Introduction
Esophageal cancer (EC) is the eighth most common
malignant tumor worldwide (1) and
the sixth leading cause of cancer-related deaths (2). The number of deaths resulting from EC
has decreased due to advances in surgery, chemotherapy and
radiation therapy; however, the results have been unsatisfactory
(1). Therefore, elucidating the
molecular mechanisms underlying EC growth and metastasis. and
developing novel targeted therapies to overcome therapeutic
resistance are crucial for improving treatment outcomes.
CD40 is a member of the tumor necrosis factor
receptor superfamily. CD40 is primarily detected on the surface of
antigen-presenting cells (APCs), such as B cells,
macrophages/monocytes and dendritic cells (3,4). CD40
activation stimulates multiple signaling pathways, including the
NF-κB and PI3K signaling pathways, leading to immune responses
(5,6). CD40 signaling polarizes macrophages to
the M1 phenotype, exerting strong antitumor effects (7), inducing dendritic cell maturation and
enhancing antigen presentation (3,4). CD40
acts as a co-stimulatory molecule when APCs present antigens to
CD4+ T lymphocytes, serving an essential role in
adaptive immunity (8). CD40 is also
expressed in various malignancies, including malignant melanoma,
gastric cancer and lung cancer, and its expression in cancer cells
such as EC cells is associated with poor prognosis (9–15).
However, to the best of our knowledge, the molecular function of
CD40 expression on the surface of EC cells and why it is a poor
prognostic factor remain unclear.
MMP-9 is involved in breaking down the extracellular
matrix (ECM) in normal physiological processes, such as embryonic
development, reproduction and tissue remodeling, as well as in
disease processes, including cancer metastasis (16,17).
MMP-9 serves a crucial role in cancer cell invasion, migration and
angiogenesis (18–20). In non-cancer cells such as human
podocytes, CD40 receptor activation induces MMP-9 secretion, which
is involved in ECM degradation (21). However, to the best of our
knowledge, this phenomenon has not been confirmed in cancer cells.
Therefore, the present study aimed to evaluate CD40
activation-induced changes in cancer cell bioactivity and MMP-9
upregulation. We hypothesized that CD40 regulates MMP-9 secretion,
which is crucial for EC cells to acquire malignant potential.
Materials and methods
Cell culture
TE-1, −4, −5, −6, −8, −9, −10, −11, −14 and −15
human esophageal squamous cancer cell lines were purchased from the
Cell Resource Center for Biomedical Research, Institute of
Development, Aging and Cancer, Tohoku University (Sendai, Japan).
All cells were cultured in RPMI-1640 medium (Thermo Fisher
Scientific, Inc.) containing 10% (v/v) FBS (Thermo Fisher
Scientific, Inc.) and 1% (v/v) penicillin-streptomycin solution
(Thermo Fisher Scientific, Inc.) at 37°C in a humidified incubator
with 5% CO2. To evaluate morphological differences among
these cell lines, cells at 50–70% confluency were fixed and stained
using a Diff-Quick staining kit (Sysmex Corporation). The cells
were sequentially immersed for 2 min each in the fixative solution,
stain solution I and stain solution II at room temperature. Stained
cells were observed under a BZ-9000 light microscope (Keyence
Corporation) at a magnification of ×20 and analyzed using the
dedicated BZ-H3C software (version 1.4.1; Keyence Corporation).
Peripheral blood mononuclear cell
(PBMC) isolation
Peripheral venous blood samples were collected from
healthy adult donors (male; median age, 38 years; age range, 36–60
years) at Hokkaido University (Sapporo, Japan) between July 2024
and September 2024. All participants provided written informed
consent prior to participation. Inclusion criteria included age ≥20
years and the ability to provide informed consent. Individuals with
a history of cancer or active malignant disease were excluded. The
present study was approved (approval no. 24-0126; approval date
July 10, 2024) by the Institutional Review Board Committee of
Hokkaido University (Sapporo, Japan). Blood collection from healthy
donors began in July 2024, and the samples were subsequently used
for experiments. PBMCs were isolated from heparinized blood samples
via density gradient centrifugation at 400 × g for 30 min at room
temperature using Ficoll-Paque Plus reagent (GE Healthcare)
according to the manufacturer's instructions.
Western blotting
Cells were lysed using an ULTRARIPA kit (BioDynamics
Laboratory, Inc.) supplemented with a Protease Inhibitor Cocktail
(Promega Corporation). Protein concentrations were determined using
the TaKaRa BCA Protein Assay Kit (Takara Bio Inc.). Equal amounts
of protein (10 µg per lane) were loaded onto 15% SDS-PAGE gels and
transferred to PVDF membranes (Bio-Rad Laboratories, Inc.). Human
PBMCs were used as positive controls. After protein transfer to the
PVDF membranes, the membranes were blocked with Tris-buffered
saline with 0.1% Tween-20 (TBST) and 5% skim milk at room
temperature for 1 h, and then incubated with primary antibodies
overnight at 4°C. A list of the antibodies used in the present
study (including dilutions, cat. nos. and suppliers) is presented
in Table I. After two washes with
TBST, the membranes were incubated at room temperature for 1 h with
HRP-conjugated secondary antibodies, including anti-rabbit IgG
(HRP-linked; cat. no. 7074; Cell Signaling Technology, Inc.) and
anti-mouse IgG (HRP-linked; cat. no. 7076; Cell Signaling
Technology, Inc.) antibodies, depending on the host species of the
primary antibody, both diluted 1:4,000 in TBST containing 5% skim
milk. Detection was performed using an ECL plus enhanced
chemiluminescent substrate (GE Healthcare) and a ChemiDoc
instrument (Bio-Rad Laboratories, Inc.). Densitometry was performed
using Image Lab software (version 6.1.0; Bio-Rad Laboratories,
Inc.). Equal loading was confirmed using actin staining.
 | Table I.Characteristics of the antibodies
used in the present study. |
Table I.
Characteristics of the antibodies
used in the present study.
| Antigen | Cat. no. | Applications | Dilution | Manufacturer |
|---|
| CD40 | ab13545 | WB; ICC | 1:1,000; 1:500 | Abcam |
|
| REA733 | FC | 1:50 | Miltenyi Biotec
GmbH |
| CD61 | REA733 | FC | 1:50 | Miltenyi Biotec
GmbH |
| CD62P | REA389 | FC | 1:50 | Miltenyi Biotec
GmbH |
| CD154 | REA238 | FC | 1:50 | Miltenyi Biotec
GmbH |
| β-actin | 8H10D10 | WB | 1:1,000 | Cell Signaling
Technology, Inc. |
| p-Akt | 4060T | WB | 1:4,000 | Cell Signaling
Technology, Inc. |
| Akt | 4691T | WB | 1:4,000 | Cell Signaling
Technology, Inc. |
| p-p44/42 MAPK
(Erk1/2) | 4370T | WB | 1:4,000 | Cell Signaling
Technology, Inc. |
| p44/42 MAPK
(Erk1/2) | 4695T | WB | 1:4,000 | Cell Signaling
Technology, Inc. |
| p-JNK | 4668T | WB | 1:4,000 | Cell Signaling
Technology, Inc. |
| JNK | 9252T | WB | 1:4,000 | Cell Signaling
Technology, Inc. |
| NF-κB p65 | 8242T | WB | 1:4,000 | Cell Signaling
Technology, Inc. |
| Histone H3 | 12648T | WB | 1:4,000 | Cell Signaling
Technology, Inc. |
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from TE cell lines or human
PBMCs using the RNeasy Mini Kit (Qiagen GmbH) according to the
manufacturer's protocol as previously described (22). cDNA was generated using 1 µg RNA per
reaction with Prime Script RT Master Mix (Takara Bio, Inc.)
according to the manufacturer's protocol. RT-qPCR was performed
using TaqMan Universal PCR Master Mix (No AmpErase UNG; Thermo
Fisher Scientific, Inc.) and TaqMan probes (human CD40,
Hs01002915_g1; human MMP-9, Hs00957562_m1; and human β-actin,
Hs99999903_m1) on a Lightcycler instrument (Roche Diagnostics). The
sequences of the primers and probes used in these assays are
proprietary and not publicly disclosed by the manufacturer. The
thermocycling conditions were as follows: Initial enzyme activation
at 95°C for 10 min, followed by 40 cycles of denaturation at 95°C
for 15 sec and annealing/extension at 60°C for 1 min. Data were
analyzed using the 2−ΔΔCq method (23), and β-actin was used as the reference
gene for normalization.
CD40 mRNA expression was evaluated using
semi-quantitative PCR. Total RNA was extracted from TE cells using
the RNeasy Mini Kit (Qiagen GmbH) and quantified
spectrophotometrically. Reverse transcription was performed using 1
µg RNA with the Prime Script RT Master Mix (Takara Bio, Inc.)
according to the manufacturer's protocol. The reverse transcription
conditions were 37°C for 15 min and 85°C for 5 sec. The PCR
reaction was performed with KOD Plus polymerase (Toyobo Co., Ltd.),
which includes dNTPs in the reaction mixture, according to the
manufacturer's instructions. The thermocycling conditions were as
follows: 94°C for 2 min; 35 cycles of 98°C for 10 sec, 64°C for 1
sec and 68°C for 28 sec; followed by a hold at 10°C. The following
primers were used: CD40 forward, 5′-AGAGTTCACTGAAACGGAATGCC-3′ and
reverse, 5′-ACAGGATCCCGAAGATGATGG-3′; and β-actin forward,
5′-CAACCGCGAGAAGATGACCC-3′ and reverse,
5′-GGAACCGCTCATTGCCAATGG-3′. PCR products were separated on 2%
agarose gels and visualized using ethidium bromide staining under
UV light with a ChemiDoc instrument (Bio-Rad Laboratories, Inc.).
Densitometric analysis was performed using Image Lab software
(version 6.1.0; Bio-Rad Laboratories, Inc.). β-actin was used as
the reference gene to confirm equal loading and for
normalization.
The Prime PCR 96-well plate array ECM Remodeling H96
(cat. no. 10025275; Bio-Rad Laboratories, Inc.) was used according
to the manufacturer's instructions to evaluate ECM-related gene
expression. PCR was performed using the Lightcycler instrument
(Roche Diagnostics) as aforementioned, and data were analyzed using
the ΔΔCq method. TE-5 and TE-10 EC cell lines were used in this
assay.
Flow cytometry
The cells were first stained for the surface
antigens CD40, CD154, CD61 and CD62P, followed by washing with
autoMACS Rinsing Solution (Miltenyi Biotec GmbH). Cells were
surface-stained for 10 min at 4°C with the following antibodies:
CD40-VioBright™ FITC (cat. no. REA733; 1:50), CD154-allophycocyanin
(cat. no. REA238; 1:50), CD61-VioBright™ 515 (cat. no. REA761;
1:50) and CD62P-phycoerythrin (cat. no. REA389; 1:500) (all from
Miltenyi Biotec GmbH). Data were acquired using a
MACSQuant® Analyzer 10 flow cytometer (Miltenyi Biotec
GmbH) and analyzed using FlowJo software (version 10.6.2; Becton,
Dickinson and Company).
Immunocytochemistry
Cells were cultured on coverslips and fixed with
3.7% formaldehyde for 15 min at 25°C. After three washes with PBS,
the cells were blocked with PBS containing 3% BSA (Thermo Fisher
Scientific, Inc.) and 10% (v/v) goat serum (Thermo Fisher
Scientific, Inc.) for 15 min at 25°C. The cells were then incubated
with human polyclonal anti-CD40 antibodies (1:500; cat. no.
ab13545; Abcam) in PBS for 1 h at 25°C. After three PBS washes, the
cells were incubated with Alexa Fluor® 488-conjugated
goat anti-rabbit antibodies (1:500; cat. no. ab150077; Abcam) for 1
h at 25°C. After three PBS washes, the coverslips were mounted on
slides using Dapi-Fluoromount-G™ (Southern Biotech), and the nuclei
were labeled by staining with 4′,6-diamidino-2-phenylindole.
Permeabilization was not performed, as CD40 is a membrane protein
with an extracellular epitope recognized by the antibody used. DAPI
counterstaining was performed according to the manufacturer's
instructions, which included incubation at room temperature for 5
min. Finally, the samples were analyzed using a confocal microscope
(BZ-9000; Keyence Corporation) with the analysis software BZ-H3C
(version 1.4.1; Keyence Corporation).
CD154 stimulation
Cells were treated with 5 µg/ml recombinant soluble
CD154 (rsCD154; ENZO MEGACD40L; Enzo Life Sciences) for 24 h in
serum-free culture medium. After incubation at 37°C, the
supernatants were harvested and centrifuged at 10,000 × g for 5 min
at 4°C. Finally, the cell pellets were lysed using RNeasy kit
(Qiagen GmbH), as described in the RT-qPCR section, for total RNA
extraction. For the dose-response assay, TE-10 cells were treated
under the same conditions with various concentrations of rsCD154
(10 ng/ml, 30 ng/ml, 100 ng/ml, 300 ng/ml, 1 µg/ml, 3 µg/ml, 10
µg/ml and 30 µg/ml) to evaluate MMP-9 mRNA expression in response
to CD154 stimulation. The expression levels of MMP-9 mRNA were
quantified using RT-qPCR as aforementioned.
Cell viability assay
The CellTiter 96 AQueous One Solution Cell
Proliferation Assay System (Promega Corporation) was used according
to the manufacturer's instructions for the cell viability assays.
Briefly, 4×103 cells/well were plated into a 96-well
plate and incubated for 48 h with or without stimulation with 5
µg/ml rsCD154 at 37°C in a humidified incubator with 5%
CO2. After 48 h of incubation, 10 µl CellTiter 96
AQueous One Solution reagent was added to each well. The absorbance
was measured at 490 nm using a SpectraMax I3 microplate reader
(Molecular Devices, LLC) after 2 h of incubation at 37°C in a
humidified 5% CO2 atmosphere. A total of five replicate
wells were used to measure cell viability.
Cell migration and invasion
assays
Transwell cell migration assays were performed using
8-µm pore size membrane chambers without Matrigel (Corning BioCoat
Control Inserts; cat. no. 354578; Corning, Inc.), while invasion
assays were performed using Matrigel-coated chambers of the same
type (Corning BioCoat Matrigel Invasion Chambers; cat. no. 354480;
Corning, Inc.). A total of 5×104 cells in 200 µl
RPMI-1640 medium containing 1% FBS and 25 pg rsCD154 were seeded in
the upper compartments, and 500 µl RPMI-1640 medium containing 10%
FBS as a chemoattractant was added to the lower compartments. After
48 h of incubation at 37°C in a humidified incubator with 5%
CO2, the non-invading cells were removed from the upper
surface of the membrane by scrubbing with cotton-tipped swabs.
Invasive cells on the membranes beneath the insert were fixed and
stained using a Diff-Quick staining kit (Sysmex Corporation), which
consists of a fixative solution, stain solution I and stain
solution II. The membranes were sequentially immersed in each
solution for 2 min at room temperature, according to the
manufacturer's instructions. Stained cells were counted using a
confocal microscope (BZ-9000; Keyence Corporation) and KEYENCE
software (BZ H3C; version 1.4.1). A total of five fields were
randomly selected, the total area of cells in each field was
measured and the average was obtained to measure the number of
invasive cells.
Gelatin zymography
Culture media from esophageal squamous cell
carcinoma (ESCC) cells stimulated with or without 5 µg/ml rsCD154
for 24 h at 37°C were analyzed for gelatin degradation using 7.5%
SDS-PAGE gels containing 0.1% (w/v) gelatin (from porcine skin type
A; MilliporeSigma). After SDS-PAGE, the gels were washed twice for
1 h in 2.5% Triton X-100 and incubated for 24 h at 37°C in an
incubation buffer [50 mM Tris (pH 7.4), 1 µM ZnCl2, 5 mM
CaCl2 and 1% Triton X-100]. The gels were stained with
0.5% Coomassie brilliant blue R250 in methanol for 1 h at room
temperature, and destained with a solution containing 40% methanol
and 10% acetic acid for 4 h at room temperature. Gelatinolytic
activity appeared as white bands on a blue background. The gels
were scanned, and the protein band intensities were determined
using a ChemiDoc image analyzer (Bio-Rad Laboratories, Inc.) and
Image Lab software (version 6.1.0; Bio-Rad Laboratories, Inc.).
CD40 small interfering RNA (siRNA)
transfection
CD40-specific and control siRNA were obtained from
Santa Cruz Biotechnology, Inc. Control siRNA-A (cat. no. sc-37007)
served as a non-targeting scrambled siRNA. CD40 siRNA (cat. no.
sc-29250) was used to specifically knock down CD40 gene expression.
The sequences of siRNAs were not provided by the supplier. TE-10
cells in the exponential growth phase were seeded into 96-well
plates at a density of 7.5×103 cells per well, cultured
for 24 h, and then transfected with 800 nM siRNA using
oligofectamine (Thermo Fisher Scientific, Inc.) and OPTI-MEM I
reduced-serum medium (Thermo Fisher Scientific, Inc.) at 37°C for 4
h, according to the manufacturer's instructions. The siRNA
concentration was determined based on prior dose-response
experiments. After transfection, the cells were further incubated
in complete growth medium at 37°C. Transfection efficiency was
assessed 72 h post-transfection via flow cytometry using
allophycocyanin-conjugated anti-CD40 antibodies for staining.
Subsequent experiments were conducted 72 h after transfection.
Control cells underwent mock transfection with control siRNA under
identical conditions.
Signaling pathway assay
TE-10 cells (1×105 cells/well) were
seeded in 12-well plates, incubated at 37°C for 24 h and
subsequently stimulated with 5 µg/ml rsCD154 for 15 or 30 min at
room temperature. Cell pellets were collected at 15 and 30 min
after stimulation. Proteins were then extracted from the cell
pellets following the aforementioned western blotting protocol. The
extracted proteins were analyzed using the antibodies listed in
Table I to evaluate the activation
of each signaling pathway involved in the CD40-CD154
interaction.
Platelet isolation
Venous blood was collected in an aplastic syringe
containing 1/10 volume of CPD buffer (16 mM citric acid, 90 mM
sodium citrate, 16 mM NaH2PO4, 142 mM
dextrose, pH 7.4). Blood samples were centrifuged at 200 × g for 20
min at 20°C. The top layer containing platelet-rich plasma was
transferred into a new tube containing 1 volume of HEP buffer (140
mM NaCl, 2.7 mM KCl, 3.8 mM HEPES, 5 mM EGTA, pH 7.4) and
prostaglandin E1 (1 µM final concentration; Cayman
Chemical Company), and centrifuged at 100 × g for 20 min at 20°C.
The supernatant was collected and placed into a new tube, which was
centrifuged at 800 × g for 20 min at 20°C. After discarding the
supernatant, the platelet pellet was washed twice with a platelet
wash buffer [10 mM sodium citrate, 150 mM NaCl, 1 mM EDTA and 1%
(w/v) dextrose, pH 7.4]. The platelet pellet was slowly resuspended
in modified Tyrode buffer (134 mM NaCl, 12 mM NaHCO3,
2.9 mM KCl, 0.34 mM Na2HPO4, 1 mM
MgCl2, 10 mM HEPES, 5 mM dextrose, 3 mg/ml BSA and 1 µM
PGE1, pH 7.4) and used as a platelet solution. Platelets
were activated by pre-warming at 37°C for 5 min, followed by the
addition of 1 U human α-thrombin (Prolytix). After thrombin
addition, the mixture was allowed to stand at room temperature for
1 min.
ELISA
The concentrations of soluble CD154 (sCD154) and
MMP-9 in the culture supernatant were measured using ELISA kits
(Quantikine® ELISA: Human CD40 Ligand/TNFSF5
Immunoassay; cat. no. DCDL40; R&D Systems, Inc.;
Quantikine® ELISA: Human MMP-9; cat. no. DMP900; R&D
Systems, Inc.) according to the respective manufacturer's
instructions. The optical density at 540 nm was determined using a
SpectraMax I3 microplate reader (Molecular Devices, LLC). All
samples were analyzed in quadruplicate.
Co-culturing TE cells and
platelets
Briefly, 7.5×104 TE-10 cells were
inoculated into 24-well plates and incubated at 37°C for 24 h.
After PBS washing, RPMI-1640 medium without FBS was added to the
cells, and 1×108 of the activated platelets suspended in
0.2 ml modified Tyrode solution were added to 0.4-µm-pore cell
culture inserts and incubated at 37°C for another 24 h. After
incubation, the culture supernatant and cell pellet were collected,
and MMP-9 mRNA levels were determined using RT-qPCR as
aforementioned. TaqMan probe-based assays were used (MMP-9:
Hs00957562_m1; β-actin: Hs99999903_m1; Thermo Fisher Scientific,
Inc.), and primer sequences were not disclosed by the supplier.
Analyzing MMP-9 and CD154
concentrations in the sera of patients undergoing EC surgery and
determining their survival period
Approval was obtained from the Institutional Review
Board of Hokkaido University Hospital (approval no. 24-0126;
approval date July 10, 2024; Sapporo, Japan). Written informed
consent was obtained from all patients who participated in this
clinical research. The present study was conducted between August
2014 and October 2017 on 49 consecutive patients who underwent
curative esophagectomy for EC at the Department of
Gastroenterological Surgery, Hokkaido University Hospital (Sapporo,
Japan). Eligible patients were aged ≥20 years and had provided
informed consent to store blood samples for research purposes.
Patients judged by the investigators to be inappropriate for
inclusion were excluded. The survival analysis included 41 men and
8 women. The median age was 67 years, with an age range of 52–85
years. Patient sera were collected either immediately before
surgery on the day of the operation or on the day prior to surgery,
and stored at −80°C until further analyses. The samples were
analyzed in August 2024. Serum MMP-9 and CD154 concentrations were
measured using ELISA kits (Quantikine ELISA Human CD40 Ligand,
Human MMP-9; R&D Systems, Inc.). Cancer recurrence and survival
were analyzed over a minimum follow-up period of 5 years after
esophagectomy. Information on cancer recurrence, survival time, TNM
classification (Union for International Cancer Control 8th edition)
(24) and histological type was
extracted from the medical records.
Statistical analysis
All statistical analyses were performed using
GraphPad Prism 9 software (Dotmatics). Pearson's χ2
test, the unpaired t-test or the Mann-Whitney U test were used as
appropriate to compare different groups. For comparisons involving
three groups, one-way ANOVA was used, followed by Bonferroni post
hoc analysis when statistical significance was detected. For
survival analysis, the log-rank (Mantel-Cox) test was used to
compare Kaplan-Meier survival curves. Data are presented as mean ±
SD, unless otherwise indicated. All experiments were performed in
triplicate (n=3) unless stated otherwise. P<0.05 was considered
to indicate a statistically significant difference.
Results
Analysis of CD40 expression on ESCC
cells
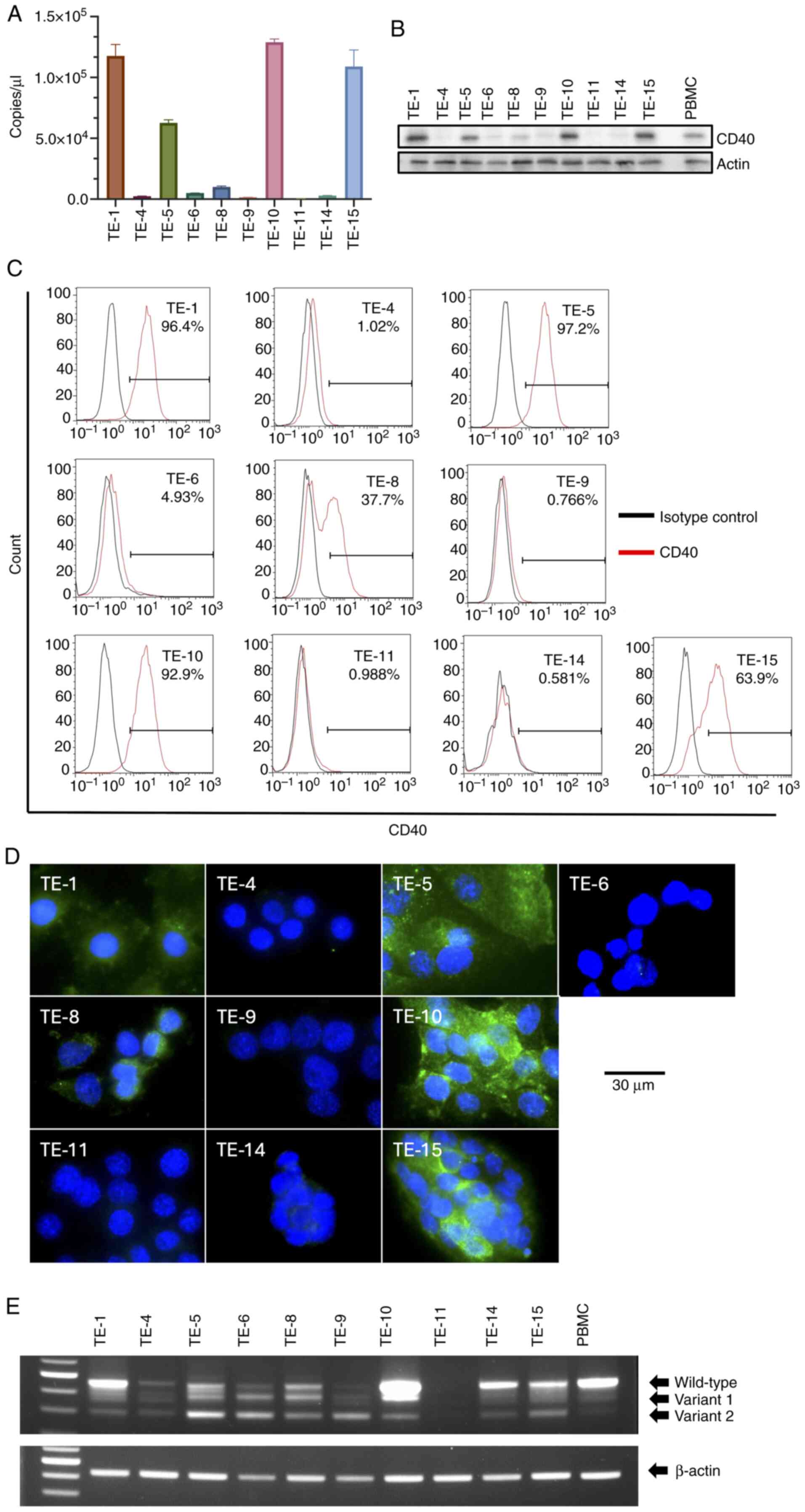
CD40 mRNA expression was analyzed in 10 TE
cell lines, among which TE-1, −5, −10 and −15 cells exhibited
relatively high expression levels (Fig.
1A). Western blotting was performed to evaluate CD40 protein
levels in TE cells. TE-1, −5, −10 and −15 cells showed clear CD40
bands (Fig. 1B). Flow cytometry and
immunocytochemistry analyses were also performed to examine the
cell surface expression of CD40 on TE cells, which revealed similar
results to those obtained by western blotting, except for TE-1
cells, in which the immunocytochemistry result differed (Fig. 1C and D). No morphological
differences were observed among the 10 types of TE cells based on
CD40 expression, as assessed by Diff-Quick staining and light
microscopy (Fig. S1). Due to
discrepancies in CD40 expression levels across the different
detection methods, such as weak or absent surface staining by
immunocytochemistry in TE-1 cells despite positive results by
RT-qPCR and western blotting, semi-quantitative PCR was also
performed to further validate mRNA expression. Human CD40 is known
to have splicing variants resulting from alternative splicing
(25). The presence of splicing
variants was confirmed in TE cells positive for CD40 mRNA
expression (Fig. 1E). Based on
these results, particular emphasis was placed on the flow cytometry
and immunocytochemistry data. TE-5 and TE-10 cells, which exhibited
high CD40 expression intensities, were selected as
CD40-overexpressing cells for subsequent experiments, while TE-4
and TE-11 cells, which had low CD40 expression intensities, were
used as low CD40 expression cells.
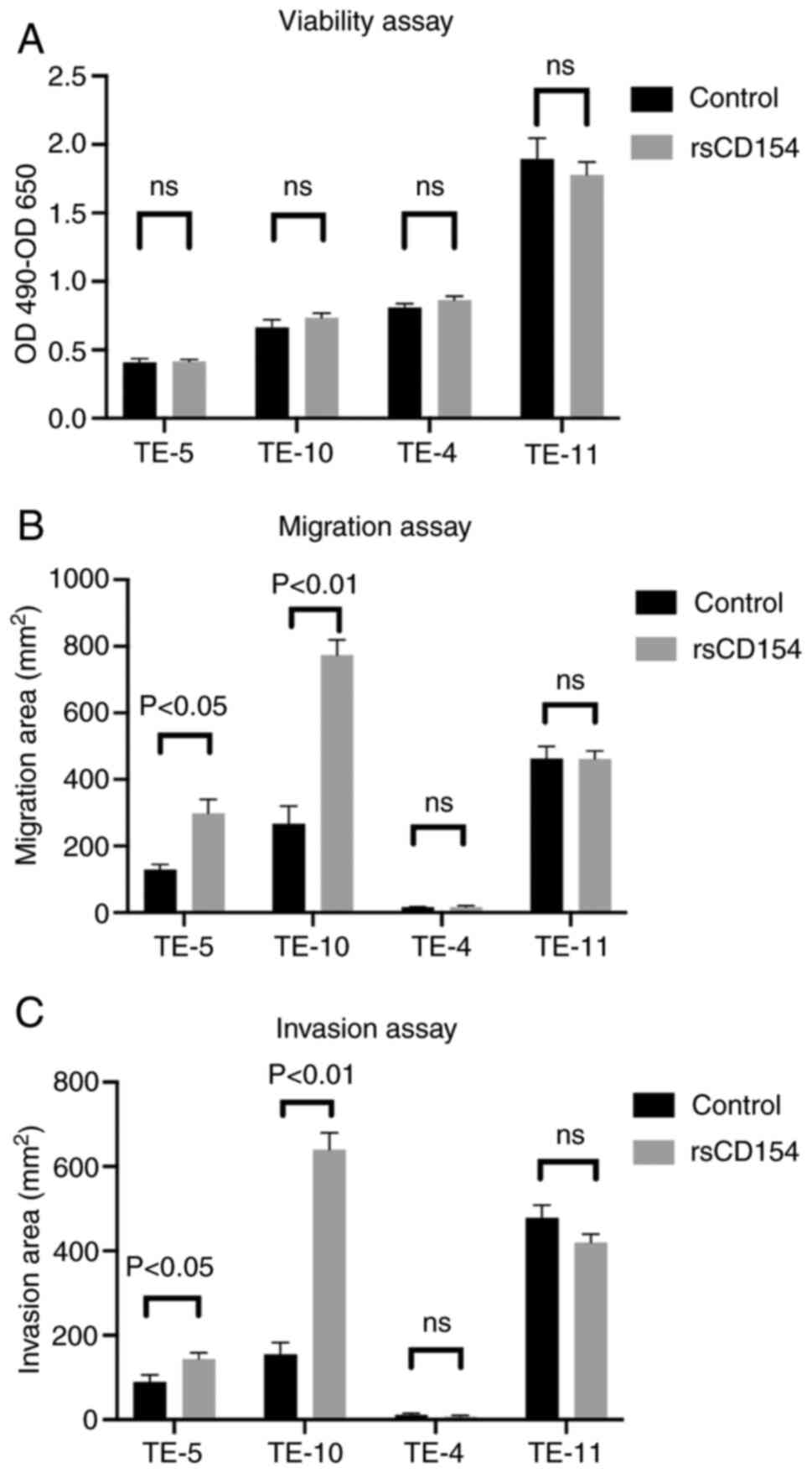
CD154 stimulation assay of TE
cells
CD40 activation was performed in vitro to
assess changes in TE cell activity and investigate the function of
CD40 in EC. In the present study, CD154, a CD40 ligand, was used to
activate CD40. The four types of TE cells showed no morphological
differences (Fig. S1); however,
their baseline viability, migration and invasion varied. Notably,
TE-11 cells exhibited high viability and invasion (Figs. 2 and S2). TE cells did not exhibit significant
changes in proliferative ability, regardless of the presence or
absence of CD40 expression (Fig.
2A). No changes in cell migration or invasion were observed in
low CD40 expression cells upon stimulation with rsCD154. By
contrast, high CD40 expression cells exhibited significantly
increased migration and invasion (Figs.
2B and C, and S2). Although
baseline differences in viability, migration and invasion were
observed among the cell lines investigated in the present study,
the results suggested that the CD40-CD154 interaction in TE cells
enhanced cell motility without changing their proliferative
capacity. These interactions were only observed in high CD40
expression cells.
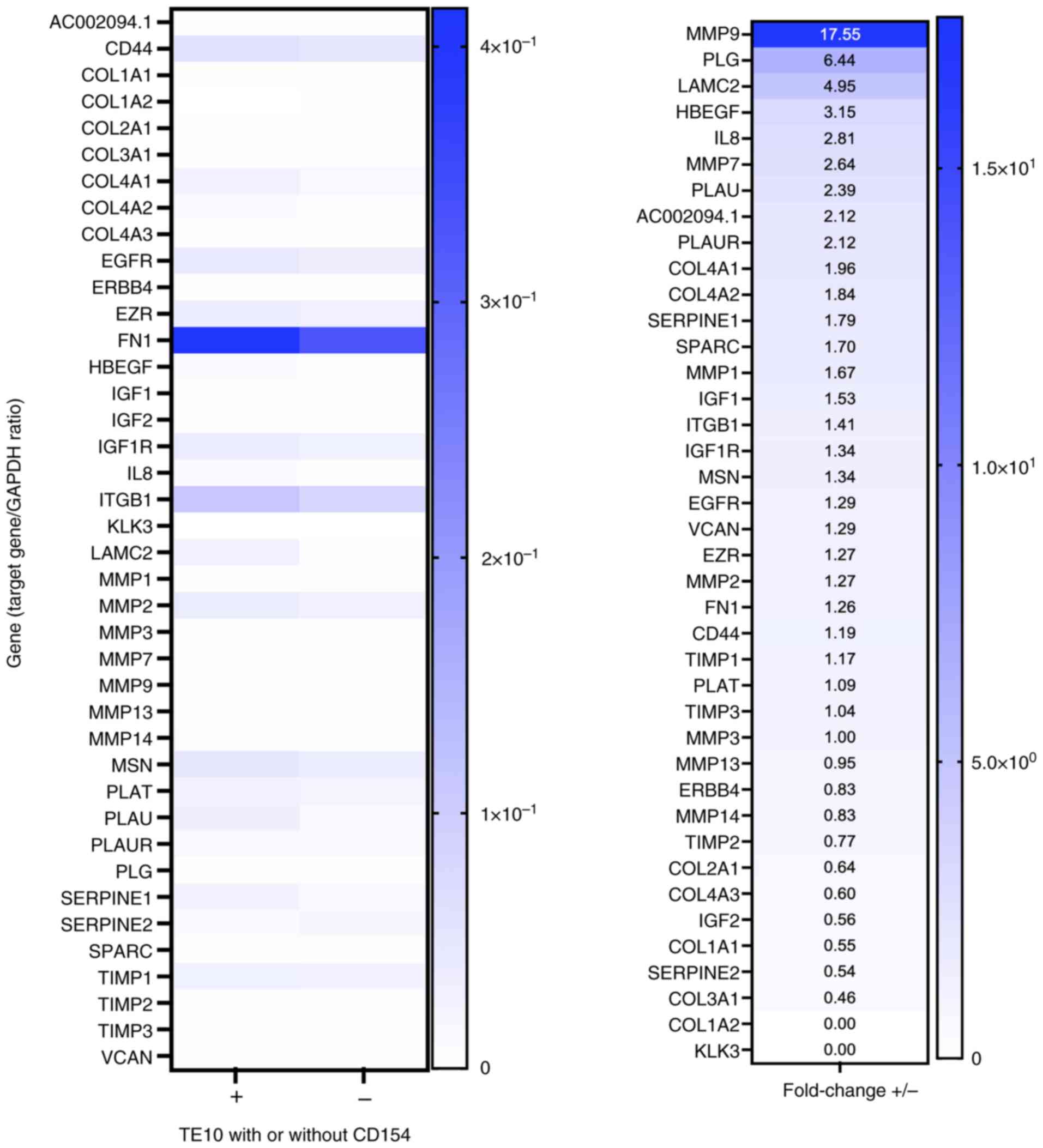
Changes in gene expression levels upon
CD40-CD154 interaction
Changes in gene expression levels in TE-10 cells due
to the CD40-CD154 interaction were examined. The genes involved in
ECM remodeling were investigated because no change in cell
viability was observed, whereas cell migration and invasion were
increased. The expression levels of 40 genes related to changes in
ECM remodeling were screened. Specifically, following stimulation
with rsCD154, the mRNA expression level of MMP-9 increased by
17.55-fold, that of plasminogen (PLG) by 6.44-fold, and that of
laminin subunit γ-2 (LAMC2) by 4.95-fold in TE-10 cells (Fig. 3). The analysis was also performed in
TE-5 cells, where a similarly strong upregulation of MMP-9 mRNA was
observed (Fig. S3). Therefore,
MMP-9 expression was investigated in subsequent experiments.
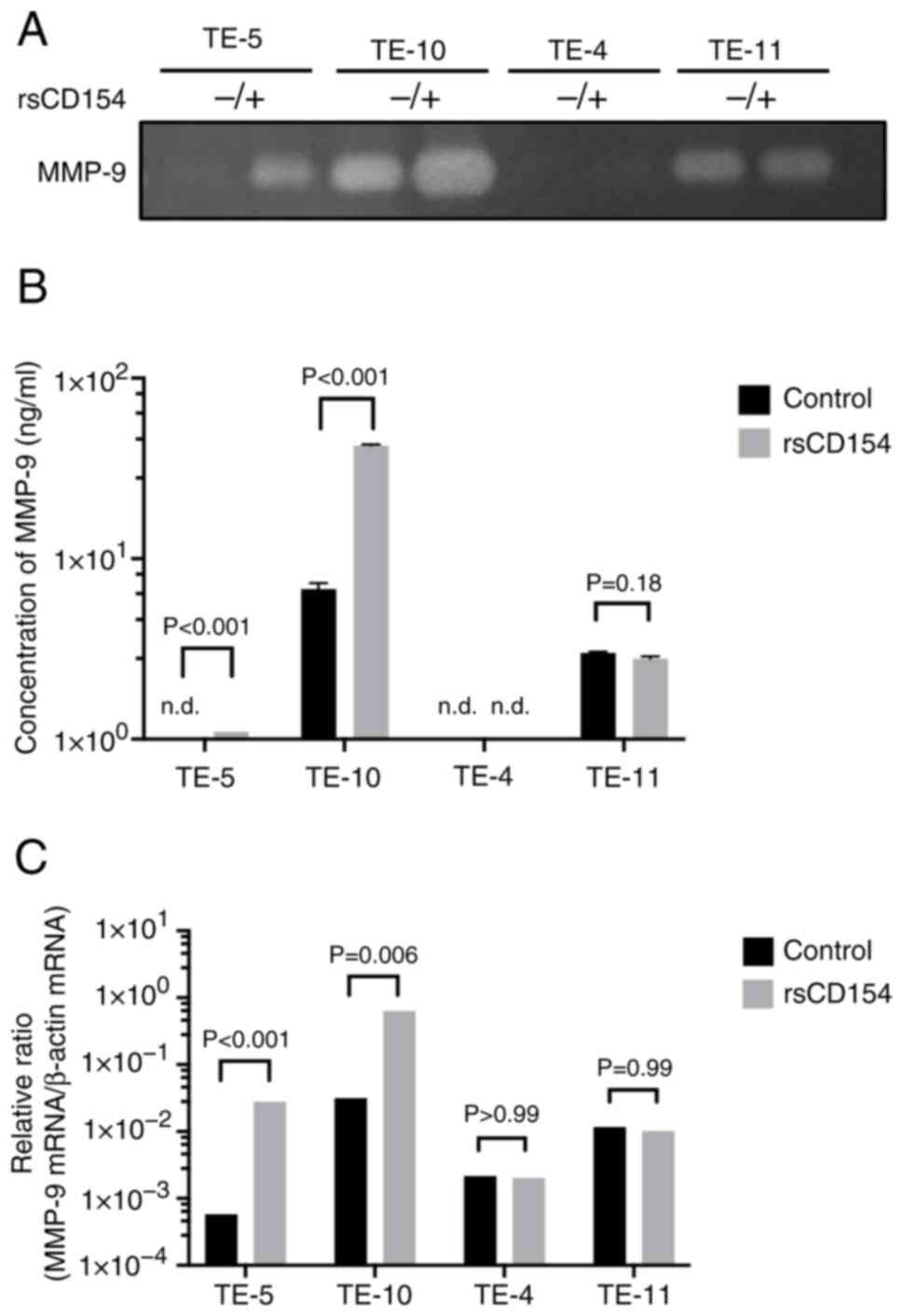
It was determined whether the CD40-CD154 interaction
induced MMP-9 secretion. rsCD154 and PBS (control) were added to TE
cells, and MMP-9 secretion in the culture supernatant was analyzed
via gelatin zymography (Fig. 4A)
and ELISA (Fig. 4B). MMP-9
upregulation was observed in the culture supernatants of high CD40
expression cells, whereas MMP-9 expression in low CD40 expression
cells did not change significantly. TE-10 and TE-11 cells, which
exhibited higher baseline invasive abilities in migration and
invasion assays, also exhibited elevated MMP-9 levels even without
sCD154 stimulation. Next, MMP-9 regulation was assessed at the mRNA
level. After rsCD154 stimulation, MMP-9 mRNA expression was
upregulated in high CD40 expression TE cells, whereas no change was
observed in low CD40 expression cells (Fig. 4C). Additionally, the upregulation of
MMP-9 mRNA induced by CD154 stimulation occurred in a
dose-dependent manner (Fig.
S4).
Changes in TE-10 cell function induced
by CD40 knockdown
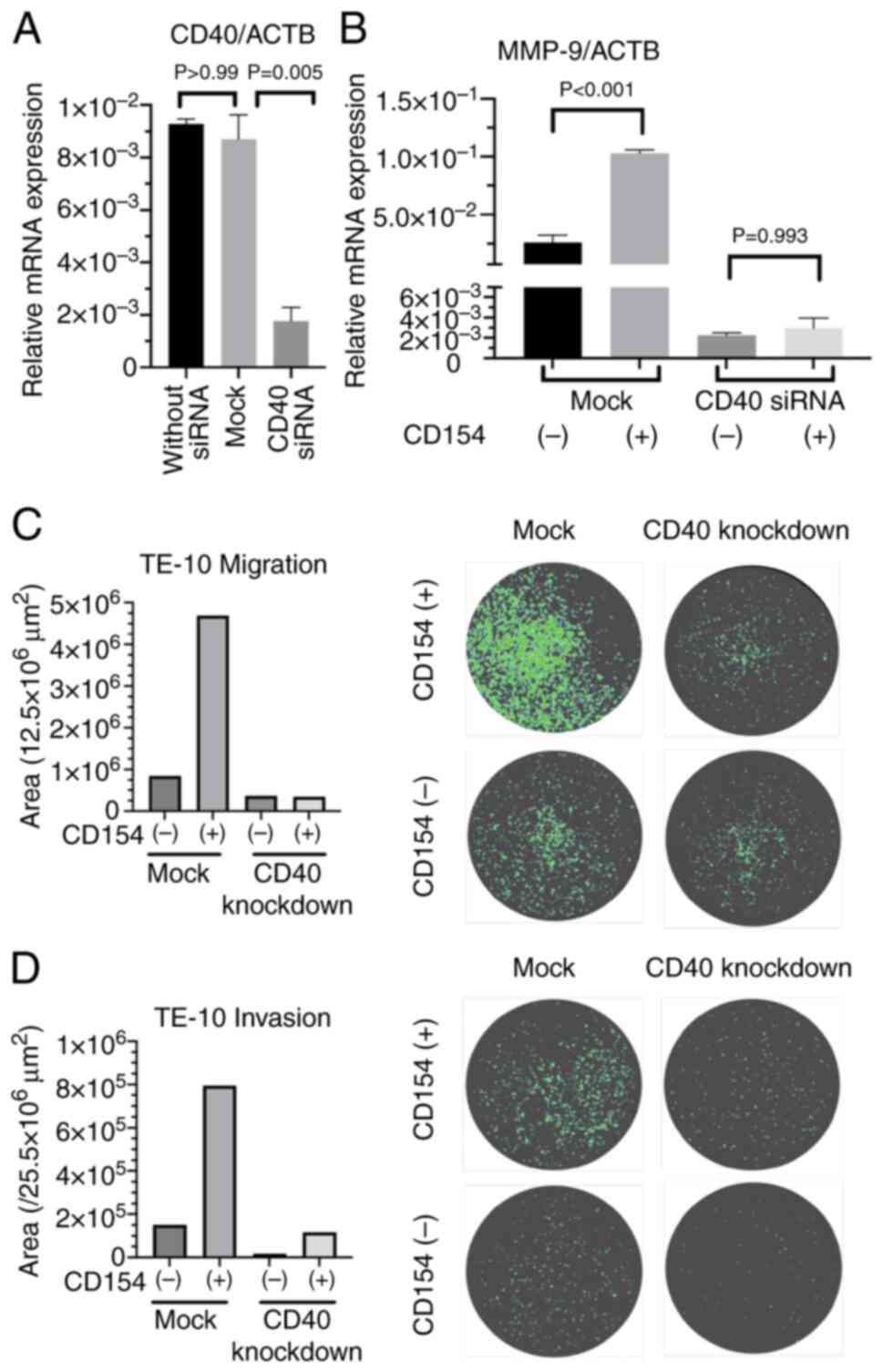
To elucidate the role of the CD40 gene in TE cells,
CD40-knockdown TE-10 cells were generated. Transfection of TE-10
cells with si-CD40 or si-control resulted in the creation of CD40
knockdown TE-10 cells and control TE-10 cells (Mock), respectively.
The knockdown efficiency was then evaluated by measuring CD40 mRNA
levels using qPCR (Fig. 5A).
Additionally, the response of these cells to sCD154 stimulation was
assessed by measuring MMP-9 mRNA levels. CD40-knockdown TE-10 cells
consistently exhibited low MMP-9 mRNA levels regardless of sCD154
stimulation (Fig. 5B). Furthermore,
migration and invasion assays were performed using CD40-knockdown
TE-10 cells. The results demonstrated that CD40-knockdown TE-10
cells exhibited reduced migratory and invasive capabilities
compared with the control cells (Fig.
5C and D).
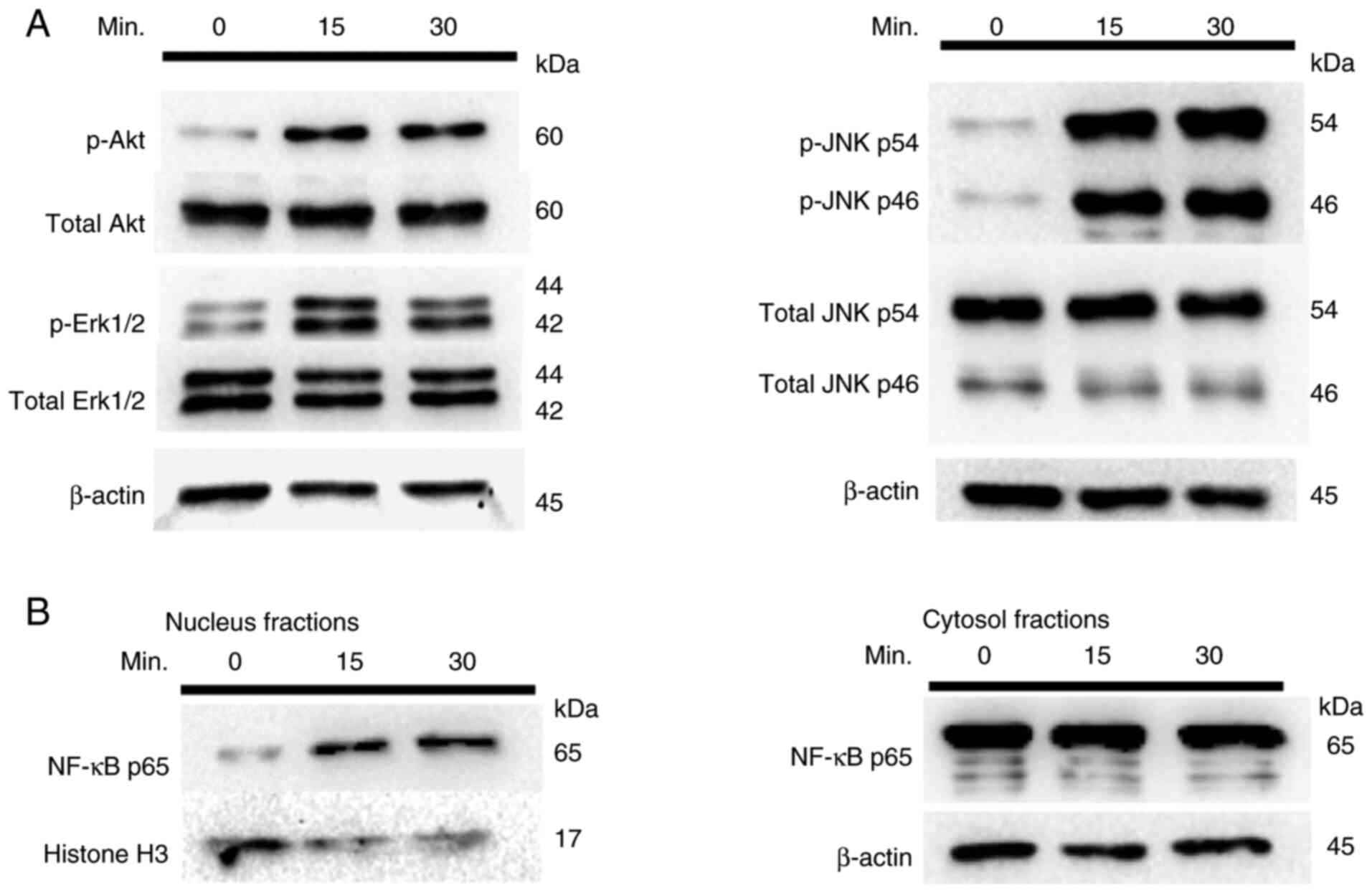
CD40 signaling pathway in TE-10
cells
Given that the CD40-CD154 interaction induces MMP-9
upregulation, the activation of PI3K, MAPK and NF-κB was
investigated following CD40 activation in TE-10 cells. As shown in
Fig. 6A, phosphorylation of Akt, a
downstream effector of PI3K, was observed in CD154-stimulated TE-10
cells, confirming PI3K activation. Furthermore, phosphorylation of
Erk1/2 and JNK, indicating MAPK pathway activation, is also shown
in Fig. 6A. Nuclear translocation
of NF-κB p65 was also observed, as shown in Fig. 6B, indicating its activation.
Platelet extraction and MMP-9
upregulation in TE cells induced by CD154-expressing human
platelets
In vivo, CD154 exists either as a
transmembrane protein on the surface of CD4+ T
lymphocytes or as a soluble form. The majority of circulating
sCD154 is derived from platelets (26), which are activated by proteinaceous
platelet-activating factors secreted by cancer cells in the tumor
microenvironment (TME). The viability and metastasis of cancer
cells are promoted by the induction of platelet aggregation
(26–28). Therefore, the relationship between
platelets and high CD40 expression EC cells was evaluated. Platelet
solutions were prepared using blood samples collected from healthy
human donors. Because platelets are activated in the TME and engage
tumor cells (29), α-thrombin was
used to activate platelets in the present study.
The purity of the generated platelet solution was
confirmed via flow cytometry for platelet marker CD61 expression
(30). Platelets were activated via
α-thrombin stimulation, and their activity was confirmed by
detecting CD62P (P-selectin) expression, an activated platelet
marker (Fig. S5).
Thrombin-activated platelets exhibited a slight increase in CD154
expression on their cell surface (Fig.
S6). In addition, sCD154 release into the platelet solution was
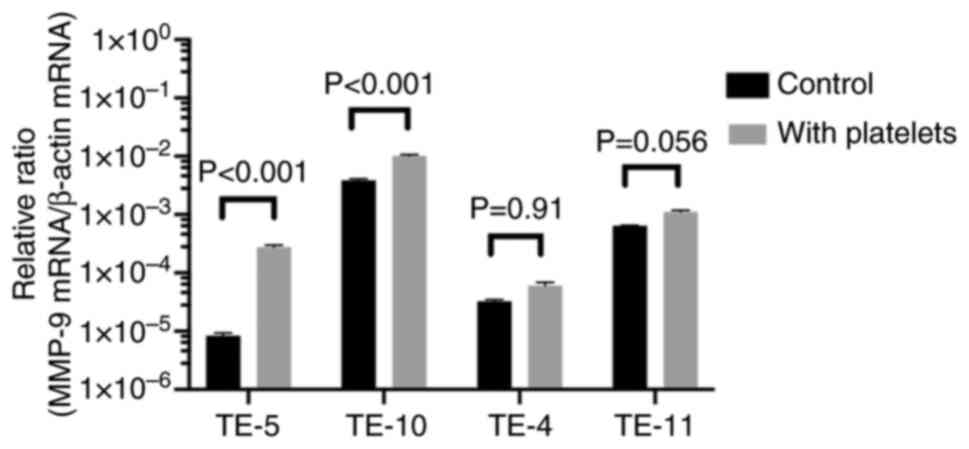
increased (Fig. S7). Next, four
types of TE cells were co-cultured with the platelet solution to
observe the reaction between the platelet solution and EC cells.
MMP-9 mRNA upregulation was confirmed in all four types of TE
cells; however, the increase was statistically significant only in
TE-5 and TE-10 cells, which exhibited high CD40 expression
(Fig. 7).
Survival analysis of patients
undergoing esophagectomy
Esophagectomies were performed on 49 patients during
the study period. The median age of the patients was 67 years, and
41 patients were male. The 3-year overall survival (OS) rate was
66.3%, and the 3-year disease-free survival (DFS) rate was 58.5%
(Table II). The MMP-9
concentration was 534.1±384.3 ng/ml (mean ± SD), and the CD154
concentration was 2.15±1.12 ng/ml (mean ± SD). In the univariate
analysis, platelet counts were significantly higher in the high
MMP-9 group, and a trend toward elevated platelet counts was
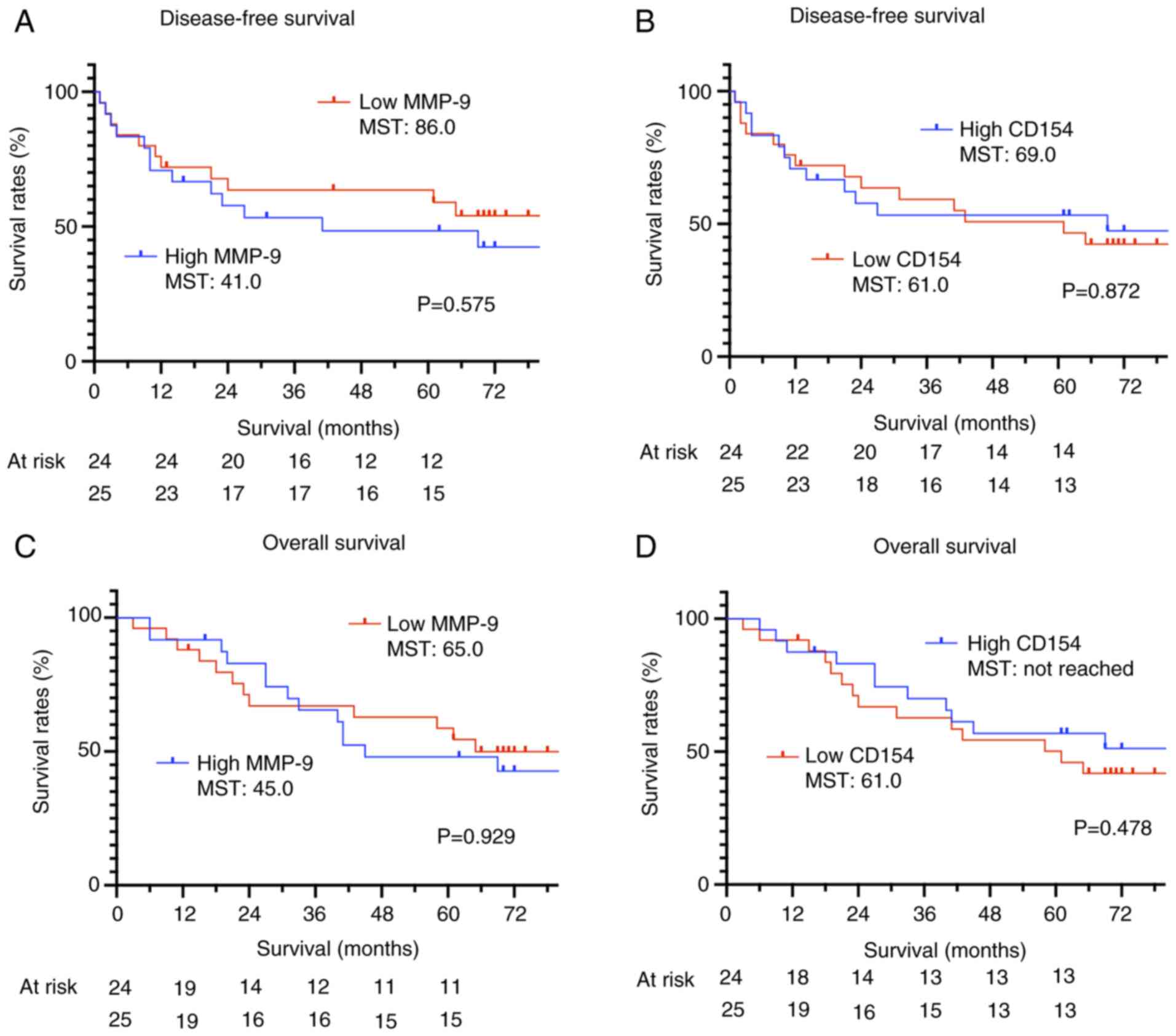
observed in the high CD154 group (Tables SI and SII). The relationship between the
survival period and the concentrations of MMP-9 and CD154 was
evaluated using Kaplan-Meier curves, with patients stratified into
high and low expression groups based on the median value. No
significant association was observed between serum MMP-9 or CD154
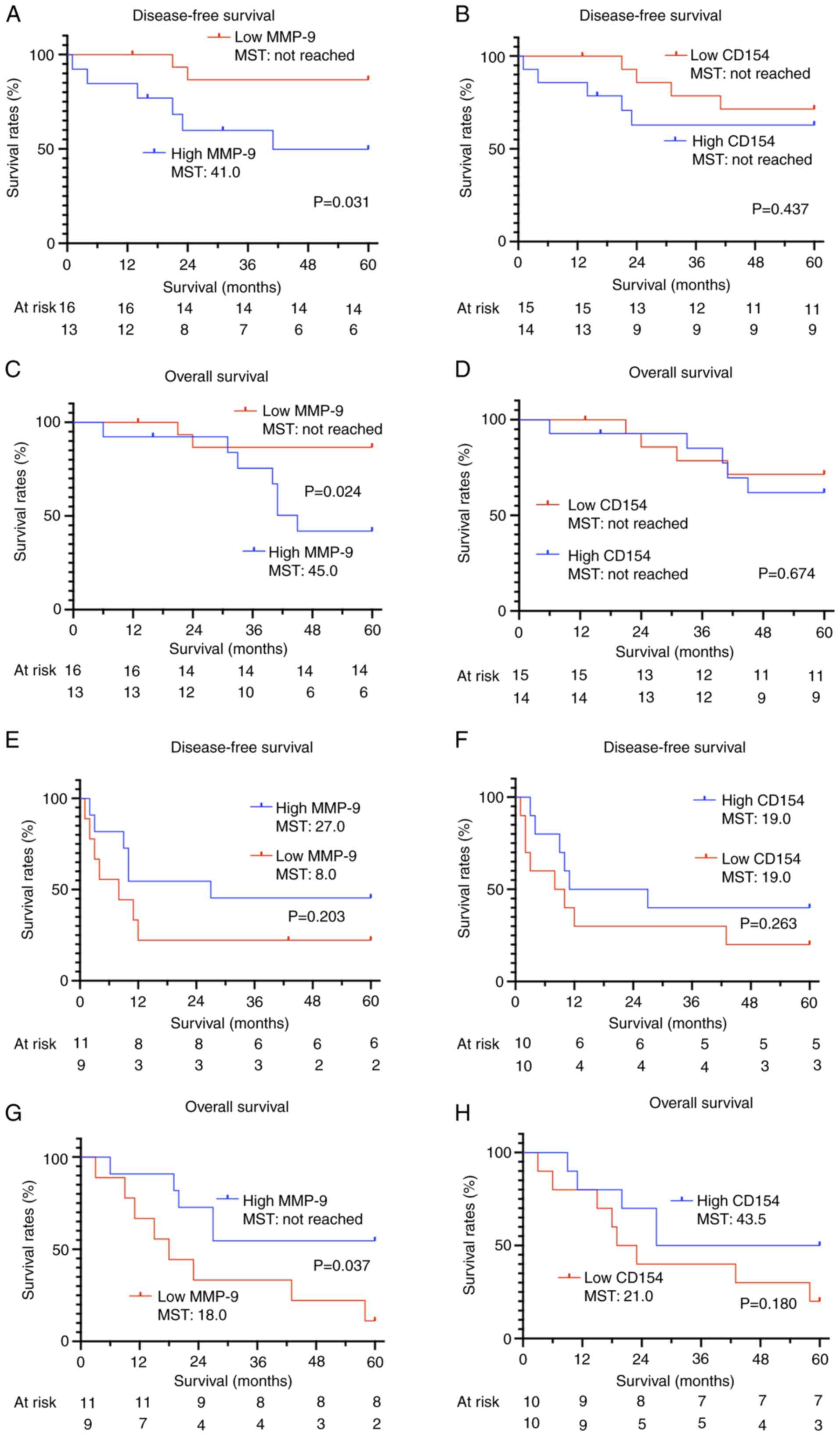
levels and OS or DFS (Fig. 8A-D).
However, when survival was analyzed based on serum MMP-9 and serum
CD154 levels stratified by pathological stage (pStage I and II–IV),
a significant improvement in prognosis was noted for patients with
low MMP-9 levels in pStage I (Fig. 9A
and C). By contrast, low MMP-9 levels were significantly
associated with reduced OS in patients with pStage II–IV (Fig. 9G). Serum CD154 levels did not
exhibit any association with survival, even when analyzed according
to pStage.
 | Table II.Patient characteristics (n=49). |
Table II.
Patient characteristics (n=49).
|
Characteristics | Value |
|---|
| Median age, years
(interquartile range) | 67 (63–74) |
| Sex, n (%) |
|
|
Male | 41 (83.7) |
|
Female | 8 (16.3) |
| Histopathological
type, n (%) |
|
|
Squamous cell carcinoma | 46 (93.9) |
|
Adenocarcinoma | 3 (6.1) |
| Pathological T
stage, UICC 8th, n (%) |
|
| T1 | 31 (63.3) |
| T2 | 2 (4.1) |
| T3 | 14 (28.6) |
| T4 | 2 (4.1) |
| Pathological N
stage, UICC 8th, n (%) |
|
| N0 | 30 (61.2) |
| N1 | 8 (16.3) |
| N2 | 7 (14.3) |
| N3 | 4 (8.2) |
| Pathological M
stage, UICC 8th, n (%) |
|
| M0 | 49 (100) |
| Pathological stage,
UICC 8th, n (%) |
|
| IA | 8 (16.3) |
| IB | 16 (32.7) |
|
IIA | 1 (2.0) |
|
IIB | 8 (16.3) |
|
IIIA | 1 (2.0) |
|
IIIB | 11 (22.4) |
|
IVA | 4 (8.2) |
| Platelet count,
×103/µl (mean ± SD) | 227.5±65.0 |
| Serum MMP-9 level,
ng/ml (mean ± SD) | 534.1±384.3 |
| Serum CD154 level,
ng/ml (mean ± SD) | 2.2±1.1 |
| 3-year overall
survival, % | 66.3 |
| 3-year disease-free
survival, % | 58.5 |
Discussion
In the present study, several notable findings were
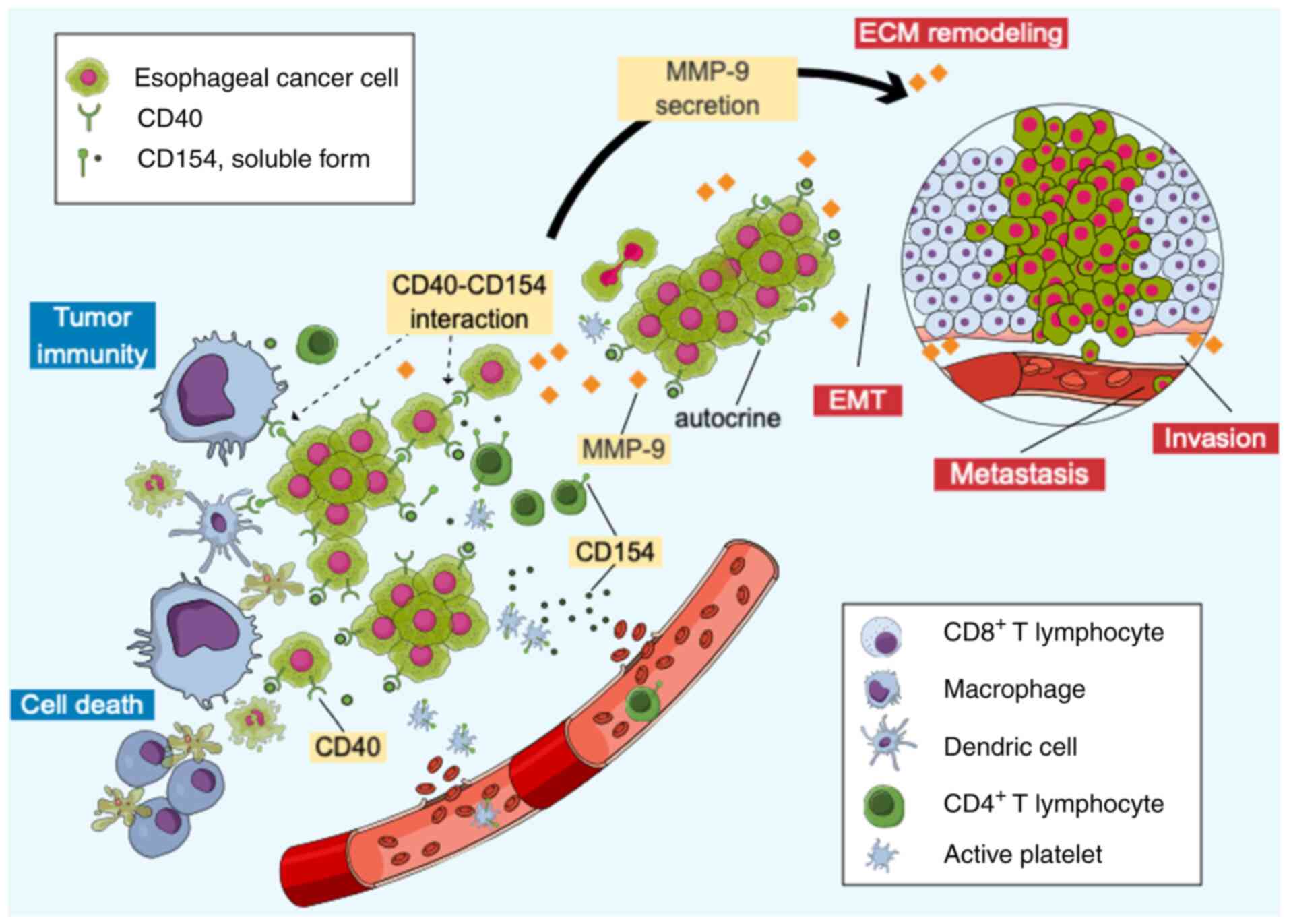
reported. First, it was demonstrated that the CD40-CD154
interaction promoted MMP-9 secretion in EC cells, increasing their
invasiveness (Fig. 10). Second,
the involvement of platelets in enhancing CD40-mediated MMP-9
secretion in EC cells was revealed. Third, the preliminary analysis
of sera from patients undergoing esophagectomy indicated a possible
association between MMP-9 levels and survival time.
CD40 is expressed on APCs and serves a crucial role
as a co-stimulatory molecule in immune responses, including
antitumor immunity. However, CD40 is also expressed in cancer
cells, where its activation increases IL-8, IL-10, IL-12p40 and
tumor growth factor-β secretion, contributing to apoptosis evasion,
cell viability and immune escape (14,31).
The present study also demonstrated that the upregulation of ECM
remodeling molecules such as MMP-9, PLG and LAMC2 via the MAPK,
NF-κB and PI3K/Akt pathways contributes to cancer invasion and
metastasis. These findings suggested that the CD40-CD154
interaction in cancer cells may create a favorable environment for
cancer cell viability. CD40 expression in cancer cells is a poor
prognostic factor; however, this may not be true for all cancer
types. For example, CD40 ligand stimulation did not alter viability
in cell lines co-expressing CD40 and CD154, whereas stimulation
with CD40- and CD154-blocking antibodies decreased cell viability
and reduced immunoregulatory cytokine secretion (IL-6, IL-10 and
IL-12p40) (14). These findings
suggest that autocrine CD40 activity is enhanced in cell lines
co-expressing CD40 and CD154, contributing to increased cancer cell
invasion.
CD40 expression in the TME affects tumor invasion,
metastasis and antitumor immunity (5). The balance between cancer cell
invasion and immunity changes depending on CD154 secretion from
host platelets, CD154 expression by the cancer cells themselves and
the response of the surrounding lymphocytes (3,4). Based
on these findings, a hypothesis was proposed to further explore the
dynamics of CD40-CD154 interactions in the TME. Specifically, these
interactions were categorized into four conceptual patterns,
according to the presence or absence of CD40 and CD154 expression
in tumor cells, as discussed subsequently.
Based on the aforementioned observations, we
hypothesized the following regarding the potential roles of
CD40-CD154 interactions in the TME: When both CD40 and CD154 are
expressed, CD40-CD154 interactions are enhanced through autocrine
signaling among cancer cells, T lymphocytes and platelets,
promoting tumor progression. Concurrently, cancer cell-derived
CD154 stimulates CD40 on APCs, enhancing tumor immunity. Therefore,
whether tumor progression or antitumor immunity becomes dominant is
determined by the relative strength of CD40-CD154-mediated cancer
cell invasion vs. immune activation. For example, CD40 and CD154
co-expression is a poor prognostic factor in melanoma (9) and renal cancer (32), indicating a tendency towards
malignancy. Second, in cases where only CD40 is expressed,
CD40-CD154 interactions are enhanced by CD154 from T lymphocytes or
platelets, promoting tumor progression without affecting tumor
immunity. Consequently, cancer cell viability is predominant.
Third, CD40-CD154 interactions do not affect tumor progression when
only CD154 is expressed, resulting in stable tumor progression.
However, CD40 expression in APCs is stimulated by cancer
cell-derived CD154, enhancing tumor immunity and resulting in a
dominant antitumor immune response. In cervical cancer, CD154
positivity is associated with improved survival (33). Lastly, when neither CD40 nor CD154
is expressed, CD40-CD154 interactions do not affect tumor
progression or immunity.
Survival analysis using serum samples from patients
with EC who underwent surgery revealed intriguing results. While no
significant association was observed between serum MMP-9 or serum
CD154 levels and survival across all patients, stage-specific
survival analysis showed a significant association between serum
MMP-9 levels and survival. In patients with stage I EC, high MMP-9
levels were associated with poor prognosis, whereas in patients
with stage II–IV EC, low MMP-9 levels were associated with poor
overall survival, but not with disease-free survival. In patients
with EC eligible for surgery (that is, those without distant
metastases), MMP-9 levels could reflect the metastatic potential of
a tumor. Conversely, in patients with established metastases, tumor
progression at metastatic sites might decrease MMP-9 levels. In the
present study, the stage I high MMP-9 group and the stage II–IV
high MMP-9 group exhibited comparable prognoses. Specifically, the
5-year OS rate was 38.5% in the stage I high MMP-9 group and 54.5%
in the stage II–IV high MMP-9 group. The 5-year DFS rates were also
similar, at 38.5 and 45.5%, respectively. Despite the difference in
staging, these groups may share similar biology in terms of
metastasis and invasion. The opposite association observed between
MMP-9 levels and survival in early- vs. advanced-stage disease may
reflect stage-specific differences in the underlying metastatic
processes (34). While further
studies are needed to confirm these observations, MMP-9 levels may
have potential as a prognostic biomarker when interpreted in the
context of disease stage.
The results of the present study were verified at
the cellular level; however, experiments using cell lines limited
to in vitro conditions may not consistently reproduce the
in vivo TME. Furthermore, the survival analysis has
limitations as it is a retrospective study conducted at a single
institution with a small number of cases.
In conclusion, the present in vitro
experiments demonstrated that CD40 expression in EC cells enhanced
migration and invasion upon CD154 stimulation, accompanied by
increased MMP-9 secretion, suggesting a potential role in promoting
tumor aggressiveness.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
KU designed and conducted the experiments, and
drafted the manuscript. TN contributed to the study design,
critically revised the manuscript for important intellectual
content and provided expert advice throughout the project. KS
contributed to conducting the experiments and compiling the data.
OS and TS assisted with the experimental procedures. SH contributed
to the study design, provided critical revisions of the manuscript
and supervised the scientific direction of the project. KU and TN
confirm the authenticity of all the raw data. All authors have read
and approved the final version of the manuscript.
Ethics approval and consent to
participate
Approval (approval no. 24-0126; approval date July
10, 2024) was obtained from the Institutional Review Board of
Hokkaido University Hospital (Sapporo, Japan). Written informed
consent was obtained from all patients participating in the present
study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
APC
|
antigen-presenting cell
|
|
DFS
|
disease-free survival
|
|
EC
|
esophageal cancer
|
|
ECM
|
extracellular matrix
|
|
ESCC
|
esophageal squamous cell carcinoma
|
|
LAMC2
|
laminin subunit γ-2
|
|
PBMC
|
peripheral blood mononuclear cell
|
|
PLG
|
plasminogen
|
|
TME
|
tumor microenvironment
|
|
RT-qPCR
|
reverse transcription-quantitative
PCR
|
|
sCD154
|
soluble CD154
|
|
rsCD154
|
recombinant sCD154
|
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pennathur A, Gibson MK, Jobe BA and
Luketich JD: Oesophageal carcinoma. Lancet. 381:400–412. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
van Kooten C and Banchereau J: CD40-CD40
ligand. J Leukoc Biol. 67:2–17. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schonbeck U and Libby P: The CD40/CD154
receptor/ligand dyad. Cell Mol Life Sci. 58:4–43. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Richards DM, Sefrin JP, Gieffers C, Hill O
and Merz C: Concepts for agonistic targeting of CD40 in
immuno-oncology. Hum Vaccin Immunother. 16:377–387. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xu W, Li Y, Yuan WW, Yin Y, Song WW, Wang
Y, Huang QQ, Zhao WH and Wu JQ: Membrane-bound CD40L promotes
senescence and initiates senescence-associated secretory phenotype
via NF-κB activation in lung adenocarcinoma. Cell Physiol Biochem.
48:1793–1803. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lim CY, Chang JH, Lee WS, Kim J and Park
IY: CD40 agonists alter the pancreatic cancer microenvironment by
shifting the macrophage phenotype toward M1 and suppress human
pancreatic cancer in organotypic slice cultures. Gut and Liver.
16:645–659. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bishop GA, Moore CR, Xie P, Stunz LL and
Kraus ZJ: TRAF proteins in CD40 signaling. Adv Exp Med Biol.
597:131–151. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
van den Oord JJ, Maes A, Stas M, Nuyts J,
Battocchio S, Kasran A, Garmyn M, De Wever I and De Wolf-Peeters C:
CD40 is a prognostic marker in primary cutaneous malignant
melanoma. Am J Pathol. 149:1953–1961. 1996.PubMed/NCBI
|
|
10
|
Sabel MS, Yamada M, Kawaguchi Y, Chen FA,
Takita H and Bankert RB: CD40 expression on human lung cancer
correlates with metastatic spread. Cancer Immunol Immunother.
49:101–108. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li R, Chen WC, Pang XQ, Hua C, Li L and
Zhang XG: Expression of CD40 and CD40L in gastric cancer tissue and
its clinical significance. Int J Mol Sci. 10:3900–3917. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ishikawa K, Miyamoto M, Yoshioka T, Kato
T, Kaji M, Ohbuchi T, Hirano S, Itoh T, Dosaka-Akita H and Kondo S:
Up-regulation of CD40 with juxtacrine activity in human nonsmall
lung cancer cells correlates with poor prognosis. Cancer.
113:530–541. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cooke PW, James ND, Ganesan R, Wallace M,
Burton A and Young LS: CD40 expression in bladder cancer. J Pathol.
188:38–43. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shoji Y, Miyamoto M, Ishikawa K, Yoshioka
T, Mishra R, Ichinokawa K, Matsumura Y, Itoh T, Shinohara T, Hirano
S and Kondo S: The CD40-CD154 interaction would correlate with
proliferation and immune escape in pancreatic ductal
adenocarcinoma. J Surg Oncol. 103:230–238. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Matsumura Y, Hiraoka K, Ishikawa K, Shoji
Y, Noji T, Hontani K, Itoh T, Nakamura T, Tsuchikawa T, Shichinohe
T and Hirano S: CD40 expression in human esophageal squamous cell
Carcinoma is associated with tumor progression and lymph node
metastasis. Anticancer Res. 36:4467–4475. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Davidson B, Reich R, Risberg B and Nesland
JM: The biological role and regulation of matrix metalloproteinases
(MMP) in cancer. Arkh Patol. 64:47–53. 2002.PubMed/NCBI
|
|
17
|
Rhee JS and Coussens LM: RECKing MMP
function: Implications for cancer development. Trends Cell Biol.
12:209–211. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Egeblad M and Werb Z: New functions for
the matrix metalloproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dagouassat M, Suffee N, Hlawaty H, Haddad
O, Charni F, Laguillier C, Vassy R, Martin L, Schischmanoff PO,
Gattegno L, et al: Monocyte chemoattractant protein-1 (MCP-1)/CCL2
secreted by hepatic myofibroblasts promotes migration and invasion
of human hepatoma cells. Int J Cancer. 126:1095–1108. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nakamura T, Kuwai T, Kim JS, Fan D, Kim SJ
and Fidler IJ: Stromal metalloproteinase-9 is essential to
angiogenesis and progressive growth of orthotopic human pancreatic
cancer in parabiont nude mice. Neoplasia. 9:979–986. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rigothier C, Daculsi R, Lepreux S, Auguste
P, Villeneuve J, Dewitte A, Doudnikoff E, Saleem M, Bourget C,
Combe C and Ripoche J: CD154 induces matrix metalloproteinase-9
secretion in human podocytes. J Cell Biochem. 117:2737–2747. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Takeuchi S, Baghdadi M, Tsuchikawa T, Wada
H, Nakamura T, Abe H, Nakanishi S, Usui Y, Higuchi K, Takahashi M,
et al: Chemotherapy-derived inflammatory responses accelerate the
formation of immunosuppressive myeloid cells in the tissue
microenvironment of human pancreatic cancer. Cancer Res.
75:2629–2640. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C (T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Brierley JD, Gospodarowicz MK and
Wittekind C, RBrierley JD, Gospodarowicz MK and Wittekind C: TNM
Classification of Malignant Tumours. 8th ed. Oxford:
Wiley-Blackwell; 2017
|
|
25
|
Tone M, Tone Y, Fairchild PJ, Wykes M and
Waldmann H: Regulation of CD40 function by its isoforms generated
through alternative splicing. Proc Natl Acad Sci USA. 98:1751–1756.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schlesinger M: Role of platelets and
platelet receptors in cancer metastasis. J Hematol Oncol.
11:1252018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Haemmerle M, Stone RL, Menter DG,
Afshar-Kharghan V and Sood AK: The platelet lifeline to cancer:
Challenges and opportunities. Cancer Cell. 33:965–983. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gay LJ and Feldin-Habermann B:
Contribution of platelets to tumour metastasis. Nat Rev Cancer.
11:123–134. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Goubran H, Sabry W, Kotb R, Seghatchian J
and Burnouf T: Platelet microparticles and cancer: An intimate
cross-talk. Transfus Apher Sci. 53:168–172. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Blair TA and Frelinger AL: 3rd: Platelet
surface marker analysis by mass cytometry. Platelets. 31:633–640.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chatzigeorgiou A, Lyberi M, Chatzilymperis
G, Nezos A and Kamper E: CD40/CD40L signaling and its implication
in health and disease. Biofactors. 35:474–483. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bussolati B, Russo S, Deambrosis I,
Cantaluppi V, Volpe A, Ferrando U and Camussi G: Expression of
CD154 on renal cell carcinomas and effect on cell proliferation,
motility and platelet-activating factor synthesis. Int J Cancer.
100:654–661. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Grazia GA, Bastos DR and Villa LL:
CD40/CD40L expression and its prognostic value in cervical cancer.
Braz J Med Biol Res. 56:e130472023. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pantel K and Brakenhoff RH: Dissecting the
metastatic cascade. Nat Rev Cancer. 4:448–456. 2004. View Article : Google Scholar : PubMed/NCBI
|