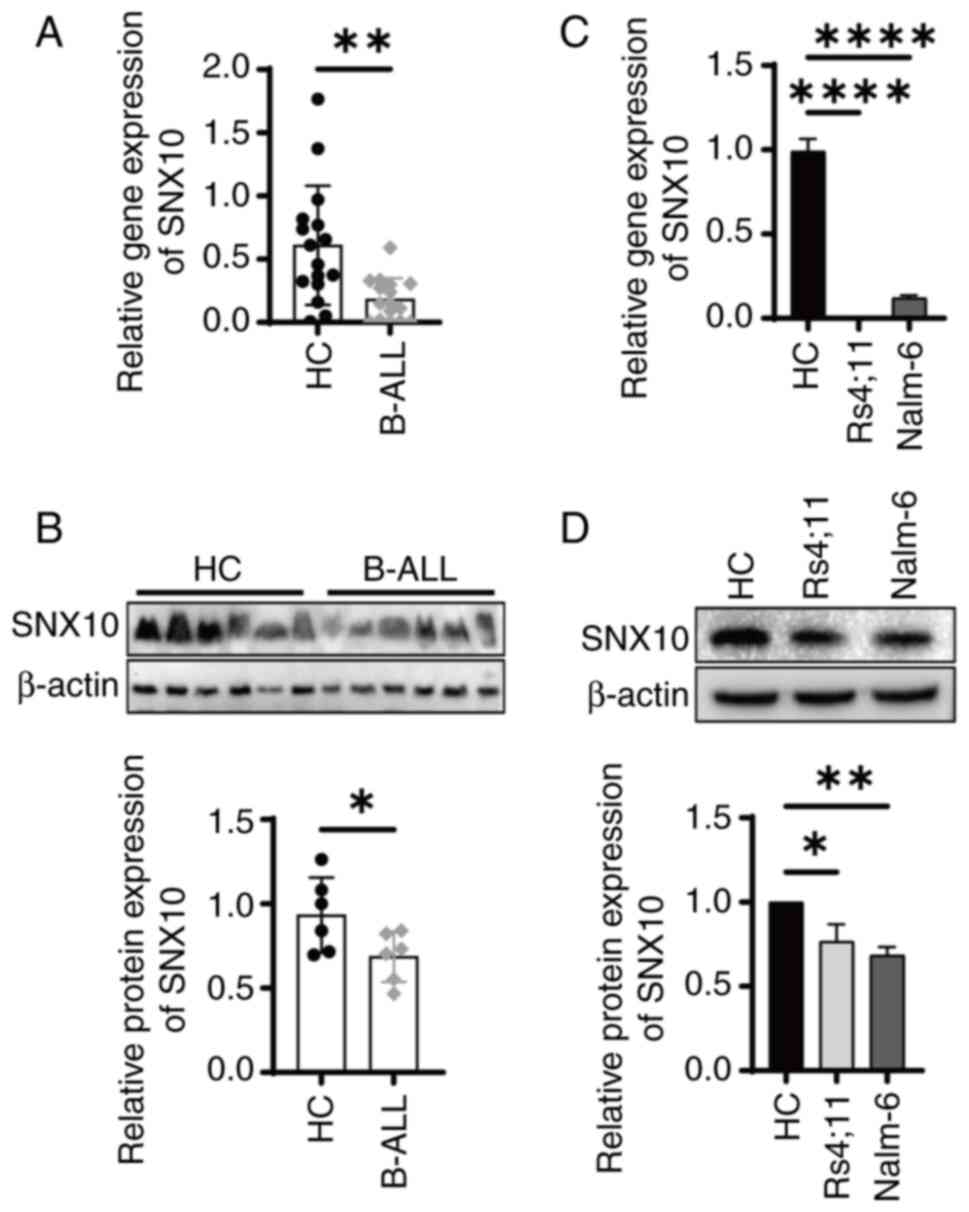
SNX10 expression is decreased in B-ALL
specimens and cell lines
SNX10 influences cell proliferation and survival by
regulating lysosomal function and vesicle transport, as well as
processes such as apoptosis and autophagy (9), which are particularly critical for
leukemia cells. However, its role in B-ALL remains to be
investigated. To examine the role of SNX10 in B-ALL, BMMCs were
collected from both patients with B-ALL and healthy individuals.
Analysis revealed a significant reduction in SNX10 mRNA (Fig. 2A, P<0.01) and protein expression
(Fig. 2B, P<0.05) in the
patients with B-ALL, consistent with the results of bioinformatics
analysis (Fig. 1E). Similar results
were obtained with B-cell precursor leukemia cell lines Nalm-6 and
RS4;11 (Fig. 2C and D).
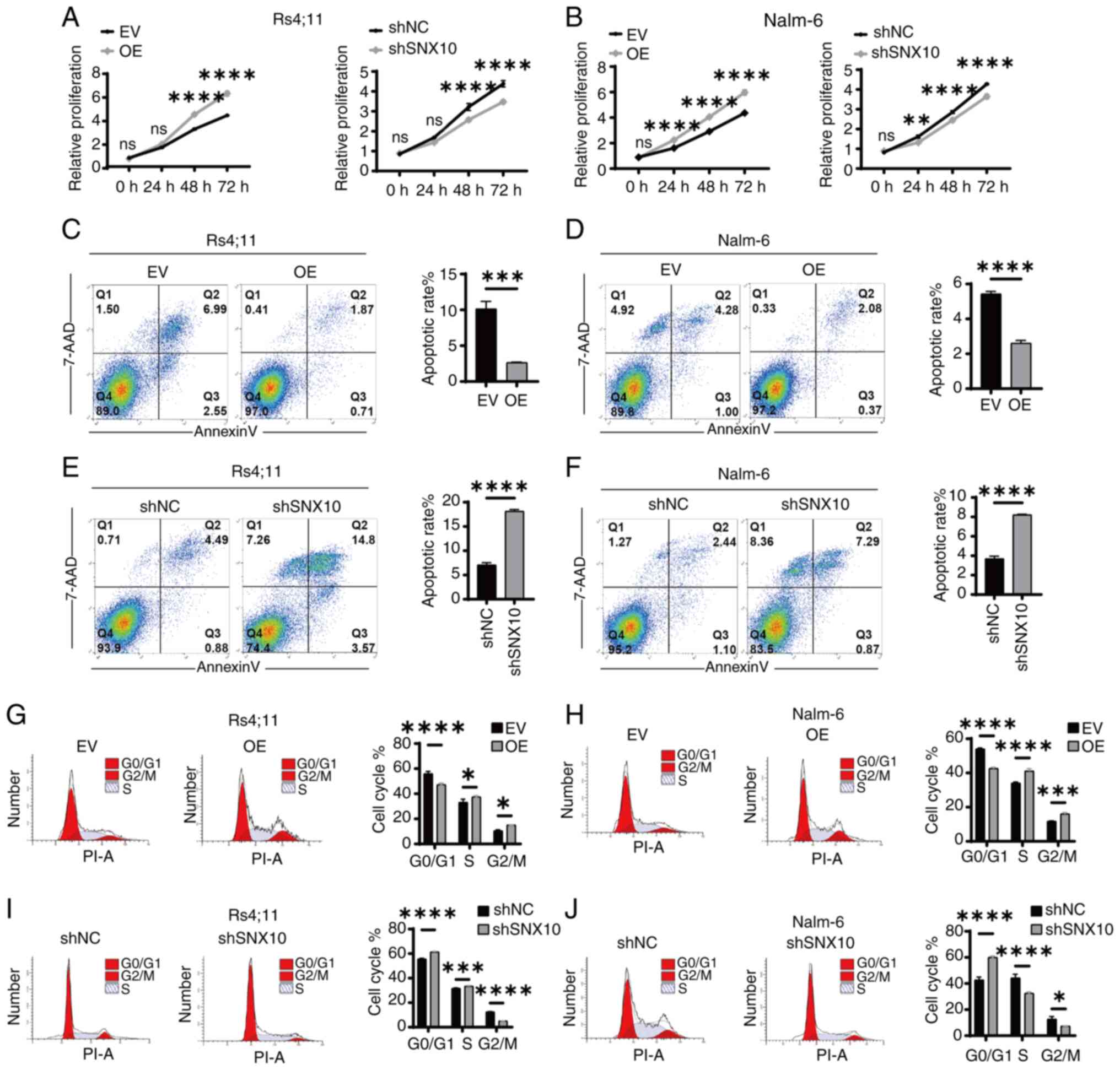
Knockdown of SNX10 inhibits the
proliferation of B-ALL cells
To further investigate the function of SNX10 in
B-ALL cells, RS4;11 and Nalm-6 SNX10 overexpression and knockdown
cells were established by lentiviral infection and confirmed
through RT-qPCR (Fig. S1A, C, E and
G) and western blotting (Fig. S1B,
D, F and H). Among all the shRNA knockdown cell lines,
shSNX10-1 exhibited the highest knockdown efficiency in both cell
types and was thus used for further experiments. Then, increased
cell proliferation was observed in both cell types with SNX10
overexpression, whereas knockdown of SNX10 expression inhibited
cell proliferation (Fig. 3A and B).
A decreased ratio of apoptosis (Fig. 3C
and D) and an increased proportion of cells in the G2/M phase
of the cell cycle (Fig. 3G and H)
were observed in these SNX10-overexpressing cells. By contrast,
SNX10-knockdown cells exhibited increased apoptosis (Fig. 3E and F) and a higher proportion of
cells arrested in the G0/G1 phase (Fig.
3I and J). These findings suggest that SNX10 plays a role in
the proliferation of B-ALL cells by affecting their apoptosis and
cell cycle progression.
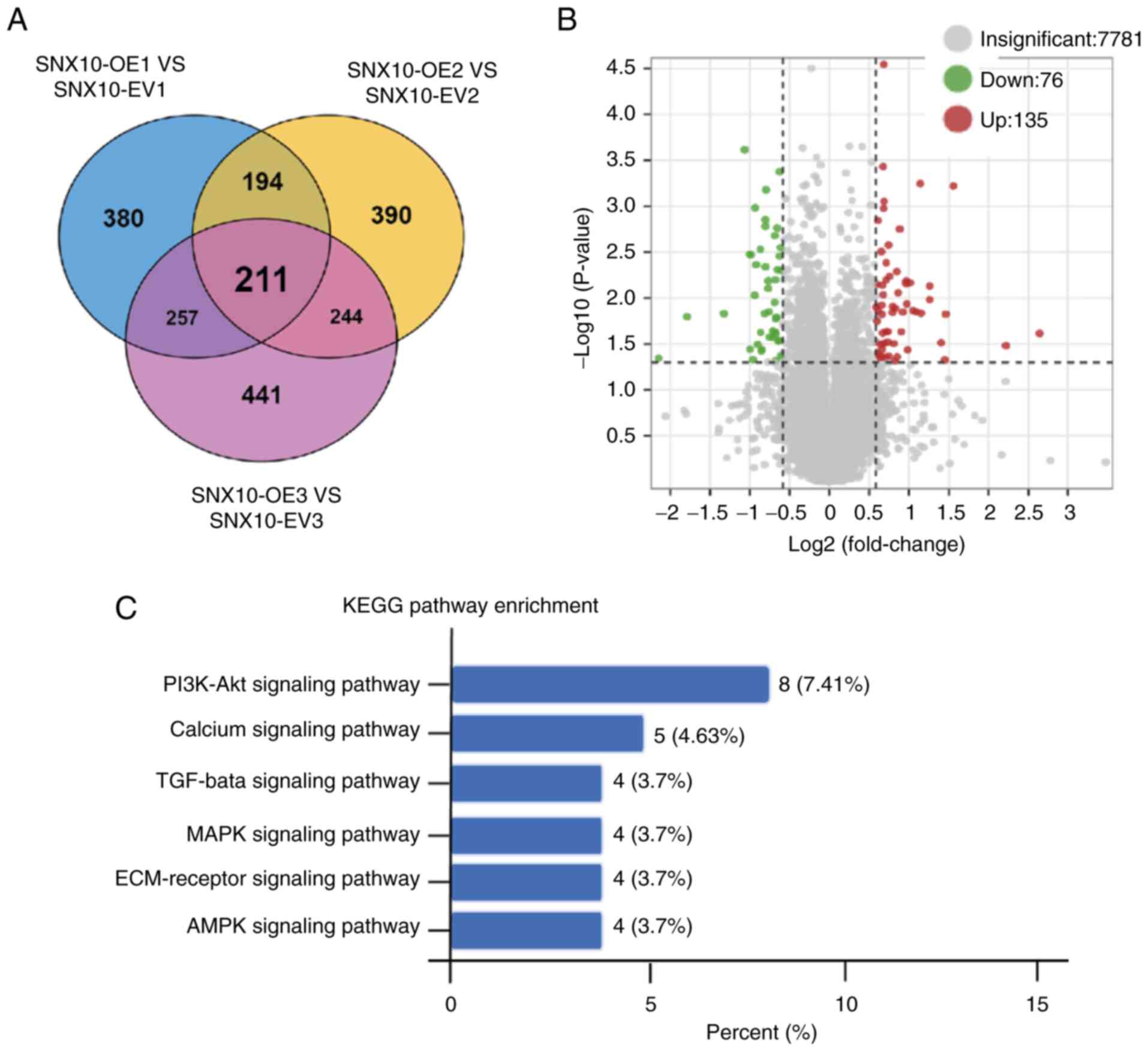
SNX10 promotes the development of
B-ALL cells via regulation of the PI3K/Akt pathway
To investigate the molecular mechanisms underlying
the role of SNX10 in B-ALL, proteomic sequencing on cytoplasmic and
membrane proteins extracted from SNX10-overexpressing Rs4;11 cells
and their corresponding EV cells were performed. A total of 211
differentially expressed proteins were identified (Fig. 4A), of which 76 were downregulated
and 135 were upregulated (Fig. 4B).
To further elucidate the pathways influenced by these
differentially expressed proteins, KEGG pathway enrichment analysis
was performed. The PI3K-Akt signaling pathway, calcium signaling
pathway, TGF-β signaling pathway, MAPK signaling pathway,
ECM-receptor signaling pathway and AMPK signaling were all enriched
(Fig. 4C). Among these, the
PI3K-Akt pathway showed the most notable changes, suggesting that
SNX10 may influence the properties of B-ALL cells via the PI3K-Akt
signaling pathway.
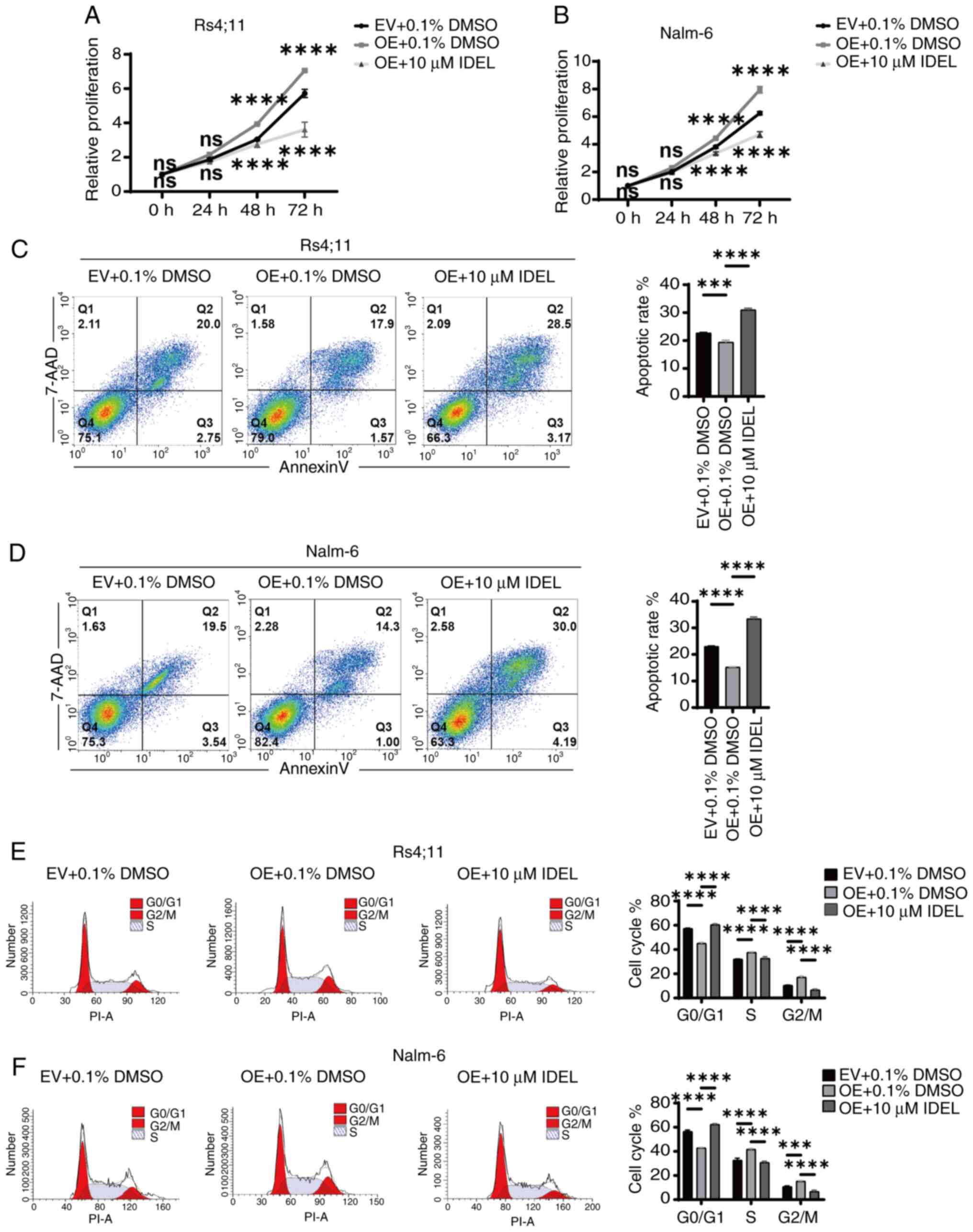
SNX10 regulates the proliferation,
apoptosis and cycle progression of B-ALL cells via the PI3K/AKT
signaling pathway
The expression of key molecules within the PI3K-Akt
signaling pathway in SNX10-overexpressing and knockdown Nalm-6 and
Rs4;11 cells was next assessed. The overexpression of SNX10
resulted in increased phosphorylation of PI3K and Akt, and
significantly elevated ratios of p-PI3K/PI3K and p-Akt/Akt in both
cell lines (Fig. 5A and B),
indicating the activation of the PI3K-Akt signaling pathway.
Conversely, SNX10 knockdown exhibited reduced phosphorylation of
PI3K and Akt proteins, resulting in a decrease in the ratios of
p-PI3K/PI3K and p-Akt/Akt (Fig. 5A and
B). To further confirm the role of SNX10 in activating the
PI3K-Akt pathway, SNX10-overexpressing cells were treated with 10
µM IDEL, a PI3K inhibitor (32),
and observed decreased phosphorylation of PI3K and Akt, and
concurrently a reduction in the ratio of p-PI3K/PI3K and p-Akt/Akt
(Fig. 5C and D). This inhibition
also counteracted the effects of SNX10 overexpression, namely,
reduced cell proliferation (Fig. 6A and
B), increased apoptosis (Fig. 6C
and D), and a decreased proportion of cells in the G2/M phase
(Fig. 6E and F). These findings
indicate that SNX10 regulates apoptosis and cell cycle progression
by modulating the PI3K-Akt signaling pathway, promoting the
proliferation of B-ALL.
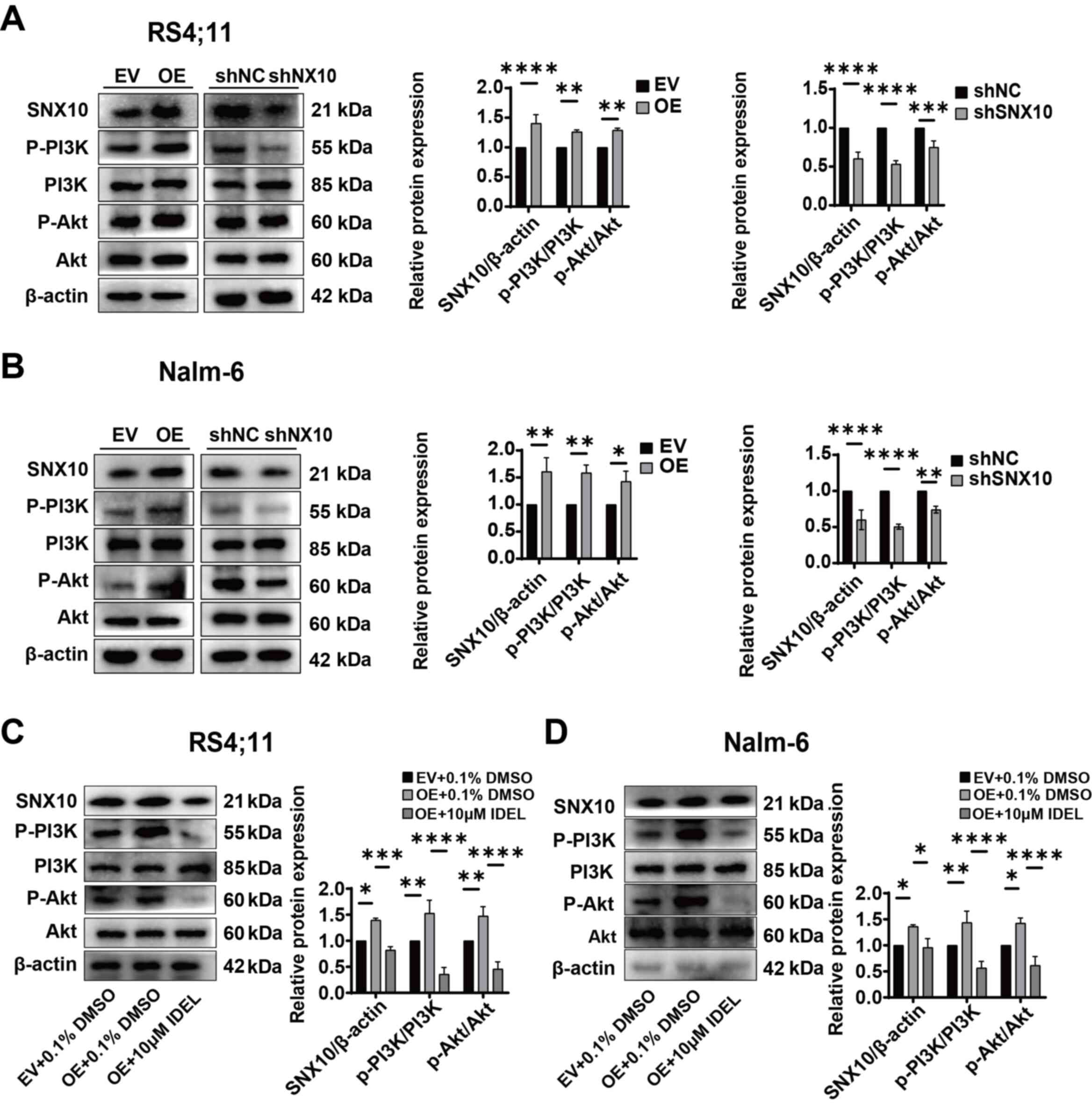 | Figure 5.SNX10 influences the activity of the
PI3K/Akt signaling pathway in B-ALL cells. The protein expression
levels of PI3K, p-PI3K, Akt and p-Akt proteins in SNX10-OE and
knockdown (A) Rs4;11 and (B) Nalm-6 cells were detected. After
treating SNX10-OE B-ALL cells with 10 µM IDEL (a PI3K inhibitor)
for 24 h, the protein expression levels of PI3K, p-PI3K, Akt and
p-Akt proteins in SNX10-OE and knockdown (C) Rs4;11 and (D) Nalm-6
cells were detected. Data are presented as the mean ± SD of three
independent repeats and compared using a one-way or two-way ANOVA.
*P<0.05, **P<0.01, ***P<0.001 and ****P<0.0001 vs. EV
group or shNC group. SNX10, sorting nexin 10; B-ALL, B-cell acute
lymphoblastic leukemia; OE, overexpression; EV, empty vector;
shRNA, short hairpin RNA; NC, negative control; IDEL, Idelalisib;
p-, phosphorylated. |
SNX10 overexpression accelerates the
development of B-ALL in a xenograft mouse model
To further validate the in vitro findings
that high levels of SNX10 promoted the growth and proliferation of
leukemia cells, a CDX xenograft model of B-ALL was established
using NOD-SCID mice via tail-vein injection of SNX10-overexpressing
RS4;11 cells or EV cells. By day 21, mice injected with the
SNX10-overexpressing cells began to display symptoms related to
B-ALL, including systemic tremors, hind limb weakness and gradual
weight loss. The OS time of these mice was notably shorter compared
with their controls (Fig. 7A,
P<0.05), consistent with the results of the bioinformatics
analysis (Fig. 1F). In addition,
the proportion of human B-ALL cells was significantly higher in the
bone marrow (Fig. 7B, P<0.0001),
peripheral blood (Fig. 7C,
P<0.0001) and lymph nodes (Fig.
7D, P<0.01) of mice injected with SNX10-overexpressing
cells. Furthermore, the liver and spleen in these mice were also
markedly enlarged in both volume (Fig.
7E and G) and weight (Fig. 7F and
H, P<0.01). Additionally, an increased proportion of
infiltrating leukemia cells was observed in the liver (Fig. 7I) and spleen (Fig. 7J) of these mice. These results
collectively suggest that elevated SNX10 levels facilitate the
proliferation, migration and infiltration of B-ALL cells, thereby
accelerating leukemia progression in vivo.
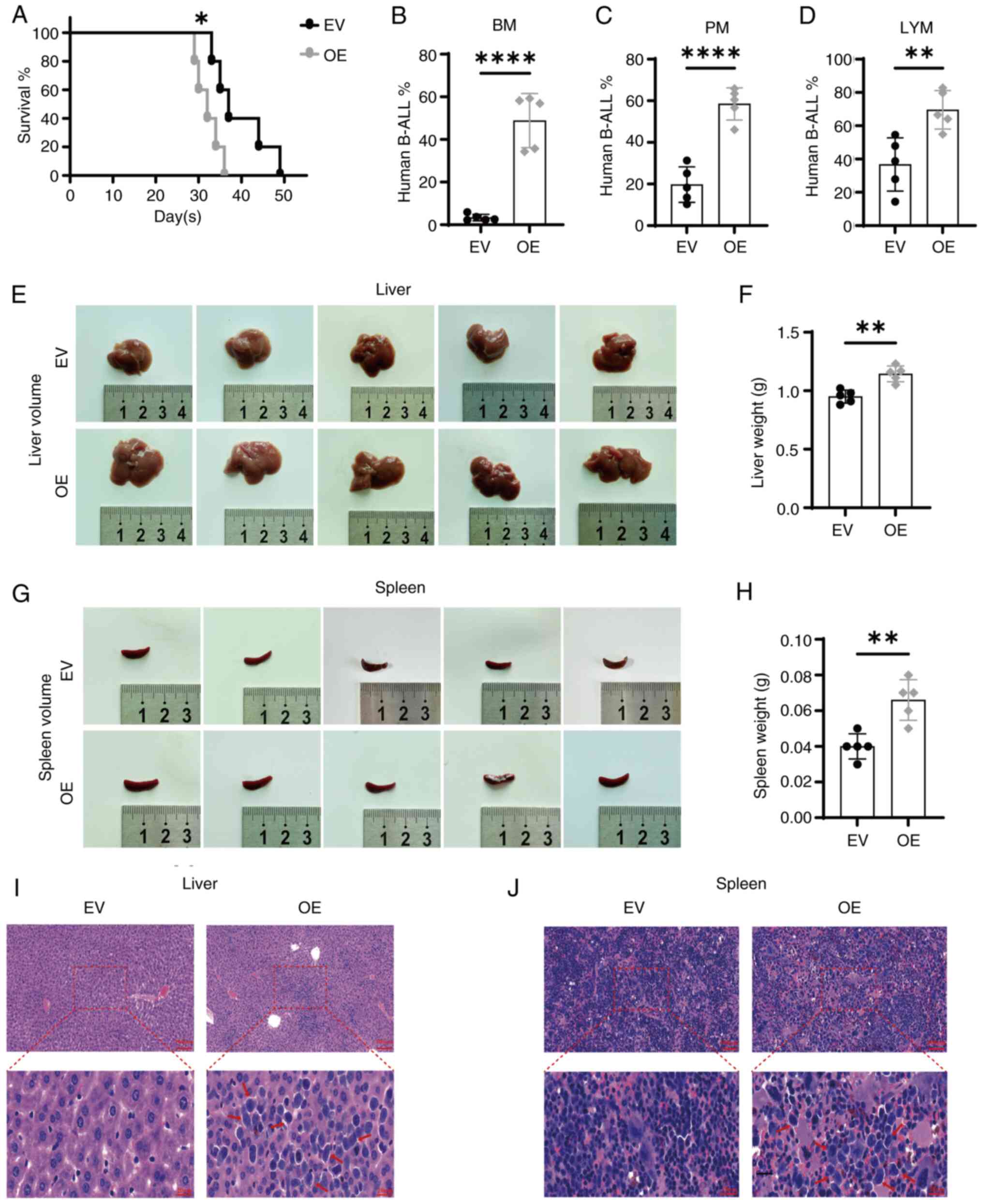 | Figure 7.Overexpression of SNX10 accelerates
the progression of B-ALL in a CDX xenograft mouse model. (A)
Survival was analyzed using the Kaplan-Meier method. The presence
of human B-ALL cells (human CD19-APC/GFP-FITC) in (B) BM, (C) PM
and (D) LYM cells were assessed using flow cytometry. The liver (E)
volume, (F) weight, and spleen (G) volume and (H) weight were
measured. Tissue sections were stained with hematoxylin-eosin, and
the leukemia infiltration areas are indicated with the red arrow,
in the (I) liver and (J) spleen. Scale bar, 100 µm (top) and 20 µm
(bottom). Data are presented as the mean ± SD of three independent
repeats and compared using a Student's t-test. *P<0.05,
**P<0.01 and ****P<0.0001 vs. EV group. SNX10, sorting nexin
10; B-ALL, B-cell acute lymphoblastic leukemia; OE, overexpression;
EV, empty vector; BM, bone marrow; PM, peripheral blood; LYM, lymph
node. |
Discussion
As a relatively recently identified cancer regulator
(13,14,35),
the specific mechanism of action of SNX10 in B-ALL has not been
assessed previously. The present study addressed this gap by
investigating the role of SNX10 in B-ALL and its potential
mechanisms. It was found that upregulated expression of SNX10
activated the PI3K-Akt signaling pathway, thereby reducing
apoptosis and increasing the proportion of cells in the G2/M phase,
thus promoting the proliferation of B-ALL cells and accelerating
disease progression. By contrast, SNX10 was generally downregulated
in the samples of patients with B-ALL, as well as in B-ALL cell
lines. Knockdown of SNX10 resulted in reduced PI3K-Akt signaling,
an increased proportion of cells in the G0/G1 phase and increased
apoptosis, thus reducing the proliferation of B-ALL cells. The
in vivo experiments further supported these findings,
illustrating that high SNX10 expression contributed to aggressive
behaviors of B-ALL.
High expression of SNX10 and poorer prognosis in
B-ALL is a trend also observed in cervical cancer (14) and glioblastoma (15). The expression levels of SNX10
differentially affect the progression and prognosis of various
tumors. For example, in osteosarcoma tissues (36), high expression of SNX10 inhibits the
proliferation, migration and invasion of tumor cells. Additionally,
low expression of SNX10 has been associated with a poor prognosis
in patients with stomach adenocarcinoma (37) and colorectal cancer (13). These observations suggest that SNX10
may act as a bifunctional gene, with its effects depending on
tissue type and cellular context. The impact of SNX10 on B-ALL also
appears to depend on its expression level within the context of the
specific disease; however, the specific molecular mechanisms
require further experimental exploration.
SNX10 has been demonstrated to be involved in
several cellular processes, including intracellular transport
(38) and signal transduction
(15), as well as in physiological
processes such as cellular absorption and secretion (10,11),
and intracellular glucose metabolism (12). In healthy populations, SNX10 is
expressed at high levels, while its expression is reduced in
patients with B-ALL, which may reflect specific metabolic or
transcriptional regulatory changes in leukemia cells. This decrease
in expression may be related to mechanisms that inhibit the
proliferation, survival, or drug resistance of leukemia cells in
vivo. Numerous studies have shown that not only tumor cells
(39) but also immune cells
(40) in the tumor microenvironment
undergo metabolic reprogramming, which may directly affect the
biological activity of tumor cells and is closely related to tumor
drug resistance and malignant progression (41). In B-ALL, the expression of SNX10 may
be influenced by the tumor microenvironment, leading to its reduced
expression in tumor cells. Although certain patients with B-ALL
exhibit relatively high levels of SNX10 expression, this high
expression does not necessarily indicate a functionally ‘healthy’
state. Conversely, aberrantly relatively upregulated SNX10
expression may be associated with cell proliferation, an
anti-apoptotic effect, or treatment resistance, resulting in a poor
prognosis. In the present study, both in vivo and in
vitro experiments demonstrated that overexpression of SNX10
modified the cell cycle of leukemia cells, suppressed apoptosis,
and promoted cell survival. Upregulated expression of SNX10 may
signify a highly active state of tumor cells, associated with
increased aggressiveness or a higher probability of recurrence.
Therefore, despite the elevated expression levels of SNX10 observed
in healthy individuals, its expression pattern and impact in
leukemia may be associated with tumor progression, exhibiting a
duality in its effects.
The present study is the first to demonstrate that
SNX10 regulates the proliferation, apoptosis and cell cycle
processes of B-ALL cells via the PI3K/Akt signaling pathway, to the
best of our knowledge. This finding suggests that SNX10 may play an
important role in the development of B-ALL and could be associated
with the onset of leukemia, resistance to treatment and a poorer
prognosis. Consequently, SNX10 has the potential to serve as a
biomarker for B-ALL and a therapeutic target for drug development.
For example, by evaluating the expression levels of SNX10 across a
range of populations of patients with B-ALL, high-risk groups can
be identified, and subsequent personalized treatment plans can be
formulated (42,43). Alternatively, by developing oral
nanoparticles of SNX10-shRNA plasmids (44,45) or
through the use of an SNX10 protein-protein interaction inhibitor
such as DC-SX029 (46) in
combination with existing treatment regimens, particularly targeted
drugs such as PI3K/Akt inhibitors (5,14,32),
it may be possible to overcome resistance and enhance therapeutic
efficacy. Therefore, further research into the specific mechanisms
of SNX10 in B-ALL cells is warranted. By exploring the interactions
between SNX10 and the PI3K/Akt signaling pathway in-depth, novel
therapeutic targets may be uncovered. Additionally, it is also
worth investigating whether the function of SNX10 is regulated by
other cellular signaling pathways, which can aid in constructing a
more comprehensive model of the pathogenesis of B-ALL and promote
the implementation of personalized treatment.
B-ALL is a genetically heterogeneous disease
(47). The limited variety of cell
lines and clinical samples utilized in the present study may hinder
the applicability and statistical relevance of the results. Future
research should aim to enhance the sample size, especially in
multicenter clinical trials, to confirm the role of SNX10 across
patients from different demographic backgrounds. Furthermore, the
effects of targeting SNX10 in normal cells were not evaluated,
necessitating further studies to assess the selectivity of its
therapeutic targets to reduce potential side effects. Additionally,
the present study used a NOD-SCID mouse model (48) to investigate the role of SNX10 in
vivo. While this model is widely used in cancer research due to
its lack of functional T and B cells (49), allowing human cell transplantation,
it may not fully recapitulate the human tumor microenvironment,
especially in terms of immune interactions. B-ALL progression and
treatment responses are complex processes influenced by the immune
system, and the absence of immune cells in NOD-SCID mice limits the
study of these interactions. Furthermore, the mouse
microenvironment differs from the human setting, potentially
affecting B-ALL cell behavior and limiting clinical relevance.
Although the NOD-SCID model is valuable for drug screening and
mechanistic research, future studies may benefit from employing
more humanized models, such as humanized mice (50) or transgenic models (51), to simulate the immune interactions
and microenvironmental conditions more accurately. Cross-validation
with multiple models may enhance the reliability of these findings
and facilitate their clinical translation.
In conclusion, the present study identified SNX10 as
a core hub gene within the B-ALL-related signaling network and
revealed its significant impact on B-ALL cell biology. Through both
in vitro and in vivo experiments, it was found that
SNX10 regulated the PI3K/Akt signaling pathway, affecting B-ALL
cell proliferation, apoptosis, cell cycle progression, and disease
progression. These findings provide novel insights into the
potential of SNX10 as a prognostic marker and therapeutic target
for the management of B-ALL, supporting further research into
SNX10-modulating therapies that could improve treatment outcomes
for patients with B-ALL.
Supplementary Material
Supporting Data
Supporting Data
Supporting Data
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science
Foundation of China (grant no. 82260324), the Science and
Technology Program of Guizhou [grant no. Qiankehe Fundamentals-ZK
(2023) General (394, 398)], the Key Lab for Chronic Disease
Biomarkers of Guizhou Medical University (grant no. 2024fy004) and
the Institutional Fund of Guizhou Medical University Affiliated
Hospital (grant no. gyfybsky-2022-40).
Availability of data and materials
The data generated in the present study may be found
in the ProteomeXchange Consortium under accession number PXD061830
or at the following URL: http://proteomecentral.proteomexchange.org/cgi/GetDataset?ID=PXD061830.
The data generated in the present study may be requested from the
corresponding author.
Authors' contributions
FY conceived and designed the study. CW and XY
performed the experiments, drafted and revised the manuscript, and
confirm the authenticity of all the raw data. XS and SY analyzed
the patient data. JL and YW assisted in performing the experiments.
TT, TW and QK contributed to the interpretation of the data,
provided critical feedback on the experimental design, and
participated in drafting and revising the manuscript to ensure
scientific accuracy and integrity. All authors read and approved
the final version of the manuscript.
Ethics approval and consent to
participate
Human studies aligned with the guidelines of The
Declaration of Helsinki and were approved (approval no. 2022328) by
the Ethical Committee of the Affiliated Hospital of Guizhou Medical
University (Guiyang, China). Animal experiments were approved
(approval no. 2402141) by the Animal Care Committee of Guizhou
Medical University (Guiyang, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Huang FL, Liao EC, Li CL, Yen CY and Yu
SJ: Pathogenesis of pediatric B-cell acute lymphoblastic leukemia:
Molecular pathways and disease treatments. Oncol Lett. 20:448–454.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Inaba H and Mullighan CG: Pediatric acute
lymphoblastic leukemia. Haematologica. 105:2524–2539. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yasuda T, Sanada M, Tsuzuki S and Hayakawa
F: Oncogenic lesions and molecular subtypes in adults with B-cell
acute lymphoblastic leukemia. Cancer Sci. 114:8–15. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zeng XL, Heneghan MB and Badawy SM:
Adherence to oral chemotherapy in acute lymphoblastic leukemia
during maintenance therapy in children, adolescents, and young
adults: A systematic review. Curr Oncol. 30:720–748. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Simioni C, Martelli AM, Zauli G, Vitale M,
McCubrey JA, Capitani S and Neri LM: Targeting the
phosphatidylinositol 3-kinase/Akt/mechanistic target of rapamycin
signaling pathway in B-lineage acute lymphoblastic leukemia: An
update. J Cell Physiol. 233:6440–6454. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Han L, Xing H, Cao W, Song Y, Jiang Z and
Yu J: Bispecific antibodies in immunotherapy for adult acute
leukemia: Latest updates from the 65th American Society of
Hematology 2023 annual meeting. Expert Opin Biol Ther. 24:221–223.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wei G, Wang J, Huang H and Zhao Y: Novel
immunotherapies for adult patients with B-lineage acute
lymphoblastic leukemia. J Hematol Oncol. 10:1502017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Krali O, Marincevic-Zuniga Y, Arvidsson G,
Enblad AP, Lundmark A, Sayyab S, Zachariadis V, Heinäniemi M,
Suhonen J, Oksa L, et al: Multimodal classification of molecular
subtypes in pediatric acute lymphoblastic leukemia. NPJ Precis
Oncol. 7:1312023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu J, Qiu H, Zhao J and Pavlos NJ: The
molecular structure and function of sorting nexin 10 in skeletal
disorders, cancers, and other pathological conditions. J Cell
Physiol. 236:4207–4215. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhu CH, Morse LR and Battaglino RA: SNX10
is required for osteoclast formation and resorption activity. J
Cell Biochem. 113:1608–1615. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ye L, Morse LR, Zhang L, Sasaki H, Mills
JC, Odgren PR, Sibbel G, Stanley JR, Wong G, Zamarioli A and
Battaglino RA: Osteopetrorickets due to Snx10 deficiency in mice
results from both failed osteoclast activity and loss of gastric
acid-dependent calcium absorption. PLoS Genet. 11:e10050572015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Feng H, Tan J, Wang Q, Zhou T, Li L, Sun
D, Fan M, Cheng H and Shen W: α-Hederin regulates glucose
metabolism in intestinal epithelial cells by increasing SNX10
expression. Phytomedicine. 111:1546772023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang S, Hu B, You Y, Yang Z, Liu L, Tang
H, Bao W, Guan Y and Shen X: Sorting nexin 10 acts as a tumor
suppressor in tumorigenesis and progression of colorectal cancer
through regulating chaperone mediated autophagy degradation of
p21Cip1/WAF1. Cancer Lett. 419:116–127. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liao D, He Y, He B, Zeng S, Cui Y, Li C
and Huang H: Inhibiting SNX10 induces autophagy to suppress
invasion and EMT and inhibits the PI3K/AKT pathway in cervical
cancer. Clin Transl Oncol. Oct 5–2024.(Epub ahead of print).
View Article : Google Scholar
|
|
15
|
Gimple RC, Zhang G, Wang S, Huang T, Lee
J, Taori S, Lv D, Dixit D, Halbert ME, Morton AR, et al: Sorting
nexin 10 sustains PDGF receptor signaling in glioblastoma stem
cells via endosomal protein sorting. JCI Insight. 8:e1580772023.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang J, Deng J, Wang K, Wang A, Chen G,
Chen Q, Ye M, Wu X, Wang X and Lin D: Tetrahydropalmatine promotes
random skin flap survival in rats via the PI3K/AKT signaling
pathway. J Ethnopharmacol. 324:1178082024. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xiao F, Zhang Z, Li L, He X and Chen Y:
LINC01370 suppresses hepatocellular carcinoma proliferation and
metastasis by regulating the PI3K/AKT pathway. Discov Oncol.
15:3262024. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu S, Xie X, Li C, Liu Z and Zuo D:
Micromolar sodium fluoride promotes osteo/odontogenic
differentiation in dental pulp stem cells by inhibiting PI3K/AKT
pathway. Arch Oral Biol. 131:1052652021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang HP, Yu ZL, Wu BB and Sun FR: PENK
inhibits osteosarcoma cell migration by activating the PI3K/Akt
signaling pathway. J Orthop Surg Res. 15:1622020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhu Y, Jing L, Li X, Zheng D, Zhou G,
Zhang Y, Sang Y, Shi Z, Sun Z and Zhou X: Decabromodiphenyl ether
disturbs hepatic glycolipid metabolism by regulating the
PI3K/AKT/GLUT4 and mTOR/PPARγ/RXRα pathway in mice and L02 cells.
Sci Total Environ. 763:1429362021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fransecky L, Mochmann LH and Baldus CD:
Outlook on PI3K/AKT/mTOR inhibition in acute leukemia. Mol Cell
Ther. 3:22015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liem M, Ang CS and Mathivanan S: Insulin
mediated activation of PI3K/Akt signalling pathway modifies the
proteomic cargo of extracellular vesicle. Proteomics.
17:16003712017. View Article : Google Scholar
|
|
23
|
Glaviano A, Foo A, Lam HY, Yap KCH, Jacot
W, Jones RH, Eng H, Nair MG, Makvandi P, Geoerger B, et al:
PI3K/AKT/mTOR signaling transduction pathway and targeted therapies
in cancer. Mol Cancer. 22:1382023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhong C, Ju G, Yang S, Zhao X, Chen J and
Li N: Total flavonoids of polygala fallax hemsl induce apoptosis of
human ectopic endometrial stromal cells through PI3K/AKT/Bcl-2
signaling pathway. Gynecol Obstet Invest. 88:197–213. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sun F, Mu C, Kwok HF, Xu J, Wu Y, Liu W,
Sabatier JM, Annweiler C, Li X, Cao Z and Xie Y: Capivasertib
restricts SARS-CoV-2 cellular entry: A potential clinical
application for COVID-19. Int J Biol Sci. 17:2348–2355. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sultana F, Morse LR, Picotto G, Liu W, Jha
PK, Odgren PR and Battaglino RA: Snx10 and PIKfyve are required for
lysosome formation in osteoclasts. J Cell Biochem. 121:2927–2937.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
He J and Li X: Identification and
validation of aging-related genes in idiopathic pulmonary fibrosis.
Front Genet. 13:7800102022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
World Medical Association, . World medical
association declaration of Helsinki: Ethical principles for medical
research involving human subjects. JAMA. 310:2191–2194. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Grüninger PK, Uhl F, Herzog H, Gentile G,
Andrade-Martinez M, Schmidt T, Han K, Morgens DW, Bassik MC, Cleary
ML, et al: Functional characterization of the PI3K/AKT/MTOR
signaling pathway for targeted therapy in B-precursor acute
lymphoblastic leukemia. Cancer Gene Ther. 29:1751–1760. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang L, Liu X, Kang Q, Pan C, Zhang T,
Feng C, Chen L, Wei S and Wang J: Nrf2 overexpression decreases
vincristine chemotherapy sensitivity through the PI3K-AKT pathway
in adult B-Cell acute lymphoblastic leukemia. Front Oncol.
12:8765562022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Holz C, Lange S, Sekora A, Knuebel G,
Krohn S, Murua Escobar H, Junghanss C and Richter A: Combined BCL-2
and PI3K/AKT pathway inhibition in KMT2A-rearranged acute
B-lymphoblastic leukemia cells. Int J Mol Sci. 24:13592023.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ma J, Chen T, Wu S, Yang C, Bai M, Shu K,
Li K, Zhang G, Jin Z, He F, et al: iProX: An integrated proteome
resource. Nucleic Acids Res. 47:D1211–D1217. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen T, Ma J, Liu Y, Chen Z, Xiao N, Lu Y,
Fu Y, Yang C, Li M, Wu S, et al: iProX in 2021: Connecting
proteomics data sharing with big data. Nucleic Acids Res.
50:D1522–D1527. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhou B, Min B, Liu W, Li Y, Zhu F, Huang
J, Fang J, Chen Q and Wu D: Construction of a five-gene-based
prognostic model for relapsed/refractory acute lymphoblastic
leukemia. Hematology. 29:24129522024. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang Y, Sun N, Zhang Z, Zhou Y, Liu H,
Zhou X, Zhang Y and Zhao Y: Overexpression pattern of miR-301b in
osteosarcoma and its relevance with osteosarcoma cellular behaviors
via modulating SNX10. Biochem Genet. 61:87–100. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang J, Wu Y, Jin HY, Guo S, Dong Z,
Zheng ZC, Wang Y and Zhao Y: Prognostic value of sorting nexin 10
weak expression in stomach adenocarcinoma revealed by weighted gene
co-expression network analysis. World J Gastroenterol.
24:4906–4919. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Battaglino RA, Jha P, Sultana F, Liu W and
Morse LR: FKBP12: A partner of Snx10 required for vesicular
trafficking in osteoclasts. J Cell Biochem. 120:13321–13329. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jiao J, Zhao Y, Li Q, Jin S and Liu Z:
LncRNAs in tumor metabolic reprogramming and tumor microenvironment
remodeling. Front Immunol. 15:14671512024. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang X, Zhang S, Xue D, Neculai D and
Zhang J: Metabolic reprogramming of macrophages in cancer therapy.
Trends Endocrinol Metab. Sep 19–2024.(Epub ahead of print).
View Article : Google Scholar
|
|
41
|
Shi R, Tang YQ and Miao H: Metabolism in
tumor microenvironment: Implications for cancer immunotherapy.
MedComm (2020). 1:47–68. 2020. View
Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gupta SK, Bakhshi S, Kumar L, Seth R and
Kumar R: IKZF1 (IKAROS) deletions in B-ALL and its clinical
correlation: A prospective study from a tertiary care centre in
Northern India. Leuk Res. 41:7–11. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Altieri F, Buono L, Lanzilli M, Mirabelli
P, Cianflone A, Beneduce G, De Matteo A, Parasole R, Salvatore M
and Smaldone G: LINC00958 as new diagnostic and prognostic
biomarker of childhood acute lymphoblastic leukaemia of B cells.
Front Oncol. 14:13881542024. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhu X and Li S: Nanomaterials in tumor
immunotherapy: New strategies and challenges. Mol Cancer.
22:942023. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bao WL, Wu Q, Hu B, Sun D, Zhao S, Shen X,
Cheng H and Shen W: Oral nanoparticles of SNX10-shRNA plasmids
ameliorate mouse colitis. Int J Nanomedicine. 16:345–357. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bao W, Liu X, You Y, Hou H, Wang X, Zhang
S, Li H, Feng G, Cao X, Jiang H, et al: Targeting sorting nexin 10
improves mouse colitis via inhibiting PIKfyve-mediated TBK1/c-Rel
signaling activation. Pharmacol Res. 169:1056792021. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Li J, Dai Y, Wu L, Zhang M, Ouyang W,
Huang J and Chen S: Emerging molecular subtypes and therapeutic
targets in B-cell precursor acute lymphoblastic leukemia. Front
Med. 15:347–371. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Baersch G, Möllers T, Hötte A,
Dockhorn-Dworniczak B, Rübe C, Ritter J, Jürgens H and Vormoor J:
Good engraftment of B-cell precursor ALL in NOD-SCID mice. Klin
Padiatr. 209:178–185. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lepus CM, Gibson TF, Gerber SA, Kawikova
I, Szczepanik M, Hossain J, Ablamunits V, Kirkiles-Smith N, Herold
KC, Donis RO, et al: Comparison of human fetal liver, umbilical
cord blood, and adult blood hematopoietic stem cell engraftment in
NOD-scid/gammac-/-, Balb/c-Rag1-/-gammac-/-, and C.B-17-scid/bg
immunodeficient mice. Hum Immunol. 70:790–802. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chen A, Neuwirth I and
Herndler-Brandstetter D: Modeling the tumor microenvironment and
cancer immunotherapy in next-generation humanized mice. Cancers
(Basel). 15:29892023. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lampreht TU, Horvat S and Cemazar M:
Transgenic mouse models in cancer research. Front Oncol. 8:2682018.
View Article : Google Scholar : PubMed/NCBI
|