Introduction
Na+/K+-ATPase plays a crucial
role in maintaining the ion balance inside and outside the cell and
mediate complex cell signal transduction by binding with the
tyrosine kinase Src family joint receptor (1,2). It is
composed of the α, β and γ subunit. The α subunit is the catalytic
subunit of the enzyme and is involved in all processes of ion
transport inside and outside the cell (3). The β subunit has three subtypes:
β1, β2 and β3. It consists of an
extracellular domain and a transmembrane helix. The α subunit
interactions jointly maintain the ion balance inside and outside
the cell (4). β1 is
expressed in all tissues and cells, β2 is expressed
mainly in the skeletal muscle and nerve cells, and β3 is
expressed in the retina, optic nerve, sciatic nerve, lung and liver
(5).
The Na+/K+-ATPase has been
implicated in the occurrence and progression of various cancers.
Ouabain, a potent inhibitor of Na+/K+-ATPase,
has shown efficacy in the treatment of heart failure. When combined
with low-dose ouabain, Na+/K+-ATPase can
activate intracellular signal transduction without affecting its
own function, leading to the apoptosis of cancer cells (3). Ouabain has also been found to promote
the phosphorylation of Src, ERK and Akt, inhibit the growth of
kidney cancer, breast cancer, and activate the proteasome in breast
cancer cells, thereby inhibiting the signal transduction of
17β-estradiol and inducing apoptosis (6,7).
Additionally, ouabain has dose- and time-dependent inhibitory
effects on the proliferation and migration of human cervical and
pancreatic cancer cells while promotes apoptosis (8). In the present study, the expression of
ATP1B2 in patients with esophageal squamous cell carcinoma (ESCC)
was evaluated and its association with the clinical pathological
indicators was explored. Changes in the proliferation, migration
and apoptosis of the ESCC cells were compared before and after
ouabain treatment and the preliminarily role of ATP1B2 in ESCC was
investigated.
Materials and methods
Clinical information
Between December 1, 2017 and December 1, 2018, a
total of 44 patients who underwent surgical treatment at Taihe
Hospital were recruited for the present study. The cohort consisted
of 37 male and 7 female patients, with an age range of 43–74 years
(median age, 56 years). The inclusion criteria are as follows:
confirmation of pathological tissue as ESCC, compliance with the
eighth edition of the AJCC International TNM staging criteria for
ESCC, no prior radiotherapy, chemotherapy, biological therapy, or
immunotherapy, ability to participate in the study, and
availability of complete medical records. The exclusion criteria
included the presence of other tumors, severe cardiovascular
diseases precluding surgery, incomplete clinical and pathological
data. The present study was approved (approval nos. 2016KS001 and
2022KS038) by the Ethics Committee of Taihe Hospital (Shiyan,
China), and informed consent was obtained from the patients and
their families.
Cells and main reagents in the
experiment
The esophageal cancer cell line EC109 was purchased
from China center for type culture collection (cat. no. GDC0207).
The esophageal cancer cell line KYSE150 and Het-1A were purchased
from National Collection of Authenticated Cell Cultures (cat. nos.
TCHu236 and GNHu51, respectively). The CEC2 cell line was gifted by
Professor Li Jinsong from Biomedical Engineering at Beijing
Institute of Technology. The main reagents used was the anti-ATP1B2
antibody (Atlas Antibacteries; http://www.atlasantibodies.com/). The additional
reagents used include DAB staining solution (MaxVision Biology;
http://www.maxim.com.cn/), Opti MEM and RPMI-1640
medium, the transfection reagent Lipofectamine 3000 (Thermo Fisher
Scientific, Inc.), fetal bovine serum (Zhejiang Tianhang
Biotechnology Co., Ltd.), TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), puromycin, propidium, ribonuclease A (Guangzhou
Saiguo Biotech Co., Ltd.), DMSO (Bioproxx; NeoFroxx), plasmid
(Geogene; www.geogene.com), trypsin and PBS
(Invitrogen; Thermo Fisher Scientific, Inc.). A reverse
transcription kit (Takara Bio, Inc.), methylcyclopentadienyl
manganese tricarbonyl (MTT) cell proliferation test kit, Hoechst
333258 (Beyotime Institute of Biotechnology), RT-qPCR kit, DEPC
(Tiangen Biotech Co., Ltd.), GoldView nucleic acid dye (Coolpo;
http://www.coolaber.com/), rabbit anti-β-actin
antibody, Goat anti-Mouse IgG (H+L)-HRP, Goat anti-Rabbit IgG
(H+L)-HRP (LABLEAD; http://www.lablead.cn/), ECL luminescence reagent
(Absin) for western blotting, and ouabain (cat. no. HY-B0542;
MedChemExpress) were used for the assays.
Immunohistochemical staining
MaxVision was used to detect the expression of the
ATP1B2 protein. In the preliminary experiment, ATP1B2 was diluted
at 1:250, 1:100, and 1:50 for staining comparison. The best
staining effect was achieved at a dilution ratio of 1:100.
Accordingly, PBS was diluted to 1:100 according to the
manufacturer's instructions. Esophageal tissue was embedded in
paraffin, fixed with Carnoy solution (Ethanol, acetic acid, and
trichloromethane mixed in a volume ratio of 6:3:1) at room
temperature for 12 h, and sliced with a thickness of 4 µm. Then, it
was dewaxed and hydrated with xylene and gradient ethanol. The
slices were placed in ETDA (pH 9.0) antigen repair buffer and
repaired with high-pressure steam for 6 min. Then, they were rinsed
with distilled water for 1 min. Thereafter, an oil pen was used to
mark the area of the tissue to be tested. Finally, the tissue was
incubated with 3% H2O2 for 10 min to block
endogenous peroxidase activity. Endogenous peroxidase blocker of
100 µl was added to the delineated area of each slice and incubated
at room temperature for 10 min. Then, the slices were rinsed three
times with PBS solution for 3 min each time. After removing PBS,
non-specific antibody blocker of 100 µl was added to the delineated
area of each slice and incubated at room temperature for 10 min,
and the slices were rinsed three times with PBS solution for 3 min
each time. After removing PBS, the primary antibody (mouse
anti-human ATP1B2 polyclonal antibody; 1:100) was added to cover
the entire tissue. Incubation was conducted in a 25°C environment
for 60 min. Thereafter, the slices were rinsed three times with PBS
solution for 3 min each time. After removing PBS, enzyme Goat
anti-Mouse IgG (H+L)-HRP of 100 µl was added to completely cover
the tissue. Incubation was conducted at room temperature for 15
min, followed by three rinses with PBS solution for 3 min each
time. After removing PBS, freshly prepared DAB color solution of
100 µl was added and incubated for 3 min at room temperature. The
tissue was rinsed with distilled water (tap water) for 1 min and
was incubated with 100 µl hematoxylin staining solution for 10 sec
at room temperature until the nucleus was stained dark. Finally,
the steps of dehydration, transparency and sealing were completed.
All slices were observed, and images were captured under the Leica
DM2500 light microscope (Leica Microsystems GmbH).
A total of five areas were randomly selected and
imaged under a microscope, and the area and integrated option
density (IOD) of the target protein in the immunohistochemical
image were calculated using the ImageJ plus 6.0 software (National
Institutes of Health). The formula was mean density=IOD/area
(9). The cutoff value of ATP1B2 was
subsequently determined based on the ROC curve. The highest Jordan
index was obtained when the relative expression of ATP1B2 was 2.1.
Using the relative expression of ATP1B2 as the critical value, the
44 cases of ESCC were divided into the high-expression group
(relative expression ≥2.1; 28 cases) and low-expression group
(relative expression <2.1;16 cases).
Cell culture, transfection and
construction of stable cell line
Immortalized epithelial esophageal cells (Het-1A)
and ESCC cells (EC109, KYSE150 and CEC2) were cryopreserved in the
laboratory. The cells used in the experiment were cultured in DMEM
supplemented with 10% fetal bovine serum and 1%
penicillin-streptomycin (penicillin 100 U/ml; streptomycin 100
µg/ml) in a 5%-CO2 incubator at 37°C.
Based on the test results of qPCR, two cell lines of
esophageal cancer EC109 and KYSE150 were selected for subsequent
experiments. Before conducting cell transfection experiments,
esophageal cancer EC109 and KYSE150 cells were observed under a
microscope after being treated with antibiotics and puromycin. The
concentrations of the two drugs gradually decrease in the order of
2, 1.5, 1, 0.5, and 0 µg/ml. Based on the standard of culturing for
3 days with the majority of cell death concentrations, the optimal
suitable concentration for EC109 cells was selected as 1 µg/ml,
while the optimal concentration for KYSE150 cells was 0.5 µg/ml.
Short hairpin (shRNA) was used to silence the ATP1B2 gene. The
construction of the plasmid was synthesized and constructed by
Shanghai Jikai Gene Chemical Technology Co., Ltd. The interfering
sequence of the shRNA of the ATP1B2 gene was shATP1B2:
5′-GAATGTAGAATGTCGCATCAA21bp-3′ (10). Cell transfection experiments were
conducted under constant temperature conditions of 25°C. Then,
incubation was performed in a 37°C incubator for 72 h. Next, the
expression of green fluorescence was examined under a microscope.
Finally, a substance containing puromycin of 1 µg/ml was used to
screen stable cell line and the maintenance concentration of
puromycin is half of the screening concentration.
Cells in the logarithmic growth phase were seeded in
a 12-well plate, and the cell density reached 60% within 24 h. The
cells were then transfected with 500 ng/well small interfering
RNA/plasmids according to the experimental instructions provided by
the Lip3000 transfection agent. After transfection, puromycin (0.5
µg/ml) was used to screen the cells and establish a stable cell
line, with the empty sh-vector served as the negative control
group.
Reverse transcription-quantitative PCR
(RT-qPCR) and western blot analysis
The ATP1B2 nucleic acid sequence template was
queried in the primer design software Primer 6.0 (NCBIGene ID: 482;
http://premiergroup.ca/), and the primer sequence
was synthesized by a manufacturing company. RT-PCR was performed to
detect the expression of the ATP1B2 gene following transfection.
The primers used are included in Table
I. In RT-qPCR, our reagents were purchased from China Tiangen
Biochemical Technology Co., Ltd. and relevant research was
conducted according to the manufacturer's instructions. Total RNA
was extracted from the samples using the TRIzol reagent, after
which the RNA concentration and purity were assessed (acceptable
260/280 nm ratio >1.9). The RNA was reverse transcribed into
complementary DNA using a reverse transcription kit (HiFi Script
CDNA Synthesis Kit) at 42°C for 50 min, followed by incubation at
85°C for 5 min and an infinite cycle at 4°C. Fluorescence-based
qPCR was performed using a US BIO-RAD C1000 PCR instrument. For
each sample, five replicate wells were used, and the reaction
conditions were as follows: 95°C for 10 min, 95°C for 15 sec, and
60°C for 1 min. A total of 39 cycles were carried out for
amplification. Gene expression was determined using the
2−ΔΔCq method for data analysis (11,12).
This experiment was repeated 6 times.
 | Table I.Primer sequences used for reverse
transcription-quantitative PCR. |
Table I.
Primer sequences used for reverse
transcription-quantitative PCR.
| Gene name | Primer sequence
(5′-3′) | Product length,
bp |
|---|
| ATP12B | F:
GTCCCAAAGCCAGCCGATGT | 240 |
|
| R:
GCCGTTCTGTCACCCAAATA |
|
| GAPDH | F:
TCGGAGTCAACGGATTTGGT | 181 |
|
| R:
TTCCCGTTCTCAGCCTTGAC |
|
| TP53 | F:
TGTGACTTGCACGTACTCCC | 199 |
|
| R:
ACCATCGCTATCTGAGCAGC |
|
| RAC1 | F:
GGTGGGAGACGGAGCTGTA | 212 |
|
| R:
AGAACACATCTGTTTGCGGA |
|
| MMP-7 | F:
AAGTGGTCACCTACAGGATCGTA | 286 |
|
| R:
CTACCATCCGTCCAGCGTTC |
|
| MMP-10 | F:
TTTGGCTCATGCCTACCCAC | 263 |
|
| R:
CAGGGGGAGGTCCGTAGAGA |
|
Western blotting was performed according to the
standard protocol. Proteins were extracted using
BBproExtra® RIPA Buffer BB-3201 and concentration
measured using BCA assay kit. A total of 40 µg of protein was
loaded per lane and subjected to SDS-PAGE (10%). Proteins were
transferred to a PVDF membrane which was blocked using 1X TBS-T and
10% skimmed milk at room temperature for 1 h. Primary antibodies
for mouse anti human ATP1B2 polyclonal antibody (1:1,000; Atlas
antibodies), rabbit anti-β-actin (1:3,000; Abcam), rabbit
anti-E-cadherin (1:1,000; Abcam), rabbit anti-N-cadherin (1:1,000;
Abcam) and rabbit anti-P53 (1:1,000; Abcam) were incubated
overnight at 4°C. Proteins were detected with ECL luminescence
reagent by Bio-Rad ChemiDoc (Bio-Rad Laboratories, Inc.). This
experiment was repeated 3 times. Densitometric analysis was
performed using ImageJ 2.0 software (National Institutes of
Health).
Measurement of cell migration and
repair ability by scratch test
Logarithmic-phase cells were suspended and seeded in
a six-well plate. After the cells had adhered to the plate, they
were cultured for 12 h to form a uniform monolayer. A 10-µl pipette
tip was used to create a vertical scratch perpendicular to the
horizontal line. The samples were then rinsed with PBS three times.
Serum-free medium was added, and the samples were incubated at 37°C
in an incubator with 5% CO2. Leica's inverted microscope
was used in wound healing assays. ImageJ 6.0 software was used to
calculate the scratch area and measure cell migration and healing
ability. The scratch area of cells was measured at 0, 6, 12 and 24
h. The calculation was cell scar healing rate=(scratch area at 0
h-scratch area at each time point) divided by scratch area at 0 h.
This experiment was repeated 3 times.
Detection of cell cycle and apoptosis
by flow cytometry
The cell cycle experiment was conducted by
centrifuging at 0.8 × g for 5 min at an indoor temperature of 25°C.
The logarithmic cells were obtained to create a cell suspension,
and the cells were accurately counted. The cell density was
adjusted to 20,000 cells/ml, after which a 2 ml/well cell
suspension was obtained and inoculated into a six-well plate. The
cells in the six-well plate were cultured in an oven at a constant
temperature of 37°C under 5% CO2. When the cell
confluence reached 80%, the cells were collected in an Eppendorf
tube. Then, 200 µl of precooled PBS and 1 µl of ribonuclease A were
added. The cells were incubated in a water bath at 37°C for 30 min.
Then, 1 µl of PI was added, and the cells were placed in a
refrigerator at 4°C in the dark for 30 min. Afterward, 300 ml of
precooled PBS was added, and the cells were filtered using a
machine. The cells were collected following the aforementioned
steps. Then, 200 µl of precooled PBS was added to the suspension
cells, and 1 µl of Hoechst 33258 dye solution was added at a
concentration of 1 µg/µl. The solutions were mixed evenly, and the
samples were incubated in an incubator at 37°C for 10 min. The dye
solution was discarded, and 2 ml of PBS was added to the samples.
The cells were rinsed and centrifuged at 0.8 × g for 5 min. These
steps were repeated twice. Then, 500 µl of PBS was added to the
cells, and PI was added. The suspension was mixed evenly, incubated
at 4°C for 15 min, and filtered. Both the cell cycle and apoptosis
were detected on the BD FACSAria III flow cytometer and analyzed by
FlowJo software (FlowJo LLC). This experiment was repeated 3
times.
MTT
A blank control group, negative control group,
ATP1B2 knockdown group and ATP1B2 overexpression group were
established with multiple wells. Cells were transfected in 96-well
plates. After 24, 48, 72 and 96 h of treatment, newly prepared 5
g/l MTT culture solution was added to each well under closed light
conditions. The cells were routinely incubated in a 37°C incubator
for 4 h. The culture plates then removed, and the supernatant was
discarded. Then, 100 µl of DMSO solution was added to each well to
dissolve the purple crystals in the cells. The samples were placed
on a shaking table and shaken at 100 times per minute for 5 min (5%
CO2 at 37°C). After mixing, a microplate reader was used
to detect the absorbance at 570 nm. The experiment was repeated
three times. This experiment was repeated 3 times.
Plate cloning experiment
During the logarithmic growth period, single-cell
suspension was prepared from EC109 and KYSE150 cells. A total of
100 cells were inoculated in each well of a six-well plate, after
which 2 ml of culture medium was added. The mixture was gently
shaken and incubated. A total of three wells were used for each
group, and the cell status was observed daily. The culture medium
was changed as needed. After 11 days of culture, the cells were
collected when colonies were visible to the naked eye. The cells
were washed with 2 ml PBS twice. Then, in an indoor environment at
25°C, 1 ml of 4% paraformaldehyde was added and cells were fixed
for 15 min. Paraformaldehyde was poured out, then rinsing with
distilled water three times. Staining was conducted using 1 ml of
crystal violet for 15 min at room temperature. Then, wells were
rinsed again with distilled water three times. Finally, the cells
were placed in a well-ventilated area and dry for 20 min. The
number of cloned cells was counted under the microscope. A colony
was defined as having >50 cells, and the colony formation rate
was calculated using the following formula: Colony formation
rate=(number of clones/number of inoculations) ×100. This
experiment was repeated 3 times.
Statistical analysis
SPSS 25.0 software (IBM Corp.) was used for
statistical analysis. Normally distributed data were expressed as
the mean ± standard deviation, while counting data were expressed
as cases and percentages. Independent sample t-test were used to
compare two groups. Analysis of variance followed by LSD test was
used for multiple groups, and chi-square test was used for
comparison between two groups. When the expected frequency in any
group was <5, Fisher's exact test was employed. Skewed
distribution data are presented as medians (lower quartile, upper
quartile). The non-parametric Wilcoxon rank sum test was used for
comparisons between two samples, and the Kruskal Wallis H test was
used for comparisons among multiple groups. A statistically
significant difference was considered at P<0.05.
Results
Expression of ATP1B2 in the ESCC and
normal adjacent tissues
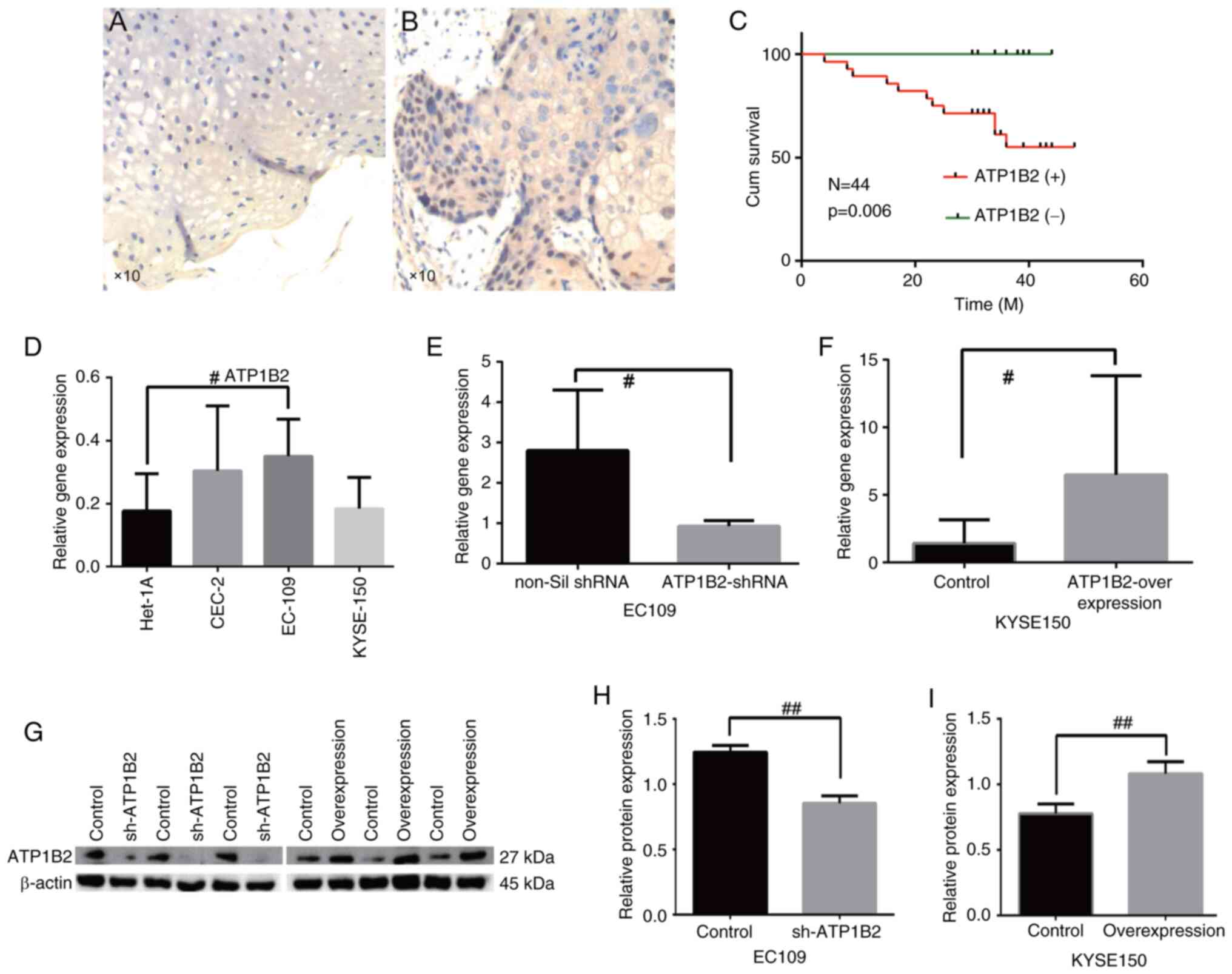
The ATP1B2 protein was primarily expressed in the
nucleus and stained brown (Fig.
1A). Some proteins are expressed in the cytoplasm and stained
light yellow (Fig. 1B). The
expression of the ATP1B2 protein in ESCC tissues was significantly
greater than that in normal adjacent tissues [3.54 (0.42, 4.89) vs.
1.40 (0.20, 2.39); P<0.01, Table
II]. The incidence of lymph node metastasis and invasion of
vessels or nerves in patients with ESCC with high ATP1B2 expression
was significantly higher than that in patients with low ATP1B2
expression (all P<0.05, Table
III). There were no significant differences in sex, age,
smoking history, drinking history, tumor stage, or maximum tumor
diameter among patients with different ATP1B2 expression levels
(P>0.05, Table III). Follow-up
was conducted for 44 patients for 4–48 months, for a median
follow-up time of 35 months. The survival time of patients with
ESCC with high AP1B2 expression was higher than that of patients
with low ATP1B2 expression (P<0.01, Table III). K-M-single-factor survival
analysis demonstrated that the survival rate of patients with ESCC
with high ATP1B2 expression was significantly lower than that of
patients with low ATP1B2 expression (P<0.01, Fig. 1C).
 | Table II.Gene expression in esophageal cancer
and adjacent tissues. |
Table II.
Gene expression in esophageal cancer
and adjacent tissues.
|
| ATP1B2 |
|
|
|
|
|---|
|
|---|
| Tissue sample | Tissue sample | Upper quartile | Lower quartile | x̄ | Z | P |
|---|
| Normal tissue | 44 | 0.2 | 2.39 | 1.470 | −4.103 | 0 |
| Esophageal squamous
cell carcinoma | 44 | 0.42 | 4.89 | 3.541 |
|
|
 | Table III.Relationship between the expression
level of ATP1B2 and clinicopathological features in patients with
esophageal squamous cell carcinoma. Clinical characteristics. |
Table III.
Relationship between the expression
level of ATP1B2 and clinicopathological features in patients with
esophageal squamous cell carcinoma. Clinical characteristics.
|
| N | (+) | (−) | χ2 | P-value |
|---|
| Sex |
|
|
|
|
|
|
Male | 37 | 24 (64.86) | 13 (35.14) | a | 0.690 |
|
Female | 7 | 4 (57.14) | 3 (42.86) |
|
|
| Age, years |
|
|
|
|
|
|
>60 | 16 | 10 (62.5) | 6 (37.5) | 0.140 | 0.910 |
|
≤60 | 28 | 18 (64.29) | 10 (35.71) |
|
|
| Smoking |
|
|
|
|
|
|
Yes | 30 | 19 (63.33) | 11 (36.67) | 0.004 | 0.950 |
| No | 14 | 9 (64.29) | 5 (35.71) |
|
|
| Drinking |
|
|
|
|
|
|
Yes | 31 | 18 (58.06) | 13 (41.94) | a | 0.314 |
| No | 13 | 10 (76.92) | 3 (23.07) |
|
|
| T stage |
|
|
|
|
|
| I +
II | 17 | 8 (47.06) | 9 (52.94) | 3.290 | 0.070 |
| III +
IV | 27 | 20 (74.07) | 7 (25.93) |
|
|
| N stage |
|
|
|
|
|
|
N0 | 17 | 15 (88.24) | 2 (11.76) | a | 0.010 |
|
N1 +
N2 | 27 | 13 (48.15) | 14 (51.85) |
|
|
| Lymph node
metastasis |
|
|
|
|
|
|
Yes | 17 | 15 (88.24) | 2 (11.76) | a | 0.010 |
| No | 27 | 13 (48.15) | 14(51.85) |
|
|
| Invasion of vessels
and nerves |
|
|
|
|
|
|
Yes | 14 | 12 (85.71) | 2 (14.29) | a | 0.049 |
| No | 30 | 16 (53.33) | 14 (46.67) |
|
|
| Tumor diameter |
|
|
|
|
|
| >3
cm | 24 | 18 (75.0) | 6 (25.0) | 2.950 | 0.086 |
| ≤3
cm | 20 | 10 (50.0) | 10 (50.0) |
|
|
| Survival
status |
|
|
|
|
|
|
Alive | 33 | 17 (51.51) | 16 (48.28) | a | 0.003 |
|
Death | 11 | 11 (100) | 0 (0) |
|
|
Expression of ATP1B2 in the ESCC
before and after shRNA transfection
The expression levels of ATP1B2 in EC109, CEC2 and
KYSE150 cells (0.35±0.12, 0.30±0.21, and 0.18±0.10) were
significantly greater than those in Het-1A (0.17±0.12), but the
difference was statistically significant only in EC109 (t=−2.59;
P=0.028; Fig. 1D). Thus, EC109, a
cell line with the highest ATP expression, was targeted for
knockdown experiments in esophageal cancer cells, while KYSE150, a
cell line with the lowest ATP expression, was selected for
overexpression experiments to optimize the intervention's impact on
the cells. In EC109 cells, the expression level of ATP1B2 mRNA in
the knockdown group was significantly lower than that in the
empty-vector group (t=−0.76; P<0.05; Fig. 1E), and the expression level of
ATP1B2 protein in the knockdown group was also significantly lower
than that in the empty-vector group (t=113.734; P<0.05, Fig. 1G and H). In KYSE150 cells, the
expression level of ATP1B2 mRNA in the overexpression group was
significantly higher than that in the empty-vector group (t=−2.31;
P<0.05; Fig. 1F), and the
expression level of ATP1B2 protein in the overexpression group was
significantly higher than that in the empty-vector group (t=−9.342;
P<0.05; Fig. 1G and I).
Effect of ATP1B2 on the biological
behavior of ESCC cells: Migration ability, cell cycle, apoptosis
and proliferation
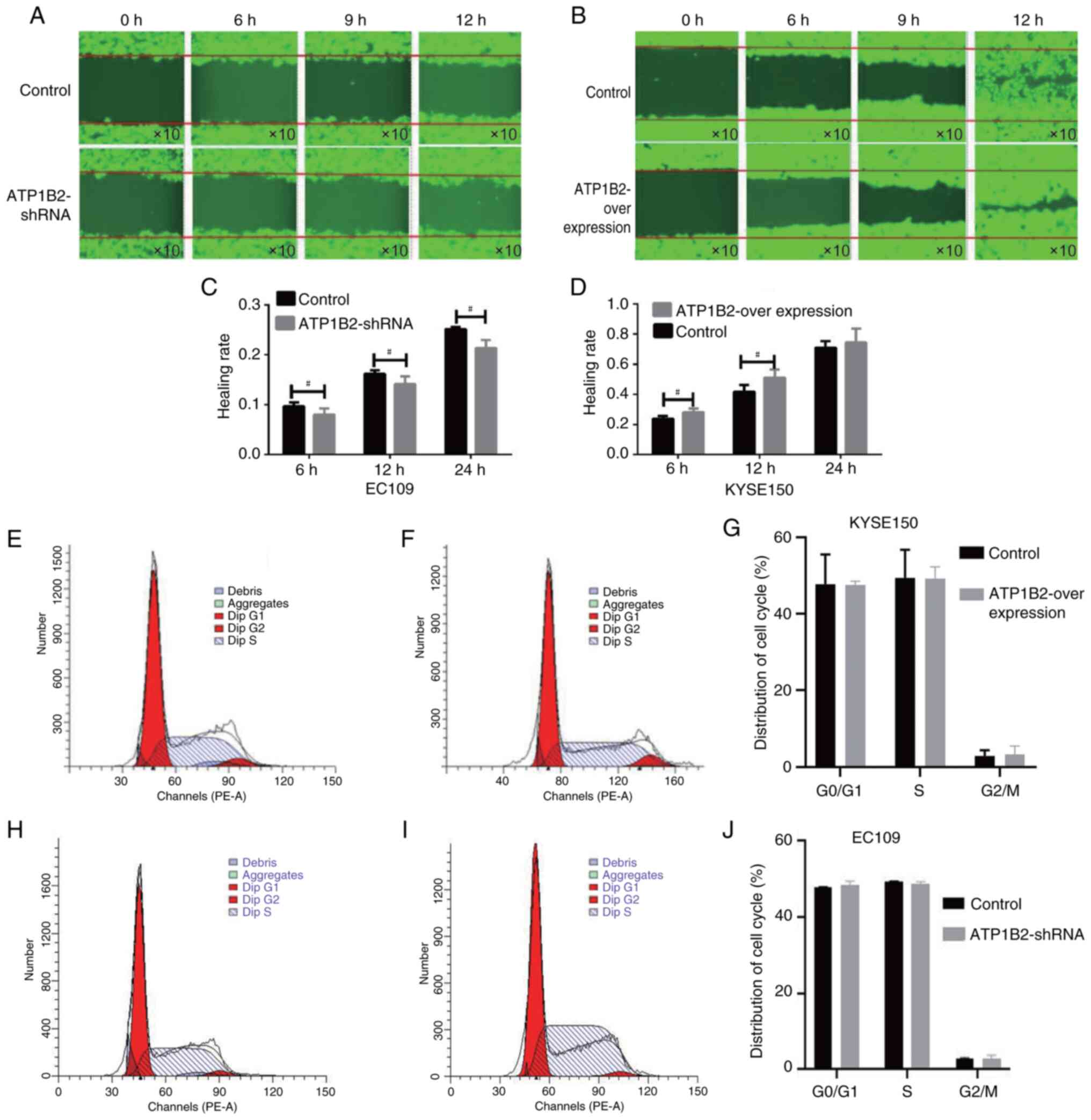
The scratch test revealed that the cell migration
ability of the ATP1B2 knockdown group was significantly lower than
that of the empty-vector control group at 6, 12 and 24 h
(10,963.25±311.53 vs. 15,854.75±2,187.5; 19,371.25±3,804.25 vs.
25,677.25±2,135.76; 33,332.25±5,237.49 vs. 39,618.5±4,191.08; all
P<0.05, Fig. 2A and C). The cell
migration ability of the ATP1B2 overexpression group was
significantly greater than that of the empty-vector control group
at 6 and 12 h (81,909,383±50,721.35 vs. 736,194±66, 803.26; 1,552,
102.6±80, 499.28 vs. 1,257,579.0±144,188.72; all P<0.05,
Fig. 2B and D). The cell cycle
analysis demonstrated that the overexpression of ATP1B2 and the
empty-vector control had no significant effect on the proportions
of KYSE150 ESCC cells in the G0 (47.58±0.94 vs.
47.74±7.74), S (49.1±3.19 vs. 49.37±7.37), or M (3.22±2.26 vs.
2.88±1.48) groups. Similarly, in the cell cycle analysis of EC109
cells in ATP1B2 knockdown group and the empty-vector control group,
there was no significant difference in G0 phase (47.94±0.08 vs.
48.43±1.04), S phase (49.24±0.28 vs. 48.67±0.66) and M phase
(2.82±0.26 vs. 2.89±0.87), but these difference were not
statistically significant (all P>0.05; Fig. 2E-J).
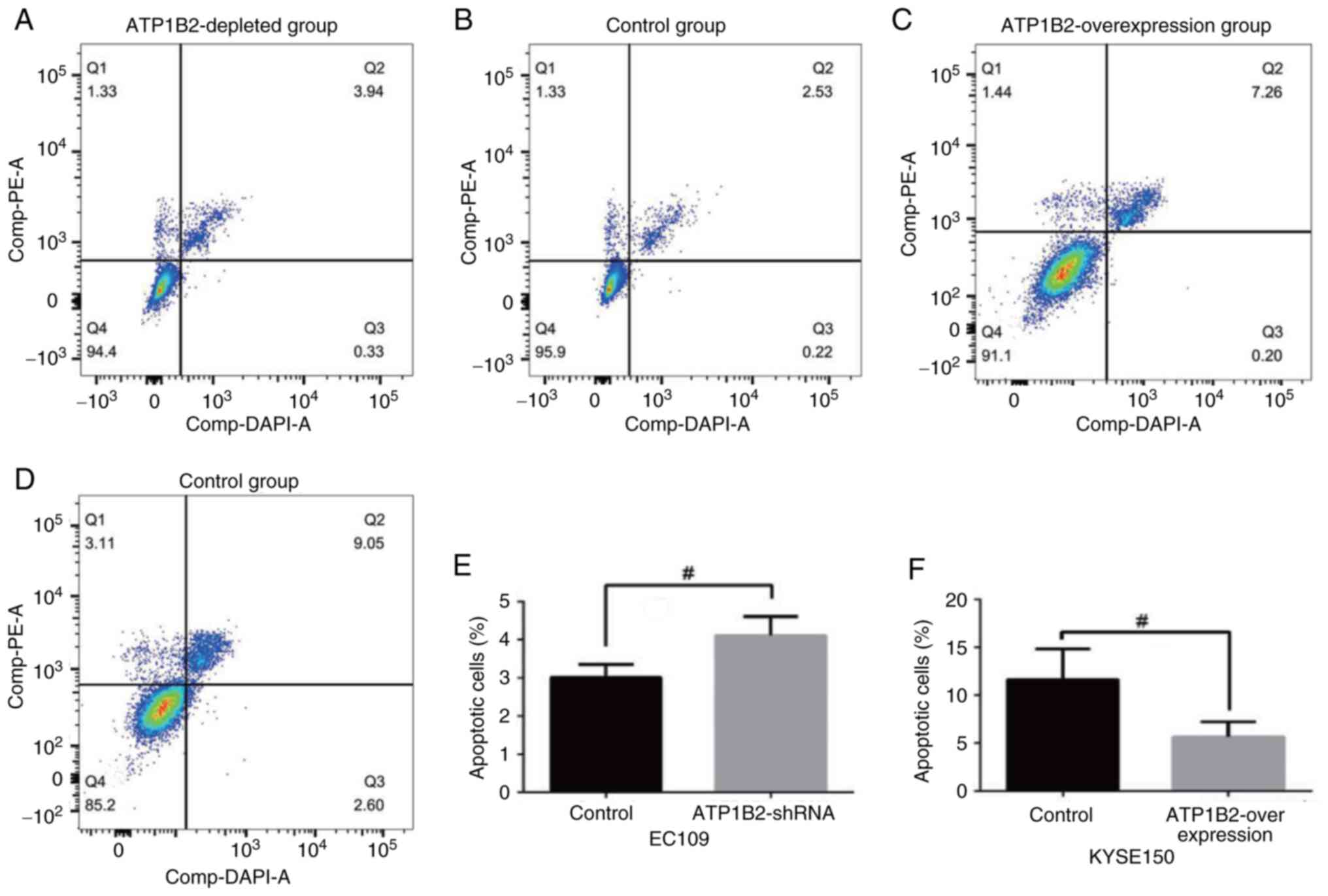
The ATP1B2 knockdown group exhibited significantly
increased apoptosis compared with the empty-vector control group in
EC109 cells (t=3.137; P<0.05; Fig.
3A, B and E). Compared with those in the empty-vector control
group, apoptosis was significantly lower in the ATP1B2
overexpression experimental group (t=−2.906; P<0.05; Fig. 3C, D and F). According to the plate
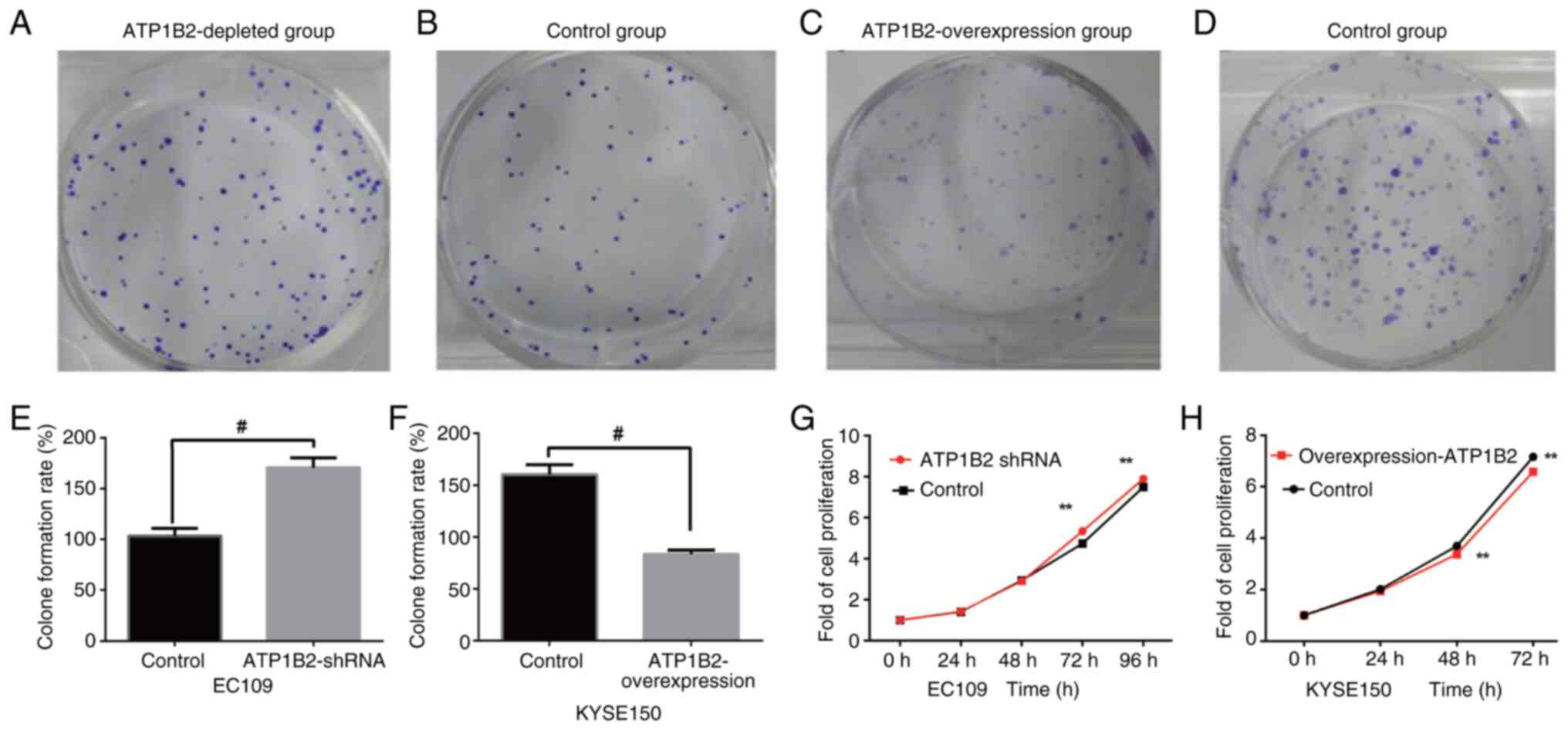
cloning results, the cell clone proliferation ability of the ATP1B2
knockdown group was significantly greater than that of the
empty-vector control group (170.67±9.50 vs. 103.33±7.64; t=9.57;
P<0.05; Fig. 4A, B and E). The
cell colony formation ability of the ATP1B2-overexpression cells
was significantly lower than that of the empty-vector control cells
(83.3±4.16 vs. 160.3±9.45; t=−12.91; P<0.05, Fig. 4C, D and F). After 72 and 96 h after
the EC109 ESCC cells were transfected, the absorbance value of the
ATP1B2 knockdown group was significantly greater than that of the
empty-vector control group (72 h, 5.34±0.17 vs. 4.74±0.16, t=−6,37;
96 h, 7.90±0.23 vs. 7.5±0.17, t=−3.50; all P<0.05; Fig. 4G). After the KYSE150 ESCC cells were
transfected for 48 and 72 h, the absorbance value of the ATP1B2
overexpression group was significantly lower than that of the
empty-vector control group (48 h, 3.38±0.16 vs. 3.69±0.27, t=−2.50;
72 h, 6.58±0.17 vs. 7.16±0.49, t=−2.77; P<0.05; Fig. 4H). Thus, it is evident that ATP
expression significantly suppresses the proliferative capacity of
esophageal cancer cells.
Determination of gene expression
related to epithelial-mesenchymal transformation (EMT) by
RT-qPCR
Following successful transfection of the plasmid,
RNA was extracted for reverse transcription. RT-qPCR revealed that
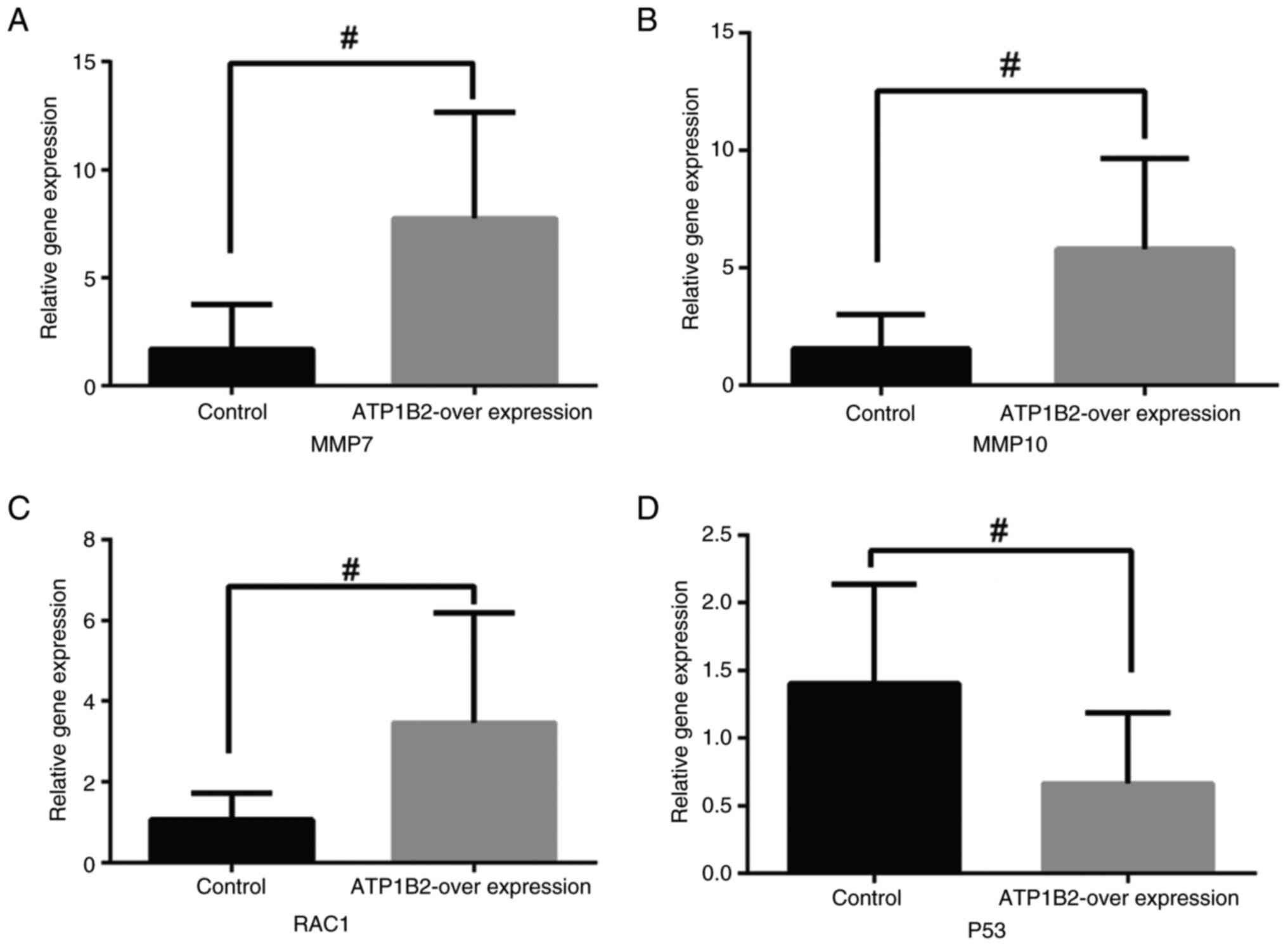
the genes RAC1, MMP7 and MMP10 were associated with EMT. The
expression of genes related to migration was significantly
upregulated in the ATP1B2 overexpression group compared with the
control group (P<0.05, Fig.
5A-C). Therefore, overexpression of ATP1B2 was observed to
promote cell migration. Additionally, the overexpression of ATP1B2
significantly inhibited the expression of P53 (P<0.05, Fig. 5D).
Expression of EMT-related proteins is
detected by western blotting
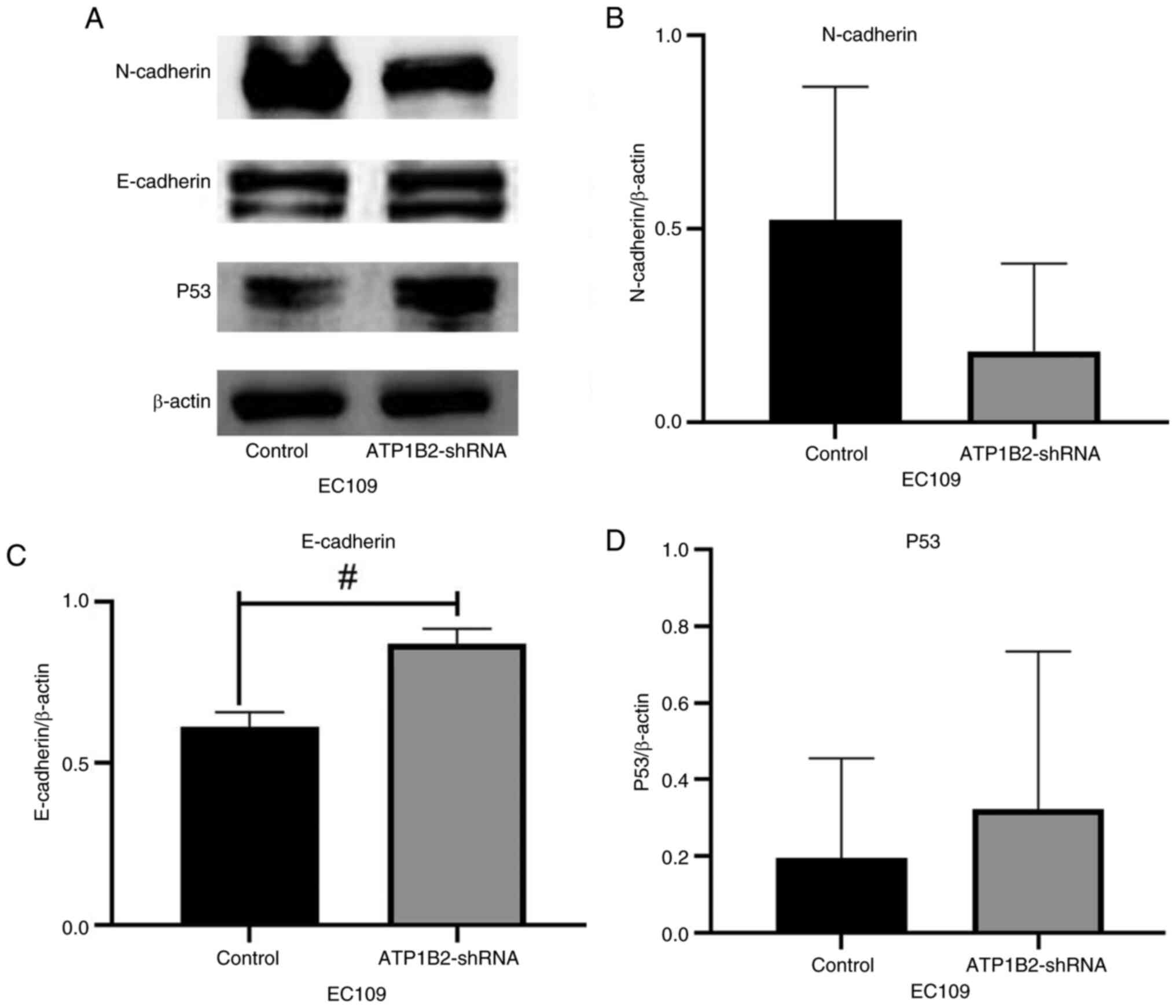
After knocking down ATP1B2, the expression levels of
E-cadherin increased in EC109 cells, and the difference was
statistically significant (P<0.05; Fig. 6A and C). Although the difference was
not statistically significant, it cannot be ignored that knocking
down ATP1B2 reduced the expression level of N-cadherin (Fig. 6A and B) whereas it increased P53
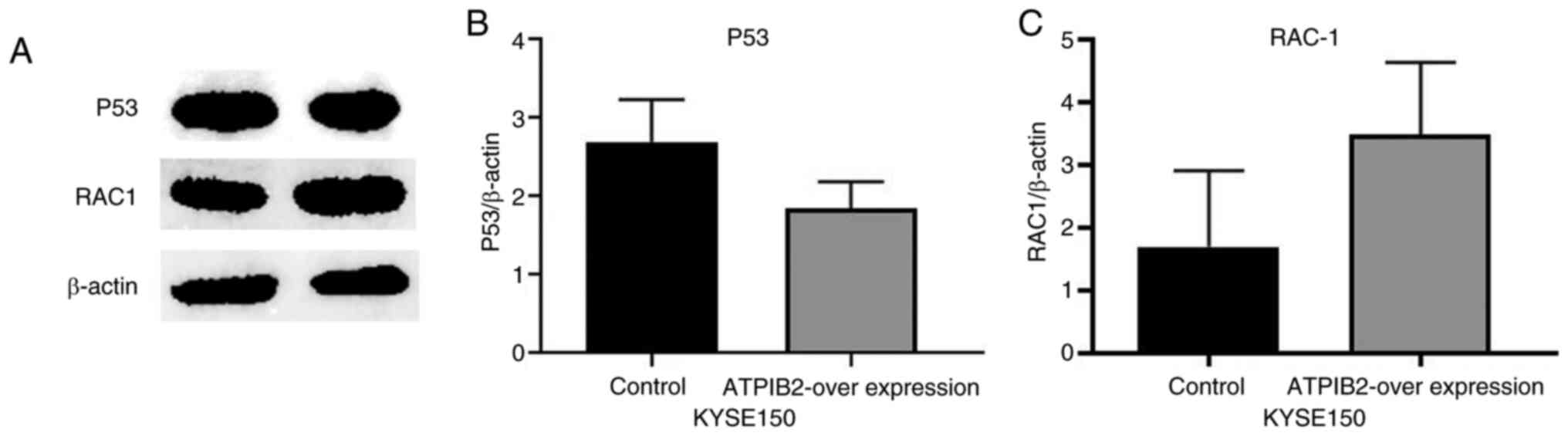
(Fig. 6A and D). In KYSE150 cells,
overexpression of ATP1B2 resulted in increased RAC1 protein
expression and decreased p53 protein expression, consistent with
trends observed in RT-qPCR analysis, although these differences
were not statistically significant (P>0.05, Fig. 7A-C).
Significantly enhanced inhibitory
effect of ouabain on cell proliferation and migration
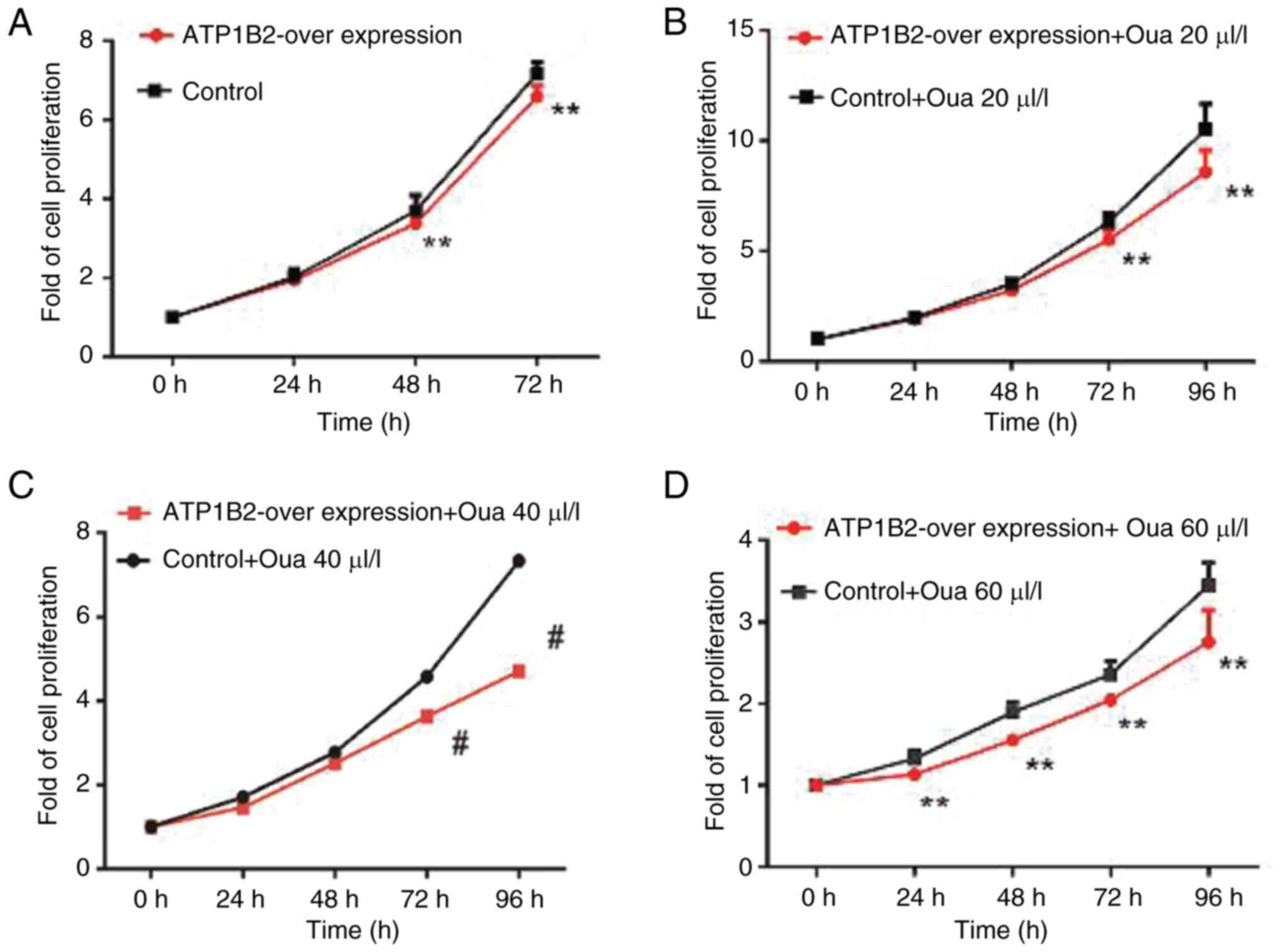
A total of three different concentrations of ouabain
(20, 40 and 60 nmol/l) were tested. MTT assays demonstrated that
ouabain significantly enhanced the inhibitory effect of
ATP1B2-overexpressing ESCC cells on proliferation in response to
increasing concentrations and durations of exposure compared with
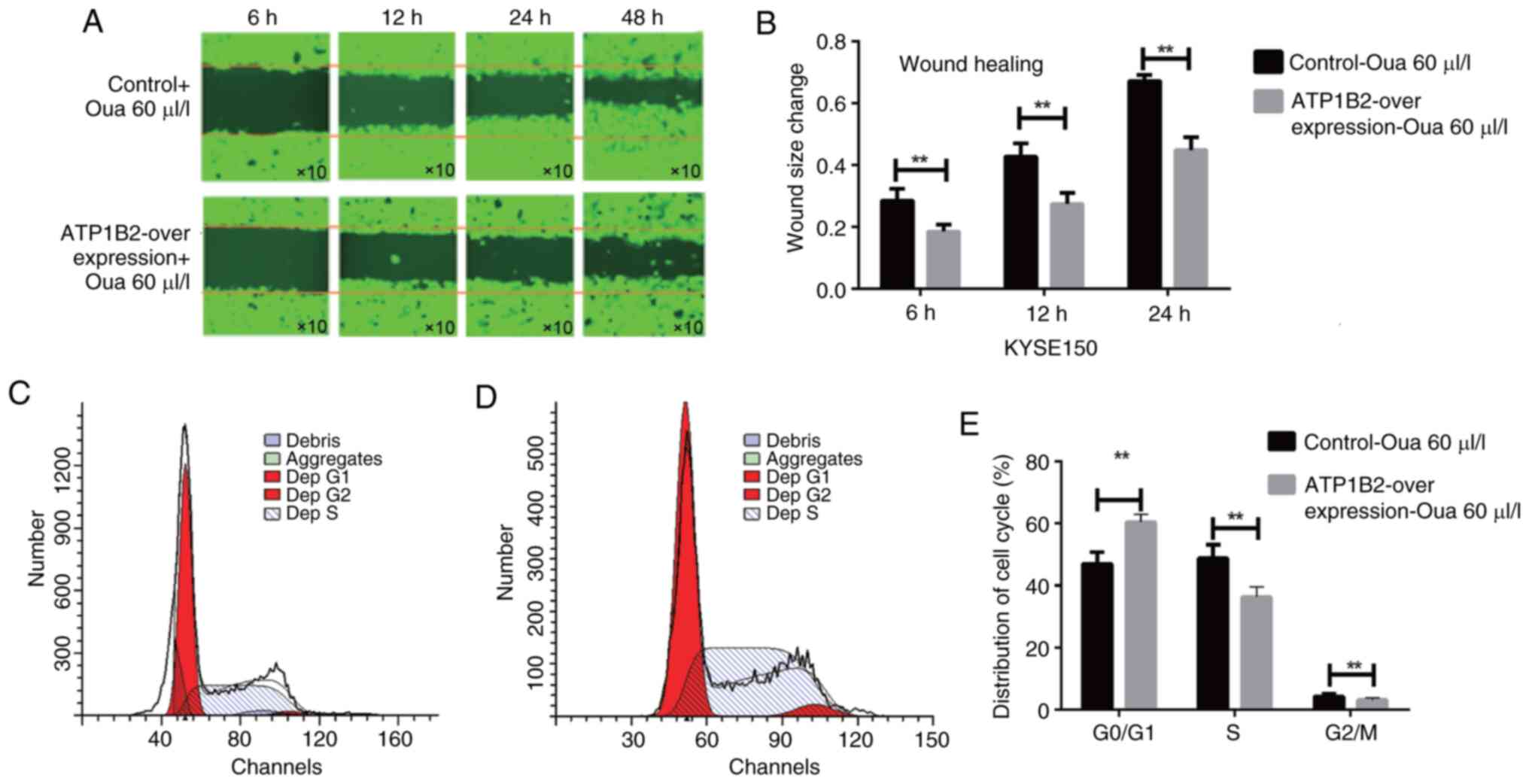
those in the control group (P<0.05; Fig. 8). In the scratch test, 60 µl/l
ouabain was added to the ATP1B2 overexpression group and the
control group containing the empty vector. The migration rate of
the cells was measured at 6, 12 and 24 h, and flow cytometry was
used to detect the cell cycle. The inhibitory effect of ouabain on
the migration ability of ATP1B2-overexpressing cells was
significantly greater than that on the migration of control cells
(P<0.01; Fig. 9A and B).
Furthermore, the inhibition effect of ouabain on
ATP1B2-overexpression ESCC cells resulted in arrest in the
G1/S phase of the cell cycle, which was also
significantly enhanced (P<0.05; Fig.
9C and D). Ultimately, the inhibition of ATP1B2 cell migration
was significantly enhanced (P<0.01; Fig. 9E).
Discussion
Gastrointestinal malignancies account for ~30% of
the global cancer incidence rate and 40% of the mortality rate,
with ESCC mortality ranks fourth (13,14).
The clinical manifestation of early ESCC is not evident, and the
diagnosis rate is low. Some patients already have lymph node
metastasis at the initial diagnosis, missing the optimal time for
surgery. Even if surgery is performed, complete removal of the
focus is not always possible, resulting in short postoperative
survival and poor quality of life (15). Therefore, it is crucial to explore
the mechanism of invasion and metastasis in ESCC for its treatment
and prognosis. The intron 17P13.1 in the ATP1B2 subunit is located
in the 3′-region of TP53 and can regulate TP53 function, increasing
the probability of ESCC (16). The
ATP1B2-PRAKCB gene fusion has been detected in intraductal
eosinophilic papillary tumors of pancreas and bile duct (17). ATP1B2 expression is greater in
glioma tissues than that in adjacent tissues. Patients with glioma
and high expression of ATP1B2 are more prone to cancer cell
infiltration and lymph node metastasis, resulting in a shorter
overall survival period and poor prognosis (10). Additionally, ATP1B2 expression in
ESCC tissues was significantly greater than that in adjacent
tissues (P<0.05). High ATP1B2 expression is closely associated
with poor prognosis in patients with ESCC and with nerve, vessel
and lymph node metastasis.
Change of ion flux can influence cell signal
transduction and cytoskeleton remodeling and support cell migration
(18). The activity of
Na+/K+-ATPase in tumor cells is greater than
that in normal cells, altering the concentration of ions inside and
outside the cells and promote cancer progression (19,20).
Cardiac glycosides can inhibit the activity of indoleamine pyrrole
2′,3′-dioxygenase1 in cancer cells by inhibiting the activity of
Na+/K+ ATPase to activate STAT1. These
findings provide insights into the immune mechanism of cancer
(21). The
Na+/K+-ATPase may serve as a potential target
for tumor therapy. ATP1B2 is overexpressed in glioma cell lines,
and knockdown of ATP1B2 inhibits cell migration and induces
apoptosis (17). Furthermore,
patients with high ATP1B2 expression in mononuclear cell lines are
more sensitive to the toxicity of marine polyether marsh (22). In the present study, ATP1B2 was
highly expressed in the ESCC cells, and ATP1B2 knockdown inhibited
cancer cell migration and induced apoptosis. Conversely, ATP1B2
overexpression promoted cell migration and inhibited apoptosis
(10). These findings align with
the observation that patients with ESCC with high ATP1B2 expression
are prone to lymph node metastasis and have a worse prognosis.
Therefore, ATP1B2 played a pivotal role in the invasion and
metastasis of ESCC.
The invasion and migration of cancer involve a
multifactor, multistep cascade reaction. Matrix-degrading protease
7 (MMP7) plays a crucial role in the degradation of the
extracellular matrix. Its expression is highly upregulated in ESCC
tissue and enhances cell migration (23). Similarly, MMP10 is overexpressed in
ESCC tissue (24). In the present
study, the expression of MMP7 and MMP10 in the experimental group
overexpressing ATP1B2 was higher than that in the empty-vector
control group. These results suggest that ATP1B2 may promote cell
migration by upregulating EMT-related genes. Wild-type P53
regulates the cell cycle through the transcription of multiple
factors, induces apoptosis of cancer cells, and prevents initiation
of cancer. P53 is a tumor suppressor gene, but the mutation rate in
ESCC cells can reach 96%, and its overexpression promotes cancer
progression with poor prognosis (25,26).
Dey et al (27) used
immunohistochemistry to detect the expression level of P53 in
cancer tissue and adjacent tissues of patients with ESCC. The
results showed that the expression level of P53 in squamous cell
carcinoma tissue was significantly higher than that in adjacent
tissues, and the expression level of P53 was directly proportional
to the size of the tumor. In the present study, it was found that
overexpression of ATP1B2 can inhibit the expression of P53 gene;
subsequent protein expression assays were also consistent with this
finding, which suggests that cell proliferation may be inhibited by
altering the expression of transcription genes in P53. After
knocking down ATP1B2, the expression of E-cadherin and P53 in
cancer cells increased, while the expression of N-cadherin
decreased, indicating that ATP1B2 may play a role in the signaling
pathway through EMT and P53. However, the detailed mechanisms
involved have not yet been thoroughly studied due to energy and
financial constraints.
As a member of the RAS family, RAC1 encodes GTPs.
When combined with GTP, ATP1B2 activates the downstream pathway of
RAC1 and plays an important role in regulating cell invasion,
migration and cytoskeleton (28).
In gastric cancer, RAC1 activation regulates tumor cell invasion
and migration (29). Overexpression
of RAC1 in ESCC is positively correlated with lymph node metastasis
and prognosis of patients with ESCC (30). In the current experiment, the
results of RT-qPCR further demonstrated that the overexpression of
ATP1B2 promoted the expression of the migration gene RAC1.
Furthermore, the corresponding protein expression levels were
consistent with this trend. These findings were consistent with the
scratch test results, in which ATP1B2 overexpression enhanced cell
migration.
The proliferation and migration effects of most
genes in tumor cells are the same. For instance, ATP1B3 knockdown
inhibited the proliferation and migration of gastric cancer cells
(31), and the knockdown of ATP1B2
inhibited the proliferation and migration of glioma cells and
promoted their apoptosis (32).
However, there are also cases where the opposite occurs. For
example, FXBO22 promoted cancer cell proliferation in primary
breast cancer but targeted SNAIL, a crucial regulator of EMT and
breast cancer metastasis, via glycogen synthase kinase 3β (33). Its migration was inhibited through
ubiquitin-mediated proteasome degradation in a
phosphorylation-dependent manner. Cheng et al (34) reported that the overexpression of
miR-210 inhibits proliferation in the ESCC EC109 cell line but
promotes cell migration and induced apoptosis. Hezova et al
(35) found that overexpressing
miR-205 in esophageal adenocarcinoma cells could restrict
proliferation and migration and induce apoptosis through EMT
process. However, the knockdown of miR-205 in ESCC inhibits cell
proliferation and migration and induces cell apoptosis by
regulating metalloproteinase 10.
In the present study, it was observed that the
overexpression of ATP1B2 enhanced cell migration and inhibited
apoptosis. Conversely, ATP1B2 knockdown decreased cell migration
and induced apoptosis, indicating that patients with ESCC are
susceptible to lymph node metastasis. However, in the proliferation
experiment, ATP1B2 knockdown promoted the proliferation of
well-differentiated ESCC EC109 cells, while ATP1B2 overexpression
inhibited the proliferation of poorly differentiated ESCC KYSE150
cells. Consequently, ATP1B2 exhibited varying proliferative
capacities in different ESCC cells. This result was further
confirmed through a plate cloning experiment, which aligned with
the MTT results.
ATP1B2 had different effects on cell proliferation
in highly differentiated ESCC cell line EC109 and the poorly
differentiated ESCC cell line KYSE150. First, only in vitro
cell experiments have been completed; the specific characteristics
of the two ESCC cells have not been fully elucidated. The cell
characteristics and involvement of other genes may have contributed
to the differential proliferative rates of ATP1B2 in the ESCC cells
EC109 and KYSE150.
However, the exact underlying mechanism remains to
be further explored. Second, gene transfer regulates numerous
signal pathways post-translationally. The activation factors differ
in the ESCC cells with varying degrees of differentiation and exert
diverse effects on proliferation. Moreover, these dual effects
illustrate the diversity of multifunctional transfer factors
involved in regulating the onset and progression of cancer,
potentially explaining the complexity of ESCC diagnosis and
treatment.
The focus of the present study was to investigate
the potential therapeutic effects of ouabain, a specific inhibitor
of Na+/K+-ATPase, on various medical
conditions, including coronary heart disease, hypertension,
neuritis, viral infections and cancer (36,37).
Previous findings revealed that ouabain can inhibit the
proliferation of multiple tumor cells, such as those of breast
cancer, lung cancer, prostate cancer, colon cancer, glioma and
leukemia (38,39). Research at Moscow State University
observed that the combination of ouabain with
Na+/K+-ATPase could inhibit cell function and
cause cell death (40).
Additionally, low-concentration ouabain combined with
Na+/K+-ATPase has no effect on cell survival
(41) but could change the
conformation of subunits of Na+/K+-ATPase and
activate multiple signal pathways (37).
Furthermore, it was found that ouabain in
combination with Na+/K+-ATPase enhanced the
phosphorylation of Tyr10 stimulated by EGF in adrenal epithelial
cells. It also increased the ADP:ATP ratio while inhibiting the
production of lactic acid and the oxygen consumption rate in a
dose- and time-dependent manner (42). Ouabain is a specific inhibitor of
Na+/K+-ATPase, which exerts cardiotonic
effect by inhibiting the efflux of Na+ and indirectly
increasing the concentration of intracellular Ca2+.
Ouabain is a Na-K-ATPase inhibitor but not a specific inhibitor of
ATP1B2. Ouabain was shown to reduce the viability of human
osteosarcoma cancer cells, induce cell chromosome aggregation,
cause DNA fragmentation damage, and promote cell apoptosis
(43). Specifically, in the context
of study, our research demonstrated that ouabain significantly
enhanced the inhibitory effect on the proliferation of ESCC cell
lines overexpressing ATP1B2. Flow cytometry revealed that at a
concentration of 60 µl/l, ouabain promoted cell cycle arrest and
G1/S phase. Therefore, ouabain could inhibit cell
proliferation and migration and promote cell cycle arrest in ESCC
cells overexpressing ATP1B2. However, the impact of ATP1B2 on the
invasive ability of ESCC cells in the present study was indirectly
validated through experiments, and no in vivo experiments
were conducted. The detailed mechanism is currently unclear and
further research is needed.
A significant association was also discovered
between the overexpression of ATP1B2 in ESCC tissues and cell
lines, lymph node metastasis and prognosis. The overexpression of
ATP1B2 promoted cell migration while inhibiting cell apoptosis.
Conversely, ATP1B2 knockdown inhibited cell migration and promoted
cell apoptosis. Interestingly, ouabain significantly inhibit the
proliferation and migration of ESCC cells overexpressing ATP1B2 and
induced cell cycle arrest at the G1/S phase. These
findings may contribute to the development of novel ATP1B2-targeted
anticancer drugs for ESCC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Health Commission of
Hubei scientific research project (grant nos. WJ2021M046 and
WJ2023Q022), the Shiyan City Science and Technology Bureau Guiding
Research Project (grant no. 21Y19), the Foundation of Taihe
Hospital (grant no. 2020JJXM032) and the Free Exploration Fund
Project of Hubei University of Medicine (grant no. FDFR201904).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
QT and XBL conceived and designed the project. FFL
and HW conducted the majority of the experiments. FFL and HW
confirm the authenticity of all the raw data. FFL, SBL and SJ were
responsible for the animal experiments and part of molecular
biology experiment. ZYG contributed to data extraction and data
analysis. FFL and XBL wrote the manuscript, SBL, XBL and QT
approved and submitted the final manuscript. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
It was decided to start a cohort study on esophageal
cancer in the northwest region of Hubei in 2016. When applying for
this project, it had already been approved by the Ethics Committee
of Taihe Hospital (approval no. 2016KS001;). Since then, our
research center has been collecting esophageal samples and
gradually conducting related research. The study protocol was
reviewed and approved by the Taihe Hospital Ethics Committee
(approval no. 2022KS038), and all patients received information
concerning their participation in the study and provided written
informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen L, Jiang P, Li J, Xie Z, Xu Y, Qu W,
Feng F and Liu W: Periplocin promotes wound healing through the
activation of Src/ERK and PI3K/Akt pathways mediated by
Na/K-ATPase. Phytomedicine. 57:72–83. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Felippe Goncalves-De-Albuquerque C,
Ribeiro Silva A, Ignacio Da Silva C, Caire Castro-Faria-Neto H and
Burth P: Na/K pump and beyond: Na/K-ATPase as a modulator of
apoptosis and autophagy. Molecules. 22:5782017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bejček J, Spiwok V, Kmoníčková E and
Rimpelová S: Na+/K+-ATPase revisited: On its mechanism of action,
role in cancer, and activity modulation. Molecules. 26:19052021.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hilbers F, Kopec W, Isaksen TJ, Holm TH,
Lykke-Hartmann K, Nissen P, Khandelia H and Poulsen H: Tuning of
the Na,K-ATPase by the beta subunit. Sci Rep. 6:204422016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rotoli D, Cejas MM, Maeso MD,
Pérez-Rodríguez ND, Morales M, Ávila J, Mobasheri A and
Martín-Vasallo P: The Na, K-ATPase β-subunit isoforms expression in
glioblastoma multiforme: Moonlighting roles. Int J Mol Sci.
18:23692017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Banerjee M, Cui X, Li Z, Yu H, Cai L, Jia
X, He D, Wang C, Gao T and Xie Z: Na/K-ATPase Y260
Phosphorylation-mediated src regulation in control of aerobic
glycolysis and tumor growth. Sci Rep. 8:123222018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Busonero C, Leone S, Bianchi F, Maspero E,
Fiocchetti M, Palumbo O, Cipolletti M, Bartoloni S and Acconcia F:
Ouabain and digoxin activate the proteasome and the degradation of
the ERα in cells modeling primary and metastatic breast cancer.
Cancers (Basel). 12:38402020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Du J, Jiang L, Chen F, Hu H and Zhou M:
Cardiac glycoside ouabain exerts anticancer activity via
downregulation of STAT3. Front Oncol. 11:6843162021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang Y, Wu C, Qin Y, Liu S and Zhang R:
Multi-angle investigation of the fractal characteristics of
nanoscale pores in the lower cambrian niutitang shale and their
implications for CH4 adsorption. J Nanosci Nanotechnol.
21:156–167. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li S, Dai Z, Yang D, Li W, Dai H, Sun B,
Liu X, Xie X, Xu R and Zhao X: Targeting β2 subunit of
Na+/K+-ATPase induces glioblastoma cell apoptosis through elevation
of intracellular Ca2. Am J Cancer Res. 9:1293–1308. 2019.PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pfaffl MW: A new mathematical model for
relative quantification in real-time RT-PCR. Nucleic Acids Res.
29:e452001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li J: Digestive cancer incidence and
mortality among young adults worldwide in 2020: A population-based
study. World J Gastrointest Oncol. 14:278–294. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu SG, Zhang WW, Sun JY, Li FY, Lin Q and
He ZY: Patterns of distant metastasis between histological types in
esophageal cancer. Front Oncol. 8:3022018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu C, Wang Z, Song X, Feng XS, Abnet CC,
He J, Hu N, Zuo XB, Tan W, Zhan Q, et al: Joint analysis of three
genome-wide association studies of esophageal squamous cell
carcinoma in Chinese populations. Nat Genet. 46:1001–1006. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Singhi AD, Wood LD, Parks E, Torbenson MS,
Felsenstein M, Hruban RH, Nikiforova MN, Wald AI, Kaya C, Nikiforov
YE, et al: Recurrent rearrangements in PRKACA and PRKACB in
intraductal oncocytic papillary neoplasms of the pancreas and bile
duct. Gastroenterology. 158:573–582.e2. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang M, James AD, Suman R, Kasprowicz R,
Nelson M, O'Toole PJ and Brackenbury WJ: Voltage-dependent
activation of Rac1 by Nav 1.5 channels promotes cell migration. J
Cell Physiol. 235:3950–3972. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Leslie TK, James AD, Zaccagna F, Grist JT,
Deen S, Kennerley A, Riemer F, Kaggie JD, Gallagher FA, Gilbert FJ
and Brackenbury WJ: Sodium homeostasis in the tumour
microenvironment. Biochim Biophys Acta Rev Cancer. 1872:1883042019.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Capatina AL, Lagos D and Brackenbury WJ:
Targeting ion channels for cancer treatment: Current progress and
future challenges. Rev Physiol Biochem Pharmacol. 183:1–43. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shandell MA, Capatina AL, Lawrence SM,
Brackenbury WJ and Lagos D: Inhibition of the Na+/K+-ATPase by
cardiac glycosides suppresses expression of the IDO1 immune
checkpoint in cancer cells by reducing STAT1 activation. J Biol
Chem. 298:1017072022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pelin M, Stocco G, Florio C, Sosa S and
Tubaro A: In vitro cell sensitivity to palytoxin correlates with
high gene expression of the Na+/K+-ATPase β2 subunit isoform. Int J
Mol Sci. 21:58332020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu YW, Peng YH, Chen B, Wu ZY, Wu JY, Shen
JH, Zheng CP, Wang SH, Guo HP, Li EM and Xu LY: Autoantibodies as
potential biomarkers for the early detection of esophageal squamous
cell carcinoma. Am J Gastroenterol. 109:36–45. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ardalan Khales S, Abbaszadegan MR, Majd A
and Forghanifard MM: TWIST1 upregulates matrix metalloproteinase
(MMP) genes family in esophageal squamous carcinoma cells. Gene
Expr Patterns. 37:1191272020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yao L, Zhong X, Huang G, Ma Q, Xu L, Xiao
H and Guo X: Investigation on the potential correlation between
TP53 and esophageal cancer. Front Cell Dev Biol. 9:7303372021.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang N, Shi J, Shi X, Chen W and Liu J:
Mutational characterization and potential prognostic biomarkers of
Chinese patients with esophageal squamous cell carcinoma.
OncoTargets Ther. 13:12797–12809. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dey B, Raphael V, Khonglah Y and Lynrah
KG: Immunohistochemical analysis of P53 and PRB in esophageal
squamous cell carcinoma. J Clin Diagn Res. 8:FC01–FC03.
2014.PubMed/NCBI
|
|
28
|
Wang C, Yan G, Zhang Y, Jia X and Bu P:
Long non-coding RNA MEG3 suppresses migration and invasion of
thyroid carcinoma by targeting of Rac1. Neoplasma. 62:541–549.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim HJ, Ryu KJ, Kim M, Kim T, Kim SH, Han
H, Kim H, Hong KS, Song CY, Choi Y, et al: RhoGDI2-mediated Rac1
recruitment to filamin a enhances Rac1 activity and promotes
invasive abilities of gastric cancer cells. Cancers (Basel).
14:2552022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang Q, Luo GY, Li Y, Shan HB, Wang HY and
Xu GL: Expression of Rac-1 related to tumor depth, lymph node
metastasis and patient prognosis in esophageal squamous cell
carcinoma. Med Oncol. 30:6892013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li L, Feng R, Xu Q, Zhang F, Liu T, Cao J
and Fei S: Expression of the β3 subunit of Na+/K+-ATPase is
increased in gastric cancer and regulates gastric cancer cell
progression and prognosis via the PI3/AKT pathway. Oncotarget.
8:84285–84299. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sun MZ, Kim JM, Oh MC, Safaee M, Kaur G,
Clark AJ, Bloch O, Ivan ME, Kaur R, Oh T, et al: Na+/K+-ATPase
β2-subunit (AMOG) expression abrogates invasion of
glioblastoma-derived brain tumor-initiating cells. Neuro Oncol.
15:1518–1531. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sun R, Xie HY, Qian JX, Huang YN, Yang F,
Zhang FL, Shao ZM and Li DQ: FBXO22 possesses both protumorigenic
and antimetastatic roles in breast cancer progression. Cancer Res.
78:5274–5286. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cheng Z, Geng H, Cheng Y, Dong N, Ning F,
Yu Z, Jian J and Chen S: Effects of MiR-210 on proliferation,
apoptosis and invasion abilities of esophageal cancer cells. J
BUON. 23:814–819. 2018.PubMed/NCBI
|
|
35
|
Hezova R, Kovarikova A, Srovnal J,
Zemanova M, Harustiak T, Ehrmann J, Hajduch M, Sachlova M, Svoboda
M and Slaby O: MiR-205 functions as a tumor suppressor in
adenocarcinoma and an oncogene in squamous cell carcinoma of
esophagus. Tumour Biol. 37:8007–8018. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Botelho AFM, Pierezan F, Soto-Blanco B and
Melo MM: A review of cardiac glycosides: Structure, toxicokinetics,
clinical signs, diagnosis and antineoplastic potential. Toxicon.
158:63–68. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tverskoi AM, Poluektov YM, Klimanova EA,
Mitkevich VA, Makarov AA, Orlov SN, Petrushanko IY and Lopina OD:
Depth of the steroid core location determines the mode of
Na,K-ATPase inhibition by cardiotonic steroids. Int J Mol Sci.
22:132682021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Rupaimoole R, Yoon B, Zhang WC, Adams BD
and Slack FJ: A High-throughput small molecule screen identifies
ouabain as synergistic with miR-34a in killing lung cancer cells.
iScience. 23:1008782020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chang YM, Shih YL, Chen CP, Liu KL, Lee
MH, Lee MZ, Hou HT, Huang HC, Lu HF, Peng SF, et al: Ouabain
induces apoptotic cell death in human prostate DU 145 cancer cells
through DNA damage and TRAIL pathways. Environ Toxicol.
34:1329–1339. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Klimanova EA, Fedorov DA, Sidorenko SV,
Abramicheva PA, Lopina OD and Orlov SN: Ouabain and
marinobufagenin: Physiological effects on human epithelial and
endothelial cells. Biochemistry (Mosc). 85:507–515. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Akimova OA, Tverskoi AM, Smolyaninova LV,
Mongin AA, Lopina OD, La J, Dulin NO and Orlov SN: Critical role of
the α1-Na(+), K(+)-ATPase subunit in insensitivity of rodent cells
to cytotoxic action of ouabain. Apoptosis. 20:1200–1110. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Petrič M, Vidović A, Dolinar K, Miš K,
Chibalin AV and Pirkmajer S: Phosphorylation of Na+,K+-ATPase at
Tyr10 of the α1-Subunit is suppressed by AMPK and enhanced by
ouabain in cultured kidney cells. J Membr Biol. 254:531–548. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yang JL, Yang MD, Chen JC, Lu KW, Huang
YP, Peng SF, Chueh FS, Liu KC, Lin TS, Chen PY and Chen WJ: Ouabain
induces DNA damage in human osteosarcoma U-2 OS cells and alters
the expression of DNA damage and DNA Repair-associated proteins. In
Vivo. 35:2687–2696. 2021. View Article : Google Scholar : PubMed/NCBI
|