Introduction
In recent years, cerebral neoplasms have garnered
heightened research interest in medical science, particularly from
those specializing in oncological research, owing to cerebral
neoplasms' recalcitrant nature and resistance to conventional
treatment modalities. Such neoplasms manifest within the encephalic
tissue and are categorized into primary cerebral tumours and
secondary encephalic metastases, constituting 1.4% of the totality
of malignant growths (1). Divergent
from the typical symptomatology associated with oncological
ailments, individuals afflicted with primary cerebral malignancies
frequently exhibit an absence of symptoms until the malignancy
presents with conspicuous clinical indications, attributable to the
cerebral organ's intricate architecture. Treatment efficacy, as a
result, is diminished when traditional oncological treatments are
employed. Statistical analysis divulges that in the US alone, an
excess of 15,000 mortalities annually are attributed to malignant
primary cerebral neoplasms. Of note, the incidence rate of this
serious condition increases drastically with advanced age, with the
average quinquennial survival rate hovering at ~36%, bereft of
viable curative strategies (2).
Gliomas, constituting a formidable 81% of malignancies within the
central nervous framework (3),
predominantly originate from astrocytic, oligodendrocytic or
ventricular meningeal progenitors, subsequently developed into
astrocytic tumors, ependymomas or oligodendroglial neoplasms.
Gliomas are classified into quartets of escalating
severity based on their anticipated survival outcomes: Grade I, II
III and IV glioma. Grades I and II represent the less aggressive
cohort, known as low-grade gliomas, whilst Grades III and IV
epitomize the more highly fatal high-grade gliomas. Prognostically,
low-grade gliomas confer a more auspicious outlook, with the
average decennial survival rate standing at 47%. By contrast, the
decennial survival likelihood for corticoblastic astrocytoma
reaches a remarkable 96%, a stark juxtaposition to diffuse
infiltrating glioma's more somber fate, which has a median survival
duration of 5.6 years for World Health Organization Grade II
astrocytomas. For patients with Grade III gliomas, the median
overall continuance is reduced to approximately three years.
Glioblastomas (GBM), predominant amongst Grade IV gliomas, are
encumbered with a median persistence of a scant 4.8 months, and a
quinary survival rate languishing at 7.2% (4).
This review explores the role of natural killer (NK)
cells in the brain tumour microenvironment, discusses the
advantages and prospects of NK cells compared to traditional
methods of treatment and investigates NK cell treatment strategies
for overcoming the blood-brain barrier (BBB) in the tumour
microenvironment by combining treatment with other cell treatments,
looking at the future directions in highly effective brain tumour
therapies.
Common treatments for gliomas
Gliomas present significant clinical challenges due
to their high rates of morbidity, recurrence and mortality. Current
standard treatments include surgical resection, radiation therapy
and chemotherapy, each with notable limitations. Surgical resection
remains the cornerstone of glioma treatment. However, complete
tumor removal is often unachievable due to the infiltrative growth
pattern of gliomas, leading to high recurrence rates. Furthermore,
craniotomy procedures bear a risk of causing permanent damage to
surrounding healthy brain tissue, potentially resulting in
significant functional impairments (5).
Radiation therapy delivers high-dose localized
irradiation using advanced modalities such as γ-knife, X-knife and
proton knife systems. Despite these technological advances,
treatment efficacy remains limited. Minniti et al (6) demonstrated that GBM (accounting for
50% of glioma cases) typically recur at or near the primary site
following radiotherapy. While medulloblastomas show notable
radiosensitivity, other glioma subtypes respond poorly to radiation
treatment. The combination of temozolomide with radiotherapy has
emerged as an improved therapeutic approach. Compared to
radiotherapy alone, this regimen demonstrates superior outcomes,
increasing 2-year survival rates from 10.9 to 27.2% and 5-year
survival rates from 1.9 to 9.8% (2). However, this benefit comes with
significant toxicity, including grade 3–4 hematological adverse
events in 7% of patients (7).
Additionally, whole-brain irradiation carries the risk of radiation
necrosis, which can profoundly impact neurological function.
Chemotherapeutic approaches have demonstrated even
greater limitations in glioma treatment compared to radiotherapy,
primarily due to the restrictive properties of the BBB. The BBB
consists of endothelial cells in the capillary wall, astrocyte ends
encasing the capillaries and pericytes embedded in the basement
membrane of the capillaries. The tight junction of endothelial
cells restricts the entry of most hydrophilic small molecules into
the brain region. Besides, there are a series of ATP-dependent
efflux transporter proteins on the luminal side of the BBB and the
polarised expression of efflux transporter proteins significantly
blocks the delivery of numerous therapeutic drugs, such as the
typical BBB efflux transporter proteins, breast cancer resistant
protein and P-glycoprotein (8,9).
Therefore, only a small number of drugs are approved for the
treatment of gliomas, and limited clinical data are available
(10). While the BBB serves the
crucial physiological function of protecting the central nervous
system (CNS) from neurotoxic substances, this protective mechanism
simultaneously prevents most conventional chemotherapeutic drugs
from reaching therapeutic concentrations in brain tumors (11). This fundamental limitation has
created an urgent need for alternative treatment strategies. Recent
advances in cancer immunotherapy have shown promise for both
hematological and solid malignancies (12,13).
After surgical removal of the primary tumour lesion, cytotoxic
T-lymphocytes (CTL) or NK cells may be infused in the local area.
In addition, it has been shown that NK cells may be attracted to
the tumour site (14).
A cell therapy based on an immunotherapy
strategy
NK cells are the main innate lymphocyte
subpopulation that eliminates tumours, not only recognising and
killing tumour cells that escape the T-cell response, but also
promoting the anti-tumour response of other immune cells, such as T
cells (15). NK cell-based cancer
therapies have been rapidly evolving in recent years and numerous
studies have demonstrated that NK cells have an important place in
the treatment of a wide range of cancers, which is now a major area
of innovation in immunotherapy (16–18).
In 2021, Shaim et al (19) assessed the abundance by analysing
excised glioma specimens from 21 patients with primary GBM and 2
patients with low-grade gliomas and found that the number of NK
cells entering the microenvironment of GBM was much greater in
high-grade gliomas. By analysing >1,746 NK cell samples from
tumour patients and >530 NK cell samples from peripheral blood
mononuclear cells of healthy donors, it was found that unedited NK
cells from healthy individuals were able to exert toxicity on
patient-derived GBM stem-like cells (GSCs) without affecting normal
brain cells (astrocytes). NK cells were first demonstrated to be
able to target and kill GSCs (GBM) in vitro. In addition to
this, in the treatment of gliomas in the brain, the BBB is another
major limitation to treatment, preventing the systemic delivery of
most targeted drugs to the brain tumour site, which makes the
treatment of brain tumours difficult. By contrast, NK cells can
cross the BBB and migrate into brain tumour tissue (1). This renders NK cell-based brain tumour
therapies to be of great potential and value.
For the past few years, NK cells have been thought
to play an immunosurveillance role in the brain and exert toxic
effects on abnormal cells (20,21).
First, activated T cells enter the CNS via lymphatic drainage
through the cerebellar vessels, choroid plexus epithelium and
peripheral regions of the barrier, and subsequently, antigen and
antigen-presenting cells in the brain interact with activated CD4+
T cells traversing the barrier, and this interaction generates an
inflammatory response that permits immune cells to enter the CNS
parenchyma. NK cells, represented by the immature CD16-CD56bright
subtype, are able to cross the BBB and choroid plexus into the CNS
and settle in substantia parenchyma induced by the chemokines
C-X3-C motif chemokine ligand CX3CL1, C-C motif chemokine ligand
(CCL)2 and C-X-C motif chemokine ligand (CXCL)10 (22).
The potential of NK cells in glioma
therapy
The ability of NK cells to cross the BBB to exert
toxic effects has brought them much attention in the treatment of
gliomas. GBM, a representative of highly malignant primary brain
cancers, has a median survival time of 14 months, a low overall
survival (OS) rate, limited therapeutic options and is highly
susceptible to recurrence after treatment (23). One of the reasons why GBM is
difficult to treat is due to its unique tumour microenvironment. In
the microenvironment of GBM, glioma cells upregulate the secretion
of immunosuppressive factors like programmed cell death 1 ligand
and indoleamine 2,3-dioxygenase (IDO), which limit the expression
of antigens (24,25). In addition, gliomas promote the
secretion of interleukin 10 (IL-10) and transforming growth factor
β (TGF-β) by glioma-associated macrophages, which reduces the
activity of immune cells (26,27).
More often, In the microenvironment, gliomas can mediate
immunosuppressive effects by eliminating cytotoxic T lymphocytes
around the tumour via regulatory T (Treg) cells (28,29).
Current approaches used for the treatment of GBM
include virotherapy, which uses released lysogenic viruses to
destroy glioma cells (30), and
dendritic cell (DC) vaccine therapy, which kills glioma cells by
exploiting the efficacy of DC antigen presentation and the ability
to induce activation of CTLs (31).
Blocking immune checkpoints, i.e., preventing glioma cells from
evading immune surveillance by inhibiting programmed cell death 1
(PD-1) (32,33), cytotoxic T-lymphocyte antigen 4
(34,35) and IDO (36,37).
Furthermore, one immunotherapy strategy involves activating type 1
T helper (Th1) cells via cytokines, thereby enhancing CTL-dependent
antitumor immunity. In addition, NK cells can be activated by IFN-α
and IFN-β to eliminate tumour cells via antibody-dependent
cytotoxicity (ADCC) (38–40). With chimeric antigen receptor
(CAR)-T therapy, IL-13Rα2, epidermal growth factor receptor vIII
and CD70 and other related antigens on gliomas can be recognised by
CTLs, and the recognition of gliomas by CTLs can be enhanced by
using these antigens as targets for transgenic CAR-T cells
(41–48). In addition, MMP-9 and TGF-β1
secreted by tumour-associated macrophages activated with
colony-stimulating factor (CSF)-1 and CCL2 promote glioma cell
invasion, so the spreading of glioma cells can also be slowed down
by inhibiting the secretion of MMP-9 and TGF-β1 by
tumour-associated macrophages (49–51).
However, despite the advances in efficacy of these strategies
compared to traditional surgical radiotherapy, the treatment of GBM
still has its own limitations and drawbacks. That is why a portion
of researchers have focused their attention on novel NK cell
therapies and have made surprising discoveries.
Previously, tumour-killing tests in immunodeficient
mice suggested NK cells may help destroy human tumour cells in
vivo (52). Castriconi et
al (53) isolated tumor cells
from surgical specimens obtained from nine glioblastoma (GBM)
patients exhibiting classic clinical and radiographic features.
Following in vitro expansion, these GBM cells were
characterized for neural stem cell marker expression,
differentiation potential and tumorigenic capacity in
immunodeficient mice. Notably, their study also revealed the
susceptibility of these stem-like GBM cells to lysis by both
resting and lymphokine-activated allogeneic and autologous NK cells
(53). In addition, the types of
receptor and ligand interactions recognised by NK-associated cancer
cells were measured, as well as the amount of. All GBM analysed in
this study showed susceptibility to NK-mediated cytotoxicity,
demonstrating that recipient mice can be cured by intra-tumoural
injection of IL-21 inducing a cytotoxic response involving NK cells
in a mouse model of GBM (54), and
discovered a new strategy that can be used to eliminate residual
cancer cells by activating NK cells. Although NK cells were not
detected in GBM tumour homogenates from primary tumours, the
ability of NK cells to reach tumours located in the CNS can be
judged by the overshoot of activated NK cells in NOD/SCID mice
carrying human GBM. Since GBM cells do not have human leukocyte
antigen class I molecules, NK cells do not show activated receptor
downregulation and dysfunction as they do in other tumours
(55,56). In addition, lymphokine-activated NK
cells are able to achieve the highest goal of conventional
therapies, i.e., killing cancer cells with stem cell-like
properties (57,58). Avril et al (59) similarly found that GSCs can be
killed by lectin-activated NK cells. More recently, cancer
stem-like cells (CSC) have been found to be present in GBM
specimens, and it is hypothesised that CSC are responsible for this
recurrence (60–62). GSCs are self-expanding in
vitro and can differentiate into CNS cells. The study by Avril
et al (59) confirmed the
sensitivity of GSCs to antibody-mediated cytotoxicity as well as to
cytotoxicity exerted by IL-2-activated NK cells and tumour-specific
T cells through experiments using the therapeutic antibody
cetuximab. More importantly, GSCs were more sensitive to NK- and
T-cell-mediated lysis relative to GBM cells cultured with the
corresponding serum obtained from the same initial tumour specimen.
In addition, for the first time, researchers demonstrated that GSCs
are sensitive to ADCC mediated by NK cells that use the anti-EGFR
antibody cetuximab. These results demonstrate the sensitivity of
GSCs to NK cytotoxicity and show the great potential of NK cells in
the treatment of gliomas.
In addition, Poli et al (63) identified a method of combining the
antibody mAb9.2.27 with overt NK cells to reduce GBM cell
proliferation and improve cell survival. Previously, studies have
shown that increased levels of neuron-glial antigen 2/chondroitin
sulfate proteoglycan 4 (NG2/CSPG4) proteoglycans on GBM cells and
angiogenic material are associated with more severe tumour
expansion (64,65). A strategy targeting NG2/CSPG4 in
combination with mAb9.2.27 and NK cells was found, using cytokines
released by NK cells to reverse the anti-inflammatory axis and, in
combination with mAb9.2.27, to eliminate tumours in a GBM animal
model. Expression of major histocompatibility complex (MHC) class
II molecules and ED1 (CD68) was upregulated in microglia from
animals co-treated with NK + mAb9.2.27 compared to controls,
enabling them to present GBM antigens (66). NK + mAb9.2.27 combination therapy
effectively inhibited tumour growth and was associated with
apoptosis and prolonged survival compared to controls. In addition,
combination therapy reduced ED2 scavenger receptor-positive
microglia, suggesting that a decrease in perivascular microglia may
promote the development of GBM (67). NK cell-mediated mAb9.2.27
immunotherapy significantly reduced the proportion of
CCR2-expressing macrophages, a pro-invasive subpopulation known to
drive glioblastoma (GBM) progression. (68). Furthermore, in vitro tests
demonstrated that mAb9.2.27 effectively reduced the
tumour-promoting effects of tumour-associated macrophages (TAM) and
microglia. To summarise, these results suggest that NK + mAb9.2.27
treatment may be a viable therapeutic strategy for the treatment of
NG2/CSPG4-expressing GBMs using NG2/CSPG4 as a therapeutic
target.
Mechanisms of NK cytotoxicity regulation in
the glioma microenvironment
Despite the efficacy of NK cell-mediated
cytotoxicity for glioma treatment, glioma cells have acquired their
own unique immune microenvironment over a long period of evolution.
The antitumor cytotoxicity of NK cells is governed by a dynamic
equilibrium between activating and inhibitory signals. Activating
receptors facilitate tumor cell recognition through antigen
detection, triggering cytotoxic responses, while inhibitory
receptors maintain immune tolerance by suppressing NK cell activity
(69). In the tumour
microenvironment, gliomas inhibit NK cell activity by inducing
upregulation of inhibitory receptors on NK cells and downregulation
of activating receptors, as well as by releasing tumour-associated
factors. Activating receptors typically bind to linker proteins
containing immunoreceptor tyrosine-activating motifs, such as
FcεRIγ, CD3ζ and DAP12, in mutant cells. Inhibitory receptors
recognise inhibitory motifs (ITIMs) with immunoreceptor tyrosines
on normal cells (70). ITIM is a
conserved amino acid sequence located in the cytoplasmic domains of
certain inhibitory receptors in immune cells. It transduces
inhibitory signals to suppress immune activation, such as blocking
B cell, T cell and NK cell responses. Binding of the activating
receptor leads to a phosphorylation response in NK cells and the
inhibitory receptor leads to a dephosphorylation response in NK
cells. At the molecular level, these counteracting receptor signals
are integrated through phosphorylation-dephosphorylation dynamics,
which bidirectionally regulate NK cell cytotoxic function by
modulating its activation-inhibition balance. The main receptors
that activate NK cells include natural cytotoxicity receptor (NCR),
CD16 and natural killer group 2D (NKG2D). The NCR family includes
three members of type I membrane-penetrating receptors known as
NKp46, NKp44 and NKp30, which are encoded by the respective NCR
genes, and play important roles in the activation of NK cells
(71). CD16, encoded by the Fc
gamma receptor IIIa gene, is a receptor for the Fc segment of
immunoglobulin G (IgG), which assists NK cells in recognising
IgG-bound cells, thus exerting ADCC (72). NKG2D, encoded by the killer cell
lectin-like receptor K1 gene, is required to bind to the junction
protein DNAX-activating protein of 10 kDa on the surface of NK
cells to stabilise the receptor complex, and its ligand is
expressed on a variety of tumour cells (73). When NK cells are stimulated and
activated by the corresponding ligands of NKG2D, ADCC,
degranulation and cytokine production of NK cells are promoted
(74).
The main inhibitory receptors include PD-1, T-cell
Ig and mucin-domain containing-3 (TIM3), ITIM structural domain
protein (TIGIT) and CD96. PD-1 is considered to be a marker of
T-cell depletion, and it can also be expressed in NK cells
(75). Hepatocellular carcinoma
cells can induce high expression of PD-1 in tumour-infiltrating NK
cells by secreting the exosome circular RNA ubiquitin-like with PHD
and ring finger domains 1, thereby suppressing their anti-tumour
immune function (76). TIM3 is also
a marker of NK cell depletion. Compared to TIM3-PD-1-NK cells,
TIM3+PD-1+ NK cells within a variety of tumours are defective in
their ability to secrete IFN-γ and granzyme B and have reduced
cytotoxicity (77). TIGIT is a
typical inhibitory receptor and T-cell Igs are as well (78), with a ligand of CD155, which has
been found to be highly expressed in a variety of tumours. CD155 is
expressed at low levels in normal tissues but is highly expressed
in numerous tumour cells, as well as primary tumour tissues. It is
associated with tumour cell proliferation and migration (Fig. 1). In addition, CD155 expression is
upregulated in tumour-associated antigen-presenting cells, which
also regulate the immune response of NK cells (79).
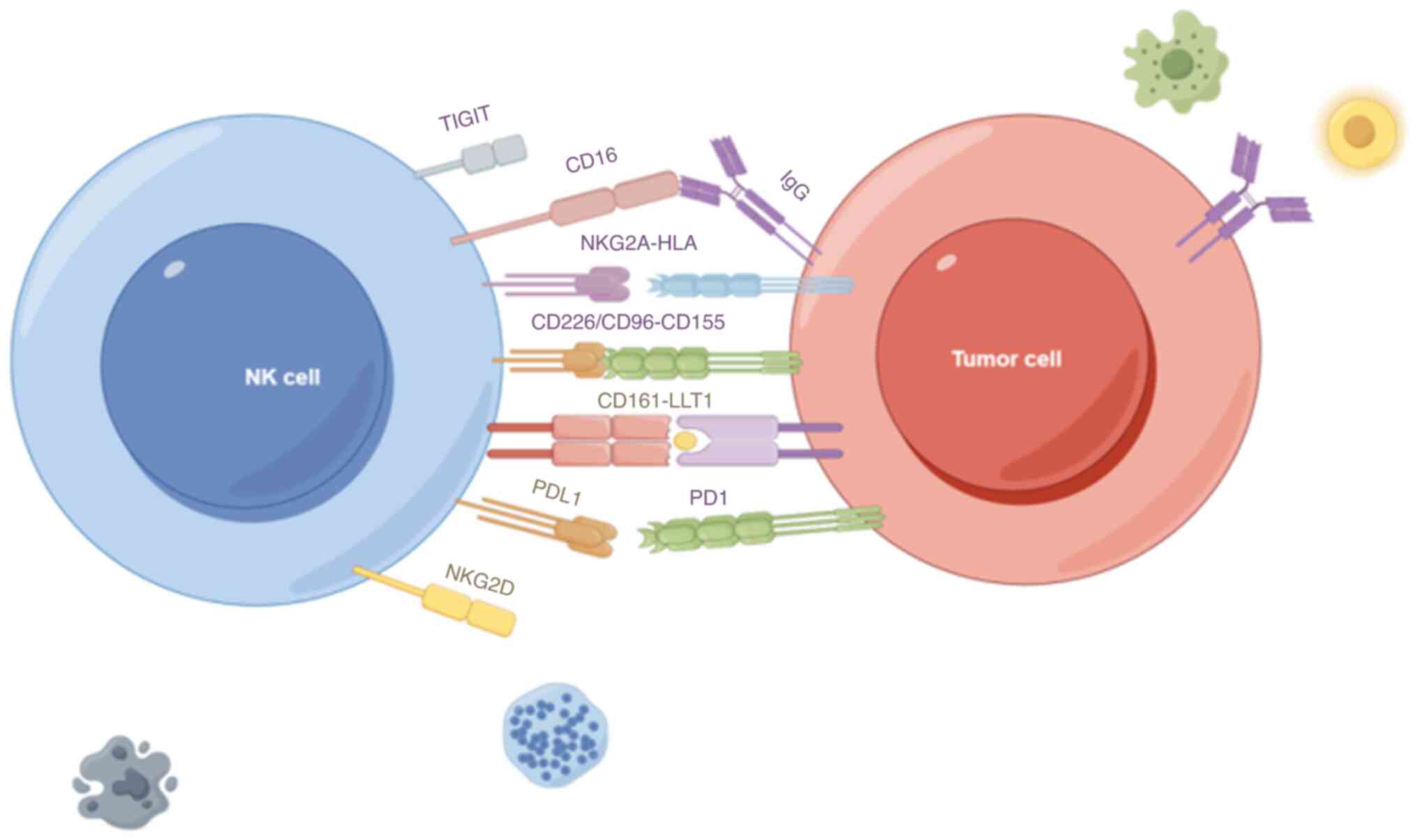 | Figure 1.Receptor interactions between NK
cells and tumour cells. Natural cytotoxic receptors on NK cells,
such as NCR, CD16 and NKG2D, stimulate NK activation by binding to
the corresponding ligands on tumours. CD16, encoded by the FCGR3A
gene, interacts with IgG to help NK cells recognise IgG-bound
cells, thus exerting antibody-dependent cell-mediated cytotoxicity.
NKG2D, encoded by the KLRK1 gene, binds to the NK cell surface
connexin DAP10 to stabilise the receptor complex. The suppressive
receptors NKG2A, PDL1 and CD155 bind to the corresponding ligands
to initiate NK cell inhibition. TIGIT, T-cell immunoreceptor with
Ig and ITIM domains; CD16, cluster of differentiation 16;
NKG2A-HLA, natural killer group 2A-human leukocyte antigen;
CD226/CD96-CD155, cluster of differentiation 226/cluster of
differentiation 96-cluster of differentiation 155; NKG2D, natural
killer group 2D; CD161-LLT1, cluster of differentiation
161-lectin-like transcript 1; PDL1, programmed death ligand 1; PD1,
programmed cell death protein 1; IgG, immunoglobulin G; NK, natural
killer. |
In addition to the regulation of receptor
signalling, the regulatory cytokines IL-2, IL-15 and type I IFN
activate NK cells by enhancing signalling downstream of the
activated receptor, while IL-21 stimulates NK cell differentiation
and IFNγ secretion. By contrast, IL-10 and TGF-β exert inhibitory
effects on NK cells (80,81). In addition, chemokines, including
CXCR3, CXCR4, CCR2-CCR5 and CX3CR1, induce NK cells to be recruited
into tissues (82). Cytokines and
growth factors such as IFNγ, TNF, granulocyte-macrophage-CSF and
CCL5, can enhance cytotoxic effects, and they can also coordinate
anti-tumour immune responses by recruiting immune cells, such as
DCs, to achieve a joint anti-tumour effect of innate and adaptive
immune responses (83).
In the glioma microenvironment, TAM in microglia or
circulating monocytes gained an anti-inflammatory and pro-tumour
M2-like phenotype under the influence of GBM-secreted factors.
These TAMs secrete immunosuppressive cytokines such as TGF-β and
IL-10, which inhibit effector immune cells and recruit regulatory T
cells (Tregs) (84,85). Furthermore, widespread PDL1
expression in GBM further dampens antitumor immunity (86,87).
The extracellular nucleosidase CD39 acts on Treg and TAM cells and
CD73 on glioma cells, and adenosine produced by extracellular ATP
produces a wide range of immunosuppressive effects, such as via the
A2A adenosine receptor, which inhibits NK cells. Cholesterol is
converted to 25-hydroxycholesterol with the help of cholesterol
25-hydroxylase and inhibits NK-cell metabolism through inhibition
of critical transcription factor sterol regulatory element binding
protein (88). In addition, oxygen
and nutrient deprivation reduces NK cell activity, and
prostaglandin E2 restricts DC recruitment by NK cells, thereby
inhibiting the joint DC-NK cell antitumour effect, whereas lactic
acid impedes NK cell activity by decreasing perforin and granzyme B
expression (89,90). In IDH-mutant gliomas, R-enantiomer
of 2-hydroxyglutarate-mediated epigenetic reprogramming suppresses
CXCL9 and CXCL10 expression, impairing the chemotaxis of
tumor-infiltrating lymphocytes, including NK cells (91,92).
CAR-NK in tumour therapy
In order to increase the ability of NK cells to kill
tumours without being inhibited by the tumour microenvironment, a
new type of NK cell, CAR-NK, was created. CAR-NK is a genetically
engineered NK cell that incorporates a chimeric antibody, CAR,
which recognises tumour cells and at the same time activates the NK
cell to kill the tumour cell. The CAR includes extracellular
recognition domains used to recognise tumour-specific antigens, as
well as a transmembrane structural domain and an intracellular
signalling structural domain, enabling NK cells to precisely and
efficiently kill tumour cells in vivo. The CAR approach uses
NK cells derived from patients with transduction of the CAR gene to
generate CAR, and after expanding CAR-NK cells in vitro,
these cells are injected back into the tumour patient. So far,
CAR-NK has shown good efficacy in the treatment of various
tumours.
In 2018, Tang et al (93) found that CD33-CAR-NK-92 cells showed
stronger cytotoxicity than CAR-T cells against CD33-positive
leukaemia cells. On the other hand, CD33-CAR-NK cells are more
effective than NK-92 cells in treating HL-60 and are safe in
patients with relapsed and refractory acute myeloid leukemia
(93). CD19-CAR-NK-92 cells
significantly suppressed tumor cell proliferation in a B-cell
lymphoma (BCL) model using NOD-SCID IL-2Rγ-deficient mice,
demonstrating the feasibility of generating CAR-engineered NK-92
cells with potent tumor-targeting capabilities (94). Furthermore, CD4-CAR-NK cells are
also selectively toxic to a wide range of CD4+ human T-cell
leukaemia and lymphoma cells (95).
Of note, with the rapid progress of research in
non-solid tumours, CAR-NK has also shown great potential in solid
tumours. Liu et al (96)
showed that EGFR-specific CAR-NK-92 cells and NK cells in
vitro inoculated with mammary carcinoma cell lines MDA-MB-231
and MDA-MB-468 showed strong cytotoxicity, with epidermal growth
factor receptor-specific CAR-NK-92 having better therapeutic
efficacy compared to parental NK cells. Furthermore, EGFR-CAR-NK-92
cells or oncolytic herpes simplex virus 1 were effective in
stopping breast tumour progression (96). Furthermore, Ng et al
(97) found that
mesothelin-positive ovarian cancer cells could be eliminated by
mesothelin-specific CAR-NK cells, whereas they did not respond to
mesothelin-negative cells. Notably, redirected NK cells were highly
potent against ovarian cancer cells in either subcutaneous or
peritoneal tumour models, demonstrating that in addition to folate
receptor α, mesothelin may be a viable target for targeted therapy
in ovarian cancer. In the same year, this was also confirmed in a
study by Ueda et al (97,98).
In a preclinical study, CAR-KHYG-1 NK cells were
used to target c-Met, folate receptor α and AXL receptors, all of
which are highly expressed in GBM cells. It has been shown that
anti-EGFR/EGFR vIII and anti-human EGFR2 (HER2) CAR-NK cells are
highly efficient against GBM and are effective in controlling tumor
growth in animal models (1). In
2023, by experimenting with diffuse intrinsic pontine glioma (DIPG)
cells and primitive pontine neural progenitor cells derived from
five patients, Zuo et al (99) conducted the first comprehensive
evaluation of GD2-CAR NK-92 cells for the treatment of DIPG, and
the anti-tumour capacity and safety of this therapeutic strategy
were confirmed. The results indicated that this novel treatment had
significant anti-tumor effects and showed no significant side
effects. Furthermore, tumor killing tests showed that GD2-CAR NK-92
cells were effective in killing high GD2-expressing DIPG cells with
less activity against low GD2-expressing DIPG cells. In animal
experiments, GD2-CAR NK-92 cells successfully inhibited tumour
growth in GD2-overexpressing DIPG xenograft mice, which survived
longer compared to controls. Nevertheless, the efficacy of this
strategy in low GD2-overexpressing DIPG tumours was not
significant. In another report, ErbB2/HER2-specific NK cells were
targeted against GBM. Following intratumoral administration of
2×106 ErbB2-specific NK-92/5.28 cells administered
weekly for 11 consecutive weeks, significant suppression of tumor
growth was observed (100). Of
note, while CAR-NK therapy has shown promising results in
preclinical studies, there are currently no large-scale clinical
trials reporting its effectiveness in treating brain tumors.
Clinical advances in NK cell therapy for
gliomas
Clinical trials investigating NK cell therapy for
gliomas remain limited, with scarce reported outcomes. As early as
2004, the clinical trial ‘Autologous NK Cell Therapy for Recurrent
Malignant Gliomas in Humans’ demonstrated that the administration
of autologous NK cells to patients with recurrent malignant gliomas
has proven to be safe and partially effective: By intravenous
administration of autologous NK cells and low-dose IFN-β, 3/9
patients had a partial response and 2/9 patients had a mild
response to treatment. No serious neurotoxicity was observed in any
patient (101).
In 2020, a Phase I clinical trial (NCT02271711) on
‘Expanded NK Cell Infusion in Treating Younger Patients With
Recurrent/Refractory Brain Tumors’ (NCT02271711) was completed at
the M.D. Anderson Cancer Center. Patients in the trial received
intravenous injections of autologous expanded NK cells over 3 min
once a week for several weeks. In the absence of disease
progression or unacceptable toxicity, treatment was repeated every
4 weeks for up to 3 courses. Patients were followed up within 30
days after completion of the study treatment. The results showed no
dose-limiting toxicity after 112 intracerebroventricular infusions
of NK cells; 8 patients progressed and 1 stabilized (102).
In the same year, a Phase II trial, the ‘STIR Trial:
Haploidentical Transplant and Donor NK cells for Solid Tumors’ was
completed. Results showed that patients tolerated NK infusion well
and had no cytokine release syndrome (CRS). The median follow-up
was 1.3 years, with 1- and 2-year OS of 64 and 40%, respectively,
for the entire cohort. The disease control rate at 6 months was 72%
(103).
In 2021, a phase I/IIa clinical trial on ‘Autologous
Adoptive Immune-cell Therapy Elicited a Durable Response With
Enhanced Immune Reaction Signatures in Patients with Recurrent GBM’
was completed (KCTOO03815). Autologous permissive immune cell
therapy elicited durable responses and enhanced immune response
characteristics in patients with recurrent GBM and no serious
adverse events were observed in the trial. The median OS was 22.5
months and median progression-free survival was 10 months. A total
of five patients survived >2 years and demonstrated durable
responses with enhanced transcriptomic profiles of immune responses
and no clinical decline (104).
In 2023, to assess the safety and feasibility of
cerebrospinal fluid infusion of activated NK cells in recurrent
gliomas to patients with drug-resistant brain tumours, a clinical
phase I trial was performed with a mean follow-up of 8.08 months
for recurrent brain tumours, GBM among them (105). Patients underwent several
injections of activated NK cells. The first dose was administered
two weeks after surgery and the other injections were frozen cells
activated after thawing. Depending on the size of the tumor,
2×106 to 100×106 NK cells were injection
straight into the tumour site. NK cells were embedded after
surgical removal of the tumour or direct injection of NK cells was
performed via vein/subcutaneous injection. At two weeks following
the completion of chemotherapy for patients with inoperable diffuse
tumours, 49.3–60×106 NK cells were injected into the
cerebrospinal fluid. Locally administered NK cells for malignant
brain tumours were ultimately shown to be safe and feasible, with
tolerance rising with increasing dose.
A phase II clinical trial of ‘Injection of Active
Allogeneic NK Cells in Patients With Gliomas’ at the Royan
Institute in Tehran, Iran is currently recruiting (NCT06687681).
The research team has conducted a Phase I clinical trial in glioma
patients, the results of which have not been disclosed.
Additionally, a Phase I clinical study on ‘Engineered NK Cells
Containing Deleted TGF-βR2 and NR3C1 for the Treatment of Recurrent
GBM’ (NCT04991870) is currently recruiting at the M.D. Anderson
Cancer Center (Respondent Party), Texas, US (Table I).
 | Table I.Clinical trials of NK cell therapy
for glioma. |
Table I.
Clinical trials of NK cell therapy
for glioma.
| NCTID | Trial
description | Year | Phase | Therapeutic
approach | Outcome | Notes | (Refs.) |
|---|
| – | Autologous NK cell
therapy for recurrent malignant gliomas in humans | 2004 | Phase I | Intravenous
infusion of autologous NK cells + low-dose interferon β | Partial response in
3/9 patients; mild response in 2/9 patients; no serious
neurotoxicity observed | – | (100) |
| NCT02271711 | Expanded NK cell
infusion in treating younger patients with recurrent/refractory
brain tumors | 2020 | Phase I | Intravenous
injection of expanded autologous NK cells, once weekly for several
weeks | 8 patients
progressed; 1 patient stabilized; no dose-limiting toxicity | – | (101) |
| – | STIR Trial:
Haploidentical transplant and donor NK cells for solid tumors | 2020 | Phase II | Infusion of
haploidentical donor NK cells | Median follow-up:
1.3 years; 1-year OS: 64%; 2-year OS: 40%; 6-month disease control
rate: 72% | No cytokine release
syndrome; well-tolerated by patients | (102) |
| KCT0003815 | Autologous adoptive
immune-cell therapy elicited a durable response with enhanced
immune reaction signatures in patients with recurrent GBM | 2021 | Phase I/IIa | Autologous
permissive immune cell therapy | Median OS: 22.5
months; median progression-free survival: 10 months; 5 patients
survived >2 years | No serious adverse
events; enhanced immune response signatures observed | (103) |
| – | Intra-lesion
injection of activated NK cells in recurrent malignant brain
tumors | 2023 | Phase I | Local
administration of activated NK cells, some via cerebrospinal
fluid | Safe and feasible;
tolerance increased with higher doses | – | (104) |
| NCT06687681 | Injection of active
allogeneic NK cells in patients with gliomas | Ongoing | Phase II | Infusion of active
allogeneic NK cells | – | Recruiting | – |
| NCT04991870 | Engineered NK cells
containing deleted TGF-βR2 and NR3C1 for the treatment of recurrent
GBM | Ongoing | Phase I | Engineered NK cells
with deleted TGF-βR2 and NR3C1 | – | Recruiting | – |
| NCT01588769 | A phase I study to
investigate the tolerability and efficacy of ALECSAT administered
to GBM multiforme patients (ALECSAT-GBM) | Completed | Phase I | ALECSAT
therapy | – | Results not
disclosed | – |
| NCT051080 | NK cell therapy for
recurrent GBM multiforme patients | Unknown | Phase I | NK cell
therapy | – | – | – |
| NCT04254419 | Intra-tumoral
injection of NK cells in high-grade gliomas (NK HGG) | Not yet
started | Phase I | Intra-tumoral
injection of NK cells | – | – | – |
| NCT01525459 | Gene expression,
immunological status and metabolome in glioma patients | Ongoing | Observational | Analysis of gene
expression, immunological status and metabolome in glioma
patients | – | – | – |
| NCT04489420 | NK cell (CYNK-001)
IV infusion or IT administration in adults with recurrent GBM | Terminated | Phase I | Intravenous or IT
administration of CYNK-001 NK cells | – | – | – |
Anti-tumor therapy with combination of
multiple immunotherapies
In addition to directly modifying NK cells through
genetic engineering, there is another approach to attenuate the
inhibitory effects of the glioma microenvironment on NK cells
through the adjuvant effects of NKT cells or drugs. NKT cells have
been shown to exert anti-tumor effects in vivo. Yamada et
al (106) found that invariant
NKT cells differentiated from induced pluripotent stem cells (iPSC)
were able to effectively activate autologous NK cells and, when
activated by ligand-pulsed DCs, were able to produce large amounts
of IFN-γ, which resulted in more effective elimination of tumors.
Furthermore, in 2024, Peng et al (107) invented a glioma immunotherapy that
enhances the tumor-killing activity of T cells and NK cells. The
study reports a nanocomposite consisting of a phospho-dendrimer
macromolecule (AK128) combined with an antibody to PD-1 (aPD1).
Since α4 and β1 integrins highly expressed on M1-type macrophage
membranes (M1m) can bind to vascular endothelial adhesion
molecule-1 on the surface of endothelial cells, the nanocomplexes
could effectively pass the BBB after being encapsulated by M1m.
Furthermore, the nanocomplexes modified by M1m had a lower monocyte
clearance and longer blood circulation time. The results showed
that after successfully crossing the BBB, AK128 in the complex
promotes rapid proliferation and activation of NK cells, whereas
the aPD-1 effectively reduced the inhibitory impact of the glioma
microenvironment on both NK cells and T cells (107).
Advantages and prospects of NK cell therapy
in glioma treatment
CAR-T as a cell therapy has been shown to play a
role in alleviating hematologic tumors and clinical studies are
currently underway for gliomas (47,108).
However, this treatment has a non-negligible drawback: The
heterogeneity of natural immune host defenses. Since T cells mainly
stimulate cytokines, including IL-1/2/6/8/10/15 and TNF-α, they are
prone to neurotoxicity and CRS even if the raw material is derived
from themselves (109). Therefore,
a homologous and safe therapeutic modality, such as NK cell
therapy, is urgently needed.
NK cells are not prone to eliciting an overactive
autoimmune response due to a lack of MHC. The feasibility and
safety of autologous NK cells for over-the-counter cell therapy of
recurrent gliomas in humans was also confirmed as early as 2004
(101). In addition, the ability
of NK cells to cross the BBB gives them a unique advantage in the
treatment of gliomas. However, NK cells are dependent on external
ILs for proliferation and activation, and they are unable to
produce IL-2 on their own as T cells do, making sustained delivery
of IL to brain tumor sites challenging in clinical therapy. This
makes it difficult for NK cells to have durability in the fight
against gliomas and makes them more prone to depletion. Therefore,
genetic engineering is needed to solve this problem.
Despite the paucity of research data on CAR-NK for
glioma treatment, the advantages of CAR-NK make it highly promising
for the treatment of glioma. First, modification of NK cells
through gene editing techniques such as CRISPR/Cas9 or through the
introduction of specific receptors (e.g. CARs) can improve their
recognition and cytotoxicity against specific tumour markers,
allowing NK cells to rapidly recognise antigens on tumour cells,
maintain a higher proliferative capacity and in vivo
persistence. It can also recognise and kill tumour cells through
natural receptors for NK cells that are independent of CAR
engineering, and are less likely to escape disease by
downregulating CAR antigens. It enables improved tumour
infiltration and is able to overcome the drug-resistant tumour
microenvironment (110). In 2024,
Shanley et al (111)
increased the killing effect and persistence of NK cells against
gliomas by engineering NK cells to express IL-21 via overexpression
of CCAAT/enhancer-binding proteins and CCAAT/enhancer-binding
protein delta in particular. The results showed that IL-21 NK cells
promoted the expression of Ki67, CD25, perforin, TNF-α and granzyme
B, achieved sufficient tumor infiltration and were able to
effectively alleviate or eliminate gliomas in vivo, as well
as maintained a potent killing ability after multiple consecutive
attacks on gliomas. In addition, the engineered NK cells enhanced
metabolic adaptation by generating ATP through the oxidative
phosphorylation pathway to counteract the metabolic inhibition of
NK cells in the glioma microenvironment (111).
Secondly, autologous immune cells from cancer
patients usually have low activity and cytotoxicity effects as well
as limited cell expansion capacity, which can be well solved by
allogeneic transfusion. CAR-NK is able to use an unlimited source
of allogeneic NKs without the fear of graft vs. host disease (GVDH)
compared to CAR-T cells. Meanwhile, the allogeneic environment
counteracts the limitation of NK cells by self MHC-I molecules,
allowing NK cells to achieve a more potent killing effect on
tumors. Clinical trials of allogeneic CAR-NK were first completed
in 2024 (NCT03056339) (112).
There were no findings of any cytokine release syndrome,
neurotoxicity or GVHD during the treatment of 37 patients with
CD19+ B-cell malignancies. The objective response rate at day 100
was 48.6% and the 1-year OS rate was 68%. Thus, the safety of
CAR-NK allogeneic transfusion was confirmed. It also demonstrates
that CAR-NK can be generated ‘off-the-shelf’ from NK cell lines or
iPSC-NK with a relatively short production time (113). Several preclinical studies have
demonstrated that iPSC-derived CAR-NK has better anti-tumor
capabilities compared to autologous CAR-NK cells (114–116).
Of note, NK cells have the ability to acquire
adaptive immunity and become memory-like NK cells in a tumor
environment or in the presence of pro-inflammatory cytokine
stimulation (117). This means
that the memory-like NK cells left behind after NK therapy
eliminate the tumour and create a long-term immune environment
against the specific tumour, enabling them to respond rapidly to
escaped cancer cells as well as those that enter dormancy that
cannot be killed by chemotherapy. The risk of tumour recurrence is
markedly reduced and the prognosis for patient survival is
improved. Several studies have shown memory-like NK cells that can
be induced by cytokines, such as IL-12, IL-15 and IL-18 (118).
Finally, in addition to the advantages of CAR-NK
itself, a large number of studies have shown that CAR-NK is able to
interact with other immune-immune cells to achieve better
therapeutic outcomes. For instance, Parihar et al (119) found that CAR-NK cells may be able
to be used in combination with T-cell-based therapies for solid
tumours. Furthermore, it has been shown that NK cells can
coordinate anti-tumour immune responses by recruiting DCs, thereby
facilitating the coupling of innate and adaptive immune responses
(120).
In clinical practice, the treatment of NK cells
delivered to the circulating medullary fluid by intraventricular
catheter and subcutaneous routes after surgical removal of gliomas
has yielded good results (105).
Besides, several clinical studies on NK-cell therapy for glioma
have demonstrated the safety of NK-cell therapy. Although clinical
studies using NK cells for glioma treatment are currently limited,
these advantages that NK autogenously possesses, as well as the
safety shown in the clinic and the potential demonstrated in
preclinical studies of CAR-NK, portend that the use of modified
CAR-NK in combination with other therapies after surgical resection
may be a major breakthrough in the treatment of malignant
gliomas.
However, although current clinical results show
improvement in clinical symptoms in patients treated with cell
therapy via NK cells, the very limited number of successfully
enrolled clinical studies means that there are still unknown
challenges in the actual treatment. A number of conditions may
affect the treatment of patients with GBM with NK cells: First, the
timing and site of NK-cell infusion have not been clarified. In
current research and clinical trials on GBM, the timing of infusion
varies, with the most efficacious infusion site and optimal
infusion regimen yet to be discovered. Second, cell counts and
infusion intervals can also affect patient outcomes. Finally, tumor
residue or residual tumor mass after surgery or tumor regrowth
after radiation/chemotherapy may reduce the effectiveness of NK
therapy. How to combine NK cells with radiotherapy, antibody drugs
and immune pathways represented by T cells/NKT cells/DC cells after
surgical resection, as well as exploring optimal combination
therapies, is a critical step in the future of NK immunotherapy for
gliomas.
Acknowledgements
The figures were generated using Figdraw (www.figdraw.com).
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
ZX, YL and CS structured the article, compiled the
review, drafted the manuscript, and reviewed and edited the
manuscript. QQ, XW and NW contributed to the conception of the
article. All authors provided contributions to the article and have
read and approved the final version submitted. Data authentication
is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Fares J, Davis ZB, Rechberger JS, Toll SA,
Schwartz JD, Daniels DJ, Miller JS and Khatua S: Advances in NK
cell therapy for brain tumors. NPJ Precis Oncol. 7:172023.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schaff LR and Mellinghoff IK: Glioblastoma
and other primary brain malignancies in adults: A review. JAMA.
329:574–587. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xu S, Tang L, Li X, Fan F and Liu Z:
Immunotherapy for glioma: Current management and future
application. Cancer Lett. 476:1–12. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ohgaki H and Kleihues P: Population-based
studies on incidence, survival rates, and genetic alterations in
astrocytic and oligodendroglial gliomas. J Neuropathol Exp Neurol.
64:479–489. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang Z, Zhao C, Zong S, Piao J, Zhao Y and
Chen X: A review on surgical treatment options in gliomas. Front
Oncol. 13:10884842023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Minniti G, Niyazi M, Alongi F, Navarria P
and Belka C: Current status and recent advances in reirradiation of
glioblastoma. Radiat Oncol. 16:362021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jatyan R, Sahel DK, Singh P, Sakhuja R,
Mittal A and Chitkara D: Temozolomide-fatty acid conjugates for
glioblastoma multiforme: In vitro and in vivo evaluation. J Control
Release. 359:161–174. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Conti A, Geffroy F, Kamimura HAS, Novell
A, Tournier N, Mériaux S and Larrat B: Regulation of P-glycoprotein
and breast cancer resistance protein expression induced by focused
ultrasound-mediated blood-brain barrier disruption: A pilot study.
Int J Mol Sci. 23:154882022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Luo H and Shusta EV: Blood-brain barrier
modulation to improve glioma drug delivery. Pharmaceutics.
12:10852020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Oberoi RK, Parrish KE, Sio TT, Mittapalli
RK, Elmquist WF and Sarkaria JN: Strategies to improve delivery of
anticancer drugs across the blood-brain barrier to treat
glioblastoma. Neuro Oncol. 18:27–36. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yamanaka R: Novel immunotherapeutic
approaches to glioma. Curr Opin Mol Ther. 8:46–51. 2006.PubMed/NCBI
|
|
12
|
Guha P, Heatherton KR, O'Connell KP,
Alexander IS and Katz SC: Assessing the future of solid tumor
immunotherapy. Biomedicines. 10:6552022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang W, Liu Y, He Z, Li L, Liu S, Jiang M,
Zhao B, Deng M, Wang W, Mi X, et al: Breakthrough of solid tumor
treatment: CAR-NK immunotherapy. Cell Death Discov. 10:402024.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ran GH, Lin YQ, Tian L, Zhang T, Yan DM,
Yu JH and Deng YC: Natural killer cell homing and trafficking in
tissues and tumors: from biology to application. Signal Transduct
Target Ther. 7:2052022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang H, Li J, Dong X, Zhou X, Zhao L, Wang
X, Rashu R, Zhao W and Yang X: NK cells contribute to protective
memory T cell mediated immunity to Chlamydia muridarum infection.
Front Cell Infect Microbiol. 10:2962020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang Q, Zhang H, Ding J, Liu H, Li H, Li
H, Lu M, Miao Y, Li L and Zheng J: Combination therapy with
EpCAM-CAR-NK-92 cells and regorafenib against human colorectal
cancer models. J Immunol Res. 2018:42635202018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Page A, Chuvin N, Valladeau-Guilemond J
and Depil S: Development of NK cell-based cancer immunotherapies
through receptor engineering. Cell Mol Immunol. 21:315–331. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Alshahrani MY, Uthirapathy S, Kumar A,
Oghenemaro EF, R R, Lal M, Arora I, Chauhan AS, Saud MJ and Hulail
HM: NK cell-based cancer immunotherapies: Current progress,
challenges and emerging opportunities. J Biochem Mol Toxicol.
38:e700442024. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shaim H, Shanley M, Basar R, Daher M,
Gumin J, Zamler DB, Uprety N, Wang F, Huang Y, Gabrusiewicz K, et
al: Targeting the αv integrin/TGF-β axis improves natural killer
cell function against glioblastoma stem cells. J Clin Invest.
131:e1421162021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Poli A, Kmiecik J, Domingues O, Hentges F,
Bléry M, Chekenya M, Boucraut J and Zimmer J: NK cells in central
nervous system disorders. J Immunol. 190:5355–5362. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Brown NF, Carter TJ, Ottaviani D and
Mulholland P: Harnessing the immune system in glioblastoma. Br J
Cancer. 119:1171–1181. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sedgwick AJ, Ghazanfari N, Constantinescu
P, Mantamadiotis T and Barrow AD: The role of NK cells and innate
lymphoid cells in brain cancer. Front Immunol. 11:15492020.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Miller CR and Perry A: Glioblastoma. Arch
Pathol Lab Med. 131:397–406. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wainwright DA, Balyasnikova IV, Chang AL,
Ahmed AU, Moon KS, Auffinger B, Tobias AL, Han Y and Lesniak MS:
IDO expression in brain tumors increases the recruitment of
regulatory T cells and negatively impacts survival. Clin Cancer
Res. 18:6110–6121. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bloch O, Crane CA, Kaur R, Safaee M,
Rutkowski MJ and Parsa AT: Gliomas promote immunosuppression
through induction of B7-H1 expression in tumor-associated
macrophages. Clin Cancer Res. 19:3165–3175. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Khan F, Lin Y, Ali H, Pang L, Dunterman M,
Hsu WH, Frenis K, Grant Rowe R, Wainwright DA, McCortney K, et al:
Lactate dehydrogenase A regulates tumor-macrophage symbiosis to
promote glioblastoma progression. Nat Commun. 15:19872024.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang Y, Liu T, Yang N, Xu S, Li X and Wang
D: Hypoxia and macrophages promote glioblastoma invasion by the
CCL4-CCR5 axis. Oncol Rep. 36:3522–3528. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chauhan P, Hu S, Sheng WS and Lokensgard
JR: Regulatory T-cells suppress cytotoxic T lymphocyte responses
against microglia. Cells. 11:28262022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
van Hooren L, Handgraaf SM, Kloosterman
DJ, Karimi E, van Mil LWHG, Gassama AA, Solsona BG, de Groot MHP,
Brandsma D, Quail DF, et al: CD103+ regulatory T cells
underlie resistance to radio-immunotherapy and impair
CD8+ T cell activation in glioblastoma. Nat Cancer.
4:665–681. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lawler SE, Speranza MC, Cho CF and Chiocca
EA: Oncolytic viruses in cancer treatment: A review. JAMA Oncol.
3:841–849. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Reardon DA and Mitchell DA: The
development of dendritic cell vaccine-based immunotherapies for
glioblastoma. Semin Immunopathol. 39:225–239. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jiacheng D, Jiayue C, Ying G, Shaohua W,
Wenhui L and Xinyu H: Research progress and challenges of the
PD-1/PD-L1 axis in gliomas. Cell Biosci. 14:1232024. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhu H, You Y, Shen Z and Shi L:
EGFRvIII-CAR-T cells with PD-1 knockout have improved anti-glioma
activity. Pathol Oncol Res. 26:2135–2141. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu F, Huang J, Liu X, Cheng Q, Luo C and
Liu Z: CTLA-4 correlates with immune and clinical characteristics
of glioma. Cancer Cell Int. 20:72020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim KS, Habashy K, Gould A, Zhao J, Najem
H, Amidei C, Saganty R, Arrieta VA, Dmello C, Chen L, et al:
Fc-enhanced anti-CTLA-4, anti-PD-1, doxorubicin, and
ultrasound-mediated blood-brain barrier opening: A novel
combinatorial immunotherapy regimen for gliomas. Neuro Oncol.
26:2044–2060. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Du L, Xing Z, Tao B, Li T, Yang D, Li W,
Zheng Y, Kuang C and Yang Q: Both IDO1 and TDO contribute to the
malignancy of gliomas via the Kyn-AhR-AQP4 signaling pathway.
Signal Transduct Target Ther. 5:102020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xing Z, Li X, He ZNT, Fang X, Liang H,
Kuang C, Li A and Yang Q: IDO1 inhibitor RY103 suppresses
Trp-GCN2-mediated angiogenesis and counters immunosuppression in
glioblastoma. Pharmaceutics. 16:8702024. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Arora J, Ayyappan S, Yin C, Smith BJ,
Lemke-Miltner CD, Wang Z, Farooq U and Weiner GJ: T-cell help in
the tumor microenvironment enhances rituximab-mediated NK-cell
ADCC. Blood. 143:1816–1824. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hofman T, Ng SW, Garcés-Lázaro I, Heigwer
F, Boutros M and Cerwenka A: IFNγ mediates the resistance of tumor
cells to distinct NK cell subsets. J Immunother Cancer.
12:e0094102024. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kurebayashi Y, Olkowski CP, Lane KC,
Vasalatiy OV, Xu BC, Okada R, Furusawa A, Choyke PL, Kobayashi H
and Sato N: Rapid depletion of intratumoral regulatory T cells
induces synchronized CD8 T- and NK-cell activation and
IFNγ-dependent tumor vessel regression. Cancer Res. 81:3092–3104.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang P, Li C, Wang Y, Chi X, Sun T, Zhang
Q, Zhang Y and Ji N: Expression features of targets for anti-glioma
CAR-T cell immunotherapy. J Neurooncol. 171:179–189. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Antonucci L, Canciani G, Mastronuzzi A,
Carai A, Del Baldo G and Del Bufalo F: CAR-T therapy for pediatric
high-grade gliomas: Peculiarities, current investigations and
future strategies. Front Immunol. 13:8671542022. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Santiago-Vicente Y, de Jesús
Castillejos-López M, Carmona-Aparicio L, Coballase-Urrutia E,
Velasco-Hidalgo L, Niembro-Zúñiga AM, Zapata-Tarrés M and
Torres-Espíndola LM: Immunotherapy for pediatric gliomas: CAR-T
cells against B7H3: A review of the literature. CNS Neurol Disord
Drug Targets. 23:420–430. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhu G, Zhang Q, Zhang J and Liu F:
Targeting tumor-associated antigen: A promising CAR-T therapeutic
strategy for glioblastoma treatment. Front Pharmacol.
12:6616062021. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hatae R, Kyewalabye K, Yamamichi A, Chen
T, Phyu S, Chuntova P, Nejo T, Levine LS, Spitzer MH and Okada H:
Enhancing CAR-T cell metabolism to overcome hypoxic conditions in
the brain tumor microenvironment. JCI Insight. 9:e1771412024.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Jin L, Ge H, Yang C, Long Y and Huang J:
CD70 as a novel target of CAR-T-cell therapy for gliomas. J Clin
Oncol. 35 (Suppl 7):S1482017. View Article : Google Scholar
|
|
47
|
Brown CE, Hibbard JC, Alizadeh D,
Blanchard MS, Natri HM, Wang D, Ostberg JR, Aguilar B, Wagner JR,
Paul JA, et al: Locoregional delivery of IL-13Rα2-targeting CAR-T
cells in recurrent high-grade glioma: A phase 1 trial. Nat Med.
30:1001–1012. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hou AJ, Shih RM, Uy BR, Shafer A, Chang
ZL, Comin-Anduix B, Guemes M, Galic Z, Phyu S, Okada H, et al:
IL-13Rα2/TGF-β bispecific CAR-T cells counter TGF-β-mediated immune
suppression and potentiate anti-tumor responses in glioblastoma.
Neuro Oncol. 26:1850–1866. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Luo L, Zhang XY, Zhen YW, Guo GC, Peng DZ,
Wei C, Pei DL, Yu B, Ji YC, Liu XZ, et al: Polo-like kinase 1 is
related with malignant characteristics and inhibits macrophages
infiltration in glioma. Front Immunol. 13:10580362022. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Tang F, Wang Y, Zeng Y, Xiao A, Tong A and
Xu J: Tumor-associated macrophage-related strategies for glioma
immunotherapy. NPJ Precis Oncol. 7:782023. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wang J, Xu L, Ding Q, Li X, Wang K, Xu S
and Liu B: Siglec15 is a prognostic indicator and a potential
tumor-related macrophage regulator that is involved in the
suppressive immunomicroenvironment in gliomas. Front Immunol.
14:10650622023. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Gerew A, Sexton S, Wasko KM, Shearman MS,
Zhang K, Chang KH and Khan SQ: Deletion of CISH and TGFβR2 in
iPSC-derived NK cells promotes high cytotoxicity and enhances in
vivo tumor killing. Blood. 138 (Suppl 1):S27802021. View Article : Google Scholar
|
|
53
|
Castriconi R, Daga A, Dondero A, Zona G,
Poliani PL, Melotti A, Griffero F, Marubbi D, Spaziante R, Bellora
F, et al: NK cells recognize and kill human glioblastoma cells with
stem cell-like properties. J Immunol. 182:3530–3539. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Daga A, Orengo AM, Gangemi RMR, Marubbi D,
Perera M, Comes A, Ferrini S and Corte G: Glioma immunotherapy by
IL-21 gene-modified cells or by recombinant IL-21 involves antibody
responses. Int J Cancer. 121:1756–63. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Murad S, Michen S, Becker A, Füssel M,
Schackert G, Tonn T, Momburg F and Temme A: NKG2C+ NK cells for
immunotherapy of glioblastoma multiforme. Int J Mol Sci.
23:58572022. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Lin Q, Wei Y, Xu G, Wang L, Ling F, Chen
X, Cheng Y and Zhou Y: Integrative multi-omic profiling of the
neoantigen landscape of glioblastoma for the development of
therapeutic vaccines reveals vast heterogeneity in immunogenic
signatures. Front Oncol. 15:15076322025. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Schulenburg A, Ulrich-Pur H, Thurnher D,
Erovic B, Florian S, Sperr WR, Kalhs P, Marian B, Wrba F, Zielinski
CC and Valent P: Neoplastic stem cells: A novel therapeutic target
in clinical oncology. Cancer. 107:2512–2520. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Guo F, Zhang Y, Bai L and Cui J: Natural
killer cell therapy targeting cancer stem cells: Old wine in a new
bottle. Cancer Lett. 570:2163282023. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Avril T, Vauleon E, Hamlat A, Saikali S,
Etcheverry A, Delmas C, Diabira S, Mosser J and Quillien V: Human
glioblastoma stem-like cells are more sensitive to allogeneic NK
and T cell-mediated killing compared with serum-cultured
glioblastoma cells. Brain Pathol. 22:159–174. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Lu Y, Wang W and Tan S: EHD1 promotes the
cancer stem cell (CSC)-like traits of glioma cells via interacting
with CD44 and suppressing CD44 degradation. Environ Toxicol.
37:2259–2268. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Wang X, Dai X, Zhang X, Li X, Xu T and Lan
Q: Enrichment of glioma stem cell-like cells on 3D porous scaffolds
composed of different extracellular matrix. Biochem Biophys Res
Commun. 498:1052–1057. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Yuan X, Curtin J, Xiong Y, Liu G,
Waschsmann-Hogiu S, Farkas DL, Black KL and Yu JS: Isolation of
cancer stem cells from adult glioblastoma multiforme. Oncogene.
23:9392–9400. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Poli A, Wang J, Domingues O, Planagumà J,
Yan T, Rygh CB, Skaftnesmo KO, Thorsen F, McCormack E, Hentges F,
et al: Targeting glioblastoma with NK cells and mAb against
NG2/CSPG4 prolongs animal survival. Oncotarget. 4:1527–1546. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Stallcup WB: NG2 proteoglycan enhances
brain tumor progression by promoting beta-1 integrin activation in
both Cis and trans orientations. Cancers (Basel). 9:312017.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Wang X, Osada T, Wang Y, Yu L, Sakakura K,
Katayama A, McCarthy JB, Brufsky A, Chivukula M, Khoury T, et al:
CSPG4 protein as a new target for the antibody-based immunotherapy
of triple-negative breast cancer. J Natl Cancer Inst.
102:1496–1512. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Aloisi F, Ria F and Adorini L: Regulation
of T-cell responses by CNS antigen-presenting cells: Different
roles for microglia and astrocytes. Immunol Today. 21:141–147.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Charles NA and Holland EC: The
perivascular niche microenvironment in brain tumor progression.
Cell Cycle. 9:3084–3093. 2010. View Article : Google Scholar
|
|
68
|
Shono K, Yamaguchi I, Mizobuchi Y, Kagusa
H, Sumi A, Fujihara T, Nakajima K, Kitazato KT, Matsuzaki K, Saya H
and Takagi Y: Downregulation of the CCL2/CCR2 and CXCL10/CXCR3 axes
contributes to antitumor effects in a mouse model of malignant
glioma. Sci Rep. 10:152862020. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Deborah EA, Nabekura T, Shibuya K and
Shibuya A: THEMIS2 impairs antitumor activity of NK cells by
suppressing activating NK receptor signaling. J Immunol.
212:1819–1828. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Panda AK, Gangaplara A, Buszko M,
Natarajan K, Boyd LF, Sharma S, Margulies DH and Shevach EM:
Cutting edge: Inhibition of the interaction of NK inhibitory
receptors with MHC class I augments antiviral and antitumor
immunity. J Immunol. 205:567–572. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Luczo JM, Ronzulli SL and Tompkins SM:
Influenza A virus hemagglutinin and other pathogen glycoprotein
interactions with NK cell natural cytotoxicity receptors NKp46,
NKp44, and NKp30. Viruses. 13:1562021. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Bailly E, Macedo C, Gu X, Hollingshead D,
Bentlejewski C, Fong E, Morel PA, Randhawa P, Zeevi A, Lefaucheur C
and Metes D: FCGR2C Q13 and FCGR3A V176
alleles jointly associate with worse natural killer cell-mediated
antibody-dependent cellular cytotoxicity and microvascular
inflammation in kidney allograft antibody-mediated rejection. Am J
Transplant. 25:302–315. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Billadeau DD, Upshaw JL, Schoon RA, Dick
CJ and Leibson PJ: NKG2D-DAP10 triggers human NK cell-mediated
killing via a Syk-independent regulatory pathway. Nat Immunol.
4:557–564. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
74
|
Lanier LL: NKG2D receptor and its ligands
in host defense. Cancer Immunol Res. 3:575–582. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Sivori S, Vacca P, Del Zotto G, Munari E,
Mingari MC and Moretta L: Human NK cells: Surface receptors,
inhibitory checkpoints, and translational applications. Cell Mol
Immunol. 16:430–441. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Zhang PF, Gao C, Huang XY, Lu JC, Guo XJ,
Shi GM, Cai JB and Ke AW: Cancer cell-derived exosomal circUHRF1
induces natural killer cell exhaustion and may cause resistance to
anti-PD1 therapy in hepatocellular carcinoma. Mol Cancer.
19:1102020. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Khan M, Arooj S and Wang H: NK cell-based
immune checkpoint inhibition. Front Immunol. 11:1672020. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Harjunpää H and Guillerey C: TIGIT as an
emerging immune checkpoint. Clin Exp Immunol. 200:108–119. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Briukhovetska D, Suarez-Gosalvez J, Voigt
C, Markota A, Giannou AD, Schübel M, Jobst J, Zhang T, Dörr J,
Märkl F, et al: T cell-derived interleukin-22 drives the expression
of CD155 by cancer cells to suppress NK cell function and promote
metastasis. Immunity. 56:143–161.e11. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Saraiva M and O'Garra A: The regulation of
IL-10 production by immune cells. Nat Rev Immunol. 10:170–181.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Devis-Jauregui L, Eritja N, Davis ML,
Matias-Guiu X and Llobet-Navàs D: Autophagy in the physiological
endometrium and cancer. Autophagy. 17:1077–1095. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Fitzgerald AA, Wang S, Agarwal V, Marcisak
EF, Zuo A, Jablonski SA, Loth M, Fertig EJ, MacDougall J, Zhukovsky
E, et al: DPP inhibition alters the CXCR3 axis and enhances NK and
CD8+ T cell infiltration to improve anti-PD1 efficacy in murine
models of pancreatic ductal adenocarcinoma. J Immunother Cancer.
9:e0028372021. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Bejarano L, C. Jordāo MJ and Joyce JA:
Therapeutic targeting of the tumor microenvironment. Cancer Discov.
11:933–959. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Klemm F, Maas RR, Bowman RL, Kornete M,
Soukup K, Nassiri S, Brouland JP, Iacobuzio-Donahue CA, Brennan C,
Tabar V, et al: Interrogation of the microenvironmental landscape
in brain tumors reveals disease-specific alterations of immune
cells. Cell. 181:1643–1660.e17. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Friebel E, Kapolou K, Unger S, Núñez NG,
Utz S, Rushing EJ, Regli L, Weller M, Greter M, Tugues S, et al:
Single-cell mapping of human brain cancer reveals tumor-specific
instruction of tissue-invading leukocytes. Cell. 181:1626–1642.e20.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Fares J, Ulasov I, Timashev P and Lesniak
MS: Emerging principles of brain immunology and immune checkpoint
blockade in brain metastases. Brain. 144:1046–1066. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Chen Z and Hambardzumyan D: Immune
microenvironment in glioblastoma subtypes. Front Immunol.
9:10042018. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Sampson JH, Gunn MD, Fecci PE and Ashley
DM: Brain immunology and immunotherapy in brain tumours. Nat Rev
Cancer. 20:12–25. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Quail DF and Joyce JA: The
microenvironmental landscape of brain tumors. Cancer Cell.
31:326–341. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Grabowski MM, Sankey EW, Ryan KJ,
Chongsathidkiet P, Lorrey SJ, Wilkinson DS and Fecci PE: Immune
suppression in gliomas. J Neurooncol. 151:3–12. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Bugide S, Gupta R, Green M and Wajapeyee
N: EZH2 inhibits NK cell-mediated antitumor immunity by suppressing
CXCL10 expression in an HDAC10-dependent manner. Proc Natl Acad Sci
USA. 118:e21027181182021. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Mangani D, Weller M and Roth P: The
network of immunosuppressive pathways in glioblastoma. Biochem
Pharmacol. 130:1–9. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Tang X, Yang L, Li Z, Nalin AP, Dai H, Xu
T, Yin J, You F, Zhu M, Shen W, et al: First-in-man clinical trial
of CAR NK-92 cells: Safety test of CD33-CAR NK-92 cells in patients
with relapsed and refractory acute myeloid leukemia. Am J Cancer
Res. 8:1083–1089. 2018.PubMed/NCBI
|
|
94
|
Oelsner S, Friede ME, Zhang C, Wagner J,
Badura S, Bader P, Ullrich E, Ottmann OG, Klingemann H, Tonn T and
Wels WS: Continuously expanding CAR NK-92 cells display selective
cytotoxicity against B-cell leukemia and lymphoma. Cytotherapy.
19:235–249. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Salman H, Pinz KG, Wada M, Shuai X, Yan
LE, Petrov JC and Ma Y: Preclinical targeting of human acute
myeloid leukemia using CD4-specific chimeric antigen receptor (CAR)
T cells and NK cells. J Cancer. 10:4408–4419. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Liu Y, Zhou Y, Huang KH, Fang X, Li Y,
Wang F, An L, Chen Q, Zhang Y, Shi A, et al: Targeting epidermal
growth factor-overexpressing triple-negative breast cancer by
natural killer cells expressing a specific chimeric antigen
receptor. Cell Prolif. 53:e128582020. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Ng YY, Tay JCK and Wang S: CXCR1
expression to improve anti-cancer efficacy of intravenously
injected CAR-NK cells in mice with peritoneal xenografts. Mol Ther
Oncolytics. 16:75–85. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Ueda T, Kumagai A, Iriguchi S, Yasui Y,
Miyasaka T, Nakagoshi K, Nakane K, Saito K, Takahashi M, Sasaki A,
et al: Non-clinical efficacy, safety and stable clinical cell
processing of induced pluripotent stem cell-derived anti-glypican-3
chimeric antigen receptor-expressing natural killer/innate lymphoid
cells. Cancer Sci. 111:1478–1490. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Zuo P, Li Y, He C, Wang T, Zheng X, Liu H,
Wu Z, Zhang J, Liao X and Zhang L: Anti-tumor efficacy of anti-GD2
CAR NK-92 cells in diffuse intrinsic pontine gliomas. Front
Immunol. 14:11457062023. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Zhang C, Burger MC, Jennewein L, Genßler
S, Schönfeld K, Zeiner P, Hattingen E, Harter PN, Mittelbronn M,
Tonn T, et al: ErbB2/HER2-specific NK cells for targeted therapy of
glioblastoma. J Natl Cancer Inst. 108:djv3752016. View Article : Google Scholar
|
|
101
|
Ishikawa E, Tsuboi K, Saijo K, Harada H,
Takano S, Nose T and Ohno T: Autologous natural killer cell therapy
for human recurrent malignant glioma. Anticancer Res. 24:1861–1871.
2004.PubMed/NCBI
|
|
102
|
Soumen K, Cooper LJN, Sandberg DI, Leena
K, Johnson JM, Rytting ME, Liu DD, Meador H, Trikha P, Nakkula RJ,
et al: Phase I study of intraventricular infusions of autologous ex
vivo expanded NK cells in children with recurrent medulloblastoma
and ependymoma. Neuro Oncol. 22:1214–1225. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Thakar MS, Browning M, Hari P, Charlson
JA, Margolis DA, Logan B, Schloemer N, Kelly ME, Newman A, Johnson
B, et al: Phase II trial using haploidentical hematopoietic cell
transplantation (HCT) followed by donor natural killer (NK) cell
infusion and sirolimus maintenance for patients with high-risk
solid tumors. J Clin Oncol. 38 (1Suppl 15):e235512020. View Article : Google Scholar
|
|
104
|
Lim J, Park YJ, Ahn JW, Sim JM, Kang SJ,
Hwang S, Chun J, Choi H, Kim SH, Chun DH, et al: Autologous
adoptive immune-cell therapy elicited a durable response with
enhanced immune reaction signatures in patients with recurrent
glioblastoma: An open label, phase I/IIa trial. PLoS One.
16:e02472932021. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Asl NS, Behfar M, Amiri RS, Mohseni R,
Azimi M, Firouzi J, Faranoush M, Izadpanah A, Mohmmad M, Hamidieh
AA, et al: Intra-lesion injection of activated Natural Killer (NK)
cells in recurrent malignant brain tumors. Int Immunopharmacol.
120:1103452023. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Yamada D, Iyoda T, Vizcardo R, Shimizu K,
Sato Y, Endo TA, Kitahara G, Okoshi M, Kobayashi M, Sakurai M, et
al: Efficient regeneration of human Vα24+ invariant
natural killer T cells and their anti-tumor activity in vivo. Stem
Cells. 34:2852–2860. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Peng Y, Zhan M, Karpus A, Zou Y, Mignani
S, Majoral JP, Shi X and Shen M: Brain delivery of biomimetic
phosphorus dendrimer/antibody nanocomplexes for enhanced glioma
immunotherapy via immune modulation of T cells and natural killer
cells. ACS Nano. 18:10142–10155. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Bagley SJ, Logun M, Fraietta JA, Wang X,
Desai AS, Bagley LJ, Nabavizadeh A, Jarocha D, Martins R, Maloney
E, et al: Intrathecal bivalent CAR T cells targeting EGFR and
IL13Rα2 in recurrent glioblastoma: Phase 1 trial interim results.
Nat Med. 30:1320–1329. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Liu S, Galat V, Galat Y, Lee YKA,
Wainwright D and Wu J: NK cell-based cancer immunotherapy: From
basic biology to clinical development. J Hematol Oncol. 14:72021.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Marofi F, Abdul-Rasheed OF, Rahman HS,
Budi HS, Jalil AT, Yumashev AV, Hassanzadeh A, Yazdanifar M,
Motavalli R, Chartrand MS, et al: CAR-NK cell in cancer
immunotherapy; A promising frontier. Cancer Sci. 112:3427–3436.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Shanley M, Daher M, Dou J, Li S, Basar R,
Rafei H, Dede M, Gumin J, Pantaleόn Garcίa J, Nunez Cortes AK, et
al: Interleukin-21 engineering enhances NK cell activity against
glioblastoma via CEBPD. Cancer Cell. 42:1450–1466.e11. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Marin D, Li Y, Basar R, Rafei H, Daher M,
Dou J, Mohanty V, Dede M, Nieto Y, Uprety N, et al: Safety,
efficacy and determinants of response of allogeneic CD19-specific
CAR-NK cells in CD19+ B cell tumors: a phase 1/2 trial.
Nat Med. 30:772–784. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Siegler EL, Zhu Y, Wang P and Yang L:
Off-the-shelf CAR-NK cells for cancer immunotherapy. Cell Stem
Cell. 23:160–161. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Lin X, Sun Y, Dong X, Liu Z, Sugimura R
and Xie G: IPSC-derived CAR-NK cells for cancer immunotherapy.
Biomed Pharmacother. 165:1151232023. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Kong D, Kwon D, Moon B, Kim DH, Kim MJ,
Choi J and Kang KS: CD19 CAR-expressing iPSC-derived NK cells
effectively enhance migration and cytotoxicity into glioblastoma by
targeting to the pericytes in tumor microenvironment. Biomed
Pharmacother. 174:1164362024. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Lupo KB, Yao X, Borde S, Wang J,
Torregrosa-Allen S, Elzey BD, Utturkar S, Lanman NA, McIntosh M and
Matosevic S: synNotch-programmed iPSC-derived NK cells usurp TIGIT
and CD73 activities for glioblastoma therapy. Nat Commun.
15:19092024. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Pal M, Schwab L, Yermakova A, Mace EM,
Claus R, Krahl AC, Woiterski J, Hartwig UF, Orange JS,
Handgretinger R and André MC: Tumor-priming converts NK cells to
memory-like NK cells. Oncoimmunology. 6:e13174112017. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Terrén I, Orrantia A, Astarloa-Pando G,
Amarilla-Irusta A, Zenarruzabeitia O and Borrego F:
Cytokine-induced memory-like NK cells: From the basics to clinical
applications. Front Immunol. 13:8846482022. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Parihar R, Rivas C, Huynh M, Omer B,
Lapteva N, Metelitsa LS, Gottschalk SM and Rooney CM: NK cells
expressing a chimeric activating receptor eliminate MDSCs and
rescue impaired CAR-T cell activity against solid tumors. Cancer
Immunol Res. 7:363–375. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Böttcher JP, Bonavita E, Chakravarty P,
Blees H, Cabeza-Cabrerizo M, Sammicheli S, Rogers NC, Sahai E,
Zelenay S and Reis e Sousa C: NK cells stimulate recruitment of
cDC1 into the tumor microenvironment promoting cancer immune
control. Cell. 172:1022–1037.e14. 2018. View Article : Google Scholar : PubMed/NCBI
|















